Abstract
Background
Postoperative air leaks are a common complication after lung surgery. They are associated with prolonged hospital stay, increased postoperative pain and treatment costs. The treatment of prolonged air leaks remains controversial. Several treatments have been proposed including different types of sealants, chemical pleurodesis, or early surgical intervention. The aim of this review was to analyze the impact of autologous blood pleurodesis in a systematic way.
Methods
A systematic review of the literature was conducted until July 2020. Studies with more than five adult patients undergoing lung resections were included. Studies in patients receiving blood pleurodesis for pneumothorax were excluded. The search strategy included proper combinations of the MeSH terms “air leak”, “blood transfusion” and “lung surgery”.
Results
Ten studies with a total of 198 patients were included in the analysis. The pooled success rate for sealing the air leak within 48 h of the blood pleurodesis was 83.7% (95% CI: 75.7; 90.3). The pooled incidence of the post‐interventional empyema was 1.5%, with a pooled incidence of post‐interventional fever of 8.6%.
Conclusions
Current evidence supports the idea that autologous blood pleurodesis leads to a faster healing of postoperative air leaks than conservative treatment. The complication rate is very low. Formal recommendations on how to perform the procedure are not possible with the current evidence. A randomized controlled trial in the modern era is necessary to confirm the benefits.
Keywords: blood patch, lobectomy, lung surgery, pleurodesis, prolonged air leak
A systematic literature review was conducted until July 2020 to detect the success rate of autologous blood pleurodesis in healing prolonged air leaks. Ten studies with a total of 198 patients were included in the meta‐analysis. The pooled success rate for sealing the air leak within 48 h of the blood pleurodesis was 83.7% (95% CI: 75.7; 90.3). The pooled incidence of the post‐interventional empyema was 1.5%.

INTRODUCTION
Postoperative air leak is one of the most common complications following lung surgery. Most patients undergoing a lung resection leave the operating theater with an air leak, which usually resolves within the first 24 h while 10%–20% of patients develop a prolonged air leak. 1 However the definition of what exactly constitutes a prolonged air leak varies; therefore, we do not really know what the true incidence is for this complication.
Based on the fact that the majority of air leaks will eventually heal spontaneously, there is no widely accepted algorithm on when and how to manage this complication. Prolonged air leaks are associated with increased postoperative pain, increased hospital stay, and an increase in other complications. Furthermore, air leaks significantly increase treatment costs. 1 , 2
It is not surprising that the definition of a prolonged air leak has changed. In the late 1990s an air leak would become a problem after the seventh postoperative day or even later than that 3 , 4 because this was around the time when the patient would be discharged home. Most recent publications focus on the treatment of postoperative air leaks after the fifth postoperative day. 5 , 6 Taking into consideration that nowadays the average length of hospital stay is under five days for an uncomplicated lobectomy, and that around 30% of the patients leave the hospital within the first three postoperative days, 7 using five days to define a prolonged air leak maybe outdated.
Several treatment options have been proposed for postoperative air leaks. Chemical pleurodesis with different agents such as talc, tetracyclines or iodine, prolonged chest tube drainage using a Heimlich valve system, insertion of endobronchial valves, or surgical intervention have been previously described. 8 , 9 , 10
Autologous blood pleurodesis (ABP) was introduced 33 years ago as a treatment option in patients with pneumothorax. 11 Several theories have tried to explain the mechanism of action; one suggested that blood initiates an intrapleural inflammatory reaction leading to pleurodesis, while another theory supported the idea of the leaking airway being directly sealed by the blood. 12 The first study in patients with postoperative air leaks after lobectomies was published almost 30 years ago. Two patients were treated successfully with ABP as a “last resort” therapy for persisting air leaks. 13 Since then, several studies have followed in this field.
The purpose of this meta‐analysis was to examine the role of autologous blood pleurodesis in patients after thoracic surgery and define the efficacy of the method and rate of postoperative complications.
METHODS
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 14 The objective of the analysis was to determine in patients following thoracic surgery with a prolonged air leak whether autologous blood pleurodesis leads to an earlier sealing of the air leaks, and the incidence of post‐interventional complications.
All studies reporting results on patients treated with autologous blood pleurodesis for postoperative air leaks were included. Only studies with more than five patients were included. This limit was introduced in order to exclude case reports and case series with a very small patient number. Reviews, technical trials reports, studies in pediatric patients and conference abstracts of unpublished data were excluded. Studies on patients undergoing autologous blood pleurodesis for pneumothorax were also not included. The reason for the exclusion of studies on pneumothorax patients was that they probably represent a different etiology of air leak.
Systematic literature research
A computer‐based literature search was performed (MG) up until the July 30, 2020 in the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews (CDSR) from The Cochrane Library, MEDLINE (1966 to present), Cinahl (1981 to present) and Web of Science (1945 to present). Reference lists of retrieved articles were scanned for further eligible trials (backward search) and citations of identified trials were checked for inclusion (forward search). Search strategies included proper combinations of the MeSH terms “lung/surgery”, “blood transfusion/autologous/autotransfusion/blood patch”, “air leak” and “pleurodesis”. The search was not limited by publication type and there were no restrictions on language. The review was registered in the PROSPERO registry for systematic reviews and meta‐analyses (CRD42021223388). The exact search strategy is available in the registry.
Two reviewers (IK and CG) independently performed the extraction of the data from the included studies. Data were extracted for following outcomes: volume of blood which was used for the pleurodesis, the way of blood application, the time point of the blood application, the handling of the drain after the pleurodesis, the time point of the cessation of the air leak, the side effects of the pleurodesis and the way the side effects were dealt with. No automatic data extraction tools were used in this study. The findings of the two independent reviewers were controlled for concordance. Disagreements were resolved with discussion and intense analysis of the trials and the data. In order to provide and analyze as much data as possible, original authors were contacted via e‐mail. In case no valid email address was available, a thorough web‐based search and contact was attempted. Only reported data were used in the analysis. No assumptions were made for missing/unclear information.
Statistical analysis
The endpoint of the analysis was when the postoperative air leak ceased. In order to consider the pleurodesis as successful, the air leak had to stop within 48 h after the intervention. The time point of 48 h after the pleurodesis was chosen for two reasons; first, it was a time point that was either directly reported or could be extracted in all the studies that we included. Second, it is clinically reasonable to expect the pleurodesis to work within 48 h.
The success rate with 95% CI per study and pooled analysis are shown in the forest plot. The heterogeneity of studies was calculated using the I2 index. The random effects model was used for analysis of pooled data that takes heterogeneity between the studies into account. The weighting of the studies, according to DerSimonian & Laird of the random model, is presented in the forest plot.
The publication bias analysis was visualized in a funnel plot and examined with the Egger's test. A subgroup analysis was performed with studies only including patients that underwent lung resections (six studies). The correlation between the success event and autologous blood pleurodesis, and the time of blood pleurodesis was examined with Spearman's correlation coefficient. Statistical analyses were completed using MedCalc software (version 19.6). A p‐value of <0.05 was considered statistically significant.
Risk of bias assessment
A risk of bias assessment was performed by two independent reviewers (IK and AA) for studies included in the review. The revised Cochrane risk‐of‐bias toll for randomized trials (RoB 2) was used for the assessment of the randomized controlled trials. 15 For the nonrandomized studies, we used the ROBINS‐I tool for assessment of the risk of bias in nonrandomized interventional studies. 16
RESULTS
The database search provided 258 references (Figure 1). After removing duplicates, 245 references were available for reviewing. Two independent reviewers (IK and CG) additionally hand‐searched the references to assess the publications for eligibility.
FIGURE 1.
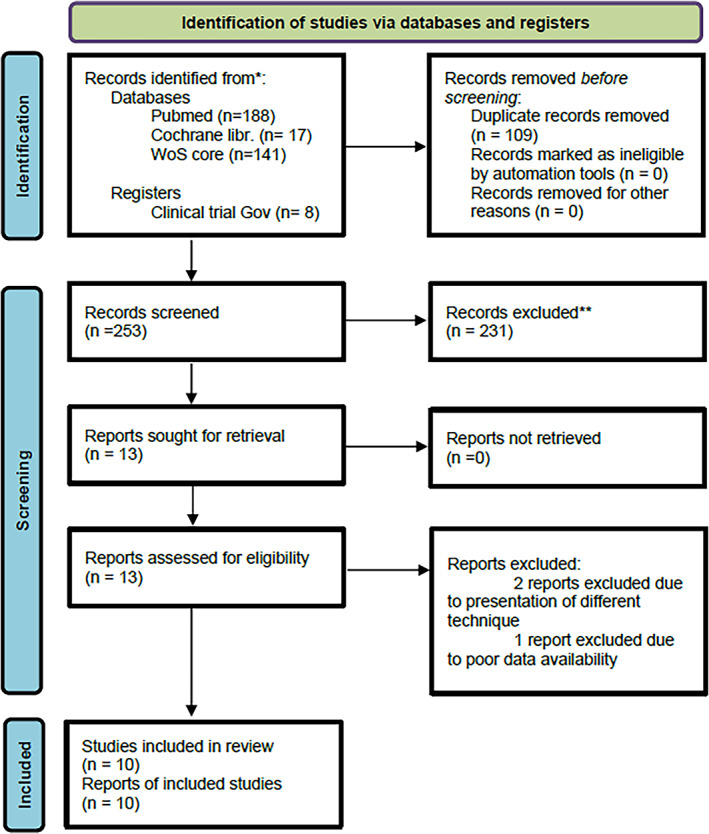
PRISMA flowchart
The citations of the initially included references were also hand‐screened in order to further identify relevant publications, through which eight additional references were found. A total of 253 abstracts and 30 full‐text articles were assessed for eligibility. Ten articles were ultimately included in the analysis (Table 1).
TABLE 1.
Included studies
| N | Author | Year | Journal | Study type |
|---|---|---|---|---|
| 1 | Yokomise | 1998 | Ann Thor Surg | Retrospective |
| 2 | Rivas de Andres | 2000 | Ann Thor Surg | Retrospective |
| 3 | Lang‐Lazdunski | 2004 | Eur J Cardiothorac Surg | Retrospective |
| 4 | Droghetti | 2006 | General Thoracic Surg | Retrospective |
| 5 | Shackcloth | 2006 | Ann Thor Surg | RCT |
| 6 | Andreetti | 2007 | General Thoracic Surg | Prospective |
| 7 | Athanassiadi | 2009 | J Thorac Cardiovasc Surg | Retrospective |
| 8 | Oliveira | 2010 | Respiration | Retrospective |
| 9 | Korasidis | 2010 | Interact Cardiovasc Thorac Surg | Retrospective |
| 10 | Dye | 2020 | Cureus | Retrospective |
We had initially included 13 studies for analysis. Two of the studies were excluded because we considered the intraoperative blood pleurodesis as a different treatment technique than the postoperative one. 17 , 18 The third study was excluded because of poor data availability. 19 In the final analysis 10 studies were therefore included.
Six of the included studies only analyzed patients who underwent lung resections and underwent an autologous blood pleurodesis for a postoperative air leak. 3 , 4 , 5 , 20 , 21 , 22 The other four studies included both patients with lung resections and patients who underwent surgery for pneumothorax, emphysema or empyema.
The 10 studies included a total of 198 patients. The timing of the blood pleurodesis in relation to the postoperative day that the procedure was performed is presented in Table 2. The amount of blood used for the pleurodesis varied between 45 and 250 ml. One study randomized patients to blood pleurodesis with 50 or 100 ml of blood and concluded that the second group had a significantly shorter drainage time. 21 In nine studies the blood was directly introduced through the pleural drain, and in one study a catheter was inserted through the pleural drain in order to allow a more targeted blood application. 23 Two studies only included patients with a confirmed “dead space” on the chest x‐ray, 3 , 22 and in a third study most patients had a “dead space”. 24 In two other studies the authors did not perform blood pleurodesis in patients with a “dead space”. 21 , 25 Four studies reported that more than one application of blood was necessary in some cases, 22 , 23 , 24 with one study reporting up to four applications in one patient. 6
TABLE 2.
Characteristics of the intervention
| Author | Patient number | Time of ABP (days) | Blood volume (ml) | Complications |
|---|---|---|---|---|
| Yokomise | 10 | 8.7 ± 4.7 | 50 | 5 fever |
| Rivas de Andres | 6 | 16.3 (mean) | 50–250 | No complications |
| Lang‐Lazdunski | 11 | 7.8 ± 2 | 50 | 2 fever + 1 pneumonia |
| Droghetti | 21 | 11 (mean) | 50–150 | 1 low grade fever |
| Shackcloth | 20 | 5–7‐9 | 120 | 2 fever, 1 empyema |
| Andreetti | 25 | 6 (mean) | 50 vs 100 | No complications |
| Athanassiadi | 20 | >7 | 60 | No complications |
| Oliveira | 27 | 10.6 (mean) | 92 | 1 fever, 1 empyema |
| Korasidis | 39 | n.a. | 100 | 6 mild fever |
| Dye | 19 | 7 (mean) | 45–120 | 1 empyema |
Regarding the handling of the pleural drain after completing the pleurodesis, five studies described raising the drain above the level of the patient, two authors raised the drain above the level of the patient and removed the suction, one study reported keeping the suction while raising the drain, and in another study the drain was clamped for 30 min and was then connected with an underwater seal. It is worth mentioning that in the last study all patients had a second drain which was left unclamped without suction. 25
In five studies the blood was introduced through the pleural drain without any processing, in one study the blood was mixed with OK432 (picibanil) 3 and in another study a pneumoperitoneum was performed one day before the pleurodesis. 22
Three studies reported no complications following the pleurodesis, another three studies reported one case of empyema each and another five studies reported a total of 17 patients that developed fever after the procedure, two of which had positive pleural cultures. The overall pooled incidence of empyema in this analysis was 1.5% with an incidence of post‐interventional fever of 8.6%.
The pooled success rate of the blood pleurodesis for stopping the air leak within 48 h after the intervention for all studies that were included was 83.7% (95% CI: 75.7; 90.3, Figure 2). Publication bias was assessed using the Egger's test (p = 0.825).
FIGURE 2.
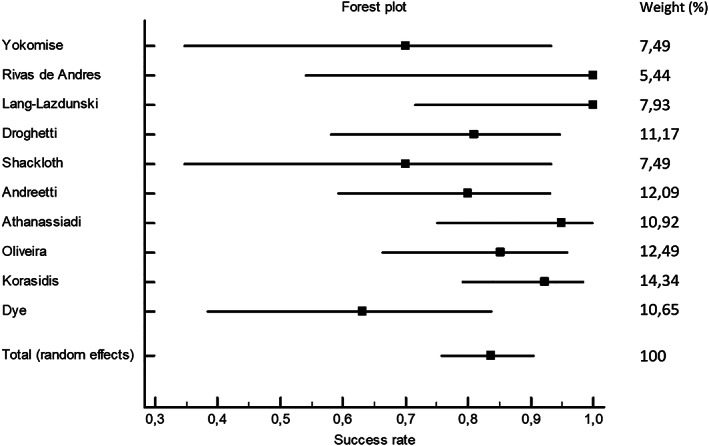
Forest plot for pooled success rate for all included studies
A second analysis was performed with only the six studies that included patients undergoing lung resections. The pooled success rate of the blood pleurodesis for stopping the air leak within 48 h after the intervention was 85.7% (95% CI: 74.4; 94.0). Publication bias was also assessed using the Egger's test (p = 0.829, Figure 3). The study by Rivas de Andres and Lang‐Lazdunski showed the same success rate, which is why there are five points in the funnel plot.
FIGURE 3.
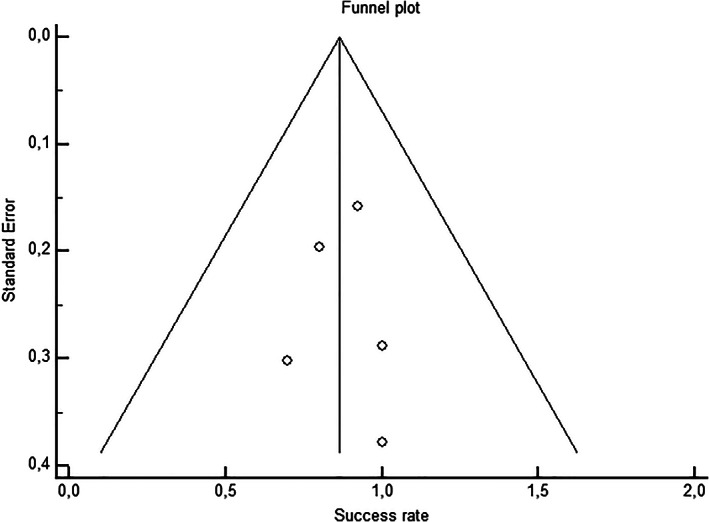
Symmetrical funnel plot showing publication bias assessment in the six studies with lung resections
Pearson's coefficient was calculated to detect a potential correlation between the amount of blood used for the pleurodesis and the success of the pleurodesis. No correlation could be detected (r = 0.049, p = 0.893).
Risk of bias assessment
The RoB 2 tool was used for the assessment of the randomized controlled trials and the ROBINS‐I tool for the nonrandomized interventional studies. The overall risk of bias was classified as moderate for one study, 20 high for one study, 5 serious for three studies 6 , 21 , 22 , 24 , 26 and critical for three studies. 3 , 4 , 23
DISCUSSION
This study showed that the success rate of autologous blood pleurodesis in sealing postoperative air leaks within 48 h was 83.7% for all the included studies and 85.7% for the studies on patients undergoing lung resections. The pooled empyema rate after the procedure was 1.5% with an incidence of post‐interventional fever of 8.6%.
Dissecting the lung tissue to expose the pulmonary artery branches will inevitably lead to postoperative air leaks. Several techniques have been developed in order to reduce the incidence of air leaks. High quality evidence suggests that the use of surgical sealants can reduce the time to drain removal and seal the air leaks faster, but does not decrease the hospital stay. 27 Fissure‐last surgery is certainly helpful in avoiding air leaks but it is not always technically possible. 28
The solution to this problem is probably the combination of atraumatic surgery, use of tissue sealants when indicated and having a strategy to deal with air leaks effectively in the postoperative period.
Autologous blood pleurodesis is a inexpensive and effective method of treating postoperative air leaks. The blood can be obtained easily, it is not considered a transfusion, does not require specific processing and has a very low peri‐interventional complication rate.
It is clear that we cannot predict exactly the natural course of each air leak, and it is difficult to suggest that the air leaks would have not healed without the intervention within the same time frame. Looking into the time point that the blood pleurodesis was performed in each study, the air leak had been present in most patients for over a week prior to the pleurodesis. In three studies the air leak was evident for almost two weeks before proceeding with the pleurodesis. Furthermore, in two studies, other measures such as tetracycline‐based pleurodesis or other forms of chemical pleurodesis had been used unsuccessfully before the blood pleurodesis. Hence, it is realistic to hypothesize that the blood pleurodesis successfully sealed the air leak in about 85% of the patients with prolonged or very prolonged air leaks.
The widespread of minimally invasive surgery, the enhanced recovery after surgery concepts and modern anesthesiology have significantly reduced the hospital stay of patients undergoing lung resections. It is therefore reasonable to suggest that the definition of a prolonged air leak should be revised. Every air leak that prevents the patient from being discharged from hospital could be a possible definition. Furthermore, the time‐point of dealing with a postoperative air leak has to be adapted accordingly since dealing with an air leak two weeks after a lobectomy is undoubtedly too late.
This meta‐analysis has several limitations. Despite the low heterogeneity of the I2 test in the included studies and the consideration of between‐study variance in the statistics, most studies represent retrospective analyses without a control group and with relevant bias. The ROBINS‐1 tool was chosen to evaluate the risk of bias for the nonrandomized trials. This tool was primarily designed to evaluate prospective nonrandomized interventional studies. However, there is no widely accepted tool to assess risk of bias in retrospective studies.
The way the blood pleurodesis was performed, the time point of the intervention, the primary operation, surgical approach and several other factors were different among the included studies which limited the generalizability of the results. As mentioned above, the time point to assess the efficacy of the pleurodesis was set at 48 h following the intervention. It is clear that this time point makes sense from the surgical point of view. However, it was chosen because it was also documented in all the included studies, which is another potential source of bias.
In conclusion, autologous blood pleurodesis appears to be an effective method to treat postoperative air leaks with a low complication rate. The current evidence is outdated for several reasons; first, air leak classification has changed since the introduction of electronic suction systems. Second, postoperative hospital stay has decreased significantly during the last decades and finally, the way the surgery itself is performed has changed. Open lobectomies are rarely required and lobar and sublobar resections through a minimally invasive approach are now the gold standard. The only randomized study in this analysis reported a significantly reduced time to drain removal and hospital stay in patients that underwent blood pleurodesis for postoperative air leaks compared with the control group. New up‐to‐date evidence is required in this field in order to clarify the role of autologous blood pleurodesis in modern thoracic surgery.
CONFLICT OF INTEREST
All authors have completed the ICMJE uniform disclosure form. The authors have no conflicts of interest to declare. EDR has no competing interests to declare.
ACKNOWLEDGMENT
Open Access funding was enabled and organized by Projekt DEAL.
Karampinis I, Galata C, Arani A, Grilli M, Hetjens S, Shackcloth M, et al. Autologous blood pleurodesis for the treatment of postoperative air leaks. A systematic review and meta‐analysis. Thorac Cancer. 2021;12:2648–2654. 10.1111/1759-7714.14138
The study has been registered in the PROSPERO registry for systematic reviews and meta‐analyses (CRD42021223388).
REFERENCES
- 1. Varela G, Jiménez MF, Novoa N, Aranda JL. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg. 2005;27:329–33. [DOI] [PubMed] [Google Scholar]
- 2. Yoo A, Ghosh SK, Danker W, Kassis E, Kalsekar I. Burden of air leak complications in thoracic surgery estimated using a national hospital billing database. Clin Outcomes Res. 2017;9:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yokomise H, Satoh K, Ohno N, Tamura K. Autoblood plus OK432 pleurodesis with open drainage for persistent air leak after lobectomy. Ann Thorac Surg. 1998;65:563–5. [DOI] [PubMed] [Google Scholar]
- 4. Rivas de Andrés JJ, Blanco S, de la Torre M. Postsurgical pleurodesis with autologous blood in patients with persistent air leak. Ann Thorac Surg. 2000;70:270–2. [DOI] [PubMed] [Google Scholar]
- 5. Shackcloth MJ, Poullis M, Jackson M, Soorae A, Page RD. Intrapleural instillation of autologous blood in the treatment of prolonged air leak after lobectomy: a prospective randomized controlled trial. Ann Thorac Surg. 2006;82:1052–6. [DOI] [PubMed] [Google Scholar]
- 6. Oliveira FH, Cataneo DC, Ruiz RL Jr, Cataneo AJ. Persistent pleuropulmonary air leak treated with autologous blood: results from a university hospital and review of literature. Respiration. 2010;79:302–6. [DOI] [PubMed] [Google Scholar]
- 7. Giambrone GP, Smith MC, Wu X, Gaber‐Baylis LK, Bhat AU, Zabih R, et al. Variability in length of stay after uncomplicated pulmonary lobectomy: is length of stay a quality metric or a patient metric?†. Eur J Cardiothorac Surg. 2016;49:e65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jabłoński S, Kordiak J, Wcisło S, Terlecki A, Misiak P, Santorek‐Strumiłło E, et al. Outcome of pleurodesis using different agents in management prolonged air leakage following lung resection. Clin Respir J. 2018;12:183–92. [DOI] [PubMed] [Google Scholar]
- 9. Travaline JM, McKenna RJ Jr, De Giacomo T, Venuta F, Hazelrigg SR, Boomer M, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest. 2009;136:355–60. [DOI] [PubMed] [Google Scholar]
- 10. Stamenovic D, Messerschmidt A, Steger V, Schneider T. New method in treatment of post‐operative air leakage with fresh frozen plasma. ANZ J Surg. 2020;90:144–9. [DOI] [PubMed] [Google Scholar]
- 11. Robinson CL. Autologous blood for pleurodesis in recurrent and chronic spontaneous pneumothorax. Can J Surg. 1987;30:428–9. [PubMed] [Google Scholar]
- 12. Rinaldi S, Felton T, Bentley A. Blood pleurodesis for the medical management of pneumothorax. Thorax. 2009;64:258–60. [DOI] [PubMed] [Google Scholar]
- 13. Dumire R, Crabbe MM, Mappin FG, Fontenelle LJ. Autologous “blood patch” pleurodesis for persistent pulmonary air leak. Chest. 1992;101:64–6. [DOI] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 16. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Catalyurek H, Silistreli E, Hepaguslar H, Kargi A, Açikel U. The role of autologous blood injection on postoperative air leak at lung resections. J Cardiovasc Surg (Torino). 2002;43:135–7. [PubMed] [Google Scholar]
- 18. Pagan V, Fontana P, Zaccaria A, Lo Giudice F, Ferronato A, Salvi R, et al. Intraoperative identification and effective “blood patch” prevention of persistent air leak in lung resections. Chir Ital. 2006;58:413–21. [PubMed] [Google Scholar]
- 19. Ploenes T, Kyritsis I, Mardanzai K, Muhmann D, Langehegermann L, Slama A, et al. A prospective study investigating blood patch pleurodesis for postoperative air leaks after pulmonary resection. J Surg Res. 2020;255:240–6. [DOI] [PubMed] [Google Scholar]
- 20. Lang‐Lazdunski L, Coonar AS. A prospective study of autologous 'blood patch' pleurodesis for persistent air leak after pulmonary resection. Eur J Cardiothorac Surg. 2004;26:897–900. [DOI] [PubMed] [Google Scholar]
- 21. Andreetti C, Venuta F, Anile M, De Giacomo T, Diso D, Di Stasio M, et al. Pleurodesis with an autologous blood patch to prevent persistent air leaks after lobectomy. J Thorac Cardiovasc Surg. 2007;133:759–62. [DOI] [PubMed] [Google Scholar]
- 22. Korasidis S, Andreetti C, D'Andrilli A, Ibrahim M, Ciccone A, Poggi C, et al. Management of residual pleural space and air leaks after major pulmonary resection. Interact Cardiovasc Thorac Surg. 2010;10:923–5. [DOI] [PubMed] [Google Scholar]
- 23. Dye K, Jacob S, Ali M, Orlando D, Thomas M. Autologous blood patching to mitigate persistent air leaks following pulmonary resection: a novel approach. Cureus. 2020;12:e7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Droghetti A, Schiavini A, Muriana P, Comel A, De Donno G, Beccaria M, et al. Autologous blood patch in persistent air leaks after pulmonary resection. J Thorac Cardiovasc Surg. 2006;132:556–9. [DOI] [PubMed] [Google Scholar]
- 25. Lang‐Lazdunski L, Barrington S, Bille A, Bondiau PY. Cyberknife radiosurgery for focal paravertebral recurrence after radical pleurectomy/decortication in malignant pleural mesothelioma. Eur J Cardiothorac Surg. 2012;41:1393–4. [DOI] [PubMed] [Google Scholar]
- 26. Athanassiadi K, Bagaev E, Haverich A. Autologous blood pleurodesis for persistent air leak. Thorac Cardiovasc Surg. 2009;57:476–9. [DOI] [PubMed] [Google Scholar]
- 27. Belda‐Sanchís J, Serra‐Mitjans M, Iglesias Sentis M, Rami R. Surgical sealant for preventing air leaks after pulmonary resections in patients with lung cancer. Cochrane Database Syst Rev. 2010;2010(1):Cd003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stamenovic D, Bostanci K, Messerschmidt A, Jahn T, Schneider T. Fissureless fissure‐last video‐assisted thoracoscopic lobectomy for all lung lobes: a better alternative to decrease the incidence of prolonged air leak? Eur J Cardiothorac Surg. 2016;50:118–23. [DOI] [PubMed] [Google Scholar]


