Abstract
Introduction
Immune cells and molecules are considered as clinical biomarkers and potential targets for immunotherapy. Analyses of the composition of peripheral blood cells hold promise for providing a basis for diagnosing and prognosis lung cancer. In this study, we assessed correlations between immune cell subset profiles in peripheral blood and disease prognosis in patients with lung cancer.
Methods
One hundred and thirteen patients with lung cancer and 99 age‐matched healthy people were enrolled in this study. The percentage and cell count of monocytes, neutrophils, T cells, B cells, natural killer (NK), and NKT cells in peripheral blood were analyzed by flow cytometry or peripheral blood analyzer. Serum cytokines and colony‐stimulating factors were detected by enzyme‐linked immunosorbent assay (ELISA).
Results
A reduction in antitumor NK cells (p < 0.0001) and an increase in the protumor MDSCs (p < 0.0001) were observed in the lung cancer patients compared with the controls. Monocyte counts were significantly higher in lung cancer patients with histories of smoking (p < 0.05) or drinking (p < 0.01) than in patients with no relevant history or healthy controls. The number of neutrophils and the neutrophil‐to‐lymphocyte ratio (NLR) were particularly higher in patients with liver metastasis (p < 0.01) compared with no metastasis patients or healthy controls. Levels of the monocyte‐derived cytokine interleukin‐6 (p < 0.05), granulocyte colony‐stimulating factor (G‐CSF) (p < 0.0001), and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) (p < 0.0001) were higher in patients than in controls. G‐CSF levels decreased during the remission phase (p < 0.05), and positively correlated with carbohydrate antigen 19–9 (p < 0.05) and gene mutation (p < 0.05).
Conclusion
Monocyte and neutrophil counts were higher in peripheral blood in lung cancer patients than in controls, especially when patients had histories of smoking, drinking, and liver metastasis. Serum levels of G‐CSF and GM‐CSF were higher in lung cancer patients, and G‐CSF levels positively correlated with disease severity.
Keywords: granulocyte colony‐stimulating factor, lung neoplasms, monocytes, peripheral blood
One hundred and thirteen patients with lung cancer and 99 age‐matched healthy people were enrolled for analysis of immune cell profiles, cytokines, and colony‐stimulating factors in peripheral blood. Monocyte and neutrophil counts were higher in lung cancer patients with histories of smoking, drinking, and liver metastasis. Serum levels of granulocyte colony‐stimulating factor (G‐CSF) and granulocyte‐macrophage colony‐stimulating factor were higher in lung cancer patients, and G‐CSF levels positively correlated with disease severity.
INTRODUCTION
Lung cancer is the most commonly diagnosed cancer and a leading cause of cancer‐related deaths worldwide, with approximately 2.5 million new cases and 1.5 million deaths annually. 1 The overall 5‐year survival rate has been estimated to be 15%, and frequently delayed diagnoses have been associated with patient dissatisfaction. 1
The human immune system is composed of numerous immune cells, T lymphocytes, B lymphocytes, and natural killer (NK) cells, forming a critical component of the antitumor immune response of the host. In addition to preventing tumor initiation and progression, immune cells and their respective factors, including myeloid‐derived suppressor cells (MDSCs), regulatory T cells, and interleukin‐10 (IL‐10), also promote tumor proliferation, infiltration, metastasis, and resistance. Immune cells are considered potential targets for both immunotherapy and as clinical biomarkers. Analyses of the composition of peripheral blood cells hold promise for providing a basis for diagnosing and staging lung cancer.
High levels of MDSCs have recently been proposed as useful biomarkers for lung cancer diagnosis and prognosis. Moreover, a high neutrophil‐to‐lymphocyte ratio (NLR) has been associated with substantially worse overall survival and progression‐free survival compared with a low NLR in lung cancer patients. 2 , 3 , 4 A high NLR at the time of diagnosis has been associated with poor performance status, advanced cancer stage, and lower response rates during immune checkpoint blockade therapy. 2 , 5 MDSCs are a heterogeneous population of immature myeloid cells that are characterized by a high degree of immunosuppressive activity. High levels of MDSCs have been observed in lung cancer patients and may be associated with poor disease prognosis. 6 , 7 , 8 Although changes in other subsets of immune cells have previously been observed during cancer development, the implications of these findings remain poorly understood. Further integrative analyses are needed to determine the potential role of immune system composition in the diagnosis of lung cancer and outcome prediction. The present study assessed profiles of subsets of immune cells in peripheral blood in lung cancer patients and possible correlations with disease prognosis.
MATERIALS AND METHODS
Ethical considerations
All patients gave written informed consent. Our work with human biological samples was approved by the ethics committee of the Peking University Health Science Center and complies with the World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects.
Study population and clinical data
We included patients with lung cancer who underwent first‐line chemotherapy at Peking University Third Hospital between June 2018 and January 2020. The inclusion criteria for the patients studied were as follows: (1) histopathological diagnosed non‐small‐cell lung carcinoma (NSCLC) or small‐cell lung carcinoma (SCLC) with a survival expectancy not less than 3 months from the time of diagnosis; (2) tumor stage and ultimate efficacy evaluated using imaging data, including that obtained by cranial magnetic resonance imaging and computed tomography (CT) of the chest and abdomen; (3) routine blood tests performed at the Clinical Laboratory Department of Peking University Third Hospital from which peripheral blood cell composition could be determined, with eligible blood samples having been collected a week prior to treatment in patients with no history of hormone therapy or co‐infections with infectious fever; (4) eligibility for chemotherapy based on indices obtained through blood routine tests and biochemistry, such as electrolytes, liver and kidney function, and myocardial enzymes; (5) an Eastern Cooperative Oncology Group performance status of ≤2; and (6) written informed consent for voluntary participation in chemotherapy and regular follow‐up. The exclusion criteria included the presence of any of the following: at least two different malignancies, blood or immune system diseases, co‐infections with hepatitis B or C virus, or a history of long‐term hormone therapy. A total of 107 patients were eligible for inclusion, and written consent, clinical information, and blood samples were collected from each patient. The main patient information, including age, sex, cell types, and smoking and drinking histories, are summarized in Table 1. Additionally, 99 age‐matched, disease‐free individuals were recruited from the Medical Examination Centre of Peking University Third Hospital to serve as healthy controls.
TABLE 1.
Patient information
| Clinical features | Cases | Percentages |
|---|---|---|
| Age | ||
| <62 | 50 | 46.7% |
| ≥62 | 57 | 53.3% |
| Gender | ||
| Male | 84 | 78.5% |
| Female | 23 | 21.5% |
| Diagnose | ||
| Squamous carcinoma | 25 | 23.6% |
| Adenocarcinoma | 43 | 40.6% |
| Small cell lung cancer | 33 | 31.1% |
| Clinical stage | ||
| I | 3 | 2.9% |
| II | 2 | 4.8% |
| III | 36 | 34.3% |
| IV | 64 | 61.0% |
| Distant metastasis | ||
| Yes | 68 | 23.6% |
| No | 21 | 76.4% |
| Liver metastasis | ||
| Yes | 18 | 20.2% |
| No | 71 | 79.8% |
| Brain metastasis | ||
| Yes | 11 | 12.4% |
| No | 78 | 87.6% |
| Family history | ||
| Yes | 32 | 36.0% |
| No | 57 | 64.0% |
| Smoking history | ||
| Yes | 66 | 67.3% |
| None | 32 | 32.7% |
| Drinking history | ||
| Yes | 49 | 50.5% |
| None | 48 | 49.5% |
We collected and recorded the clinical and treatment data of all patients. The clinical data comprised age, gender, tumor stage, pathological tumor type, and epidermal growth factor receptor gene mutation status, whereas the treatment data included the therapeutic responses following both the second and fourth treatment cycles as well as the imaging data obtained during the treatment period. Baseline peripheral blood samples were collected prior to treatment, and subsequent samples were obtained on day 14 ± 2 after two and four cycles of treatment. The neutrophil, lymphocyte, monocyte, and eosinophil counts were measured by peripheral blood analyzer (sysmex 800i) according to the guidelines of the Peking University Third Hospital. Sera were collected and stored at −20°C for subsequent enzyme‐linked immunosorbent assay (ELISA). Fresh peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque (Solarbio) density gradient centrifugation and cryopreservation using fetal bovine serum containing 10% dimethyl sulfoxide for subsequent flow cytometry analysis.
The gene mutations were detected for correlation with immune cells and cytokines, including that epidermal growth factor receptor (EGFR), K‐ras, breast cancer gene (BRCA), and MET were detected by next‐generation sequencing (NGS), anaplastic lymphoma kinase (ALK) were detected by fluorescence in situ hybridization (FISH), and human epidermal growth factor receptor3 (HER3) were detected by polymerase chain reaction (PCR).
Evaluation criteria
Tumor staging was carried out according to the standards of the International Association for the Study of Lung Cancer, 7th edition. 9 As per the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1), the efficacy assessment index included short‐ and long‐term clinical outcomes, the latter of which was further classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). 10 According to the RECIST 1.1 guidelines, all cases of PR and CR were confirmed 4 weeks later.
Follow‐up assessments
Regular follow‐ups were conducted for all patients between June 2018 and the end of January 2020 via clinical visits or telephone. The follow‐up rate was 100%.
Flow cytometry
To analyze the composition of the immune cells in the peripheral blood, we resuscitated the frozen PBMCs and labeled them as the following antibodies: PE anti‐human CD56 (NCAM; BioLegend, catalog number: 318306); CD4 Monoclonal Antibody (OKT4 [OKT‐4]), PerCP‐Cyanine5.5, eBioscience (Invitrogen, catalog number: 45‐0048‐42); PE‐Cy7 Mouse Anti‐Human CD11b (BD Pharmingen, catalog number: 557743); APC anti‐human CD19 (BioLegend, catalog number: 302212); Alexa Fluor 700 anti‐human CD3 (BioLegend, catalog number: 344822), APC/Cyanine7 anti‐human CD45 (BioLegend, catalog number: 304014), BV421 Mouse Anti‐Human HLA‐DR (BD Horizon, catalog number: 562804), Brilliant Violet 510 anti‐human CD33 (BioLegend, catalog number: 303422), Brilliant Violet 605 anti‐human CD8a (BioLegend, catalog number: 301040). CD45+CD11b+ cells were identified as monocytes, CD45+HLA‐DR−CD11b+CD33+ cells were identified as MDSCs, CD45+CD3+CD56− cells were identified as T cells, CD45+CD3−CD56+ cells were identified as NK cells, CD45+CD3+CD56+ cells were identified as natural killer T (NKT) cells, CD45+CD3+CD56−CD4+CD8− cells were identified as CD4+ T cells, CD45+CD3+CD56−CD4−CD8+ cells were identified as CD8+ T cells, and CD45+CD19+ cells were identified as B cells. Samples were acquired using the BD FACSCanto instrument (BD Biosciences), and the results were analyzed using the FlowJo software V10 (BD Life Sciences, Ashland, OR, USA).
Enzyme‐linked immunosorbent assay
We assessed the serum IL (interleukin)‐6, tumor necrosis factor (TNF)‐α, IL‐1 beta, IL‐12, and IL‐10 levels of both the patients and healthy controls using commercially available sandwich ELISA kits (eBioscience) in accordance with the instructions of the manufacturer. With respect to the ELISA kits, the catalog numbers for IL‐6, TNF‐α, IL‐10, IL‐12, and IL‐1 beta were 88‐7066, 88‐7346, 88‐7106, 88‐7126, and 88‐7261, respectively. Serum granulocyte colony‐stimulating growth factor (G‐CSF) and granulocyte monocyte colony‐stimulating growth factor (GM‐CSF) concentrations were measured using another commercially available ELISA kit (PeproTech). The catalog numbers for G‐CSF were 500‐P43 and 500‐P43Bt, whereas those of GM‐CSF were 500‐P33 and 500‐P33Bt. The binding was visualized using o‐phenylenediamine dihydrochloride substrate, and the reaction was terminated by adding 2 N H2SO4. Absorbance was then measured at 492 nm.
Statistical analysis
All statistics were analyzed using SPSS version 27.0 (SPSS) and graphed using Prism software version 9 (GraphPad). The measurement data were expressed as mean deviation (). For data with normal distributions, the t‐test was used; if the data were non‐normally distributed or variances were not homogeneous, the nonparametric test method was used. Enumeration data were expressed as rates (%) and analyzed using either the chi‐square or Fisher's exact test. The Pearson correlation test was used to analyze the dependency between variables. Receiver operating characteristic (ROC) curves were generated, and the areas under the receiver curves (AUC) were calculated to evaluate the diagnostic accuracy of the index. All statistical tests were two‐sided with a test level of α = 0.05, and p < 0.05 was considered a statistically significant difference.
RESULTS
Elevations of monocytes and MDSCs in peripheral blood in lung cancer patients
PBMCs were isolated from both lung cancer patients and healthy controls. Their respective cell profiles were analyzed by cell surface markers using either flow cytometry or a peripheral blood analyzer. Monocytes comprise a substantial proportion of PBMCs and are characterized by high heterogeneity. MDSCs play an essential role in tumor development and progression. Using both CD45 and CD11b as monocyte markers and CD11b+HLA‐DR−CD33+ as an MDSC marker, we analyzed the proportions of monocytes and MDSCs in peripheral blood in patients and healthy controls (Figure 1(a)).
FIGURE 1.
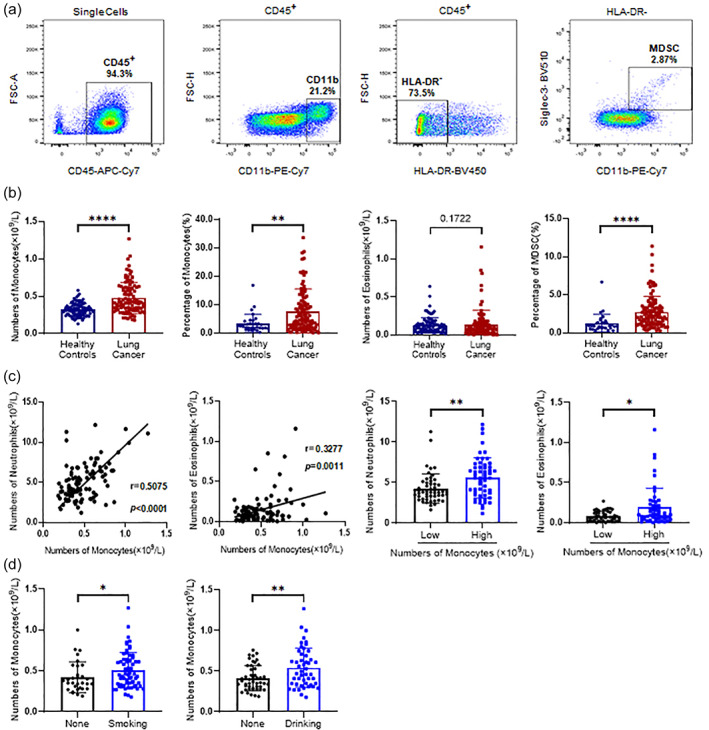
Differences in peripheral blood mononuclear cell (PBMC) counts between lung cancer patients and healthy controls. (a) Flow cytometry was performed to detect myeloid cell subsets in PBMCs and analyze the proportions of monocytes (CD11b+) and myeloid‐derived suppressor cells (MDSCs; HLA‐DR−CD11b+CD33+) among CD45+ cells. The results are presented using scatter plots. (b) Differences in the proportions of monocytes, eosinophils, and MDSCs in PBMCs between lung cancer patients and healthy controls. (c) Correlations between monocyte and eosinophil counts in PBMCs in lung cancer patients. (d) Correlations between histories of smoking and drinking and monocyte counts in PBMCs in lung cancer patients. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Both the proportion and total number of monocytes in peripheral blood in lung cancer patients significantly increased compared with healthy controls (Figure 1(b)). The number of eosinophils did not significantly differ between groups. The proportions of MDSCs in peripheral blood in lung cancer patients were significantly higher compared with healthy controls.
Correlations between monocyte levels, neutrophil and eosinophil counts, and smoking and drinking
We analyzed correlations between high monocyte levels and other indicators and patients' histories of smoking and drinking. The number of monocytes correlated with neutrophils and eosinophils (p < 0.0001 and p = 0.0011, respectively). When patients with lung cancer were further subdivided into two groups according to monocyte counts, patients with higher monocyte counts had higher levels of neutrophils and eosinophils (p < 0.01 and p < 0.05, respectively; Figure 1(c)).
Monocyte counts were higher in smokers than in never‐smokers (p < 0.05) and higher in drinkers than in nondrinkers (p < 0.01), indicating potential effects of smoking and drinking on blood monocyte counts (Figure 1(d) and Table 2).
TABLE 2.
Distribution of clinical characteristics stratified by monocyte levela
| Clinical features | Cases | Monocyte (n = 107) b | p value a | |
|---|---|---|---|---|
| <0.42 | ≥0.42 | |||
| Age | 107 | 62.65 ± 8.67 | 60.58 ± 9.47 | 0.241 |
| Gender | 0.391 | |||
| Male | 84 | 39 (46.4%) | 45 (53.6%) | |
| Female | 23 | 13 (56.5%) | 10 (43.5%) | |
| Smoking history | 0.062 | |||
| Yes | 66 | 28 (42.4%) | 38 (57.6%) | |
| None | 32 | 20 (62.5%) | 12 (37.5%) | |
| Neutrophil | 107 | 4.27 ± 1.79 | 5.59 ± 2.48 | 0.002 |
| Eosinophil | 98 | 0.08 ± 0.06 | 0.19 ± 0.24 | 0.002 |
Data are presented as n (%) and meanSD. P value as determined by 2‐tailed paired Student t test and χ2 test.
Data are grouped as cutoff (Monocyte < 0.42 or Monocyte ≥ 0.42), which is determined according to the ROC curves.
Elevations of neutrophils in peripheral blood in lung cancer patients
The number of neutrophils positively correlated with both monocyte and eosinophil counts. No difference in the number of eosinophils was observed between lung cancer patients and healthy controls, therefore we analyzed neutrophils. We analyzed the number of neutrophils and lymphocytes and the NLR in peripheral blood in lung cancer patients and healthy controls using a peripheral blood analyzer. Both the number of neutrophils and NLR were significantly higher in lung cancer patients than in controls, whereas the number of lymphocytes was significantly lower in patients than in controls (Figure 2(a) and Table 3). We plotted ROC curves to evaluate the diagnostic value of these findings and found that neutrophils (AUC = 0.717), lymphocytes (AUC = 0.775), and the NLR (AUC = 0.869) may be used as independent diagnostic markers of lung cancer (Figure 2(b)), which is consistent with previous reports. We also analyzed correlations between neutrophil counts and disease severity. Lung cancer patients with liver metastasis had fewer lymphocytes (p < 0.05) and a higher NLR (p < 0.01), with no significant difference in the number of neutrophils compared with controls (Figure 2(c)). These findings suggest that a higher NLR may increase the likelihood of liver metastasis.
FIGURE 2.
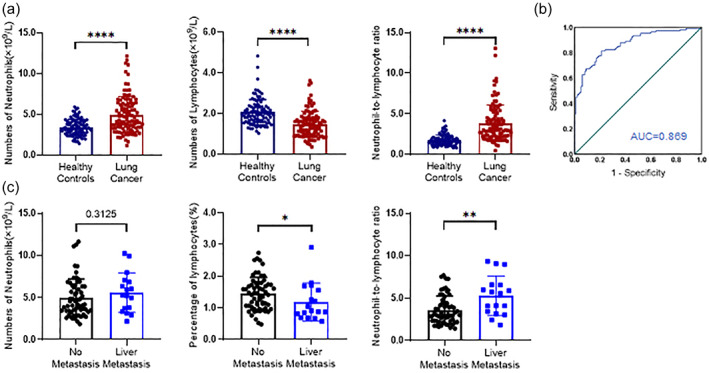
Differences in the neutrophil‐to‐lymphocyte ratio (NLR) of peripheral blood mononuclear cells between lung cancer patients and healthy controls. (a) Correlations between monocyte and neutrophil counts in lung cancer patients. The efficacy of their combination as a clinical diagnostic index was determined using receiver‐operating characteristic (ROC) curves. SPSS 27.0 software was used for the analysis. (b) Differences in neutrophil counts, lymphocyte counts, and the NLR between lung cancer patients and healthy controls. (c) The efficacy of neutrophil counts, lymphocyte counts, and the NLR as clinical diagnostic indices was determined using ROC curves and analyzed using SPSS 27.0 software. (d) Correlations between liver metastasis, neutrophil and lymphocyte counts, and the NLR in lung cancer patients. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
TABLE 3.
Distribution of clinical characteristics stratified by NLR level a
| Clinical features | Cases | NLR (n = 107) b | p value a | |
|---|---|---|---|---|
| <3.12 | ≥3.12 | |||
| Age | 107 | 62.17 ± 8.26 | 61.00 ± 9.93 | 0.510 |
| Gender | 0.853 | |||
| Male | 84 | 42 (50.0%) | 42 (50.0%) | |
| Female | 23 | 12 (52.2%) | 11 (47.8%) | |
| Liver metastasis | 0.004 | |||
| Yes | 18 | 3 (16.7%) | 15 (83.3%) | |
| No | 71 | 39 (54.9%) | 32 (45.1%) | |
Data are presented as n (%) and mean SD. p value as determined by two‐tailed paired Student t‐test and χ 2 test.
Data are grouped as cut‐off (NLR < 3.12 or NLR ≥ 3.12), which is determined according to the ROC curves.
Changes in the proportion of lymphocytes in peripheral blood in lung cancer patients
Monocytes, neutrophils, and lymphocytes are critical components of peripheral blood cells. Lymphocytes, including T lymphocytes, B lymphocytes, NK cells, and NKT cells, play an essential role in both anti‐ and protumor processes. Natural killer cells comprise a group of large granular lymphocytes that produce effectors to kill virus‐infected and tumorous cells. Natural killer T cells strengthen the function of the immune system by producing interferon β or participating directly in killing cancer cells (Figure 3(a)). Compared with healthy subjects, the proportion of B lymphocytes (CD45+CD19+) was significantly higher in lung cancer patients (p < 0.0001), whereas the proportion of NK cells (CD45+CD3−CD56+) was significantly lower in patients compared with healthy controls (p < 0.0001). No difference in the percentage of T lymphocytes (CD45+CD3+CD56−) or NKT cells (CD45+CD3+CD56+) was found between lung cancer patients and healthy controls (Figure 3(b)).
FIGURE 3.
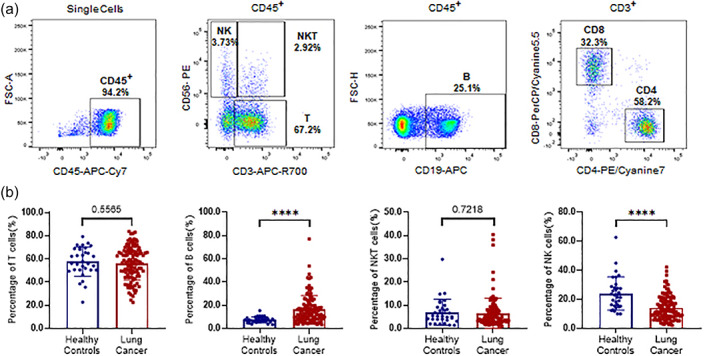
Differences in lymphocytes counts of peripheral blood mononuclear cells (PBMCs) in peripheral blood in lung cancer patients and healthy controls. (a) Flow cytometry was performed to detect lymphocyte subsets in PBMCs and to analyze the proportions of T cells (CD3+CD56−), B cells (CD19+), natural killer T (NKT) cells (CD3+CD56+), and natural killer (NK) cells (CD3−CD56−) among CD45+ cells and the proportions of CD4+ T cells and CD8+ T cells among T cells. The results are presented using scatter plots. (b) Differences in T cells (CD3+CD56−), B cells (CD19+), NKT cells (CD3+CD56+), and NK cells (CD3−CD56−) among CD45+ cells and the proportions of CD4+ T cells and CD8+ T cells among T cells between lung cancer patients and healthy controls. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Elevations of serum monocyte‐related cytokines in lung cancer patients
Both the proportion and number of monocytes in peripheral blood were significantly higher in lung cancer patients than in healthy controls (Figure 1). We used ELISA kits to further analyze the serum expression of several cytokines, including TNF‐α, IL‐1β, IL‐6, IL‐10, and IL‐12. Levels of monocyte‐derived serum cytokine IL‐6 were significantly higher in lung cancer patients than in controls (p < 0.05). No significant changes in TNF‐α, IL‐1β, IL‐10, or IL‐12 were observed (Figure 4).
FIGURE 4.
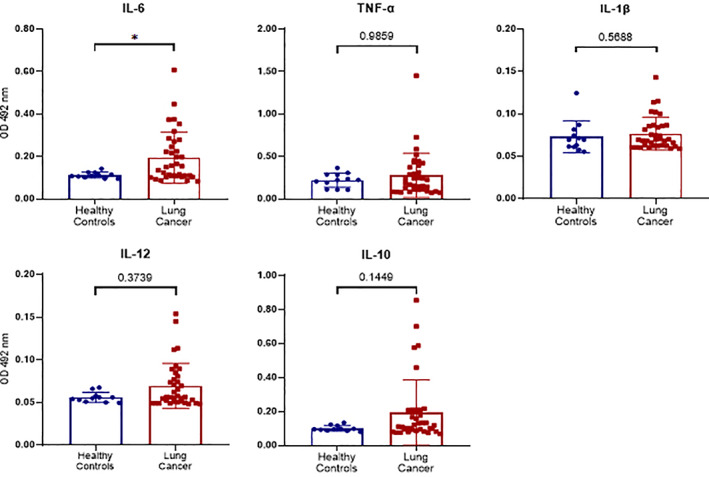
Differences in the serum expression of monocyte‐associated cytokines between lung cancer patients and healthy controls. Serum levels of IL‐6, TNF‐α, IL‐1β, IL‐12, and IL‐10 in healthy controls and lung cancer patients were determined by enzyme‐linked immunosorbent assays, and absorbance was read at 492 nm using an enzyme‐labeling instrument. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Increase in serum colony‐stimulating factor in lung cancer patients
The elevations of monocyte and neutrophil counts indicated the expansion of myeloid cells in peripheral blood in lung cancer patients. The elevation of myeloid cell counts in these patients may have been attributable to increases in either proliferation in circulation or mobilization in bone marrow, the latter of which is induced by colony‐stimulating factor (CSF). Colony‐stimulating factor may promote the differentiation and proliferation of corresponding cells and enhance their function.
To further investigate the mechanism that underlies the increase in myeloid cell counts, we analyzed whether G‐CSF and GM‐CSF were directly related to the mobilization of myeloid cells in bone marrow. Serum levels of G‐CSF and GM‐CSF in both lung cancer patients and healthy controls were determined using ELISA kits (Figure 5(a)). Serum levels of G‐CSF (p < 0.0001) and GM‐CSF (p < 0.0001) were significantly higher in lung cancer patients than in healthy controls. The ROC analysis showed that both G‐CSF and GM‐CSF may be potential independent diagnostic indicators for lung cancer patients. The AUCs for G‐CSF and GM‐CSF were 0.688 and 0.749, respectively (Figure 5(b)).
FIGURE 5.
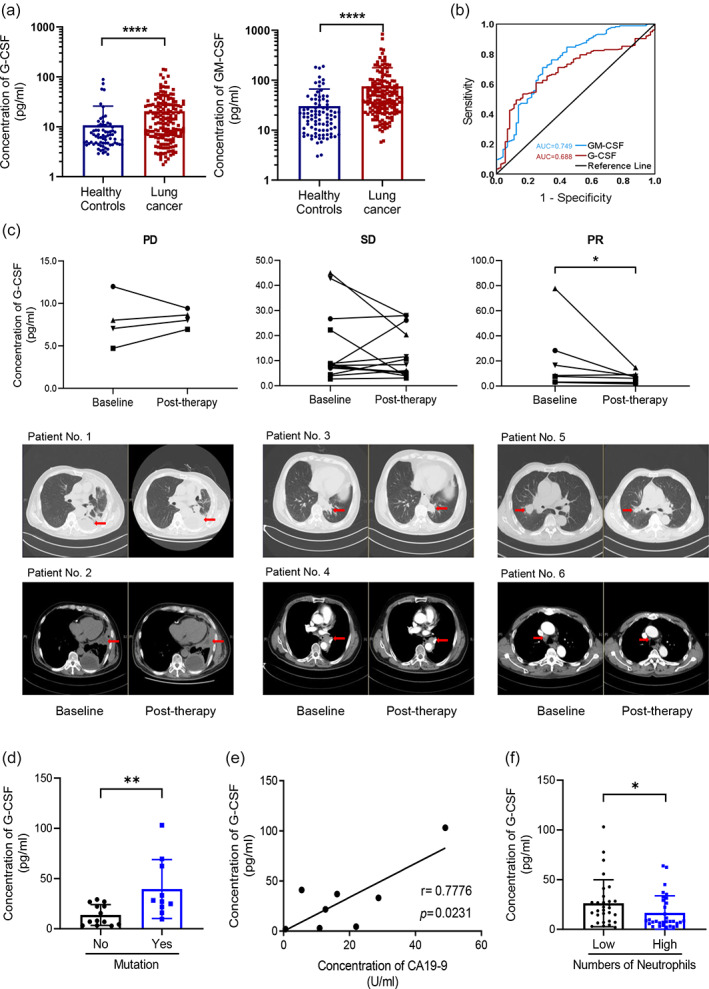
Differences in the serum expression of colony‐stimulating factor (CSF) between lung cancer patients and healthy controls. (a) Serum levels of granulocyte CSF (G‐CSF) and granulocyte‐monocyte CSF (GM‐CSF) in healthy controls and lung cancer patients were detected by enzyme‐linked immunosorbent assays, and absorbance was read at 492 nm using an enzyme‐labeling instrument. The concentrations were calculated according to the standard curve. (b) The efficacy of G‐CSF and GM‐CSF as clinical diagnostic indices was determined using receiver‐operating characteristic curves and analyzed using SPSS 27.0 software. (c) Short‐term therapeutic effects of serum G‐CSF concentrations and corresponding computed tomography images of the lungs in lung cancer patients with progressive disease, stable disease, or partial response status in the short‐term. (d–f) correlations between the occurrence of gene mutation status, CA19‐9 concentration, neutrophil count, and G‐CSF concentration. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Correlation between G‐CSF levels and disease severity
To further investigate the correlation between serum levels of CSF and disease severity in lung cancer patients, we used the treatment effect, determined by CT, to further divide lung cancer patients into PD, SD, and PR subgroups. The levels of CSF were assessed both before and after treatment in each subject (Figure 5(c)). Serum G‐CSF levels decreased in patients in the PR phase (p < 0.05), whereas G‐CSF levels did not significantly change during the PD and SD phases.
Further analyses of the gene mutation (Figure 5(d)) and tumor antigen (Figure 5(e)) findings revealed that G‐CSF levels increased in patients with the gene mutation (p < 0.05). However, because of the relatively small sample size in our study, the relationship between G‐CSF and gene mutations warrants further investigation. Several lung cancer markers, including CEA, cyfra 21–1, NSE, SCC, GRP, and CA19‐9, were detected and analyzed for correlation with immune factors. The level of CA19‐9 positively correlated with G‐CSF levels (p < 0.05, r = 0.7776), which was consistent with the ROC curve, showing a decrease during partial remission. Consequently, G‐CSF may be useful for lung cancer diagnosis and prognosis. One unexpected finding was that patients with greater neutrophil counts also had lower serum levels of G‐CSF (p < 0.05; Figure 5(f)), thus contradicting the observation that both G‐CSF and neutrophil counts increased in lung cancer patients. Whether or not this was attributable to negative feedback remains unclear.
DISCUSSION
The present study investigated circulating immune cell phenotypes in patients with lung cancer. Our findings revealed a reduction of antitumor NK cell and an increase in the protumor MDSCs in lung cancer patients compared with healthy controls. The levels of monocyte and neutrophil myeloid cells both increased, a finding that was particularly pronounced in patients with a history of smoking and drinking, and in patients in whom liver metastasis was observed. Myeloid‐specific G‐CSF and GM‐CSF expression increased, which correlated with disease severity, and decreased during partial remission, which positively correlated with both gene mutation and tumor antigen CA19‐9.
Natural killer cells are essential, innately cytotoxic cells with the ability to eliminate cancer cells, making them the first line of defense with regard to antitumor immunity. Ottonello et al. reported that NK cell expression decreased in patients with NSCLC, and higher NK cell levels were associated with longer survival times. 11 Lower NK cell counts were also observed in patients with colorectal cancer 12 and hepatocellular carcinoma. 13 MDSCs are a heterogeneous population of cells that are characterized by their immunosuppressive properties and association with poor clinical outcomes. MDSCs have been reported to significantly increase in patients with NSCLC and have been associated with a worse prognosis. 6 , 7 , 8 In the present study, we systematically analyzed immune profiles of lung cancer patients while carefully avoiding subset bias. We found that NK cell expression decreased (Figure 3) and MDSC expression increased (Figure 1). These findings suggest an association between circulating cell phenotypes and an immunocompromised state, the latter of which hampers the killing of nascent tumor cells and promotes tumor escape.
Smoking is well known to negatively affect lung function 14 and decrease survival time. 15 However, the effect of smoking on immune cell profiles remains poorly understood. The association between smoking and monocyte counts has been debated. In a study that compared 31 smokers with 33 nonsmokers 16 and another study that compared 30 smokers with 36 nonsmokers, 17 smokers all exhibited significantly higher total circulating levels of white blood cells, lymphocytes, monocytes, and neutrophils. A third study of 298 nonatopic smokers, 136 never‐smokers, 160 smokers who had quit, and 30 continuing smokers found that monocyte counts were higher in never‐smokers than in smokers. Moreover, heavy smokers exhibited less of an increase in monocyte counts compared with light smokers. 14 Additionally, smokers exhibited significant increases in C‐reactive protein fibrinogen, IL‐6, and carcinoembryonic antigen levels, 16 , 17 thereby confirming the correlation between smoking history and inflammation.
Few reports have investigated correlations between drinking and lung cancer, and still fewer studies have examined correlations between drinking and monocytes. In a study of 97 excessive drinkers and 51 controls, increases in levels of lipopolysaccharides and other markers of monocyte activation, including sCD14 and sCD163, were observed in excessive drinkers. 18 Excessive drinkers were previously shown to exhibit the early immune activation of messenger ribonucleic acid transcripts of the peripheral blood monocytes IL‐6 and IL‐10 but not circulating IL‐6 or IL‐10. 19
Notably, we found that both neutrophil and monocyte myeloid cell counts were elevated in peripheral blood in lung cancer patients compared with healthy controls. Furthermore, a correlation with disease severity was also found. The higher myeloid cell counts may have been attributable to an increase in proliferation in the circulation or an elevation of bone marrow mobilization, the latter of which is induced by CSF. G‐CSF and GM‐CSF are cytokines that stimulate bone marrow to produce myeloid cells, such as monocytes and neutrophils. Preliminary studies reported that high serum levels of G‐CSF or GM‐CSF may be useful for lung cancer diagnosis. 20 , 21 , 22 In the present study, increases in both myeloid cells and myeloid cell‐specific CSFs were detected using several types of cell subsets and CSFs. These findings indicate the important role of G‐CSF/GM‐CSF‐induced myeloid cell mobilization in the pathogenesis of lung cancer.
Colony‐stimulating factor promotes tumor progression via either an autocrine or paracrine mechanism. Various solid, advanced malignancies simultaneously produce G‐CSF and express its receptor, thus enabling the autocrine proliferation of tumor cells. 21 CSF1 has been previously shown to increase A549 proliferation and invasion. An in vivo study reported that CSF1 knockdown mice exhibited smaller osteolytic lesions and tumor volume compared with controls. 23 Mechanistic studies proposed that G‐CSF triggers the G‐CSFR/JAK/STAT3 signaling pathway, leading to the epithelial‐mesenchymal transition in NSCLCs. 20 Moreover, tumor‐secreted G‐CSF and GM‐CSF may act as paracrine factors in the tumor microenvironment, thereby promoting mobilization into tumor sites via the recruitment of immature and mature neutrophils that function as MDSCs, in turn intensifying the immunosuppressive milieu of the tumor microenvironment. 20 , 21 , 24 G‐CSF‐triggered increases in immature myeloid cells in bone marrow, blood, and the spleen have been found to be predictive of poor disease prognosis. 21
Although CSF is strongly related to cancer progression, G‐CSF is seldom discussed from the perspective of cancer therapy. In what may be the only report that addressed this issue, G‐CSF was reportedly used during cancer therapy to ameliorate neutropenia, a severe adverse effect of chemotherapy, to promote granulocytes. 20 In mouse models, recombinant GM‐CSF 25 and the overexpression of G‐CSF 26 both induced the expansion of circulating neutrophils. Moreover, the ablation of G‐CSF with anti‐G‐CSF antibodies prevented neutrophil accumulation in the blood circulation. 26 Nevertheless, these methods are not specifically related to cancer therapy. G‐CSF is involved in the occurrence, development, and diagnosis of tumors and may be considered a potential therapeutic target, but further research is needed to confirm this possibility.
The negative correlation between G‐CSF and neutrophil counts was difficult to explain (Figure 5(f)). Endogenous G‐CSF is believed to be responsible for neutrophilia in cancer patients, but the underlying mechanism remains unknown. 26 A few case reports have discussed the link between high endogenous serum G‐CSF levels and high white blood cell counts, consisting primarily of neutrophils. Nonetheless, evidence of the role of endogenous serum G‐CSF as a dominant driver of neutrophilia in cancer patients remains weak. 26
The main limitation of the present study was its small sample size. The inclusion of several pathological types of lung cancer also prevented us from drawing definitive conclusions about these different pathologies. Future studies with larger samples of specific pathological types should be conducted.
Altogether, we found a significant increase in the number of both monocyte and neutrophil myeloid cells in peripheral blood in lung cancer patients. This finding was especially pronounced in lung cancer patients with a history of smoking and drinking, and in patients in whom liver metastasis was detected. Serum levels of G‐CSF and GM‐CSF were also elevated in lung cancer patients. Furthermore, G‐CSF levels correlated with disease severity in this population. The increases in levels of myeloid cells and myeloid cell‐specific CSF support the critical role of myeloid cells in the diagnosis and pathogenesis of lung cancer.
CONCLUSIONS
Our findings revealed a reduction of antitumor NK cells and an increase in the protumor of MDSCs in lung cancer patients compared with healthy controls. Monocyte and neutrophil counts were higher in peripheral blood in lung cancer patients than in healthy controls, especially when these patients had histories of smoking, drinking, and liver metastasis. Serum levels of G‐CSF and GM‐CSF were higher in lung cancer patients, and G‐CSF levels positively correlated with disease severity.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant No. 81972041) and the Beijing Natural Science Foundation (grant No. 7172112).
Yin W, Lv J, Yao Y, Zhao Y, He Z, Wang Q, et al. Elevations of monocyte and neutrophils, and higher levels of granulocyte colony‐stimulating factor in peripheral blood in lung cancer patients. Thorac Cancer. 2021;12:2680–2690. 10.1111/1759-7714.14103
Funding information National Natural Science Foundation of China, Grant/Award Number: 81972041; Natural Science Foundation of Beijing Municipality, Grant/Award Number: 7172112
REFERENCES
- 1. Siegel R, Miller K, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Kang M et al. The prognostic impact of the neutrophil‐to‐lymphocyte ratio in patients with small‐cell lung cancer. Br J Cancer. 2014;111:452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liang H, Gao K, Jia R, Li J, Wang C. Prognostic significance of the combination of preoperative fibrinogen and the neutrophil‐lymphocyte ratio in patients with non‐small cell lung cancer following surgical resection. Oncol Lett. 2019;17:1435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diem S et al. Neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) as prognostic markers in patients with non‐small cell lung cancer (NSCLC) treated with nivolumab. Lung cancer. 2017;111:176–81. [DOI] [PubMed] [Google Scholar]
- 5. Ren F, Zhao T, Liu B, Pan L. Neutrophil‐lymphocyte ratio (NLR) predicted prognosis for advanced non‐small‐cell lung cancer (NSCLC) patients who received immune checkpoint blockade (ICB). OncoTargets Ther. 2019;12:4235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamauchi Y, Safi S, Blattner C, Rathinasamy A, Umansky L, Juenger S, et al. Circulating and tumor myeloid‐derived suppressor cells in resectable non‐small cell lung cancer. Am J Respir Crit Care Med. 2018;198:777–87. [DOI] [PubMed] [Google Scholar]
- 7. Barrera L, Montes‐Servín E, Hernandez‐Martinez JM, Orozco‐Morales M, Montes‐Servín E, Michel‐Tello D, et al. Levels of peripheral blood polymorphonuclear myeloid‐derived suppressor cells and selected cytokines are potentially prognostic of disease progression for patients with non‐small cell lung cancer. Cancer Immunol Immunother. 2018;67:1393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vetsika E et al. A circulating subpopulation of monocytic myeloid‐derived suppressor cells as an independent prognostic/predictive factor in untreated non‐small lung cancer patients. J Immunol Res. 2014;2014:659294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Detterbeck FC, Boffa DJ, Tanoue LT, Wilson LD. Details and difficulties regarding the new lung cancer staging system. Chest. 2010;137:1172–80. [DOI] [PubMed] [Google Scholar]
- 10. Nishino M, Jackman DM, Hatabu H, Yeap BY, Cioffredi LA, Yap JT, et al. New response evaluation criteria in solid tumors (RECIST) guidelines for advanced non‐small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. Am J Roentgenol. 2010;195:W221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ottonello S, Genova C, Cossu I, Fontana V, Rijavec E, Rossi G, et al. Association between response to Nivolumab treatment and peripheral blood lymphocyte subsets in patients with non‐small cell lung cancer. Front Immunol. 2020;11:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tang F, Tie Y, Tu C, Wei X. Surgical trauma‐induced immunosuppression in cancer: recent advances and the potential therapies. Clin Transl Med. 2020;10:199–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu HZ, Deng W, Li JL, Tang YM, Zhang LT, Cui Y, et al. Peripheral blood lymphocyte subset levels differ in patients with hepatocellular carcinoma. Oncotarget. 2016;7:77558–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen EJ, Pedersen B, Narvestadt E, Dahl R. Blood eosinophil and monocyte counts are related to smoking and lung function. Respir Med. 1998;92:63–9. [DOI] [PubMed] [Google Scholar]
- 15. Tamminga M et al. Immune microenvironment composition in non‐small cell lung cancer and its association with survival. Clin Transl Immunol. 2020;9:e1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luetragoon T, Rutqvist LE, Tangvarasittichai O, Andersson BÅ, Löfgren S, Usuwanthim K, et al. Interaction among smoking status, single nucleotide polymorphisms and markers of systemic inflammation in healthy individuals. Immunology. 2018;154:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elisia I, Lam V, Cho B, Hay M, Li MY, Yeung M, et al. The effect of smoking on chronic inflammation, immune function and blood cell composition. Sci Rep. 2020;10:19480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liangpunsakul S, Toh E, Ross RA, Heathers LE, Chandler K, Oshodi AP, et al. Quantity of alcohol drinking positively correlates with serum levels of endotoxin and markers of monocyte activation. Sci Rep. 2017;7:4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walline CC, Blum JS, Linton T, Mangiacarne D, Liangpunsakul S. Early activation of peripheral monocytes with hallmarks of M1 and M2 monocytic cells in excessive alcohol drinkers: a pilot study. J Invest Med. 2018;66:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cui YH, Suh Y, Lee HJ, Yoo KC, Uddin N, Jeong YJ, et al. Radiation promotes invasiveness of non‐small‐cell lung cancer cells through granulocyte‐colony‐stimulating factor. Oncogene. 2015;34:5372–82. [DOI] [PubMed] [Google Scholar]
- 21. Wu HL, Wu YM, Chen JT, Chang KY, Cherng YG, Lin SP, et al. A comparison of inflammation markers for predicting oncological outcomes after surgical resection of non‐small‐cell lung cancer: a validated analysis of 2,066 patients. Sci Rep. 2020;10:19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stathopoulos GP, Armakolas A, Tranga T, Marinou H, Stathopoulos J, Chandrinou H. Granulocyte colony‐stimulating factor expression as a prognostic biomarker in non‐small cell lung cancer. Oncol Rep. 2011;25:1541–4. [DOI] [PubMed] [Google Scholar]
- 23. Hung JY, Horn D, Woodruff K, Prihoda T, LeSaux C, Peters J, et al. Colony‐stimulating factor 1 potentiates lung cancer bone metastasis. Lab Invest. 2014;94:371–81. [DOI] [PubMed] [Google Scholar]
- 24. Waight JD et al. Tumor‐derived G‐CSF facilitates neoplastic growth through a granulocytic myeloid‐derived suppressor cell‐dependent mechanism. PLoS One. 2011;6:e27690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Quail DF, Olson OC, Bhardwaj P, Walsh LA, Akkari L, Quick ML, et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM‐CSF. Nat Cell Biol. 2017;19:974–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lecot P, Sarabi M, Pereira Abrantes M, Mussard J, Koenderman L, Caux C, et al. Neutrophil heterogeneity in cancer: from biology to therapies. Front Immunol. 2019;10:2155. [DOI] [PMC free article] [PubMed] [Google Scholar]


