Abstract
Extracellular matrix (ECM) formation and remodeling are critical processes for proper morphogenesis, organogenesis, and tissue repair. The proinflammatory cytokine tumor necrosis factor alpha (TNF-α) inhibits ECM accumulation by stimulating the expression of matrix proteolytic enzymes and by downregulating the deposition of structural macromolecules such as type I collagen. Stimulation of ECM degradation has been linked to prolonged activation of jun gene expression by the cytokine. Here we demonstrate that TNF-α inhibits transcription of the gene coding for the α2 chain of type I collagen [α2(I) collagen] in cultured fibroblasts by stimulating the synthesis and binding of repressive CCAAT/enhancer proteins (C/EBPs) to a previously identified TNF-α-responsive element. This conclusion was based on the concomitant identification of C/EBPβ and C/EBPδ as TNF-α-induced factors by biochemical purification and expression library screening. It was further supported by the ability of the C/EBP-specific dominant-negative (DN) protein to block TNF-α inhibition of α2(I) collagen but not TNF-α stimulation of the MMP-13 protease. The DN protein also blocked TNF-α downregulation of the gene coding for the α1 chain of type I collagen. The study therefore implicates repressive C/EBPs in the TNF-α-induced signaling pathway that controls ECM formation and remodeling.
Tumor necrosis factor alpha (TNF-α) is a 17-kDa cytokine released by activated macrophages that locally elicits a wide spectrum of metabolic responses and cellular activities (20). The pleiotropic properties of TNF-α are signaled through alternative routes that culminate in diversified responses, such as cell proliferation, differentiation, or death (20). These ill-defined intracellular pathways in part influence gene expression by modulating the activity and synthesis of several transcription factors. Relevant examples include the activation of AP1 and NF-κB in the regulation of cell death and cell survival, the induction of IRF1 in the stimulation of the alpha and beta interferon genes, and the undefined pathway that inhibits C/EBP expression in an experimental model of inflammation-induced atrophy of adipose tissue (3, 6, 17).
Aside from cachexia, TNF-α is believed to be a key player in inflammatory disorders such as rheumatoid arthritis and osteoarthritis (20). Extracellular matrix (ECM) degradation is the hallmark of these conditions and an important component in morphogenesis, organogenesis, and tissue remodeling, as well as in wound healing and tissue repair. TNF-α stimulates ECM degradation by inducing the production of stromal collagenases. The stimulation is the result of TNF-α prolonged activation of jun gene expression with the consequent binding of the AP1 complex to the responsive elements in the collagenase promoters (4). TNF-α reduces ECM deposition by inhibiting the synthesis of structural components. They include elastin, osteocalcin, and type I collagen, the major structural component of connective tissue (10, 15, 19). TNF-α also counteracts transforming growth factor β (TGF-β) stimulation of type I collagen gene expression in cell cultures (11). The last finding reflects the functionally antagonistic nature of these cytokines and represents a useful paradigm to study the complex cellular signals that regulate ECM formation and remodeling in vivo.
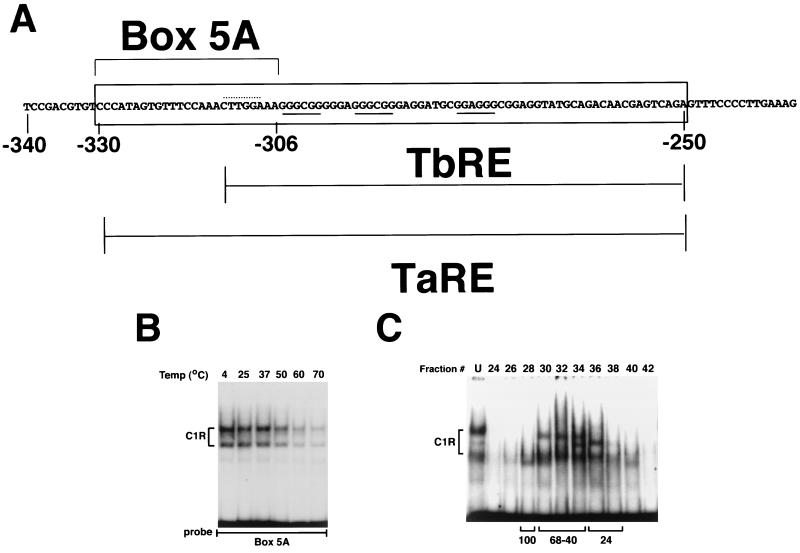
To elucidate the molecular mechanism of TNF-α action on ECM remodeling, we have been studying the cytokine regulation of the human α2(I) collagen (COL1A2) gene. We have shown that the opposing stimuli of TNF-α and TGF-β converge on the same transcriptional complex (cytokine responsive complex [CyRC]) that is bound to the −330 to −235 upstream sequence (Fig. 1A) (8). The −330 to −235 sequence is sufficient to mediate the antagonistic effects of the cytokines, for it confers TGF-β and TNF-α responsiveness to the otherwise unresponsive thymidine kinase promoter. DNA binding assays and cell transfection experiments have also separated the TGF-β-responsive element from the TNF-α responsive element (Fig. 1A). Indeed, we were able to show that TNF-α, but not TGF-β, increases protein binding to nucleotides −330 to −306 (box 5A [Fig. 1A]).
FIG. 1.
Purification and characterization of C1R. (A) Schematic representation of the TNF-α-responsive element. The −340 to −235 nucleotide sequence of the COL1A2 gene is shown along with the location of the TGF-β-responsive element (TbRE), TNF-α-responsive element (TaRE), and box 5A. The C/EBP and Sp1/Sp3 binding sites are highlighted by the dotted and continuous lines, respectively. (B) C1R is a heat-resistant protein. Nuclear extracts from NIH 3T3 cells were incubated at the indicated temperatures for 10 min, followed by EMSA using box 5A as the probe. (C) Gel filtration chromatography of denatured nuclear proteins. Aliquots (30 μl) from fractions were examined for box 5A binding activity by EMSA following renaturation. The migration of size markers (in kilodaltons) is indicated below the autoradiograph. U, unfractionated sample.
Based on the above results, we have hypothesized that the antagonistic signals of the cytokines act on the CyRC by influencing the activity of the complex in opposite ways and, in the case of TNF-α, by inducing the binding of a negative factor (C1R) to box 5A (8). Here we report the characterization of the DNA-binding C1R protein. Our findings establish a biochemical and genetic link between the C/EBPs and the regulation of the type I collagen genes by TNF-α.
MATERIALS AND METHODS
Cell culture, plasmids, and reagents.
Fibroblasts were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (HyClone Inc., Logan, Utah) and antibiotics (penicillin [50 U/ml] and streptomycin [50 μg/ml]). Human recombinant TNF-α (Boehringer Mannheim Corp., Indianapolis, Ind.) was used at a final concentration of 20 ng/ml. Unless otherwise indicated, cells were placed in medium containing 0.1% fetal bovine serum 12 h before TNF-α addition. The COL1A2 promoter-chloramphenicol acetyltransferase (CAT) reporter plasmid C/EBP/CAT and the A-CREB and A-C/EBP constructs have been described before (8, 16). A-C/EBP differs from A-ZIP/F in that contains only the C/EBPα leucine zipper and the acidic extension DPLEQRAEELARENEELEKAEELEQENAE, in addition to the FLAG epitope (1). Bacterially expressed recombinant proteins were purified over heparin columns as described before (16, 14). Antibodies specific for C/EBPα, C/EBPβ, and C/EBPδ and high-affinity recognition sequences for C/EBP, CREB, and CTF/NF-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Characterization of C1R and DNA binding assays.
The C1R protein was partially purified by DNA affinity chromatography from nuclear extracts prepared from 600 150-mm-diameter culture dishes of NIH 3T3 fibroblasts (9). Southwestern screening was performed as described by Vinson et al. (22), using 106 independent phage clones engineered in the λgt 11 expression vector by random priming of human fibroblast RNA. Nuclear extracts were purified from control and TNF-α-treated cells and used in electrophoretic mobility shift assay (EMSA) with the oligonucleotide corresponding to box 5A of COL1A2 (−330 to −296) or with a high-affinity recognition sequence for CREB according to published conditions (8). Unlabeled competitors were added in the amounts indicated in the figure legends. When appropriate, nuclear extracts were preincubated with antibodies before addition of the labeled probe. DNA-protein complexes were separated from unbound material on a 5% polyacrylamide gel and then visualized by autoradiography. In some experiments, recombinant A-C/EBP or A-CREB proteins were mixed at the concentrations indicated in the figure legends with 5 μg of nuclear extracts obtained from TNF-α-treated NIH 3T3 cells and used in EMSA with the box 5A or CREB oligonucleotide as a probe. To estimate the molecular weight of C1R, 500 μg of nuclear extracts was subjected to gel filtration chromatography using a Sephacryl S-300 column, and the collected fractions were analyzed by EMSA using the box 5A probe (9).
Cell transfections.
Conditions for the preparation and transfection of plasmid DNA into NIH 3T3 cells or HepG2 cells by the calcium phosphate method were described before (8). In the titration test, 8 μg of the C/EBPα expression vector was cotransfected in HepG2 cells with 0.3 μg of the C/EBP/CAT reporter plasmid along with increasing amounts of the dominant-negative (DN) expression plasmids. A-CREB or A-C/EBP expression plasmids were cotransfected with 10 μg of the −378COL1A2/CAT reporter constructs and with 2 μg of pSVLUC, a luciferase-expressing vector under the control of the simian virus 40 promoter. Eighteen hours after transfection, cells were washed twice with phosphate-buffered saline, placed in medium containing 0.5% fetal bovine serum for 2 h, and then treated with TNF-α for 18 h. Transfections were performed multiple times in duplicate. CAT activities were normalized against the activity of pSVLUC. The statistical value of the data was evaluated by the Mann-Whitney U test. Stable transfectants were selected by culturing the cells for about 3 weeks in the presence of G418 (400 μg/ml).
Northern blot analysis and in vitro transcription assay.
Total RNA was used for Northern blot hybridization to probes for α1(I) and α2(I) collagen, MMP-13, and glyceraldehyde-3-phosphate dehydrogenase (7). Rates of COL1A2 transcription were determined as previously described (7). Quantitative data were obtained with the aid of the computer program Adobe Photoshop (Adobe Systems Inc., Mountain View, Calif.). All experiments were performed in triplicate. Cells were harvested at various time points after TNF-α treatment and used to obtain RNA or nuclear extracts as described above. Cell viability, determined by the trypan blue exclusion test, was always higher than 90%.
Western blot analysis.
Nuclear extracts, at the concentrations indicated in the figure legends, were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 12.5% gels and transferred onto a nitrocellulose membrane. Membranes were probed with C/EBPβ or C/EBPδ antibodies at a final dilution of 1:1,000, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Santa Cruz Biotechnology) diluted 1:3,000. C/EBPs were detected with an enhanced chemiluminescence system (Renaissance; NEN Life Science Products, Boston, Mass.), as recommended by the manufacturer. Conditions used to assess the expression of FLAG epitope in cells stably transfected with the different DN proteins were described before (14, 16). Proteins resolved by SDS-PAGE were visualized by silver staining.
RESULTS AND DISCUSSION
C1R was biochemically purified by anion-exchange DNA affinity chromatography using multimers of box 5A (Fig. 1A). The partially purified protein (∼6,000-fold) was found to be a heat-resistant polypeptide with an estimated molecular mass of ≥40 kDa under denaturing conditions (Fig. 1B and C). These features, and the presence of the CTTGGA sequence on the coding strand of box 5A (Fig. 1A), strongly suggested that C1R may be a leucine zipper protein (13). Parallel Southwestern screening of a fibroblast cDNA expression library with box 5A provided independent support for this postulate. The screen identified six positive DNA-binding recombinants. Three of them code for C/EBPβ, -γ, and -δ; the others code for non-leucine zipper proteins and are likely to represent false positives.
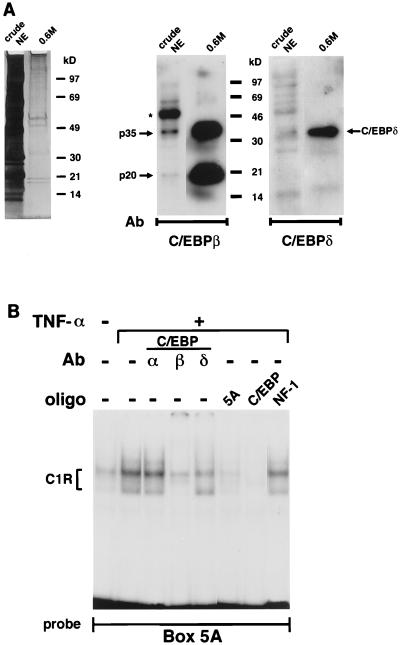
The results of the biochemical purification and the Southwestern screening concurred in suggesting that C1R is probably the same complex as C/EBP. Additional data confirmed this conclusion. First, Western blot analysis showed a substantial increase of C/EBPβ and -δ in the partially purified sample compared to the original nuclear extracts (Fig. 2A). Second, preincubation of nuclear extracts from TNF-α-treated mouse fibroblasts with C/EBP-specific antibodies revealed the ability of the C/EBPβ and C/EBPδ antisera, but not of the anti-C/EBPα antibody, to interfere with C1R formation (Fig. 2B). The same results were obtained with nuclear extracts from human fibroblasts (data not shown). Third, C1R formation was competed by box 5A and the C/EBP consensus sequence but not by the recognition site for CTF/NF-1 (Fig. 2B). This last finding is important in view of previous reports that implicated CTF/NF-1 in mediating TNF-α and TGF-β modulation of collagen synthesis (2, 18).
FIG. 2.
C/EBPs bind to box 5A. (A) Left, silver staining of a 4 to 15% gradient SDS-Tris-HCl polyacrylamide gel loaded with 25 μg of crude nuclear extract (crude NE) or approximately 10 ng of partially purified C1R fraction (0.6M). Middle, Western blot analysis of the samples shown on the left probed with the anti-C/EBPβ antibody (Ab) (12.5% gel). Arrows indicate positions of the p20 and p35 C/EBPβ isoforms. The larger 45-kDa band (asterisk) is due to the antibody cross-reactivity with actin. Right, Western blot analysis of the same sample probed with the anti-C/EBPδ antibody. Relative mobilities of the molecular weight standards are marked. (B) EMSA of nuclear extracts from control and TNF-α-treated NIH 3T3 cells assayed with box 5A. Some of the samples were preincubated with antibodies (Ab) against C/EBPα, C/EBPβ, and C/EBPδ or with a 100-fold molar excess of unlabeled box 5A and high-affinity recognition sequences for C/EBP or CTF/NF-1. oligo, oligonucleotide.
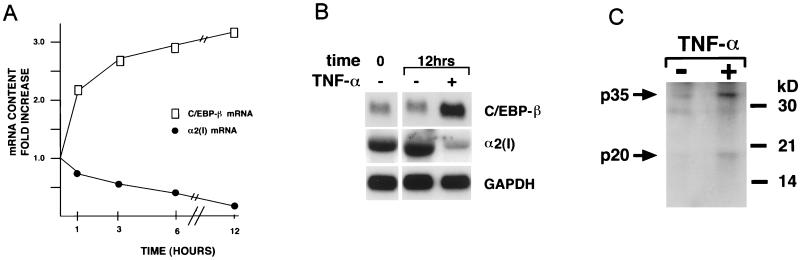
Northern blot hybridizations established the existence of an inverse relationship between steady-state levels of C/EBPβ mRNA and accumulation of α2(I) collagen transcripts in TNF-α-treated fibroblasts (Fig. 3). A smaller increase was also observed for C/EBPγ, C/EBPδ, and CHOP mRNAs (data not shown). Two isoforms of C/EBPβ (p35 and p20) have been described (5). The smaller isoform has been shown to act as a DN inhibitor of C/EBP-mediated transcription by virtue of missing the transactivating domain, whereas the larger one has been associated with gene transactivation (5). Western blot analysis confirmed the role of TNF-α in C/EBPβ stimulation by documenting the increase of both activator and repressor isoforms of the transcription factor (Fig. 3). It is interesting that the p20 and p35 isoforms showed ∼10- and 6-fold, respectively, increases over the amounts of the same proteins in the untreated sample. Whether this result indicates the slight preference of TNF-α to stimulate the repressor as opposed to the activator isoform of C/EBPβ remains to be determined. More generally, the precise mechanism underlying TNF-α induction of C/EBP repressive activity on collagen transcription is the focus of ongoing investigations. Here, we sought to provide functional support for the DNA binding evidence.
FIG. 3.
TNF-α stimulates C/EBPβ mRNA expression. (A) Time course accumulation of α2(I) collagen and C/EBPβ mRNAs after TNF-α administration to NIH 3T3 cultures. Values are expressed as fold increase with respect to untreated cells. The graph summarizes the data from three independent tests. Human dermal fibroblasts yielded the same results except that the mRNA kinetics were delayed by about 1 h. (B) Representative Northern blot of cells obtained before adding TNF-α (time 0) and of control and TNF-α-treated (12 h) cells. The probes used are indicated to the right. (C) Western analysis of nuclear proteins from cells cultured for 9 h with and without TNF-α and probed against the anti-C/EBPβ antibody. Arrows indicate positions of the p20 and p35 C/EBPβ isoforms. Relative mobilities of the molecular weight standards are marked.
Overexpression of DN molecules provides an effective tool with which to investigate the in vivo functions of transcription factors. The design of the construct can allow the study of both induction and repression of transcription and can overcome the problem of functional redundancy among related gene products. The approach has been successfully implemented to create DNs to distinct members of the basic-leucine zipper (bZIP) class of transcription factors. They include A-CREB, which prevents the basic region of CREB from binding to DNA; GBF-F, which changes the specificity of C-EBP binding to DNA; and A-ZIP/F, which inhibits bZIP transcription factors binding to DNA by forming stable heterodimers (1, 14, 16).
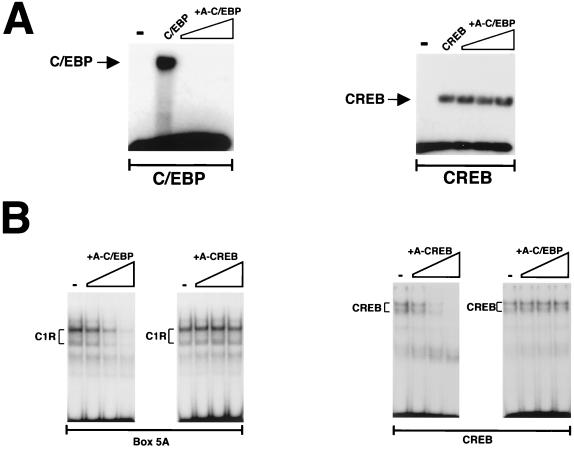
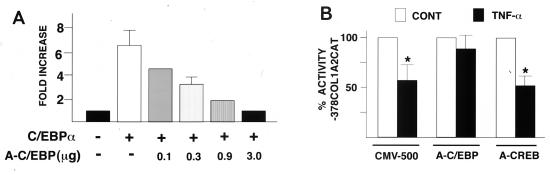
In this study we used A-C/EBP, a novel design of the A-ZIP/F type that heterodimerizes promiscuously with all C/EBPs and only with them. The specificity of A-C/EBP was tested by challenging the binding of recombinants C/EBP and CREB to the respective recognition sequences with increasing amounts of recombinant A-C/EBP. The disappearance of the C/EBP, but not the CREB complex, demonstrated the specificity of A-C/EBP (Fig. 4A). As previously reported by Moitra et al. (14) for the prototype A-ZIP/F, A-C/EBP ablated DNA binding at 1-fold molar equivalent, conceivably as a result of preferential formation of heterodimers (14). The same kind of test was repeated to assess the efficacy of A-C/EBP in our system. Crude nuclear extracts were preincubated with recombinant A-C/EBP or recombinant A-CREB, and C1R formation was examined by the EMSA. Preincubation with increasing amounts of A-C/EBP effectively prevented C1R formation but not CREB binding; likewise, increasing amounts of A-CREB acted only on the formation of the CREB complex (Fig. 4B). The results therefore demonstrated the specificity of the DN molecules against the respective bZIP proteins. Most importantly, they ruled out the possibility that C1R was the heterodimerization product of CREB and C/EBP (21).
FIG. 4.
A-C/EBP inhibits C1R binding. (A) One, 10-, and 100-fold molar equivalents of A-C/EBP were added to the incubation of recombinant C/EBP (left) or CREB (right) with the cognate recognition sequence. (B) EMSA of 5-μg aliquots of nuclear extracts incubated with 1, 10, and 100 nM (final concentration) purified recombinant A-C/EBP or A-CREB and box 5A (left) or a high-affinity recognition sequence for CREB (right).
The next set of experiments examined A-C/EBP ability to relieve COL1A2 transcription from TNF-α-induced repression. The inhibitory activity of A-C/EBP was first titrated in transient cotransfection assays with the C/EBPα expression vector and a reporter construct harboring the C/EBP binding site (Fig. 5A). Based on these results, 5 μg of A-C/EBP was transiently cotransfected with the −378COL1A2/CAT promoter construct in fibroblasts cultured with or without TNF-α. Controls included the empty CMV-500 vector and the A-CREB plasmid. Consistent with the in vitro binding test, only A-C/EBP abrogated TNF-α-induced downregulation of the COL1A2 promoter (Fig. 5B). This result functionally links C/EBP binding with TNF-α activity. To provide further support for this correlate, we repeated the analysis with cells stably transfected with the DN plasmids and examined the activities of several endogenous genes, including the genes coding for α1(I) and α2(I) collagen and the metalloproteinase MMP-13.
FIG. 5.
A-CEBP inhibits C1R activity. (A) Cotransfection in HepG2 cells of the C/EBP/CAT reporter gene and the C/EBPα and A-C/EBP expression plasmids. (B) Transient cotransfections of the −378COL1A2/CAT and A-C/EBP expression plasmids in fibroblasts cultured with or without TNF-α. Controls included transfections with the CMV-500 empty vector or with the A-CREB construct. Values are expressed relative to those of the transfections of the −378COL1A2/CAT construct without TNF-α treatment. Asterisks indicate values that are statistically different from those of the controls (CONT) (Mann-Whitney U test, P < 0.05).
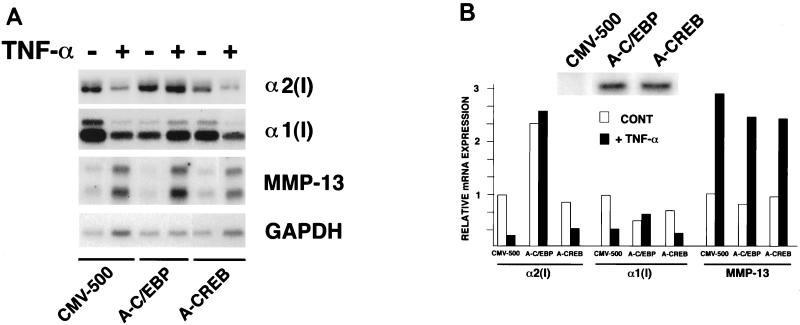
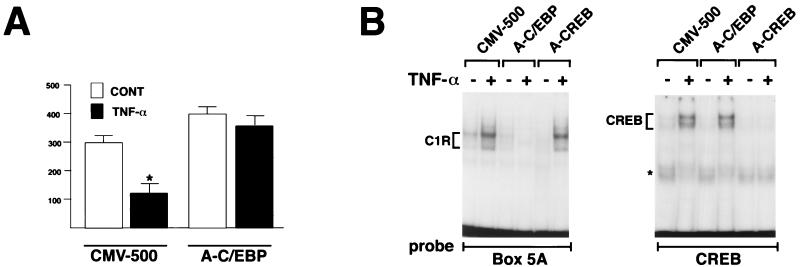
Steady-state levels of α2(I) collagen mRNA remained virtually unaltered in TNF-α-treated cells that express A-C/EBP (Fig. 6). By contrast, α2(I) collagen mRNA levels were reduced after TNF-α treatment in cells stably transfected with CMV-500 or A-CREB (Fig. 6). A run-on transcription assay showed that A-C/EBP block of COL1A2 inhibition by TNF-α occurs at the transcriptional level (Fig. 7A). The EMSA confirmed the specificity of A-C/EBP to inhibit C/EBP binding to box 5A (Fig. 7B). A-C/EBP had also an effect on the coordinately expressed α1(I) collagen (COL1A1) gene. As for COL1A2, stable expression of A-C/EBP, but not A-CREB, led to block of TNF-α-induced downregulation of COL1A1 (Fig. 6). Additionally, COL1A1 expression was reproducibly less in the TNF-α-untreated A-C/EBP transfectants than in the CMV-500 counterparts (Fig. 6). This result is opposite what is observed with the COL1A2 gene; together, the data may suggest that distinct mechanisms underlie C/EBP regulation of the type I collagen genes. Consistent with the established involvement of AP1 in the upregulation of metalloproteinases (4), A-C/EBP had no effect on TNF-α stimulation of MMP-13 (Fig. 6). This last result and previous work by Brenner et al. (4) thus indicate that the opposing effects of TNF-α on collagen downregulation (matrix deposition) and metalloproteinase upregulation (matrix degradation) are apparently transduced through distinct signaling pathways.
FIG. 6.
A-C/EBP inhibits TNF-α repression of type I collagen. (A) Northern analysis of total RNA extracted from control and TNF-α-treated NIH 3T3 cells harboring stably integrated copies of the empty CMV-500 vector, A-C/EBP, or A-CREB. The probes used are indicated on the right. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. (B) Summary of the Northern data. The Western blot against the anti-FLAG epitope (inset) documents the levels of A-C/EBP and A-CREB produced by the stable transfectants. CONT, control.
FIG. 7.
A-C/EBP acts at the transcriptional level. (A) Run-on transcription assay of the endogenous COL1A2 gene in NIH 3T3 stably transfected with either the empty vector (CMV-500) or the A-C/EBP expression vector. Cells were cultured for 12 h with or without TNF-α addition. Values are expressed as arbitrary units of COL1A2 expression. The histograms summarize the data (average ± standard deviation) of three independent tests. The asterisk indicates a value statistically different from that of the controls (CONT) (Mann-Whitney U test, P < 0.05). (B) EMSA of nuclear extracts purified from the CMV500, A-C/EBP, and A-CREB samples cultured for 24 h with or without TNF-α and assayed with box 5A (left) or high-affinity recognition sequences for CREB (right); the asterisk indicates a nonspecific band.
In summary, this study provides evidence for involvement of C/EBPs in ECM remodeling and implicates specifically this small group of bZIP proteins in mediating TNF-α inhibition of matrix accumulation. Our conclusion is based on the following observations. First, TNF-α induces production of C/EBPβ. Second, the cytokine induction translates into increased C/EBPβ binding to the TNF-α responsive element of the COL1A2 gene. Third, A-C/EBP relieves the COL1A2 gene from TNF-α inhibition by sequestering all C/EBPs from DNA binding. It is therefore plausible to conclude that TNF-α downregulates COL1A2 transcription at least in part by inducing C/EBP molecules with repressing activity. C/EBP-induced repression could be the result of higher binding affinity of the p35-p20 heterodimer or could be dictated by protein interactions taking place within the context of the CyRC. Although only suggestive, the greater abundance of p20 than of p35 after TNF-α treatment seems to favor the former possibility.
The precise mechanism responsible for the generation of the C/EBPβ isoforms is still controversial. Two recent reports have raised the possibility that these C/EBP isoforms are the products of proteolysis rather than translational initiation, as previously thought (5, 12, 23). C/EBPβ participation in TNF-α-induced inhibition of type I collagen expression is not indispensable, in that other C/EBPs compensate for its loss in c/ebpβ−/− fibroblasts (data not shown). Our study demonstrates two additional new points which are of relevance to the understanding of ECM remodeling and the etiopathogenesis of diseased conditions. First, TNF-α downregulates the type I collagen genes coordinately through the action of the bZIP proteins. Second, the opposing effects of TNF-α on ECM deposition and degradation are transduced through distinct nuclear signals which utilize C/EBPs and AP1, respectively.
ACKNOWLEDGMENTS
P. Greenwel and S. Tanaka made equal contributions to this work.
We thank K. Calame, P. Johnson, D. Ron, U. Schibler, and S. Shapiro for generously providing invaluable materials for this study and V. Vinson for critical review of the manuscript. We also thank K. Johnson for typing the manuscript and R. Ni and C. Else for excellent technical assistance.
This work was supported by grants AR38648 and AA12196 from the National Institutes of Health and by the Arthritis Foundation.
REFERENCES
- 1.Ahn S, Olive M, Aggarwal S, Krylov D, Ginty D D, Vinson C. A dominant negative inhibitor of CREB reveals that is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alevizopoulos A, Mermod N. Antagonistic regulation of a proline-rich transcription factor by transforming growth factor β and tumor necrosis factor α. J Biol Chem. 1996;271:29672–29681. doi: 10.1074/jbc.271.47.29672. [DOI] [PubMed] [Google Scholar]
- 3.Baker S J, Reddy E P. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17:3261–3270. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]
- 4.Brenner D A, O'Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumor necrosis factor-α. Nature. 1989;337:661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- 5.Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–576. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- 6.Fujita T, Reis L F, Watanabe N, Kimura Y, Taniguchi T, Vilcek J. Induction of the transcription factor IRF-1 and interferon mRNAs by cytokines and activators of the second messenger pathways. Proc Natl Acad Sci USA. 1989;86:9936–9940. doi: 10.1073/pnas.86.24.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenwel P, Hu W, Kohanski R D, Ramirez F. Tyrosine dephosphorylation of nuclear proteins mimics transforming growth factor β1 stimulation of α2(I) collagen gene expression. Mol Cell Biol. 1995;15:6813–6819. doi: 10.1128/mcb.15.12.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inagaki Y, Truter S, Tanaka M, Di Liberto M, Ramirez F. Overlapping pathways mediate the opposing actions of tumor necrosis factor-α and transforming growth factor-β on α2(I) collagen gene transcription. J Biol Chem. 1995;263:13909–13915. doi: 10.1074/jbc.270.7.3353. [DOI] [PubMed] [Google Scholar]
- 9.Kadonaga J T, Tjian R. Affinity purification of sequence-specific DNA-binding proteins. Proc Natl Acad Sci USA. 1986;83:5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahari V M, Chen Y R, Bashir M M, Rosenbloom J, Uitto J. Tumor necrosis factor-α down-regulates human elastin gene expression. J Biol Chem. 1992;267:26134–26141. [PubMed] [Google Scholar]
- 11.Kahari V M, Chen Y Q, Su M W, Ramirez F, Uitto J. Tumor necrosis factor α and interferon-γ suppress the activation of human type I collagen gene expression by transforming growth factor-β1. J Clin Investig. 1990;86:1489–1495. doi: 10.1172/JCI114866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lincoln A J, Monczak Y, Williams S C, Johnson P F. Inhibition of CCAAT/enhancer-binding protein α and β translation by upstream open reading frames. J Biol Chem. 1998;273:9552–9560. doi: 10.1074/jbc.273.16.9552. [DOI] [PubMed] [Google Scholar]
- 13.McKnight S L. CCAAT/enhancer binding protein. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 771–795. [Google Scholar]
- 14.Moitra J, Mason M M, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, Reitman M L, Vinson C. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanes M S, Rubin J, Titus G, Hendy G, Catherwood B D. Tumor necrosis factor alpha inhibits 1,25 dihydroxy-vitamin D3-stimulated bone Gla protein synthesis in rat osteosarcoma cells (ROS 17/2.8) by a pretranslational mechanism. Endocrinology. 1991;128:2577–2584. doi: 10.1210/endo-128-5-2577. [DOI] [PubMed] [Google Scholar]
- 16.Olive M, Williams S C, Dezan C, Johnson P F, Vinson C. Design of a C/EBP-specific, dominant-negative bZIP protein with both inhibitory and gain-of-function properties. J Biol Chem. 1996;271:2040–2047. doi: 10.1074/jbc.271.4.2040. [DOI] [PubMed] [Google Scholar]
- 17.Ron D, Braiser A R, McGehee R E, Habener J F. Tumor necrosis factor-induced reversal of adipocytic phenotype of 3T3-L1 cells is preceded by loss of nuclear CCAAT/enhancer binding protein (C/EBP) J Clin Investig. 1992;89:223–233. doi: 10.1172/JCI115566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi P, Karsenty G, Roberts A B, Roche N S, Sporn M B, de Crombrugghe B. A nuclear factor 1 binding site mediates the transcriptional activation of a type I collagen promoter by transforming growth factor β. Cell. 1988;52:405–414. doi: 10.1016/s0092-8674(88)80033-3. [DOI] [PubMed] [Google Scholar]
- 19.Solis-Herruzo J A, Brenner D A, Chojkier M. Tumor necrosis factor α inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J Biol Chem. 1988;263:5841–5845. [PubMed] [Google Scholar]
- 20.Tracey K J, Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- 21.Vallejo M, Ron D, Miller C P, Habener J F. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CCAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci USA. 1993;90:4679–4683. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinson C R, LaMarco K L, Johnson P F, Landschulz W H, McKnight S L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988;2:801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- 23.Welm A L, Timchenko N A, Darlington G J. C/EBPα regulates generation of C/EBPβ isoforms through activation of specific proteolytic cleavage. Mol Cell Biol. 1999;19:1695–1704. doi: 10.1128/mcb.19.3.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]