Abstract
Objectives
Various drug‐sensitivity markers have been reported to be associated with tumor progression and chemotherapy resistance. Detailed expression profiles of sensitivity markers for cytotoxic chemotherapy in pulmonary large cell neuroendocrine carcinoma (LCNEC) remain unclear. Herein, we aimed to clarify the correlation between the expression of drug‐sensitivity markers and clinicopathological features, prognostic impact, and status of tumor immunity in patients with LCNEC.
Methods
We retrospectively analyzed the correlation between clinicopathological features and the expression of drug‐sensitivity‐related markers, including vascular endothelial growth factor 2 (VEGFR2), thymidylate synthase (TS), tubulin beta 3 class III (TUBB3), topoisomerase I (Topo‐I), and Topo‐II in 92 surgically resected LCNEC samples. Furthermore, we examined the prognostic significance of expression of these and their correlation with the immune cell status.
Results
Overall, high expression of TS, TUBB3, VEGFR2, Topo‐I, and Topo‐II was detected in 50 (54%), 31 (34%), 23 (25%), 65 (71%), and 36 (39%) samples, respectively. Univariate and multivariate analyses revealed that advanced pathological T and N factors, positive lymphatic permeation, and Topo‐II expression were independent unfavorable prognosticators for recurrence‐free survival, and advanced pathological T and N factors, Topo‐II positive expression, and TS positive expression were independent unfavorable prognosticators for overall survival. In terms of correlation with immune cell status, higher expression of VEGFR2 was closely linked to negative PD‐L1 expression.
Conclusions
These findings suggest that elevated Topo‐II and TS expression may contribute to poor outcomes through protumoral biology in patients with LCNEC, and elevated VEGFR2 expression might negatively impact tumor immune reactions in LCNEC.
Keywords: chemosensitivity, large cell neuroendocrine carcinoma, lung cancer, thymidylate synthase, topoisomerase II
High expression of chemosensitive markers such as Topo‐II and TS may be responsible for the poor prognosis of LCNEC patients. Furthermore, VEGFR2 expression is negatively correlated with antitumor immune response and may not be just a chemosensitive marker. The expression of chemosensitive markers is an important prognostic factor for LCNEC and may be a biomarker of resistance to overall treatment of lung cancer, such as immune checkpoint inhibitors.

Abbreviations and acronyms
- CI
Confidence interval
- Foxp3
Forkhead box protein P3
- HR
Hazard ratio
- LCNEC
Large cell neuroendocrine carcinoma
- ND
Nodal dissection
- NSCLC
Non‐small cell lung cancer
- OS
Overall survival
- PD‐L1
Programmed cell death ligand 1
- RFS
Recurrence‐free survival
- SCLC
Small cell carcinoma
- TS
Thymidylate synthase
- Topo‐I
Topoisomerase I
- Topo‐II
Topoisomerase II
- TUBB3
Tubulin beta 3 class III
- VEGFR2
Vascular endothelial growth factor receptor 2
INTRODUCTION
Large cell neuroendocrine carcinoma (LCNEC) is a relatively rare tumor, accounting for approximately 1.3–4% of all lung cancers. 1 , 2 Notably, LCNEC has been treated as a subtype of non‐small‐cell lung cancer (NSCLC). Conversely, LCNEC is currently classified as a neuroendocrine carcinoma in addition to small cell carcinoma (SCLC) and carcinoid tumor. Although LCNEC is resistant to cytotoxic chemotherapy, chemotherapeutic regimens for both NSCLC and SCLC could be universally applicable to LCNEC. Clinically, there is accumulating evidence regarding the efficacy of immune checkpoint inhibitors in LCNEC. 3 , 4 However, from the perspective of genomic profiles, the frequency of driver gene alterations in LCNEC is much lower than that in adenocarcinoma, 5 and cytotoxic chemotherapy remains the main therapeutic option for treating patients with advanced LCNEC.
Previous studies have reported that various proteins are involved in the sensitivity to cytotoxic chemotherapy in lung cancer. In NSCLC, fluorinated pyrimidine anticancer agents such as 5‐fluorouracil (5‐FU), TS‐1, and pemetrexed, are key drugs for cytotoxic chemotherapy. Thymidylate synthase (TS), an enzyme that catalyzes the methylation of deoxyuridine monophosphate to deoxythymidine monophosphate, 6 , 7 is a target molecule of fluorinated pyrimidine anticancer agents; the efficacy of these agents, as well as their prognosis, are reportedly associated with TS expression. 8 In addition, antimicrotubule agents, such as taxanes, are crucial cytotoxic chemotherapeutic regimens for NSCLC. Reportedly, the expression of tubulin beta 3 class III (TUBB3) correlates with the response to antimicrotubule agents in NSCLC. 9 Previous clinical studies have shown that NSCLC patients with higher TUBB3 levels are more resistant to paclitaxel/vinorelbine‐based chemotherapy than NSCLC patients with low TUBB3 levels. 10 Vascular endothelial growth factor receptor 2 (VEGFR2) is an important protein for tumor angiogenesis as a receptor of VEGF‐A, and ramucirumab is a humanized IgG1 monoclonal antibody that targets the extracellular domain of VEGFR2. 11 , 12 In terms of neuroendocrine histology, topoisomerase (Topo)‐I and Topo‐II are key enzymes as targets of SCLC. Topoisomerase plays a vital role in cellular reproduction and DNA organization, and Topo‐II is a target for Topo‐II inhibitors, including etoposide and anthracyclines such as amrubicin. 13 , 14 High expression of Topo‐II is also known to be a potential biomarker for predicting response to anthracycline chemotherapy. 15 Topo‐I is a target for Topo‐I inhibitors such as irinotecan or nogitecan, which are key drugs for treating SCLC. However, owing to the relatively rare histology of LCNEC, the expression profiles of these chemosensitive markers and, in turn, the prognostic impacts of the expression of these in terms of LCNEC have not been well‐investigated. Currently, platinum‐based combination chemotherapy, including etoposide or irinotecan, is ordinarily administered to patients with recurrent or advanced LCNEC based on the therapeutic strategy for SCLC. Owing to limited efficacy, the outcome of patients receiving such regimens is dismal, necessitating the selection of an appropriate chemotherapeutic agent. To resolve the chemotherapeutic efficacy of LCNEC, we need to elucidate the underlying mechanism through which targeting molecules are associated with tumor progression and survival. Moreover, there are no reports on the correlation between the expression of these biomarkers and the status of the tumor immune microenvironment, such as programmed cell death ligand 1 (PD‐L1), CD4, CD8, and forkhead box protein P3 (Foxp3) expression.
Accordingly, the aim of this study was to clarify the expression of chemosensitivity‐related markers, such as VEGFR2, TS, TUBB3, Topo‐I, and Topo‐II, in LCNEC, and establish a correlation between the expression of these and clinicopathological features and their prognostic impact, using resected surgical specimens. We attempted to clarify the correlation between the expression of biomarkers related to chemosensitivity and immune cell status.
METHODS
Patients
The present study was a multi‐institutional joint retrospective study conducted by researchers at Gunma University Hospital, Gunma Prefectural Cancer Center, Maebashi Red Cross Hospital, National Hospital Organization Takasaki General Medical Center, and National Hospital Organization Shibukawa Medical Center. The research protocol was approved by the institutional review board of each participating institution, according to the Helsinki Declaration.
The data and tissue samples of 92 patients who underwent surgical resection of LCNEC between April 2000 and March 2016 were collected as previously described. 16 The histology of LCNEC was confirmed at Gunma University according to World Health Organization criteria. The stages of pathological tumor‐node metastasis were established using the International System for Staging Lung Cancer adopted by the American Joint Committee on Cancer and the Union Internationale Center le Cancer. The TNM stage was determined according to the eighth edition of the TNM classification.
Immunohistochemistry
Immunohistochemistry was performed as described previously 17 , 18 using the following antibodies: rabbit monoclonal anti‐Topo‐I (1:100; Abcam), rabbit polyclonal anti‐Topo‐II (1:100, ab180393; Abcam), rabbit monoclonal anti‐VEGFR2 (1:400; Abcam), mouse monoclonal anti‐TUBB3 (1:500; MMS‐435P; Convance), rabbit polyclonal anti‐TS (1:1600, clone RTSSA; Taiho Pharmaceutical), and rabbit polyclonal anti‐Ki67 (1:100, ab180393; Abcam). Cells were considered positive for Topo‐I, Topo‐II, and Ki67 if positive staining was present in the nuclei and deemed positive for TUBB3 and VEGFR2 if positive staining was present in the cytoplasm. TS levels were determined based on the presence or absence of nuclear or cytoplasmic staining. For each protein, the percentage of positive cells was evaluated using a semiquantitative scoring method, and samples were scored according to the percentage of positive cells: 1, <10%; 2, 10% to <25%; 3, 25% to <50%; 4, ≥50%. We compared survival data between groups showing high and low expression, with various cut‐off scores for the expression of each protein. In the current study, Topo‐I and Topo‐II expression was defined as high if the tumors contained cancer cells with a staining score ≥3, with low expression defined as scores ˂3. VEGFR2 expression was defined as high if the tumors contained cancer cells with a staining ≥2, with low expression defined as a score of 1. TUBB3 and TS expression was deemed high if the tumors contained cancer cells with a staining score ≥4, with low expression defined as scores ˂4. The percentage of Ki67‐positive cells was calculated for each sample, and the median value was used as the cut‐off score. The expression of immune markers such as PD‐L1, CD8, CD4, and Foxp3 was assessed as previously described. 16 Tissue sections were examined in a blinded fashion using light microscopy by two authors (Y.O. and K.K.). In case of any discrepancies, both researchers evaluated the slides simultaneously until agreement was reached on the final assessment. Neither researcher had any knowledge of the patient's outcome.
Statistical analysis
Recurrence‐free survival (RFS) was defined as the time from the date of surgery to the date of recurrence or death from any cause. Overall survival (OS) was defined as the time interval between the date of tumor resection and the date of death from any cause or censored date. For univariable analyses, survival rates were estimated using the Kaplan–Meier method, and differences in survival between subgroups were compared using the log‐rank test. Multivariate analyses were performed using the Cox proportional hazards model. Forward and backward stepwise procedures were performed to determine the prognostic effects of the combined factors. A chi‐squared test was performed to evaluate the relationship between categorical variables, and Student's t‐test was used to evaluate continuous variables. All reported p values were two‐sided, with a significance level of ˂0.05. All statistical analyses were performed using SPSS Statistics 24 statistical software (SPSS II for Windows, standard version 20.0; SPSS Inc.).
RESULTS
Patient demographics and immunohistochemical findings
Table 1 presents the patient characteristics according to chemosensitive markers. The median age was 74 years (range 36–88 years) and 80 patients (87%) were men. A history of smoking was observed in 88 patients (96%). Pure LCNEC histology was observed in 74 patients (80%), and combined histology with histology other than LCNEC was observed in 18 patients (20%). Patient characteristics limited to pure LCNEC according to chemosensitive markers are shown in Table A1. The median age was 74 years (range 36–88 years) and 64 (86%) were men. The patient background was nearly identical to that of all patients with LCNEC. Immunohistochemical examination of 92 primary samples from LCNEC patients was performed, and representative findings for each marker are shown in Figure 1. The score distribution for each marker based on histology is presented in Table A2. High expression of TS, TUBB3, VEGFR2, Topo‐I, and Topo‐II was observed in 50 (54%), 31 (34%), 23 (25%), 65 (71%), and 36 (39%) samples and 42 (57%), 23 (31%), 19 (26%), 50 (68%), and 29 (39%) pure LCNEC samples, respectively. Mean scores for each marker of all samples are as follows (mean ± standard deviation): TS 3.3 ± 0.92, TUBB3 3.0 ± 0.94, VEGFR2 1.3 ± 0.61, Topo‐I 3.0 ± 1.1, and Topo‐II 2.2 ± 0.95. For tumor cells, the median Ki67 staining rate was 28%. The positive expression of Topo‐I was significantly associated with lower T and N factors and a lower Ki‐67 labeling index, whereas positive Topo‐II expression was strongly associated with a higher Ki‐67 labeling index. Positive VEGFR2 expression was significantly related to lymphatic permeation and pleural invasion, but not vascular invasion. Moreover, positive TS expression was significantly correlated with smoking and vascular invasion. None of the examined characteristics correlated with TUBB3 expression.
TABLE 1.
Patient characteristics according to chemosensitive markers
| Different variables | Total | Topoisomerase I | Topoisomerase II | VEGFR2 | TUBB3 | TS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 92 | High N = 65 | Low N = 27 | p | High N = 36 | Low N = 56 | p | High N = 23 | Low N = 69 | p | High N = 31 | Low N = 61 | p | High N = 50 | Low N = 42 | p | |
| Age | ||||||||||||||||
| ≤74/>74 years | 52/40 | 36/29 | 16/11 | 0.81 | 22/14 | 30/26 | 0.52 | 11/12 | 41/28 | 0.34 | 20/11 | 32/29 | 0.37 | 25/25 | 27/15 | 0.21 |
| Gender | ||||||||||||||||
| Male/female | 80/12 | 55/10 | 25/2 | 0.49 | 31/5 | 49/7 | >0.99 | 20/3 | 60/9 | >0.99 | 28/3 | 52/9 | 0.74 | 44/6 | 36/6 | 0.76 |
| Smoking | ||||||||||||||||
| Yes/no | 88/4 | 63/2 | 25/2 | 0.57 | 34/2 | 54/2 | 0.64 | 21/2 | 67/2 | 0.25 | 29/2 | 59/2 | 0.60 | 50/0 | 38/4 | 0.04 |
| Histology | ||||||||||||||||
| LCNEC/combined LCNEC | 74/18 | 50/15 | 24/3 | 0.25 | 29/7 | 45/11 | >0.99 | 19/4 | 55/14 | >099 | 22/9 | 52/9 | 0.16 | 42/8 | 32/10 | 0.43 |
| Pathological stage | ||||||||||||||||
| IA/IB‐ | 29/63 | 19/46 | 10/17 | 0.47 | 10/26 | 19/37 | 0.54 | 6/17 | 23/46 | 0.52 | 11/20 | 18/43 | 0.56 | 14/36 | 15/27 | 0.43 |
| T factor | ||||||||||||||||
| T1/T2, T3 or T4 | 40/52 | 40/25 | 0/27 | <0.01 | 22/14 | 18/38 | 0.09 | 6/17 | 34/35 | 0.05 | 15/16 | 25/36 | 0.51 | 19/31 | 21/21 | 0.29 |
| N factor | ||||||||||||||||
| N0/N1, N2 or N3 | 69/23 | 53/12 | 16/11 | 0.03 | 25/11 | 44/12 | 0.33 | 18/5 | 51/18 | 0.78 | 25/6 | 44/17 | 0.45 | 38/12 | 31/11 | 0.81 |
| Lymphatic permeation | ||||||||||||||||
| Yes/no | 61/31 | 46/19 | 15/12 | 0.22 | 25/11 | 36/20 | 0.65 | 20/3 | 41/28 | 0.02 | 19/12 | 42/19 | 0.49 | 29/21 | 32/10 | 0.07 |
| Vascular invasion | ||||||||||||||||
| Yes/NO | 67/25 | 49/16 | 18/9 | 0.44 | 29/7 | 38/18 | 0.23 | 20/3 | 47/22 | 0.11 | 24/7 | 43/18 | 0.62 | 33/17 | 34/8 | 0.01 |
| Pleural invasion | ||||||||||||||||
| Yes/no | 36/56 | 29/36 | 7/20 | 0.10 | 15/21 | 21/35 | 0.82 | 14/9 | 22/47 | 0.02 | 10/21 | 26/35 | 0.73 | 20/30 | 16/26 | >0.99 |
| Ki‐67 labeling index | ||||||||||||||||
| High/low | 45/47 | 38/27 | 7/20 | <0.01 | 27/9 | 18/38 | <0.01 | 12/11 | 33/36 | 0.81 | 18/27 | 27/34 | 0.69 | 27/23 | 18/24 | 0.31 |
| Adjuvant therapy | ||||||||||||||||
| Yes/no | 22/70 | 15/50 | 7/20 | 0.79 | 9/27 | 13/43 | >0.99 | 6/17 | 16/53 | 0.78 | 8/23 | 14/47 | >0.99 | 9/41 | 13/29 | 0.01 |
Note: Bold values shown p < 0.05. Abbreviations: LCNEC, large cell neuroendocrine carcinoma; TUBB3, class III tubulin beta; TS, thymidine synthase; VEGFR2, vascular endothelial growth factor receptor 2.
FIGURE 1.
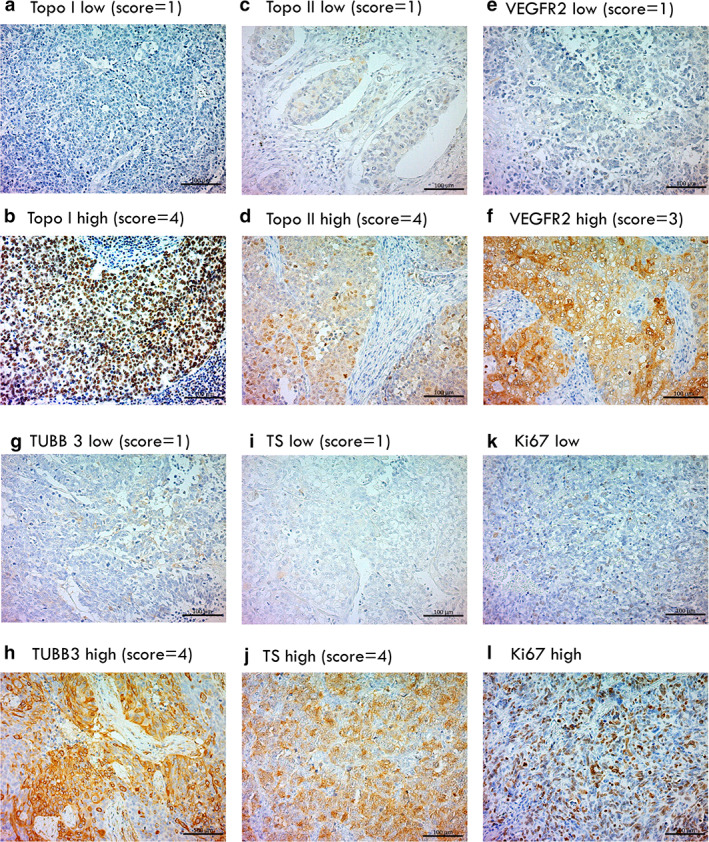
Representative immunohistochemical findings of LCNEC. Topo‐I low (a) and topo‐I high (b). Topo‐II low (c), topo‐II high (d), VEGFR2 low (e), VEGFR2 high (f), TUBB3 low (g), TUBB3 high (h), TS low (i), TS high (j), Ki67 low (k), and Ki67 high (l). LCNEC, large cell neuroendocrine carcinoma; Topo‐I, topoisomerase I; Topo‐II, topoisomerase II; TS, thymidylate synthase; VEGFR2, vascular endothelial growth factor 2; TUBB3, tubulin beta 3 class III
Postoperative survival was measured using the date of surgery as the start date, with a median follow‐up period of 1113 days (range 16–5131 days).
Univariate and multivariate analysis
For all patients, the median RFS and OS were 475 and 641 days, respectively. Of the 92 patients, 36 had recurrences and 38 died after the initial surgery. Table 2 presents the univariate and multivariate analyses of RFS after surgery. Univariate analyses identified that advanced pathological T and N factors, positive lymphatic permeation, high Topo‐II expression, and high VEGFR2 expression were unfavorable prognostic factors for RFS. Advanced pathological T and N factors, positive lymphatic permeation, and high expression of Topo‐II were independent worse prognosticactors for RFS. Table A3 shows the univariate and multivariate analyses of RFS when tumors were limited to pure LCNEC histology (n = 72) without any combined histology. On assessing tumors with pure LCNEC histology, advanced T factor, positive lymphatic permeation, and high Topo‐II expression were found to be independent unfavorable prognostic factors for RFS.
TABLE 2.
Univariable and multivariable analyses for RFS (all cases)
| Different variables | Total | Univariable | Multivariable | ||
|---|---|---|---|---|---|
| N = 92 | 5‐y RFS rate (%) | p | Hazard ratio (95% CI) | p | |
| Age | |||||
| <75 years | 52 | 50.4 | – | ||
| ≥75 years | 40 | 37.2 | 0.164 | – | – |
| Sex | |||||
| Male | 80 | 40.9 | – | ||
| Female | 12 | 63.6 | 0.203 | – | – |
| Smoking | |||||
| Yes | 88 | 43.4 | – | ||
| No | 4 | NR | 0.798 | – | – |
| Histology | |||||
| LCNEC | 74 | 45.4 | – | ||
| Combined LCNEC | 18 | 37.9 | 0.509 | – | – |
| T factor | |||||
| T1 | 40 | 59.9 | 1 | ||
| T2‐4 | 52 | 31.0 | 0.003 | 2.75 (1.46–5.19) | 0.002 |
| N factor | |||||
| N0 | 69 | 49.8 | 1 | ||
| N1‐3 | 23 | 24.9 | 0.038 | 1.89 (1.02–3.50) | 0.043 |
| Lymphatic permeation | |||||
| Yes | 61 | 33.4 | 1 | ||
| No | 31 | 62.7 | 0.020 | 2.01 (1.01–4.01) | 0.048 |
| Vascular invasion | |||||
| Yes | 67 | 38.7 | – | ||
| No | 25 | 55.0 | 0.192 | – | – |
| Ki‐67 labeling index | |||||
| High | 45 | 40.6 | – | ||
| Low | 47 | 45.6 | 0.611 | – | – |
| Adjuvant therapy | |||||
| Yes | 22 | 45.8 | – | ||
| No | 70 | 42.7 | 0.814 | – | – |
| Topoisomerase I | |||||
| High | 65 | 44.3 | – | ||
| Low | 27 | 42.2 | 0.974 | – | – |
| Topoisomerase II | |||||
| High | 36 | 24.7 | 2.35 (1.29–4.29) | ||
| Low | 56 | 54.8 | 0.006 | 1 | 0.005 |
| VEGFR2 | |||||
| High | 23 | 24.2 | 1.73 (0.92–3.26) | ||
| Low | 69 | 49.5 | 0.011 | 1 | 0.090 |
| TUBB3 | |||||
| High | 31 | 56.6 | |||
| Low | 61 | 36.5 | 0.105 | ||
| TS | |||||
| High | 50 | 33.4 | |||
| Low | 42 | 57.9 | 0.052 | ||
Note: Bold values shown p < 0.05. Abbreviations: CI, confidence interval; DFS, disease free survival; LCNEC, large cell neuroendocrine carcinoma; MST, median survival time; NR, not reached; OS, overall survival; RFS, recurrence‐free survival; TUBB3, class III tubulin beta; TS, thymidine synthase; VEGFR2, vascular endothelial growth factor receptor 2.
Table 3 shows univariable and multivariable analyses of OS after lung resection in LCNEC. Univariate analyses revealed that advanced pathological T and N factors, high Topo‐II expression, high VEGFR2 expression, low TUBB3 expression, and high TS expression were identified as unfavorable prognostic factors for OS. Among them, advanced pathological T and N factors, as well as high Topo‐II expression and high TS expression, were shown to be independent prognostic factors for predicting worse outcomes. For OS limited to pure LCNEC, older age, advanced pathological T and N factors, high VEGFR2 expression, and high TS expression were identified as significant unfavorable prognostic factors in multivariable analyses (Table A4). Figure 2 shows survival differences based on the expression of chemosensitive markers.
TABLE 3.
Univariable and multivariable analyses for OS (all cases)
| Different variables | Total | Univariable | Multivariable | ||
|---|---|---|---|---|---|
| N = 92 | 5‐y OS rate (%) | p | Hazard ratio (95% CI) | p | |
| Age | |||||
| <75 years | 52 | 54.7 | – | ||
| ≥75 years | 40 | 48.9 | 0.129 | – | – |
| Sex | |||||
| Male | 80 | 48.4 | – | ||
| Female | 12 | 71.4 | 0.264 | – | – |
| Smoking | |||||
| Yes | 88 | 50.2 | – | ||
| No | 4 | NR | 0.301 | – | – |
| Histology | |||||
| LCNEC | 74 | 49.4 | – | ||
| Combined LCNEC | 18 | 60.1 | 0.513 | – | – |
| T factor | |||||
| T1 | 40 | 67.0 | 1 | ||
| T2‐4 | 52 | 39.4 | 0.010 | 2.55 (1.17–5.58) | 0.019 |
| N factor | |||||
| N0 | 69 | 57.7 | 1 | ||
| N1‐3 | 23 | 31.3 | 0.031 | 2.50 (1.22–5.12) | 0.012 |
| Lymphatic permeation | |||||
| Yes | 61 | 42.6 | – | ||
| No | 31 | 67.2 | 0.076 | – | – |
| Vascular invasion | |||||
| Yes | 67 | 47.7 | – | ||
| No | 25 | 58.5 | 0.718 | – | – |
| Ki‐67 labeling index | |||||
| High | 45 | 47.8 | – | ||
| Low | 47 | 53.3 | 0.623 | – | – |
| Adjuvant therapy | |||||
| Yes | 22 | 61.2 | – | ||
| No | 70 | 47.9 | 0.127 | – | – |
| Topoisomerase I | |||||
| High | 65 | 54.5 | – | ||
| Low | 27 | 44.3 | 0.468 | – | – |
| Topoisomerase II | |||||
| High | 36 | 29.5 | 2.41 (1.16–5.01) | ||
| Low | 56 | 64.5 | 0.001 | 1 | 0.018 |
| VEGFR2 | |||||
| High | 23 | 36.6 | 1.98 (0.94–4.18) | ||
| Low | 69 | 55.7 | 0.007 | 1 | 0.073 |
| TUBB3 | |||||
| High | 31 | 64.6 | 1 | ||
| Low | 61 | 43.4 | 0.024 | 2.19 (0.96–5.02) | 0.064 |
| TS | |||||
| High | 50 | 39.1 | 2.40 (1.10–5.22) | ||
| Low | 42 | 67.8 | 0.007 | 1 | 0.028 |
Note: Bold values shown p < 0.05. Abbreviations: CI, confidence interval; DFS, disease free survival; LCNEC, large cell neuroendocrine carcinoma; MST, median survival time; NR, not reached; OS, overall survival; TS, thymidine synthase; TUBB3, class III tubulin beta; VEGFR2, vascular endothelial growth factor receptor 2.
FIGURE 2.

Recurrence‐free survival (RFS) and overall survival (OS) curves according to expression levels of topo‐I (a, b), topo‐II (c, d), VEGFR2 (e, f), TUBB3 (g, h), TS (i, j), and Ki67 (k, l). Topo‐I, topoisomerase I; topo‐II, topoisomerase II; TS, thymidylate synthase; VEGFR2, vascular endothelial growth factor 2; TUBB3, tubulin beta 3 class III
Figure 3 shows the survival differences according to the combination of significant prognostic markers. Patients with LCNEC expressing both high Topo‐II and high VEGFR2 showed significantly shorter RFS times than those in the other groups (Figure 3a). In terms of OS, patients with LCNEC expressing both low Topo‐II and low VEGFR2 or both low Topo‐II and low TS revealed significantly longer OS times than the other groups (Figure 3b,c). In combination with Topo‐II and TUBB3, patients with LCNEC expressing high Topo‐II and low TUBB3 presented significantly shorter OS than the other groups (Figure 3d).
FIGURE 3.

Survival differences according to the combination of significant prognostic markers. RFS according to the combination of topo‐II and VEGFR2 (a). OS according to the combination of topo‐II and VEGFR2 (b) or topo‐II and TS (c). OS according to the combination of topo‐II and TUBB (d). Topo‐II, topoisomerase II; TS, thymidylate synthase; VEGFR2, vascular endothelial growth factor 2; TUBB3, tubulin beta 3 class III; RFS, recurrence‐free survival; OS, overall survival
Correlation between immune cell infiltration and expression of chemosensitivity markers
In terms of the association with immune cell status, we observed that a higher expression of VEGFR2 was closely linked to lower PD‐L1 expression in both all LCNEC and pure LCNEC (Tables 4 and A5). Higher Topo‐I expression was associated with higher Foxp3 expression in both all LCNEC and pure LCNEC (Tables 4 and A5). Furthermore, higher TUBB3 expression was significantly associated with higher Foxp3 expression, and higher TS expression was associated with higher CD4 expression in pure LCNEC (Table A5).
TABLE 4.
Correlation between immune cell infiltrations with expression of chemosensitive markers
| Different variables | Total | Topoisomerase I | Topoisomerase II | VEGFR2 | TUBB3 | TS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 92 | High N = 65 | Low N = 27 | p | High N = 36 | Low N = 56 | p | High N = 23 | Low N = 69 | p | High N = 31 | Low N = 61 | p | High N = 50 | Low N = 42 | p | |
| PD‐L1 expression | ||||||||||||||||
| High/low | 69/23 | 49/16 | 20/7 | 0.90 | 27/9 | 42/14 | 1.0 | 13/10 | 56/13 | 0.018 | 26/5 | 43/18 | 0.16 | 39/11 | 30/12 | 0.47 |
| CD8 expression in whole tumor | ||||||||||||||||
| High/low | 43/49 | 32/33 | 11/16 | 0.46 | 18/18 | 25/31 | 0.62 | 9/14 | 40/29 | 0.12 | 16/15 | 27/34 | 0.50 | 24/26 | 19/23 | 0.79 |
| CD8 expression in cancer nest | ||||||||||||||||
| High/low | 24/68 | 20/45 | 4/23 | 0.11 | 10/26 | 14/42 | 0.77 | 8/15 | 16/53 | 0.27 | 9/22 | 15/46 | 0.65 | 14/36 | 10/32 | 0.65 |
| CD8 expression in cancer stroma | ||||||||||||||||
| High/low | 50/42 | 39/26 | 11/16 | 0.09 | 19/17 | 31/25 | 0.81 | 8/15 | 35/34 | 0.23 | 20/11 | 30/31 | 0.16 | 22/28 | 22/20 | 0.73 |
| CD4 expression in whole tumor | ||||||||||||||||
| High/low | 74/18 | 51/14 | 23/4 | 0.46 | 27/9 | 47/9 | 0.29 | 20/3 | 54/15 | 0.36 | 26/5 | 48/13 | 0.55 | 43/7 | 31/11 | 0.14 |
| CD4 expression in cancer nest | ||||||||||||||||
| High/low | 15/77 | 10/55 | 5/22 | 0.71 | 6/30 | 9/47 | 0.94 | 4/19 | 11/58 | 0.87 | 5/26 | 10/51 | 0.97 | 11/39 | 4/38 | 0.11 |
| CD4 expression in cancer stroma | ||||||||||||||||
| High/low | 76/16 | 52/13 | 24/3 | 0.31 | 27/9 | 49/7 | 0.12 | 21/2 | 55/14 | 0.20 | 26/5 | 50/11 | 0.82 | 43/7 | 33/9 | 0.35 |
| Foxp3 expression | ||||||||||||||||
| High/low | 42/50 | 34/31 | 8/19 | 0.047 | 15/21 | 27/29 | 0.54 | 13/10 | 29/40 | 0.23 | 18/13 | 24/37 | 0.09 | 20/30 | 22/20 | 0.24 |
Note: Bold values shown p < 0.05. Abbreviations: LCNEC, large cell neuroendocrine carcinoma; TS, thymidine synthase; TUBB3, class III tubulin beta; VEGFR2, vascular endothelial growth factor receptor 2.
DISCUSSION
This is the first study to conduct multivariate analyses to examine the prognostic impact of chemosensitivity‐related markers in pulmonary LCNEC. Herein, we reveal that high Topo‐II expression is an independent unfavorable prognostic factor for both OS and RFS in patients with LCNEC. Furthermore, we demonstrate that high TS expression is an independent unfavorable prognostic factor for OS after surgery. These results suggest that the expression levels of these chemosensitivity‐related proteins contribute to worse survival postsurgery, mediated via the progression of cancer.
Additionally, we revealed that high Topo‐II expression is an independent prognosticator of LCNEC in large samples after surgical resection, consistent with various malignancies, including SCLC. 19 , 20 , 21 , 22 Xue et al. have shown that Topo‐II inhibition inhibits NSCLC growth, invasion, and migration in vitro and tumor growth in vivo, which supports our results that high expression of Topo‐II is a prognostic factor after surgical resection. 23 In terms of the effect of Topo‐II expression on anticancer drug therapy, several previous reports have shown that high Topo‐II expression may contribute to a superior response to Topo‐II inhibitors in breast cancer and NSCLC. 13 , 24 , 25 , 26 Reportedly, patients with SCLC expressing high Topo‐II present longer progression free survival with amrubicin therapy than those with SCLC expressing low Topo‐II 13 ; however, this is not significant in NSCLC. 22 Currently, there is no data on whether high Topo‐II expression is correlated with the efficacy of Topo‐II inhibitors in LCNEC. Unfortunately, only 12 patients received standard platinum‐based chemotherapy for postoperative recurrence, thus we were unable to assess the correlation between Topo‐II expression and the efficacy of Topo‐II inhibitors. Makino et al. reported that LCNEC expresses Topo‐II more frequently than pulmonary adenocarcinoma, suggesting that Topo‐II is an important protein of LCNEC. 27 The results of our study indicate that high Topo‐II expression indicates a poor prognosis in patients with LCNEC through its molecular function in cancer DNA replication.
Regarding the prognostic impact of TS expression, high TS expression was found to be significantly correlated with a worse prognosis in various malignancies. 8 , 28 , 29 , 30 A previous study has revealed that the efficacy of pemetrexed or 5‐FU treatment is associated with TS expression levels, and higher TS expression is associated with resistance to these treatments. 31 , 32 Several reports on TS expression have focused on LCNEC histology. 7 , 33 , 34 Reportedly, expression levels of TS mRNA and TS protein are significantly higher in LCNEC than in squamous cell carcinoma and adenocarcinoma, 7 and Nagasaki et al. have shown the same tendency of expressional differences among histological types. 33 These results suggest the low efficacy of pemetrexed in patients with LCNEC presenting high TS expression, although multiple genes have been shown to be involved in the efficacy of pemetrexed. 34 A previous study failed to reveal the prognostic significance of the expression of TS in 46 patients with neuroendocrine carcinoma 33 ; however, our study, using a larger sample size, showed that high TS expression was an independent prognostic factor in 92 patients with LCNEC.
In the subgroup analysis of only pure LCNEC, high VEGFR2 expression was found to be an independent prognostic indicator of OS. Moreover, high VEGFR2 expression was a significantly worse prognosticator of both OS and RFS in all LCNEC patients, although not independently. Currently, there are no reports on the prognostic impact of angiogenic marker expression in LCNEC. Imai et al. have revealed the same prognostic tendency of VEGFR2 expression in pulmonary pleomorphic carcinoma, 17 indicating that VEGFR2 levels may have prognostic value in identifying patients who may respond to antiangiogenic therapies, such as bevacizumab and ramucirumab. As reported in the REVEL trial, ramucirumab, a monoclonal VEGFR‐2 antibody, improves survival in combination with docetaxel compared with docetaxel alone, following first‐line platinum‐based chemotherapy for both squamous and non‐squamous NSCLC. 35 Further studies are needed to determine whether the anticancer effect of ramucirumab is uniform among histological grades, even in LCNEC.
In the present study, we observed a significant association between chemosensitivity‐related markers and immune cell infiltration, and high VEGFR2 expression was closely linked to lower PD‐L1 expression. As VEGF upregulation promotes the presence of regulatory T cells, VEGFR2 expression might negatively impact tumor immune reactions. 36 , 37 , 38 , 39 In patients with LCNEC, VEGFR2 may be a negative marker of the tumor immune environment, suppressing the efficacy of immune checkpoint inhibitors. TS and TUBB3 suggest a positive impression of the tumor immune reaction. Further studies are needed to clarify the relationship between these markers and the tumor immune environment.
This study had several limitations. First, the results of our study were analyzed using surgically resected specimens, hence we did not observe the actual effect of anticancer drugs and their correlation with each protein. Therefore, whether or not the expression of chemosensitive markers is predictive of therapeutic outcomes remains unclear. Second, we did not detect any differences in the expressional status of histology other than in LCNEC. Third, although we included more than 90 LCNEC samples in this study, the total number of samples was still not substantially large.
CONCLUSIONS
In conclusion, we revealed the prognostic impact of chemosensitivity‐related protein expression in LCNECs. High Topo‐II expression and high TS expression were independent unfavorable prognosticators of LCNEC. These results imply that the expression of these chemosensitivity‐related proteins contributes to worse survival postsurgery, possibly mediated by the progression of cancer. In addition, high VEGFR2 expression was associated with worse prognosis and lower expression of immune cells such as PD‐L1 and CD8, suggesting that VEGFR2 might have a negative function in tumor immune reactions.
CONFLICT OF INTEREST
All authors participated in this study and agreed with the content of this paper. K.K. has received research grants and honoraria for lectures from Ono Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb. The authors declare no conflict of interest.
CONTRIBUTORS
The authors confirm contribution to the paper as follows. Study conception and design: Y.O., K.K., T.Y., and K.S. Data collection: Y.O., O.K., M.K., H.I., R.O., T.I., T.K., S.N., and T.N. Analysis and interpretation of results: Y.O., K.K., B.E.‐O., and T.O. Draft manuscript preparation: Y.O. and K.K. All authors reviewed the results and approved the final version of the manuscript.
ETHICS STATEMENT
(1) This material is the original work of the authors, which has not been previously published elsewhere. (2) The paper is not currently being considered for publication elsewhere. (3) The paper reflects the authors' own research and analysis in a truthful and complete manner. (4) The paper credits the meaningful contributions of co‐authors and co‐researchers. (5) The results are appropriately placed in the context of prior and existing research. (6) All sources used are properly disclosed. Literally copying of text must be indicated by using quotation marks and providing proper reference. (7) All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content. No author has any financial or other relationship that could lead to a conflict of interest. The research was approved by the Institutional Review Board.
Supporting information
Appendix S1. Supporting information
ACKNOWLEDGMENTS
The English in this document has been checked by a professional editor, a native English speaker. We would like to thank Editage (www.editage.com) for editing the English. This work was supported in part by JSPS KAKENHI (Grant Number JP20K09175).
Ohtaki Y, Kaira K, Yajima T, Erkhem‐Ochir B, Kawashima O, Kamiyoshihara M, et al. Comprehensive expressional analysis of chemosensitivity‐related markers in large cell neuroendocrine carcinoma of the lung. Thorac Cancer. 2021;12:2666–2679. 10.1111/1759-7714.14102
Funding information JSPS KAKENHI, Grant/Award Number: JP20K09175
Contributor Information
Kyoichi Kaira, Email: kkaira1970@yahoo.co.jp.
Toshiki Yajima, Email: yohtaki@gunma-u.ac.jp.
DATA AVAILABILITY STATEMENT
We do not have any shared data.
REFERENCES
- 1. Committee for Scientific Affairs TJAfTS , Shimizu H, Okada M, Tangoku A, Doki Y, Endo S, et al. Thoracic and cardiovascular surgeries in Japan during 2017: annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2020;68:414–49. [DOI] [PubMed] [Google Scholar]
- 2. Fasano M, Della Corte CM, Papaccio F, Ciardiello F, Morgillo F. Pulmonary large‐cell neuroendocrine carcinoma: from epidemiology to therapy. J Thorac Oncol. 2015;10:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Komiya T, Ravindra N, Powell E. Role of immunotherapy in stage IV large cell neuroendocrine carcinoma of the lung. Asian Pac J Cancer Prev. 2021;22:365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherman S, Rotem O, Shochat T, Zer A, Moore A, Dudnik E. Efficacy of immune check‐point inhibitors (ICPi) in large cell neuroendocrine tumors of lung (LCNEC). Lung Cancer. 2020;143:40–6. [DOI] [PubMed] [Google Scholar]
- 5. George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, et al. Integrative genomic profiling of large‐cell neuroendocrine carcinomas reveals distinct subtypes of high‐grade neuroendocrine lung tumors. Nat Commun. 2018;9:1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reidy‐Lagunes D. Thymidylate synthase expression in high‐grade neuroendocrine and non‐small cell lung cancer histotypes—is it ready for primetime? J Surg Oncol. 2010;102:10. [DOI] [PubMed] [Google Scholar]
- 7. Ibe T, Shimizu K, Nakano T, Kakegawa S, Kamiyoshihara M, Nakajima T, et al. High‐grade neuroendocrine carcinoma of the lung shows increased thymidylate synthase expression compared to other histotypes. J Surg Oncol. 2010;102:11–7. [DOI] [PubMed] [Google Scholar]
- 8. Kaira K, Ohde Y, Nakagawa K, Okumura T, Murakami H, Takahashi T, et al. Thymidylate synthase expression is closely associated with outcome in patients with pulmonary adenocarcinoma. Med Oncol. 2012;29:1663–72. [DOI] [PubMed] [Google Scholar]
- 9. Seve P, Reiman T, Dumontet C. The role of betaIII tubulin in predicting chemoresistance in non‐small cell lung cancer. Lung Cancer. 2010;67:136–43. [DOI] [PubMed] [Google Scholar]
- 10. Zhang HL, Ruan L, Zheng LM, Whyte D, Tzeng CM, Zhou XW. Association between class III beta‐tubulin expression and response to paclitaxel/vinorebine‐based chemotherapy for non‐small cell lung cancer: a meta‐analysis. Lung Cancer. 2012;77:9–15. [DOI] [PubMed] [Google Scholar]
- 11. Miettinen M, Rikala MS, Rys J, Lasota J, Wang ZF. Vascular endothelial growth factor receptor 2 as a marker for malignant vascular tumors and mesothelioma: an immunohistochemical study of 262 vascular endothelial and 1640 nonvascular tumors. Am J Surg Pathol. 2012;36:629–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clarke JM, Hurwitz HI. Targeted inhibition of VEGF receptor 2: an update on ramucirumab. Expert Opin Biol Ther. 2013;13:1187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miura Y, Kaira K, Sakurai R, Sunaga N, Saito R, Hisada T, et al. High expression of topoisomerase‐II predicts favorable clinical outcomes in patients with relapsed small cell lung cancers receiving amrubicin. Lung Cancer. 2018;115:42–8. [DOI] [PubMed] [Google Scholar]
- 14. Ceppi P, Longo M, Volante M, Novello S, Cappia S, Bacillo E, et al. Excision repair cross complementing‐1 and topoisomerase IIalpha gene expression in small‐cell lung cancer patients treated with platinum and etoposide: a retrospective study. J Thorac Oncol. 2008;3:583–9. [DOI] [PubMed] [Google Scholar]
- 15. Bartlett JM, McConkey CC, Munro AF, Desmedt C, Dunn JA, Larsimont DP, et al. Predicting anthracycline benefit: TOP2A and CEP17‐not only but also. J Clin Oncol. 2015;33:1680–7. [DOI] [PubMed] [Google Scholar]
- 16. Ohtaki Y, Kaira K, Atsumi J, Nagashima T, Kawashima O, Ibe T, et al. Prognostic significance of PD‐L1 expression and tumor infiltrating lymphocytes in large cell neuroendocrine carcinoma of lung. Am J Transl Res. 2018;10:3243–53. [PMC free article] [PubMed] [Google Scholar]
- 17. Imai H, Shimizu K, Kawashima O, Endoh H, Imaizumi K, Goto Y, et al. Clinical significance of various drug‐sensitivity markers in patients with surgically resected pulmonary pleomorphic carcinoma. Cancers (Basel). 2019;11:1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaira K, Takahashi T, Murakami H, Shukuya T, Kenmotsu H, Ono A, et al. The role of betaIII‐tubulin in non‐small cell lung cancer patients treated by taxane‐based chemotherapy. Int J Clin Oncol. 2013;18:371–9. [DOI] [PubMed] [Google Scholar]
- 19. Ferrandina G, Petrillo M, Carbone A, Zannoni G, Martinelli E, Prisco M, et al. Prognostic role of topoisomerase‐IIalpha in advanced ovarian cancer patients. Br J Cancer. 2008;98:1910–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doussis‐Anagnostopoulou IA, Vassilakopoulos TP, Thymara I, Korkolopoulou P, Angelopoulou MK, Siakantaris MP, et al. Topoisomerase IIalpha expression as an independent prognostic factor in Hodgkin's lymphoma. Clin Cancer Res. 2008;14:1759–66. [DOI] [PubMed] [Google Scholar]
- 21. Wong N, Yeo W, Wong WL, Wong NL, Chan KY, Mo FK, et al. TOP2A overexpression in hepatocellular carcinoma correlates with early age onset, shorter patients survival and chemoresistance. Int J Cancer. 2009;124:644–52. [DOI] [PubMed] [Google Scholar]
- 22. Sakurai R, Kaira K, Miura Y, Sunaga N, Saito R, Oyama T, et al. Clinical significance of topoisomerase‐II expression in patients with advanced non‐small cell lung cancer treated with amrubicin. Thorac Cancer. 2020;11:426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xue X, Sun DF, Sun CC, Liu HP, Yue B, Zhao CR, et al. Inhibitory effect of riccardin D on growth of human non‐small cell lung cancer: in vitro and in vivo studies. Lung Cancer. 2012;76:300–8. [DOI] [PubMed] [Google Scholar]
- 24. O'Malley FP, Chia S, Tu D, Shepherd LE, Levine MN, Bramwell VH, et al. Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J Natl Cancer Inst. 2009;101:644–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pritchard KI, Messersmith H, Elavathil L, Trudeau M, O'Malley F, Dhesy‐Thind B. HER‐2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol. 2008;26:736–44. [DOI] [PubMed] [Google Scholar]
- 26. Yan S, Shun‐Chang J, Li C, Jie L, Ya‐Li L, Ling‐Xiong W. Topoisomerase II alpha expression and the benefit of adjuvant chemotherapy for postoperative patients with non‐small cell lung cancer. BMC Cancer. 2010;10:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Makino T, Mikami T, Hata Y, Otsuka H, Koezuka S, Isobe K, et al. Comprehensive biomarkers for personalized treatment in pulmonary large cell neuroendocrine carcinoma: a comparative analysis with adenocarcinoma. Ann Thorac Surg. 2016;102:1694–701. [DOI] [PubMed] [Google Scholar]
- 28. Kaira K, Serizawa M, Koh Y, Miura S, Kaira R, Abe M, et al. Expression of thymidylate synthase, orotate phosphoribosyltransferase and dihydropyrimidine dehydrogenase in thymic epithelial tumors. Lung Cancer. 2011;74:419–25. [DOI] [PubMed] [Google Scholar]
- 29. Shimizu A, Kaira K, Okubo Y, Utsumi D, Bolag A, Yasuda M, et al. Expression of thymidylate synthase (TS) and its prognostic significance in patients with cutaneous angiosarcoma. Neoplasma. 2017;64:916–21. [DOI] [PubMed] [Google Scholar]
- 30. Shimizu A, Kaira K, Yasuda M, Asao T, Ishikawa O. Prognostic significance of thymidylate synthase (TS) expression in cutaneous malignant melanoma. Neoplasma. 2016;63:282–7. [DOI] [PubMed] [Google Scholar]
- 31. Hanauske AR, Eismann U, Oberschmidt O, Pospisil H, Hoffmann S, Hanauske‐Abel H, et al. In vitro chemosensitivity of freshly explanted tumor cells to pemetrexed is correlated with target gene expression. Invest New Drugs. 2007;25:417–23. [DOI] [PubMed] [Google Scholar]
- 32. Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298–304. [DOI] [PubMed] [Google Scholar]
- 33. Nagasaki T, Tsuchiya T, Tagawa T, Honda S, Yamasaki N, Miyazaki T, et al. Analysis of 5‐fluorouracil‐related enzymes in pulmonary neuroendocrine carcinoma: differences in biological properties compared to epithelial carcinoma. Clin Lung Cancer. 2010;11:412–22. [DOI] [PubMed] [Google Scholar]
- 34. Hou J, Lambers M, den Hamer B, den Bakker MA, Hoogsteden HC, Grosveld F, et al. Expression profiling‐based subtyping identifies novel non‐small cell lung cancer subgroups and implicates putative resistance to pemetrexed therapy. J Thorac Oncol. 2012;7:105–14. [DOI] [PubMed] [Google Scholar]
- 35. Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second‐line treatment of stage IV non‐small‐cell lung cancer after disease progression on platinum‐based therapy (REVEL): a multicentre, double‐blind, randomised phase 3 trial. Lancet. 2014;384:665–73. [DOI] [PubMed] [Google Scholar]
- 36. Yang J, Yan J, Shao J, Xu Q, Meng F, Chen F, et al. Immune‐mediated antitumor effect by VEGFR2 selective inhibitor for gastric cancer. Onco Targets Ther. 2019;12:9757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti‐PD‐L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9(385):eaak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Terme M, Pernot S, Marcheteau E, Sandoval F, Benhamouda N, Colussi O, et al. VEGFA‐VEGFR pathway blockade inhibits tumor‐induced regulatory T‐cell proliferation in colorectal cancer. Cancer Res. 2013;73:539–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information
Data Availability Statement
We do not have any shared data.


