Abstract
Background
The risk of cancer treatment‐related acute exacerbation (AE) in patients with lung cancer and mild interstitial lung disease (ILD) on imaging, classified as indeterminate for usual interstitial pneumonia (UIP), has not previously been clarified.
Methods
We retrospectively reviewed the clinical records of 27 patients with lung cancer and ILD who were diagnosed and treated from April 2016 to March 2021.
Results
Among the 27 patients, 21 were classified as indeterminate for UIP and six as UIP/probable UIP; furthermore, 10 (46.6%) and three (50%) patients from each group, respectively, developed treatment‐related AEs. No significant difference was observed regarding the incidence of AEs between the two groups. However, significantly more patients in the AE group received immune checkpoint inhibitors (ICIs) compared to the non‐AE group (p = 0.021). Multivariate analysis revealed that the use of ICIs was a significant independent risk factor for treatment‐related AEs.
Conclusions
Lung cancer patients with mild ILD suggestive of indeterminate for UIP and UIP patterns are at an increased risk for treatment‐related AEs. Furthermore, ICI use is an independent risk factor for AEs in patients with lung cancer complicated by ILD, and ICIs should be used with great caution.
Keywords: exacerbation, immune checkpoint inhibitors, interstitial lung disease, lung cancer, radiotherapy
Patients with mild ILD and showing an indeterminate for UIP pattern on imaging are at an increased risk for cancer treatment‐related AEs, similar to those showing the UIP/probable UIP pattern. Furthermore, ICI use is an independent risk factor for AEs in patients with lung cancer complicated by ILD and should be used with great caution.
INTRODUCTION
The association between idiopathic pulmonary fibrosis (IPF) and lung cancer has already been established. 1 In clinical practice, chemotherapeutic drugs, surgery, and radiotherapy are associated with an increased risk of fatal acute exacerbations in patients with IPF and lung cancer, which present difficulties in the management of these patients.
Previous studies have reported the possible utility of the usual interstitial pneumonia (UIP) pattern on computed tomography (CT) images in determining the risk of acute exacerbation (AE) in chemotherapy‐related interstitial lung disease (ILD). 2 , 3 However, the problem lies in the difficulty in distinguishing the UIP pattern in patients with limited findings of a honeycomb lung. 4
The 2018 revision of the International Guidelines for the diagnosis of IPF classified the CT findings of IPF into four categories: “UIP,” “probable UIP,” “indeterminate for UIP,” and “alternative diagnosis”. 5 Among these, indeterminate for UIP is defined as ILD without honeycomb lung with slight reticular shadows in the dorsal predominance of the bilateral lower lung fields. However, a previous study reported that approximately 30% of patients classified as indeterminate for UIP were pathologically associated with a UIP pattern. 6 On the other hand, a previous study found that ILDs classified as indeterminate for UIP have been discriminated by UIP patterns. 7
Therefore, in actual clinical practice, ILDs classified as indeterminate for UIP may be judged to have a lower AE risk than ILDs with a typical UIP pattern. However, it remains unclear if patients with IPF showing mild reticular shadows on imaging studies, such as indeterminate for UIP, have a decreased risk of treatment‐related AEs compared to patients with a typical UIP pattern.
Therefore, this study aimed to determine the difference in the risk of developing AE and prognosis between patients with lung cancer and ILD with either indeterminate UIP or UIP/probable UIP pattern.
To the best of our knowledge, this is the first study to evaluate the risk factors for AEs associated with lung cancer treatment complicated by ILD based on the new IPF classification. The results of this study may lead to a more accurate prediction of the risk of AEs due to lung cancer treatment complicated by ILD.
METHODS
From April 2016 to March 2021, we retrospectively selected patients with ILD with advanced lung cancer (stage IIIB, IV) classified as either non‐small cell (NSCLC) or small cell lung cancer (SCLC). Data such as age, sex, smoking history, performance status (PS), histological type and clinical stage of lung cancer, surfactant protein‐D (SP‐D), Krebs von den Lungen‐6 (KL‐6), percent lung capacity (%VC), percent diffusing capacity of the lung carbon monoxide (%DLCO), and treatment details, were collected. This study was approved by the Review Committee of Kanazawa Medical University Hospital (approval no. I637).
Two respiratory physicians and a radiologist used chest high‐resolution CT (HRCT) prior to initiating cancer treatment to classify the imaging patterns of ILD into the following four categories based on the American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Association guidelines for IPF diagnosis. 5 (1) UIP (subpleural and basal predominance of honeycomb lung with or without bronchiectasis). (2) Probable UIP (reticular pattern with subpleural and basilar predominance and traction bronchiectasis). (3) Indeterminate for UIP (subpleural, basal predominance of the lung with subtle reticulation). (4) Alternative diagnosis (the distribution of the lesions is not subpleural or basal lung predominant [peribronchial vascular predominance, perilymphatic predominance, upper to middle lung field predominance], and the imaging features are cysts, marked mosaic attenuation, predominant ground‐glass opacity [GGO], profuse micronodules, centrilobular nodules, nodules, consolidation).Disagreements were settled after a consensus among the reviewers regarding the HRCT findings.
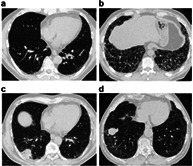
Representative CT images determined as UIP, probable UIP, indeterminate for UIP, and alternative diagnosis are shown in Figure 1. There were 13 patients with ILD classified as alternative diagnosis, including nine with predominantly subpleural and basilar GGOs, three with predominantly upper lobe GGOs, and one with perilymphatic predominance shadows.
FIGURE 1.
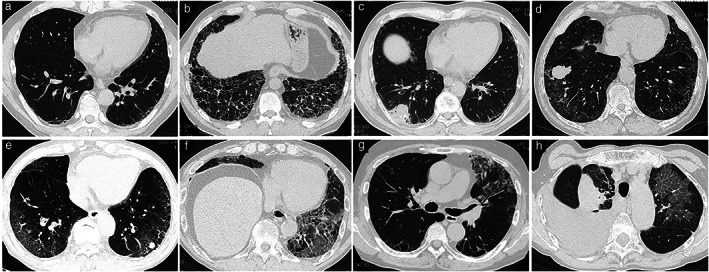
High‐resolution computed tomography (HRCT) image of the chest. (a) Pretreatment chest HRCT image showing very slight reticular shading with subpleural and basal lung predominance (indeterminate for usual interstitial pneumonia [I‐UIP] pattern). (b) Pretreatment chest HRCT image showing honeycomb lung with subpleural and basal lung predominance (usual interstitial pneumonia [UIP]). (c) Pretreatment chest HRCT image showing reticular lesions and traction bronchiectasis with subpleural and basal lung predominance (probable UIP). (d) Pretreatment chest HRCT image showing predominant ground‐glass opacities (GGOs) with subpleural and basal lung predominance (alternative diagnosis). (e) Pretreatment chest HRCT image showing predominant GGOs with subpleural and basal lung predominance (alternative diagnosis). (f) Pretreatment chest HRCT image showing predominant GGOs with subpleural and basal lung predominance (alternative diagnosis). (g) Pretreatment chest HRCT image showing predominant GGOs with upper to middle lung field predominance (alternative diagnosis). (h) Pretreatment chest HRCT image showing predominant GGOs with perilymphatic predominance (alternative diagnosis)
Previous studies have found a decreased risk for AE among patients with ILD and a non‐UIP pattern 2 , 3 ; therefore, patients with ILDs classified as alternative diagnosis were excluded from the study. Additionally, patients with the following characteristics were excluded from the study: absence of active cancer treatment and presence of ILD complications including collagen diseases or pneumoconiosis. Patients who were indeterminate for UIP were defined as the I‐UIP group, and patients with UIP/probable UIP were defined as the UIP group. Additionally, the incidence of AE and its impact on prognosis were examined.
The diagnostic criteria for AEs in IPF in Japan were used to diagnose AE; this included: (1) worsening of unexplained dyspnea within 1 month; (2) a significant decrease in arterial oxygen partial pressure (>10 mmHg under the same conditions); (3) new radiographic alveolar infiltrates; and (4) an absence of an alternative explanation, such as infection, pulmonary embolism, pneumothorax, or heart failure. 8 , 9
Cancer treatment‐related AEs were defined as those occurring within 8 weeks of the end of chemotherapy or within 6 months of the start of radiation therapy. 3 , 10
The severity of AE‐ILD was graded using the Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
All analyses were performed using SPSS 26.0 (SPSS Inc.). Statistical significance was set at p < 0.05. Categorical variables with predictive frequencies >5 and <5 were analyzed using the Chi‐square test and Fisher's exact test, respectively. Continuous variables were analyzed using the unpaired t‐test. Additionally, multivariate analysis was performed using logistic regression.
Survival curves were generated using the Kaplan–Meier method, and the analysis covered the period from the initiation of lung cancer treatment to death or discontinuation. Survival analysis was performed in mid‐April 2021. The log‐rank test was used to analyze the difference in survival due to differences in imaging patterns or the presence of AEs. A risk rate of <5% was considered statistically significant.
RESULTS
Patient background
Among 69 patients initially considered, 27 were included in the final analysis (Figure 2). All patients were Japanese, with a mean age of 72.4 (range: 59–85) years. Most patients were male and had a history of smoking. Furthermore, 21 patients were classified as the I‐UIP group and six as the UIP group. The patient characteristics are shown in Table 1. Two patients (9.5%) in the I‐UIP group achieved a PS of 3 on the Eastern Cooperative Oncology Group PS; the remaining patients mostly achieved a PS of 0 or 1. None of the patients in the UIP group received radiotherapy.
FIGURE 2.
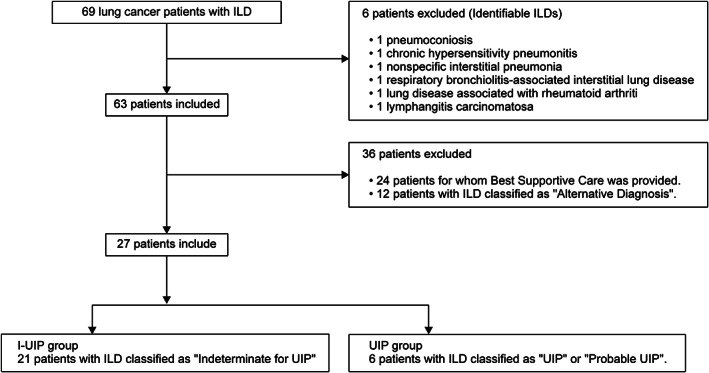
Flowchart of the study. Initially, 69 patients with lung cancer and interstitial lung disease (ILD) were considered for inclusion in this study. Among them, six patients with identifiable ILD, 24 patients who received only ideal supportive care, and 12 patients with ILD identified as alternative diagnosis were excluded. Finally, 27 patients were included in the study
TABLE 1.
Patient characteristics
| Characteristics | I‐UIP | UIP | p‐value |
|---|---|---|---|
| I‐UIP vs. UIP | |||
| Total n | 21(77.8%) | 6(22.2%) | |
| Age (years) | 72 (59–85) | 74 (62–77) | 0.603 |
| Sex (male/female) | (20/1) | (6/0) | 1.000 |
| Smoking history (Never/prior current) | (1/20) | (0/6) | 1.000 |
| ECOG PS (0–1/2–4) | (19/2) | (6/0) | 1.000 |
| Tumor type (NSCLC/SCLC) | (16/5) | (5/1) | 1.000 |
| Clinical stages (III/IV) | (6/15) | (2/4) | 1.000 |
| AE (+/−) | (10/11) | (3/3) | 1.000 |
| Treatment cycle (1, 2/3>) | (16/5) | (5/1) | 1.000 |
| PD‐1/PD‐L1 inhibitors (+/−) | (11/10) | (3/3) | 1.00 |
| Thoracic radiation (+/−) | (6/16) | (0/6) | 0.284 |
| SP‐D (ng/ml) | 124.9 (17.2–327) | 156.6 (63.5–232) | 1.000 |
| KL‐6 (U/ml) | 891.3 (157–5996) | 1215 (563–2770) | 0.480 |
| %VC (%) | 99.4(83.8–114.1) | 99.4(83.8–114.1) | 0.177 |
| %DLCO (%) | 69.5 (47.7–95.9) | 56.5 (30.3–74) | 0.146 |
Abbreviations: %DLCO, percent diffusing capacity of the lung carbon monoxide; %VC, percent lung capacity; AE, acute exacerbation; ECOG, Eastern Cooperative Oncology Group; I‐UIP, indeterminate for UIP group; KL‐6, Krebs von den Lungen‐6; NSCLC, non‐small cell lung carcinoma; PD‐1, programmed cell death‐1; PD‐L1, programmed death‐ligand 1; PS, performance status; SCLC, small cell lung carcinoma; SP‐D, surfactant protein‐D; UIP, UIP/probable UIP group.
No significant difference was observed regarding the age, sex, smoking history, PS, histological type, and clinical stage of lung cancer, SP‐D, KL‐6, %VC, %DLCO, immune checkpoint inhibitor (ICI) use, or treatment cycle between the two groups. Furthermore, no significant difference was observed in the incidence of AEs between the two groups.
Treatment regimen for patients
The initial treatment for each group is shown in Table 2. Among 27 patients, the first‐line treatment regimen used in eight (29.6%) was cisplatin (CDDP)/carboplatin (CBDCA) with etoposide, seven (25.9%) were administered with CDDP/CBDCA and paclitaxel (PTX)/nab PTX, and four (14.8%) with pembrolizumab.
TABLE 2.
First‐line treatment in patients in the indeterminate for UIP and UIP/probable UIP groups
| First‐line treatment | ||||
|---|---|---|---|---|
| ALL | I‐UIP | UIP | p‐value | |
| I‐UIP vs. UIP | ||||
| CDDP (CBDCA) + etoposide | 8 (29.6%) | 6 | 2 | 1.000 |
| CDDP (CBDCA) + PTX (nab‐PTX) | 7 (25.9%) | 6 | 1 | 0.633 |
| CBDCA + S‐1 | 1 (3.8%) | 0 | 1 | 0.222 |
| CDDP (CBDCA) + PEM + BEV | 3 (11.1%) | 3 | 0 | 1.000 |
| CBDCA + PEM | 1 (3.8%) | 1 | 0 | 1.000 |
| Pembrolizumab | 4 (14.8%) | 2 | 2 | 0.204 |
| Erlotinib | 1 (3.8%) | 1 | 0 | 1.000 |
| Radiation + CBDCA + PTX (nab‐PTX) | 2 (7.4%) | 2 | 0 | 1.000 |
Abbreviations: BEV, bevacizumab; CBDCA, carboplatin; CDDP, cisplatin; I‐UIP, “Indeterminate for UIP” group; PEM, pemetrexed; PTX, paclitaxel; S‐1, tegafur/gimeracil/oteracil; UIP, “UIP/Probable UIP” group.
No treatment bias was observed between the groups; however, pemetrexed (PEM) therapy and radiotherapy were avoided as initial treatment in the UIP group at the discretion of the attending physician.
Among the 27 patients with lung cancer and ILD, 13 had cancer treatment‐related AEs (Table 3). ICIs were the most common cause of treatment‐related AEs, causing the condition in eight patients, followed by five patients who underwent chemotherapy (38.4%), and four (30.8%) who underwent radiotherapy.
TABLE 3.
Main cancer treatments associated with the development of AEs and outcomes
| Cause of AE | Case | Regimen (treatment cycle) | Group | Treatment | AE grade | All (%)N = 13 |
|---|---|---|---|---|---|---|
| Chemotherapy | 5 (38.4%) | |||||
| Not including ICIs | 1 | CBDCA + nab‐PTX (first) | I‐UIP | Drug withdrawal | 2 | |
| 2 | PEM (fourth) | I‐UIP | MP pulse therapy | 5 | ||
| 3 | DTX + RAM (second) | UIP | MP pulse therapy | 5 | ||
| ICIs | 8 (61.5%) | |||||
| Chemotherapy combo | 4 | CBDCA + PEM + pembrolizumab (first) | I‐UIP | PSL 60 mg/day | 4 | |
| 5 | CBDCA + PTX + atezolizumab (3rd) | I‐UIP | Drug withdrawal | 2 | ||
| ICI monotherapy | 6 | Pembrolizumab (first) | I‐UIP | Drug withdrawal | 3 | |
| 7 | Pembrolizumab (second) | I‐UIP | Drug withdrawal | 1 | ||
| 8 | Pembrolizumab (first) | UIP | Drug withdrawal | 2 | ||
| 9 | Nivolumab (second) | I‐UIP | Steroid pulse | 4 | ||
| 10 | Nivolumab (first) | UIP | Drug withdrawal | 2 | ||
| 11 | Durvalumab (second) | I‐UIP | PSL 35 mg/day | 3 | ||
| Radiation | 4 (30.8%) | |||||
| Chemoradiotherapy | 11 | Radiotherapy; 60 Gy | I‐UIP | PSL 35 mg/day | 3 | |
| 12 | Radiotherapy; 50 Gy | I‐UIP | MP pulse therapy | 4 | ||
| Radiotherapy alone | 13 | Palliative irradiation; 36 Gy | I‐UIP | PSL 30 mg/day | 3 | |
| 2 | Palliative irradiation; 30 Gy | I‐UIP | MP pulse therapy | 5 |
Abbreviations: AE, acute exacerbation; CBDCA, carboplatin; PTX, paclitaxel; DTX, docetaxel; ICIs, immune checkpoint inhibitors; I‐UIP, indeterminate for UIP group; MP pulse therapy, methylprednisolone pulse therapy (methylprednisolone 1 g/day for 3 days); PEM, pemetrexed; PSL, prednisolone; RAM, ramucirumab; UIP, “UIP/Probable UIP” group.
Two patients in the I‐UIP group developed grade 4 AEs with high mortality risk (patients 4 and 9).
A patient in the I‐UIP group who received PEM as fourth‐line therapy (Patient 2) and a patient in the UIP group who received docetaxel + ramucirumab as second‐line therapy (Patient 3) died despite treatment with methylprednisolone pulse therapy.
Radiotherapy in patients
Radiotherapy alone or in combination with other therapies was administered to six patients in the I‐UIP group wherein four developed AEs (66.7%).
Two patients with stage IIIA disease were treated with radical radiation therapy plus chemotherapy to cure the disease (Patients 11 and 12). Patient 11 was treated with 60 Gy radiation therapy and chemotherapy (CBDCA + nab‐PTX weekly) followed by maintenance therapy with ICIs (durvalumab) and developed grade 3 AEs. Patient 12 was treated with 50 Gy radiation therapy and chemotherapy (CBDCA + PTX weekly) with no pharmacological maintenance therapy but developed grade 4 AEs.
Palliative irradiation, in which the primary lesion was not included in the irradiation field (lung was included in the irradiation field), was performed in four patients. Among these four patients, one (Patient 2) received 30 Gy of palliative irradiation for metastatic disease of the thoracic spine, followed by fourth‐line PEM monotherapy, and developed Grade 5 AEs. Patient 13 received 36 Gy of palliative irradiation for malignant airway stenosis and developed grade 3 AEs. On the other hand, no AEs were observed in a patient who received 30 Gy of palliative irradiation for metastatic disease of the thoracic spine and two patients who received 30 Gy of palliative irradiation for tumor‐induced superior vena cava syndrome.
Comparison of patients who developed AE
The baseline characteristics of patients who developed AEs of ILD (with AE‐ILD) and those who did not (without AE‐ILD) are compared in Table 4. The two groups had similar characteristics; however, the number of patients who received ICIs was significantly increased in the with AE‐ILD group compared to the without AE‐ILD group (p = 0.021). A bias was observed regarding the histology (SCLC and NSCLC) between the with AE‐ILD and without AE‐ILD groups. In particular, patients with SCLC were not included in the with AE‐ILD group (p = 0.016). A trend was observed regarding more treatment cycles in the with AE‐ILD group compared to the without AE‐ILD group; however, no significant difference was observed (p = 0.077).
TABLE 4.
Patient characteristics of patients with and without AEs of ILD associated with cancer treatment
| Characteristics | With AE‐ILD | Without AE‐ILD | p‐value |
|---|---|---|---|
| Total n | 13 (%) | 14 (%) | |
| Age (years) | 71.2 (59–85) | 73.6 (63–82) | 0.369 |
| Sex (male/female) | (13/0) | (13/1) | 1.000 |
| Smoking history (never/prior current) | (0/13) | (1/13) | 1.000 |
| ECOG PS (0–1/2–4) | (13/0) | (13/1) | 0.450 |
| Tumor type (NSCLC/SCLC) | (13/0) | (8/6) | 0.016 a |
| Clinical stages (III/IV) | (4/9) | (4/10) | 1.000 |
| Treatment cycle (1,2/3>) | (8/5) | (13/1) | 0.077 |
| PD‐1/PD‐L1 inhibitors (+/−) | (10/3) | (4/10) | 0.021 a |
| Thoracic radiation (+/−) | (9/4) | (12/2) | 0.385 |
| SP‐D (ng/ml) | 127.1 (17.2–213) | 141.1 (35.9–327) | 0.786 |
| KL‐6 (U/ml) | 1184.6 (389–5996) | 778.6 (157–1422) | 0.422 |
| %VC (%) | 93.2 (69.7–110.2) | 92.7 (67.5–125.8) | 0.933 |
| %DLCO (%) | 65.2 (30.3–95.9) | 65.3 (52.7–80.1) | 0.352 |
Fisher's exact test.
Abbreviations: %DLCO, percent diffusion capacity of the lung; %VC, percent lung capacity; AE, acute exacerbation; ECOG, Eastern Cooperative Oncology Group; ILD, interstitial lung disease; KL‐6, Krebs von den Lungen‐6; NSCLC, non‐small cell lung carcinoma; PD‐1, programmed cell death‐1; PD‐L1, programmed death‐ligand 1; PS, performance status; SCLC, small cell lung carcinoma; SP‐D, surfactant protein‐D.
Representative CT images in the with AE‐ILD and without AE‐ILD groups are shown in Figure 3.
FIGURE 3.
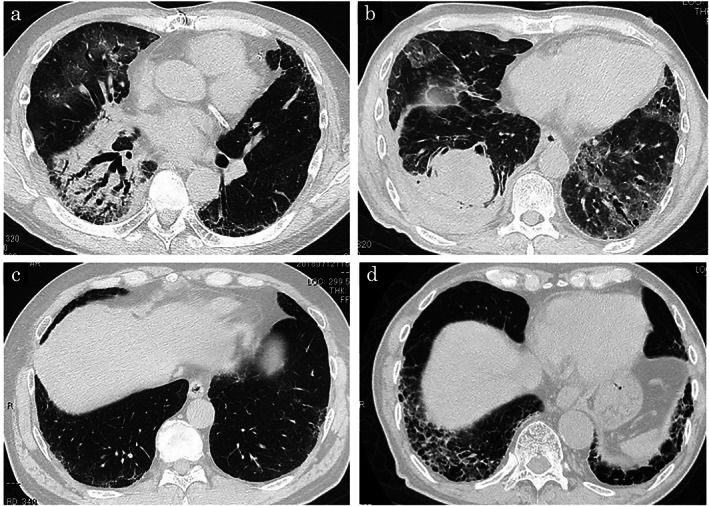
High‐resolution computed tomography (HRCT) image of the chest. (a and b) Representative HRCT findings in the with AE‐ILD group. (c and d) Representative HRCT findings in the without AE‐ILD group. (a) Case 11 in Table 3. A new consolidation and ground glass‐opacity (GGO) was found in the predominant right lung, which was considered to be acute exacerbation (AE) caused by immune checkpoint inhibitors (ICIs) or radiation. (b) Case 10 in Table 3. A new GGO was observed in both lung fields which were considered to be AEs caused by ICIs. (c) Patients with complications of interstitial lung disease (ILD) were determined to be indeterminate for usual interstitial pneumonia (UIP). This patient did not develop AE‐ILD during treatment. (d) Patients with complications of ILD were determined to be UIP. This patient did not develop AE‐ILD during treatment
Univariate and multivariate analysis
Univariate and multivariate analyses were conducted to determine the risk factors for AE of ILD. (Table 5).
TABLE 5.
Univariable and multivariable analysis of risk factors of acute exacerbation of ILD
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p‐value | OR | p‐value | |
| Treatment cycle (1–2 vs. ≥3) | 8.125 (0.798–82.731) | 0.077 | 2.528 (0.185–34.523) | 0.487 |
| ICIs (yes or no) | 8.333 (1.470–47.226) | 0.017 | 13.774 (1.104–171.810) | 0.042 |
| Radiation (yes or no) | 1.800 (0.249–12.988) | 0.560 | 8.494 (0.517–139.652) | 0.11 |
| ILD pattern (UIP vs. I‐UIP) | 1.100 (0.179–6.755) | 0.918 | 1.315 (0.147–11.733) | 0.806 |
| Age (≥75 years vs. <75 years) | 2.880 (0.603–13.749) | 0.1850 | ||
| Stage (III vs. IV) | 1.111 (0.213–5.802) | 0.9010 | ||
| PEM (yes or no) | 3.900 (0.351–43.364) | 0.2680 | ||
| DTX (yes or no) | 1.091 (0.130–9.124) | 0.936 | ||
| KL‐6 (≥500 vs. <500) | 1.250 (0.239–6.633) | 0.7930 | ||
Abbreviations: AE, acute exacerbation; CI, confidence interval; DTX, docetaxel; ICIs, immune checkpoint inhibitors; ILD, interstitial lung disease; KL‐6, Krebs von den Lungen‐6; OR, odds ratio; PEM, pemetrexed; UIP, UIP/Probable UIP group.
ICI use and the treatment cycle were selected as candidate risk factors. Additionally, radiotherapy use, 11 UIP pattern on HRCT, 2 , 3 KL‐6 concentration, 12 and PEM 13 and DTX 14 administration were associated with an increased risk of AE and were considered as candidate risk factors. Other variables used were age, gender, and stage.
Among these variables, univariate analysis showed that ICI use was a significant predictor of AE of ILD (odds ratio [OR] 13.774; 95% CI: 1.104–171.810; p = 0.042). This was confirmed by multivariate analysis that identified ICI use as an independent risk factor for AE (OR: 13.8, 95% CI: 1.11–171.1; p = 0.04).
The survival curves of patients with and without AE‐ILD are shown in Figure 4. There was no statistically significant difference between the two groups (p = 0.90, log‐rank test).
FIGURE 4.
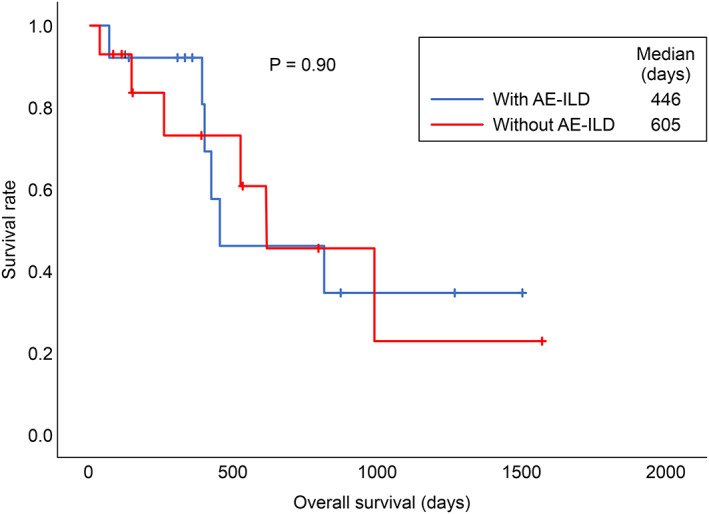
Overall survival of patients with and without acute exacerbations (AEs) of interstitial lung disease (ILD). The median survival time of patients in the with AE‐ILD group was 406 days, compared to 605 days for patients in the without AE‐ILD group. There was no statistically significant differences between the two groups (p = 0.90, log‐rank test)
DISCUSSION
Previous studies have reported that patients with a UIP/possible UIP pattern on CT imaging are at an increased risk of cancer chemotherapy‐related AE and worse prognosis compared to other types of ILD. 2 , 3 , 15 Therefore, it is important to differentiate between UIP/IPF and other ILDs in lung cancer patients with ILD in order to determine the prognosis and treatment.
The presence of a honeycomb lung with a predominantly basal lung distribution can be used to identify the UIP pattern. However, its presence is inconsistently identified among clinicians, which may be further complicated when there is concomitant emphysema. 4 , 16 Therefore, this indicates the heterogeneity of the group of diseases with non‐UIP patterns including IPF with atypical imaging findings. In particular, the IPFs classified as indeterminate for UIP are ILDs with no honeycomb lung on imaging; therefore, previous studies have classified this group of IPF as ILDs with a non‐UIP pattern. 6 This suggests the decreased likelihood of treatment‐related AEs in patients with IPFs classified as indeterminate for UIP compared to those with the typical UIP pattern. However, we found no significant difference regarding the incidence of AE between the I‐UIP and UIP groups indicating a similar risk for AE between the two groups.
Compared to other types of IP patterns, the UIP pattern exhibits no response to steroidal and immunosuppressive drugs and demonstrates a higher mortality rate during AEs. 3 However, the risk of lung cancer treatment complicated by indeterminate for UIP should not be underestimated. Patients and their families should be fully informed of the benefits and risks of the treatment.
In the present study, an increased frequency of ICI administration was observed in the with AE‐ILD group compared to the without AE‐ILD group. Furthermore, the administration of ICIs was identified as an independent risk factor for AEs. Clinical trials of ICIs have excluded patients with pre‐existing ILD; therefore, data regarding their efficacy and safety are lacking. In principle, the administration of ICIs to lung cancer patients with ILD is avoided because of concerns regarding AEs due to immune activation. 17 A previous study found that patients with NSCLC with ILD treated with ICIs had an increased incidence of drug‐induced lung injury with increased severity compared to the non‐ILD group. 18 , 19 Additionally, another study found that among 49 patients with lung cancer and ILD, 15 (30.6%) developed drug‐induced lung injury wherein three (6%) died due to ICI treatment. 20 This suggests the high risk of ICI use in patients with lung cancer and ILD similar to anticancer drug therapy.
In the present study, no significant difference was observed regarding the survival rates between the with AE‐ILD and without AE‐ILD groups; however, the prognosis was not solely defined by the presence or absence of AEs. In terms of prognosis, complicated lung cancers consist of a heterogeneous group of diseases (adenocarcinoma, squamous cell carcinoma, NSCLC not otherwise specified, and polymorphous carcinoma) with varying treatment response; therefore, it is expected that differences in the primary tumor grade and treatment response will have a significant impact. In addition, serious immune‐related adverse events (irAEs) are life‐threatening adverse events that require treatment discontinuation and high‐dose immunosuppressive therapy. Furthermore, discontinuation of treatment with ICIs due to irAEs has been reported to have a negative impact on survival in NSCLC, 21 and the development of irAEs may lead to shortened overall survival in NSCLC. Further studies with a larger number of patients will be needed to clarify the relationship between the impact of AEs and the prolonged prognostic effect of treatment in lung cancer patients with ILD.
In the present study, a bias was observed regarding the histology (SCLC and NSCLC) between the with AE‐ILD and without AE‐ILD groups. In particular, patients with SCLC were not included in the with AE‐ILD group, which may be due to the avoidance of ICI use in patients with SCLC since it was not approved at the start of the study. Now that ICI therapy in SCLC patients has been approved, a concomitant increase in ICI therapy is expected in patients with SCLC and ILD.
Furthermore, no significant difference was observed regarding the presence of radiation between the with AE‐ILD group and without AE‐ILD group; however, no patients in the UIP group received radiation. Our results suggest that radiation to the lung field, even with an indeterminate for UIP pattern on imaging, can cause fatal AEs; therefore, careful attention should be provided to AEs, even when palliative radiation is used. However, radiotherapy is expected to prolong the prognosis of patients with curative potential, such as stage III NSCLC and LD‐SCLC, and in patients with oncological emergencies. Additionally, it is advisable to select patients with lung cancer and ILD for radiotherapy and provide a sufficient explanation of the beneficial and adverse effects.
This study has several limitations. First, the sample size was small and the statistical power may not have been sufficient to detect the difference between the UIP and I‐UIP groups; in particular, the UIP group was very small with only six patients. In the future, analysis of a larger number of patients is required. However, we believe that it is also important to accumulate evidence of AE risk from cancer treatment in lung cancers complicated by ILD by gathering results through small clinical studies. Second, chronic hypersensitivity pneumonitis and ILD associated with rheumatoid arthritis often appear as a UIP pattern resembling IPF on imaging. 22 , 23 In this study, we tried to exclude diseases with identifiable causes, such as collagen diseases and pneumoconiosis; however, inadequate history, clinical examination, and evaluation may have led to the inclusion of ILD due to collagen diseases and chronic hypersensitivity pneumonitis in the UIP group. However, surgical lung biopsy cannot be performed sufficiently for patients with lung cancer and ILD due to its invasiveness. Therefore, it is important to evaluate the risk of AEs by examining imaging findings based on the new IPF classification prior to initiating cancer treatment. Third, there was a lack of histopathological diagnosis of AEs. However, other causes of AE have been ruled out including infections by bacteriological tests and heart failure by physical examination and echocardiography. The diagnosis of AE may be made reliably through the evaluation of the clinical and imaging course, which seems to accurately represent the actual clinical situation.
Fourth, this was a small retrospective study, where various biases and confounding factors are expected to exist. Schoenfeld et al. reported that the receptor occupancy time of anti‐PD‐(L)1 antibodies lasts for several months, 24 and therefore, irAEs may occur even several months after the end of treatment. In other words, even in cases where chemotherapy or radiotherapy is considered to be the cause of cancer treatment‐related AEs, it is difficult to completely deny the possibility of irAEs when an ICI was administered as pretreatment.
Fifth, although the logistic analysis to identify risk factors for AE‐ILD associated with cancer treatment showed that a history of ICI administration could be an independent risk factor for AE in both the UIP and I‐UIP groups, conditions considered to be ILD other than IPF—alternative diagnosis—were excluded in this study. Therefore, the effect of the existing ILD subtypes other than IPF was not investigated. As GGOs were reported to be an independent risk factor for ICI‐ILD in previous reports, 25 , 26 it is possible that there are populations with high AE risk among the ILDs classified as alternative diagnosis. To clarify the risk of AEs due to ICI in lung cancer complicated by ILDs, a large‐scale study including ILD patients with GGO‐dominant imaging patterns will be necessary. However, GGO‐predominant ILDs include a heterogeneous group of diseases composed of various conditions with different risks of ILD acute exacerbation, such as nonspecific interstitial pneumonia (NSIP), smoking‐related idiopathic interstitial pneumonias (IIPs), and respiratory bronchiolitis‐ILD. 27 Therefore, it may be difficult to evaluate the GGO‐predominant group as a single entity. Ideally, ILDs with a definitive diagnosis based on clinical, radiological, and histological findings before cancer treatment should be analyzed.
In conclusion, lung cancer patients with mild ILD showing bilateral lower lobe predominant distribution and classified in the indeterminate for UIP group (I‐UIP) and those with a typical UIP pattern are at an increased risk of developing cancer treatment‐related AE. Furthermore, ICI use is an independent risk factor for AEs in patients with lung cancer complicated by ILD and should therefore be used with great caution.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Takahara Y, Tanaka T, Ishige Y, Shionoya I, Yamamura K, Sakuma T, et al. Risk factors for acute exacerbation in lung cancer complicated by interstitial lung disease with slight reticular shadows. Thorac Cancer. 2021;12:2758–2766. 10.1111/1759-7714.14121
REFERENCES
- 1. Kato E, Takayanagi N, Takaku Y, Kagiyama N, Kanauchi T, Ishiguro T, et al. Incidence and predictive factors of lung cancer in patients with idiopathic pulmonary fibrosis. ERJ Open Res. 2018;4:00111–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asai N, Katsuda E, Hamanaka R, Kosaka K, Matsubara A, Nishimura M, et al. The ATS/ERS/JRS/ALAT statement “IPF by HRCT” could predict acute exacerbation of interstitial lung disease in non‐small cell lung cancer. Tumori. 2017;103:60–5. [DOI] [PubMed] [Google Scholar]
- 3. Kenmotsu H, Naito T, Kimura M, Ono A, Shukuya T, Nakamura Y, et al. The risk of cytotoxic chemotherapy‐related exacerbation of interstitial lung disease with lung cancer. J Thorac Oncol. 2011;6:1242–6. [DOI] [PubMed] [Google Scholar]
- 4. Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–44. [DOI] [PubMed] [Google Scholar]
- 5. Raghu G, Remy‐Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–68. [DOI] [PubMed] [Google Scholar]
- 6. Ogawa K, Takahashi Y, Murase K, Hanada S, Uruga H, Takaya H, et al. Treatment outcome of patients with unresectable stage III non‐small cell lung cancer and interstitial pneumonia. Respir Investig. 2019;57:388–94. [DOI] [PubMed] [Google Scholar]
- 7. Lynch DA, Sverzellati N, Travis WD, Brown KK, Colby TV, Galvin JR, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir. Med. 2018;6:138–53. [DOI] [PubMed] [Google Scholar]
- 8. Kondoh Y, Taniguchi H, Kawabata Y, Yokoi T, Suzuki K, Takagi K. Acute exacerbation in idiopathic pulmonary fibrosis: analysis of clinical and pathological findings in three cases. Chest. 1993;103:1808–12. [DOI] [PubMed] [Google Scholar]
- 9. Akira M, Hamada H, Sakatani M, Kobayashi C, Nishioka M, Yamamoto S. CT findings during phase of accelerated deterioration in patients with idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 1997;168:79–83. [DOI] [PubMed] [Google Scholar]
- 10. Minami‐Shimmyo Y, Ohe Y, Yamamoto S, Sumi M, Nokihara H, Horinouchi H, et al. Risk factors for treatment‐related death associated with chemotherapy and thoracic radiotherapy for lung cancer. J Thorac Oncol. 2012;7:177–82. [DOI] [PubMed] [Google Scholar]
- 11. Makimoto T, Tsuchiya S, Hayakawa K, Saitoh R, Mori M. Risk factors for severe radiation pneumonitis in lung cancer. Jpn J Clin Oncol. 1999;29:192–7. [DOI] [PubMed] [Google Scholar]
- 12. Kohno N, Kyoizumi S, Awaya Y, Fukuhara H, Yamakido M, Akiyama M. New serum indicator of interstitial pneumonitis activity. Sialylated carbohydrate antigen KL‐6. Chest. 1989;96:68–73. [DOI] [PubMed] [Google Scholar]
- 13. Kato M, Shukuya T, Takahashi F, Mori K, Suina K, Asao T, et al. Pemetrexed for advanced non‐small cell lung cancer patients with interstitial lung disease. BMC Cancer. 2014;10:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kenmotsu H, Naito T, Mori K, Ko R, Ono A, Wakuda K, et al. Effect of platinum‐based chemotherapy for non‐small cell lung cancer patients with interstitial lung disease. Cancer Chemother Pharmacol. 2015;75:521–6. [DOI] [PubMed] [Google Scholar]
- 15. Omori T, Tajiri M, Baba T, Ogura T, Iwasawa T, Okudela K, et al. Pulmonary resection for lung cancer in patients with idiopathic interstitial pneumonia. Ann Thorac Surg. 2015;100:954–60. [DOI] [PubMed] [Google Scholar]
- 16. Akira M, Inoue Y, Kitaichi M, Yamamoto S, Arai T, Toyokawa K. Usual interstitial pneumonia and nonspecific interstitial pneumonia with and without concurrent emphysema: thin–section CT findings. Radiology. 2009; 251: 271–9. [DOI] [PubMed] [Google Scholar]
- 17. Otsubo K, Okamoto I, Hamada N, Nakanishi Y. Anticancer drug treatment for advanced lung cancer with interstitial lung disease. Respir Investig. 2018;56:307–11. [DOI] [PubMed] [Google Scholar]
- 18. Shibaki R, Murakami S, Matsumoto Y, Yoshida T, Goto Y, Kanda S, et al. Association of immune‐related pneumonitis with the presence of preexisting interstitial lung disease in patients with non‐small lung cancer receiving anti‐programmed cell death 1 antibody. Cancer Immunol Immunother. 2020;69:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakanishi Y, Masuda T, Yamaguchi K, Sakamoto S, Horimasu Y, Nakashima T, et al. Pre‐existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor‐induced interstitial lung disease in non‐small cell lung cancer. Respir Investig. 2019;57:451–9. [DOI] [PubMed] [Google Scholar]
- 20. Tasaka Y, Honda T, Nishiyama N, Tsutsui T, Saito H, Watabe H, et al. Non‐inferior clinical outcomes of immune checkpoint inhibitors in non‐small cell lung cancer patients with interstitial lung disease. Lung Cancer. 2021;155:120–6. [DOI] [PubMed] [Google Scholar]
- 21. Naqash AR, Ricciuti B, Owen DH, Florou V, Toi Y, Cherry C, et al. Outcomes associated with immune‐related adverse events in metastatic non‐small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother. 2020;69:1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiba S, Tsuchiya K, Akashi T, Ishizuka M, Okamoto T, Furusawa H, et al. Chronic hypersensitivity pneumonitis with a usual interstitial pneumonia–like pattern: correlation between histopathologic and clinical findings. Chest. 2016;149:1473–81. [DOI] [PubMed] [Google Scholar]
- 23. Park JH, Kim DS, Park IN, Jang SJ, Kitaichi M, Nicholson AG, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease‐related subtypes. Am J Respir Crit Care Med. 2007;175:705–11. [DOI] [PubMed] [Google Scholar]
- 24. Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, et al. Severe immune‐related adverse events are common with sequential PD‐(L)1 blockade and osimertinib. Ann Oncol. 2019;30:839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nishiyama N, Honda T, Sema M, Kawahara T, Jin Y, Natsume I, et al. The utility of ground‐glass attenuation score for anticancer treatment‐related acute exacerbation of interstitial lung disease among lung cancer patients with interstitial lung disease. Int J Clin Oncol. 2020;25:282–91. [DOI] [PubMed] [Google Scholar]
- 26. Shimoji K, Masuda T, Yamaguchi K, Sakamoto S, Horimasu Y, Nakashima T, et al. Association of preexisting interstitial lung abnormalities with immune checkpoint inhibitor–induced interstitial lung disease among patients with nonlung cancers. JAMA Netw Open. 2020;3:e2022906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elizabeth AB, Beckford R, Hadley R, Flaherty KR. Idiopathic non‐specific interstitial pneumonia. Respirology. 2016;21:256–68. [DOI] [PubMed] [Google Scholar]


