Summary
Background
The effectiveness of heterologous prime-boost Coronavirus disease 2019 (Covid-19) vaccination is currently unknown.
Methods
From individuals vaccinated with two doses against Covid-19 in Sweden until July 5, 2021 (N=3,445,061), we formed a study cohort including 94,569 individuals that had received heterologous ChAdOx1 nCoV-19 / BNT162b2 prime-boost vaccination, 16,402 individuals that received heterologous ChAdOx1 nCoV-19 / mRNA-1273 prime-boost vaccination, and 430,100 individuals that received homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 prime-boost vaccination. In addition, 180,716 individuals were selected who were unvaccinated at the date of vaccination in the corresponding case. Unvaccinated individuals were censored at first dose of any vaccine. Baseline was the date of the second dose of any vaccine, with the same date in the corresponding unvaccinated individual. The outcome included incident symptomatic Covid-19 infection occurring >14 days after baseline.
Findings
During a mean follow-up time of 76 (range 1-183) days, symptomatic Covid-19 infection was confirmed in 187 individuals with heterologous vaccine schedules (incidence rate: 2.0/100,000 person-days) and in 306 individuals from the unvaccinated control group (incidence rate: 7.1/100,000 person-days). The adjusted vaccine effectiveness was 67% (95% CI, 59-73, P<0.001) for heterologous ChAdOx1 nCoV-19 / BNT162b2 prime-boost vaccination, and 79% (95% CI, 62-88, P<0.001) for heterologous ChAdOx1 nCoV-19 / mRNA-1273 prime-boost vaccination. When combined and analysed together, the two heterologous vaccine schedules had an effectiveness of 68% (95% CI, 61-74, P<0.001) which was significantly greater (Pinteraction<0.001) than the 50% effectiveness for homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 (95% CI, 41-58, P<0.001).
Interpretation
The findings of this study suggest that the use of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination is an effective alternative to increase population immunity against Covid-19, including against the Delta variant which dominated the confirmed cases during the study period. These findings could have important implications for vaccination strategies and logistics, and consequently in the battle against the Covid-19 pandemic.
Research in context.
Evidence before this study
After the rare thromboembolic events coupled to the first dose of vector-based vaccines towards Covid-19, heterologous vaccine schedules have been proposed as an option. Pre-clinical and clinical studies have suggested that heterologous vaccine schedules using ChAdOx1 nCoV-19 as the first dose, and either the BNT162b2 or mRNA-1273 as the second dose, elicit a stronger immune response compared with a homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 vaccine schedule. However, data on the potential effectiveness of heterologous vaccine schedules against symptomatic Covid-19 infection is lacking.
Added value of this study
In this study, heterologous Covid-19 vaccination using ChAdOx1 nCoV-19 as a first dose followed by either the BNT162b2 or mRNA-1273 as the second dose, was associated with 67% to 79% effectiveness against symptomatic Covid-19-infection. The effectiveness of the two heterologous schedules combined was significantly higher compared with the 50% effectiveness from homologous vaccination using ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19.
Implications of all the available evidence
The use of heterologous vaccine schedules appears to be an effective alternative for increasing population immunity against Covid-19, including against the Delta variant which dominated the confirmed cases during the study period. These results could have important implications for vaccination strategies and consequently in the battle against the pandemic.
Alt-text: Unlabelled box
1. Introduction
Since the World Health Organization (WHO) issued its first emergency use validation for a vaccine against Coronavirus disease 2019 (Covid-19) [1], the efficacy of many different vaccines has been determined in clinical trials [2,3,4,5,6], with recent nationwide studies showing a high effectiveness of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca), BNT162b2 (Pfizer-BioNTech), and mRNA-1273 (Moderna) vaccines in real-world settings [7,8,9,10,11,12].
However, after the European Medicines Agency linked the ChAdOx1 nCoV-19 vaccine with rare, yet severe and sometimes fatal, adverse thromboembolic events mainly in younger people [13], which was recently confirmed in a nationwide study from Denmark and Norway [14], many countries in Europe halted their distribution of this vaccine to either parts or all of their population [15,16,17]. Consequently, several of these countries are now recommending that first-dose recipients of the vector-based ChAdOx1 nCoV-19 vaccine henceforth should be offered an mRNA vaccine such as the BNT162b2 or mRNA-1273 for their second dose [17,18], known as a heterologous prime-boost vaccine schedule.
Recent studies suggest that individuals given ChAdOx1 nCoV-19 as the first dose followed by either the BNT162b2 or mRNA-1273 as the second dose, experience a robust cellular and immune response [19,20,21,22], which appears to be of at least similar or even greater magnitude compared with individuals given a homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 schedule [20,22,23,24]. Yet, there is to our knowledge no data regarding effectiveness of heterologous ChAdOx1 nCoV-19 / mRNA prime-boost vaccination against symptomatic Covid-19 infection. Furthermore, according to some evidence, there also seems to be a potentially short-lived increase in systemic reactogenicity from heterologous ChAdOx1 nCoV-19 / mRNA schedules [20,23,25], although this was not seen in a recent study where the interval between the first and second dose was longer [22]. Regardless, there is to our knowledge no data regarding the safety of heterologous vaccine schedules in terms of any potential risks of thromboembolic events. Thus, such nationwide studies conducted in real-world settings are urgently needed [26,27]. Therefore, in the present nationwide cohort study, we investigate the effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden. We also report cases of Covid-19 hospitalisation and risk of thromboembolic events.
2. Methods
2.1. Study design and cohort
This study was approved by the Swedish Ethical Review Authority (number 495/2021), who waived the requirement of obtaining informed consent given the retrospective nature of the study. The main cohort considered for inclusion included all individuals that received heterologous ChAdOx1 nCoV-19 / BNT162b2 prime-boost vaccination (N=94,569), all individuals that received heterologous ChAdOx1 nCoV-19 / mRNA-1273 prime-boost vaccination (N=16,402), and all individuals that received homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 prime-boost vaccination (N=430,100) in Sweden until July 5, 2021. Each vaccinated individual was matched with one individual on birth year, sex and municipality. These individuals were randomly sampled from the total population of Sweden by Statistics Sweden, which is a government agency for national statistics. This matching was done at the date of the first dose of vaccine in the corresponding vaccinated individuals. Baseline for both individuals in the matched pairs was the date of the second dose of vaccine in the vaccinated individual, and several vaccinated individuals were allowed to be matched to the same unvaccinated individual. As reference, we also present estimates of vaccine effectiveness for individuals that received homologous BNT162b2 / BNT162b2 prime-boost vaccination (N=2,065,831) and homologous mRNA-1273 / mRNA-1273 prime-boost vaccination (N=248,234). Data on individuals vaccinated against Covid-19 were collected from the Swedish Vaccination Register, managed by the Public Health Agency of Sweden [28,29]. All health care providers in Sweden are obliged to report to this register according to Swedish law, with a 100% coverage of the total population.
In the analyses, from a total of 2,583,626 unique matched unvaccinated individuals, 1,696,415 were excluded because they received the first dose before their assigned baseline date, and an additional 4,231 individuals were excluded because they died before their assigned baseline.
2.2. Exposure and outcome
In the analyses, the evaluated exposure was the different combinations of vaccines. The outcomes included two severities of Covid-19 infection, occurring >14 days after baseline. The first outcome was any confirmed symptomatic Covid-19 infection until August 23, 2021, and the second outcome was hospitalisation for Covid-19 until July 30, 2021. Symptomatic Covid-19 infection was confirmed in 94.4% of the cases using polymerase chain reaction, and in 4.8% by sequencing, according to the SmiNet registry [29], managed by the Public Health Agency of Sweden. All health care providers are obliged to report to this register according to Swedish law, as Covid-19 is considered a public health hazard. The outcome of symptomatic Covid-19 infection was defined on the basis that in Sweden, health authorities has stated that citizens should be tested if they experience any symptoms of Covid-19. Thus, asymptomatic disease is likely to represent a minority of cases collected in the SmiNet register. Covid-19 hospitalisations were traced in the Swedish National Inpatient Register using the International Classification of Disease (ICD, version 10) code U071. Finally, using the National Inpatient Register and the National Outpatient Register for specialist care we traced adverse events in terms of incident cases of thrombocytopenia, thrombosis and embolus until August 8, 2021, using ICD-10 codes (Supplemental Table 1).
2.3. Covariates
From Statistics Sweden we obtained information on whether the individuals were born in Sweden or not, birth year, birth month, and sex for all individuals [30]. From Statistics Sweden, we also obtained information about highest education during year 2019. Data regarding diagnoses, prescription medications, and living conditions was obtained from national registries managed by the Swedish National Board of Health and Welfare (www.socialstyrelsen.se). From the Swedish National Patient Register and National Outpatient Register for specialist care, diagnoses from 1998 and 2001 and later, respectively, were obtained, based on ICD-10 codes. Prescription medications from 2018 and later were obtained from the Prescribed Drug Register using Anatomic Therapeutic Chemical classification system codes. These three registers are complete for all specialist care and medications prescribed in Sweden for the years selected. Diagnoses and medications (Supplemental Table 1) were selected based on results from a previous nationwide study in a similar population where risk factors for different severities of Covid-19 were investigated [31].
2.4. Statistical analysis
Time-to-event for the outcome of incident symptomatic Covid-19 infection based on vaccination schedule (homologous/heterologous) was illustrated using cumulative incidence curves with 95% confidence intervals (CI), estimated using the Kaplan-Meier method. To compare the risk of symptomatic Covid-19 infection based on the exposure of vaccination (vaccinated/unvaccinated), Cox regression was used to calculate hazard ratios (HR), with robust standard errors to acknowledge the matching calculated using the VCE procedure and ROBUST option in Stata. The adjusted HR was used to calculate adjusted vaccine effectiveness for each vaccination schedule using the following formula: Adjusted vaccine effectiveness = (1-adjusted HR) x 100%. In the analysis, follow-up time in cases was counted until date of confirmed Covid-19 infection, death, or end of possible follow-up time, as specified above, whichever came first. In unvaccinated matched individuals, time was counted until first dose of vaccination, death, or end of possible follow-up time, whichever came first, with the same baseline date as in the corresponding vaccinated individual. The proportional hazard assumption was checked using log minus log plots and not violated. The first model was adjusted for age. The second model included the additional covariates sex, baseline time (number of days between each individual's baseline date and the earliest baseline date in the cohort), homemaker service (yes/no), education (six categories), whether the family members were born in Sweden or not, and nine diagnoses at baseline (yes/no). Fully adjusted models are presented if not specified otherwise. To determine whether the associations differed according to subgroups, an interaction term was created and added to the fully adjusted regression model. All analyses were performed in SPSS v27.0 for Mac (IBM Corp, Armonk, NY, USA), and Stata v16.1 for Mac (Statcorp, College Station, Texas, USA). A two-sided P-value <0.05 or HR with 95% CIs not crossing one were considered significant.
2.5. Role of the funding source
The present study was not funded.
3. Results
3.1. Study cohort
In the main analysis, a total of 541,071 vaccinated individuals and 180,716 unvaccinated matched individuals with a maximum possible follow-up time until August 23, 2021, were included (Table 1). A total of 94,569 individuals received heterologous ChAdOx1 nCoV-19 / BNT162b2 vaccination, 16,402 individuals received heterologous ChAdOx1 nCoV-19 / mRNA-1273 vaccination, while 430,100 individuals received homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 vaccination. Vaccinated individuals were in general older and had more comorbidities compared with corresponding unvaccinated individuals in the control group, across all vaccine schedules (Table 1). Vaccinated individuals were also more commonly born in Sweden and had a higher level of education. Moreover, individuals receiving heterologous ChAdOx1 nCoV-19 / mRNA vaccination were younger with fewer comorbidities compared with individuals that received homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 vaccination.
Table 1.
Baseline characteristics of the cohort according to vaccine schedule
| ChAdOx1 nCoV-19 / BNT162b2 |
ChAdOx1 nCoV-19 / mRNA-1273 |
ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 |
||||
|---|---|---|---|---|---|---|
| Vaccinated individuals | Unvaccinated individuals | Vaccinated individuals | Unvaccinated individuals | Vaccinated individuals | Unvaccinated individuals | |
| N=94,569 | N=60,190 | N=16,402 | N=10,984 | N=430,100 | N=109,542 | |
| Baseline date, mean | 28/5/2021 | 27/5/2021 | 28/5/2021 | 27/5/2021 | 10/6/2021 | 3/6/2021 |
| Age, mean, SD | 44.4±13.5 | 36.1±18.5 | 43.8±13.0 | 39.2±11.2 | 67.2±12.8 | 56.4±18.4 |
| Female sex, N (%) | 72,547 (76.7%) | 44,758 (74.4%) | 12,589 (76.8%) | 8,206 (74.7%) | 235,708 (54.8%) | 67,823 (61.9%) |
| Homemaker service, N (%) | 326 (0.3%) | 92 (0.2%) | 21 (0.1%) | 27 (0.2%) | 2,911 (0.7%) | 2,967 (2.7%) |
| Born in Sweden, N (%) | 75,825 (80.2%) | 43,822 (72.8%) | 13,518 (82.4%) | 7,819 (71.2%) | 380,147 (88.4%) | 76,756 (70.1%) |
| Highest education, N (%) | ||||||
| Elementary school < 9yrs | 1,474 (1.6%) | 2,113 (3.5%) | 158 (1.0%) | 372 (3.4%) | 39,897 (9.3%) | 11,243 (10.3%) |
| Elementary school 9yrs | 6,778 (7.2%) | 6,256 (10.4%) | 827 (5.0%) | 996 (9.1%) | 40,671 (9.5%) | 12,297 (11.2%) |
| Secondary school, 2 yrs | 17,710 (18.7%) | 8,021 (13.3%) | 2,369 (14.4%) | 1,401 (12.8%) | 121,208 (28.2%) | 24,758 (22.6%) |
| Secondary school, >2 yrs | 23,083 (24.4%) | 17,148 (28.5%) | 3,191 (19.5%) | 2,987 (27.2%) | 66,560 (15.5%) | 21,354 (19.5%) |
| University education | 42,898 (45.4%) | 24,645 (40.9%) | 9,474 (57.8%) | 4,843 (44.1%) | 158,780 (36.9%) | 35,589 (32.5%) |
| Unknown | 2,626 (2.8%) | 2,007 (3.3%) | 383 (2.3%) | 385 (3.5%) | 2,984 (0.7%) | 4,301 (3.9%) |
| Diagnoses at baseline, N (%) | ||||||
| Myocardial infarction | 553 (0.6%) | 122 (0.2%) | 69 (0.4%) | 22 (0.2%) | 18,678 (4.3%) | 3,075 (2.8%) |
| Stroke | 540 (0.6%) | 155 (0.3%) | 77 (0.5%) | 19 (0.2%) | 12,847 (3.0%) | 2,746 (2.5%) |
| Diabetes | 4,701 (5.0%) | 1,464 (2.4%) | 680 (4.1%) | 242 (2.2%) | 57,141 (13.3%) | 10,105 (9.2%) |
| Hypertension | 14,569 (15.4%) | 3,716 (6.2%) | 2,419 (14.7%) | 650 (5.9%) | 215,234 (50.0%) | 30,557 (27.9%) |
| Kidney failure | 576 (0.6%) | 161 (0.3%) | 83 (0.5%) | 30 (0.3%) | 7,043 (1.6%) | 1,577 (1.4%) |
| Chronic obstructive pulmonary disease | 454 (0.5%) | 125 (0.2%) | 61 (0.4%) | 27 (0.2%) | 11,277 (2.6%) | 2,552 (2.3%) |
| Asthma | 5,165 (5.5%) | 2,868 (4.8%) | 942 (5.7%) | 492 (4.5%) | 17,764 (4.1%) | 4,670 (4.3%) |
| Covid-19 | 14,365 (15.2%) | 8,652 (14.4%) | 2,653 (16.2%) | 1,587 (14.4%) | 22,300 (5.2%) | 11,954 (10.9%) |
| Cancer | 2,171 (2.3%) | 704 (1.1%) | 326 (2.0%) | 113 (1.0%) | 40,494 (9.4%) | 5,456 (5.0%) |
3.2. Vaccine effectiveness against symptomatic Covid-19-infection
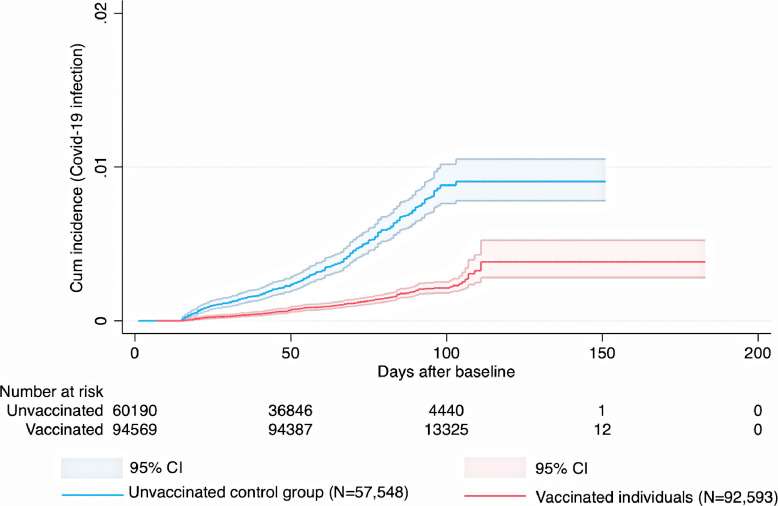
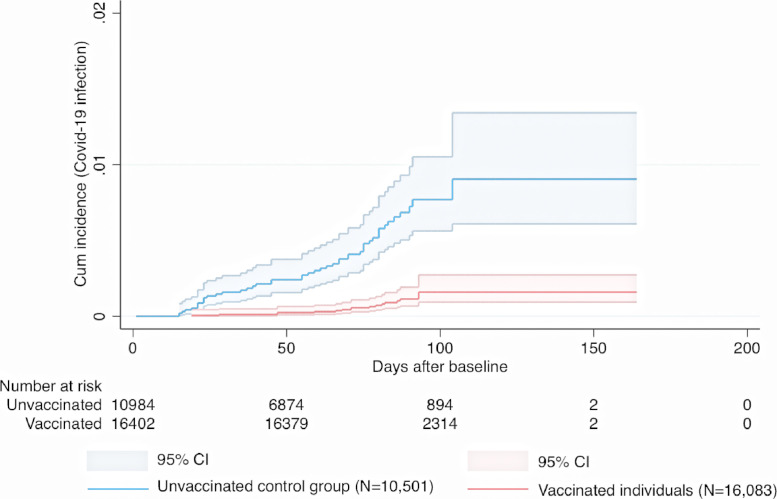
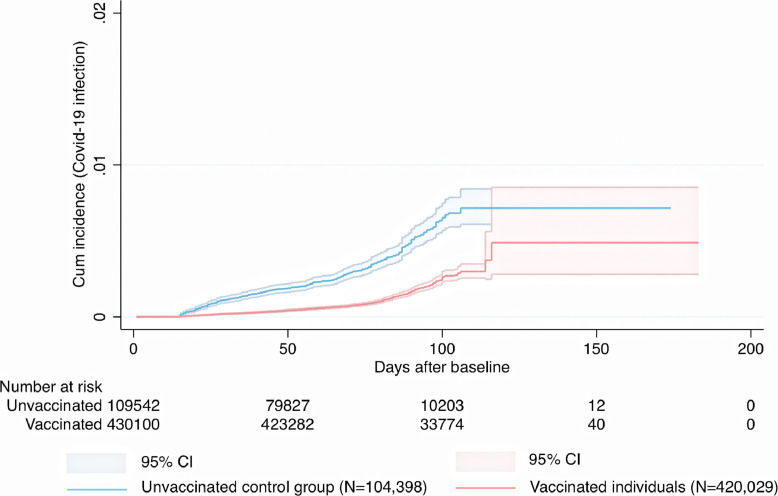
Cumulative incidence of symptomatic Covid-19 infection for the heterologous ChAdOx1 nCoV-19 / mRNA vaccine schedules and for the homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 schedule is shown in (Fig. 1, Fig. 2, Fig. 3). During a mean follow-up time of 76 (range 1-183) days, symptomatic Covid-19 infection was confirmed in 187 individuals with heterologous vaccine schedules (incidence rate [IR], 2.0/100,000 person-days) and in 306 individuals from the unvaccinated control group (IR, 7.1/100,000 person-days) (Fig. 1, Fig. 2, Fig. 3). When the heterologous vaccine schedules were analysed separately, the adjusted vaccine effectiveness was 67% (95% CI, 59-73, P<0.001) for heterologous ChAdOx1 nCoV-19 / BNT162b2 vaccination, and 79% (95% CI, 62-88, P<0.001) for heterologous ChAdOx1 nCoV-19 / mRNA-1273 vaccination (Table 2). When these two heterologous schedules were combined and analysed together, they had an effectiveness of 68% (95% CI, 61-74, P<0.001) which was significantly greater (Pinteraction<0.001) than the 50% (95% CI, 41-58, P<0.001) effectiveness for homologous ChAdOx nCoV-19 / ChAdOx1 nCoV-19 vaccination (Table 2). Next, it was found that age was not associated with the estimated effectiveness for the two heterologous vaccine schedules (Pinteraction=0.18 and Pinteraction=0.11), but with the estimated effectiveness for the homologous ChAdOx1 nCoV-19 vaccine schedule (Pinteraction<0.001), where higher age was associated with lower estimated effectiveness. Further, in an analysis including only vaccinated individuals, higher number of days between first and second dose was not associated with lower risk of Covid-19 infection during follow-up in individuals that received heterologous ChAdOx1 nCoV-19 / BNT162b2 vaccination (HR, 1.00, 95% CI, 0.99-1.01, P=0.93, heterologous ChAdOx1 nCoV-19 / mRNA-1273 vaccination (HR, 1.03, 95% CI, 0.99-1.07, P=0.18), or homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 vaccination (HR, 1.00, 95% CI, 0.99-1.01, P=0.48), after adjustment for all covariates.
Fig. 1.
Cumulative incidence and 95% confidence intervals for symptomatic Covid-19 infection in individuals given a heterologous ChAdOx1 nCoV-19 / BNT162b2 schedule and in corresponding controls.
Fig. 2.
Cumulative incidence and 95% confidence intervals for symptomatic Covid-19 infection in individuals given a heterologous ChAdOx1 nCoV-19 / mRNA-1273 schedule, and in corresponding controls.
Fig. 3.
Cumulative incidence and 95% confidence intervals for symptomatic Covid-19 infection in individuals given a homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 schedule.
Table 2.
Vaccine effectiveness of different vaccine schedules against symptomatic Covid-19 infection during follow-up
| Vaccine schedule | Incident symptomatic Covid-19 infection (N) |
Mean date of infection | Follow-up (days) |
Incidence rate/100,000 person-days |
Adjusted for age | Fully adjusted model* | Fully adjusted vaccine effectiveness (95% CI)* | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | Unvaccinated | HR (95% CI) | HR (95% CI) | |||
| ChAdOx1 nCoV-19 / BNT162b2 | 170 | 259 | 21 July, 2021 | 85.9 | 60.1 | 2.1 | 7.2 | 0.32 (0.26-0.39) | 0.33 (0.27-0.41) | 67% (59-73) |
| ChAdOx1 nCoV-19 / mRNA-1273 | 17 | 47 | 22 July, 2021 | 86.5 | 61.3 | 1.2 | 7.0 | 0.21 (0.12-0.37) | 0.21 (0.12-0.38) | 79% (62-88) |
| ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 | 446 | 323 | 19 July, 2021 | 73.0 | 61.8 | 1.4 | 4.8 | 0.48 (0.41-0.56) | 0.50 (0.42-0.59) | 50% (41-58) |
| BNT162b2 / BNT162b2 | 5,113 | 10,188 | 9 May, 2021 | 101.7 | 67.2 | 2.4 | 16.6 | 0.16 (0.16-0.17) | 0.22 (0.21-0.22) | 78% (78-79) |
| mRNA-1273 / mRNA-1273 | 312 | 889 | 26 May, 2021 | 99.1 | 63.7 | 1.3 | 13.5 | 0.12 (0.10-0.13) | 0.13 (0.12-0.16) | 87% (84-88) |
Adjusted for age, sex, baseline date for vaccination, home maker service, place of birth, education, and diagnoses according to Table 1.
Finally, as reference, we also estimated the adjusted vaccine effectiveness for individuals that received homologous BNT162b2 / BNT162b2 vaccination (78%, 95% CI, 78-79, P<0.001) or homologous mRNA-1273 / mRNA-1273 vaccination (87%, 95% CI, 84-88, P<0.001) (Table 2).
3.3. Sensitivity analysis
In a sensitivity analysis, the risk of symptomatic Covid-19 infection was compared directly for individuals given the homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 schedule, to that of individuals given any of the heterologous ChAdOx1 nCoV-19 / mRNA vaccine schedules, thus, excluding the unvaccinated controls. After adjustment for differences in age (Table 1), the heterologous ChAdOx1 nCoV-19 / mRNA vaccine schedules were associated with 37% lower risk of symptomatic Covid-19 infection (HR, 0.63, 95% CI, 0.53-0.76, P<0.001), which remained after adjustment for all covariates (HR, 0.64, 95% CI, 0.53-0.76, P<0.001).
3.4. Covid-19 hospitalisations
During a mean follow-up time of 67 (range 1-214) days, there were a total of 19 cases of Covid-19 hospitalisation in the heterologous cohorts and in the homologous ChAdOx1 nCoV-19 cohort. Of these, 16 were unvaccinated individuals, two were individuals given the homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 vaccine schedule, and one was an individual given the heterologous ChAdOx1 nCoV-19 / BNT162b2 vaccine schedule.
3.5. Risk of thrombocytopenia, thrombosis, or embolus during follow-up
In total, 439 individuals had a first diagnosis of thrombocytopenia after baseline (Supplemental Table 2). The IR was between 0 to 0.18/100,000 person-days according to the different vaccine schedules, with no significant differences according to vaccine schedule, or among unvaccinated individuals after adjustment for age (P>0.05 for all comparisons). A first diagnosis of venous thrombosis was diagnosed in 250 individuals after baseline, also with a similar IR of 0 to 0.10/100,000 person-days according to vaccine schedule or unvaccinated individuals (P>0.05 for comparison after adjustment for age). Finally, 226 individuals were diagnosed with a first arterial embolus during follow up, with a similar IR of 0 to 0.12/100,000 person-days according to vaccine schedule or among unvaccinated individuals (P>0.05 for all comparisons after adjustment for age).
4. Discussion
In this nationwide cohort study, we investigated the effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination in Sweden. The results showed that heterologous ChAdOx1 nCoV-19 / BNT162b2 and heterologous ChAdOx1 nCoV-19 / mRNA-1273 had 67% and 79% effectiveness against symptomatic Covid-19 infection, respectively. Also, using ChAdOx1 nCoV-19 as the first dose and an mRNA vaccine as the second dose was associated with a significantly higher effectiveness compared with the 50% effectiveness from homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 vaccination.
Prior to this study, there has been no large-scale, real-world evidence supporting the use of heterologous prime-boost vaccination against Covid-19. Consequently, the WHO and other experts has stated an interest in studies investigating the effectiveness of heterologous vaccine schedules against clinical Covid-19 related outcomes in nationwide settings [26,27]. We estimated that Covid-19 vaccination using ChAdOx1 nCoV-19 as the first dose, followed by either the BNT162b2 or mRNA-1273 as the second dose, was 67% and 79% effective, respectively, against symptomatic Covid-19 infection. The results were similar before and after adjustments for covariates. These results are of interest to put into perspective based on results from clinical trials and observational effectiveness studies on homologous vaccine schedules. In a clinical trial, Polack and colleagues showed that homologous BNT162b2 prime-boost vaccination had a 95% efficacy against laboratory confirmed Covid-19 [3]. The same vaccine schedule has also been associated with an effectiveness of 90% to 95% in nationwide mass vaccination settings [7,9,32]. Similar protective effects from homologous mRNA-1273 prime-boost vaccination was seen in a clinical trial [4] as well as in nationwide observational studies [10,11]. The protection from homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 prime-boost vaccination has generally been lower. Voyse and colleagues [2], showed that two standard doses of ChAdOx1 nCoV-19 reduced the risk of Covid-19 infection with 62%. In general, the effectiveness associated with the homologous vaccine schedules were lower in our study compared to in previous studies, which could be influenced by longer follow-up times and the concomitant later dominance of the Delta variant of the virus. In addition, the infection pressure was low during follow-up, which may have attenuated the estimated effects seen both for homologous ChAdOx1 nCoV-19 / ChAdOx1 nCoV-19 vaccines schedule, as well as for the heterologous vaccine schedules. Regardless, the effectiveness of heterologous vaccine schedules observed in the present study are similar to the mean of the estimated effects associated with the different homologous vaccine schedules in the present study. In addition, these findings have support from a recent observational study where the effects of a heterologous compared with a homologous vaccine schedule was evaluated in 88 health care workers after a first dose of ChAdOx1 nCoV-19 [20]. Compared with at baseline, levels of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) trimeric spike protein antibodies and receptor binding domain antibodies increased more than 100 times in individuals given the mRNA-1273 as second dose compared with 5 times in individuals choosing ChAdOx1 nCoV-19 for their second dose. Similar effects were seen recent studies for heterologous ChAdOx1 nCoV-19 / BNT162b2 vaccination [19,33]. In battle against the Covid-19 pandemic, the results of the present study regarding effectiveness against symptomatic infection add to the earlier small-scale evidence and support implementation of heterologous vaccine schedules during mass vaccination campaigns. The clinical implications relate to reducing the risk of supply shortages and facilitating logistics, which could play a major role for national, regional, and global vaccination plans. Essentially, this could help speed up vaccinations, which in turn would aid in ensuring that a high level of population immunity in is achieved in the shortest time possible.
The primary reason for the halted use of the ChAdOx1 nCoV-19 vaccine in many countries is the rare, yet very severe adverse events in the form of thrombocytopenia and thromboembolic events coupled to this vaccine [13]. It is not previously known from either clinical trials or observational studies whether using ChAdOx1 nCoV-19 as the first dose followed by another type of vaccine, such as an mRNA vaccine, as the second dose, increases the risk of these adverse events. In the present cohort, the incidence of these diagnoses during follow-up was low, regardless of vaccine schedule and similar to that in the matched, unvaccinated individuals.
The present study has important strengths including the large and unique cohort studied, with more than 2.8 million individuals given different type of vaccine schedules in a real-world setting, as well as the nationwide coverage with respect to Covid-19 cases and vaccinations, together increasing the generalisability of the results to other populations. We also adjusted our analyses for a great number of covariates based on a recent study in a similar population, where risk factors for Covid-19 was evaluated in a nationwide cohort [31]. Further, because the majority of confirmed Covid-19 cases in the present study were identified as the Delta (B.1.617.2) variant, according to data collected at the Public Health Agency of Sweden [34], the present study including its findings are timely and relevant given the ongoing dominance of the Delta variant in many countries. The present study also has limitations. First, the observational design precludes causal inferences from being made. Moreover, the different vaccine schedules were not at random during follow-up, but strongly associated with the detection of thromboembolic events from use of the ChAdOx1 nCoV-19 vaccine in younger individuals, resulting in a higher mean age of individuals that received homologous ChAdOx1 nCoV-19 vaccine schedules. Thus, the different vaccine schedules were not random in time. A strength of the present study is therefore the use of unvaccinated individuals as controls, with the same baseline date as the corresponding vaccinated individual. Another limitation is that although the associations found in the present study has support from recent studies as discussed above, and despite adjustment for many potential confounders, the possibility of unmeasured and residual confounding remains. Further, given the short follow-up time, we were unable to investigate whether the effectiveness of heterologous vaccine schedules decreased over time. Finally, Covid-19 infections severe enough to result in hospitalisations were rare and the effectiveness could therefore not be estimated with desirable precision, which remains the primary aim of the vaccines. However, investigating the effectiveness against symptomatic infection should not be overlooked, given the strong association with more severe disease.
To summarise, heterologous prime-boost vaccination using ChAdOx1 nCoV-19 as the first dose and either BNT162b2 or mRNA-1273 as the second dose was associated with 67% and 79% effectiveness against symptomatic Covid-19 infection. Furthermore, there was a very low incidence of thromboembolic adverse events during follow-up associated with these heterologous schedules. The results of this study support the use of heterologous vaccine schedules as an effective alternative to increase population immunity against Covid-19, which could have important implications for vaccination strategies and consequently in the battle against the pandemic.
5. Contributors
Concept and design: PN, MB
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: PN, MB.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: PN.
Data availability: All authors.
Supervision: PN, AN.
6. Data availability statement
The data files used for the present study is publicly unavailable according to regulations under Swedish law. However, all data used for the present study can be applied for from the National Board of Health and Welfare, Statistics Sweden, and the Public Health Agency of Sweden.
Declaration of interests
None.
Footnotes
Funding: None.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100249.
Appendix. Supplementary materials
References
- 1.World Health Organization. WHO issues its first emergency use validation for a COVID-19 vaccine and emphasizes need for equitable global access. December 30, 2020. Available from:https://www.who.int/news/item/31-12-2020-who-issues-its-first-emergency-use-validation-for-a-covid-19-vaccine-and-emphasizes-need-for-equitable-global-access. Accessed June 22, 2021.
- 2.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA. 2021;326(1):35–45. doi: 10.1001/jama.2021.8565. Pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. The Lancet. 2021;397(10287):1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung H, He S, Nasreen S, et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374:n1943. doi: 10.1136/bmj.n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021 doi: 10.1038/s41591-021-01446-y. [DOI] [PubMed] [Google Scholar]
- 12.Thompson MG, Burgess JL, Naleway AL, et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers - Eight U.S. Locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency. AstraZeneca's COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. April 7, 2021. Available from: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood. Accessed June 22, 2021.
- 14.Pottegard A, Lund LC, Karlstad O, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. doi: 10.1136/bmj.n1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Public Health Agency of Sweden. AstraZeneca vaccine paused as a precautionary measure pending EMA investigation. March 16, 2021. Available from:https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2021/mars/astra-zenecas-vaccin-pausas-som-en-forsiktighetsatgard-i-vantan-pa-emas-utredning/. Accessed June 22, 2021. .
- 16.Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. Bmj-Brit Med J. 2021:372. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 17.European Centre for Disease Prevention and Control. Overview of EU/EEA country recommendations on COVID-19 vaccination with Vaxzevria, and a scoping review of evidence to guide decision-making. May 18, 2021. Available from:https://www.ecdc.europa.eu/sites/default/files/documents/Overview%20EU%20EEA%20country%20recommendations%20on%20COVID-19%20vaccination%20Vaxzevria%20and%20scoping%20review%20of%20evidence.pdf. Accessed June 22, 2021.
- 18.Public Health Agency of Sweden. Recommendation for an age limit of 65 for AstraZeneca's vaccine remains. April 20, 2021. Available from:https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2021/april/rekommendation-om-aldersgrans-pa-65-ar-for-astrazenecas-vaccin-kvarstar/. Accessed June 22, 2021.
- 19.Borobia AM, Carcas AJ, Perez-Olmeda M, et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–130. doi: 10.1016/S0140-6736(21)01420-3. Pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Normark J, Vikström L, Gwon Y-D, et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. New England Journal of Medicine. 2021 doi: 10.1056/NEJMc2110716. PMC8314734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenbusch M, Schumacher S, Vogel E, et al. Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. Lancet Infect Dis. 2021;21(9):1212–1213. doi: 10.1016/S1473-3099(21)00420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillus D, Schwarz T, Tober-Lau P, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barros-Martins J, Hammerschmidt SI, Cossmann A, et al. Humoral and cellular immune response against SARS-CoV-2 variants following heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. medRxiv. 2021 doi: 10.1038/s41591-021-01449-9. 2021.06.01.21258172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groß R, Zanoni M, Seidel A, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity. medRxiv. 2021 doi: 10.1016/j.ebiom.2021.103761. 2021.05.30.21257971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw RH, Stuart A, Greenland M. Heterologous prime-boost COVID-19 vaccination: initial reactogenicity data. Lancet. 2021;397(10289):2043–2046. doi: 10.1016/S0140-6736(21)01115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duarte-Salles T, Prieto-Alhambra D. Heterologous vaccine regimens against COVID-19. Lancet. 2021;398(10295):94–95. doi: 10.1016/S0140-6736(21)01442-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Evaluation of COVID-19 vaccine effectiveness. Interim guidance. March 17, 2021. Available from:https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccine_effectiveness-measurement-2021.1. Accessed June 22, 2021.
- 28.Public Health Agency of Sweden. The National Vaccination Register. Available from: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/vaccinationer/nationella-vaccinationsregistret/. Accessed 16 June, 2021.
- 29.Public Health Agency of Sweden. SmiNet. Available from:https://www.folkhalsomyndigheten.se/smittskydd-beredskap/overvakning-och-rapportering/sminet/. Accessed 17 June, 2021.
- 30.The Statistics Sweden Database. The official agency for goverment statistics. Sweden. Available at: https://www.scb.se/en/.
- 31.Bergman J, Ballin M, Nordstrom A, Nordstrom P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: a nationwide study. Eur J Epidemiol. 2021;36(3):287–298. doi: 10.1007/s10654-021-00732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. New England Journal of Medicine. 2021;385(2):187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Shaw R, Stuart A, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. August 6, 2021 doi: 10.1016/S0140-6736(21)01694-9. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Public Health Agency of Sweden. Statistics on SARS-CoV-2 variants of concern. 2021. Available from: https://www.folkhalsomyndigheten.se/smittskydd-beredskap/utbrott/aktuella-utbrott/covid-19/statistik-och-analyser/sars-cov-2-virusvarianter-av-sarskild-betydelse/ Accessed 27 July, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data files used for the present study is publicly unavailable according to regulations under Swedish law. However, all data used for the present study can be applied for from the National Board of Health and Welfare, Statistics Sweden, and the Public Health Agency of Sweden.