Abstract
The severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) keeps on destroying normal social integrity worldwide, bringing about extraordinary medical services, cultural and financial interruption. Individuals with diabetes have been demonstrated to be at higher risk of complications and even death when exposed to SARS-CoV-2. Regardless of pandemic scale infection, there is presently limited comprehension on the potential impact of SARS-CoV-2 on individuals with diabetes. Human serum albumin (HSA) is the most abundant circulating plasma protein in human serum and attracted more interest from researchers because most susceptible to non-enzymatic glycation reactions. Albumin down-regulates the expression of ACE2 that is the target receptor of COVID-19. Hypoalbuminemia, coagulopathy, and vascular disease have been connected in COVID-19 and appear to predict outcomes independent of age and morbidity. This review discusses the most recent evidence that the ACE/ACE2 ratio could influence by human serum albumin both the susceptibility of individuals to SARS-CoV-2 infection and the outcome of the COVID-19 disease.
Abbreviations: ACE, angiotensin (Ang)-converting enzyme; ACE2, angiotensin-converting-enzyme 2; ARB, angiotensin receptor blockers; ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; CVD, cardiovascular diseases; DM, diabetes mellitus; EGCG, epigallocatechin gallate; HMA, human mercaptalbumin; HNA, human non-mercaptalbumin; HSA, human serum albumin; ROS, reactive oxygen species; SARSCoV-2, severe acute respiratory syndrome coronavirus 2
Keywords: Diabetes, Human serum albumin, Hypoalbuminemia, COVID-19
1. Introduction
Albumin is an essential transport and drug binding protein for various substances in plasma and maintains the osmotic pressure of blood [1]. Low serum albumin concentrations can be caused by liver impairments during acute phase inflammatory processes, or by increased excretion through the kidney [2]. Decreased levels of serum albumin are reported in patients with severe forms of myocardial infarction, stroke, hip fracture, malignancy, and renal disease, [3], [4], [5]. Hypoalbuminemia has been associated with morbidity and mortality in hospitalized patients [5], [6]. In the course of the coronavirus disease-2019 (COVID-19) pandemic, research continuously reveals effects and characteristics of the disease-related to hypoalbuminemia. The patients suffering from myocardial infarction, stroke, hip fracture, malignancy, and renal disease were likely to be admitted to the ICU due to a severe form of COVID-19. A German study found low serum albumin concentrations alongside increased albumin concentration in urine [7]. Small abnormal albumin excretion, known as microalbuminuria, serves as an indicator for the temporary overload of the glomerular filtration and is an early indicator for nephropathies [8].
Clinically, albumin is a critical biomarker used to evaluate liver function [9]. Numerous factors, including nutritional states, oncotic pressure, and hormonal factors, regulate albumin synthesis [10]. During critical illness, inflammatory mediators decrease albumin synthesis in order to prioritize the synthesis of other acute phase reactants. Additionally, these mediators increase vascular permeability allowing albumin to escape to the extravascular space, which may also lead to low serum albumin levels [11]. In diabetes, the concentration of albumin in blood is decreased, and administration of insulin is required to prevent hypoalbuminemia [12]. Early biochemical investigations have shown that insulin stimulates albumin production in the liver by activating gene transcription [13], [14]. Patients with stress hyperglycaemia, without a history of diabetes, are related to an increase in COVID-19 infection severity and mortality as found in those with established diabetes [15]. Moreover, in these peoples, increased levels of inflammatory biomarkers, hypercoagulopathy as well as leukocytosis, and neutrophilia were additionally comparable to those found in patients with established diabetes [8], [16], suggesting that hyperglycaemia may reflect results of a counter-regulatory state during severe COVID-19 infection [17].
2. Low human serum albumin level in non-surviving COVID-19 patients
The half-life of HSA is about 19 days and its decrease production in the liver by 10–15% after 50 years of age [18]. Free albumin up to 80% is found within the interstitial spaces where it is assumed to perform the transport function for fatty acids, bilirubin, and hormones as well as for many drugs [19]. Free fatty acids (FFA), SARS coronavirus-2 (SARS-CoV-2) virions and melatonin are transported through bound albumin, as are called glycolates [18]. Albumin role is well accepted in the regulation of hormones [14]. Testosterone is suspected of playing a critical role in driving the pronounced excess of COVID-19 lethality in male patients [20], and low testosterone levels predict adverse clinical outcomes [21]. Critically ill male COVID-19 patients suffer from severe testosterone deficiency, which may or may not be caused by the disease [22]. Albumin research provided new insights such as the characterization of the pleiotropic effects of albumin (which surpass its oncotic properties) and the concept of effective albumin concentration. Albumin has the capability to binds mostly non-specifically with each ligand having a different affinity for competing sites [24], [25]. Glycated albumin has long been known to affect platelet aggregation a factor of clotting [26].
3. Hypoalbuminemia: an indicator of the severity and prognosis of COVID-19 patients
Lower serum albumin can be indicative of malnutrition, underlying disease, or infectious processes [27]. Albumin was also regarded as a reliable indicator of the prognosis of patients with severe COVID-19 infection [28]. Hypoalbuminemia is very frequently noticed in patients with conditions like diabetes, hypertension and chronic heart failure, and these patients are statistically most vulnerable to SARS-CoV-2 infection [29]. For each unit increase in serum albumin, the chance of death reduced 14% [30]. Patients with concomitant hypoalbuminemia have higher mortality rates and longer hospital stays [31]. In a recent study on COVID-19 patients, hypoalbuminemia was found to be an independent risk factor for death, and the risk of death in patients with hypoalbuminemia was 6.394 times higher than that in patients with normal albumin [32]. Huang et al. found that lower albumin levels on admission can predict the outcome of COVID-19 independent of other known indicators such as lymphocyte count or co-morbidities [33]. Coagulopathy and vascular disease have been linked in COVID-19 patients because albumin encompasses anticoagulant properties and heparin-like action [34]. Reduction in colloid oncotic pressure due to hypoalbuminemia contributes to lung injury, renal failure might be mitigated as a known factor in sepsis and acute respiratory distress syndrome (ARDS) [35], [36].
4. SARS-CoV-2 and connection with Cys34 of HSA
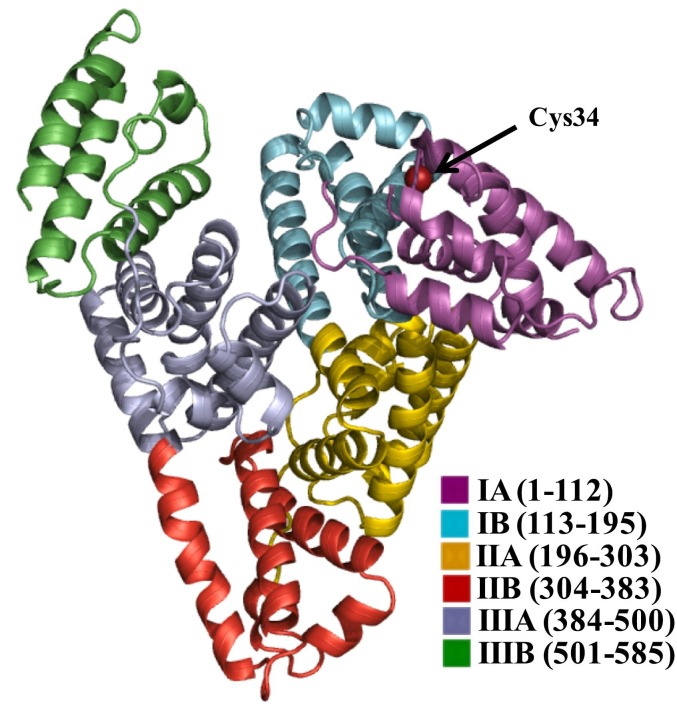
Cys34 is one of the most reactive thiol group in serum and the level of oxidized albumin are an oxidative stress marker (Fig. 1 ). Thus, the level of Cys34 cysteinylated albumin is significantly increased in patients with diabetes mellitus (DM), liver, and kidney diseases [37]. Cys34 is one of the most important “scavengers” of reactive oxygen species (ROS), nitrogen species (RNS), and nitric oxide (NO). HSA is available in two forms: reduced albumin known as ‘human mercaptalbumin’ (HMA) and oxidized albumin known as ‘human non-mercaptalbumin’ (HNA) [38]. An unnecessary excessive increase in ROS level due to the function of the immune cells (frequently macrophages and neutrophils) against virus infection has been identified in patients with COVID-19 [39]. Then again, infection with SARS-CoV-2 can lead to the damage of some cells and release of their genetic material which in turn can initiate the epithelial cells, toxic stress responses, accumulation of water in the lungs, uncontrolled inflammatory responses, increased ROS production, and consequently generation of many disease in patients [40].
Fig. 1.
Three-dimensional crystal structure of HSA. The subdomains of HSA are rendered with different colors (subdomain IA, in dark purple; subdomain IB, in cyan; subdomain IIA, in yellow; subdomain IIB, in red; subdomain IIIA, in gray; subdomain IIIB, in forest green). The site of redox modification (Cys34) is shown in red color ball. To create the figure, a crystal structure of HSA from the PDB database (1AO6) was used. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
For that reasons concerning the variables which influence the oxidized condition of serum albumin in COVID-19 ill patients, albumin may further be more oxidized, once as a positive input cycle induce the synthesis of cytokines by activated leukocytes in the lung, increment the discharge of inflammatory cytokines and later start to initiate cytokine storm which can eventually cause the development of worse state or even death in these patients. These points are important for researchers to consider HNA levels in COVID-19 patients. It has been accounted for that there is an association between HNA level and expansion in the risk of death from cardiovascular disease (CVD), aging, diabetes, and other basic illness [41], [42]. On the other hand, because of contribution in the progression of cytokine storm, the level of HNA in serum of COVID-19 patients may be a positive indicator of mortality and intensity of infection, particularly in patients with basic diseases for example CVD, diabetes, aging and other inflammatory diseases [43].
It should be noticed that as a result of a decrease in the HSA level of COVID-19 patients, serum albumin therapy might be proposed as an alternative by scientists. However, it is important to say that reports show that there is practically 57% heterogeneity on the oxidized state of cys-34 in albumin preparation by industrial production [44]. This change fundamentally reduces the antioxidant activity of albumin and diminishes its binding ability to drugs [45]. On the other hand, the utilization of serum albumin has been related to the deposition of fatal inflammatory responses in animal models [46]. In this manner, COVID-19 patients may be injected with reduced albumin, which may increase the survival rate of these patients at least the applied drugs may not be sufficiently transferred by albumin. On the basis of clinical study, we propose that checking HNA and HMA levels in the serum of patients may give new diagnostic, symptomatic and therapeutic opportunities to combat the COVID-19 battle. In its severity of worsening, HNA correlated with inflammatory responses and disease progressionas well as death in critically sick patients. A systematic review study on laboratory publications of people with COVID-19 has recently published that 75.8% of patients had decreased amounts of HSA [47]. Nevertheless, there isn't any study that has measured the HNA in these patients yet. However, the clinical measurement can be easily done by using the high-performance liquid chromatography method on whole blood samples [48] and the ratio of HNA may be a sign of systemic redox change in COVID-19 patients. It is only a proposal and clinical trials are needed to examine the level of HNA in the serum of patients with COVID-19 [49].
5. Binding of SARS-CoV-2 to albumin
The nature of the SARS-CoV-2 virion-albumin binding site is obscure but some permanent binding cannot be discounted as proposed in Fig. 2 . At the cell level, the SARS-CoV-2 infection enters the body and uses the cell's machinery to reproduce itself [31]. Spike glycoprotein is a trimeric transmembrane protein that adopts multiple folded conformation and each monomer comprises two functional S1 and S2 subunits [50]. This permits proteolytic digestion and processing by host cell proteases (TMPRSS2 and furin) at last prompting to internalization of the virion [51]. The S1 subunit contains a receptor binding domain (RBD) that is involved in the binding to the host cell receptor [52]. However, the S2 subunit is involved in the fusion of the viral particle and host cell membrane [53]. The interaction between the SARS-CoV-2 surface spike protein (S1) and its receptor angiotensin-converting enzyme-2 (ACE2) serves in the penetration and replication of the virus particle into host cells [54]. One single virion can utilize the mechanisms innately accessible to reproduce itself in a single cell [55]. The damage to the cell can only be caused by instability of the cellular mechanisms by depletion of nutrients necessary for replication of the virus and the wellbeing of the cell [56]. Depletion is most likely to occur by expenditure within the cell due to repeated virion manufacture and ejection [18]. The maintenance of the cell is the critical factor and is determined by the delivery of nutrients and the rate they are used. The degrees of supplements provided by the blood decide this balance.
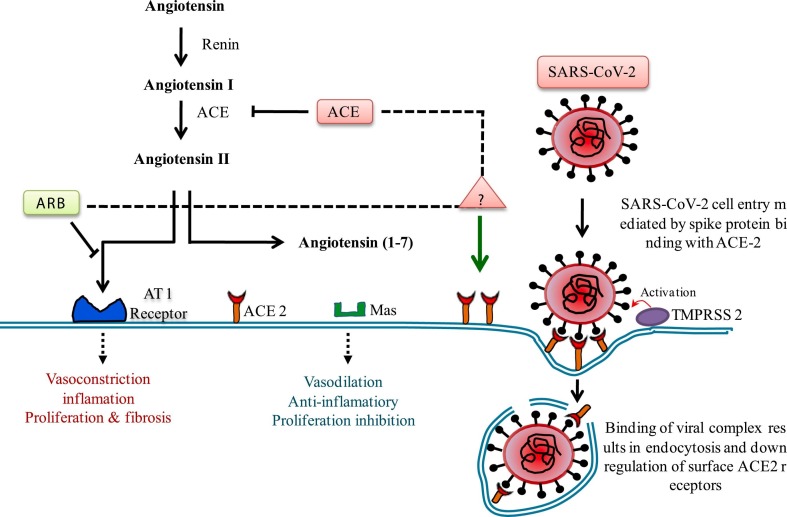
Fig. 2.
The function of ACE2 in RAS and SARS-CoV2 infection. ACE2 catalyzes angiotensin II into angiotensin 1–7 which exerts anti-inflammatory vasoprotective effects. Conflicting studies have proposed that ACEi and ARBs may interfere with ACE2 action and expression leading to significant interest in the function of ACEi and ARBs in regulation of COVID-19 disease. ACE2 is situated on cell membrane and mediates entry of SARS-CoV-2 by binding with spike glycoprotein and resulting in endocytosis of viral complex and down regulation of ACE2 level.
6. Angiotensin converting enzyme 2 (ACE2) as a viral recognition receptor
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have different mechanisms in the pathogenesis of COVID-19. As depicted in Fig. 2, ACEIs in clinical use do not directly affect angiotensin-converting enzyme (ACE2) activity [57]. ACE2 catalyzes the conversion of AngII to Ang-(1–7), therapeutic ACE2 or ACE2-Fc fusion protein can change the equilibrium from AngII-mediated stimulation of angiotensin type I (AT1) receptor to AT2 as well as Mas receptor activation, which may decrease pulmonary dysfunction due to AT1 related inflammatory reactions, lung edema and ARDS [58], [59], [60]. Since there is no clinical data available on ACE2-determined antiviral treatments, the theories about helpful renin-angiotensin-aldosterone system (RAAS) inhibition depend on perceptions of COVID-19 patients who are on existing ACEI or angiotensin receptor blocker (ARB) medicines [61]. The overall agreement is that patients should proceed with RAAS barricade treatment for treating co-morbidities during recovering from viral infection [62].
7. Effect of SARS CoV-2 on blood glucose level
ACE2 has been involved in heart function, hypertension, and diabetes, with its belongings being interceded, to a limited extent to change angiotensin II over to angiotensin 1–7. Unexpectedly, ACE2 additionally serves as the cellular passage point for SARS-CoV-2 [63], [64]. ACE2 receptors are expressed in pancreatic islets and infection with SARS-CoV-2 has been believed to cause hyperglycaemia in individuals without previous diabetes. Hyperglycaemia preserves during a 3-year subsequent follow-up period after recovery from SARS-CoV-2 indicating transient damage to pancreatic β-cell [65]. ACE2 is expressed not only in type I and II alveolar epithelial cells in the lungs and upper respiratory tract, but also few different other areas like the heart, endothelium, renal tubular epithelium, intestinal epithelium, and pancreas [66]. S-glycoprotein present on the surface of SARS CoV-2 binds to ACE2 and causes a conformational change in the S-glycoprotein. Cellular entry of the virus infection triggers an inflammatory response with the recruitment of T-helper cells which produce interferon-γ. This leads to the recruitment of other inflammatory cells leading to a ‘cytokine storm’ which could lead to organ damage and multi-organ failure seen in severe sickness.
8. Albumin regulation on the ACE2
Serum albumin downregulates the expression of the ACE2 receptors [67] and has been appeared to improve the ratio of arterial partial pressure of oxygen/fraction of inspired oxygen in patients with acute respiratory distress syndrome when 24 h after treatment and with an effect that persisted for at least seven days [68]. COVID-19 patients also have some indications of unhealthiness such as malnutrition, decreased serum albumin level and impaired liver and kidney function [69]. In addition, researchers who have studied the clinical attributes of COVID-19 patients have confirmed again and again that lower serum albumin levels were related to an increased risk of death, even to suggest that “albumin therapy might be a potential remedy”. The clinical characteristics of COVID-19 patients who have lower serum albumin levels were related to an increased risk of death [70]. Hypoproteinaemia was characterized as blood albumin of less than 25 g/L.
To efficiently treat the SARS-CoV-2 infection, we are proposing the strategy of concurrent targeting on the viral particle extracellularly and intracellularly. To guarantee the viable and effective internalization of the medications with demonstrated actions against the viral protease, polymerase, and RNA, serum albumin could be utilized as an excellent delivery vehicle [71]. It emphasizes the significance of examining the possible impacts of serum albumin in the clinical results of COVID-19 patients [72]. Besides, serum albumin should be utilized to expand the cellular internalization and improve the pharmacological impacts. Besides, serum albumin should be utilized to augment cellular internalization and improve the pharmacological effects. A comparable strategy based on the proposed idea was used in the influenza virus-infected mouse liver cells, where it yielded a positive result [73].
9. Human serum albumin and COVID-19
The immunity conferred on the young must be because of the difference in the systemic environment. Obesity results in a rise in the plasma-free fatty acids that are bound to albumin thus reducing the unbound portion [74]. In COVID-19 patients, the level of serum albumin is decreased and the body requires more serum albumin [69]. In cases of influenza and other respiratory viruses supplementation with bovine serum albumin was used as a transport medium in community-wide surveillance of febrile respiratory disease for influenza viruses in order to attenuate the enhanced production of ROS such as hydroxyl radicals by neutrophils during an influenza viral infection [75], [76]. This suggests that there may be mechanisms other than a hepatocellular injury that explains the profound hypoalbuminemia seen in COVID-19. ALT and AST slightly increased in COVID-19 patients [77]. Inflammation has been shown to cause the escape of serum albumin into interstitial space due to increased capillary permeability, and eventually lead to increased volume distribution of albumin [78] (Fig. 3 ). As there is no specific treatment for the systemic inflammation in COVID-19 until now, an albumin treatment with low side-effect could be a potential approach [33]. SARS-CoV-2 infection is intervened by the binding of its spike (S) protein to a cell receptor on its target host cells, and an ongoing study proved that ACE2 is a useful functional receptor for SARS-CoV-2 S protein binding [63], [64]. An examination of ACE2 protein localization in 15 human organs found that ACE2 was plentiful in the epithelia of the lung and small digestive tract, where SARS-CoV-2 may enter [79]. Another examination of 72 human tissues by Harmer and his colleagues [80] confirmed ACE2 mRNA expression in the bronchus, lung parenchyma, ileum, testis, and cardiovascular, renal, and gastrointestinal tissues, and pancreas.
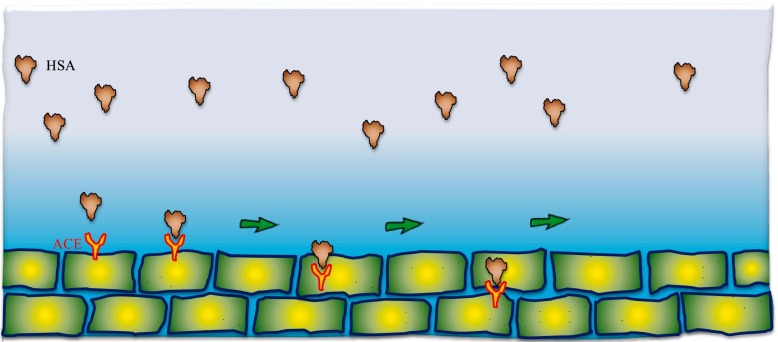
Fig. 3.
The clinical binding mechanism of ACE inhibitor action by HSA is a physiologically important angiotensin I converting enzyme.
10. Therapeutic strategy of COVID-19 infection
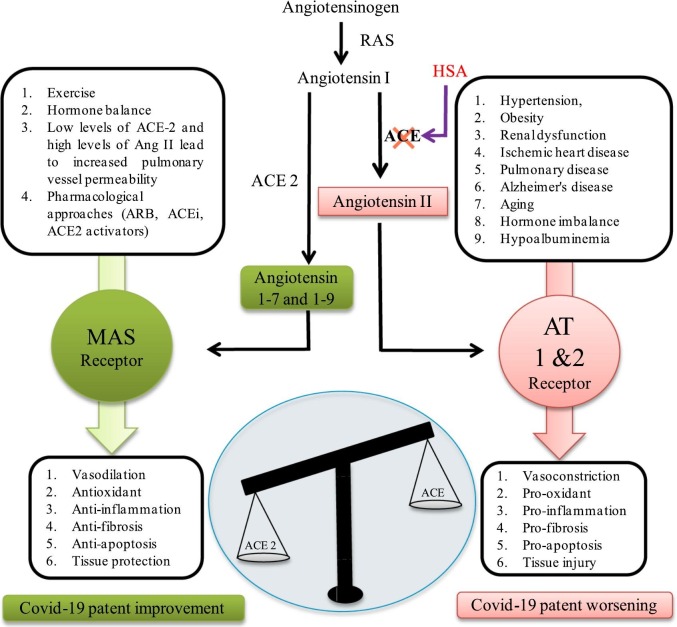
Liu et al. performed a small study in Shenzhen China, on twelve patients eight males and seven older than 60 years. They found that lab parameters related to terrible prognosis were diminished albumin concentrations, lymphopaenia, elevated C-reactive protein (CRP) and lactate dehydrogenase (LDH) concentrations. They estimated angiotensin II concentrations by enzyme-linked immunosorbent assay (ELISA) and found them to be increased. This increased angiotensin II was related to a high viral load and lung injury recommending an imbalanced renin angiotensin system (RAS) (Fig. 4 ). The researchers, therefore, questioned the possible utility of ACE inhibitor or angiotensin receptor blocker (ARB) as possible treatment modalities [81].
Fig. 4.
The increased ACE/ACE2 proportions have high risk of worse outcomes in COVID-19 infection. In physiological conditions, the ACE metabolizes angiotensin I (Ang I) to angiotensin II (Ang II), consequently leading to increased inflammation ACE 2 inactivates Ang I by generating angiotensin 1–7 and 1–9 (Ang 1–7, 1–9), which at that point binds with the G-protein-coupled receptor Mas. This interaction process is known to be vasoprotective, since it antagonizes the actions of Ang I. However, SARS-CoV-2 downregulates the expression of ACE2, thus leading to RAAS over activation and to increased lung damage and edema.
The ongoing studies support the serum albumin therapy of COVID-19 patients since there is a complex relation of albumin concentration and ACE2 receptor expression in the cells, ACE2 receptors are crucial in mediating the virus infection [82]. If the albumin is utilized to stabilize and deliver the epigallocatechin gallate (EGCG) and curcumin for targeting the intracellular virus components in combination with the medication that could block the virus fusion and/or entry to a cell, this strategy might represent an effective method of treating the SARS CoV-2 infection [83], [84], [85].
Besides giving corona virus its characteristic name, spike glycoprotein receptor binding domains (RBDs) bind to ACE2 surface receptors with high affinity, mediating viral entry into the host cell and enhancing the pathogenicity of SARS-CoV-2 [86], [87]. After binding to ACE2, SARS-CoV-2 invades the host cell through endocytosis, leading to the subsequent down regulation of surface ACE2 receptor [88]. ACE2 is a type I membrane-bound receptor with a single active site and is abundant in mucosal epithelial cells within lung alveolar tissue, as well as the heart, kidney, intestine and blood vessels [89], [90]. Angiotensin-converting-enzyme 2 is likewise an endogenous inhibitor of the RAS through degradation of angiotensin II into angiotensin 1–7 [91]. Angiotensin 1–7 subsequently binds to the protein-coupled receptor Mas and applied vasodilatory, antithrombotic, anti-proliferative and anti-oxidative impacts [92].
11. Possible link between diabetes and COVID-19
ACE2 and inhibition of the RAS in healthy individuals, circulating ACE2 is low [93]. However, circulating ACE2 is increased in individuals with diabetes, hypertension and nephropathy [91], [94]. This has been credited to a compensatory component to represent overactivity of angiotensin II and the RAS in individuals with diabetes [95]. Critically there is currently no clinical information in individuals to show that a change in ACE2 activity and susceptibility to SARS-CoV-2 infection. Numerous individuals with cardiovascular complications and diabetes are prescribed ACEi or angiotensin II receptor blockers (ARBs) for cardiovascular [96] and renoprotective impacts [95], [97]. There has been a hypothesis that the utilization of ACEi and ARBs may upregulate ACE2 [98]. An animal study demonstrate that binding of angiotensin II to angiotensin type I (AT1) receptor-activated MAP kinase-phosphotase signaling pathways, which decreased ACE2 mRNA gene expression and activity [99]. Therefore it is recommended that ACEi may increase ACE2 by inhibiting the formation of angiotensin II and therefore its negative regulation of ACE2 (Fig. 4). In other studies, it is suggested that angiotensin II binds to the AT1 receptor, which mediates internalization of ACE2 and degradation by lysosomes. This process was inhibited by the ARB and losartan [100]. While some animal studies have shown upregulation of ACE2 gene expression in response to ACE inhibition [101], [102], normotensive models were used and these studies neglected to explore whether increased ACE2 gene expression corresponded with increased ACE2 action. Other animal studies have conflictingly indicated no increase in ACE2 gene expression or activity levels with ACEi or ARBs [103], [104]. Until now, human studies have shown no proof of upregulation of ACE2 activity in patients on ACEi or ARBs [91], [105].
12. Alterations of the immune system in people with diabetes and SARS-CoV-2 infection
Diabetes mellitus is described by a chronic poor-grade inflammatory state initiated by overabundance of fat tissue [106]. Increased levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), CRP, ROS, and plasminogen activator inhibitor have been shown in adipose tissue of obese mice [107], [108]. These inflammatory cytokines are accepted to inhibit insulin signaling by serine phosphorylation of insulin receptor substrate through activation of inhibitor of nuclear factor kappa-B kinase (IKK-β) and c-Jun N-terminal kinase I (JNK1) mediators [109]. Activated IKKb also results in the transcription of various other inflammatory genes [109]. Notwithstanding the pathogenesis of insulin resistance and type 2 diabetes, increased proinflammatory macrophages, chemokines, cytokines and proteases contribute to the development of diabetes-related nephropathy, retinopathy, neuropathy and cardiovascular infection [110]. People with diabetes are at increased risk of SARS-CoV-2 infection due to dysregulation of the innate and humoral immune system. Previous studies demonstrated that hyperglycemia upregulates adhesion molecules (intracellular cell attachment molecule-1, vascular cell attachment molecule-1, eselectin and CD11b) on endothelial cells and neutrophils [111], [112], which is believed to diminish neutrophil chemotaxis at sites of SARS-CoV-2 infection [113]. Acute hyperglycaemia likewise disables neutrophil phagocytosis and bactericidal activity [111], [114], [115], respiratory burst capacity [116], [117] and development of granular elastase and myeloperoxidase extracellular traps leading to susceptibility to infection [118]. Acute hyperglycaemia has additionally been related to decreased complement fixation and opsonization of microorganisms [119]. Suppression of cytokine synthesis by peripheral blood mononuclear cells and monocytes isolated from people with diabetes has been accounted for [70]. These inflammatory mediators are important for inducing the adaptive immune response and therefore may explain the increased susceptibility to invading pathogens in people with diabetes [109]. This evidence suggests that insulin treatment may reestablish immune function by improving phagocytosis and chemotaxis, bactericidal capacities of neutrophils [120], [121] and consequently supporting the significance of sufficient blood glucose management during COVID-19.
13. Therapies for COVID-19 in people with diabetes
Clinical groups ought to guarantee sufficient glycaemic control in patients with diabetes with Coronavirus. This requires considering all potential ramifications that treatments for Coronavirus may create when utilized in patients with diabetes. Treatment with chloroquine or hydroxychloroquine can cause hypoglycaemia, especially in patients on insulin or sulfonylureas, in light of their consequences for insulin discharge, debasement, and activity [122]. Then again, antiviral medications, for example, lopinavir and ritonavir could prompt hyperglycaemia and intensify glycaemic control [123]. These agents can cause hepatic and muscle poisonousness so an alert is suggested when they are utilized in mix with statins and patients with greasy liver sickness [124]. Glucocorticoids have been utilized in patients with COVID-19 with extreme intense respiratory pain disorder as suggestive and mitigating therapy [92]. Their utilization, in any case, can compound insulin obstruction, support gluconeogenesis, intensify glycaemic control, and cause stamped hyperglycaemia. As known, glucocorticoids apply their hyperglycaemic impacts by decreasing insulin affectability and insulin discharge, and by meddling with GLP-1 impacts and improving the creation of glucagon.
14. Conclusion
This review article conveys the blueprint of serum albumin glycation responses including point of interest physiopathology of diabetes infection and SARS-CoV-2. Low serum albumin on presentation in COVID-19 infection is associated with a higher incidence of serious outcomes like kidney injury, cardiac injury, hypercoagulability, post-viral physical debility, and encephalopathy; and higher mortality. In light of discussed facts supplemented by the recent studies, it is highly prescribed to use serum albumin as a therapeutic material, stabilizer, and transporter of the drugs (such as known antiviral drugs and traditional molecules) that could effectively target the extracellular and intracellular viral components in the therapy of patients infected with SARS-CoV-2.
Acknowledgments
This study is supported by the internal fund of the Nano Diagnostics & Devices (NDD), Gumi-si, Gyeongbuk, Republic of Korea.
References
- 1.Rabbani G., Lee E.J., Ahmad K., Baig M.H., Choi I. Binding of tolperisone hydrochloride with human serum albumin: effects on the conformation, thermodynamics, and activity of HSA. Mol. Pharm. 2018;15(4):1445–1456. doi: 10.1021/acs.molpharmaceut.7b00976. [DOI] [PubMed] [Google Scholar]
- 2.Levitt D.G., Levitt M.D. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016;9:229–255. doi: 10.2147/IJGM.S102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong W., Lin S., Zippi M., Geng W., Stock S., Basharat Z., Cheng B., Pan J., Zhou M. Serum albumin is independently associated with persistent organ failure in acute pancreatitis. Can. J. Gastroenterol. Hepatol. 2017;2017:5297143. doi: 10.1155/2017/5297143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien S.C., Chen C.Y., Lin C.F., Yeh H.I. Critical appraisal of the role of serum albumin in cardiovascular disease. 2017;5:31. doi: 10.1186/s40364-017-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akirov A., Masri-Iraqi H., Atamna A., Shimon I. Low albumin levels are associated with mortality risk in hospitalized patients. Am. J. Med. 2017;130(12) doi: 10.1016/j.amjmed.2017.07.020. 1465 e11-1465 e19. [DOI] [PubMed] [Google Scholar]
- 6.Zheng C.M., Wu C.C., Lu C.L., Hou Y.C., Wu M.S., Hsu Y.H., Chen R., Chang T.J., Shyu J.F., Lin Y.F., Lu K.C. Hypoalbuminemia differently affects the serum bone turnover markers in hemodialysis patients. Int. J. Med. Sci. 2019;16(12):1583–1592. doi: 10.7150/ijms.39158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werion A., Belkhir L., Perrot M., Schmit G., Aydin S., Chen Z., Penaloza A., De Greef J., Yildiz H., Pothen L., Yombi J.C., Dewulf J., Scohy A., Gerard L., Wittebole X., Laterre P.F., Miller S.E., Devuyst O., Jadoul M., Morelle J. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98(5):1296–1307. doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liew A., Bavanandan S., Prasad N., Wong M.G., Chang J.M., Eiam-Ong S., Hao C.-M., Lim C.Y., Lim S.K., Oh K.H., Okada H., Susantitaphong P., Lydia A., Tran H.T.B., Villanueva R., Yeo S.C., Tang S.C.W. Asian Pacific society of nephrology clinical practice guideline on diabetic kidney disease. Nephrology. 2020;25(S2):12–45. doi: 10.1111/nep.13785. [DOI] [PubMed] [Google Scholar]
- 9.Fanali G., di Masi A., Trezza V., Marino M., Fasano M., Ascenzi P. Human serum albumin: from bench to bedside. Mol. Asp. Med. 2012;33(3):209–290. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Sakuma K., Ohyama T., Sogawa K., Fujii-Kuriyama Y., Matsumura Y. Low protein–high energy diet induces repressed transcription of albumin mRNA in rat liver. J. Nutr. 1987;117(6):1141–1148. doi: 10.1093/jn/117.6.1141. [DOI] [PubMed] [Google Scholar]
- 11.Soeters P.B., Wolfe R.R., Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. J. Parenter. Enter. Nutr. 2018;43(2):181–193. doi: 10.1002/jpen.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang D.C., Xu X., Ferrante A.W., Krakoff J. Reduced plasma albumin predicts type 2 diabetes and is associated with greater adipose tissue macrophage content and activation. Diabetol. Metab. Syndr. 2019;11(1):14. doi: 10.1186/s13098-019-0409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawaguchi Y., Sawa J., Hamai C., Kumeda Y. Differential effect of hypoalbuminemia on hypoglycemia on type 2 diabetes patients treated with insulin glargine 300 U/ml and insulin degludec. Diabetes Ther. 2019;10(4):1535–1541. doi: 10.1007/s13300-019-0654-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q., Lu M., Monks B.R., Birnbaum M.J. Insulin is required to maintain albumin expression by inhibiting forkhead box O1 protein. J. Biol. Chem. 2016;291(5):2371–2378. doi: 10.1074/jbc.M115.677351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang W., Li C., Wang Z., Wang H., Zhou N., Jiang J., Ni L., Zhang X.A., Wang D.W. Decreased serum albumin level indicates poor prognosis of COVID-19 patients: hepatic injury analysis from 2,623 hospitalized cases. Sci. China Life Sci. 2020;63(11):1678–1687. doi: 10.1007/s11427-020-1733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beshbishy A.Magdy, Hetta H.F., Hussein D.E., Saati A.A., C C.U., Rivero-Perez N., Zaragoza-Bastida A., Shah M.A., Behl T., Batiha G.E. Factors associated with increased morbidity and mortality of obese and overweight COVID-19 patients. Biology (Basel) 2020;9(9) doi: 10.3390/biology9090280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., Freedberg D.E., Kirtane A.J., Parikh S.A., Maurer M.S., Nordvig A.S., Accili D., Bathon J.M., Mohan S., Bauer K.A., Leon M.B., Krumholz H.M., Uriel N., Mehra M.R., Elkind M.S.V., Stone G.W., Schwartz A., Ho D.D., Bilezikian J.P., Landry D.W. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26(7):1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson A.S., Fatemi R., Winlow W. SARS-CoV-2 bound human serum albumin and systemic septic shock. Front. Cardiovasc. Med. 2020;7(153) doi: 10.3389/fcvm.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabbani G., Baig M.H., Lee E.J., Cho K.W., Ma J.Y., Choi I. Biophysical study on the interaction between eperisone hydrochloride and human serum albumin using spectroscopic, calorimetric, and molecular docking analyses. Mol. Pharma. 2017;14(5):1656–1665. doi: 10.1021/acs.molpharmaceut.6b01124. [DOI] [PubMed] [Google Scholar]
- 20.Pozzilli P., Lenzi A. Commentary: testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giagulli V.A., Guastamacchia E., Magrone T., Jirillo E., Lisco G., De Pergola G., Triggiani V. Worse progression of COVID-19 in men: is testosterone a key factor? Andrology. 2021;9(1):53–64. doi: 10.1111/andr.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hackett G., Kirby M. Testosterone deficiency in men infected with COVID-19. Trends Urol.Men's Health. 2020;11(6):7–10. [Google Scholar]
- 24.Larsen M.T., Kuhlmann M., Hvam M.L., Howard K.A. Albumin-based drug delivery: harnessing nature to cure disease. Mol. Cell. Ther. 2016;4:3. doi: 10.1186/s40591-016-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabbani G., Ahn S.N. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: a natural cargo. Int. J. Biol. Macromol. 2019;123:979–990. doi: 10.1016/j.ijbiomac.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X., Li Q., Tu C., Zeng Y., Ye Y. High glycated albumin is an independent predictor of low response to clopidogrel in ACS patients: a cross-sectional study. Cardiovasc. Diabetol. 2020;19(1):171. doi: 10.1186/s12933-020-01146-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allard L., Ouedraogo E., Molleville J., Bihan H., Giroux-Leprieur B., Sutton A., Baudry C., Josse C., Didier M., Deutsch D., Bouchaud O., Cosson E. Malnutrition: percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients. 2020;12(12) doi: 10.3390/nu12123679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin S., Li W., Shi X., Wu Y., Wang C., Shen J., Pang R., He B., Zhao J., Qiao Q., Luo T., Guo Y., Yang Y., Han Y., Wu Q., Wu J., Dai W., Zhang L., Chen L., Xue C., Jin P., Gan Z., Ma F., Xia X. 3044 Cases reveal important prognosis signatures of COVID-19 patients. Comput. Struct. Biotechnol. J. 2021;19:1163–1175. doi: 10.1016/j.csbj.2021.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J.T., Sheng W.H., Fang C.T., Chen Y.C., Wang J.L., Yu C.J., Chang S.C., Yang P.C. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg. Infect. Dis. 2004;10(5):818–824. doi: 10.3201/eid1005.030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin. Nutr. 2001;20(3):265–269. doi: 10.1054/clnu.2001.0438. [DOI] [PubMed] [Google Scholar]
- 32.Chen C., Zhang Y., Zhao X., Tao M., Yan W., Fu Y. Hypoalbuminemia - an indicator of the severity and prognosis of COVID-19 patients: a multicentre retrospective analysis. Infect. Drug Resist. 2021;14:3699–3710. doi: 10.2147/IDR.S327090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J., Cheng A., Kumar R., Fang Y., Chen G., Zhu Y., Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J. Med. Virol. 2020;92(10):2152–2158. doi: 10.1002/jmv.26003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joorgensen K.A., Stoffersen E. Heparin like activity of albumin. Thromb. Res. 1979;16(3–4):569–574. doi: 10.1016/0049-3848(79)90105-1. [DOI] [PubMed] [Google Scholar]
- 35.Iriyama H., Abe T., Kushimoto S., Fujishima S., Ogura H., Shiraishi A., Saitoh D., Mayumi T., Naito T., Komori A., Hifumi T., Shiino Y., Nakada T.-A., Tarui T., Otomo Y., Okamoto K., Umemura Y., Kotani J., Sakamoto Y., Sasaki J., Shiraishi S.-I., Takuma K., Tsuruta R., Hagiwara A., Yamakawa K., Masuno T., Takeyama N., Yamashita N., Ikeda H., Ueyama M., Fujimi S., Gando S., Tasaki O., Mizobata Y., Funakoshi H., Okuyama T., Yamashita I., Kanai T., Yamada Y., Aibiki M., Sato K., Yamashita S., Yoshida K., Kasaoka S., Kon A., Rinka H., Kato H., Okudera H., Narimatsu E., Fujiwara T., Sugita M., Shichinohe Y., Nakae H., Iiduka R., Nakamura M., Murata Y., Sato Y., Ishikura H., Myojo Y., Tsujita Y., Kinoshita K., Yamaguchi H., Sakurai T., Miyatake S., Saotome T., Yasuda S., Mizushima Y., J.F.g. on behalf of Risk modifiers of acute respiratory distress syndrome in patients with non-pulmonary sepsis: a retrospective analysis of the FORECAST study. J. Intensive Care. 2020;8(1):7. doi: 10.1186/s40560-020-0426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herlekar R., Sur Roy A., Matson M. Hypoalbuminaemia in COVID-19 infection: a predictor of severity or a potential therapeutic target? J. Med. Virol. 2021;93(1):83–84. doi: 10.1002/jmv.26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagumo K., Tanaka M., Chuang V.T., Setoyama H., Watanabe H., Yamada N., Kubota K., Matsushita K., Yoshida A., Jinnouchi H., Anraku M., Kadowaki D., Ishima Y., Sasaki Y., Otagiri M., Maruyama T. Cys34-cysteinylated human serum albumin is a sensitive plasma marker in oxidative stress-related chronic diseases. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mera K., Anraku M., Kitamura K., Nakajou K., Maruyama T., Otagiri M. The structure and function of oxidized albumin in hemodialysis patients: its role in elevated oxidative stress via neutrophil burst. Biochem. Biophys. Res. Commun. 2005;334(4):1322–1328. doi: 10.1016/j.bbrc.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 39.Nasi A., McArdle S., Gaudernack G., Westman G., Melief C., Rockberg J., Arens R., Kouretas D., Sjölin J., Mangsbo S. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS-CoV-2 in an ageing population, consider N-acetylcysteine as early therapeutic intervention. Toxicol. Rep. 2020;7:768–771. doi: 10.1016/j.toxrep.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schönrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020;77 doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kadota K., Yui Y., Hattori R., Murohara Y., Kawai C. Decreased sulfhydryl groups of serum albumin in coronary artery disease. Jpn. Circ. J. 1991;55(10):937–941. doi: 10.1253/jcj.55.937. [DOI] [PubMed] [Google Scholar]
- 42.Michelis R., Kristal B., Zeitun T., Shapiro G., Fridman Y., Geron R., Sela S. Albumin oxidation leads to neutrophil activation in vitro and inaccurate measurement of serum albumin in patients with diabetic nephropathy. Free Radic. Biol. Med. 2013;60:49–55. doi: 10.1016/j.freeradbiomed.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Rahmani-Kukia N., Abbasi A., Pakravan N., Hassan Z.M. Measurement of oxidized albumin: an opportunity for diagnoses or treatment of COVID-19. Bioorg. Chem. 2020;105 doi: 10.1016/j.bioorg.2020.104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bar-Or D., Bar-Or R., Rael L.T., Gardner D.K., Slone D.S., Craun M.L. Heterogeneity and oxidation status of commercial human albumin preparations in clinical use. Crit. Care Med. 2005;33(7):1638–1641. doi: 10.1097/01.ccm.0000169876.14858.91. [DOI] [PubMed] [Google Scholar]
- 45.Klammt S., Brinkmann B., Mitzner S., Munzert E., Loock J., Stange J., Emmrich J., Liebe S. Albumin binding capacity (ABiC) is reduced in commercially available human serum albumin preparations with stabilizers. Z. Gastroenterol. 2001;39(Suppl. 2):24–27. doi: 10.1055/s-2001-919056. [DOI] [PubMed] [Google Scholar]
- 46.Humpert P.M., Lukic I.K., Thorpe S.R., Hofer S., Awad E.M., Andrassy M., Deemer E.K., Kasper M., Schleicher E., Schwaninger M., Weigand M.A., Nawroth P.P., Bierhaus A. AGE-modified albumin containing infusion solutions boosts septicaemia and inflammation in experimental peritonitis. J. Leukoc. Biol. 2009;86(3):589–597. doi: 10.1189/jlb.1008646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutierrez-Ocampo E., Villamizar-Pena R., Holguin-Rivera Y., Escalera-Antezana J.P., Alvarado-Arnez L.E., Bonilla-Aldana D.K., Franco-Paredes C., Henao-Martinez A.F., Paniz-Mondolfi A., Lagos-Grisales G.J., Ramirez-Vallejo E., Suarez J.A., Zambrano L.I., Villamil-Gomez W.E., Balbin-Ramon G.J., Rabaan A.A., Harapan H., Dhama K., Nishiura H., Kataoka H., Ahmad T., Sah R. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med. Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yasukawa K., Shimosawa T., Okubo S., Yatomi Y. A simple, rapid and validated high-performance liquid chromatography method suitable for clinical measurements of human mercaptalbumin and non-mercaptalbumin. Ann. Clin. Biochem. 2018;55(1):121–127. doi: 10.1177/0004563217693257. [DOI] [PubMed] [Google Scholar]
- 49.Focosi D., Anderson A.O., Tang J.W., Tuccori M. Convalescent plasma therapy for COVID-19: state of the art. Clin. Microbiol. Rev. 2020;33(4) doi: 10.1128/CMR.00072-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barnes C.O., Jette C.A., Abernathy M.E., Dam K.-M.A., Esswein S.R., Gristick H.B., Malyutin A.G., Sharaf N.G., Huey-Tubman K.E., Lee Y.E., Robbiani D.F., Nussenzweig M.C., West A.P., Bjorkman P.J. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588(7839):682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bestle D., Heindl M.R., Limburg H., Van Lam van T., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O., Rohde C., Klenk H.D., Garten W., Steinmetzer T., Bottcher-Friebertshauser E. TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells. Life Sci. Alliance. 2020;3(9) doi: 10.26508/lsa.202000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antivir. Res. 2020;178 doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Papa G., Mallery D.L., Albecka A., Welch L.G., Cattin-Ortola J., Luptak J., Paul D., McMahon H.T., Goodfellow I.G., Carter A., Munro S., James L.C. Furin cleavage of SARS-CoV-2 spike promotes but is not essential for infection and cell-cell fusion. PLoS Pathog. 2021;17(1) doi: 10.1371/journal.ppat.1009246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2020;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Astuti I. Ysrafil, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetol. Metab. Syndr. 2020;14(4):407–412. doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L., Xie X., Tu Z., Fu J., Xu D., Zhou Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021;6(1):255. doi: 10.1038/s41392-021-00679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brojakowska A., Narula J., Shimony R., Bander J. Clinical implications of SARS-CoV-2 interaction with renin angiotensin system: JACC review topic of the week. J. Am. Coll. Cardiol. 2020;75(24):3085–3095. doi: 10.1016/j.jacc.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Namsolleck P., Moll G.N. Does activation of the protective renin-angiotensin system have therapeutic potential in COVID-19? Mol. Med. 2020;26(1):80. doi: 10.1186/s10020-020-00211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magalhaes G.S., Rodrigues-Machado M.D.G., Motta-Santos D., Campagnole-Santos M.J., Santos R.A.S. Activation of ang-(1–7)/Mas receptor is a possible strategy to treat coronavirus (SARS-CoV-2) infection. Front. Physiol. 2020;11:730. doi: 10.3389/fphys.2020.00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao Y.L., Du Y., Zhang C., Cheng C., Yang H.Y., Jin Y.F., Duan G.C., Chen S.Y. Role of renin-angiotensin system in acute lung injury caused by viral infection. Infect. Drug Resist. 2020;13:3715–3725. doi: 10.2147/IDR.S265718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kai H., Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens. Res. 2020;43(7):648–654. doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elrashdy F., Redwan E.M., Uversky V.N. Why COVID-19 transmission is more efficient and aggressive than viral transmission in previous coronavirus epidemics? Biomolecules. 2020;10(9) doi: 10.3390/biom10091312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bornstein S.R., Dalan R., Hopkins D., Mingrone G., Boehm B.O. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020;16(6):297–298. doi: 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.-L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D., van der Voort P.H., Mulder D.J., van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J. Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu B.C., Gao J., Li Q., Xu L.M. Albumin caused the increasing production of angiotensin II due to the dysregulation of ACE/ACE2 expression in HK2 cells. Clin. Chim. Acta. 2009;403(1–2):23–30. doi: 10.1016/j.cca.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moon S.S., Lee K., Park J., Yun S., Lee Y.S., Lee D.S. Clinical characteristics and mortality predictors of COVID-19 patients hospitalized at nationally-designated treatment hospitals. J. Korean Med. Sci. 2020;35(36) doi: 10.3346/jkms.2020.35.e328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mani Mishra P., Uversky V.N., Nandi C.K. Serum albumin-mediated strategy for the effective targeting of SARS-CoV-2. Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bedock D., Bel Lassen P., Mathian A., Moreau P., Couffignal J., Ciangura C., Poitou-Bernert C., Jeannin A.C., Mosbah H., Fadlallah J., Amoura Z., Oppert J.M., Faucher P. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin. Nutr. ESPEN. 2020;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanaka R., Ishima Y., Enoki Y., Kimachi K., Shirai T., Watanabe H., Chuang V.T., Maruyama T., Otagiri M. Therapeutic impact of human serum albumin-thioredoxin fusion protein on influenza virus-induced lung injury mice. Front. Immunol. 2014;5:561. doi: 10.3389/fimmu.2014.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arner P., Ryden M. Fatty acids, obesity and insulin resistance. Obes Facts. 2015;8(2):147–155. doi: 10.1159/000381224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersen J.T., Pehrson R., Tolmachev V., Daba M.B., Abrahmsen L., Ekblad C. Extending half-life by indirect targeting of the neonatal fc receptor (FcRn) using a minimal albumin binding domain. J. Biol. Chem. 2011;286(7):5234–5241. doi: 10.1074/jbc.M110.164848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka R., Ishima Y., Maeda H., Kodama A., Nagao S., Watanabe H., Chuang V.T., Otagiri M., Maruyama T. Albumin fusion prolongs the antioxidant and anti-inflammatory activities of thioredoxin in mice with acetaminophen-induced hepatitis. Mol. Pharm. 2014;11(4):1228–1238. doi: 10.1021/mp400690v. [DOI] [PubMed] [Google Scholar]
- 77.Clark R., Waters B., Stanfill A.G. Elevated liver function tests in COVID-19: causes, clinical evidence, and potential treatments. Nurse Pract. 2021;46(1):21–26. doi: 10.1097/01.NPR.0000722316.63824.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spada A., Emami J., Tuszynski J.A., Lavasanifar A. The uniqueness of albumin as a carrier in nanodrug delivery. Mol. Pharm. 2021;18(5):1862–1894. doi: 10.1021/acs.molpharmaceut.1c00046. [DOI] [PubMed] [Google Scholar]
- 79.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 81.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suh S.H., Ma S.K., Kim S.W., Bae E.H. Angiotensin-converting enzyme 2 and kidney diseases in the era of coronavirus disease 2019. Korean J. Intern. Med. 2021;36(2):247–262. doi: 10.3904/kjim.2020.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J., Song D., Wang S., Dai Y., Zhou J., Gu J. Antiviral effect of epigallocatechin gallate via impairing porcine circovirus type 2 attachment to host cell receptor. Viruses. 2020;12(2) doi: 10.3390/v12020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen D.-Y., Shien J.-H., Tiley L., Chiou S.-S., Wang S.-Y., Chang T.-J., Lee Y.-J., Chan K.-W., Hsu W.-L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010;119(4):1346–1351. [Google Scholar]
- 85.Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al-Rasadi K., Banach M., Sahebkar A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020;34(11):2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu. Rev. Virol. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chowdhury R., Boorla V.S., Maranas C.D. Computational biophysical characterization of the SARS-CoV-2 spike protein binding with the ACE2 receptor and implications for infectivity. Comput. Struct. Biotechnol. J. 2020;18:2573–2582. doi: 10.1016/j.csbj.2020.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N. Engl. J. Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/Angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 91.Ramchand J., Patel S.K., Srivastava P.M., Farouque O., Burrell L.M. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bader M. ACE2, angiotensin-(1–7), and mas: the other side of the coin. Pflugers Arch. 2013;465(1):79–85. doi: 10.1007/s00424-012-1120-0. [DOI] [PubMed] [Google Scholar]
- 93.Lew R.A., Warner F.J., Hanchapola I., Yarski M.A., Ramchand J., Burrell L.M., Smith A.I. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp. Physiol. 2008;93(5):685–693. doi: 10.1113/expphysiol.2007.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soro-Paavonen A., Gordin D., Forsblom C., Rosengard-Barlund M., Waden J., Thorn L., Sandholm N., Thomas M.C., Groop P.H. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J. Hypertens. 2012;30(2):375–383. doi: 10.1097/HJH.0b013e32834f04b6. [DOI] [PubMed] [Google Scholar]
- 95.Brenner B.M., Cooper M.E., de Zeeuw D., Keane W.F., Mitch W.E., Parving H.H., Remuzzi G., Snapinn S.M., Zhang Z., Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 96.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart outcomes prevention evaluation study investigators. Lancet. 2000;355(9200):253–259. [PubMed] [Google Scholar]
- 97.Lewis E.J., Hunsicker L.G., Clarke W.R., Berl T., Pohl M.A., Lewis J.B., Ritz E., Atkins R.C., Rohde R., Raz I. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 98.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gallagher P.E., Ferrario C.M., Tallant E.A. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am. J. Physiol. Cell Physiol. 2008;295(5):C1169–C1174. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deshotels M.R., Xia H., Sriramula S., Lazartigues E., Filipeanu C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64(6):1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 102.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 103.Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S., Tikellis C., Grant S.L., Lew R.A., Smith A.I., Cooper M.E., Johnston C.I. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005;26(4):369–375. doi: 10.1093/eurheartj/ehi114. discussion 322-4. [DOI] [PubMed] [Google Scholar]
- 104.Burchill L.J., Velkoska E., Dean R.G., Griggs K., Patel S.K., Burrell L.M. Combination renin-angiotensin system blockade and angiotensin-converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin. Sci. (Lond.) 2012;123(11):649–658. doi: 10.1042/CS20120162. [DOI] [PubMed] [Google Scholar]
- 105.Walters T.E., Kalman J.M., Patel S.K., Mearns M., Velkoska E., Burrell L.M. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017;19(8):1280–1287. doi: 10.1093/europace/euw246. [DOI] [PubMed] [Google Scholar]
- 106.Kulcsar K.A., Coleman C.M., Beck S.E., Frieman M.B. Comorbid diabetes results in immune dysregulation and enhanced disease severity following MERS-CoV infection. JCI Insight. 2019;4(20) doi: 10.1172/jci.insight.131774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 108.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 109.Berbudi A., Rahmadika N., Tjahjadi A.I., Ruslami R. Type 2 diabetes and its impact on the immune system. Curr. Diabetes Rev. 2020;16(5):442–449. doi: 10.2174/1573399815666191024085838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meshkani R., Vakili S. Tissue resident macrophages: key players in the pathogenesis of type 2 diabetes and its complications. Clin. Chim. Acta. 2016;462:77–89. doi: 10.1016/j.cca.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 111.Delamaire M., Maugendre D., Moreno M., Le Goff M.C., Allannic H., Genetet B. Impaired leucocyte functions in diabetic patients. Diabet. Med. 1997;14(1):29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 112.Morigi M., Angioletti S., Imberti B., Donadelli R., Micheletti G., Figliuzzi M., Remuzzi A., Zoja C., Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J. Clin. Invest. 1998;101(9):1905–1915. doi: 10.1172/JCI656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alon R., Sportiello M., Kozlovski S., Kumar A., Reilly E.C., Zarbock A., Garbi N., Topham D.J. Leukocyte trafficking to the lungs and beyond: lessons from influenza for COVID-19. Nat. Rev. Immunol. 2020;21(1):49–64. doi: 10.1038/s41577-020-00470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kinoshita M., Nakashima H., Nakashima M., Koga M., Toda H., Koiwai K., Morimoto Y., Miyazaki H., Saitoh D., Suzuki H., Seki S. The reduced bactericidal activity of neutrophils as an incisive indicator of water-immersion restraint stress and impaired exercise performance in mice. Sci. Rep. 2019;9(1):4562. doi: 10.1038/s41598-019-41077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neethi Raj P., Shaji B.V., Haritha V.H., Anie Y. Neutrophil secretion modulates neutrophil and monocyte functions during hyperglucose and/or hyperinsulin conditions in vitro. J. Cell. Immunother. 2018;4(2):65–70. [Google Scholar]
- 116.Fuji S., Loffler J., Savani B.N., Einsele H., Kapp M. Hyperglycemia as a possible risk factor for mold infections-the potential preventative role of intensified glucose control in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;52(5):657–662. doi: 10.1038/bmt.2016.306. [DOI] [PubMed] [Google Scholar]
- 117.Sanaei Dashti A., Taheri S., Jouybar R., Hashemnia M., Karimi A., Shoja S.A. Respiratory burst process in diabetic children, Iran. J. Pediatr. 2016;26(3) doi: 10.5812/ijp.3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klebanoff S.J., Kettle A.J., Rosen H., Winterbourn C.C., Nauseef W.M. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013;93(2):185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tentolouris A., Thanopoulou A., Tentolouris N., Eleftheriadou I., Voulgari C., Andrianakos A., Sfikakis P.P. Low prevalence of rheumatoid arthritis among patients with pre-existing type 2 diabetes mellitus. Ann. Transl. Med. 2018;6(20):399. doi: 10.21037/atm.2018.09.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang P., Wang X., Wang D., Shi Y., Zhang M., Yu T., Liu D., Gao M., Zhang X., Liu Y. Topical insulin application accelerates diabetic wound healing by promoting anti-inflammatory macrophage polarization. J. Cell Sci. 2020;133(19) doi: 10.1242/jcs.235838. [DOI] [PubMed] [Google Scholar]
- 121.Bartlett D.B., Slentz C.A., Willis L.H., Hoselton A., Huebner J.L., Kraus V.B., Moss J., Muehlbauer M.J., Spielmann G., Muoio D.M., Koves T.R., Wu H., Huffman K.M., Lord J.M., Kraus W.E. Rejuvenation of neutrophil functions in association with reduced diabetes risk following ten weeks of low-volume high intensity interval walking in older adults with prediabetes - a pilot study. Front. Immunol. 2020;11:729. doi: 10.3389/fimmu.2020.00729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Unubol M., Ayhan M., Guney E. Hypoglycemia induced by hydroxychloroquine in a patient treated for rheumatoid arthritis. J. Clin. Rheumatol. 2011;17(1):46–47. doi: 10.1097/RHU.0b013e3182098e1f. [DOI] [PubMed] [Google Scholar]
- 123.Paengsai N., Jourdain G., Salvadori N., Tantraworasin A., Mary J.Y., Cressey T.R., Chaiwarith R., Bowonwatanuwong C., Bhakeecheep S., Kosachunhanun N. Recommended first-line antiretroviral therapy regimens and risk of diabetes mellitus in HIV-infected adults in resource-limited settings, open forum. Infect. Dis. 2019;6(10) doi: 10.1093/ofid/ofz298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bruno R., Sacchi P., Maiocchi L., Patruno S., Filice G. Hepatotoxicity and antiretroviral therapy with protease inhibitors: a review. Dig. Liver Dis. 2006;38(6):363–373. doi: 10.1016/j.dld.2006.01.020. [DOI] [PubMed] [Google Scholar]