Abstract
The purpose of this study was to assess the impact of measures designed to mitigate the spread of coronavirus disease 2019 (COVID-19) on worldwide cancer screening. We systematically searched PubMed, Ovid MEDLINE, the Cochrane COVID-19 Study Register, ClinicalTrials.gov, and EMBASE without language restrictions for studies published between January 1, 2021, and February 10, 2021. Studies selected for full-text review contained data on patients screened for any type of cancer during the COVID-19 pandemic and comparison data from a time interval just prior to the pandemic. Data were obtained through dual extraction. All the included studies were assessed for quality and risk of bias. A meta-analysis was performed on 13 studies: 7 on screening mammography, 5 on colon cancer screening, and 3 on cervical cancer screening. Two of our studies reported on more than one type of cancer screening. The screening outcomes were reported as pooled incidence rate ratios using the inverse variance method and random effects models. All studies included in our meta-analysis reported the number of patients screened for cancer in defined time intervals before and during the COVID-19 pandemic. We found that the pooled incidence rate ratios were significantly lower for screening during the COVID-19 pandemic for breast cancer (0.63; 95% CI, 0.53 to 0.77; P<.001), colon cancer (0.11; 95% CI, 0.05 to 0.24; P<.001), and cervical cancer (0.10; 95% CI, 0.04 to 0.24; P<.001). These findings may add further morbidity and mortality to this public health crisis.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; IRR, incidence rate ratio
In order to call countries into action to reduce transmission of coronavirus disease 2019 (COVID-19) among their citizens, the World Health Organization declared this novel viral illness a pandemic on March 11, 2020. Across the world, governments enforced quarantines, asking their citizens to stay home and avoid contact with others because epidemiological studies revealed that stay-at-home orders effectively reduced the case rates and hospitalizations associated with the virus.1,2 Although these lockdowns may have led to a reduction in viral transmission, the impacts on public health initiatives such as cancer screening are still being determined. As a result of the new measures to mitigate the spread of COVID-19, routine visits to physicians decreased substantially. Data document that the number of primary care visits in the United States decreased by 50% in the second quarter (April 1 to June 30) of 2020 compared with average levels from 2018-2019 for this same period of time.3 Primary care physicians attempted to provide care by implementing telemedicine, which comprised 35% of visits in the second quarter of 2020 compared with 1% of visits in all of 2018-2019.3 This marked reduction in office visits likely had a substantial impact on preventive services, including cancer screening.
In April 2020, the Center for Medicare and Medicaid Services recommended considering postponing nonurgent services, including preventive care visits and screening examinations.4 This recommendation affected ambulatory screenings, including colonoscopies, mammograms, Papanicolaou smears, and low-dose chest computed tomography. Modeling studies predict increased rates of tumor up-staging as well as higher disease-specific morbidity and mortality due to decreased and delayed cancer screening in 2020.5,6 The aim of our systematic review was to examine the impact the COVID-19 pandemic has had on worldwide cancer screening or secondary prevention.
Methods
Search Strategy, Study Selection, and Inclusion Criteria
We began our systematic review by writing a study question and protocol (Supplemental Appendix, available at https://www.mcpiqojournal.org), which was registered through PROSPERO (ID: CRD42021241831) on March 11, 2021. Our systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.7
We developed our search strategies using medical subject heading terms and text words that were selected based on common indexing practices. Search terms were compiled and tested repeatedly to produce sensitive searches to capture potentially relevant publications. We searched the following databases: PubMed, Ovid MEDLINE, the Cochrane COVID-19 Study Register, ClinicalTrials.gov, and EMBASE, without language restriction, for studies published between January 1, 2021, and February 10, 2021. Searches were performed employing the following terms: cancer screening, lung cancer screening, mammography, breast cancer screening, colonoscopy, colon and rectum cancer, cervical cancer screening, Papanicolaou or PAP testing, prostate-specific antigen or PSA, prostate cancer screening, COVID-19, SARS-CoV-2 [severe acute respiratory syndrome coronavirus 2], and 2019 novel coronavirus. Our search was augmented by author and reference tracking to identify additional studies.
Included in our analysis were retrospective observational studies of cohorts or cancer registries. We chose studies that included the numbers of screened patient populations both just prior to and during the pandemic (specifically, the years 2019 and 2020). If studies only contained screening rates, we attempted to obtain the absolute numbers of patients screened by contacting the authors for unpublished data. Studies were excluded if these data were not available. Also excluded were studies that did not record the number of patients screened for any cancers both during the year 2019 and in the pandemic lockdown period of 2020. Abstract-only publications were excluded because study design and methods of data acquisition may not be able to be evaluated and reconciled in such publications. We decided by consensus to exclude outlying results in the statistical analysis of our meta-analysis.
Data Collection and Quality Assessment
We collected initial references in citation files (using Covidence software), removed duplicates, and began our screening process for titles and abstracts against eligibility criteria. Two reviewers (M.M. and R.A.S.) independently reviewed abstracts for inclusion in the initial screening phase, followed by the full-text screening phase of our systematic review. Studies were selected for full-text review if they contained data on patients screened for any type of cancer during the COVID-19 pandemic and contained comparison data from a time interval just prior to the pandemic. Disagreements among reviewers in the initial abstract screening phase and full-text review were resolved by consensus by 2 reviewers (M.M. and R.A.S.). Disagreements among reviewers in the full-text screening phase were reconciled by discussion and consensus with a third reviewer (B.P.).
Two reviewers (M.M. and R.A.S.) evaluated all selected studies from phase 2 of screening independently for inclusion in data extraction. Data extracted from studies included study description (research setting), methods used to record screening rates, and comparison data of screening rates before 2019. Two reviewers (M.M. and R.A.S.) also independently extracted data from the included studies and performed an assessment of the quality and risk of bias of all included studies using the Newcastle-Ottawa Quality Assessment Tool for the Observational, Cohort and Cross-Sectional Studies available from the National Institutes of Health.8 The quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.9
Statistical Analyses
A meta-analysis was performed for 3 outcomes: changes in screening rates for breast cancer, colon cancer, and cervical cancer. Data on lung cancer screening were insufficient to be included in our meta-analysis. The comparison groups were the time periods before and during the COVID-19 pandemic. The screening outcomes were reported as incidence rates, and the pooled effect size reported in this analysis is the incidence rate ratio (IRR). The IRR was calculated from each study using the number of patients screened in time intervals (days) before and after the start of the COVOD-19 pandemic. The individual study IRRs are unadjusted, and both the IRRs and the pooled IRR were calculated using the default assumption form the meta::metainc function in R statistical software, version 4.9-6 (R Foundation for Statistical Computing). The pooled IRR statistic for breast cancer screening was based on 7 studies; data for colon cancer screening was based on 5 studies; and the analysis of cervical cancer screening was based on 3 studies. Two of our studies reported on more than one type of cancer screening. The pooled IRRs were calculated using the inverse variance method, and random effects models were presented. Random effects models were used because the intention of our meta-analysis was to generalize the results beyond the included studies given the universality of our data and the heterogeneity of our study of several patient populations. For consistency, the same model was employed for all 3 outcomes. Heterogeneity between studies was assessed using the χ2 test and I2 statistic. Leave-one-out analyses were performed to calculate pooled estimates to determine if studies with high influence were impacting the significance of the results.
Results
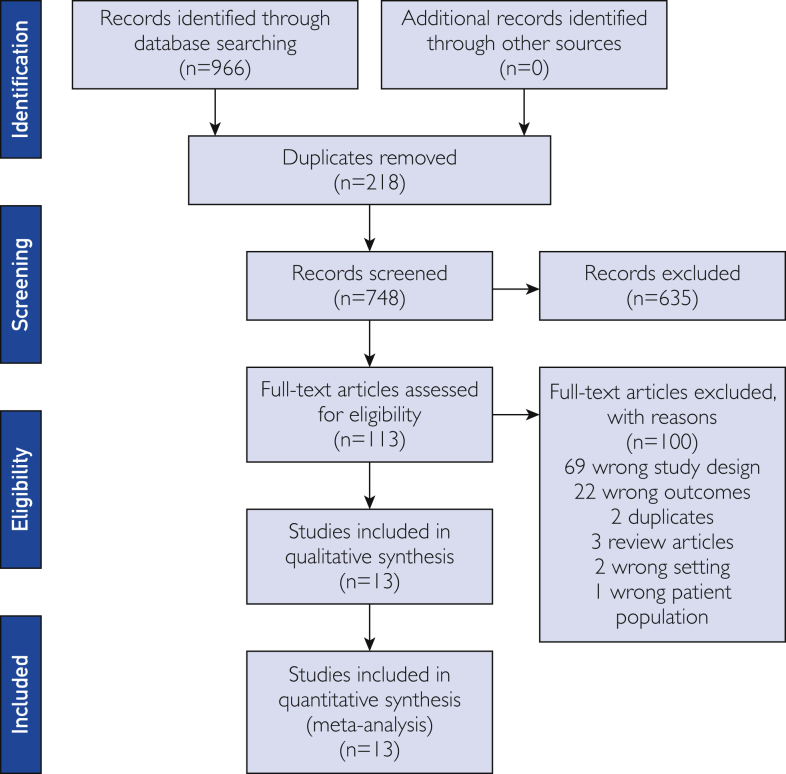
Our database search and study selection process are outlined in the flow diagram (Figure 1). We identified 748 articles for our systematic review with 113 identified as eligible for full-text review. Eleven of these studies met our inclusion criteria and provided the numbers of patients screened before and after lockdown measures were instituted.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 These studies met inclusion criteria for our meta-analysis, with their characteristics described in Table 1.10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 We included 5 studies that examined colon cancer screening, 7 studies of breast cancer screening, and 3 of cervical cancer screening in our statistical analysis (Table 1). Two of our studies reported on more than one type of cancer screening.11,13
Figure1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram.
Table 1.
Study Characteristics.
| Reference, year | Setting and database | Study design | Screening method | Pre-COVID-19 pandemic study interval (d) | COVID-19 pandemic study interval (d) |
|---|---|---|---|---|---|
| Breast cancer | |||||
| Mantellini et al,13 2020 | Italy, National Database (20/21 regions reporting) | Retrospective observational study | Mammography | 151 | 152 |
| Sutherland et al,14 2020 | New South Wales, Australia, multiple databases | Retrospective observational study | Mammography | 122 | 122 |
| Chou et al,15 2021 | Taiwan, public academic hospital electronic records | Retrospective observational study | Mammography | 154 | 154 |
| Tsai et al,16 2021 | Taiwan, Kaohsiung City Community Hospital | Retrospective observational study | Mammography | 59 | 60 |
| Song et al,17 2021 | Database from 34 US states from insurance claims data | Retrospective observational study | Mammography | 800 | 149 |
| Tsai et al,18 2020 | Taiwan National screening database | Retrospective observational study | Mammography | 119 | 120 |
| Colon cancer screening | |||||
| Challine et al,10 2021 | France, National database | Retrospective observational study | Colonoscopy | 75 | 58 |
| Gorin et al,11 2021 | University of Michigan, US, ambulatory medicine clinics | Retrospective observational study | Colonoscopy | 52 | 52 |
| Lantinga et al,12 2021 | Netherlands, multicenter database | Retrospective observational study | Colonoscopy | 62 | 62 |
| Mantellini et al,13 2020 | Italy, National database (20/21 regions reporting) | Retrospective observational study | Colonoscopy | 151 | 152 |
| Chiriac et al,19 2021 | Romania, St. Spiridon Emergency Hospital electronic records | Retrospective observational study | Colonoscopy | 199 | 199 |
| Cervical cancer | |||||
| Gorin et al,11 2021 | University of Michigan, US, ambulatory medicine clinic | Retrospective observational study | Cervical cytology (Papanicolaou testing) and HPV testing | 52 | 52 |
| Mantellini et al,13 2020 | Italy, 20/21 regions database | Retrospective observational study | Cervical cytology (Papanicolaou testing) | 151 | 152 |
| Miller et al,20 2021 | Southern California, integrated health care system | Retrospective observational study | Cervical cytology (Papanicolaou testing) and/or HPV testing | 78 | 85 |
COVID-19, coronavirus disease 2019; HPV, human papillomavirus; US, United States.
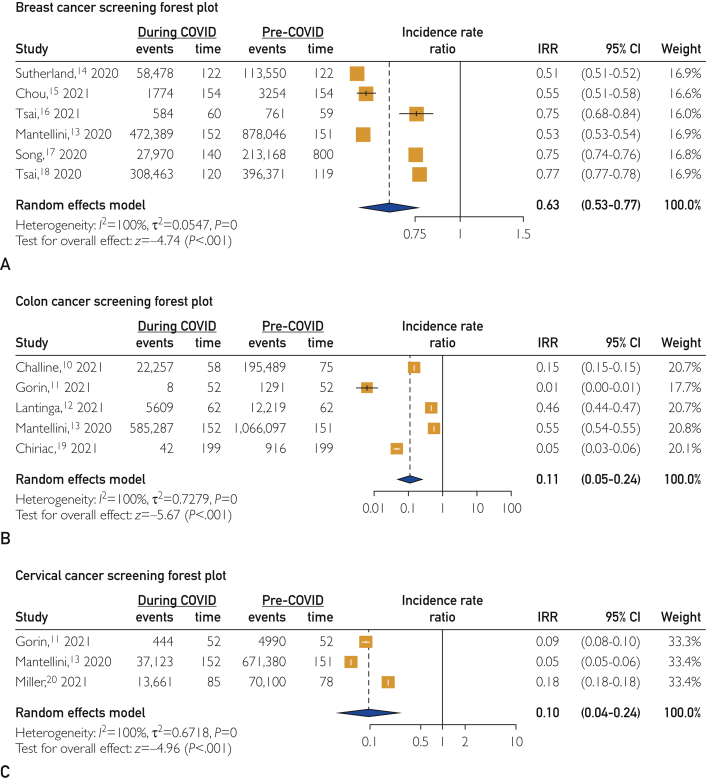
The result of pooling studies that tracked breast cancer screening before and during the COVID-19 pandemic revealed a significantly lower rate of breast cancer screening during the pandemic than before the pandemic, with a pooled IRR of 0.63 (95% CI, 0.53 to 0.77; P<.001) (Figure 2). The 2021 study by Gorin et al11 was removed from this analysis because it was identified as an outlier. Results of leave-one-out influence analyses revealed that the significance of the pooled estimate does not change after omitting any of the studies.
Figure 2.
Outcome measures. A, Breast cancer screening forest plot. B, Colon cancer screening forest plot. C, Cervical cancer screening forest plot. COVID, coronavirus disease 2019; IRR, incidence rate ratio.
The result of pooling the studies assessing colon cancer screening before and during the COVID-19 pandemic revealed a significantly lower rate of colon cancer screening during the pandemic than before the pandemic (pooled IRR, 0.11; 95% CI, 0.05 to 0.24; P<.001) (Figure 2). Results of leave-one-out influence analyses revealed that the significance of the pooled estimate did not change after omitting any of the studies.
The result of pooling the studies that evaluated cervical cancer screening before and during the COVID-19 pandemic revealed a significantly lower rate of cervical cancer screening during the pandemic than before the pandemic (pooled IRR, 0.10; 95% CI, 0.04 to 0.24; P<.001) (Figure 2). Results of leave-one-out influence analyses revealed that the significance of the pooled estimate does not change after omitting any of the studies.
Because there were only 2 studies devoted to the evaluation of lung cancer screening during the COVID-19 pandemic, we were unable to perform a meta-analysis on these data.
Discussion
The COVID-19 pandemic has become one of the most widespread challenges to worldwide public health in the past century. The magnitude of disease and mortality associated with this novel disease led to suspension of routine health care, including age-appropriate cancer screening. Our meta-analysis pooled data from 11 studies that assessed cancer screening data from a variety of settings: 6 on breast cancer, 5 on colon cancer, and 3 on cervical cancer (2 of the studies11, 13 examied more than one type of cancer screening - Figure 2). Our analysis documented a significant decrease in the incidence of screening for all 3 cancer types during the pandemic. Compared with the baseline before the pandemic, screening mammography declined to 63% (95% CI, 0.53 to 0.77; P<.001), screening colonoscopy decreased to 11% (95% CI, 0.05 to 0.24), and cervical cancer screening diminished to 10% (95% CI, 0.04 to 0.24; P<.001). The greater decline for colonoscopy and cervical cancer screening may be attributable to more invasive screening techniques for these cancers compared with that for breast cancer.
The most concerning potential effect of a decrease in cancer screening is an increase in cancer mortality. Mortality data regarding decreased screening during the COVID-19 pandemic is not yet available. The magnitude of a potential increase in mortality will likely add to the global public health burdens of this pandemic. For most of the 20th century, cancer mortality has risen. Overall cancer mortality has decreased every year since 1991, however, and from 1991 to 2018, cancer mortality decreased by 30%.21 Screening in the United States as of 2018 has been documented to prevent 10,179 breast cancer deaths over the lifetime of a cohort of 50-year-old women, 74,470 colon cancer deaths in a cohort of 50-year-old men and women, and 27,166 cervical cancer deaths in a cohort of 21-year-old women.22 It seems reasonable to expect an increase in cancer-specific mortality because of the decrease in cancer screening rates; however, it remains unclear to what degree this may occur. The abrupt decrease in cancer screening resulting from the lockdowns during the pandemic was unprecedented. Global effects on future cancer mortality due to the pandemic have widespread public health ramifications that are yet to be determined.
Another important feature of our analysis is the consistent results across a wide spectrum of health care settings. We analyzed studies performed in multiple countries, including the United States, Italy, Taiwan, the Netherlands, France, and Romania. The data that researchers collected came from a range of sources as well. For instance, some examined hospital records from single or multiple hospitals.12, 15, 16, 19 Others mined regional or national health care databases.10, 13, 14, 17, 18, 20 One study used data from insurance company claims for screening procedures.17 Notably, the reported decreases in cancer screening rates were consistently large within each cancer type; the range in the decline of screening mammography rates was 51% to 77%, screening colonoscopies decreased to 1% to 55%, and cervical cancer screening was 5% to 18% of the pre–COVID-19 pandemic rates. The consistency of these results is support for generalizability of our findings across various health care settings.
Limitations of our study are reflective of our study design. Our study question could only be answered by collecting and analyzing data from retrospective observational studies of cohorts of patients or data collection from patient registries. Since all of the studies were retrospective and unblinded, the potential exists for a risk of bias in the assessment of outcomes and data reporting (Table 2). However, because the outcomes are highly objective, we assess the risk of reporting or selection bias to be low. None of the studies included a method for ensuring that any patients were not counted more than once in the data registries. The certainty of evidence was evaluated with the GRADE approach for all 3 of our studied outcomes.9 Using this approach, we determined the quality of evidence to be high for diminished colon cancer screening and moderate for the diminution of breast and cervical cancer screening during the beginning of the COVID-19 pandemic. We reviewed several studies that reported only the rates in which cancer screening was reduced during the pandemic.6,23, 24, 25, 26, 27, 28, 29 Without data on the numbers of patients screened, we were unable to include these studies in our statistical analysis. In an attempt to collect more data, we contacted the corresponding authors of these studies twice and received data from only one, which became an included study.20 Bias due to confounding seems unlikely in our studies, although covariate analysis was not performed in any of our included studies and an inference cannot be made regarding screening disparities (Table 2). Including data from an insurance registry is a potential confounder for 2 of our included studies.11, 17,20 The short time period of diminished screening for cancer during the COVID-19 pandemic lockdown periods has unclear long-term implications.
Table 2.
Modified Newcastle-Ottawa Quality Assessment Scorea
| Reference, year | Selection |
Comparability | Outcome | |
|---|---|---|---|---|
| Representativeness of exposed cohort | Ascertainment of exposure | |||
| Challine et al,10 2021 | 1 | 1 | 1 | 1 |
| Gorin et al,11 2021 | 1 | 1 | 0 | 1 |
| Lantinga et al,12 2021 | 1 | 1 | 0 | 1 |
| Mantellini et al,13 2020 | 1 | 1 | 1 | 1 |
| Sutherland et al,14 2020 | 1 | 1 | 0 | 1 |
| Chou et al,15 2021 | 1 | 1 | 0 | 1 |
| Tsai et al,16 2021 | 1 | 1 | 1 | 1 |
| Song et al,17 2021 | 1 | 1 | 1 | 1 |
| Tsai et al,18 2020 | 1 | 1 | 1 | 1 |
| Chiriac et al,19 2021 | 1 | 1 | 1 | 1 |
| Miller et al,20 2021 | 1 | 1 | 0 | 1 |
A score of 1 is equal to 1 star and signifies a low risk of bias.
In conclusion, we found high-quality evidence for diminished screening of colon cancer and a moderate quality of evidence for diminished screening of both breast and cervical cancers across a spectrum of health care systems in several different countries during the COVID-19 pandemic. With current cases of COVID-19 in the hundreds of millions worldwide, the complete public health ramifications of this novel viral illness remain to be fully understood and elucidated. The effects of the pandemic will likely be lengthy and manifest in changing the epidemiology of many concomitant disease processes. A downstream result of the pandemic may be an increased incidence of advanced-stage tumors as well as a rise in cancer-specific mortality.
Footnotes
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at https://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Sen S., Karaca-Mandic P., Georgiou A. Association of stay-at-home orders with COVID-19 hospitalizations in 4 states. JAMA. 2020;323(24):2522–2524. doi: 10.1001/jama.2020.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyu W., Wehby G.L. Comparison of estimated rates of coronavirus disease 2019 (COVID-19) in border counties in Iowa without a stay-at-home order and border counties in Illinois with a stay-at-home order. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander G.C., Tajanlangit M., Heyward J., Mansour O., Qato D.M., Stafford R.S. Use and content of primary care office-based vs telemedicine care visits during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Medicare and Medicaid Services Non-emergent, elective medical services and treatment recommendations. Published April 7, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- 5.Ricciardiello L., Ferrari C., Cameletti M., et al. Impact of SARS-CoV-2 pandemic on colorectal cancer screening delay: effect on stage shift and increased mortality. Clin Gastroenterol Hepatol. 2021;19(7):1410–1417.e9. doi: 10.1016/j.cgh.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinmohamed A.G., Cellamare M., Visser O., et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Netherlands. J Hematol Oncol. 2020;13(1):147. doi: 10.1186/s13045-020-00984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis; 2021. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed September 15, 2021.
- 9.Murad M.H. Clinical practice guidelines: a primer on development and dissemination. Mayo Clic Proc. 2017;92(3):423–433. doi: 10.1016/j.mayocp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Challine A., Lazzati A., Dousset B., Voron T., Parc Y., Lefevre J.H. Colorectal screening: we have not caught up; a surge of colorectal cancer after the coronavirus disease 2019 (COVID-19) pandemic [editorial]? Surgery. 2021;169(4):991–993. doi: 10.1016/j.surg.2020.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorin S.N.S., Jimbo M., Heizelman R., Harmes K.M., Harper D.M. The future of cancer screening after COVID-19 may be at home [correction published online ahead of print in Cancer. 2021 Aug 6:10.1002/cncr.33519. doi: 10.1002/cncr.33519] Cancer. 2021;127(4):498–503. doi: 10.1002/cncr.33274. [DOI] [PubMed] [Google Scholar]
- 12.Lantinga M.A., Theunissen F., Ter Borg P.C.J., Bruno M.J., Ouwendijk R.J.T., Siersema P.D. Trans.IT Foundation Study Group. Impact of the COVID-19 pandemic on gastrointestinal endoscopy in the Netherlands: analysis of a prospective endoscopy database. Endoscopy. 2021;53(2):166–170. doi: 10.1055/a-1272-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantellini P., Battisti F., Armaroli P., et al. Oncological organized screening programmes in the COVID-19 era: an Italian survey on accrued delays, reboot velocity, and diagnostic delay estimates [in Italian] Epidemiol Prev. 2020;44(5-6, suppl 2):344–352. doi: 10.19191/EP20.5-6.S2.136. [DOI] [PubMed] [Google Scholar]
- 14.Sutherland K., Chessman J., Zhao J., et al. Impact of COVID-19 on healthcare activity in NSW, Australia. Public Health Res Pract. 2020;30(4):3042030. doi: 10.17061/phrp3042030. [DOI] [PubMed] [Google Scholar]
- 15.Chou C.-P., Pan H.-B., Yang T.-L., Chiang C.-L., Huang J.-S., Tsai M.-Y. Impact of the COVID-19 pandemic on the volume of mammography examinations in southern Taiwan. Breast J. 2021;27(1):89–91. doi: 10.1111/tbj.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai H.-J., Chang Y.-L., Chen F.-M. The feasibility and necessity of cancer screening events in the community during the COVID-19 pandemic in Taiwan. J Med Screen. 2021;28(1):55–56. doi: 10.1177/0969141320941055. [DOI] [PubMed] [Google Scholar]
- 17.Song H., Bergman A., Chen A.T., et al. Disruptions in preventive care: mammograms during the COVID-19 pandemic. Health Serv Res. 2021;56(1):95–101. doi: 10.1111/1475-6773.13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai H.-Y., Chang Y.-L., Shen C.-T., Chung W.-S., Tsai H.-J., Chen F.-M. Effects of the COVID-19 pandemic on breast cancer screening in Taiwan. Breast. 2020;54:52–55. doi: 10.1016/j.breast.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiriac S., Stanciu C., Cojocariu C., et al. The impact of the COVID-19 pandemic on gastrointestinal endoscopy activity in a tertiary care center from northeastern Romania. Healthcare (Basel) 2021;9(1):100. doi: 10.3390/healthcare9010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller M.J., Xu L., Qin J., et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21-65 years in a large integrated health care system—southern California, January 1-September 30, 2019, and January 1-September 30, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(4):109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall I.J., Tangka F.K.L., Sabatino S.A., Thompson T.D., Graubard B.I., Breen N. Patterns and trends in cancer screening in the United States. Prev Chronic Dis. 2018;15:E97. doi: 10.5888/pcd15.170465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma K.P., Grosse S.D., Maciosek M.V., et al. Preventing breast, cervical, and colorectal cancer deaths: assessing the impact of increased screening. Prev Chronic Dis. 2020;17:E123. doi: 10.5888/pcd17.200039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakouny Z., Paciotti M., Schmidt A.L., Lipsitz S.R., Choueiri T.K., Trinh Q.-D. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. 2021;7(3):458–460. doi: 10.1001/jamaoncol.2020.7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang M., Yeung T., Shepard J.O., et al. Operational challenges of a low-dose CT lung cancer screening program during the coronavirus disease 2019 pandemic. Chest. 2021;159(3):1288–1291. doi: 10.1016/j.chest.2020.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel S., Issaka R.B., Chen E., Somsouk M. Colorectal cancer screening and COVID-19 [letter] Am J Gastroenterol. 2021;116(2):433–434. doi: 10.14309/ajg.0000000000000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gawron A.J., Kaltenbach T., Dominitz J.A. The impact of the coronavirus disease-19 pandemic on access to endoscopy procedures in the VA healthcare system. Gastroenterology. 2020;159(4):1216–1220.e1. doi: 10.1053/j.gastro.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng S.-M., Yang K.-C., Chan W.P., et al. Impact of the COVID-19 pandemic on a population-based breast cancer screening program. Cancer. 2020;126(24):5202–5205. doi: 10.1002/cncr.33180. [DOI] [PubMed] [Google Scholar]
- 28.Patt D., Gordan L., Diaz M., et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizuno R., Ganeko R., Takeuchi G., et al. The number of obstructive colorectal cancers in Japan has increased during the COVID-19 pandemic: a retrospective single-center cohort study. Ann Med Surg (Lond) 2020;60:675–679. doi: 10.1016/j.amsu.2020.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.