Abstract
Introduction
The US Food and Drug Administration issued safety warnings about neuropathy in 2013 and dysglycemia in 2018 caused by fluoroquinolone use, mainly based on case reports and case series. We conducted this systematic review to evaluate the safety of fluoroquinolones in diabetic patients by investigating their dysglycemic and neuropathic effects.
Methods
PubMed, Scopus, and Google Scholar were searched for randomized controlled trials and observational studies published from inception till September 2019 evaluating the safety of fluoroquinolones. Efficacy studies of fluoroquinolones reporting these adverse effects were also included. Primary outcomes were hypoglycemia, hyperglycemia, and neuropathy among patients with or without diabetes and treated with fluoroquinolones compared with placebo or other antibiotics. The Cochrane Collaboration tool for randomized controlled trials and modified Newcastle–Ottawa quality-assessment scale were used for assessment of the included studies.
Results and Discussion
A total of 725 studies were identified in the initial search. After screening of titles and abstracts and full-text review, 16 articles fulfilled the inclusion criteria. The sampled patients were aged 30–78 years. Hyperglycemia was reported in 1,588 patients that received fluoroquinolone among eight studies with 4,663 patients, and hypoglycemia was reported in 2,179 patients that received fluoroquinolones among eleven studies with 6,208 patients. Dysglycemia was not generally associated with diabetes mellitus per se. Nevertheless, patients with more comorbidities, especially those with chronic kidney disease, receiving antidiabetics and/or steroids had more glycemic events when treated with fluoroquinolones.
Conclusion
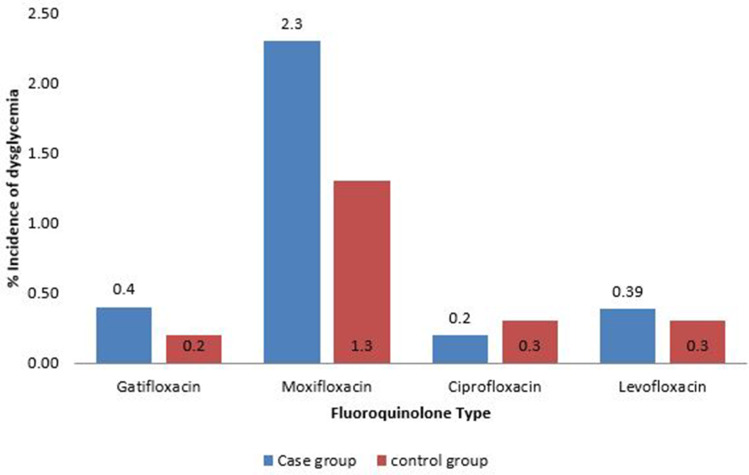
Moxifloxacin was found to be associated the most and ciprofloxacin the least with dysglycemia. fluoroquinolones must be used with great caution among diabetic patients who have comorbidities and are receiving antidiabetics and/or steroids. Further evidence is required from studies on neuropathy caused by fluoroquinolones.
Keywords: gatifloxacin, moxifloxacin, ciprofloxacin, levofloxacin, hyperglycemia, hypoglycemia, safety, adverse drug reaction
Introduction
Researchers have continued to investigate the safety of fluoroquinolones for over two decades. Physicians commonly advocate the use of these antibacterials when there is no available alternative.1 The recommendations regarding these antibacterials have been made because of the adverse effects that have come from recent warnings for common conditions, such as acute bacterial infections of sinuses and bronchi, as well as uncomplicated urinary tract infections. The most commonly reported psychiatric adverse effects include confusion, hallucinations, agitation, delirium, insomnia, aand drowsiness. McGarvey et al recommended that fluoroquinolone be discontinued at the first sign of tendon inflammation to avoid subsequent rupture.2 The US Food and Drug Administration (FDA) issued safety warnings about neuropathy in 2013 and dysglycemia in 2018 caused by fluoroquinolone use, mainly based on case reports and case series. In 2018, an FDA review found that fluoroquinolone antibiotics can also increase the occurrence of rare but serious events of ruptures or tears in the main artery of the body — the aorta.3–6
Though fluoroquinolones have proved to be effective in treating infections, there are concerns about their adverse effects on human beings. There have been some unfavorable responses in studies on fluoroquinolones during the final phases of clinical trials.7 Most of these trials have reported adverse effects of fluoroquinolone on the hepatic system, central nervous system, gastrointestinal tract, skin, and musculoskeletal system.8 Modification of fluoroquinolone structures has also been found to have adverse drug reactions that can cause dysglycemia and neuropathy.9,10 The structure of fluoroquinolones is lipoidal in nature, hence accounting for an increased affinity for bones and cartilage. This explains the effect of fluoroquinolones on the musculoskeletal system. Studies have also found risks of dysglycemia and neuropathy among patients receiving fluoroquinolone antibacterials.11 Dysglycemia and neuropathy are two effects that are also regarded as complications associated with diabetes mellitus. Diabetic patients are already at risk of these effects and subsequent complications.
This study sought to evaluate the safety of fluoroquinolones for diabetic patients by exploring dysglycemic and neuropathic adverse effects reported in research compared to other antibiotics. Our findings make several contributions toward understanding dysglycemia and neuropathy as part of the effects regarded as complications associated with diabetes mellitus.
Methods
Literature Search
This systematic research followed PRISMA (Preferred Reporting Items for Systematic Review and Meta-analysis) protocols.12 Two researchers (AA and YZ) independently performed an exclusive literature search using the Google Scholar, PubMed, and Scopus databases from inception till September 2019. The key search terms used are shown in Supplementary Information 1. We limited the search to literature published in English and international scientific journals. We opted to exclude conference abstracts, because they present findings of preliminary analyses that eventually appear as full-text publications. In addition, we screened all the reference lists of relevant systematic reviews and meta-analyses to help us identify any additional eligible publications that could have been missed during the initial search.
Study Selection and Data Extraction
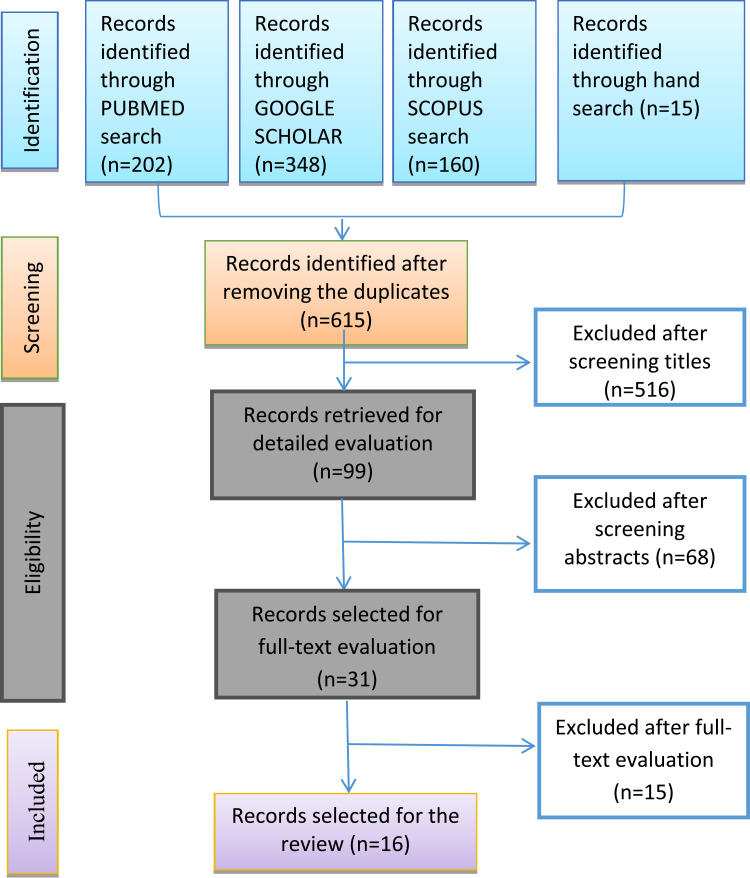
Studies eligible for inclusion in the review were any research (original research, systematic reviews) involving the use of fluoroquinolones for any infection/any duration/any age category/any ethnic group comparing the efficacy or safety with placebo or other antibiotics, and if they were conducted with or without a focus on adverse events and not necessarily conducted only in a diabetic population. We excluded all studies that involved the use of fluoroquinolones in pregnancy, economic evaluations, those that did not report adverse events related to neuropathy and/or dysglycemia, and those that did not report any adverse effects at all. Ultimately, 16 studies met our inclusion criteria.13–28 (Figure 1).
Figure 1.
PRISMA flow diagram of study selection.
Notes: Adapted from: Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.12 Creative Commons Attribution Non-commercial License.
The two researchers independently extracted data from the selected studies using a predefined data-extraction form, and resolved any discrepancies in the extracted data through discussion. The data-extraction form recorded basic characteristics of the studies, including authors, year of publication, study design, setting, number of patients in each arm, number of diabetic patients in each arm, means or medians of baseline characteristics, comorbidities, use of other medications, target infections, controls or comparators, dose and frequency of fluoroquinolone used, and the number of adverse events in each arm. Due to the heterogeneity of the included studies, it was not deemed appropriate to perform a meta-analysis.29
Risk of bias in the studies was assessed using the Cochrane Collaboration’s tool30 for randomized trials and the Newcastle–Ottawa Scale31 for observational studies. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology32 was used to determine the level of evidence for each study.
Results
A total of 725 studies were identified. After screening titles and abstracts, 31 were selected, with a total of 16 fulfilling the inclusion criteria. In these, the patient ages ranged 30–78 years. Hyperglycemia was reported in 1,588 patients that received fluoroquinolones in eight studies on a total of 4,663 patients, whereas hypoglycemia was reported in 2,179 patients that received fluoroquinolones in eleven studies on a total 6,208 patients. Summaries of the extracted information are included in Supplementary Information 2. Patients with more comorbidities, such as chronic kidney disease compared with just diabetic mellitus per se, tend to have higher risk of dysglycemia.17 Nevertheless, four studies indicated that receiving antidiabetic was associated with increased risk of dysglycemia,14,17,23,26 and two studies found the use of steroids resulted in more glycemic events when treated with fluoroquinolones than controls.17,24
Dysglycemia associated with fluoroquinolones in diabetic and nondiabetic patients is shown in Table 1. Results of the Newcastle–Ottawa scale are shown in Table 2. Results of the modified Cochrane Collaboration tool to assess risk of bias for randomized controlled trials is reported in Table 3. Figure 2 shows the incidence of dysglycemia associated with different fluoroquinolones.
Table 1.
Dysglycemia associated with fluoroquinolones in diabetic and nondiabetic patients
| Diabetic patients | Nondiabetic patients | |||
|---|---|---|---|---|
| Hypoglycemia | Hyperglycemia | Hypoglycemia | Hyperglycemia | |
| Gatifloxacin | 231 | 513 | 17 | 107 |
| Moxifloxacin | 78 | 43 | 2 | 11 |
| Ciprofloxacin | 488 | 156 | 14 | 30 |
| Levofloxacin | 579 | 186 | 15 | 40 |
| Total | 1,376 | 898 | 48 | 188 |
Table 2.
Newcastle–Ottawa quality-assessment scale scores
| Reference | Score | |
|---|---|---|
| 1 | Park-Wyllie et al 200627 | 8 out of 8 |
| 2 | Mohr et al 200824 | 8 out of 8 |
| 3 | Mohr et al 200523 | 8 out of 8 |
| 4 | Graumlich et al 200517 | 7 out of 8 |
| 5 | Etminanet et al | 7 out of 8 |
| 6 | Schelleman et al 201028 | 8 out of 8 |
| 7 | Parekh et al 201426 | 8 out of 8 |
| 8 | Aspinall et al 200913 | 8 out of 8 |
| 9 | Chou et al 201314 | 8 out of 8 |
| 10 | LaPlante et al 200820 | 7 out of 8 |
| 11 | Lodise et al 200721 | 7 out of 8 |
| 12 | Onyenwenyi et al 200725 | 8 out of 8 |
| 13 | Haerian et al 200718 | 7 out of 8 |
Notes: 1–5, case–control studies; 6–12, cohort studies.
Table 3.
Modified Cochrane Collaboration tool to assess risk of bias for randomized controlled trials
| Study | Selection bias | Reporting bias | Other bias | Performance bias | Detection bias | Attrition bias | |
|---|---|---|---|---|---|---|---|
| Random-sequence generation | Allocation concealment | Selective reporting | Blinding (participants and personnel) | Blinding (outcome assessment) | Incomplete outcome data | ||
| Gajjar et al, 2000 16 | Low | Low | Low | Low | Low | Low | Low |
| Jawahar et al, 2013 19 | Low | High | Low | Low | Low | Low | Low |
| Merle et al, 2014 22 | Low | High | Low | Low | High | Low | High |
Figure 2.
Incidence of dysglycemia associated with fluoroquinolones.
Discussion
We carried out a systematic review of the extant literature to understand the two complications of dysglycemia and neuropathy associated with diabetes mellitus. Diabetic patients are already at risk of these effects and subsequent complications. Therefore, we evaluated the safety of fluoroquinolones for diabetic patients by exploring dysglycemic and neuropathic effects of fluoroquinolones reported in research studies. This study summarized and evaluated evidence presented in 16 studies on dysglycemia and two on neuropathy. The findings we compiled from different studies helped to show that fluoroquinolones were associated with increased risk of hyperglycemia and hypoglycemia. Furthermore, our findings revealed variabilities in such risks depending on the type of fluoroquinolone administered to diabetes mellitus and those without diabetes. For example, our findings showed that levofloxacin was associated with a very high level of hypoglycemic diabetic patients, followed by ciprofloxacin and gatifloxacin. In line with our expectations, a majority of patients experiencing adverse events after being treated with sulfonylurea and insulin were at high risk of suffering of abnormal fluctuations in their glucose homeostasis. Our findings support a study conducted by Chou et al14 that found that moxifloxacin had a very high association with risk of hypoglycemia, followed by levofloxacin and ciprofloxacin. In addition, the study collected a large sample that formed their cohort population from a national database. The authors sought to investigate the risks associated with contracting dysglycemia among most of the new patients who had had antibiotics administered.14 Furthermore, it is critical to point out that the study focused only on patients that had received first episode. They did this in order to try to avoid cases of selection bias as well as diluted risk.14
There is a higher risk of hypoglycemia than hyperglycemia.33 The extant literature shows that the risks associated with hypoglycemia seem to be more associated with levofloxacin than ciprofloxacin or gatifloxacin. A case–control research also showed that there was a slight increase in the risk of hypoglycemia related to diabetic patients administered levofloxacin. However, the same risks did not manifest with moxifloxacin or ciprofloxacin.34 Another cohort study in a Veterans Affairs health-care facility indicated that the probability of diabetic patients experiencing severe hypoglycemia seemed to be greater among those administered levofloxacin than patients receiving azithromycin. However, the findings also showed a contrast in risk among those administered with ciprofloxacin.13
The effect of fluoroquinolones on secretion of insulin has also received attention among studies, showing that fluoroquinolones cause severe hypoglycemia by causing an increase in insulin secreted in the body. The body secrets insulin through adenosine triphosphate–sensitive K+-blockade pathways within β cells in the pancreas.35 Chou et al14 reiterated that such an effect might fail to be clinically significant among all the patients with physiological mechanisms regulating the flow levels of glucose in the blood. However, there is still a lack of clarity concerning the mechanisms of hyperglycemia. In a study conducted by Francis and Higgins,11 overexposure might have been a major contributing factor in terms of failure of specialists to adjust the amount of dosage administered to diabetic patients suffering renal insufficiency. The findings presented in our study and other research,1,10 as well as the significant common mechanisms associated with hypoglycemia, point to the need for caution when administering certain fluoroquinolones. Other studies have also reported differences in risk of other adverse effects from the same group of antibiotics.35
According to findings focusing on empirical in vitro animal research, it is clear that fluoroquinolones have a high chance of causing hypoglycemia. They increases insulin levels released in the blood through ATP-sensitive K+-blockade passage, depending on dosage.36 Another similar study showed that the insulinotropic effect of fluoroquinolones occurred because of stimulatory effects of β-cell nutrients instead of initial secretion of insulin. The findings showed that gatifloxacin provided the highest insulinotropic efficacy, followed by moxifloxacin and ciprofloxacin.37
Our results are also consistent with past studies. For instance, a retrospective review of medical records concerning glycemia among hospitalized patients administered with fluoroquinolones, such as gatifloxacin, levofloxacin, ceftriaxone, or ciprofloxacin, revealed that dysglycemia events were more probable among patients administered gatifloxacin. The associated risks were 3.29 for gatifloxacin and 1.55 for levofloxacin. Studies have not shown any difference between gatifloxacin and levofloxacin. However, it is critical to point out that in our study, we combined both hypoglycemia and hyperglycemia when researching meta-analyses. Within the controls (no diabetes), it was clear that the odds of gatifloxacin inducing hypo- and hyperglycemia were higher than other drugs, followed by levofloxacin. Our findings support Wang et al, who found that among elderly inpatients administered gatifloxacin or levofloxacin, the former was independently associated with hyperglycemia than hypoglycemia.38
We found two studies that focused on understanding the neuropathic effects of fluoroquinolones: difficulties experienced when diagnosing patients with fluoroquinolones related to neuropathy was because of delayed array of symptoms, confusion, and diffusion.39,40 While patients administered fluoroquinolones showed fewer side effects than those administered first-generation fluoroquinolones, eg, gastrointestinal disturbances and nausea, 0.8%–1.7% experienced adverse reactions associated with the peripheral and central nervous systems. These included agitation, delusions, psychosis, drowsiness, dizziness, and headache. Moreover, the effects extended to peripheral sensory disturbances. Of note, another study discovered contrasting findings from the aforementioned. It was suggested that patients administered fluoroquinolones experienced mild and short-term events in the nervous system. For example, 80% reported incidences of organ systems in their nerves with symptoms starting as early as 24 hours postdosage. Half the cases indicated that symptoms lasted for >1 year.41 They concluded there was a high level of association between fluoroquinolones and long-term effects on the peripheral nervous system among diabetic patients. This included 55% peripheral neuropathy symptoms, and 75% central nervous system. More than 80% of patients experienced long-term sequelae associated with fluoroquinolones.41
We believe that our findings have clinical implications specifically in ambulatory care settings where alternative drug therapies can be considered in patients at risk of dysglycemia due to fluoroquinolones, especially moxifloxacin, or more aggressive monitoring of blood glucose should be conducted if fluoroquinolones have to be used in high-risk patients. However, blood glucose is generally more closely monitored and conveniently managed in inpatient settings. Future research should explore the relationship between clinical effects and quality of fluoroquinolone products, because literature has uncovered that the quality of ciprofloxacin and levofloxacin could be compromised.42,43
Limitations
There is a caveat for any researcher that would like to use our findings as a reference point. The fact that our methodology relied on meta-analysis of of different databases resulted in problems with coding qualitative data and missing data.40 In addition, while several events could have occurred in hospitals, it was difficult for our team to validate such types of diagnoses of hypoglycemia and hyperglycemia among diabetic and nondiabetic patients. We also failed to access medical records and data from laboratories. Even though the events reported in other literature failed to show severity of conditions with hospitalization, our secondary research failed to understand the degree and duration of glycemia. This could have contributed to a possible association of gatifloxacin with both hypoglycemia and hyperglycemia among the diabetic patients. Nonetheless, we used strict criteria to achieve accuracy in the results. The severity of adverse effects from fluoroquinolones can influence insulin in the bloodstream of diabetic patients. As such, incorporating nondiabetic patients in randomized controlled trials could produce confounders. Moreover, we found only one study focusing on neuropathy, which is insufficient to draw strong conclusions related to adverse effects of fluoroquinolones This necessitates further research.
Conclusion
This study sought to evaluate the safety of fluoroquinolones for diabetic patients by exploring their dysglycemic and neuropathic effects of fluoroquinolones reported in the research. We conducted a systematic review and used the PRISMA approach to select eligible articles for a meta-analysis of randomized controlled trials and observational studies published from inception until September 2019 examining the adverse effects of fluoroquinolones on diabetic patients. Our findings suggest a strong association between certain fluoroquinolones and adverse effects of both dysglycemia and neuropathy. Dysglycemia was not generally associated with diabetes.
Patients with more comorbidities, such as chronic kidney diseases, receiving antibiotics and/or steroids had more glycemic events when treated with fluoroquinolones. Moxifloxacin had the strongest relationship with dysglycemia and ciprofloxacin the weakest. We recommend that health facilities take caution when administering fluoroquinolones to diabetic patients. This should extend to patients with comorbidities and those receiving diabetics and/or steroids.
Acknowledgment
Abdulrhman Althaqafi and Long Chiau Ming contributed equally as first authors.
Funding Statement
This study was conducted without any financial support.
Disclosure
All authors declare that they have no conflicts of interest. The authors are responsible for the content and writing of the article.
References
- 1.Jones SC, Sorbello A, Boucher RM. Fluoroquinolone-associated myasthenia gravis exacerbation: evaluation of postmarketing reports from the US FDA adverse event reporting system and a literature review. Drug Saf. 2011;34(10):839–847. doi: 10.2165/11593110-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 2.McGarvey WC, Singh D, Trevino SG. Partial Achilles tendon ruptures associated with fluoroquinolone antibiotics: a case report and literature review. Foot Ankle Int. 1996;17(8):496–498. doi: 10.1177/107110079601700811 [DOI] [PubMed] [Google Scholar]
- 3.Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open. 2015;5(11):e010077. doi: 10.1136/bmjopen-2015-010077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee CC, Lee MG, Hsieh R, et al. Oral fluoroquinolone and the risk of aortic dissection. J Am Coll Cardiol. 2018;72(12):1369–1378. doi: 10.1016/j.jacc.2018.06.067 [DOI] [PubMed] [Google Scholar]
- 5.Lee CC, Lee MT, Chen YS, et al. Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Intern Med. 2015;175(11):1839–1847. doi: 10.1001/jamainternmed.2015.5389 [DOI] [PubMed] [Google Scholar]
- 6.Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ. 2018;360:k678. doi: 10.1136/bmj.k678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathews B, Thalody AA, Miraj SS, Kunhikatta V, Rao M, Saravu K. Adverse effects of fluoroquinolones: a retrospective cohort study in a South Indian tertiary healthcare facility. Antibiotics. 2019;8(3):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis G, Juhasz A, Smith E. Detection of antibacterial-like activity on a silica surface: fluoroquinolones and their environmental metabolites. Environ Sci Pollut Res Int. 2011;19(7):2795–2801. doi: 10.1007/s11356-012-0781-8 [DOI] [PubMed] [Google Scholar]
- 9.Cowling T, Farrah K. Fluoroquinolones for the treatment of other respiratory tract infections: a review of clinical effectiveness, cost-effectiveness, and guidelines. Fluoroquinolones for the treatment of other respiratory tract infections: a review of clinical effectiveness, cost-effectiveness, and guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2019. [PubMed] [Google Scholar]
- 10.Gorelik E, Masarwa R, Perlman A, et al. Fluoroquinolones and cardiovascular risk: a systematic review, meta-analysis and network meta-analysis. Drug Saf. 2019;42(4):529–538. doi: 10.1007/s40264-018-0751-2 [DOI] [PubMed] [Google Scholar]
- 11.Francis JK, Higgins E. Permanent peripheral neuropathy: a case report on a rare but serious debilitating side-effect of fluoroquinolone administration. J Investig Med High Impact Case Rep. 2014;2(3):2324709614545225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aspinall SL, Good CB, Jiang R, McCarren M, Dong D, Cunningham FE. Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis. 2009;49(3):402–408. doi: 10.1086/600294 [DOI] [PubMed] [Google Scholar]
- 14.Chou HW, Wang JL, Chang CH, Lee JJ, Shau WY, Lai MS. Risk of severe dysglycemia among diabetic patients receiving levofloxacin, ciprofloxacin, or moxifloxacin in Taiwan. Clin Infect Dis. 2013;57(7):971–980. doi: 10.1093/cid/cit439 [DOI] [PubMed] [Google Scholar]
- 15.Etminan M, Brophy JM, Samii A. Oral fluoroquinolone use and risk of peripheral neuropathy: a pharmacoepidemiologic study. Neurology. 2014;83(14):1261–1263. doi: 10.1212/WNL.0000000000000846 [DOI] [PubMed] [Google Scholar]
- 16.Gajjar DA, LaCreta FP, Kollia GD, et al. Effect of multiple-dose gatifloxacin or ciprofloxacin on glucose homeostasis and insulin production in patients with noninsulin-dependent diabetes mellitus maintained with diet and exercise. Pharmacotherapy. 2000;20(6 Pt 2):76s–86s. doi: 10.1592/phco.20.8.76S.35182 [DOI] [PubMed] [Google Scholar]
- 17.Graumlich JF, Habis S, Avelino RR, et al. Hypoglycemia in inpatients after gatifloxacin or levofloxacin therapy: nested case-control study. Pharmacotherapy. 2005;25(10):1296–1302. doi: 10.1592/phco.2005.25.10.1296 [DOI] [PubMed] [Google Scholar]
- 18.Haerian H, McHugh P, Brown R, Somes G, Solomon SS. Gatifloxacin produces both hypoglycemia and hyperglycemia: a retrospective study. Am J Med Sci. 2008;335(2):95–98. doi: 10.1097/MAJ.0b013e31812f65fc [DOI] [PubMed] [Google Scholar]
- 19.Jawahar MS, Banurekha VV, Paramasivan CN, et al. Randomized clinical trial of thrice-weekly 4-month moxifloxacin or gatifloxacin containing regimens in the treatment of new sputum positive pulmonary tuberculosis patients. PLoS One. 2013;8(7):e67030. doi: 10.1371/journal.pone.0067030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaPlante KL, Mersfelder TL, Ward KE, Quilliam BJ. Prevalence of and risk factors for dysglycemia in patients receiving gatifloxacin and levofloxacin in an outpatient setting. Pharmacotherapy. 2008;28(1):82–89. doi: 10.1592/phco.28.1.82 [DOI] [PubMed] [Google Scholar]
- 21.Lodise T, Graves J, Miller C, Mohr JF, Lomaestro B, Smith RP. Effects of gatifloxacin and levofloxacin on rates of hypoglycemia and hyperglycemia among elderly hospitalized patients. Pharmacotherapy. 2007;27(11):1498–1505. doi: 10.1592/phco.27.11.1498 [DOI] [PubMed] [Google Scholar]
- 22.Merle CS, Fielding K, Sow OB, et al. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med. 2014;371(17):1588–1598. doi: 10.1056/NEJMoa1315817 [DOI] [PubMed] [Google Scholar]
- 23.Mohr JF, McKinnon PS, Peymann PJ, Kenton I, Septimus E, Okhuysen PC. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacotherapy. 2005;25(10):1303–1309. doi: 10.1592/phco.2005.25.10.1303 [DOI] [PubMed] [Google Scholar]
- 24.Mohr JF, Peymann PJ, Troxell E, Lodise TP, Ostrosky-Zeichner L. Risk factors for hyperglycemia in hospitalized adults receiving gatifloxacin: a retrospective, nested case-controlled analysis. Clin Ther. 2008;30(1):152–157. doi: 10.1016/j.clinthera.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 25.Onyenwenyi AJ, Winterstein AG, Hatton RC. An evaluation of the effects of gatifloxacin on glucose homeostasis. Pharm World Sci. 2008;30(5):544–549. doi: 10.1007/s11096-008-9205-8 [DOI] [PubMed] [Google Scholar]
- 26.Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med. 2014;174(10):1605–1612. doi: 10.1001/jamainternmed.2014.3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park-Wyllie LY, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354(13):1352–1361. doi: 10.1056/NEJMoa055191 [DOI] [PubMed] [Google Scholar]
- 28.Schelleman H, Bilker WB, Brensinger CM, Wan F, Hennessy S. Anti-infectives and the risk of severe hypoglycemia in users of glipizide or glyburide. Clin Pharmacol Ther. 2010;88(2):214–222. doi: 10.1038/clpt.2010.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. 2014;20(2):123–129. doi: 10.1111/1469-0691.12494 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson J, Welch V, Losos M, Tugwell P. The Newcastle–Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses; 2011. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed December 15, 2020.
- 32.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 33.Garber SM, Pound MW, Miller SM. Hypoglycemia associated with the use of levofloxacin. Am J Health Syst Pharm. 2009;66(11):1014–1019. doi: 10.2146/ajhp080105 [DOI] [PubMed] [Google Scholar]
- 34.Micheli L, Sbrilli M, Nencini C. Severe hypoglycemia associated with levofloxacin in Type 2 diabetic patients receiving polytherapy: two case reports. Int J Clin Pharmacol Ther. 2012;50(4):302–306. doi: 10.5414/CP201594 [DOI] [PubMed] [Google Scholar]
- 35.Anderson VR, Perry CM. Levofloxacin: a review of its use as a high-dose, short-course treatment for bacterial infection. Drugs. 2008;68(4):535–565. doi: 10.2165/00003495-200868040-00011 [DOI] [PubMed] [Google Scholar]
- 36.Lewis G, Juhasz A, Smith E. Environmental metabolites of fluoroquinolones: synthesis, fractionation and toxicological assessment of some biologically active metabolites of ciprofloxacin. Environ Sci Pollut Res Int. 2011;19(7):2697–2707. doi: 10.1007/s11356-012-0766-7 [DOI] [PubMed] [Google Scholar]
- 37.Ghaly H, Kriete C, Sahin S, et al. The insulinotropic effect of fluoroquinolones. Biochem Pharmacol. 2009;77(6):1040–1052. doi: 10.1016/j.bcp.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 38.Wang SH, Xie YC, Jiang B, et al. [Fluoroquinolone associated myasthenia gravis exacerbation: clinical analysis of 9 cases]. Zhonghua Yi Xue Za Zhi. 2013;93(17):1283–1286. Chinese. [PubMed] [Google Scholar]
- 39.Hedenmalm K, Spigset O. Peripheral sensory disturbances related to treatment with fluoroquinolones. J Antimicrob Chemother. 1996;37(4):831–837. doi: 10.1093/jac/37.4.831 [DOI] [PubMed] [Google Scholar]
- 40.Golomb BA, Koslik HJ, Redd AJ. Fluoroquinolone-induced serious, persistent, multisymptom adverse effects. BMJ Case Rep. 2015;2015:bcr2015209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen JS. Peripheral neuropathy associated with fluoroquinolones. Ann Pharmacother. 2001;35(12):1540–1547. doi: 10.1345/aph.1Z429 [DOI] [PubMed] [Google Scholar]
- 42.Izadi E, Afshan G, Patel RP, et al. Levofloxacin: insights into antibiotic resistance and product quality. Front Pharmacol. 2019;10:881. doi: 10.3389/fphar.2019.00881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma D, Patel RP, Zaidi STR, Sarker MMR, Lean QY, Ming LC. Interplay of the quality of ciprofloxacin and antibiotic resistance in developing countries. Front Pharmacol. 2017;8:546. doi: 10.3389/fphar.2017.00546 [DOI] [PMC free article] [PubMed] [Google Scholar]