Abstract
Purpose
To evaluate the safety and efficacy of dexamethasone intravitreal implant 0.7 mg (DEX) compared with laser photocoagulation in patients with diabetic macular edema (DME).
Patients and Methods
This Phase 3, multicenter, randomized, efficacy evaluator–masked, parallel-group, 12-month clinical study enrolled adults in China and the Philippines with reduced visual acuity secondary to fovea-involved DME in the study eye. Participants were randomized 1:1 to study eye treatment with laser photocoagulation every 3 months as needed (n = 139) or DEX every 5 months (n = 145). The main efficacy measures were best-corrected visual acuity (BCVA), central retinal thickness (CRT), and leakage area. The primary endpoint was the average change in BCVA from baseline over 12 months (area-under-the-curve method). Preplanned subgroup analyses evaluated outcomes in Chinese patients.
Results
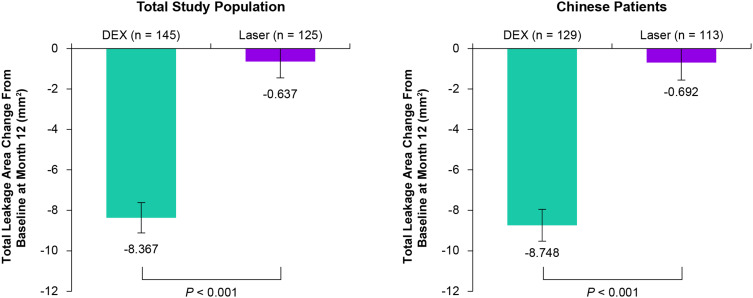
Mean average change in BCVA from baseline during the study (letters) was 4.3 with DEX (n = 145) versus 1.4 with laser (n = 127) overall (P = 0.001) and 4.6 with DEX (n = 129) versus 0.6 with laser (n = 113) in Chinese patients (P < 0.001). At Month 12, mean change in CRT from baseline was −209.5 μm with DEX versus −120.3 μm with laser (P < 0.001) and mean change in total leakage area from baseline was −8.367 mm2 with DEX versus −0.637 mm2 with laser (P < 0.001). The most common treatment-emergent adverse events in the DEX group were increased intraocular pressure and cataract.
Conclusion
DEX administered every 5 months provided significantly greater improvement in BCVA, CRT, and total leakage area compared with laser treatment. DEX demonstrated an acceptable safety profile, consistent with an intraocular corticosteroid, and similar to that reported in completed global registration studies.
Keywords: central retinal thickness, corticosteroid, dexamethasone, diabetic retinopathy, drug delivery device, laser photocoagulation, randomized controlled trial, macular edema, visual acuity
Introduction
Diabetic macular edema (DME) is a common manifestation of diabetic retinopathy (DR) and an increasingly frequent cause of vision loss and blindness because of the rising global prevalence of diabetes.1 It is estimated that from 2015 to 2040, the number of individuals with diabetes worldwide will rise from 415 million to 642 million.2 Approximately one-third of individuals with diabetes have signs of DR,3 and among individuals with DR, approximately one-fifth have DME.4
In China, the prevalence of diabetes has been estimated to be 11.6% in adults ages 18 and over.5 A cross-sectional study involving 17,985 adults aged 18–79 years in Beijing reported that the prevalence of DR was 1.5% for the total study population and 8.1% for individuals with diabetes.6 In a study that evaluated optical coherence tomography (OCT) images of eyes with DR in Shanghai, DME was present in 30.5% (46/151) of eyes with DR.7
The current first-line standard of care therapy for center-involved DME is intravitreal injections of an anti-vascular endothelial growth factor inhibitor (anti-VEGF).8,9 Anti-VEGF injections can reduce edema, improve visual acuity, and prevent further vision loss in patients with DME, and their ocular safety profile is favorable.8 However, some patients do not respond adequately to anti-VEGF injections.10,11 Furthermore, the need for frequent anti-VEGF injections can be burdensome for patients and healthcare systems.12–14 In real-world clinical practice, patients typically are monitored and receive anti-VEGF injections with lower frequency than in clinical trials, resulting in less favorable visual outcomes.15
Focal/grid laser photocoagulation and corticosteroids are also used in the treatment of center-involved DME. Laser photocoagulation helps prevent moderate vision loss, but it is often ineffective in improving visual acuity in patients with DME,16 and persistent and recurrent macular edema after laser therapy is common.17 Corticosteroids are a rational choice for DME treatment because they inhibit multiple inflammatory pathways involved in the pathophysiology of DME, including expression of VEGF and other cytokines and proinflammatory mediators, recruitment and activation of leukocytes, and changes in endothelial tight junction proteins that lead to capillary leakage, fluid accumulation, and macular thickening.18–20 Intravitreal injections of triamcinolone acetonide are widely used for treatment of posterior segment inflammatory diseases, and triamcinolone acetonide injections at 4-month intervals have been shown to improve visual acuity in patients with center-involved DME.21 However, effective intravitreal treatment of DME with corticosteroids that are more water-soluble requires the use of a sustained-release delivery system, because they have a short half-life in the vitreous.22 Biodegradable, sustained-release dexamethasone intravitreal implant 0.7 mg (DEX; Ozurdex; Allergan [an AbbVie company], Irvine, CA) releases the potent corticosteroid dexamethasone into the vitreous and provides therapeutic drug levels at posterior segment target tissues for several months.23
DEX has become a well-recognized treatment for DME. In global registration studies (the MEAD studies), an average of four to five injections of DEX over 3 years improved visual and anatomic outcomes relative to sham procedure in patients with DME.23 Randomized controlled studies have further demonstrated similar improvements in visual acuity with DEX and anti-VEGF injections in patients with DME, but rates of vision loss may be higher in patients treated with DEX because of the occurrence of cataract.24,25 Other prospective, randomized studies showed decreased retinal thickness26 or both decreased retinal thickness and improved visual acuity27 when DEX was used as an adjunct to anti-VEGF therapy in patients with DME. Finally, in a large, retrospective, registry study, DEX was safe and effective in the real-world treatment of patients with DME in Israel and seven European countries.28 Side effects of intravitreal corticosteroid therapy (ie, increases in intraocular pressure and cataract) can limit the usefulness of this approach to treatment of DME.22 However, intravitreal corticosteroids continue to have a role in the treatment of DME in patients with an inadequate response to anti-VEGF therapy, and they may also be useful as first-line therapy in selected patients.9,29,30
The objective of the present study was to evaluate the safety and efficacy of DEX compared with laser photocoagulation in patients with DME. This randomized controlled trial (RCT) was undertaken to gain Chinese regulatory agency approval of DEX for treatment of DME in China, and for this reason, approximately 90% of the study population was Chinese (which satisfied the requirement for approval in China), and subgroup analysis of outcomes in Chinese patients was performed. Laser photocoagulation was the active comparator for DEX because at the time of the study initiation, laser photocoagulation was the standard of care for DME in China—no anti-VEGF therapies had yet been approved in China for treatment of DME. This is the first reported RCT comparing DEX with laser photocoagulation for treatment of DME, as well as the first RCT evaluating DEX for treatment of DME in Chinese patients.
Materials and Methods
This 12-month, Phase 3, randomized, multicenter, parallel-group comparison study was conducted at 18 ophthalmology clinical practices (16 in China and 2 in the Philippines). The study adhered to the tenets of the Declaration of Helsinki and was conducted in compliance with Good Clinical Practice, and Institutional Review Board (IRB)/Ethics Committee approval was obtained at each site. The study is registered at ClinicalTrials.gov (NCT02121262).
The study participants were adults with fovea-involved DME and associated visual acuity loss in at least 1 eye (the study eye). Key inclusion criteria included age of 18 years or older; study eye best-corrected visual acuity (BCVA), measured with the Early Treatment Diabetic Retinopathy Study (ETDRS) method, of 34–70 letters inclusive (~20/200 to 20/40 Snellen equivalent); and macular thickening by optical coherence tomography (OCT), defined as central retinal thickness in the 1-mm central macular subfield (CRT) of ≥300 μm on Spectralis (Heidelberg) OCT, ≥275 μm on Cirrus (Zeiss) OCT, or ≥250 μm on Stratus III (Zeiss) OCT, as read by the investigator. All OCT instruments were certified by the reading center. Because CRT measurements differ among machine types, the cut-off in CRT used to define macular thickening for each type of instrument was based on the CRT in normal eyes as measured by the instrument,31,32 and for each patient, the same instrument was used for all OCT assessments throughout the duration of the study.
Key exclusion criteria included ocular disease other than DME in the study eye that in the opinion of the investigator could confound assessment of the macula or affect central vision; intraocular pressure (IOP) of ≥22 mmHg or a diagnosis of glaucoma in the study eye; use of laser photocoagulation to the retina, intravitreal anti-VEGF, or topical, intraocular, intravitreal (except triamcinolone), or periocular corticosteroid in the study eye within 3 months prior to screening; use of intravitreal triamcinolone within 6 months prior to screening, and use of DEX within 9 months prior to screening. A complete listing of all eligibility criteria for the study is provided in Appendix 1. If both eyes were eligible for the study, the eye with the worse visual acuity was selected as the study eye.
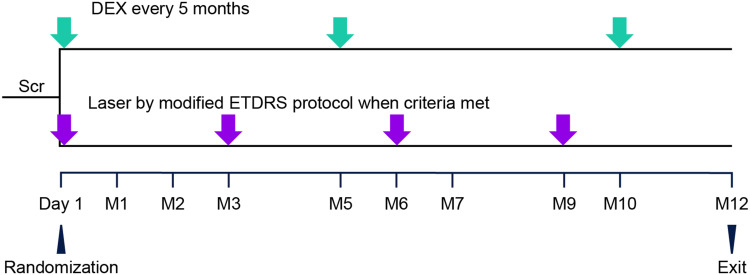
Study visits included a screening visit (Day −14 to −2) to evaluate patient eligibility, the randomization visit (Day 1; baseline and initial treatment visit), assessment visits with or without retreatment (Months 1, 2, 3, 5, 6, 7, 9, and 10), and the Month 12 exit visit. After baseline evaluations on Day 1, eligible patients were randomized in a 1:1 ratio to treatment with laser photocoagulation every 3 months as needed or DEX every 5 months (Figure 1). The randomization scheme was computer-generated and provided by the sponsor. An automated interactive voice response system/interactive web response system was used to manage the randomization and treatment assignments and provide sites with a specific medication kit number for each randomized patient. The randomization was stratified at each site by the study eye baseline BCVA (34–49 and 50–70 letters).
Figure 1.
Schematic of the study design. Randomization (1:1) was stratified at each site by the baseline BCVA in the study eye (34–49 letters vs 50–70 letters). DEX was administered intravitreally every 5 months with a 22-gauge single-use applicator. Laser photocoagulation was administered on Day 1, with retreatment administered if the investigator judged that the patient might benefit and the CRT was ≥300 μm on Spectralis OCT, ≥275 μm on Cirrus OCT, or ≥250 μm on Stratus III OCT.
Abbreviations: BCVA, best-corrected visual acuity; CRT, central retinal thickness; DEX, dexamethasone intravitreal implant 0.7 mg; ETDRS, Early Treatment Diabetic Retinopathy Study; M, month; OCT, optical coherence tomography; Scr, screening from Day −14 to Day −2.
Retinal laser photocoagulation was administered to study eyes in the laser group on Day 1 using the modified ETDRS protocol33 that is commonly used in clinical practice. Repeat treatments could be administered at Months 3, 6, and 9 if there was macular thickening in the study eye (defined as CRT of ≥300 μm on Spectralis OCT, ≥275 μm on Cirrus OCT, or ≥250 μm on Stratus III OCT) and in the opinion of the investigator, the patient may have benefited from retreatment. DEX was administered to study eyes in the DEX group on Day 1 and Months 5 and 10. The implant was injected into the vitreous through the pars plana using a single-use applicator system and sterile technique, as described previously.34 Escape treatment according to standard of care as determined by the investigator could be administered to study eyes with a decrease in BCVA from baseline of >10 letters at two consecutive visits. No type of treatment was specifically permitted or prohibited for use as escape therapy.
Efficacy measures included BCVA on an ETDRS chart, CRT on OCT, and the total area of macular leakage on fluorescein angiography (FA). A central reading center (University of Wisconsin Fundus Photograph Reading Center) quantified CRT and macular leakage from OCT and FA images. Safety measures included treatment-emergent adverse events (AEs), IOP, BCVA, biomicroscopy, and ophthalmoscopy.
The study personnel who collected the BCVA (primary efficacy) data were masked to the treatment assignment of patients, and evaluators at the reading center were also masked. The investigators and patients were aware of the treatment assignment, however, because of the differences in the administration procedures for DEX and laser.
The primary endpoint was the average change in BCVA from baseline in the study eye over the 12-month study period using observed data and an area-under-the-curve (AUC) approach. Preplanned secondary efficacy endpoints included the proportion of patients with ≥15-letter improvement in BCVA from baseline at Month 12, the change in CRT from baseline at Month 12 on OCT, and the change in the total area of leakage from baseline at Month 12 on FA.
Statistical analysis of the data was performed using SAS software (SAS Institute Inc, Cary, NC) and a 2-sided significance level of 0.05. Efficacy measures were evaluated in the modified intent-to-treat (mITT) population of all randomized and treated patients who had at least one postbaseline BCVA assessment. Any efficacy data collected after use of escape therapy were excluded from analysis. Safety measures were evaluated in the safety population of all patients who received at least one administration of the study treatment.
The primary efficacy endpoint of average change from baseline in BCVA over 12 months was analyzed using observed values with an analysis of covariance (ANCOVA) model including the treatment group as the main effect and the baseline BCVA score as the covariate. The proportion of patients with ≥15-letter improvement in BCVA from baseline at Month 12 was analyzed using the Cochran–Mantel–Haenszel test with stratification by baseline BCVA category (≤49 or ≥50 letters) within each study site and last observation carried forward (LOCF) for missing values. The change in CRT from baseline at Month 12 was analyzed using an ANCOVA model with treatment group and baseline BCVA category as main effects, baseline CRT as the covariate, and LOCF for missing values. The change in the total leakage area from baseline at Month 12 was analyzed using an ANCOVA model with treatment group and the baseline BCVA category as main effects, baseline total leakage area as the covariate, and LOCF for missing values. An additional analysis evaluated the change in BCVA from baseline at follow-up visits using a mixed-effects model for repeated measures and observed values in an unstructured covariance matrix; the model included treatment group, baseline BCVA, visit, visit-by-baseline BCVA interaction, and treatment-by-visit interaction as covariates. Subgroup analysis evaluated efficacy outcomes for patients in China.
The sample size calculation used a projected average BCVA change from baseline of approximately 4.5 letters for DEX and 1.5 letters for laser, based on data from studies of DEX and laser in DME.23,35,36 Assuming a standard deviation of eight letters and a 15% dropout rate, enrollment of 356 patients was planned to provide 90% power to detect a 3-letter difference between treatment groups in the mean average BCVA change from baseline. Enrollment was stopped in December 2018 because there was a global recall of DEX that would delay continued enrollment, and it was believed that the 284 patients who had been enrolled would provide adequate statistical power. Enrollment of 284 patients provided an estimated power of 82.8% to detect a three-letter difference between treatment groups in the mean average BCVA change from baseline, assuming a 15% dropout rate.
Results
The study was initiated on January 8, 2016 and completed on November 1, 2019. A total of 284 patients were enrolled in the study and randomized to DEX or laser treatment. Patient flow through the study is shown in Appendix 2. All patients randomized to DEX treatment received DEX on Day 1. However, 10 patients in the laser group did not receive a laser procedure on Day 1, primarily because of personal reasons; these patients were excluded from the mITT and safety analysis populations. Overall, 93.1% (135/145) of patients in the DEX group and 77.7% (108/139) in the laser group completed the study. The rate of discontinuations was higher in the laser group mainly because of discontinuations for personal reasons; the most common reasons for discontinuation were personal reasons (n = 19), AEs (n = 6), and lack of efficacy (n = 3) in the laser group and personal reasons (n = 5) and AEs (n = 3) in the DEX group.
Demographics and baseline disease characteristics for the mITT population were similar and well balanced between treatment groups (Table 1). The mean age for the mITT population (n = 272) was 59.3 years. All patients were Asian, and 242 (89%) were Chinese. Most of the study eyes (77%) were phakic. Baseline characteristics in the Chinese subgroup were also well balanced between treatment groups (Appendix 3), and study completion rates in the Chinese subgroup were similar to those in the mITT population (119 of 129 patients, 92.2% for DEX and 94 of 125 patients, 75.2% for laser).
Table 1.
Baseline Patient and Study Eye Characteristics (mITT Population)
| Parameter | DEX (N = 145) | Laser (N = 127) |
|---|---|---|
| Age, mean (SD), years | 59.1 (8.1) | 59.5 (7.7) |
| Male gender, n (%) | 81 (55.9) | 58 (45.7) |
| Race: Asian, n (%) | 145 (100) | 127 (100) |
| Ethnicity, n (%) | ||
| Chinese | 129 (89.0) | 113 (89.0) |
| Other | 16 (11.0) | 14 (11.0) |
| Lens status, n (%) | ||
| Phakic | 109 (75.2) | 100 (78.7) |
| Pseudophakic | 36 (24.8) | 27 (21.3) |
| Duration of DME, median (25%, 75% percentile), months | 7.1 (0.7, 20.0) |
6.9 (0.6, 21.8) |
| BCVA, mean (SD), letters | 55.7 (11.4) | 55.1 (10.3) |
| 34–49 letters, n (%) | 43 (29.7) | 33 (26.0) |
| 50–70 letters, n (%) | 102 (70.3) | 94 (74.0) |
| CRT, mean (SD), µm | 491.2 (160.8) | 482.1 (154.4) |
| Total macular leakage area, mean (SD), mm2 | 29.6 (10.4) | 30.1 (9.6) |
| IOP, mean (SD), mmHg | 15.2 (3.3) | 15.3 (2.9) |
Abbreviations: BCVA, best-corrected visual acuity; CRT, central retinal thickness; DEX, dexamethasone intravitreal implant 0.7 mg; DME, diabetic macular edema; IOP, intraocular pressure; mITT, modified intent-to-treat; SD, standard deviation.
Analysis using the safety population of all treated patients showed that patients in the DEX group received a mean of 2.8 intravitreal DEX injections and patients in the laser group received a mean of 2.5 laser photocoagulation treatments during the study. In the DEX group, 80.7% (117/145) of patients received all 3 planned DEX injections. In the laser group, 72.1% (93/129) of patients received at least 2 laser treatments, 46.5% (60/129) of patients received at least 3 laser treatments, and 27.1% (35/129) of patients received the protocol-specified maximum of 4 laser treatments. Eleven patients received escape therapy (6 in the DEX group, 5 in the laser group). The most used escape therapy was intravitreal ranibizumab.
The number of study treatments administered to patients in the Chinese subgroup safety population was similar. Within the Chinese subgroup, patients who were randomized to DEX treatment received a mean of 2.8 intravitreal DEX injections during the study, and 82.9% (107/129) of patients received all 3 planned DEX injections. Patients in the Chinese subgroup who were randomized to laser treatment received a mean of 2.5 laser photocoagulation treatments during the study.
Efficacy
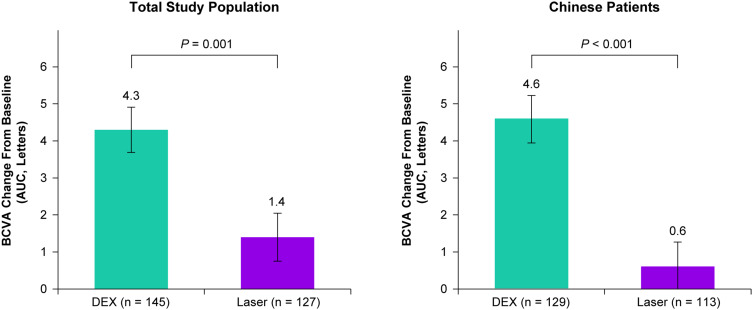
DEX met the primary efficacy endpoint and was superior to laser in the mean average change in BCVA from baseline over 12 months (Figure 2). The least squares (LS) mean (standard error, SE) average change in BCVA from baseline over 12 months was 4.3 (0.61) letters in the DEX group compared with 1.4 (0.65) letters in the laser group (P = 0.001). DEX also demonstrated superiority to laser in the mean average change in BCVA from baseline over 12 months in the Chinese patient subgroup, with a LS mean (SE) average change of 4.6 (0.62) letters with DEX and 0.6 (0.66) letters with laser (P < 0.001) (Figure 2). Analyses of the primary endpoint using the per-protocol patient population were confirmatory (see Appendix 4).
Figure 2.
Mean average change in BCVA from baseline over 12 months (primary endpoint) in the total study population and the Chinese patient subgroup. Values shown are least squares means ± standard errors from an analysis of covariance model using observed values in the mITT population with treatment group as the main effect and baseline BCVA as the covariate.
Abbreviations: AUC, area under the curve; BCVA, best-corrected visual acuity; DEX, dexamethasone intravitreal implant 0.7 mg; mITT, modified intent-to-treat.
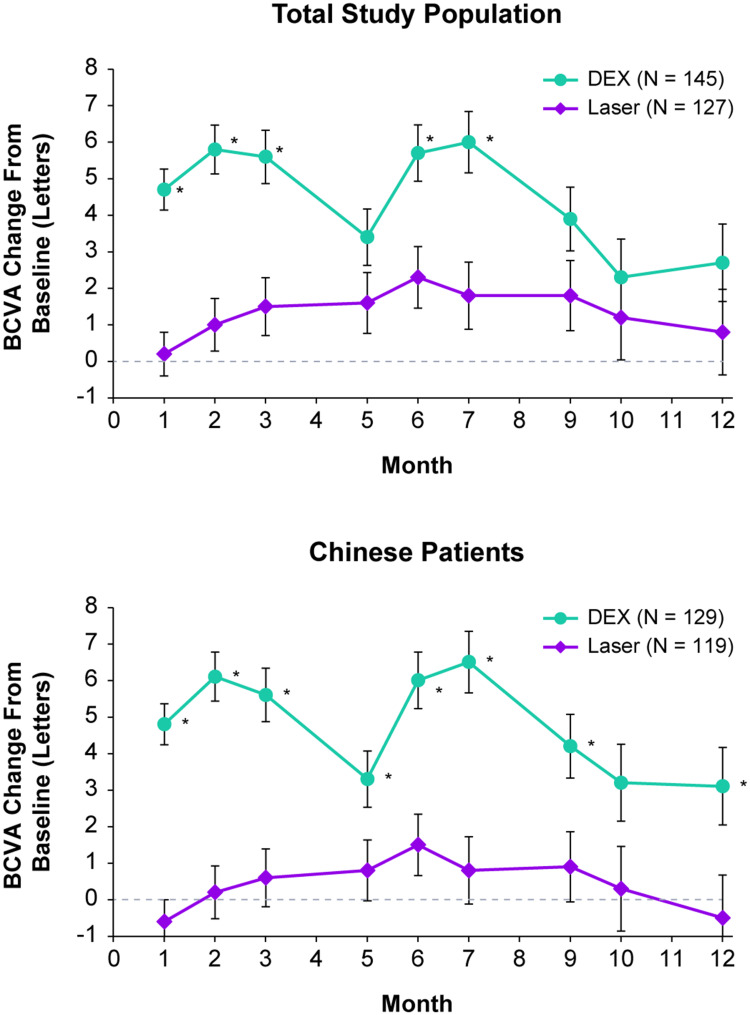
The mean improvement in BCVA from baseline was significantly greater with DEX compared with laser at Months 1, 2, 3, 6, and 7 (P ≤ 0.003) (Figure 3). For the Chinese patient subgroup, the mean improvement in BCVA from baseline was significantly greater with DEX compared with laser at Months 1, 2, 3, 5, 6, 7, 9, and 12 (ie, all postbaseline visits except Month 11) (P ≤ 0.036) (Figure 3). However, the proportion of patients with ≥15-letter improvement in BCVA from baseline at Month 12 (analysis using the mITT population with LOCF for missing values) was similar between treatment groups both overall (11.7% with DEX and 10.2% with laser) and in the Chinese patient subgroup (11.6% with DEX and 8.8% with laser).
Figure 3.
Mean change in BCVA from baseline in the total study population and the Chinese patient subgroup. Values shown are least squares means ± standard errors from a mixed-effects model for repeated measures that used observed values in an unstructured covariance matrix and fixed covariates of treatment group, baseline BCVA, visit, visit-by-baseline BCVA interaction, and treatment-by-visit interaction. *P ≤ 0.036 vs laser.
Abbreviations: BCVA, best-corrected visual acuity, DEX, dexamethasone intravitreal implant 0.7 mg.
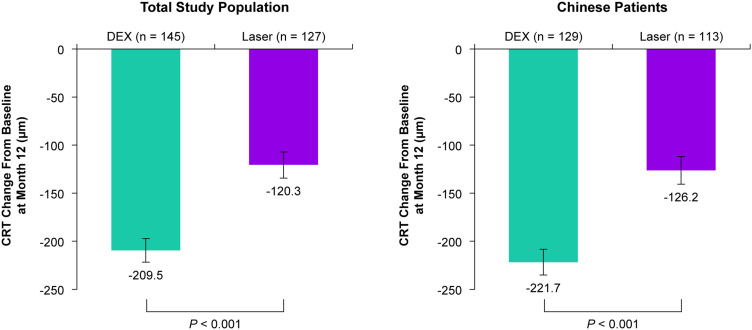
Anatomic outcomes at Month 12 were superior with DEX treatment compared with laser. At Month 12, DEX provided significantly larger mean reductions in CRT from baseline compared with laser in both the total study population and the Chinese patient subgroup (P < 0.001) (Figure 4). Furthermore, at Month 12, DEX reduced the total leakage area from baseline significantly more than laser in both the total study population and the Chinese patient subgroup (P < 0.001) (Figure 5).
Figure 4.
Mean change from baseline in CRT at Month 12 in the total study population and the Chinese patient subgroup. Values shown are least squares means ± standard errors from an analysis of covariance model in the mITT population using LOCF for missing values with treatment group and baseline BCVA categories as main effects and baseline CRT as the covariate.
Abbreviations: BCVA, best-corrected visual acuity; CRT, central retinal thickness; DEX, dexamethasone intravitreal implant 0.7 mg; LOCF, last observation carried forward; mITT, modified intent-to-treat.
Figure 5.
Mean change from baseline in the total leakage area at Month 12 in the total study population and the Chinese patient subgroup. Values shown are least squares means ± standard errors from an analysis of covariance model in the mITT population using LOCF for missing values with treatment group and baseline BCVA categories as main effects and baseline total leakage area as the covariate.
Abbreviations: BCVA, best-corrected visual acuity; CRT, central retinal thickness; DEX, dexamethasone intravitreal implant 0.7 mg; LOCF, last observation carried forward; mITT, modified intent-to-treat.
Safety
Ocular AEs in the study eye were more frequent in the DEX group than in the laser group because of the occurrence of increased IOP and cataract (Table 2), which are expected with intraocular corticosteroid treatment.22 The AEs were generally mild to moderate in severity, did not lead to study discontinuation, and were not serious. The occurrence of serious AEs and discontinuations because of AEs was similar between the treatment groups. Three deaths occurred; all were considered to be unrelated to treatment (2 patients in the DEX group with myocardial infarction and 1 patient in the laser group with liver ascites/cancer/cirrhosis).
Table 2.
Treatment-Emergent AEs in Study Eyes (Safety Population)
| AE, n (%)* | DEX (N = 145) | Laser (N = 129) |
|---|---|---|
| Overall† | 93 (64.1) | 45 (34.9) |
| Increased IOP | 49 (33.8) | 4 (3.1) |
| Cataract | 22 (15.2) | 8 (6.2) |
| Visual impairment | 8 (5.5) | 15 (11.6) |
| Conjunctivitis | 11 (7.6) | 1 (0.8) |
| Ocular hypertension | 10 (6.9) | 1 (0.8) |
| Dry eye | 8 (5.5) | 7 (5.4) |
| Vitreous hemorrhage | 6 (4.1) | 6 (4.7) |
Notes: *All individual treatment-emergent AEs reported in the study eye of ≥3% of patients in either treatment group are listed. All AEs were reported by the investigators based on their clinical expertise and judgement, †Any treatment-emergent AE in the study eye.
Abbreviations: AE, adverse event; DEX, dexamethasone intravitreal implant 0.7 mg; IOP, intraocular pressure.
Approximately one-third of patients in the DEX group had a clinically significant increase in IOP in the study eye during the study (Table 3). The increases in IOP typically were managed with topical IOP-lowering medication: 34.5% (50/145) of patients in the DEX group used IOP-lowering medications during the study. One patient (0.7%) had a procedure (trabeculectomy) to treat elevated IOP in the study eye. The mean IOP in study eyes peaked at 2 months after each DEX injection and returned to near baseline levels before the next injection (Appendix 5).
Table 3.
IOP Safety Parameters in Study Eyes (Safety Population)
| Parameter, n (%)* | DEX (N = 145) | Laser (N = 129) |
|---|---|---|
| AE* | 59 (40.7) | 5 (3.9) |
| IOP at any time during the study | ||
| ≥25 mmHg | 49 (33.8) | 6 (4.7) |
| ≥35 mmHg | 3 (2.1) | 0 (0) |
| Increase of ≥10 mmHg from baseline | 48 (33.1) | 2 (1.6) |
| Use of IOP-lowering topical medication during the study | 50 (34.5) | 3 (2.3) |
| Surgical procedure during the study to lower IOP | 1 (0.7)† | 0 (0) |
Notes: *Any treatment-emergent AE related to elevated IOP, †Trabeculectomy.
Abbreviations: AE, treatment-emergent adverse event; DEX, dexamethasone intravitreal implant 0.7 mg; IOP, intraocular pressure.
In study eyes that were phakic at baseline, the incidence of cataract-related AEs was 21.1% (23/109) in the DEX group and 7.8% (8/102) in the laser group. The incidence of cataract surgery during the study in eyes that were phakic at baseline was 6.4% (7/109) in the DEX group and 2.0% (2/102) in the laser group.
Biomicroscopy and ophthalmoscopy findings were generally similar between the treatment groups, but a larger proportion of patients in the laser group (17.1%) compared with the DEX group (1.4%) had a 2-grade or larger increase from baseline in the severity of macular scar in the study eye, likely related to the laser photocoagulation procedure.
Safety findings in the Chinese subgroup were similar to those in the total study population.
Discussion
This study demonstrated superior efficacy of DEX administered every 5 months compared with laser photocoagulation in patients with DME. The safety profile of DEX was consistent with its safety profile in the global registration studies;23 no new risks were reported during the study. Most of the patients who participated in the study were Chinese, and efficacy and safety outcomes in the Chinese subgroup were similar to those in the total study population.
The mean gains in BCVA of approximately six letters observed in the DEX group after each DEX injection were similar to those observed during the first year of treatment in the global registration studies of DEX for DME treatment.23 Furthermore, the smaller mean gains in BCVA observed in the laser group were consistent with previous reports of BCVA improvement in patients with fovea-involved DME treated with laser photocoagulation.21,37 In the first year of the VISTA and VIVID global randomized studies comparing aflibercept with laser for treatment of DME, patients in the laser treatment groups received a mean of 2.7 and 2.1 laser photocoagulation treatments and had mean gains in BCVA from baseline at Week 52 of 0.2 letters and 1.2 letters, respectively.37
The mean BCVA improvement from baseline after the third DEX injection was less than after the first and second injections, probably because of cataract development in some patients. The incidence of cataract with DEX treatment has been shown to increase after repeated injections and over longer periods of treatment.23 In the MEAD global registration studies of DEX versus sham for treatment of DME, the mean gain in BVCA from baseline after DEX injections was stable in patients with baseline pseudophakic eyes but decreased over time in the total study population.23 In baseline phakic eyes, loss in BCVA was observed after AE reports of cataract, and BCVA improvement from baseline was restored after cataract surgery.23 Similar to the MEAD studies, approximately three-quarters of patients in the present study were phakic at baseline, and therefore, the mean BCVA improvement was expected to lessen over time because of cataract development. Some patients in the present study had cataract removal at the last study visit, so BCVA improvement after the surgery could not be captured. The number of pseudophakic study eyes was relatively small, but results of an exploratory subgroup analysis of BCVA improvement in pseudophakic and phakic study eyes were consistent with the suggestion that cataract development affected the observed BCVA improvement at the end of the study: the LS mean BCVA change from baseline in DEX-treated eyes at Month 12 was +4.7 letters in pseudophakic eyes versus +2.0 letters in phakic eyes. The observed BCVA improvement with DEX at the end of the study also may have been affected by the global recall of DEX medical supplies in December 2018, as only 102 of the 145 patients (70.3%) randomized to DEX received an injection within the Month 10 visit window as specified per protocol, primarily because of a lack of medical supplies.
We used a 5-month treatment interval for DEX in this study because previous studies have suggested that patients may benefit from receiving DEX more frequently than every 6 months.23,35 The AEs that occurred (increased IOP and cataract) were expected with intraocular corticosteroid treatment, and the observed safety profile of DEX was consistent with that reported in previous studies of DEX treatment for DME23,28,38 and other indications.39,40 Typically the increases in IOP in DEX-treated eyes were managed with topical IOP-lowering medication, and after IOP control was achieved, patients received subsequent DEX injections as planned. Only 1 (0.7%) DEX-treated patient required trabeculectomy, consistent with findings from the global registration studies in which 2 (0.6%) DEX-treated patients required trabeculectomy.17
A study limitation is that because of the differences in the nature of the intervention, the patients were not masked to their assigned treatment. Ten patients assigned to laser treatment did not receive the study procedure, primarily for personal reasons, and it is possible that these patients refused treatment because they became aware of their treatment assignment and did not want to undergo laser. Also, because of the product recall, the study enrollment was lower than planned. However, the study still was adequately powered to demonstrate superiority of DEX to laser in efficacy outcomes in the total patient population, as well as in the Chinese subgroup. Some patients did not receive all three protocol-specified DEX injections because of a lack of medical supplies, but 80.7% of patients in the DEX group received three injections as planned.
Intravitreal anti-VEGF agents are currently the first-line therapy for center-involving DME. However, corticosteroids are recommended for patients who have an inadequate response to anti-VEGF therapy,9 and studies have shown treatment benefits in patients who switched to DEX treatment after a poor initial response to anti-VEGF therapy.41,42 DEX can also be considered for initial therapy in selected patients (eg, patients who are pseudophakic, pregnant, or not good candidates for anti-VEGF therapy).30 The results of this study demonstrate that DEX is effective for the treatment of DME in Chinese patients. It is expected that DEX will be used to treat DME in Chinese patients who have an inadequate response to anti-VEGF therapy; DEX may also be considered for initial therapy in some Chinese patients with DME.
Conclusions
This study conducted in China and the Philippines confirmed the efficacy and safety of DEX for treatment of DME in the study population. Subgroup analysis of outcomes in Chinese patients supported the recent approval of DEX for treatment of DME in China. DEX administered every 5 months in patients with DME significantly improved BCVA and CRT and reduced the total leakage area compared with laser photocoagulation. The safety profile of DEX was acceptable and consistent with that reported in global registration studies. The most common AEs were increased IOP and cataract.
Acknowledgments
Writing and editorial assistance was provided to the authors by Kate Ivins, PhD, of Evidence Scientific Solutions, Inc (Philadelphia, Pennsylvania) and funded by AbbVie. All authors met the ICMJE authorship criteria. Neither honoraria nor payments were made for authorship.
Funding Statement
This study was sponsored by Allergan prior to its acquisition by AbbVie Inc. The study sponsor participated in the design of the study; data management, analysis, and interpretation; and the preparation, review, and approval of the manuscript.
Abbreviations
AUC, area-under-the-curve; AE, treatment-emergent adverse event; BCVA, best-corrected visual acuity; CRT, central retinal thickness in the 1-mm central macular subfield; DEX, dexamethasone intravitreal implant 0.7 mg; DME, diabetic macular edema; DR, diabetic retinopathy; ETDRS, Early Treatment Diabetic Retinopathy Study; FA, fluorescein angiography; IOP, intraocular pressure; mITT, modified intent-to-treat; OCT, optical coherence tomography; VEGF, vascular endothelial growth factor.
Data Sharing Statement
Allergan will share de-identified patient-level data and study-level data, including protocols and clinical study reports, for Phase II, III, or IV trials completed after 2008 that are registered to ClinicalTrials.gov or EudraCT and have received regulatory approval in the USA and/or the EU in a given indication when the primary manuscript from the trial has been published. To request access to the data, the researcher must sign a data use agreement, and any shared data are to be used for non-commercial purposes. More information can be found on http://www.allerganclinicaltrials.com/.
Ethics Approval and Informed Consent
Institutional Review Board (IRB)/Ethics Committee approval was obtained at each site by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University; Ethics Committee of Beijing Hospital; Ethics Committee of Beijing Tongren Hospital; Ethics Committee of Peking Union Medical College Hospital; Ethics Committee of Peking University First Hospital; Ethics Committee of Peking University People’s Hospital; Ethics Committee of Renmin Hospital of Wuhan University; Ethics Committee of The Eye and ENT Hospital of Fudan University; Ethics Committee of The Eye Hospital of Wenzhou Medical University; Ethics Committee of The First Hospital of Nanjing Medical University; Ethics Committee of The Second Hospital of Jilin University; Ethics Committee of The Second Xiangya Hospital of Central South University; Ethics Committee of Tianjin Eye Hospital; Ethics Committee of Tianjin Medical University Eye Hospital, Ethics Committee of West China Hospital, Sichuan University; Ethics Committee of Zhongshan Ophthalmic Center, Sun Yat-sen University; Makati Medical Center Institutional Review Board; and St. Cabrini Medical Center – Asian Eye Institute Ethics Review Committee. All patients provided written informed consent.
Disclosure
Wenbin Wei, Youxin Chen, Bojie Hu, Mingwei Zhao, Mei Han, and Hong Dai have no financial relationships to disclose. Harvey S Uy has received research funding from and is a consultant for Allergan (an AbbVie company). Xian-Yan Li and Michelle Y Chen were employees of AbbVie at the time of this work. Kate Wang, Jenny Jiao, and Jean Lou are full-time employees of AbbVie Inc. The authors report no other conflicts of interest in this work. This work was presented in part at the Chinese Ophthalmological Society (COS) 25th Virtual Congress, November 19–22, 2020.
References
- 1.Sabanayagam C, Yip W, Ting DS, Tan G, Wong TY. Emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol. 2016;23(4):209–222. doi: 10.1080/09286586.2016.1193618 [DOI] [PubMed] [Google Scholar]
- 2.Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024 [DOI] [PubMed] [Google Scholar]
- 3.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2(1):17. doi: 10.1186/s40662-015-0026-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. doi: 10.1001/jama.2013.168118 [DOI] [PubMed] [Google Scholar]
- 6.Cui J, Ren JP, Chen DN, et al. Prevalence and associated factors of diabetic retinopathy in Beijing, China: a cross-sectional study. BMJ Open. 2017;7(8):e015473. doi: 10.1136/bmjopen-2016-015473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang N, Xu X, Zou H, Zhu J, Wang W, Ho PC. The status of diabetic retinopathy and diabetic macular edema in patients with type 2 diabetes: a survey from Beixinjing district of Shanghai city in China. Ophthalmologica. 2008;222(1):32–36. doi: 10.1159/000109276 [DOI] [PubMed] [Google Scholar]
- 8.Virgili G, Parravano M, Evans JR, Gordon I, Lucenteforte E. Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis. Cochrane Database Syst Rev. 2018;10(10):Cd007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regillo CD, Callanan DG, Do DV, et al. Use of corticosteroids in the treatment of patients with diabetic macular edema who have a suboptimal response to anti-VEGF: recommendations of an expert panel. Ophthalmic Surg Lasers Imaging Retina. 2017;48(4):291–301. doi: 10.3928/23258160-20170329-03 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039 [DOI] [PubMed] [Google Scholar]
- 11.Bressler SB, Qin H, Beck RW, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol. 2012;130(9):1153–1161. doi: 10.1001/archophthalmol.2012.1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sivaprasad S, Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin Ophthalmol. 2016;10:939–946. doi: 10.2147/OPTH.S100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss M, Sim DA, Herold T, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti–vascular endothelial growth factor therapy in daily practice. Retina. 2018;38(12):2293–2300. doi: 10.1097/IAE.0000000000001892 [DOI] [PubMed] [Google Scholar]
- 14.Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the Diabetic Retinopathy Clinical Research Network Comparative Effectiveness Trial. JAMA Ophthalmol. 2016;134(8):888–896. doi: 10.1001/jamaophthalmol.2016.1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holekamp NM, Campbell J, Almony A, et al. Vision outcomes following anti-vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol. 2018;191:83–91. doi: 10.1016/j.ajo.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 16.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103(12):1796–1806. doi: 10.1001/archopht.1985.01050120030015 [DOI] [PubMed] [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98(5 Suppl):766–785. doi: 10.1016/S0161-6420(13)38011-7 [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Liu H, Rojas M, Caldwell RW, Caldwell RB. Anti-inflammatory therapy for diabetic retinopathy. Immunotherapy. 2011;3(5):609–628. doi: 10.2217/imt.11.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciulla TA, Harris A, McIntyre N, Jonescu-Cuypers C. Treatment of diabetic macular edema with sustained-release glucocorticoids: intravitreal triamcinolone acetonide, dexamethasone implant, and fluocinolone acetonide implant. Expert Opin Pharmacother. 2014;15(7):953–959. doi: 10.1517/14656566.2014.896899 [DOI] [PubMed] [Google Scholar]
- 21.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447–1459. doi: 10.1016/j.ophtha.2008.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitcup SM, Cidlowski JA, Csaky KG, Ambati J. Pharmacology of corticosteroids for diabetic macular edema. Invest Ophthalmol Vis Sci. 2018;59(1):1–12. doi: 10.1167/iovs.17-22259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyer DS, Yoon YH, Belfort R Jr., et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. doi: 10.1016/j.ophtha.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 24.Gillies MC, Lim LL, Campain A, et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology. 2014;121(12):2473–2481. doi: 10.1016/j.ophtha.2014.07.002 [DOI] [PubMed] [Google Scholar]
- 25.Callanan DG, Loewenstein A, Patel SS, et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):463–473. doi: 10.1007/s00417-016-3472-1 [DOI] [PubMed] [Google Scholar]
- 26.Maturi RK, Glassman AR, Liu D, et al. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR Network Phase 2 randomized clinical trial. JAMA Ophthalmol. 2018;136(1):29–38. doi: 10.1001/jamaophthalmol.2017.4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaya M, Kocak N, Ozturk T, Bolluk V, Ayhan Z, Kaynak S. Intravitreal ranibizumab and dexamethasone implant injections as primary treatment of diabetic macular edema: simultaneously double protocol. Eye (Lond). 2021;35(3):777–785. doi: 10.1038/s41433-020-0949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenblatt A, Udaondo P, Cunha-Vaz J, et al. A collaborative retrospective study on the efficacy and safety of intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema: the European DME Registry Study. Ophthalmology. 2020;127(3):377–393. doi: 10.1016/j.ophtha.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 29.He Y, Ren XJ, Hu BJ, Lam WC, Li XR. A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol. 2018;18(1):121. doi: 10.1186/s12886-018-0779-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urbančič M, Gardašević Topčić I. Dexamethasone implant in the management of diabetic macular edema from clinician’s perspective. Clin Ophthalmol. 2019;13:829–840. doi: 10.2147/OPTH.S206769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grover S, Murthy RK, Brar VS, Chalam KV. Comparison of retinal thickness in normal eyes using Stratus and Spectralis optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(5):2644–2647. doi: 10.1167/iovs.09-4774 [DOI] [PubMed] [Google Scholar]
- 32.Sabouri MR, Kazemnezhad E, Hafezi V. Assessment of macular thickness in healthy eyes using cirrus HD-OCT: a cross-sectional study. Med Hypothesis Discov Innov Ophthalmol. 2016;5(3):104–111. [PMC free article] [PubMed] [Google Scholar]
- 33.Romero-Aroca P, Reyes-Torres J, Baget-Bernaldiz M, Blasco-Suñe C. Laser treatment for diabetic macular edema in the 21st century. Curr Diabetes Rev. 2014;10(2):100–112. doi: 10.2174/1573399810666140402123026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117(6):1134–1146.e1133. doi: 10.1016/j.ophtha.2010.03.032 [DOI] [PubMed] [Google Scholar]
- 35.Callanan DG, Gupta S, Boyer DS, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120(9):1843–1851. doi: 10.1016/j.ophtha.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 36.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031 [DOI] [PubMed] [Google Scholar]
- 37.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi: 10.1016/j.ophtha.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 38.Singer MA, Dugel PU, Fine HF, Capone A Jr., Maltman J. Real-world assessment of dexamethasone intravitreal implant in DME: findings of the prospective, multicenter REINFORCE study. Ophthalmic Surg Lasers Imaging Retina. 2018;49(6):425–435. doi: 10.3928/23258160-20180601-07 [DOI] [PubMed] [Google Scholar]
- 39.Li X, Wang N, Liang X, et al. Safety and efficacy of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in Chinese patients: randomized, sham-controlled, multicenter study. Graefes Arch Clin Exp Ophthalmol. 2018;256(1):59–69. doi: 10.1007/s00417-017-3831-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tufail A, Lightman S, Kamal A, et al. Post-marketing surveillance study of the safety of dexamethasone intravitreal implant in patients with retinal vein occlusion or noninfectious posterior segment uveitis. Clin Ophthalmol. 2018;12:2519–2534. doi: 10.2147/OPTH.S181256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Busch C, Fraser-Bell S, Iglicki M, et al. Real-world outcomes of non-responding diabetic macular edema treated with continued anti-VEGF therapy versus early switch to dexamethasone implant: 2-year results. Acta Diabetol. 2019;56(12):1341–1350. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz-Medrano J, Rodríguez-Leor R, Almazán E, et al. Results of dexamethasone intravitreal implant (Ozurdex) in diabetic macular edema patients: early versus late switch. Eur J Ophthalmol. 2021;31(3):1135–1145. doi: 10.1177/1120672120929960 [DOI] [PubMed] [Google Scholar]