Abstract
Purpose
To report patient expectations, visual performance and satisfaction with AcrySof IQ PanOptix multifocal intraocular lens in a heterogeneous patient group. Additionally, determine if identifiable pre-operative characteristics can predict post-operative satisfaction.
Methods
Data were prospectively collected for 67 consecutive patients (134 eyes) who underwent bilateral Panoptix implantation in a private ophthalmology clinic. A pre-operative questionnaire was completed regarding vision satisfaction, visual phenomena and expectations. Routine clinical parameters were collected 1 month and follow-up questionnaire administered 3 months post-operatively.
Results
Post-operative unaided distance vision was 20/20 (0.01 ± 0.10) and binocular near vision 20/25-2 (N5; 0.14 ± 0.06). Patients satisfied with vision increased from 6% (n=4) unaided and 48% (n=32) aided pre-operatively to 94% (n=63) unaided post-operatively (p<0.001). There was marked increase in frequency of halo from 14% (n=9) to 69% (n=46; p<0.001) but no corresponding increase in how bothersome this symptom was (p=0.193) nor the frequency of other visual phenomena. Worse post-operative vision and fluctuating vision were associated with lower post-operative satisfaction. There was no difference in satisfaction or residual astigmatism in those implanted with toric lenses. A total of 96% (n=64) of patients were spectacle-free at 3-months and would recommend this procedure to others.
Conclusion
This study supports the trend towards increased patient expectations of multifocal intraocular lenses, matched by excellent visual outcomes and satisfaction. Visual phenomena may be less troubling with new multifocal lenses than previously reported. A pre-operative questionnaire may be a useful education tool but could not isolate pre-operative characteristics that predict post-operative satisfaction.
Keywords: multifocal, intraocular lens, cataract, refractive lens exchange, satisfaction
Introduction
Increasing patient expectation of spectacle independence after cataract surgery is driving new designs of multifocal intraocular lens (IOL). The AcrySof IQ PanOptix (Alcon Laboratories Inc., Fort Worth, TX, USA) is a single-piece diffractive IOL based on a quadrifocal design. It redistributes the 120 cm focal point to the distance, functionally acting as a trifocal with a closer intermediate focus at 60 cm,1 which may improve satisfaction given that modern visual demands include computers and hand-held tablets.2
A growing body of evidence describes excellent vision outcomes for the non-toric Panoptix TFNT00 in select patient groups.2–13 Existing studies, utilising strict inclusion criteria that consider only ideal multifocal IOL candidates, also report remarkable satisfaction rates. A recent large prospective, multi-centre trial sponsored by Alcon (manufacturer of Panoptix) reported that 93.8% (n=121) of their 129 patients implanted with Panoptix model TFNT00 were satisfied or very satisfied with their overall vision, 99.2% (n=126) would choose to have the same lenses implanted again and 98.4% (n=125) would recommend it to their family or friends.12 Other studies have shown slightly more modest but still very high satisfaction rates, along with spectacle independence in the range of 89–100%.4,7,8,10,13,24 However, improvements in lens design and predictability of refractive outcome have led to an expanding range of patients considered suitable for multifocal IOLs, but few studies have reported outcomes in this broader cohort of patients reflective of modern ophthalmology practice. Additionally, few studies have explored pre-operative patient expectations and correlated these with post-operative outcomes and satisfaction.21
Patients with astigmatism present a refractive challenge for multifocal IOLs and in the literature are often excluded. Small misalignment of a toric IOL leads to significant loss of effective cylinder power,14 and even small amounts of residual astigmatism can significantly impact satisfaction.15,16 A small number of studies suggest equivalent vision outcomes with the toric Panoptix lenses TFNT20-60,17–20 with the proportion of toric lenses implanted in existing studies ranging from 20% to 33.6%.17–19 The low proportion of studies including toric lenses is despite large population samples suggesting that more than 40% of patients planned for cataract surgery have at least 0.75 dioptres of corneal astigmatism.15
Visual disturbances such as halo and glare have been cited as a significant source of dissatisfaction following the implantation of multifocal IOLs.22 A 2016 Cochrane review concluded that adverse subjective visual phenomena were more prevalent and more troublesome in patients with multifocal compared with monofocal IOLs (RR for glare 1.41, 95% CI 1.03–1.93).23 Reported rates of visual phenomena range significantly depending on patient group and phrasing of the question, with incidence of halo after multifocal lens implantation ranging from 43.4% to 95%.2,8,10,13,24 However, asked how bothersome this symptom is, it is typically reported as not bothersome, with one recent study involving non-toric Panoptix IOLs reporting incidence of halo 43.4% (n=30) and glare 14.4% (n=10), but only 1 patient (1.4%) finding each of these visual phenomena troubling.13
This prospective consecutive case series aimed to enhance understanding of patient expectations prior to implantation of Panoptix multifocal IOL as well as visual performance and patient satisfaction post-operatively, in a heterogenous patient group typical of that encountered in clinical practice. Identifiable factors that influence surgical outcome and satisfaction may have implications for patient selection.
Methods
Data were prospectively collected for 67 consecutive patients (134 eyes) who underwent bilateral implantation with the Alcon Panoptix multifocal IOL from July 2018 to March 2020 with a single surgeon at a private ophthalmology practice in Australia. Patients planned for this operation completed an in-house pre-operative questionnaire, which included self-reported satisfaction with current vision, presence of visual phenomena and expectations regarding surgical outcome. Routine pre-operative clinical data were obtained, with similar clinical parameters collected 1 month post-operatively. Distance vision was measured on a Snellen chart at 20 feet and near vision in N-point format at habitual working distance, with both converted to LogMAR for analysis. A follow-up phone interview was administered at 3 months post-operatively, using standardised script and questionnaire. Local Research Ethics Committee approval (Metro South Hospital and Health Service) was granted to prospectively collect and analyse the data, and all patients were given written information and signed a consent form to participate. This study was performed in accordance with the principles stated in the Declaration of Helsinki.
All consenting patients who completed the pre-operative questionnaire and underwent bilateral implantation of Panoptix multifocal IOLs were included in this study. To reflect the wide range of patients in private clinical practice, patients with prior refractive surgery such as laser-assisted in-situ keratomileusis (LASIK) as well as other ocular surgery such as pterygium excision and other ocular comorbidities not precluding a good potential visual outcome were included. There was no specific exclusion based on refractive error, and toric models of the Panoptix (TFNT20-60) were used as appropriate at the discretion of the treating surgeon. Patients without potential for good binocular visual acuity such as a history of dense amblyopia or co-existing advanced macular or optic nerve disease were not considered for multifocal IOL insertion and as such not involved in the study. A small subset of patients subsequently underwent an additional procedure within the post-operative follow-up period, such as insertion of a secondary IOL, and were included in analysis, with the final questionnaire administered 3 months after any secondary procedure.
For statistical analysis, continuous data were presented as mean and standard deviation (SD) and categorical variables as a percentage. Variables considered in analysis included: age, gender, primary motivation for surgery (refractive lens exchange vs treat visually significant cataract), pupil size, pre- and post-operative uncorrected monocular distance and binocular near vision (at habitual working distance), distance subjective refraction (including sphere and cylinder) and visual acuity, IOL model implanted and responses to pre- and post-operative questionnaires. Standard statistical analysis was performed using SPSS version 27.0. Pre- and post-operative comparisons were made using paired t-test for parametric data and Wilcoxon rank sum test for non-parametric data. Analysis between patient groups was performed using independent t-test for parametric data and Kruskal–Wallis test for non-parametric data. For all statistical tests, the same level of significance was used (p<0.05).
Results
Demographics and Clinical Outcomes
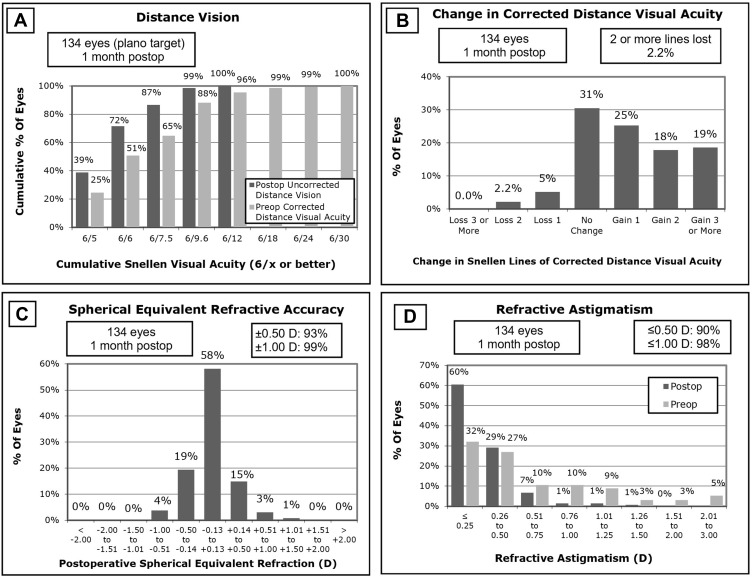
A total of 67 patients (134 eyes) were implanted with Panoptix multifocal IOLs during the study period. Table 1 shows baseline patient characteristics identified pre-operatively. Table 2 outlines pre- and post-operative vision and refraction. Three eyes underwent a second procedure with insertion of a secondary IOL in the 3-month post-operative follow-up period due to residual refractive error, all of which had previously undergone LASIK. Six eyes underwent early YAG capsulotomy, 7 eyes had in-office lens rotation performed in the early post-operative period, and 1 eye was treated for clinically significant CMO. Figure 1 reports standard surgical refractive outcome parameters.
Table 1.
Baseline Characteristics (n=67 Patients/134 Eyes)
| Age, years (SD) | 61 (8.3) |
| Female gender, n (%) | 32 (48%) |
| Currently employed, n (%) | 39 (58%) |
| RLE, n (%) | 30 (45%) |
| IOL mean power, dioptres (SD) | 19.9 (4.2) |
| Range, DS | 6.5 to 34.0 |
| Pre-operative mean sphere, DS (SD) | 0.38 (2.97) |
| Range, DS | −9.50 to +8.75 |
| Pre-operative mean cylinder, DC (SD) | 0.71 (0.65) |
| Range, DC | 0.00 to −3.00 |
| Prior ocular surgery: n eyes (%) | |
| ● LASIK | 10 (7.5%) |
| ● Pterygium excision | 8 (6.0%) |
| ● Retinal detachment repair | 2 (1.5%) |
| ● Primary repair of PEI (non-central full-thickness corneal laceration) | 1 (0.7%) |
| Toric IOL lenses implanted, n eyes (%) | 88 (66%) |
| ● TFNT20 (0.75 DC) | 48 (36%) |
| ● TFNT30 (1.50 DC) | 23 (17%) |
| ● TFNT40 (2.25 DC) | 11 (8.2%) |
| ● TFNT50 (3.00 DC) | 2 (1.5%) |
| ● TFNT60 (3.75 DC) | 4 (3.0%) |
Abbreviations: SD, standard deviation; RLE, refractive lens exchange; IOL, intraocular lens; DS, dioptres sphere; DC, dioptres cylinder; LASIK, laser-assisted in-situ keratomileusis; PEI, penetrating eye injury.
Table 2.
Pre-Operative and 1-Month Post-Operative Clinical Parameters (n=67)
| Parameter | Pre-Operative | Post-Operative | P value |
|---|---|---|---|
| Mean Snellen Acuity LogMAR (SD) | |||
| RE UDVA | 20/60−2 0.54 (0.49) | 20/20 0.01 (0.11) | <0.001 |
| LE UDVA | 20/60−2 0.55 (0.48) | 20/20 0.01 (0.10) | <0.001 |
| RE CDVA | 20/25+1 0.08 (0.16) | 20/20+2 −0.04 (0.07) | 0.004 |
| LE CDVA | 20/25+1 0.09 (0.17) | 20/20+2 −0.04 (0.07) | 0.001 |
| Binocular UNVA | 20/100+1 0.69 (0.22) | 20/25−2 0.14 (0.06) | <0.001 |
| Mean (SD) | |||
| RE spherical equivalent DS | −0.03 (3.13) | −0.04 (0.28) | 0.988 |
| Range DS | −9.75 to +8.125 | −1.00 to +0.75 | |
| LE spherical equivalent DS | 0.10 (3.03) | 0.00 (0.30) | 0.781 |
| Range DS | −9.875 to +8.50 | −0.75 to +1.125 | |
| RE cylinder DC | −0.73 (0.62) | −0.31 (0.31) | <0.001 |
| Range DC | −3.00 to 0.00 | −1.50 to 0.00 | |
| LE cylinder DC | −0.69 (0.68) | −0.29 (0.29) | <0.001 |
| Range DC | −3.00 to 0.00 | −1.25 to 0.00 | |
Abbreviations: RE, right eye; LE, left eye; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; UNVA, uncorrected near visual acuity; DS, dioptres sphere; DC, dioptres cylinder; SD, standard deviation.
Figure 1.
Vision and refractive outcomes. (A) Distance vision; (B) Change in corrected distance visual acuity; (C) Spherical equivalent refractive accuracy; (D) Refractive astigmatism.
Patient Expectation
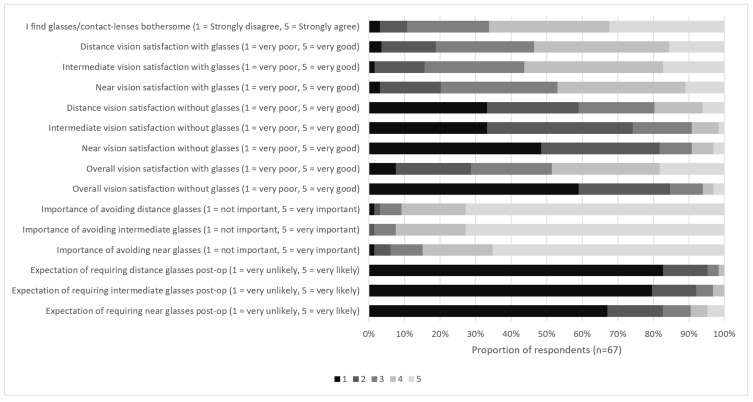
Patient dissatisfaction with existing corrective lenses was high, with 64.1% (n=43) of patients agreeing or strongly agreeing that they find glasses/contact-lenses bothersome. Overall satisfaction with vision on a scale of 1 (very dissatisfied) to 10 (very satisfied) was 6.1 ± 2.4 with spectacles and 2.6 ± 1.1 without, which improved to 8.6 ± 1.2 (p<0.001) post-operatively. Motivation to avoid spectacles was high for all distances, with 91% (n=61) ranking this as important or very important for distance and intermediate vision, and 85% (n=56) for near vision. Expectation of being spectacle-free was also high with 95% (n=64), 92% (n=62) and 83% (n=55) of patients indicating they felt they were very unlikely or unlikely to require spectacles post-operatively for distance, intermediate and near vision, respectively. A total of 90% (n=57) of respondents indicated they were willing to accept greater perception of halo in exchange for being more likely to see distance, intermediate and near without spectacles. The majority of patients (61%, n=39) were not willing to increase their likelihood of requiring spectacles for near vision in exchange for less halo or other visual phenomena. When specifically asked about the distance they would be most willing to wear spectacles after surgery, 43 patients (64%) indicated near vision, 2 patients (3%) intermediate vision and 16 patients (24%) distance vision. Six patients (9%) declined to answer the question. Individual responses to the pre-operative questionnaire are displayed in Figure 2.
Figure 2.
Pre-operative questionnaire responses.
Visual Phenomena
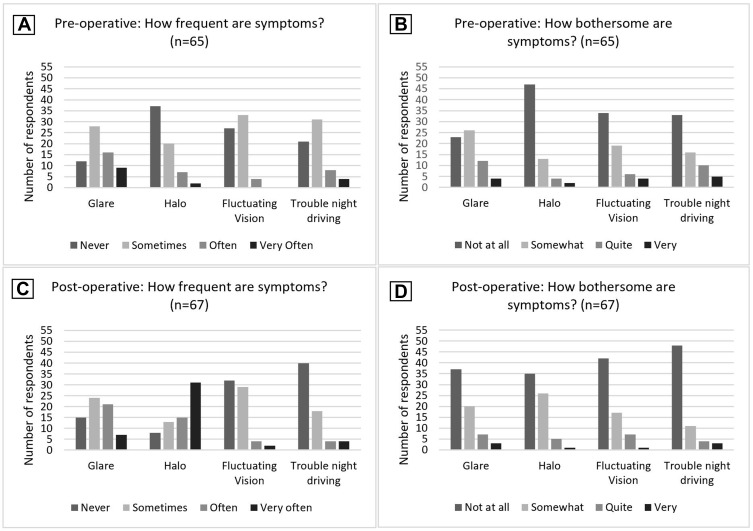
Using a self-developed questionnaire, a range of questions were asked pre- and post-operatively regarding not just frequency but also how bothersome a range of visual phenomena were. Patients indicated frequency (never = 0, sometimes = 1, often = 2, very often = 3) and how bothersome (not at all = 0, somewhat = 1, quite = 2, very = 3) the visual phenomena of glare, halo, fluctuating vision and trouble night driving were, with the mean scores summarised in Table 3 and individual responses in Figure 3. Pre-operatively, 37% (n=25) of patients reported noticing glare often or very often, and 25% (n=16) found this quite or very bothersome, compared with 43% (n=28) post-operatively noticing glare often or very often, and a reduced proportion of 15% (n=10) finding it quite or very bothersome. Halo had a marked increase in frequency from 14% (n=9) patients reporting this often or very often pre-operatively compared to 69% post-operatively (n=46), although there was no corresponding increase in how bothersome this symptom was, with 9% (n=6) of patients pre- and post-operatively describing halo as quite or very bothersome.
Table 3.
Pre-Operative and 3-Month Post-Operative Symptom Scores (n=67)
| Symptom Score (0–3 Scale) | Pre-Operative Mean (SD) | Post-Operative Mean (SD) | P value |
|---|---|---|---|
| Glare: Frequency | 1.34 (0.94) | 1.30 (0.94) | 0.715 |
| How bothersome? | 0.95 (0.89) | 0.64 (0.85) | 0.021 |
| Halo: Frequency | 0.61 (0.80) | 2.03 (1.07) | <0.001 |
| How bothersome? | 0.41 (0.74) | 0.58 (0.70) | 0.193 |
| Fluctuating Vision: Frequency | 0.64 (0.60) | 0.64 (0.73) | 0.881 |
| How bothersome? | 0.68 (0.90) | 0.51 (0.75) | 0.204 |
| Trouble driving at night: Frequency | 0.92 (0.84) | 0.58 (0.86) | 0.057 |
| How bothersome? | 0.98 (0.80) | 0.42 (0.81) | 0.053 |
Notes: Frequency: never = 0, sometimes = 1, often = 2, very often = 3. Bothersome: not at all = 0, somewhat = 1, quite = 2, very = 3.
Figure 3.
Pre- and post-operative symptom questionnaire. (A) Pre-operative frequency of symptoms; (B) Pre-operative how bothersome are symptoms; (C) Post-operative frequency of symptoms; (D) Post-operative how bothersome are symptoms.
Patient Satisfaction
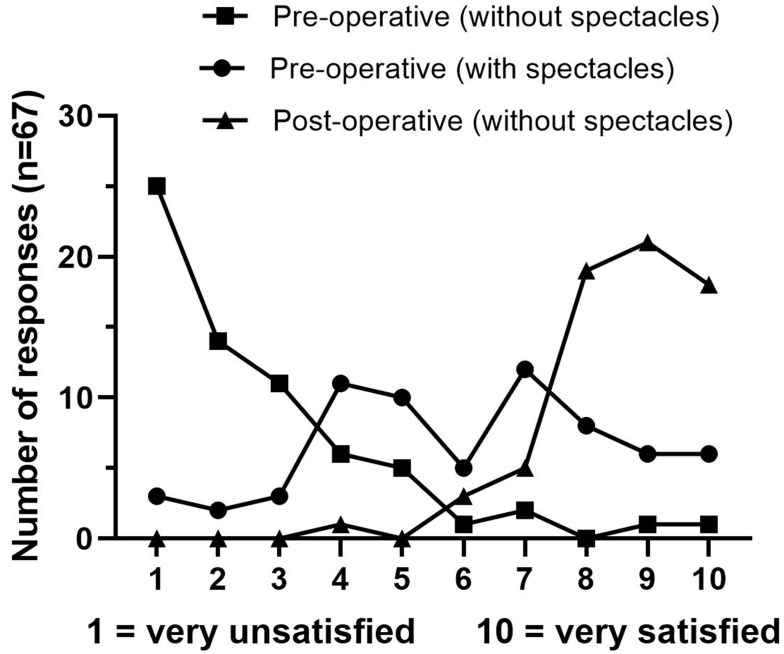
Frequency of individual global vision satisfaction scores are outlined in Figure 4. Patients satisfied or very satisfied with their overall vision increased from 6% (n=4) unaided and 48% (n=32) aided to 94% (n=63) unaided post-operatively (p<0.001). Overall, 92.5% (n=62), 95.5% (n=64) and 86.6% (n=58) of patients described their post-operative vision as good or excellent for distance, intermediate and near vision, respectively.
Figure 4.
Global vision satisfaction score.
At follow-up, 95.5% (n=64)of patients were not wearing spectacles, with 3 patients using them for near vision only. Similarly, 95.5% (n=64) of patients stated they would choose the same lens again, with the same number reporting they would recommend multifocal IOLs to others. Of the 3 patients who indicated they would not have chosen the same lens again, all were males aged in their 60s who had the non-toric TFNT00 implanted, of which 1 was using reading glasses. This patient had a mild hyperopic refractive surprise, but declined an additional procedure to correct this. The second patient was a low myope referred for RLE with asymmetric corneal astigmatism (possible subclinical keratoconus) who had -0.75 dioptres of residual astigmatism in the dominant eye post-operatively and also ultimately elected not to undergo a top-up procedure. The third patient identified shooting at night as a key visual need and indicated he would not accept more halos in exchange for good distance/intermediate/near vision in the pre-operative questionnaire, suggesting high visual demands and high expectations. A combination of dry eye and fluctuating vision despite active treatment, leading to post-operative unaided vision R 20/25 (0.10) L 20/30 (0.20), likely contributed to those expectations not being met.
To further explore factors that contribute to post-operative satisfaction, self-ranked global vision satisfaction scores were divided into high (≥8/10, n=58) and moderate-to-low satisfaction (<8/10, n=9). Worse UDVA in either eye (R 20/25 vs 20/20, p=0.016; L 20/25+1 vs 20/20, p=0.013) and presence of (0.87 ± 0.29 vs 0.66 ± 0.09, p=0.002) and bothersome (0.83 ± 0.28 vs 0.68 ± 0.09, p=0.002) fluctuating vision were significantly correlated with those in the lower satisfaction group. There was no significant difference in satisfaction scores in those who had previously undergone LASIK (n=5), noting 3 of those 5 patients underwent insertion of a secondary IOL to correct residual refractive error. Those primarily referred for refractive lens exchange (RLE) rather than to treat visually significant cataract were as expected significantly younger (mean age 56 y vs 65 y, p<0.001) and had better pre-operative visual acuity (R 20/20−1 vs 20/30+2, p<0.001, L 20/20 vs 20/30+1, p=0.001); however, there was no difference in post-operative vision, experience of visual phenomena or self-reported overall vision satisfaction between these groups. Overall post-operative vision satisfaction was not significantly correlated with any single pre-operative question regarding expectation of surgical outcome.
Toric vs Non-Toric Lenses
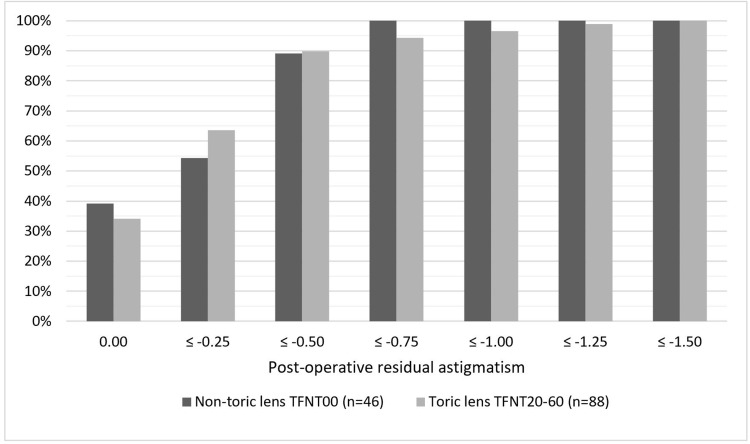
Clinical outcomes of those who received toric lenses is summarised in Table 4. Toric lens recipients had worse average pre-operative visual acuity and higher pre-operative refractive astigmatism, but no difference post-operatively in unaided vision, overall satisfaction, or residual astigmatism. The distribution of residual astigmatism is displayed graphically in Figure 5. There was also no difference in anticipated spectacle independence or in pre- or post-operative experience of visual phenomena between the toric and non-toric groups. There was no association between residual astigmatism and global satisfaction in this cohort; however, the amount of residual astigmatism in all patients was very low, with 96.3% (n=129) of eyes having ≤0.75 dioptres of residual cylinder post-operatively compared to 59.0% (n=79) pre-operatively.
Table 4.
Toric vs Non-Toric IOL Groups
| Parameter | Mean Snellen,LogMAR (SD) | P value | |
|---|---|---|---|
| Toric IOL (n=88 Eyes) | Non-Toric IOL (n=46 Eyes) | ||
| Pre-operative binocular UDVA | 20/80 0.60 (0.55) | 20/40−2 0.45 (0.28) | 0.647 |
| Pre-operative cylinder (DC) | −0.87 (0.70) | −0.40 (0.37) | <0.001 |
| Pre-operative CDVA | 20/25 0.11 (0.18) | 20/20−2 0.04 (0.11) | 0.018 |
| Post-operative binocular UDVA | 20/20 0.01 (0.10) | 20/20−1 0.02 (0.12) | 0.669 |
| Post-operative cylinder (DC) | −0.31 (0.31) | −0.29 (0.27) | 0.875 |
| Post-operative CDVA | 20/20+2 –0.04 (0.07) | 20/20+1 –0.03 (0.07) | 0.412 |
| Post-operative vision satisfaction (1 = very dissatisfied, 10 = very satisfied) |
8.8 (0.98) | 8.3 (1.5) | 0.122 |
Abbreviations: IOL, intraocular lens; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; DS, dioptres sphere; DC, dioptres cylinder; SD, standard deviation.
Figure 5.
Post-operative residual astigmatism by model implanted.
Discussion
This study confirms excellent visual outcomes of the Panoptix multifocal IOL with UDVA 20/20 (0.01) and binocular UNVA 20/32+2 (better than N5, 0.14) across a heterogeneous group of patients with varying expectations, motivations for surgery, pre-operative refractive error and ocular comorbidity.
The high rate of toric IOL implantation in this cohort (66% of all lenses) reflects an understanding of the importance of correction of even low astigmatism to maximise satisfaction, and the resultant 96.3% (n=129) of patients with ≤0.75 dioptres of residual cylinder post-operatively is consistent with recent results using the same lens.19 Also consistent with the few existing studies,17–19 there was no difference in post-operative vision, residual astigmatism or overall vision satisfaction between patients implanted with the toric and non-toric models of Panoptix lenses. Misalignment of toric multifocal IOLs in particular has a greater impact on resultant vision, and of note 7 lenses in this cohort (5.2%) underwent rotation post-operatively to maximise visual outcome.
This study offers a unique perspective in understanding patient expectations prior to multifocal IOL implantation. This diverse patient group had high expectations regarding vision and spectacle independence post-operatively. In 2014, it was reported that up to 80% of patients indicated that they would accept reading glasses after multifocal IOL insertion16 compared with this study in which only 39% indicated that they would accept the possibility of reading glasses in exchange for fewer visual phenomena. This, along with the other questionnaire results regarding anticipated spectacle independence, supports the concept that patient expectations continue to increase. A very high proportion of patients themselves (90%; n=57) indicated a willingness to tolerate more halo or other visual phenomena in exchange for a greater chance of being spectacle-free.
The use of the pre-operative questionnaire has been suggested as a tool to screen potential multifocal IOL candidates for suitability.16 There was no single question in our questionnaire that predicted post-operative vision or satisfaction. However, poor candidates for multifocal IOLs, such as those most concerned about any perceived vision compromise, may have already been screened out by using this questionnaire. In this way, the questionnaire itself can act as a tool to educate patients and manage expectations regarding potential surgical outcomes.
The findings of this study challenge the traditional belief that increased perception of glare and other visual phenomena is a significant disadvantage of multifocal IOLs. As a prospective study, patient symptoms and satisfaction with vision pre- and post-operatively could be directly compared. A significant proportion of patients pre-operatively already complained of glare symptoms, even in those primarily undergoing the procedure for supposed refractive purposes rather than to treat visually significant cataract. Furthermore, despite high rates of glare on symptom surveys including this study, with 43% reported noticing glare often or very often, whether this is bothersome to the patient is often not reported in studies, and the percentage is usually significantly lower – in this cohort only 15% found it quite or very bothersome post-operatively, which was not significantly changed from pre-operatively.
Previous conclusions that multifocal IOLs cause significantly more symptomatic glare relied on studies of older multifocal lens designs and generally reported low-quality evidence, with the lower limit of the confidence ratio just 1.03.23 The only significant increase in frequency of visual phenomena in this cohort was halo, from 14% (n=9) pre-operatively to 69% (n=46) post-operatively, which agrees with previous conclusions that halo is the most frequent visual phenomenon noted after multifocal IOL implantation (RR 3.58, 95% CI 1.99–6.46).23 However, in this patient group, this symptom was not significantly more troubling to patients post-operatively.
Overall patient satisfaction and spectacle independence post-operatively was very high, and self-ranked vision satisfaction significantly improved, consistent with prior studies. While 3 patients (4.5%) were using spectacles for near vision 3 months post-operatively, none were wearing distance or intermediate vision spectacles, which may reflect the emphasis of the Panoptix on a closer intermediate working distance. There was no single pre-operative parameter that predicted global post-operative satisfaction score. As expected, better post-operative unaided vision was associated with higher satisfaction, emphasising the importance of consistent, accurate refractive outcomes and low threshold to use toric multifocal IOL options where appropriate.
This study was conducted in a private ophthalmology practice and designed to reflect the wide range of patients in clinical practice who elect to undergo multifocal IOL implantation. This provides strengths including a diverse patient group with minimal exclusion criteria. However, it also introduces limitations including a patient cohort that has already been screened to some extent for multifocal IOL suitability. Clinical parameters were measured in a clinically convenient format rather than using tools specifically designed for research; for example, distance vision was measured on a Snellen chart and near vision on N-point near chart, only later converted to LogMAR for analysis. Additionally, the relatively short follow-up time of 3 months meant that longer-term complications, such as true rates of posterior capsular opacification, were not captured. Neuroadaptation has been suggested to lessen the effects of visual phenomena over time; however, a number of studies with longer follow-up have not shown significant changes in perception of this at 6 months and longer, compared to 3 months, suggesting that the majority of adaptation likely happens within the first 3 months.16 Finally, the 3-month questionnaire was completed by phone instead of self-directed by the patient. We aimed to minimise the potential bias this could introduce by using the same interviewer for all cases and using scripted questions without leading statements.
Conclusion
The results of this study support the trend of increasing patient expectation of spectacle independence and high visual performance with new multifocal IOLs. These expectations were matched by excellent visual outcomes and high rates of post-operative satisfaction with the Panoptix multifocal IOL. Toric models were associated with equivalent outcomes to their non-toric counterpart. Patients already experience visual phenomena such as glare pre-operatively, and these were not significantly more bothersome post-operatively. Halo was the only visual phenomenon with a marked increase in frequency, although this symptom was not significantly more bothersome. The established thinking regarding the extent to which multifocal IOLs are associated with troubling visual phenomena should be revisited with new lens designs.
A pre-operative questionnaire has been suggested as a way to identify patients who may be dissatisfied after multifocal IOL implantation.16 While a questionnaire may be a useful education tool, this study confirms the difficulty in identifying isolated pre-operative characteristics that predict post-operative satisfaction. As expectations and the range of patients considered for multifocal IOLs continue to expand, the clinical judgement of the surgeon in patient and lens selection remains as important as ever in delivering a satisfactory outcome.
Acknowledgments
The authors acknowledge and thank all patients who participated in this study. No author has any financial affiliation with Alcon or proprietary interest in any materials in this study.
Funding Statement
No financial support was applied for nor received.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sudhir RR, Dey A, Bhattacharrya S, Bahulayan A. AcrySof IQ PanOptix intraocular lens versus extended depth of focus intraocular lens and trifocal intraocular lens: a clinical overview. Asia Pac J Ophthalmol. 2019;8(4):335–349. doi: 10.1097/APO.0000000000000253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gundersen KG, Potvin R. Trifocal intraocular lenses: a comparison of the visual performance and quality of vision provided by two different lens designs. Clin Ophthalmol. 2017;11:1081–1087. doi: 10.2147/OPTH.S136164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escandón-García S, Ribeiro FJ, McAlinden C, Queirós A, González-Méijome JM. Through-focus vision performance and light disturbances of 3 new intraocular lenses for presbyopia correction. J Ophthalmol. 2018;2018:6165493. doi: 10.1155/2018/6165493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mencucci R, Favuzza E, Caporossi O, Savastano A, Rizzo S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1913–1922. doi: 10.1007/s00417-018-4052-3 [DOI] [PubMed] [Google Scholar]
- 5.Alió JL, Plaza-Puche AB, Alió Del Barrio JL, et al. A clinical outcomes with a diffractive trifocal intraocular lens. Eur J Ophthalmol. 2018;28(4):419–424. doi: 10.1177/1120672118762231 [DOI] [PubMed] [Google Scholar]
- 6.Ruiz-Mesa R, Abengozar-Vela A, Aramburu A, Ruiz-Santos M. Comparison of visual outcomes after bilateral implantation of extended range of vision and trifocal intraocular lenses. Eur J Ophthalmol. 2017;27(4):460–465. doi: 10.5301/ejo.5000935 [DOI] [PubMed] [Google Scholar]
- 7.Cochener B, Boutillier G, Lamard M, Auberger-Zagnoli C. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34(8):507–514. doi: 10.3928/1081597X-20180530-02 [DOI] [PubMed] [Google Scholar]
- 8.Kohnen T, Herzog M, Hemkeppler E, et al. Visual performance of a quadrifocal (trifocal) intraocular lens following removal of the crystalline lens. Am J Ophthalmol. 2017;184:52–62. doi: 10.1016/j.ajo.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 9.Monaco G, Gari M, Di Censo F, Poscia A, Ruggi G, Scialdone A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43(6):737–747. doi: 10.1016/j.jcrs.2017.03.037 [DOI] [PubMed] [Google Scholar]
- 10.García-Pérez JL, Gros-Otero J, Sánchez-Ramos C, Blázquez V, Contreras I. Short term visual outcomes of a new trifocal intraocular lens. BMC Ophthalmol. 2017;17(1):72. doi: 10.1186/s12886-017-0462-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawless M, Hodge C, Reich J, et al. Visual and refractive outcomes following implantation of a new trifocal intraocular lens. Eye Vis. 2017;4(1):10. doi: 10.1186/s40662-017-0076-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modi S, Lehmann R, Maxwell A, et al. Visual and patient-reported outcomes of a diffractive trifocal intraocular lens compared with those of a monofocal intraocular lens. Ophthalmology. 2021;128(2):197–207. [DOI] [PubMed] [Google Scholar]
- 13.Donmez O, Asena BS, Kaskaloglu M, Akova YA. Patients satisfaction and clinical outcomes of binocular implantation of a new trifocal intraocular lens. Int Ophthalmol. 2020;40(5):1069–1075. doi: 10.1007/s10792-020-01390-9 [DOI] [PubMed] [Google Scholar]
- 14.Ma JJK, Tseng SS. Simple method for accurate alignment in toric phakic and aphakic intraocular lens implantation. J Cataract Refract Surg. 2008;34(10):1631–1636. doi: 10.1016/j.jcrs.2008.04.041 [DOI] [PubMed] [Google Scholar]
- 15.Ferrer-Blasco T, Montes-Mico R, Peixoto-de-Matos SC, Gonzalez-Meijome JM, Cervino A. Prevalence of corneal astigmatism before cataract surgery. J Cataract Refract Surg. 2009;35(1):70–75. doi: 10.1016/j.jcrs.2008.09.027 [DOI] [PubMed] [Google Scholar]
- 16.Mester U, Vaterrodt T, Goes F, et al. Impact of personality characteristics on patient satisfaction after multifocal intraocular lens implantation: results from the “Happy Patient Study”. J Refract Surg. 2014;30(10):674–678. doi: 10.3928/1081597X-20140903-05 [DOI] [PubMed] [Google Scholar]
- 17.Rementería-Capelo LA, Contreras I, García-Pérez JL, Blázquez V, Ruiz-Alcocer J. Visual quality and patient satisfaction with a trifocal intraocular lens and its new toric version. J Cataract Refract Surg. 2019;45(11):1584–1590. doi: 10.1016/j.jcrs.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 18.Hamdi IM. Subjective perception of trifocal IOL performance, including toric models. Clin Ophthalmol. 2019;13:1955–1961. doi: 10.2147/OPTH.S223062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carreño E, Carreño EA, Carreño R, Carreño M, López V, Potvin R. Refractive and visual outcomes after bilateral implantation of a trifocal intraocular lens in a large population. Clin Ophthalmol. 2020;14:369–376. doi: 10.2147/OPTH.S238841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaylock JF, Hall B. Astigmatic results of a diffractive trifocal toric IOL following intraoperative aberrometry guidance. Clin Ophthalmol. 2020;14:4373–4378. doi: 10.2147/OPTH.S285711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang SY, Stem MS, Oren G, Shtein R, Lichter PR. Patient-centered and visual quality outcomes of premium cataract surgery: a systematic review. Eur J Ophthalmol. 2017;27(4):387–401. doi: 10.5301/ejo.5000978 [DOI] [PubMed] [Google Scholar]
- 22.de Vries NE, Webers CA, Touwslager WR, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37(5):859–865. doi: 10.1016/j.jcrs.2010.11.032 [DOI] [PubMed] [Google Scholar]
- 23.de Silva SR, Evans JR, Kirthi V, Ziaei M, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2016;12:Cd003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Böhm M, Hemkeppler E, Herzog M, et al. Comparison of a panfocal and trifocal diffractive intraocular lens after femtosecond laser-assisted lens surgery. J Cataract Refract Surg. 2018;44(12):1454–1462. doi: 10.1016/j.jcrs.2018.07.060 [DOI] [PubMed] [Google Scholar]
- 25.Garzón N, Poyales F, de Zárate BO, Ruiz-García JL, Quiroga JA. Evaluation of rotation and visual outcomes after implantation of monofocal and multifocal toric intraocular lenses. J Refract Surg. 2015;31(2):90–97. doi: 10.3928/1081597X-20150122-03 [DOI] [PubMed] [Google Scholar]