Abstract
Background
Breast cancer is one of the most common cancers among women. Surgical removal of the cancer is the mainstay of treatment; however, tumour handling during surgery can cause microscopic dissemination of tumour cells and disease recurrence. The body's hormonal response to surgery (stress response) and general anaesthesia may suppress immunity, promoting tumour dissemination. Paravertebral anaesthesia numbs the site of surgery, provides good analgesia, and blunts the stress response, minimising the need for general anaesthesia.
Objectives
To assess the effects of paravertebral anaesthesia with or without sedation compared to general anaesthesia in women undergoing breast cancer surgery, with important outcomes of quality of recovery, postoperative pain at rest, and mortality.
Search methods
On 6 April 2020, we searched the Specialised Register of the Cochrane Breast Cancer Group (CBCG); CENTRAL (latest issue), in the Cochrane Library; MEDLINE (via OvidSP); Embase (via OvidSP); the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal; and ClinicalTrials.gov for all prospectively registered and ongoing trials.
Selection criteria
We included randomised controlled trials (RCTs) conducted in adult women undergoing breast cancer surgery in which paravertebral anaesthesia with or without sedation was compared to general anaesthesia. We did not include studies in which paravertebral anaesthesia was given as an adjunct to general anaesthesia and then this was compared to use of general anaesthesia.
Data collection and analysis
Two review authors independently extracted details of trial methods and outcome data from eligible trials. When data could be pooled, analyses were performed on an intention‐to‐treat basis, and the random‐effects model was used if there was heterogeneity. When data could not be pooled, the synthesis without meta‐analysis (SWiM) approach was applied. The GRADE approach was used to assess the certainty of evidence for each outcome.
Main results
Nine studies (614 participants) were included in the review. All were RCTs of parallel design, wherein female patients aged > 18 years underwent breast cancer surgery under paravertebral anaesthesia or general anaesthesia.
None of the studies assessed quality of recovery in the first three postoperative days using a validated questionnaire; most assessed factors affecting quality of recovery such as postoperative analgesic use, postoperative nausea and vomiting (PONV), hospital stay, ambulation, and patient satisfaction.
Paravertebral anaesthesia may reduce the 24‐hour postoperative analgesic requirement (odds ratio (OR) 0.07, 95% confidence interval (CI) 0.01 to 0.34; 5 studies, 305 participants; low‐certainty evidence) compared to general anaesthesia. Heterogeneity (I² = 70%) was attributed to the fixed dose of opioids and non‐steroidal analgesics administered postoperatively in one study (70 participants), masking a difference in analgesic requirements between groups.
Paravertebral anaesthesia probably reduces the incidence of PONV (OR 0.16, 95% CI 0.08 to 0.30; 6 studies, 324 participants; moderate‐certainty evidence), probably results in a shorter hospital stay (mean difference (MD) ‐79.39 minutes, 95% CI ‐107.38 to ‐51.40; 3 studies, 174 participants; moderate‐certainty evidence), and probably reduces time to ambulation compared to general anaesthesia (SWiM analysis): percentages indicate vote counting based on direction of effect (100%, 95% CI 51.01% to 100%; P = 0.125; 4 studies, 375 participants; moderate‐certainty evidence).
Paravertebral anaesthesia probably results in higher patient satisfaction (MD 5.52 points, 95% CI 1.30 to 9.75; 3 studies, 129 participants; moderate‐certainty evidence) on a 0 to 100 scale 24 hours postoperatively compared to general anaesthesia.
Postoperative pain at rest and on movement was assessed at 2, 6, and 24 postoperative hours on a 0 to 10 visual analogue scale (VAS). Four studies (224 participants) found that paravertebral anaesthesia as compared to general anaesthesia probably reduced pain at 2 postoperative hours (MD ‐2.95, 95% CI ‐3.37 to ‐2.54; moderate‐certainty evidence). Five studies (324 participants) found that paravertebral anaesthesia may reduce pain at rest at 6 hours postoperatively (MD ‐1.54, 95% CI ‐3.20 to 0.11; low‐certainty evidence). Five studies (278 participants) found that paravertebral anaesthesia may reduce pain at rest at 24 hours postoperatively (MD ‐1.19, 95% CI ‐2.27 to ‐0.10; low‐certainty evidence). Differences in the methods of two studies (119 participants) and addition of clonidine to the local anaesthetic in two studies (109 participants), respectively, contributed to the heterogeneity (I² = 96%) observed for these two outcomes.
Two studies (130 participants) found that paravertebral anaesthesia may reduce pain on movement at 6 hours (MD‐2.57, 95% CI ‐3.97 to ‐1.17) and at 24 hours (MD ‐2.12, 95% CI ‐4.80 to 0.55; low‐certainty evidence). Heterogeneity (I² = 96%) was observed for both outcomes and could be due to methodological differences between studies.
None of the studies reported mortality related to the anaesthetic technique. Eight studies (574 participants) evaluated adverse outcomes with paravertebral anaesthesia: epidural spread (0.7%), minor bleeding (1.4%), pleural puncture not associated with pneumothorax (0.3%), and Horner's syndrome (7.1%). These complications were self‐limiting and resolved without treatment.
No data are available on disease‐free survival, chronic pain, and quality of life.
Blinding of personnel or participants was not possible in any study, as a regional anaesthetic technique was compared to general anaesthesia. Risk of bias was judged to be serious, as seven studies had concerns of selection bias and three of detection bias.
Authors' conclusions
Moderate‐certainty evidence shows that paravertebral anaesthesia probably reduces PONV, hospital stay, postoperative pain (at 2 hours), and time to ambulation and results in greater patient satisfaction on the first postoperative day compared to general anaesthesia. Paravertebral anaesthesia may also reduce postoperative analgesic use and postoperative pain at 6 and 24 hours at rest and on movement based on low‐certainty evidence. However, RCTs using validated questionnaires are needed to confirm these results. Adverse events observed with paravertebral anaesthesia are rare.
Plain language summary
Comparison of paravertebral anaesthesia to general anaesthesia for breast cancer surgery
What is the issue?
Breast cancer is one of the most common cancers in women. Surgical removal of the tumour is the main treatment; however, dissemination of cancer cells may occur due to tumour handling during surgery. The body's response to surgery (stress response or release of hormones and mediators) as well as drugs used to produce general anaesthesia (complete loss of consciousness), such as opioids and inhalational anaesthetics, can also depress immunity and promote cancer cell survival.
Paravertebral anaesthesia acts by numbing the nerves on one side of the body and can allow surgery to be performed without general anaesthesia. The lightly sedated participant can report pain, cold, thirst, or hunger during or after surgery, allowing timely treatment. This, combined with pain relief produced by paravertebral anaesthesia, has the potential to improve immunity, reduce surgical stress, and improve quality of life and cancer‐free survival.
Review question
We collected and analysed data from all relevant studies to ascertain whether paravertebral anaesthesia would provide better quality of recovery and pain relief at rest and on movement, and to determine whether paravertebral anaesthesia would have any affect on mortality, compared to general anaesthesia.
Study characteristics
We included randomised controlled trials in which participants in one group received paravertebral anaesthesia with or without sedation and participants in the other group received general anaesthesia during breast cancer surgery. We did not include studies in which paravertebral anaesthesia was administered in addition to general anaesthesia and then this was compared to the use of general anaesthesia.
Key results
Nine studies involving 614 participants were included. Four studies were conducted in Europe, three in Asia, and one each in Africa and North America. All studies took place in medical college hospitals with no external funding.
When reviewing evidence on the quality of recovery, these studies reported various outcomes. It was found that paravertebral anaesthesia:
• may reduce 24‐hour postoperative analgesic use (61 per 1000 versus 480 per 1000 in the general anaesthesia group);
• probably reduces the incidence of postoperative nausea and vomiting (72 per 1000 versus 340 per 1000 in the general anaesthesia group);
• probably reduces pain at 2 hours (by 2.95 points on a 0 to 10 point scale) and may reduce pain at 24 hours at rest (0.21 points on a 0 to 10 point scale) compared to general anaesthesia; and
• may reduce pain on movement at 6 hours and at 24 hours post surgery (at 6 hours by 2.57 points; at 24 hours by 2.12 points on a scale of 0 to 10) compared to general anaesthesia.
No study reported on death with each anaesthetic technique. None of the included studies reported on disease‐free survival, chronic pain, or quality of life after breast cancer surgery, probably because these outcomes were not envisaged or planned.
Reports of adverse events with paravertebral block were rare. Out of 290 participants who received paravertebral anaesthesia, two developed epidural spread, four had minor bleeding, and one had pleural puncture. Seventeen of 240 participants developed Horner's syndrome. All adverse events were self‐limited and resolved without treatment.
Quality of the evidence
Most evidence was judged to be of moderate certainty, meaning that we are moderately confident in the reported result. In some cases, evidence was seen to be of low certainty, meaning that our confidence in the reported result is limited.
Summary of findings
Summary of findings 1. Paravertebral anaesthesia with or without sedation compared to general anaesthesia for women undergoing breast cancer surgery.
| Paravertebral anaesthesia with or without sedation compared to general anaesthesia for women undergoing breast cancer surgery | ||||||
| Patient or population: women undergoing breast cancer surgery Setting: international Intervention: paravertebral anaesthesia with or without sedation Comparison: general anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | №. of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Risk with general anaesthesia | Risk with paravertebral anaesthesia with or without sedation | |||||
| Postoperative analgesic requirement Assessed with: number of patients who required postoperative analgesia Follow‐up: 1 day | Study population | OR 0.07 (0.01 to 0.34) | 305 (5 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 480 per 1000 | 61 per 1000 (9 to 239) | |||||
| Postoperative nausea and vomiting (PONV) Assessed with: number of patients who had persistent nausea or retching or vomiting requiring treatment Follow‐up: 1 day | Study population | OR 0.16 (0.08 to 0.30) | 323 (6 RCTs) | ⊕⊕⊕⊝ MODERATEc | ||
| 340 per 1000 | 72 per 1000 (40 to 134) | |||||
| Postoperative pain at 2 hours Assessed with: VAS 0 to 10 scale | Mean postoperative pain at 2 hours was 0 | MD 2.95 lower (3.37 lower to 2.54 lower) | ‐ | 224 (4 RCTs) | ⊕⊕⊕⊝ MODERATEd | |
| Postoperative pain at 24 hours on rest Assessed with: 0 to 10 VAS scale | Mean postoperative pain at 24 hours on rest was 0 | MD 0.21 lower (0.42 lower to 0 ) | ‐ | 169 (3 RCTs) | ⊕⊕⊝⊝ LOWe,f | |
| Postoperative pain at 6 hours on movement Assessed with: 0 to 10 VAS scale | Mean postoperative pain at 6 hours on movement was 0 | MD 2.57 lower (3.97 lower to 1.17 lower) | ‐ | 130 (2 RCTs) | ⊕⊕⊝⊝ LOWg,h | |
| Postoperative pain at 24 hours on movement Assessed with: 0 to 10 VAS scale | Mean postoperative pain at 24 hours on movement was 0 | MD 2.12 lower (4.8 lower to 0.55 higher) | ‐ | 130 (2 RCTs) | ⊕⊕⊝⊝ LOWg,h | |
| Mortality | Study population | not estimable | (9 RCTs) | ‐ | No RCT has studied this outcome; therefore no data are available | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aData from five studies were analysed to assess this outcome (Das 2012; Naja 2003; Pusch 1999; Sundarathiti 2015; Terheggen 2002). Blinding of participants was not possible as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in four studies (Das 2012; Naja 2003; Pusch 1999; Terheggen 2002), allocation concealment in two studies (Pusch 1999; Sundarathiti 2015), and blinding of outcome assessors in one study led to downgrading of risk of bias to serious (Naja 2003).
bHeterogeneity was observed for this outcome (I² = 70%) that included five RCTs; with the exclusion of 1 study, Sundarathiti 2015, heterogeneity decreased to 0%, probably because in this study, all patients in both groups received analgesics (Ultracet and Celebrex twice a day) irrespective of pain; only when they needed additional postoperative analgesia were morphine boluses administered. This routine administration of analgesics could have masked the difference in analgesic requirements between groups. In the other four studies, analgesics were administered only when patients expressed that they were in pain.
cData from six studies were analysed to assess this outcome (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Tedore 2011; Terheggen 2002). Blinding of participants or personnel was not possible, as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in five studies (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), allocation concealment in two studies (Paleczny 2005; Pusch 1999), and blinding of outcome assessors in two studies led to downgrading of risk of bias to serious (Naja 2003; Paleczny 2005).
dData from four studies were analysed to assess this outcome (Das 2012; Paleczny 2005; Pusch 1999; Terheggen 2002). Blinding of participants or personnel was not possible as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in all four studies (Das 2012; Paleczny 2005; Pusch 1999; Terheggen 2002), allocation concealment in two studies (Paleczny 2005; Pusch 1999), and blinding of outcome assessors in one study led to downgrading of risk of bias to serious (Paleczny 2005).
eData from five studies were analysed to assess this outcome (Das 2012; Naja 2003; Paleczny 2005; Sundarathiti 2015; Tedore 2011). Blinding of participants was not possible, as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it was acceptable. Uncertainty about random sequence generation in three studies (Das 2012; Naja 2003; Paleczny 2005), allocation concealment in two studies (Paleczny 2005; Sundarathiti 2015), and blinding of outcome assessors in two studies led to downgrading of risk of bias to serious (Naja 2003; Paleczny 2005).
fData from five studies were used to evaluate this outcome (Das 2012; Naja 2003; Paleczny 2005; Sundarathiti 2015; Tedore 2011). We noted heterogeneity for this outcome (I² = 96%). On performing subgroup analysis and excluding two studies (Naja 2003; Paleczny 2005), where a potent adjunctive drug (alpha‐2 agonist clonidine) was added to the local anaesthetic solution used for paravertebral anaesthesia, we found a reduction in heterogeneity.
gData from two studies were used to analyse this outcome (Naja 2003; Sundarathiti 2015). Blinding of participants or personnel was not possible, as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review; therefore it is acceptable. Uncertainty about random sequence generation and blinding of outcome assessors in one study ‐ Naja 2003 ‐ and allocation concealment in the other study ‐ Sundarathiti 2015 ‐ led to downgrading of evidence to serious.
hSignificant heterogeneity was noted in the results and sensitivity analysis could not be performed, as only two studies could be included in assessing this outcome (Naja 2003; Sundarathiti 2015). Both studies showed reduction in pain on movement with the paravertebral block, but one study showed a greater reduction in pain probably due to methodological differences (Naja 2003). The use of clonidine as an adjunct to local anaesthetic as well as multiple injections at each paravertebral level could have contributed to this effect (Naja 2003). In the other study, only local anaesthetic was administered in the paravertebral space, injection was performed at one level, a catheter was inserted 8 cm into the space, and drug was injected through the catheter at this point, as well as on withdrawing the catheter by 2 cm and then 4 cm (single‐injection, multi‐level block) (Sundarathiti 2015).
Background
Description of the condition
According to worldwide public health data, breast cancer is one of the most common cancers among women. More than one million women are diagnosed with the disease globally per year (Ban 2014; Coughlin 2009; Ferlay 2015; Youlden 2012). Before the global surveys of cancer, breast cancer was considered to be a disease of high‐income countries; however since then, its incidence in low‐ and middle‐income countries has increased (Ferlay 2015).
Women living in low‐ and middle‐income countries have higher mortality than those living in high‐income countries because they reach hospital at later stages of disease due to lack of facilities for screening and early diagnosis (Coughlin 2009; Ferlay 2015; Youlden 2012). Cancer that has spread to distant organs of the body (i.e. metastasis) is the main cause of death among women with breast cancer (Weigelt 2005).
Surgery is the mainstay of breast cancer treatment (Niwa 2013; Sessler 2008). However, despite the apparent complete surgical removal of a tumour, some residual disease may remain. Handling of the tumour during surgery may release cancer cells into the circulation, which results in the spread of cancer to other parts of the body (Coffey 2003). Suppression of a woman's immune system during surgery and under general anaesthesia may also contribute to cancer spread and recurrence at a later date (Coffey 2003; Niwa 2013; Tedore 2015).
Description of the intervention
Paravertebral block is a regional anaesthesia technique. It involves injecting local anaesthetic drug into the space alongside the vertebral column (i.e. paravertebral space) where the spinal nerves emerge from the intervertebral foramina (Karmakar 2001; Richardson 1998). Paravertebral block results in a sympathetic and sensory nerve block only on the side where the local anaesthetic is deposited. This technique can be performed as a single injection or as multiple injections. In either case, as the block occurs only on the side where the anaesthetic is injected (i.e. unilateral), it may produce minimal physiological changes in haemodynamic and respiratory variables and temperature changes (Karmakar 2001; Richardson 1998).
A single‐injection paravertebral block as an adjunct to general anaesthesia has been used to provide pain relief (Boughey 2009; Kairaluoma 2004; Moller 2007). The number of nerve segments blocked by a single injection can vary and depends on the volume of local anaesthetic injected. On average, eight sympathetic and five sensory nerve segments are estimated to be blocked with 15 mL of local anaesthetic (Cheema 1995). Some studies have used a single‐level paravertebral injection at the third to fourth thoracic level to provide surgical anaesthesia for breast surgery (Pusch 1999; Terheggen 2002).
In contrast, multiple (multi‐level) paravertebral block involves injecting 3 mL to 5 mL of local anaesthetic at each paravertebral space from the seventh cervical or the first thoracic vertebral level to the sixth thoracic vertebral level; this can provide reliable surgical anaesthesia (Dabbagh 2007; Greengrass 1996; Naja 2003; Weltz 1995; Wu 2015), along with excellent perioperative analgesia, following breast surgery (Abdallah 2014; Wu 2015). Multiple‐injection or single‐injection paravertebral block may minimise the perioperative use of long‐acting opioids such as morphine that promote angiogenesis (i.e. growth of new blood vessels) and survival of circulating tumour cells (Ecimovic 2011; Kairaluoma 2004; Moller 2007; Wu 2015).
Therefore, paravertebral block with or without sedation can be used as a stand‐alone technique to provide anaesthesia for breast surgery without the need for administering general anaesthesia (Dabbagh 2007; Greengrass 1996; Naja 2003; Pusch 1999; Weltz 1995).
How the intervention might work
General anaesthesia (GA) for breast cancer surgery can be provided via inhaled anaesthetic drugs or intravenous infusion of propofol (propofol‐based total intravenous anaesthesia (TIVA)) (Tedore 2015; Vaghari 2014). Regardless of whether an inhalational or intravenous technique is used, opioid medication is given to provide pain relief unless a local anaesthetic block is administered. Inhaled anaesthetic drugs and opioids such as morphine are the more common methods of providing general anaesthesia for women undergoing breast cancer surgery as compared to propofol‐based TIVA (Tedore 2015; Vaghari 2014).
Retrospective reviews and in vitro studies have shown that inhaled anaesthetic drugs and opioids suppress the body’s immune response and therefore may promote tumour spread and recurrence following surgery (Buckley 2014; Deegan 2009; Deegan 2010; Ecimovic 2011; Ecimovic 2013; Exadaktylos 2006; Jaura 2014). In contrast, retrospective evidence indicates that intravenous propofol‐based TIVA may have a protective effect against tumour spread (Gottschalk 2010; Vaghari 2014). Irrespective of the general anaesthesia technique used (inhalational or intravenous), the body responds to the pain and trauma of surgery by releasing hormones and mediators that suppress the body's immunity (surgical stress response) (Gottschalk 2010; Tedore 2015; Vaghari 2014).
Regional anaesthesia techniques produce a dense sensory block that is more effective in blunting this surgical stress response than general anaesthesia via opioids (Cata 2011; Tedore 2015). Paravertebral block has been shown to obtund the stress response to surgery (O'Riain 2005). In addition, factors such as perioperative hypothermia, hypoglycaemia, nausea and vomiting following surgery, and resulting delayed oral intake can contribute to immunosuppression. These are more likely to occur in unconscious patients under general anaesthesia than in lightly sedated patients under regional anaesthesia, who can communicate and maintain a patent airway without the need for intubation or supra‐laryngeal airway devices (Gottschalk 2010; Tedore 2015; Vaghari 2014).
Regional anaesthesia could result in better‐preserved immune function and lymphocyte (white blood cell) activity with reduced chance of tumour spread and recurrence compared to general anaesthesia (O'Riain 2005; Wu 2015). Local anaesthetic drugs used during regional anaesthesia have also been shown to cause direct inhibition of tumour cell multiplication and migration and increased tumour cell breakdown (Cassinello 2015; Ramirez 2015; Votta‐Velis 2015). Surgery under paravertebral anaesthesia (with or without sedation) could result in awake, pain‐free, comfortable patients with normal body temperature and minimal postoperative nausea and vomiting. Some studies have shown that paravertebral block even in combination with GA is likely to result in better quality recovery (Abdallah 2014; Buckenmaier 2010), improved quality of life (Karmakar 2014), and decreased chronic pain at six months (Chiu 2014).
Why it is important to do this review
Several systematic reviews have assessed the use of paravertebral block during surgery, but these reviews have included mixed populations (i.e. not specifically women with breast cancer) and non‐randomised, retrospective, and case series studies (Schnabel 2010; Tahiri 2011; Wu 2015). These reviews have included studies that compared paravertebral block and general anaesthesia to conventional general anaesthesia alone (Schnabel 2010; Tahiri 2011; Wu 2015).
This Cochrane Review aimed to assess whether paravertebral block as the main anaesthetic technique with or without sedation improved quality of recovery in the initial postoperative period (one to three days), reduced acute pain (at 2 hours, at 6 hours, and at 24 hours post surgery on the first postoperative day), and reduced chronic pain (3 to 12 months post surgery). It also assessed mortality associated with either anaesthetic technique and whether paravertebral anaesthesia compared to general anaesthesia is associated with improved quality of life (1 to 2 weeks, 3 to 12 months) and improved disease‐free survival in women undergoing breast cancer surgery.
Objectives
To assess the effects of paravertebral anaesthesia with or without sedation compared to general anaesthesia in women undergoing breast cancer surgery, with important outcomes of quality of recovery, postoperative pain at rest, and mortality.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that examined paravertebral anaesthesia with or without sedation compared to general anaesthesia for breast cancer surgery.
Types of participants
We included women (over 18 years of age) with early or locally advanced breast cancer scheduled for breast cancer surgery, irrespective of whether they had received chemotherapy in the past.
Types of interventions
Intervention
Paravertebral anaesthesia with or without sedation was the intervention.
Comparator
General anaesthesia (including opioids and inhaled anaesthetic drugs or propofol‐based TIVA) was the comparator.
We planned to include studies that used truncal regional anaesthesia block in addition to paravertebral block.
We excluded studies in which paravertebral block was used as an adjunct to general anaesthesia and then this was compared to general anaesthesia alone.
Types of outcome measures
Primary outcomes
Quality of recovery during the initial postoperative period (1 to 3 days) assessed by outcomes such as incidence of postoperative nausea and vomiting, postoperative analgesic requirement, hospital stay, time to ambulation, and patient satisfaction
Postoperative pain at rest at 2 hours, at 6 hours, and at 24 hours on the first postoperative day measured on a visual analogue scale (VAS) with a range of 0 to 10, or 0 to 100, or a numerical rating scale (NRS) with a similar range
Postoperative pain on movement at 2 hours, at 6 hours, and at 24 hours on the first postoperative day measured on a VAS with a range of 0 to 10, or 0 to 100, or an NRS with a similar range
Mortality related to the anaesthetic technique, if any
Secondary outcomes
Adverse events due to paravertebral block (i.e. epidural block denoted by hypotension, bilateral block, pleural tap, Horner's syndrome, bleeding, or neurological deficit)
Disease‐free survival (often defined as time to first detection of local recurrence or distant metastasis, or progression‐free survival, time to progression, or time to treatment failure)
Chronic pain that persisted beyond the immediate postoperative period (i.e. at 3 to 12 months)
Quality of life at 1 to 2 weeks and at 3 to 12 months postoperatively assessed by a validated questionnaire
Search methods for identification of studies
Electronic searches
We searched the following databases on 6 April 2020.
Cochrane Breast Cancer Group (CBCG) Specialised Register. We will apply the search strategies used by the CBCG and outlined in the CBCG module (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). In addition, we will extract and will consider for inclusion trials with the key words breast cancer surgery, conduction anaesthesia, paravertebral anaesthesia, paravertebral block, and nerve block.
Central Register of Controlled Trials (CENTRAL; latest issue), in the Cochrane Library. See Appendix 1.
MEDLINE (via OvidSP) from 1966 to the present (see Appendix 2).
Embase (via OvidSP) from 1947 to the present (see Appendix 3).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/Default.aspx), for all prospectively registered and ongoing trials (see Appendix 4).
ClinicalTrials.gov (http://clinicaltrials.gov/; see Appendix 5).
Searching other resources
Bibliographic searching
We screened the reference lists of identified relevant trials or reviews. We obtained a copy of the full article for each reference reporting a potentially eligible trial. When this was not possible, we tried to contact the study authors to request additional information.
Data collection and analysis
Selection of studies
Two review authors (AC and ARC) independently screened all titles and abstracts for eligibility. We obtained and assessed the full articles of all potentially eligible RCTs for relevance based on the pre‐specified inclusion criteria. Each review author independently documented the reason for excluding a study. We resolved any disagreement by discussion with a third review author (HP), who decided on inclusion or exclusion of the study. We compiled a list of all eligible studies and recorded the study selection process in the PRISMA flow diagram. If further information was required to make a decision about trial inclusion, AC contacted the authors of the relevant trial.
We did not impose any language restrictions and included all peer‐reviewed journals and trial registries. We obtained the articles and requested translation through Cochrane TaskExchange or through our network.
We recorded in the Characteristics of excluded studies table studies that were excluded from the review.
Data extraction and management
Two review authors (AC and ARC) independently extracted data onto a standard data extraction form. We resolved any disagreements through consultation with a third review author (HP). When additional information was required, AC contacted the corresponding author of the relevant trial.
For studies with more than one publication, data were extracted from the most recent publications and records related to the same study were combined under the overall trial ID.
Assessment of risk of bias in included studies
We minimised selection bias by including RCTs.
We used the recommended Cochrane ʻRisk of bias' tool to assess risk of bias in the following domains (Higgins 2011).
Random sequence generation (selection bias).
Concealment of the allocation sequence (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Other potential sources of bias, which we assessed by comparing groups at baseline with respect to staging of disease and by noting any difference in treatment administered except for the intervention.
After reviewing the methods used, we decided whether risk of bias for a particular domain was ‘high’, ‘low’, or ‘unclear’ (insufficient data available to decide whether risk of bias existed). This was done only after we contacted the study authors to request additional information. Two review authors (AC and ARC) assessed risk of bias. In the case of any conflict of opinion, we resolved disagreements through consultation with a third review author (HP).
We noted financial or logistical support provided for each included study.
Measures of treatment effect
For continuous data, such as factors that determine quality of recovery (at 1 to 3 days), acute postoperative pain scales on the first postoperative day, and quality of life (at 1 to 2 weeks, and at 3 to 12 months postoperatively), we calculated the mean difference (MD) if data were on the same scale, or the standardised mean difference (SMD) if data were on different scales.
For dichotomous outcomes for which either one of two outcomes was possible, such as adverse events (epidural spread, pneumothorax, Horner's syndrome, bleeding) or no adverse events; chronic pain at 3 to 12 months, or no chronic pain, we planned to calculate the risk ratio (RR) and the 95% confidence interval (CI) from a fixed‐effect or random‐effects model analysis, depending on the presence of heterogeneity. We reported ratios of treatment effects for response so that RRs less than 1.0 favour the paravertebral group and RRs greater than 1.0 favour the general anaesthesia group.
For time‐to‐event outcomes, such as mortality related to the anaesthetic technique and disease‐free survival, we had planned to express results as hazard ratios (HRs). If we could not obtain the HR and associated variance directly from the trial publication, we had planned to obtain the data indirectly using methods described in Parmar 1998, or to extract them from published Kaplan‐Meier curves. However, none of the studies included in the review reported time‐to‐event outcomes.
In the case where studies did not report the effect measure, we cross‐checked all data presented and concluded that these data could not be converted to a suitable format for performing a meta‐analysis. Therefore we applied the synthesis without meta‐analysis (SWiM) approach.
Unit of analysis issues
We included RCTs that used a parallel group design.
Dealing with missing data
We performed quantitative analysis on an intention‐to‐treat (ITT) basis and contacted trial authors to request missing data. However, some studies excluded a small number of patients who were randomised but did not start their allocated treatment (often referred to as modified ITT (mITT) analysis). We included mITT results when ITT results were not available. We had planned to discuss the impact of missing data and imputation methods in the Discussion section of the review.
We performed sensitivity analysis to explore the consistency of effect size measures in trials with low versus high risk of bias, and we investigated the impact of any missing data by using the imputation method described above.
Assessment of heterogeneity
We used the Q statistic to test statistical heterogeneity between trials and considered P ≤ 0.05% as indicating significant heterogeneity. We used the I² statistic to assess the magnitude of heterogeneity, interpreting the I² statistic as follows (Cochran 1954; Higgins 2003).
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: considerable heterogeneity.
We used a random‐effects model analysis if the value of the I² statistic was greater than 50% (Higgins 2011).
We used unadjusted effect estimates for the meta‐analysis of RCTs.
For results synthesised by the SWiM approach, we assessed the clinical and methodological variability of all studies included in all SWiM analyses. When variability was found, data presented in the SWiM analysis were organised so as to show the variation (Campbell 2020).
Assessment of reporting biases
As more than 10 studies for any outcome were not available, we could not test for funnel plot asymmetry to ascertain reporting bias. However, in future updates, we plan to test for funnel plot asymmetry if more than 10 studies (for each outcome) are included in the meta‐analysis (Egger 1997).
Data synthesis
We quantitatively reviewed the included data and combined data by intervention, outcome, and population, if appropriate, using Review Manager 5 (RevMan 5) software (RevMan 2014).
For dichotomous outcomes, we expressed pooled estimates as RRs and used a fixed‐effect model in the absence of significant statistical heterogeneity (I² < 50%) (Mantel 1959). In the case of significant heterogeneity, we used the random‐effects analysis (DerSimonian 1986).
For continuous outcomes, we expressed pooled estimates of the MD and used a fixed‐effect analysis (i.e. inverse variance method) in the absence of significant statistical heterogeneity (I² < 50%). In the case of heterogeneity, we used a random‐effects analysis (i.e. inverse variance method with the variance including between‐study variation).
In future versions of this review, for time‐to‐event outcomes such as mortality and disease‐free survival, we plan to use the HR and generic inverse variance depending on available data (Parmar 1998).
For outcomes for which numerical data were lacking or outcomes had been measured in diverse ways and only direction of effect was available, vote counting based on direction of effect was used and results reported. When possible, this was presented alongside results of the meta‐analysis (McKenzie 2019).
This method does not provide information on the magnitude of effect nor take into consideration the relative size of studies. P values were calculated via sign test and binomial probability; 95% CIs were calculated by Wilson's interval method (Brown 2001).
We prepared SWiM tables showing direction of effect based on vote counting with study ID, direction of effect as reported by study authors, and estimated risk of bias.
ʻSummary of findings' table
Two review authors (AC and ARC) used the principles of the GRADE system to assess the overall quality of evidence associated with specific outcomes (Guyatt 2011; Schünemann 2011).
We constructed a ʻSummary of findings' table using GRADEpro GDT software (GRADEpro GDT 2015). The GRADE approach appraises the quality of a body of evidence for each outcome based on five domains: (1) risk of bias of included studies (methodological quality), (2) inconsistency (i.e. heterogeneity), (3) indirectness (relevance to the review question), (4) imprecision (i.e. confidence intervals), and (5) risk of publication bias.
We listed the following main outcomes in the ʻSummary of findings' table and assessed the quality of evidence.
Analgesic requirement on the first postoperative day.
Postoperative nausea and vomiting on the first postoperative day.
Postoperative pain on the first postoperative day at 2 hours at rest based on a VAS with a range of 0 to 10.
Postoperative pain on the first postoperative day at 24 hours at rest based on a VAS with a range of 0 to 10.
Postoperative pain on the first postoperative day at 6 hours on movement based on a VAS with a range of 0 to 10.
Postoperative pain on the first postoperative day at 24 hours on movement based on a VAS with a range of 0 to 10.
Mortality related to either anaesthetic technique.
Other outcomes such as disease‐free survival and quality of life at 1 to 2 weeks and at 3 to 12 months postoperatively assessed by a validated questionnaire were not reported by any study and therefore are not listed in the 'Summary of findings' table.
For results summarised by vote counting when pooled estimation of data was not possible, we applied GRADE domains (methodological limitations of studies or risk of bias, indirectness, imprecision, inconsistency, and likelihood of publication bias) guided by Murad 2017.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analysis was not possible, as no study had reported these outcomes. We conducted an additional subgroup analysis on the use of local anaesthetic alone or in combination with adjuncts such as the alpha‐2 agonist drug clonidine in patients receiving paravertebral anaesthesia because adding clonidine to local anaesthetic can prolong the duration of analgesia as compared to that produced by local anaesthetic alone (Pöpping 2009).
Sensitivity analysis
We planned to perform sensitivity analyses to explore the consistency of effect size measures in trials with low versus high risk of bias. We considered studies at high or unclear risk of bias in two or more domains to be at high risk of bias. We interpreted absence of blinding in outcome assessment, such as incidence of mortality from medical records, as showing low risk of bias. However, absence of blinding in patient‐reported outcomes was taken as showing high risk of bias; an exception was blinding of participants and personnel (performance bias). This was not possible as participants in one group received paravertebral anaesthesia for breast cancer surgery and participants in the other group received general anaesthesia. This comparison was the main question of the review and therefore was acceptable. However, bias in any other domain, especially selection, detection, attrition, reporting, or other bias, was taken to show high risk of bias.
Results
Description of studies
Results of the search
We identified a total of 773 records through electronic database searches and one additional record by bibliographic searches. After we had removed 45 duplicate records, 729 records remained. After title and abstract screening, 683 records were found to be irrelevant and were excluded. The full text of 46 records was assessed for eligibility; 37 records did not fulfil the eligibility criteria and were excluded. Nine records fulfilled the criteria of our review, and we quantitatively analysed data from seven studies. See Figure 1.
1.
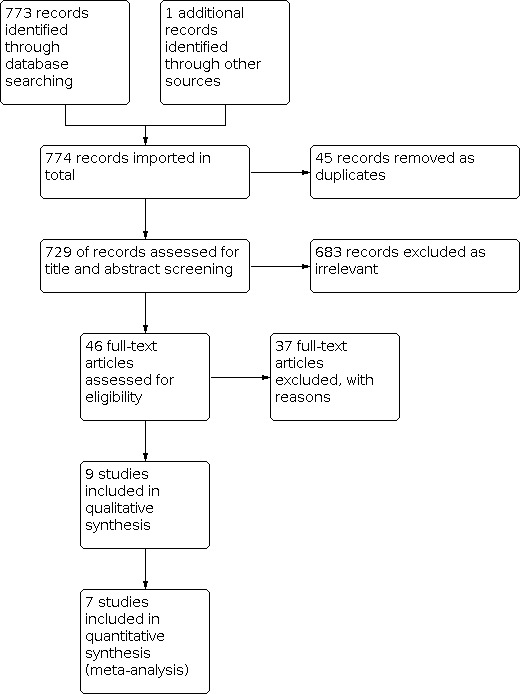
Study flow diagram.
We contacted the lead authors of all included studies by email to request missing data. The authors of two studies confirmed that their studies did not study the quality of recovery by using a validated questionnaire, mortality related to the anaesthetic technique, disease‐free survival, chronic pain persisting 3 to 12 months postoperatively, or quality of life at 1 to 2 weeks and at 3 to 12 months with a validated questionnaire (Sundarathiti 2015; Tedore 2011).
The corresponding authors of seven studies did not respond to our request and did not provide further information (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Terheggen 2002).
See Characteristics of included studies and Characteristics of excluded studies.
Included studies
We included in our review nine studies with 614 participants (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). All were RCTs of parallel design wherein participants underwent breast cancer surgical procedures under paravertebral anaesthesia or general anaesthesia. All studies were carried out in adult female patients aged > 18 years with American Society of Anaesthesiologists physical status 1 to 3.
One study was published in the Polish language (Paleczny 2005), and another one in the Russian language (Shkol'nik 2006); both were translated to English. All other studies were published in English.
Participants
Two studies were carried out among patients undergoing ambulatory breast cancer surgery with or without sentinel lymph node biopsy or axillary lymph node dissection (Tedore 2011; Terheggen 2002). Two studies included patients undergoing mastectomy for breast cancer without axillary clearance (Das 2012; Megahed 2010). Two studies included patients undergoing only major breast cancer surgery (i.e. modified radical mastectomy (MRM) ‐ Paleczny 2005 ‐ or radical/reconstructive breast cancer surgery ‐ Shkol'nik 2006). The remaining studies included all types of breast cancer surgery ranging from lumpectomy to modified radical mastectomy (Naja 2003; Pusch 1999; Sundarathiti 2015).
One study was carried out only on patients with type 2 diabetes who were undergoing mastectomy for breast cancer to assess perioperative P selectin levels as a predictor of thrombotic events (Megahed 2010). Another study estimated serum cortisol levels in paravertebral and general anaesthesia groups (Shkol'nik 2006).
Interventions
Pre‐medication was administered to participants in the paravertebral group before block was performed in eight studies as intravenous midazolam (Naja 2003; Paleczny 2005; Pusch 1999; Sundarathiti 2015), intramuscular midazolam (Megahed 2010), or a combination of intravenous midazolam and fentanyl (Das 2012; Shkol'nik 2006; Tedore 2011). In only one study involving day case procedures, no pre‐medication was administered for the block (Terheggen 2002).
During the intraoperative period (i.e. during surgery), sedation was offered to participants in the paravertebral group in all nine studies either as propofol infusion alone (Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), propofol infusion in combination with fentanyl boluses (Das 2012; Shkol'nik 2006; Tedore 2011), or propofol infusion with a single ketamine bolus (Sundarathiti 2015). In one study, despite sedation offered to all participants, two‐thirds of participants did not opt to receive it (Naja 2003). In all studies, care was taken to maintain 'conscious' sedation (i.e. participants were sedated but could respond to verbal commands and could breathe spontaneously).
Six participants in three studies (one participant in Naja 2003, two in Paleczny 2005, three in Pusch 1999) required supplementary analgesia. This involved local anaesthetic infiltration in Naja 2003 and single‐dose opioids in Paleczny 2005 and Pusch 1999 on axillary dissection in the paravertebral group. In addition, two participants in Das 2012 required supplementary local anaesthetic infiltration in the surgical site intraoperatively due to inconsistent effect in blocked dermatomes even without axillary dissection.
Excluded studies
We excluded Sultan 2013 despite inclusion of a paravertebral anaesthesia group and a general anaesthesia group of participants undergoing breast cancer surgery because study authors assessed only cytokines using either technique and did not provide quantitative or descriptive information on any of the outcomes mentioned in our review.
Risk of bias in included studies
The characteristics of included studies for assessment of risk of bias are shown in Figure 2 and in Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Overall, one study was found to be of high methodological quality with the exception of high performance bias (Tedore 2011). Three studies had at least one more domain with high risk of bias (Das 2012; Sundarathiti 2015; Terheggen 2002). The remaining studies had unclear bias in more than two domains (Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006), as detailed below.
Allocation
We judged six studies as having unclear risk of bias for random sequence generation (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002). Five studies showed uncertainty of allocation concealment (Megahed 2010; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015). We found one study to be of high methodological quality with no risk of selection bias (Tedore 2011).
Blinding
Performance bias was high in the nine studies, as participants in one group received paravertebral anaesthesia for breast cancer surgery and those in the other group received general anaesthesia; therefore blinding of participants was not possible. As this comparison was the main question of the review, this bias was considered acceptable by all review authors.
In addition, in four studies, we detected uncertainty in blinding of outcome assessors (Megahed 2010; Naja 2003; Paleczny 2005; Shkol'nik 2006).
Incomplete outcome data
We found no evidence of attrition bias in the nine included studies.
Selective reporting
We found that all planned outcomes were reported in the studies. Study authors mentioned all outcomes in their methods and reported advantages and disadvantages of both paravertebral and general anaesthesia. However, only two studies had prospectively registered protocols on the trial registries (Sundarathiti 2015; Tedore 2011); none of the other studies had trial registration records.
Other potential sources of bias
We detected no other potential sources of bias.
Effects of interventions
See: Table 1
See Table 1.
Quality of recovery during the initial postoperative period (1 to 3 days)
No study assessed quality of recovery in the initial 1 to 3 days using a validated questionnaire. However, many studies compared outcomes that affect quality of recovery following breast cancer surgery. These outcomes are postoperative pain, need for postoperative analgesics, postoperative nausea and vomiting, ambulation, hospital stay, and patient satisfaction. Postoperative pain is one of the primary outcomes and is described separately.
Postoperative analgesic use
Nine studies in total reported this outcome, five of which provided data suitable for meta‐analysis (Das 2012; Naja 2003; Pusch 1999; Sundarathiti 2015; Terheggen 2002); four provided data used for synthesis without meta‐analysis ((SWiM) (Megahed 2010; Paleczny 2005; Shkol'nik 2006; Tedore 2011).
Meta‐analysis
Data from five studies were used to analyse the number of participants who required postoperative analgesia in the first 24 hours post surgery in the paravertebral and general anaesthesia groups (Das 2012; Naja 2003; Pusch 1999; Sundarathiti 2015; Terheggen 2002). Data regarding the number of participants requiring rescue non‐steroidal anti‐inflammatory drugs (NSAIDs) were obtained from one study (Das 2012), three studies reported the number of patients requiring both NSAIDs and opioids (Naja 2003; Pusch 1999; Terheggen 2002), and another study reported the number of participants requiring rescue opioid in the postoperative period (Sundarathiti 2015).
Evidence shows that paravertebral anaesthesia may reduce the need for postoperative analgesia on the first postoperative day compared to general anaesthesia (odds ratio (OR) 0.07, 95% confidence interval (CI) 0.01 to 0.34; 305 participants; low‐certainty evidence; Analysis 1.1). Considerable heterogeneity was noted for this outcome (I² = 70%). When we excluded one study (Sundarathiti 2015), heterogeneity decreased to zero and the difference among participants requiring postoperative analgesics was OR 0.03 (95% CI 0.01 to 0.09), probably because in this study, all participants in both groups were administered fixed doses of postoperative analgesics (synthetic opioid and NSAID) twice a day, and only participants who still had pain were administered rescue analgesia with morphine boluses. This routine administration of analgesics to all participants could have masked the difference in analgesic requirements between groups, notwithstanding the fact that participants underwent more extensive breast surgery with axillary clearance in this study. In the other four included studies, analgesics were administered only when participants expressed that they were in pain.
1.1. Analysis.

Comparison 1: Postoperative outcomes, Outcome 1: Postoperative analgesic requirement
SWiM approach
Data from four of the nine studies used this approach because numerical data were lacking (Megahed 2010), and because outcomes were measured in different ways (i.e. total doses instead of number of participants) (Paleczny 2005; Shkol'nik 2006; Tedore 2011). In addition, two studies described postoperative opioid use (Megahed 2010; Tedore 2011), one reported postoperative NSAID (ketoprofen) use (Paleczny 2005), and another analysed total perioperative opioid (fentanyl) use in the two groups (Shkol'nik 2006). Therefore, vote counting based on direction of effect was performed for data from these studies. Three of the four studies found a decrease in analgesic use in the paravertebral group (Megahed 2010; Paleczny 2005; Shkol'nik 2006), whereas one study found comparable 24‐hour analgesic consumption between the two groups (Tedore 2011), indicating that paravertebral anaesthesia may reduce the postoperative analgesic requirement compared to general anaesthesia. In the SWiM analysis, percentages indicate vote counting based on direction of effect (75%, 95% CI 30% to 95%; P = 0.625; 310 participants; low‐certainty evidence; Figure 4). Due to inconsistency and concerns of risk of bias, we downgraded the certainty of evidence to low.
4.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Evidence from the meta‐analysis and from the SWiM analysis were concordant.
Postoperative nausea and vomiting (PONV)
Nine studies in total reported this outcome, six of which had data suitable for meta‐analysis (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Tedore 2011; Terheggen 2002); three used the SWiM approach (Megahed 2010; Shkol'nik 2006; Sundarathiti 2015).
Meta‐analysis
Data from six studies show the number of participants with vomiting and persistent nausea or retching in the first 24 hours postoperatively that required treatment with antiemetics (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Tedore 2011; Terheggen 2002). Paravertebral anaesthesia probably reduces the incidence of PONV in the first 24 hours post surgery compared to general anaesthesia (OR 0.16, 95% CI 0.08 to 0.30; 324 participants; moderate certainty of evidence; Analysis 1.2). No heterogeneity was observed for this outcome. Five studies had serious risk of bias (selection bias in five studies: Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002; detection bias in two studies: Naja 2003; Paleczny 2005). Only one study had low risk of bias (Tedore 2011). Performance bias was present in all studies, but as the meta‐analysis compared two anaesthetic techniques, this was considered acceptable.
1.2. Analysis.

Comparison 1: Postoperative outcomes, Outcome 2: Postoperative nausea and vomiting
SWiM approach
Data from three studies could not be pooled in meta‐analysis because they were presented narratively; therefore vote counting based on direction of effect was assessed (Megahed 2010; Shkol'nik 2006; Sundarathiti 2015). Data from these studies show that paravertebral anaesthesia probably decreases PONV compared to general anaesthesia (percentages indicate vote counting based on direction of effect (100%, 95% CI 44% to 100%; P = 0.25; 290 participants; moderate‐certainty evidence; Figure 5). All three studies had concerns of selection bias, and two studies also had concerns of detection bias (Megahed 2010; Shkol'nik 2006), downgrading the certainty of evidence to moderate.
5.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Evidence from the meta‐analysis was concordant with evidence from the SWiM analysis.
Hospital stay
Three studies estimated time to discharge from the hospital (Naja 2003; Sundarathiti 2015; Tedore 2011). Sundarathiti 2015 assessed time to readiness for discharge using the Post Anaesthetic Discharge Scoring System (PADSS), but all participants were discharged 2 days after surgery based on a fixed surgical protocol. Analysis of data from these studies revealed that hospital stay was probably shorter in the paravertebral group as compared to the general anaesthesia group (mean difference (MD) ‐79.39 minutes, 95% CI ‐107.38 to ‐51.40; 174 participants; moderate‐certainty evidence; Analysis 1.3). No heterogeneity was observed for this outcome. Two studies had selection bias (Naja 2003; Sundarathiti 2015), and one study had low risk of selection bias (Tedore 2011).
1.3. Analysis.

Comparison 1: Postoperative outcomes, Outcome 3: Hospital stay
Ambulation
Four studies reported this outcome (Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006). Data could not be pooled for meta‐analysis for this outcome, as two studies gave a narrative description of earlier ambulation in the paravertebral group (Naja 2003; Shkol'nik 2006), and two studies described outcomes in diverse ways (Paleczny 2005; Pusch 1999). Paleczny 2005 reported reduced time to standing in the paravertebral block group compared to the general anaesthesia group, and Pusch 1999 reported that a greater number of participants in the general anaesthesia group had painful restricted movement (21/42 participants) compared to none (0/44 participants) in the paravertebral group at 24 hours post surgery. Therefore the SWiM approach with vote counting based on direction of effect was used for this outcome. It was found that paravertebral anaesthesia probably reduced time to ambulation compared to general anaesthesia (percentages indicate vote counting based on direction of effect: 100%, 95% CI 51% to 100%; P = 0.125; 375 participants; moderate‐certainty evidence; Figure 6). Four studies had concerns of selection bias (Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006) and three studies showed uncertainty in detection bias (Naja 2003; Paleczny 2005; Shkol'nik 2006), which led to downgrading of evidence certainty to moderate.
6.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Patient satisfaction
Six studies reported this outcome; three studies had data suitable for meta‐analysis (Das 2012; Tedore 2011; Terheggen 2002), and three had data that could be used for SWiM (Paleczny 2005; Shkol'nik 2006; Sundarathiti 2015).
Meta‐analysis
Analysis of data from three studies revealed that participants receiving paravertebral anaesthesia probably had higher patient satisfaction at 24 hours post surgery compared to participants receiving general anaesthesia (MD 5.52 points, 95% CI 1.30 to 9.75 on a 0 to 100 scale; 129 participants; moderate‐certainty evidence; Analysis 1.4) (Das 2012; Tedore 2011; Terheggen 2002). No heterogeneity was observed for this outcome, and one study had low risk of bias (Tedore 2011), whereas the other two studies had selection bias (Das 2012; Terheggen 2002).
1.4. Analysis.

Comparison 1: Postoperative outcomes, Outcome 4: Patient satisfaction score
SWiM approach
Due to narrative descriptions or missing data, data from three studies were analysed using vote counting based on direction of effect. Two of the three studies reported higher satisfaction in the paravertebral anaesthesia group compared to the general anaesthesia group (percentages indicate vote counting based on direction of effect: 66.7%, 95% CI 20.8% to 94%; P = 1; 299 participants; Figure 7). There were concerns of selection bias in all three studies (Paleczny 2005; Shkol'nik 2006; Sundarathiti 2015), and two studies indicated detection bias (Paleczny 2005; Shkol'nik 2006). Therefore paravertebral anaesthesia may be associated with higher patient satisfaction compared to general anaesthesia (low‐certainty evidence).
7.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Evidence from the meta‐analysis was concordant with evidence from the SWiM analysis. The level of certainty of evidence for the meta‐analysis was moderate, and that for the SwiM analysis was low.
Postoperative pain at rest on the first postoperative day
Nine studies assessed postoperative pain on the first postoperative day (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). Data from one study could not be included because units of analysis were not clearly stated (Shkol'nik 2006). One study involving participants undergoing minor day case breast cancer surgery assessed pain scores up to 2 hours postoperatively or until discharge (Terheggen 2002), another assessed pain scores up to 12 hours postoperatively (Pusch 1999), three assessed pain up to 24 hours (Das 2012; Sundarathiti 2015; Tedore 2011), and another three studies assessed pain up to 3 to 5 days postoperatively (Naja 2003; Paleczny 2005; Shkol'nik 2006).
Pain was assessed at 2 hours, at 6 hours, and at 24 hours at rest on the first postoperative day.
Pain at 2 hours postoperatively
Pain at 2 hours postoperatively was assessed by four studies (Das 2012; Paleczny 2005; Pusch 1999; Terheggen 2002). It was found that the paravertebral technique probably reduced pain compared to general anaesthesia (MD ‐2.95, 95% CI ‐3.37 to ‐2.54 on a 0 to 10 VAS; 224 participants; moderate‐certainty evidence; Analysis 1.5). No heterogeneity was observed for this outcome; all four studies had concerns of selection bias, and one study also had detection bias (Paleczny 2005).
1.5. Analysis.

Comparison 1: Postoperative outcomes, Outcome 5: Postoperative pain (VAS) at 2 hours
Pain at 6 hours postoperatively
Pain at rest at 6 hours postoperatively was assessed by five studies (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Sundarathiti 2015). It was found that use of paravertebral anaesthesia may reduce pain (MD ‐1.54, 95% CI ‐3.20 to 0.11 on a 0 to 10 VAS; 324 participants; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: Postoperative outcomes, Outcome 6: Postoperative pain (VAS) at 6 hours at rest
We noted heterogeneity for this outcome (I² = 96%). On performing sensitivity analysis, we found that two studies contributed to the heterogeneity (Das 2012; Naja 2003), possibly because participants in Das 2012 at 6, 12, and 24 hours postoperatively had comparable VAS scores in the paravertebral and general anaesthesia groups due to higher analgesic consumption in the general anaesthesia group. In contrast, participants in Naja 2003 had low VAS scores in the paravertebral group, perhaps owing to the admixture of clonidine with the local anaesthetic solution used for paravertebral block. Clonidine is a potent alpha‐2 agonist that prolongs the effects of a block. Contrasting VAS scores in these two studies could have contributed to the heterogeneity. On excluding these studies, we found that heterogeneity was reduced to 0 and evidence showed that use of paravertebral anaesthesia may reduce pain (MD ‐1.57, 95% CI ‐2.46 to ‐0.68 on the 0 to 10 VAS).
Four studies showed uncertainty about random sequence generation (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999), three studies about allocation concealment (Paleczny 2005; Pusch 1999; Sundarathiti 2015), and two studies about blinding of the outcome assessor (Naja 2003; Paleczny 2005), which led to downgrading of risk of bias to serious. The certainty of evidence was downgraded to low.
Pain at 24 hours postoperatively
Pain at 24 hours at rest was assessed by five studies (Das 2012; Naja 2003; Paleczny 2005; Sundarathiti 2015; Tedore 2011).
Meta‐analysis
It was found that use of paravertebral anaesthesia may result in reduced pain (MD ‐1.19, 95% CI ‐2.27 to ‐0.10 on a 0 to 10 VAS; 278 participants; low‐certainty evidence; Analysis 1.7). We noted heterogeneity for this outcome (I² = 96%). On performing subgroup analysis and excluding two studies in which a potent adjunctive drug (clonidine) was added to the local anaesthetic solution used for paravertebral anaesthesia (Naja 2003; Paleczny 2005), we found a reduction in heterogeneity. Analysis of data from the remaining three homogenous studies showed that paravertebral anaesthesia may reduce pain (MD ‐0.21, 95% CI ‐0.42 to 0.00; 169 participants) (Das 2012; Sundarathiti 2015; Tedore 2011).
1.7. Analysis.

Comparison 1: Postoperative outcomes, Outcome 7: Postoperative pain (VAS) at 24 hours at rest
Of studies included for this outcome, only one had low risk of bias (Tedore 2011), whereas two others showed uncertainty in selection bias (Das 2012; Sundarathiti 2015); the remaining two studies showed uncertainty in two domains (Naja 2003; Paleczny 2005).
SWiM approach
Two studies provided evidence that paravertebral block probably reduces postoperative pain at 24 hours at rest compared to general anaesthesia (percentages indicate vote counting based on direction of effect: 100%, 95% CI 34.24% to 100%; P = 0.5; 220 participants; Figure 8) (Megahed 2010; Shkol'nik 2006). Both studies had uncertainty in selection bias and in detection bias. The certainty of evidence was downgraded to moderate.
8.

a = Random sequence generation (selection bias), b = Concealment of the allocated sequence (selection bias), c = Blinding of participants and personnel (performance bias), d = Blinding of outcome assessment (detection bias), e = Incomplete outcome data (attrition bias), f = Selective outcome reporting (reporting bias), g = Other potential sources of bias.
Evidence from the meta‐analysis was concordant with evidence from the SwiM analysis. The certainty of evidence for the meta‐analysis was low, and that for the SWiM analysis was moderate.
Postoperative pain on movement on the first postoperative day
We assessed pain on movement at 2, 6, and 24 hours on the first postoperative day.
Pain on movement at 2 hours
None of the included studies reported this outcome at this time point.
Pain on movement at 6 hours
Pain on movement at 6 hours was assessed by two studies (Naja 2003; Sundarathiti 2015). It was found that paravertebral anaesthesia may reduce pain on movement at 6 hours (MD‐2.57, 95% CI ‐3.97 to ‐1.17; 130 participants; low‐certainty evidence; Analysis 1.8). Considerable heterogeneity was noted for this outcome (I² = 96%). Sensitivity analysis could not be performed, but heterogeneity could be attributed to methodological differences. One study included multiple‐level paravertebral block (Naja 2003), whereas the other study involved inserting a paravertebral catheter at one level (fourth thoracic vertebral level), advancing it 8 cm into the space, injecting local anaesthetic at that depth, and withdrawing the catheter by 2 cm twice, so as to administer local anaesthetic drug at multiple levels (single injection, multi‐level block) (Sundarathiti 2015). In addition, a lower VAS score may have been observed in one study (Naja 2003), as the potent alpha‐2 agonist clonidine was added to the local anaesthetic solution. This is known to prolong the duration of analgesia produced by a regional anaesthesia block.
1.8. Analysis.

Comparison 1: Postoperative outcomes, Outcome 8: Postoperative pain (VAS) at 6 hours on movement
In both studies, there were concerns regarding selection bias, and one study had concerns of detection bias (Naja 2003); therefore the certainty of evidence was downgraded to low.
Pain on movement at 24 hours
Pain on movement at 24 hours was assessed by two studies (Naja 2003; Sundarathiti 2015), which showed that pain in the paravertebral group may be reduced compared to pain in the general anaesthesia group (MD ‐2.12, 95% CI ‐4.80 to 0.55 on a 0 to 10 VAS; 130 participants; low‐certainty evidence; Analysis 1.9). Considerable heterogeneity was noted for this outcome (I² = 96%). Sensitivity analysis could not be performed, but heterogeneity may be due to methodological differences in the two studies, as explained above.
1.9. Analysis.

Comparison 1: Postoperative outcomes, Outcome 9: Postoperative pain (VAS) at 24 hours on movement
Uncertainty about random sequence generation and blinding of outcome assessors in Naja 2003 and allocation concealment in Sundarathiti 2015 led to downgrading of risk of bias to serious, and the certainty of evidence to low.
Shkol'nik 2006 indicated that paravertebral block probably reduces pain on movement at 24 postoperative hours.
Mortality related to either anaesthetic technique
This outcome was not reported by any of the included studies.
Adverse events due to paravertebral block
Eight studies comprising 574 participants evaluated adverse outcomes due to paravertebral anaesthesia compared to general anaesthesia (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002).
Epidural spread and bilateral block
All eight studies evaluated the incidence of bilateral block indicative of epidural spread of local anaesthetic administered in the paravertebral space (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). Two of 290 participants who received the paravertebral block developed a bilateral block, indicating an incidence of 0.7% (Analysis 2.1). One of these participants developed mild breathlessness and hypotension lasting for five minutes that responded to 500 mL fluid bolus administration. This participant was subsequently administered general anaesthesia on request (Terheggen 2002). The second participant developed epidural spread with paraparesis lasting 280 minutes and Horner's syndrome lasting 170 minutes, which resolved spontaneously (Pusch 1999). In Shkol'nik 2006, two patients developed hypotension without epidural spread or bilateral block, which study authors attributed to use of two‐injection paravertebral technique with large volumes of local anaesthetics (10 to 15 mL) at each level. Study authors subsequently changed to using multi‐level paravertebral technique with 4 to 5 mL local anaesthetic at each level and found no hypotension.
2.1. Analysis.

Comparison 2: Adverse events, Outcome 1: Epidural spread
A pooled estimate of this adverse event was not possible; this adverse event can be observed only with a regional anaesthesia technique involving injections in the proximity of the vertebral canal and is not seen with general anaesthesia.
As there was uncertainty about random sequence generation in five studies (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), allocation concealment in four studies (Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015), and blinding of outcome assessors in three studies (Naja 2003; Paleczny 2005; Shkol'nik 2006), the certainty of evidence was downgraded to moderate.
Horner's syndrome
Six studies comprising 475 participants assessed the incidence of Horner's syndrome following paravertebral anaesthesia (Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Tedore 2011; Terheggen 2002). Seventeen of the total 240 participants developed this complication, indicating an incidence of 7.1%. Eleven participants had received multiple‐level paravertebral blocks from cervical seventh to thoracic sixth vertebral level (Shkol'nik 2006), and six participants received a single‐level paravertebral block at the thoracic 3/4 vertebral level (Paleczny 2005; Pusch 1999). A pooled estimate of this adverse event was not possible, as this complication is possible only with a regional anaesthesia technique involving injections in the proximity of the upper thoracic vertebrae. Therefore it occurred in the paravertebral group ‐ not in the general anaesthesia group.
As there was uncertainty about random sequence generation in four studies (Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), about allocation concealment in three studies (Paleczny 2005; Pusch 1999; Shkol'nik 2006), and about blinding of outcome assessors in three studies (Naja 2003; Paleczny 2005; Shkol'nik 2006), the certainty of evidence was downgraded to moderate.
Bleeding
Eight studies comprising 574 participants evaluated the incidence of bleeding following paravertebral block (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). Four of the 290 participants in the paravertebral group developed minor bleeding during paravertebral block, indicating an incidence of 1.4%. No participants needed interventions/transfusion for minor bleeding. A pooled estimate of this adverse event was not possible, as this complication is possible only with a regional anaesthesia technique involving injections. Therefore it is observed only in the paravertebral group ‐ not in the general anaesthesia group.
Amongst the eight included studies, seven studies had selection bias (five studies had uncertainty in random sequence generation ‐ Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002 ‐ and four studies in allocation concealment ‐ Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015). Three studies had uncertainty in blinding of outcome assessors (Naja 2003; Paleczny 2005; Shkol'nik 2006). This led to downgrading of risk of bias to serious and certainty of evidence to moderate.
Pleural tap
Eight studies comprising 574 participants evaluated the incidence of pleural tap with the paravertebral anaesthetic technique (Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Tedore 2011; Terheggen 2002). One of the 290 participants in the paravertebral group developed this complication, indicating an incidence of 0.3% for this complication with the paravertebral technique. The participant was kept in the post‐anaesthesia care unit (PACU) for 195 minutes before discharge to home. No radiological features of pneumothorax or respiratory difficulty developed during 2 days of follow‐up. No patient in the general anaesthesia group developed this complication, which is possible only with a regional anaesthesia technique involving injections in the proximity of the pleura.
There were concerns of selection bias in seven studies ‐ Das 2012; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Terheggen 2002 ‐ and detection bias in three studies (Naja 2003; Paleczny 2005; Shkol'nik 2006). This led to downgrading of risk of bias to serious and certainty of evidence to moderate.
Disease‐free survival (often defined as time to first detection of local recurrence or distant metastasis, or progression‐free survival, time to progression, or time to treatment failure)
This outcome was not reported by any of the included studies.
Chronic pain that persists beyond the immediate postoperative period (i.e. at 3 to 12 months)
This outcome was not reported by any of the included studies.
Quality of life at 1 to 2 weeks and at 3 to 12 months postoperatively assessed by a validated questionnaire
This outcome was not reported by any of the included studies.
Postoperative surgical considerations
Technical difficulties with paravertebral block included multiple needle passes after single skin pricks in six patients; another patient described moderate pain at the time of the block, requiring supplementary doses of fentanyl and local anaesthetic (Das 2012). Sundarathiti 2015 described difficulty in catheter insertion in 20% of included participants.
In two cases of paravertebral block failure ‐ one case each in Das 2012 and Paleczny 2005 ‐ both cases needed conversion to general anaesthesia for surgery.
Haemodynamic parameters were found to be comparable between groups in four studies (Das 2012; Naja 2003; Pusch 1999; Sundarathiti 2015). Three studies reported lower, more stable haemodynamic parameters in the paravertebral group than in the general anaesthesia group (Megahed 2010; Paleczny 2005; Shkol'nik 2006). Shkol'nik 2006 reported a decrease in ventricular extrasystoles and an improved ejection fraction in the paravertebral group. In addition, one of these studies ‐ Shkol'nik 2006 ‐ found reduced plasma cortisol levels, and another ‐ Megahed 2010 ‐ found reduced soluble P‐selectin levels in the paravertebral group compared to the general anaesthesia group.
Paleczny 2005 reported one participant developing laryngeal spasm after extubation in the general anaesthesia group. Sundarathiti 2015 found that 54.3% of participants in the general anaesthesia group developed sore throat after tracheal intubation.
Discussion
Summary of main results
Paravertebral anaesthesia probably reduces postoperative nausea and vomiting (PONV), hospital stay, and time to ambulation, resulting in greater patient satisfaction on the first postoperative day compared to general anaesthesia. Paravertebral anaesthesia may also reduce postoperative analgesic use. However, randomised controlled trials (RCTs) using validated questionnaires are needed to confirm these results.
Paravertebral anaesthesia probably reduces postoperative pain at 2 hours, and it may reduce pain at rest and on movement at 6 and 24 hours, postoperatively. No comment can be made regarding mortality associated with the anaesthetic technique.
Adverse events observed with paravertebral anaesthesia are rare. None of the other secondary outcomes, such as disease‐free survival, chronic pain persisting from 3 to 12 months, or quality of life described on a validated questionnaire, were assessed by any of the included studies.
Overall completeness and applicability of evidence
The study population included adult participants from Europe, Asia, Africa, and North America who underwent different types of breast cancer surgery under all techniques of paravertebral block compared to general anaesthesia.
Participants in eight RCTs were administered sedation while paravertebral block was performed. Skin infiltration with local anaesthetic and pre‐medication before any block are standard practices to decrease patient discomfort during the block procedure; they are usually administered after monitoring of vitals and when oxygen supplementation has commenced. Usually multiple‐level blocks are thought to provide more reliable surgical anaesthesia, but in two studies (Paleczny 2005; Pusch 1999), the study authors administered a greater volume of local anaesthetic (0.3 mL/kg) as a single injection at the thoracic fourth vertebral level.
In this current review, it was not possible to ascertain which technique of paravertebral block was more effective (i.e. single‐injection, multiple‐injection, or catheter‐based techniques), as only two block failures occurred (two studies each reported one block failure ‐ Das 2012; Paleczny 2005). One RCT included a single‐level paravertebral injection (Paleczny 2005), and the other a multiple‐injection technique (Das 2012). Both cases required conversion to general anaesthesia. None of the catheter‐related paravertebral blocks resulted in block failure. A failure rate ranging from 10.7% to 23.7% has been reported in the literature (Lonnqvist 1995; Najarian 2003). In this current review, failure rate and need for supplementation were low.
None of the included studies reported mortality following either anaesthetic technique, possibly because most of the included RCTs were small and mortality related to general anaesthesia for surgical patients is rare (estimated as 8.2 deaths per million hospital surgical discharges, 95% confidence interval (CI) 7.4 to 9.0). Specifically for women, it has been estimated as 6.2 deaths per million hospital surgical discharges (95% CI 5.5 to 7.0; Li 2009). In the medical literature, we found no report of mortality attributed to paravertebral anaesthesia.
As none of the studies assessed important long‐term outcomes, such as mortality, disease‐free survival, chronic pain, or quality of life following breast cancer surgery, additional large multi‐centre trials are needed.
Quality of the evidence
We selected RCTs including participants undergoing paravertebral or general anaesthesia for breast cancer surgery. As this included comparison of two techniques of providing anaesthesia (i.e. regional anaesthesia compared to general anaesthesia), blinding of participants or personnel was not possible; therefore all studies had high risk of performance bias. However as this was the main question of the review, it was considered acceptable after discussion with all review authors. Tedore 2011 had low risk of bias in all other domains. Among the remaining eight studies (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015; Terheggen 2002), all had uncertainty in selection bias. Three of the nine studies reported on the method of randomisation (Shkol'nik 2006; Sundarathiti 2015; Tedore 2011), and four studies clearly stated the method of allocation concealment (Das 2012; Naja 2003; Tedore 2011;Terheggen 2002). In addition, low detection bias or blinding of outcome assessors was clearly stated in five studies (Das 2012; Pusch 1999; Sundarathiti 2015; Tedore 2011; Terheggen 2002).
We used the GRADE approach to estimate the certainty of evidence for all outcomes studied. We found the certainty of evidence for outcomes of PONV and pain at rest at 2 hours to be of moderate certainty, primarily due to risk of bias (selection and/or detection bias). We found the certainty of evidence for postoperative analgesic use and pain at rest at 24 hours and pain on movement at 6 and 24 hours to be of low certainty. This was due primarily to risk of bias (selection, detection) and heterogeneity between studies due to methodological differences.
Data used for synthesis without meta‐analysis (SWiM) for quality of recovery outcomes of postoperative analgesic use and patient satisfaction were also downgraded to low certainty because of serious risk of bias and inconsistent results (Murad 2017). However, for other quality of recovery outcomes, namely, PONV and ambulation, descriptive data were downgraded by one level to moderate certainty because of risk of bias, as included studies consistently favoured the intervention group on vote counting and results showed no inconsistency.
Similarly, unpooled data on adverse events (epidural spread, Horner's syndrome, pleural tap, and bleeding) were downgraded to moderate certainty because of risk of bias. Therefore, we are uncertain about the estimates.
Potential biases in the review process
In an attempt to minimise bias, we followed the guidelines recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Eligibility for inclusion and exclusion and risk of bias of studies were assessed by two review authors independently.
Selection bias was observed in six studies due to uncertainty in random sequence generation (Das 2012; Megahed 2010; Naja 2003; Paleczny 2005; Pusch 1999; Terheggen 2002), and in five studies due to allocation concealment (Megahed 2010; Paleczny 2005; Pusch 1999; Shkol'nik 2006; Sundarathiti 2015). Therefore except for Tedore 2011, all studies had uncertain selection bias. Performance bias was high in all studies, as participants in one group received paravertebral anaesthesia for breast cancer surgery, and those in the other group received general anaesthesia; therefore blinding of participants or personnel was not possible. As this comparison was the main question of the review, this bias was considered acceptable by all review authors. In addition, in four studies, uncertainty in blinding of outcome assessors was detected (Megahed 2010; Naja 2003; Paleczny 2005; Shkol'nik 2006). No attrition, reporting, or other bias was noted in any of the studies.
Agreements and disagreements with other studies or reviews
Several systematic reviews have assessed the use of paravertebral block during surgery, but these reviews included mixed populations (i.e. not specifically women with breast cancer) and non‐randomised, retrospective, and case series studies (Schnabel 2010; Tahiri 2011; Wu 2015). These reviews included studies that compared paravertebral block and general anaesthesia to conventional general anaesthesia alone (Schnabel 2010; Tahiri 2011; Wu 2015). Similar to our findings, these two other meta‐analyses found improvement in parameters that determined the quality of recovery (Schnabel 2010; Tahiri 2011). Schnabel 2010 reported reduced requirement for postoperative analgesia and reduced incidence of PONV in the paravertebral group compared to the general anaesthesia group. Tahiri 2011 found lesser incidence of PONV, decreased hospital length of stay, and greater patient satisfaction with paravertebral anaesthesia.
Two other systematic reviews studied all modalities of analgesia for breast cancer surgery, including different agents and regional anaesthesia techniques (paravertebral block included) (Cheng 2017; Jacobs 2020). They included RCTs and series (Cheng 2017), or RCTs and meta‐analyses (Jacobs 2020), as well as participants who received general anaesthesia with paravertebral block in the intervention arm. They too found that paravertebral block as a single injection or as a continuous infusion was associated with reduced acute postoperative pain, need for opioids, and occurrence of postoperative nausea and vomiting when compared with opioid‐based analgesics alone. Another systematic review found a marginal decrease in hospital stay in the paravertebral anaesthesia group, which review authors stated was not clinically relevant (standardised mean difference ‐0.60 hour, 95% CI ‐1.13 to ‐0.06; P = 0.028) and with significant heterogeneity (I² = 94.37%) (Terkawi 2015). These findings are consistent with the findings of our review, but we found no heterogeneity. In general, hospital stay is affected by the extent of surgery and hospital policy following breast cancer surgery. In one of the included studies in this review (Sundarathiti 2015), participants were kept in hospital for 2 days as per hospital policy despite fulfilling criteria for discharge earlier (this time was taken into account in the analysis). Two other studies had shorter hospital stay in the paravertebral group, which they surmised could contribute to considerable cost savings (Naja 2003; Tedore 2011).
Adverse events due to paravertebral block were reported in two meta‐analyses (Schnabel 2010; Tahiri 2011). Similar to our findings, Schnabel 2010 and Tahiri 2011 reported a very low incidence of pleural tap: Schnabel 2010 reported a risk ratio (RR) of 0.01 (95% CI 0.02 to 0.03) for pleural tap; Tahiri 2011 reported two cases of pneumothorax in 11 included studies comprising 618 mixed population participants and retrospective data. A low incidence of Horner's syndrome was reported by Schnabel 2010 (RR 0.05; 95% CI 0.02 to 0.09) in contrast to 7.1% (17/240 participants) incidence estimated in this meta‐analysis. We estimated the incidence of epidural spread to be 2 of 290 (0.7%), whereas Tahiri 2011 reported the most common adverse events to be hypotension/bradycardia (n = 12) followed by epidural spread (n = 5) among the 618 mixed participant data analysed. However in both meta‐analyses, there were no long‐term sequelae of reported adverse events.
The overall complication rate reported with paravertebral block has been reported as hypotension 4.6%, vascular puncture 3.8%, pleural puncture 1.1%, pneumothorax 0.5%, and a block failure rate of 10.1% (Lonnqvist 1995). The complication rate in the present review was lower than that reported in the study for vascular puncture, hypotension, and pleural tap, probably because the study included participants of all age groups (adults and children) undergoing all types of surgery (thoracic, abdominal, lower limb, and chronic pain procedures), whereas the present review included RCTs administering paravertebral block for breast cancer surgery to adult women alone. As breast cancer surgery requiring axillary clearance requires a higher‐level paravertebral block (C7/T1), this is more likely to block the sympathetic ganglia at the T1 level, resulting in Horner's syndrome, compared to the lower thoracic or lumbar level for other surgeries.
A recent large multi‐centre RCT including 2132 participants (women aged < 85 years undergoing breast cancer surgery) assessed the incidence of breast cancer recurrence following surgery under paravertebral analgesia. This study did not fulfil our inclusion criteria, as the intervention group received general anaesthesia (total intravenous anaesthesia with propofol) in addition to paravertebral block. This study found that the incidence of cancer recurrence was comparable between the paravertebral combined with general anaesthesia group and the general anaesthesia group at 36 months' follow‐up (10% of participants in either group). The incidence of chronic pain at 6 months and at 12 months (52%; 27% to 28% of participants, respectively) and the incidence of neuropathic pain at the same time points (10% and 7% of participants, respectively) were also comparable between groups (Sessler 2019). This study had several limitations: in 17% of participants in the paravertebral group, block was performed under sevoflurane anaesthesia. Sevoflurane has been shown in an vitro study to be associated with depressed natural killer cell activity (Buckley 2014), as well as higher mortality in cancer patients (Wigmore 2016). In addition, morphine, a drug known to promote angiogenesis and tumour cell recurrence in rats, was used as the primary postoperative analgesic (Sessler 2019). Sixty per cent of participants in this RCT were from the same country. Therefore, results of this RCT cannot be used to ascertain effects of paravertebral anaesthesia on breast cancer recurrence and other long‐term outcomes, which was the goal of this review.
Authors' conclusions
Implications for practice.
No study assessed quality of recovery in the first 1 to 3 days following surgery using a validated questionnaire. However, studies did show that paravertebral anaesthesia at 24 hours post surgery probably decreased PONV, reduced hospital stay, was associated with faster time to ambulation, and resulted in higher patient satisfaction compared to general anaesthesia. Analgesic use may also be decreased with this technique.
The paravertebral technique was probably associated with reduced pain up to 2 hours postoperatively as compared to general anaesthesia. Paravertebral anaesthesia may also reduce pain at rest at 6 and 24 hours postoperatively. These effects were more marked in studies that used the alpha‐2 agonist drug clonidine as an additive to local anaesthetics for the block. Paravertebral anaesthesia may also reduce pain on movement at 6 and 24 hours postoperatively, but as these outcomes were assessed by a limited number of studies, more large multi‐centre studies of uniform design are needed to ascertain these outcomes.
All adverse effects that occurred with the paravertebral technique, such as Horner’s syndrome, epidural spread, bloody tap, or pleural injection, were self‐limited and did not require any treatment. However, for participants undergoing limited day case procedures, these side effects may cause anxiety and may prolong hospital stay. Therefore, paravertebral anaesthesia may be preferred for more extensive breast surgeries, such as simple, modified radical, or radical mastectomy with or without axillary dissection.
No study assessed mortality related to the anaesthetic technique, disease‐free survival, chronic pain at 3 to 12 months postoperatively, or quality of life at 1 to 2 weeks and at 3 to 12 months postoperatively. Therefore, no comments can be made in relation to these outcomes.
Implications for research.
Many studies compared paravertebral analgesia combined with general anaesthesia to general anaesthesia alone rather than comparing paravertebral anaesthesia alone to general anaesthesia alone. Therefore a limited number of studies could be included in this review.
In addition, studies that compared paravertebral anaesthesia to general anaesthesia had methodological differences. Some studies followed patients for only 2 hours or until discharge, whereas others followed them for 5 to 6 days. There were differences in the procedures performed as well; some assessed the effect of paravertebral block for minor breast surgery without axillary clearance, and others assessed the effect of the block on simple, modified, or radical mastectomy with axillary clearance. There were differences in the blocks performed (i.e. multiple‐level or single‐level with or without catheters). Many studies did not assess pain on movement in these patients for the first 24 hours, although this may be an important outcome that may show superiority of the paravertebral technique over general anaesthesia.
Many important outcomes, such as disease‐free survival, mortality, chronic pain, and quality of life, were not assessed by any of the included studies. Therefore further research in the form of large multi‐centre RCTs of uniform design is needed to assess the effects of paravertebral block on acute postoperative pain at rest and on movement on the first postoperative day, the quality of recovery using validated questionnaires on the first to third postoperative days, mortality with either technique, disease‐free survival, the incidence of chronic pain, and quality of life as described by a validated questionnaire.
History
Protocol first published: Issue 2, 2018 Review first published: Issue 2, 2021
Acknowledgements
We would like to thank Melina Willson (Managing Editor) for her constant, tireless help right from title registration to protocol development; Nicholas Wilcken and Annabel Goodwin (Co‐ordinating Editors) for their help in improving the manuscript; and Ava Grace Tan‐Koay/Slavica Berber for assistance in developing a search strategy. We would also like to acknowledge our peer reviewers ‐ Sam Egger (Statistical Editor), Matthew Rucklidge (Expert Clinical Reviewer), Sara Yaron (Consumer), and Dana Stewart (Consumer) ‐ who assisted during preparation of the protocol for this systematic review.
We would like to acknowledge our peer reviewers: Theresa Moore (Cochrane Methodological Editor), Laurence Gluch (Expert Clinical Reviewer), and Dana Stewart (Consumer) for their help during preparation of this review.
We would like to thank Tashtanbekova Cholpon Bolotbekovna, Junior Researcher Scientist, Educational‐Scientific Center Evidence‐Based Medicine Cochrane Russia, Institute of Medicine and Biology, at Kazan Federal University, for help in translating the Shkol'nik 2006 study from the Russian language to the English language for the purpose of this review.
We would like to thank Paulina Sitarek, Junior Associate with Rasiewicz Oleksyn & Associates, Warsaw, Mazowieckie, Poland, for confirming translation of the Paleczny 2005 study from the Polish language to the English language. Initial translation was done using Google Translate software.
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 breast near cancer* #3 breast near neoplasm* #4 breast near carcinom* #5 breast near tumour* #6 breast near tumor* #7 breast near malignan* #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Anesthesia, Conduction] explode all trees #10 MeSH descriptor: [Anesthesia, Local] explode all trees #11 regional and anesthesia #12 regional and anaesthesia #13 regional and analgesia #14 local and anesthesia #15 local and anaesthesia #16 local and analgesia #17 paravertebral and anesthesia #18 paravertebral and anaesthesia #19 paravertebral and analgesia #20 conduction and anesthesia #21 conduction and anaesthesia #22 MeSH descriptor: [Nerve Block] explode all trees #23 paravertebral and block #24 nerve block #25 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 #26 #8 and #25
Appendix 2. MEDLINE
| 1 | randomised controlled trial.pt. |
| 2 | controlled clinical trial.pt. |
| 3 | randomized.ab. |
| 4 | placebo.ab. |
| 5 | Clinical Trials as Topic/ |
| 6 | randomly.ab. |
| 7 | trial.ti. |
| 8 | (crossover or cross‐over).tw. |
| 9 | Pragmatic Clinical Trials as Topic/ |
| 10 | pragmatic clinical trial.pt. |
| 11 | or/1‐10 |
| 12 | exp Breast Neoplasms/ |
| 13 | (breast adj6 cancer$).tw. |
| 14 | (breast adj6 neoplasm$).tw. |
| 15 | (breast adj6 carcinoma$).tw. |
| 16 | (breast adj6 tumo?r$).tw. |
| 17 | or/12‐16 |
| 18 | exp Anesthesia, Conduction/ |
| 19 | exp Anesthesia, Local/ |
| 20 | ((regional or local or conduction) adj6 (an?esth* or analges*)).tw. |
| 21 | (paravertebral* adj6 (an?esth* or analges*)).tw. |
| 22 | exp Nerve Block/ |
| 23 | ((paravertebral* or nerve) adj6 block*).tw. |
| 24 | or/18‐23 |
| 25 | 11 and 17 and 24 |
| 26 | animals/ not (humans/ and animals/) |
| 27 | 25 not 26 |
Appendix 3. Embase
| Searches | |
| 1 | Randomized controlled trial/ |
| 2 | Controlled clinical study/ |
| 3 | Random$.ti,ab. |
| 4 | randomization/ |
| 5 | intermethod comparison/ |
| 6 | placebo.ti,ab. |
| 7 | (compare or compared or comparison).ti. |
| 8 | (open adj label).ti,ab. |
| 9 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. |
| 10 | double blind procedure/ |
| 11 | parallel group$1.ti,ab. |
| 12 | (crossover or cross over).ti,ab. |
| 13 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. |
| 14 | (assigned or allocated).ti,ab. |
| 15 | (controlled adj7 (study or design or trial)).ti,ab. |
| 16 | (volunteer or volunteers).ti,ab. |
| 17 | trial.ti. |
| 18 | or/1‐17 |
| 19 | exp breast/ |
| 20 | exp breast disease/ |
| 21 | (19 or 20) and exp neoplasm/ |
| 22 | exp breast tumor/ |
| 23 | exp breast cancer/ |
| 24 | exp breast carcinoma/ |
| 25 | (breast$ adj5 (neoplas$ or cancer$ or carcin$ or tumo$ or metasta$ or malig$)).ti,ab. |
| 26 | or/21‐25 |
| 27 | exp regional anesthesia/ |
| 28 | exp local anesthesia/ |
| 29 | ((regional or local) adj6 (an?esth* or analges*)).tw. |
| 30 | (paravertebral* adj6 (an?esth* or analges*)).tw. |
| 31 | (conduction adj6 anesth*).tw. |
| 32 | exp nerve block/ |
| 33 | ((paravertebral* or nerve) adj6 block*).tw. |
| 34 | or/27‐33 |
| 35 | 18 and 26 and 34 |
| 36 | limit 35 to (human and (conference abstracts or embase)) |
Appendix 4. WHO ICTRP
Basic search:
1. Breast cancer AND paravertebral anaesthesia 2. Breast cancer AND regional anaesthesia 3. Breast cancer AND local anaesthesia 4. Breast surgery AND paravertebral anaesthesia 5. Breast surgery AND regional anaesthesia 6. Breast surgery AND local anaesthesia
Advanced search:
1. Condition: Breast cancer or breast neoplasm or breast surgery Intervention: Paravertebral anaesthesia or regional anaesthesia or local anaesthesia or conduction anaesthesia or nerve block or paravertebral block Recruitment status: All
2. Condition: Breast cancer or breast neoplasm or breast surgery Intervention: paravertebral analgesia or regional analgesia or local analgesia or conduction analgesia Recruitment status: All
Appendix 5. ClinicalTrials.gov
Basic search:
1. Condition or disease: Breast cancer Other terms: paravertebral anaesthesia
2. Condition or disease: Breast cancer Other terms: regional anaesthesia
3. Condition or disease: Breast cancer Other terms: local anaesthesia
4. Condition or disease: Breast surgery Other terms: paravertebral anaesthesia
5. Condition or disease: Breast surgery Other terms: regional anaesthesia
6. Condition or disease: Breast surgery Other terms: local anaesthesia
Advanced search:
1. Condition or disease: Breast cancer OR breast neoplasm OR breast surgery Intervention: Paravertebral anaesthesia OR regional anaesthesia OR local anaesthesia OR conduction anaesthesia Study type: All studies Study results: All studies
2. Condition or disease: Breast cancer OR breast neoplasm OR breast surgery Intervention: Paravertebral analgesia OR regional analgesia OR local analgesia OR conduction analgesia Study type: All studies Study results: All studies
3. Condition or disease: Breast cancer OR breast neoplasm OR breast surgery Intervention: Nerve block OR paravertebral block Study type: All studies Study results: All studies
Data and analyses
Comparison 1. Postoperative outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Postoperative analgesic requirement | 5 | 305 | Odds Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.34] |
| 1.2 Postoperative nausea and vomiting | 6 | 324 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.08, 0.31] |
| 1.3 Hospital stay | 3 | 174 | Mean Difference (IV, Random, 95% CI) | ‐79.39 [‐107.38, ‐51.40] |
| 1.4 Patient satisfaction score | 3 | 129 | Mean Difference (IV, Fixed, 95% CI) | 5.52 [1.30, 9.75] |
| 1.5 Postoperative pain (VAS) at 2 hours | 4 | 224 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐3.37, ‐2.54] |
| 1.6 Postoperative pain (VAS) at 6 hours at rest | 5 | 324 | Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐3.20, 0.11] |
| 1.7 Postoperative pain (VAS) at 24 hours at rest | 5 | 278 | Mean Difference (IV, Random, 95% CI) | ‐1.19 [‐2.27, ‐0.10] |
| 1.8 Postoperative pain (VAS) at 6 hours on movement | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐2.57 [‐3.97, ‐1.17] |
| 1.9 Postoperative pain (VAS) at 24 hours on movement | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐4.80, 0.55] |
Comparison 2. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Epidural spread | 8 | 574 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Das 2012.
| Study characteristics | ||
| Methods | Prospective, observer‐blinded, parallel‐arm randomised controlled trial (RCT) wherein 1 arm received conventional general anaesthesia (GA) and the other arm received multi‐injection thoracic paravertebral (PVB) anaesthesia for patients undergoing breast cancer surgery | |
| Participants | 60, ASA physical status I and II, 18 to 65 years old, female; patients scheduled to undergo unilateral breast surgery with or without axillary clearance were enrolled in the study | |
| Interventions |
PVB group (n = 29)
GA group (n = 30)
|
|
| Outcomes | Primary outcome • Comparison of the efficacy of thoracic PVB block to GA for breast surgery with duration of postoperative pain relief the primary endpoint (time from last suture to first analgesic administered when VAS ≥ 4) Secondary outcomes • Total dose of analgesic (tramadol and diclofenac sodium) consumed in the first 24 hours • Number of patients experiencing PONV • Any complications • Quality of anaesthesia judged by surgeon • Satisfaction with anaesthetic technique judged by patient |
|
| Notes | No external funding received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation has not been specified |
| Allocation concealment (selection bias) | Low risk | Patients were allocated using the sealed envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible because of the nature of interventions (1 group got GA and the other got PVB) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | VAS was assessed by a resident not involved in the study. Patient anaesthesia records were not made available to the resident for the first 24 hours |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 patient in the PVB group was excluded because of inadequate block that necessitated conversion to GA |
| Selective reporting (reporting bias) | Low risk | Complications were looked for and reported |
| Other bias | Low risk | No other risk of bias was apparent |
Megahed 2010.
| Study characteristics | ||
| Methods | Prospective, parallel‐arm, randomised controlled trial (RCT) where 1 arm received thoracic paravertebral block (PVB) with sedation and the other arm received general anaesthesia (GA) for patients undergoing breast cancer surgery | |
| Participants | 40 females with type 2 diabetes mellitus were included in this study; 20 patients were randomised to each group | |
| Interventions |
PVB group (n = 20)
GA group (n = 20)
|
|
| Outcomes | Primary outcome • Evaluation of preoperative, intraoperative (15 minutes after skin incision), and postoperative (1 hour) plasma levels of sP‐selectin as a predictor of thrombotic events in diabetic patients undergoing breast cancer surgery with either technique of anaesthesia Secondary outcomes • Plasma creatine kinase (CK) and CK‐MB levels preoperatively and at 6 hours postoperatively • Time to first request for analgesic • Total analgesic consumed in first 24 hours • Postoperative complications |
|
| Notes | Study fulfils the inclusion criteria but has studied only patients with type 2 diabetes Pain scores have not been evaluated in the study No external funding was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were randomly allocated to 2 groups; however the exact method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment has not been described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible due to the nature of interventions (1 group received PVB and the other received GA) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details regarding blinding were not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all recruited patients were analysed |
| Selective reporting (reporting bias) | Low risk | Complications of both techniques were looked for |
| Other bias | Low risk | Other risk of bias was not apparent |
Naja 2003.
| Study characteristics | ||
| Methods | Prospective, parallel‐arm RCT where 1 arm received nerve stimulator‐guided PVB with and without sedation and the other arm received GA for patients undergoing breast cancer surgery | |
| Participants | 60 patients, with 30 patients in each group scheduled to undergo breast cancer surgery | |
| Interventions |
PVB group (n = 30)
GA group (n = 30)
|
|
| Outcomes | Primary outcome • Postoperative pain assessed using ‐ visual analogue scale (VAS) 0 to 100 mm; at rest and on movement at 6, 12, 24, 36 hours, and on 2nd, 3rd, 4th, and 5th days ‐ postoperative consumption of supplemental analgesics Secondary outcomes • PONV‐free patients • Duration of hospital stay • Patient and surgeon satisfaction (only in PVB group) |
|
| Notes | VAS values mentioned in the study were given as median and range. We tried to contact study authors multiple times to obtain the mean and SD values of VAS, as well as additional information; however we received no response Median and range values were used to derive mean and SD values based on equations mentioned in Hozo 2005 No external funding was received Hospital stay was expressed as mean ± SD in days; these data were converted into minutes by multiplying with 1440. This was done to enable the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation has not been specified |
| Allocation concealment (selection bias) | Low risk | Patients were allocated using the sealed envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible because of the nature of interventions (1 group got GA and the other got PVB) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details regarding who collected outcome data were not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 60 patients were included in the study, and outcome data were recorded from all 60 patients |
| Selective reporting (reporting bias) | Low risk | Both benefits and complications were reported |
| Other bias | Low risk | No other risk of bias was apparent |
Paleczny 2005.
| Study characteristics | ||
| Methods | Prospective, parallel‐arm RCT where 1 arm received GA and the other arm received thoracic PVB with sedation for patients undergoing breast cancer surgery | |
| Participants | 50 total, with 25 patients in each group; > 18 years of age, scheduled to undergo breast cancer surgery | |
| Interventions |
PVB group (n = 24)
GA group (n = 25)
|
|
| Outcomes | Compare effects of 2 different techniques (GA vs PVB with sedation) in patients undergoing Patey's mastectomy Pain was assessed using a 0 to 10 numerical pain rating scale at 60 minutes, 6, 24, 48, and 72 hours after surgery |
|
| Notes | Study authors were contacted multiple times for information regarding other outcomes but we received no response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors stated that patients were randomly divided to 2 equal groups; however details of the method of randomisation were not described |
| Allocation concealment (selection bias) | Unclear risk | This was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible as 1 group got GA and the other got PVB |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details regarding who collected outcome data were not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total of 50 patients were included; data for 49 patients were collected as 1 block was unsuccessful |
| Selective reporting (reporting bias) | Low risk | Complications and benefits of both techniques have been reported |
| Other bias | Low risk | No other risk of bias was apparent |
Pusch 1999.
| Study characteristics | ||
| Methods | Prospective, parallel‐arm RCT where 1 arm received GA and the other arm received thoracic PVB with sedation for patients undergoing breast cancer surgery | |
| Participants | Total of 86 patients included with 42 patients in GA group and 44 patients in PVB group, scheduled to undergo breast cancer surgery | |
| Interventions |
PVB group (n = 44)
GA group (n = 42)
|
|
| Outcomes | • To assess advantage of single‐injection PVB as compared to GA with respect to the following: ‐ Postoperative analgesia ‐ Painful restricted movement at 1, 6, and 24 hours after surgery ‐ Postoperative vomiting |
|
| Notes | Attempts to contact study authors were made multiple times but no response was obtained VAS scores were plotted as a 2D bar diagram depicting mean along with SEM (standard error of mean) in the article. Exact values for mean VAS scores and SEM were obtained from the figure using data extraction software: WebPlotdigitizer. Standard deviation (SD) was then derived from SEM and sample size (n) using the following equation: SD = SEM √n No external funding was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors stated that patients were randomly allocated to 2 groups; however details of the method of randomisation were not described |
| Allocation concealment (selection bias) | Unclear risk | This was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible as 1 group got GA and the other got PVB |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded nurses in the recovery room and in the general ward assessed pain and administered medications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total of 86 patients were included and outcome data were analysed from the same |
| Selective reporting (reporting bias) | Low risk | Complications and benefits of both techniques have been reported |
| Other bias | Low risk | No other risk of bias was apparent |
Shkol'nik 2006.
| Study characteristics | ||
| Methods | Prospective, parallel‐arm RCT where 1 arm received GA and the other arm received thoracic PVB with sedation for patients undergoing breast cancer surgery | |
| Participants | Total of 180 female patients with confirmed diagnosis of breast cancer ASA physical status 2 to 3 Aged 30 to 82 years Undergoing radical or reconstructive breast surgery |
|
| Interventions |
PVB group (n = 90)
GA group (n = 90)
|
|
| Outcomes | Primary outcome • Evaluation of efficacy and safety of multi‐injection PVB with sedation with traditional GA for breast cancer Secondary outcomes • VAS (0 to 100 mm) at rest and on movement at 1, 3, 6 hours and 1, 2, 3, 4 postoperative days • Variations in haemodynamics (heart rate, blood pressure, ejection fraction) and plasma cortisol levels throughout the intraoperative period • Complications noted in both intervention groups • Total intraoperative fentanyl consumption |
|
| Notes | The original article was written in Russian and was translated to English by Tashtanbekova Cholpon Bolotbekovna Figures in the article depict VAS scores at rest and on movement at 24 hours, but it was not specified if values were in the mean ± SD or median ± range. Therefore these data could not be used for final quantitative analysis No external funding was received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated to 2 groups; however the exact method of randomisation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Details were not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible due to the nature of interventions (1 group received PVB and the other received GA) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details were not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were obtained from all 180 patients included |
| Selective reporting (reporting bias) | Low risk | Both benefits and complications have been mentioned |
| Other bias | Low risk | No other risk of bias was apparent |
Sundarathiti 2015.
| Study characteristics | ||
| Methods | Prospective, parallel‐arm, single‐blinded RCT where 1 arm received GA and the other arm received thoracic PVB with sedation for patients undergoing breast cancer surgery | |
| Participants | Total of 70 patients, with 35 in each group scheduled to undergo breast cancer surgery | |
| Interventions |
PVB group (n = 35)
GA group (n = 35)
|
|
| Outcomes | Primary outcome • Quality of postoperative analgesia (number of patients requiring additional morphine) Secondary outcomes • Postoperative pain scores until 24 hours postop • Total dose of rescue IV morphine • Time to readiness for discharge • Incidence of PONV • Patient satisfaction. |
|
| Notes | Study authors were contacted and additional information was received. But on further contact for quality of recovery outcomes, study authors did not provide information No external funding was received Hospital stay was expressed as mean ± SD in hours; these data were converted into minutes by multiplying with 60. This was done to enable the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block‐of‐4 method was used |
| Allocation concealment (selection bias) | Unclear risk | This was not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinded nurses in the recovery room and in the general ward assessed pain and administered medications |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data were assessed by residents and nurses who were blinded to treatment groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data for all 70 patients were included |
| Selective reporting (reporting bias) | Low risk | Complications and benefits of both techniques have been reported |
| Other bias | Low risk | No other risk of bias was apparent |
Tedore 2011.
| Study characteristics | ||
| Methods | Prospective, parallel‐arm RCT where patients in 1 arm received general anaesthesia (GA) and patients in the other arm received ultrasound‐assisted PVB with sedation in patients undergoing ambulatory breast cancer surgery | |
| Participants | A total of 40 patients, with 18 in the GA group and 22 in the PVB group, were included | |
| Interventions |
PVB group (n = 22)
GA group (n = 18)
|
|
| Outcomes | Primary outcome • Time to home discharge readiness Secondary outcomes • VAS, pain assessment (0 to 10) at 30, 60, 90, and 120 minutes after arrival • Narcotic consumption • Incidence of nausea and vomiting • Patient satisfaction |
|
| Notes | Unpublished study; study was sent to us by study authors as details on trial registry fulfilled our inclusion criteria and full‐text review of the study revealed that study fulfilled inclusion criteria in the meta‐analysis. Study authors were contacted for information on some other parameters; however this was not available Funding was received from the Department of Anesthesiology, New York Presbyterian Hospital Weill Cornell Medical College (institute where the study was carried out) Data for hospital stay and patient satisfaction score were expressed as median (IQR); these were converted to mean ± SD via Wan's method (Wan 2014) Patient satisfaction score was expressed on a 0 to 10 scale; this was converted to a 0 to 100 scale by multiplying by 10. This was done to enable the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table was used |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were prepared by a research assistant who was not involved in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded anaesthesiologist and PACU nurse recorded the data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 40 patients were included for outcome data |
| Selective reporting (reporting bias) | Low risk | Complications and benefits of both techniques have been reported |
| Other bias | Low risk | No other risk of bias was apparent |
Terheggen 2002.
| Study characteristics | ||
| Methods | Prospective, parallel‐arm RCT where 1 arm received GA and the other arm received thoracic PVB with sedation for patients undergoing minor breast cancer surgery | |
| Participants | A total of 30 patients, with 15 patients in each group scheduled to undergo elective, outpatient, minor breast cancer surgery such as lumpectomy, quadrantectomy (with or without sentinel lymph node procedure), and radiograph wire‐localised breast biopsy | |
| Interventions |
PVB group (n = 15)
GA group (n = 15)
|
|
| Outcomes | Primary outcome • Severity of postoperative pain using 0 to 100 mm visual analogue scale (VAS) at 15, 30, 45, 60, 90, 120 minutes and at time of discharge Secondary outcomes • Incidence of PONV • Time until discharge • Patient satisfaction |
|
| Notes | Attempts to contact study authors were made multiple times, but no response was obtained VAS scores were plotted as a 2D bar diagram depicting mean, along with SEM (standard error of mean) in the article. Exact values for mean VAS scores and SEM were obtained from the figure using data extraction software: WebPlotdigitizer. Standard deviation (SD) was then derived from SEM and sample size (n) using the following equation: SD = SEM √n No external funding was received Patient satisfaction score was expressed on a 0 to 3 scale as mean ± SD; this was converted to a 0 to 100 scale by multiplying with 33.3. This was done to enable the meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of method of randomisation were not specified |
| Allocation concealment (selection bias) | Low risk | Patients were allocated using the sealed envelope technique |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible as 1 group got GA and the other got PVB |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | VAS for pain and PONV were assessed by a trained recovery nurse who was not informed about the technique used Patient satisfaction was elicited the next day after the operation by telephoning the patient |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up and data were analysed |
| Selective reporting (reporting bias) | Low risk | Complications and benefits of both techniques have been reported |
| Other bias | Low risk | No other risk of bias was apparent |
2D: two‐dimensional. ASA: American Society of Anaesthesiologists. CK‐MB: creatine kinase‐MB. GA: general anaesthesia. IQR: interquartile range. LA: local anaesthetic. LMA: laryngeal mask airway. MAP: mean arterial pressure. OT: Operation theatre. PACU: post‐anaesthesia care unit. PONV: postoperative nausea and vomiting. PVB: paravertebral anaesthesia. RCT: randomised controlled trial. SD: standard deviation. SEM: standard error of the mean. VAS: visual analogue scale.
Characteristics of excluded studies [ordered by year]
| Study | Reason for exclusion |
|---|---|
| Sultan 2013 | The study was excluded despite having a paravertebral anaesthesia group and a general anaesthesia group because study authors assessed cytokines with either technique only after breast cancer surgery, and there was no quantitative or descriptive mention of the outcomes mentioned in our review |
Differences between protocol and review
The primary outcome of quality of recovery during the initial postoperative period (1 to 3 days) using validated questionnaires was not reported by any study. However, studies assessed the factors that determine quality of recovery such as postoperative analgesic use, PONV, duration of hospital stay, time to ambulation, and patient satisfaction. Therefore these reported outcomes were included in the review to determine quality of recovery over 1 to 2 postoperative days.
The outcome of postoperative pain at rest on the first postoperative day using a visual analogue scale (VAS) with a range of 0 to 10, or 0 to 100, or a numerical rating scale (NRS) with a similar range, was assessed as ‘Postoperative pain at rest on the first postoperative day at 2 hours, 6 and 24 hours’ so as to better assess this outcome.
The outcome of postoperative pain on movement on the first postoperative day using a visual analogue scale (VAS) with a range of 0 to 10, or 0 to 100, or a numerical rating scale (NRS) with a similar range, was specified as ‘Postoperative pain on movement on the first postoperative day at 2 hours, 6 and 24 hours’ so as to better assess this outcome.
The secondary outcome of 'Adverse events due to the paravertebral block (i.e. hypotension, bilateral block, pneumothorax, Horner's syndrome, bleeding, neurological deficit)' was changed to ‘Adverse events due to the paravertebral block (i.e. epidural spread and bilateral block, Horner's syndrome, bleeding, pleural tap)'. Pleural tap is more likely to occur following paravertebral block and may not be followed by pneumothorax.
We planned to conduct a subgroup analysis to assess the effect of (a) breast tumour receptor status on the clinical outcome of disease‐free survival, and (b) primary surgery compared to surgery following chemotherapy on clinical outcomes, namely, quality of recovery, disease‐free survival, and quality of life. This was not possible due to the paucity of literature. Instead, we performed a subgroup analysis on paravertebral block with local anaesthetics alone or with the additive drug clonidine.
We planned to conduct a sensitivity analysis for ‘absence of blinding in patient‐reported outcomes’. However, blinding of participants and personnel was not possible in any study, as one group of participants had received general anaesthesia and the other paravertebral anaesthesia. As this was the main question of the review, this was considered to be acceptable by all review authors.
For outcomes for which numerical data were lacking or for outcomes that had been measured in diverse ways, vote counting based on the direction of effect was used, and results were reported using the synthesis without meta analysis (SWiM) approach. As far as possible, this was done alongside the meta‐analysis.
Contributions of authors
AC, HP, AS, and MK drafted the protocol. All review authors read and approved the final review version.
Sources of support
Internal sources
-
All India Institute of Medical Sciences, New Delhi, India
Tertiary level hospital and research institute
External sources
None, India
Declarations of interest
AC has registered for PhD thesis titled "Effect of paravertebral anaesthesia on quality of life scores and circulating tumour cells in breast cancer patients". AC has no known conflicts of interest.
ARC has no known conflicts of interest.
HP has no known conflicts of interest.
RS is co‐guide for the above mentioned thesis. RS has no known conflicts of interest.
MKA has no known conflicts of interest.
AS is co‐guide of the above mentioned PhD thesis. AS has no known conflicts of interest.
MK has no known conflicts of interest.
New
References
References to studies included in this review
Das 2012 {published data only}
- Das S, Bhattacharya P, Mandal MC, Mukhopadhyay S, Basu SR, Mandol BK. Multiple-injection thoracic paravertebral block as an alternative to general anaesthesia for elective breast surgeries: a randomised controlled trial. Indian Journal of Anaesthesia 2012;56(1):27-33. [DOI: 10.4103/0019-5049.93340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Megahed 2010 {published data only}
- Megahed N, Basha H, Abdel Hamid Sh, Ali A, El-Banawy H. Pre-, intra- and post-operative plasma levels of soluble P-selectin in diabetics under thoracic paravertebral block versus general anaesthesia. Egypt Journal of Immunology 2010;17(1):49-58. [MEDLINE: ] [PubMed] [Google Scholar]
Naja 2003 {published data only}
- Naja MZ, Ziade MF, Lonnqvist PA. Nerve stimulator guided paravertebral blockade versus general anaesthesia for breast surgery: a prospective randomized trial. European Journal of Anaesthesiology 2003;20(11):897-903. [DOI: 10.1017/s0265021503001443] [DOI] [PubMed] [Google Scholar]
Paleczny 2005 {published data only}
- Paleczny J, Loniewska-Paleczny E, PyszM, Hura G. Comparison of the use of thoracic paravertebral block and general anesthesia in breast surgery. Borgis- Anestezjologia Intensywna Terapia 2005;1:12-6. [Google Scholar]
Pusch 1999 {published data only}
- Pusch F, Freitag H, Weinstabl C, Obwegeser R, Huber E, Wildling E. Single-injection paravertebral block compared to general anaesthesia in breast surgery. Acta Anaesthesiologica Scandinavica 1999;43(7):770-4. [DOI: 10.1034/j.1399-6576.1999.430714.x] [DOI] [PubMed] [Google Scholar]
Shkol'nik 2006 {published data only}
- Shkol'nik L D, Vasil'ev VIu, Soboleva LV. Multi-injection thoracic paravertebral anesthesia during breast cancer operations. Anesteziologiia i Reanimataologiia 2006;4:80-5. [MEDLINE: ] [PubMed] [Google Scholar]
Sundarathiti 2015 {published data only}
- Sundarathiti P, Bormann B, Suvikapakornkul R, Lertsithichai P, Arnuntasupakul V. Paravertebral catheter for three-level injection in radical injection in radical mastectomy: a randomised controlled study. PLoS One 2015;10(6):e0129539. [DOI: 10.1371/journal.pone.0129539. eCollection 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tedore 2011 {unpublished data only}
- Tedore TR, Nowak EJ, Tousimis E, Guheen C, Bhadra P. Comparison of paravertebral block with general anesthesia in patients undergoing breast cancer surgery.
Terheggen 2002 {published data only}
- Terheggen MA, Wille F, Borel Rinkes IH, Ionescu TI, Knape JT. Paravertebral blockade for minor breast surgery. Anesthesia & Analgesia 2002;94(2):355-9. [DOI: 10.1097/00000539-200202000-00023] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Sultan 2013 {published data only}
- Sultan SS. Paravertebral block can attenuate cytokine response when it replaces general anesthesia for cancer breast surgeries. Saudi Journal of Anaesthesia 2013;7(4):373-7. [DOI: 10.4103/1658-354X.121043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abdallah 2014
- Abdallah FW, Morgan PJ, Cil T, McNaught A, Escallon JM, Semple JL, et al. Ultrasound-guided multilevel paravertebral blocks and total intravenous anesthesia improve the quality of recovery after ambulatory breast tumor resection. Anesthesiology 2014;120(3):703-13. [DOI: 10.1097/ALN.0000436117.52143.bc] [DOI] [PubMed] [Google Scholar]
Ban 2014
- Ban KA, Godellas CV. Epidemiology of breast cancer. Surgical Oncology Clinics of North America 2014;23(3):409-22. [DOI: 10.1016/j.soc.2014.03.011] [DOI] [PubMed] [Google Scholar]
Boughey 2009
- Boughey JC, Goravanchi F, Parris RN, Kee SS, Frenzel JC, Hunt KK, et al. Improved postoperative pain control using thoracic paravertebral block for breast operations. Breast Journal 2009;15(5):483-8. [DOI: 10.1111/j.1524-4741.2009.00763.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brown 2001
- Brown LD, Cai TT, DasGupta A. Interval estimation for a binomial proportion. Statistical Science 2001;16:101–17. [Google Scholar]
Buckenmaier 2010
- Buckenmaier CC 3rd, Kwon KH, Howard RS, McKnight GM, Shriver CD, Fritz WT, et al. Double-blinded, placebo-controlled, prospective randomized trial evaluating the efficacy of paravertebral block with and without continuous paravertebral block analgesia in outpatient breast cancer surgery. Pain Medicine 2010;11(5):790-9. [DOI: 10.1111/j.1526-4637.2010.00842.x] [DOI] [PubMed] [Google Scholar]
Buckley 2014
- Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. British Journal of Anaesthesia 2014;113(Suppl 1):i56-62. [DOI: 10.1093/bja/aeu200] [DOI] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368(l6890):1-6. [DOI: 10.1136/bmj.l6890] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassinello 2015
- Cassinello F, Prieto I, Olmo M, Rivas S, Strichartz GR. Cancer surgery: how may anesthesia influence outcome? Journal of Clinical Anesthesia 2015;27(3):262-72. [DOI: 10.1016/j.jclinane.2015.02.007] [DOI] [PubMed] [Google Scholar]
Cata 2011
- Cata JP, Gottumukkala V, Sessler DI. How regional analgesia might reduce postoperative cancer recurrence. European Journal of Pain Supplements 2011;5:345–55. [Google Scholar]
Cheema 1995
- Cheema SPS, Ilsley D, Richardson J, Sabanathan S. A thermographic study of paravertebral analgesia. Anaesthesia 1995;50(2):118-21. [DOI: 10.1111/j.1365-2044.1995.tb15092.x] [DOI] [PubMed] [Google Scholar]
Cheng 2017
- Cheng GS, Ilfeld BM. An evidence-based review of the efficacy of perioperative analgesic techniques for breast cancer-related surgery. Pain Medicine 2017;18(7):1344-5. [DOI: 10.1093/pm/pnw172] [DOI] [PubMed] [Google Scholar]
Chiu 2014
- Chiu M, Bryson GL, Lui A, Watters JM, Taljaard M, Nathan HJ. Reducing persistent postoperative pain and disability 1 year after breast cancer surgery: a randomized, controlled trial comparing thoracic paravertebral block to local anesthetic infiltration. Annals of Surgical Oncology 2014;21(3):795-801. [DOI: 10.1245/s10434-013-3334-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochran 1954
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10(1):101-29. [Google Scholar]
Coffey 2003
- Coffey JC, Wang JH, Smith MJ, Bouchier-Hayes D, Cotter TG, Redmond HP. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncology 2003;4(12):760-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coughlin 2009
- Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiology 2009;33(5):315-8. [DOI: 10.1016/j.canep.2009.10.003.] [DOI] [PubMed] [Google Scholar]
Dabbagh 2007
- Dabbagh A, Elyasi H. The role of paravertebral block in decreasing postoperative pain in elective breast surgeries. Medical Science Monitor 2007;13(10):CR464-7. [PMID: ] [PubMed] [Google Scholar]
Deegan 2009
- Deegan CA, Murray D, Doran P, Ecimovic P, Moriarty DC, Buggy DJ. Effect of anaesthetic technique on oestrogen receptor-negative breast cancer cell function in vitro. British Journal of Anesethesia 2009;103(5):685-90. [DOI: 10.1093/bja/aep261] [DOI] [PubMed] [Google Scholar]
Deegan 2010
- Deegan CA, Murray D, Doran P, Moriarty DC, Sessler DI, Mascha E, et al. Anesthetic technique and the cytokine and matrix metalloproteinase response to primary breast cancer surgery. Regional Anesthesia and Pain Medicine 2010;35(6):490-5. [DOI: 10.1097/AAP.0b013e3181ef4d05] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
Ecimovic 2011
- Ecimovic P, Murray D, Doran P, McDonald J, Lambert DG, Buggy DJ. Direct effect of morphine on breast cancer cell function in vitro: role of the NET1 gene. British Journal of Anaesthesia 2011;107(6):916-23. [DOI: 10.1093/bja/aer259] [DOI] [PubMed] [Google Scholar]
Ecimovic 2013
- Ecimovic P, McHugh B, Murray D, Doran P, Buggy DJ. Effects of sevoflurane on breast cancer cell function in vitro. Anticancer Research 2013;33(10):4255-60. [PMID: PMID: 24122989] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta-analysis:principles and procedures. BMJ 1997;315(7121):1533–7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Exadaktylos 2006
- Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 2006;105(4):660-4. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2015
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359-86. [DOI: 10.1002/ijc.29210] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gottschalk 2010
- Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesthesia and Analgesia 2010;110(6):1636-43. [DOI: 10.1213/ANE.0b013e3181de0ab6] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 13 February 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Greengrass 1996
- Greengrass R, O'Brien F, Lyerly K, Hardman D, Gleason D, D'Ercole F, et al. Paravertebral block for breast cancer surgery. Canadian Journal of Anaesthesia 1996;43(8):858-61. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026.] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [DOI: 10.1186/1471-2288-5-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2020
- Jacobs A, Lemoine A, Joshi GP, Van de Velde M, Bonnet F, PROSPECT Working Group Collaborators. PROSPECT guideline for oncological breast surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia 2020;75(5):664-73. [DOI: 10.1111/anae.14964] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaura 2014
- Jaura AI, Flood G, Gallagher HC, Buggy DJ. Differential effects of serum from patients administered distinct anaesthetic techniques on apoptosis in breast cancer cells in vitro: a pilot study. British Journal of Anaesthesia 2014;113(Suppl 1):i63-7. [DOI: 10.1093/bja/aet581] [DOI] [PubMed] [Google Scholar]
Kairaluoma 2004
- Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Single injection paravertebral block before general anaesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsy. Anesthesia and Analgesia 2004;99(6):1837-43. [DOI: 10.1213/01.ANE.0000136775.15566.87] [PMID: ] [DOI] [PubMed] [Google Scholar]
Karmakar 2001
- Karmakar MK. Thoracic paravertebral block. Anesthesiology 2001;95(3):771–80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Karmakar 2014
- Karmakar MK, Samy W, Li JW, Lee A, Chan WC, Chen PP, et al. Thoracic paravertebral block and its effects on chronic pain and health-related quality of life after modified radical mastectomy. Regional Anesthesia and Pain Medicine 2014;39(4):289-98. [DOI: 10.1097/AAP.0000000000000113] [DOI] [PubMed] [Google Scholar]
Li 2009
- Li G, Warner M, Lang BH, Huang L, Sun LS. Epidemiology of anesthesia-related mortality in the United States, 1999-2005. Anesthesiology 2009;110(4):759-65. [DOI: 10.1097/aln.0b013e31819b5bdc] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lonnqvist 1995
- Lönnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade. Failure rate and complications. Anaesthesia 1995;50(9):813-5. [DOI: 10.1111/j.1365-2044.1995.tb06148.x] [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719–48. [PubMed] [Google Scholar]
McKenzie 2019
- McKenzie JE, Brennan SE. Chapter 12. Synthesizing and presenting findings using other methods. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. Cochrane, 2019 edition. New York: John Wiley & Sons, Ltd., Updated July 2019. [Google Scholar]
Moller 2007
- Moller JF, Nikolajsen L, Rodt SA, Ronning H, Carlsson PS. Thoracic paravertebral block for breast cancer surgery: a randomized double-blind study. Anesthesia and Analgesia 2007;105(6):1848-51. [DOI: 10.1213/01.ane.0000286135.21333.fd] [PMID: ] [DOI] [PubMed] [Google Scholar]
Murad 2017
- Murad MH, Mustafa RA, Schünemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. BMJ Evidence-Based Medicine 2017;22(3):85-7. [DOI: 10.1136/ebmed-2017-110668] [DOI] [PMC free article] [PubMed] [Google Scholar]
Najarian 2003
- Najarian MM, Johnson JM, Landercasper J, Havlik P, Lambert PJ, McCarthy D. Paravertebral block: an alternative to general anesthesia in breast cancer surgery. American Surgeon 2003;69(3):213–8. [PMID: ] [PubMed] [Google Scholar]
Niwa 2013
- Niwa H, Rowbotham DJ, Lambert DG, Buggy DJ. Can anesthetic techniques or drugs affect cancer recurrence in patients undergoing cancer surgery? Journal of Anesthesia 2013;27(5):731-41. [DOI: 10.1007/s00540-013-1615-7] [DOI] [PubMed] [Google Scholar]
O'Riain 2005
- O'Riain SC, Buggy DJ, Kerin MJ, Watson RW, Moriarty DC. Inhibition of the stress response to breast cancer surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2. Anesthesia and Analgesia 2005;100(1):244-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815–34. [DOI] [PubMed] [Google Scholar]
Pöpping 2009
- Pöpping DM, Elia N, Marret E, Wenk M, Tramèr MR. Clonidine as an adjuvant to local anesthetics for peripheral nerve and plexus blocks: a meta-analysis of randomized trials. Anesthesiology 2009;111(2):406-15. [DOI: 10.1097/ALN.0b013e3181aae897] [DOI] [PubMed] [Google Scholar]
Ramirez 2015
- Ramirez MF, Tran P, Cata JP. The effect of clinically therapeutic plasma concentrations of lidocaine on natural killer cell cytotoxicity. Regional Anesthesia and Pain Medicine 2015;40(1):43-8. [DOI: 10.1097/AAP.0000000000000191] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 1998
- Richardson J, Lönnqvist PA. Thoracic paravertebral block. British Journal of Anaesthesia 1998;81(2):230–8. [DOI: 10.1093/bja/81.2.230] [DOI] [PubMed] [Google Scholar]
Schnabel 2010
- Schnabel A, Reichl SU, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: a meta-analysis of randomized controlled trials. British Journal of Anaesthesia 2010;105(6):842-52. [DOI: 10.1093/bja/aeq265] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12. Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sessler 2008
- Sessler DI, Ben-Eliyahu S, Mascha EJ, Parat MO, Buggy DJ. Can regional analgesia reduce the risk of recurrence after breast cancer? Methodology of a multicenter randomized trial. Contemporary Clinical Trials 2008;29(4):517-26. [DOI: 10.1016/j.cct.2008.01.002] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sessler 2019
- Sessler DI, Pei L, Huang Y, Fleischmann E, Marhofer P, Kurz A, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet 2019;394(10211):1807-15. [DOI: 10.1016/S0140-6736(19)32313-X] [DOI] [PubMed] [Google Scholar]
Tahiri 2011
- Tahiri Y, Tran DQ, Bouteaud J, Xu L, Lalonde D, Luc M, et al. General anaesthesia versus thoracic paravertebral block for breast surgery: a meta-analysis. Journal of Plastic, Reconstructive & Aesthetic Surgery 2011;64(10):1261-9. [DOI: 10.1016/j.bjps.2011.03.025] [DOI] [PubMed] [Google Scholar]
Tedore 2015
- Tedore T. Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. British Journal of Anaesthesia 2015;115(Suppl 2):ii34-45. [DOI: 10.1093/bja/aev375] [DOI] [PubMed] [Google Scholar]
Terkawi 2015
- Terkawi AS, Tsang S, Sessler DI, Terkawi RS, Nunemaker MS, Durieux ME, et al. Improving analgesic efficacy and safety of thoracic paravertebral block for breast surgery: a mixed-effects meta-analysis. Pain Physician 2015;18(5):E757-80. [PMID: ] [PubMed] [Google Scholar]
Vaghari 2014
- Vaghari BA, Ahmed OI, Wu CL. Regional anesthesia analgesia: relationship to cancer recurrence and infection. Anesthesiology Clinics 2014;32(4):841-51. [DOI: 10.1016/j.anclin.2014.08.004] [DOI] [PubMed] [Google Scholar]
Votta‐Velis 2015
- Votta-Velis EG, Piegeler T, Minshall RD, Aguirre J, Beck-Schimmer B, Schwartz DE, et al. Regional anaesthesia and cancer metastases: the implication of local anaesthetics. Acta Anaesthesiologica Scandinavica 2013;57(10):1211-29. [DOI: 10.1111/aas.12210] [DOI] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14(135):1-13. [http://www.biomedcentral.com/1471-2288/14/135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weigelt 2005
- Weigelt B, Peterse JL, van't Veer LJ. Breast cancer metastasis: markers and models. Nature Reviews Cancer 2005;5(8):591-602. [DOI: 10.1038/nrc1670] [PMID: ] [DOI] [PubMed] [Google Scholar]
Weltz 1995
- Weltz CR, Greengrass RA, Lyerly HK. Ambulatory surgical management of breast carcinoma using paravertebral block. Annals of Surgery 1995;222(1):19-26. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wigmore 2016
- Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology 2016;124(1):69-79. [DOI: 10.1097/ALN.0000000000000936] [DOI] [PubMed] [Google Scholar]
Wu 2015
- Wu J, Buggy D, Fleischmann E, Parra-Sanchez I, Treschan T, Kurz A, et al. Thoracic paravertebral regional anesthesia improves analgesia after breast cancer surgery: a randomized controlled multicentre clinical trial. Canadian Journal of Anaesthesia 2015;62(3):241-51. [DOI: 10.1007/s12630-014-0285-8] [DOI] [PubMed] [Google Scholar]
Youlden 2012
- Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiology 2012;36(3):237-48. [DOI: 10.1016/j.canep.2012.02.007] [DOI] [PubMed] [Google Scholar]


