Abstract
CD4+ and CD8+ T cells play specific roles during an immune response. Different molecular mechanisms could regulate the proliferation, death, and effector functions of these two subsets of T cells. The p38 mitogen-activated protein (MAP) kinase pathway is induced by cytokines and environmental stress and has been associated with cell death and cytokine expression. Here we report that activation of the p38 MAP kinase pathway in vivo causes a selective loss of CD8+ T cells due to the induction of apoptosis. In contrast, activation of p38 MAP kinase does not induce CD4+ T-cell death. The apoptosis of CD8+ T cells is associated with decreased expression of the antiapoptotic protein Bcl-2. Regulation of the p38 MAP kinase pathway in T cells is therefore essential for the maintenance of CD4/CD8 homeostasis in the peripheral immune system. Unlike cell death, gamma interferon production is regulated by the p38 MAP kinase pathway in both CD4+ and CD8+ T cells. Thus, specific aspects of CD4+ and CD8+ T-cell function are differentially controlled by the p38 MAP kinase signaling pathway.
CD4+ and CD8+ T cells perform distinct functions to mediate the immune response. The commitment of CD4 and CD8 lineages occurs during T-cell development in the thymus, and it is maintained throughout the life spans of the T cells in the peripheral immune system. CD4+ CD8+ double-positive (DP) thymocytes differentiate into mature CD4+ or CD8+ thymocytes depending on the respective T-cell receptor (TCR) specificity for major histocompatibility complex (MHC) class II or class I, respectively (positive selection). Mature CD4+ and CD8+ thymocytes leave the thymus and migrate to the peripheral immune system, becoming naive CD4+ and CD8+ T cells. Although both CD4+ and CD8+ T cells undergo clonal expansion in response to antigens, naive CD4+ T cells differentiate into helper effector cells while naive CD8+ T cells become cytotoxic cells. Effector CD4+ T cells rapidly produce large amounts of cytokine in response to an antigen. While CD8+ T cells can also secrete cytokines (e.g., gamma interferon [IFN-γ]), their major role in the immune response appears to be cytotoxic activity, mediated by secreted proteins, such as perforin and granzyme.
The divergent functions of CD4+ and CD8+ T cells suggest that distinct signaling requirements and molecular mechanisms could mediate the activation of each subset in response to antigens or environmental stimuli. Several examples of these differential controls have been described. Costimulations through 4-1BB (a new member of the tumor necrosis factor [TNF] receptor family) and CD28 are complementary to one another by activating CD8+ and CD4+ T cells, respectively (49). Signaling through the Fas ligand appears to be required for CD8+ T-cell proliferation but not for CD4+ T-cell proliferation (52).
The numbers of CD4+ and CD8+ cells in the periphery remain constant under normal conditions, but the presence of specific pathological environments can modulate the CD4/CD8 homeostasis by preferentially affecting one of these subsets. For instance, human immunodeficiency virus infection is characterized by a prolonged decline in the number of CD4+ T cells (11, 47). As the infection progresses, a decline of CD8+ T-cell numbers, which appears to be mediated by membrane-bound TNF-α expressed on macrophages, is also observed (20, 32). Thus, environmental stimuli can differentially regulate CD4/CD8 homeostasis.
p38 mitogen-activated protein (MAP) kinase can be activated by multiple stimuli, such as proinflammatory cytokines (e.g., interleukin-1β [IL-1β] and TNF-α), hematopoietic growth factors (e.g., colony-stimulatory factor-1, granulocyte/macrophage colony-stimulatory factor, and IL-3), lipopolysaccharide, and environmental stress (12, 13, 18, 28, 40, 46). p38 MAP kinase activation is mediated by phosphorylation on Thr and Tyr by the dual-specificity MAP kinase kinases MKK3, MKK4, and MKK6 (8, 19, 34, 41). Several transcription factors (ATF-2, Elk-1, CHOP, MEF2C, and SAP-1) and downstream protein kinases (eukaryotic initiation factor 4E protein kinases Mnk1 and Mnk2, PRAK, MSK1, and MAPKAP kinase 2 and 3) are substrates for p38 MAP kinase (6, 8, 13, 14, 17, 33, 36, 40, 41, 46, 56–58). Activation of the p38 MAP kinase pathway has been associated with cell death, proliferation, and cytokine expression (28, 30, 44, 61).
In this study, we examined the role of the p38 MAP kinase pathway in the expression of cytokines and death of CD8+ T cells. p38 MAP kinase plays an important role in the production of IFN-γ by CD4+ and CD8+ T cells. However, activation of p38 MAP kinase in vivo causes a selective loss of the CD8 lineage, while the number of CD4+ T cells is not affected. Activation of the p38 MAP kinase pathway induces spontaneous apoptotic CD8+ T-cell death, which is associated with decreased levels of Bcl-2. Thus, p38 MAP kinase plays a critical role in the homeostasis of CD4+ and CD8+ T cells in the peripheral immune system.
MATERIALS AND METHODS
Transgenic mice.
The MKK6(Glu) and dn p38 transgenic mice have been described previously (44). In both transgenic models the expression of MKK6(Glu) and the dominant-negative (dn) p38 MAP kinase was driven by the distal lck promoter (59). These transgenic mice have been backcrossed with B10.BR mice (Jackson Laboratory, Bar Harbor, Maine).
Cell preparation and surface staining.
The distribution of major cell populations in the thymus, spleen, and lymph nodes was examined by cell surface staining and flow cytometry (EPICS; Coulter), with phycoerythrin (PE)-conjugated anti-CD4 monoclonal antibodies (MAb), a red613-conjugated anti-CD8 MAb, and a fluorescein isothiocyanate-conjugated anti-CD45R/B220 MAb (Pharmingen, San Diego, Calif.). Additional surface markers were stained with PE-conjugated anti-CD44 (Caltag, Burlingame, Calif.), biotinylated anti-TCR(H57), anti-CD69, or anti-CD25 MAb followed by a red670-conjugated streptavidin (Pharmingen).
Total CD8+ T cells were isolated from spleen and lymph nodes by negative selection with anti-NK (NK1.1; Pharmingen), anti-CD4 (GK 1.5), anti-Mac1 (Pharmingen), and anti-MHC class II MAbs to label NK, macrophages, CD4+ cells, and B cells, respectively, followed by depletion with magnetic beads (Perceptive Biosystems, Framingham, Mass.) as described previously (24, 42, 43). Total CD4+ T cells were similarly isolated from spleen and lymph nodes with anti-NK1.1, anti-CD8, anti-Mac1, and anti-MHC class II MAbs. Splenocytes were derived from syngeneic antigen-presenting cells (APC).
Proliferation and measurement of cytokine production.
Enzyme-linked immunosorbent assays (ELISA) were performed with purified anti-IFN-γ MAb (2 μg/ml) as the primary (capture) antibody, biotinylated anti-IFN-γ MAb as the secondary (detection) antibody, horseradish peroxidase-conjugated avidin D (2.5 μg/ml; Vector Laboratories, Burlingame, Calif.), and peroxidase substrate and reaction stop solutions (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) following the recommended protocol (Pharmingen). Recombinant mouse IFN-γ (Gibco-BRL, Gaithersburg, Md.) was used as a standard. The proliferative response was determined after 3 days by measurement of [3H]thymidine incorporation (Amersham Corp.) for 18 h.
Viability and cell death.
CD8+ cells were cultured under various conditions. The number of live cells was determined by Trypan Blue staining. Purified CD8+ cells were stained with PE-conjugated anti-CD4 and red613-conjugated anti-CD8 MAb, fixed in 1% paraformaldehyde, permeabilized in 70% ethanol, and assayed for apoptosis via terminal deoxynucleotidyltransferase-mediated fluorescein isothiocyanate-dUTP incorporation, as described by the manufacturer (Pharmingen).
Reverse transcriptase PCR (RT-PCR).
Total RNA was extracted with the Ultraspec RNA isolation system (Biotex Laboratories) as recommended by the manufacturer. First-strand cDNA was obtained by reverse transcription as described previously (43) with total RNA (2 μg). cDNA was used to determine Bcl-2 and hypoxanthine guanine phosphoribosyltransferase (HPRT) (26, 63) gene expression by PCR with previously described primers.
p38 MAP kinase assays.
Cells were lysed with buffer A (20 mM Tris [pH 7.5] 10% glycerol, 1% Triton X-100, 0.137 M NaCl, 25 mM β-glycerophosphate, 2 mM EDTA, 0.5 mM dithiothreitol, 1 mM sodium orthovanadate, 2 mM sodium pyrophosphate, 10 μg of leupeptin/ml, 1 mM phenylmethylsulfonyl fluoride) as described previously (7, 43). Endogenous p38 MAP kinase was immunoprecipitated with anti-p38 polyclonal antibody (40) prebound to protein A-Sepharose. The immunoprecipitates were washed twice with buffer A and twice with kinase buffer (25 mM HEPES [pH 7.4], 25 mM β-glycerophosphate, 25 mM MgCl2, 0.5 mM dithiothreitol, 0.1 mM sodium orthovanadate). The protein kinase reactions were initiated by addition of 1 μg of recombinant substrate protein (glutathione S-transferase-ATF2) and 50 μM [γ-32P]ATP (10 Ci/mmol). The reactions were terminated after 30 min at 30°C by addition of Laemmli sample buffer. Phosphorylation of the substrate protein was examined after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by autoradiography and PhosphorImager analysis (Molecular Dynamics Inc.).
Western blot analysis.
Proteins were fractionated by SDS-PAGE, electrophoretically transferred to an Immobilon-P membrane (Millipore Inc.), and probed for p38 with an anti-p38 polyclonal antibody (Santa Cruz Biotechnology). Antibodies used to detect Bcl-2 family proteins were mouse anti-Bcl-XL, hamster anti-Bcl-2, and rabbit anti-Bax (Pharmingen). Immunocomplexes were detected by chemiluminescence (Renaissance; NEN).
Reagents.
Reagents used for T-cell culture included phorbol myristate acetate (PMA) and ionomycin (Sigma Chemical Co., St. Louis, Mo.), concanavalin A (Boehringer Gmblt, Mannheim, Germany), IL-2 (R & D Systems, Minneapolis, Minn.), SB203580 (Vertex Pharmaceuticals, Inc., Cambridge, Mass.), zVAD-fmk (Enzyme Systems Products, Livermore, Calif.), and anti-IFN-γ MAb (Pharmingen).
RESULTS
Regulation of the p38 MAP kinase signaling pathway in CD4+ and CD8+ T cells.
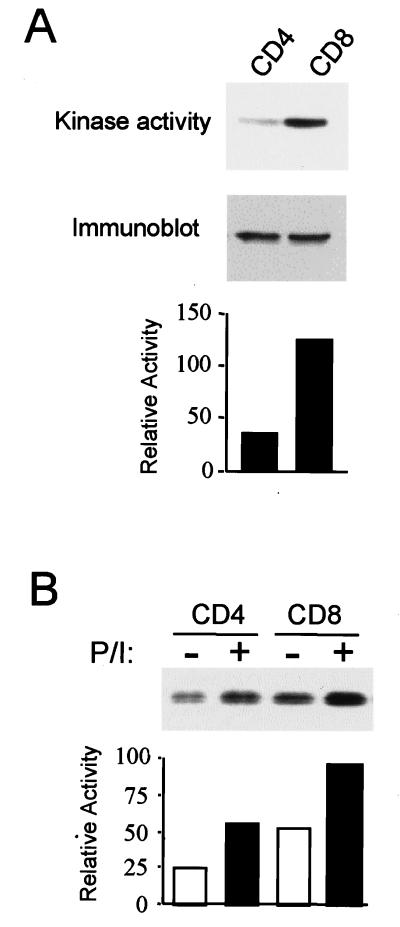
CD8+ and CD4+ T cells have different effector functions during an immune response, suggesting that intracellular signaling pathways and gene expression patterns may also differ between the two T-cell populations. We have recently shown that the p38 MAP kinase signaling pathway plays an important role in the production of IFN-γ by effector CD4+ Th1 cells but does not affect CD4+ T-cell expansion. To investigate the role of p38 MAP kinase in CD8+ T cells, we first examined the activity of p38 MAP kinase in both CD4+ and CD8+ T-cell subsets by an in vitro assay with ATF-2 as the substrate. The level of p38 MAP kinase activity detected in CD8+ T cells was consistently higher (two- to three-fold) than the level of activity detected in CD4+ T cells (Fig. 1A). Immunoblot analysis, however, showed that the amounts of p38 MAP kinase protein in CD4+ and CD8+ T cells were similar (Fig. 1A). Thus, the elevated p38 MAP kinase activity observed in CD8+ T cells was not caused by an increased p38 MAP kinase protein expression. Increased p38 MAP kinase activity was also observed in CD8+ T cells stimulated with PMA and ionomycin compared to activity in stimulated CD4+ T cells (Fig. 1B). Together, these results suggested that this signaling pathway may be differentially regulated in CD8+ and CD4+ T cells.
FIG. 1.
Regulation of p38 MAP kinase in CD4+ and CD8+ T cells. (A) CD4+ and CD8+ T cells freshly isolated from spleens and lymph nodes from wild-type mice were lysed. p38 MAP kinase activity was measured in an immune complex assay with glutathione S-transferase ATF2 as a substrate in the presence of [γ-32P]ATP. The phosphorylated ATF2 was detected after SDS-PAGE by autoradiography (top) and was quantitated by PhosphorImager analysis (bottom). p38 MAP kinase protein levels were examined by immunoblot analysis of whole extracts (middle). (B) CD4+ and CD8+ T cells were incubated with medium alone (−) or with PMA (5 ng/ml) plus ionomycin (250 ng/ml) (P/I) for 30 min. p38 MAP kinase activity was assayed as described for panel A. The phosphorylated ATF2 was detected after SDS-PAGE by autoradiography (top) and was quantitated by PhosphorImager analysis (bottom). The results are representative of three (A) and two (B) experiments.
Activation of p38 MAP kinase in vivo causes a specific loss of CD8+ T cells in the peripheral immune system.
To investigate the specific role of p38 MAP kinase in CD4+ and CD8+ T-cell function, we examined these two populations by using transgenic mice in which the p38 MAP kinase pathway was constitutively activated in vivo. We have developed transgenic mice (44) expressing a constitutively activated form of MKK6, a MAP kinase kinase that selectively phosphorylates and activates p38 MAP kinase (19, 34, 41). These mice express an MKK6 mutant in which the amino acids at the activating sites of phosphorylation, Ser207 and Thr211, were replaced by Glu [MKK6(Glu)] (41, 44). The expression of MKK6(Glu) was targeted to peripheral T cells and certain thymocyte populations by using the distal lck promoter. These mice have been previously used to confirm the role of p38 MAP kinase in the production of IFN-γ by CD4+ Th1 cells (44).
Two lines of MKK6(Glu) transgenic mice showed a selective reduction in the percentage of CD8+ T cells in the total peripheral lymphocyte population (Fig. 2A). Results of successive experiments (n = 4) revealed a diminution of CD8+ T cells, both as a percentage of total T cells (Fig. 2B) and as an absolute CD8+ T-cell number (Fig. 2C). CD4+ T-cell or B-cell populations were not significantly altered.
FIG. 2.
Selective loss of CD8+ T cells in the MKK6(Glu) transgenic mice. (A) Cells from spleens or lymph nodes from NLC and MKK6(Glu) transgenic (Tg+) mice from lines 15 and 12 were stained with anti-CD4 and anti-CD8 MAb and analyzed by flow cytometry. Numbers represent the percentages of cells in each gate. One representative experiment is shown. (B) Cells from spleens or lymph nodes (LN) from NLC or MKK6(Glu) transgenic mice (line 15) were stained with anti-CD4, anti-CD8, and anti-CD45R(B220) MAb and analyzed by flow cytometry. Values represent the average percentages of CD8+ T, CD4+ T, and B (B220+) cells from four experiments. (C) Absolute numbers of CD4+ T cells and CD8+ T cells in lymph nodes from NLC littermate control and MKK6(Glu) transgenic mice. The means and standard deviations from four independent experiments are presented. (D) Thymocytes from NLC mice and MKK6(Glu) transgenic mice were stained and analyzed as described for panel A. (E) CD4+ and CD8+ T cells freshly isolated from spleens and lymph nodes from NLC and MKK6(Glu) transgenic mice were lysed, and whole extracts were assayed for p38 MAP kinase activity as described for Fig. 1A. The phosphorylated ATF2 was detected after SDS-PAGE by autoradiography (top) and quantitated by PhosphorImager analysis (bottom). The data shown are representative of three experiments.
Thymic development in the MKK6(Glu) transgenic mice was not modified, as evidenced by normal distribution of CD4− CD8− double-negative (DN), DP, CD4+, and CD8+ thymocytes (Fig. 2D). The absolute number of cells in each of these populations was also normal (data not shown), and no difference in the expression of the heat-stable antigen, CD44, CD25, CD69, and TCR was observed (data not shown). These data indicated that activation of MKK6 caused a specific reduction of the peripheral CD8+ T cells without disturbing thymocyte development significantly.
To confirm the constitutive activation of p38 MAP kinase in the MKK6(Glu) transgenic mice, we examined p38 MAP kinase activity in CD4+ and CD8+ T-cell populations isolated from control and MKK6(Glu) transgenic mice. The levels of p38 MAP kinase activity in both CD4+ and CD8+ T cells from these mice were augmented compared to the levels of activity detected in CD4+ and CD8+ T cells from negative-littermate control (NLC) mice, respectively (Fig. 2E). Thus, the expression of the MKK6(Glu) transgene has led to the activation of p38 MAP kinase in both CD4+ and CD8+ populations, although only the CD8+ T-cell number was reduced in the MKK6(Glu) transgenic mice.
The p38 MAP kinase pathway negatively regulates the proliferative response in CD8+ T cells.
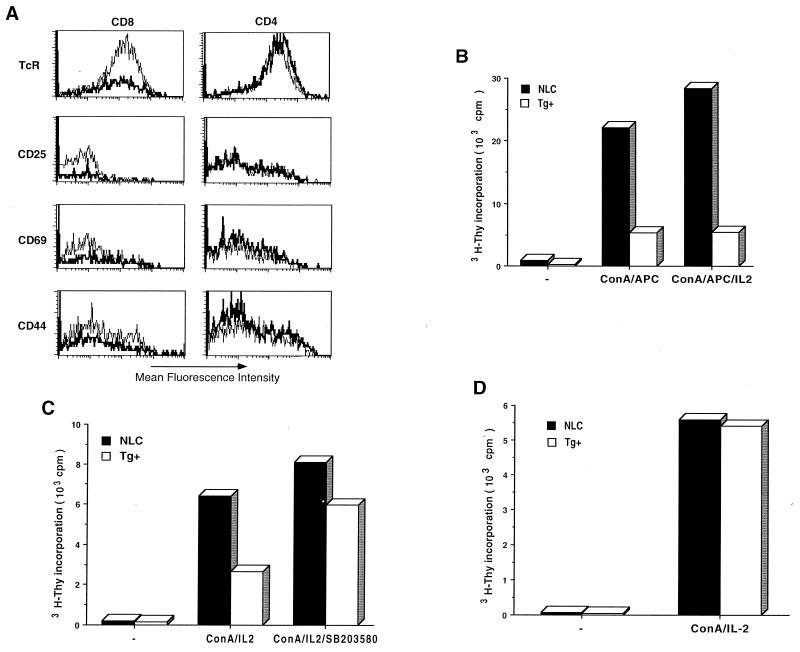
An analysis of TCR and cell surface activation markers (CD25, CD69, and CD44) by flow cytometry showed normal expression of these molecules in CD4+ T cells from the MKK6(Glu) transgenic mice (Fig. 3A). No significant difference in the levels of expression of CD25 and CD44 activation markers in the residual CD8+ T cells present in the MKK6(Glu) mice compared to those in control CD8+ T cells was observed (Fig. 3A). The expression of the TCR was slightly reduced, and that of CD69 was slightly upregulated, on MKK6(Glu) CD8+ T cells (Fig. 3A). Furthermore, the expression of the memory marker CD45RB was normal in these cells (data not shown), suggesting that the CD8+ T cells present in the MKK6(Glu) transgenic mice did not represent a population of activated or memory cells.
FIG. 3.
Characterization of cell surface marker expression and proliferative activity in MKK6(Glu) CD8+ T cells. (A) Lymph node cells from NLC (light line) and MKK6(Glu) transgenic (dark line) mice were stained with anti-CD4 and anti-CD8 MAb in combination with anti-TCR, anti-CD25, anti-CD69, or anti-CD44 MAb. Histograms represent the expression of the indicated cell surface marker in CD8+ or CD4+ T-cell populations. (B) CD8+ T cells (5 × 104 cells/well) were isolated from NLC and MKK6(Glu) transgenic (Tg+) mice and incubated with medium alone (−) or stimulated with ConA (2.5 μg/ml) and APC (5 × 104) in the absence (ConA/APC) or presence (ConA/APC/IL-2) of IL-2 (30 U/ml). Proliferation was determined after 3 days by [3H]thymidine incorporation. The data shown are representative of four experiments. (C) CD8+ T cells (5 × 104 cells/well) were isolated from NLC and MKK6(Glu) transgenic mice and incubated with medium alone (−) or stimulated with ConA (2.5 μg/ml) plus IL-2 (30 U/ml), in the presence (ConA/IL2/SB203580) or absence (ConA/IL2) of the p38 MAP kinase inhibitor SB203580 (1 μM). Proliferation was determined as described for panel B. (D) CD4+ T cells (5 × 104 cells/ml) isolated from NLC littermate control or MKK6(Glu) transgenic mice were cultured in medium alone (−) or ConA (2.5 μg/ml) and IL-2 (30 U/ml) (ConA/IL-2). The proliferative response was determined as for panel B.
To determine the proliferative response of the CD8+ T cells remaining in the MKK6(Glu) transgenic mice, equal numbers of purified CD8+ T cells from NLC and MKK6(Glu) transgenic mice were stimulated with concanavalin A (ConA) in the presence of wild-type APC. Interestingly, low levels of proliferation were detected in CD8+ T cells isolated from MKK6(Glu) transgenic mice (Fig. 3B). Addition of IL-2 did not restore the proliferative ability of CD8+ of T cells (Fig. 3B), indicating that IL-2 production was not the major defect in these cells. To demonstrate that the hypoproliferation of CD8+ T cells from the MKK6(Glu) transgenic mice was caused by the activation of p38 MAP kinase, we examined the effect of the specific p38 MAP kinase inhibitor SB203580 (28, 54, 60, 64). CD8+ T cells were stimulated with ConA and IL-2 in the presence or absence of SB203580. In these experiments, APC were not added in order to avoid indirect effects of the drug on these cells. The presence of SB203580 restored the proliferative capacity of CD8+ T cells from the MKK6(Glu) transgenic mice (Fig. 3C) and did not inhibit the proliferation of control cells. An analysis of the CD4+ T-cell subset indicated that CD4+ T cells from the MKK6(Glu) transgenic mice proliferate similarly to control CD4+ T cells (Fig. 3D).
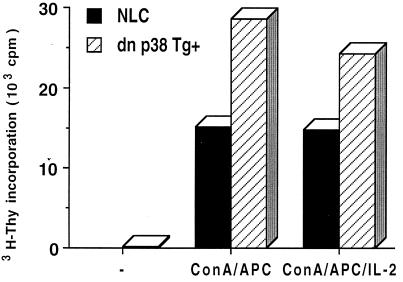
We have previously shown that inhibition of the p38 MAP kinase pathway by expression of dn p38 MAP kinase in transgenic mice did not affect the proliferation of CD4+ T cells (44). To further demonstrate the specific role of p38 MAP kinase in CD8+ T cells, we isolated CD8+ T cells from NLC and dn p38 transgenic mice and stimulated them with ConA and APC in the presence or absence of IL-2. In correlation with the studies of the MKK6(Glu) transgenic mice, CD8+ T cells from the dn p38 transgenic mice had an increased proliferative response (Fig. 4). Together, these results indicated that p38 MAP kinase selectively interfered with the proliferation of CD8+ T cells but that it did not affect CD4+ T-cell proliferation.
FIG. 4.
Increased proliferation of CD8+ T cells from the dn p38 transgenic mice. CD8+ T cells (5 × 104 cells/well) were isolated from NLC and dn p38 transgenic (dn p38 Tg+) mice and incubated with medium alone (−) or stimulated with ConA (2.5 ng/ml) and APC (5 × 104) in the absence (ConA/APC) or presence (ConA/APC/IL-2) of IL-2 (30 U/ml). Proliferation was determined as described for Fig. 3B. The data are representative of two experiments.
Activation of the p38 MAP kinase pathway induces apoptosis selectively in CD8+ T cells but not in CD4+ T cells.
The low number of CD8+ T cells in the MKK6(Glu) transgenic mice could be caused by a direct inhibition of CD8+ T-cell proliferation. However, no difference in BrdU incorporation in vivo was observed in these mice (data not shown). The activation of the p38 MAP kinase pathway has been associated with the induction of apoptosis (62), indicating that an increased death of CD8+ T cells could be an alternative cause for the loss of this population in the MKK6(Glu) transgenic mice. Supporting this hypothesis, reduced numbers of MKK6(Glu) CD8+ T cells were recovered from cultures after stimulation with ConA for 48 h (Fig. 5A), whereas the presence of the p38 MAP kinase inhibitor during stimulation increased the viability of these cells (Fig. 5A).
FIG. 5.
Activation of p38 MAP kinase induces cell death selectively in CD8+ T cells. (A) CD8+ T cells were stimulated with ConA (2.5 μg/ml) in the presence or the absence of the p38 MAP kinase inhibitor SB203580 (1 μM). The viability of the cells at 48 h was determined by Trypan Blue staining. (B) CD4+ and CD8+ T cells (4 × 105 cells) were isolated from NLC and MKK6(Glu) transgenic (Tg+) mice and incubated in medium alone. Cells were harvested at various points, and viability was determined by staining with Trypan Blue. (C) Apoptosis of freshly isolated CD8+ and CD4+ T cells (day 0) or CD8+ and CD4+ T cells incubated in medium alone for 1 day was determined by TUNEL assay. Numbers represent the percentages of TUNEL-positive cells. One representative experiment of three is shown for each cell type. TdT, terminal deoxynucleotidyltransferase. (D) CD8+ T cells were isolated from the MKK6(Glu) transgenic mice and incubated in the presence of dimethyl sulfoxide (0.5%) (−) or zVAD (50 μM) for 24 h. Apoptosis was determined by TUNEL assay. (E) CD8+ T cells from NLC mice and the dn p38 transgenic mice were incubated in the presence of medium alone (medium) or ConA plus IL-2 (ConA) for the indicated times. Cell viability was determined by Trypan Blue staining.
We examined the rate of spontaneous death of CD8+ T cells isolated from the MKK6(Glu) transgenic and NLC mice in vitro upon incubation in medium alone. The number of live MKK6(Glu) CD8+ T cells recovered after 24 h was low, and very few MKK6(Glu) CD8+ T cells survived at 48 h, compared with CD8+ T cells from control mice (Fig. 5B). In contrast, the survival of CD4+ T cells from the MKK6(Glu) transgenic mice was comparable to the survival of CD4+ T cells from control mice (Fig. 5B).
An analysis of apoptosis by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay in freshly isolated lymphocytes showed an increased fraction of apoptotic CD8+ T cells in vivo from the MKK6(Glu) transgenic mice (Fig. 5C). Incubation in medium alone for 24 h resulted in increased apoptotic death of MKK6(Glu) CD8+ T cells compared to that of control CD8+ T cells (Fig. 5C). Similar results were obtained by an analysis of forward- and side-scatter parameters to measure apoptosis by flow cytometry (data not shown). In contrast, no difference in the number of apoptotic CD4+ T cells in the MKK6(Glu) transgenic mice compared to that in NLC mice was observed (Fig. 5C). These results demonstrated that activation of the p38 MAP kinase pathway in vivo selectively induced apoptosis in CD8+ T cells but not in CD4+ T cells.
Caspases constitute a family of cysteine proteases that are activated during programmed cell death (29). To determine whether the caspase pathway was involved in the p38 MAP kinase-induced CD8+ T-cell death, we examined the effect of the caspase inhibitor zVAD-fmk. CD8+ T cells from the MKK6(Glu) transgenic mice were incubated in vitro in the presence of zVAD or dimethyl sulfoxide alone, and apoptosis was examined by TUNEL after 24 h. The presence of zVAD completely prevented the death of the MKK6(Glu) CD8+ T cells (Fig. 5D), indicating that the induction of apoptosis by p38 MAP kinase in CD8+ T cells was mediated by caspases.
The increased cell death observed in MKK6(Glu) CD8+ T cells suggested that hyperproliferation of CD8+ T cells from the dn p38 transgenic mice could be due to increased resistance to activation-induced cell death. We therefore examined the survival of CD8+ T cells from negative-control mice and dn p38 transgenic mice upon stimulation with ConA plus IL-2. After stimulation but prior to cell expansion, an increased number of live dn p38 CD8+ T cells was recovered compared with the number of wild-type CD8+ T cells (Fig. 5E). Compared to CD8+ T cells from the MKK6(Glu) transgenic mice, CD8+ T cells from the dn p38 transgenic mice were more resistant to spontaneous cell death during the incubation in medium alone (Fig. 5E). These results suggested that the p38 MAP kinase pathway is involved in cell death induced during CD8+ T-cell activation.
Bcl-2 expression is negatively regulated by the p38 MAP kinase pathway in CD8+ T cells.
Several molecular mechanisms are involved in T-cell death. The expression of Fas ligand in activated T cells leads to induced cell death. JNK, another member of the MAP kinase family, has been implicated in the upregulation of Fas ligand expression on specific cell types (10, 25). No Fas ligand expression was detected by cell surface staining and RT-PCR in CD8+ T cells from the MKK6(Glu) transgenic mice (data not shown), indicating that activation of p38 MAP kinase did not upregulate Fas ligand expression in these cells. In addition, similar levels of Fas were expressed in CD8+ T cells from control and MKK6(Glu) transgenic mice (data not shown).
The Bcl-2 protein family comprises multiple members that act to mediate or protect cells from apoptotic death (4). Bcl-2 itself confers resistance to cell death and is expressed at low levels in immature DP thymocytes but is upregulated in mature single-positive thymocytes and peripheral T cells (15, 21, 55). We examined the expression of Bcl-2 in T cells from the MKK6(Glu) transgenic mice. CD4+ and CD8+ T cells isolated from NLC and MKK6(Glu) transgenic mice were lysed, and whole extracts were used for Bcl-2 detection by Western blot analysis. Strikingly, the level of Bcl-2 in CD8+ T cells from the MKK6(Glu) transgenic mice was diminished compared to its expression in control CD8+ T cells (Fig. 6A), while its expression in CD4+ T cells was not affected.
FIG. 6.
Diminished Bcl-2 protein levels in CD8+ T cells by activation of p38 MAP kinase. (A) CD4+ and CD8+ T cells freshly isolated from spleens and lymph nodes from NLC and MKK6(Glu) transgenic mice (Tg+) were lysed. Whole extracts were assayed for Bcl-2, Bax, and Bcl-XL expression by immunoblot analysis. Total thymocyte (Thy) extracts from control mice were used as controls for Bcl-XL expression. (B) cDNA was generated from total RNA obtained from freshly isolated CD8+ T cells from NLC and MKK6(Glu) transgenic mice. Bcl-2 gene expression was determined by PCR with 3 (top) or 2 μl (middle) of the cDNA reaction mixture. HPRT expression was determined by using 2 μl of the cDNA mixture.
Bcl-xL is another antiapoptotic protein whose expression is inverse to the expression of Bcl-2 in thymocytes and T cells. Peripheral T cells express Bcl-2 but not Bcl-xL, while high levels of Bcl-xL and low levels of Bcl-2 have been found in immature DP thymocytes (16, 31). In correlation, Bcl-xL was undetectable in CD4+ and CD8+ T cells from control mice (Fig. 6A). No upregulation in CD8+ T cells from the MKK6(Glu) transgenic mice was observed (Fig. 6A), indicating that the low level of expression of Bcl-2 in these cells was not compensated for by an increase in Bcl-xL.
The balance between proapoptotic and antiapoptotic Bcl-2 family members is a determinant for cell death or survival. We therefore examined the expression of the proapoptotic Bax molecule, which is also involved in cell death (38). Similar levels of Bax were found in control and MKK6(Glu) CD8+ T cells (Fig. 6A). Together, these data suggested that the selective death of CD8+ T cells in the MKK6(Glu) transgenic mice in vivo could be due to an unbalanced ratio of pro- and antiapoptotic Bcl-2 family members.
Recently, several molecular mechanisms have been described as being involved in the regulation of Bcl-2 protein and gene expression (4). To determine whether activation of p38 MAP kinase may regulate transcription of the bcl-2 gene, we examined bcl-2 mRNA levels by RT-PCR. No significant difference in bcl-2 gene expression between CD8+ T cells from NLC and MKK6(Glu) transgenic mice was observed (Fig. 6B). Similar results were obtained by microarray analyses (data not shown), indicating that activation of p38 MAP kinase did not affect bcl-2 gene expression but rather regulated Bcl-2 protein levels in CD8+ T cells.
Regulation of IFN-γ production by p38 MAP kinase in CD8+ T cells.
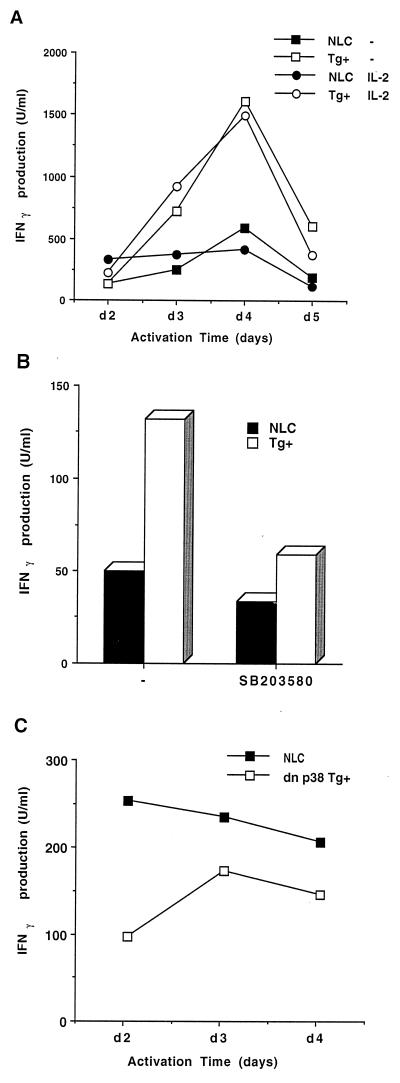
We have recently demonstrated that the activation of p38 MAP kinase increases IFN-γ production during differentiation of CD4+ T cells (44). IFN-γ is also produced by activated CD8+ T cells as an effector molecule. The current results indicate that activation of p38 MAP kinase causes apoptosis in CD8+ T cells but not in CD4+ T cells. We therefore examined the effect of p38 MAP kinase activation on the production of IFN-γ in CD8+ T cells. IFN-γ production in MKK6(Glu) and control CD8+ T cells was determined at different times after activation with ConA in the presence or absence of IL-2. Despite the inability of CD8+ T cells from the MKK6(Glu) transgenic mice to proliferate, these cells produced large amounts of IFN-γ compared to control CD8+ T cells (Fig. 7A). The presence of SB203580 inhibited the overproduction of IFN-γ by CD8+ T cells from the MKK6(Glu) transgenic mice (Fig. 7B). These results indicate that persistent activation of p38 MAP kinase also potentiated IFN-γ production by antigen-stimulated CD8+ T cells.
FIG. 7.
Regulation of IFN-γ production by p38 MAP kinase in CD8+ T cells. (A) CD8+ T cells (106 cells/ml) from NLC and MKK6(Glu) transgenic (Tg+) mice were isolated and activated with ConA (2.5 μg/ml) and APC (5 × 105 cells/ml) in the absence (−) or presence (IL-2) of IL-2 (30 U/ml). The supernatants were harvested at different periods of time upon stimulation and were analyzed by ELISA to determine IFN-γ production. (B) CD8+ T cells (106 cells/ml) were isolated and activated with ConA (2.5 μg/ml) in the absence (−) or presence of SB203580 (1 μM). The supernatants were harvested 3 days poststimulation and were analyzed by ELISA to determine IFN-γ production. (C) CD8+ T cells (106 cells/ml) from NLC and dn p38 transgenic mice were isolated and activated with ConA and APC in the absence or presence of IL-2 (30 U/ml). The supernatants were harvested at different periods of time upon stimulation and were analyzed by ELISA to determine IFN-γ production.
Activation of p38 MAP kinase is required for IFN-γ expression in effector CD4+ Th1 cells (44). To determine whether activation of the p38 MAP kinase pathway was also required for induction of IFN-γ in CD8+ T cells, we examined CD8+ T cells from the dn p38 transgenic mice. CD8+ T cells from control and dn p38 transgenic mice were stimulated with ConA for different periods of time. The production of IFN-γ was lower in CD8+ T cells from the dn p38 transgenic mice than in CD8+ T cells from control animals (Fig. 7C). Thus, the p38 MAP kinase pathway is required for IFN-γ expression in both CD4+ and CD8+ T cells but induces cell death selectively in CD8+ T cells.
DISCUSSION
The molecular mechanisms that control commitment to the CD4 or CD8 lineage, effector function, and homeostasis of CD4+ and CD8+ T cells represent an aspect of the T-cell response that remains unclear. Despite the presence of the TCR in both CD4 and CD8 subsets, distinct sources of costimulation and intracellular signaling pathways can control the activation, survival, and death of CD4+ and CD8+ T cells. Here, we demonstrate that the p38 MAP kinase signaling pathway is implicated in the control of IFN-γ production in both CD4+ and CD8+ T cells but that it regulates apoptosis selectively in CD8+ T cells and not in CD4+ T cells.
IFN-γ is an effector cytokine produced by several cell types, such as CD4+ and CD8+ T cells (1). Little is known about the molecular mechanisms that regulate the expression of this cytokine in different types of effector cells. It has recently been shown that TCR-mediated IFN-γ production is dependent on Stat4 in CD4+ T cells but not in CD8+ T cells (3). We have previously shown the importance of the p38 MAP kinase pathway on the production of IFN-γ in CD4+ Th1 effector cells (44). Inhibition of p38 MAP kinase reduces the production of IFN-γ, while activation of this pathway increases IFN-γ production in CD4+ Th1 cells. In contrast, IL-4 production by CD4+ Th2 cells is not affected by p38 MAP kinase (44). In this study, we have shown that activation of the p38 MAP kinase pathway results in an elevated TCR-mediated IFN-γ production by CD8+ T cells, while p38 MAP kinase inhibition reduced IFN-γ production in these same cells. Thus, the p38 MAP kinase pathway plays a key role in the control of IFN-γ gene expression in both CD4+ and CD8+ T-cell populations.
The p38 MAP kinase pathway has been implicated in several biological processes, including proliferation, cell death, and cytokine expression; however, its role in cell death remains unclear. p38 MAP kinase has been shown to be necessary for apoptosis in the PC12 neuronal cell line (62). Activation of MKK6 induces cell death in Jurkat T cells, although this effect is not mediated by p38 MAP kinase (22). Our results demonstrate that in vivo expression of a constitutively activated MKK6 in transgenic mice causes apoptosis of CD8+ T cells but not CD4+ T cells. Inhibition of p38 MAP kinase rescues MKK6(Glu) CD8+ T cells from death. Different intracellular signaling pathways therefore control cell death and survival in these two T-cell populations. The selective induction of apoptosis in CD8+ T cells by p38 MAP kinase indicates that this pathway is critical to maintaining normal CD4/CD8 homeostasis.
Several studies have shown that the disruption of specific signaling pathways leads to changes in peripheral CD4/CD8 homeostasis. The expression of an activated form of the cell surface receptor Notch in thymocytes leads to both an increase of the CD8 lineage and a decrease of the CD4 lineage (45). Expression of a mutant form of IκBα in the thymus causes a reduction of CD8+ T cells in both the periphery and the thymus (2). Activation of the ERK MAP kinase pathway favors the differentiation to the CD4 lineage in the thymus and periphery (48). An increased CD4/CD8 ratio was observed for peripheral organs and the thymuses of mice deficient in Jak3 (37, 39, 50, 53). In these mouse models, impairment of thymic maturation and lineage commitment appears to be the cause of the changes in the CD4/CD8 homeostasis. In contrast, in our study, we show that the persistent activation of p38 MAP kinase results in the specific loss of CD8+ T cells in the peripheral immune system, while thymic development does not appear to be affected.
It has been shown that the in vitro overexpression of wild-type MKK6 in fetal thymus organ culture due to retroviral infection causes deletion of DP thymocytes (51). We did not observe an impairment of DP thymocyte development in the MKK6(Glu) transgenic mice, likely because the level of expression of the MKK6(Glu) transgene in DP thymocytes driven by the distal lck promoter is lower than the level of retrovirus-mediated expression. Moreover, the distal lck promoter does not drive expression in DN thymocytes (59). However, using recently generated transgenic mice that express MKK6(Glu) in all thymocyte populations under the control of the proximal lck promoter, we have shown that activation of p38 MAP kinase is required for early stages of DN thymocyte differentiation (8a).
Bcl-2 and Bcl-xL are antiapoptotic components which display an inverse pattern of expression during lymphocyte development. Within the thymus, Bcl-2 is expressed only in a few DP thymocytes, but it is widely expressed in mature CD4+ and CD8+ thymocytes and peripheral T cells (15, 21, 55). Alternatively, Bcl-xL is present in DP thymocytes but absent from mature single-positive thymocytes and resting peripheral T cells (16, 31). We have shown that activation of p38 MAP kinase results in a decreased expression of Bcl-2 that is not compensated for by increased amounts of Bcl-xL in CD8+ T cells. In contrast, Bcl-2 levels in CD4+ T cells were not affected by p38 MAP kinase activation. Bax is a proapoptotic member of the Bcl-2 family which heterodimerizes with Bcl-2 and homodimerizes with itself (38). A high Bax/Bcl-2 ratio accelerates cell death. Activation of p38 MAP kinase does not affect the amount of Bax present in CD8+ T cells, but the diminished Bcl-2 level in these cells increases the Bax/Bcl-2 ratio, and that could increase the rate of apoptosis. Thus, the downregulation of Bcl-2 constitutes a potential mechanism for induction of apoptosis by the p38 MAP kinase pathway in specific mammalian cells. In correlation with the decreased level of Bcl-2 and the loss of the CD8 lineage in the MKK6(Glu) transgenic mice, lower percentages of CD8+ T cells and normal CD4+ T cells have also been observed in Bcl-2-deficient mice (35). Interestingly, Bcl-2-deficient CD8+ T cells die more quickly than Bcl-2-deficient CD4+ T cells, supporting the model of different regulatory mechanisms for CD4+ and CD8+ T-cell death.
Despite the low level of Bcl-2 protein present in CD8+ T cells from the MKK6(Glu) transgenic mice, we did not observe a significant difference in the expression of the bcl-2 gene. This suggests that p38 MAP kinase could regulate Bcl-2 levels by posttranscriptional mechanisms. Several posttranslational mechanisms have been found to be involved in the regulation of Bcl-2 function (4). Recently, it has been shown that phosphorylation by the ERK pathway prevents ubiquitination-dependent degradation of Bcl-2 in endothelial cells (9). In addition, the level of Bcl-2 protein is regulated by caspase-mediated cleavage, and the caspase cleavage fragment of Bcl-2 appears to cause the release of cytochrome c (5, 23, 27). We have shown that p38 MAP kinase-induced CD8+ T-cell apoptosis is mediated by caspases. It is therefore possible that the decreased Bcl-2 level may be mediated by activation of the caspase pathway in these cells.
Our results suggest that regulation of p38 MAP kinase due to antigenic or environmental stimuli could affect the survival of CD8+ T cells in the periphery. Recently, it has been reported that the decreased number of CD8+ T cells observed in advanced AIDS patients is due to increased apoptosis mediated by the interaction between macrophage-bound TNF-α and a TNF-α receptor on CD8+ T cells (20). In addition, CD8+ cells from human immunodeficiency virus-infected individuals display reduced levels of Bcl-2, while Bcl-2 expression on CD4+ T cells is normal. It is possible that activation of the p38 MAP kinase signaling pathway by membrane-bound TNF-α could downregulate Bcl-2 in CD8+ T cells, rendering these cells highly susceptible to apoptosis.
Our studies demonstrate that the p38 MAP signaling pathway can control both cell death and cytokine production during an immune response. However, the specific function of this pathway depends on the cell type; it regulates IFN-γ expression in both CD4+ and CD8+ T cells and promotes death selectively in CD8+ T cells. The p38 MAP kinase pathway therefore plays a important regulatory role in the function and fate of CD4+ and CD8+ T cells.
ACKNOWLEDGMENTS
We thank R. A. Flavell for kindly providing the transgenic mice and helpful discussion, and M. S.-S. Su for kindly providing SB203580, D. T. Zapton for expert technical assistance, and C. Charland for flow cytometry analysis and helpful discussion.
This work was supported in part by the Howard Hughes Medical Institute Research Resource Program for Medical Schools and Arthritis Foundation Research grants (M.R.) and grants CA 65861 and CA72009 (R.J.D.). D.C. is a recipient of the Vermont EPSCoR Graduate Research Fellowship. R.J.D. is an Investigator of the Howard Hughes Medical Institute.
C.M. and H.E. contributed equally to this work.
REFERENCES
- 1.Billiau A. Interferon-g: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 2.Boothby M R, Mora A L, Scherer D C, Brockman J A, Ballard D W. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor(NF)-kappaB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter L L, Murphy K M. Lineage-specific requirements for signal transducer and activator of transcription (Stat)4 in interferon γ production from CD4+ versus CD8+ T cells. J Exp Med. 1999;189:1355–1360. doi: 10.1084/jem.189.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao D T, Korsmeyer S J. Bcl-2 family: regulators of cell death. Ann Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 5.Cheng E H, Kirsch D G, Clem R J, Ravi R, Kastan M B, Bedi A, Ueno K, Hardwick J M. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 6.Deak M, Clifton A D, Lucocq L M, Alessi D R. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dérijard B, Hibi M, Wu I-H, Barret T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 8.Dérijard B, Rainjeaud J, Barret T, Wu I-H, Han J, Ulevitch R J, Davis R J. Independent human MAP kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:683–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 8a.Diehl, N. L., H. Enslen, K. A. Fortner, C. Merrit, N. Stetson, C. Charland, R. A. Flavell, R. J. Davis, and M. Rincón. Activation of the p38 MAP kinase pathway arrests cell cycle progression and differentiation of immature thymocytes in vivo. J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 9.Dimmeler S, Breitschopf K, Haendeler J, Zeiher A M. Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteosome pathway. J Exp Med. 1999;189:1815–1822. doi: 10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faris M, Kokot N, Latinis K, Kasibhatla S, Green D R, Koretzky G A, Nel A. The c-Jun N-terminal kinase cascade plays a role in stress-induced apoptosis in Jurkat cells by up-regulating Fas ligand expression. J Immunol. 1998;160:134–144. [PubMed] [Google Scholar]
- 11.Fauci A S. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 12.Foltz I N, Lee J C, Young P R, Schrader J W. Hemopoietic growth factors with the exception of interleukin-4 activate the p38 mitogen-activated protein kinase pathway. J Biol Chem. 1997;272:3296–3301. doi: 10.1074/jbc.272.6.3296. [DOI] [PubMed] [Google Scholar]
- 13.Freshney N W, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of the Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 14.Fukunaga R, Hunter T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997;16:1921–1933. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gratiot-Deans J, Ding L, Turka L A, Nuñez G. bcl-2 proto-oncogene expression during human T cell development. Evidence for biphasic regulation. J Immunol. 1993;151:83–91. [PubMed] [Google Scholar]
- 16.Grillot D A, Merino R, Nuñez G. Bcl-XL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med. 1995;182:1973. doi: 10.1084/jem.182.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han J, Jian Y, Li Z, Kravchenko V V, Ulevitch R J. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- 18.Han J, Lee J-D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Lee J-D, Jiang Y, Li Z, Feng L, Ulevitch R J. Characterization of the structure and function of a novel MAP kinases kinases (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 20.Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O'Brian W A, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189–194. doi: 10.1038/26026. [DOI] [PubMed] [Google Scholar]
- 21.Hockenbery D M, Zutter M, Hickey W, Nahm M, Korsmeyer S J. Bcl-2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci USA. 1991;88:6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang S, Jiang Y, Li Z, Nishida E, Mathias P, Lin S, Ulevitch R J, Nemerow G R, Han J. Apoptosis signaling pathway in T cells is composed of ICE/Ced-3 family proteases and MAP kinase kinase 6b. Immunity. 1997;6:739–749. doi: 10.1016/s1074-7613(00)80449-5. [DOI] [PubMed] [Google Scholar]
- 23.Johnson B W, Boise L H. Bcl-2 and caspase inhibition cooperate to inhibit tumor necrosis factor-α-induced cell death in a Bcl-2 cleavage-independent fashion. J Biol Chem. 1999;274:18552–18558. doi: 10.1074/jbc.274.26.18552. [DOI] [PubMed] [Google Scholar]
- 24.Kamogawa Y, Minasi L-A E, Carding S, Bottomly K, Flavell R A. The relationship of IL-4 and IFNγ-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 1993;75:985–995. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- 25.Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green D R. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1998;1:543–541. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- 26.Kihara-Negishi F, Yamada T, Kubota Y, Kondoh N, Yamamoto H, Abe M, Shirai T, Hashimoto Y, Oikawa T. Down-regulation of c-myc and bcl-2 gene expression in PU.1-induced apoptosis in murine erythroleukemia cells. Int J Cancer. 1998;76:523–530. doi: 10.1002/(sici)1097-0215(19980518)76:4<523::aid-ijc14>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Kirsch D G, Doseff A, Chau B N, Lim D-S, de Souza-Pinto N C, Hansford R, Kastan M B, Lazebnik Y A, Hardwick J M. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochorme c. J Biol Chem. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- 28.Lee J C, Laydon T, McDonnell P C, Gallagher T F, Kumar S, Gree D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 29.Los M, Wesselborg S, Schulze-Osthoff K. The role of caspases in development, immunity, and apoptotic signal transduction: lessons from knockout mice. Immunity. 1999;10:629–639. doi: 10.1016/s1074-7613(00)80062-x. [DOI] [PubMed] [Google Scholar]
- 30.Lu H T, Yang D D, Wysk M, Gatti E, Mellman I, Davis R J, Flavell R A. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma A, Pena J C, Chang B, Margosian E, Davidson L, Alt F W, Thompson C B. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci USA. 1995;92:4763–4767. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Margolick J B, Muñoz A, Donnenberg A D, Park L P, Galai N, Giorgi J V, O'Gorman M R, Ferbas J. Failure of T-cell homeostasis preceding AIDS in HIV-1 infection. The Multicenter AIDS Cohort Study. Nat Med. 1995;1:674–680. doi: 10.1038/nm0795-674. [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin M M, Kumar S, McDonnell P C, Van Horn S, Lee J C, Livi G P, Young P R. Identification of mitogen-activated protein (MAP) kinase-activated protein kinase-3, a novel substrate of CSBP p38 MAP kinase. J Biol Chem. 1996;271:8488–8492. doi: 10.1074/jbc.271.14.8488. [DOI] [PubMed] [Google Scholar]
- 34.Moriguchi T, Kuroyanigi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida B, Hagiwara M. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 35.Nakayama K, Negishi I, Kuida K, Sawa H, Loh D Y. Targeted disruption of Bcl-2 αβ in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.New L, Jiang Y, Zhao M, Liu K, Zhu W, Flood L J, Kato Y, Parry G C, Han J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nosaka T, van Deursen J M, Tripp R A, Thierfelder W E, Witthuhn B A, McMickle A P, Doherty P C, Grosveld G C, Ihle J N. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 38.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 39.Park S Y, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 40.Raingeaud J, Gupta S, Roger J, Dickens M, Han J, Ulevitch R J, Davis R J. Pro-inflammatory cytokines and environmental stress cause p38 MAP kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 41.Raingeaud J, Whitmarsh A J, Barret T, Dérijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rincón M, Anguita J, Nakamura T, Fikrig E, Flavell R A. IL-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rincón M, Dérijard B, Chow C-W, Davis R J, Flavell R A. Reprogramming the signaling requirement for AP-1 (activator protein-1) activation during differentiation of precursor CD4+ T-cells into effector Th1 and Th2 cells. Genes Funct. 1997;1:51–68. doi: 10.1046/j.1365-4624.1997.00007.x. [DOI] [PubMed] [Google Scholar]
- 44.Rincón M, Enslen H, Raingeaud J, Recht M, Zapton T, Su M S-S, Penix L A, Davis R J, Flavell R A. Interferon-γ expression by Th1 effector T cells mediated by the p38 MAP kinase signaling pathway. EMBO J. 1998;17:2817–2829. doi: 10.1093/emboj/17.10.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robey E, Chang D, Itano A, Cado D, Alesander H, Lans D, Weinmaster G, Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- 46.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 47.Schnittman S M, Fauci A S. Human immunodeficiency virus and acquired immunodeficiency syndrome: an update. Adv Intern Med. 1994;39:305–355. [PubMed] [Google Scholar]
- 48.Sharp L L, Schwarz D A, Bott C M, Marshall C J, Hedrick S M. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7:609–618. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- 49.Shuford W W, Klussman K, Tritchler D D, Loo D T, Chalupny J, Siadak A W, Brown T J, Emswiler J, Raecho H, Larsen C P, Pearson T C, Ledbetter J A, Aruffo A, Mitler R S. 4-1BB costimulatory signals preferentially induce CD8+ T cells proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sohn S J, Forbush K A, Nguyen N, Witthuhn B, Nosoka T, Ihle J N, Perlmutter R M. Requirement for Jak3 in mature T cells: its role in regulation of T cell homeostasis. J Immunol. 1998;160:2130–2138. [PubMed] [Google Scholar]
- 51.Sugawara T, Moriguchi T, Nishida E, Takahama Y. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998;9:565–574. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki I, Fink P J. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187:123–128. doi: 10.1084/jem.187.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomis D C, Gurniak C B, Tivol E, Sharpe A H, Berg L J. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 54.Tong L, Pav S, White D M, Rogers S, Crane K M, Cywin C L, Brown M L, Pargellis C A. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nat Struct Biol. 1997;4:311–316. doi: 10.1038/nsb0497-311. [DOI] [PubMed] [Google Scholar]
- 55.Veis D J, Sentman C L, Bach E A, Korsmeyer S J. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 56.Wang X-Z, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- 57.Waskiewicz A J, Flynn A, Proud C G, Cooper J A. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitmarsh A J, Yang S H, Su M S, Sharrocks A D, Davis R J. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wildin R S, Garvin A M, Pawar S, Lewis D B, Abraham K M, Forbush K A, Ziegler S F, Allen J M, Perlmutter R M. Developmental regulation of lck gene expression in T lymphocytes. J Exp Med. 1991;173:383–393. doi: 10.1084/jem.173.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilson K P, McCaffrey P G, Hsiao K, Pazhanisamy S, Galullo V, Bemis G W, Fitzgibbon M J, Caron P R, Murcko M A, Su M S. The structural basis for the specificity of pyridinylimidazole inhibitors of p38 MAP kinase. Chem Biol. 1997;4:423–431. doi: 10.1016/s1074-5521(97)90194-0. [DOI] [PubMed] [Google Scholar]
- 61.Wysk M, Yang D D, Lu H T, Flavell R A, Davis R J. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc Natl Acad Sci USA. 1999;96:3763–3768. doi: 10.1073/pnas.96.7.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Opposing effects of ERK and JNK-p38 MAP kinase on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 63.Yang D D, Kuan C-Y, Whitmarsh A J, Rincón M, Zheng T S, Davis R J, Rakic P, Flavell R A. Absence of excitotoxicity-induced apoptosis in the hippocampus of mice lacking the Jnk3 gene. Nature. 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 64.Young P R, McLaughlin M M, Kumar S, Kassis S, Doyle M L, McNulty D, Gallagher T F, Fisher S, McDonnell P C, Carr S A, Huddleston M J, Seibel G, Porter T G, Livi G P, Adams J L, Lee J C. Pyridinyl imidazole inhibitors of p38 mitogen-activated protein kinase bind in the ATP site. J Biol Chem. 1997;272:12116–12121. doi: 10.1074/jbc.272.18.12116. [DOI] [PubMed] [Google Scholar]