Abstract
The golden standard to treat acute pain is by intravenous drug delivery of opioids such as fentanyl or morphine. Intravenous drug delivery requires the placement of an intravenous (IV) port, which can cause infections, dislodgments, and distress to the patients, and therefore a non-invasive method is desirable. Pulmonary drug delivery is a non-invasive method that has been shown to be a good alternative to intravenous administration. New devices have been investigated for treating acute pain by delivering fentanyl by heat. The pure drug, fentanyl, is applied onto a surface which is then heated up to 350 °C and inhaled, resulting in no formation of degradation products. Furthermore, forced degradation of fentanyl has been studied which showed that longer heating time and higher temperatures will result in the formation of degradation products. The evidence indicates that heat can be used to deliver drugs to the lungs where fast onset reaction can be obtained giving fast and non-invasive pain relief.
Keywords: Fentanyl, Inhalation, Acute pain
Graphical abstract
Canwedeliverdrugsin a fast an non-invasivewayby usingheat? –A reviewstudy Yes, by targetingthe lungs!
1. Introduction
Fentanyl was introduced in 1962 and is a potent synthetic opioid that is an antagonist on the μ-receptor (Stanley et al., 2008). Due to its fast onset reaction (1–2 min) and high potency, only a small amount is needed for effect, making it a perfect candidate for treating pain (Vallejo et al., 2011). Treating acute pain involves intravenous injection or insertion of an IV port which can be an unpleasant experience for the patients (Helm et al., 2015). To target the lungs, could be the answer to treat acute pain in a non-invasive way. Pulmonary drug delivery has been used in many pharmaceutical fields such as treating asthma. New studies have shown an increased interest in pulmonary drug delivery for pain relief due to its fast onset reaction and non-invasive method (Macleod et al., 2012; Mather et al., 1998). Various devices such as dry powder inhalers, metered dosed inhalers, and nebulizers are used to deliver the drug to the lungs. However, all these conventional devices have major limitations including humidity sensitivity and possible dosing errors. This has resulted in a new device emerging that is based on heat which allows the drug to be released when the heat is applied (Dinh et al., 2011; Myers et al., 2021). The aim of this review is to examine the current field of inhaled fentanyl and the possibility of using heat.
2. Literature search
A systematic search was conducted in the following databases: PubMed, Web of Science, and Scopus with the following search criteria: fentanyl AND inhalation AND poorly soluble drugs AND analogs AND inhalation devices AND degradation of fentanyl. Cite references were also used to find additional articles. Only articles written in English were considered for this review.
3. Treating acute pain
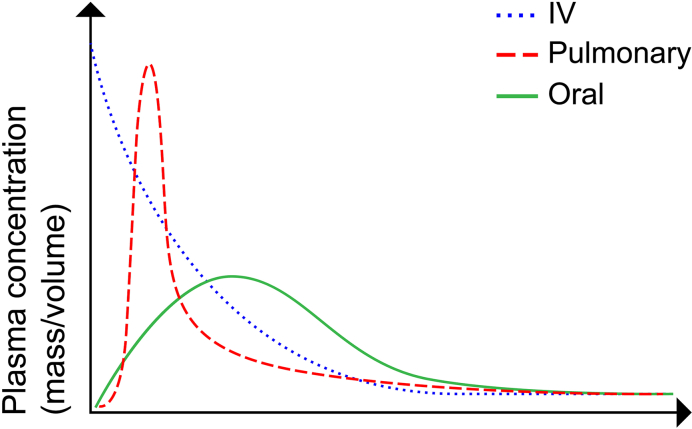
70% of all visits to the emergency room are due to acute pain (Berben et al., 2008). Treating pain has always been a fundamental goal in patient care, and different opioids have been the main tool to reach that goal. The most common treatment for acute pain is the intravenous administration of opioids such as fentanyl and morphine. Intravenous administration requires the placement of an IV port which can be highly unpleasant to the patient and in the worst case even complications such as infections or dislodgements (Helm et al., 2015). Other common ways to treat acute pain are intranasal, sublingual, and pulmonary drug delivery which bypasses the first-pass metabolism and reaches the central nervous system. Oral administration of fentanyl goes through the first pass metabolism and therefore has a lower bioavailability. Moreover, oral administration results in a significantly lower plasma concentration and requires a longer time to reach maximal plasma concentration compared to intravenous and pulmonary drug delivery, Fig. 1. Previous studies have shown that nebulized fentanyl can be a good alternative to IV administration (Mather et al., 1998; Thompson and Thompson, 2016). Mather et al. compared nebulized fentanyl with IV administrated fentanyl in 15 volunteers over 8 weeks (Mather et al., 1998). The plasma concentration profile showed that IV administrated fentanyl reached a maximum concentration of around 2 to 4 min while pulmonary administration reached the maximum concentration around 4 to 9 min. However, the volunteers reported feeling pain relief almost immediately after both inhalation and intravenous administration. Nebulizers have proven to be a promising candidate for pulmonary drug delivery but it still has some limitations such as manufacturing, variability in devices and the size which reduces mobility (Thompson and Thompson, 2016).
Fig. 1.
Illustration of plasma concentration with a different way of treatment.
4. Pulmonary drug delivery
Pulmonary drug delivery is a non-invasive method that has the ability to absorb drugs for both local and systematic delivery. The pulmonary route has some advantages over the more conventional methods such as oral drug delivery which has increased the interest in finding new therapies such as diabetes, vaccines, pain, to target the lungs. The pulmonary route offers many advantages such as a large surface area (70–140 m2 in adults) for absorption, high bioavailability, rapid uptake by the alveoli, rich blood supply, and the drugs will not be exposed to extreme pH or any type of metabolism in the pulmonary track (Ali, 2010). By using pulmonary drug delivery systematic side effects can be avoided due to the rapid onset action and the possibility of using a smaller dose.
The gas exchange occurs at the alveolar epithelium and is crucial for the delivery of drug molecules (Osman et al., 2018; Scheuch and Siekmeier, 2007; Siekmeier and Scheuch, 2008). The epithelium has a thickness of 0.1–0.2 μm, meaning that the distance to the blood is 0.5–1.0 μm resulting in short drug transportation from epithelium to the bloodstream (Patton and Byron, 2007; Wolff, 1998). The epithelium is lined with a pulmonary surfactant which consists of a mixture of proteins and lipids (Guagliardo et al., 2018). The epithelium consists of type I and type II cells where type I is responsible for the gas exchange and type II acts as a progenitor cell and can help to repopulate the epithelium. Type II cells are also responsible for transporting drugs by endocytosis (Patton, 1996; Takano et al., 2015). However, to reach the alveoli different parameters need to be considered; formulation, particle size, and device.
4.1. Poorly soluble drugs
The low solubility of drugs results in a challenge when it comes to developing a new pharmaceutical product where approximately 90% of new drugs have been classified as poorly soluble in water (Rodriguez-Aller et al., 2015). There are different strategies to increase the solubility for low soluble drugs such as using surfactants and lipids or modification of the solid-state by increasing the structural disorder by transforming the drug crystal into polymorph, salt, or amorphous form (Rodriguez-Aller et al., 2015). The alveoli have a natural surfactant layer which makes it possible to solubilize poorly soluble drugs where several studies have attempted to target the surfactant layer in the alveoli (Guagliardo et al., 2018).
Liposomes are mostly developed for intravenous delivery but few liposomes are in clinical development for pulmonary delivery. Liposomes are small particles (100 nm) that possess unique properties which make them suitable for pulmonary drug delivery. Due to their small particle size, they can penetrate leaking tumor vessels and accumulate in cancerous tissue where they can release the drug to the site. Only one study reported inhaled fentanyl liposomes where intravenous administration of fentanyl was compared in vivo to inhaled fentanyl liposomes (Hung et al., 1995). The result showed it is possible to deliver fentanyl by using liposomes for encapsulating, however, it gave a prolonged release compared to the instant delivery by the intravenous delivery. Bigger progress has been made in chemotherapy and liposomes where several products are under clinical trials (Guma et al., 2014; Rudokas et al., 2016a; Skubitz and Anderson, 2000). By targeting lung cancer through inhalation, systematic adverse effects of chemotherapy can be avoided. Guma et al. investigated the effect of inhaled liposomal interleukin-2 (IL-2) on pulmonary metastases from osteosarcoma in dogs which showed that inhaled IL-2 was effective against the metastases (Guma et al., 2014). The effect of IL-2 on pulmonary metastases was also investigated in-vivo by Skubitz et al. where they showed anti-tumor activity in patients after administration of the drug (Skubitz and Anderson, 2000).
Another approach to delivering poorly soluble drugs is to use micelles that have a lipophilic core and hydrophilic shell, Fig. 2. The use of micelles for pulmonary drug delivery has mostly included treatments for asthma (Pellosi et al., 2018; Sahib et al., 2012; Triolo et al., 2017). Sahib et al. successfully loaded nanomicelles with beclomethasone dipropionate (BDP), a steroid for treating asthma. They reported a high deposition of BDP in vivo after pulmonary drug delivery. Budesonide, another common drug to treat asthma, has been loaded into micelles for pulmonary drug delivery by Pellosi et al. (Pellosi et al., 2018). The micelles were mixed with a polymer, Pluronic, which can be modified to impart cell targeting properties (Pellosi et al., 2016). The budesonide-loaded Pluronic-micelles were shown to be much more effective in vivo in controlling the inflammatory process compared to conventional suspensions.
Fig. 2.
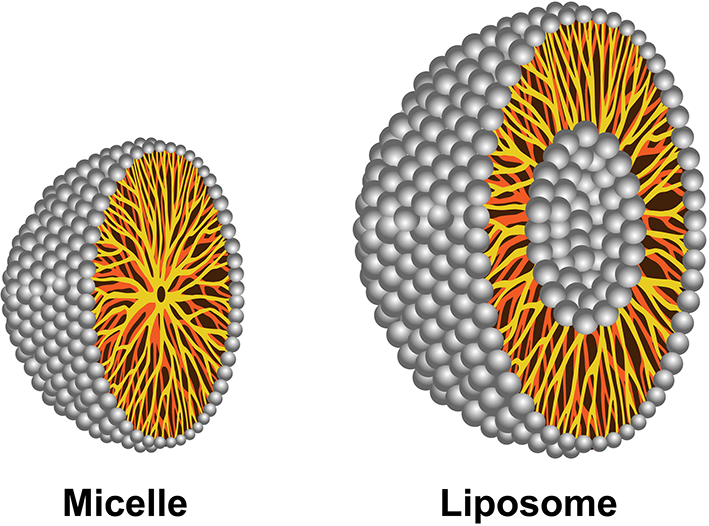
Illustration of micelle with a monolayer and liposome with a double phospholipid payer.
Even though liposomes and micelles are promising formulations for pulmonary drug delivery of poorly soluble drugs, they still have some major limitations regarding pharmaceutical manufacturing; (i) reproducibility, (ii) stability of the encapsulated drug, and (iii) shelf-life stability (Sercombe et al., 2015a).
4.2. Particle size and devices
The particle size for inhalation is referred to as mass median aerodynamic diameter (MMAD) and plays an important role in delivering the particles to the lungs. Studies have shown that MMAD should have a range of 1–3 μm in order to reach the lung (Lipworth et al., 2014). Particles with a size greater than 5 μm tend not to pass the oropharyngeal cavity (Heyder et al., 1986). Traditionally, particles with an MMAD smaller than 1 μm have not been considered until recently. New studies have investigated the effect of smaller particles <1 μm and the distribution to the deep lung (Hodges et al., 2002). It has been shown that smaller particles have a higher probability to reach the deep lung and the outer alveoli, making it possible to deliver the drug to the active site while larger particles cannot reach as far as the smaller particles.
To obtain the desired particle size the drug formulation is very important. Different types of formulations demand different types of devices where the most common devices are the dry powder inhaler (DPI), metered dosed inhaler (MDI), and nebulizer. DPI and MDI are common devices for the treatment of asthma. DPI has preloaded doses in the inhaler which are controlled by a rotating disk and the dose is inhaled as a dry powder. MDI has a pressurized canister with a predetermined dose that releases as a spray from the canister and becomes an aerosol by a volume expansion. A nebulizer is a larger device that generates an aerosol from a liquid and therefore can only be used in one place, such as in hospitals. These devices possess limitations such as limited variation of drugs, humidity sensitivity (PDI), possible dosing errors (MDI), and portability issues (nebulizers), Table 1. Due to the limitation of the traditional devices new types of devices have been emerging on the market.
Table 1.
Advantages and limitations for each inhalation device.
| Device | Advantage | Limitation | Reference |
|---|---|---|---|
| Nebulizer | Easy to use. The dose can be adjusted to the patient. |
A large device that requires power. Requires a minimum volume of 2 mL. Longer user time. |
(Johns and Roberts, 2007; Thompson and Thompson, 2016) |
| Dry powder inhaler | Simple and easy to use. | Sensitive to humidity. Requires respiratory coordination. |
(GK, 1991) |
| Metered dosed inhaler | Allows micro pulverization. | Requires high inspiratory flow. Possible dosing errors. |
(GK, 1991) |
| Staccato | Allows inhalation of pure drugs. Portable. |
Usage of heat to release the drug might lead to degradation. | (Dinh et al., 2011) |
5. A new generation of inhalers
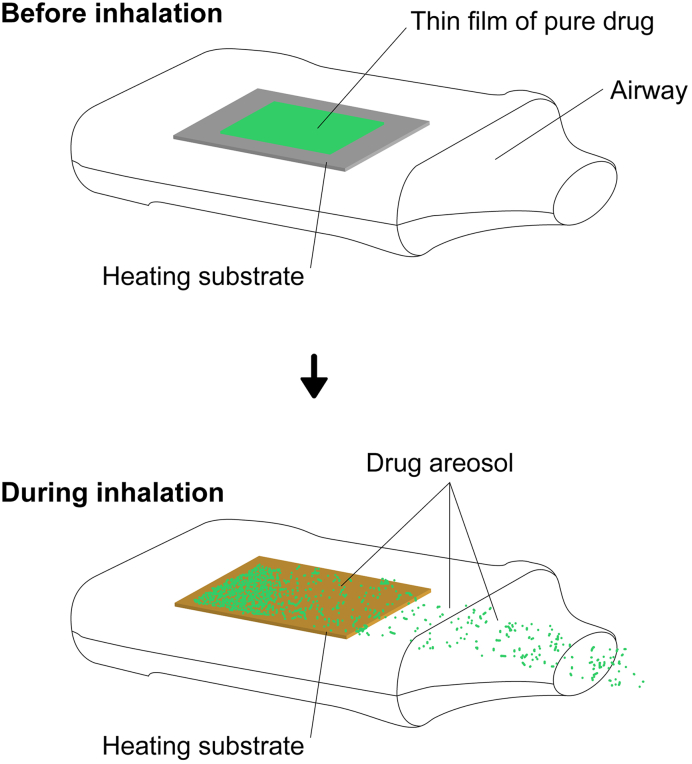
Currently, some new devices are on the market and under development to deliver highly potent drugs via inhalations. Alexza pharmaceutical is developing an inhaler, Staccato®, which has a thin coating of a drug on a metal plate that heats up for 1 min at ~350 °C releasing the drug from the plate, making it possible for the patient to inhale it, Fig. 3 (Myers et al., 2021). In Staccato® the heat is used to release the drug and no excipients are used, i.e. the pure drug is applied onto the metal plate. Currently, Staccato® in combination with the drug loxapine is approved by the European Medicine Agency for the treatment of schizophrenia or bipolar I disorder (CHMP, 2012). Other substances such as fentanyl in combination with the device are under clinical trials (Macleod et al., 2012). Furthermore, in vivo testing has shown very high bioavailability for the inhaled drug and that the full dose has been detected in the plasma concentration (Macleod et al., 2012).
Fig. 3.
Staccato device showing the release of drug when the heat is applied. Adapted with modifications (Dinh et al., 2011).
6. Usage of heat and its complications
Subjecting molecules to heat usually results in the degradation of the molecular structure. Thermal analysis has been crucial in drug development to determine the molecules' stability and formation of unwanted degradants (Fang et al., 2015). However, despite the complication of using heat for drug delivery, the interest has increased over the last decade resulting in the development of new devices (Myers et al., 2021). Thorough investigations need to be done when using heat for drug delivery to assure the safety of the patients. The formed degradation product or the alternative molecules can in some cases be more toxic than the original molecule and some might not have any effect at all (Armenian et al., 2018).
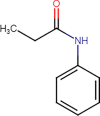
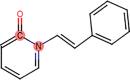
Forced Degradation of fentanyl has previously been investigated in different media; heat, UV, and acid (Garg et al., 2010; Lambropoulos et al., 1999). Rabinowitz et al. showed that the degradation of fentanyl on a hot plate for five minutes at 300 °C resulted in unknown peaks and a total degradation rate of 30% (Rabinowitz et al., 2004). A more thorough investigation of fentanyl degradations products has been assessed by Garg et al. who studied fentanyl degradation in UV, acid, base, heat, and oxidation (Garg et al., 2010). The heating test was performed for five minutes at 350 °C. The sample was heated in a glass tube and was allowed to cool down to room temperature for further analysis. The analysis showed the presence of five different degradants which were identified as; norfentanyl (NRF), propionanilide (PRP), 1-Phenethylpyridinium salt (1-PEP), 1-Phenethyl-1H-pyridin-2-one (1-PPO), and 1-Styryl-1H-pyridin-2-one (1-SPO), Table 2. NRF and PRP are considered to be the two most common thermal degradants of fentanyl which are not more potent than fentanyl (Armenian et al., 2018). Furthermore, NRF is a major urinary metabolite that is formed when fentanyl is metabolized by the CYP3A4 enzyme (Feierman and Lasker, 1996; Tateishi et al., 1996). MacLeod et al. investigated the possibility of using inhaled fentanyl by generating heated aerosols in healthy volunteers (Macleod et al., 2012). The volunteers used a predosed and handheld device Staccato® where the fentanyl is dosed on a thin foil, Fig. 2. The foil was heated up to 350 °C releasing the fentanyl and making it possible for the volunteers to inhale it. The time of inhalation was set to five seconds and the obtained results showed a high bioavailability of 100% which can be directly compared to IV administration of drugs. The plasma concentrations were analyzed for analogs and degradants, which showed that no analogs or degradants could be observed.
Table 2.
Compounds obtained after forced degradation of fentanyl with heat.
| Compound | NRF Mw 232 g/mol |
PRP Mw 149 g/mol |
1-PPO Mw 199 g/mol |
1-SPO Mw 197 g/mol |
1-PEP Mw 184 g/mol |
|---|---|---|---|---|---|
| Structure |  |
 |
 |
 |
 |
The release of fentanyl by using heat has been investigated previously by us (Vazda et al., 2021). Fentanyl was dosed onto calcium sulfate ceramic and released when heated. The heating was done in a modified commercial device (PAX-3) where the evaporated drug was collected by a syringe. A temperature of 350 °C was used and heating was done for 1 and 5 min, which showed that it was possible to release and collect fentanyl by applying heat. Unknown peaks were detected, indicating the formation of possible degradants. However, the degradants were not assessed due to the lack of instrumentation.
7. Discussion
Curing acute pain has been one of the main goals in patient care. The golden standard for treating acute pain is by intravenous delivery of opioids such as fentanyl or morphine. The insertion of an IV port might cause the patient distress etc. Previous studies have shown that nebulized fentanyl is a good candidate for non-invasive treatment for acute pain. Even though the nebulizer is a potential candidate it still has some limitations such as manufacturing, variability in devices, and the size which reduces mobility (Thompson and Thompson, 2016).
Some studies have reported fentanyl's bioavailability which was significantly lower compared to intravenous administration (Higgins et al., 1991; WORSLEY et al., 1990). The pulmonary administration of morphine has also been compared to the intravenous administration which also showed low bioavailability (17–59%) (Chrubasik et al., 1988; Dershwitz et al., 2000). A more recent study by MacLeod et al. reported a bioavailability close to 100% which is significantly higher than the previous studies (Macleod et al., 2012). A possible explanation for the low bioavailability might be the design of delivery systems. Macleod et al. used the Staccato® device in their study which used heat to release fentanyl and be inhaled by the patient, Fig. 2. The substrate was heated to 350 °C allowing the drug to be released and inhaled. Using heat at those high temperatures, to release drugs can be problematic since it can destroy or alter the molecule. Forced degradation of fentanyl has been studied resulting in the formation of various degradation products, Table 2. The degradation test was performed at 350 °C for five minutes compared to the in vivo study where the patients inhaled fentanyl after short heating of 0.5 s. This indicates that it is possible to use heat but only for a shorter period that will not result in degradation products.
The biggest progress in pulmonary drug delivery, other than asthma, has been with the treatment of lung cancer (Kumar et al., 2020; Rudokas et al., 2016b). Systematic effects can be avoided by targeting lung cancer via pulmonary drug delivery. The cytotoxic drugs are embedded in liposomes which are then dispersed into the lungs by an inhaler. The main limitations and challenges with liposomes are pharmaceutical manufacturing and reproducibility (Sercombe et al., 2015b). Since cytotoxic drugs are classified as poorly soluble drugs, the heat might be a possible solution to deliver the drugs to the site of action.
8. Conclusion
Using heat to deliver drugs to the pulmonary system might be a good supplement for more common treatments such as intravenous, sublingual, buccal, nasal, or oral administration. Even though heating drugs result in degradation products when heated over a longer period, it still shows that it is possible to release the drug without the formation of degradants. Different approaches have been made to target the lungs which resulted in a difference in bioavailability indicating that the biggest source of error is the device/inhaler. Further research needs to be conducted to explore the possibilities of using other drugs for drug release by heat and to investigate device liability and reproducibility.
Author contribution
HE conceived the idea, AV collected literature data and wrote the manuscript, HE and WX reviewed the manuscript and provided comments. All authors approved the final version.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgment
Funding from the faculty is gratefully acknowledged.
References
- Ali M. Handbook of Non-Invasive Drug Delivery Systems. 2010. Chapter 9 - pulmonary drug delivery. [DOI] [Google Scholar]
- Armenian P., Vo K.T., Barr-Walker J., Lynch K.L. Fentanyl, fentanyl analogs and novel synthetic opioids: a comprehensive review. Neuropharmacology. 2018 doi: 10.1016/j.neuropharm.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Berben S.A.A., Meijs T.H.J.M., van Dongen R.T.M., van Vugt A.B., Vloet L.C.M., Mintjes-de Groot J.J., van Achterberg T. Pain prevalence and pain relief in trauma patients in the Accident & Emergency department. Injury. 2008;39:578–585. doi: 10.1016/j.injury.2007.04.013. [DOI] [PubMed] [Google Scholar]
- CHMP . 2012. Committee for Medicinal Products for Human Use (CHMP) Assessment Report. [Google Scholar]
- Chrubasik J., Wust H., Friedrich G., Geller E., Chrubasik Joachim, Wust Hans, Eran Geller F. Absorption and bioavailability of nebulized morphine. Br. J. Anaesth. 1988 doi: 10.1093/bja/61.2.228. [DOI] [PubMed] [Google Scholar]
- Dershwitz M., Walsh J.L., Morishige R.J., Connors P.M., Rubsamen R.M., Shafer S.L., Rosow C.E. Pharmacokinetics and pharmacodynamics of inhaled versus intravenous morphine in healthy volunteers. Anesthesiology. 2000;93:619–628. doi: 10.1097/00000542-200009000-00009. [DOI] [PubMed] [Google Scholar]
- Dinh K., Myers D.J., Glazer M., Shmidt T., Devereaux C., Simis K., Noymer P.D., He M., Choosakul C., Chen Q., Cassella J.V. In vitro aerosol characterization of Staccato® Loxapine. Int. J. Pharm. 2011;403:101–108. doi: 10.1016/j.ijpharm.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Fang M., Ivanisevic J., Benton H.P., Johnson C.H., Patti G.J., Hoang L.T., Uritboonthai W., Kurczy M.E., Siuzdak G. Thermal Degradation of Small Molecules: a Global Metabolomic Investigation. Anal. Chem. 2015;87:10935–10941. doi: 10.1021/acs.analchem.5b03003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierman D.E., Lasker J.M. Metabolism of fentanyl, a synthetic opioid analgesic, by human liver microsomes: Role of CYP3A4. Drug Metab. Dispos. 1996;24:932–939. [PubMed] [Google Scholar]
- Garg A., Solas D.W., Takahashi L.H., Cassella J.V. Forced degradation of fentanyl: Identification and analysis of impurities and degradants. J. Pharm. Biomed. Anal. 2010;53:325–334. doi: 10.1016/j.jpba.2010.04.004. [DOI] [PubMed] [Google Scholar]
- GK, C Dry powder inhalers: advantages and limitations. J. Aerosol Med. 1991;4:151–156. doi: 10.1089/JAM.1991.4.151. [DOI] [PubMed] [Google Scholar]
- Guagliardo R., Pérez-Gil J., De Smedt S., Raemdonck K. Pulmonary surfactant and drug delivery: focusing on the role of surfactant proteins. J. Control. Release. 2018 doi: 10.1016/j.jconrel.2018.10.012. [DOI] [PubMed] [Google Scholar]
- Guma S.R., Lee D.A., Ling Y., Gordon N., Kleinerman E.S. Aerosol interleukin-2 induces natural killer cell proliferation in the lung and combination therapy improves the survival of mice with osteosarcoma lung metastasis. Pediatr. Blood Cancer. 2014;61:1362–1368. doi: 10.1002/pbc.25019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm R.E., Klausner J.D., Klemperer J.D., Flint L.M., Huang E. Accepted but unacceptable: peripheral IV catheter failure. J. Infus. Nurs. 2015;38:189–203. doi: 10.1097/NAN.0000000000000100. [DOI] [PubMed] [Google Scholar]
- Heyder J., Gebhart J., Rudolf G., Schiller C.F., Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005-15 μm. J. Aerosol Sci. 1986;17:811–825. doi: 10.1016/0021-8502(86)90035-2. [DOI] [Google Scholar]
- Higgins M.J., Asbury A.J., Brodie M.J. Inhaled nebulised fentanyl for postoperative analgesia. Anaesthesia. 1991;46:973–976. doi: 10.1111/j.1365-2044.1991.tb09862.x. [DOI] [PubMed] [Google Scholar]
- Hodges C.C., Lloyd P.M., Mufson D., Rogers D.D., Wensley M.J. 2002. Delivery of Aerosols Containing Small Particles through an Inhalation Route. [Google Scholar]
- Hung O.R., Whynot S.C., Varvel J.R., Shafer S.L., Mezei M. Pharmacokinetics of inhaled liposome-encapsulated fentanyl. Anesthesiology. 1995;83:277–284. doi: 10.1097/00000542-199508000-00007. [DOI] [PubMed] [Google Scholar]
- Johns R., Roberts C.M. Nebulisers: their effectiveness, indications and limitations. Prescriber. 2007;18:16–28. doi: 10.1002/PSB.18. [DOI] [Google Scholar]
- Kumar M., Jha A., Mishra B. Targeted drug nanocrystals for pulmonary delivery: a potential strategy for lung cancer therapy. Expert Opin. Drug Deliv. 2020 doi: 10.1080/17425247.2020.1798401. [DOI] [PubMed] [Google Scholar]
- Lambropoulos J., Spanos G.A., Lazaridis N.V., Ingallinera T.S., Rodriguez V.K. Development and validation of an HPLC assay for fentanyl and related substances in fentanyl citrate injection. USP. J. Pharm. Biomed. Anal. 1999;20:705–716. doi: 10.1016/S0731-7085(99)00077-1. [DOI] [PubMed] [Google Scholar]
- Lipworth B., Manoharan A., Anderson W. Unlocking the quiet zone: the small airway asthma phenotype. Lancet Respir. Med. 2014 doi: 10.1016/S2213-2600(14)70103-1. [DOI] [PubMed] [Google Scholar]
- Macleod D.B., Habib A.S., Ikeda K., Spyker D.A., Cassella J.V., Ho K.Y., Gan T.J. Inhaled fentanyl aerosol in healthy volunteers: pharmacokinetics and pharmacodynamics. Anesth. Analg. 2012;115:1071–1077. doi: 10.1213/ANE.0b013e3182691898. [DOI] [PubMed] [Google Scholar]
- Mather L.E., Woodhouse A., Ward M.E., Farr S.J., Rubsamen R.A., Eltherington L.G. Pulmonary administration of aerosolised fentanyl: pharmacokinetic analysis of systemic delivery. Br. J. Clin. Pharmacol. 1998;46:37–43. doi: 10.1046/j.1365-2125.1998.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers D., Kubel K., Cassella J. 2021. Use of Antistatic Materials in the Airway for thermal Aerosol Condensation Process. US 2021/0052830 A1. [Google Scholar]
- Osman N., Kaneko K., Carini V., Saleem I. Carriers for the targeted delivery of aerosolized macromolecules for pulmonary pathologies. Expert Opin. Drug Deliv. 2018 doi: 10.1080/17425247.2018.1502267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J.S. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 1996;19:3–36. doi: 10.1016/0169-409X(95)00113-L. [DOI] [Google Scholar]
- Patton J.S., Byron P.R. Inhaling medicines: Delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 2007 doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- Pellosi D.S., Moret F., Fraix A., Marino N., Maiolino S., Gaio E., Hioka N., Reddi E., Sortino S., Quaglia F. Pluronic® P123/F127 mixed micelles delivering sorafenib and its combination with verteporfin in cancer cells. Int. J. Nanomedicine. 2016;11:4479–4494. doi: 10.2147/IJN.S103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellosi D.S., d’Angelo I., Maiolino S., Mitidieri E., D’emmanuele di Villa Bianca R., Sorrentino R., Quaglia F., Ungaro F. In vitro/in vivo investigation on the potential of Pluronic® mixed micelles for pulmonary drug delivery. Eur. J. Pharm. Biopharm. 2018;130:30–38. doi: 10.1016/j.ejpb.2018.06.006. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J.D., Wensley M., Lloyd P., Myers D., Shen W., Lu A., Hodges C., Hale R., Mufson D., Zaffaroni A. Fast onset medications through thermally generated aerosols. J. Pharmacol. Exp. Ther. 2004;309:769–775. doi: 10.1124/jpet.103.062893. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Aller M., Guillarme D., Veuthey J.L., Gurny R. Strategies for formulating and delivering poorly water-soluble drugs. J. Drug Deliv. Sci. Technol. 2015;30:342–351. doi: 10.1016/j.jddst.2015.05.009. [DOI] [Google Scholar]
- Rudokas M., Najlah M., Alhnan M.A., Elhissi A. Medical Principles and Practice. S. Karger AG; 2016. Liposome delivery systems for inhalation: a critical review highlighting formulation issues and anticancer applications; pp. 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudokas M., Najlah M., Alhnan M.A., Elhissi A. Medical Principles and Practice. S. Karger AG; 2016. Liposome delivery systems for inhalation: a critical review highlighting formulation issues and anticancer applications; pp. 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahib M.N., Darwis Y., Peh K.K., Abdulameer S.A., Fung Tan Y.T. Incorporation of beclomethasone dipropionate into polyethylene glycol-diacyl lipid micelles as a pulmonary delivery system. Drug Dev. Res. 2012;73:90–105. doi: 10.1002/ddr.21000. [DOI] [Google Scholar]
- Scheuch G., Siekmeier R. Novel approaches to enhance pulmonary delivery of proteins and peptides. J. Physiol. Pharmacol. 2007;58:615–625. [PubMed] [Google Scholar]
- Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015 doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015 doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siekmeier R., Scheuch G. Systemic treatment by inhalation of macromolecules - principles, problems, and examples. J. Physiol. Pharmacol. 2008;59(Suppl 6):53–79. [PubMed] [Google Scholar]
- Skubitz K.M., Anderson P.M. Inhalational interleukin-2 liposomes for pulmonary metastases: a phase I clinical trial. Anti-Cancer Drugs. 2000;11:555–563. doi: 10.1097/00001813-200008000-00006. [DOI] [PubMed] [Google Scholar]
- Stanley T.H., Egan T.D., Van Aken H. A tribute to Dr. Paul A. J. Janssen: entrepreneur extraordinaire, innovative scientist, and significant contributor to anesthesiology. Anesth. Analg. 2008;106:451–462. doi: 10.1213/ane.0b013e3181605add. table of contents. [DOI] [PubMed] [Google Scholar]
- Takano M., Kawami M., Aoki A., Yumoto R. Vol. 12. 2015. Receptor-Mediated Endocytosis of Macromolecules and Strategy to Enhance their Transport in Alveolar Epithelial Cells; pp. 813–825. [DOI] [PubMed] [Google Scholar]
- Tateishi T., Krivoruk Y., Ueng Y.F., Wood A.J.J., Guengerich F.P., Wood M. Identification of human liver cytochrome P-450 3A4 as the enzyme responsible for fentanyl and sufentanil N-dealkylation. Anesth. Analg. 1996;82:167–172. doi: 10.1097/00000539-199601000-00031. [DOI] [PubMed] [Google Scholar]
- Thompson J.P., Thompson D.F. Nebulized Fentanyl in Acute Pain: a Systematic Review. Ann. Pharmacother. 2016;50:882–891. doi: 10.1177/1060028016659077. [DOI] [PubMed] [Google Scholar]
- Triolo D., Craparo E.F., Porsio B., Fiorica C., Giammona G., Cavallaro G. Polymeric drug delivery micelle-like nanocarriers for pulmonary administration of beclomethasone dipropionate. Colloids Surf. B: Biointerfaces. 2017;151:206–214. doi: 10.1016/j.colsurfb.2016.11.025. [DOI] [PubMed] [Google Scholar]
- Vallejo R., Barkin R.L., Wang V.C. Pharmacology of opioids in the treatment of chronic pain syndromes. Pain Physician. 2011;14:E343–E360. [PubMed] [Google Scholar]
- Vazda A., Xia W., Engqvist H. Int. Conf. Mater. Sci. Eng; 2021. Evaporation of Fentanyl from Ceramics for Pulmonary Drug Delivery: A Pilot Study. Accepted. [Google Scholar]
- Wolff R.K. Safety of inhaled proteins for therapeutic use. J. Aerosol Med. Depos. Clear. Eff. Lung. 1998 doi: 10.1089/jam.1998.11.197. [DOI] [PubMed] [Google Scholar]
- WORSLEY M.H., MACLEOD A.D., BRODIE M.J., ASBURY A.J., CLARK C. Inhaled fentanyl as a method of analgesia. Anaesthesia. 1990;45:449–451. doi: 10.1111/j.1365-2044.1990.tb14331.x. [DOI] [PubMed] [Google Scholar]