Abstract
Plant-specialized secondary metabolites have ecological functions in mediating interactions between plants and their entophytes. In this study, high-throughput gene sequencing was used to analyze the composition and abundance of bacteria from Ginkgo leaves at five different sampling times. The results indicated that the bacterial community structure varied during leaf developmental stage. Bacterial diversity was observed to be the highest at T2 stage and the lowest at T1 stage. Proteobacteria, Firmicutes, Actinobacteria, Chloroflexi, Cyanobacteria, and Bacteroidetes were found as the dominant phyla. The major genera also showed consistency across sampling times, but there was a significant variation in their abundance, such as Bacillus, Lysinibacillus, and Staphylococcus. Significant correlations were observed between endophytic bacteria and flavonoids. Especially, Staphylococcus showed a significant positive correlation with quercetin, and changes in the abundance of Staphylococcus also showed a strong correlation with flavonoid content. In order to determine the effect of flavonoids on endophytic bacteria of Ginkgo leaves, an extracorporeal culture of related strains (a strain of Staphylococcus and a strain of Deinococcus) was performed, and it was found that the effect of flavonoids on them remained consistent. The predicted result of Tax4Fun2 revealed that flavonoids might lead to a lower abundance of endophytic microorganisms, which further proved the correlation between bacterial communities and flavonoids. This study provided the first insight into the bacterial community composition during the development of Ginkgo leaves and the correlation between the endophytic bacteria and flavonoids.
Keywords: flavonoids, bacterial endophyte communities, Ginkgo biloba, community assembly, function prediction
Introduction
Endophytes, as a group of microorganisms present in the survival history of the host plant, are a variety of endophytic bacteria and fungi that establish a mutualistic beneficial relationship with the host plant (Brader et al., 2017). In order to better adapt to the host plant environment, certain physiological and biochemical characteristics of plant endophytes are assimilated, such as the secondary metabolites (Qin et al., 2009; Cui et al., 2012; Cao et al., 2016). Therefore, endophytes have important application value, such as plant disease resistance and agricultural production (Ramamoorthy et al., 2001; Merzaeva and Shirokikh, 2010; Pham et al., 2017). As an important part of host biology, the research on endophytic bacteria has become a hot topic in recent years. It is therefore necessary to study plant endophytes and their relationship and mechanisms of influence with the host plant.
Many factors can affect the endophytic community of plants, including temperature (Latz et al., 2021), precipitation (Huang, 2020), and soil property (Lehtonen et al., 2005). It has been reported that temporal changes can cause changes in the community composition of the host endophytic bacteria named temporal patterns (Jackson and Denney, 2011). Besides, some studies have shown that the reasons for the change of endophytic microbial community may be related to the change of endophytic environment (Fort et al., 2021). Plants express different functional traits at different stages of growth, including the production of specific secondary metabolites that may guide the assembly of endophytic microbial communities (Chaparro et al., 2014; Wagner et al., 2016). These studies suggest that plant secondary metabolites can mediate interactions between hosts and endophytic microbes. However, the specific influencing factors and mechanisms of endophytic microbial community changes need to be further studied.
Ginkgo biloba L. are deciduous trees of Ginkgo Ginkgoaceae, which has a high medicinal value. Ginkgo extract has been used by the ancients as an herbal remedy for cardiovascular and bronchial diseases (Dubber and Kanfer, 2006; Pereira et al., 2013). Flavonoids as the main active substances in Ginkgo are a kind of polyphenolic compound, which have antioxidant and free radical-scavenging effects (Singh et al., 2008). Flavonoids may shape the assembly of endophytic microbial communities. However, most of the studies only focused on the screening of endophytic bacteria and their flavonoid production performance (Yuan et al., 2019), but neglected the exploration of plant endophytes and their relationship with the host plant and flavonoids.
Ginkgo leaves contain a large number of active, medicinally valuable compounds. In addition, internal phytochemical profiles vary at various developmental stages in Ginkgo leaves. In this study, three wild female Ginkgo trees were selected, and the compositional shifts in endophytic bacterial community of wild Ginkgo leaves from April to August were assessed. We hypothesized that (i) the composition and diversity of the endophytic bacterial community of Ginkgo leaves were variable and patterned with the development of Ginkgo leaves and (ii) flavonoids might influence the abundance and community composition of endophytic bacteria in Ginkgo leaves. This experiment can support the subsequent search of functional microorganisms and guide the cultivation, protection, and increase of resource utilization of Ginkgo.
Materials and Methods
Site Description and Sampling Methods
The Ginkgo leaf samples were taken from three wild Ginkgo trees in 2019 on Yuelu Mountain (28°10′30′′ N, 112°55′20′′ E) in Changsha, Hunan province (Supplementary Table 1). Yuelu Mountain belongs to the subtropical monsoon humid climate area. It is cloudy and rainy in spring and summer. The annual average temperature is 17.2°C, and the annual average precipitation is 1,200–1,400 mm. Yuelu Mountain is a rare “5A” urban mountain scenic area, and the ecological environment of this scenic area is gradually being affected by largely unplanned tourist activity and urbanization. The wild Ginkgo trees on the Yuelu Scenic Spot are limited, and the selected three wild Ginkgo trees are growing well and protected from damage (namely, S1, S2, and S3). Leaf samples were collected monthly from when the leaves began to grow in April to August. The leaves began to turn yellow in September. The canopy of each tree was divided into three sections, and 200 g of healthy leaves were collected from 5 to 7 m above the ground as one sample from each section. In total, 45 leaf samples were obtained from three trees at five time points (Supplementary Table 2; T1: Apr 1st, T2: May 1st, T3: Jun 1st, T4: Jul 1st, and T5: Aug 1st). To minimize the effects of other factors, all samples were collected aseptically wearing sterile gloves on a sunny day between 9:00 a.m. and 1:00 p.m. Leaves were cut off from each tree, immediately put in a sterile plastic bag, and transported to the laboratory, within 1 h. Environmental temperature and fresh weight of a leaf was recorded at the same time. After being transported to the lab, each sample was divided into three parts for phytochemical analysis, endophyte isolation, and DNA extraction. The parts for DNA extraction were surface sterilized and frozen in liquid nitrogen and then stored at −80°C. The process of surface disinfection was as follows: the sample was firstly flushed cleanly with tap water, then secondly immersed in 75% ethanol for 1 min, thirdly rinsed in sterile deionized water after, fourthly immersed in 8% NaClO for 10 min, then fifthly rinsed in sterile deionized water for five times, and finally dried with sterile filter paper. After surface sterilization, part of the sample was reserved for the isolation of endophytic bacteria, and the rest were triturated in liquid nitrogen and stored at −80°C until DNA extraction. Leaf moisture content was determined by drying the leaves at 70°C to constant weight.
Phytochemical Analysis of G. biloba Leaves
The washed fresh leaves were ground in liquid nitrogen, and 200 mg of the ground powder in liquid nitrogen was added to 30 ml of 70% ethanol, vortexed and homogenized for 1 min, and then subjected to an hour ultrasonic extraction for the flavonoids according to research (Zou et al., 2021). The mixture was centrifuged at 13,000 rpm for 10 min at 4°C and then 1 ml of the supernatant was dried in nitrogen at 37°C. The residue was dissolved in 1 ml of methanol and centrifuged at 13,000 rpm for 10 min at 4°C. The supernatant was passed through a 0.22-μm filter membrane and then diluted to 10 ml. The high-performance liquid chromatography (HPLC) system was used for the determination of the flavonoid content (including quercetin, kaempferol, and isorhamnetin) (Shimadzu, Japan). A C18 column (217 mm × 2.1 mm, 1.7 μm, Waters, Milford, United States) was used for the separation, and the column temperature was controlled at 25°C. Flavonoids were analyzed with 0.05 M sodium acetate (A) and 90% acetonitrile (B) comprising the mobile phase. The gradient elution condition was optimized as follows: 0–20 min, B: 5–70%; 20–23 min, B: 70–5%; 23–25 min, B: 5%. A flow rate of 1.0 ml/min was used. Standard curves were established by a series concentration of the corresponding standard (ChemFaces, Wuhan, China) at 360 nm. Then, the content of total flavonoids was calculated by the following formula (the coefficient in the formula is the average conversion factor between flavonol glycosides and flavonoid glycosides) (Hasler et al., 1992):
Chlorophyll A and chlorophyll B were determined by acetone decolorization (Arnon, 1949). Malondialdehyde (MDA) in leaves was determined by the thiobarbituric acid method (Reitznerova et al., 2017). Superoxide dismutase (SOD) activity from leaf samples was extracted using Plant tissue Cu2+Zn2+—SOD assay kit (Nanjing, Jiancheng, China).
DNA Extraction, Sequencing, and Analysis of the Endophytic Bacterial Community in G. biloba Leaves
Genomic DNA of endophytic bacteria from leaf samples was extracted using DNeasy® Plant Mini Kit (250) (Qiagen, Inc., Dusseldorf, Germany). The V4–V5 hypervariable regions of the 16S rDNA were amplified using the 799F (5′-AACMGGATTAGATACCCKG-3′)/1115R (5′-AGGGTTGCGCTCG TTG-3′) (Chelius and Triplett, 2001; Redford and Fierer, 2009). The amplified DNA was determined by running the samples on a 1.2% agarose gel and then purifying using the Gel Extraction Kit D2500 (OMEGA Bio-tek, Norcross, Georgia, United States).
High-throughput sequencing of the library preparation products was conducted on an Illumina Hiseq platform (Hiseq PE250). Gene sequencing data of raw 16S rRNA were processed using the Galaxy pipeline established by Zhou’s lab1 at Oklahoma University (OU), United States. To ensure the quality of the sequences, both forward and reverse reads were trimmed based on sequence quality scores using Btrim (Kong, 2011; Deng et al., 2012). After trimming, the forward and reverse reads with 50–250 bp overlapping were combined to obtain longer sequences. Also, unqualified sequences were removed if they were too short or contained an undetermined base “N.” Following this, potential chimeric sequences were detected and removed by UCHIME. Sequences were then clustered into operational taxonomic units (OTUs) at 97% sequence similarity using UCLUST (Edgar et al., 2011). Finally, the RDP Classifier was used to assign 16S rRNA sequences to the bacterial taxa2 with a minimal 50% confidence estimate. Also, it was necessary to filter OTUs classified as mitochondrion or chloroplast in data outputted from Galaxy pipeline and those unclassified OTUs (Li and Godzik, 2006; Wang et al., 2007). All sequences produced from Illumina sequencing had been uploaded to the sequence read archive (SRA) of NCBI database with numbers from SRR13946092 to SRR13946136.
Alpha diversity indexes, including Shannon index (H) and Pielou evenness (J), were calculated on Institute for Galaxy| Denglab pipeline.3 Non-metric multidimensional scaling (NMDS) was used for observing the changes of bacterial community composition along with the leaf growth. The analysis of similarities (ANOSIM) was performed to analyze the significance of clustering. Redundancy analysis (RDA), variance partitioning analysis (VPA), and Mantel test were carried out on the R platform, with the “Vegan” Package, for correlating between bacterial community and all the environmental factors. For RDA and VPA, correlated variables were removed [variance inflation factor (VIF) > 10] using variance inflation factors test. Tax4Fun2 was used to predict the relative abundance of functional genes of each sample (Asshauer et al., 2015).
Isolation of Endophytes in G. biloba Leaves and Their Flavonoid Resistance
The surface-sterilized leaves were physically crushed using phosphate-buffered saline (PBS). Then, the suspension obtained was spread on R2A medium agar plates (0.5 g/l yeast extract, 0.5 g/l peptone, 0.5 g/l casein hydrolysate, 0.5 g/l glucose, 0.5 g/l soluble starch, 0.3 g/l dipotassium hydrogen phosphate, 0.024 g/l anhydrous magnesium sulfate, 0.3 g/l sodium pyruvate, and 15 g/l agar). All colonies were streaked for consecutive times, and finally, 11 bacterial strains were obtained. The identification of the strains was carried out by BIG (Shenzhen, China). The sequences obtained were analyzed using NCBI BLASTn search. Among them, two strains, including a strain of Staphylococcus and a strain of Deinococcus, were selected for flavonoid resistance analysis based on a correlation analysis between endophytic bacteria and flavonoids. The isolates were inoculated in 50 ml R2A medium at 30°C and 200 rpm to obtain the seed liquid with OD600 ≈ 0.9. The seed liquid was then diluted 100-fold with fresh R2A medium containing a gradually increased concentration of flavonoids according to the concentration of flavonoids in leaves. Total flavonoid content (quercetin/kaempferol/isorhamnetin = 1:2:1) was uniformly set: 0, 152.7, 305.4, 458.1, and 610.8 mg/l. The absorbance of bacterial liquid (OD600) was measured every 6 h.
Results
Phytochemical Characteristics of G. biloba Leaves in Leaf Developmental Period
During the annual developmental period, the average weight of Ginkgo leaves increased gradually from April to August (Supplementary Table 3). The fresh weight of a leaf was 0.37–0.46 g at the T1 stage and increased rapidly by 0.18–0.21 g at the T2 stage and 0.17–0.26 g at the T3 stage. Then, the fresh weight of Ginkgo leaves remains relatively stable at T4 and T5. Thus, based on changes in leaf weight, we divided the growth process of the leaves into three stages, namely, T1 stage: leaf bud formation; T2 and T3 stages: rapid growth stage of leaves; and T4 and T5 stages: late growth stage of leaves.
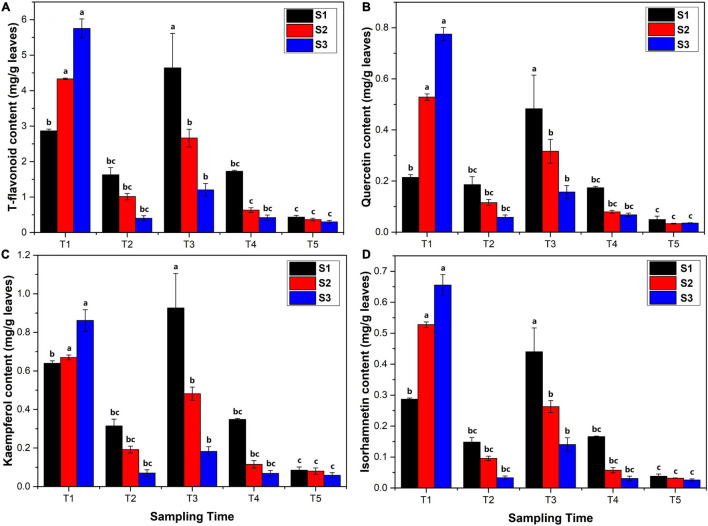
The content of T-flavonoids, quercetin, kaempferol, and isorhamnetin, in Ginkgo leaves were determined by HPLC analysis (Figure 1). The results showed that the T-flavonoid content was 2.864–5.753 mg/g in the new leaves at the T1 stage (Figure 1A), followed by a sharply decreased trend at the T2 stage (0.404–1.630 mg/g) with the rapid growth of leaves. The content of flavonoids significantly increased to 1.078–5.764 mg/g in June at the T3 stage and then decreased gradually to 0.434–0.301 mg/g at the T5 stage. The temporal variation pattern of quercetin, kaempferol, and isorhamnetin content was similar to T-flavonoid content. The quercetin content was 0.202–0.791 mg/g in April, 0.049–0.222 mg/g in May, 0.139–0.635 mg/g in June, 0.062–0.179 mg/g in July, and 0.031–0.065 mg/g in August, respectively (Figure 1B). The kaempferol content was 0.626–0.926 mg/g in April, 0.049–0.355 mg/g in May, 0.165–1.132 mg/g in June, 0.056–0.352 mg/g in July, and 0.049–0.103 mg/g in August, respectively (Figure 1C). The isorhamnetin content was 0.282–0.693 mg/g in April, 0.026–0.165 mg/g in May, 0.125–0.529 mg/g in June, 0.026–0.167 mg/g in July, and 0.023–0.046 mg/g in August, respectively (Figure 1D).
FIGURE 1.
Flavonoid content (mg/kg leaves) analysis in Ginkgo biloba L. extracts determined by high-performance liquid chromatography (HPLC) using gradient elution at 360 nm. (A) The content of total flavonoids. (B) The content of quercetin. (C) The content of kaempferol. (D) The content of isorhamnetin. The error bar was plotted based on the standard deviation of replicate samples.
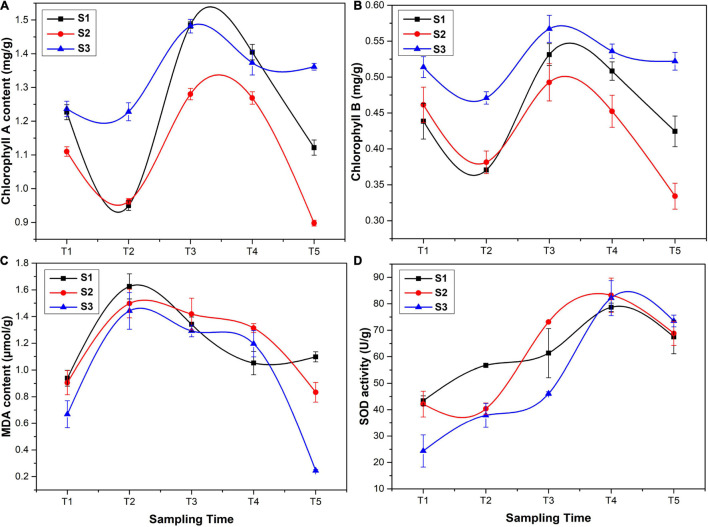
The results of phytochemical analysis of Ginkgo leaves are shown in Figure 2. The chlorophyll A and chlorophyll B content was firstly decreased in May, then reached the maximum in June, and gradually declined in July and August, which was similar to the changes of flavonoid content (Figures 2A,B). The maximum content of chlorophyll A and chlorophyll B at the T3 stage was 1.280–1.486 and 0.493–0.531 mg/g, respectively. MDA is a crucial product of membrane lipid peroxidation, and its accumulation can aggravate damage and lead to aging and resistance physiology (Liu et al., 2021). The MDA content was first increased at the T2 stage and then declined. The highest content of MDA was 1.442–1.626 μmol/g at the T2 stage (Figure 2C). SOD is considered as one of the most effective antioxidant enzymes for all the aerobic organisms, which catalyzes and reduces O2– to H2O2, thus minimizing the risk of hydroxide (OH–) formation (Liling et al., 2021). The activity of SOD showed an increasing trend with the maximum value at the T4 stage (78.671–83.245 U/g) (Figure 2D), followed by a slight decrease at the T5 stage.
FIGURE 2.
Phytochemical analysis of G. biloba leaves: (A) chlorophyll A content, (B) chlorophyll B content, (C) malondialdehyde (MDA) content, and (D) superoxide dismutase (SOD) activity.
Endophytic Bacterial Diversity and Community Composition in G. biloba Leaves
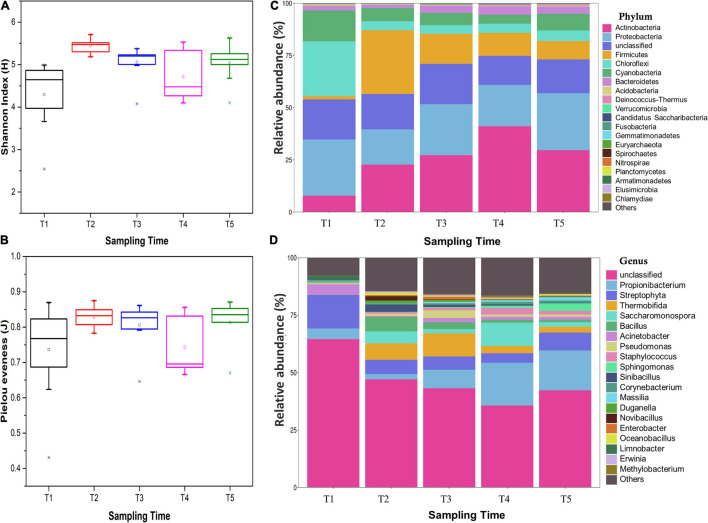
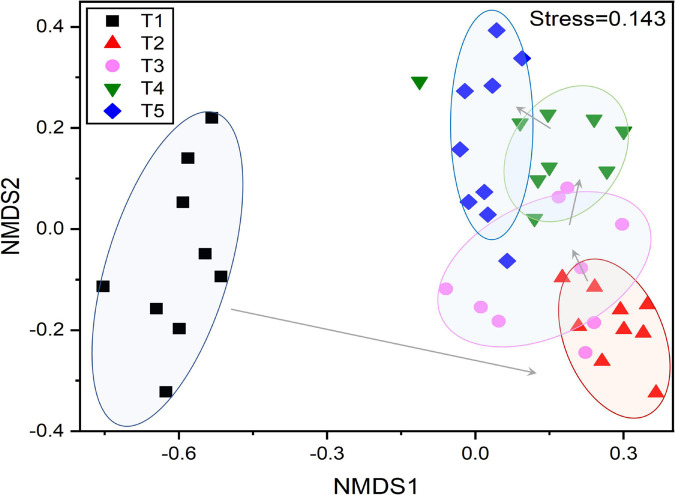
A total of 116,218 high-quality 16S rRNA gene sequences were obtained. By UCLUST at the 97% similarity, 576 OTUs were obtained based on 16S Greengene database, and the rarefaction curves showed that the sequencing depth was sufficient for further analysis (Supplementary Figure 1). Venn diagram showed that all the samples shared 20 core OTUs (Supplementary Figure 2). The results of alpha diversity indexes showed that the Shannon index and Pielou evenness of endophytic community in leaves were slightly lower at the T1 stage and then kept a relatively stable level at T2–T5 stages (Figures 3A,B). NMDS analysis indicated that the bacterial communities in leaves were dynamic and time-varying (Figure 4). Temporal patterns were obvious. The replicates of leaf samples at the same time point were well clustered. The ANOSIM test on Bray–Curtis matrix also showed that the endophytic communities were significantly different among the T1–T5 groups (Supplementary Table 4, p < 0.05). Most notably, the endophytic communities at the T1 stage were completely separated from the other four groups on the first axis, and the endophytic communities at the other four stages were gradually changed along with the second axis.
FIGURE 3.
Diversity indexes of endophytic bacterial communities within an individual tree: (A) Shannon index, (B) Pielou evenness, (C) endophytic bacterial relative abundance at the phylum level, and (D) endophytic bacterial relative abundance at the genus level. All indexes were means with standard deviations (SDs) calculated from nine replicates. The different lower case letters following the indexes indicate a significant difference (P< 0.05) between trees.
FIGURE 4.
Non-metric multidimensional scaling (NMDS) based on Bray–Curtis distances showing the changes in the composition and β-diversity of the endophytic bacterial community in G. biloba leaves at different development stages.
The endophytic bacterial community in Ginkgo leaves was mainly composed of Proteobacteria (10.42–43.98%), Firmicutes (0.86–38.42%), Actinobacteria (4.19–49.22%), Chloroflexi (1.80–48.97%), Cyanobacteria (3.04–21.18%), and Bacteroidetes (0.69–5.70%) (Supplementary Figure 3 and Supplementary Table 5). The average relative abundance of endophytic bacterial community at phylum level is shown in Figure 3C. Most of those phyla showed different abundance in different groups, e.g., the phyla Actinobacteria was significantly increased from the T1 stage (7.86%) to the T4 stage (41.09%) and then decreased at the T5 stage (29.65%). Chloroflexi and Cyanobacteria were significantly more abundant in the T1 group (26.2 and 14.84%, respectively) than the other four groups (4.12–5.00% and 4.25–7.99%, respectively), while Firmicutes showed a significantly lower relative abundance in T1 samples (1.60%). Then, Firmicutes was gradually decreased from the T2 stage (30.49%) to the T5 stage (8.84%). When referring to genus level, 576 genera were identified from all sequences and the most abundant 20 genera were shown (Figure 3D and Supplementary Figure 4). The main genera were Acinetobacter (0.28–12.77%), Bacillus (0.02–7.99%), Propionibacterium (1.44–32.29%), Pseudomonas (0.07–6.26%), and Staphylococcus (0.11–6.97%). Most of those genera in T1 samples had significantly different abundances from others. For example, the genera Bacillus, Saccharomonospora, Sinibacillus, and Thermobifida were less than 0.1% in the bacterial community of T1 samples, while they reached over 3.17–10.08% in T2 and T3 samples and then decreased to 0.24–2.53% in T5 samples. Genera Streptophyta and Acinetobacter were significantly higher in the T1 group (14.62 and 4.45%, respectively) than the other four groups (4.13–7.82% and 0.37–1.90%, respectively). In addition, some genera, including Propionibacterium, Sphingomonas, Corynebacterium, Massilia, and Staphylococcus, were gradually increased from the T1 stage to T5 stage. These results indicated that changes in endophytic bacterial community composition accompanied the development of the leaves.
Linkages Between Phytochemical Parameters and Endophytic Bacterial Abundances in G. biloba Leaves
The relationship between endophytic bacterial populations and flavonoids as well as physicochemical parameters was analyzed by the Spearman correlation test and RDA. The phylum Actinobacteria was significantly negatively correlated with three flavonol glycosides, while Cyanobacteria and Chloroflexi were significantly positively correlated with three flavonol glycosides (Table 1, P < 0.05). Actinobacteria was positively correlated with chlorophyll B, SOD, and temperature and negatively correlated with leaf water content (Table 2, P < 0.05). Cyanobacteria showed an opposite relationship with chlorophyll B, SOD, temperature, and leaf water content. Firmicutes was positively related to MDA (r = 0.74, p < 0.001), and Bacteroidetes was positively related to SOD and temperature. At genus level, most of the genera, including Aerococcus, Blastococcus, Chryseobacterium, Deinococcus, Lysinibacillus, Methylobacterium, Saccharomonospora, etc., were significantly positively correlated with three flavonol glycosides, while Staphylococcus and Propionibacterium were negatively correlated with three flavonol glycosides (Supplementary Table 6, P < 0.05). In addition, most of the genera were mainly negatively influenced by SOD and MDA and positively influenced by temperature (Supplementary Table 7).
TABLE 1.
Spearman correlation test showing the relative abundance of endophytic bacterial phyla association with three flavonol glycosides.
| Phylum | Quercetin |
Kaempferol |
Isorhamnetin |
|||
| r | p | r | p | r | p | |
| Acidobacteria | 0.093 | 0.544 | 0.111 | 0.468 | 0.170 | 0.264 |
| Actinobacteria | –0.537 | 0.000 | –0.574 | 0.000 | –0.600 | 0.000 |
| Bacteroidetes | –0.050 | 0.746 | –0.110 | 0.471 | –0.147 | 0.333 |
| Candidatus saccharibacteria | –0.030 | 0.849 | –0.058 | 0.705 | –0.002 | 0.989 |
| Chloroflexi | 0.304 | 0.043 | 0.372 | 0.012 | 0.432 | 0.003 |
| Cyanobacteria | 0.286 | 0.057 | 0.319 | 0.033 | 0.333 | 0.026 |
| Deinococcus–thermus | –0.009 | 0.955 | –0.014 | 0.925 | 0.052 | 0.734 |
| Firmicutes | –0.147 | 0.334 | –0.115 | 0.452 | –0.220 | 0.142 |
| Fusobacteria | –0.034 | 0.823 | –0.075 | 0.622 | –0.025 | 0.872 |
| Gemmatimonadetes | –0.014 | 0.930 | –0.043 | 0.780 | –0.023 | 0.882 |
| Nitrospirae | –0.043 | 0.780 | –0.079 | 0.605 | –0.025 | 0.872 |
| Proteobacteria | –0.115 | 0.451 | –0.126 | 0.408 | –0.079 | 0.603 |
| Verrucomicrobia | –0.017 | 0.913 | –0.049 | 0.750 | 0.009 | 0.954 |
Significant differences (P < 0.05) are indicated in bold.
TABLE 2.
Spearman correlation test showing the relative abundance of endophytic bacterial phyla association with physical and chemical parameters.
| Phylum | MDA | Chlorophyll A | Chlorophyll B | SOD | Water | Temperature |
| Acidobacteria | –0.265 | –0.034 | –0.113 | –0.052 | 0.018 | –0.121 |
| Actinobacteria | 0.205 | 0.284 | 0.353* | 0.657*** | −0.507*** | 0.631*** |
| Bacteroidetes | –0.272 | 0.128 | 0.120 | 0.525*** | –0.213 | 0.376* |
| Candidatus saccharibacteria | –0.056 | 0.030 | –0.003 | –0.054 | 0.146 | 0.052 |
| Chloroflexi | −0.242** | –0.000 | –0.059 | –0.143 | 0.457** | −0.581*** |
| Cyanobacteria | –0.387 | –0.266 | −0.313* | −0.356* | 0.369* | −0.543*** |
| Deinococcus–Thermus | –0.238 | 0.286 | 0.259 | 0.342* | –0.122 | 0.233 |
| Firmicutes | 0.740*** | 0.013 | 0.083 | 0.064 | –0.125 | 0.122 |
| Fusobacteria | –0.130 | 0.076 | 0.050 | 0.054 | –0.086 | 0.186 |
| Gemmatimonadetes | 0.011 | 0.009 | –0.012 | –0.052 | –0.106 | 0.103 |
| Nitrospirae | –0.084 | –0.012 | –0.038 | –0.085 | –0.29 | 0.106 |
| Proteobacteria | –0.468 | –0.046 | –0.097 | –0.048 | 0.008 | 0.019 |
| Verrucomicrobia | –0.113 | –0.004 | –0.034 | –0.072 | 0.014 | –0.236 |
Significant differences are indicated in bold.
*Indicates significant differences (P < 0.05);
**indicates significant differences (P < 0.01);
***indicated significant differences (P < 0.001).
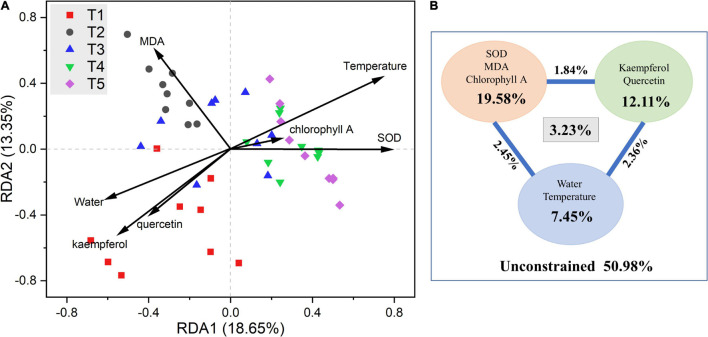
RDA and VPA plots after removing all correlated variables are shown in Figure 5. Among which, chlorophyll B, T-flavonoid, and isorhamnetin were removed because of their VIF > 10. The results of the RDA showed that six of the seven selected physicochemical variables were significantly correlated with the microbial community structure (Supplementary Tables 8, 9). Kaempferol, quercetin, and leaf water content mainly influenced the microbial community structure in the T1 period. Endophytic bacterial communities in T2 and T3 periods were mainly impacted by MDA, and bacterial communities in T4 and T5 periods were mainly impacted by temperature and SOD. For VPA, variables were classified into three groups: flavonoids (kaempferol and quercetin), phytochemical properties (SOD, MDA, and chlorophyll A), and environmental parameters (leaf water content and temperature). VPA showed that these seven variables constrained 49.12% of the total community distribution. The flavonoids constrained 12.11% of the community distribution, while phytochemical properties and environmental parameters constrained 19.58 and 7.45%, respectively.
FIGURE 5.
(A) Redundancy analysis (RDA) and (B) variance partitioning analysis (VPA) of the relationships between endophytic bacterial community and environmental variables in G. biloba leaves.
Isolation of Endophytes in G. biloba Leaves and Their Flavonoid Resistance
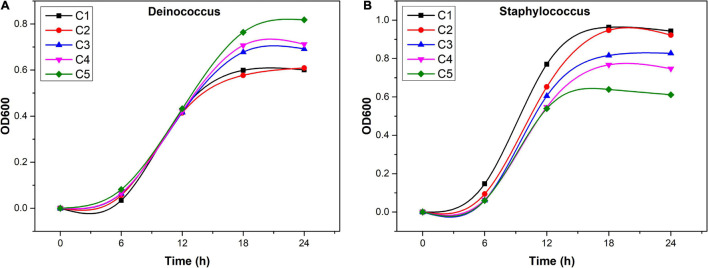
In total, 11 isolates were obtained, including Microbacterium, Deinococcus, Paenibacillus, Bacillus, Staphylococcus, Achromobacter, Burkholderia, and Massilia, and the specific details are shown in Supplementary Table 10. Among which, only Deinococcus and Staphylococcus were shown to significantly correlate with at least one of the three flavonol glycosides (Supplementary Table 6). Deinococcus was positively correlated with three flavonol glycosides, and Staphylococcus was negatively correlated with quercetin and other two flavonol glycosides (not significant). We monitored the growth of selected endophytic strains in R2A media supplemented with flavonoids (Figure 6). After 18-h culture, Deinococcus and Staphylococcus reached stationary growth phase. The maximum OD600 of Deinococcus increased from 0.58 (0 mg/l) to 0.85 (610.8 mg/l) with the increase of flavonoids, while OD600 of Staphylococcus decreased from 0.97 (0 mg/l) to 0.59 (610.8 mg/l) with the increase of flavonoids.
FIGURE 6.
Growth of Staphylococcus (A) and Deinococcus (B) in R2A medium with additional flavonoids (C1: 0 mg/l, C2: 152.7 mg/l, C3: 305.4 mg/l, C4: 458.1 mg/l, and C5: 610.8 mg/l).
Functional Gene Prediction by Tax4Fun2
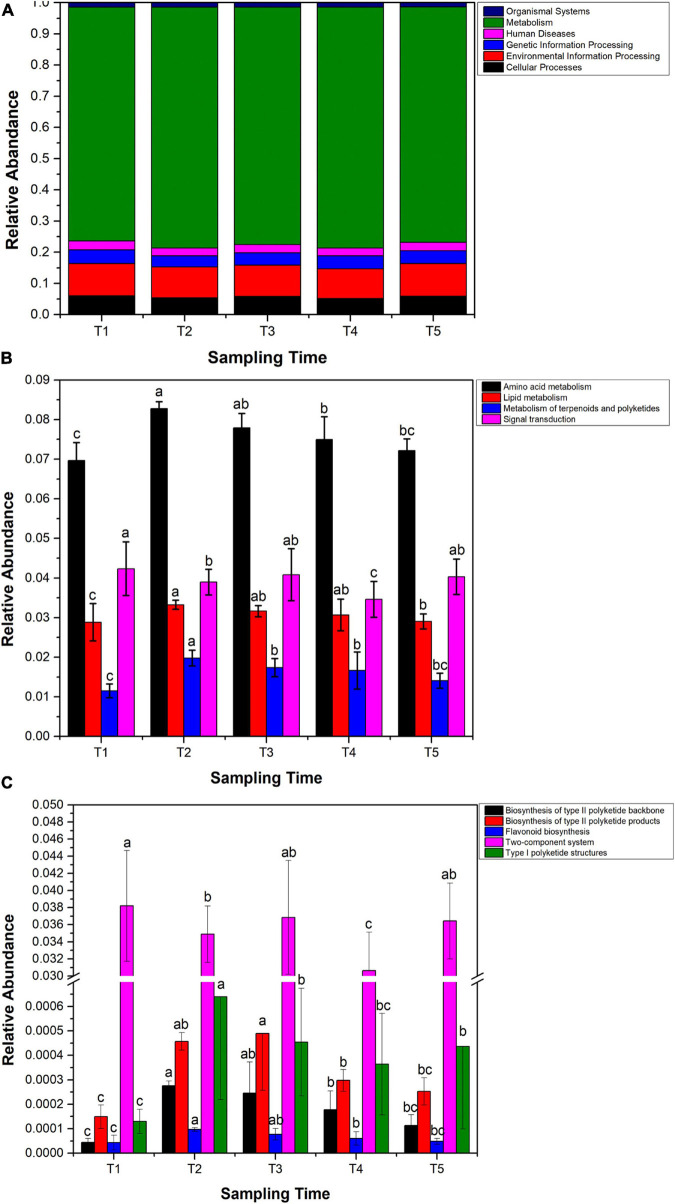
By gene function analysis, seven ortholog taxa at level I had been annotated in the Ginkgo endophytic community. The relative abundance of these ortholog taxa tended to be similar in five sample groups. Most of these function genes were metabolism, accounting for nearly 75% of all genes (Figure 7A). At level II, the relative abundance of four functional genes including amino acid metabolism, lipid metabolism, metabolism of terpenoids and polyketides, and signal transduction reached the highest at the T2 stage, and then gradually decreased until T5. However, the relative abundance of signal transduction showed an almost opposite trend (Figure 7B). Further analysis at level III showed related genes of polyketide, including biosynthesis of type II polyketide backbone, biosynthesis of type II polyketide products, flavonoid biosynthesis, and type I polyketide structures, reaching the highest abundance at the T2 stage and then gradually decreasing from the T2 stage to T5 stage.
FIGURE 7.
Functional genes analyzed by Tax4Fun2. (A) Relative abundance of predicted functional genes at level I. (B) Relative abundance variation of genes involved in amino acid metabolism, lipid metabolism, metabolism of terpenoids and polyketides, and signal transduction. (C) Changes of relative abundance of predicted genes in the polyketone metabolism, flavonoid biosynthesis, and two-component systems. Samples with different letters indicate that there are significant differences between samples (p < 0.05).
Discussion
Leaf Developmental Stage Strongly Influenced Phytochemical Characteristics in G. biloba Leaves
Ginkgo is one of the most important medicinal plants, and flavonoids are the main medicinal active substances in Ginkgo. According to our results, the content of three flavonol glycosides rapidly changed along with leaf developmental stages. Previous studies showed that the synthesis and accumulation of secondary metabolites in Ginkgo were a temporal variational process (Lin et al., 2020; Wang et al., 2020; Tetali et al., 2021). This temporal variational process may be related to the growth differentiation balance (GDB) of plants (Herms, 1999; Karasov et al., 2017). The temporal pattern of flavonoid variation in Ginkgo leaves was the result of competition between resources for growth and resources for defense. Based on changes in flavonoid content and the increase in biomass of Ginkgo leaves, it was found that there was a sudden decline in flavonoid content during the phase of rapid growth of Ginkgo leaves at T1–T2 stages and a rise in flavonoid content when growth slowed down at T2–T3 stages. Later when Ginkgo is starting to bear fruit, most of the resources obtained was used for the growth of the fruit, which led to a steady decrease in flavonoid content (Lindroth and Peterson, 1988; Lin et al., 2020).
As a reference value for the temporal variation in flavonoid content of Ginkgo leaves, the variation in physicochemical parameters aided in explaining the reason for the variation in flavonoids. The increased chlorophyll content enhanced the photosynthesis intensity, resulting in an increase in flavonoid content to some extent (Torres et al., 2021). The antioxidant mechanism of Ginkgo explains to some extent the reasons for the relevant changes in in flavonoid content (in terms of changes in MDA content and SOD activity). The antioxidant response of Ginkgo leaves was mainly non-enzymatic antioxidant first and followed by enzymatic reactions in the early stage, which resulted in the higher flavonoid content and lower SOD activity (Li et al., 2021). With the growth of Ginkgo leaves, rapid changes in flavonoid content were due to the depletion of flavonoids and the large allocation of resources to leaf growth. Coupled with changes in ambient temperature and leaf water content, plant cell membrane oxidation increased, leading to an increase in MDA content, while SOD activity increased with the antioxidant response mechanism (Baisak and Acharya, 1994).
Leaf Developmental Stage Strongly Influenced the Assembly of Endophytic Microbiome in G. biloba Leaves
The study of endophytic bacteria in Ginkgo leaves is of great value to the follow-up study of medicinal components and the exploitation of medicinal component-producing microbial resources. Our results demonstrate that endophytic microbiome assembly in Ginkgo leaves was mainly influenced by leaf developmental stage regardless of individual species (Figure 4). The regionality caused the formation of core members of endophytic bacteria in the host plant, but developmental stages caused substantial changes in endophytic bacterial diversity (Figure 3) and community structure (Figure 4) in Ginkgo leaves (Huang, 2019). Because for individual species, the effect of time on endophytic bacterial community assembly was much stronger than spatial locations (Figure 4), although spatial locations are well-known to make a great contribution to bacterial community composition (Meyer and Leveau, 2012; Leff et al., 2015). It was suggested that time itself usually is not a driver of community assembly processes (Hui et al., 2017). Microbial communities, like plant and animal communities, are dynamic and exhibit temporal patterns that can reflect underlying biotic and abiotic processes (Banning et al., 2011; Blasko et al., 2015; Wu et al., 2015). On the other hand, the functional capacity of bacterial species is key to their recruitment by hosts, and Burke et al. (2011) proposed that bacterial community assembly is associated with function rather than the taxonomy (Burke et al., 2011; Grady et al., 2019). For example, changes in the abundance of Bacillus demonstrated its growth-promoting effect, as the proportion of Bacillus increased rapidly during the rapid growth period of Ginkgo leaves (Compant et al., 2005). Lysinibacillus is a genus of endophytic bacteria capable of inducing defense responses in host plants. However, the results showed that it was not found in the samples of T1. This could be that Lysinibacillus belongs to a group of soil bacteria that are usually found in roots and therefore would not have been found in the early leaves (Passera et al., 2021). Furthermore, based on the results of alpha diversity analysis and beta diversity analysis, the endophytic bacterial community of Ginkgo leaves was not a temporally linear succession process. Therefore, it suggested that certain factors dominated the assembly of the endophytic bacterial community under temporal turnover, such as secondary metabolites. Moreover, this influential nature decreased with time, and changes in the endophytic bacterial community stabilized, which is consistent with the results we obtained (Grady et al., 2019).
Flavonoids Attributed to the Assembly of Endophytic Bacterial Communities in G. biloba Leaves
Secondary metabolites are unique natural compounds that can, to some extent, guide the assembly of specific bacterial communities (Sasse et al., 2018; Stringlis et al., 2018). A shred of evidence showed that salicylic acid causes alterations in the response of bacterial communities in the Arabidopsis root zone to signals (Lebeis et al., 2015). A comparison of bacterial profiles in Arabidopsis roots also shows that the triterpene biosynthetic network can regulate the establishment of specific microbiota (Lebeis et al., 2015; Huang et al., 2019). In this study, both phytochemical characteristics and endophytic bacterial community of the Ginkgo leaves varied with developmental stages. It was suggested that phytochemical characteristics of the host Ginkgo affected the endophytic bacterial community. For example, the genus Staphylococcus, as in the results of this study, was low in abundance in the pre-growth phase and increased in abundance in the late growth phase of Ginkgo leaves, because of the effect of changes in flavonoid content (Osorio et al., 2021). Otherwise, the results of the alpha diversity analysis of the endophytic bacterial community of Ginkgo leaves showed that the factors influencing the diversity of the endophytic bacterial community were related to flavonoids, as the trends of changes in the two clearly corresponded to the opposite. Moreover, the results of the beta diversity analysis showed that the endophytic communities in the early stages were more different, which was caused by the greater variation in flavonoid content in the early stages. The results for RDA were similar, with the endophytic bacterial community being most associated with flavonoids in the early stages, and its influence diminishing over time (Figure 5). This weakening effect over time confirmed the result that flavonoids could influence the assembly of endophytic bacterial communities in Ginkgo leaves.
Host plant metabolites can also selectively regulate the growth of bacteria from different taxa by acting as antibiotics or proliferating agents, and plant exudation traits and microbial substrate uptake traits could interact to yield specific patterns of microbial community assembly (Zhalnina et al., 2018). For example, scopoletin selectively inhibits soil-borne fungal pathogens, while growth-promoting rhizobacteria is highly tolerant to scopoletin (Stringlis et al., 2018). Flavonoids selectively inhibit the growth of bacteria by acting as antibiotics (Cushnie and Lamb, 2005). As shown by the results of the Spearman correlation analysis, flavonoids did have an inhibitory effect on the genera of bacteria that were significantly negatively correlated with flavonoids in this study, such as Propionibacterium, Saccharomyces, and Staphylococcus (Supplementary Table 6). However, some microbes are highly tolerant to antibiotics and join forces to trigger flavonoid metabolism (Cui et al., 2012; Cao et al., 2016), as we observed that some genera, including Deinococcus, Propionibacterium, Saccharomonospora, Staphylococcus, etc., had significant correlations with flavonol glycosides in this study (Supplementary Table 6). Our culture results showed that the increase of flavonoid content could promote the accumulation of Deinococcus (Figure 6A), which might be the result of amylosucrase produced by Deinococcus being able to utilize quercetin (Rha et al., 2020). Moreover, Deinococcus was also found to produce flavonoid, which may contribute to the anti-oxidative damage ability of this species (Sajjad et al., 2017). Instead, the inhibitory effect of flavonoids on Staphylococcus was found to be enhanced with the increase of concentration (Figure 6B). Previous studies speculated that the inhibitory effect of flavonoids might be realized by interfering with the entry of nutrients and metabolites into bacteria (Tanaka et al., 2004). The results suggested that flavonoids play an important role in the assembly of endophytic communities (Figure 5).
Generally speaking, microorganisms select for their environment mainly in terms of the set of functions (Hammesfahr et al., 2011). Therefore, functional predictions were made about endophytic bacterial communities and found that flavonoids affected endophytic bacterial community assembly at the molecular level (Figure 7). Based on the results of temporal changes in flavonoid content and temporal changes in the abundance of certain genes in the endophytic bacterial community (Figures 7B,C), it can be hypothesized that the microbes colonized in the plant might be affected by flavonoids produced by the host. In general, environmental stress is an important reason for the high abundance of early microbial environmental information-processing genes (Debroas et al., 2009). Signal transduction, an important molecular response mechanism for cells to process stimulus signals, is one of the means by which bacteria process environmental information (Cascales et al., 2014). In this study, the results revealed that the abundance of signal transduction genes in the endophytic bacterial community of Ginkgo leaves was higher in the pre-growth phase of Ginkgo leaves. This may be the result of a response to environmental stress in the pre-growth phase of Ginkgo leaves, including the effect of high concentrations of flavonoids. Amino acid metabolism and lipid metabolism are metabolic processes that are essential for the survival of microorganisms (Cai et al., 2012; Parsons and Rock, 2013). The results of this study found that the trend in gene abundance for these two metabolisms in the endophytic bacterial community of Ginkgo leaves was opposite to the trend in flavonoid content. This is consistent with the report of Zeng et al. (2020) that flavonoids can regulate endophytic bacterial communities by inhibiting amino acid metabolism and lipid metabolism in endophytic bacteria. Endophytes coexist with their hosts, and they adapt to each other and coevolve. It has been shown that genes and abilities that evolve in one lineage are usually acquired steadily by another lineage (Papke and Gogarten, 2012). Direct gene transfer between species has occurred in all major taxa and seems to occur more frequently in prokaryotes (Moran, 2007). Genes including flavonoid synthesis and polyketide metabolism were observed in endophytic bacteria of Ginkgo leaves. The results showed that flavonoid synthesis in the host likely influences the expression of genes related to flavonoid synthesis in the endophytic bacteria. It is like a complementary process (Beekwilder et al., 2007; Ma et al., 2009).
Conclusion
This study provided the first insight into the bacterial communities at different growth stages in Ginkgo leaves. There were significant differences in bacterial communities among different times under the influence of different growth stages of Ginkgo leaves and flavonoids. A strong correlation between the endophytic bacteria and flavonoids was found by the Spearman correlation analysis. In vitro cultivation experiments further provided evidence for the effects of flavonoids on microorganisms. The predicted results of Tax4Fun2 further demonstrated that flavonoids might affect the variation of bacterial communities in Ginkgo leaves at different growth periods. All in all, this experiment can support the subsequent search of functional microorganisms and guide the cultivation, protection, and increase of resource utilization of Ginkgo.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, SRR13946092–SRR13946136.
Author Contributions
YD, HH, KZ, SF, SZ, YL, and XL conceived and designed the work. YD, HH, KZ, SF, and FL performed the experiments. YD and HH analyzed the data and wrote the manuscript. YD revised the manuscript, reanalyzed the data, and plotted. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
Funding
This work was supported by the following grants: the Chinese National Science and Technology Support Program (2013BAC09B00), the National Natural Science Foundation of China (302001124 and 31570113), and the Graduate Research and Innovation Project (1053320170629) in Central South University, China.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.698703/full#supplementary-material
References
- Arnon D. I. (1949). COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 24 1–15. 10.1104/pp.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asshauer K. P., Wemheuer B., Daniel R., Meinicke P. (2015). Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31 2882–2884. 10.1093/bioinformatics/btv287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisak R. R. D., Acharya P. B. (1994). Altermation in the activities of active oxygen scavenging enzymes of wheat leaves subjiected to water stress. Plant Cell Physiol. 35 489–495. [Google Scholar]
- Banning N. C., Gleeson D. B., Grigg A. H., Grant C. D., Andersen G. L., Brodie E. L., et al. (2011). Soil microbial community successional patterns during forest ecosystem restoration. Appl. Environ. Microbiol. 77 6158–6164. 10.1128/AEM.00764-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekwilder J., I, van der Meer M., Sibbesen O., Broekgaarden M., Qvist I., Mikkelsen J. D., et al. (2007). Microbial production of natural raspberry ketone. Biotechnol. J. 2 1270–1279. 10.1002/biot.200700076 [DOI] [PubMed] [Google Scholar]
- Blasko R., Bach L. H., Yarwood S. A., Trumbore S. E., Hogberg P., Hogberg M. N. (2015). Shifts in soil microbial community structure, nitrogen cycling and the concomitant declining N availability in ageing primary boreal forest ecosystems. Soil Biol. Biochem. 91 200–211. 10.1016/j.soilbio.2015.08.041 [DOI] [Google Scholar]
- Brader G., Compant S., Vescio K., Mitter B., Trognitz F., Ma L. J., et al. (2017). Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Annu. Rev. Phytopathol. 55 61–83. 10.1146/annurev-phyto-080516-035641 [DOI] [PubMed] [Google Scholar]
- Burke C., Steinberg P., Rusch D., Kjelleberg S., Thomas T. (2011). Bacterial community assembly based on functional genes rather than species. Proc. Natl. Acad. Sci. U.S.A. 108 14288–14293. 10.1073/pnas.1101591108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H. L., Li H. D., Yan X. Z., Sun B., Zhang Q., Yan M., et al. (2012). Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperidone. J. Proteome Res. 11 4338–4350. 10.1021/pr300459d [DOI] [PubMed] [Google Scholar]
- Cao L.-L., Zhang Y.-Y., Liu Y.-J., Yang T.-T., Zhang J.-L., Zhang Z.-G., et al. (2016). Anti-phytopathogenic activity of sporothriolide, a metabolite from endophyte Nodulisporium sp A21 in Ginkgo biloba. Pesticide Biochem. Physiol. 129 7–13. 10.1016/j.pestbp.2015.10.002 [DOI] [PubMed] [Google Scholar]
- Cascales E., Tschauner K., Hörnschemeyer P., Müller V. S., Hunke S. (2014). Dynamic interaction between the CpxA sensor kinase and the periplasmic accessory protein CpxP mediates signal recognition in E. coli. PLoS One 9:e107383. 10.1371/journal.pone.0107383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparro J. M., Badri D. V., Vivanco J. M. (2014). Rhizosphere microbiome assemblage is affected by plant development. ISME J. 8 790–803. 10.1038/ismej.2013.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelius M., Triplett E. (2001). The diversity of archaea and bacteria in association with the roots of Zea mays L. Microb. Ecol. 41 252–263. 10.1007/s002480000087 [DOI] [PubMed] [Google Scholar]
- Compant S., Duffy B., Nowak J., Clement C., Barka E. A. (2005). Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 71 4951–4959. 10.1128/AEM.71.9.4951-4959.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Yi D., Bai X., Sun B., Zhao Y., Zhang Y. (2012). Ginkgolide B produced endophytic fungus (Fusarium oxysporum) isolated from Ginkgo biloba. Fitoterapia 83 913–920. 10.1016/j.fitote.2012.04.009 [DOI] [PubMed] [Google Scholar]
- Cushnie T. P. T., Lamb A. J. (2005). Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 26 343–356. 10.1016/j.ijantimicag.2005.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debroas D., Humbert J.-F., Enault F., Bronner G., Faubladier M., Cornillot E. (2009). Metagenomic approach studying the taxonomic and functional diversity of the bacterial community in a mesotrophic lake (Lac du Bourget - France). Environ. Microbiol. 11 2412–2424. 10.1111/j.1462-2920.2009.01969.x [DOI] [PubMed] [Google Scholar]
- Deng Y., He Z., Xu M., Qin Y., Van Nostrand J. D., Wu L., et al. (2012). Elevated carbon dioxide alters the structure of soil microbial communities. Appl. Environ. Microbiol. 78 2991–2995. 10.1128/AEM.06924-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubber M. J., Kanfer I. (2006). Application of reverse-flow micellar electrokinetic chromatography for the simultaneous determination of flavonols and terpene trilactones in Ginkgo biloba dosage forms. J. Chromatogr. A 1122 266–274. 10.1016/j.chroma.2006.04.062 [DOI] [PubMed] [Google Scholar]
- Edgar R. C., Haas B. J., Clemente J. C., Quince C., Knight R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27 2194–2200. 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort T., Pauvert C., Zanne A. E., Ovaskainen O., Caignard T., Barret M., et al. (2021). Maternal effects shape the seed mycobiome in Quercus petraea. New Phytol. 230 1594–1608. 10.1111/nph.17153 [DOI] [PubMed] [Google Scholar]
- Grady K. L., Sorensen J. W., Stopnisek N., Guittar J., Shade A. (2019). Assembly and seasonality of core phyllosphere microbiota on perennial biofuel crops. Nat. Commun. 10:4135. 10.1038/s41467-019-11974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammesfahr U., Bierl R., Thiele-Bruhn S. (2011). Combined effects of the antibiotic sulfadiazine and liquid manure on the soil microbial-community structure and functions. J. Plant Nutr. Soil Sci. 174 614–623. 10.1002/jpln.201000322 [DOI] [Google Scholar]
- Hasler A., Gross G. A., Meier B., Sticher O. (1992). Complex flavonol glycosides from the leaves of Ginkgo biloba. Phytochemistry 31 1391–1394. 10.1016/0031-9422(92)80298-S [DOI] [PubMed] [Google Scholar]
- Herms D. A. (1999). Physiological and abiotic determinants of competitive ability and herbivore resistance. Phyton Ann. Rei Bot. 39 53–64. [Google Scholar]
- Huang A. C., Jiang T., Liu Y.-X., Bai Y.-C., Reed J., Qu B., et al. (2019). A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 364:eaau6389. 10.1126/science.aau6389 [DOI] [PubMed] [Google Scholar]
- Huang Y. (2019). Illumina-based Analysis of endophytic bacterial diversity of four Allium species. Sci. Rep. 9:15271. 10.1038/s41598-019-51707-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.-L. (2020). Effect of host, environment and fungal growth on fungal leaf endophyte communities in Taiwan. J. Fungi 6:244. 10.3390/jof6040244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui N., Jumpponen A., Francini G., Kotze D. J., Liu X., Romantschuk M., et al. (2017). Soil microbial communities are shaped by vegetation type and park age in cities under cold climate. Environ. Microbiol. 19 1281–1295. 10.1111/1462-2920.13660 [DOI] [PubMed] [Google Scholar]
- Jackson C. R., Denney W. C. (2011). Annual and seasonal variation in the phyllosphere bacterial community associated with leaves of the Southern Magnolia (Magnolia grandiflora). Microb. Ecol. 61 113–122. 10.1007/s00248-010-9742-2 [DOI] [PubMed] [Google Scholar]
- Karasov T. L., Chae E., Herman J. J., Bergelson J. (2017). Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 29 666–680. 10.1105/tpc.16.00931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. (2011). Btrim: a fast, lightweight adapter and quality trimming program for next-generation sequencing technologies. Genomics 98 152–153. 10.1016/j.ygeno.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Latz M. A. C., Kerrn M. H., Sorensen H., Collinge D. B., Jensen B., Brown J. K. M., et al. (2021). Succession of the fungal endophytic microbiome of wheat is dependent on tissue-specific interactions between host genotype and environment. Sci. Total Environ. 759:143804. 10.1016/j.scitotenv.2020.143804 [DOI] [PubMed] [Google Scholar]
- Lebeis S. L., Paredes S. H., Lundberg D. S., Breakfield N., Gehring J., McDonald M., et al. (2015). Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 349 860–864. 10.1126/science.aaa8764 [DOI] [PubMed] [Google Scholar]
- Leff J. W., Del Tredici P., Friedman W. E., Fierer N. (2015). Spatial structuring of bacterial communities within individual Ginkgo biloba trees. Environ. Microbiol. 17 2352–2361. 10.1111/1462-2920.12695 [DOI] [PubMed] [Google Scholar]
- Lehtonen P., Helander M., Saikkonen K. (2005). Are endophyte-mediated effects on herbivores conditional on soil nutrients? Oecologia 142 38–45. 10.1007/s00442-004-1701-5 [DOI] [PubMed] [Google Scholar]
- Li P., Sun J., Xie X., Li Z., Huang B., Zhang G., et al. (2021). Stress response and tolerance to perfluorooctane sulfonate (PFOS) in lettuce (Lactuca sativa). J. Hazardous Mater. 404:124213. 10.1016/j.jhazmat.2020.124213 [DOI] [PubMed] [Google Scholar]
- Li W., Godzik A. (2006). Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22 1658–1659. 10.1093/bioinformatics/btl158 [DOI] [PubMed] [Google Scholar]
- Liling S., Roska T. P., Arfiansyah R., Maryam F., Nainu F. (2021). Pharmacological effect of Muntingia calabura leaves on the expression of sod1 and sod2 in Drosophila. Biointerface Res. Appl. Chem. 11 12985–12992. 10.33263/BRIAC115.1298512992 [DOI] [Google Scholar]
- Lin Y., Lou K., Wu G., Wu X., Zhou X., Feng Y., et al. (2020). Bioactive metabolites in of Ginkgo biloba leaves: variations by seasonal, meteorological and soil. Braz. J. Biol. 80 790–797. 10.1590/1519-6984.220519 [DOI] [PubMed] [Google Scholar]
- Lindroth R. L., Peterson S. S. (1988). Effects of plant phenols of performance of southern armyworm larvae. Oecologia 75 185–189. 10.1007/BF00378595 [DOI] [PubMed] [Google Scholar]
- Liu P., Cai Y., Zhang J., Wang R., Li B., Weng Q., et al. (2021). Antifungal activity of liquiritin in Phytophthora capsici comprises not only membrane-damage-mediated autophagy, apoptosis, and Ca2+ reduction but also an induced defense responses in pepper. Ecotoxicol. Environ. Saf. 209:111813. 10.1016/j.ecoenv.2020.111813 [DOI] [PubMed] [Google Scholar]
- Ma L.-Q., Guo Y.-W., Gao D.-Y., Ma D.-M., Wang Y.-N., Li G.-F., et al. (2009). Identification of a Polygonum cuspidatum three-intron gene encoding a type III polyketide synthase producing both naringenin and p-hydroxybenzalacetone. Planta 229 1077–1086. 10.1007/s00425-009-0899-1 [DOI] [PubMed] [Google Scholar]
- Merzaeva O. V., Shirokikh I. G. (2010). The production of auxins by the endophytic bacteria of winter rye. Appl. Biochem. Microbiol. 46 44–50. 10.1134/S0003683810010072 [DOI] [PubMed] [Google Scholar]
- Meyer K. M., Leveau J. H. J. (2012). Microbiology of the phyllosphere: a playground for testing ecological concepts. Oecologia 168 621–629. 10.1007/s00442-011-2138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N. A. (2007). Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl.Acad. Sci.U.S.A. 104 8627–8633. 10.1073/pnas.0611659104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio M., Carvajal M., Vergara A., Butassi E., Zacchino S., Mascayano C., et al. (2021). Prenylated flavonoids with potential antimicrobial activity: synthesis, biological activity, and in silico study. Int. J. Mol. Sci. 22:5472. 10.3390/ijms22115472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke R. T., Gogarten J. P. (2012). Ecology. How bacterial lineages emerge. Science 336 45–46. 10.1126/science.1219241 [DOI] [PubMed] [Google Scholar]
- Parsons J. B., Rock C. O. (2013). Bacterial lipids: metabolism and membrane homeostasis. Prog. Lipid Res. 52 249–276. 10.1016/j.plipres.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passera A., Rossato M., Oliver J. S., Battelli G., Shahzad G.-I. R., Cosentino E., et al. (2021). Characterization of Lysinibacillus fusiformis strain S4C11: In vitro, in planta, and in silico analyses reveal a plant-beneficial microbe. Microbiol. Res. 244:126665. 10.1016/j.micres.2020.126665 [DOI] [PubMed] [Google Scholar]
- Pereira E., Barros L., Ferreira I. C. F. R. (2013). Chemical characterization of Ginkgo biloba L. and antioxidant properties of its extracts and dietary supplements. Ind. Crops Prod. 51 244–248. 10.1016/j.indcrop.2013.09.011 [DOI] [Google Scholar]
- Pham V. T. K., Rediers H., Ghequire M. G. K., Nguyen H. H., De Mot R., Vanderleyden J., et al. (2017). The plant growth-promoting effect of the nitrogen-fixing endophyte Pseudomonas stutzeri A15. Arch. Microbiol. 199 513–517. 10.1007/s00203-016-1332-3 [DOI] [PubMed] [Google Scholar]
- Qin J.-C., Zhang Y.-M., Hu L., Ma Y.-T., Gao J.-M. (2009). Cytotoxic metabolites produced by alternaria No.28, an endophytic fungus isolated from Ginkgo biloba. Natural Prod. Commun. 4 1473–1476. 10.1177/1934578X0900401106 [DOI] [PubMed] [Google Scholar]
- Ramamoorthy V., Viswanathan R., Raguchander T., Prakasam V., Samiyappan R. (2001). Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Protection 20 1–11. 10.1016/S0261-2194(00)00056-9 [DOI] [Google Scholar]
- Redford A. J., Fierer N. (2009). Bacterial succession on the leaf surface: a novel system for studying successional dynamics. Microb. Ecol. 58 189–198. 10.1007/s00248-009-9495-y [DOI] [PubMed] [Google Scholar]
- Reitznerova A., Sulekova M., Nagy J., Marcincak S., Semjon B., Certik M., et al. (2017). Lipid peroxidation process in meat and meat products: a comparison study of malondialdehyde determination between modified 2-thiobarbituric acid spectrophotometric method and reverse-phase high-performance liquid chromatography. Molecules 22:1988. 10.3390/molecules22111988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha C. S., Kim H. G., Baek N. I., Kim D. O., Park C. S. (2020). Amylosucrase from Deinococcus geothermalis can be modulated under different reaction conditions to produce novel quercetin 4 ′-O-alpha-d-isomaltoside. Enz. Microb. Technol. 141:109648. 10.1016/j.enzmictec.2020.109648 [DOI] [PubMed] [Google Scholar]
- Sajjad W., Ahmad M., Khan S., Ilyas S., Hasan F., Celik C., et al. (2017). Radio-protective and antioxidative activities of astaxanthin from newly isolated radio-resistant bacterium Deinococcus sp. strain WMA-LM9. Ann. Microbiol. 67 443–455. 10.1007/s13213-017-1269-z [DOI] [Google Scholar]
- Sasse J., Martinoia E., Northen T. (2018). Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 23 25–41. 10.1016/j.tplants.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Singh B., Kaur P., Singh G. R. D., Ahuja P. S. (2008). Biology and chemistry of Ginkgo biloba. Fitoterapia 79 401–418. 10.1016/j.fitote.2008.05.007 [DOI] [PubMed] [Google Scholar]
- Stringlis I. A., Yu K., Feussner K., de Jonge R., van Bentum S., Van Verk M. C., et al. (2018). MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl.Acad. Sci.U.S.A. 115 E5213–E5222. 10.1073/pnas.1722335115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H., Sato M., Oh-Uchi T., Yamaguchi R., Etoh H., Shimizu H., et al. (2004). Antibacterial properties of a new isoflavonoid from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Phytomedicine 11 331–337. 10.1078/0944711041495137 [DOI] [PubMed] [Google Scholar]
- Tetali S. D., Acharya S., Ankari A. B., Nanakram V., Raghavendra A. S. (2021). Metabolomics of Withania somnifera (L.) Dunal: advances and applications. J. Ethnopharmacol. 267 113469. 10.1016/j.jep.2020.113469 [DOI] [PubMed] [Google Scholar]
- Torres N., Martinez-Luscher J., Porte E., Yu R., Kurtural S. K. (2021). Impacts of leaf removal and shoot thinning on cumulative daily light intensity and thermal time and their cascading effects of grapevine (Vitis vinifera L.) berry and wine chemistry in warm climates. Food Chem. 343:128447. 10.1016/j.foodchem.2020.128447 [DOI] [PubMed] [Google Scholar]
- Wagner M. R., Lundberg D. S., del Rio T. G., Tringe S. G., Dangl J. L., Mitchell-Olds T. (2016). Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 7:12151. 10.1038/ncomms12151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xiong F., Yang L., Xiao Y., Zhou G. (2020). A seasonal change of active ingredients and mineral elements in Root of Astragalus membranaceus in the qinghai-tibet plateau. Biol. Trace Elem. Res. 199 3950–3959. 10.1007/s12011-020-02486-0 [DOI] [PubMed] [Google Scholar]
- Wang Q., Garrity G. M., Tiedje J. M., Cole J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73 5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Haack S. E., Lin W., Li B., Wu L., Fang C., et al. (2015). Soil microbial community structure and metabolic activity of Pinus elliottii plantations across different stand ages in a subtropical area. PLoS One 10:e0135354. 10.1371/journal.pone.0135354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z., Tian Y., He F., Zhou H. (2019). Endophytes from Ginkgo biloba and their secondary metabolites. Chin. Med. 14:51. 10.1186/s13020-019-0271-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S.-L., Li S.-Z., Xiao P.-T., Cai Y.-Y., Chu C., Chen B.-Z., et al. (2020). Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 6:eaax6208. 10.1126/sciadv.aax6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhalnina K., Louie K. B., Hao Z., Mansoori N., da Rocha U. N., Shi S., et al. (2018). Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 3 470–480. 10.1038/s41564-018-0129-3 [DOI] [PubMed] [Google Scholar]
- Zou K., Liu X., Hu Q., Zhang D., Fu S., Zhang S., et al. (2021). Root endophytes and Ginkgo biloba are likely to share and compensate secondary metabolic processes, and potentially exchange genetic information by LTR-RTs. Front. Plant Sci. 12:704985. 10.3389/fpls.2021.704985 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, SRR13946092–SRR13946136.