Abstract
Background
Youth with asthma commonly have suboptimal adherence to inhaled corticosteroids (ICS). It is critical to systematically evaluate the effectiveness of ICS adherence promotion interventions and discern which techniques are most effective.
Objective
This study aims to (1) quantify the extent to which interventions improve ICS adherence in pediatric asthma, (2) explore differences in effect size estimates based on intervention and study characteristics, and (3) characterize the risk of bias across interventions.
Methods
We conducted literature searches across five databases. Included studies quantitatively measured ICS adherence as an intervention outcome among youth (<18 years old) diagnosed with asthma and were published after 1997. We analyzed aggregate effect sizes and moderator variables using random-effects models and characterized risk of bias using the Cochrane Collaboration tool.
Results
Thirty-three unique studies met inclusion criteria. At post-intervention, the aggregate effect size for pediatric ICS adherence promotion interventions was small but significant (n = 33, g = 0.39, 95% confidence interval [CI] = 0.24–0.54); however, the aggregate effect size at follow-up was not statistically significant (n = 6, g = 0.38, 95% CI = −0.08 to 0.83). Method of adherence measurement and intervention format were significant moderators. Most interventions had a high risk of performance bias and an unclear risk of bias in one or more domains.
Conclusions
ICS adherence promotion interventions are effective among youth with asthma. Additional longitudinal research is needed to quantify a more precise measure of intervention effectiveness over time, and moderators of intervention effectiveness should be reassessed as the literature base expands.
Keywords: adherence, adolescent, asthma, child, meta-analysis, self-management
Introduction
Asthma-related morbidity has increased over the past 40 years despite the use of daily inhaled corticosteroids (ICS) for those with persistent subtypes (Serebrisky & Wiznia, 2019; Vrijens et al., 2016). ICS adherence is a cornerstone of national guidelines (NAEPP, 2007); however, youth take approximately half of their recommended ICS doses (McQuaid et al., 2003). Suboptimal adherence is associated with more poorly controlled asthma as well as increased risk of asthma exacerbations, school absenteeism, emergency room visits, and mortality (Engelkes et al., 2015).
Rates of ICS adherence among youth have remained low throughout the past two decades despite the development of numerous interventions. Existing interventions utilize a variety of approaches, ranging from in-person to technology only to a combination of the two; they also vary in number of contacts, include different recipients, and occur in various settings (e.g., automated text message reminders for 30 days [Kenyon et al., 2019] or a single in-person asthma day camp for youth [Horner et al., 2016]). Examining time-based trends among ICS adherence interventions published between 1985 to 2012 demonstrates no systematic improvements in intervention effectiveness (Bender, 2016). The persistently low ICS adherence rates among youth prompted a recent editorial to describe the field of adherence research in pediatric asthma as “stuck” (Bender, 2016). As such, it is critical to systematically evaluate the effectiveness of interventions to promote ICS adherence and discern which techniques are most effective for encouraging behavior change.
Prior meta-analyses indicate that adherence promotion interventions across chronic illness populations can effectively improve adherence to treatment regimens (Graves et al., 2010; Kahana et al., 2008; Pai & McGrady, 2014; Wu & Pai, 2014) as well as more distal health outcomes, including patient quality of life, family functioning, and healthcare utilization rates (McGrady et al., 2015). Two systematic reviews have focused specifically on pediatric asthma; one reviewed adherence interventions within a severe asthma population (Boutopoulou et al., 2018) and the other examined digital interventions (Ramsey et al., 2020). Both reviews suggested that interventions can improve ICS adherence; however, these systematic reviews had narrower scopes and their results may not be generalizable to youth with non-severe asthma or to non-digital interventions. Additionally, the authors provided narrative summaries of the included studies, but did not conduct quantitative analyses. There is still a need to establish an effect size for general ICS adherence promotion interventions in pediatric asthma that can help the field power trials and evaluate the overall clinical importance of available intervention technologies.
The purpose of the current meta-analysis is to quantify the extent to which interventions improve ICS adherence in pediatric asthma. Secondary aims include exploring differences in effect size estimates based on intervention and study characteristics, including the method of measuring adherence; participant age; intervention length, recipient, location, target, and type; as well as treatment decay over time. Our tertiary aim was to characterize the risk of bias across adherence promotion interventions in pediatric asthma. Findings from the current study will address a crucial gap in the literature and provide empirical evidence to inform the development of more effective adherence promotion interventions within pediatric asthma. Results will also help characterize the risk of bias within existing interventions and inform recommendations to improve the quality of future studies.
Methods
Literature Search and Article Selection
We conducted initial literature searches in August 2017 via PubMed/MEDLINE, PsycINFO, CINAHL, Cochrane Central, and Web of Science with the assistance of a research librarian. An updated search was conducted in July 2019. Search terms included a combination of the following keywords: asthma, intervention, treatment, adherence, compliance, self-management, and youth (see Supplementary Table S1). Initial search results were limited to peer-reviewed English-language publications spanning youth with asthma aged birth through 17 years. We included studies that quantitatively measured ICS adherence as an intervention outcome; both randomized controlled trials (RCTs) and non-RCTs were considered. We excluded articles that did not include an intervention on adherence and/or self-management, did not quantitatively assess ICS adherence, or did not have adequate data to calculate a meaningful effect size (e.g., dichotomous measures of adherence). Dissertation studies, correlational studies, and review articles were also excluded. To ensure results aligned with current clinical practice guidelines (NAEPP, 2007), we excluded studies published before 1998. More specifically, across all databases, initial searches were limited to articles published from 1998 to Present (August 2017) and updated searches included those published from 2017 to Present (July 2019).
Two authors (A.F. and R.S.) double-screened an initial 10 abstracts and were 100% in agreement regarding which records to include. The two authors then independently screened abstracts and reviewed full-text articles. Random number generation was used to select 20% of the reviewed articles for double coding. Interrater reliability for article inclusion was 97%. Discrepancies were resolved via discussion with senior authors (D.F. and C.C.C.). We conducted forward and backward citation searches on included articles; this process involved reviewing the reference list of each article as well as newer works that cited each article.
We did not include studies with longitudinal follow-ups of previously published studies in the aggregate post-intervention analyses. Instead, we examined these as moderators of the effect of intervention follow-up length as well as in the aggregate follow-up analyses. We chose the most stringent intervention comparison group for studies with multiple treatment arms (e.g., chose education-only attention-control groups as the comparator over usual care groups) due to limitations of the software used for analyses.
Data Extraction and Analysis Plan
Extraction Procedures
We created a standardized codebook based on review of extant literature; the codebook was not previously piloted. Using this codebook, three authors (A.F., R.S., and A.O.) extracted participant, study, and intervention characteristics from all included articles. We double coded each article and resolved disagreements through discussion with senior authors (D.F. and C.C.C.) until we achieved 100% agreement. Extracted study and intervention characteristics included brief descriptions of the study design, intervention, control group, intervention frequency and length, intervention participants (e.g., child, caregiver, or provider), and main adherence findings. Extracted sample characteristics included sample size, sex, race, ethnicity, and age (M, SD, and range). We prioritized extracting sample-wide demographics; however, if that data were not available, we reported the characteristics of the intervention group. We combined sex and gender into one category and dichotomized sex as male or female unless otherwise stated; therefore, if a study only reported the percent of the sample that was male, we assumed the remaining portion was female. Although we extracted race and ethnicity separately, we reported this as racial/ethnic majority due to the limited studies that provided both the race and ethnicity of their sample. Overlapping samples were identified based on study characteristics (e.g., inclusion criteria, intervention description, number of participants), shared authors, and references to prior publications. We extracted data exclusively from the included articles and did not contact authors to supply missing data due to time constraints. We followed the PRISMA checklist (see Supplementary Table S2).
Outcome Extraction
We extracted and coded variables measuring ICS adherence as an outcome of an intervention designed to impact adherence (specifically) or asthma self-management (broadly). Outcomes included ordinal and continuous measures of adherence (i.e., percent adherence over time). Dichotomous measures of adherence (i.e., percent of participants who were above an ICS adherence threshold) were not included because this measurement method did not enable us to assess effect magnitude.
Aggregate Effect Size Calculation
We used statistical results that were reported as, or could be converted into, standardized mean difference values in analyses (e.g., p-values, F statistics, t tests, sample sizes, and nonparametric tests). A random effects model was tested using Comprehensive Meta-Analysis, version 2.2.064 (Biostat Inc.). We examined the overall effectiveness of interventions across studies at post-intervention (i.e., pre-intervention to immediately following intervention) and follow-up (i.e., pre-intervention to the furthest timepoint after post-intervention). Effect sizes were expressed as a standardized mean difference (i.e., Hedges g), where 0.20–0.49, 0.50–0.79, and ≥0.80 were interpreted as small, medium, and large effects, respectively (Cohen, 1988; Hedges, 1981). We also quantified the amount of heterogeneity and systematic variability in effect sizes between studies (e.g., Q).
Moderator Coding and Analysis
Three authors (A.F., R.S., and A.O.) double coded a priori moderator variables. We coded a total of 10 moderators. Categorical moderators (n = 9) included intervention type (education only or other), intervention target (adherence as a primary or secondary outcome), intervention format (in-person only, combined in-person plus technology-assisted, or technology only), intervention location (single setting or multiple settings), intervention length (single session or multiple sessions), caregiver involvement in the intervention (yes or no), provider involvement in the intervention (yes or no), child age (early childhood, school-aged, or adolescent), and type of adherence measurement (subjective report, electronic monitoring, or pharmacy data). Moderators were chosen based on review of extant literature, which included established relationships between developmental characteristics and ICS use (McQuaid et al., 2003), the emergence of new areas of research (i.e., technology-based interventions; Ramsey et al., 2020) and support for specific intervention components (Kahana et al., 2008). We dichotomized moderators to increase interpretability of findings. We measured intervention decay over time continuously via length of time since intervention. We assessed moderator variables using the random effects analog to Lipsey and Wilson’s (2001) analysis of variance technique. We entered continuous moderators into a fixed effect meta-regression. We initially intended to compare the effectiveness of interventions across early childhood, school-age, and adolescence subgroups. However, we deviated from our planned protocol and collapsed early childhood (n = 3) and school age (n = 18) into a single preadolescence subgroup to increase the power of detecting differences from the adolescence subgroup (n = 8).
Risk of Bias and Fail-Safe N
We assessed risk of bias via the Cochrane Collaboration risk of bias tool (Higgins et al., 2019), which categorizes risk based on (1) selection bias due to random sequence generation and allocation concealment, (2) performance bias due to blinding of participants and personnel, (3) detection bias due to blinding of outcome assessment, (4) attrition bias due to completeness of outcome data, and (5) reporting bias due to selective reporting. We assigned categorical risk ratings of high, unclear, or low for each domain. In accordance with published recommendations (Higgins et al., 2019), we calculated risk of selection and reporting bias at the study level. Risk of performance, detection, and attrition bias were assessed at the adherence outcome level for each study. Two authors (A.F. and R.S.) conducted initial risk of bias screening; we resolved disagreements through discussion with senior authors (D.F. and C.C.C.). We provided a narrative summary of findings across studies; however, we included all articles that met inclusion criteria in our analysis, regardless of risk of bias, given that risk of bias is often high among pediatric clinical trials (Crocetti et al., 2010). We also calculated a fail-safe number given that, for a variety of reasons, some studies that contain null findings may not appear in published literature (Rosenthal, 1979).
Results
Studies Retrieved
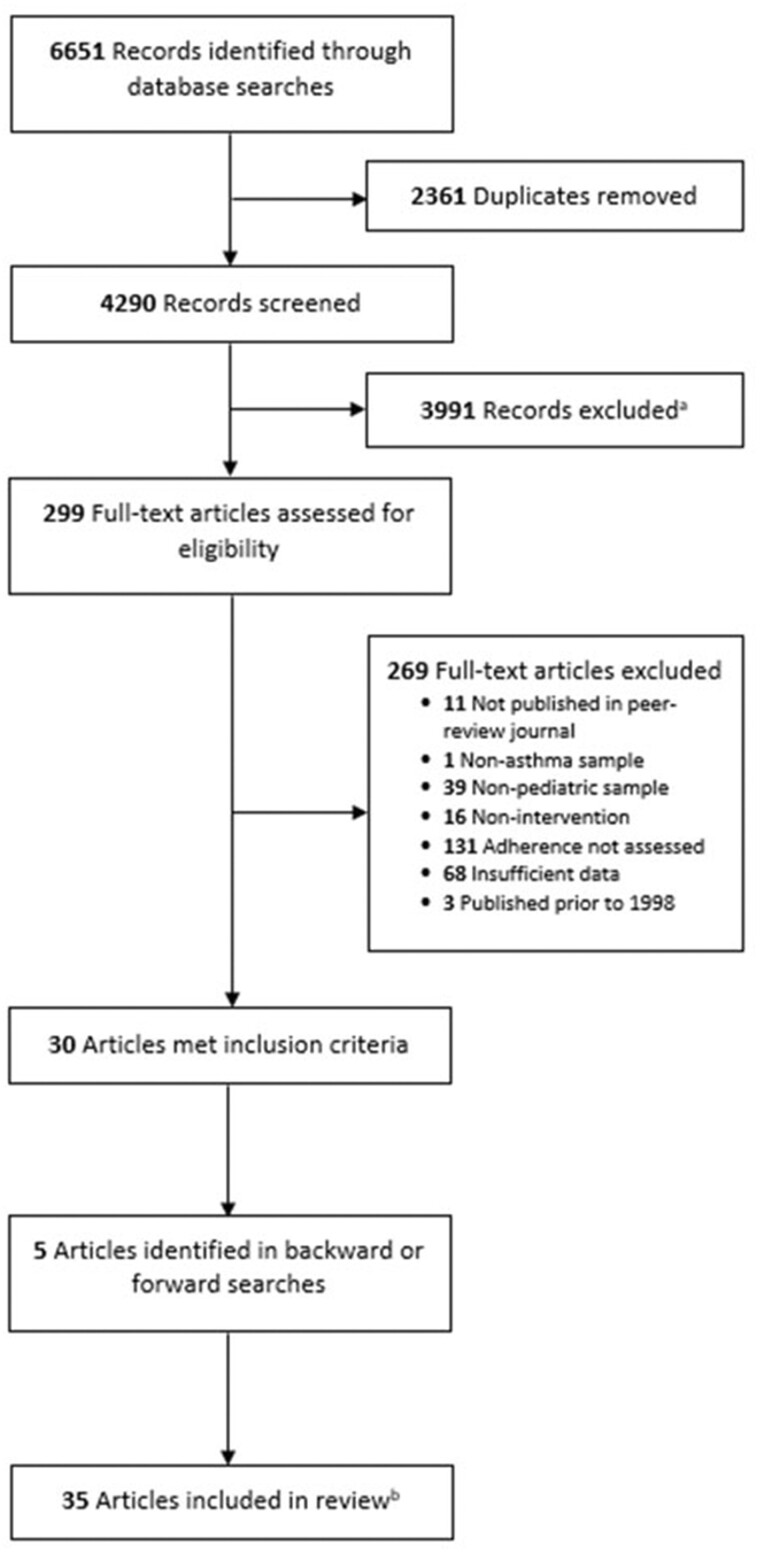
We identified 6651 records through database searches. After removal of duplicates, 4290 records remained. From this, we screened 299 articles for full-text review. Thirty articles met inclusion criteria. We conducted forwards/backwards searches, which yielded an additional five articles. The final sample consisted of 35 articles representing 33 unique studies (see Figure 1). Two articles (Hederos et al., 2009; Naar et al., 2018) presented longitudinal follow-up of previously published studies (Hederos et al., 2005; Naar-King et al., 2014).
Figure 1.
PRISMA flow diagram. PRISMA flowchart detailing process of study selection.
aAbstracts not published in English or peer-reviewed journals, that did not include youth with asthma, and that did not describe a self-management intervention or measure ICS adherence were excluded. bTwo articles presented longitudinal follow-up of previously published studies.
Participant, Study, and Intervention Characteristics
Of the 33 unique studies, 28 studies utilized an RCT design, two used pre-post study designs, and others employed longitudinal crossover (n = 1), non-randomized experimental versus control (n = 1), or two-sample comparison designs (n = 1). The most common control groups were usual care (n = 14), asthma education (n = 9), and attention control (n = 8). Three studies (Duncan et al., 2013; Horner et al., 2016; Otsuki et al., 2009) contained three treatment arms. We analyzed data from 4469 participants across 33 studies. The median sample size was 78 (range = 8 to 899). Participant age ranged from 3 months to 22 years old; most samples (62.1%) had an average age between 6 and 12 years old. Of participants with reported sex, 2806 (58.8%) were male. Seven studies (21.2%) did not describe the race or ethnicity of their sample. Of participants with reported race or ethnicity, 1871 (44.3%) were Black/African American, 1026 (24.3%) were White/Caucasian, 489 (11.6%) were Hispanic/Latino, 365 (8.6%) were Dutch, 54 (1.3%) were Asian, and 419 (9.9%) were another racial or ethnic group or not described. Broadly, the most common interventions included asthma education (n = 10), technology feedback/electronic monitoring (n = 4), text message-based asthma management reminders (n = 4), spirometry/peak flow monitoring reports (n = 2), parental support group meetings (n = 2), written action plans (n = 2), and multisystemic therapy (n = 2). See Table I for study and participant characteristics.
Table I.
Characteristics of Included Studies
| Author (year) |
Study design; control group format | Intervention description | Intervention frequency and length | N | Sample age (years) M ± SD (range); racial/ethnic majority group; majority sex |
Measure of adherence | Main adherence findings |
|---|---|---|---|---|---|---|---|
| Abramson (2015) |
Cluster RCT; Usual care |
3-month spirometry with reports returned to the practice and regular medical review | 4 visits over a 12-month period | 72 |
Unable to extract M ± SD (8–17)a; Not reported; Unable to extract |
Subjective | Mean adherence improved over 12 months in the intervention group; however, there was no improvement in the control group (mean difference = 0.62, p = .14). |
| Bender (2015) |
Pragmatic RCT; Usual care |
Speech recognition telephone calls to parents were triggered when an ICS refill was due or overdue | As indicated for 24 months | 899 |
8.2b ± 0.13 (3–12)a; 56.3% White; 64.2% male |
Pharmacy refills | Adherence was higher in the intervention group than the usual care group, p < .001. |
| Britto (2017) |
Longitudinal Crossover Study; Usual care during nonintervention period |
Participants created and scheduled personalized text messages to be sent to their phones for tailored asthma management reminders | Access to asthma management messages for 3 months | 22 |
Unable to extract M ± SD (12–22)a; Unable to extract; Unable to extract |
Electronic monitor | Intervention improved ICS adherence by 2.75% per month, p < .01. |
| Burgess (2010) |
RCT; Attention control |
Participants, their parents, and physicians received feedback from Smartinhaler on the child's adherence over the past month | Monthly visits for 4 months | 26 |
9.1b (6–13); Not reported; 69.2% male |
Electronic monitor | Adherence was significantly higher in the intervention group, p < .01. |
| Butz (2006) |
RCT; Asthma education control |
Home-based asthma education, including symptom recognition, appropriate medication practice and healthcare use | 6 home visits of 1-h sessions over 6 months | 181 |
4.5 ± 2.1 (2–9); 89.1% African American; 65.6% male |
Pharmacy refills | Mean number of ICS prescriptions were significantly higher for the control group, p = .02. |
| Butz (2010) |
RCT; Asthma education control |
Caregiver-clinician asthma communication skills education, assistance in arranging clinician visits, and health educator attending child's primary care visits | 4 home visits over an 8-week period plus attendance at primary care clinic visit over 6 months | 156 |
8.0 ± 1.9 (6–12); 92.6% African American; 60.6% male |
Pharmacy refills | Intervention participants trended towards a higher ICS to total asthma medication ratio at 12 months, p = .07. |
| Butz (2014) |
RCT; Attention control consisting of home asthma education |
Home asthma education visits, nurse attendance at child's PCP visit, and delivering clinician feedback letter | 3 nurse visits over 4 months | 274 |
(3–10)a; 95.7% African American; 59.3% male |
Pharmacy refills | No significant difference in mean change in ICS pharmacy refills from baseline to 12 months, p = .66. |
| Chan (2003) |
Experimental versus control; Office-based traditional education control |
Internet-based education and video monitoring system using store-and-forward technology | Education given at 2,6,12, and 24 weeks | 10 |
7.6 ± 2.0 (6–17)a; Not reported; 50% male, 50% female |
Pharmacy refills | Adherence and refill rate for ICS was comparable and acceptable in both groups. |
| Chan (2015) |
RCT; Attention control (no audiovisual reminders) |
Electronic monitoring with audiovisual reminders enabled | Follow-up visits every 2 months for 6 months | 220 |
8.9b ± 2.5 (6–15)a; 62.2% Non-European; 51.4% male |
Electronic monitor | Median percentage ICS adherence was 84% in the intervention group, compared to 30% in control group. |
| Davis (2019) |
RCT; Usual care |
Question prompt list and short video about asthma self-management | Single session before seeing provider | 296 |
13.1b ± 2.9 (11–17)a; 63.7% Non-African American; 58.9% male |
Subjective | Group assignment was not associated with either caregiver or youth reported adherence. |
| Ducharme (2011) |
Single-masked RCT; Usual care (unformatted prescription) |
Written action plan plus formatted prescription provided during acute asthma care visit in ED | Single session intervention | 211, 98c |
Median Age = 4b (1–17)a; 62.1% White; 60.3% male |
Electronic monitor | Both groups had similar decline in adherence during the initial 14 days. Intervention group had significantly higher adherence between days 15–28. |
| Duncan (2013) |
3-arm RCTd; Asthma-education attention control |
Parent–youth teamwork intervention focused on sharing responsibility for asthma management | 4 sessions every 2–3 weeks spanning a 2-month period | 29 |
11.1 ± 1.9 (9–15); 84.3% Caucasian; 59.4% male |
Electronic monitor | Intervention produced significantly higher rates of ICS adherence compared to control. |
| Farber and Oliveria (2004) |
RCT; Usual care |
Basic asthma education and written asthma self-management plan as part of an ED visit | Single session and 3 brief follow-up phone calls within 3 months | 50 |
7.5 (2–18); Not reported, authors note sample was predominantly African American; Not reported |
Pharmacy refills | Intervention improved number of medication dispensing events compared to control group, p = .004. |
| Feldman (2012) |
Non-randomized controlled study; Usual care (youth unable to see peak expiratory flow (PEF) feedback) |
Children predicted their PEF and viewed their actual PEF values afterwards | Twice daily for 6 weeks | 85 |
10.8b ± 0.2 (7–15); 52.1% Puerto Rican; 57.3% male |
Electronic monitor | Children in PEF-feedback group had better ICS adherence compared to children in PEF-feedback group, p < .01. |
| Garbutt (2015) |
Pre-post; None |
A peer-trainer provided tailored education, skill training, goals setting, and targeted asthma management as well as parenting support | Weekly to biweekly phone calls over 6 months | 8 |
Unable to extract M ± SD (3–6); 50% Caucasian, 50% African American; Not reported |
Unable to extract | ICS adherence went from 72% to 100%, p = .013. |
| Gustafson (2012) |
RCT; Usual care plus asthma information control |
eHealth program and monthly telephone call from asthma nurse case manager | Yearlong program with monthly telephone calls | 259 |
7.7b ± 2.6 (4–12); 56.8% African American; 61.1% male |
Unable to extract | Adherence did not change significantly within or between groups. |
| Harrington (2018) |
Pilot prospective RCT; Usual care (family administered ICS at home 2x/day) |
School nurse administered morning ICS | 60 days | 46 |
8.2 ± 2.5 (K-8th grade); 91.3% Non-Hispanic African American; 56.5% male |
Subjective | No significant differences between groups, p = .06. |
| Hederos (2005)e |
Prospective RCT; Usual care |
Parental support group meetings with providers following initial diagnosis | 4 group meetings over 6-month period | 60 |
2.3b (0–6)a; Not reported; 60.0% male |
Subjective + Electronic monitor | Intervention group had higher objective adherence, p = .06. No significant group differences based on parent report of adherence. |
| Hederos (2009)e |
Prospective RCT; Usual care |
Parental support group meetings with providers following initial diagnosis |
4 group meetings over 6-month period | 54 |
8.3b (6–13)a; Not reported; 61.1% male |
Subjective | Doctor-reported adherence was higher in for the intervention group, p < .001. However, parent report indicated no between group differences. |
| Horner (2016) |
3-arm RCTd; Attention control (general health education) |
Single asthma day camp with brief didactic presentations following Asthma Plan for Kids curriculum | Single day (4 hours of total content) | 173 |
8.8b ± 1.2 (2nd–5th grade); 60.7% Hispanic; 58.4% male |
Unable to extract | No significant changes over time or group effects for ICS adherence. |
| Jan (2007) |
RCT; Usual care plus educational handouts and written asthma diary |
Internet-based multimedia asthma education with interactive asthma monitoring system | 12-week access to web-based program | 153 |
10.9b ± 2.5 (6–12); Not reported; 61.6% female |
Unclear | Rates of ICS adherence were higher in the intervention group than control, p < .05. |
| Johnson (2016) |
RCT; Usual care |
Subjects received SMS reminders at user-defined medication administration times | 3-week access to scheduled SMS reminders | 65 |
14.2b ± 1.8 (12–17)a; 51.7% Non-African American; 50.6% male |
Subjective | Intervention patients had a significant improvement in self-reported 7-day adherence, compared to controls, p = .01. |
| Kenyon (2019) |
RCT; Attention control (2 reminders to sync sensor) |
Automated text message reminders to take ICS | Daily texts for 30 days | 32 |
5.9 ± 2.1 (2–13); 85.4% African American; 53.7% male |
Electronic monitor | No significant differences in mean adherence between intervention and control group. |
| Kosse (2019) |
Cluster RCT; Usual care |
Smartphone app, including asthma-related educational/motivational movies, medication reminder alarm, peer & pharmacist chat, adherence monitoring | 6-month access to smartphone app | 234 |
15.1 ± 1.9 (12–18)a; 97.9% Dutch; 52.6% female |
Subjective | Non-significant improvement in adherence in the intervention group compared to the control, p = .25. |
| Koumpagioti (2020) |
RCT; Usual care |
Asthma care educational program aimed to develop self-management skills, self-responsibility, and self-efficacy among newly diagnosed youth | 45–60 min interactive session | 78 |
8.4b ± 2.8 (4–16)a; Not reported; 64.1% male |
Electronic monitor | Median percentage ICS adherence was significantly higher in the intervention compared to the control, p < .001. |
| Morton (2017) |
RCT; Usual care (adherence monitoring alone) |
Electronic monitoring with daily reminder alarms with feedback in the clinic regarding ICS use | Daily reminders plus routine clinic visits every 3 months for 1 year | 77 |
10.4b ± 2.9 (6–16)a; 60.0% White British; 56.2% male |
Electronic monitor | Average adherence was significantly higher in the intervention compared to the control, p ≤ .001. |
| Mosnaim (2013) |
RCT; Attention control (doctor-recorded messages only) |
Coping peer group support sessions using motivational interviewing approaches and peer-recorded asthma messages delivered via mp3 players | Weekly support sessions and access to mp3s over 10 weeks | 46 |
13.4 (11–16); 86.8% Black; 52.9% female |
Electronic monitor | No significant group differences in median adherence, p = .93. |
| Naar (2018)e |
RCT; Attention control (weekly in-home family supportive counseling) |
Multisystemic therapy-healthcare intervention | Access to MST therapists for 6 months | 167 |
13.3b ± 1.3 (at baseline—see Naar-King et al., 2014) (12–16)a; 100% African American; 61.1% male |
Subjective | Intervention group demonstrated significantly greater improvements in adherence compared to the control, p = .03. |
| Naar-King (2014)e |
RCT; Attention control (weekly in-home family supportive counseling) |
Multisystemic therapy-healthcare intervention | Access to MST therapists for 6 months | 167 |
13.3b ± 1.3 (12–16)a; 100% African American; 61.1% male |
Subjective | Intervention group demonstrated significantly greater improvements in adherence compared to the control, p = .03. |
| Otsuki (2009) |
3-arm RCTd; Home-based asthma education only |
Home-based asthma education combined with medication adherence feedback | 5 30- to 45- min home visits | 167 |
6.5b ± 3.3 (2–12)a; 98.8% African American; 63.5% male |
Pharmacy refills, Subjective | No significant differences between groups. |
| Riekert (2011) |
Pre-post; None |
Motivational interviewing-based asthma self-management program | 5 30- to 40-min home visits | 37 |
12.5 ± 1.6 (10–16)a; 100% African American; 59.5% male |
Subjective | No significant changes in mean ratings of ICS adherence based on either parent or youth-report. |
| Rohan (2013) |
RCT; Usual care |
Provider-delivered problem solving and adherence feedback | 2–3 sessions (average length = 15 min) during routine medical care | 11 |
8.5 ± 3.1 (5–14); 63.6% African American; 72.7% male |
Electronic monitor | Intervention significantly increased adherence rates during the intervention period for all but one patient. |
| van Es (2001) |
RCT; Usual care |
Individual and group sessions with asthma nurse aimed to increase social support, enhance self-efficacy, and stimulate positive attitude | 4.30-min individual visits with asthma nurse and 3.90-min group sessions over a year period | 86 |
13.6b ± 1.4 (11–18); 75.0% Caucasian; 51.8% male |
Subjective | No significant differences post-intervention. Intervention group had higher adherence than the control group at 24 months, p = .05. |
| Vasbinder (2016) |
RCT; Real-time medication monitoring without SMS reminders |
Real-time medication monitoring with “time-tailored” SMS medication reminders when participants were at risk of missing a dose | As needed SMS reminders over a year period | 209 |
7.8b ± 2.2 (4–11)a; 65.1% Dutch; 62.7% male |
Electronic monitor | Mean adherence was higher in the intervention group, mean difference 12.0% (95% CI 6.7–17.7%). |
| Wiecha (2015) |
Pilot RCT; Usual care plus asthma education manual and peak flow meter |
Interactive educational website designed to promote adherence, enhance family-provider teamwork, and increase physician awareness of the child's asthma status | access to website for 6 months; feedback provided to family and provider every 2 months | 30 |
11.9b ± 2.0 (8–16); 58.6% Black; 58.6% male |
Electronic monitor | Mean change in adherence was positive for the intervention group and negative for the control group; however, this difference was not statistically significant, p = .46. |
Note. N = number of participants with adherence data; Not reported = Original study authors did not report this information; Unable to extract = Data could not be interpreted for the current study. aRecruited age range; bintervention group only, unable to calculate for full sample; cTwo hundred eleven participants were only prescribed ICS for 14 days, while 98 participants were prescribed ICS for >14 days; dFor Duncan et al. (2013) and Otsuki et al. (2009), we compared their primary multi-component interventions to their education-only attention-control groups. Horner et al. (2016) examined outcomes of the same intervention across two different settings and compared each to an attention control group; we compared their community-based day camp intervention, described as their primary intervention, to their attention control group. eTwo articles (Hederos et al., 2009; Naar et al., 2018) presented longitudinal follow-up of previously published studies (Hederos et al., 2005; Naar-King et al., 2014). The boldface text is used to indicate statistically significant results.
Aggregate Effect Size
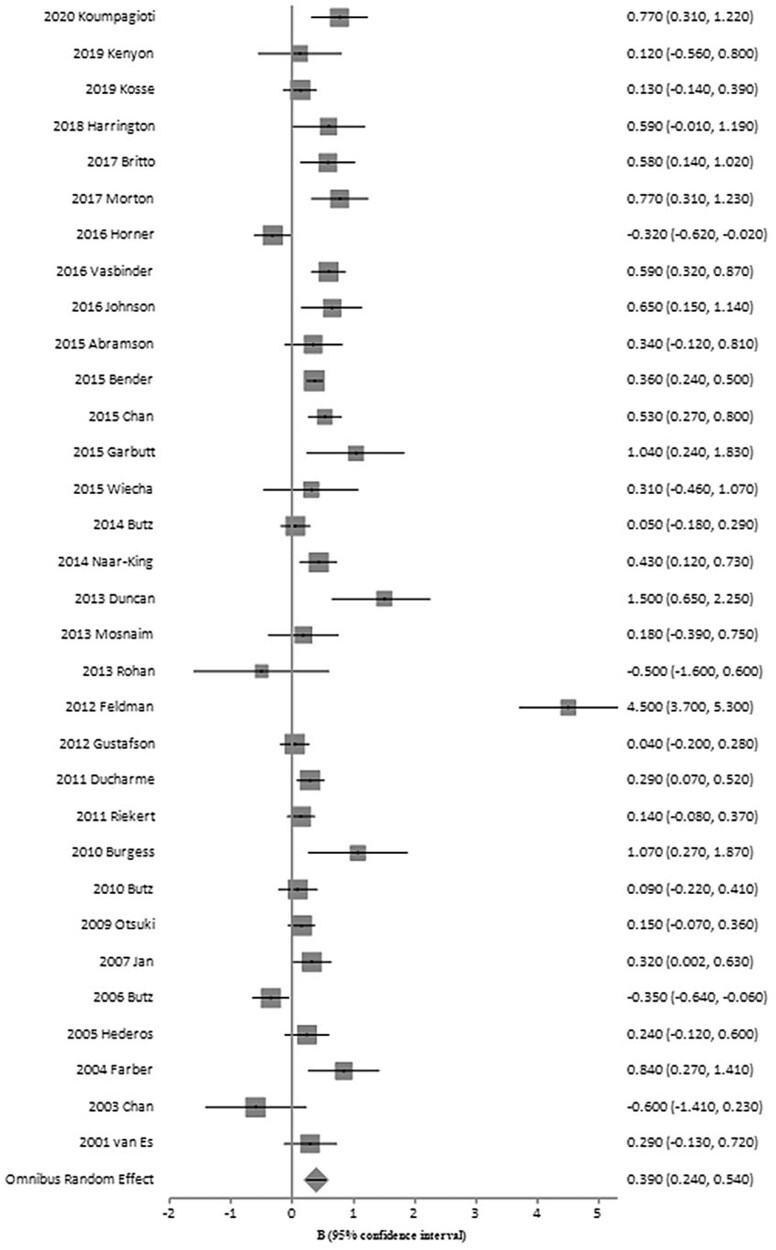
The aggregate post-intervention effect size for pediatric adherence promotion interventions was small, but significant (n = 33, g = 0.39, SE = 0.08, 95% confidence interval [CI] = 0.24–0.54) as displayed in Figure 2. However, we observed significant heterogeneity in the effect sizes (Q = 216.73, p < .001), which suggests that potential moderators of intervention effectiveness can be analyzed. A small portion of studies also included a follow-up period (n = 6); however, the overall effect was not statistically significant (g = 0.38, SE = 0.23, 95% CI = −0.08 to 0.83).
Figure 2.
Summary of post-intervention effect sizes for included studies. Forest plot of standard mean differences in ICS adherence across interventions. Square size corresponds to individual study weight. Horizontal lines indicate 95% confidence interval.
Coded Moderators
Supplementary Table S3 presents potential moderators of intervention effectiveness for each study. Table II provides the aggregate effect sizes within each moderator subgroup. Adherence measurement emerged as a significant moderator in the random effects analysis (Q = 8.34, p = .02). Interventions utilizing electronic measurements yielded significantly larger aggregate effect sizes (n = 13, Cohen d = 0.80, 95% CI = 0.41–1.19) than those using pharmacy refills (n = 6, Cohen d = 0.10, 95% CI = −0.20 to 0.40) and subjective report (n = 8, Cohen d = 0.24, 95% CI = 0.08–0.40). Intervention format was also a significant moderator (Q = 6.78, p = .03). Although post hoc analyses indicated the groups were not statistically different, technology only interventions (n = 11, Cohen d = 0.66, 95% CI = 0.38–0.93), as well as combined in-person plus technology interventions (n = 9, Cohen d = 0.43, 95% CI = 0.14–0.73) appeared to produce larger aggregate effect sizes compared to in-person only interventions (n = 13, Cohen d = 0.17, 95% CI = −0.07 to 0.41). The length of time to follow-up significantly impacted treatment effectiveness (Q = 6.95, p < .01), such that for each passing year an increase of 0.11 effect size was observed. However, this entire finding was driven by the inclusion of one study with a 6-year follow-up and high risk of detection bias (Hederos et al., 2009). If this study was removed from the analysis, a significant decay in treatment effect was observed (Q = 7.06, p < .01), where effect sizes were estimated to decrease by 0.44 each year. Assessing adherence as a primary target (Q = .99, p = .32), involvement of caregivers (Q = 1.09, p = .30) or providers (Q = .32, p = .57) as intervention recipients, participant mean age (Q = 2.97, p = .09), intervention location (Q = .07, p = .79), length of intervention (Q = 1.49, p = .22), and use of education only intervention techniques (Q = 3.45, p = .06) did not moderate effectiveness.
Table II.
Summary of Mean Effect Sizes by Moderator
| Moderator category | Number of studies | Mean weighted-effect size | 95% confidence interval | Q | p |
|---|---|---|---|---|---|
| Adherence measurement | 8.34 | .02 | |||
| Electronic | 13 | 0.80 | 0.41–1.19 | ||
| Pharmacy refill | 6 | 0.10 | −0.20–0.40 | ||
| Subjective | 8 | 0.24 | 0.08–0.40 | ||
| Intervention format | 6.78 | .03 | |||
| In-person only | 13 | 0.17 | −0.07–0.41 | ||
| Combined in-person plus technology | 9 | 0.43 | 0.14–0.73 | ||
| Technology only | 11 | 0.66 | 0.38–0.93 | ||
| Intervention target | 0.99 | .32 | |||
| Primary | 25 | 0.44 | 0.26–0.62 | ||
| Secondary | 8 | 0.25 | −0.06–0.57 | ||
| Caregiver recipient of intervention | 1.09 | .30 | |||
| Yes | 25 | 0.44 | 0.26–0.62 | ||
| No | 8 | 0.25 | −0.07–0.56 | ||
| Provider recipient of intervention | 0.32 | .57 | |||
| Yes | 5 | 0.27 | −0.18–0.72 | ||
| No | 28 | 0.41 | 0.24–0.58 | ||
| Participant mean age | 2.97 | .09 | |||
| Adolescent | 8 | 0.24 | 0.08–0.41 | ||
| Pre-adolescent | 21 | 0.50 | 0.26–0.74 | ||
| Intervention type | 3.45 | .06 | |||
| Education only | 1 | −0.35 | −1.14–0.44 | ||
| Other | 32 | 0.42 | 0.26–0.57 | ||
| Intervention location | 0.07 | .79 | |||
| Single location | 9 | 0.14 | −0.10–0.38 | ||
| Multiple locations | 2 | 0.07 | −0.37–0.51 | ||
| Intervention length | 1.49 | .22 | |||
| Single session | 4 | 0.16 | −0.25–0.57 | ||
| Multiple sessions | 29 | 0.43 | 0.26–0.60 |
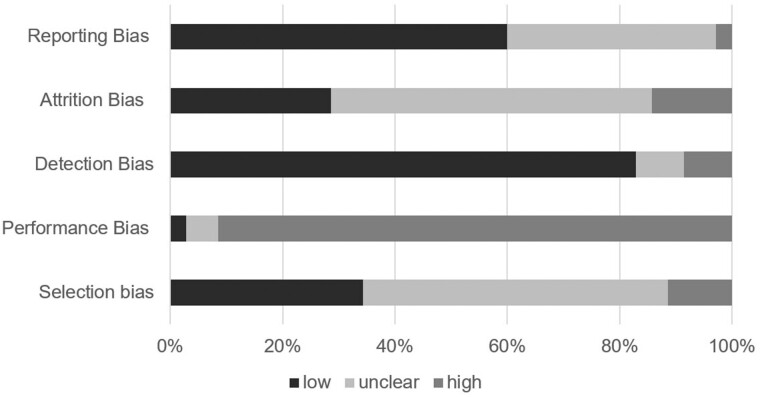
Risk of Bias and Fail-Safe N
We rated most studies as having high risk for performance bias (n = 32, 91%) due to a lack of blinding participants and personnel to participant allocation. Outcome assessors were often blinded and, as a result, we coded many studies as having low risk for detection bias (n = 29, 83%). Over half of studies (n = 21, 60%) had a low risk of selectively reporting outcomes. Many studies did not include enough information to adequately judge selection bias (n = 19, 54%) or attrition bias (n = 20, 57%). Figure 3 illustrates the aggregate risk of bias ratings across included studies. Table III provides the ratings for each individual study. Based on the effect size observed for the omnibus test, 937 null findings are needed to overturn these results, indicating low risk of publication bias (Rosenthal, 1979).
Figure 3.
Aggregate risk of bias ratings across studies. Stacked bar graph illustrating percent of studies rated as having low, unclear, or high risk of bias.
Table III.
Risk of Bias Ratings by Study
| Author (year) | Selection bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias |
|---|---|---|---|---|---|
| Abramson (2015) | Low | High | Low | Unclear | Low |
| Bender (2015) | Unclear | High | Low | Low | Low |
| Britto (2017) | Low | High | Low | High | Unclear |
| Burgess (2010)) | Unclear | High | Low | Unclear | Unclear |
| Butz (2006) | Unclear | High | Low | Unclear | Unclear |
| Butz (2010) | Unclear | High | Low | Unclear | Low |
| Butz (2014) | Low | High | Low | Unclear | Low |
| Chan (2003) | Unclear | High | Low | Unclear | High |
| Chan (2015) | Low | Unclear | Low | Low | Low |
| Davis (2019) | Unclear | High | Low | Unclear | Low |
| Ducharme (2011) | Low | Low | Low | Unclear | Unclear |
| Duncan (2013) | Unclear | High | Low | Low | Low |
| Farber and Oliveria (2004) | Low | Unclear | Low | Unclear | Unclear |
| Feldman (2012) | High | High | Low | Unclear | Low |
| Garbutt (2015) | High | High | Unclear | Unclear | Low |
| Gustafson (2012) | Unclear | High | Low | High | Low |
| Harrington (2018) | Low | High | Low | Unclear | Unclear |
| Hederos (2005) | Unclear | High | High | Unclear | Unclear |
| Hederos (2009) | Unclear | High | High | Unclear | Unclear |
| Horner (2016) | Unclear | High | Low | Unclear | Unclear |
| Jan (2007) | Unclear | High | Unclear | Unclear | Low |
| Johnson (2016) | Low | High | Unclear | High | Low |
| Kenyon (2019) | Unclear | High | Low | Unclear | Low |
| Kosse (2019) | Unclear | High | Low | Low | Low |
| Koumpagioti (2020) | Unclear | High | Low | High | Unclear |
| Morton (2017) | Low | High | Low | Low | Low |
| Mosnaim (2013) | Low | High | Low | Low | Low |
| Naar (2018) | Unclear | High | Low | Low | Low |
| Naar-King (2014) | Unclear | High | Low | Low | Low |
| Otsuki (2009) | Low | High | Low | Low | Low |
| Riekert (2011) | High | High | High | Unclear | Low |
| Rohan (2013) | Unclear | High | Low | High | Unclear |
| van Es (2001) | Unclear | High | Low | Unclear | Unclear |
| Vasbinder (2016) | Low | High | Low | Low | Low |
| Wiecha (2015) | High | High | Low | Unclear | Unclear |
Discussion
The purpose of this meta-analysis was to determine the extent to which interventions improve ICS adherence in pediatric asthma. Aggregating post-intervention effects across the 33 included studies revealed a small but significant effect of interventions to improve pediatric ICS adherence. Our findings are consistent with previous meta-analyses examining adherence promotion interventions for youth with a variety of chronic illnesses (Graves et al., 2010; Kahana et al., 2008; Pai & McGrady, 2014; Wu & Pai, 2014) and suggest that these interventions may be a viable method for increasing ICS adherence among youth with asthma.
Intervention format significantly moderated treatment effectiveness. The aggregate effects of both technology only and combined in-person plus technology-assisted interventions on ICS adherence differed significantly from zero, suggesting that incorporating technological components into interventions may be beneficial. Results contradict findings from a 2008 meta-analysis indicating that technology only interventions produced minimal shifts in adherence behaviors among youth with chronic health conditions (Kahana et al., 2008). This discrepancy may be due to technological advances over the past decade, such as the proliferation of smartphones (Smith, 2015). Our findings align with a recent review of digital interventions targeting pediatric asthma management which demonstrated their potential for improving adherence, increasing quality of life, and reducing rates of healthcare utilization (Ramsey et al., 2020). Notably, digital intervention components can also be responsive to the recent call to differentially address distinct types of non-adherence and improve access to tailored intervention content (Van Boven et al., 2015).
Interventions that utilized electronic measures of adherence also had larger effect sizes than those that utilized pharmacy refill data or subjective reports of adherence. While limitations exist for all adherence measurement tools, electronic monitoring of inhaler actuation is considered the criterion standard of measuring ICS adherence (Sumino & Cabana, 2013). The possibility exists that more accurate measures of adherence may be more responsive to change brought about by effective intervention protocols; subjective measures may have reported higher baseline adherence rates (Desager et al., 2018) and therefore had less room for improvement. These findings may be also partially attributable to well-funded studies being more likely to utilize costly electronic monitors and having greater resources to allocate to interventions. These explanations are speculative in nature and should be further explored within future research.
Several study characteristics did not moderate intervention effectiveness, including participant mean age, the inclusion of caregivers or providers as intervention recipients, and intervention target, type, location, or length. These findings may suggest that a wide range of interventions can be effective for a wide range of patients. However, moderation analyses examining intervention type and location fell below the recommended four studies per subgroup (Fu et al., 2011), thus limiting the power to assess differences in intervention effectiveness between groups. For example, the education only subgroup consisted of a single study with a negative effect size. Although comparing education only interventions to other interventions did not yield any significant group differences within the current meta-analysis, findings from prior reviews of adherence promotions within other chronic illnesses suggest that education interventions alone are insufficient and produce negligible changes in adherence behaviors (Dean et al., 2010; Kahana et al., 2008). We also acknowledge that there are potential confounds between moderators; for instance, the benefit of caregiver involvement may covary with child age, intervention format, or disease control. Finally, our inclusive categorical coding scheme may have weakened our ability to detect precise group differences as many moderator codes were dichotomized (e.g., caregiver involvement versus no caregiver involvement, single versus multiple intervention sessions).
Eighteen percent of studies (6/33) in the present meta-analysis measured adherence after a follow-up period ranging from three months to over 5 years. Notably, the overall intervention effect at follow-up became nonsignificant, suggesting that adherence promotion interventions do not consistently result in long-term gains in ICS adherence. The relatively small number of participants involved in the follow-up analyses (n = 676) as well as the heterogeneity within studies likely contributed to the unstable confidence interval. To fully characterize the intervention effect at follow-up, we would need approximately three times as many participants enrolled in high-quality trials. In the full sample of studies with follow-up periods (n = 6), treatment effects initially appeared to significantly increase over time. However, excluding the study with a high rating of detection bias produced a significant decay in treatment effect. This observed decay is consistent with prior reports of intervention effects across various pediatric chronic illnesses diminishing over time (Kahana et al., 2008) as well as with the established triangular relapse pattern, where participants experience positive behavior change during interventions and then decline toward baseline at post-intervention (Wood & Neal, 2016). Future meta-analyses should quantify a more precise measure of intervention effectiveness over time and reassess treatment decay as new longitudinal studies are added to the literature base.
This meta-analysis should be interpreted within the context of several limitations as well as the quality of included studies. Some individual studies were limited by methodological weakness, including high rates of participant attrition or missing adherence data (14%), inadequate randomization or failure to conceal group allocations (11%), and failure to blind outcome assessors (9%). Most interventions had a high risk of performance bias (91%) and an unclear risk of bias in one or more domains (88%). Although participant knowledge of assigned interventions is considered a high risk of performance bias and can affect behavioral outcomes, such as adherence, we recognize that blinding participants is not feasible in all behavioral interventions. Additional limitations are related to exclusion criteria and the generalizability of findings. Excluding binary measures of adherence reduced the number of studies included in this meta-analysis; however, given the lack of empirical support for establishing a single cut-point indicating adherence versus non-adherence as well as the variance in cut-point values (e.g., 80% vs. 50%; Gellad et al., 2017), we opted to prioritize obtaining a more stringent measure of intervention success using ordinal and continuous measures of adherence. We did not contact authors to supply missing data, which may have reduced the number of included studies and the number of participants with reported demographic information. Furthermore, the exclusion of articles not published in English and grey literature (e.g., conference abstracts, dissertations) may reduce generalizability of findings and increase publication bias, respectively. However, the fail-safe n calculation indicates publication bias is not likely to be a significant concern in this case. Lastly, given the limited statistical power to assess proposed moderators, moderation effects should be interpreted with caution and could change with the addition of new studies.
Results of this meta-analysis highlight key methodological concerns to consider when developing future ICS adherence promotion interventions. To reduce bias, future interventions should randomize participants, conceal group allocations when possible, develop analysis plans that address missing data, and use intent-to-treat analyses (Higgins et al., 2019). We also encourage the use of CONSORT checklists, participant flow diagrams, and reporting of key demographic characteristics to improve the transparency and interpretability of findings (Raad et al., 2008; Schulz et al., 2010). When feasible, studies should utilize electronic monitoring of inhaler actuation to assess ICS adherence (Sumino & Cabana, 2013) at a minimum of two post-intervention time-points to quantify a more precise measure of intervention effectiveness over time. As recommended by Pai and McGrady (2014), timing of assessments should be assigned based on theoretical or logistical rationale (e.g., every 6 months (NAEPP, 2007)).
In conclusion, our findings suggest that adherence promotion interventions are a viable method for improving ICS adherence among youth with asthma. As illustrated in Figure 2, intervention effectiveness appears stagnant over the past two decades despite substantial scientific advances. It appears that the field of pediatric asthma adherence literature has indeed become “stuck” (Bender, 2016) and that novel intervention and methodological approaches are necessary to optimize interventions.
These challenges related to maintaining long-term improvements in ICS adherence suggest we need to be even more effortful with intervention. One pathway to improving efficiency at the group level is to ensure interventions only include “active ingredients” or effective behavior change techniques. Future studies should consider utilizing factorial designs to assess the effects of individual intervention components and ultimately streamline the intervention for use in real-world settings (Collins, 2018). An additional pathway to getting “unstuck” includes utilizing a precision medicine approach and tailoring interventions to the unique needs of each participant (Nahum-Shani et al., 2018). Findings from this meta-analysis suggest that the incorporation of technological components may also increase intervention effectiveness. Another important consideration is a family’s ability to access medical care and fill ICS prescriptions (Canino et al., 2009). Multi-level interventions that address healthcare system-level factors are needed and have demonstrated success in improving ICS adherence, lung functioning, and healthcare utilization rates (Naar et al., 2018; Naar-King et al., 2014).
It is also important to acknowledge that the nonsignificant effect at follow-up highlights the need for future interventions to encourage long-term adherence. Low adherence cannot be “cured” (Nieuwlaat et al., 2014). Even successful interventions do not consistently result in long-term gains in ICS adherence after supports are withdrawn, which suggests that interventions should be maintained for as long as necessary. Because many of the successful interventions were complex, included technological components, and relied on frequent patient contact, they may be costly and require substantial effort. As such, interventions should utilize cost-effective approaches that can be integrated into the existing healthcare system (Nieuwlaat et al., 2014) to ensure the successful uptake by clinicians, payers, and policymakers (Wilson et al., 2019). This is also a signal that, consistent with clinical practice guidelines (NAEPP, 2007), providers should assess ICS adherence at every patient visit.
Future meta-analyses should reassess moderators of intervention effectiveness and treatment decay as well as examine more distal health outcomes beyond ICS adherence as the pediatric asthma literature base expands. For example, McGrady et al.’s (2015) meta-analysis indicates that pediatric adherence promotion interventions lead to clinically meaningful improvements in patient quality of life and healthcare utilization rates within general chronic illness populations. Quantifying the extent to which pediatric asthma interventions improve lung functioning, quality of life, and healthcare system usage would further demonstrate the clinical value of these interventions.
Supplementary Data
Supplementary data can be found at: https://academic.oup.com/jpepsy.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge reference and liaison librarian, Mary Edwards, of UF Health Science Center Libraries for her guidance on the search strategy and systematic review process.
Funding
This work was supported by a career development award from the National Heart, Lung, and Blood Institute (K23HL139992 to R.R.). Funders had no role in this meta-analysis.
Conflicts of interest: None declared.
References
- Abramson M. J., Schattner R. L., Holton C., Simpson P., Briggs N., Beilby J., Nelson M. R., Wood-Baker R., Thien F., Sulaiman N. D., Colle E. D., Wolfe R., Crockett A. J., Massie R. J. (2015). Spirometry and regular follow-up do not improve quality of life in children or adolescents with asthma: cluster randomized controlled trials. Pediatric Pulmonology, 50, 947–954. 10.1002/ppul.23096 [DOI] [PubMed] [Google Scholar]
- Bender B. G. (2016). Nonadherence to asthma treatment: getting unstuck. The Journal of Allergy and Clinical Immunology. In Practice, 4, 849–851. 10.1016/j.jaip.2016.07.007 [DOI] [PubMed] [Google Scholar]
- Bender B. G., Cvietusa P. J., Goodrich G. K., Lowe R., Nuanes H. A., Rand C., Shetterly S., Tacinas C., Vollmer W. M., Wagner N., Wamboldt F. S., Xu S., Magid D. J. (2015). Pragmatic trial of health care technologies to improve adherence to pediatric asthma treatment a randomized clinical trial. JAMA Pediatrics, 169, 317–323. 10.1001/jamapediatrics.2014.3280 [DOI] [PubMed] [Google Scholar]
- Boutopoulou B., Koumpagioti D., Matziou V., Priftis K. N., Douros K. (2018). Interventions on adherence to treatment in children with severe asthma: a systematic review. Frontiers in Pediatrics, 6, 232. 10.3389/fped.2018.00232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto M. T., Rohan J. M., Dodds C. M., Byczkowski T. L. (2017). A randomized trial of user-controlled text messaging to improve asthma outcomes: a pilot study. Clinical Pediatrics, 56, 1336–1344. 10.1177/0009922816684857 [DOI] [PubMed] [Google Scholar]
- Burgess S. W., Sly P. D., Devadason S. G. (2010). Providing feedback on adherence increases use of preventive medication by asthmatic children. Journal of Asthma, 47, 198–201. 10.3109/02770900903483840 [DOI] [PubMed] [Google Scholar]
- Butz A. M., Halterman J., Bellin M., Kub J., Tsoukleris M., Frick K. D., Thompson R. E., Land C., Bollinger M. E. (2014). Improving preventive care in high risk children with asthma: lessons learned. The Journal of Asthma : Official Journal of the Association for the Care of Asthma, 51, 498–507. 10.3109/02770903.2014.892608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz A. M., Tsoukleris M. G., Donithan M., Hsu V. D., Zuckerman I., Mudd K. E., Thompson R. E., Rand C., Bollinger M. E. (2006). Effectiveness of nebulizer use-targeted asthma education on underserved children with asthma. Archives of Pediatrics & Adolescent Medicine, 160, 622–628. 10.1001/archpedi.160.6.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz A., Kub J., Donithan M., James N. T., Thompson R. E., Bellin M., Tsoukleris M., Bollinger M. E. (2010). Influence of caregiver and provider communication on symptom days and medication use for inner-city children with asthma. The Journal of Asthma, 47, 478–485. 10.3109/02770901003692793 [DOI] [PubMed] [Google Scholar]
- Canino G., McQuaid E. L., Rand C. S. (2009). Addressing asthma health disparities: a multilevel challenge. The Journal of Allergy and Clinical Immunology, 123, 1209–1217. 10.1016/j.jaci.2009.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. H. Y., Stewart A. W., Harrison J., Camargo C. A., Black P. N., Mitchell E. A. (2015). The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. The Lancet Respiratory Medicine, 3, 210–219. 10.1016/S2213-2600(15)00008-9 [DOI] [PubMed] [Google Scholar]
- Chan D. S., Callahan C. W., Sheets S. J., Moreno C. N., Malone F. J. (2003). An Internet-based store-and-forward video home telehealth system for improving asthma outcomes in children. American Journal of Health-System Pharmacy, 60, 1976–1981. 10.1093/ajhp/60.19.1976 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd edn). Lawrence Earlbaum Associates. [Google Scholar]
- Collins L. M. (2018). Optimization of behavioral, biobehavioral, and biomedical interventions: the Multiphase Optimization Strategy (MOST) (1st edn). Springer International Publishing. http://www.springernature.com/series/3463 [Google Scholar]
- Crocetti M. T., Amin D. D., Scherer R. (2010). Assessment of risk of bias among pediatric randomized controlled trials. Pediatrics, 126, 298–305. 10.1542/peds.2009-3121 [DOI] [PubMed] [Google Scholar]
- Davis S. A., Carpenter D., Lee C., Garcia N., Reuland D. S., Tudor G., Loughlin C. E., Sleath B. (2019). Effect of an asthma question prompt list and video intervention on adolescents’ medication adherence 12 months later. Annals of Pharmacotherapy, 53, 683–689. 10.1177/1060028019831259 [DOI] [PubMed] [Google Scholar]
- Dean A., Walters J., Hall A. (2010). A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Archives of Disease in Childhood, 95, 717–723. 10.1136/adc.2009.175125 [DOI] [PubMed] [Google Scholar]
- Desager K., Vermeulen F., Bodart E. (2018). Adherence to asthma treatment in childhood and adolescence–a narrative literature review. Acta Clinica Belgica, 73, 348–355. 10.1080/17843286.2017.1409684 [DOI] [PubMed] [Google Scholar]
- Ducharme F. M., Zemek R. L., Chalut D., McGillivray D., Noya F. J. D., Resendes S., Khomenko L., Rouleau R., Zhang X. (2011). Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. American Journal of Respiratory and Critical Care Medicine, 183, 195–203. 10.1164/rccm.201001-0115OC [DOI] [PubMed] [Google Scholar]
- Duncan C. L., Hogan M. B., Tien K. J., Graves M. M., Chorney J. M., Zettler M. D., Koven L., Wilson N. W., Dinakar C., Portnoy J. (2013). Efficacy of a parent-youth teamwork intervention to promote adherence in pediatric asthma. Journal of Pediatric Psychology, 38, 617–628. 10.1093/jpepsy/jss123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelkes M., Janssens H. M., De Jongste J. C., Sturkenboom M. C. J. M., Verhamme K. M. C. (2015). Medication adherence and the risk of severe asthma exacerbations: a systematic review. The European Respiratory Journal, 45, 396–407. 10.1183/09031936.00075614 [DOI] [PubMed] [Google Scholar]
- Farber H. J., Oliveria L. (2004). Trial of an asthma education program in an inner-city pediatric emergency department. Pediatric Asthma, Allergy & Immunology, 17, 107–115. 10.1089/0883187041269913 [DOI] [Google Scholar]
- Feldman J. M., Kutner H., Matte L., Lupkin M., Steinberg D., Sidora-Arcoleo K., Serebrisky D., Warman K. (2012). Prediction of peak flow values followed by feedback improves perception of lung function and adherence to inhaled corticosteroids in children with asthma. Thorax, 67, 1040–1045. 10.1136/thoraxjnl-2012-201789 [DOI] [PubMed] [Google Scholar]
- Fu R., Gartlehner G., Grant M., Shamliyan T., Sedrakyan A., Wilt T. J., Griffith L., Oremus M., Raina P., Ismaila A., Santaguida P., Lau J., Trikalinos T. A. (2011). Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. Journal of Clinical Epidemiology, 64, 1187–1197. 10.1016/j.jclinepi.2010.08.010 [DOI] [PubMed] [Google Scholar]
- Garbutt J. M., Sylvia S., Rook S., Schmandt M., Ruby-Ziegler C., Luby J., Strunk R. C. (2015). Peer training to improve parenting and childhood asthma management skills: A pilot study. Annals of Allergy, Asthma & Immunology, 114, 148–149. 10.1016/j.anai.2014.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellad W. F., Thorpe C. T., Steiner J. F., Voils C. I. (2017). The myths of medication adherence. Pharmacoepidemiology and Drug Safety, 26, 1437–1441. 10.1002/pds.4334 [DOI] [PubMed] [Google Scholar]
- Graves M. M., Roberts M. C., Rapoff M., Boyer A. (2010). The efficacy of adherence interventions for chronically ill children: A meta-analytic review. Journal of Pediatric Psychology, 35, 368–382. 10.1093/jpepsy/jsp072 [DOI] [PubMed] [Google Scholar]
- Gustafson D., Wise M., Bhattacharya A., Pulvermacher A., Shanovich K., Phillips B., Lehman E., Chinchilli V., Hawkins R., Kim J. S. (2012). The effects of combining web-based eHealth with telephone nurse case management for pediatric asthma control: A randomized controlled trial. Journal of Medical Internet Research, 14, e101. 10.2196/jmir.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington C. B., Langhans E., Shelef D. Q., Savitz M., Whitmore C., Teach S. J. (2018). A pilot randomized trial of school-based administration of inhaled corticosteroids for at-risk children with asthma. The Journal of Asthma, 55, 145–151. 10.1080/02770903.2017.1323915 [DOI] [PubMed] [Google Scholar]
- Hederos C. A., Janson S., Hedlin G. (2005). Group discussions with parents have long-term positive effects on the management of asthma with good cost-benefit. Acta Paediatrica, 94, 602–608. 10.1111/j.1651-2227.2005.tb01946.x [DOI] [PubMed] [Google Scholar]
- Hederos C. A., Janson S., Hedlin G. (2009). Six-year follow-up of an intervention to improve the management of preschool children with asthma. Acta Paediatrica, 98, 1939–1944. 10.1111/j.1651-2227.2009.01477.x [DOI] [PubMed] [Google Scholar]
- Hedges L. V. (1981). Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational Statistics, 6, 107–128. 10.3102/10769986006002107 [DOI] [Google Scholar]
- Higgins J. P. T., Savović J., Page M., Elbers R., Sterne J. (2019). Chapter 8: assessing risk of bias in a randomized trial. In Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M., Welch V. (Eds.), Cochrane Handbook for Systematic Reviews of Interventions (6.0). Cochrane Collaboration. www.training.cochrane.org/handbook [Google Scholar]
- Horner S. D., Brown A., Brown S. A., Rew D. L. (2016). Enhancing asthma self-management in rural school-aged children: A randomized controlled trial. The Journal of Rural Health, 32(3), 260–268. 10.1111/jrh.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan R. L., Wang J. Y., Huang M. C., Tseng S. M., Su H. J., Liu L. F. (2007). An internet-based interactive telemonitoring system for improving childhood asthma outcomes in Taiwan. Telemedicine and e-Health, 13, 257–268. 10.1089/tmj.2006.0053 [DOI] [PubMed] [Google Scholar]
- Johnson K. B., Patterson B. L., Ho Y. X., Chen Q., Nian H., Davison C. L., Slagle J., Mulvane S. A. (2016). The feasibility of text reminders to improve medication adherence in adolescents with asthma. Journal of the American Medical Informatics Association, 23, 449–455. 10.1093/jamia/ocv158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana S., Drotar D., Frazier T. (2008). Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. Journal of Pediatric Psychology, 33, 590–611. 10.1093/jpepsy/jsm128 [DOI] [PubMed] [Google Scholar]
- Kenyon C. C., Gruschow S. M., Quarshie W. O., Griffis H., Leach M. C., Zorc J. J., Bryant-Stephens T. C., Miller V. A., Feudtner C. (2019). Controller adherence following hospital discharge in high risk children: A pilot randomized trial of text message reminders. The Journal of Asthma, 56(1), 95–103. 10.1080/02770903.2018.1424195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosse R. C., Bouvy M. L., de Vries T. W., Koster E. S. (2019). Effect of a mHealth intervention on adherence in adolescents with asthma: A randomized controlled trial. Respiratory Medicine, 149, 45–51. 10.1016/j.rmed.2019.02.009 [DOI] [PubMed] [Google Scholar]
- Koumpagioti D., Boutopoulou B., Priftis K. N., Douros K. (2020). Effectiveness of an educational program for children and their families on asthma control treatment adherence. Journal of Asthma, 57, 567–573. 10.1080/02770903.2019.1585873 [DOI] [PubMed] [Google Scholar]
- Lipsey M. W., Wilson D. B. (2001). Practical meta-analysis. (Vol. 49). Sage Publications, Inc. [Google Scholar]
- McGrady M. E., Ryan J. L., Gutiérrez-Colina A. M., Fredericks E. M., Towner E. K., Pai A. L. H. (2015). The impact of effective paediatric adherence promotion interventions: systematic review and meta-analysis. Child: Care, Health and Development, 41, 789–802. 10.1111/cch.12271 [DOI] [PubMed] [Google Scholar]
- McQuaid E., Kopel S. J., Klein R. B., Fritz G. K. (2003). Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. Journal of Pediatric Psychology, 28, 323–333. 10.1093/jpepsy/jsg022 [DOI] [PubMed] [Google Scholar]
- Morton R. W., Elphick H. E., Rigby A. S., Daw W. J., King D. A., Smith L. J., Everard M. L. (2017). STAAR: A randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma. Thorax, 72, 347–354. 10.1136/thoraxjnl-2015-208171 [DOI] [PubMed] [Google Scholar]
- Mosnaim G., Li H., Martin M., Richardson D. J., Belice P. J., Avery E., Ryan N., Bender B., Powell L. (2013). The impact of peer support and mp3 messaging on adherence to inhaled corticosteroids in minority adolescents with asthma: A randomized, controlled trial. The Journal of Allergy and Clinical Immunology: In Practice, 1, 485–493. 10.1016/j.jaip.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar S., Ellis D., Cunningham P., Pennar A. L., Lam P., Brownstein N. C., Bruzzese J. M. (2018). Comprehensive community-based intervention and asthma outcomes in African American adolescents. Pediatrics, 142, e20173737. 10.1542/peds.2017-3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naar-King S., Ellis D., King P. S., Lam P., Cunningham P., Secord E., Bruzzese J. M., Templin T. (2014). Multisystemic Therapy for high-risk African American adolescents with asthma: A randomized clinical trial. Journal of Consulting and Clinical Psychology, 82, 536–545. 10.1037/a0036092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I., Smith S. N., Spring B. J., Collins L. M., Witkiewitz K., Tewari A., Murphy S. A. (2018). Just-in-Time Adaptive Interventions (JITAIs) in mobile health: key components and design principles for ongoing health behavior support. Annals of Behavioral Medicine, 52, 446–462. 10.1007/s12160-016-9830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Asthma Education and Prevention Program. Third Expert Panel on the Diagnosis and Management of Asthma. (2007). Guidelines for the Diagnosis and Management of Asthma. In NIH Publication No 08-5846. 10.1016/j.jaci.2007.09.043 [DOI]
- Nieuwlaat R., Wilczynski N., Navarro T., Hobson N., Jeffery R., Keepanasseril A., Agoritsas T., Mistry N., Iorio A., Jack S., Sivaramalingam B., Iserman E., Mustafa R. A., Jedraszewski D., Cotoi C., Haynes R. B. (2014). Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews, 11,CD000011. 10.1002/14651858.CD000011.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki M., Eakin M. N., Rand C. S., Butz A. M., Hsu V. D., Zuckerman I. H., Ogborn J., Bilderback A., Riekert K. A. (2009). Adherence feedback to improve asthma outcomes among inner-city children: A randomized trial. Pediatrics, 124, 1513–1521. 10.1542/peds.2008-2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai A. L. H., McGrady M. (2014). Systematic review and meta-analysis of psychological interventions to promote treatment adherence in children, adolescents, and young adults with chronic illness. Journal of Pediatric Psychology, 39, 918–931. 10.1093/jpepsy/jsu038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raad J. M., Bellinger S., McCormick E., Roberts M. C., Steele R. G. (2008). Reporting practices of methodological information in four journals of pediatric and child psychology. Journal of Pediatric Psychology, 33, 688–693. 10.1093/jpepsy/jsm130 [DOI] [PubMed] [Google Scholar]
- Ramsey R. R., Plevinsky J. M., Kollin S. R., Gibler R. C., Guilbert T. W., Hommel K. A. (2020). Systematic review of digital interventions for pediatric asthma management. The Journal of Allergy and Clinical Immunology. In Practice, 8, 1284–1293. 10.1016/j.jaip.2019.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riekert K. A., Borrelli B., Bilderback A., Rand C. S. (2011). The development of a motivational interviewing intervention to promote medication adherence among inner-city, African-American adolescents with asthma. Patient Education and Counseling, 82(1), 117–122. 10.1016/j.pec.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan J. M., Drotar D., Perry A. R., McDowell K., Malkin J., Kercsmar C. (2013). Training health care providers to conduct adherence promotion in pediatric settings: An example with pediatric asthma. Clinical Practice in Pediatric Psychology, 1, 314–325. 10.1037/cpp0000036 [DOI] [Google Scholar]
- Rosenthal R. (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86, 638–641. 10.1037/0033-2909.86.3.638 [DOI] [Google Scholar]
- Schulz K. F., Altman D. G., Moher D. (2010). CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ (Clinical Research ed.), 340, c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serebrisky D., Wiznia A. (2019). Pediatric asthma: A global epidemic. Annals of Global Health, 85(1), 6. 10.5334/aogh.2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. (2015). Chapter one: A portrait of smartphone ownership. In U.S. Smartphone Use in 2015. Pew Research Center. https://www.pewresearch.org/internet/2015/04/01/chapter-one-a-portrait-of-smartphone-ownership/
- Sumino K., Cabana M. D. (2013). Medication adherence in asthma patients. Current Opinion in Pulmonary Medicine, 19(1), 49–53. 10.1097/MCP.0b013e32835b117a [DOI] [PubMed] [Google Scholar]
- Van Boven J. F. M., Trappenburg J. C. A., Van Der Molen T., Chavannes N. H. (2015). Towards tailored and targeted adherence assessment to optimise asthma management. Primary Care Respiratory Medicine, 25(1), 1–6. 10.1038/npjpcrm.2015.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Es S. M., Nagelkerke A. F., Colland V. T., Scholten R. J. P. M., Bouter L. M. (2001). An intervention programme using the ASE-model aimed at enhancing adherence in adolescents with asthma. Patient Education and Counseling, 44, 193–203. 10.1016/S0738-3991(00)00195-6 [DOI] [PubMed] [Google Scholar]
- Vasbinder E. C., Goossens L. M. A., Rutten-Van Mölken M. P. M. H., De Winter B. C. M., Van Dijk L., Vulto A. G., Blankman E. I. M., Dahhan N., Veenstra-Van Schie M. T. M., Versteegh F. G. A., Wolf B. H. M., Janssens H. M., Van Den Bemt P. M. L. A. (2016). E-Monitoring of Asthma Therapy to Improve Compliance in children (e-MATIC): A randomised controlled trial. The European Respiratory Journal, 48, 758–767. 10.1183/13993003.01698-2015 [DOI] [PubMed] [Google Scholar]
- Vrijens B., Dima A. L., Van Ganse E., van Boven J. F. M., Eakin M. N., Foster J. M., de Bruin M., Chisholm A., Price D. (2016). What we mean when we talk about adherence in respiratory medicine. The Journal of Allergy and Clinical Immunology. In Practice, 4, 802–812. 10.1016/j.jaip.2016.05.019 [DOI] [PubMed] [Google Scholar]
- Wiecha J. M., Adams W. G., Rybin D., Rizzodepaoli M., Keller J., Clay J. M. (2015). Evaluation of a web-based asthma self-management system: A randomised controlled pilot trial. BMC Pulmonary Medicine, 15(1), 1–10. 10.1186/s12890-015-0007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. K., Christensen A., Jacobsen P. B., Kaplan R. M. (2019). Standards for economic analyses of interventions for the field of health psychology and behavioral medicine. Health Psychology 38, 669–671. 10.1037/hea0000770 [DOI] [PubMed] [Google Scholar]
- Wood W., Neal D. (2016). Healthy through habit: interventions for initiating and maintaining health behavior change. Behavioral Science & Policy, 2(1), 71–83. [Google Scholar]
- Wu Y., Pai A. L. H. (2014). Health care provider-delivered adherence promotion interventions: A meta-analysis. Pediatrics, 133, e1698–e1707. 10.1542/peds.2013-3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.