Abstract
Objective
Pediatric brain tumor survivors (PBTS) experience deficits in social functioning. Facial expression and identity recognition are key components of social information processing and are widely studied as an index of social difficulties in youth with autism spectrum disorder (ASD) and other neurodevelopmental conditions. This study evaluated facial expression and identity recognition among PBTS, youth with ASD, and typically developing (TD) youth, and the associations between these face processing skills and social impairments.
Methods
PBTS (N = 54; ages 7–16) who completed treatment at least 2 years prior were matched with TD (N = 43) youth and youth with ASD (N = 55) based on sex and IQ. Parents completed a measure of social impairments and youth completed a measure of facial expression and identity recognition.
Results
Groups significantly differed on social impairments (p < .001), with youth with ASD scoring highest followed by PBTS and lastly TD youth. Youth with ASD performed significantly worse on the two measures of facial processing, while TD youth and PBTS were not statistically different. The association of facial expression recognition and social impairments was moderated by group, such that PBTS with higher levels of social impairment performed worse on the expression task compared to TD and ASD groups (p < .01, η2 = 0.07).
Conclusions
Variability in face processing may be uniquely important to the social challenges of PBTS compared to other neurodevelopmental populations. Future directions include prospectively examining associations between facial expression recognition and social difficulties in PBTS and face processing training as an intervention for PBTS.
Keywords: brain tumor, face processing, pediatric cancer, social cognition, social competence
Introduction
Pediatric brain tumor survivors (PBTS) are at risk for significant psychosocial challenges associated with their disease and treatments. The treatments that have improved 5-year survival rates over the past 30 years (Ostrom et al., 2019), are often multi-modal and include surgical resection, chemotherapy, and radiation therapy. These treatments and other tumor sequelae (e.g., hydrocephalus, seizures) impact the developing brain through white matter disruption (King et al., 2019), resulting in significant neurocognitive, neurological, and sensori-perceptual deficits (Packer et al., 2003; Robinson et al., 2010). A significant consequence of these sequelae are difficulties with social acceptance compared to healthy peers and other illness groups (Hocking et al., 2015; Schulte et al., 2019). PBTS experience greater social isolation and are more likely to not have close friends (Salley et al., 2015; Schulte et al., 2018; Vannatta et al., 1998). While these issues contribute to PBTS having the poorest quality of life among childhood cancer survivors (Zeltzer et al., 2009), the mechanisms of these challenges are poorly understood.
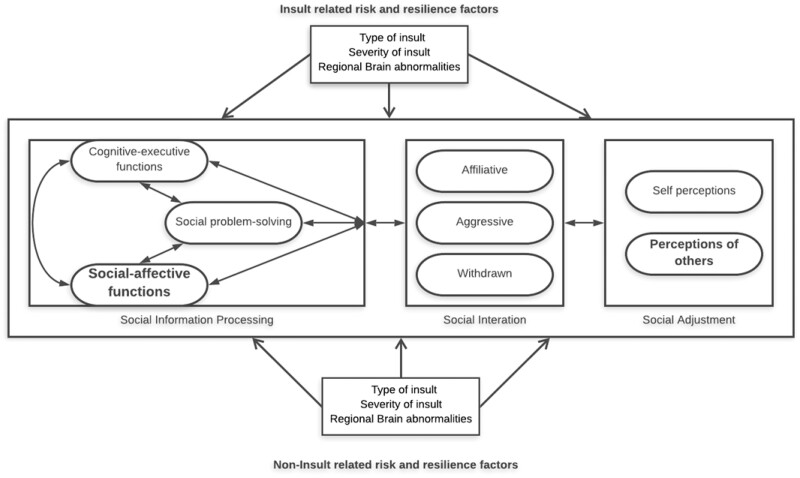
A model of social competence for children with brain disorder (Yeates et al., 2007) offers a framework for understanding the social functioning of PBTS (Hocking et al., 2015). The model is informed by social neuroscience and developmental psychology and highlights the impact of central nervous system abnormalities or insults on social information processing (SIP) abilities, which in turn influence social interactions, social adjustment, and both self and other’s perceptions of social acceptance. SIP offers domains for study in PBTS and are comprised of social-problem solving, social-affective functioning, and cognitive-executive functions. Social problem-solving involves interpreting social cues and implementing responses. Social-affective function includes processes such as emotion regulation abilities and face processing.
Face processing is an important aspect of SIP that informs the quality of social interactions, and hence social adjustment. Facial expressions provide nonverbal information that is crucial for understanding and responding appropriately in social situations (Erickson & Schulkin, 2003). Within the model of social competence (Yeates et al., 2007), face processing is a vital component of social problem-solving. Difficulties with recognizing identities and expressions likely affect later social problem-solving steps, including identification of goals for interactions and the selection of various responses to achieve those goals (Yeates et al., 2007).
Due to their importance in social interactions, face processing has been studied in several groups with known social functioning difficulties, including youth with autism spectrum disorder (ASD; Nomi & Uddin, 2015; Tanaka et al., 2012) and PBTS (Bonner et al., 2008; Hocking et al., 2020a). A series of studies have demonstrated that, compared to youth without ASD who are matched for age and general intelligence (IQ), youth with ASD have deficits in identifying faces across changes in orientation and expression (Wolf et al., 2008), as well as expressions of emotion (Parish-Morris et al., 2013; Tanaka et al., 2012). Furthermore, face processing was related to autism symptom severity (Tanaka et al., 2012) and social skills in youth with ASD (Parish-Morris et al., 2013). In the two studies that have evaluated face processing in PBTS, survivors showed more difficulty identifying adult facial expressions than youth with juvenile rheumatoid arthritis (Bonner et al., 2008) and worse face processing was related to worse parent-rated social adjustment (Bonner et al., 2008) and difficulty naming a friend (Hocking et al., 2020a).
The poor social outcomes of PBTS warrant more research that evaluates SIP in PBTS compared to different groups and determines associations between aspects of the social competence model, in order to identify mechanisms for these outcomes. Such research will elucidate potential targets for interventions to enhance social adjustment. Given the primacy of face processing in social interactions and its relevance in youth with social impairments, this SIP domain is the focus of this study. The aims of this study were: (1) to compare the face processing and social adjustment outcomes of PBTS, youth with ASD and typically developing (TD) youth; (2) to determine associations between face processing and social adjustment outcomes; and (3) to evaluate whether these associations are moderated by group. It was hypothesized that (a) PBTS and youth with ASD would have comparable face processing that were significantly worse than TD youth; and that (b) the associations between facial processing and social adjustment would be moderated by group, with significant associations for PBTS and youth with ASD.
Methods
Participants
Participants included three groups of English-speaking youth between the ages of 6 and 17: PBTS, youth with ASD and TD youth. Inclusion criteria for the PBTS included being 6–17 years of age, received any combination of resection, chemotherapy, and/or cranial radiation therapy, diagnosed at least 5 years ago, and completed all tumor-directed treatments at least 2 years prior. Exclusion criteria for PBTS included only receiving a biopsy or being monitored, any genetic condition affecting neurocognitive functioning (e.g., Down Syndrome), cognitive or significant developmental delay prior to brain tumor diagnosis, no tumor-directed treatment, and vision problems that cannot be corrected through corrective lenses (e.g., field cuts) that would preclude engagement in the eye-tracking protocol associated with the larger study. Of the 207 potential participants contacted, 97 were screened, and 90 met inclusion criteria. Information from screening was confirmed via medical record review. Fifty-four participants completed the study. Reasons for not participating included lack of time. There were no differences in race or sex between participants and non-participants. See Table 1 for medical history information on the PBTS participants. Average age of diagnosis was 6.1 (SD = 3.25, range = 1.21–12.10) and survivors were 8.75 years from diagnosis (SD = 2.68, range = 5.34–16.83). Most survivors (80%) underwent resection, half received radiation therapy, and 43.6% received multi-modal therapy.
Table I.
Participant Characteristics
| Variables | Brain tumor (n = 54) | Autism spectrum (n = 55) | Typically developing (n = 43) | Test statistic |
|---|---|---|---|---|
| n (%) or M ± SD | n (%) or M ± SD | n (%) or M ± SD | ||
| Age in years | 13.72 ± 3.15 | 12.56 ± 2.37 | 12.36 ± 2.64 | F(2, 152) = 3.57* |
| IQ | 99.73 ± 21.16 | 96.49 ± 27.84 | 103.05 ± 15.52 | F(2, 151) = 1.20 |
| Female sex | 23 (42.6%) | 19 (34.5%) | 12 (27.9%) | [2, N = 152] = 2.29 |
| Race | [8, N = 150] = 17.12* | |||
| Caucasian | 40 (74.1%) | 45 (81.8%) | 22 (51.2%) | |
| African-American | 7 (13.0%) | 6 (10.9%) | 14 (32.6%) | |
| Multi-ethnic | 4 (7.4% | 3 (5.5%) | 4 (9.3%) | |
| Asian | 3 (5.6%) | 1 (1.8%) | 0 (0%) | |
| Other | 0 (0%) | 0 (0%) | 1 (2.3%) | |
| Unreported | 0 (0%) | 0 (0%) | 2 (4.7%) | |
| Hispanic/Latinx | [4, N = 122] = 10.60* | |||
| Hispanic/Latinx | 2 (3.6%) | 2 (3.6%) | 7 (16.3%) | |
| Caregiver education | [4, N = 122] = 6.24 | |||
| High school or less | 15 (27.3%) | 3 (5.5%) | 7 (16.3%) | |
| Some college | 11 (20.1%) | 7 (12.7%) | 4 (9.3%) | |
| At least college graduate | 28 (50.9%) | 25 (45.4%) | 22 (51.3%) | |
| Age at tumor diagnosis in years | 6.1 ± 3.25 | |||
| Tumor types | ||||
| Low grade glioma | 6 | |||
| Medulloblastoma | 9 | |||
| Ependymoma | 5 | |||
| Craniopharyngioma | 4 | |||
| Other* | 25 | |||
| Treatment | ||||
| Surgery only | 21 (39.6%) | |||
| Radiation only | 3 (5.7%) | |||
| Chemo only | 5 (9.4%) | |||
| Surgery + radiation | 7 (13.2%) | |||
| Surgery + chemo | 2 (3.8%) | |||
| Radiation + chemo | 1 (1.9%) | |||
| All three | 14 (26.4%) |
Data from youth with ASD and TD youth came from studies conducted at the Center for Autism Research (CAR) at the Children’s Hospital of Philadelphia. Youth with ASD needed to meet diagnostic criteria for ASD on the Autism Diagnostic Observation Schedule—Generic (ADOS-G; Lord et al., 2000) and the Autism Diagnostic Interview—Revised (ADI-R; Rutter et al., 2003) with consensus diagnostic agreement between at least two clinicians. Exclusion criteria for both youth with ASD and TD youth included vision problems that cannot be corrected with lenses and a history of traumatic brain injury or other neurological abnormality. Exclusion criteria for the TD youth included (a) delays suggestive of autism-like impairments on screening by study personnel; (b) a first- or second-degree relative with ASD; and (c) a DSM-IV-TR Axis I disorder or significant symptoms of Attention-Deficit/Hyperactivity Disorder or mood, anxiety, substance-related, or conduct disorders. ASD and TD participants were selected from the larger CAR database using a nearest neighbor matching algorithm using the R program “matchit,” including age, sex, and IQ as matching variables. The algorithm selected participants from the larger dataset that most closely matched the PBTS dataset in a multidimensional Euclidean space constructed from the specific matching variables (age, sex, and IQ, each weighted equally). This procedure is retrospective, and does not guarantee a non-significant difference between the final groups on each of the matching variables—it only finds the best match from the larger sample to the target sample.
Procedures
All procedures were approved by our institutional review board and written informed consent (and child assent) was obtained. Potentially eligible PBTS were identified through tumor registry and electronic medical records and sent a letter describing the study. Study personnel contacted these families via phone to provide more information about the study and conduct a verbal screening to determine eligibility. Those meeting eligibility criteria and interested in participating were invited to a one-time, in-person evaluation. The measures for this evaluation mirrored those given to youth with ASD and TD youth as part of a larger CAR study comparing these groups on a variety of domains. Potential PBTS participants were recruited to compare with the ASD and TD samples with a target goal of 50. Parents completed a demographics form and questionnaires while youth completed the assessment. Participants received a $50 gift card.
Measures
Cognitive Ability
General cognitive ability was measured using the Differential Ability Scales, Second Edition (DAS-II; Elliott, 2007). The DAS-II evaluates verbal and nonverbal reasoning abilities and provides a General Conceptual Ability score, which is a norm-referenced overall cognitive ability score that can be considered a measure of overall IQ due to its high correlation with other IQ tests (Elliott, 2007). Participants completed the following subtests: Recall of Designs, Word Definitions, Pattern Construction, Matrices, Verbal Similarities, and Sequencing and Quantitative Reasoning. Standard scores have a mean of 100 (±15).
Face Processing
Face processing was measured using two subtests of the computerized Let’s Face It! (LFI) Skills Battery (Wolf et al., 2008). The full LFI battery consists of 11 subtests that are guided by contemporary theories of face perceptual processes and assess the perception of (1) facial identity and (2) facial expressions. These constructs were validated in a principle components analysis in a sample of 128 TD children and adolescents (Wolf et al., 2008). The current study used the Identity Across Expression and Expression Across Identity subtests of the “Match Maker Expression” section. Identity Across Expression evaluated participants’ ability to recognize facial identities across changes in expression (happy, angry, sad, disgusted, and fear). In Expression Across Identity, participants match facial expressions for basic emotions (happy, angry, sad, disgust, frighten) across changes in facial identity without explicit labels. A face depicting an emotion is presented for one second followed by three probe faces of different identities and participants select the probe face that matches the emotion of the initially presented face. The initial face and the three probe faces are shown simultaneously while the participant selects. There are 30 total trials with six trials per emotion. This study evaluated task accuracy; with higher scores indicating better performance. The Match Maker Expression battery has demonstrated robust differences in face processing between youth with ASD and TD youth (effect sizes of 0.4 to 1.0) and has strong associations with measures of social behavior in youth with ASD (Tanaka et al., 2012). Reliability is high (split half reliabilities > .75), and there are large normative data sets (by sex and IQ) for ages 6 to 18. Accuracy data across emotions on the tasks was not available for the youth with ASD and TD youth.
Social Adjustment
The Social Responsiveness Scale, Second Edition (SRS-2; Constantino & Gruber, 2012) is a 65-item parent-report measure that evaluates the frequency of reciprocal social behaviors, communication, and repetitive and stereotypic behaviors of a child. It yields an age-standardized Total T score that provides an index of social impairments (m = 50, SD = 10). The SRS has strong internal consistency (Cronbach’s alpha = .97), test–retest reliability, and inter-rater reliability and has strong associations with other measures of social difficulties (Constantino & Gruber, 2012). Higher Total T scores indicate more impairments.
Statistical Analyses
Descriptive statistics summarized demographic and medical variables and were compared between the three groups. Groups were compared on demographic and sample characteristics using chi-square analyses (e.g., sex, ethnicity) and ANOVAs (e.g., age, IQ). For PBTS participants, t-tests compared scores on the SRS-2 and LFI subtests based on tumor treatment variables (e.g., surgery vs. no surgery). Pearson correlation analyses evaluated associations between continuous variables. Variables that emerged as significantly different between groups were included as covariates in subsequent analyses. ANCOVAs compared groups on the two face processing domains (LFI Identity Across Expression and LFI Expression Across Identity) and social impairments (SRS-2). General linear model (GLM) analyses predicting social impairments evaluated models that included group (PBTS, ASD, TD), face processing domain, and the interaction between group and face processing domain, as a test of moderation, while statistically accounting for participant age and IQ. Two separate GLM models were conducted, one for each face processing domain. There were few cases of missing data across the measures. When missing data did arise, the participant with missing data was excluded from the analysis.
Results
Participants
Table 1 provides demographic information on the three groups. The groups did not differ in terms of sex, [2, N = 152] = 2.29, p = .32. Participant IQ was in the average range (see Table 2) and did not differ across groups, F(2, 151) = 1.20, p > .30, ηp2 = 0.016. Groups differed in terms of current age, F(2, 152) = 3.57, p < .05, ηp2 = 0.05, with a trend for PBTS participants to be older than TD participants using Bonferroni correction, p = .054. The groups also differed in terms of race, [8, N = 150] = 17.12, p < .05, with more Black participants in the TD group (31.4%) compared to the PBTS (13.0%) or ASD (10.9%) participants. Groups also differed in terms of participants from Hispanic/Latino backgrounds, [2, N = 140] = 6.80, p < .05, with more Hispanic/Latino participants in the TD group (n = 7) compared to the PBTS (n = 2) or ASD (n = 2) groups. Groups did not differ in terms of highest level of parental education.
Table II.
Group Comparisons on Main Variables
| Measure | Brain tumor |
Autism spectrum |
Typically developing |
F(2,149) | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | ||
| SRS-2 total T score | 52.76b | 9.95 | 75.58a | 9.77 | 42.63c | 3.96 | 192.88*** |
| Let’s face it: percent correct | |||||||
| Expression Across Identity | 82.8%ab | 14.2 | 76.5%c | 17.8 | 87.13%ad | 9.8 | 9.871*** |
| Identity Across Expression | 74.4%ab | 18.1 | 66.2%c | 20.1 | 80.0%ad | 15.6 | 9.394*** |
p < .001.
Indicate in a row without a common superscript, means differ (p < .05) as analyzed from post-hoc Bonferroni tests.
Individual Difference Analyses (Age, IQ, and Social Impairments)
In Pearson correlation analyses (Table 3), younger age at time of participation was associated with poorer performance on the facial identity and facial expression subtests of the LFI. Participant IQ also was related to performance on both LFI subtests. Given the significant difference in age across groups, age was included as a covariate in group comparisons of the LFI variables. Group comparisons did not include IQ as a covariate given that the groups did not significantly differ on IQ. LFI performance on the facial identity and facial expression subtests were significantly associated with SRS-2 scores. For PBTS participants, those treated with chemotherapy (n = 22; m = 0.71, SD = 0.15) performed worse on the facial identity task compared to those who were not treated with chemotherapy (n = 31; m = 0.83, SD = 0.14), t [51] = 2.91, p < .01. Also, for PBTS, older age at diagnosis was related to better performance on the facial identity task, with a trend for older age at diagnosis to be related to better accuracy on the facial expression task (r = .27, p = .055). Secondary analyses indicated that compared to PBTS who did not receive chemotherapy, those treated with chemotherapy were significantly younger at the time of study participation (m = 12.37, SD = 3.49 vs. m = 14.69, SD = 2.65, t[52] = 2.82, p < .01) and time of brain tumor diagnosis (m = 4.77, SD = 3.10 vs. m = 7.04, SD = 3.06, t[51] = 2.65, p < .05). There were not differences in IQ based on chemotherapy exposure (m = 95.65, SD = 14.30) or not (m = 103.90, SD = 17.46, t[52] = 1.85, p > .05).
Table III.
Pearson Correlations Between LFI Variables and Social Impairments
| Variable | n | M | SD | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|---|
| 1. IQ | 152 | 99.73 | 21.16 | — | |||||
| 2. SRS total T score | 152 | 58.15 | 16.22 | −0.209** | — | ||||
| 3. LFI ID across expression | 152 | 0.74 | 0.18 | 0.52** | −0.349** | — | |||
| 4. LFI expression across ID | 151 | 0.83 | 0.14 | 0.43** | −0.42** | 0.63** | — | ||
| 5. Age at evaluation | 153 | 12.91 | 2.77 | 0.195* | −0.57 | 0.426** | 0.223** | — | |
| 6. Age at diagnosisa | 53 | 6.10 | 3.25 | 0.299** | −0.097 | 0.478** | 0.265 | 0.654** | — |
p < .05.
p < .01.
Age at diagnosis refers to the PBTS sample only.
Social Impairments by Group
PBTS level of social impairment on the SRS-2 was generally within normal limits (Table 2) with 9.25% falling within the mildly impaired range or higher. There were significant group differences on social impairments, F(2, 151) = 192.88, p < .001, ηp2 = 0.72, with each group significantly different from one another at p < .001 in post hoc analyses using Bonferroni corrections. Youth with ASD had the highest level of social impairments, followed by PBTS.
Face Processing by Group
Groups differed on their performance on the facial identity and facial expression subtests of the LFI while statistically accounting for age, F(2, 152) = 10.82, p < .001, ηp2 = 0.13, and F(2, 151) = 9.25, p < .001, ηp2 = 0.11, respectively. In post hoc analyses using Bonferroni corrections, youth with ASD performed significantly worse on both LFI subtests than the TD youth and PBTS at the p < .01 level. PBTS and TD youth did not significantly differ on the LFI tasks.
GLM Analyses Testing Moderation
Facial Identity
The overall GLM (Table 4) predicting social impairments on the SRS-2 from group membership, facial identity recognition ability, and the interaction between group membership and facial identity, while statistically accounting for age and IQ, was significant F(7, 152) = 59.43, p < .001, ηp2 = 0.74. However, no variables in the model emerged as a significant predictor of social impairments, with group membership nearing significance, p = .06.
Table IV.
General Linear Models Predicting Social Impairments from Facial Expression Recognition and Facial Identity Recognition
| General linearized models: Outcome variable-SRS total T score | |||
|---|---|---|---|
| Variable | F | p | η 2 |
| Model 1: Facial expression recognition | |||
| Participant age | 0.127 | 0.722 | 0.001 |
| Diagnosis | 174.5 | 0.000 | 0.71 |
| IQ | 1.392 | 0.24 | 0.010 |
| LFI: Expression Across Identity | 8.4 | 0.004 | 0.06 |
| Diagnosis × Expression Across Identity | 5.4 | 0.005 | 0.07 |
| Model 2: Facial identity recognition | |||
| Participant age | 0.180 | 0.672 | 0.001 |
| Diagnosis | 170.82 | 0.000 | 0.703 |
| IQ | 3.53 | 0.06 | 0.024 |
| LFI: Identity Across Expression | 0.496 | 0.483 | 0.003 |
| Diagnosis × Identity Across Expression | 2.94 | 0.056 | 0.039 |
Facial Expression
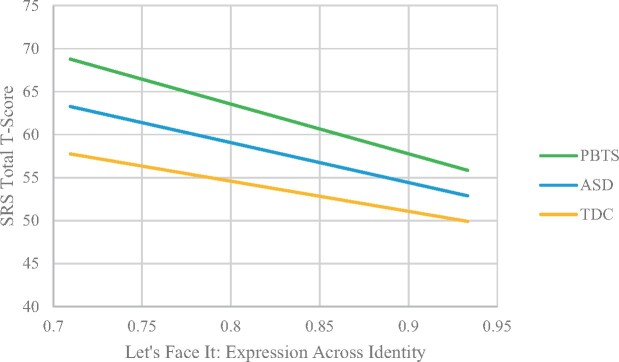
The GLM model (Table 5) predicting social impairments from group membership, facial expression recognition accuracy, and the interaction between diagnosis and facial identity, while controlling for age and IQ, also was significant F(7, 151) = 65.71, p < .001, ηp2 = 0.76. There was a significant interaction between group membership and LFI Facial Expression accuracy, p < . 01. At lower levels of facial expression recognition accuracy, PBTS had higher levels of social impairments on the SRS-2 compared to the other groups (Figure 2).
Figure 2.
SRS-2 total and expression across identity moderated by diagnosis.
Figure 1.
Yeates model of social competence (Yeates et al., 2007).
Discussion
Significant late effects associated with pediatric brain tumors, including social difficulties, are well documented. These deficits include being less likely to identify a close friend (Hocking et al., 2020a) and having lower rates of social acceptance (Salley et al., 2015; Vannatta et al., 1998). Given that face processing difficulties may play a significant role in these social challenges (Bonner et al., 2008), this study evaluated social impairments and facial expression and identity recognition in PBTS compared to youth with ASD and TD youth. Groups differed on social impairments, with PBTS showing more impairment than TD youth and less impairment than youth with ASD. While the three groups differed in both facial expression and identity recognition abilities, PBTS and TD youth performed similarly. However, greater social impairments were seen for PBTS at lower levels of facial expression recognition accuracy compared to the other groups. This differential association between facial expression recognition and social impairments suggests a potential mechanism for social challenges that is particularly relevant for PBTS. These findings are consistent with prior face processing research with PBTS (Bonner et al., 2008; Hocking et al., 2020a) and offer directions for future research.
PBTS demonstrated more social impairments than TD youth and less than youth with ASD. This is consistent with previous research where PBTS show more social adjustment difficulties than their TD siblings (Schulte et al., 2018). Notably, both PBTS and TD youth had scores within the average range on the SRS-2, but PBTS scores were higher, indicating relatively more social impairment. The SRS-2 measures social impairment severity within ASD. While PBTS have shown similarities to youth with ASD across elements of social behavior and SIP (e.g., social attention) (Hocking et al., 2020b), it is not surprising that their overall level of social impairment is less than youth with ASD, a group characterized by social deficits. Also, it is an unresolved question whether measures developed for youth with ASD are appropriate to evaluate PBTS. Future research could address this by using multiple methods for assessing PBTS social functioning, including those designed for youth with ASD.
PBTS performed similarly to TD youth on measures of facial identity and expression recognition, while youth with ASD performed significantly worse than the other two groups on these tasks. Additionally, group membership did not moderate the association between social impairment and ability to identify individuals. These findings on facial expression recognition contrast with our hypotheses and findings from a prior study with PBTS (Bonner et al., 2008). The discrepancy in these studies could be due to differing face processing measures. The LFI is widely used to identify emotional processing deficits in youth with ASD (Tanaka et al., 2010; Wolf et al., 2008), suggesting that clinical use of the LFI in PBTS may be beneficial.
While PBTS did not differ from TD participants on the facial expression recognition task, the association between facial expression recognition and social impairments was moderated by group, such that PBTS with poorer task performance showed greater social impairments. This suggests that difficulties with facial expression recognition may have more relevance on PBTS social behavior than they do for youth with ASD and TD youth. Different neurodevelopmental groups rely on different skills for appropriate social interaction (Tanaka et al., 2012), and perhaps the more global SIP impairment seen in youth with ASD was not measured in this study. Additionally, PBTS were an average of 8.75 years from diagnosis; youth with ASD have had their entire lives to use coping mechanisms in social interactions, and may not rely as heavily on affect recognition. Facial expression recognition may be of unique import for survivor social behavior because they may not have developed compensatory strategies like youth with ASD.
While there may be a difference in the mechanism by which youth with ASD and PBTS process and use facial expression information, it is an integral component of SIP and social interaction. Prior trials in youth with ASD found that face processing training improved facial expression recognition (Tanaka et al., 2012). Similarly, face processing training could prove helpful for PBTS and warrants further study. Also, survivor socialization could be emphasized to parents in order to increase opportunities for face processing and potentially strengthen this skill.
Strengths of this study include the novelty in using the LFI in PBTS and comparing PBTS to youth with ASD and TD youth. However, study limitations should be considered. First, data are cross-sectional and focus on long-term survivors of pediatric brain tumors. Future studies should prospectively evaluate SIP and examine predictive associations between SIP and later social adjustment. Second, the sample of PBTS is heterogeneous with a variety of diagnoses and treatment histories, limiting our ability to evaluate how these variables impact outcomes. PBTS treated with chemotherapy had worse performance on the LFI Identity Across Expression test compared to those not treated with chemotherapy, though this may be due to demographic differences among these two treatment groups since those treated with chemotherapy were younger at diagnosis and at study participation. Third, the groups differed in terms of age. However, we included age and IQ as covariates in all analyses. Next, data on the error rates for facial expression recognition for different emotions is not presented here due to these data not being available for youth with ASD and TD youth. Lastly, the PBTS and ASD groups were not racially representative and more diverse samples are needed to evaluate the potential effects of culture on face processing and the presentation of social impairments.
In conclusion, PBTS displayed similar levels of social impairment and face processing abilities to TD youth. However, group membership moderated the association between facial expression recognition and social impairment. Future studies of SIP in PBTS should prospectively evaluate the influence of face processing on social adjustment and whether or not facial expression recognition training lessens social impairment.
Funding
This research was supported by the National Cancer Institute of the National Institutes of Health (K07CA178100), the Intellectual and Developmental Disabilities Research Center at Children’s Hospital of Philadelphia and the University of Pennsylvania (U54HD086984), and Children’s Hospital of Philadelphia.
Conflicts of interest: None declared.
References
- Bonner M. J., Hardy K. K., Willard V. W., Anthony K. K., Hood M., Gururangan S. (2008). Social functioning and facial expression recognition in survivors of pediatric brain tumors. Journal of Pediatric Psychology, 33, 1142–1152. http://jpepsy.oxfordjournals.org/content/33/10/1142.full.pdf [DOI] [PubMed] [Google Scholar]
- Constantino J. N., Gruber C. P. (2012). (SRS™-2) Social Responsiveness Scale™ (2nd edn). Western Psychological Services. [Google Scholar]
- Elliott C. D. (2007). Differential Ability Scales-Second edition (DAS-II). Harcourt Assessment. [Google Scholar]
- Erickson K., Schulkin J. (2003). Facial expressions of emotion: A cognitive neuroscience perspective. Brain and Cognition, 52(1), 52–60. [DOI] [PubMed] [Google Scholar]
- Hocking M. C., McCurdy M., Turner E., Kazak A. E., Noll R. B., Phillips P., Barakat L. P. (2015). Social competence in pediatric brain tumor survivors: application of a model from social neuroscience and developmental psychology. Pediatric Blood & Cancer, 62, 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking M. C., Noll R. B., Kazak A. E., Brodsky C., Phillips P., Barakat L. P. (2020a). Friendships in pediatric brain tumor survivors and non-central nervous system tumor survivors. Journal of Pediatric Psychology, 45, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking M. C., Parish-Morris J., Schultz R. T., Minturn J., Brodsky C., Shabason E., Herrington J. (2020b). Diminished social attention in pediatric brain tumor survivors: using eye tracking technology during naturalistic social perception. Neuropsychology, 34, 350–358. 10.1037/neu0000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. Z., Ailion A. S., Fox M. E., Hufstetler S. M. (2019). Neurodevelopmental model of long-term outcomes of adult survivors of childhood brain tumors. Child Neuropsychology, 25(1), 1–21. [DOI] [PubMed] [Google Scholar]
- Lord C., Risi S., Lambrecht L., Cook E. H., Leventhal B. L., DiLavore P. C., Pickles A., Rutter M. (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders, 30, 205–223. [PubMed] [Google Scholar]
- Nomi J. S., Uddin L. Q. (2015). Face processing in autism spectrum disorders: from brain regions to brain networks. Neuropsychologia, 71, 201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom Q. T., Cioffi G., Gittleman H., Patil N., Waite K., Kruchko C., Barnholtz-Sloan J. S. (2019). CBTRUS Statistical Report: primary brain other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro-oncology, 21(Supplement_5), v1–v100. 10.1093/neuonc/noz150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer R. J., Gurney J. G., Punyko J. A., Donaldson S. S., Inskip P. D., Stovall M., Yasui Y., Mertens A., Sklar C. A., Nicholson H. S., Zeltzer L. K., Neglia J. P., Robison L. L. (2003). Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood Cancer Survivor Study. Journal of Clinical Oncology, 21, 3255–3261. http://jco.ascopubs.org/content/21/17/3255.long [DOI] [PubMed] [Google Scholar]
- Parish-Morris J., Chevallier C., Tonge N., Letzen J., Pandey J., Schultz R. T. (2013). Visual attention to dynamic faces and objects is linked to face processing skills: a combined study of children with autism and controls. Frontiers in Psychology, 4, 185. 10.3389/fpsyg.2013.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson K. E., Kuttesch J. F., Champion J. E., Andreotti C. F., Hipp D. W., Bettis A., Barnwell A., Compas B. E. (2010). A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatric Blood & Cancer, 55, 525–531. [DOI] [PubMed] [Google Scholar]
- Rutter M., Le Couteur A., Lord C. (2003). ADI-R Autism Diagnostic Interview Revised. Manual. Western Psychological Services. [Google Scholar]
- Salley C. G., Hewitt L. L., Patenaude A. F., Vasey M. W., Yeates K. O., Gerhardt C. A., Vannatta K. (2015). Temperament and social behavior in pediaric brain tumor survivors and comparison peers. Journal of Pediatric Psychology, 40, 297–308. 10.1093/jpepsy/jsuo83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte F., Brinkman T. M., Li C., Fay-McClymont T., Srivastava D. K., Ness K. K., Howell R. M., Mueller S., Wells E., Strother D., Lafay-Cousin L., Leisenring W., Robison L. L., Armstrong G. T., Krull K. R. (2018). Social adjustment in adolescent survivors of pediatric central nervous system tumors: a report from the Childhood Cancer Survivor Study. Cancer, 124, 3596–3608. 10.1002/cncr31593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte F., Kunin-Batson A. S., Olson-Bullis B. A., Banerjee P., Hocking M. C., Janzen L., Kahalley L. S., Wroot H., Forbes C., Krull K. R. (2019). Social attainment in survivors of pediatric central nervous system tumors: a systematic review and meta-analysis from the Children’s Oncology Group. Journal of Cancer Survivorship, 13, 921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J. W., Wolf J. M., Klaiman C., Koenig K., Cockburn J., Herlihy L., Brown C., Stahl S., Kaiser M. D., Schultz R. T. (2010). Using computerized games to teach face recognition skills to children with autism spectrum disorder: the Let’s Face It! program. Journal of Child Psychology and Psychiatry, 51, 944–952. 10.1111/j.1469-7610.2010.02258.x [DOI] [PubMed] [Google Scholar]
- Tanaka J. W., Wolf J. M., Klaiman C., Koenig K., Cockburn J., Herlihy L., Brown C., Stahl S. S., South M., McPartland J. C., Kaiser M. D., Schultz R. T. (2012). The perception and identification of facial emotions in individuals with autism spectrum disorders using the Let's Face It! Emotion Skills Battery. Journal of Child Psychology and Psychiatry, 53, 1259–1267. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3505257/pdf/nihms395695.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannatta K., Gartstein M. A., Short A., Noll R. B. (1998). A controlled study of peer relationships of children surviving brain tumors: teacher, peer, and self ratings. Journal of Pediatric Psychology, 23, 279–287. http://jpepsy.oxfordjournals.org/content/23/5/279.full.pdf [DOI] [PubMed] [Google Scholar]
- Wolf J. M., Tanaka J. W., Klaiman C., Cockburn J., Herlihy L., Brown C., South M., McPartland J., Kaiser M. D., Phillips R., Schultz R. T. (2008). Specific impairment of face-processing abilities in children with autism spectrum disorder using the Let's Face It! skills battery. Autism Research, 1, 329–340. http://onlinelibrary.wiley.com/store/10.1002/aur.56/asset/56_ftp.pdf?v=1&t=ioegf8i3&s=b64d46dc305554d5f21c9ce8943f61e0a82cc0a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates K. O., Bigler E. D., Dennis M., Gerhardt C. A., Rubin K. H., Stancin T., Taylor H. G., Vannatta K. (2007). Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin, 133, 535–556. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2841002/pdf/nihms179971.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltzer L., Recklitis C. J., Buchbinder D., Zebrack B., Casillas J. N., Tsao J. C. I., Lu Q., Krull K. R. (2009). Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology, 27, 2396–2404. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2677925/pdf/zlj2396.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]