Abstract
The improvement of the science and art of surgery began over 150 years ago. Surgical core tasks, “cutting and sewing” with hand and direct contact with the organs, have remained the same. However, in the 21st century, there has been a shifting paradigm in the methodology of surgery. The joint union between innovators, engineers, industry, and patient demands resulted in minimally invasive surgery (MIS). This method has influenced the techniques in every aspect of abdominal surgery, such as surgeons are not required to direct contact or see the structures on which they operate. Advances in the endoscope, imaging, and improved instrumentations convert the essential open surgery into the endoscopic method. Furthermore, computers and robotics show a promising future to facilitate complex procedures, enhance accuracy in microscale operations, and develop a simulation to improve the ability to face sophisticated approaches. MIS has been replacing open surgery due to improved survival, fewer complications, and rapid recoveries in recent years. Minimally invasive surgery's further research in diagnostic and therapeutic modalities is under investigation to achieve genuinely “noninvasive” surgery. Thus, MIS has gained interest in recent days and has been improving with promising outcomes.
Keywords: Minimally invasive surgery, Laparoscopic surgery, Advances in surgery
Highlights
-
•
Minimally invasive surgery has interfered with multiple aspects of the surgical approach.
-
•
Advancement in the endoscope, imaging, and other instrumentations shifting the current methodological conventional surgery.
-
•
The benefit over risk is the promising primary outcome to achieve an exceptional quality of life.
Later in 1910, a Swedish internist called Hans Christian Jacobaeus reported a laparoscopy technique by examined 17 patients with ascites. Two years later, Jacobaeus described liver pathology, peritoneal tuberculosis, and tumors in 115 patients. The first laparoscopic procedure in the United States (US) happened in 1911 by Bertram Bernheim at John Hopkins University. An inspection using a 12 mm proctoscope through an epigastric incision to visualize the abdominal cavity. The advancement of endoscopy and laparoscopy shifted quickly in 1957 when Basil Hirschowitz, an American gastroenterologist, developed a fiberoptic gastroscope which was applicable, affordable, and less fragile than the previous technology [3,6].
1. History
The earliest endoscopic examination happened in 1901 by Dimitri Oskarovich Ott of Petrograd, Rusia. He performed an endoscopic examination of the abdominal cavity through a vaginal incision using a mirror and speculum. Then, George Kellig of Dresden, Germany, is recognized as the first live endoscopic canine using Nitze cystoscope. The publication called this procedure celioscopy and described by creating pneumoperitoneum and entry into the abdominal cavity [3,5,6] [1,2,4].
Erich Muhe from Germany done the first laparoscopic cholecystectomy (LC) in 1985 but was officially recognized by the German Surgical Society in 1993 due to a skeptical mindset in the earlier years. Philippe Mouret performed the following success in Lyon, France. Mouret did the first video-assisted laparoscopic cholecystectomy. In 1988, this method was done first time in the US by Barry McKernan and William Saye. Later, in the five following years, the National Institutes of Health declared laparoscopic cholecystectomy as the choice for uncomplicated cholelithiasis [3,7,8]. In Indonesia, Ibrahim Ahmadsyah, a digestive surgeon, performed the first laparoscopic cholecystectomy in 1991 [9].
2. Types of minimally invasive surgery
2.1. Laparoscopic cholecystectomy
For decades, LC has become the preferred treatment for cholelithiasis, which a trained surgeon performs [10,11]. A retrospective study analyzed 1878 patients who underwent LC and observed its complications. The LC was successful in 1774 patients (94%) with postoperative morbidity of 1.5% and mortality of 0% [12]. However, the incidence of bile duct injury remained higher than in open surgery. Still, higher advantages are offered in LC with less postoperative pain, shorter hospital stays, and increased patient satisfaction [[13], [14], [15]].
The guideline of LC is now well-defined, with providing adequate training for the general surgeons. In the fact of the history of laparoscopic, LC became the pioneer of advances in laparoscopic surgery. Further innovations are expected with several updates in instrumentations, robotics, and telemedicine [10].
2.2. Laparoscopic appendectomy
Semm introduced the earliest laparoscopic appendectomy, followed by a report of 70 female patients with laparoscopic appendectomy, which Schreiber did [16,17]. Since then, numerous reports have been published from personal series until meta-analysis. A Consensus Conference has obtained results of laparoscopic appendectomy, which are similar to the open technique. However, its main advantages are precise diagnosis, lower wound infection, and rapid recovery time, but with several considerations such as longer operation time and higher hospital cost. Thus, laparoscopic appendectomy becomes the favorite choice of any condition of appendicitis, while surgeon training skills should be improved for minimalizing iatrogenic complications [10,18].
2.3. Laparoscopic hernia repair
Firstly, laparoscopic hernia repair was described in 1993 by Leblanc and Booth [19]. The basic concepts of laparoscopic hernia repair are underlaid mesh positioning and distribute intraabdominal pressure across the area of the overlapping mesh (Fig. 1). The advantages include shortened hospitalization, minimal dissection, and lower wound infection. In the beginning, laparoscopic hernia repair is used to be the standard approach of incarcerated hernias. Nowadays, the indications of laparoscopic hernia repair expand to symptomatic relief, prevent complications, and treat acute complications [[20], [21], [22]].
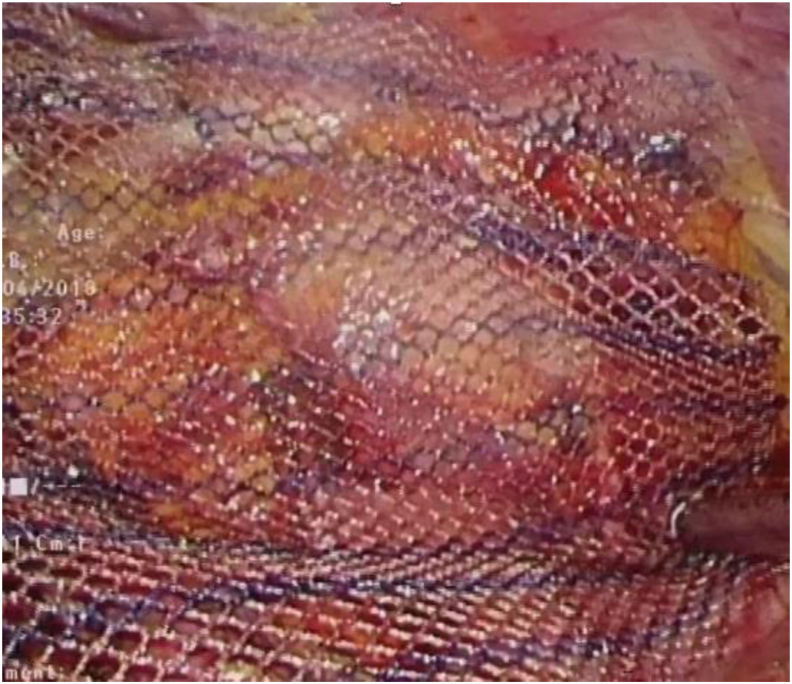
Fig. 1.
Laparoscopic view of fixating a mesh in hernia repair.
The recurrence rate of laparoscopic hernia repair is acceptably low than open repair, between 0 and 17% in mid-to-long-term follow-up [20]. Seroma formations become the most common complication after the procedure. Seroma can be identified in 100% of patients with ultrasonography at one small prospective study. Providentially, most seroma resolved without intervention [23]. Surgical site infections (SSI) significantly reduced in the laparoscopic approach with rates of 1.1% compared to 10% in open repairs. This superiority is acclaimed from the smaller incisions, less direct contact exposure, minimal dissection, and less exposed tissues [24].
Several techniques have been evolved over the years. For example, transabdominal preperitoneal hernia (TAPP) repair requires preperitoneal mesh placement to reduce intense inflammatory reaction due to direct contact of the mesh with intraabdominal viscera. Prasad et al. reported no major complications with several complications such as 6% seroma, 4.4% postoperative urinary retention, 4.4% SSI, 2.9% bowel injury, and 3% recurrence rate [25]. Another method, laparoscopic retro rectus repair is adapted by Miserez and Penninckx in the early 2000s. This technique requires no mesh in the visceral cavity, releasing myofascial from the posterior rectus sheath, reducing tension from the midline closure. The feasibility in 15 patients resulted without any major complications and one recurrence at 5.5 months [26]. Newest method reported in 2003; robotic hernia repair has gained interest in recent years [27]. One study reported worse outcomes in infection and minor complications compared to laparoscopic hernia repair [28]. Otherwise, a comparative review showed superiority in the length of hospitalization and SSI [29]. Besides current concerns such as hospital cost and operative time, potential benefits of robotic hernia repair are ease of closure, ease of mesh placement, and reduced mesh fixation [[27], [28], [29], [30]].
Another most used technique in hernia repair called totally extraperitoneal (TEP) hernia repair was introduced by Dulucq in 1991. This procedure creates a tension-free mesh reinforcement of the groin through laparoscopic surgery [31,32]. The main issue of TEP is peritoneal leakage with CO2 loss to the peritoneal cavity and subsequent compression to preperitoneal dissection space, and the incidence rate is up to 50%. Though, several studies reported an assuring result of the TEP approach. A meta-analysis comparing TEP and TAPP approach concluded TEP has superiority in the length of hospitalization over TAPP. Otherwise, the clinical outcomes and efficacy remain equivalent in both groups [33]. Tamme et al. [31] described a 5203 TEP in 3868 patients over the 7.5 years that TEP considered has a low complication rate with low recurrence rate. Another study showed a lower incidence of postoperative edema and subcutaneous emphysema in the TEP group over the TAPP group. Meanwhile, both groups resulted in zero recurrences of the hernia in two years follow-up [34].
2.4. Laparoscopic bariatric surgeries
Laparoscopic sleeve gastrectomy (LSG) is recognized as an innovative and novel surgical approach for managing obesity [35]. Gagner et al. performed the first LSG in 1999 [36]. The stomach's greater curvature is resected to produce a narrow and tubular stomach (Fig. 2); thus, the hormone ghrelin, which stimulates hunger, is removed. This technique attracts attention due to anastomosis and bypass of the intestinal are not required [37]. Moreover, laparoscopic Roux-en-Y gastric bypass (LRYGB) complies with the most common technique in bariatric surgery besides LSG. This procedure creates a small gastric pouch and creates an antecolic end-to-side gastrojejunostomy, as either a linea or a circular anastomosis [38,39]. As LRYGB effectively alleviates type 2 diabetes (T2DM), it is also a gold standard surgery in obese patients [40]. The other options in bariatric surgeries include biliopancreatic diversion (BPD) and biliopancreatic diversion with duodenal switch (BPD/DS). Both procedures require a double stage operation which initiates with sleeve gastrectomy and leaving the pylorus intact. Duodenum is disconnected, and the gaster is anastomosed with the distal of the small bowel, called the duodenal switch. Then, the biliopancreatic limb is connected to the proximal ileocecal valve; thus, rapid absorption is achieved. In total, the procedure leaves a 400 ml gastric pouch and 50 cm alimentary tract [41].
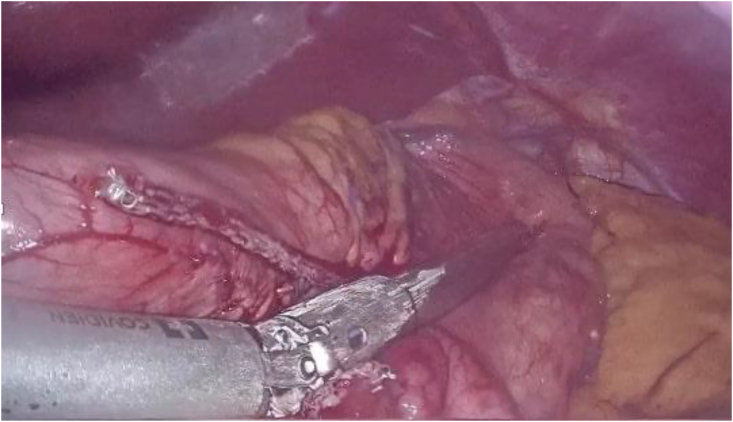
Fig. 2.
Transecting the greater curvature during LSG.
A retrospective study reported 148 patients who underwent LSG had 2.9% major complications, including one leak and one hemorrhage [42]. Baltasar et al. [37] reported a 3.2% mortality rate and 63.1% excess BMI loss from 4 to 27 months. Some disadvantages of LSG include second-stage operation with higher BMI, higher potential of leaks, and inadequate weight loss. Although, LSG is a relatively safe and ideal surgical option for weight reduction with BMI >55 and morbid obesity. Long-term outcomes and LSG roles should be done, and this technique remains an investigational procedure due to poorly defined guidelines [35,37,42]. A comparative meta-analysis of LSG and LRYGB disclosed an advantage in the LRYGB group regarding estimated weight loss (%EWL), remission of dyslipidemia, and hypertension at five-year follow-up [40]. Supporting meta-analysis by Salminen et al. [38] stated a five-year follow-up of LRYGB had superior %EWL over LSG. Meanwhile, in terms of complication rates between the two groups in the prospective study, they found no significant differences [43]. Despite the superiority of each approach, both LSG and LRYGB offer a safe procedure followed by satisfying results and resolution of comorbidities [44].
On the other hand, several techniques were developed as primary and revisional surgery. Single-anastomosis duodeno-ileal switch (SADIS) fundamentally is an alteration of BPD/DS and introduced by Sánchez-Pernaute in 2007 [45]. It is deliberated a less complicated technique with a reduced number of anastomosis and has a comparable outcome of weight loss than BPD/DS. Another effective option is the one anastomosis gastric bypass/mini gastric bypass (OAGB-MBG), which shows a beneficial outcome in long-term observation. This technique was initiated by Rutledge as MBG and afterward altered as OAGB by Carbajo in 2005 [46]. Many studies compared these techniques in terms of efficacy and outcomes. Bashah et al. [47] reported both SADIS and OAGB-MBG were effective as revisional procedures with equivalent results in BMI loss, nutritional deficiency, and comorbidities’ remission. However, SADIS appears superior to cause fewer upper gastrointestinal complications. In another comparative study, OAGB-MBG is favorable for patients with early-onset DM type II. Resolution of DM in OAGB-MBG occurred in 60% of patients after 12 months until 65% in 15 months. Instead, 75% and 80% in SADIS patients after 12 months and 15 months, respectively [48]. Therefore, both procedures are beneficial and promising operations for bariatric surgery.
2.5. Natural orifice transluminal endoscopic surgery
Natural orifice transluminal endoscopic surgery (NOTES), “scarless surgery”, or “incision-less surgery”, is considered a novel surgical method in recent years and a new chapter of minimally invasive surgery [49]. The very beginning of NOTES was performed by Kalloo et al. [50] on swine in 2000. Later in 2004, the first human transgastric NOTES appendectomy happened in India by Rao and Reddy [51]. In Indonesia, the author performed the first transvaginal hybrid cholecystectomy and expanded the other three cases through transvaginal and transgastric (Fig. 3) [52].
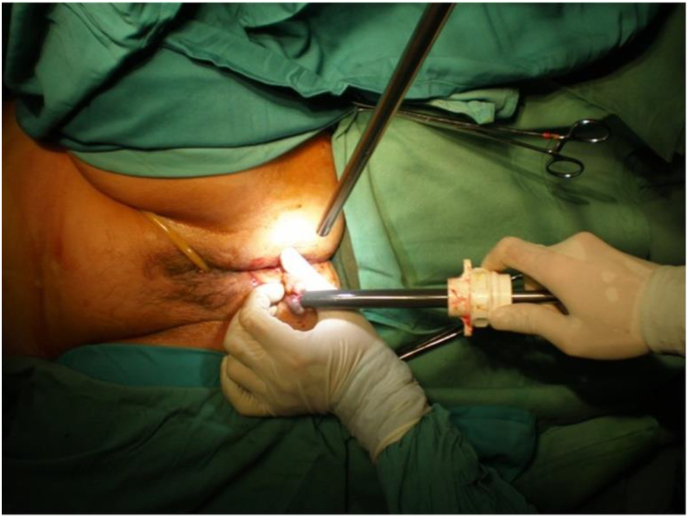
Fig. 3.
The vaginal access in NOTES cholecystectomy.
Based on personal experiences, every route of access has benefits and drawbacks. For example, transvaginal access is easy to perform, uncomplicated sutures (conventional sutures), ease to close, and minimal complications. Therefore, this route is only applied to a subset of patients (adult female only). Instead, transgastric access is easy to perform with precautions; however, the current instrument's primary concern is closure, and complications are fatal (bleeding and peritonitis). A specially dedicated gastric closure device might solve these problems. In recent years, the current challenges of NOTES are the safety and optimal procedure of peritoneal access, including prevention of infection due to high risk of contamination. Another concern about the closure device availability and throughout the years, medical industries and researchers collaborated to develop various clipping and suturing devices. Although these developments are in an animal study, the outcome is quite promising and proved safe and feasible [53].
Hence, NOTES represents a breakthrough and innovative era of minimal access surgery based on the current technique of endoscopy and laparoscopy. Researchers are continuing to improve NOTES through the development of the equipment for safety and feasibility. Later, NOTES may be a promising option to substitute the conventional approach and operate a complex procedure with precise, accurate, and excellent outcomes [53,54].
2.6. Single-incision laparoscopic surgery
To date, there is not a definite name for the relatively novel minimally invasive technique. In this article, the author will use single-incision laparoscopic surgery (SILS). One of the earliest names is single-port access (SPA) surgery. Other industries or individuals suggested various names included one-port umbilical surgery (OPUS), transumbilical endoscopy surgery (TUES), embryonic NOTES (eNOTES), natural orifice transumbilical surgery (NOTUS), single laparoscopic port procedure (SLPP), single-port laparoscopic (SPL), and single laparoscopic incision transabdominal (SLIT) surgery. The recent names came at a consensus with laparoendoscopic single-site (LESS) surgery and single-instrument port laparoscopic surgery (SIMPL) [55,56]. Due to a lack of a definitive name, the future of SILS might be a potential advance in single-incision surgery, and further research is needed to standardize the SILS.
Several studies compared single-incision and laparoscopic cholecystectomy (Fig. 4) due to many potential advantages. Besides the higher costs, longer operation time, and complicated technique, SILS showed a safe and feasible procedure with reduced postoperative pain and improved cosmetic results [57,58].
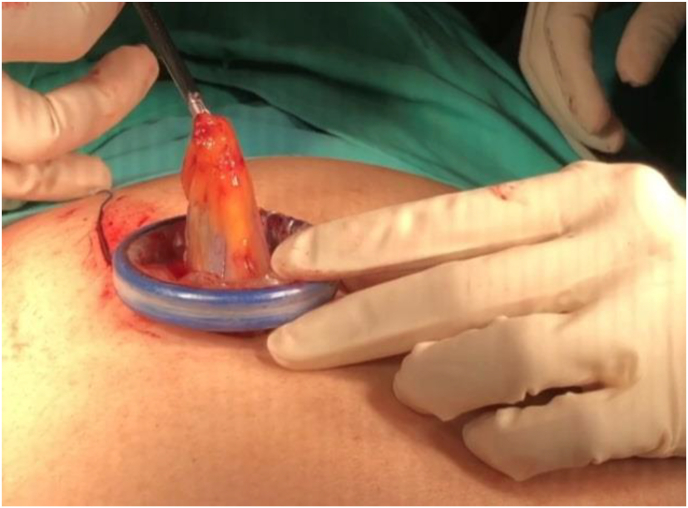
Fig. 4.
Retrieving the gall bladder from SILS cholecystectomy.
Several meta-analyses comparing SILS and conventional laparoscopic appendectomy analyzed an operation time, complications, wound infection, and length of hospitalization. They concluded there were no significant differences between both groups in the treatment of appendicitis. Thus, SILS appendectomy did not appear superior nor beneficial to conventional laparoscopic appendectomy; nonetheless, SILS appendectomy is technically feasible, safe, and reliable [[59], [60], [61]].
Hernia repair with SILS was introduced in 2005 by Chawla et al. [62] from India. A comparison study between SILS hernia repair and the traditional approach reported a superior result in postoperative pain, hospitalization stay, and improved cosmetics with the SILS technique. There was no difference in comorbidities and length of operation time [63]. Therefore, a matched comparison by Rajapandian et al. concluded no difference in cosmetic outcome and postoperative pain in both SILS and conventional laparoscopic hernia repair groups [64].
The concept of single-incision laparoscopy surgery is a potential and emerging technique in the future, like used to a laparoscopy surgery back in two decades. The aims of the new approach are feasibility, safety, and clinical benefits. As a surgeon, cosmetic improvement is not the primary concern of SILS. A new method and the learning curve should be a new beginning for every laparoscopic surgeon and continue to investigate for improving outcomes. The current limitation of SILS comes from technical issues such as triangulation, retraction, inline vision, instrument crowding, ergonomic, instrumentation, cost, and safety. Further developments are widely available in these areas to overcome the limitations. More studies with new updates and wider spread are needed to lower costs and show the pluses of SILS compared to the conventional approach.
3. Trans-anal endoscopic microsurgery and trans-anal minimally invasive surgery
Before the 1980s, surgeons performed local excision (LE) of distal rectal tumors through a posterior parasacral incision, transsphincteric, and transanal. Conversely, those techniques are related to higher complications such as rectocutaneous fistule and anal incontinence. Alongside numerous limitations and adverse events, Gerhard Buess introduced the first trans-anal endoscopic microsurgery (TEM) in 1983, purposely extending the LE to the proximal rectum. Compared to conventional LE, TEM has superior resection quality, decreased recurrence, and enhanced survival rate. Nevertheless, TEM compels a rigid proctoscope, laparoscopic camera, and specialized instruments; thus, the complexity of the procedure and high-cost rate burdened both surgeons and patients [65].
Technology undergoes various evolvements alongside surgical skills with today's advancement in minimally invasive surgery. The recent approach was developed by Atallah et al. [66] in 2009 called trans-anal minimally invasive surgery (TAMIS). This method generates co-existing laparoscopic instruments, including 360° high-definition optics and triangulated instruments; hence, TAMIS is believed to hold improved resection quality and increase disease-free survival.
Several literatures compared these techniques in numerous aspects such as morbidity, complications, recurrence rate, and re-operative repairment. Melin et al. [65] reported equivalent outcomes despite the latest approach of TAMIS. Broader visualization and flexibility of the instruments without repositioning are beneficial for TAMIS. These advantages allow obtaining a larger specimen and decreasing the operation time. Other statements by Stipa et al. [67] concluded an equally effective of specimen quality and perioperative complications, although TAMIS provided a lesser operative time and setup time. The main concern of both techniques is suturing. An endo-GIA stapler, intra-, and extra-corporeal suture-tying have been advised to overcome the difficulties. The clashing of instruments and inadequate tension of suturing remain burdened by the surgeons to succeed in the optimal result [68].
Consequently, low comparability of TEM and TAMIS with insignificant differences become a debate to many authors. The current claim of TAMIS as the alternative option might be too optimistic. Future developments are still desirable for enhancing the clinical outcomes, decreasing the unreasonable cost, and flexibility as the operator. This elaboration is relatively impressive progress that parallels high-speed technology innovation and might be a potential for the robotic method or non-invasive surgery.
3.1. Robotic surgery
A trend to shift minimally invasive towards “noninvasive” surgery is growing these days. As a result, the development of robotic surgery is on the lane alongside the central core of surgery: improved patient safety and outcomes. Robotics surgery was applied as a military project by the National Aeronautics and Space Administration (NASA) in 1970 for providing healthcare to astronauts in spacecraft without a surgeon's presence [69]. For the last four decades, robotic surgery rapidly grows in medical industries, and perfection is on track.
The concept of robotic surgery might be beyond human capabilities and unreasonable a century year ago. This irrationality shifted in 1985 by Kwoh et al. [70] performed computed tomography (CT) guided neurosurgical biopsy with PUMA 200. This system was adopted by Davies et al. [71] to perform transurethral resection of the prostate (TURP), later called PROBOT. However, the limitation of PROBOT was not eligible for dynamic surgical targets (e.g., gastrointestinal surgery). Parallel development with ROBODOC system in 1992, the first robotic surgery with the US Food and Drug Administration (FDA) approved, the machine designated for arthroplasty and widely adopted in the US and Europe [72].
Two surgical telemanipulators were invented and approved by the FDA in the following years: the Zeus and the da Vinci system. The Zeus, composed of three arms consisting of two arms, acted as the surgeon's hands. The third hand was a voice-controlled camera navigator called Automated Endoscopic System for Optimal Positioning (AESOP) [73]. Firstly used in a fallopian tube anastomosis at Cleveland Clinic, US in July 1998. Later, Zeus was widely used in digestive surgery, including appendectomy, cholecystectomy, bariatric, hernioplasty, splenectomy, and colectomy [74]. The da Vinci was performed to take a cholecystectomy in Belgium in 1997 by Jacques Himpens and Guy Cardiere [75]. Subsequently, the success of the previous surgery began to attract another surgery in Germany that performed mitral valve replacement by Carpentier et al. [76] Later, the Zeus and the da Vinci system were merged in 2003 when Computer Motion and Intuitive Surgical merged. The following upgrade version of the da Vinci made this system the most widely used robotic surgery worldwide. The adjustment happened in 2014; the da Vinci Xi is composed of four mounted robotic arms. This upgraded version has adjustable finger loops, adjustable intraocular distance, and a padded headrest based on the surgeon's need. The high accuracy precision is achieved through three-dimensional (3D) visualization, tremor avoidance, motion scaling, and advanced user interface. Yet, the lack of haptic feedback is the major drawback of this system [69,77].
Nowadays, many robotic competitors are widely available and developing at different stages (Table 1). The advantages of robotic surgery are beneficial and overcome the barriers of laparoscopic surgery. Robotic surgery minimizes iatrogenic complications, improves visualization, eliminates hand tremor, specifies the position, and handles micro-anastomoses. The limitations of this advancement focus on high expenses, lack of benefits, and haptic feedback. The first transcontinental robot-assisted remote telesurgery was done in 2002 by Marescaux et al. [78] who performed cholecystectomy from New York in patient over France. The operation was carried out in 54 min without difficulties and complications. A two-center collaborative and retrospective study comparing robotic-assisted and laparoscopic cholecystectomy reported no significant clinical differences. However, the pain score at the discharge was lower in the robotic-assisted group (p = 0.010) [79]. Kim et al. [80] conducted a prospective multicenter comparative study to compare short-term outcomes of robotic and laparoscopic gastrectomy. The results showed no significant differences in mortality, open conversion rates, blood loss, diet build-up, or hospitalization days. The analysis indicated robotic gastrectomy was not superior to the laparoscopic approach. Another retrospective study of long-term outcomes in groups between robotic and laparoscopic gastrectomy elaborated a similar result with no promising improvement and outcomes in robotic gastrectomy groups [81]. The latest telerobotic spinal surgery using the 5th generation (5G) network in 12 patients happened in China by Tian et al. [82] They concluded 5G remote robot-assisted spinal surgery was safe and feasible with zero intraoperative complications.
Table 1.
The current available digestive robotic platforms [77].
| Device | Company | Type | Feature | FDA Status, Phase |
|---|---|---|---|---|
| da Vinci | Intuitive Surgical Inc | Laparoscopy | Tremor filtration | Approved, Commercially available |
| FreeHand v1.2 | Freehand 2010 Ltd | Laparoscopy | Laser guidance | Approved, Commercially available |
| Invendoscopy E200 System | Invendo Medical GmbH | Colonoscopy | Aseptic single-use | Approved, Commercially available |
| Senhance | TransEnterix | Laparoscopy | Haptic feedback, eye-sensing camera | Approved, FDA anticipated |
| NeoGuide Colonoscope | Intuitive Surgical Inc | Colonoscopy | 3D mapping | Approved, Acquired |
| MiroSurge | DLR Robotics | Laparoscopy | Haptic feedback | NA, Commercially available |
| ViaCath System | BIOTRONIK | NOTES | Haptic feedback | NA, Commercially available |
| MASTER | Nanyang Technological University | NOTES | Haptic feedback, reconstruction navigation | NA, Clinical trial |
| SPORT™ Surgical System | Titan Medical Inc | SILS | Multi-articulated instruments | NA, FDA pending |
| SurgiBot | TransEnterix | SILS | Internal triangulation | NA, FDA resubmission |
| Versius Robotic System | Cambridge Medical Robotics | Laparoscopy | Haptic feedback | NA, Cadaveric trial |
| Einstein Surgical Robot | Medtronic | Laparoscopy | Unreported | Unreported |
NA, not approved.
Evolving robotic platforms are continuing to grow as the medical needs. Engineers and developers are applying the newest updates to expand robotic capabilities. These trends tend to shift upward alongside human objective for converting the conventional approach to fully robotic surgery in current practice. Numerous studies indicated a further enhancement to generate the strength power of recent technologies for improving surgical outcomes. In the future, several anticipations are expected in different aspects. The breaking of high cost is the primary concern of recent robotic-assisted surgery, followed by specific robotic training and fundamental guidelines initiated by the robotic surgical society. Hence, the fate of robotic surgery depends on overcoming limitations to prove its feasibility, safety, cost reduction, and clinical benefits.
Reprinted by permission from [Springer Nature Customer Service Centre GmbH]: [Springer Nature][Surgical Endoscopy][Review of emerging surgical robotic technology, Brian S. Peters, Priscila R. Armijo, Crystal Krause, Songita A. Choudhury, Dmitry Oleynikov], [Copyright 2018]
3.2. Virtual- and augmented-reality
In recent years, an exponential interest in virtual reality (VR) and augmented reality (AR) has been in the medical field. These technologies have been applied in other industries, including telecommunication, aviation, aerospace, games, etc. Although the introduction of VR and AR is considered as a newborn in the medical era. Virtual reality is a computer-generated artificial technology for merging images and environments with real-time interaction [83]. Meanwhile, AR overlays generated data over a real or live image to enrich the actual image [84]. These technologies offer enormous interaction and unify the fragments between the actual and digital worlds. The succeeding advancement enhances digital healthcare and clinical practice, leading to improved patient safety and outcome.
The similarities of VR and AR lie in their fundamental science to deliver three-dimensional (3D) digital experiences. Virtual reality employs a 3D environment generated by computers and alters human sensory perception using a head-mounted display (HMD), stereo units, and data gloves [85]. On the other hand, AR generates a digital image on actual imagery captured by a camera and projected by a computer or a video projector [86].
However, the differences between VR and AR are in the delivery of 3D digital experiences. Virtual reality provides a fully immersive experience through an HMD with a virtual interactive environment. On the other side, a holographic or transparent display overlays with the real world and is visible together to create a breathtaking digital experience. In addition, the digital display of both technologies delivers various information of patient's condition, anatomical abnormalities, and detailed measurement. These benefits allow the surgeon to explore and analyze the patient's current issue, thus increasing surgeon accuracy, efficiency, and safety to enhance health outcomes.
The projection use of VR and AR provides a multidimensional exploration of medical data. They can reconstruct and visualize patient issues and later simulate the procedure with 3D digital experiences. The first live broadcast of VR has successfully done in 2016 in the Royal College Hospital by Shafi Ahmed, an oncology surgeon [87]. Augmented reality has also found the clinical application in pancreatic and hepatobiliary surgery, which happened in 2013 by Onda et al. [88], and concluded AR could calculate an accurate dissection and identify lesions while preserving the adjacent organs and vessels [89].
Followed by the newest technology of the 5G connection, the prospect of VR and AR will accelerate and undoubtedly shift the surgical approach into an entirely virtual procedure. The integrated data center within VR and AR brings the capability to transform healthcare service into a digitalization era. Three-dimensional reconstructed patient information alongside virtual simulation of surgical approaches and outcomes possibilities nearly in the hand of the medical period. Future studies and improvements aim to maximize current technologies, including robotic hand gestures, haptic feedback, and virtual display, to accommodate current issues in these areas. Thus, the advancement of VR and AR is more compelling by their improved skills, which might be followed by other technologies to become easily accessible, affordable, efficient, and improve the aims of surgical advancement, the quality of life for every patient.
3.3. Three-dimensional printing
Three-dimensional printing describes the addition of multiple layering of materials through a conversion from digital images to a 3D printed object [90,91]. This invention was patented by Charles Hull in 1984 as a method used for rapid prototyping [92]. The exponential increment use of 3D printing in surgery happened in 2013. The application in recent years has spread the wings even further, from anatomical practice until specific patient's surgical instruments [93]. Moreover, 3D printing has been used in several medical specialties, includes gastroenterology surgery, cardiothoracic surgery, cardiology, paediatric, neurosurgery, oral & maxillofacial surgery, orthopaedic surgery, plastic surgery, vascular surgery, and others [94].
The application of 3D printing in medical fields has each aspect of benefit on both sides. It is used as preoperative planning for presurgical simulation; therefore, integrating the current patient's issue and imaging information will impact the best treatment possible. Preoperative planning may reduce perioperative time, days of hospitalization, and healthcare costs due to higher operative time efficiency in the operating theater (OR). In the perioperative procedure, 3D printing works as customization of specific surgical instruments and prostheses. These benefits allow the reduction of cost and offer particular needs in diverse approaches [94,95].
In colorectal surgery, a comparison study of superior mesenteric vascular 3D printed models in 22 patients who underwent preoperative planning in the right hemicolectomy showed an accurate measurement among the 3D printed models and the actual anatomy during operation; thus, this study concluded a promising adjunctive technology to preoperative planning and perioperative navigation [90]. Zein et al. [96] proved the accuracy of the 3D printed liver as the preoperative planning prior to actual liver transplantation. This method allows the surgeon to recognize the current patient's anatomical variety and ensure the procedure's safety.
Three-dimensional printing is also improving medical education through capabilities to create various anatomical differences. These benefits accelerate medical students to understand and learn regardless of their institutions with the possibility to transfer 3D printed models [94]. Thus, every institution has the same level of opportunity to acquire equal knowledge. Many advantages are being offered from 3D printing besides in the surgical field. Patient education, forensic practice, bioprinting, personalized 3D printed drug, and customizing synthetic organs describe the wide range of 3D printing uses in medical industries and discover the impending excitement of the new era.
3.4. Artificial intelligence
Artificial intelligence (AI) has penetrated and predisposed medical healthcare in recent years. Frankly, AI is a machined-based algorithm with reasoning and cognitive ability to perform daily human bases such as problem-solving, object recognition, word recognition, and decision making [97]. The wave of enthusiasm in AI escalates medical health professionals' role in reducing human errors during the examination, diagnosing, and treating of patients. As a role, Cornell University reported an outstanding accuracy using a deep learning algorithm in detecting lymph node metastases in breast cancer [98].
The involvement of AI in surgery accommodates surgeons in complex decisions, including multimodal therapy, surgical timing, and type of surgery option. Moreover, surgeons are expected to provide surgical risks prior to surgery and the likelihood of mortality and morbidity in every decision [99]. Another impact of AI also significant on an image-based procedures such as endoscopy and radiology. A digestive surgeon may rely on AI-assisted endoscopy to analyze and recognize gastrointestinal neoplasia during endoscopy alongside integrated data information of lesions to classify the degree of cancers and provide an evidence-based optimal therapy [100]. Byrne et al. [101] demonstrated an AI-assisted colonoscopy to identify colorectal polyps and differentiate hyperplastic polyps and adenomatous polyps. The result of AI-assisted colonoscopy was impressive with high sensitivity of 98% and specificity of 83%.
Later, the surgeons can augment live decision-making during operation based on real-time AI analysis of intraoperative progress with vital signs, anatomical tracking, time decision, and live video to calculate the current percentage of adverse events, mortality, and morbidity. Followed by postoperative data, which integrated with the patient's condition to estimate vital signs, evaluate postoperative needs, recurrency rate, and potential adverse events [97,102]. Chen et al. [103] developed an AI-based multimodal risk assessment model for surgical site infection (AMRAMS) for inpatients undergoing an operation. They compared them with the national nosocomial infections surveillance (NNIS) risk index. The result based on the deep learning method had significant advantages in accuracy and might be a potential tool to predict surgical site infection outcomes.
Although the hype of AI in the medical industry might be an own pitfall due to higher expectations. This technology cannot yield answers to all problems and is not entirely operated without human interference. Future expectations to replace surgeons in every aspect of a patient's decision may be quite exaggerating in the meantime and cannot be sidestepped in the future. Human judgment in the medical area still in the upper hand beside the advancement of AI technology in recent years. Nonetheless, AI is quiet at a young age for the current situation. The future might be a turnover for AI to generate an evidence-based, real-time clinical judgment and optimize patient safety and quality of life.
4. Conclusion
In the future, there will be further advancements in technologies in surgery that will alter current practice. It might be challenging to predict the forthcoming precisely for the next decade. The major development of concurrent surgical ability will not be a total upside-down from semi-assisted until fully autonomous. The second generation of laparoscopic, robotic, AI, 3D printing, VR, and AR might serve as an advanced human-computer interface, working in interdependence with surgeons and achieving better results. Thus, surgeons, scientists, and engineers must collaborate to revolutionize the current work to develop another breakthrough to improve patient's circumstances and cost-effectiveness.
Ethical approval
Author does not require ethical approval in this article.
Sources of funding
Author delcares no funding of this article.
Author contribution
Author contributes in study concept, data collection, and writing the paper of this article.
Registration of research studies
Author does not need registration of research studies.
Consent
Author does not need consent in this article.
Guarantor
Reno Rudiman acts as the author and the guarantor of this article. Author is fully responsible for the work, the conduct of the study, data accessibility and decision to publish.
Declaration of competing interest
Author declares no conflict of interest.
Acknowledgements
The author wishes to thank Ricarhdo Valentino Hanafi for preparing the manuscript of this article.
References
- 1.Mack M.J. Minimally invasive and robotic surgery. J. Am. Med. Assoc. 2001;285:568–572. doi: 10.1001/jama.285.5.568. [DOI] [PubMed] [Google Scholar]
- 2.Fuschs K.H. Minimally invasive surgery. Endoscopy. 2002;34:154–159. doi: 10.1055/s-2002-19857. [DOI] [PubMed] [Google Scholar]
- 3.Harrell A.G., Heniford B.T. Minimally invasive abdominal surgery: lux et veritas past, present, and future. Am. J. Surg. 2005;190:239–243. doi: 10.1016/j.amjsurg.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Robinson T.N., Stiegmann G.V. Minimally invasive surgery. Endoscopy. 2004:48–51. doi: 10.1055/s-2004-814113. [DOI] [PubMed] [Google Scholar]
- 5.Lee-Kong S., Feingold D.L. The history of minimally invasive surgery. Semin. Colon Rectal Surg. 2013:3–6. [Google Scholar]
- 6.Litynski G.S. Laparoscopy - the early attempts: spotlighting georg kelling and hans christian jacobaeus. ournal Soc Laparoendosc Surg. 1997:83–85. [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds W. The first laparoscopic cholecystectomy. J. Soc. Laparoendosc. Surg. 2001:89–94. [PMC free article] [PubMed] [Google Scholar]
- 8.Gollan J.L., Bulkley G.B., Diehl A.M. Gallstones and laparoscopic cholecystectomy. J. Am. Med. Assoc. 1993:1018–1024. [Google Scholar]
- 9.Budiarti A.G., Muhar A.M. Changes in glutamic oxaloacetic transaminase serum (sgot) and glutamic pyruvic transaminase serum (sgpt) value in laparoscopic cholecystectomy patient at usu hospital. Sumatera Med J. 2020:1–5. [Google Scholar]
- 10.Périssat J., Collet D., Monguillon N. Advances in laparoscopic surgery. Digestion. 1998;59:606–618. doi: 10.1159/000007535. [DOI] [PubMed] [Google Scholar]
- 11.Cuschieri A. Laparoscopic surgery: current status, issues and future developments. Surgeon. 2005:125–130. doi: 10.1016/s1479-666x(05)80032-0. [DOI] [PubMed] [Google Scholar]
- 12.Tuveri M., Tuveri A. Laparoscopic cholecystectomy: complications and conversions with the 3-trocar technique. Surg. Laparosc. Endosc. Percutaneous Tech. 2007:380–384. doi: 10.1097/SLE.0b013e3180dca5d6. [DOI] [PubMed] [Google Scholar]
- 13.Connor S., Garden O.J. Bile duct injury in the era of laparoscopic cholecystectomy. Br. J. Surg. 2006;93:158–168. doi: 10.1002/bjs.5266. [DOI] [PubMed] [Google Scholar]
- 14.Ford J.A., Soop M., Du J., BPT Loveday, Rodgers M. Systematic review of intraoperative cholangiography in cholecystectomy. Br. J. Surg. 2012:160–167. doi: 10.1002/bjs.7809. [DOI] [PubMed] [Google Scholar]
- 15.Lee W.J., Chan C.P., Wang B.Y. Recent advances in laparoscopic surgery. Asian J. Endosc. Surg. 2013:1–8. doi: 10.1111/ases.12001. [DOI] [PubMed] [Google Scholar]
- 16.Semm K. vol. 15. Endoscopy; 1983. pp. 59–64. (Endoscopy Appendectomy). [DOI] [PubMed] [Google Scholar]
- 17.Schreiber J.H. Early experience with laparoscopic appendectomy in women. Surg. Endosc. 1987:211–216. doi: 10.1007/BF00591150. [DOI] [PubMed] [Google Scholar]
- 18.Himal H.S. Minimally invasive (laparoscopic) surgery: the future of general surgery. Surg Endosc Other Interv Tech. 2002;16:1647–1652. doi: 10.1007/s00464-001-8275-7. [DOI] [PubMed] [Google Scholar]
- 19.LeBlanc K., Booth W. Laparoscopic repair of incisional abdominal hernias using expanded polytetrafluoroethylene: preliminary findings. Surg. Laparosc. Endosc. 1993:39–41. [PubMed] [Google Scholar]
- 20.Otero J., Huber A.T., Heniford B.T. Laparoscopic hernia repair. Adv. Surg. 2019;53:1–19. doi: 10.1016/j.yasu.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Pechman D.M., Cao L., Fong C., Thodiyil P., Surick B. Laparoscopic versus open emergent ventral hernia repair: utilization and outcomes analysis using the ACSNSQIP database. Surg. Endosc. 2018;32:4999–5005. doi: 10.1007/s00464-018-6312-z. [DOI] [PubMed] [Google Scholar]
- 22.Colavita P.D., Tsirline V.B., Belyansky I., Walters A.L., Lincourt A.E., Sing R.F. Prospective, long-term comparison of quality of life in laparoscopic versus open ventral hernia repair. Ann. Surg. 2012:714–723. doi: 10.1097/SLA.0b013e3182734130. [DOI] [PubMed] [Google Scholar]
- 23.Susmallian S., Gewurtz G., Ezri T., Charuzi I. Seroma after laparoscopic repair of hernia with PTFE patch: is it really a complication? Hernia. 2001;5:139–141. doi: 10.1007/s100290100021. [DOI] [PubMed] [Google Scholar]
- 24.Franklin M., Dorman J., Glass J., Balli J., Gonzalez J. Laparoscopic ventral and incisional hernia repair. Surg. Laparosc. Endosc. 1998;8:294–299. [PubMed] [Google Scholar]
- 25.Prasad P., Tantia O., Patle N.M., Khanna S., Sen B. Laparoscopic transabdominal preperitoneal repair of ventral hernia: a step towards physiological repair. Indian J. Surg. 2011;73:403–408. doi: 10.1007/s12262-011-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miserez M., Penninckx F. Endoscopic totally preperitoneal ventral hernia repair: surgical technique and short-term results. Surg Endosc Other Interv Tech. 2002;16:1207–1213. doi: 10.1007/s00464-001-9198-z. [DOI] [PubMed] [Google Scholar]
- 27.Ballantyne G.H., Hourmont K., Wasielewski A. Telerobotic laparoscopic repair of incisional ventral hernias using intraperitoneal prosthetic mesh. J. Soc. Laparoendosc. Surg. 2003;7:7–14. [PMC free article] [PubMed] [Google Scholar]
- 28.Armijo P., Pratap A., Wang Y., Shostrom V., Oleynikov D. Robotic ventral hernia repair is not superior to laparoscopic: a national database review. Surg. Endosc. 2018;32:1834–1839. doi: 10.1007/s00464-017-5872-7. [DOI] [PubMed] [Google Scholar]
- 29.Altieri M.S., Yang J., Xu J., Talamini M., Pryor A., Telem D.A. Outcomes after robotic ventral hernia repair: a study of 21,565 patients in the State of New York. Am. Surg. 2018;84:902–908. [PubMed] [Google Scholar]
- 30.Coakley K.M., Sims S.M., Prasad T., Lincourt A.E., Augenstein V.A., Sing R.F. A nationwide evaluation of robotic ventral hernia surgery. Am. J. Surg. 2017;214:1158–1163. doi: 10.1016/j.amjsurg.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 31.Tamme C., Scheidbach H., Hampe C., Schneider C., Köckerling F. Totally extraperitoneal endoscopic inguinal hernia repair (tep): results of 5,203 hernia repairs. Surg Endosc Other Interv Tech. 2003;17:190–195. doi: 10.1007/s00464-002-8905-8. [DOI] [PubMed] [Google Scholar]
- 32.Leibl B.J., Jäger C., Kraft B., Kraft K., Schwarz J., Ulrich M. Laparoscopic hernia repair - tapp or/and tep? Langenbeck's Arch. Surg. 2005;390:77–82. doi: 10.1007/s00423-004-0532-5. [DOI] [PubMed] [Google Scholar]
- 33.Bracale U., Melillo P., Pignata G., Salvo E Di, Rovani M., Merola G. Which is the best laparoscopic approach for inguinal hernia repair: tep or tapp? A systematic review of the literature with a network meta-analysis. Surg. Endosc. 2012;26:3355–3366. doi: 10.1007/s00464-012-2382-5. [DOI] [PubMed] [Google Scholar]
- 34.Vǎrcuş F., Duţǎ C., Dobrescu A., Lazǎr F., Papurica M., Tarta C. Laparoscopic repair of inguinal hernia tep versus tapp. Chir. 2016;111:308–312. [PubMed] [Google Scholar]
- 35.Shi X., Karmali S., Sharma A.M., Birch D.W. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes. Surg. 2010;20:1171–1177. doi: 10.1007/s11695-010-0145-8. [DOI] [PubMed] [Google Scholar]
- 36.Ren C.J., Patterson E., Gagner M. Early results of laparoscopic biliopancreatic diversion with duodenal switch: a case series of 40 consecutive patients. Obes. Surg. 2000;10:514–523. doi: 10.1381/096089200321593715. [DOI] [PubMed] [Google Scholar]
- 37.Baltasar A., Serra C., Pérez N., Bou R., Bengochea M., Ferri L. Laparoscopic sleeve gastrectomy: a multi-purpose bariatric operation. Obes. Surg. 2005;15:1124–1128. doi: 10.1381/0960892055002248. [DOI] [PubMed] [Google Scholar]
- 38.Salminen P., Helmio M., Ovaska J., Juuti A., Leivonen M., Peromaa-Haavisto P. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-y gastric bypass onweight loss at 5 years among patients with morbid obesity. J. Am. Med. Assoc. 2018;319:241–254. doi: 10.1001/jama.2017.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdeen G., le Roux C.W. Mechanism underlying the weight loss and complications of roux-en-y gastric bypass: review. Obes. Surg. 2016;26:410–421. doi: 10.1007/s11695-015-1945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu L., Huang X., Li S., Mao D., Shen Z., Khadaroo P.A. A meta-analysis of the medium- and long-term effects of laparoscopic sleeve gastrectomy and laparoscopic roux-en-y gastric bypass. BMC Surg. 2020;20:1–10. doi: 10.1186/s12893-020-00695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hng K.N., Ang Y.S. Overview of bariatric surgery for the physician. Clin Med J R Coll Physicians London. 2012;12:435–440. doi: 10.7861/clinmedicine.12-5-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalor P.F., Tucker O.N., Szomstein S., Rosenthal R.J. Complications after laparoscopic sleeve gastrectomy. Surg. Obes. Relat. Dis. 2008:33–38. doi: 10.1016/j.soard.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Lynn W., Ilczyszyn A., Rasheed S., Davids J., Aguilo R., Agrawal S. Laparoscopic roux-en-y gastric bypass is as safe as laparoscopic sleeve gastrectomy: results of a comparative cohort study. Ann Med Surg. 2018;35:38–43. doi: 10.1016/j.amsu.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Albeladi B., Bourbao-Tournois C., Huten N. Short- and midterm results between laparoscopic roux-en-y gastric bypass and laparoscopic sleeve gastrectomy for the treatment of morbid obesity. J Obes. 2013:1–6. doi: 10.1155/2013/934653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shoar S., Poliakin L., Rubenstein R. Single anastomosis duodeno-ileal switch (SADIS): a systematic review of efficacy and safety. Obes. Surg. 2018:104–113. doi: 10.1007/s11695-017-2838-8. [DOI] [PubMed] [Google Scholar]
- 46.Kermansaravi M., Shahmiri S.S., DavarpanahJazi A.H. One anastomosis/mini-gastric bypass (OAGB/MGB) as revisional surgery following primary restrictive bariatric procedures: a systematic review and meta-analysis. Obes. Surg. 2021:370–383. doi: 10.1007/s11695-020-05079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bashah M., Aleter A., Baazaoui J. Single anastomosis duodeno-ileostomy (SADI-S) versus one anastomosis gastric bypass (OAGB-MGB) as revisional procedures for patients with weight recidivism after sleeve gastrectomy: a comparative analysis of efficacy and outcomes. Obes. Surg. 2020:4715–4723. doi: 10.1007/s11695-020-04933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bypass D., Abbas A., Moustafa S. Comparative study between single anastmosis gastric bypass as regard remission of type-2 DM after application of DIAREM scoring system. Med. Sci. 2020:4098–4107. [Google Scholar]
- 49.Li S., ling, Zhao E., Zhao L., Wang Z., kai, Li W. Transvaginal natural orifice transluminal endoscopic surgery in the diagnosis of ascites of unknown origin. Gastrointest. Endosc. 2019;89:872–877. doi: 10.1016/j.gie.2018.09.047. [DOI] [PubMed] [Google Scholar]
- 50.Kalloo A.N., Singh V.K., Jagannath S.B., Niiyama H., Hill S.L., Vaughn C.A. Flexible transgastric peritoneoscopy: a novel approach to diagnostic and therapeutic interventions in the peritoneal cavity. Gastrointest. Endosc. 2004;60:114–117. doi: 10.1016/s0016-5107(04)01309-4. [DOI] [PubMed] [Google Scholar]
- 51.Rao P., Reddy N. Per oral transgastric endoscopic appendectomy in human. Proc 45th Annu Conf Soc Gastrointest Endosc India. 2004:28–29. [Google Scholar]
- 52.Rudiman R., Wiradisuria E. Initial experience with laparoscopic-assisted transvaginal cholecystectomy: a hybrid approach to natural orifice surgery. Int. Surg. 2009;94:258–261. [PubMed] [Google Scholar]
- 53.Yip H.C., Chiu P.W.Y. Recent advances in natural orifice transluminal endoscopic surgery. Eur J Cardio-thoracic Surg. 2015;49:i25–30. doi: 10.1093/ejcts/ezv364. [DOI] [PubMed] [Google Scholar]
- 54.Moreira-Pinto J., Lima E., Correia-Pinto J., Rolanda C. Natural orifice transluminal endoscopy surgery: a review. World J. Gastroenterol. 2011;17:3795–3801. doi: 10.3748/wjg.v17.i33.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romanelli J.R., Earle D.B. Single-port laparoscopic surgery: an overview. Surg. Endosc. 2009;23:1419–1427. doi: 10.1007/s00464-009-0463-x. [DOI] [PubMed] [Google Scholar]
- 56.Rudiman R., Winata A.A. Single-port laparoscopic surgery: a mini review. World J. Laparosc. Surg. 2018;11:149–150. [Google Scholar]
- 57.Markar S.R., Karthikesalingam A., Thrumurthy S., Muirhead L., Kinross J., Paraskeva P. Single-incision laparoscopic surgery (SILS) vs. conventional multiport cholecystectomy: systematic review and meta-analysis. Surg. Endosc. 2012;26:1205–1213. doi: 10.1007/s00464-011-2051-0. [DOI] [PubMed] [Google Scholar]
- 58.Saad S., Strassel V., Sauerland S. Randomized clinical trial of single-port, minilaparoscopic and conventional laparoscopic cholecystectomy. Br. J. Surg. 2013;100:339–349. doi: 10.1002/bjs.9003. [DOI] [PubMed] [Google Scholar]
- 59.Concha J.A.M., Cartes-velásquez R., Delgado C.M. Single-incision laparoscopic appendectomy versus conventional laparoscopy in adults: a systematic review. Acta Cir. Bras. 2014;29:826–831. doi: 10.1590/S0102-86502014001900010. [DOI] [PubMed] [Google Scholar]
- 60.Li P., Chen Z.H., Li Q.G., Qiao T., Tian Y.Y., Wang D.R. Safety and efficacy of single-incision laparoscopic surgery for appendectomies: a meta-analysis. World J. Gastroenterol. 2013;19:4072–4082. doi: 10.3748/wjg.v19.i25.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pisanu A., Porceddu G., Reccia I., Saba A., Uccheddu A. Meta-analysis of studies comparing single-incision laparoscopic appendectomy and conventional multiport laparoscopic appendectomy. J. Surg. Res. 2013;183:e1–11. doi: 10.1016/j.jss.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 62.Chawla S., Lal P., Ganguly P.K., Arora M.P., Hadke N.S. Endoscope-assisted inguinal hernia repair. J. Soc. Laparoendosc. Surg. 2005;9:42–46. [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai A.Y., Selzer D.J. Single-port laparoscopic surgery. Adv. Surg. 2010;44:1–27. doi: 10.1016/j.yasu.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 64.Rajapandian S., Bhushan C., Sabnis S.C., Jain M., Raj P.P., Paratasharthi R. Single incision multiport versus conventional laparoscopic inguinal hernia repair: a matched comparison. J. Minimal Access Surg. 2018;14:44–51. doi: 10.4103/jmas.JMAS_30_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melin A.A., Kalaskar S., Taylor L. Transanal endoscopic microsurgery and transanal minimally invasive surgery: is one technique superior? Am. J. Surg. 2016;212:1063–1067. doi: 10.1016/j.amjsurg.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 66.Atallah S., Albert M., Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg. Endosc. 2010;24:2200–2205. doi: 10.1007/s00464-010-0927-z. [DOI] [PubMed] [Google Scholar]
- 67.Stipa F., Tierno S.M., Russo G. Trans-anal minimally invasive surgery (TAMIS) versus trans-anal endoscopic microsurgery (TEM): a comparative case–control matched-pairs analysis. Surg. Endosc. 2021:1–6. doi: 10.1007/s00464-021-08494-y. [DOI] [PubMed] [Google Scholar]
- 68.Rimonda R., Arezzo A., Arolfo S. Transanal minimally invasive surgery (TAMIS) with SILS port versus transanal endoscopic microsurgery (TEM): a comparative experimental study. Surg. Endosc. 2013;27:3762–3768. doi: 10.1007/s00464-013-2962-z. [DOI] [PubMed] [Google Scholar]
- 69.Diana M., Marescaux J. Robotic surgery. Br. J. Surg. 2015;102:15–28. doi: 10.1002/bjs.9711. [DOI] [PubMed] [Google Scholar]
- 70.Kwoh Y.S., Hou J., Jonckheere E., Hayati S. A robot with improved absolute positioning accuracy for ct guided stereotactic brain surgery. IEEE Trans. Biomed. Eng. 1988;35:153–160. doi: 10.1109/10.1354. [DOI] [PubMed] [Google Scholar]
- 71.Davies B.L., Hibberd R.D., Ng W.S., Timoney A.G., Wickham J.E.A. The Development of a surgeon robot for prostatectomies. J Eng Med. 1991;205:35–38. doi: 10.1243/PIME_PROC_1991_205_259_02. [DOI] [PubMed] [Google Scholar]
- 72.Lanfranco A.R., Castellanos A.E., Desai J.P., Meyers W.C. Robotic surgery: a current perspective. Ann. Surg. 2004;239:14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lane T. A short history of robotic surgery. Ann. R. Coll. Surg. Engl. 2018;100:5–7. doi: 10.1308/rcsann.supp1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leal Ghezzi T., Campos Corleta O. 30 years of robotic surgery. World J. Surg. 2016;40:2550–2557. doi: 10.1007/s00268-016-3543-9. [DOI] [PubMed] [Google Scholar]
- 75.Himpens J., Leman G., Cadiere G. Telesurgical laparoscopic cholecystectomy. Surg. Endosc. 1998;12:1091. doi: 10.1007/s004649900788. [DOI] [PubMed] [Google Scholar]
- 76.Carpentier A., Loulmet D., Aupècle B., Kieffer J.-P., Tournay D., Guibourt P. First computer assisted open heart operation. Med. Sci. 1998;321:437–442. doi: 10.1016/s0764-4469(98)80309-0. [DOI] [PubMed] [Google Scholar]
- 77.Peters B.S., Armijo P.R., Krause C., Choudhury S.A., Oleynikov D. Review of emerging surgical robotic technology. Surg. Endosc. 2018;32:1636–1655. doi: 10.1007/s00464-018-6079-2. [DOI] [PubMed] [Google Scholar]
- 78.Marescaux J., Leroy J., Rubino F., Smith M., Vix M., Simone M. Transcontinental robot-assisted remote telesurgery, feasibility and potential applications. Ann. Surg. 2002;235:487–492. doi: 10.1097/00000658-200204000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jang E.J., Roh Y.H., Kang C.M., Kim D.K., Park K.J. Single-port laparoscopic and robotic cholecystectomy in obesity (>25 kg/m2) J. Soc. Laparoendosc. Surg. 2019;23:1–7. doi: 10.4293/JSLS.2019.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim H Il, Han S.U., Yang H.K., Kim Y.W., Lee H.J., Ryu K.W. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann. Surg. 2016;263:103–109. doi: 10.1097/SLA.0000000000001249. [DOI] [PubMed] [Google Scholar]
- 81.Obama K., Kim Y.M., Kang D.R., Son T., Kim H Il, Noh S.H. Long-term oncologic outcomes of robotic gastrectomy for gastric cancer compared with laparoscopic gastrectomy. Gastric Cancer. 2018;21:285–295. doi: 10.1007/s10120-017-0740-7. [DOI] [PubMed] [Google Scholar]
- 82.Tian W., Fan M., Zeng C., Liu Y., He D., Zhang Q. Telerobotic spinal surgery based on 5g network: the first 12 cases. Neurospine. 2020;17:114–120. doi: 10.14245/ns.1938454.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khor W.S., Baker B., Amin K., Chan A., Patel K., Wong J. Augmented and virtual reality in surgery-the digital surgical environment: applications, limitations and legal pitfalls. Ann. Transl. Med. 2016;4:1–10. doi: 10.21037/atm.2016.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao C., Cerfolio R.J. Virtual or augmented reality to enhance surgical education and surgical planning. Thorac. Surg. Clin. 2019;29:329–337. doi: 10.1016/j.thorsurg.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 85.Sampogna G., Pugliese R., Elli M., Vanzulli A., Forgione A. Routine clinical application of virtual reality in abdominal surgery. Minim Invasive Ther. Allied Technol. 2017;26:135–143. doi: 10.1080/13645706.2016.1275016. [DOI] [PubMed] [Google Scholar]
- 86.Vávra P., Roman J., Zonča P., Ihnát P., Němec M., Kumar J. Recent development of augmented reality in surgery: a review. J Healthc Eng. 2017. 2017:1–9. doi: 10.1155/2017/4574172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McConaghie G. Virtual surgery aiming at real results. Bull. Roy. Coll. Surg. Engl. 2016;98:264–265. [Google Scholar]
- 88.Onda S., Okamoto T., Kanehira M., Fujioka S., Suzuki N., Hattori A. Short rigid scope and stereo-scope designed specifically for open abdominal navigation surgery: clinical application for hepatobiliary and pancreatic surgery. J Hepatobiliary Pancreat Sci. 2013;20:448–453. doi: 10.1007/s00534-012-0582-y. [DOI] [PubMed] [Google Scholar]
- 89.Fida B., Cutolo F., di Franco G., Ferrari M., Ferrari V. Augmented reality in open surgery. Updates Surg. 2018;70:389–400. doi: 10.1007/s13304-018-0567-8. [DOI] [PubMed] [Google Scholar]
- 90.Papazarkadas X., Spartalis E., Patsouras D., Ioannidis A., Schizas D., Georgiou K. vol. 33. 2019. The role of 3D printing in colorectal surgery: current evidence and future perspectives; pp. 297–302. (Vivo (Brooklyn)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Segaran N., Saini G., Mayer J.L., Naidu S., Patel I., Alzubaidi S. Application of 3d printing in preoperative planning. J. Clin. Med. 2021;10:917. doi: 10.3390/jcm10050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hull C.W. United States Pat; 1986. pp. 1–16. (Apparatus for Production of Three-Dimensonal Objects by Stereolithography). [Google Scholar]
- 93.Hoang D., Perrault D., Stevanovic M., Ghiassi A. Today surgical applications of three-dimensional printing: a review of the current literature & how to get started. Ann. Transl. Med. 2016;4:1–19. doi: 10.21037/atm.2016.12.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aimar A., Palermo A., Innocenti B. The role of 3d printing in medical applications: a state of the art. J Healthc Eng. 2019:1–10. doi: 10.1155/2019/5340616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shilo D., Emodi O., Blanc O., Noy D., Rachmiel A. Printing the future: updates in 3d printing for surgical applications. Rambam Maimonides Med J. 2018;9 doi: 10.5041/RMMJ.10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zein N.N., Hanouneh I.A., Bishop P.D., Samaan M., Eghtesad B., Quintini C. Three-dimensional print of a liver for preoperative planning in living donor live transplantation. Liver Transplant. 2013;19:1304–1310. doi: 10.1002/lt.23729. [DOI] [PubMed] [Google Scholar]
- 97.Hashimoto D.A., Rosman G., Rus D., Meireles O.R. Artificial intelligence in surgery: promises and perils. Ann. Surg. 2018;268:70–76. doi: 10.1097/SLA.0000000000002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bejnordi B.E., Veta M., Van Diest P.J., Van Ginneken B., Karssemeijer N., Litjens G. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. J. Am. Med. Assoc. 2017;318:2199–2210. doi: 10.1001/jama.2017.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mirnezami R., Ahmed A. Surgery 3.0, artificial intelligence and the next-generation surgeon. Br. J. Surg. 2018;105:463–465. doi: 10.1002/bjs.10860. [DOI] [PubMed] [Google Scholar]
- 100.Sung J.J., Poon N.C. Artificial intelligence in gastroenterology: where are we heading? Front. Med. 2020;14:511–517. doi: 10.1007/s11684-020-0742-4. [DOI] [PubMed] [Google Scholar]
- 101.Byrne M.F., Chapados N., Soudan F., Oertel C., Pérez M.L., Kelly R. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut. 2019;68:94–100. doi: 10.1136/gutjnl-2017-314547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hashimoto D.A., Ward T.M., Meireles O.R. The role of artificial intelligence in surgery. Adv. Surg. 2020;54:89–101. doi: 10.1016/j.yasu.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 103.Chen W., Lu Z., You L., Zhou L., Xu J., Chen K. Artificial intelligence-based multimodal risk assessment model for surgical site infection (amrams): development and validation study. JMIR Med Informatics. 2020;8 doi: 10.2196/18186. [DOI] [PMC free article] [PubMed] [Google Scholar]