Abstract
Retinoic acid receptor β (RARβ) plays a critical role in mediating the anticancer effects of retinoids. Expression of RARβ is highly induced by retinoic acid (RA) through a RA response element (βRARE) that is activated by heterodimers of RARs and retinoid X receptors (RXRs). However, RARβ induction is often lost in cancer cells despite expression of RARs and RXRs. In this study, we provide evidence that orphan receptor COUP-TF is required for induction of RARβ expression, growth inhibition, and apoptosis by RA in cancer cells. Expression of COUP-TF correlates with RARβ induction in a variety of cancer cell lines. In addition, stable expression of COUP-TF in COUP-TF-negative cancer cells restores induction of RARβ expression, growth inhibition, and apoptosis by RA, whereas inhibition of COUP-TF by expression of COUP-TF antisense RNA represses the RA effects. In a transient transfection assay, COUP-TF strongly induced transcriptional activity of the RARβ promoter in a RA- and RARα-dependent manner. By mutation analysis, we demonstrate that the effect of COUP-TF requires its binding to a DR-8 element present in the RARβ promoter. The binding of COUP-TF to the DR-8 element synergistically increases the RA-dependent RARα transactivation function by enhancing the interaction of RARα with its coactivator CREB binding protein. These results demonstrate that COUP-TF, by serving as an accessory protein for RARα to induce RARβ expression, plays a critical role in regulating the anticancer activities of retinoids.
Retinoids, a class of natural and synthetic vitamin A analogs, exert profound effects on many biological processes, including cell proliferation and differentiation, vision, reproduction, morphogenesis, and pattern formation, both in normal and transformed cells (9, 30). They have been well recognized as promising agents for the prevention of human cancers (9, 13, 30, 58). Their therapeutic potential has also received a great amount of attention, since all-trans-retinoic acid (RA) showed dramatic antitumor effects in patients with acute promyelocytic leukemia (58). However, retinoid resistance associated with many different types of cancer has prevented the further application of retinoids (13, 58).
The effects of retinoids are mainly mediated by two classes of nuclear receptors, the RA receptors (RARs) and retinoid X receptors (RXRs) (18, 34, 67). 9-cis-RA is a high-affinity ligand for both RARs and RXRs, whereas all-trans-RA is a ligand for only RARs. RARs and RXRs are encoded by three distinct genes (α, β, and γ) and are members of the steroid/thyroid hormone receptor superfamily, which function as ligand-activated transcription factors (18, 34, 67). RARs and RXRs primarily function as RXR-RAR heterodimers that bind to a variety of RA response elements (RAREs) and regulate their transactivation function (18, 34, 67). Transcriptional activation by retinoid receptors requires a carboxy-terminal helical region, termed activation function-2, that forms part of the ligand-binding pocket and that undergoes a conformational change required for the recruitment of coactivator proteins, such as CREB binding protein (CBP) (17). This appears to provide a direct link to the core transcriptional machinery and modulates chromatin structure (62).
In addition to retinoid receptors, a number of orphan receptors whose ligands are unknown have been implicated in the regulation of the retinoid response (25, 47). One of the factors is COUP-TF. COUP-TF is encoded by two distinct genes, COUP-TFI (ear-3) (36, 56) and COUP-TFII (ARP-1) (22). Both genes show an exceptionally high degree of homology, and their expression patterns often overlap, suggesting that they may serve redundant functions (47). However, each factor possesses its own distinct expression profile during development (47). A null mutation of COUP-TFI resulted in defects in neurogenesis, axon guidance, and arborization (46), whereas deletion of COUP-TFII resulted in striking defects in angiogenesis, vascular remodeling, and fetal heart development (41). COUP-TF was originally shown to stimulate gene transcription (40). However, subsequent work has demonstrated that COUP-TF can repress the transcription induced by a number of nuclear receptors including RARs, thyroid hormone receptors, and vitamin D receptor (3, 20, 24, 53, 59). It has been recently reported that COUP-TFs can function as positive regulators for many different genes. In the arrestin gene promoter, a DR-7 element mediates the positive transcriptional effect of COUP-TF (32), while in the other genes, such as the trout estrogen receptor (23), the phosphoenolpyruvate carboxykinase (10), the vHNF1 (45), the human immunodeficiency virus long terminal repeat (49), and the NGFI-A (44) genes, the positive-transcription function of COUP-TF is mediated through its interaction with other transcriptional factors. For the NGFI-A gene, COUP-TF enhances transcription by recruiting coactivator SRC-1 through its interaction with SP-1 (44).
Retinoids exert their anticancer activities mainly through their abilities to modulate the growth, differentiation, and apoptosis of cancer cells. Recent studies have indicated that RARβ plays a critical role in mediating the growth-inhibitory effect of retinoids in many different types of cancer cells, including those of breast cancer (27, 29), lung cancer (28), ovarian cancer (42), prostate cancer (2), neuroblastoma (6), renal cell carcinoma (11), pancreatic cancer (16), liver cancer (26), and head and neck cancer (70). Expression of RARβ in RARβ-negative cancer cells restored RA-induced growth inhibition and apoptosis, whereas inhibition of RARβ expression in RARβ-positive cancer cells abolished RA effects (27–29). In addition, transgenic mice expressing RARβ antisense sequences showed increased incidence of lung tumor (1), whereas suppression of RARβ expression was responsible for diminished anticancer activities of retinoids in animals (57). Moreover, up-regulation of RARβ is associated with a positive clinical response to retinoids in patients with premalignant oral lesions (31, 50).
The finding that a deletion of the short arm of chromosome 3p24, close to where the RARβ gene maps, occurs with high frequency in human tumors (21, 38) led to studies on abnormalities of the RARβ gene and its expression. These studies revealed a high frequency of abnormal expression of the RARβ gene, but not of genes of the other RAR subtypes, in human cancer cell lines and primary human cancer tissues (8, 14, 15, 39, 55, 68, 69). Thus, the loss of RARβ may be a key mechanism by which cancer cells escape normal growth control and a contributing factor in cancer development (35, 63). Indeed, it has been shown that loss of RARβ is an early event in carcinogenesis (43, 60, 63, 64) and may be involved in liver cancer development (4).
How RARβ expression is regulated and how its expression is lost in cancer cells remain largely unknown and are subjects of intensive study. Expression of RARβ is highly induced by RA through a RARE (βRARE) present in its promoter (5, 12, 51) that is activated by RAR-RXR heterodimers (54, 65). However, we and others have recently demonstrated that expression of RARs and RXRs is not sufficient to render RARβ expression responsive to RA (19, 37, 55, 68). In the majority of lung cancer cell lines, RARβ expression could not be induced by RA, despite expression of RARs and RXRs (68). Similarly, retinoid refractoriness occurs during lung carcinogenesis despite the expression of functional retinoid receptors (19). Thus, factors other than RARs and RXRs are required for RA to induce RARβ expression.
We have previously demonstrated that expression of COUP-TF is positively correlated with RARβ induction and growth inhibition by RA in lung cancer cell lines (61). In this study, we further investigated the effect of COUP-TF in the regulation of RA-dependent RARβ induction and the underlying molecular mechanism. Our results demonstrate that COUP-TF is required for RA to induce RARβ expression, growth inhibition, and apoptosis in cancer cells. In addition, our studies showed that COUP-TF could strongly induce transcriptional activity of the RARβ promoter in a RA- and RARα-dependent manner through its binding to a DR-8 element present in the promoter. Furthermore, we observed that COUP-TF, through its interaction with RARα, strongly enhanced the interaction of RARα with its coactivator CBP, demonstrating that COUP-TF induces RARβ promoter transcription by acting as an accessory protein for RARα to recruit its coactivator.
MATERIALS AND METHODS
Cell culture.
CV-1, HeLa, MDA-MB231, and MDA-MB468 cells were grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (FCS). Calu-6, HT-1376, J82, SK-MES-1, and 5637 cells were maintained in minimal essential medium supplemented with 10% FCS. H292, H520, H460, H596, H441, and H661 cells were grown in RPMI 1640 supplemented with 10% FCS. A-549 cells were maintained in F12 medium supplemented with 10% FCS.
Plasmid constructions.
The RARβ promoter reporter (−745RARβCAT) has been described previously (12). The coding sequence of RARα was inserted into the multiple cloning sites of the eukaryotic expression vector pECE (65). The insertion of COUP-TFI cDNA into pRC/CMV vector (Invitrogen, San Diego, Calif.) in sense and antisense orientations followed the procedure described previously (29). The RARβ promoter deletion mutants were created by cloning BamHI-flanked PCR products derived from −745RARβ promoter into the pBluescript chloramphenicol acetyltransferase (CAT) plasmid (12). The forward primers were as follows: for the −516RARβ promoter, CATGGATCCTAGCCATTCTCGTTCTACAGT; for the −300RARβ promoter, CATGGATCCAGAAGTTGGTGCTCAACGTGA; for the −126RARβ promoter, CATGGATCCAGCTCTGTGAGAATCCTGGG. The oligonucleotide CATGGATCCTACCCCGACGGTGCCCAGA was used as the reverse primer for the above mutants. The −60RARβ promoter mutant was constructed by subcloning a SmaI/BamHI-flanked fragment from the RARβ promoter into the pBluescript CAT plasmid. The COUP-TF-RE and βRARE mutant constructs were generated by ligating the mutated RARβ promoter PCR products into the pBluescript CAT vector. The following primers were used to incorporate the appropriate mutations: TCCCCCGGGCTGCTAACCTTCAAATGACCCAAGTGACATCACCAA for COUP-TF-RE/M1, TCCCCCGGGCTGCTAACCTTCAAATGACCCAACTAGTCGAGCATCACCAA for COUP-TF-RE/M2, TCCCCCGGGTAGAACTCACCGAAAGTTCAC for βRARE/M1, and TCCCGGGTAGGGTTCACCGAGTTCAC for βRARE/M2. The COUP-TF-RE-tk-CAT reporter was obtained by inserting the DR-8 synthetic oligonucleotide into the BamHI site of pBL-CAT2 (65). For COUP-TFII receptor mutations, pBSCOUP-TFII deletion mutants were obtained by cloning PCR products from COUP-TFII into pBluescript (Stratagene). The oligonucleotide CATCGAGTGCGTGAGACGGGAAGCGGTG was used as the forward primer, while the reverse primers were as follows: GCCATGTCGACTCAGTTAAAACTGCTGCC for pBS-COUP-TFII/Δ7, TGCTGGTCGACTATAACATATCCCGGATG for pBS-COUP-TFII/Δ14, and ACCTGTCGACTAGACGAAAAACAATTGC for pBS COUP-TFII/Δ30. pBS-COUP-TFII/Δ80 was obtained by digesting pBS-COUP-TFII with HindIII, whereas pBS-COUP-TFII/Δ108 was obtained by digesting pBS-COUP-TFII with HindII. pBS-COUP-TFII/Δ179 was generated by subcloning an EcoRI/PstI COUP-TFII fragment into EcoRI/PstI-digested pBluescript. The orientations and sequences of all mutants were confirmed by DNA sequencing. To generate COUP-TFIIΔDBD, the N terminus coding sequence of COUP-TFII was amplified by PCR with a primer (CCGCTTCCCGTCTCACGCACTCGATGTG) corresponding to COUP-TFII cDNA sequences that preceded the DNA binding domain, whereas the C terminus coding sequence of COUP-TFII was amplified with a primer (CATCGAGTGCGTGAGACGGGAAGCGGTG) corresponding to COUP-TFII cDNA sequences right after the DNA binding domain. The resulting PCR products were then ligated to generate a COUP-TFII mutant with the DNA binding domain deleted. The COUP-TFII cDNA deletion mutants were also cloned into pCDNA3 (Invitrogen) for a transient transfection assay.
Preparation of COUP-TF proteins.
Receptor proteins for COUP-TFI and COUP-TFII were synthesized by an in vitro transcription and translation system using rabbit reticulocyte lysate (Promega) as described previously (65). The relative amounts of the translated proteins were determined by using [35S]methionine-labeled protein in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), quantitating the amount of incorporated radioactivity and normalizing it relative to the content of methionine in each protein.
Transient and stable transfection assay.
CV-1 cells were plated at 105 cells per well in a 24-well plate 16 to 24 h before transfection, as described previously (66). For cancer cells, 5 × 105 cells were seeded in a six-well plate. A modified calcium phosphate precipitation procedure was used for transient transfection and is described elsewhere (66). Briefly, 700 ng of reporter plasmid, 100 ng of β-galactosidase (β-Gal) expression vector (pCH 110; Pharmacia), and various amounts of COUP-TF expression vector and RARα were mixed with carrier DNA (pBluescript) to 1,000 ng of total DNA per well. CAT activity was normalized for transfection efficiency to the corresponding β-Gal activity. For stable transfection, the pRc/CMV-COUP-TFI recombinant plasmid was stably transfected into MDA-MB231 cells, and pRC/CMV-antisense-COUP-TFI was stably transfected into J82 cells by the calcium phosphate precipitation method, and stable clones were screened with G418 (GIBCO BRL, Grand Island, N.Y.) as described previously (29). Southern blotting and Northern blotting were used to determine the integration and expression of transfected cDNA, respectively.
Gel retardation assay.
Oligonucleotides used for the gel retardation assay are described in the text. For the protein-DNA binding assay, in vitro-translated protein (1 to 4 μl, depending on the translation efficiency) was incubated with the 32P-labeled oligonucleotides in a 20-μl reaction mixture containing 10 mM HEPES buffer, pH 7.9, 50 mM KCl, 1 mM dithiothreitol, 2.5 mM MgCl2, 10% glycerol, and 1 μg of poly(dI-dC) at 25°C for 20 min. Unprogrammed reticulocyte lysate was used to maintain equal protein concentrations in all reaction mixtures. Each reaction mixture was then loaded on a 5% nondenaturing polyacrylamide gel containing 0.5× TBE (1× TBE is 0.089 M Tris-borate, 0.088 M boric acid, and 0.002 M EDTA).
GST pull-down assay.
To prepare the glutathione S-transferase (GST)-receptor fusion protein, the receptor cDNA (COUP-TFI or RARα) was cloned in frame into the expression vector pGEX-2T (Pharmacia). The fusion protein was expressed in bacteria by the procedure provided by the manufacturer and was analyzed by a gel retardation assay and Western blotting (data not shown). To analyze the interaction between RARα and COUP-TFI and between COUP-TFI and CBP, the fusion protein was immobilized to glutathione-Sepharose beads. For a control, the vector protein (GST), prepared under the same conditions, was also immobilized. The beads were preincubated with bovine serum albumin (1 mg/ml) at room temperature for 5 min. 35S-labeled, in vitro-synthesized receptor proteins (2 to 5 μl, depending on translation efficiency) were then added to the beads. The beads were then continuously rocked for 1 h at 4°C in a final volume of 200 μl in EBC buffer (140 mM NaCl, 0.5% NP-40, 100 mM NaF, 200 μM sodium orthovanadate, and 50 mM Tris, pH 8.0). After being washed five times with NETN buffer (100 mM NaCl, 1 mM EDTA, 20 mM Tris [pH 8.0], 0.5% NP-40), the bound proteins were analyzed by SDS-PAGE.
Growth inhibition assays.
To determine the effect of all-trans-RA on the viability of the stable transfectants, cells were seeded at 1,000 per well in a 96-well plate and treated with various concentrations of all-trans-RA for 6 days. Media were changed every 48 h. The number of viable cells was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay as described previously (29). For the anchorage-independent growth assay, 30,000 cells/60-mm-diameter dish in culture medium containing 10% FCS, 0.3% agar (Difco, Detroit, Mich.), and 10−7 M RA were plated onto an already-hardened 0.6% agar underlayer in medium supplemented with 10% FCS. The plates were incubated for 21 days in a 5% CO2 incubator. Colonies with more than 40 cells were counted with a microscope.
Apoptosis assay.
Cells were treated with or without all-trans-RA (10−6 M). Forty-eight hours later, cells were trypsinized, washed with phosphate-buffered saline (PBS; pH 7.4), and fixed in 1% formaldehyde in PBS. After being washed in PBS, cells were resuspended in 70% ice-cold ethyl alcohol and immediately stored at −20°C overnight. Cells were then labeled with biotin-16-dUTP by terminal deoxynucleotidyltransferase (TdT) and stained with avidin-fluorescein isothiocyanate (Boehringer, Mannheim, Germany). Fluorescently labeled cells were analyzed with a FACScater-plus as described previously (29). Representative histograms are shown.
Northern blotting.
For Northern blot analysis, total RNAs were prepared with an RNeasy Mini Kit (Qiagen, Hilden, Germany). Thirty micrograms of total RNA from different cell lines treated with or without all-trans-RA (10−6 M) was analyzed by Northern blotting. An EcoRI fragment in the ligand binding domain of RARβ or a PstI fragment in the ligand binding domain of COUP-TFI cDNA was used as a probe to study the expression of RARβ or COUP-TFII, respectively. The PstI fragment could detect the expression of COUP-TFI and COUP-TFII due to the high degree of homology of both receptor genes in the region. To determine that equal amounts of RNA were used, the expression of β-actin was studied.
RESULTS
Correlation between COUP-TF expression and RARβ induction by RA.
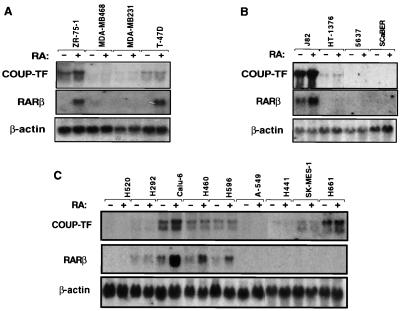
We recently showed that expression of COUP-TF is required for RA sensitivity in certain lung cancer cell lines (61). To examine to what degree COUP-TF is involved in the regulation of RARβ expression, we analyzed the expression of COUP-TF in various cancer cell lines, including breast cancer cell lines ZR-75-1, MDA-MB468, MDA-MB231, and T-47D, bladder cancer cell lines J82, HT-1376, 5637, and SCaBER, and lung cancer cell lines H520, H292, Calu-6, H460, H596, A-549, H441, SK-MES-1, and H661. A PstI fragment in the ligand binding domain of COUP-TF was used as a probe. The probe could detect transcripts for COUP-TFI and COUP-TFII. When the expression of COUP-TFs was compared with RA-induced RARβ expression, we found a perfect correlation between COUP-TFI expression and the ability of RA to induce RARβ in breast cancer cell lines (Fig. 1). COUP-TFI was expressed in ZR-75-1 and T-47D cell lines, in which RARβ expression was highly induced by RA. In contrast, COUP-TF transcripts were not detected in MDA-MB468 and MDA-MB231 cell lines that did not show a clear induction of RARβ by RA. In bladder cancer cell lines, RARβ was highly induced by RA in COUP-TFI-positive J82 cells but not in COUP-TFI-negative 5637 and SCaBER cells. Although HT-1376 cells expressed COUP-TFI, we did not observe any RARβ expression. This is due to lack of RARα expression in these cells (see Discussion). A correlation was also observed in lung cancer cell lines, except SK-MES-1 and H661 lines. COUP-TFI and COUP-TFII were highly expressed in RARβ-inducible cell lines Calu-6, H460, and H596 but not in RARβ-noninducible H520, H292, A549, and H441 cell lines. These observations suggest that expression of COUP-TF may be required for RA to induce RARβ expression in different types of cancer cells.
FIG. 1.
Correlation between COUP-TF expression and RARβ induction by RA in human breast cancer (A), bladder cancer (B), and lung cancer (C) lines. Total RNAs were prepared from the indicated cancer cell lines treated with or without all-trans-RA (10−6 M) for 24 h and analyzed for the expression of COUP-TFI and RARβ. For a control, the expression of β-actin is shown.
Levels of COUP-TFI expression modulate RA-dependent RARβ expression, growth inhibition, and apoptosis.
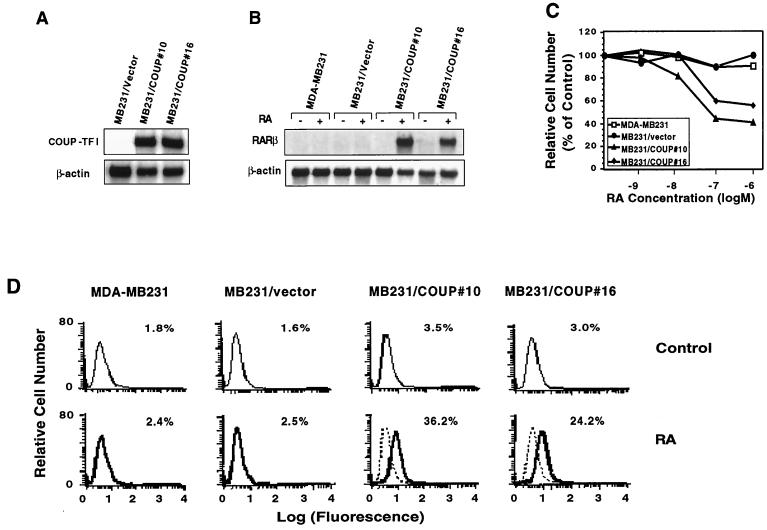
To further determine the requirement of COUP-TF for RA-dependent activation of RARβ gene expression, we stably expressed COUP-TFI in COUP-TF-negative MDA-MB231 breast cancer cells. Two stable clones (MB231/COUP#10 and MB231/COUP#16) that expressed a high level of transfected COUP-TFI (Fig. 2A) were subjected to an analysis of RARβ gene expression in the absence or presence of RA. Under the conditions used, we did not detect any expression of the RARβ transcript in the parental MDA-MB231 cells in either the absence or presence of RA (Fig. 2B), consistent with our previous observation (29). However, RA treatment strongly induced RARβ expression in the COUP-TFI-stable clones, not in the MDA-MB231 cells transfected with the empty vector (MB231/vector) (Fig. 2B). This suggests that expression of COUP-TFI confers on MDA-MB231 cells sensitivity to RA regulation of RARβ expression. When the effect of the stable transfection of COUP-TFI on growth of MDA-MB231 cells was analyzed, we observed that RA, which was unable to regulate the growth of the parental MDA-MB231 cells, could strongly inhibit the growth of the clones carrying stably transfected COUP-TFI, with about 41 and 54% inhibition in MB231/COUP#16 and MB231/COUP#10 cells, respectively, when they were treated with 10−6 M RA for 6 days (Fig. 2C). The growth-inhibitory effect of RA is specific to COUP-TFI expression since RA had no effect on the growth of MB231/vector cells. We also examined the effect of COUP-TFI expression on apoptosis induction by RA. The TdT assay showed extensive DNA fragmentation in MB231/COUP#10 and MB231/COUP#16 cells but not in MDA-MB231 and MB231/vector cells. About 2% apoptotic cells were detected in cultures of MDA-MB231 and MB231/vector cells when they were treated with 10−6 M RA for 2 days. However, the same RA treatment resulted in about 36 and 24% apoptotic cells in cultures of MB231/COUP#10 and MB231/COUP#16 cells, respectively (Fig. 2D), suggesting that expression of COUP-TFI could allow RA to induce apoptosis of MDA-MB231 cells. Thus, expression of COUP-TFI in COUP-TF-negative cancer cells can restore their response to RA effects on RARβ expression, growth inhibition, and apoptosis.
FIG. 2.
Stable expression of COUP-TF in COUP-TF-negative, RA-resistant MDA-MB231 cells enhances the effect of RA on RARβ induction, growth inhibition, and apoptosis induction. (A) Expression of transfected COUP-TFI in MDA-MB231 cells. COUP-TFI was stably transfected into MDA-MB231 cells, and expression of transfected COUP-TFI was determined by Northern blotting. For comparison, cells transfected with the empty vector (MB231/vector) were used. (B) Expression of RARβ gene in MDA-MB231 cells and MDA-MB231/COUP-TF stable clones. Total RNAs were prepared from MDA-MB231, its COUP-TFI stable clones (MB231/COUP#16 and MB231/COUP#10), and MDA-MB231 transfected with the empty vector (MB231/vector). Cells were treated with or without all-trans-RA (10−6 M) for 24 h and analyzed for the expression of RARβ by Northern blotting. In the control, the expression of β-actin is shown. (C) Growth-inhibitory effect of RA in MDA-MB231 and MDA-MB231/COUP-TF stable clones. Cells were seeded at 1,000 cells per well in a 96-well plate and treated with the indicated concentrations of all-trans-RA for 6 days. The numbers of viable cells were determined by the MTT assay. (D) Effect of RA on apoptosis of MDA-MB231 cells and MDA-MB231/COUP-TF stable clones. Cells were treated with or without all-trans-RA (10−6 M) for 48 h, and DNA fragmentations were then determined by the TdT assay. Representative histograms show relative apoptotic cell numbers.
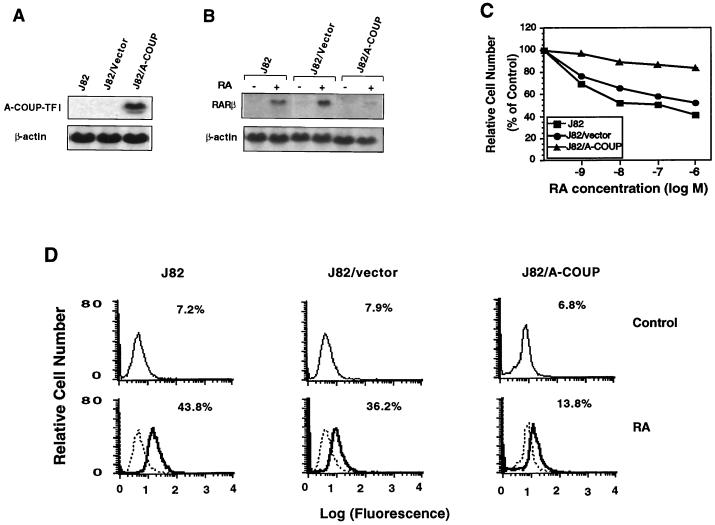
To further study the role of COUP-TF, we stably expressed COUP-TFI antisense cDNA in COUP-TF-positive J82 bladder cancer cells. A stable clone that expressed a high level of COUP-TFI antisense RNA (J82/A-COUP) (Fig. 3A) was analyzed for the effect of RA on RARβ expression (Fig. 3B), growth inhibition (Fig. 3C), and apoptosis induction (Fig. 3D). RARβ expression was highly induced by RA in J82 cells. However, the ability of RA to induce RARβ expression was significantly reduced in J82/A-COUP cells but not in J82 cells expressing the empty vector (J82/vector) (Fig. 3B). Thus, inhibition of COUP-TF expression reduced the ability of RA to induce RARβ expression. When the effect of RA on growth inhibition was analyzed, we observed that RA could effectively inhibit the growth of J82 cells, with 55% inhibition when they were treated with 10−6 M RA for 6 days. A similar growth-inhibitory effect of RA (46% inhibition) was also observed in J82/vector cells. However, the effect of RA was dramatically reduced in J82/A-COUP cells, with only 12% inhibition upon RA treatment (Fig. 3C). Consistent with the role of RARβ in apoptosis induction, we observed that RA strongly induced apoptosis of J82 and J82/vector cells, with 43.8 and 36.2% apoptotic cells, respectively, as determined by the TdT assay (Fig. 3D). However, the apoptosis-inducing effect of RA was significantly repressed by COUP-TFI antisense RNA expression, as only 13.8% apoptotic cells were detected among J82/A-COUP cells. Together, these data demonstrate that inhibition of COUP-TFI expression by COUP-TFI antisense RNA represses the ability of RA to induce RARβ expression, growth inhibition, and apoptosis in COUP-TF-positive cancer cells.
FIG. 3.
Inhibition of COUP-TF expression by stable expression of COUP-TF anti-sense RNA in COUP-TF-positive J82 cells represses effect of RA on RARβ expression, growth inhibition, and apoptosis induction. (A) Expression of transfected COUP-TFI antisense RNA in J82 cells. COUP-TFI cDNA was stably transfected into J82 cells, and expression of transfected COUP-TFI antisense RNA in a selected stable clone (J82/A-COUP) was analyzed by Northern blotting. For comparison, the parental J82 cells and J82 cells stably transfected with the empty vector (J82/vector) were used. (B) Inhibition of RARβ induction by RA by stable expression of COUP-TFI antisense RNA in J82 cells. Total RNAs were prepared from J82, J82/vector, and J82/A-COUP cells treated with or without all-trans-RA (10−6 M) for 24 h and analyzed for the expression of RARβ by Northern blotting. The expression of β-actin is shown for the control. (C) Inhibition of RA-induced growth inhibition by stable expression of COUP-TF antisense RNA in J82 cells. Cells were seeded at 1,000 cells per well in a 96-well plate and treated with the indicated concentrations of all-trans-RA for 6 days. The numbers of viable cells were determined by the MTT assay. (D) Inhibition of RA-induced apoptosis of J82 cells by COUP-TF antisense RNA. Cells were treated with or without all-trans-RA (10−6 M) for 48 h, and DNA fragmentations were then determined by the TdT assay. Representative histograms show relative apoptotic cell numbers.
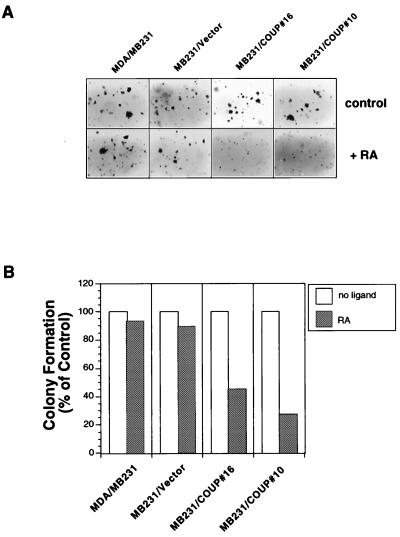
To further characterize the effect of the transfected COUP-TFI gene, the COUP-TFI-transfected MDA-MB231 cells were analyzed for their anchorage-independent growth in soft agar. As shown in Fig. 4, the growth of MDA-MB231 and MB231/vector cells in soft agar was not clearly affected by RA treatment. However, RA dramatically inhibited the growth of MB231/COUP#10 and MB231/COUP#16 cells. These data suggest that expression of COUP-TFI could confer on RA the ability to inhibit the transforming activity of cancer cells.
FIG. 4.
Inhibition of anchorage-independent growth of MDA-MB231 cells by COUP-TF gene expression. (A) Visualization of colonies formed in the soft agar by parental MDA-MB231, MB231/COUP#10, MB231/COUP#16, and MB231/vector cells in the presence or absence of RA (10−7 M). (B) Quantitation of colonies formed by parental MDA-MB231, MB231/COUP#10, MB231/COUP#16, and MB231/vector cells. Colonies formed by MB231/COUP#10, MB231/COUP#16, MB231/vector, and parental MDA-MB231 cells in the presence or absence of all-trans-RA (10−7 M) were scored and expressed as percentages of colonies formed by cells treated with control solvent.
COUP-TF enhances RARβ promoter activity in a RARα- and RA-dependent manner.
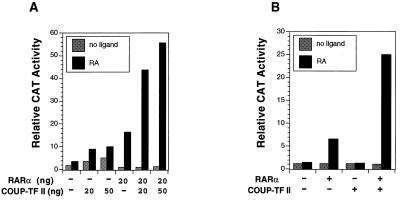
To investigate the possibility that COUP-TFI induced RARβ expression by activating RARβ promoter transcription, a reporter construct that contains the RARβ promoter sequences from −745 to +162 linked to the CAT reporter gene (−745RARβCAT) (12) was transiently transfected into CV-1 cells. Cotransfection of the RARα expression vector induced RARβ promoter activity in response to RA (Fig. 5A), while cotransfection of the COUP-TFII expression vector slightly enhanced the reporter gene activity. However, when both COUP-TFII and RARα were cotransfected, a synergistic induction of RARβ promoter activity in response to RA was observed. Induction of RARβ promoter activity by RARα was enhanced from 16-fold to 44-fold when 20 ng of COUP-TFII was cotransfected, while a 56-fold induction was observed when 50 ng of COUP-TFII was cotransfected. A similar observation was also made with COUP-TFI (data not shown). We also investigated the effect of COUP-TFII in HT-1376 bladder cancer cells, which express a low level of COUP-TF (Fig. 1) and an undetectable level of RARα (our unpublished result). Cotransfection of the RARα expression vector slightly induced RARβ promoter activity in response to RA in these cells, while cotransfection of COUP-TFII did not show any effect on RARβ promoter activity (Fig. 5B). The lack of COUP-TFII activity is likely due to loss of RARα expression in these cells (see Discussion). When COUP-TFII was cotransfected with RARα, the RARα-induced RARβ activity was strongly increased, compared to a 5-fold induction of RARβ promoter activity by RARα alone and a 24-fold induction when both RARα and COUP-TFII were present. Similar results were also obtained when COUP-TFI was used and in other cancer cell lines, such as HeLa cells and MDA-MB231 cells (data not shown). Together, our data demonstrate that expression of COUP-TF is required for efficient induction of RARβ promoter activity by RA in a RARα-dependent manner.
FIG. 5.
COUP-TF enhances RARβ promoter activity in a RARα- and RA-dependent manner. (A) Activation of RARβ promoter activity by COUP-TF in CV-1 cells. RARβ promoter reporter (−745RARβCAT; 700 ng) was cotransfected with the indicated amounts of expression vector for COUP-TFII and RARα into CV-1 cells. Cells were treated with or without all-trans-RA (10−6 M) for 24 h and assayed for CAT activity. (B) Activation of RARβ promoter in HT-1376 bladder cancer cells by COUP-TF. Cells were transfected with 1,500 ng of −745RARβCAT reporter gene together with expression vectors for RARα (300 ng) and/or COUP-TFII (300 ng). Cells were treated with or without all-trans-RA (10−6 M) and 24 h later were assayed for CAT activity. Data shown represent the means of three independent experiments.
A DR-8 element in the RARβ promoter mediates the COUP-TF effect.
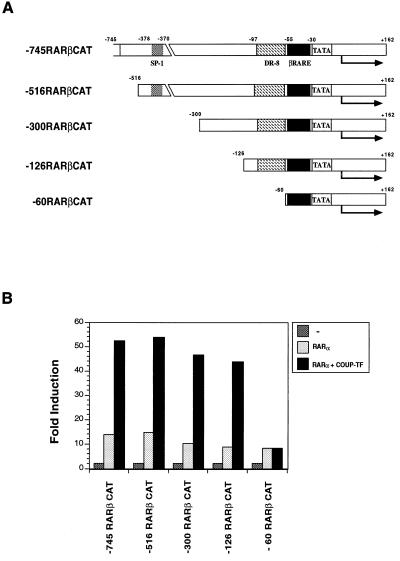
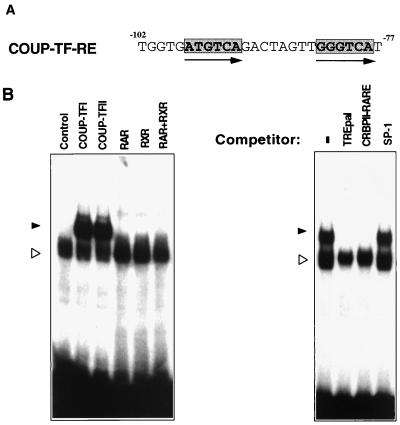
To identify DNA sequences responsible for the COUP-TF effect in the RARβ promoter, we generated a series of 5′ deletion mutants of the RARβ promoter (Fig. 6A). The resulting RARβ promoter fragments were fused to the CAT reporter gene and analyzed for the effect of COUP-TFII in enhancing RA-induced RARα activity in CV-1 cells (Fig. 6B). Deletion of 229 bp from the 5′ end of the RARβ promoter (−516RARβCAT) did not have any effect on the ability of COUP-TFII to enhance RARβ activity. Recently, it was shown that COUP-TF could positively regulate gene transcription through an SP-1 site (44, 49). There is an SP-1 site (from −378 to −370) in the RARβ promoter that could bind the SP-1 protein (data not shown). However, COUP-TFII retained its ability to induce RARα activity in −300RARβCAT and −126RARβCAT reporters in spite of deletion of the SP-1 binding site. This suggests that the effect of COUP-TFII on the RARβ promoter is not mediated through the SP-1 binding site. Further deletion of 66 bp from −126RARβCAT, however, completely abolished the COUP-TFII effect (Fig. 6B). This suggests that position −126 to −60 of the RARβ promoter contains a COUP-TF response element (COUP-TF-RE). Inspection of the fragment between positions −126 and −60 revealed a direct repeat of AGGTCA-like motifs with 8-bp spacing (DR-8) located from −99 to −78 (Fig. 7A). COUP-TF is known to bind to a variety of nuclear receptor response elements containing AGGTCA-like motifs arranged in different orientations with different spacings (53). We therefore examined whether the DR-8 element in the RARβ promoter could bind to COUP-TF. An oligonucleotide containing the sequence was synthesized and analyzed by gel retardation assay for its COUP-TF binding activity. As shown in Fig. 7B, in vitro-synthesized COUP-TFI or COUP-TFII formed a strong complex with the sequence. For comparison, RARα, RXRα, or the RARα-RXRα heterodimer did not exhibit any binding. The complex formed by COUP-TFI could be abolished by a 50-fold-excess amount of TREpal or CRBPII-RARE, which are known to bind with high affinity to COUP-TFs (53), while the same amount of SP-1 oligonucleotide did not show any effect on the binding. Thus, COUP-TF may exert its effect on the RARβ promoter through its binding to the DR-8 element.
FIG. 6.
Identification of COUP-TF response element in the RARβ promoter. (A) Schematic representation of the RARβ promoter deletion mutants. The Sp-1 binding site, βRARE, and the TATA box are indicated. (B) Effect of COUP-TFII on RA-induced RARα transactivation activity of various RARβ promoter deletion mutants. CV-1 cells were transfected with 700 ng of CAT reporter gene containing the indicated RARβ promoter fragment together with expression vectors for COUP-TFII (50 ng) and RARα (20 ng). Cells were then treated with or without all-trans-RA (10−6 M) and 24 h later were assayed for CAT activity. −, without receptor cotransfection. Data shown represent the means of three independent experiments.
FIG. 7.
Analysis of COUP-TF binding on the COUP-TF-RE in the RARβ promoter. (A) Sequence of the COUP-TF-RE. Arrows indicate the AGGTCA-like core motifs. (B) Binding of COUP-TF on the COUP-TF-RE. In vitro-synthesized COUP-TFI or COUP-TFII was incubated with 32P-labeled oligonucleotide containing the COUP-TF-RE and analyzed by a gel retardation assay. For comparison, the binding of RARα, RXRα, and the RARα-RXRα heterodimer was analyzed. For the competition assay, a 50-fold excess amount of the indicated oligonucleotide was used. −, without competitor; solid arrowheads, specific COUP-TF binding complex; open arrowheads, nonspecific binding complex.
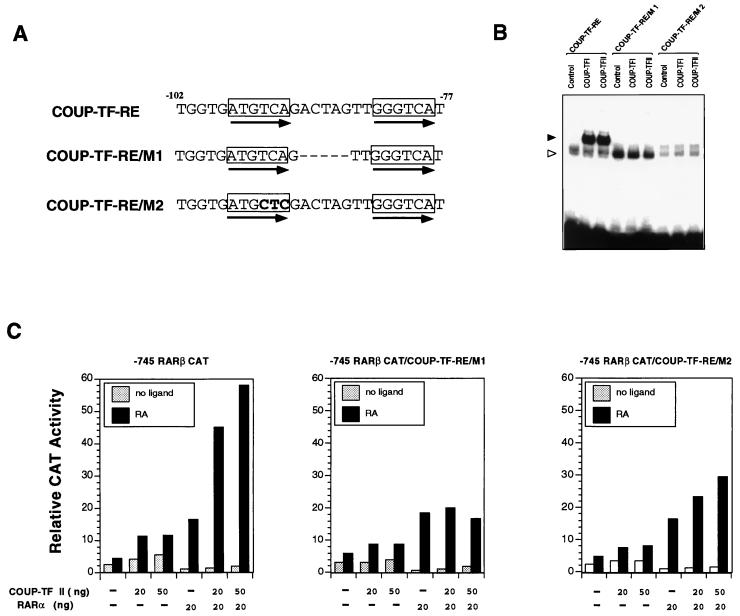
To study the role of the COUP-TF binding sequence, we investigated the effect of COUP-TF-RE mutations on COUP-TF activity in a RARβ promoter. The COUP-TF-RE was mutated by changing either the spacing between two core motifs (COUP-TF-RE/M1) or the core motif sequences (COUP-TF-RE/M2) (Fig. 8A). The mutated COUP-TF-REs were first analyzed for their binding to COUP-TF proteins. Unlike their binding to the wild-type COUP-TF-RE, the binding of COUP-TFI or COUP-TFII to the mutated COUP-TF-REs was largely impaired (Fig. 8B). We then introduced the same mutations into the −745RARβCAT reporter by PCR. The resulting RARβ promoter reporter mutants, −745RARβ/COUP-TF-RE/M1/CAT and −745RARβ/COUP-TF-RE/M2/CAT, were then analyzed by transient transfection assay for the effect of COUP-TF-RE mutations on COUP-TFII activity. As shown in Fig. 8C, mutations of COUP-TF-RE did not affect the ability of RARα to induce reporter transcription since cotransfection of RARα showed similar degrees of induction of transcription of both the mutated RARβ promoter and the wild-type promoter (compare with Fig. 5). However, the enhancing effect of COUP-TFII on RARα activity was largely reduced in the mutated RARβ promoter reporters. These results demonstrate that the DR-8 element is required for COUP-TF to enhance RA- and RARα-dependent activation of RARβ promoter transcription.
FIG. 8.
COUP-TF-RE is required for positive regulation of RARβ promoter activity by COUP-TF. (A) Mutations of the COUP-TF-RE. Depicted are the COUP-TF-RE sequence and its mutations (boldfaced). (B) The mutated COUP-TF-REs failed to bind to the COUP-TF protein. The indicated COUP-TF-RE mutant was synthesized and analyzed for its binding to COUP-TF by the gel retardation assay. In vitro-synthesized COUP-TF protein was incubated with 32P-labeled COUP-TF-RE or the mutated COUP-TF-RE and analyzed by a gel retardation assay. Solid arrowhead, specific COUP-TAF binding complex; open arrowhead, nonspecific binding. (C) Mutations of COUP-TF-RE abolished the effect of COUP-TF on the RARα transactivation function in the RARβ promoter. The COUP-TF-RE in the RARβ promoter was mutated by PCR. The resulting RARβ promoter mutants (−745RARβCAT/COUP-TF-RE/M1 and −745RARβCAT/COUP-TF-RE/M2) were analyzed by transient transfection assay in CV-1 cells for their effect on COUP-TF activity. CV-1 cells were transfected with the 700-ng reporter gene together with the indicated amounts of expression vectors for RARα and COUP-TFII. Cells were treated with or without all-trans-RA (10−6 M) and 24 h later were assayed for CAT activity. CAT activity was normalized for transfection efficiency to the corresponding β-Gal activity. Data shown represent the means of three independent experiments.
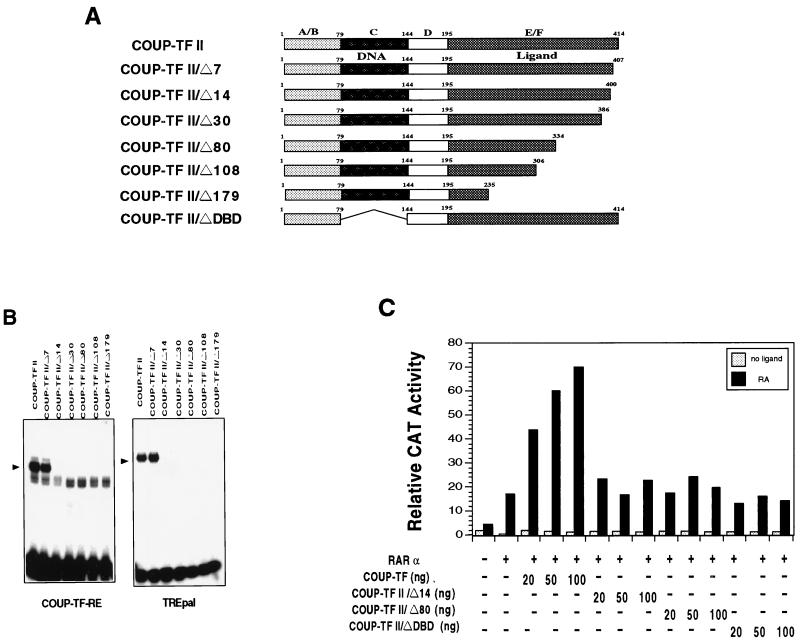
DNA binding of COUP-TF is required for its transactivation function.
To determine which region of COUP-TF is required for the binding of the DR-8 element and the activation of the RARβ promoter, a number of COUP-TFII mutants were generated (Fig. 9A) and analyzed for their binding to the element (Fig. 9B) and their enhancing effect on RARα activity in the −745RARβCAT reporter (Fig. 9C). Deletion of seven amino acids from the C-terminal end of the COUP-TFII protein did not affect the binding of COUP-TF-RE (Fig. 9B). The same mutant also bound strongly to TREpal as the wild-type receptor. However, removal of an additional seven amino acid residues resulted in a mutant (COUP-TFII/Δ14) that could not bind either to COUP-TF-RE or to TREpal. As expected, other mutants analyzed, including COUP-TFII/Δ30, COUP-TFII/Δ80, COUP-TFII/Δ108, and COUP-TFII/Δ179 (Fig. 9A), did not exhibit any detectable binding on both elements (Fig. 9B). When the effect of these mutants on RARα transactivation function in −745RARβCAT was analyzed, we observed that removal of as few as 14 amino acid residues from the C-terminal end of the COUP-TFII protein completely abolished the enhancing effect of COUP-TFII (Fig. 9C). Other C-terminal deletion mutants, which could not bind to the DR-8 element, also failed to enhance RARα activity (data not shown). A mutant with the DNA binding domain deleted (COUP-TF/ΔDBD) was also unable to enhance RARα activity (Fig. 9C). Thus, the DNA binding of COUP-TF is essential for its enhancing effect on RARα activity.
FIG. 9.
DNA binding of COUP-TF is required for its enhancing effect on RARα activity in the RARβ promoter. (A) Schematic representation of COUP-TF mutants. The DNA binding domain and the ligand domain of COUP-TFII as well as the A/B, C, D, and E/F domains are indicated. (B) Binding of the COUP-TFII mutants to COUP-TF-RE and TREpal. In vitro-synthesized COUP-TFII or the indicated COUP-TFII deletion mutant was incubated with 32P-labeled COUP-TF-RE or TREpal as indicated and analyzed by a gel retardation assay. The arrowheads indicate the specific COUP-TF binding complexes. (C) Effect of the COUP-TFII mutants on RARα activity in the RARβ promoter. CV-1 cells were transfected with 700 ng of −745RARβCAT reporter gene together with the expression receptor for RARα (20 ng) and the indicated amount of COUP-TFII or COUP-TFII deletion mutants. Cells were treated with or without all-trans-RA (10−6 M) for 24 h and were assayed for CAT activity. Data shown represent the means of three independent experiments.
βRARE is required for the DR-8 element to confer the RA- and RARα-dependent transactivation function of COUP-TF.
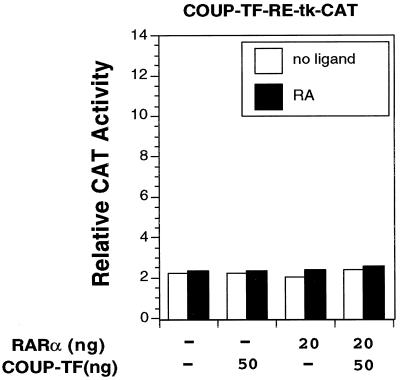
To further determine the role of the DR-8 element in mediating the COUP-TF transactivation function, we cloned the sequence into the pBLCAT2 plasmid, which contains the thymidine kinase (TK) promoter linked with the CAT reporter gene (65). The resulting construct, COUP-TF-RE-tk-CAT, was transiently transfected into CV-1 cells. Surprisingly, we did not see any effect of COUP-TFII on reporter transcription either in the absence or presence of RA, indicating that the binding of COUP-TFII could not transactivate the reporter (Fig. 10). Cotransfection of the RARα expression vector did not have any effect on the reporter activity, either in the absence or presence of RA, consistent with our gel retardation assay showing lack of RARα binding to the DR-8 element (Fig. 7B). Furthermore, cotransfection of COUP-TFII and RARα expression vectors failed to activate reporter transcription. These data demonstrate that the DR-8 element alone is not sufficient to confer the RA- and RARα-dependent transactivation function of COUP-TF observed in the RARβ promoter.
FIG. 10.
COUP-TF-RE alone is not sufficient to confer the activation function of COUP-TF. COUP-TF-RE was cloned into pBLCAT2, which contained the TK promoter linked with the CAT gene. The resulting reporter construct (COUP-TF-RE-tk-CAT) was analyzed for its response to the COUP-TF effect by transient transfection assay. The reporter gene (100 ng) was cotransfected with the indicated amounts of expression receptor for RARα and COUP-TFII into CV-1 cells. After transfection, cells were treated with or without all-trans-RA (10−6 M) and 24 h later were assayed for CAT activity. Data shown represent the means of three independent experiments.
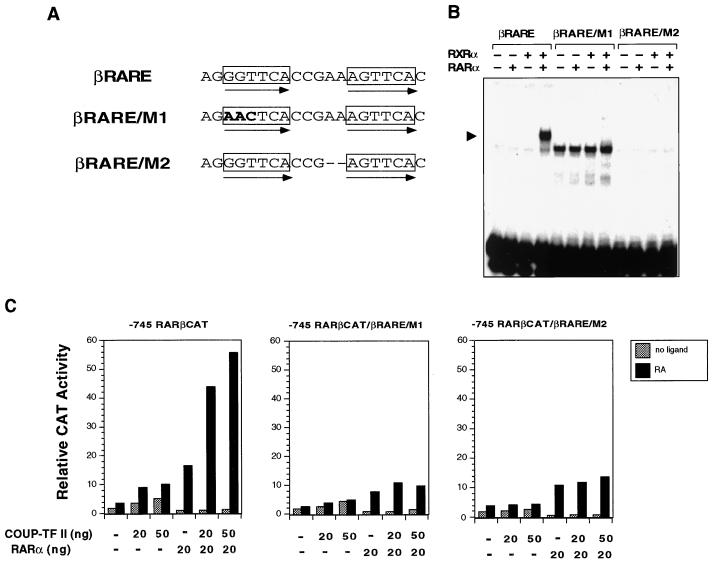
The above data suggest that sequences other than the DR-8 element in the RARβ promoter are required for the RA- and RARα-dependent transactivation function of COUP-TFs. We therefore analyzed the involvement of βRARE by mutational analysis since it is the only known sequence in the RARβ promoter that mediates the RA effect (12). Two βRARE mutations were made by changing either the core motif sequences (βRARE/M1) or the spacing between two core motifs (βRARE/M2) (Fig. 11A). Both βRARE mutants failed to bind to RARα, RXRα, or RARα-RXRα heterodimers in the gel retardation assay (Fig. 11B). The RARβ promoter reporter constructs with mutated βRARE, −745RARβCAT/βRARE/M1 and −745RARβCAT/βRARE/M2, were then generated by PCR and analyzed by a transient transfection assay (Fig. 11C). We first examined the effect of RARα on the mutated RARβ promoter. Compared to that of the parental RARβ promoter (−745RARβCAT), the transactivation function of RARα was significantly reduced in the mutated RARβ promoters. Interestingly, RARα could still induce transcriptional activities of both RARβ promoter mutants, although its binding on the mutated βRAREs was completely abolished (Fig. 11B). Since we did not observe any effect of RARα on the mutated βRAREs when they were fused to the TK promoter (data not shown), the observed effect of RARα is likely due to the presence of another RARE in the RARβ promoter (our unpublished observation). Nevertheless, when the effect of COUP-TFII on both promoter mutants was analyzed, we did not observe any enhancement of RARα activity, suggesting that intact βRARE, capable of binding with the RARα-RXRα heterodimer, is essential for the transactivation function of COUP-TF. Thus, both βRARE and the DR-8 element are required for the RA- and RARα-dependent transactivational function of COUP-TF in the RARβ promoter.
FIG. 11.
βRARE in the RARβ promoter is required for the enhancing effect of COUP-TF on RARα activity in the RARβ promoter. (A) Schematic representation of the βRARE mutations. Depicted are wild-type βRARE in the RARβ promoter and its mutations (boldface). Arrows indicate the care motifs of the βRARE. (B) Effect of βRARE mutations on retinoid receptor binding. In vitro-synthesized RARα and RXRα were incubated with 32P-labeled βRARE or the mutated βRARE and analyzed by a gel retardation assay. The arrowhead indicates specific RAR-RXR heterodimer binding. (C) Mutations of the βRARE impair the enhancing effect of COUP-TF on RARα activity in the RARβ promoter. Mutations in the βRARE of the RARβ promoter were introduced by PCR as described in Materials and Methods. The resulting RARβ promoter mutants (−745RARβCAT/βRARE/M1 and −745RARβCAT/βRARE/M2) were analyzed by transient transfection assay for the effect of COUP-TF. CV-1 cells were transfected with 700 ng of reporter gene together with the indicated amounts of expression vectors for COUP-TFII and RARα. Cells were then treated with or without all-trans-RA (10−6 M) and 24 h later were assayed for CAT activity. Data shown represent the means of three independent experiments.
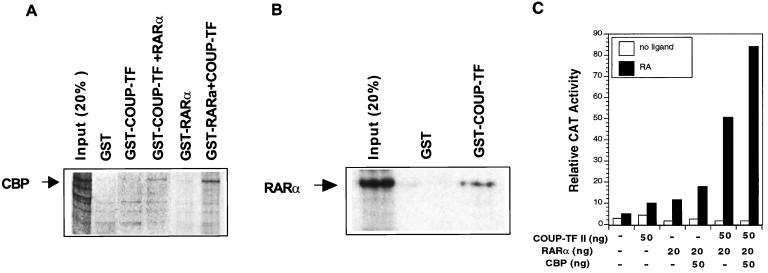
COUP-TF enhances recruitment of CBP by RARα.
The transactivation function of nuclear receptors requires their interaction with receptor coactivators (62), such as CBP (17). The transactivation function of COUP-TF could be due to its interaction with CBP. We therefore investigated whether COUP-TFI could interact with CBP by the GST pull-down assay. Under the conditions used, we did not observe a clear interaction between COUP-TFI and CBP (Fig. 12A), consistent with a previous report (44). This demonstrates that the transactivation function of COUP-TFI is unlikely to be mediated through its direct interaction with CBP. The facts that the transactivation function of COUP-TF is RARα and RA dependent and requires βRARE suggest that the effect of COUP-TF may be mediated by RARα, which is known to interact with CBP (17). We then incubated GST–COUP-TFI with CBP in the presence of in vitro-synthesized RARα. As shown in Fig. 12A, a significant amount of CBP was pulled down by the GST–COUP-TFI fusion protein when RARα was present. We also determined whether COUP-TF could enhance the interaction between RARα and CBP. As shown in Fig. 12A, CBP was slightly pulled down by the GST-RARα fusion protein. However, when GST-RARα was mixed with in vitro-synthesized COUP-TFI protein, a significant amount of CBP was pulled down. The observation that both RARα and COUP-TFI are required for their maximum interaction with CBP suggests that COUP-TFI may interact with RARα. Indeed, in vitro-synthesized RARα was efficiently pulled down by GST–COUP-TFI, but not by the GST control protein (Fig. 12B), demonstrating that COUP-TFI could directly interact with RARα. Thus, COUP-TF may facilitate the RARα-CBP interaction by interacting with RARα, resulting in a RARα conformation favorable for CBP interaction.
FIG. 12.
COUP-TF enhances recruitment of CBP by RARα. (A) COUP-TF enhances interaction of RAR and CBP. COUP-TFI or RARα protein was synthesized in bacteria with pGEX-2T (Pharmacia). The GST–COUP-TFI or GST-RARα fusion protein was immobilized on glutathione-Sepharose beads, while the same amount of GST was also immobilized on beads as a control. 35S-labeled CBP was then mixed with beads and in vitro-synthesized RARα (for GST–COUP-TFI) or in vitro-synthesized COUP-TFI (for GST-RARα) in the presence of 10−6 M all-trans-RA. After extensive washing, the bound proteins were analyzed by SDS-PAGE. The input proteins are shown for comparison. (B) COUP-TFI interacts with RARα. To analyze the interaction between COUP-TF and RARα, 35S-labeled RARα was mixed with GST–COUP-TFI fusion protein on beads. After extensive washing, the bound proteins were analyzed by SDS-PAGE. As a comparison, the input proteins are shown. (C) COUP-TF increases the effect of CBP on RARα activity. The indicated amounts of expression vector for RARα, COUP-TFII, and CBP were cotransfected with RARβ promoter into CV-1 cells. Cells were treated with or without all-trans-RA (10−6 M) for 24 h and then were assayed for CAT activity. The corresponding β-Gal activity was normalized as a control.
We next analyzed whether COUP-TF could influence the transcriptional effect of CBP on RARα activity in CV-1 cells. Cotransfection of the CBP expression vector slightly enhanced RARα transcriptional activity in the RARβ promoter. However, when COUP-TFII and RARα were cotransfected, the effect of CBP on RARβ promoter activity was greatly increased (Fig. 12C). Such an effect of COUP-TF on CBP activity, together with our GST pull-down results, strongly suggests that COUP-TF, through its binding to the DR-8 element and interacting with RARα, may function as a bridge protein to facilitate the interaction between βRARE binding receptors and CBP, resulting in an enhanced RA-dependent transcription of the RARβ promoter.
DISCUSSION
RARβ plays a crucial role in the mediating growth-inhibitory effect of retinoids, and lack of its expression contributes to retinoid resistance in many different types of cancer cells. Expression of RARβ is induced by RA through binding of RAR-RXR heterodimers to the βRARE in the RARβ promoter. However, expression of RAR and RXR in cancer cells is not sufficient to account for induction of RARβ by RA. Here, we provide convincing evidence that orphan receptor COUP-TF is required for RA to induce RARβ expression, growth inhibition, and apoptosis in cancer cells and that loss of COUP-TF expression is the main factor contributing to the lack of RARβ expression in cancer cells. In addition, we show that COUP-TF synergistically increases the RA-dependent RARα transactivation function in the RARβ promoter through its binding to a DR-8 element in the promoter, which enhances the interaction of RARα with its coactivator CBP.
Expression of COUP-TF is required for efficient RARβ induction by RA in cancer cells.
Several lines of evidence provided in this study demonstrate that expression of COUP-TF is required for efficient RARβ induction by RA in cancer cells. First, expression of COUP-TF is positively correlated with induction of RARβ by RA in breast cancer, lung cancer, and bladder cancer cell lines (Fig. 1). A perfect correlation was observed in breast cancer cell lines, while a close correlation was found in lung cancer and bladder cancer cell lines. This observation is consistent with previous studies (33, 47, 48) demonstrating that RARβ and COUP-TFII are coexpressed in motor neurons. In addition, our stable expression of COUP-TFI in COUP-TF-negative MDA-MB-231 breast cancer cells (Fig. 2) showed that expression of COUP-TFI could restore the ability of RA to induce RARβ expression. Furthermore, inhibition of COUP-TF expression by stable expression of COUP-TFI antisense RNA in COUP-TF-positive J82 cancer cells reduced the ability of RA to induce RARβ expression (Fig. 3). Finally, our transient transfection assays clearly demonstrated that COUP-TF could activate the RARβ promoter in response to RA when RARα was expressed (Fig. 5). Thus expression of COUP-TF is required for RA to effectively induce RARβ expression, and loss of COUP-TF in cancer cells may be one of the important mechanisms responsible for the lack of RARβ expression in cancer cells.
Effect of COUP-TF on growth inhibition and apoptosis induction by RA.
RA is known to inhibit the growth of and induce apoptosis in cancer cells, in part due to its induction of RARβ (27–29). Our observation that COUP-TF expression is required for RARβ induction by RA implies that COUP-TF is also involved in RA-induced growth inhibition and apoptosis signaling. This is clearly shown by our stable transfection of COUP-TFI in COUP-TF-negative MDA-MB231 cells (Fig. 2). MDA-MB231 breast cancer cells are RA resistant due to lack of RARβ induction by RA (29). When COUP-TFI was stably expressed in these cells, their growth was strongly inhibited by RA (Fig. 2C). Furthermore, the cells underwent extensive apoptosis in response to RA treatment (Fig. 2D). The observation that RARβ was strongly induced in the COUP-TFI stable clones (Fig. 2B) suggests that the observed growth inhibition and apoptosis induction are likely mediated by the induced RARβ expression. The involvement of COUP-TF in the regulation of RA-dependent growth inhibition and apoptosis induction is further supported by our stable expression of COUP-TFI antisense RNA in COUP-TF-positive J82 bladder cancer cells (Fig. 3). Expression of COUP-TFI antisense RNA in this RA-sensitive cell line dramatically reduced the ability of RA to induce growth inhibition (Fig. 3C) and apoptosis of the cells (Fig. 3D), which was accompanied by a reduced level of RARβ expression (Fig. 3B). Together, our results demonstrate that COUP-TF may play a critical role in the regulation of the growth-inhibitory effect of retinoids in cancer cells.
Transactivation function of COUP-TF requires both COUP-TF-RE and βRARE in the RARβ promoter.
By mutation analysis, we demonstrated that a DNA sequence comprising two AGGTCA-like core motifs arranged as a direct repeat with 8-bp spacing (the DR-8 element) is required for the COUP-TF effect. The DR-8 element bound strongly with COUP-TF (Fig. 7). Interestingly, the binding of COUP-TF did not repress the RARβ promoter. Instead, it is essential for the transactivation function of COUP-TF. Deletion of the sequence from the RARβ promoter completely abolished the effect of COUP-TF on RARβ promoter activity (Fig. 6). In addition, mutations in the DR-8 element that impaired the binding of COUP-TF reduced the COUP-TF effect on the enhancement of RARα activity (Fig. 8). The requirement of COUP-TF DNA binding is also supported by our COUP-TF mutational analysis showing that deletion of as few as 14 amino acid residues from the C-terminal end of COUP-TF abolished both its DNA binding and transactivation function (Fig. 9). Furthermore, COUP-TF with the DNA binding domain deleted was unable to enhance RARβ promoter activity (Fig. 9). Thus, unlike its binding to RAREs, which results in repression of RARE activity (3, 20, 53, 59), the binding of COUP-TF to the DR-8 element in the RARβ promoter is required for COUP-TF to enhance RA-dependent RARα activity. Interestingly, the binding of COUP-TF to a DR-7 element in the arrestin gene promoter could mediate the positive transcriptional effect of COUP-TF (32). Thus, whether COUP-TF exerts transactivation or transrepression function is largely dependent on the configuration of its DNA binding sequences.
RARE alone is sufficient to confer the repressive effect of COUP-TF on the element (3, 20, 53, 59). However, our results demonstrate that the DR-8 element in the RARβ promoter alone is not sufficient to confer the transactivation function of COUP-TF. When it was fused to the TK promoter, the DR-8 element could not be activated by COUP-TF in the presence or absence of RARα (Fig. 10). Thus, the effect of COUP-TF is specific to the RARβ promoter. By mutational analysis (Fig. 11), we demonstrated that the βRARE in the RARβ promoter is required for DR-8 to mediate the effect of COUP-TF. The observation that βRARE is required for the positive transactivation function of COUP-TF is interesting in light of the previous observation that COUP-TF is an effective negative regulator of RAREs (3, 20, 53, 59). Recently, Folkers et al. reported (7) that COUP-TF could inhibit the activity of βRARE and the RARβ promoter due to its binding to the element. However, the notion that COUP-TF acts as a negative regulator of the βRARE was based mainly on transient transfection assays where COUP-TF might be overexpressed. In fact, induction of βRARE and RARβ promoter activity was observed when a low concentration of COUP-TF was used (7). This is consistent with our previous study (61) showing that COUP-TF, at low concentrations, enhanced the RA sensitivity of the βRARE through its binding to the element, which prevents RA-independent activation of the βRARE. These observations demonstrate that COUP-TF at appropriate concentrations, which are likely to occur in most cells, does not act as a negative regulator of the βRARE and the RARβ promoter. This is well supported by observations that RARβ and COUP-TF are coexpressed in various cancer cell lines (Fig. 1) and motor neurons (33, 48). Thus our present data, together with our previous findings (61), demonstrate that COUP-TF plays a critical role in the regulation of RA-dependent RARβ expression by acting as an activator and sensitizer through its binding to COUP-TF-RE and βRARE, respectively.
COUP-TF acts as an accessory protein for RARα.
Results provided in this study demonstrate a new mechanism for positive gene regulation by COUP-TF. Unlike the effect of COUP-TF on vHNF1 (45), human immunodeficiency virus long terminal repeat (49), and NGFI-A (44), which does not require COUP-TF DNA binding, DNA binding of COUP-TF is essential for COUP-TF to positively regulate RARβ gene expression (Fig. 8 and 9). The positive transcriptional regulation observed in the NGFI-A gene is mediated through a direct transactivation function of COUP-TF through its recruitment of receptor coactivator SRC-1 (44). However, the effect of COUP-TF on the RARβ promoter is not mediated by a direct transactivation function of COUP-TF since cotransfection of COUP-TF could not activate transcription of the COUP-TF-RE-tk-CAT reporter (Fig. 10). Our observation that cotransfection of COUP-TF alone could not activate the RARβ promoter in HT-1376 cells (Fig. 5) due to the lack of RARα expression in the cells (data not shown) demonstrates that the transactivation function of COUP-TF in the RARβ promoter is likely mediated by RARα. This is further supported by our finding that positive gene regulation by COUP-TF was RARα dependent (Fig. 5). Indeed, COUP-TF did not show a clear interaction with the receptor coactivator CBP (Fig. 12), consistent with a previous report (44). However, COUP-TF strongly enhances the interaction between RARα and CBP (Fig. 12A). This may explain the enhancement of the transcriptional effect of CBP by COUP-TF on RA-dependent RARα activity (Fig. 12C). It is likely that COUP-TF, through its direct interaction with RARα (Fig. 12B), may induce a RARα conformation change that is more favorable for its interaction with CBP. Thus, COUP-TF functions as an accessory protein for RARα to activate the RARβ promoter. It may also be considered a member of the RARα coactivator complex on the RARβ promoter.
In summary, studies described here reveal a novel mechanism by which COUP-TF positively regulates RA-induced RARβ expression through its ability to interact with RARα and the DR-8 element in the RARβ promoter. The observation that the expression of COUP-TF is crucial for RARβ expression and anticancer activities of RA further enhances our understanding of the retinoid signaling in cancer cells. This becomes especially apparent since retinoid resistance is frequently observed in various types of cancer cells despite expression of functional retinoid receptors (19, 52, 68). Our finding that COUP-TF is not expressed in a majority of RA-resistant cancer cell lines (Fig. 1) suggests that the loss of COUP-TF may represent one of the important mechanisms for retinoid resistance in cancer cells. It also indicates that an understanding of how COUP-TF expression is lost may be crucial for understanding defects associated with retinoid resistance in cancer cells. The fact that loss of RARβ expression is an early event in breast carcinogenesis (60, 63) implies that COUP-TF may also play a role in cancer development.
ACKNOWLEDGMENTS
We thank L. Frazer for preparation of the manuscript and Q. Wu for technical assistance.
This work was in part supported by grants to X.-K.Z. from the National Institute of Health (CA60988 and CA51933), the Tobacco-Related Disease Research Program of California (6RT-0168), the California Breast Cancer Research Program (3PB-0018), and the U.S. Army Medical Research Program (DAMD17-4440).
REFERENCES
- 1.Berard J, Laboune F, Mukuna M, Masse S, Kothary R, Bradley W E C. Lung tumors in mice expressing an antisense RARβ2 transgene. FASEB J. 1996;10:1091–1097. doi: 10.1096/fasebj.10.9.8801172. [DOI] [PubMed] [Google Scholar]
- 2.Campbell M J, Park S, Uskokovic M R, Dawson M I, Koeffler H P. Expression of retinoic acid receptor-beta sensitizes prostate cancer cells to growth inhibition mediated by combinations of retinoids and a 19-nor hexafluoride vitamin D3 analog. Endocrinology. 1998;139:1972–1980. doi: 10.1210/endo.139.4.5943. [DOI] [PubMed] [Google Scholar]
- 3.Cooney A J, Tsai S Y, O'Malley B W, Tsai M-J. Chicken ovalbumin upstream promoter transcription factor (COUP-TF) dimers bind to different GGTCA response elements, allowing COUP-TF to repress hormonal induction of the vitamin D3, thyroid hormone, and retinoic acid receptors. Mol Cell Biol. 1992;12:4153–4163. doi: 10.1128/mcb.12.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejean A, Bougueleret L, Grzeschik K-H, Tiollais P. Hepatitis B virus DNA integration in a sequence homologous to c-erb-A and steroid receptor genes in a hepatocellular carcinoma. Nature. 1991;322:70–72. doi: 10.1038/322070a0. [DOI] [PubMed] [Google Scholar]
- 5.de The H, Vivanco-Ruiz M M, Tiollais P, Stunnenberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor β gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari N, Pfahl M, Levi G. Retinoic acid receptor gamma1 (RARγ1) levels control RARβ2 expression in SK-N-BE2(c) neuroblastoma cells and regulate a differentiation-apoptosis switch. Mol Cell Biol. 1998;18:6482–6492. doi: 10.1128/mcb.18.11.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folkers G E, van der Burg B, van der Saag P T. Promoter architecture, cofactors, and orphan receptors contribute to cell-specific activation of the retinoic acid receptor beta2 promoter. J Biol Chem. 1998;273:32200–32212. doi: 10.1074/jbc.273.48.32200. [DOI] [PubMed] [Google Scholar]
- 8.Gebert J F, Moghal N, Frangioni J V, Sugarbaker D J, Neel B G. High frequency of retinoic acid receptor β abnormalities in human lung cancer. Oncogene. 1991;6:1859–1868. [PubMed] [Google Scholar]
- 9.Gudas L J, Sporn M B, Roberts A B. Cellular biology and biochemistry of the retinoids. In: Sporn M B, Roberts A B, Goodman D S, editors. The retinoids. 2nd ed. New York, N.Y: Raven Press; 1994. pp. 443–520. [Google Scholar]
- 10.Hall R K, Sladek F M, Granner D K. The orphan receptors COUP-TF and HNF-4 serve as accessory factors required for induction of phosphoenolpyruvate carboxykinase gene transcription by glucocorticoids. Proc Natl Acad Sci USA. 1995;92:412–416. doi: 10.1073/pnas.92.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman A D, Engelstein D, Bogenrieder T, Papandreou C N, Stecklman E, Dave A, Motzer R J, Dmitrovsky E, Albino A P, Nanus D M. Expression of retinoic acid receptor beta in human renal cell carcinomas correlates with sensitivity to the antiproliferative effects of 13-cis-retinoic acid. Clin Cancer Res. 1996;2:1077–1082. [PubMed] [Google Scholar]
- 12.Hoffmann B, Lehmann J M, Zhang X-K, Hermann T, Graupner G, Pfahl M. A retinoic acid receptor-specific element controls the retinoic acid receptor-β promoter. Mol Endocrinol. 1990;4:1727–1736. doi: 10.1210/mend-4-11-1727. [DOI] [PubMed] [Google Scholar]
- 13.Hong W K, Itri L M. Retinoids and human cancer. In: Sporn M B, Roberts A B, Goodman D S, editors. The retinoids. 2nd ed. New York, N.Y: Raven Press; 1994. pp. 597–630. [Google Scholar]
- 14.Houle B, Ledue F, Bradley W E C. Implication of RARβ in epidermoid (squamous) carcinoma cells. Exp Cell Res. 1991;195:163–170. [Google Scholar]
- 15.Hu L, Crowe D L, Rheinwald J G, Chambon P, Gudas L. Abnormal expression of retinoic acid receptors and keratin 19 by human oral and epidermal squamous cell carcinoma cell lines. Cancer Res. 1991;51:3972–3981. [PubMed] [Google Scholar]
- 16.Kaiser A, Herbst H, Fisher G, Koenogsmann M, Berdel W E, Riecken E O, Rosewicz S. Retinoic acid receptor beta regulates growth and differentiation in human pancreatic carcinoma cells. Gastroenterology. 1997;113:920–929. doi: 10.1016/s0016-5085(97)70188-4. [DOI] [PubMed] [Google Scholar]
- 17.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S-C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 18.Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83:859–869. doi: 10.1016/0092-8674(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y-H, Dohi D F, Han G R, Zou C-P, Oridate N, Walsh G L, Nesbitt J C, Xu X-C, Hong W K, Lotan L, Kurie J M. Retinoid refractoriness occurs during lung carcinogenesis despite functional retinoid receptors. Cancer Res. 1995;55:5603–5610. [PubMed] [Google Scholar]
- 20.Kliewer S A, Kazuhiko U, Heyman R A, Mangelsdorf D J, Dyck J A, Evans R M. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci USA. 1992;89:1448–1452. doi: 10.1073/pnas.89.4.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kok K, Osinga J, Carritt B, Davis M B, van der Hout A H, van der Veen A Y, Landsvater R M, de Leij L F M H, Berendsen H H, Fostmus P E, Poppema S, Buys C H C M. Deletion of a DNA sequence at the chromosomal region 3p21 in all major types of lung cancer. Nature. 1987;330:578–581. doi: 10.1038/330578a0. [DOI] [PubMed] [Google Scholar]
- 22.Ladias J A, Karathanasis S K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991;251:561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- 23.Lazennec G, Kern L, Valotaire Y, Salbert G. The nuclear orphan receptors COUP-TF and ARP-1 positively regulate the trout estrogen receptor gene through enhancing autoregulation. Mol Cell Biol. 1997;17:5053–5066. doi: 10.1128/mcb.17.9.5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee M-O, Liu Y, Zhang X-K. A retinoic acid response element that overlaps an estrogen response element mediates multihormonal sensitivity in transcriptional activation of the lactoferrin gene. Mol Cell Biol. 1995;15:4194–4207. doi: 10.1128/mcb.15.8.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leng X, Cooney J, Tsai S Y, Tsai M-J. Molecular mechanisms of COUP-TF-mediated transcriptional repression: evidence for transrepression and active repression. Mol Cell Biol. 1996;16:2332–2340. doi: 10.1128/mcb.16.5.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Wan Y J. Differentiation and antiproliferation effects of retinoic acid receptor beta in hepatoma cells. Cancer Lett. 1998;124:205–211. doi: 10.1016/s0304-3835(97)00475-8. [DOI] [PubMed] [Google Scholar]
- 27.Li X-S, Shao Z-M, Shekh M H, Eiseman J L, Sentz D, Jetten A M, Chen J-H, Dawson M I, Aisner S, Rishi A K, Gutierrez P, Schnapper L, Fontana J A. Retinoic acid nuclear receptor β inhibits breast carcinoma anchorage independent growth. J Cell Physiol. 1995;165:449–458. doi: 10.1002/jcp.1041650302. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Dawson M I, Agadir A, Lee M O, Jong L, Hobbs P D, Zhang X-K. Regulation of RARβ expression by RAR- and RXR-selective retinoids in human lung cancer cell lines: effect on growth inhibition and apoptosis induction. Int J Cancer. 1998;75:88–95. doi: 10.1002/(sici)1097-0215(19980105)75:1<88::aid-ijc14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Lee M-O, Wang H-G, Li Y, Hashimoto Y, Klaus M, Reed J C, Zhang X-K. Retinoic acid receptor β mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol Cell Biol. 1996;16:1138–1149. doi: 10.1128/mcb.16.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotan R. Effect of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1981;605:33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- 31.Lotan R, Xu X-C, Lippman S M, Ro J Y, Lee J S, Lee J J, Hong W K. Suppression of retinoic acid receptor-β in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332:1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- 32.Lu X P, Salbert G, Pfahl M. An evolutionary conserved COUP-TF binding element in a neural-specific gene and COUP-TF expression patterns support a major role for COUP-TF in neural development. Mol Endocrinol. 1994;8:1774–1788. doi: 10.1210/mend.8.12.7708064. [DOI] [PubMed] [Google Scholar]
- 33.Lutz B, Kuratani S, Cooney A J, Wawersik S, Tsai S Y, Eichele G, Tsai M-J. Developmental regulation of the orphan receptor COUP-TFII gene in spinal motor neurons. Development. 1994;120:25–36. doi: 10.1242/dev.120.1.25. [DOI] [PubMed] [Google Scholar]
- 34.Mangelsdorf D J, Evans R M. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 35.Minna J D, Mangelsdorf D J. Retinoic acid receptor expression abnormalities in lung cancer: important clues or major obstacles? J Natl Cancer Inst. 1997;89:602–604. doi: 10.1093/jnci/89.9.602. [DOI] [PubMed] [Google Scholar]
- 36.Miyajima N, Kadowaki Y, Fukushige S, Shimizu S, Semba K, Yamanashi Y, Matsubara K, Toyoshima K, Yamamoto T. Identification of two novel members of erb A superfamily by molecular cloning: the gene products of the two are highly related to each other. Nucleic Acids Res. 1988;16:11057–11074. doi: 10.1093/nar/16.23.11057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moghal N, Neel B G. Evidence for impaired retinoic acid receptor-thyroid hormone receptor AF-2 cofactor activity in human lung cancer. Mol Cell Biol. 1995;15:3945–3959. doi: 10.1128/mcb.15.7.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naylor S L, Johoson B E, Minna J D, Sakaguchi A Y. Loss of heterozygosity of chromosome 3p markers in small cell lung cancer. Nature. 1987;329:451–453. doi: 10.1038/329451a0. [DOI] [PubMed] [Google Scholar]
- 39.Nervi C, Volleberg T M, George M D, Zelent A, Chambon P, Jetten A M. Expression of nuclear retinoic acid receptors in normal tracheobronchial cells and in lung carcinoma cells. Exp Cell Res. 1991;195:163–170. doi: 10.1016/0014-4827(91)90512-s. [DOI] [PubMed] [Google Scholar]
- 40.Pastorcic M, Wang H, Elbrecht A, Tsai S Y, Tsai M-J, O'Malley B W. Control of transcription initiation in vitro requires binding of a transcriptional factor to the distal promoter of the ovalbumin gene. Mol Cell Biol. 1986;6:2784–2791. doi: 10.1128/mcb.6.8.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira F A, Qiu Y, Zhou G, Tsai M J, Tsai S Y. The orphan nuclear receptor COUP-TFII is required for angiogenesis and heart development. Genes Dev. 1999;13:1037–1049. doi: 10.1101/gad.13.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pergolizzi R, Appierto V, Crosti M, Cavadini E, Cleris L, Guffanti A, Formelli F. Role of retinoic acid receptor overexpression in sensitivity to fenretinide and tumorigenicity of human ovarian carcinoma cells. Int J Cancer. 1999;81:829–834. doi: 10.1002/(sici)1097-0215(19990531)81:5<829::aid-ijc26>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Picard E, Seguin C, Monhoven N, Rochette-Egly C, Siat J, Borrelly J, Martinet Y, Martinet N, Vignaud J M. Expression of retinoid receptor genes and protein in non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:1059–1066. doi: 10.1093/jnci/91.12.1059. [DOI] [PubMed] [Google Scholar]
- 44.Pipaón C, Tsai S Y, Tsai M-J. COUP-TF upregulates NGFI-A gene expression through an Sp1 binding site. Mol Cell Biol. 1999;19:2734–2745. doi: 10.1128/mcb.19.4.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Power S C, Cereghini S. Positive regulation of the vHNF1 promoter by the orphan receptors COUP-TF1/Ear3 and COUP-TFII/Arp1. Mol Cell Biol. 1996;16:778–791. doi: 10.1128/mcb.16.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu Y, Pereira F A, DeMayo F J, Lydon J P, Tsai S Y, Tsai M J. Null mutation of mCOUP-TFI results in defects in morphogenesis of the glossopharyngeal ganglion, axonal projection, and arborization. Genes Dev. 1997;11:1925–1937. doi: 10.1101/gad.11.15.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu Y, Tsai S Y, Tsai M-J. COUP-TF. An orphan member of the steroid/thyroid hormone receptor superfamily. Trends Endocrinol Metab. 1994;5:234–239. doi: 10.1016/1043-2760(94)p3081-h. [DOI] [PubMed] [Google Scholar]
- 48.Reuberte E, Friederich V, Chambon P, Morriss-Kay G. Retinoic acid receptors and cellular retinoid binding proteins. III. Their differential transcript distribution during mouse nervous system development. Development. 1993;118:267–282. doi: 10.1242/dev.118.1.267. [DOI] [PubMed] [Google Scholar]
- 49.Rohr O, Aunis D, Schaeffer E. COUP-TF and Sp1 interact and cooperate in the transcriptional activation of the human immunodeficiency virus type 1 long terminal repeat in human microglial cells. J Biol Chem. 1997;272:31149–31155. doi: 10.1074/jbc.272.49.31149. [DOI] [PubMed] [Google Scholar]
- 50.Shin D M, Xu X C, Lippman S M, Lee J S, Batsakis J G, Ro J Y, Martin J W, Hittelman W N, Lotan R, Hong W K. Accumulation of P53 protein and retinoic acid receptor beta in retinoid chemoprevention. Clin Cancer Res. 1997;3:875–880. [PubMed] [Google Scholar]
- 51.Sucov H M, Murakami K K, Evans R M. Characterization of an autoregulated response element in the mouse retinoic acid receptor type β gene. Proc Natl Acad Sci USA. 1990;87:5392–5396. doi: 10.1073/pnas.87.14.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swisshelm K, Ryan K, Lee X, Taou H C, Peacocke M, Sager R. Down-regulation of retinoic acid receptor β in mammary carcinoma cell lines and its up-regulation in senescing normal mammary epithelial cells. Cell Growth Differ. 1994;5:133–141. [PubMed] [Google Scholar]
- 53.Tran P, Zhang X-K, Salbert G, Hermann T, Lehmann J M, Pfahl M. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol. 1992;12:4666–4676. doi: 10.1128/mcb.12.10.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valcarcel R, Holz H, Jimenez C G, Barettino D, Stunnenberg H G. Retinoid-dependent in vitro transcription mediated by the RXR/RAR heterodimer. Genes Dev. 1994;8:3068–3079. doi: 10.1101/gad.8.24.3068. [DOI] [PubMed] [Google Scholar]
- 55.van der Leede B M, van den Brink C E, van der Saag P T. Retinoic acid receptor and retinoid X receptor expression in retinoic acid-resistant human tumor cell lines. Mol Carcinog. 1993;8:112–122. doi: 10.1002/mc.2940080208. [DOI] [PubMed] [Google Scholar]
- 56.Wang L-H, Tsai S Y, Cook R G, Beattie W G, Tsai M-J, O'Malley B W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989;340:163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- 57.Wang X D, Liu C, Bronson R T, Smith D E, Krinsky N I, Russel M. Retinoid signaling and activator protein-1 expression in ferrets given beta-carotene supplements and tobacco smoke. J Natl Cancer Inst. 1999;91:60–66. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]
- 58.Warrell R P, Jr, De The H, Wang Z-Y, Degos L. Acute promyelocytic leukemia. N Eng J Med. 1993;329:177–189. doi: 10.1056/NEJM199307153290307. [DOI] [PubMed] [Google Scholar]
- 59.Widom R L, Rhee M, Karathansis S K. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXRα and retinoic acid. Mol Cell Biol. 1992;12:3380–3389. doi: 10.1128/mcb.12.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Widschwendter M, Berger J, Daxenbichler E, Muller-Holzner A, Widschwendter A, Mayr C, Marth C, Zeimet A G. Loss of retinoic acid receptor expression in breast cancer and morphologically normal adjacent tissue but not in the normal breast tissue distant from the cancer. Cancer Res. 1997;57:4158–4161. [PubMed] [Google Scholar]
- 61.Wu Q, Li Y, Liu R, Agadir A, Lee M-O, Liu Y, Zhang X-K. Modulation of retinoic acid sensitivity in lung cancer cells through dynamic balance of orphan receptors nur77 and COUP-TF and their heterodimerization. EMBO J. 1997;16:1656–1669. doi: 10.1093/emboj/16.7.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu L, Glass C K, Rosenfeld M G. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 63.Xu X-C, Sneige N, Liu X, Nandagiri R, Lee J J, Lukmanji F, Hortoobagyi G, Lippman S M, Dhingra K, Lotan R. A progressive decrease in nuclear retinoic acid receptor acid receptor β message RNA levels during breast carcinogenesis. Cancer Res. 1997;57:4992–4996. [PubMed] [Google Scholar]
- 64.Xu X-C, Sozzi G, Lee J S, Lee J J, Pastorino S P, Kurie J M, Hong W K, Lotan R. Suppression of retinoic acid receptor β in non-small-cell lung cancer in vivo: implications for lung cancer development. J Natl Cancer Inst. 1996;89:624–629. doi: 10.1093/jnci/89.9.624. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X-K, Hoffmann B, Tran P, Graupner G, Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992a;355:441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- 66.Zhang X-K, Lehmann J, Hoffmann B, Dawson M, Cameron J, Graupner G, Hermann T, Tran P, Pfahl M. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature. 1992b;358:587–591. doi: 10.1038/358587a0. [DOI] [PubMed] [Google Scholar]
- 67.Zhang X-K, Pfahl M. Regulation of retinoid and thyroid hormone action through homodimeric and heterodimeric receptors. Trends Endocrinol Metab. 1993;4:156–162. doi: 10.1016/1043-2760(93)90105-n. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X-K, Liu Y, Lee M-O, Pfahl M. A specific defect in the retinoic acid receptor associated with human lung cancer cell lines. Cancer Res. 1994;54:5663–5669. [PubMed] [Google Scholar]
- 69.Zhang X-K, Liu Y, Lee M-O. Retinoid receptors in human lung cancer and breast cancer. Mutation Res. 1996;350:267–277. doi: 10.1016/0027-5107(95)00102-6. [DOI] [PubMed] [Google Scholar]
- 70.Zou C P, Hong W K, Lotan R. Expression of retinoic acid receptor beta is associated with inhibition of keratinization in human head and neck squamous carcinoma cells. Differentiation. 1999;64:123–132. doi: 10.1046/j.1432-0436.1999.6420123.x. [DOI] [PubMed] [Google Scholar]