Abstract
Combating the COVID-19 pandemic has raised the demand for and disposal of personal protective equipment in the United States. This work proposes a novel waste personal protective equipment processing system that enables energy recovery through producing renewable fuels and other basic chemicals. Exergy analysis and environmental assessment through a detailed life cycle assessment approach are performed to evaluate the energy and environmental sustainability of the processing system. Given the environmental advantages in reducing 35.42% of total greenhouse gas emissions from the conventional incineration and 43.50% of total fossil fuel use from landfilling processes, the optimal number, sizes, and locations of establishing facilities within the proposed personal protective equipment processing system in New York State are then determined by an optimization-based site selection methodology, proposing to build two pre-processing facilities in New York County and Suffolk County and one integrated fast pyrolysis plant in Rockland County. Their optimal annual treatment capacities are 1,708 t/y, 8,000 t/y, and 9,028 t/y. The proposed optimal personal protective equipment processing system reduces 31.5% of total fossil fuel use and 35.04% of total greenhouse gas emissions compared to the personal protective equipment incineration process. It also avoids 41.52% and 47.64% of total natural land occupation from the personal protective equipment landfilling and incineration processes.
Keywords: PPE processing system, Process design, Life cycle assessment, Techno-economic analysis, GHG emissions, Fossil fuel reduction
Abbreviations: CAPEX, Capital expenditure; GAO US, Government Accountability Office; GHG, Greenhouse gas; GWP, Global warming potential; HEPA, High-Efficiency Particulate Arrestance; HEX, Heat exchangers; HP, High-pressure steam; LCA, Life cycle assessment; LCI, Life cycle inventory; LP, Low-pressure steam; MEA, Monoethanolamine; MILP, Mixed-integer linear programming; MINLP, Mixed-integer nonlinear programming; MP, Mid-pressure steam; MSDS, Material Safety Data Sheet; NMVOC, Non-methane volatile organic compound; NPV, Net present value; NYS, New York State; O&M, Operation and maintenance cost; OPEX, Operating expenditure; PSA, Pressure-swing adsorption; PPE, Personal protective equipment; SD, Solid waste disposal fee MUSD; TEA, Techno-economic analysis; fec, Feedstock cost MUSD; inc, revenue from downstream products MUSD; obj, Annualized cost MUSD; omc, Operation and maintenance cost MUSD; stor, The total storage cost MUSD; tci, Total capital cost MUSD; tran, Total transportation cost MUSD; uc, Total utility cost MUSD
1. Introduction
1.1. Foreword
A surge of COVID-19 pandemic in the US has put new strains on the supply of personal protective equipment (PPE) [1]. While coronavirus prompts medical centres to find ways to reuse PPE amid shortages [2], the demand continues to run ahead of the total supply. Various companies, including 3 M Corporation, ramp up their PPE production to handle this shortage [3]. Typical PPE, including face shields, surgical masks, surgical gowns, N95 respirators [4], and surgical gloves, are mainly made from plastics [5]. Notably, their monomeric ingredients, such as propylene, are produced from non-renewable fossil resources, including crude oil or shale gas [6]. During the production of these compounds, large amounts of electricity and heating energy generated from fossil fuels are consumed to maintain operating conditions. In this regard, large-scale production of PPE can lead to high consumption of fossil fuels and resources that contributes to fossil fuel depletion.
Due to the low recyclability of plastic compounds [7], mismanaged PPE in landfill or disposal processes can lead to severe plastic pollution [8,9] and can damage the niche from soil [10] to marine ecosystems [11]. Viruses on waste PPE can also pose a threat to public health [12]. These concerns about plastic pollution and viral infection can be overcome by employing an incineration process to treat waste PPE and kill the virus at high-temperature conditions [13]. Given the high calorific value of plastic compounds, combusting waste PPE can produce high-temperature energy to generate electricity [14] and mitigates fossil fuel depletion [15]. This process can also emit large amounts of greenhouse gases (GHGs), contributing to climate change [16]. A viable strategy is to propose a waste PPE treatment method with low GHG emissions and fossil fuel depletion alleviation.
1.2. Literature review
Effective treatment and environmental sustainability are two vital issues to be addressed when designing the waste PPE processing system. An effective PPE conversion can tackle the technical challenge of high treatment capacity caused by the increasing PPE production [5]. Plastic recycling processes can effectively treat the waste PPE due to their similar compositions [8].
Current investigations on plastic recycling processes are categorized into mechanical and chemical pathways [17]. The mechanical recycling process that aims to produce virgin plastics can hardly handle plastic compound mixture within waste PPE [18] because their recycling efficiency is highly dependent on the purity of waste plastic [19]. On the other hand, chemical recycling processes are more versatile in treating complex plastic mixture [20] and relevant waste, such as electronic waste [21] and waste plastics from American Plastic Council [22]. Among chemical recycling technologies [23], thermal degradation technologies, including pyrolysis [24], are technically viable in treating waste plastics, including polyethylene [25] and their mixture [26] to produce clean fuels [27], such as pyrolysis oil [28]. Fast and thermal plasma pyrolysis were investigated in existing studies by showing high conversions of plastic waste and medical waste treatment [29]. The disadvantage of the thermal plasma pyrolysis is the high energy use and low yield of value-added pyrolysis oil [30] compared to the fast pyrolysis process when treating plastic compounds and their mixture. Fast pyrolysis, on the other hand, could produce a higher yield of hydrocarbon products involving pyrolysis oil than other chemical recycling processes [31]. If applied in treating waste PPE, pyrolysis may have high economic viability and technical feasibility, as proven by experimental studies of pyrolyzing respirators (hydrocarbon yield: 40%) [32] and gloves (pyrolysis oil yield: 35%) [33]. However, the environmental performance of pyrolyzing PPE should also be evaluated and quantified despite the proposed economic potentials in the waste PPE pyrolysis.
The life cycle assessment (LCA) approach [34], which was widely applied to medical waste management and processing [35], is a powerful tool to systematically evaluate and quantify the environmental performance of waste PPE processing. Various life cycle impact assessment (LCIA) methods, including the Eco – indicator 99 used for evaluating the environmental performances of cascade absorption-compression refrigeration [36] and fuel cells [37], are applied in the LCA for interpreting the detailed life cycle inventory data into quantified life cycle assessment results. There are some relevant studies [38] before the COVID-19 pandemic on quantifying the environmental performance of PPE disinfection pathways through various life cycle inventory analysis (LCIA) methods corresponding to GHG emissions and fossil fuel depletion [39]. However, they did not account for a complete assessment of life-cycle environmental COVID-19 pandemic impacts from waste PPE due to the absence of PPE manufacturing in these LCA studies.
Kumar et al. [39] recently incorporated pandemic-related PPE manufacturing within the system boundary to evaluate the environmental performances of waste PPE incineration and landfill processes. Their environmental sustainability is hampered by the high GHG emissions and human toxicity. Pyrolysis technology, on the other hand, could lead to lower GHG emissions [40] than the incineration process and enabled fossil fuel depletion alleviation by manufacturing hydrocarbon products to reduce fossil fuel use [41]. Yet, there are very few technology designs and corresponding LCA results of waste PPE processing systems based on the pyrolysis technology.
1.3. Research challenges and proposed innovations
The reviewed studies did not account for the environmentally sustainable waste PPE processing system and corresponding LCA, which is the knowledge gap to fill in this work. Several research challenges need to be addressed. The first one is to design a PPE processing system with safe operations and effective PPE conversion for energy recovery. The viral infection of waste PPE is reduced through sterilization in the pre-processing facility. The waste PPE are converted into various hydrocarbon products for energy recovery through fast pyrolysis and different downstream processing units. GHG emissions are minimized via cutting the flue gas emissions and avoiding fossil fuel use from generating energy offsite. The second challenge is to build life cycle inventories (LCIs) used in the LCA on the proposed PPE processing systems. Mass and energy balance relationships are regarded as the primary sources of LCA on proposed PPE processing systems. Critical parameters of these LCIs, such as the utility usage, are absent in the literature and can be obtained by performing rigorous process simulations. The third challenge is to develop an optimization model to determine the number, sizes, and locations of establishing the facilities within the proposed optimal PPE processing system [42].
This work presents a novel waste PPE processing system to effectively treat PPE for energy recovery with low GHG emissions and fossil fuel depletion alleviation. The processing system integrates the pre-processing facility [43] and eleven downstream unit processes to produce disinfected PPE particles and convert them into various value-added products. These products are then supplied as renewable fuels and basic chemicals for refineries, chemical plants, and metallurgy plants to reduce fossil fuel use. A detailed LCA is performed to evaluate the environmental sustainability of the proposed PPE processing system in terms of GHG emissions, fossil fuel depletion, and other environmental impacts. Detailed LCIs used in this LCA are built through compiling high-fidelity process simulation results and Ecoinvent V3.7 [44] database. Economic performances of the proposed integrated PPE processing system, the PPE incineration process, and the PPE landfill process are calculated through the techno-economic analysis (TEA) [42]. Two case studies are considered for illustrating environmental advantages and determining the optimal number, sizes, and locations of establishing the facilities within the proposed PPE processing system. The proposed PPE processing system, PPE incineration process, and PPE landfill process aim to treat the waste PPE with an annual treatment capacity estimated based on the US Government Accountability Office (GAO) report of the 90-day PPE storage amount starting from July 30, 2020 [45]. In the first case study, the environmental performances of the proposed integrated PPE processing system, PPE incineration process, and PPE landfill process are compared to illustrate the environmental advantages of establishing the proposed PPE processing system in terms of environmental sustainability. Given these environmental advantages, a mixed-integer nonlinear programming (MINLP) model that considers PPE processing pathways, as well as waste PPE and product distributions in counties, is developed for site selection to minimize the annualized cost of the proposed PPE processing system. The environmental performances of the proposed optimal PPE processing system, incineration process, and landfill process are compared to demonstrate the environmental advantages of the proposed optimal PPE processing system.
The remaining sections are organized as follows: The design and analysis of the waste PPE processing system, the proposed design of the PPE processing system, the LCA and TEA methodologies, as well as the optimization model for site selection are presented in Section 2. The environmental advantages of establishing the proposed optimal PPE processing system in NYS are illustrated in Section 3. By presenting all the above sections and corresponding analyses, this work proposes an efficient process design of the proposed PPE processing system and evaluates its energy use and environmental advantages over PPE landfill and incineration processes in terms of climate change alleviation fossil fuel use reduction, and natural land preservation.
2. Methodology
2.1. Process design and analysis of the waste PPE processing system
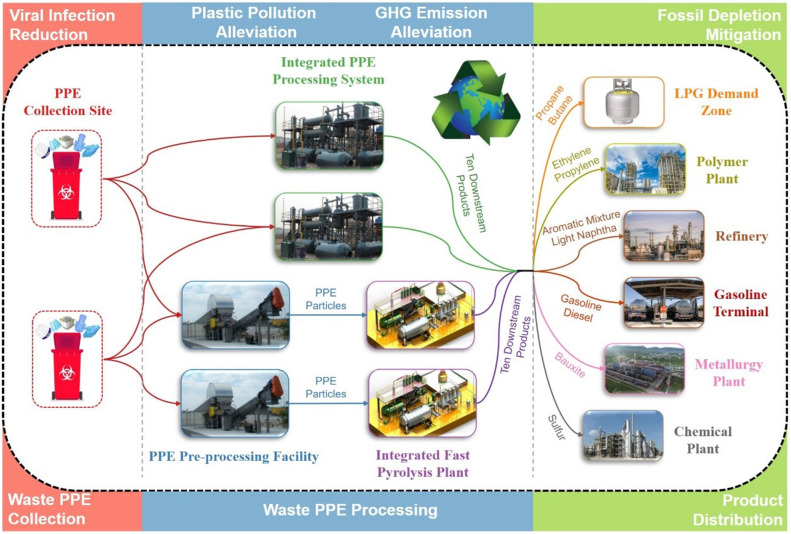
This work develops an advanced waste PPE processing system and analyzes its potential in mitigating plastic pollution with low GHG emissions and fossil fuel depletion alleviation. Two processing pathways shown in Fig. 1 , namely centralized and distributed-centralized pathways, are employed to treat the waste PPE. In the centralized pathway, the waste PPE collected from counties are directly shipped to the proposed integrated PPE processing system for treatment. In the distributed-centralized pathway, the waste PPE are firstly collected and transported to pre-processing facilities distributed in various counties for sterilization. All processed PPE from these pre-processed facilities are shipped to a centralized, integrated fast pyrolysis plant for PPE conversion. Both processing pathways can manufacture ten downstream products that are shipped and sold to refineries, chemical plants, and metallurgy plants as renewable fuels and basic chemicals.
Fig. 1.
The network structure of the waste PPE processing system includes two PPE processing pathways, namely the centralized pathway and the distributed-centralized pathway.
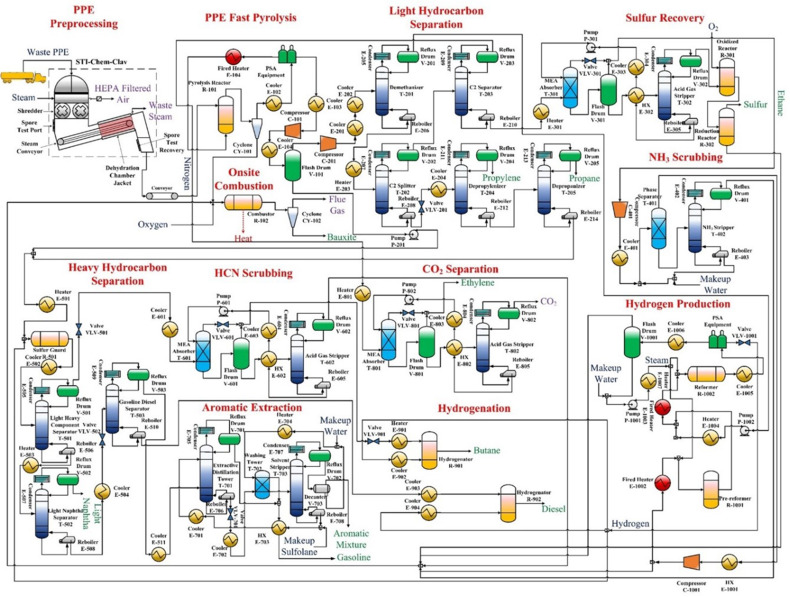
The process flowsheet of the integrated PPE processing system is shown in Fig. 2 . Compared to the integrated PPE processing system, the integrated fast pyrolysis plant includes all processing sections except for the pre-processing facility. Heating utilities, including low-pressure steam (LP), mid-pressure steam (MP), high-pressure steam (HP), fired heat, and cooling water (CW), are heat integrated to reduce GHG emissions and fossil fuel use from offsite energy production. The cooling water can be treated through equalization, anaerobic digestion, and aerobic digestion processes and regenerated in the cooling tower with an efficiency of 97.89% [46].
Fig. 2.
Process flowsheet of the proposed integrated PPE processing system.
The waste PPE starts being processed in a pre-processing facility to reduce the viral infection on the PPE and produce PPE particles used in the downstream fast pyrolysis process. The STI-Chem-Clav is applied to disinfect the waste PPE and shred, sterilize, and dehydrate it into particles in insulated, high temperature, and automatic operating conditions [47]. The pre-processed PPE particles are then transported externally to the integrated fast pyrolysis plant or directly conveyed to the fast pyrolizer within the proposed integrated PPE processing system to be thermally cracked into gaseous products and char [48]. The precooled gaseous products separated from a cyclone are treated by the PSA unit while the char is fed into the combustor to generate high-temperature heating energy. The remaining stream is split into a light stream to be fed into the light component separation section to produce propane and propylene and a heavy stream to produce C4+ hydrocarbons. Both manufacturing processes avoid the offsite production of these monomeric basic chemicals, and the fossil fuel use for energy generation in this offsite production can be reduced.
In the light component separation section, the light stream from PPE fast pyrolysis section produces methane in −118 °C under 2 MPa in the demethanizer. Its overhead gas and the raffinate are sent to the C2 separator and the C2 splitter. The raffinate stream of the C2 splitter is separated into propylene in 26 °C under 1.2 MPa from the depropylenizer, propane in 31 °C under 1.2 MPa, and heavy streams in 139 °C under 1.2 MPa from the depropanizer. The CO2 stream is split from the ethylene stream in the CO2 separation section, while the ethylene stream is precooled and pressurized into liquid before transportation. The hydrogen sulfide within the ethane stream, which can deactivate hydrotreating catalysts [49], requires to be separated and oxidized via the sulfur recovery section. The separated H2S is treated in the oxidization reactor in 1,000 °C under 100 kPa and reduction in 220 °C under 100 kPa reactors to produce sulfur [50]. The output ethane stream is then fed to the NH3 scrubbing processing section to split ammonia further, and the split NH3 in 117.5 °C under 200 kPa is fed into the combustor.
The heavy hydrocarbon separation section can produce light naphtha from a mixture of heavy streams in the PPE fast pyrolysis section and depropanizer. At starters, the organic sulfur compounds within the heavy stream mixture are converted in the sulfur guard at 350 °C under 760 kPa into hydrocarbons by Raney Ni catalyst [51] to avoid its deactivation to the hydrogenation catalyst [52]. The output stream is fed into the light-heavy component separator in 80 °C under 760 kPa to separate the C4 stream in 61.3 °C under 760 kPa, while the raffinate in 141.8 °C under 760 kPa is split into light naphtha in 111.7 °C under 760 kPa in the light naphtha separator in 145.7 °C under 760 kPa. The HCN within the C4 stream is separated via the HCN scrubbing section to protect human health. Meanwhile, the raffinate from the light naphtha separator in 237.3 °C under 760 kPa is then sent to the gasoline diesel separator in 136.7 °C under 100 kPa to separate gasoline compounds in 115.6 °C under 100 kPa and diesel compounds in 150 °C under 100 kPa. The gasoline compounds are then fed into the liquid-liquid extractive distillation-based aromatic extraction section to produce the aromatic blend [53]. The aromatic mix is supplied to the refinery as an aromatic ingredient, which can avoid the offsite production of the aromatic blend. The diesel compounds and treated C4 stream is mixed with preheated hydrogen and fed into the hydrogenator at 150 °C under 100 kPa to be converted by the NiMo catalyst [54]. The use of hydrogen in this section is produced from the hydrogen production section, where the methane is converted to avoid its direct GHG emissions [55].
All the waste flow is mixed with the char and other unconverted chemicals and then sent to the combustor. Direct emissions, such as unconverted methane as the GHG, HCN as the poisonous pollutant, and NH3 as the air pollutant, can be avoided in the onsite combustion section. This section can also produce heat to maintain the high-temperature operating conditions within the proposed integrated PPE processing system, which reduces the offsite heat production from fossil fuels, including natural gas. The solid phase is sold as a bauxite product to metallurgy sites.
2.2. LCA approach
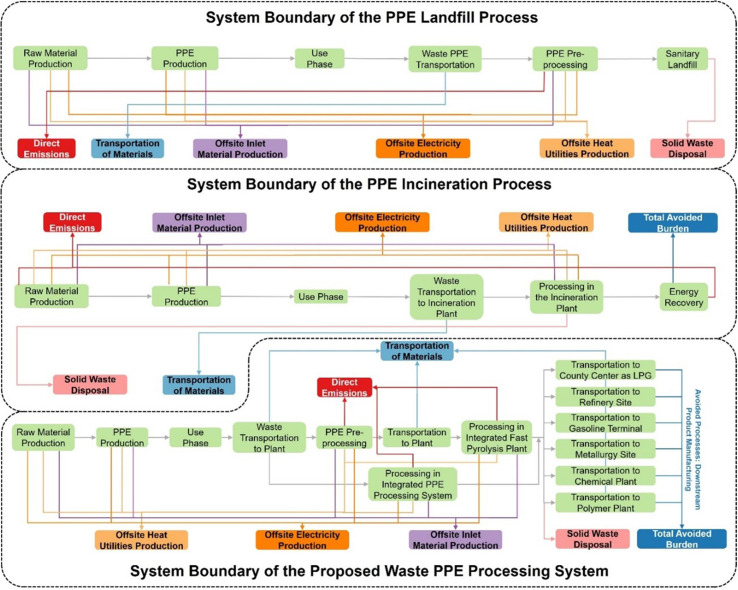
To evaluate the environmental advantage of the proposed PPE processing system, we employ an LCA approach to evaluate, quantify, and compare the environmental impacts from the proposed PPE processing system, PPE incineration process, and PPE landfill process. The LCA of this work aims to evaluate the proposed PPE processing system's environmental advantages by quantifying and comparing the GHG emissions and other impacts from three PPE treatment processes, as shown in Fig. 3 . Six life cycle stages are included within the system boundaries: offsite inlet material production, waste PPE processing, offsite heat utility production, transportation of materials, offsite electricity production, and solid waste disposal. All transportation within the system boundary is external transportation from one facility to another by repurposed trucks, of which distances are employed for calculating the transportation fee in TEA and the site selection optimization model. If the site selections of these facilities within the proposed PPE processing system are determined, the emissions from material transportation can be evaluated. The system boundaries also involve various avoided processes [56], of which the emissions (avoided burden) are subtracted from the total emissions [57].
Fig. 3.
System boundaries of three PPE treatment processes, namely the proposed integrated processing system and incineration and landfill processes with various life cycle stages. A series of lime blocks denote the processes from producing raw materials for PPE production to the external transportation of downstream products. The grey ticks represent the mass flows, while blocks in other colors denote the emissions and avoided burden. The orange, dark orange, violet, and pink blocks represent various life cycle stages corresponding to indirect emissions, while the red blocks denote the total direct emissions. The ticks in other colors indicate emission flow corresponding to blocks in the same colors. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Since various downstream products are produced from the proposed waste PPE processing system, the avoid processes are the production of these products. The applications of these products are given in Table 1 . For the PPE incineration process, the high-temperature energy produced from the waste PPE combustion can be used for generating mid-voltage electricity (energy recovery), which avoids the emissions of mid-voltage electricity production [58]. Like the landfilled waste plastic, the landfilled waste PPE produces negligible landfill gas, and it is not economically viable to use this gas to generate electricity [59]. No avoided process is included in the proposed PPE landfill processing system. The waste PPE can be converted into products, used for electricity generation, or landfilled in these three PPE treatment processes. In these regards, the functional unit is defined as 1 t of PPE treated, which is not only proportional to the amounts of products or energy produced from these proposed PPE treatment processes but also avoids the problem that the composition of the waste PPE mixture varies by counties [60]. This functional unit is adopted to quantify the environmental performances.
Table 1.
Detailed application for downstream products.
| Products | Application | Products | Application |
|---|---|---|---|
| Ethylene | A basic chemical used in the polymer industry | Light Naphtha | An organic solvent used in the refinery |
| Propylene | A basic chemical used in the polymer industry | Aromatic Mixture | A basic chemical used in the refinery |
| Propane | LPG fuel in the county | Gasoline | Fuel in the gasoline terminal |
| Butane | LPG fuel in the county | Diesel | Fuel in the gasoline terminal |
| Bauxite | A basic chemical used in the metallurgy plant | Sulfur | A basic chemical in the chemical plant |
Mass and energy balance relationships within the given system boundary are extracted as the source of LCI data [61] from the high-fidelity process simulations and compile as LCI data via the Ecoinvent V3.7 Database [62]. We employ the Peng-Robinson thermodynamic package to perform process simulations because of its wide application in the literature on waste plastics treatment [63]. Aspen HYSYS is adopted to perform the process simulation for the sulfur recovery, HCN scrubbing, NH3 scrubbing, CO2 separation, and aromatic extraction sections due to the accurate and complete thermodynamic parameters used in MEA absorption and sulfonate extraction [64]. To extract the unavailable product distribution of pyrolyzing waste PPE, relevant premises and corresponding assumptions are required for performing process simulations. The product distribution of pyrolyzing five types of PPE, namely face shields, surgical gowns, surgical masks, respirators, and surgical gloves, is assumed to be the average chemical composition for pyrolyzing each type of PPE. Due to the similar chemical compositions of the plastic mixture and the PPE, the product distribution obtained from the fast pyrolysis of a specific type of PPE is postulated to be the weighted average chemical composition from thermally-cracking each of the corresponding plastic compounds, as demonstrated in Westcherout et al. [65]. The chemical composition of each type of PPE is extracted from their related Material Safety Data Sheets (MSDSs), patents, or experimental measurement; see Ref. [66] for face shields [67], for facemasks [68], for respirators, and [33] for gloves. The atomic composition of char is determined by the difference in chemical compositions between pyrolysis products and the waste PPE feedstock; see Ref. [69] for pyrolyzing PET [70], for pyrolyzing polyisoprene (PI) [71], for pyrolyzing polypropylene (PP) and pyrolyzing PE [72], for pyrolyzing polyurethane (PU), and [33] for pyrolyzing gloves. For the proposed integrated PPE processing system, operating conditions, such as reflux ratios, are postulated to remain unchanged with the treatment capacities [73], while the total utility consumption is proportional to the PPE processing mass flow rate [74].
The global warming potential (GWP) indicator over 100 y (GWP100) is employed as a life cycle impact assessment (LCIA) method to interpret the aforementioned LCI data into quantified GHG emissions from these proposed PPE processing systems via specified characterization factors. Specifically, the GWP100 of methane (CH4) is 28 because the greenhouse impact caused by the methane emission is equivalent to 28 times that of CO2 in the time horizon of 100 y [75]. In addition to global warming, another two LCIA methods, namely ReCiPe and ILCD 2.0 approaches, are employed to evaluate environmental performances from these proposed PPE treatment processes in terms of air, water, land, and resource. The ILCD 2.0 approach, which has three major impact categories corresponding to climate change, is adopted in evaluating the life cycle environmental impacts in terms of 19 impact categories [76]. The environmental performances for each of the three proposed PPE processing systems are also evaluated through the ReCiPe approach from the hierarchic perspective [77], which is widely used in LCAs of medical waste processing systems [38]. The specific characterization factors of these LCIA methods are extracted from the Ecoinvent V3.7 database. For offsite electricity production, the specified characterization factor provided in the US e-GRID [78] is employed to evaluate environmental impact in a specific state because the energy source of electricity generation varies by state.
The interpretation phase aims to demonstrate the environmental advantages of the proposed waste PPE processing system by comparing its LCA results with those of PPE incineration and PPE landfill processes. Their GWP-based LCA results are firstly presented as impact breakdowns for comparing their GHG emissions. By summarizing and presenting the ILCD and ReCiPe results in heat maps, the environmental advantages of the proposed integrated and optimal PPE processing systems are evaluated in terms of each impact category. Impact breakdowns on ILCD and ReCiPe bases are also presented to illustrate the environmental hotspot within the proposed PPE processing system.
2.3. TEA methodology
In the TEA, the capital (CAPEX) and operating expenditure (OPEX) are calculated to estimate and compare the annualized cost for establishing each of these proposed PPE processing systems. The CAPEX includes the total equipment installation cost and indirect capital cost, and the working capital cost and the land cost [79]. For the OPEX calculation of the proposed waste PPE processing system and the PPE incineration process, costs for transportation, feedstock, utility, operation and maintenance (O&M), and solid waste disposal fee are all considered. For the PPE landfill processing system, the OPEX is estimated, excluding the solid waste disposal fee. The revenues of the proposed integrated PPE processing system and the incineration process are calculated using market prices for downstream products and the mid-voltage electricity price in the US. The discount rate is 10%, and the annualized cost equals the summation of expenses (annualized CAPEX and total OPEX) subtracted by the total revenue [42].
Since the proposed PPE processing system can fast pyrolyzing waste PPE like plastic mixture, the project lifetime of the proposed PPE processing system is assumed to be 20 y according to relevant literature that studied for waste plastic fast pyrolysis [52,54]. The unit transportation costs of the waste PPE and pre-processed PPE are assumed to be the same as those for transporting regular medical waste [80] and municipal solid waste [81].
2.4. Optimization model for the site selection
Considering the environmental advantages of the proposed PPE processing system, the annualized cost (obj in MUSD) of the proposed waste PPE processing system should be minimized. According to the TEA approach, the annualized cost depends on the transportation cost (tran in MUSD) calculated by the external transportation distance and total capital investment (tci in MUSD) evaluated by treatment capacity. The annualized cost is the summation of transportation cost, utility cost (uc in MUSD), feedstock cost (fec in MUSD), O&M cost (omc in MUSD), storage cost (stor in MUSD), solid waste disposal fee (SD in MUSD), minus the income (inc in MUSD). In these regards, a site selection optimization model is developed that aims to minimize the annualized cost through determining the optimal number, sizes, locations of establishing the facilities within the proposed waste PPE processing system and sites of consuming downstream products in NYS. The optimization model is subject to constraints corresponding to feedstock distribution, pre-processing facility, integrated fast pyrolysis plant, proposed integrated PPE processing system, product distribution, and TEA. The general optimization model is shown as follows.
min
s.t. Feedstock distribution constraints.
Pre-processing facility constraints.
Integrated fast pyrolysis plant constraints.
Integrated PPE processing system constraints.
Product distribution constraints.
TEA constraints
The capital cost is modeled via separable concave terms to account for the economy of scale, while other constraints are in linear form for all variables' input-output relationships. Therefore, this optimization model is formulated as a nonconvex MINLP problem [82]. This problem can be reformulated into a relaxed mixed-integer linear programming (MILP) problem by successive piecewise linearization. The reformulated optimization problem is solved iteratively following the branch-and-refine algorithm [83]. The global optimal solution is guaranteed to be found within finite iterations.
3. Results and discussion
3.1. Case study 1: environmental performances of the proposed integrated PPE processing system
This section presents a case study employing the proposed LCA methodologies to evaluate the environmental advantages of developing the proposed integrated PPE processing system to reduce GHG emissions and alleviate other environmental impacts. Since NYS residents are mandated to wear PPE, such as facemask, in public to reduce viral infection [84], the consumption and disposal of PPE statewide are consequently increased [85]. The waste PPE are treated in medical waste disposal sites. The transportation distance of waste PPE is assumed to be the distance between the county center and the medical waste disposal site [60]. The proposed PPE processing system is also postulated near the medical waste disposal site due to its similar functions.
Exergy analysis is performed to evaluate the energy recovery efficiency of the proposed PPE processing system. Table 2 shows the exergy input and output flows corresponding to PPE, makeup water, gaseous basic chemicals, and downstream products. The total exergy efficiency is 80%, and the heating utility is the major contributor to the total exergy flow. A high heating energy input and complexity in heat integration can cause heat loss and lead to exergy loss. Reducing this exergy loss requires implementing the process design optimization and novel heat integration methodology that future studies aim to investigate.
Table 2.
The exergy flow of the proposed integrated and optimal PPE processing system and the exergy efficiency is 80%.
| Exergy Input |
Exergy Output |
||
|---|---|---|---|
| Input | Exergy Flows (J/s) | Output | Exergy Flows (J/s) |
| N2 | 1,548 | C2H4 | 57,319 |
| O2 | 1,925 | C3H6 | 39,891 |
| PPE | 503,390 | C3H8 | 10,065 |
| H2O | 1,423 | C4H10 | 13,383 |
| LP Steam | 842,702 | Light Naphtha | 12,401 |
| MP Steam | 732,229 | Aromatic Mix | 34,466 |
| HP Steam | 641,043 | Gasoline | 4,044 |
| Cooling Water | 740,546 | Diesel | 37,195 |
| Electricity | 66,086 | Sulfur | 18 |
| Bauxite | 89,757 | ||
| Offgas | 2,542,131 | ||
In the proposed integrated PPE processing system, the waste PPE is converted into various products sent to counties or processing plants in NYS. The counties and plant locations are given in Table 3 . Locations of all existing processing plants for consuming downstream products, medical waste disposal sites, incineration sites, and landfill sites in NYS are extracted from the data on NYS's Department of Environmental Conservation [86] and existing chemical processing plants in Google Map. The annual amounts of the waste PPE over counties are assumed to be proportional to their population. The yearly total waste PPE amount is estimated by the GAO report of the 90 day PPE storage amount starting from July 30, 2020 [45].
Table 3.
Chemical products manufactured from the proposed integrated PPE processing system.
| Products | Location of Processing Plant/County | Products | Location of Processing Plant/County |
|---|---|---|---|
| Ethylene | Orange County | Light Naphtha | Ulster County |
| Propylene | Putnam County | Aromatic Mixture | Nassau County |
| Propane | Rockland County | Gasoline | Albany County |
| Butane | Orange County | Diesel | Dutchess County |
| Bauxite | Washington County | Sulfur | Nassau County |
3.1.1. GHG emissions reduction
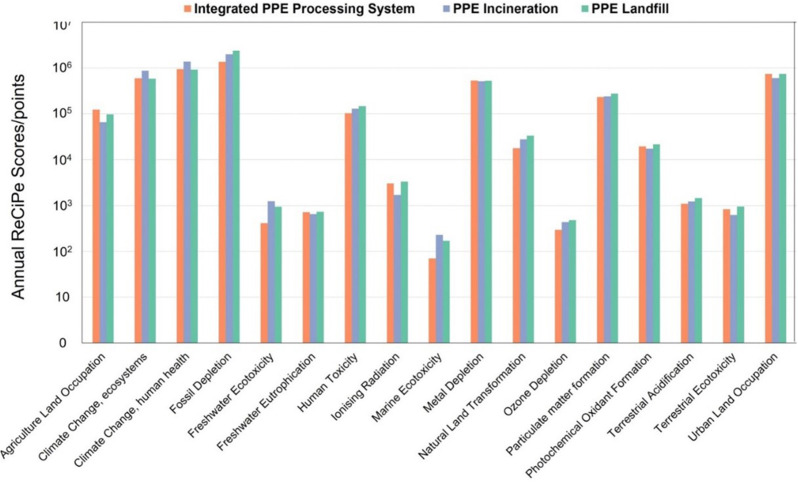
Comparison of unit GWPs and climate change-related environmental impacts from the proposed integrated PPE processing system, the PPE incineration process, and the PPE landfill process illustrates the environmental advantages of the proposed PPE processing system in terms of the GHG footprints [34]. Fig. 4 and Fig. 5 show that the proposed integrated PPE processing system alleviates climate change through a 35.42%, 35.06%, and 34.64% decrement in total GHG emissions (3.72 t CO2-eq/t PPE), those in terms of human health and ecosystem, and climate change from fossil than the PPE incineration process (total GHG emissions: 5.76 t CO2-eq/t PPE), respectively. These GHG emissions are major contributors to the overall environmental impacts from both the proposed PPE processing system and PPE incineration. When treating waste PPE in the proposed integrated PPE processing system, the carbon within PPE is allocated to hydrocarbon products and reduces carbon combustion and its direct carbon emissions, including various GHGs (including CO2) that cause climate change. Consequently, the proposed integrated PPE processing system has an environmental advantage over the PPE incineration process in terms of alleviating GHG emissions.
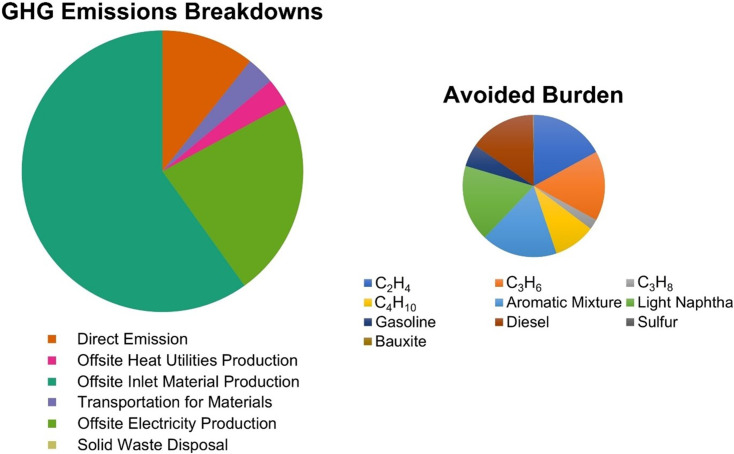
Fig. 4.
Breakdowns of the GHG emissions of the proposed integrated PPE processing system and the avoided burden calculated by the LCA approach in this work [44], calculated from GWP100 based on Intergovernmental Panel on Climate Change (IPCC) 2013 [75].
Fig. 5.
Annual ReCiPe scores of the proposed integrated PPE processing system and PPE incineration and PPE landfill processes are based on the LCA results calculated in this work [44].
Moreover, the GHG emissions avoided from onsite product manufacturing (0.54 t CO2-eq/t PPE) can offset those from direct emissions (0.46 t CO2-eq/t PPE). This GHG emissions reduction (0.07 t CO2-eq/t PPE) results from the onsite production of various hydrocarbon products, which substitutes their offsite production and avoids the fossil fuel use in their offsite production, such as heating energy production in naphtha cracking to produce ethylene. Nevertheless, this reduction in GHG emissions can not offset those from the heat production (0.13 t CO2-eq/t PPE) to maintain high-temperature conditions in the combustor and various reboilers, which is not included in the landfill process. The unit GWP of the proposed integrated PPE processing system is slightly higher than that of the landfill process (3.70 t CO2-eq/t PPE).
3.1.2. Environmental advantages of alleviating other environmental impacts
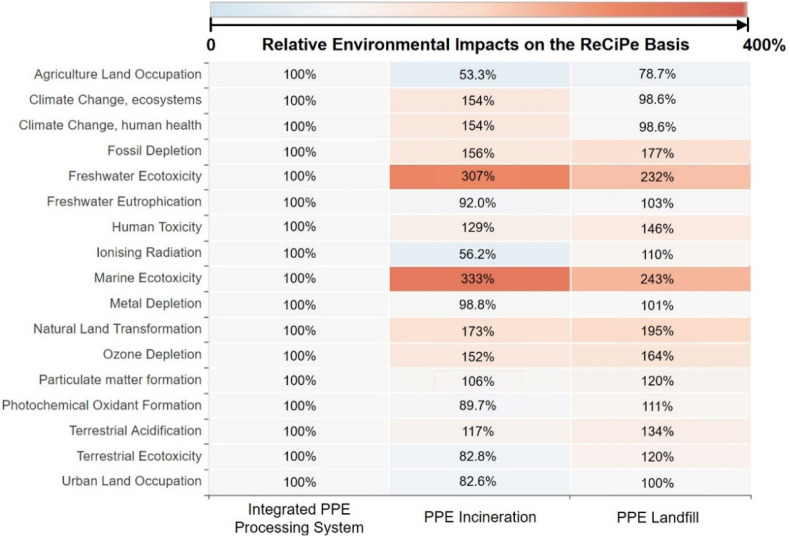
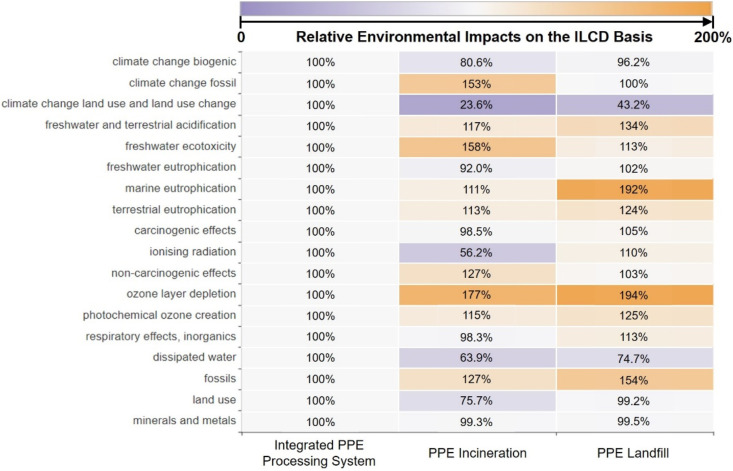
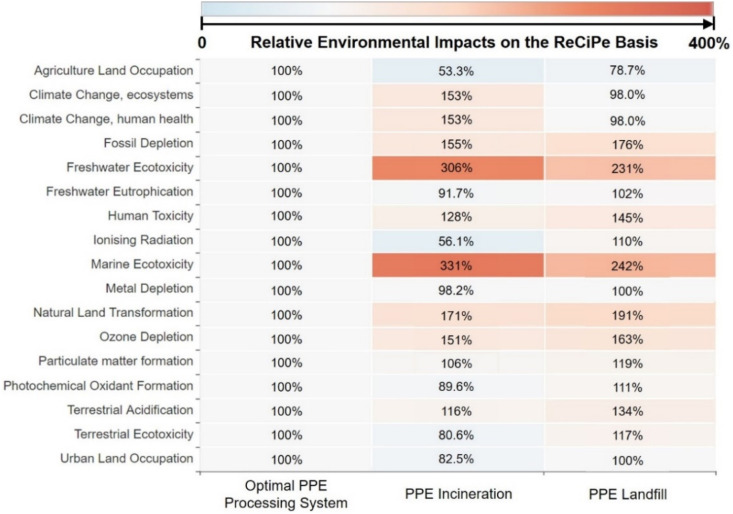
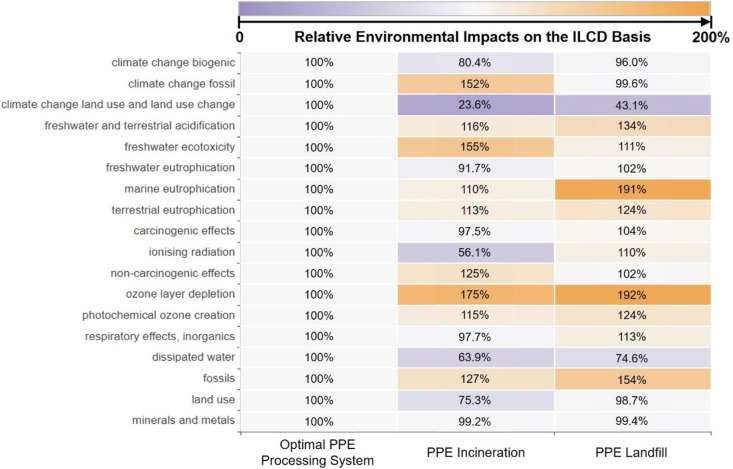
Environmental advantages of the proposed integrated PPE processing system are also pronounced in terms of human toxicity, ozone depletion, particulate matter formation, and ecotoxicity problems. Compared to the PPE incineration process that emits non-methane volatile organic compounds (NMVOCs), as shown in Fig. 6 and Fig. 7 , the total human toxicity, ozone depletion, freshwater ecotoxicity, and marine ecotoxicity problems posed by the proposed integrated PPE processing system are reduced by 22.48%, 34.21%, 67.43%, and 69.97%, respectively. The landfill process, on the other hand, emitted metal components, including iron within the waste PPE, that poses toxicity to freshwater and marine ecosystems [87] and human health [88]. This leads to 132%, 143%, and 46% higher environmental impacts on freshwater ecotoxicity, marine ecotoxicity, and human toxicity compared to the proposed PPE processing system. Conversely, the onsite manufacturing of downstream products in the proposed integrated PPE processing system, which offsets exterior production, can alleviate the total marine eutrophication, terrestrial eutrophication, ozone depletion, natural land transformation, particulate matter formation, terrestrial acidification, photochemical ozone creation, and respiratory by 17.7%, 18.3%, 15.9%, 29.0%, 15.9%, 18.3%, 18.3%, and 12.3%. Although the environmental drawbacks of these proposed PPE processing systems are pronounced in terms of fossil fuel use, natural land transformation, and dissipated water, they can be offset by the onsite manufacturing within the proposed integrated PPE processing system given in Fig. 5. Overall, onsite manufacturing products and reduction of toxic and NMVOC emissions lead to the environmental advantages of the proposed PPE processing system over the other two PPE treatment processes in terms of various impact categories.
Fig. 6.
The heat map reflects the ReCiPe-based relative environmental impacts from PPE processing systems based on the LCA results calculated in this work. The number denotes the ratio of quantified environmental impacts from the proposed integrated PPE processing system and PPE incineration and PPE landfill processes relative to the proposed integrated PPE processing system in terms of each impact category [44].
Fig. 7.
The heat map illustrates the ILCD-based relative environmental impacts from PPE processing systems based on the LCA results calculated in this work. The number denotes the ratio of quantified environmental impacts from the proposed integrated PPE processing system and PPE incineration and PPE landfill processes relative to the proposed integrated PPE processing system in terms of each impact category [44].
3.1.3. Sensitivity analysis
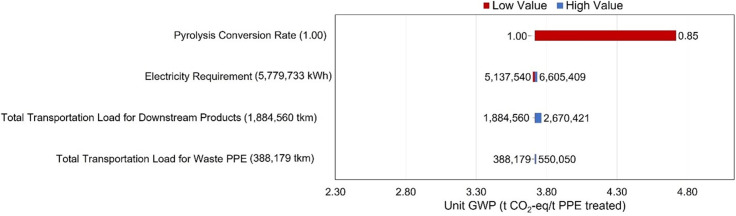
Environmental sensitivity analysis results, which is the unit GWPs, evaluate the most influential operating parameters on the environmental performance of the proposed integrated PPE processing system. Transportation loads used in this sensitivity analysis are proportional to the corresponding transportation distances, of which incremental value is evaluated from the ratio of road distance with a straight-line distance between two US sites [89]. Fig. 8 shows the most influential operating parameter as the conversion rate of waste PPE, and an 0.85 conversion rate can increase the unit GWP of the proposed integrated PPE processing system to 4.72 t CO2-eq/t PPE treated. Therefore, maintaining environmental sustainability requires a high conversion rate on fast pyrolyzing PPE. Waste PPE transportation load, on the other hand, is the least effective because when the transportation load increases to 550,050 tkm, the unit GWP only decreases to 3.73 CO2-eq/t PPE treated. Other transportation loads and electricity requirements are also relatively ineffective operating parameters on environmental sustainability.
Fig. 8.
Environmental sensitivity analysis results of the proposed integrated PPE processing system evaluated by the LCA approach in this work [44]. High and low values of the operating parameters are presented in the heads and tails of corresponding bar charts. The blue chart denotes the effect of lowering values of operating parameters, while the dark red bar chart represents the effect of higher values. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. Case study 2: the proposed optimal PPE processing system
Reducing GHG emissions and fossil fuel depletion as environmental hotspots is the primary environmental advantage of the centralized pathway within the proposed integrated PPE processing system. The distributed-centralized approach in the proposed waste PPE processing system, as described in Section 2, can also be employed to treat waste PPE through 11 processing sections with the aforementioned environmental advantage. The main differences between these two pathways lie in the total external transportation distance and sites to build facilities and facilities' treatment capacities. To increase their economic feasibility, an optimization-based site selection methodology is then proposed, and the MINLP problem described in Section 3.3 is solved to minimize the annualized cost and determine the optimal number, sizes, and locations of establishing the facilities within the proposed waste PPE processing system in New York State. The sites and locations for receiving downstream products are also predicted. Notably, the site selection of the proposed PPE processing system changes the total environmental impacts through only total transportation loads. Since the total GHG emissions will increase by 1.5% when the total transportation load enhances by 41.70%, the change in total transportation loads causes low variation in total GHG emissions. Moreover, the transportation of materials only poses 1.44% of total fossil depletion. Therefore, the total environmental performances will not be much varied when optimizing the site selection, so the environmental advantages of the proposed optimal PPE processing system in reducing fossil depletion and climate are not changed. Overall, we could enhance the economic feasibility of the proposed PPE processing system by the optimization-based site selection methodology to minimize the annualized cost while maintaining environmental sustainability.
The computational experiment was performed on a DELL OPTIPLEX 7040 desktop with Intel(R) Core (TM) i7-6700 CPU @ 3.40 GHz and 32 GB RAM. The mathematical formulation of the optimization problem and related solution process is coded in GAMS 24.8.3 [90], using CPLEX 12.7 as the MILP optimizer [91]. The tolerance of the branch-and-refine algorithm is set as 10−4. The reformulated optimization problem is comprised of 66,960 variables and 70,360 constraints. The problem is converged within finite iterations.
3.2.1. Site selection within the proposed optimal PPE processing system
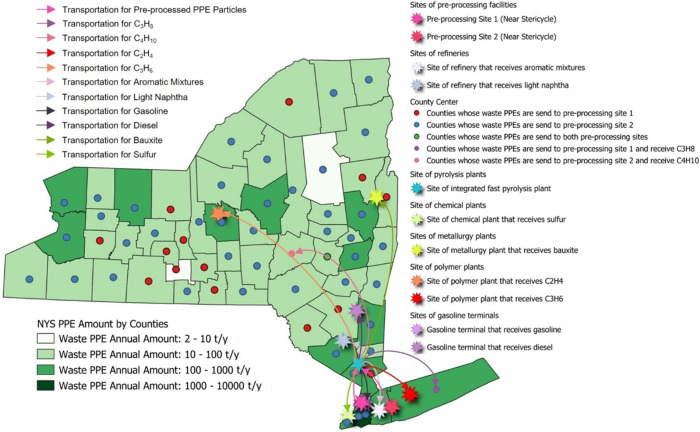
The optimal site selection of the proposed PPE processing system is presented in Fig. 9 . Two PPE pre-processing facilities with an annual treatment capacity of 1,708 t/y and 8,000 t/y are proposed to be established near Stericycle in New York County and Stericycle in Suffolk County, respectively, to effectively treat the waste PPE collected from counties within NYS. The waste PPE are shredded, sterilized, and dehydrated in two proposed pre-processing facilities. The pre-processed PPE particles are transported by repurposed trucks to the integrated fast pyrolysis plant in Rockland County with an annual treatment capacity of 9,027.7 t. The downstream products manufactured from this plant are then transported to processing plants or counties for use, as given in Table 4 .
Fig. 9.
The spatial information of the proposed optimal PPE processing system is shown in the density map. The site selections are extracted from the optimization results evaluated in this work. The dots in red, blue, and green denote the counties that send waste PPE to pre-processing site one, pre-processing site two, and both pre-processing sites, respectively. The stars in colors represent the locations of pre-processing facilities, integrated fast pyrolysis plants, and demand zones for various downstream products. In contrast, the ticks in corresponding colors show the external transportation of the pre-processed PPE particles and downstream products by repurposed trucks [86]. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 4.
Chemical products manufactured from the proposed optimal PPE processing system and locations of the processing plant based on the optimization results.
| Products | Location of Processing Plant/County | Products | Location of Processing Plant/County |
|---|---|---|---|
| Ethylene | Onondaga County | Light Naphtha | Orange County |
| Propylene | Suffolk County | Aromatic Mixture | Nassau County |
| Propane | Suffolk County | Gasoline | Dutchess County |
| Butane | Otsego County | Diesel | Queens County |
| Bauxite | Richmond County | Sulfur | Washington County |
3.2.2. Economic and environmental performances of the proposed optimal PPE processing system
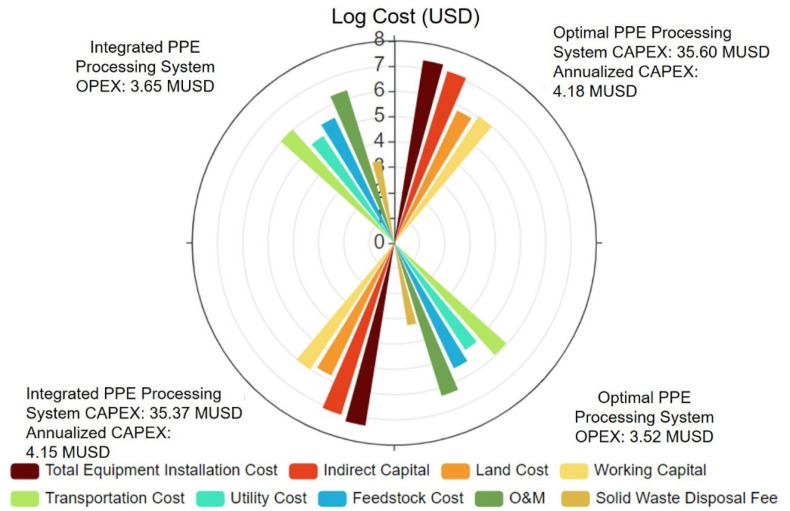
We display and compare the economic breakdowns of the proposed optimal PPE processing system and the integrated PPE processing system proposed in the first case study. Because the unit transportation cost of waste PPE (1.01 USD/tkm) is much higher than transporting pre-processed PPE, Fig. 10 shows a lower total transportation cost of the proposed optimal PPE processing system (0.93 MUSD) than that of the proposed integrated PPE processing system (1.08 MUSD) with more waste PPE transportation load and less total transportation load. The total capital investment, which is the summation of the discounted annualized capital cost, is calculated by the total equipment installation cost and indirect capital cost, and the working capital cost and the land cost. These capital costs are proportional to the equipment procurement (pect (MUSD)) or installation cost (capt (MUSD)), both of which are scaled by their corresponding processing capacities (cap (t/y)), base-case cost (ECCB (MUSD) and ECCPE (MUSD)), and scaling factors (SCF) smaller than one, as given by Eqs. (1), (2). Consequently, the equipment procurement and installation costs for the pre-processing facilities within both the proposed integrated and optimal PPE processing systems are calculated accordingly and presented in Table 5 . It shows a lower capital cost for the pre-processing facility within the integrated PPE processing system. Therefore, the capital cost can be saved when establishing a single pre-processing facility rather than two facilities with the same total capacity.
Fig. 10.
CAPEX and OPEX breakdowns of the proposed optimal PPE processing system and proposed integrated PPE processing system extracted from the TEA results calculated in this work.
Table 5.
Equipment procurement and installation costs for pre-processing facilities within the proposed integrated and optimal PPE processing system.
| Proposed PPE Processing System | Pre-processing Facilities Sites | Equipment Procurement Cost (MUSD) | Equipment Installation Cost (MUSD) |
|---|---|---|---|
| Optimal | New York County | 0.46 | 0.48 |
| Suffolk County | 1.59 | 1.66 | |
| Integrated | Rockland County | 2.05 | 2.14 |
The proposed integrated and optimal PPE processing systems incorporate the integrated fast pyrolysis plants that have the same processing capacities, so they have the same equipment procurement and installation costs and the annualized capital cost of the proposed optimal PPE processing system (4.18 MUSD) is slightly higher than that of the proposed integrated PPE processing system (4.15 MUSD). However, the higher transportation cost can offset the capital cost saving of the proposed integrated PPE processing system, which leads to a higher annualized cost. These high annualized capital investments for both proposed PPE processing systems can be reduced by optimizing process designs and heat integration, while the economic feasibility can also be maintained by enhancing income from manufacturing downstream products with the higher market price. This can be realized by implementing advanced plastic waste upcycling technology into existing waste PPE treatment processes.
| (1) |
| (2) |
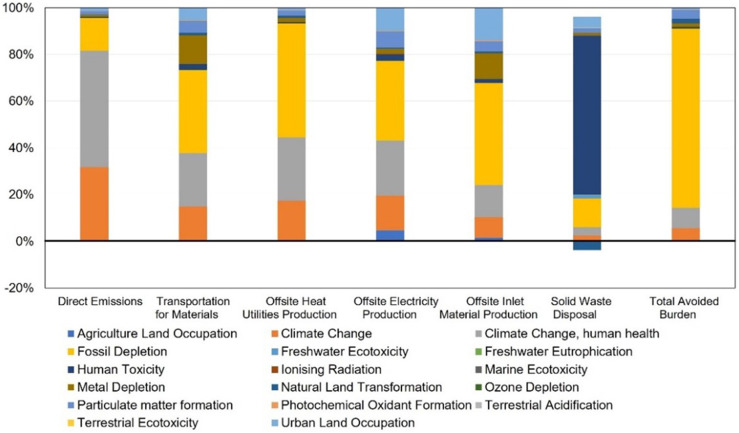
Both proposed PPE processing systems are close in utility use and have the same offsite material production process, but the proposed optimal PPE processing system has the higher total transportation load (2.59 M tkm) and results in a slightly higher unit GWP (3.74 t CO2-eq/t PPE), which is 35.04% lower than the PPE incineration process and closed to the PPE landfill processing system (3.70 t CO2-eq/t PPE). Fig. 11 shows the same environmental advantages of both proposed PPE processing systems in terms of fossil fuel depletion alleviation, climate change mitigation, and natural land perseverance. On the other hand, the proposed optimal PPE processing system requires various distillation columns that occupy more land and consume more water than the PPE incineration and PPE landfill processes, resulting in higher land use and dissipated water (see Fig. 12 ). Meanwhile, fossil depletion is another environmental drawback, which is caused by a high fossil fuel consumption in external truck transportation, inlet material, as well as hydrocarbon, heat, and electricity production. This environmental problem can be alleviated through the onsite manufacturing of products (shown as the total avoided burden in Fig. 13 ) within the proposed optimal PPE processing system.
Fig. 11.
The heat map reflects the ReCiPe-based relative environmental impacts from PPE processing systems based on the LCA results calculated in this work. The number denotes the ratio of quantified environmental impacts from the proposed optimal PPE processing system, PPE incineration, and PPE landfill processes relative to the proposed optimal PPE processing system in terms of each impact category [44].
Fig. 12.
The heat map reflects the ILCD-based relative environmental impacts from PPE processing systems based on the LCA results calculated in this work. The number denotes the ratio of quantified environmental impacts from the proposed optimal PPE processing system, PPE incineration, and PPE landfill processes relative to the proposed optimal PPE processing system in terms of each impact category [44].
Fig. 13.
ReCiPe-based environmental impacts of all life cycle stages within the proposed optimal PPE processing system based on the LCA results calculated in this work [44].
4. Conclusion
The first and efficient waste PPE processing system that includes twelve processing sections was proposed to treat PPE for energy recovery with low GHG emissions and fossil fuel depletion alleviation. This environmental advantage was then analyzed by a detailed LCA approach. An optimization-based site selection methodology determined the optimal number, sizes, and locations of establishing facilities within the PPE processing system. Two pre-processing facilities in New York County and Suffolk County and one integrated fast pyrolysis plant located in Rockland County were proposed to be built with their optimal annual treatment capacities of 1,708 t/y, 8,000 t/y, and 9,028 t/y. The key findings corresponding to the environmental advantages were then drawn as follows:
-
•
Onsite hydrocarbon production from and heat integration of the proposed optimal PPE processing system reduced GHG emissions and fossil fuel use compared to the PPE incineration process.
-
•
The use of the proposed optimal PPE processing system avoided PPE from being landfilled, thus cutting toxic chemical emissions and preserving the natural land and ecosystem.
-
•
Implementing the proposed optimal PPE processing system impaired the use of the PPE incineration process and reduced the NMVOC emissions that cause ozone depletion and particulate matter formation.
-
•
The onsite manufacturing of basic chemicals and renewable fuels could alleviate the fossil depletion, land use, and dissipated water as three environmental burdens posed by the proposed optimal PPE processing system.
Future research should investigate fossil fuel use and GHG emissions reduction through developing advanced PPE processing technologies with low energy consumption and flue gas output by referring to studies proposed by Taniguchi et al. for degrading PET [92] and Zafar et al. for degrading PU [93]. Moreover, a lower annualized cost by reducing total capital investment or enhancing income from downstream products paved the wide application of PPE processing in the real world. Existing plastic waste upcycling studies provided insights on gaining higher revenue from low-value plastic waste [94], while the capital cost could be reduced through optimization on process designs. Nevertheless, fulfilling these research gaps requires preliminary environmental and economic assessment results on the proposed PPE processing system that this study aimed to present.
Author contribution
XZ and FY developed the models, conducted the simulations and analyzed the results. XZ, JJK, and FY wrote the manuscript. All authors reviewed the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This Engaged Cornell work was supported in part by an Engaged Research Grant, and by the Cornell Atkinson Center for Sustainability. One author contribution was funded by the gratefully acknowledged project "Sustainable Process Integration Laboratory – SPIL" funded by EU CZ Operational Programme Research and Development, Education, Priority1: Strengthening capacity for quality research (Grant No. CZ.02.1.01/0.0/0.0/15_003/000045).
References
- 1.Hufford A. Face Masks Are Again in Short Supply as Covid-19 Cases Surge. Coronavirus: The Wall Street Journal, https://www.wsj.com/articles/face-masks-are-again-in-short-supply-as-covid-19-cases-surge-11604499588; [accessed 4 November 2020].
- 2.Card K.J., Crozier D., Dhawan A., Dinh M., Dolson E., Farrokhian N., et al. medRxiv; 2020. UV sterilization of personal protective equipment with idle laboratory biosafety cabinets during the Covid-19 pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hufford A. Mask Makers Work With FEMA, Get Antitrust Protection. Coronavirus: The Wall Street Journal, https://www.wsj.com/articles/mask-makers-work-with-fema-get-antitrust-protection-11610712009; [accessed 15 January 2021].
- 4.Tao Y., You F. Can decontamination and reuse of N95 respirators during COVID-19 pandemic provide energy, environmental, and economic benefits? Appl Energy. 2021;304:117848. doi: 10.1016/j.apenergy.2021.117848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prata J.C., Silva A.L., Walker T.R., Duarte A.C., Rocha-Santos T. COVID-19 pandemic repercussions on the use and management of plastics. Environ Sci Technol. 2020;54:7760–7765. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- 6.Bruijnincx P.C., Weckhuysen B.M. Shale gas revolution: an opportunity for the production of biobased chemicals? Angew Chem Int Ed. 2013;52:11980–11987. doi: 10.1002/anie.201305058. [DOI] [PubMed] [Google Scholar]
- 7.Vanapalli K.R., Sharma H.B., Ranjan V.P., Samal B., Bhattacharya J., Dubey B.K., et al. Challenges and strategies for effective plastic waste management during and post COVID-19 pandemic. Sci Total Environ. 2020;750:141514. doi: 10.1016/j.scitotenv.2020.141514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klemeš J.J., Van Fan Y., Tan R.R., Jiang P. Minimising the present and future plastic waste, energy and environmental footprints related to COVID-19. Renew Sustain Energy Rev. 2020;127:109883. doi: 10.1016/j.rser.2020.109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.You S., Sonne C., Ok Y.S. COVID-19's unsustainable waste management. Science. 2020;368:1438. doi: 10.1126/science.abc7778. [DOI] [PubMed] [Google Scholar]
- 10.Fadare O.O., Okoffo E.D. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci Total Environ. 2020;737:140279. doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma H.B., Vanapalli K.R., Cheela V.S., Ranjan V.P., Jaglan A.K., Dubey B., et al. Challenges, opportunities, and innovations for effective solid waste management during and post COVID-19 pandemic. Resour Conserv Recycl. 2020;162:105052. doi: 10.1016/j.resconrec.2020.105052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nzediegwu C., Chang S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour Conserv Recycl. 2020;161:104947. doi: 10.1016/j.resconrec.2020.104947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva A.L.P., Prata J.C., Walker T.R., Campos D., Duarte A.C., Soares A.M., et al. Rethinking and optimising plastic waste management under COVID-19 pandemic: policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci Total Environ. 2020;742:140565. doi: 10.1016/j.scitotenv.2020.140565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fazeli A., Bakhtvar F., Jahanshaloo L., Che Sidik N.A., Bayat A.E. Malaysia׳s stand on municipal solid waste conversion to energy: a review. Renew Sustain Energy Rev. 2016;58:1007–1016. [Google Scholar]
- 15.Townsend T.G. Environmental issues and management strategies for waste electronic and electrical equipment. J Air Waste Manag Assoc. 2011;61:587–610. doi: 10.3155/1047-3289.61.6.587. [DOI] [PubMed] [Google Scholar]
- 16.Klemeš J.J., Van Fan Y., Jiang P. The energy and environmental footprints of COVID-19 fighting measures–PPE, disinfection, supply chains. Energy. 2020;211:118701. doi: 10.1016/j.energy.2020.118701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ragaert K., Delva L., Van Geem K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017;69:24–58. doi: 10.1016/j.wasman.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 18.Qureshi M.S., Oasmaa A., Pihkola H., Deviatkin I., Tenhunen A., Mannila J., et al. Pyrolysis of plastic waste: opportunities and challenges. J Anal Appl Pyrol. 2020;152:104804. [Google Scholar]
- 19.Wong S., Ngadi N., Abdullah T.A.T., Inuwa I.M. Current state and future prospects of plastic waste as source of fuel: a review. Renew Sustain Energy Rev. 2015;50:1167–1180. [Google Scholar]
- 20.Rahimi A., García J.M. Chemical recycling of waste plastics for new materials production. Nature Reviews Chemistry. 2017;1 [Google Scholar]
- 21.Garlapati V.K. E-waste in India and developed countries: management, recycling, business and biotechnological initiatives. Renew Sustain Energy Rev. 2016;54:874–881. [Google Scholar]
- 22.Munir D., Irfan M.F., Usman M.R. Hydrocracking of virgin and waste plastics: a detailed review. Renew Sustain Energy Rev. 2018;90:490–515. [Google Scholar]
- 23.George N., Kurian T. Recent developments in the chemical recycling of postconsumer poly (ethylene terephthalate) waste. Ind Eng Chem Res. 2014;53:14185–14198. [Google Scholar]
- 24.Unnisa S.A., Hassanpour M. Development circumstances of four recycling industries (used motor oil, acidic sludge, plastic wastes and blown bitumen) in the world. Renew Sustain Energy Rev. 2017;72:605–624. [Google Scholar]
- 25.Lopez G., Artetxe M., Amutio M., Bilbao J., Olazar M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew Sustain Energy Rev. 2017;73:346–368. [Google Scholar]
- 26.Panda A.K., Singh R.K., Mishra D.K. Thermolysis of waste plastics to liquid fuel: a suitable method for plastic waste management and manufacture of value added products—a world prospective. Renew Sustain Energy Rev. 2010;14:233–248. [Google Scholar]
- 27.Ma C., Yu J., Wang B., Song Z., Xiang J., Hu S., et al. Chemical recycling of brominated flame retarded plastics from e-waste for clean fuels production: a review. Renew Sustain Energy Rev. 2016;61:433–450. [Google Scholar]
- 28.Kunwar B., Cheng H.N., Chandrashekaran S.R., Sharma B.K. Plastics to fuel: a review. Renew Sustain Energy Rev. 2016;54:421–428. [Google Scholar]
- 29.Huang H., Tang L. Treatment of organic waste using thermal plasma pyrolysis technology. Energy Convers Manag. 2007;48:1331–1337. [Google Scholar]
- 30.Gholizadeh M., Li C., Zhang S., Wang Y., Niu S.L., Li Y.J., et al. Progress of the development of reactors for pyrolysis of municipal waste. Sustainable Energy & Fuels. 2020;4:5885–5915. [Google Scholar]
- 31.Lam S.S., Wan Mahari W.A., Ok Y.S., Peng W., Chong C.T., Ma N.L., et al. Microwave vacuum pyrolysis of waste plastic and used cooking oil for simultaneous waste reduction and sustainable energy conversion: recovery of cleaner liquid fuel and techno-economic analysis. Renew Sustain Energy Rev. 2019;115:109359. [Google Scholar]
- 32.Zhu H., Yan J., Jiang X., Lai Y., Cen K. Study on pyrolysis of typical medical waste materials by using TG-FTIR analysis. J Hazard Mater. 2008;153:670–676. doi: 10.1016/j.jhazmat.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Kaminsky W., Mennerich C., Zhang Z. Feedstock recycling of synthetic and natural rubber by pyrolysis in a fluidized bed. J Anal Appl Pyrol. 2009;85:334–337. [Google Scholar]
- 34.Van Fan Y., Jiang P., Klemeš J.J., Liew P.Y., Lee C.T. Integrated regional waste management to minimise the environmental footprints in circular economy transition. Resour Conserv Recycl. 2021;168:105292. [Google Scholar]
- 35.Chen L., Sun J.M., Huang Z.W. Trans Tech Publ; 2014. Research on the whole-progress management of medical waste with life cycle assessment. Advanced Materials Research; pp. 900–904. [Google Scholar]
- 36.Mousavi S.A., Mehrpooya M. A comprehensive exergy-based evaluation on cascade absorption-compression refrigeration system for low temperature applications - exergy, exergoeconomic, and exergoenvironmental assessments. J Clean Prod. 2020:246. [Google Scholar]
- 37.Mehrpooya M., Ansarinasab H., Mousavi S.A. Life cycle assessment and exergoeconomic analysis of the multi-generation system based on fuel cell for methanol, power, and heat production. Renew Energy. 2021;172:1314–1332. [Google Scholar]
- 38.Soares S.R., Finotti A.R., Prudêncio da Silva V., Alvarenga R.A.F. Applications of life cycle assessment and cost analysis in health care waste management. Waste Manag. 2013;33:175–183. doi: 10.1016/j.wasman.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Kumar H., Azad A., Gupta A., Sharma J., Bherwani H., Labhsetwar N.K., et al. COVID-19 Creating another problem? Sustainable solution for PPE disposal through LCA approach. Environ Dev Sustain. 2020:1–15. doi: 10.1007/s10668-020-01033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Salem S., Lettieri P., Baeyens J. Recycling and recovery routes of plastic solid waste (PSW): a review. Waste Manag. 2009;29:2625–2643. doi: 10.1016/j.wasman.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Scott D., Czernik S., Piskorz J., Radlein D.S.A. Fast pyrolysis of plastic wastes. Energy & Fuels. 1990;4:407–411. [Google Scholar]
- 42.Zhao N., You F. Food-energy-water-waste nexus systems optimization for New York State under the COVID-19 pandemic to alleviate health and environmental concerns. Appl Energy. 2021;282:116181. [Google Scholar]
- 43.Nutsch A., Spire M. Kansas State University; Manhattan, KS, USA: 2004. Carcass disposal: a comprehensive review. national agricultural biosecurity center. [Google Scholar]
- 44.Ecoinvent Ecoinvent V3.6. Zurich, Switzerland. https://www.ecoinvent.org/database/older-versions/ecoinvent-36/ecoinvent-36.html accessed.
- 45.Management Waste. s Department of Environmental Conservation: New York State's Department of Environmental Conservation; USA: 2021. New York state. [Google Scholar]
- 46.Phillips S., Aden A., Jechura J., Dayton D., Eggeman T. National Renewable Energy Lab.(NREL); Golden, CO (USA): 2007. Thermochemical ethanol via indirect gasification and mixed alcohol synthesis of lignocellulosic biomass. [Google Scholar]
- 47.Alternative regulated medical waste treatment technologies New York state approved treatment systems. 2017. USA. [Google Scholar]
- 48.Czajczyńska D., Anguilano L., Ghazal H., Krzyżyńska R., Reynolds A.J., Spencer N., et al. Potential of pyrolysis processes in the waste management sector. Thermal Science and Engineering Progress. 2017;3:171–197. [Google Scholar]
- 49.Jones S.B., Zhu Y., Anderson D.B., Hallen R.T., Elliott D.C., Schmidt A.J., et al. Pacific Northwest National Lab.(PNNL); Richland, WA (USA): 2014. Process design and economics for the conversion of algal biomass to hydrocarbons: whole algae hydrothermal liquefaction and upgrading. [Google Scholar]
- 50.Goar B. American Institute of Chemical Engineers; New York, NY, USA: 1986. Sulfur recovery technology. [Google Scholar]
- 51.Hauptmann H., Walter W.F. The action of Raney nickel on organic sulfur compounds. Chem Rev. 1962;62:347–404. [Google Scholar]
- 52.Swanson R.M., Platon A., Satrio J.A., Brown R.C., Hsu D.D. National Renewable Energy Lab. (NREL); Golden, CO (USA): 2010. Techno-economic analysis of biofuels production based on gasification. [Google Scholar]
- 53.Meyers R.A. McGraw-Hill; New York, USA: 2004. Handbook of petroleum refining processes. [Google Scholar]
- 54.Gracida-Alvarez U.R., Winjobi O., Sacramento-Rivero J.C., Shonnard D.R. System Analyses of high-value chemicals and fuels from a waste high-density polyethylene refinery. Part 1: conceptual design and techno-economic assessment. ACS Sustainable Chem Eng. 2019;7:18254–18266. [Google Scholar]
- 55.Yang M., You F. Modular methanol manufacturing from shale gas: techno-economic and environmental analyses of conventional large-scale production versus small-scale distributed, modular processing. AIChE J. 2018;64:495–510. [Google Scholar]
- 56.Thomassen M.A., Dalgaard R., Heijungs R., De Boer I. Attributional and consequential LCA of milk production. Int J Life Cycle Assess. 2008;13:339–349. [Google Scholar]
- 57.Roberts K.G., Gloy B.A., Joseph S., Scott N.R., Lehmann J. Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential. Environ Sci Technol. 2010;44:827–833. doi: 10.1021/es902266r. [DOI] [PubMed] [Google Scholar]
- 58.Petrakli F., Gkika A., Bonou A., Karayannis P., Koumoulos E.P., Semitekolos D., et al. End-of-Life recycling options of (nano) enhanced CFRP composite prototypes waste—a life cycle perspective. Polymers. 2020;12:2129. doi: 10.3390/polym12092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eriksson O., Finnveden G. Plastic waste as a fuel-CO 2-neutral or not? Energy Environ Sci. 2009;2:907–914. [Google Scholar]
- 60.Yue D., Kim M.A., You F. Design of sustainable product systems and supply chains with life cycle optimization based on functional unit: general modeling framework, mixed-integer nonlinear programming algorithms and case study on hydrocarbon biofuels. ACS Sustainable Chem Eng. 2013;1:1003–1014. [Google Scholar]
- 61.Tian X., Meyer T., Lee H., You F. Sustainable design of geothermal energy systems for electric power generation using life cycle optimization. AIChE J. 2020;66 [Google Scholar]
- 62.Moreno Ruiz E., Valsasina L., FitzGerald D., Symeonidis A., Müller J., Minas N., et al. Ecoinvent Association. Zürich; Switzerland: 2020. Documentation of changes implemented in Ecoinvent database v3. 7. [Google Scholar]
- 63.Fivga A., Dimitriou I. Pyrolysis of plastic waste for production of heavy fuel substitute: a techno-economic assessment. Energy. 2018;149:865–874. [Google Scholar]
- 64.HYSYS A. Aspen Technology Inc; Burlington, MA, USA: 2010. Aspen HYSYS customization guide. [Google Scholar]
- 65.Westerhout R.W.J., Van Koningsbruggen M.P., Van Der Ham A.G.J., Kuipers J.A.M., Van Swaaij W.P.M. Techno-economic evaluation of high temperature pyrolysis processes for mixed plastic waste. Chem Eng Res Des. 1998;76:427–439. [Google Scholar]
- 66.Desy R.O. Google Patents; 1999. Disposable face shield. [Google Scholar]
- 67.Xue J., Casar D.C. Google Patents; 2001. Face masks having an elastic and polyolefin thermoplastic band attached thereto by heat and pressure. [Google Scholar]
- 68.https://multimedia.3m.com/mws/mediawebserver?mwsId=SSSSSu9n_zu8l00xlYtUP8_e4v70k17zHvu9lxtD7xtBevSSSSSS Article Information Sheet. 3M Corporation. accessed.
- 69.Martin-Gullon I., Esperanza M., Font R. Kinetic model for the pyrolysis and combustion of poly-(ethylene terephthalate)(PET) J Anal Appl Pyrol. 2001;58:635–650. [Google Scholar]
- 70.Reshetnikov S.M., Reshetnikov I.S. Oxidation kinetic of volatile polymer degradation products. Polym Degrad Stabil. 1999;64:379–385. [Google Scholar]
- 71.Jung S.-H., Cho M.-H., Kang B.-S., Kim J.-S. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process Technol. 2010;91:277–284. [Google Scholar]
- 72.Font R., Fullana A., Caballero J., Candela J., Garcıa A. Pyrolysis study of polyurethane. J Anal Appl Pyrol. 2001;58:63–77. [Google Scholar]
- 73.Bora R.R., Tao Y., Lehmann J., Tester J.W., Richardson R.E., You F. Techno-economic feasibility and spatial analysis of thermochemical conversion pathways for regional poultry waste valorization. ACS Sustainable Chem Eng. 2020;8:5763–5775. [Google Scholar]
- 74.Gong J., You F. Consequential life cycle optimization: general conceptual framework and application to algal renewable diesel production. ACS Sustainable Chem Eng. 2017;5:5887–5911. [Google Scholar]
- 75.Hartmann D., Tank A., Rusticucci M. IPCC fifth assessment report, climate change 2013: the physical science basis. IPCC Ar5. 2013;5:31–39. [Google Scholar]
- 76.Fazio S., Castellani V., Sala S., Schau E., Secchi M., Zampori L., et al. Supporting information to the characterisation factors of recommended EF life cycle impact assessment methods. New models and differences with ILCD. EUR. 2018:28888. [Google Scholar]
- 77.Goedkoop M., Heijungs R., Huijbregts M., De Schryver A., Struijs J., Van Zelm R. The hague. Ministry of VROM ReCiPe; 2009. A life cycle impact assessment method which comprises harmonised category indicators at the midpoint and the endpoint level. [Google Scholar]
- 78.Emissions & Generation Resource Integrated Database (eGRID). United States Environmental Protection Agency, https://www.epa.gov/egrid/emissions-generation-resource-integrated-database-egrid; [accessed 28 January 2020].
- 79.Gong J., You F. A new superstructure optimization paradigm for process synthesis with product distribution optimization: application to an integrated shale gas processing and chemical manufacturing process. AIChE J. 2018;64:123–143. [Google Scholar]
- 80.Allen T.G., Criner G.K. 2002. TB184: least-cost options for the collection, treatment, and disposal of biomedical waste in Maine. [Google Scholar]
- 81.Duffy DP. From Transfer Station to MRF. MSW Management, https://www.mswmanagement.com/collection/article/13004365/from-transfer-station-to-mrf; [accessed 1 March 2009].
- 82.Gong J., You F. Global optimization for sustainable design and synthesis of algae processing network for CO2 mitigation and biofuel production using life cycle optimization. AIChE J. 2014;60:3195–3210. [Google Scholar]
- 83.You F., Grossmann I.E. Stochastic inventory management for tactical process planning under uncertainties: MINLP models and algorithms. AIChE J. 2011;57:1250–1277. [Google Scholar]
- 84.The COVID Tracking Project. The Atlantic Monthly Group, https://covidtracking.com/data/state/new-york; [accessed 12 December 2020].
- 85.Reopening: What You Need to Know. New York State Government, USA https://forward.ny.gov/reopening-what-you-need-know; [accessed 12 December 2020].
- 86.Waste Management. New York state's Department of Environmental Conservation: New York State's Department of Environmental Conservation. 2021. USA. [Google Scholar]
- 87.Aziz H.A., Yusoff M.S., Adlan M.N., Adnan N.H., Alias S. Physico-chemical removal of iron from semi-aerobic landfill leachate by limestone filter. Waste Manag. 2004;24:353–358. doi: 10.1016/j.wasman.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Zaman A.U. Comparative study of municipal solid waste treatment technologies using life cycle assessment method. Int J Environ Sci Technol. 2010;7:225–234. [Google Scholar]
- 89.Boscoe F.P., Henry K.A., Zdeb M.S. A nationwide comparison of driving distance versus straight-line distance to hospitals. Prof Geogr. 2012;64 doi: 10.1080/00330124.2011.583586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosenthal R. September 2016. GAMS–A user's guide. USA. [Google Scholar]
- 91.Gamrath G., Fischer T., Gally T., Gleixner A.M., Hendel G., Koch T., et al. 2016. The SCIP optimization suite 3.2. [Google Scholar]
- 92.Taniguchi I., Yoshida S., Hiraga K., Miyamoto K., Kimura Y., Oda K. Biodegradation of PET: current status and application aspects. ACS Catal. 2019;9:4089–4105. [Google Scholar]
- 93.Zafar U., Nzeram P., Langarica-Fuentes A., Houlden A., Heyworth A., Saiani A., et al. Biodegradation of polyester polyurethane during commercial composting and analysis of associated fungal communities. Bioresour Technol. 2014;158:374–377. doi: 10.1016/j.biortech.2014.02.077. [DOI] [PubMed] [Google Scholar]
- 94.Weckhuysen B.M. Creating value from plastic waste. Science. 2020;370:400–401. doi: 10.1126/science.abe3873. [DOI] [PubMed] [Google Scholar]