Abstract
Background
The effect of the COVID pandemic on stroke network performance is unclear, particularly with consideration of drip&ship vs. mothership models.
Aims
We systematically reviewed and meta-analyzed variations in stroke admissions, rate and timing of reperfusion treatments during the first wave COVID pandemic vs. the pre-pandemic timeframe depending on stroke network model adopted.
Summary of findings
The systematic review followed registered protocol (PROSPERO-CRD42020211535), PRISMA and MOOSE guidelines. We searched MEDLINE, EMBASE, and CENTRAL until 9 October 2020 for studies reporting variations in ischemic stroke admissions, treatment rates, and timing in COVID (first wave) vs. control-period. Primary outcome was the weekly admission incidence rate ratio (IRR = admissions during COVID-period/admissions during control-period). Secondary outcomes were (i) changes in rate of reperfusion treatments and (ii) time metrics for pre- and in-hospital phase. Data were pooled using random-effects models, comparing mothership vs. drip&ship model. Overall, 29 studies were included in quantitative synthesis (n = 212,960). COVID-period was associated with a significant reduction in stroke admission rates (IRR = 0.69, 95%CI = 0.61–0.79), with higher relative presentation of large vessel occlusion (risk ratio (RR) = 1.62, 95% confidence interval (CI) = 1.24–2.12). Proportions of patients treated with endovascular treatment increased (RR = 1.14, 95%CI = 1.02–1.28). Intravenous thrombolysis decreased overall (IRR = 0.72, 95%CI = 0.54–0.96) but not in the mothership model (IRR = 0.81, 95%CI = 0.43–1.52). Onset-to-door time was longer for the drip&ship in COVID-period compared to the control-period (+32 min, 95%CI = 0–64). Door-to-scan was longer in COVID-period (+5 min, 95%CI = 2–7). Door-to-needle and door-to-groin were similar in COVID-period and control-period.
Conclusions
Despite a 35% drop in stroke admissions during the first pandemic wave, proportions of patients receiving reperfusion and time-metrics were not inferior to control-period. Mothership preserved the weekly rate of intravenous thrombolysis and the onset-to-door timing to pre-pandemic standards.
Keywords: Stroke, mothership, drip and ship, stroke network, COVID
Introduction
Stroke care is based on fast rescue, rapid assessment, quick transportation according to local stroke network model and standardized management.1,2 Rapid definition of stroke syndromes and reperfusion of salvageable tissue is mandatory to increase chances of living in functional independence later in life.1–3
In December 2019, the emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), in China, gradually evolved into a pandemic, with the death toll steadily increasing. The outbreak has forced national and local authorities to adapt emergency services to the need of the hour, and to impose global and unprecedented restrictions, from social distancing to national lockdown. The impact of such regulations on people perception of health problems and on their seeking of medical care has yet to be fully understood. Preliminary reports highlighted a potential contraction in presentation to the emergency department (ED) for acute time-dependent diseases, including acute ischemic stroke.4–9 However, large studies also reported higher rates of stroke admissions during the pandemic,4,10 potentially attributable to a higher risk of stroke with SARS-CoV-2 infection.11,12 Therefore, we lack conclusive data to quantify the variation in stroke admissions and time metrics of treatments provided.
The impact of the organization of the stroke network on the management of acute ischemic stroke under such unprecedented circumstances has been unexplored. Mothership model, based on direct admission to a comprehensive stroke center, and drip&ship (D&S) model, based on first assessment in spoke centers and transfer to a hub center, can critically impact time metrics.2,13 Therefore, the stroke network model might have consistently contributed to the variations in performance of the pre- and in-hospital service through the pandemic. As the pandemic still spreads, with countries still knee-deep in second waves, understanding how our stroke networks have adapted on a global scale seems crucial to program what to do in the near future, and advocate with health institutions for local needs.
Here, we provide a systematic review and meta-analysis of stroke admissions, rate, and timing of reperfusion treatments during the first phase of COVID-19 pandemic in comparison to a standard-of-care timeframe. We included stroke network organization as a variable to define the potential benefit of a chosen paradigm in such peculiar circumstances.
Materials and methods
Search strategy
The methods and guidelines of this study-level meta-analysis followed the PRISMA 14 and MOOSE 15 guidelines. The study protocol was registered with PROSPERO (CRD42020211535, principal investigator (PI) – M Romoli). We systematically searched Pubmed, EMBASE, and Cochrane CENTRAL for studies addressing ischemic stroke admissions, treatment, and time metrics across the COVID-19 pandemic (COVID-period) published from 2 June 2019 to 9 October 2020. Such timeframe was used to ensure a strict focus on the first phase of the pandemic, allowing for variations according to spread across countries. We used the following keywords in addition to Medical Subject Headings terms to build up the search protocol: ischemic stroke, cerebrovascular disease, cerebrovascular accident, coronavirus, severe acute respiratory syndrome coronavirus 2, COVID, COVID19, COVID-19, novel coronavirus, nCoV, SARS-CoV2 (Supplementary Material). We used forward and backward citation tracking to improve the results.
Eligibility criteria
We included all studies, prospective or retrospective, reporting original data on variations in stroke epidemiology, admissions, and stroke network performance over the COVID-period. We limited the studies to English language and excluded case reports, small case series (<30), conference proceedings/reviews. Studies not reporting data on consecutive stroke admissions or timing of rescue and treatment were excluded, as those including data for cumulative cerebrovascular diseases (e.g. hemorrhagic/ischemic stroke, stroke/TIA) or figure-only dissertation of undisclosed data. We also excluded duplicates of the same dataset and studies reporting stroke rates in patients diagnosed with SARS-CoV2. Four reviewers screened available literature (LP, MG, SF, FG) and selected studies according to pre-specified criteria; disagreement between reviewers was resolved by the corresponding author (MR).
Data extraction and subgroup analysis
Four reviewers (LP, MG, SF, FG) independently evaluated the titles and abstracts of retrieved articles and screened for the full texts based on predefined criteria. Disagreements were resolved by the corresponding author. We collected data concerning design, stroke admissions, setting, population served, timeframes explored, baseline characteristics including National Institute of Health Stroke Scale (NIHSS) and treatment timing. We contacted authors of included studies reporting number of strokes in the two periods to improve results regarding stroke network organization, population served, and total number of stroke admissions and treatments provided. We reported the lack of data, when appropriate. When multiple distinct time periods were used as control-periods, whenever possible we selected data from the previous year to reduce the influence of seasonal variability; alternatively, summary and average measures from control-periods were used. 16 Subgroup analysis was planned according to the stroke network model. Studies reporting data from multiple centers were considered according to the predominant network model whenever center-specific data were not available. For D&S and mothership definition, whenever a comprehensive stroke center received patients already treated with intravenous thrombolysis (IVT) to perform endovascular treatment (EVT), the paradigm was defined as D&S.
Publication bias and risk of bias assessment
Publication bias was measured with the Egger’s regression test, and visualized in case of significant findings by funnel plots. Critical appraisal and methodological quality assessment was performed with the Newcastle-Ottawa scale (NOS), as previously validated and reported,2,17 and included assessment of selection of cohort explored, control cohort, length and adequacy of observation, and comparability of cohorts.2,18 We summarized the assessment as low, moderate, or high risk of bias. 17
Outcomes
The primary outcome was variation in weekly admission rate of stroke admissions during the COVID-period compared to the control-period. Study-dependent timeframes were used for all outcomes, as the pandemic evolved progressively but not contemporarily in all countries. The secondary outcomes included: (i) changes in weekly IVT and EVT rates, (ii) the proportion of IVT and EVT over those admitted, (ii) changes in time metrics for pre-hospital and in-hospital phase during COVID-period vs. control-period. Last-known-well/onset-to-door, door-to-needle, door-to-scan, door-to-groin, onset-to-needle, and door-to-reperfusion were extracted and calculated. Primary and secondary outcomes were also compared according to the stroke network model adopted (D&S vs. mothership).
Statistical analysis
We pooled data from COVID-period vs. control-period according to original study definition. Outcome heterogeneity was evaluated with Cochrane’s Q-test and I2 statistic. An overall p-value of <.05 was considered statistically significant.
Stroke admission and reperfusion rate ratio: We calculated weekly incidence rates for admissions (IR) and reperfusion treatments (IR-IVT; IR-EVT) for all stroke centers with disclosed estimates of population served, using number of weeks of each timeframe to standardize result (IR = stroke/100,000/week). We pooled IR through meta-analysis of event count, deriving IR estimates for each timeframe (IRCOVID, IRcontrol). We then pooled the incidence rate ratio for stroke admissions (IRR = IRCOVID/IRcontrol), 19 IVT and EVT through meta-analysis, displaying respective estimates for D&S and mothership models. Random-effects model (DerSimonian and Laird method) was preferred to account for heterogeneity in catchment area, design, and outcome assessment, as we previously reported.2,18 Combinatorial meta-analysis was implemented for the stroke admissions, IVT, and EVT IRR, and reported graphically. 2 Meta-regression analysis was performed whenever trends of pooled estimates differed depending on network model, and if more than five studies were available overall.
Reperfusion treatments: We used meta-analysis of proportions to estimate the rate of IVT and EVT among all those admitted during each timeframe; our aim was to identify fluctuations in treatment rates rather than absolute reduction of treatments. Risk ratio (RR) was used to pool estimates, considering the control-period as baseline and the COVID-period as experimental. RR and respective 95% confidence interval (CI) were systematically calculated according to network model (D&S vs. mothership). We planned sensitivity analysis (i) excluding studies with unknown stroke network organization and (ii) including studies with prospective design only.
Time measures: Continuous variables, including stroke severity, as defined by NIHSS, and time metrics, were expressed as means and standard deviations. Onset/last-known-well-to-door, onset-to-needle, door-to-needle, door-to-scan, door-to-groin, and door-to-reperfusion were extracted. In cases where multiple time measures were available, time measures for IVT were selected for stroke-to-door and door-to-needle time, with time measures for door-to-groin were derived from EVT data. Mean and variance were calculated according to reported methodology whenever medians and IQR were available. 20 We calculated mean difference (MD) in time (minutes) and NIHSS score (points) through meta-analysis of continuous outcome based on random-effect model, reporting MD and 95%CI. Meta-regression analysis was performed whenever differences in trend of pooled estimates emerged depending on network model, and if more than five studies were available overall. Sensitivity analysis involved: (i) analysis excluding studies with unknown stroke network organization and (ii) analysis including studies with prospective design only.
Data analysis was performed with R3.3.1.
Results
Literature review and characteristics of included studies
Of 461 records identified with systematic search, 110 fully assessed (Supplemental Figure I for PRISMA flow-chart, Supplemental Table I for excluded studies). Twenty-nine studies were included in qualitative synthesis (Table 1). Overall, data regarding 212,960 patients were collected from three continents (Europe, Asia, America). Mean length of the COVID-period was 7.2 ± 2.9 weeks vs. 11.2 ± 12.3 weeks for control-period, with the latter consisting of standard workflow data from 2019 in 16/29 studies (55.2%). COVID-period varied according to the spread of the pandemic, starting from January in studies performed in eastern countries,21,22 March in European countries,4,23 and up to May in western countries24–26 (Table 1). All studies focused on stroke admissions, none having appropriate catchment methods or pursuit to provide incidence data. Five studies had prospective data collection,21,27–30 while most of the studies were retrospective in nature. Four studies used data collected for the American Heart Association Get-with-the-guideline study.10,24,31,32 Two studies reported data from electronic-based registries, with aggregated data available only16,25; two studies were reperfusion registries27,33 and were therefore included for time metrics. Nine studies reported data on stroke networks organized according to a preferred mothership model4,21–23,28,32,34–36 (Table 1).
Table 1.
Characteristics of the included studies
| Reference | Year | Region (country) | Design | Setting | COVID start | COVID end | Stroke network model | Population served |
|---|---|---|---|---|---|---|---|---|
| Agarwal et al. 24 | 2020 | New York City (USA) | Retrospective a | Single center | 1 Mar 2020 | 15 May 2020 | Unknown | Unknown |
| Baracchini et al. 38 | 2020 | Veneto (Italy) | Retrospective | Single center | 1 Feb 2020 | 31 Mar 2020 | Drip&ship | Unknown |
| de Havenon et al. 16 | 2020 | USA | Retrospective | Multicenter | 1 Feb 2020 | 31 Mar 2020 | Unknown | Unknown |
| Diegoli et al. 28 | 2020 | Joinville (Brazil) | Prospective | Multicenter | 17 Mar 2020 | 15 Apr 2020 | Mothership | 590,466 |
| Esenwa et al. 39 | 2020 | New York City (USA) | Retrospective | Multicenter | 1 Jan 2020 | 25 Feb 2020 | Drip&ship | 2,000,000 |
| Hsiao et al. 40 | 2020 | Ohio, Kentucky, Indiana (USA) | Retrospective | Multicenter | 9 Mar 2020 | 12 Apr 2020 | Drip&ship | 2,100,000 |
| Huang et al. 37 | 2020 | Mayo Clinic (USA) | Retrospective | Multicenter | 11 Mar 2020 | 09 Apr 2020 | Drip&ship | Unknown |
| Jasne et al. 32 | 2020 | Connecticut (USA) | Retrospective a | Multicenter | 1 Mar 2020 | 28 Apr 2020 | Mothership b | Unknown |
| Kerleroux et al. 27 c | 2020 | France | Prospective | Multicenter national level | 15 Feb 2020 | 30 Mar 2020 | Unknown | Unknown |
| Mehrpour et al. 41 | 2020 | Iran | Retrospective | Single center | 15 Feb 2020 | 15 Apr 2020 | Drip&ship | 2,000,000 |
| Montaner et al. 29 | 2020 | Seville (Spain) | Prospective | Multicenter | Unknown | Unknown | Drip&ship | 2,450,000 |
| Naccarato et al. 6 | 2020 | Trieste (Italy) | Retrospective | Single center | 9 Mar 2020 | 09 Apr 2020 | Drip&ship | 373,803 |
| Nguyen-Huynh et al. 10 | 2020 | Northern California (USA) | Retrospective a | Multicenter | 15 Mar 2020 | 09 May 2020 | Drip&ship | 4,400,000 |
| Onteddu et al. 25 | 2020 | USA | Retrospective | TriNetX platform | 20 Jan 2020 | 16 May 2020 | Unknown | Unknown |
| Paliwal et al. 34 | 2020 | Singapore | Retrospective | Single center | 7 Feb 2020 | 30 Apr 2020 | Mothership | 1,300,000 |
| Pandey et al. 19 | 2020 | Michigan and North-West Ohio (USA) | Retrospective | Multicenter | 1 Mar 2020 | 31 Mar 2020 | Unknown | Unknown |
| Perry et al. 35 | 2020 | North Central London (UK) | Retrospective | Single center | 1 Apr 2020 | 11 May 2020 | Mothership | 1,270,000 |
| Pop et al. 36 | 2020 | Alsace (France) | Retrospective | Multicenter | 1 Mar 2020 | 31 Mar 2020 | Mothership | 1,900,000 |
| Rudilosso et al. 30 | 2020 | Barcelona (Spain) | Prospective | Single center | 1 Mar 2020 | 31 Mar 2020 | Drip&ship d | 2,740,000 |
| Saxhaug Kristoffersen et al. 23 | 2020 | Akershus University Hospital (Norway) | Retrospective, observational | Single center | 13 Mar 2020 | 30 Apr 2020 | Mothership | 570,000 |
| Schirmer et al. 31 | 2020 | USA | Retrospective a | Multicenter | 1 Mar 2020 | 28 Mar 2020 | Unknown | Unknown |
| Siegler et al. 42 | 2020 | Cooper University Hospital (NJ, USA) | Retrospective | Single center | 1 Mar 2020 | 15 Apr 2020 | Drip&ship | 2,500,000 |
| Strasser et al. 26 | 2020 | Florida (USA) | Retrospective | Single center | 1 Mar 2020 | 30 Apr 2020 | Unknown | Unknown |
| Tejada Meza et al. 5 | 2020 | Aragon (Spain) | Retrospective | Multicenter | 14 Mar 2020 | 19 Apr 2020 | Drip&ship e | 1,319,291 |
| Tejada Meza et al. 43 | 2020 | Aragon (Spain) | Retrospective | Multicenter | 09 Mar 2020 | 03 May 2020 | Drip&ship e | 11,500,000 |
| Teo et al. 22 | 2020 | Hong Kong | Retrospective | Single center | 23 Jan 2020 | 24 Mar 2020 | Mothership | 500,000 |
| Yang et al. 21 | 2020 | Beijing (China) | Prospective | Single center | 23 Jan 2020 | 07 Mar 2020 | Mothership | Unknown |
| Zhao et al. 33 c | 2020 | China | Retrospective | Multicenter (China Registry) | 01 Jan 2020 | 29 Feb 2020 | Unknown | Unknown |
| Zini et al. 4 | 2020 | Bologna (Italy) | Retrospective | Single center | 01 Mar 2020 | 30 Apr 2020 | Mothership | 1,100,000 |
Get-with-the-guidelines AHA study (except Aragon, mothership).
Network based on drip&ship, reported data only for comprehensive stroke center mothership admissions (own catchment area).
Endovascular treatment registry.
Except direct admission to comprehensive stroke center with mothership paradigm in limited central area.
Except Aragon (mothership).
Publication bias, risk of bias, and heterogeneity assessment
Funnel plots for studies reporting stroke admissions and reperfusion treatment ratio in the two periods explored are displayed in Supplemental Figure II. Egger’s regression test did not detect publication bias (pEgger = .71, .27, and .96, respectively). Bias assessment demonstrated an overall good quality for included studies: 25/29 had low risk of bias (NOS score ≥ 7), 5 had low to moderate quality mainly in relation to selection and comparability bias (Supplemental Table II). Duration of observation for COVID-period was <5 weeks in nine studies.6,19,28–31,35–37 The study of lowest quality was excluded from quantitative analysis due to undisclosed data and ascertainment methods. 38
The overall estimates for the primary outcome, weekly IVT/EVT IRR and proportion of patients receiving IVT had low heterogeneity (I2 = 0%, p > .10; Figures 1 and 2, Supplemental Figure IV). Heterogeneity was mild for estimates of proportions of EVT among patients admitted (I2 = 40%, pheterogeneity = .03, Figure 3) and onset-to-door time (I2 = 62%, pheterogeneity < .01, Figure 4). In both cases, heterogeneity mainly derived from studies with unknown network organization (I2 > 70%, pheterogeneity < .05, Figures 3 and 4), with estimates from D&S and mothership studies showing low heterogeneity (Figures 3 and 4, Supplemental Figure VIII). Door-to-groin had mild heterogeneity (I2 = 52%, pheterogeneity = .04), with low heterogeneity in D&S stratum and mild heterogeneity in mothership stratum (I2 = 65%, pheterogeneity = .02, Supplemental Figure XIII). Analysis of the remaining time metrics was conducted under low heterogeneity.
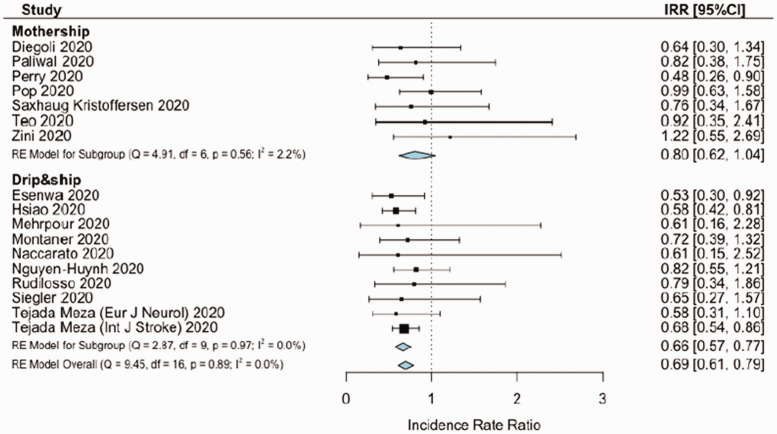
Figure 1.
Meta-analysis of incidence rate ratio, comparing stroke admissions during COVID-period vs. control-period, depending on stroke network model.
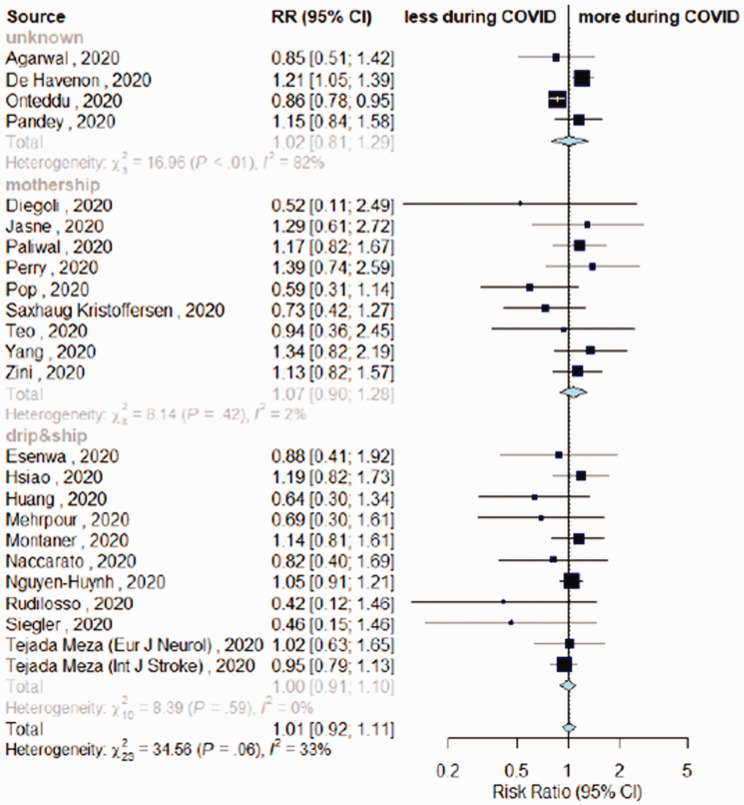
Figure 2.
Differences in rate of intravenous thrombolysis (IVT) among admitted patients in COVID-19 vs. control-period, depending on stroke network model adopted.
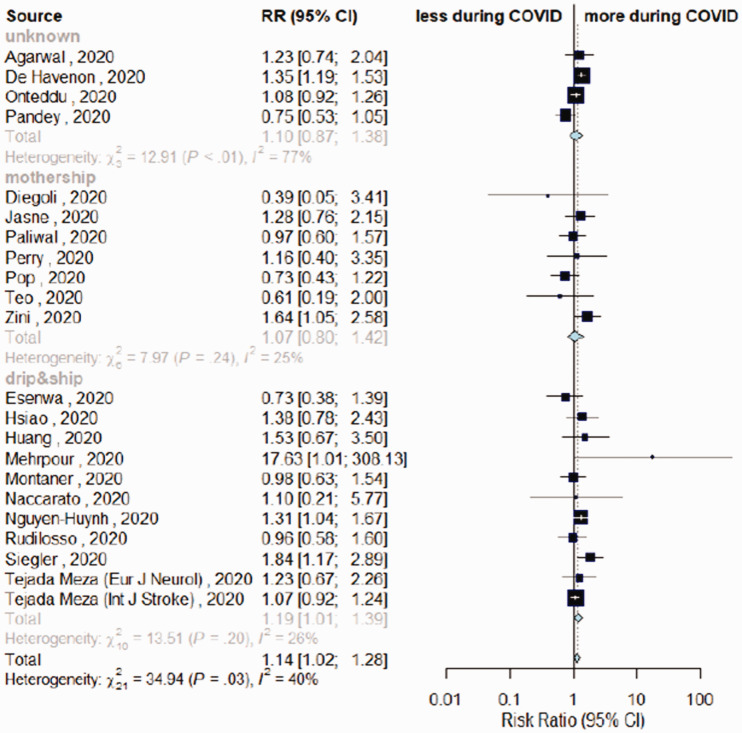
Figure 3.
Differences in rate of endovascular thrombectomy (EVT) among patients admitted during COVID-19 vs. control-period, depending on stroke network model adopted.
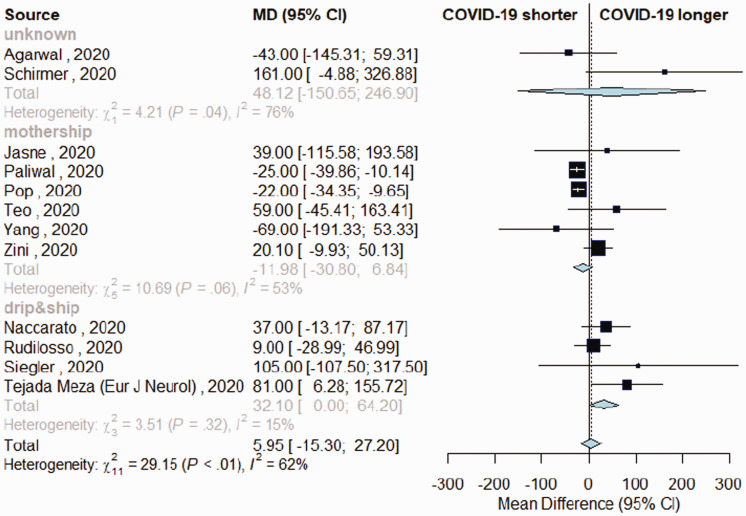
Figure 4.
Variation in onset-to-door time in patients with acute ischemic stroke admitted during COVID-19 and control-period, depending on stroke network model adopted.
Outcome of meta-analysis
Stroke admission and reperfusion treatment rate ratios
Seventeen studies reported data on stroke admissions during the pandemic and respective catchment areas, for a global served population of 38.6 million inhabitants across three continents (n = 38,613,560). COVID-period was associated with a significant reduction in stroke admission rate (IRR = 0.69, 95%CI = 0.61–0.79). COVID-period was associated with a slight trend in reduction of stroke admissions for mothership paradigm (IRR = 0.8, 95%CI = 0.61–1.04), while the reduction seemed significant for D&S model (IRR = 0.66, 95%CI = 0.57–0.77). Sensitivity combinatorial analysis did not highlight potential clusters or outliers (Supplemental Figure III). IVT weekly rates decreased during COVID-period (IRR = 0.72, 95%CI = 0.54–0.96). IVT weekly rates remained stable to pre-pandemic levels in mothership paradigm, while IVT weekly rates significantly decreased in D&S model (IRR-IVT = 0.7, 95%CI = 0.5–0.96) (Supplemental Figure IV). EVT rates were unchanged during COVID-period compared to control-period, independently from stroke network model (IRR-EVT = 0.79, 95%CI = 0.58–1.08) (Supplemental Figure IV). Mean NIHSS had a minor increase during COVID-period only in mothership studies (MD = 0.82, 95%CI = 0.05–1.58, Supplemental Figure V). Among people admitted with acute ischemic stroke in each timeframe, large vessel occlusions (LVO) were more frequent during COVID-period compared to control-period (RR = 1.62, 95%CI = 1.24–2.12, Supplemental Figure VI).
Reperfusion treatments
Twenty-four studies reported IVT among patients admitted during COVID- and control-period (n = 160,554).4–6,10,16,19,21–25,28–30,32,34–37,39,40–43 The proportion of people receiving IVT among those admitted for acute ischemic stroke was similar across timeframes (RR = 1.01, 95%CI = 0.92–1.11, Figure 2, Supplemental Figure VII).
Twenty-three studies reported EVT among patients admitted during the two timeframes.4–6,10,16,19,21,22,24,25,28–30,32,34–37,39,40,41–43 A higher proportion of people among those admitted received EVT during COVID-period compared to control-period (RR = 1.14, 95%CI = 1.02–1.28, Figure 3). A significant higher proportion of patients treated with EVT emerged during COVID-timeframe with D&S (RR = 1.19, 95%CI = 1.01–1.39), although not differing from mothership model (RR = 1.07, 95%CI = 0.80–1.42, pmeta-regression = 0.52). Sensitivity analysis removing studies with unknown network organization confirmed a marginal increase in EVT rates during COVID-period (RR = 1.12, 95%CI = 0.99–1.27; Supplemental Figure VIII).
Time metrics
Comparing onset-to-door time, no substantial differences emerged between periods (Figure 4). However, mothership and D&S models differed, with the latter associated with longer interval (MD +32 min, 95%CI = 0–64) and the former having non-significant shorter interval during COVID-period (MD –12 min, 95%CI = (-30)-(+7) minutes, Supplemental Figure IX). The mothership model had a significant impact on shortening onset-to-door time, as confirmed by meta-regression analysis (p = .03, Supplemental Table III). Door-to-scan time was longer during COVID-period in both models (MD = 5 min, 95%CI = 2–7, Supplemental Figure X). Door-to-needle, door-to-groin, onset-to-needle, and door-to-recanalization time were similar across timeframes (Supplemental Figures XI to XV), although for the latter data were not available from studies using D&S model.
Discussion
This meta-analysis compared stroke network performance before and during the COVID-19 pandemic, revealing that, despite a substantial reduction in stroke admissions, the time metrics and the proportion of patients undergoing reperfusion treatments remained unchanged compared to pre-pandemic period. Stroke admissions dropped by 35%, a substantial reduction with slight impact from the stroke network model adopted, with the mothership model having marginally lower decline compared to D&S. At the same time, the proportion of people admitted with LVO increased, justifying the hypothesis that people with transient ischemic attack or very minor stroke might have avoided in-person consultation due to safety concerns.4,7,40,44 To this regard, considering that the proportion of patients undergoing reperfusion treatments and their time metrics were not reduced during the pandemic, stroke awareness campaigns seem to represent a priority to prompt people with minor stroke to seek medical attention. The only study that reported an increase in stroke admission rates during COVID-period was performed in Emilia-Romagna, a region advertising a stroke awareness campaign (https://salute.regione.emilia-romagna.it/campagne/ictus-vedo-riconosco-chiamo) immediately before the pandemic, providing a potential proof of concept. 4
While proportions of patients treated with IVT among all those admitted with stroke were similar across timeframes, EVT increased during COVID-period. This should be put in context of a 60% relative increase in LVO presentation during the pandemic, potentially in relation to the avoidance of searching hospital/medical attention during the COVID-period in case of minor symptoms. Both mothership and D&S models had higher proportions of EVT, although mothership model associated with shorter onset-to-door time compared to D&S. The increase in rate of LVO and EVT might be attributable to several factors, first of all from the adoption of protocols in line with the DEFUSE345 and DAWN 46 trials. To this extent, the marginal increase in mean NIHSS during COVID-period supports a difference in diagnostic approach rather than only in stroke severity. At the same time, the potential vascular complications of SARS-CoV-211 and the reduced compliance with antithrombotic medications during the pandemic47,48 might have further contributed to the higher risk of LVO. Regarding IVT variations, it might be useful to highlight that mothership preserved the rates of treatment during the COVID-period, as opposed to D&S, which faced a 30% reduction in weekly IVT rates. This result seems in line with a lower reduction in stroke admissions with mothership paradigm compared to D&S, which might have contributed to preserving the overall weekly IVT rates.
The longer onset-to-door time found in the D&S model has to be analyzed with caution. The stroke network model adopted depends on geography and logistics. Several hospitals hosting stroke spoke centers have been redeployed to COVID-hospitals, which might have impacted on time metrics of the stroke network, as well as on people safety concerns in accessing the ED. 49 Mothership model might be more elastic compared to D&S given the fact that it usually deals with higher volumes, such as those happening during the pandemic, and might therefore more easily maintain the standards for timing of rescue and treatment.2,4 To this regard, we previously reported shorter timing for rescue and treatment after transitioning from a D&S to a mothership model during the pandemic, 50 an option that might therefore be taken into account during the second wave. In this meta-analysis, the door-to-needle and onset-to-needle time did not differ between timeframes as well as between network models. However, data were available for few studies, and the door-to-recanalization time was only available for mothership-based studies. Therefore, how a delay in onset-to-door can translate into longer onset-to-treatment time during the pandemic has yet to be defined. To this regard, it seems critical to organize both COVID-free and COVID-positive pathways for stroke care to deliver treatment with the same time metrics regardless of the infective status of the patient.4,8,11 Brain imaging represents a critical stage to limit delays, and availability of advanced CT imaging in both pathways might be crucial to limit door-to-scan time.4,50
Limitations of this meta-analysis can be seen in the heterogeneity of the study included, which span geographically and in time, following the first pandemic wave. However, the variation in stroke admissions and treatments is in line with reports from national-level surveys,44,51 lending weight to our results. The local changes to the availability of stroke beds might have impacted on stroke care, but our approach, considering stroke admissions and relative rates of reperfusion treatments, limited the bias in our results. A second limitation derives from the stroke network model adopted, which was not available in some studies. To this extent, sensitivity analysis has been provided to support the findings, with little differences emerging for main estimates. Third, onset-to-needle time was available for few studies, preventing the identification of subtle delays in treatment delivery. However, results seem to have little heterogeneity, and the adherence to the rate of reperfusion treatments provided during normal conditions also during COVID-period suggests that little if no impact remained from pre-hospital delay on treatment delivery. Fourth limitation, our systematic review did not include treatment outcome, a limitation that limits our conclusions to stroke networks rather than on stroke care. However, preliminary findings suggests that, when treatment delivery matches standards, the benefit on functional outcome is maintained.9,52 Finally, our results derived from the first pandemic wave only, therefore representing a “worse-case scenario” which might not be applicable to future waves. Overall, as we are still facing and are expected to face in the near future other pandemic waves, with further personal and medical burden, the picture emerging from this meta-analysis lends support to our efforts to ensure high-quality acute stroke care in global healthcare systems.
Conclusions
The main finding of this meta-analysis is that, despite contraction in admissions, stroke networks have been able to deliver similar rates of treatment to those provided in pre-pandemic period, with similar timing, with both D&S and mothership models. Such findings suggest that stroke networks can deal with the pandemic wave, and that neurologists should advocate with local institutions to allocate appropriate resources to keep stroke networks in function, or adapt to local circumstances. Few studies included in this systematic review reported that Healthcare Authorities refrained from limiting the resources and capacity of stroke units.4,27,30 The results of this meta-analysis are critical to sustain and encourage such attitude.
Supplemental Material
Supplemental material, sj-pdf-1-wso-10.1177_17474930211041202 for Stroke network performance during the first COVID-19 pandemic stage: A meta-analysis based on stroke network models by Michele Romoli, Paolo Eusebi, Stefano Forlivesi, Mauro Gentile, Fabrizio Giammello, Laura Piccolo, David Giannandrea, Simone Vidale, Marco Longoni, Matteo Paolucci, Jessica Hsiao, Emily Sayles, Leonard LL Yeo, Espen Saxhaug Kristoffersen, Angel Chamorro, Liqun Jiao, Pooja Khatri, Georgios Tsivgoulis, Maurizio Paciaroni and Andrea Zini in International Journal of Stroke
Acknowledgements
The authors would like to thank all professionals involved in stroke care during the pandemic. The authors would also like to thank the authors of all studies included in this review, which greatly helped sharing their data, with particular regard to: Prof Tejada Meza (Hospital Macarena, Sevilla, Spain), Prof Siegler (Cooper University Hospital, NJ, USA), Prof Huang (Mayo Clinic, Jacksonville, USA), Prof Esenwa (Montefiore Medical Center, NY, USA), Prof Farahani (Teheran Imam Hossein Hospital, Iran), Prof Diegoli (Hospital São José, Joinville/SC, Brasil), Dr Schirmer (Geisinger Health System, Pennsylvania, USA), Prof Kui Kai Lao (University of Hong Kong, Hong Kong), Dr Perry (University College Hospital, London), Prof Jasne (Yale Medicine, USA) for sharing details regarding their original studies.
Declaration of conflicting interests: The authors have no conflict of interest related to this work. Relevant disclosures outside the submitted work: PK declares grants from the National Institutes of Health, Nervive, and Cerenovus to her department, and payments to her department from Bayer and Genentech for her role as principal investigator for clinical trials; AZ declares speaker and consulting fees from Boehringer-Ingelheim, Cerenovus and advisory board from Boehringer-Ingelheim and Stryker and declares grant from the Italian Ministry of Health as principal investigator for clinical trial (RF-2019-12370834). All other authors have nothing to declare.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
These authors are senior authors.
ORCID iDs
Michele Romoli https://orcid.org/0000-0001-8009-8543
Fabrizio Giammello https://orcid.org/0000-0002-2828-1305
David Giannandrea https://orcid.org/0000-0001-5931-8986
Simone Vidale https://orcid.org/0000-0003-1426-0885
Espen Saxhaug Kristoffersen https://orcid.org/0000-0002-8999-5424
Angel Chamorro https://orcid.org/0000-0003-0493-5340
Liqun Jiao https://orcid.org/0000-0003-4982-6295
Georgios Tsivgoulis https://orcid.org/0000-0002-0640-3797
Andrea Zini https://orcid.org/0000-0003-1486-4507
References
- 1.Campbell BCV, Khatri P. Stroke. Lancet 2020; 396: 129–142. [DOI] [PubMed] [Google Scholar]
- 2.Romoli M, Paciaroni M, Tsivgoulis G, et al. Mothership versus drip-and-ship model for mechanical thrombectomy in acute stroke: a systematic review and meta analysis for clinical and radiological outcomes. J Stroke 2020; 22: 317–323. [DOI] [PMC free article] [PubMed]
- 3.Tsivgoulis G, Katsanos AH, Schellinger PD, et al. Successful reperfusion with intravenous thrombolysis preceding mechanical thrombectomy in large vessel occlusions. Stroke 2018; 49: 232–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zini A, Romoli M, Gentile M, et al. The stroke mothership model survived during COVID-19 era: an observational single center study in Emilia Romagna, Italy. Neurol Sci 2020; 41: 3395–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tejada Meza H, Lambea Gil Á, Sancho Saldaña A, et al. Ischaemic stroke in the time of coronavirus disease 2019. Eur J Neurol 2020; 27: 1788–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naccarato M, Scali I, Olivo S, et al. Has COVID-19 played an unexpected “stroke” on the chain of survival? J Neurol Sci 2020; 414: 116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsivgoulis G, Katsanos AH, Ornello R, et al. Ischemic stroke epidemiology during the COVID-19 pandemic: navigating uncharted waters with changing tides. Stroke 2020; 51: 1924–1926. [DOI] [PMC free article] [PubMed]
- 8.Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke 2020. doi:10.1161/STROKEAHA.120.029838. [DOI] [PMC free article] [PubMed]
- 9.Kerleroux B, Fabacher T, Bricout N, et al. Mechanical thrombectomy for acute ischemic stroke amid the COVID-19 outbreak. Stroke 2020. doi:10.1161/STROKEAHA.120.030373. [DOI] [PubMed]
- 10.Nguyen Huynh MN, Tang XN, Vinson DR, et al. Acute stroke presentation, care, and outcomes in community hospitals in Northern California during the COVID-19 pandemic. Stroke 2020; 51: 2918–2924. [DOI] [PMC free article] [PubMed]
- 11.Katsanos AH, Palaiodimou L, Zand R, et al. The impact of SARS-CoV-2 on stroke epidemiology and care: a meta analysis. Ann Neurol 2020; 0–3. [DOI] [PMC free article] [PubMed]
- 12.Romoli M, Jelcic I, Bernard-Valnet R, et al. A systematic review of neurological manifestations of SARS CoV 2 infection: the devil is hidden in the details. Eur J Neurol 2020; 27: 1712–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holodinsky JK, Williamson TS, Kamal N, et al. Drip and ship versus direct to comprehensive stroke center. Stroke 2017; 48: 233–238. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed]
- 15.Stroup DF, Berlin JA, Morton SC, et al. Meta analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283: 2008–2012. [DOI] [PubMed] [Google Scholar]
- 16.de Havenon A, Ney J, Callaghan B, et al. A rapid decrease in stroke, acute coronary syndrome, and corresponding interventions at 65 United States hospitals following emergence of COVID-19. medRxiv. Prepr Serv Heal Sci. Epub ahead of print 2020. doi:10.1101/2020.05.07.20083386.
- 17.Luchini C, Stubbs B, Solmi M, et al. Assessing the quality of studies in meta analyses: advantages and limitations of the Newcastle Ottawa Scale. World J Meta Analysis 2017; 5: 80. [Google Scholar]
- 18.Vidale S, Romoli M, Consoli D, et al. Bridging versus direct mechanical thrombectomy in acute ischemic stroke: a subgroup pooled meta analysis for time of intervention, eligibility, and study design. Cerebrovasc Dis 2020; 1–10. [DOI] [PubMed]
- 19.Pandey AS, Daou BJ, Tsai JP, et al. Letter: COVID-19 pandemic – the bystander effect on stroke care in Michigan. Neurosurgery 2020; 2020–2022. [DOI] [PMC free article] [PubMed]
- 20.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang B, Wang T, Chen J, et al. Impact of the COVID-19 pandemic on the process and outcome of thrombectomy for acute ischemic stroke. J Neurointerv Surg 2020; 1–5. [DOI] [PubMed]
- 22.Teo K-C, Leung WCY, Wong Y K, et al. Delays in stroke onset to hospital arrival time during COVID-19. Stroke 2020. doi:10.1161/STROKEAHA.120.030105. [DOI] [PMC free article] [PubMed]
- 23.Saxhaug Kristoffersen E, Holt Jahr S, Thommessen B, et al. Effect of COVID-19 pandemic on stroke admission rates in a Norwegian population. Acta Neurol Scand 2020; 0–2. [DOI] [PMC free article] [PubMed]
- 24.Agarwal S, Scher E, Rossan-Raghunath N, et al. Acute stroke care in a New York City comprehensive stroke center during the COVID-19 pandemic. J Stroke Cerebrovasc Dis 2020; 29: 105068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onteddu SR, Nalleballe K, Sharma R, et al. Underutilization of Healthcare for strokes during the COVID-19 outbreak. Int J Stroke 2020. doi:10.1177/1747493020934362. [DOI] [PubMed]
- 26.Strasser S, Miskolczi L, Cunha J, et al. COVID-19 impact on stroke presentations. J Stroke Cerebrovasc Dis 2020; 29: 105077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerleroux B, Fabacher T, Bricout N, et al. Mechanical thrombectomy for acute ischemic stroke amid the COVID-19 outbreak. Stroke. Epub ahead of print 20 May 2020. doi:10.1161/STROKEAHA.120.030373. [DOI] [PubMed]
- 28.Diegoli H, Magalhães PSC, Martins SCO, et al. Decrease in hospital admissions for transient ischemic attack, mild, and moderate stroke during the COVID-19 Era. 2020; 51: 2315–2321. [DOI] [PMC free article] [PubMed]
- 29.Montaner J, Barragán-Prieto A, Pérez Sánchez S, et al. Break in the stroke chain of survival due to COVID-19. Stroke 2020. doi:10.1161/STROKEAHA.120.030106. [DOI] [PMC free article] [PubMed]
- 30.Rudilosso S, Laredo C, Vera V, et al. Acute stroke care is at risk in the era of COVID-19: experience at a comprehensive stroke center in Barcelona. Stroke 2020. doi:10.1161/STROKEAHA.120.030329. [DOI] [PMC free article] [PubMed]
- 31.Schirmer CM, Ringer AJ, Arthur AS, et al. Delayed presentation of acute ischemic strokes during the COVID-19 crisis. J Neurointerv Surg 2020; 1–4. [DOI] [PubMed]
- 32.Jasne AS, Chojecka P, Maran I, et al. Stroke code presentations, interventions, and outcomes before and during the COVID-19 pandemic. Stroke 2020; 51: 2664–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J, Li H, Kung D, et al. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke 2020. doi:10.1161/STROKEAHA.120.030225. [DOI] [PMC free article] [PubMed]
- 34.Paliwal PR, Tan BYQ, Leow AST, et al. Impact of the COVID-19 pandemic on hyperacute stroke treatment: experience from a comprehensive stroke centre in Singapore. J Thromb Thrombolysis 2020; 2019. [DOI] [PMC free article] [PubMed]
- 35.Perry R, Banaras A, Werring DJ, et al. What has caused the fall in stroke admissions during the COVID-19 pandemic? J Neurol 2020; 1–2. [DOI] [PMC free article] [PubMed]
- 36.Pop R, Quenardelle V, Hasiu A, et al. Impact of the Covid‐19 outbreak on acute stroke pathways – insights from the Alsace region in France. Eur J Neurol. Epub ahead of print 2020. doi:10.1111/ene.14316. [DOI] [PMC free article] [PubMed]
- 37.Huang JF, Greenway MRF, Nasr DM, et al. Telestroke in the Time of COVID-19: the Mayo Clinic experience. Mayo Clin Proc 2020; 95: 1704–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baracchini C, Pieroni A, Viaro F, et al. Acute stroke management pathway during Coronavirus 19 pandemic. Neurol Sci.. Epub ahead of print 2020. doi:10.1007/s10072 020 04375 9. [DOI] [PMC free article] [PubMed]
- 39.Esenwa C, Parides MK, Labovitz DL. The effect of COVID-19 on stroke hospitalizations in New York City. J Stroke Cerebrovasc Dis 2020; 29: 105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsiao J, Sayles E, Antzoulatos E, et al. Effect of COVID-19 on emergent stroke care: a regional experience. Stroke 2020. doi:10.1161/STROKEAHA.120.030499. [DOI] [PMC free article] [PubMed]
- 41.Mehrpour M, Shuaib A, Farahani M, et al. EXPRESS: COVID-19 and stroke in Iran; a case series, and effects on stroke admissions. Int J Stroke 2020. doi:10.1177/1747493020937397. [DOI] [PMC free article] [PubMed]
- 42.Siegler JE, Heslin ME, Thau L, et al. Falling stroke rates during COVID-19 pandemic at a Comprehensive Stroke Center: Cover title: Falling stroke rates during COVID- 19. J Stroke Cerebrovasc Dis 2020. doi:10.1016/j.jstrokecerebrovasdis.2020.104953. [DOI] [PMC free article] [PubMed]
- 43.Tejada Meza H, Lambea Gil Á, Saldaña AS, et al. Impact of COVID-19 outbreak on ischemic stroke admissions and in-hospital mortality in North West Spain. Int J Stroke 2020. doi:10.1177/1747493020938301. [DOI] [PMC free article] [PubMed]
- 44.Sacco S, Ricci S, Ornello R, et al. Reduced admissions for cerebrovascular events during COVID-19 outbreak in Italy. Stroke 2020; 51: 3746–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 47.Hermans C, Lambert C. Impact of the COVID-19 pandemic on therapeutic choices in thrombosis-hemostasis. J Thromb Haemost 2020; 18: 1794–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poli D, Tosetto A, Palareti G, et al. Managing anticoagulation in the COVID-19 era between lockdown and reopening phases. Intern Emerg Med 2020; 15: 783–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zedde M, Pezzella FR, Paciaroni M, et al. Stroke care in Italy: an overview of strategies to manage acute stroke in COVID-19 time. Eur Stroke J 2020; 5: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paolucci M, Biguzzi S, Cordici F, et al. Impact of COVID-19 pandemic on acute stroke care: facing an epidemiological paradox with a paradigm shift. Neurol Sci. Epub ahead of print 2020. doi:10.1007/s10072 020 04914 4. [DOI] [PMC free article] [PubMed]
- 51.Nogueira RG, Qureshi MM, Abdalkader M, et al. Global impact of COVID-19 on stroke care and IV thrombolysis. Neurology 2021; 96: e2824–e2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang B, Wang T, Chen J, et al. Management of acute ischemic stroke under routine infection prevention practices for COVID-19. J Neurointerv Surg 2020. doi:10.1136/neurintsurg 2020 016803. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-wso-10.1177_17474930211041202 for Stroke network performance during the first COVID-19 pandemic stage: A meta-analysis based on stroke network models by Michele Romoli, Paolo Eusebi, Stefano Forlivesi, Mauro Gentile, Fabrizio Giammello, Laura Piccolo, David Giannandrea, Simone Vidale, Marco Longoni, Matteo Paolucci, Jessica Hsiao, Emily Sayles, Leonard LL Yeo, Espen Saxhaug Kristoffersen, Angel Chamorro, Liqun Jiao, Pooja Khatri, Georgios Tsivgoulis, Maurizio Paciaroni and Andrea Zini in International Journal of Stroke