Abstract
Background:
Microglia activation-induced neuroinflammation may contribute to the etiology of depression. Podocarpus nagi containing high concentration of isoginkgetin could effectively treat mental diseases in ancient times. However, the therapeutic role, peculiarly in the brain–immune modulation in depression is still unclear. This study aimed to determine effects of isoginkgetin on lipopolysaccharide (LPS)-induced depression-like changes. Furthermore, its modulation on the p38/nuclear factor-kappa B (NF-κB) pathway in LPS-activated microglia was evaluated.
Methods:
Adult Kunming mice were intraperitoneally injected vehicle or isoginkgetin (4 mg/kg) daily for 14 days before saline or LPS (0.83 mg/kg) administration. Depression-like behavior, neurotransmitter levels, and markers of neuroinflammation were determined. Isoginkgetin effect on LPS-induced microglial activation was then assessed in BV2 cells. Finally, conditioned medium (CM) derived from isoginkgetin-treated BV2 cells was co-cultured with SH-SY5Y cells for 24 h. Cell viability and apoptosis were evaluated.
Results:
LPS significantly induced helplessness and anxiety, which were associated with decreased 5-HT, noradrenaline, and dopamine concentrations. Meanwhile, LPS increased microglia M1 hallmark Iba1 expression and serum interleukin (IL)-1β concentration. These changes were attenuated by isoginkgetin treatment. In vitro, isoginkgetin markedly suppressed the production of IL-1β, IL-6, tumor necrosis factor-alpha, cyclooxygenase-2, inducible nitric oxide, and reactive oxygen species, which are released from LPS-stimulated BV2 cells. More interestingly, CM from isoginkgetin-treated BV2 cells significantly alleviated SH-SY5Y cell apoptosis and restored cell viability compared to LPS-treated group through the inhibition of p38/NF-κB signaling pathway.
Conclusion:
These data demonstrate that isoginkgetin is an effective therapeutic agent for depression-like behaviors and neuropathological changes via potent anti-inflammatory property.
Keywords: Depression, lipopolysaccharide, neuroinflammation, isoginkgetin, p38/NF-κB signaling pathway
Introduction
Accumulating evidence reveals that inflammation plays a crucial role in the etiology of depression (Furtado and Katzman, 2015; Miller and Raison, 2016). Activation of peripheral innate immune cells elicits the secretion of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α, which can pass the blood–brain barrier into the central nervous system (CNS), and activate microglial cells. Then, microglia further produce proinflammatory cytokines and trigger neuroinflammation (Brites and Fernandes, 2015; Henry et al., 2008). In such condition, sickness behaviors and other symptoms of depression occur in susceptible patients (Dantzer et al., 2008). Similar results were reported in rodent models of depression. These animals exhibited depression-like behavior with high expression of IL-1β, IL-6, and cyclooxygenase (COX)-2, increased nitric oxide synthase (iNOS) and reactive oxygen species (ROS) levels in the prefrontal cortex and hippocampus (Adzic et al., 2018; Jeon and Kim, 2016; Yang et al., 2013). Therefore, the prevention of inflammatory disturbances can be a therapeutic approach in the treatment of depression.
With regard to inflammation-induced animal models of depression, lipopolysaccharide (LPS) was popularly used to study the relationship between neuroinflammation and depression, as well as new treatments. Mounting evidence revealed that LPS can activate various inflammatory pathways associated with a series of behavioral alterations, including depressed mood, fatigue, and psychomotor slowing in humans, as well as anhedonia, lethargy, anxiety, and cognitive impairment in rodents (Dinel et al., 2014; Maes et al., 2012; Mello et al., 2013). Furthermore, several studies reported that LPS-treated mice exhibited abnormal expression of proinflammatory cytokines and increased oxidative damage, which are similar to those observed in depressed patients (Han et al., 2019; Sulakhiya et al., 2016).
Due to poor efficacy and side effects of current antidepressant treatments, searching for natural anti-inflammatory drugs has received a great attention. Isoginkgetin, a member of the flavonoid family, is a natural compound that was first discovered in the traditional Chinese medicine Ginkgo (Briancon-Scheid et al., 1983). Many studies have demonstrated that isoginkgetin inhibited TNF-α, IL-6, and prostaglandin E2 productions and reduced mRNA expression of inducible NO synthase and COX-2 in LPS-activated RAW 264.7 macrophage cells (Li et al., 2019). These results suggested that isoginkgetin may be a promising candidate for the anti-inflammatory agents. More importantly, Chinese Yao folk medicine Podocarpus nagi, enriched with high concentration of isoginkgetin, was found to be effective in the treatment of mental diseases in ancient times (Abdillahi et al., 2010). However, isoginkgetin therapeutic effects, peculiarly in the brain–immune modulation in depression treatment, is still unclear.
At molecular level, NF-κB and mitogen-activated protein kinases (MAPKs) are two major signaling pathways involved in inflammation. Upon activation, NF-κB p65 expression is translocated from cytoplasm to the nucleus, where it induces the transcription of inflammatory target genes, such as iNOS, COX-2, and TNF-α. Moreover, the MAPK family, comprising three main members, including c-Jun N-terminal kinase (JNK), ERK, and p38 MAPK were reported to play important roles in LPS-induced neuroinflammation. Once activated, ERK, JNK, and p38 MAPKs are phosphorylated at specific sites and induce expressions of a series of inflammatory mediators. However, whether isoginkgetin treats mental disease via the modulation of MAPK or NF-κB pathway is unknown.
Thus, the present study evaluated the effect of isoginkgetin on the depression-like behaviors, abnormal monoamine neurotransmitter synthesis and metabolism, and proinflammatory cytokine changes in LPS-induced model of depression. Furthermore, the p38 MAPK/NF-κB signaling pathway in LPS-induced activation of microglia cells were studied.
Materials and methods
Animals
Adult male Kunming mice (age 8–10 weeks, 30–50 g) were purchased from Changsha Tianqin Biotechnology Co., Ltd., Changsha, China. Animals were housed two mice per cage, with a temperature of 23 ± 1°C and a humidity level of 50 ± 10%. Standard rodent chow and water were available ad libitum, and mice were maintained on a 12 h light/dark cycle (lights on at 7:00 and off at 19:00). Upon arrival, mice were acclimatized to the laboratory for 7 days. Animal experimental procedure was approved by the Laboratory Animal Care and Use Committees of Guangdong Ocean University and met the National Institutes of Health guidelines for the care and use of laboratory animals.
Experimental procedure
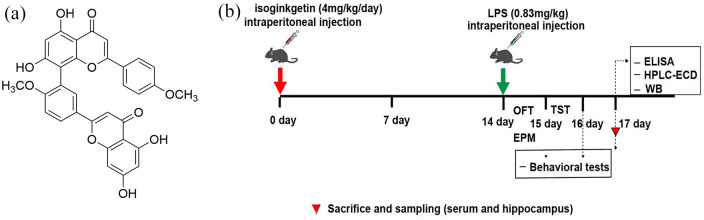
To choose the optimal dose for the treatment, three concentrations of isoginkgetin (2 mg/kg, 4 mg/kg, and 8 mg/kg) were tested in a preliminary experiment. The structural formula is 4H-1-benzopyran-4-one,8-[5-(5,7-dihydroxy-4-oxo-4H-1-benzopyran-2-yl)-2-methoxyphenyl]-5,7-dihydroxy-2-(4-methoxyphenyl) (Figure 1(a)). The result showed that high dose of isoginkgetin (8 mg/kg) was toxic to mice, whereas low dose of isoginkgetin (2 mg/kg) only slightly alleviated depressive-like behavior. Thus, on the basis of these results, isoginkgetin 4 mg/kg was selected in subsequent animal experiments. About 40 mice were randomly divided into four experimental groups as: (1) control + saline; (2) isoginkgetin (4 mg/kg); (3) LPS; and (4) LPS + isoginkgetin (4 mg/kg). Isoginkgetin administered at a dose of 4 mg/kg was injected daily for 14 days by intraperitoneally (i.p.) prior to LPS (0.83 mg/kg) administration. After 24 h of LPS or saline i.p. administration, depression-like behaviors were tested. Then mice were sacrificed after completing behavioral tests. Serum cytokine concentrations were evaluated by enzyme-linked immunosorbent assay (ELISA) kits. Neurotransmitter and metabolite levels were measured by high-performance liquid chromatography (HPLC) with electrochemical detector (ECD), and microglial M1 hallmark Iba1 protein expression and inflammation-related NF-κB and MAPK signaling pathway in hippocampal tissue were detected by Western blotting (WB) (Figure 1).
Figure 1.
The structure of isoginkgetin and the design for the in vivo experiment: (a) the structure of isoginkgetin and (b) the design for the in vivo experiment. EPM: elevated plus maze; OFT: open field test; TST: tail suspension test.
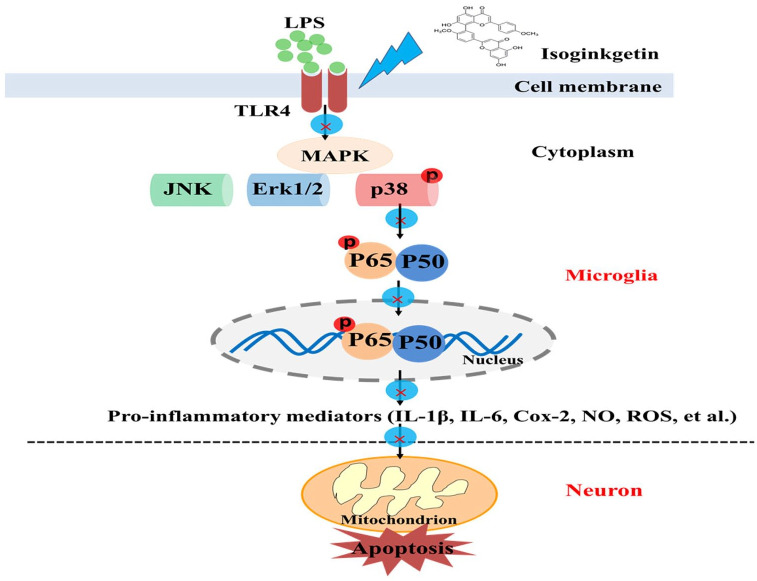
In the in vitro experiment, activation of BV2 microglia was induced by 20 ng/ml LPS in the presence of isoginkgetin for 24 h. Proinflammatory mediators (i.e., IL-1β, IL-6, and Cox-2) were tested at both transcriptional and protein levels. Intracellular ROS and NO were also measured according to the manufacturer’s instruction. Furthermore, the supernatant from isoginkgetin-treated BV2 cells, as a conditioned medium (CM), were co-culture with neuronal cells (SH-SY5Y cells) for 24 h. Cell viability and apoptosis were then measured by CCK8 assay and flow cytometry, respectively. Finally, we hypothesized that the underlying molecular mechanism by which isoginkgetin protected neurons from inflammation-induced apoptosis was through the p38/NF-κB signaling pathway (Figure 2).
Figure 2.
Schematic illustration of potential signaling pathways associated with the anti-inflammatory effects of isoginkgetin on microglia-induced neuroinflammation after lipopolysaccharide (LPS) stimulation.
Behavioral tests
Elevated plus maze test
Anxiety-related behavior was measured by elevated plus maze (EPM) test as previously described (Zhang et al., 2019). Briefly, the maze consisted of two open arms (50 cm), two closed arms (50 cm), and a central platform (10 cm). The apparatus was composed of black plastic and raised 60 cm above the floor. The maze was lit by a 40-W bulb positioned 150 cm above the center. Then, mice were placed on the central platform facing the same open arm. The number of entries and the time spent on open and closed arms were recorded blindly according to the SuperMaze behavior analysis system (Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China) for 5 min.
“Open-field” test
Open-field test OFT was developed for the assessment of rodent locomotor, exploration, and anxiety-like behavior in a novel environment. The open field consisted of four adjacent activity chambers (40 cm × 40 cm × 40 cm) surrounded by walls. A 60-W white bulb was positioned 1 m above the center of the apparatus (Zhang et al., 2019). Briefly, mice were placed with heads toward the walls of the apparatus. Total distance traveled, the number of rearing, and entries into the central zone were recorded using the SuperMaze behavior analysis system (Shanghai Xinruan Information Technology Co., Ltd., Shanghai, China) for 3 min by two highly trained observes blinded to the treatment groups.
Tail suspension test
Tail suspension test (TST) is widely used to assess hopeless and desperate behaviors in rodents (Gu et al., 2018). Briefly, mice with a medical tape placed 1 cm from the tip of the tail were hung on the suspension test instrument holder for 5 min, approximately 20 cm away from the ground. The immobility time was recorded by an infrared camera.
Detection of cytokine concentrations in the serum
Blood samples were collected immediately after mice were decapitated and naturally coagulated at room temperature for 20 min. Then serum were collected by centrifugation at 1000 rpm for 10 min at room temperature. Proinflammatory cytokines (IL-1β) and anti-inflammatory cytokines (IL-10) were determined using the commercial ELISA Kits (Meimian Biotechnology, Yancheng, China) in accordance with the manufacturer’s protocols.
Determination of neurotransmitter and metabolite levels
Hippocampal samples were rapidly removed from the cranium and separated on ice plate. They were weighted in a 1.5-ml centrifuge tube and added tissue lysis buffer to homogenize for 15 min on ice to aid the precipitation of proteins and cell debris. Homogenized samples were centrifuged at 12,000 rpm for 5 min at 4°C, and resulting supernatant was collected in a 1.5-ml centrifuge tube. An equal volume of perchloric acid (HClO4) solution was added to the supernatant for 10 min to break sample protein at 4°C. After supernatant was centrifuged again at 14,000 rpm, 4°C for 15 min, the clear supernatant was collected in a 1.5-ml centrifuge tube. The supernatant was passed through Millipore syringe filters (Merck Millipore, Darmstadt, Germany) before injecting in to HPLC. The measurement of monoamines and metabolites included serotonin (5-HT), dopamine (DA), noradrenaline (NE), and their metabolites 5-hydroxyindoleacetic acid (5-HIAA), 3,4-dihydroxyphenylacetic acid (DOPAC), and 3-methoxy-4-hydroxyphenyglycol (MHPG) by HPLC with ECD.
Cell culture and treatments
BV2 murine microglial and human neuroblastoma SH‑SY5Y cells were obtained from the Institute of Biochemistry and Cell Biology (Shanghai, China). BV2 cells were maintained in high glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin/penicillin. SH-SY5Y cells were maintained in MEM/F12 supplemented with 15% FBS and 1% streptomycin/penicillin. They were maintained in humidified 5% CO2/95% air environment at 37°C.
Treatments
Isoginkgetin (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China) was dissolved in dimethyl sulfoxide (DMSO) to a concentration of 1 mM and aliquots stored in −20°C. In the in vitro experiment, cells were treated with in the absence or presence of LPS (20 ng/ml).
Changes in BV2 cell viability after different doses of isoginkgetin treatment
BV2 cells were treated with different doses (0, 0.1, 0.5, 1, 2, and 4 µM) of isoginkgetin. After 24 h of co-culture, the cell viability was assessed by CCK8 solution (Beyotime, Shanghai, China). The absorbance at 450 nm was detected using the spectrophotometer (Thermo Fisher Scientific, San Jose, CA, USA).
Based on the above results, 0.1 µM and 0.5 µM of isoginkgetin were selected for the subsequent experiments. BV2 cells were pretreated with isoginkgetin for 1 h and then stimulated with LPS (20 ng/mL) in the presence or absence of isoginkgetin for 24 h. The proliferation was assessed by CCK8 solution. The details of the measurement are the same as described above.
Nitrite measurement
BV2 cells were seeded at a concentration of 1 × 105 cells/well into 12-well plates and grown at 37°C for 24 h. Thereafter, cells were pretreated with isoginkgetin (0.1 µM and 0.5 µM) for 1 h and then stimulated with LPS in the presence or absence of isoginkgetin for 24 h. Levels of nitrite in culture media were measured using Griess reagent (Promega Corp., Madison, WI, USA) according to the manufacturer’s instructions. The optical density value was detected by microplate reader at 550 nm of absorbance. NaNO2 was used as the standard to calculate NO2− concentrations.
ROS measurement
The accumulation of intracellular ROS was detected by using Reactive Oxygen Species Assay Kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. In brief, BV2 cells were pretreated with isoginkgetin for 1 h and then stimulated with LPS (20 ng/ml) in the presence or absence of isoginkgetin for 24 h. Following collected and washed with serum-free cell culture medium twice, cells were incubated with dichlorodihydrofluorescein diacetate (DCFH-DA) at 37°C for 30 min in the dark. After washed three times with serum-free cell culture medium to fully remove DCFH-DA that did not enter the cells, ROS levels were detected by an FACSCanto™ II flow cytometry (BD Biosciences, San Jose, CA, USA).
Co-culture of BV2 CM with SH-SY5Y cells
BV2 cells were seeded in a 96-well plate at a density of 1 × 104 cells/well and pretreated with isoginkgetin for 6 h, then the culture supernatants were discarded to eliminate any direct effect of isoginkgetin on SH-SY5Y neurons. Cells were further incubated with LPS for 24 h in the absence of isoginkgetin. The culture media were collected from the dishes and centrifuged to remove the detached cells as CM. Briefly, five groups were set up as (1) CM from control BV2 cells (control-CM); (2) CM from LPS-treated BV2 cells (LPS-CM); (3) CM from BV2 cells stimulated by LPS after isoginkgetin pretreatment (Iso/LPS-CM); (4) CM from BV2 cells treated with isoginkgetin (Iso-CM), and (5) LPS added to control-CM (control-CM+LPS). SH-SY5Y cells were treated with various-treated CM for next 24 h, and neuronal viability was measured by CCK8 assay.
Apoptosis assay of SH-SY5Y cells
Cell apoptosis was assessed by PE Annexin V Apoptosis Detection Kit I (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instructions. Briefly, SH-SY5Y cells were exposed to the CM for 24 h. Cells were collected and then resuspended in 100 µl binding buffer, stained with Annexin V-PE and 7-AAD at room temperature in the dark for 15 min. Following 400 µl binding buffer was added to the suspension, the stained cells were analyzed using a FACSCanto II flow cytometer (BD Biosciences, San Jose, CA, USA). Cell apoptosis rate was quantified in three times independent experiments.
Quantitative reverse transcription-polymerase chain reaction
The quantitative reverse transcription-polymerase chain reaction (qRT-PCR) assay was performed to measure inflammatory gene expression levels in BV2 cells cultured with isoginkgetin and LPS. Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and cDNA was prepared from 1 µg of total RNA using reverse transcription kit (Takara, Dalian, China) at 37°C for 15 min, followed by an 85°C incubation for 5 s. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control. The reaction was performed in a LightCycler® 480 II Real-time PCR Instrument (Roche, Basel, Switzerland) under the following cycling conditions: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 20 s. The relative quantification of gene expression was presented with the values of 2−ΔΔCT, which were normalized to GAPDH mRNA expression level.
Western blotting
Total cell lysates and hippocampal tissues were homogenized in the RIPA (Thermo Fisher Scientific, San Jose, CA, USA) buffer and then centrifuged at 12,000 × g for 15 min to remove the debris. Protein concentrations were determined using a BCA Protein Assay Kit (Beyotime, Shanghai, China). Total protein (10 µg) was separated on 10% or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (MilliporeSigma, Burlington, MA, USA). The membranes were then blocked in Tris-buffered saline (TBS) containing 5% non-fat dry milk for 1 h, following which they were incubated with primary antibodies against phosphorylated p38 (p-p38), total p38, phosphorylated Erk (p-Erk), total Erk, phosphorylated JNK (p-JNK), total JNK, NF-κB, Iba1, COX-2, Lamin B, and Actin (all from Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4°C. The membranes were then washed with TBST and incubated for 1 h with secondary antibodies. The antibody-reactive bands were detected using the Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific, San Jose, CA, USA). Quantification of band intensity was analyzed using the Image J software (version 1.48; National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were presented as mean ± SEM and analyzed by IBM SPSS 22.0 software (IBM Corp., Armonk, NY, USA). For the dose–response analyses, isoginkgetin/LPS effects were determined by one-way analysis of variance (ANOVA), followed by the Dunnett’s post hoc analyses in case of significant main effects (p < 0.05). For the in vivo study of isoginkgetin treatment against LPS, the possible interaction between both treatment factors was measured by two-way ANOVA with the Bonferroni post hoc test if main or interaction effects were significant (p < 0.05).
Results
Isoginkgetin attenuated LPS-induced depression-like behavior
“Open field” test
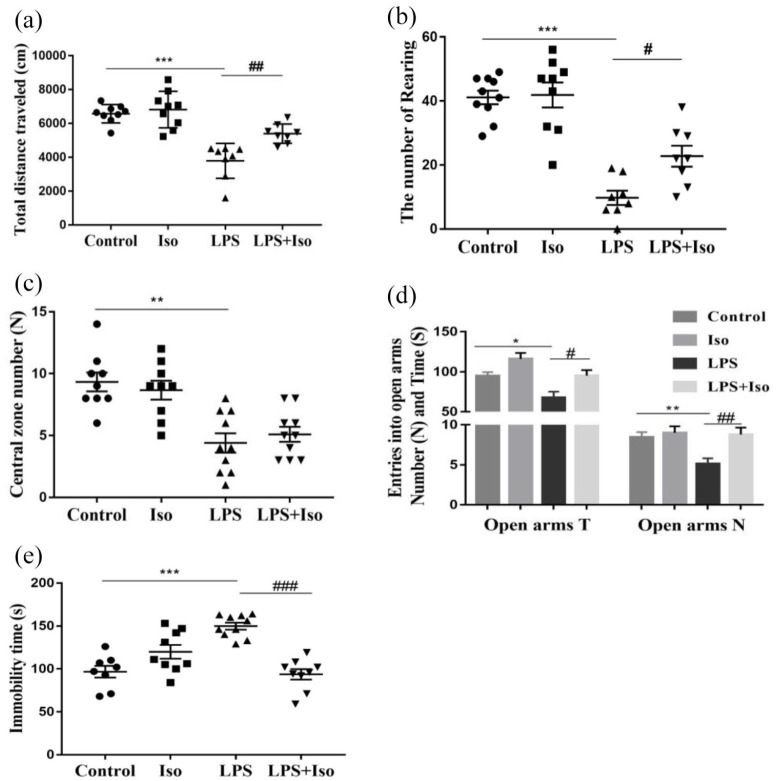
Two-way ANOVA analysis showed that the interaction between LPS and isoginkgetin was also significantly different in terms of total distance traveled (F1, 30 = 5.548, p < 0.05) and rearing numbers (F1, 30 = 3.212, p < 0.05). However, isoginkgetin treatment by itself (no LPS) had no effect on these behaviors. The post hoc test revealed that isoginkgetin significantly reversed LPS-induced hypoactivity in terms of total distance traveled (p < 0.01) and rearing numbers (p < 0.05)), whereas the reduction in the number of entries into the central zone was not significantly improved (Figure 3(a)–(c)).
Figure 3.
Isoginkgetin attenuated LPS-induced depression-like behavioral changes: (a) total distance traveled in the OFT, (b) the number of rearing in the OFT, (c) the number of entries into the central zone in the OFT, (d) entry number into and the time spent on open arms in the EPM, and (e) total immobility time in the TST. *p < 0.05, **p < 0.01, ***p < 0.001 versus control group; #p < 0.05, ##p < 0.01, ###p < 0.001 versus LPS group. EPM: elevated plus maze; LPS: lipopolysaccharide; Iso: isoginkgetin; OFT: open-field test; TST: tail suspension test.
EPM test
The interaction between LPS and isoginkgetin was significantly different in terms of the number of entries into the open arms (F1, 33 = 5.836, p < 0.05). The post hoc test revealed that isoginkgetin significantly reversed LPS-induced decrease in the number of entries into the open arms (p < 0.05) and the time spent on open arms (p < 0.01). Meanwhile, there is no significant difference between isoginkgetin treatment alone and control group (Figure 3(d)).
Tail suspension test
As shown in Figure 2(e), two-way ANOVA analysis suggested that a significant interaction between LPS and isoginkgetin (F1, 32 = 39.180, p < 0.001) in immobility times was found. The post hoc test further revealed that LPS significantly increased the immobility time (p < 0.001), whereas isoginkgetin treatment reversed this change (p < 0.001).
Serum cytokine concentrations
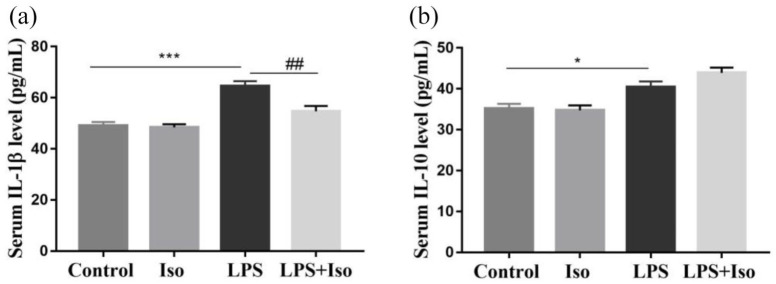
Two-way ANOVA indicated that a significant interaction between LPS and isoginkgetin (F1, 24 = 7.405, p < 0.05) in IL-1β level was found. The post hoc test revealed that isoginkgetin significantly reversed LPS-increased IL-1β level. Meanwhile, a slightly but significantly increased IL-10 level was found in LPS group, which was not attenuated by isoginkgetin treatment (Figure 4).
Figure 4.
Effects of isoginkgetin on the concentration of pro- and anti-inflammatory cytokine (IL-1 and IL-10) in the serum: (a) IL-1β and (b) IL-10 levels. *p < 0.05, ***p < 0.001 versus control group; ##p < 0.01 versus LPS group. LPS: lipopolysaccharide; Iso: isoginkgetin.
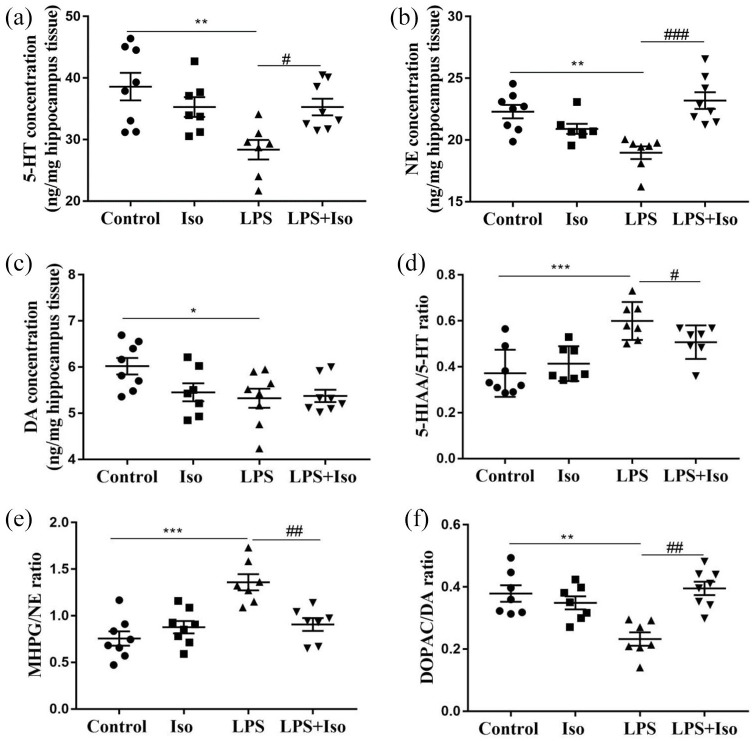
Isoginkgetin normalized hippocampal neurotransmitter contents and their metabolites
The interaction between LPS and isoginkgetin was significantly different in terms of 5-HT (F1, 26 = 8.582, p < 0.01) and NE levels (F1, 26 = 25.437, p < 0.001) (Figure 5(a) and (b)). The post hoc test showed that isoginkgetin significantly attenuated LPS-decreased 5-HT and NE levels, whereas DA level was not significantly changed (Figure 5(c)). No significant changes were observed in isoginkgetin treatment by itself. In addition, the ratios of 5-HIAA/5-HT and MHPG/NE were significantly increased by LPS, but not by isoginkgetin treatment. However, there was a significant interaction between LPS and isoginkgetin (5-HIAA/5-HT: F1, 26 = 4.503, p < 0.05; MHPG/NE: F1, 26 = 15.963, p < 0.001). The post hoc test showed that isoginkgetin significantly attenuated LPS-induced increase in 5-HIAA/5-HT ratio (p < 0.05) and MHPG/NE ratio (p < 0.01), respectively (Figure 5(d) and (e)). Contrary to these results, DOPAC/DA ratio (p < 0.01) was lower in the LPS group compared with control group, which was restored by isoginkgetin treatment (p < 0.01) (Figure 5(f)).
Figure 5.
Effects of isoginkgetin on the concentration of neurotransmitters and their metabolites in the hippocampus of mice with or without LPS administration: (a) 5-HT, (b) NE, (c) DA concentrations, (d) the ratio of 5-HIAA/5-HT, (e) the ratio of MHPG/NE and (f) the ratio of DOPAC/DA. *p < 0.05, **p < 0.01, ***p < 0.001 versus control group; #p < 0.05, ##p < 0.01, ###p < 0.001 versus LPS group. DA: dopamine; DOPAC: 3,4-dihydroxyphenylacetic acid; 5-HIAA: 5-hydroxyindoleacetic acid; LPS: lipopolysaccharide; Iso: isoginkgetin; MHPG: 3-methoxy-4-hydroxyphenyglycol; NE: noradrenaline.
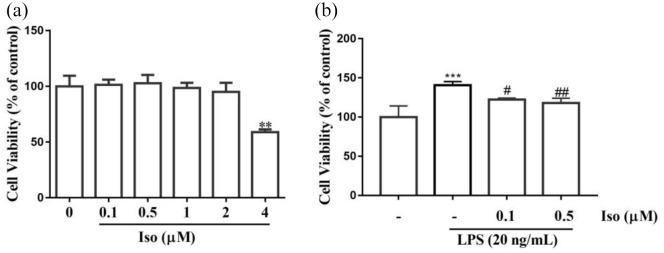
Isoginkgetin reduced BV2 cell viability after LPS stimulation
BV2 cells were treated with different concentrations of isoginkgetin for 24 h to screen a safe dose range (0.1–4 µM). The results showed that isoginkgetin had no cytotoxicity on BV2 cells at the concentration of 0 to 2 µM (Figure 6(a)), whereas isoginkgetin at 4 µM exerted significant toxicity on BV2 cells. Therefore, low and medium isoginkgetin (0.1 µM and 0.5 µM) were selected as the following in vitro experiments. Isoginkgetin pretreatment was carried out for 1 h prior to LPS (20 ng/ml) stimulation. Then, BV2 cells were treated with LPS in the presence of isoginkgetin for 24 h. The results showed that exposure to LPS significantly increased the cell viability as compared to the control group (p < 0.001). The post hoc test showed that both doses of isoginkgetin significantly reduced cell proliferation in LPS-treated group (from 140.55 ± 4.77% to 122.5 ± 3.07%, and to 118.02 ± 6.13%, respectively) (Figure 6(b)).
Figure 6.
Effects of isoginkgetin on the BV2 cell viability with or without lipopolysaccharide (LPS) stimulation. BV2 cells were seeded in 96-well plates (5000 cells/well) and then treated with isoginkgetin at various concentration (0, 0.1, 0.5, 1, 2, 4 μM) with or without LPS stimulation. Cell viability was determined by CCK8 assay. Data were presented as means ± SEM from three independent experiments in triplicate. Data was evaluated by one-way ANOVA, followed by the Dunnett’s post hoc analyses in case of significant main effects (p < 0.05): (a) different concentrations of isoginkgetin respond to BV2 cell viability and (b) isoginkgetin significantly attenuated LPS-induced BV2 cell viability. Data are presented as means ± SEM from three independent experiments in triplicate. ***p < 0.001 versus control group; #p < 0.05, ##p < 0.01 versus LPS group. Iso: isoginkgetin.
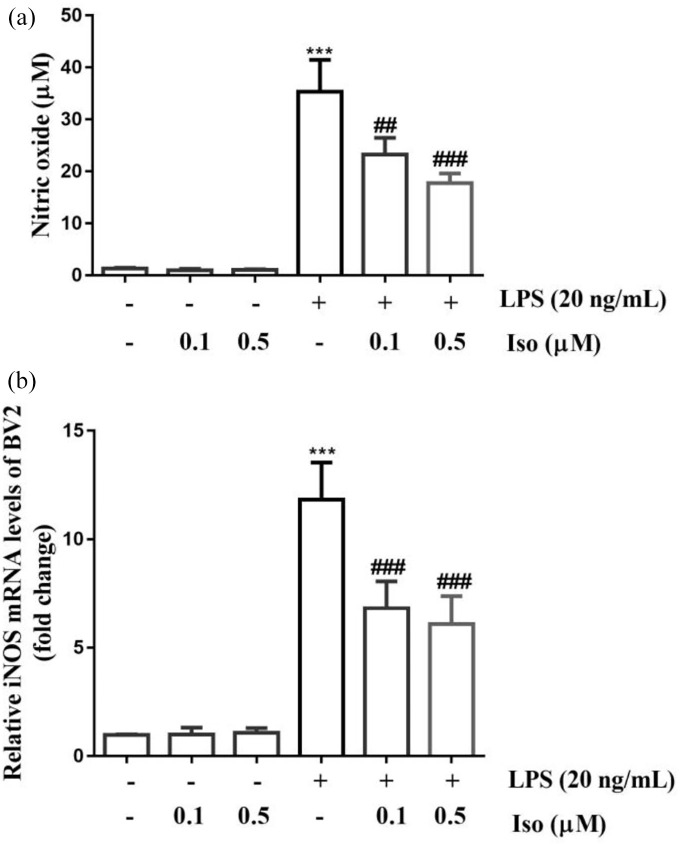
Isoginkgetin reduced NO production and iNOS expression in LPS-activated BV2 cells
Compared to unstimulated cells, LPS treatment significantly increased NO production (p < 0.01) (Figure 7(a)), which was largely suppressed by isoginkgetin treatment in a dose-dependent manner. Furthermore, isoginkgetin significantly reversed iNOS gene expression following LPS stimulation (p < 0.001) (Figure 7(b)).
Figure 7.
Isoginkgetin reduced NO production and iNOS mRNA expression in lipopolysaccharide (LPS)-activated BV2 cells. Cells were treated with isoginkgetin (0.1 μM and 0.5 μM) for 1 h and then incubated with or without LPS (20 ng/mL) for 24 h: (a) the NO concentration in the supernatant was measured by the Griess reagent and (b) iNOS mRNA levels were determined by qRT-PCR. ***p < 0.001 versus control group; #p < 0.05, ##p < 0.01, ###p < 0.001 versus LPS group. Iso: isoginkgetin; iNOS: increased nitric oxide synthase; qRT-PCR: quantitative reverse transcription-polymerase chain reaction.
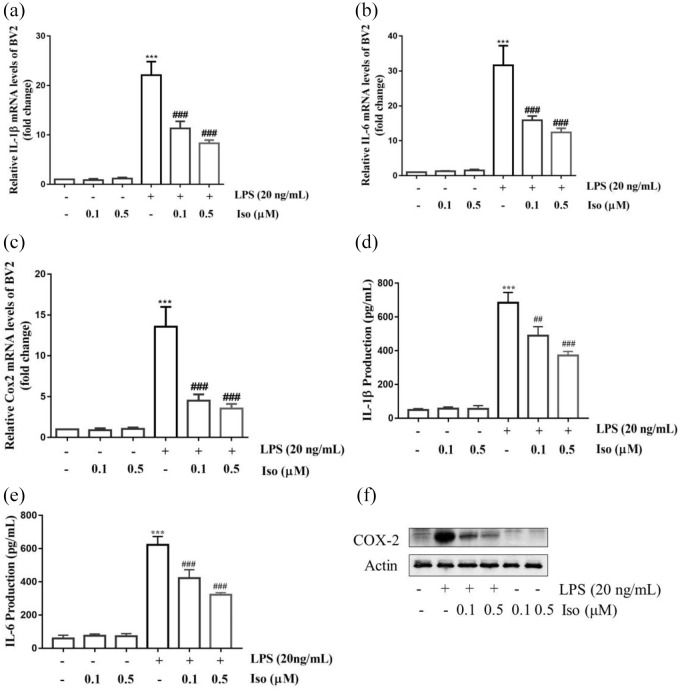
Isoginkgetin inhibited LPS-induced the release of pro-inflammatory mediators in BV2 cells
Isoginkgetin significantly inhibited mRNA expression of pro-inflammatory mediators, IL-1β, IL-6, and COX-2 in a dose-dependent manner (p < 0.001) (Figure 8(a)–(c)). In parallel, an ELISA assay showed that the IL-1β level in supernatant without LPS stimulation was 48.44 ± 4.35 pg/ml. Isoginkgetin reduced the IL-1β level from 683.56 ± 30.68 pg/ml (LPS group) to 489.11 ± 26.46 pg/ml and 372.22 ± 11.80 pg/ml at 0.1 µM and 0.5 µM doses, respectively, in BV2 cell supernatant following 24 h of treatment (Figure 8(d)). Similarly, LPS increased the IL-6 production from 59.04 ± 9.86 pg/ml to 622.09 ± 25.36 pg/ml compare with the control group. This was reduced to 422.96 ± 25.09 pg/ml and 322.38 ± 6.37 pg/ml with isoginkgetin treatments at 0.1 µM and 0.5 µM doses, respectively (Figure 8(e)). Isoginkgetin by itself did not significantly affect proinflammatory cytokines (IL-1β and IL-6) concentrations. Furthermore, COX-2 protein expression was detected by the WB assay. The result showed that markedly increased COX-2 protein level in LPS-induced BV2 cells. Pretreatment with isoginkgetin significantly inhibited the expression in dose-dependent manner (Figure 8(f)).
Figure 8.
Isoginkgetin inhibited lipopolysaccharide (LPS)-induced pro-inflammatory mediator release in BV2 cells. The mRNA level of IL-1β (a), IL-6 (b), and COX-2 (c) were assessed by qRT-PCR, (d) IL-1β and (e) IL-6 secretion in culture media were measured by ELISA kits, and (f) the protein levels of COX-2 were assessed by WB. Actin was used as an internal control. ***p < 0.001 versus control group; ##p < 0.01, ###p < 0.001 versus LPS group. COX-2: cyclooxygenase-2; Iso: isoginkgetin; qRT-PCR: quantitative reverse transcription-polymerase chain reaction; WB: Western blotting.
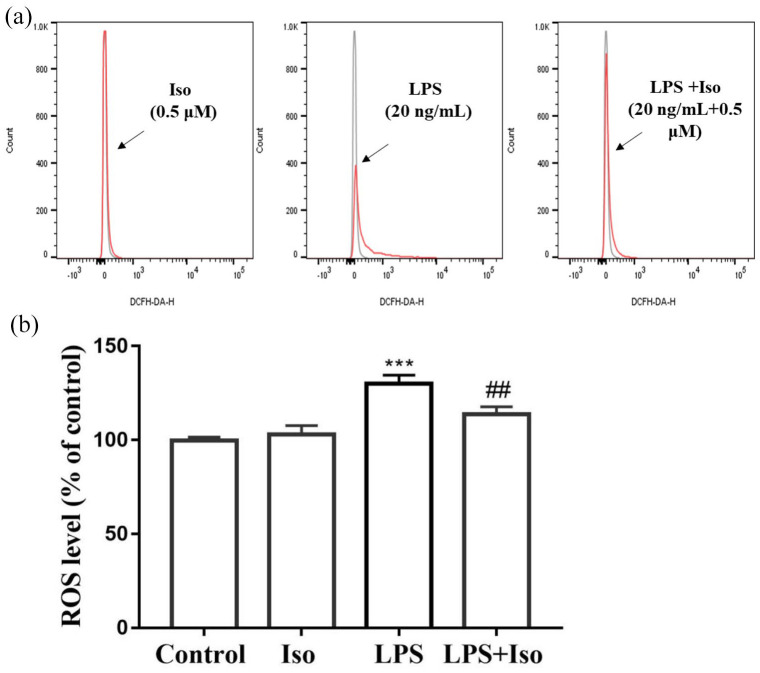
Isoginkgetin blocked LPS-induced ROS production in BV2 cells
As shown in Figure 9, LPS treatment led to an increase in ROS production in BV2 cells (p < 0.001). The post hoc Bonferroni tests showed that isoginkgetin significantly attenuated the effect of LPS (P < 0.01), whereas isoginkgetin by itself did not affect ROS production.
Figure 9.
Isoginkgetin blocked LPS-induced ROS production in BV2 cells: (a) BV2 cells were treated with isoginkgetin in the presence or absence of LPS. Intracellular ROS was measured by flow cytometry. (b) Quantification of ROS level presented as percent change of mean fluorescence. ***p < 0.001 versus control group; ##p < 0.01 versus LPS group. Iso: isoginkgetin; LPS: lipopolysaccharide; ROS: reactive oxygen species.
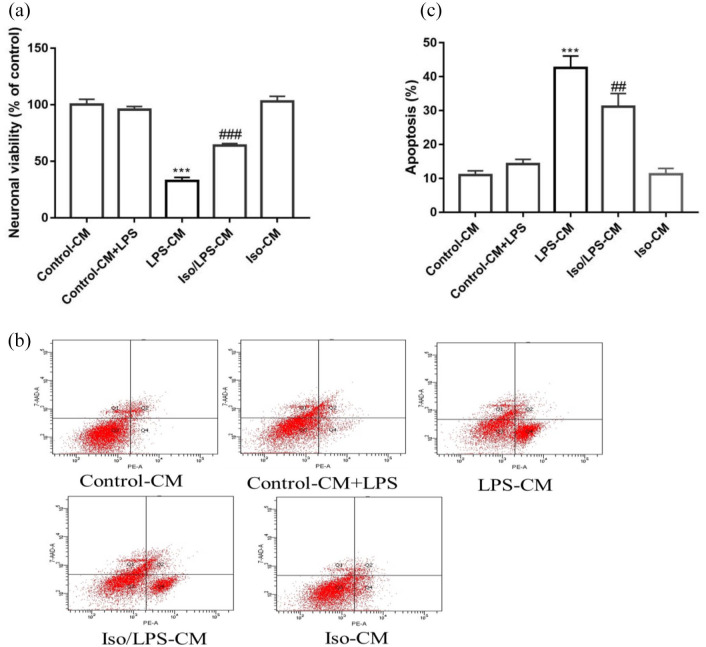
Neuroprotective effects of isoginkgetin against microglia CM-induced neuronal cell death and apoptosis
LPS-CM administration markedly decreased SH-SY5Y cell viability (from 95.53 ± 1.48% to 32.55 ± 1.56%), compared with control-CM group, whereas Iso/LPS-CM treatment markedly improved cell viability to 63.80 ± 0.69% (p < 0.001) (Figure 10(a)). Similarly, Iso/LPS-CM treatment (31.13 ± 3.90%) protected SH-SY5Y cells from LPS-CM-induced cell apoptosis (42.57 ± 3.54%) (p < 0.01) (Figure 10(b) and (c)).
Figure 10.
Neuroprotective effects of isoginkgetin against microglia CM-induced neuronal cell death and apoptosis: (a) cell viability was assessed by CCK8 assay, (b) cell apoptosis was detected by flow cytometry, (c) summarized data on apoptosis rate (%) assessed by flow cytometry. Groups are divided as CM from control BV2 cells (control-CM); LPS added to CM from control BV-2 cells (control-CM + LPS); CM from LPS-treated BV2 cells (LPS-CM); CM from BV2 cells stimulated with LPS after isoginkgetin pretreatment (Iso/LPS-CM); CM from BV2 cells treated with isoginkgetin (Iso-CM). ***p < 0.001 versus control-CM group; ###p < 0.001 versus LPS-CM group. CM: condition medium; Iso: isoginkgetin; LPS: lipopolysaccharide.
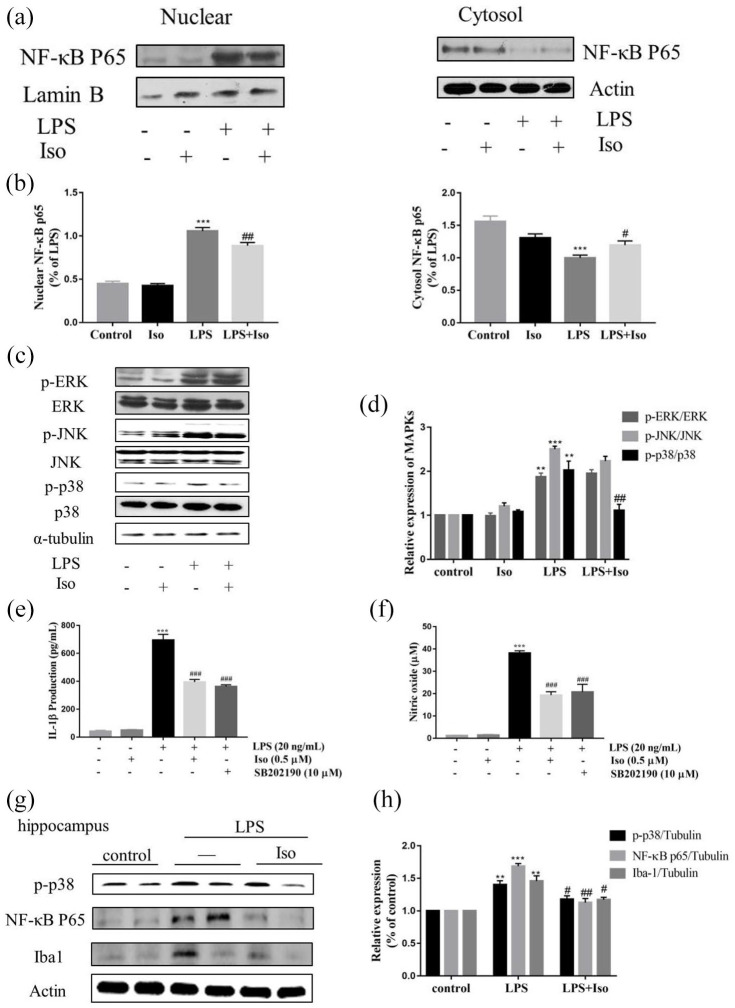
The molecular mechanism by which isoginkgetin reduced neuroinflammation
As shown in Figure 11(a) and (b), compared to group with LPS stimulation, NF-κB p65 translocation from the cytoplasm to nucleus was significantly reduced after isoginkgetin treatment. Accordingly, LPS markedly increased the phosphorylation of p38 (p < 0.01), ERK (p < 0.01), and JNK (p < 0.001) (Figure 11(c) and (d)). By contrast, pretreatment with isoginkgetin significantly inhibited LPS-induced phosphorylation of p38 (p < 0.01), but not ERK and JNK. Then, BV2 cells were pretreated with a specific p38 inhibitor, SB202190 (10 µM), for 2 h and incubated with LPS for 24 h. In consistent with above results from isoginkgetin treatment, p38 inhibitor SB202190 significantly decreased the LPS-induced IL-1β production (p < 0.001) and NO release (p < 0.001) in BV2 cells (Figure 11(e) and (f)).
Figure 11.
Molecular mechanisms underlying the anti-inflammatory effects of isoginkgetin: (a) nuclear and cytosolic expression of NF-κB in BV2 cells, (b) quantification of nuclear and cytosol NF-κB protein expression, (c) p38, ERK1/2 and JNK phosphorylation level in BV-2 cells, (d) quantification of p-ERK/ERK, p-JNK/JNK and p-p38/p38 protein expression, (e) IL-1β production and (f) NO release in BV2 cell supernatant, (g) protein levels of p-p38, NF-κB, Iba1 in mice hippocampal tissue, and (h) quantification of p-p38, NF-κB, Iba1 protein expression by densitometric analysis. IL: interleukin; Iso: isoginkgetin; JNK: Jun N-terminal kinase; NF-κB: nuclear factor-kappa B. SB202190, p38 inhibitor.
Furthermore, molecular study in mouse hippocampus showed that ionized calcium binding adaptor molecule 1 (Iba1) protein expression, a hallmark of microglial activation was significantly increased after LPS injection, whereas isoginkgetin treatment markedly reduced Iba1 expression (p < 0.05). In correlation, the expressions of p-p38 (p < 0.05) and NF-κB p65 (p < 0.01) were significantly decreased by isoginkgetin treatment compared to LPS group (Figure 11(g) and (h)).
Discussion
Acute LPS was popularly used as a sickness model to mimic excessive inflammatory response in depression due to LPS cannot be chronically administrated after conforming LPS-complex upon once injection. Many changes-induced by LPS are similar to those observed in depressed patients, including behavior, neurotransmitters, the HPA axis, neuroinflammatory response, and even some cellular and molecular aspects (Mello et al., 2013; Zhang et al., 2014). These changes can be attenuated by clinical effective antidepressant treatments. The present study for the first time demonstrated that isoginkgetin was able to prevent LPS-induced anxiety- and depression-like behaviors, such as increased immobility time in the TST, decreased number of entries into and time spent on the open arms in the EPM, decreased the numbers of rearing, central zone exploration and locomotor activity in OFT. These anxiolytic and antidepressant effects were accompanied by inhibiting proinflammatory cytokines (IL-1β) production in the periphery and microglial M1 hallmark Iba1 expression in the hippocampus. Furthermore, isoginkgetin significantly restored neurotransmitter levels (5-HT and NE) in hippocampal tissue of LPS-induced depression model. Furthermore, the present study in vitro demonstrated that isoginkgetin could inhibit the release of inflammatory mediators (i.e., IL-1β, IL-6, COX-2, and iNOS) and decreasing the neuroinflammation by downregulating p38/NF-κB signaling pathway in microglia cells (BV2 cells), thereby protected neuronal cells (SH-SY5Y cells) from activated microglia-induced toxicity and apoptosis, which may contribute to its improvement of LPS-induced depression-like behavior in mice.
It is well known that depression results from monoamine neurotransmitter deficiencies, such as 5-HT, NE, and DA, which are in charges of mood, pleasure, stress response, etc. (Calapai et al., 2001; Yang et al., 2019). The present study showed that LPS administration decreased neurotransmitters 5-HT, NE, and DA levels in the hippocampus. However, isoginkgetin treatment significantly reversed 5-HT and NE levels but no effect on DA level in LPS-induced mice. It is possible that the dose and time of isoginkgetin treatment exert different effects on monoamine neurotransmitters in LPS-induced depression animal model (Yoshitake et al., 2010). The mechanism was known as LPS induced an increase in proinflammatory mediators with the capacity to activate indoleamine 2,3-dioxygenase (IDO). IDO can split tryptophan, a serotonin precursor, to kynurenine, leading to monoamine neurotransmitter deficiency. We and others have reported that proinflammatory cytokines can directly enhance metabolism of 5-HT, NE, and DA thereby can reduce concentrations of these monoamines to develop depression (Felger and Lotrich, 2013; Miller et al., 2009; Song and Wang, 2011). In this study, both IL-1β and IL-10 levels in the serum were increased in LPS-induced mice. Isoginkgetin treatment significantly reduced IL-1β level while further increased anti-inflammatory IL-10 level. These data indicate that the effect of isoginkgetin on abnormal behavior may be via anti-inflammation. Then, the present study further explored cellular and molecular mechanisms of isoginkgetin anti-inflammatory biomarkers and pathways in LPS-induced microglia activation, as an in vitro model. BV2 microglia is a murine immortalized microglia cell line that was proven as a valid substitute for primary microglia. BV2 cells stimulated by LPS in vitro often resemble the microglial response in vivo to some extent (Henn et al., 2009). In this study, the results firstly demonstrated that isoginkgetin inhibited proinflammatory cytokines IL-1β and IL-6 gene expression at both transcriptional and protein levels in LPS-activated BV2 cells. Then, COX-2, a key enzyme for the production of a series of inflammatory cytokine found in depression and anxiety (Gamble-George et al., 2016), was overexpressed, which were again attenuated by isoginkgetin treatment in BV2 cells. Second, at molecular level, the present study demonstrated that NF-κB p65 expression was almost complete translocation from the cytoplasm to the nucleus following LPS stimulation, whereas isoginkgetin treatment attenuated LPS-induced translocation of NF-κB p65. NF-κB is a pivotal transcription factor that regulates the expression of various inflammatory genes, such as iNOS, COX-2, IL-1β, and IL-6 observed in the pathogenesis of the inflammatory process (Zhang et al., 2018). Moreover, the present study determined the inhibition of NF-κB activation by isoginkgetin was mediated through the MAPKs pathway. MAPKs are a group of signaling molecules known to mediate inflammatory signals that trigger the transcription of inflammatory genes involved in the control of cellular responses to proinflammatory cytokines and stress (Tan et al., 2017). The MAPKs family is composed of the ERK, JNK, and p38 MAPK pathway. Once activated, MAPKs modulate the functional responses of cells through phosphorylation of numerous transcription factors and activation of other kinases (Supriady et al., 2015). The study demonstrated that isoginkgetin could significantly attenuate LPS triggered phosphorylation of p38 MAPK, without affecting ERK and JNK. This may suggest that isoginkgetin selectively inhibited the upstream kinase p38 MAPK, but not the other two genes. Finally, to further confirm p38 involvement in the production of proinflammatory mediators, the preset study utilized a specific p38 inhibitor, SB202190 to inhibit the production of IL-1β and NO, which gained similar results to isoginkgetin effects. These results demonstrated that the anti-inflammatory effect of isoginkgetin was through downregulating p38/NF-κB signaling pathway. Third, since oxidant production is usually triggered by inflammatory response, which contributes to neuronal apoptosis in depression (Bakunina et al., 2015), changes in activation of oxidative and nitrosative stress were selected as biomarkers in the present study (Anderson and Maes, 2014; Moylan et al., 2014). The results showed that the intracellular ROS and NO production were increased in LPS-activated microglia cells, which were reversed by isoginkgetin administration.
Fourth, the present study demonstrated that isoginkgetin treatment markedly improved SH-SY5Y cell viability and protected cells from LPS-CM-induced cell apoptosis. Interestingly, LPS direct administration neither reduced SH-SY5Y cell viability nor induced apoptosis, which indicated that SH-SY5Y cell death was induced by inflammatory factors released from LPS-activated microglial. Taken together, these results suggest that the neuroprotective effect of isoginkgetin was through attenuating the microglial-mediated neuroinflammation.
In the end, several limitations in the present study should be raised. First, only effect of isoginkgetin on microglial M1 phenotypic hallmark (Iba1), but not M2 phenotypic biomarkers were evaluated. Second, although the anti-inflammatory effect of isoginkgetin was shown via p38/NF-κB signaling pathway, the other inflammatory signaling pathways (such as JAK/STAT and PI3K/AKT) may be also involved, which needs further verification. Third, although BV2 mouse cell line is the most well characterized and widely used cells for studying neuroinflammatory mechanisms, primary microglia are the best cells to study the function and characters of inflammatory response in the brain (Sarkar et al., 2018). Finally, LPS as an inducer is not naturally reasonable for clinical research. The chronic stress model should be used as a further research (Willner, 2017). We will use this as a further research in the follow-up research.
Conclusion
In summary, the present study demonstrated that isoginkgetin could inhibit the production of peripheral inflammatory cytokines, decrease the neuroinflammation by downregulating p38/NF-κB signaling pathway in microglia, and protect neuronal cells from activated microglia-induced toxicity and apoptosis (Figure 2), which may contribute to it attenuated LPS-induced depression- and anxiety-like behavior and restored neurotransmitter synthesis and metabolism in mice. These data suggest that isoginkgetin may be a potential antidepressant candidate.
Footnotes
Author contributions: CS designed and conceptualized the study, edited, and reviewed the manuscript. PL performed the experiments and wrote the manuscript. FZ performed the animal experiments. YL and CZ collected and analyzed the experimental data. ZY and YZ reviewed the manuscript and suggested some important corrections in the manuscript. All authors read and approved the final manuscript.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by Guangdong Provincial Special Fund for Promoting Economic Development Project (Marine Economic Development Purpose) (Yue Natural Resources Contract No. [2019]015), Guangdong Provincial Science and Technology Planning Project of Guangdong Province, China (2016B020235001), Shenzhen Dapeng District Industrial Development Special Funds for science and technology support project (KY20180201), and Project of Enhancing School with Innovation of Guangdong Ocean University (GDOU2013050102).
Ethical approval and informed consent: Animal experimental procedure was approved by the Laboratory Animal Care and Use Committees of Guangdong Ocean University and met the National Institutes of Health guidelines for the care and use of laboratory animals.
ORCID iD: Cai Song  https://orcid.org/0000-0002-4349-5918
https://orcid.org/0000-0002-4349-5918
References
- Abdillahi H, Stafford G, Finnie J, et al. (2010) Ethnobotany, phytochemistry and pharmacology of Podocarpus sensu latissimo (sl). S Afr J Bot 76: 1–24. [Google Scholar]
- Adzic M, Brkic Z, Mitic M, et al. (2018) Therapeutic strategies for treatment of inflammation-related depression. Curr Neuropharmacol 16: 176–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Maes M. (2014) Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: Treatment implications. Curr Pharm Des 20: 3812–3847. [DOI] [PubMed] [Google Scholar]
- Bakunina N, Pariante CM, Zunszain PA. (2015) Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 144: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briancon-Scheid F, Lobstein-Guth A, Anton R. (1983) HPLC separation and quantitative determination of biflavones in leaves from Ginkgo biloba. Planta Med 49: 204–207. [DOI] [PubMed] [Google Scholar]
- Brites D, Fernandes A. (2015) Neuroinflammation and depression: Microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci 9: 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calapai G, Crupi A, Firenzuoli F, et al. (2001) Serotonin, norepinephrine and dopamine involvement in the antidepressant action of hypericum perforatum. Pharmacopsychiatry 34: 45–49. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, et al. (2008) From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinel A-L, André C, Aubert A, et al. (2014) Lipopolysaccharide-induced brain activation of the indoleamine 2, 3-dioxygenase and depressive-like behavior are impaired in a mouse model of metabolic syndrome. Psychoneuroendocrinology 40: 48–59. [DOI] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE. (2013) Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 246: 199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado M, Katzman MA. (2015) Examining the role of neuroinflammation in major depression. Psychiatry Res 229: 27–36. [DOI] [PubMed] [Google Scholar]
- Gamble-George JC, Baldi R, Halladay L, et al. (2016) Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. Elife 5: e14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Li Y, Tang H, et al. (2018) Endogenous omega (n)-3 fatty acids in Fat-1 mice attenuated depression-like behavior, imbalance between microglial M1 and M2 phenotypes, and dysfunction of neurotrophins induced by lipopolysaccharide administration. Nutrients 10: 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JH, Lee YS, Im JH, et al. (2019) Astaxanthin ameliorates lipopolysaccharide-induced neuroinflammation, oxidative stress and memory dysfunction through inactivation of the signal transducer and activator of transcription 3 pathway. Mar Drugs 17: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn A, Lund S, Hedtjärn M, et al. (2009) The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 26: 83–94. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, et al. (2008) Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation 5: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SW, Kim YK. (2016) Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J Psychiatry 6: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Li B, Hou Y, et al. (2019) Anti-inflammatory effects of chemical components from Ginkgo biloba L. male flowers on lipopolysaccharide-stimulated RAW264.7 macrophages. Phytother Res 33: 989–997. [DOI] [PubMed] [Google Scholar]
- Maes M, Berk M, Goehler L, et al. (2012) Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello BSF, Monte AS, McIntyre RS, et al. (2013) Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J Psychiatr Res 47: 1521–1529. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. (2009) Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65: 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. (2016) The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat Rev Immunol 16: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan S, Berk M, Dean OM, et al. (2014) Oxidative & nitrosative stress in depression: Why so much stress? Neurosci Biobehav Rev 45: 46–62. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Malovic E, Sarda D, et al. (2018) Characterization and comparative analysis of a new mouse microglial cell model for studying neuroinflammatory mechanisms during neurotoxic insults. Neurotoxicology 67: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Wang H. (2011) Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry 35: 760–768. [DOI] [PubMed] [Google Scholar]
- Sulakhiya K, Keshavlal GP, Bezbaruah BB, et al. (2016) Lipopolysaccharide induced anxiety-and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci Lett 611: 106–111. [DOI] [PubMed] [Google Scholar]
- Supriady H, Kamarudin MNA, Chan CK, et al. (2015) SMEAF attenuates the production of pro-inflammatory mediators through the inactivation of Akt-dependent NF-κB, p38 and ERK1/2 pathways in LPS-stimulated BV-2 microglial cells. J Funct Foods 17: 434–448. [Google Scholar]
- Tan S, Wang Y, Chen K, et al. (2017) Ketamine alleviates depressive-like behaviors via down-regulating inflammatory cytokines induced by chronic restraint stress in mice. Biol Pharm Bull 40: 1260–1267. [DOI] [PubMed] [Google Scholar]
- Willner P. (2017) The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress 6: 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Hong T, Shen J, et al. (2013) Ketamine exerts antidepressant effects and reduces IL-1β and IL-6 levels in rat prefrontal cortex and hippocampus. Exp Ther Med 5: 1093–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Zhang M-Q, Xue Y, et al. (2019) Dietary of n-3 polyunsaturated fatty acids influence neurotransmitter systems of rats exposed to unpredictable chronic mild stress. Behav Brain Res 376: 112172. [DOI] [PubMed] [Google Scholar]
- Yoshitake T, Yoshitake S, Kehr J. (2010) The Ginkgo biloba extract EGb 761® and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br J Pharmacol 159: 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Zhang Y-P, Li Y-Y, et al. (2019) Minocycline ameliorates depressive behaviors and neuro-immune dysfunction induced by chronic unpredictable mild stress in the rat. Behav Brain Res 356: 348–357. [DOI] [PubMed] [Google Scholar]
- Zhang L, Previn R, Lu L, et al. (2018) Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res Bull 142: 352–359. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu L, Peng YL, et al. (2014) Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci Ther 20: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]