Abstract
Background:
Newly approved, drug-modifying therapies are associated with still unknown adverse events, although clinical trials leading to approval have strict inclusion and exclusion criteria and analyse safety and efficacy.
Objectives:
The aim of this study was to analyse the eligibility of multiple sclerosis (MS) patients treated in routine care into the phase III clinical trial of the respective drug.
Methods:
In total, 3577 MS patients with 4312 therapies were analysed. Patients with primary-progressive MS were excluded. Inclusion and exclusion criteria of phase III clinical trials in relapsing–remitting MS were adopted and subsequently applied. A comparison in clinical and sociodemographic characteristics was made between patient who met the criteria and those who did not.
Results:
83% of registered patients would not have been eligible to the respective phase III clinical trial. Relapse was the single most frequent criterion not fulfilled (74.7%), followed by medication history (21.2%).
Conclusion:
The majority of MS patients treated in routine care would not have met clinical trials criteria. Thus, the efficacy and safety of therapies in clinical trials can differ from those in the real world. Broader phase III inclusion criteria would increase their eligibility and contribute to a better generalizability of the results in clinical trials.
Keywords: Multiple sclerosis, multiple sclerosis disease therapy, registry, phase III clinical trials, generalizability, drug approval, pharmacoepidemiology, drug safety
Introduction
Multiple sclerosis (MS) is a neurodegenerative demyelinating and chronic inflammatory disease of the central nervous system that is associated with a variety of symptoms affecting the motor, sensory, visual and autonomic systems. 1 MS shows a broad variability in prevalence across the globe, from high proportions in Europe and North America (>100/100,000 inhabitants) to a low prevalence in Africa and Eastern Asia (2/100,000 inhabitants). 2 The prevalence of MS in Germany is comparable to other European countries and shows a constant increase in the past decades. 3
The high MS prevalence and the potential severe consequences require appropriate treatments.4,5 While there is no curative MS treatment, patients are treated with different disease-modifying therapies (DMTs). DMTs show various modes of action, route and frequency of administration, treatment adherence, adverse effects and toxicity. 6 With the approval of interferon beta (IFNβ) and glatiramer acetate for the treatment of relapsing–remitting MS (RRMS), the modern era of MS treatment had started in 1990s. Natalizumab was the first monoclonal antibody used in the MS therapy approved in Germany in 2006. Since then, the development of MS DMTs experienced a strong progress. An important step was the approval of oral medications, first fingolimod, followed by teriflunomide, dimethyl fumarate and cladribine. Ocrelizumab was the first DMT, recently approved for the therapy of primary-progressive MS (PPMS). 7 Siponimod was recently approved for the treatment of secondary-progressive MS.
The fast-growing drug market for MS results in many new treatment options and thus includes chances and risks for MS patients. Phase I and II studies are essential parts of clinical drug development. Phase III clinical studies are of crucial importance in drug approval, since they show the efficacy of a drug for patients selected by inclusion and exclusion criteria. In addition, phase III studies provide safety data for many common adverse events. 8 Generalizability describes the transferability of efficacy and safety results from clinical trial patients to patients receiving treatment in clinical routine. 9 Thus, generalizability addresses the effectiveness of a specific intervention in the setting of routine care, sometimes assessed in phase IV studies, post-authorization safety studies (PASS) or registries. These studies enable the assessment of rarer adverse events.
The aim of this study was to evaluate the proportion of MS patients in routine care fulfilling inclusion and exclusion criteria of phase III clinical trials and compare those with and without regarding clinical and sociodemographic characteristics.
Methods
Data source and study population
For this study, data from the German MS Register (GMSR) initiated by the Federal Association of the German Multiple Sclerosis Society (acronym: DMSG) were analysed. The GMSR collects health-related information for MS patients using a web-based documentation system (EDC). Further details of the GMSR have been described elsewhere. 10
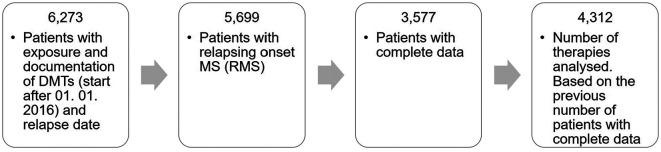
In brief, patients who started a treatment between 1 January 2016 and 31 December 2019 with a DMT (name of the active substance, product name, start date, end date and therapy duration), complete data on gender, age, age at onset, EDSS (expanded disability status scale) score, clinical course and relapse data were analysed. Since exact number of T2 lesions is not collected in GMSR, magnetic resonance imaging (MRI) was not taken into consideration. Initially, 6273 patients with exposure and documentation of selected DMTs were included of which 5699 had a relapsing MS onset. In total, 2122 patients were excluded because of lacking information on clinical data (Figure 1). Although 574 patients with PPMS are in the register, we excluded the PPMS patients due to the small number of patients with full data sets. Exposure with the following DMTs were assessed: ocrelizumab, cladribine, daclizumab, dimethyl fumarate, teriflunomide, alemtuzumab, fingolimod, natalizumab, mitoxantrone, glatiramer acetate, peginterferon β-1a, interferon β-1a and interferon β-1b. Due to the short time on the market, siponimod was not included.
Figure 1.
Data flowchart.
DMT: disease-modifying therapies.
Based on the ‘Summary of Product Characteristics’ published by the European Medicines Agency and a PubMed literature search for each active substance, reports on phase III clinical trials providing information about clinical efficacy and safety were selected. This included the appendix and protocol as well the ClinicalTrials.gov summary. From these documents, patient in- and exclusion criteria for the respective trial were extracted. Summary of Product Characteristics (SCM) references used in this analysis are listed in the Supplementary File.
Data analysis
In case a patient in the register was treated with several drugs over time, the assessment of in- and exclusion criteria was done for every DMT separately. A patient may thus be included in multiple analyses, for each DMT-treatment with the corresponding data. First, the percentage of patients with eligibility to clinical trial criteria across different DMTs was described. Next, the proportion of patients fulfilling each criterion of phase III clinical trials for every selected DMT was analysed. Finally, treated patients fulfilling all recruitment inclusion criteria for a specific drug were compared with those who did not with regard to clinical and sociodemographic characteristics.
For continuous variables, between group comparisons were performed using the Student test and for categorical variables the chi-square test. A p value of <0.05 was considered statistically significant. Analyses were performed using STATA/SE 13.0.
Results
Demographic and clinical characteristics
In total, 3577 individual patients with 4312 therapies were analysed (Figure 1). Demographic and clinical characteristics are summarized in Table 1. 73.4% of the patients were female, mean age was 39.7 years (standard deviation (SD) = 11.6) with a range from 14.6 to 76.0 years. The majority (93.5%) suffered from RRMS, 4% from secondary-progressive MS (SPMS) and 2.5% from a clinically isolated syndrome (CIS).
Table 1.
Demographic and clinical characteristics of the GMSR population and analysed patients.
| Characteristics | GMSR patients a | Analysed patients |
|---|---|---|
| No. of patients | 5699 | 3577 |
| No. of DMT, b mean (SD) | 1.34 (0.74) | 1.21 (0.44) |
| Female (%) | 73.30 | 73.39 |
| Age (years), c mean (SD) | 40.69 (11.58) | 39.68 (11.58) |
| Age onset (years), mean (SD) | 31.322 (10.28) | 30.91 (10.16) |
| DMT duration (days), mean (SD) | 541.71 (427.5) | 564.31 (390.90) |
| EDSS score, mean (SD) | 2.32 (1.93) | 2.06 (1.79) |
| No. of relapses 12, d mean (SD) | 0.28 (0.57) | 0.31 (0.59) |
| No. of relapses 24, e mean (SD) | 0.35 (0.71) | 0.40 (0.73) |
| RRMS (%) | 91.55 | 93.49 |
GMSR: German multiple sclerosis register; DMT: disease-modifying therapy; EDSS: expanded disability status scale; RRMS: relapsing–remitting multiple sclerosis.
First documented DMT started after 1 January 2016.
Number of DMTs during study period of 1 January 2016 to 31 December 2019 per patient.
At treatment start during study period of 1 January 2016 to 31 December 2019.
Since 2015.
Since 2014.
Selection criteria
Table 2 shows a summary of the selected phase III trials for each DMT.11–30 Age, EDSS score, clinical course and relapses were the most frequent inclusion criteria, whereas medication history was a common exclusion criterion. In most clinical trials, patients were included if they were aged between 18 and 55 years; had a RRMS clinical course with at least two relapses within the last 2 years prior to randomization or one relapse in the year before and/or an MRI scan of the brain showing abnormalities consistent with multiple sclerosis (e. g. at least one gadolinium-enhancing lesion 0–6 weeks to randomization). This inclusion criterion (MRI scan) was not applied due to the limited data. A further selection criterion was an EDSS score between 0.0 and 5.0. A detailed description of the inclusion criteria is shown in Table 3. Table 4 shows the medication history requirements for clinical trial entrance.
Table 2.
Summary of phase III clinical trials proving information on safety and efficacy for disease-modifying therapies in multiple sclerosis patients.
| Active substance | Name | Pharmacotherapeutic group a | EU approval | Trial name | Trial published | Trial reference |
|---|---|---|---|---|---|---|
| Ocrelizumab | Ocrevus | Immunosuppressant | 2018 | OPERA I and II | 2017 | 11 |
| Cladribine | Mavenclad | Immunosuppressant | 2017 | CLARITY | 2010 | 12 |
| Daclizumab | Zinbryta | Immunosuppressant | 2016 | DECIDE | 2015 | 13 |
| SELECT | 2013 | 14 | ||||
| Dimethyl fumarate | Tecfidera | Antineoplastic and immunomodulating agents | 2014 | CONFIRM | 2012 | 15 |
| DEFINE | 2012 | 16 | ||||
| Teriflunomide | Aubagio | Selective immunosuppressant | 2013 | TOWER | 2014 | 17 |
| TEMSO | 2011 | 18 | ||||
| Alemtuzumab | Lemtrada | Selective immunosuppressant | 2013 | CARE MS I | 2012 | 19 |
| CARE MS II | 2012 | 20 | ||||
| Fingolimod | Gilenya | Immunosuppressant | 2011 | FREEDOM MS | 2010 | 21 |
| TRANSFORM | 2010 | 22 | ||||
| Natalizumab | Tysabri | Selective immunosuppressant | 2006 | AFFIRM | 2006 | 23 |
| Mitoxantrone | Novantrone | Antineoplastic agent | 2003 | MIMS | 2002 | 24 |
| Glatiramer acetate | Copaxone | Immunostimulants | 2001 | European/Canadian GA study | 2001 | 25 |
| Copolymer 1MS study | 1995 | 26 | ||||
| Peginterferon β-1a | Plegridy | Immunostimulants | 2014 | ADVANCE | 2014 | 27 |
| Interferon β-1a | Rebif | Immunostimulants | 1998 | STUDY NS26321 | 1998 | 28 |
| Interferon β-1a | Avonex | Immunostimulants | 1997 | PRISM | 1995 | 29 |
| Interferon β-1b | Betaferon | Immunostimulants | 1995 | IFNB multiple sclerosis study | 1993 | 30 |
According to Summary of Product Characteristics (SCM) of the European Medicines Agency.
Table 3.
Inclusion criteria for phase III clinical trials providing information on clinical efficacy and safety of disease-modifying therapies for multiple sclerosis patients.
| DMT | Age | EDSS score | Clinical course | Relapses |
|---|---|---|---|---|
| Ocrelizumab | 18–55 | 0.0–5.0 | RRMS | At least two relapses within the last 2 years prior to screening or one relapse in the year before screening. No relapse 30 days before trial entry |
| Cladribine | 18–65 | 0.0–5.5 | RRMS | At least one relapse within 12 months before study entry. No relapse within 28 days before study entry |
| Daclizumab | 18–55 | 0.0–5.0 | RRMS | At least one relapse in the 12 months before randomization |
| Dimetyl fumarate | 18–55 | 0.0–5.0 | RRMS | At least one relapse within the 12 months before randomization |
| Teriflunomide | 18–55 | 0.0–5.5 | RRMS/SPMS | At least one relapse in the 12 months or at least two relapses in the 24 months before randomization. No relapse within 30 days before randomization |
| Alemtuzumab | 18–55 | 0.0–5.5 | RRMS | At least two relapses in the previous 2 years and at least one in the previous year |
| Fingolimod | 18–55 | 0.0–5.5 | RRMS | At least one relapse during the previous year or at least two relapses during the previous 2 years |
| Natalizumab | 18–50 | 0.0–5.0 | RRMS | At least one relapse within the 12 months before the study began. No relapse within 50 days before the first administration of the study drug |
| Mitoxantrone | 18–55 | 3.0–6.0 | RRMS/SPMS | No relapses 8 weeks before randomization |
| Glatiramer acetate | 18–50 | 0.0–5.0 | RRMS | At least two relapses in the 2 years prior to entry, onset of the first relapse at least 1 year before randomization |
| Peginterferon β-1a | 18–65 | 0.0–5.0 | RRMS | At least two relapses within the last 3 years with at least one occurring in the last 12 months |
| Interferon β-1a | 18–55 | 0.0–5.0 | RRMS | At least two relapses in the previous 2 years |
| Interferon β-1b | 18–55 | 0.0–5.5 | RRMS | At least two relapses in the previous 2 years |
DMT: disease-modifying therapy; EDSS score: expanded disability status scale score; RRMS: relapsing–remitting multiple sclerosis; SPMS: secondary-progressive multiple sclerosis.
Table 4.
Medication history criteria for entrance to a phase III clinical trials providing information on clinical efficacy and safety of disease-modifying therapies for multiple sclerosis patients.
| DMT | Medication history |
|---|---|
| Ocrelizumab | No treatment with β interferons, glatiramer acetate or other immunomodulatory therapies within 4 weeks prior to baseline. No previous treatment with B-cell targeted therapies (rituximab, ocrelizumab) or with alemtuzumab, cladribine, mitoxantrone, daclizumab and teriflunomide. No treatment with cyclophosphamide, azathioprine, methotrexate or natalizumab within 24 months prior to screening. Patients previously treated with natalizumab will be eligible for trial only if duration of treatment was <1 year. No treatment with fingolimod within 24 weeks prior to screening |
| Cladribine | For patient who had received a DMT, a washout period of at least 3 months before study entry was required. No prior immunosuppressive treatment. No prior natalizumab treatment |
| Daclizumab | No prior treatment with caldribine. No prior treatment with mitoxantrone, cyclophosphamide, fingolimod or natalizumab within 1 year prior to randomization. No treatment with glatiramer acetate within the 30 days prior to randomization |
| Dimethyl fumarate | No previous treatment with glatiramer acetate or cladribine. No prior treatment with mitoxantrone within 1 year prior to randomization. No prior treatment with natalizumab within the 6 months prior to randomization. No prior treatment with interferons within the 3 months prior to randomization. No treatment with steroids or oral corticosteroids within 50 days prior to randomization |
| Teriflunomide | No prior use of glatiramer acetate or interferons in the 3 months prior to randomization. No prior or use of natalizumab, cladribine or mitoxantrone |
| Alemtuzumab | No treatment within the previous 6 months with natalizumab or methotrexate |
| Fingolimod | No requirements |
| Natalizumab | No treatment with mitoxantrone or cyclophosphamide within the previous year. No treatment with interferons, azathioprine, glatiramer acetate or immune globulins within the previous 6 months |
| Mitoxantrone | No treatment with glucocorticosteroids for at least 8 weeks before baseline. No previous therapy with interferons, glatiramer acetate or mitoxantrone |
| Glatiramer acetate | No requirements |
| Peginterferon β-1a | Subjects must have discontinued interferon treatment 6 months prior to baseline. No prior treatment with caldribine, fingolimod or mitoxantrone (1 year prior to baseline) and glatiramer acetate (4 week prior to baseline) |
| Interferon β-1a | No prior treatment with interferon or with other immunomodulatory or immunosuppressive treatments in the preceding 12 months |
| Interferon β-1b | No requirements |
Post-approval compliance with selected clinical trial criteria
Across all analysed drugs, 714 (16.56%) treatments were done in patients who would have been included into a phase III clinical trial if all prespecified analysis criteria were applied. If the selection criterion ‘relapse’ was dropped, 67.5% (2909) would have fulfilled all other prespecified analysis criteria and would therefore been included into the respective phase III clinical trial.
Thus, ‘relapse’ was the selection criterion with the highest exclusion rate (74.7%) across the different DMTs, followed by ‘medication history’ (21.2%). 11.8% of the analysed patients did not meet the criterion ‘age’, while 7.2% of the patients did not conform with the ‘EDSS score’ criterion. ‘Clinical course’ (5.4%) leads to the lowest exclusion rate (Figure 2).
Figure 2.
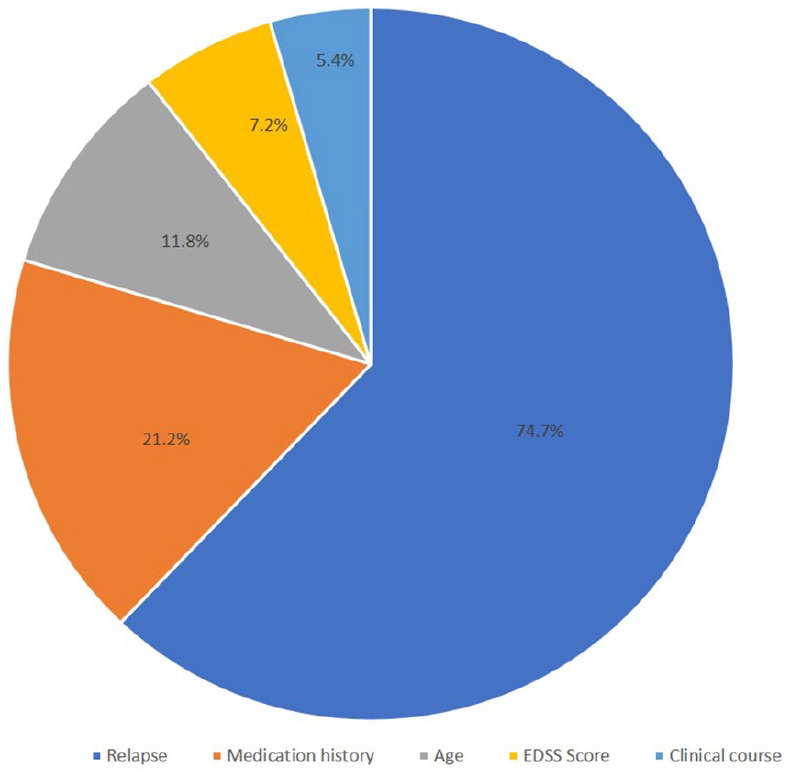
Selected clinical trial criteria and exclusion rates.
Table 5 shows the percentage of patients in this routine care register who would have been selected for a phase III clinical trial of their respective drug. 21.7% of patients treated with alemtuzumab and 20.8% of patients receiving natalizumab fulfilled all five selection criteria, showing the highest concordance with clinical trial criteria, while ocrelizumab (7.6%) and interferon (4.0%) patients had the lowest concordance. 27.7% of fingolimod and 20.22% of glatiramer acetate patients fulfilled all four admission criteria.
Table 5.
Percentage of GMSR patients fulfilling phase III clinical trial selection criteria.
| DMT | Patients (N) | Therapies (N) | Percentage of patients fulfilling each criterion | Percentage of patients fulfilling all criteria | Percentage of patients fulfilling four criteria a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age | EDSS score | Relapse | Clinical course | Medication history | |||||
| Ocrelizumab | 580 | 580 | 85.17 | 77.07 | 22.76 | 86.55 | 62.76 | 7.59 | 39.48 |
| Cladribine | 136 | 136 | 99.26 | 90.44 | 33.82 | 92.65 | 50.00 | 12.50 | 41.18 |
| Daclizumab | 171 | 171 | 86.55 | 84.21 | 29.24 | 94.15 | 81.87 | 20.47 | 60.23 |
| Dimethyl fumarate | 643 | 661 | 92.74 | 98.18 | 22.39 | 96.97 | 83.96 | 15.73 | 74.58 |
| Teriflunomide | 521 | 528 | 84.66 | 96.40 | 20.82 | 97.73 | 85.23 | 14.77 | 67.61 |
| Alemtuzumab | 166 | 166 | 96.99 | 93.37 | 31.33 | 99.40 | 83.73 | 21.69 | 76.51 |
| Fingolimod | 601 | 606 | 89.11 | 95.21 | 32.84 | 97.03 | n/a | 27.72 | 84.65 |
| Natalizumab | 418 | 423 | 88.42 | 93.38 | 33.33 | 97.40 | 85.34 | 20.80 | 68.32 |
| Mitoxantrone | 37 | 37 | 59.64 | 62.16 | 91.89 | 100.00 | 94.59 | 37.84 | 40.53 |
| Glatiramer acetate | 547 | 554 | 81.95 | 98.38 | 26.53 | 92.24 | n/a | 20.22 | 75.09 |
| Interferons | 441 | 450 | 92.00 | 96.89 | 7.56 | 93.33 | 82.89 | 4.00 | 69.11 |
DMT: disease-modifying therapy; EDSS score: expanded disability status scale score.
Percentage of patients fulfilling all criteria when selection criterion relapse was excluded.
When excluding the selection criterion, ‘relapse’ patients with alemtuzumab (76.5%) and dimethyl fumarate (74.6%) therapy had the highest concordance with the four selection criteria of the respective phase III clinical trials, while those treated with cladribine (41.2%) and ocrelizumab (39.5%) showed the lowest.
Comparison of patients treated with DMTs post approval
Table 6 shows the comparison of MS patients with and without fulfillment of the respective phase III criteria. In general, more women than men did not meet the selection criteria. Across all DMTs, with exception of cladribine, patients not fulfilling all criteria were older than patients fulfilling all criteria. With regard to treatment duration, patients on alemtuzumab not fulfilling all inclusion criteria were treated longer (on average 167 days) than those who met all criteria. This difference equals 111 days in patients receiving natalizumab. Statistical significant differences between MS patients with and without fulfillment of the respective phase III criteria are marked in Table 6.
Table 6.
Difference (Δ) between patients who would not have been accepted into phase III clinical trial compared patients fulfilling all admission criteria.
| Mean Δ | Ocrelizumab | Cladribine | Daclizumab | Dimethyl fumarate | Teriflunomide | Alemtuzumab | Fingolimod | Natalizumab | Mitoxantrone | Glatiramer acetate | Interferons |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients (therapies) a | 536 (536) | 119 (119) | 135 (135) | 546 (557) | 447 (450) | 130 (130) | 437 (438) | 331 (335) | 23 (23) | 436 (442) | 425 (432) |
| Female (%) | 0.63** | 5.04 | 4.39 | −0.20 | 5.85 | −5.18 | 5.57 | 4,05 | −1.86 | −0.53* | 12.5 |
| Age (years) | 3.98* | −6.47* | 6.59** | 2.38* | 3.61** | 3.21 | 3.66*** | 1.93 | 11.29*** | 3.21*** | 2.48 |
| Age onset (years) | 0.68 | −3.63 | 1.95 | 0.54 | 1.77 | 3.47* | 1.79 | 1.23 | 7.44* | 4.22*** | 2.78 |
| DMT duration (days) | 40.10 | 74.39 | −4.91 | 10.79 | −16.62 | 167.89* | 98.25** | 111.18* | −68.35 | −60.09 | 66.26 |
| DMTs, No | −0.23 | −0.07 | 0.51 | −0.05 | 0.04 | −0.20** | −0.22*** | −0.18* | −0.35 | 0.07 | −0.09 |
| EDSS score | 0.79* | −0.56 | 1.11** | 0 | 0.51** | 0 | 0.30 | 0 .36 | 0.84 | 0.13 | 0.37 |
| Relapses 12, No. | −0.89*** | −1.06*** | −1.19*** | −1.17*** | −1.05*** | −1.37*** | −1.22*** | −1.14*** | 0.36*** | −1.13*** | −1.54*** |
| Relapses 24, No. | −0.99*** | −1.17*** | −1.29*** | 1.28*** | −1.10*** | −1.52*** | −1.54*** | −1.29*** | 0.44*** | −1.21*** | −1.76*** |
DMT: disease-modifying therapy; EDSS: expanded disability status scale; No.: number.
Number of patients (therapies) missed at least one selection criterion; *p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
In this analysis, we assessed the transferability of clinical trial in- and exclusion criteria to MS patients in routine clinical care using a large MS register in Germany. We showed that the majority of patients treated with DMT in routine care after approval of a DMT would not have met the inclusion criteria for phase III clinical trials leading to approval of the respective drug. This was mainly due one criterion, relapses. Omitting this criterion would increase the proportion of patients fulfilling inclusion criteria to about two-thirds (67.5%).
Phase III clinical trials mainly include patients who experienced at least two relapses in the 2 years prior or at least one relapse during the first year prior to randomization. Since the reduction of the annualized relapse rate (ARR) is a common endpoint in phase-III studies in MS, there is rational for an indication of disease activity. But, not fulfilling this criterion does not imply potential risks since a low relapse rate is an indication for a successful, rather stable disease under current therapy. 31 On the other extreme, patients might receive a DMT too early, inducing potential risks. The omission of the criterion ‘relapse’ increased the final eligibility for all patients, irrespective of the DMTs, with the exception of mitoxantrone, for which this criterion was defined differently than in other clinical trials. Next to ‘relapse’, about one in five patients did not fulfill the criterion requirement of ‘medication history’. Most of the clinical trials required at least a washout period before randomization; in some trials, a prior DMT therapy acted as an exclusion criterion. Current national guidelines suggest to switch to a second-line therapy if a patient has at least one relapse under DMT or does not respond to the current DMT (e.g. shown by new MRI activity). 32 As a result, most patients receive more than one DMT over the disease course and therefore a treatment history without MS DMT is rare. Furthermore, certain DMTs are known to result in rebound relapses if a treatment is stopped without an appropriate DMT switch. 33 Thus, washout periods as imposed in phase III clinical trials are difficult to achieve in clinical routine. Both might have contributed to the high non-fulfillment of this criterion.
Several studies investigated clinical trial generalizability in different disease indications, for instance, cardiovascular diseases, cancer and lung diseases. According to the review of He et al., 9 who assessed generalizability of clinical trials in the big data era, 59.9% (111) of the published studies concluded that the evaluated trials are not generalizable, whereas 29.4% concluded that they are. 11.2% (21) of authors reported mixed results. This study for MS patients is in concordance with similar studies in other disease. No generalizability studies of clinical trials for DMTs in MS have been published yet.
The severity of disease, pharmacological response and adverse events show a decent variability in different patient populations. Children, elderly, both men and women as well as individuals with different genotypes show divergent pharmacokinetic and pharmacodynamic profiles. 34 Thus, therapy should be adapted especially in term of dose adjustment and safety. Most phase III clinical trials exclude patients younger than 18 and older than 55 years. Thus, post-approval studies providing information on clinical safety and efficacy in these population groups are rare. Consequently, potential risks for drug safety in these groups are less known. But different characteristics of patients who do not fulfill inclusion criteria will have different importance when inferences on effects and risks of a new drug are made to those not included in the clinical trial. Patients not fulfilling all criteria in our register were on average older than those fulfilling all inclusion criteria. Similarly, in the groups with at least one selection criterion, missing women were more often included than men, indicating a limitation of gender generalizability.
Our study has several limitations. Several patients were excluded because of incomplete documentation, resulting in a smaller sample size for some DMTs which could imply a generalizability issue for those DMTs. Some of the phase III clinical trials applied MRI of the brain indicating MS comparable abnormalities (e. g. at least one gadolinium-enhancing lesion 0–6 weeks to randomization) as an inclusion criterion. Because of incomplete MRI data, this criterion was not applied to the GMSR patients, a further limitation of our study. However, this mirrors the real world, since MRIs are not standardized and can only be compared to a trial situation to a limited extent because different images are evaluated by different radiologists. 35 An additional limitation is the lack of treatment-related outcomes (e.g. adverse events) in the register. Thus, we could not investigate differences in outcomes between patient who met the criteria and those who did not.
In conclusion, we found that the majority of MS patients treated with a DMT in routine care would not have been included into the clinical trial that led to the approval of the respective drug. Regulatory agencies and clinical trial investigators may change the approach in trial design in order to ensure a more representative patient population enrolment. By contrast, more heterogeneous trials with lower probability of an endpoint occurrence may require larger sample sizes increasing the costs of trials and decelerate the progression of drug development due to recruitment concerns.
A priori generalizability studies based on registry data and trial design information might be a chance for investigators to adapt studies before start. Posteriori generalizability studies, like the present one, may benefit from data linkage, bringing together primary registry and secondary data (for instance, data from health insurances) in order to describe the clinical care routine and the treated population even better. An integration of a priori generalizability studies, a posteriori generalizability studies, and randomized clinical trials would result in an improvement in the drug life cycle in consideration for a safe and efficient drug therapy. Another approach in the implementation of clinical trials in clinical care routine could be the establishment of registry-based randomized clinical trials.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_1352458520985118 for Effect of applying inclusion and exclusion criteria of phase III clinical trials to multiple sclerosis patients in routine clinical care by Kris Oliver Jalusic, David Ellenberger, Paulus Rommer, Alexander Stahmann, Uwe Zettl and Klaus Berger in Multiple Sclerosis Journal
Acknowledgments
The authors would like to thank all patients who have given their informed consent to be included in GMSR. Furthermore, this study would not have been possible without the efforts of the centres participating in the registries. They thank all members of staff in all contributing centres for their continued efforts. Centres that provided data for this study are listed below. Medizinische Versorgungszentren, Altenburger Land GmbH, Altenburg. Gemeinschaftspraxis Dr. med. A. Safavi and M. Schädel, Alzenau. Neurozentrum Alzey, Dr. Loos/Dr. Bernhard, Alzey. Rhein-Mosel-Fachklinik Andernach, Zentrum für Psychiatrie, Psychotherapie und Neurologie, Andernach. DRK Kamillus Klinik, Neurologische Abteilung, Asbach. Praxis Dr. Hofmann, Aschaffenburg. Zentrum für Neurologie, Psychiatrie und Psychotherapie Asperg, Asperg. HELIOS Klinikum Aue, Klinik für Neurologie, Aue. Universitätsklinikum Augsburg, Neurologische Klinik mit klinischer Neurophysiologie, Augsburg. Dr. Schöll, Dr. Steidl & Kollegen, Bad Homburg. Caritas-Krankenhaus Bad Mergentheim gGmbH, Klinik für Neurologie, Bad Mergentheim. Rhön-Klinikum Campus Bad Neustadt, Klinik für Neurologie, Bad Neustadt a. d. Saale. Johanniter Ordenshäuser, Neurologische Abteilung, Bad Oeynhausen. MediClin Reha-Zentrum Bad Orb, Neuro-orthopädisches Reha-Zentrum, Bad Orb. Agaplesion Ev. Bathildis Krankenhaus, Neurologische Ambulanz, Bad Pyrmont. Segeberger Kliniken GmbH, Neurologisches Zentrum, Bad Segeberg. Neurologisches Rehabilitationszentrum Quellenhof, Bad Wildbad. Wicker-Klinik, Neurologische Abteilung, Bad Wildungen. Dr. Becker Kiliani-Klinik, Neurologische Abteilung, Bad Windsheim. Hardtwaldklinik I, Neurologisches Zentrum, Werner Wicker GmbH & Co. KG, Bad Zwesten. Neurologische Klinik Selzer GmbH, Baiersbronn. Kallmann Neurologie–Multiple Sklerose Zentrum Bamberg (MSZB), Dr. med. Boris-A. Kallmann, Bamberg. Praxisklinik für Neurologie, Psychiatrie, Psychosomatische Medizin und Psychotherapie, Dres. Käfferlein, Dengler, Jung und Schreiber, Bamberg. Klinik Hohe Warte Bayreuth, Neurologische Klinik, Bayreuth. Marianne-Strauß-Klinik, Behandlungszentrum Kempfenhausen für Multiple Sklerose Kranke gemeinnützige GmbH, Berg. Charité–Universitätsmedizin Berlin, Ambulanz für Multiple Sklerose und Neuroimmunologie, Berlin. Alexianer St. Joseph-Krankenhaus Berlin-Weißensee, Klinik für Neurologie/MS-Ambulanz §116 B, Berlin. Jüdisches Krankenhaus Berlin, Akademisches Lehrkrankenhaus der Charite, Zentrum für Multiple Sklerose, Berlin. Vivantes Klinikum Neukölln–Klinik für Neurologie, Spezialambulanz für Multiple Sklerose, Berlin. Neurologische Praxis, MD Turki Akil, Berlin. Evangelisches Krankenhaus Bethel gGmbH, Klinik für Neurologie, Bielefeld. Neurozentrum Bielefeld-Brackwede, Dres. med. J. und M. Böhringer, Dr. J. Katzmann, F. Sudfeldt, Bielefeld. Medical Park Loipl, Bischofswiesen. Neurologische Praxis, Böblingen. Gemeinschaftspraxis Dres. med. Niederhofer, Kauermann, Küper, Bochum. Berufsgenossenschaftliches Universitätsklinikum Bergmannsheil gGmbH, Neurologische Klinik und Poliklinik, Bochum. St. Josef-Hospital, Klinikum der Ruhr-Universität Bochum, Klinik für Neurologie, Bochum. Neurologische Facharztpraxis Dr. I. Nastos, Bochum. Gemeinschaftspraxis Kausch & Lippert, Bogen. Neurologische Gemeinschaftspraxis, Bonn. Neurologisches Rehabilitationszentrum Godeshöhe e.V., Bonn. Asklepios Fachklinikum Brandenburg, Klinik für Neurologie, Brandenburg. Neurozentrum Schlosscarree, Dr. med. Ekkehard Klippel, Braunschweig. Neurologie am Ziegenmarkt, Braunschweig. Städtisches Klinikum Braunschweig gGmbH, Neurologische Klinik, Braunschweig. Klinikum Bremerhaven Reinkenheide, Neurologische Tagesklinik, Bremerhaven. Carl-Thiem Klinikum Cottbus, Klinik für Neurologie, Cottbus. Dr. med. Martin Delf, Facharzt für Neurologie, Dahlwitz-Hoppegarten bei Berlin. VAMED Rehaklinik Damp, Abteilung Neurologie, Damp. Bezirksklinikum Mainkofen, Neurologische Klinik, Deggendorf. Neurologische Gemeinschaftspraxis Dillingen, Dillingen. Praxis für Neurologie Leclaire/Rotermund, DOC Center Dortmund, Dortmund. Multiple Sklerose Zentrum Dresden, Neurologische Uniklinik Dresden, Dresden. Christophorus–Kliniken GmbH, Neurologische Klinik Dülmen, Dülmen. Medizinisches Versorgungszentrum Düren–Lendersdorf, Praxis Dr. Brand, Düren. Heinrich-Heine-Universität Düsseldorf, Westdeutsches MS-Zentrum Düsseldorf, Düsseldorf. Martin Gropius Krankenhaus Eberswalde, Klinik für Neurologie, Eberswalde. Neurologie im MVZ und Klinikum Eisenach, Eisenach. Praxis Dr. Kirchhöfer, Erfurt. HELIOS Klinikum Erfurt GmbH, Klinik Für Neurologie, Erfurt. Universitätsklinikum Erlangen, Klinik für Neurologie, Erlangen. Dr. med. Edgar Bollensen, Facharzt für Neurologie und Psychiatrie, Eschwege. Universitätsklinikum Essen, Klinik für Neurologie, MS-Ambulanz, Essen. Alfried-Krupp-Krankenhaus, Klinik für Neurologie, Essen. Praxis Nervenstark, Essen. Zentrum für ambulante Neurologie, Essen. Fachklinik Feldberg GmbH, Klinik am Haussee, Feldberger Seenlandschaft. Universitätsklinikum Frankfurt, Klinik für Neurologie, Frankfurt/Main. Krankenhaus Nordwest GmbH, Neurologische Klinik, Frankfurt/Main. Kliniken Schmieder Gailingen, Neurologisches Fach- und Rehabilitationskrankenhaus, Gailingen. MVZ Gelderland, Dr. med. Peter Asmus, Geldern. SRH Waldklinikum Gera GmbH, Klinik für Neurologie, Gera. Gemeinschaftspraxis Dres. Ivancic & Kollegen, Gersthofen. Kath. Kliniken Emscher-Lippe GmbH, St. Barbara-Hospital, Neurologische Abteilung, Gladbeck. Dr. med. Kornelia Seidel, Praxis für Neurologie und Psychiatrie, Gladenbach. Universitätsmedizin Göttingen, Klinik für Neurologie, Göttingen. Universitätsmedizin Greifswald, Klinik und Poliklinik für Neurologie, Greifswald. VAMED Klinik Hagen-Ambrock GmbH, Klinik für Neurologie, Hagen. Städtisches Krankenhaus Martha-Maria Halle-Dölau gGmbH, Klinik für Neurologie, Halle. Lars Daume, Facharzt für Neurologie, Halle. Gemeinschaftspraxis Dres. Schult/Löffler-Wulff, Hamburg. Praxisgemeinschaft Neurologie–Psychiatrie, Elias/Elias-Hamp und Hebell-Siewers, Hamburg. RehaCentrum Hamburg GmbH, Fachbereich Neurologie, Hamburg. Klinikum Hanau GmbH, Klinik für Neurologie, Hanau. Universitätsklinikum Heidelberg, Neurologische Klinik, Heidelberg. Medizinisches Versorgungszentrum, Zentrum für Sozialpsychiatrie und Nervenheilkunde GmbH, Hemmoor. Klinik Hennigsdorf, Oberhavel Kliniken GmbH, Neurologische Abteilung, Hennigsdorf. Klinikum Herford, Klinik für Neurologie/MS-Ambulanz, Herford. Medizinisches Versorgungszentrum Herne, Herne. Haus- und Facharztzentrum Laucherttal/Alb, Praxis für Neurologie, Hettingen. Celenus Klinik für Neurologie Hilchenbach, Hilchenbach. HELIOS Fachkliniken Hildburghausen, Klinik für Neurologie, Hildburghausen. Praxis für Neurologie, Doctor-medic Claudia Man, Höxter. Klinikum Ibbenbüren, Klinik für Neurologie, Ibbenbüren. MVZ Immenstadt Allgäu GmbH, Neurologie, Psychiatrie, Psychotherapie, Immenstadt. Alexianer Misericordia GmbH–Augustahospital Anholt, Klinik für Neurologie, Isselburg-Anholt. Neurozentrum am Klosterforst, Itzehoe. Universitätsklinikum Jena, Neurologische Klinik, Jena. Neurozentrum Kaltenkirchen, Kaltenkirchen. ZNS Kamen, Neurologisch-Psychiatrische Praxisgemeinschaft, Kamen. Städtisches Klinikum Karlsruhe gGmbH, Neurologische Klinik -Haus D-, Karlsruhe. Neurologische Gemeinschaftspraxis Kassel Vellmar, Lassek/Dr. Ammerbach/Dr. Fetzer/Fischer, Kassel. Universitätsklinikum Schleswig-Holstein, Ambulanz und Tagesklinik für Neuroimmunologie und Multiple Sklerose, Kiel. Heilig Geist-Krankenhaus, Klinik für Neurologie, Köln. Praxis rechts vom Rhein, Dr. med. Jan-Dirk Seifert und PD Dr. med. Hela-Felicitas Petereit, Köln. NeuroMed Campus Nelles, Scharpegge, Haupt, Scharwat, Fachärzte für Neurologie, Schmerztherapie, Köln. Universitätsklinikum Köln, Klinik und Poliklinik für Neurologie, Köln. Kliniken Schmieder Konstanz, Neurologisches Fach- und Rehabilitationskrankenhaus, Konstanz. Facharztpraxis für Neurologie und Psychiatrie, im Facharztzentrum am Klinikum Konstanz, Konstanz. Praxisgemeinschaft Neurologie am Zoo, Krefeld. Gemeinschaftspraxis Dr. med. B. Wittmann & P. Rieger, Landshut. Praxis Dr. Pfeffer & Dr. Staudinger-Pfeffer, Praxis für Neurologie und Psychiatrie, Landshut. Ärztehaus Stötteritz, Neurologische Praxis, Leipzig. hygieia.net Leipzig, Leipzig. Klinikum Lippe-Lemgo, Neurologische Klinik, Lemgo. Helios Klinik Lengerich GmbH, Abteilung Neurologie, Lengerich. Märkische Kliniken GmbH, Klinikum Lüdenscheid, Klinik für Neurologie, Lüdenscheid. Neuropsychiatricum, Dr. Deibel/Dr. Fischer/Dr. Klenk/Dr. Kohlmaier/Dr. Stenzel, Ludwigshafen. St.-Marien-Hospital GmbH, Neurologische Klinik, Lünen. Gemeinschaftspraxis Katte, Vogelsang-Dietz, Graf, Lünen. Neurologische Praxis U. Kullik, Lutherstadt Eisleben. MS-Spezialambulanz Stephanik, Universitätsklinikum Magdeburg, Magdeburg. MEDIAN Klinik NRZ Magdeburg, Magdeburg. Universitätsklinikum Marburg, Klinik für Neurologie, Marburg. Neurologie in Meerbusch, Meerbusch. Gesundheitszentrum Glantal, Meisenheim. Neurologische Gemeinschaftspraxis, Memmingen, Memmingen. Neurologische Gemeinschaftspraxis im medicentrum Mönchengladbach, Mönchengladbach. Fachübergreifende Gemeinschaftspraxis Neurologie & Radiologie, Mosbach. Ökumenisches Hainich Klinikum gGmbH, Klinik für Neurologie, Mühlhausen. Neuro-Psychiatrisches Zentrum Riem, München. Neurologie am Ludgeriplatz, Münster. Herz-Jesu-Krankenhaus Hiltrup GmbH, Klinik für Neurologie mit klinischer Neurophysiologie, Münster. Gemeinschaftspraxis Rickert/Enck/Jansen, Münster. Gemeinschaftspraxis Dres. Wiborg, Kramer, Brummer, Neu-Ulm. Praxis Dr. Bergmann & Kollegen, Praxis für Neurologie und Psychotherapie, Neuburg. Ruppiner Kliniken GmbH, Klinik für Neurologie, MS-Ambulanz, Neuruppin. Gemeinschaftspraxis Dr. Rieth, Saur, Dr. Pfister, Neusäß. Nervenärztliche Gemeinschaftspraxis Nürnberg, Nürnberg. Gemeinschaftspraxis Dres. Uhlig/Windsheimer, Nürnberg. Praxis Dres. Schlüter, Beckmann, Berufsausübungsgemeinschaft, Öhringen. Neurologie an der Hase, Holger Lorenzen, Dr. med. Edzard Ites, Stephan Ulf Sylvester, Jens Gläscher, Dr. med. Elisabeth Rehkopf, Osnabrück. MVZ für Neurologie der Paracelsus-Klinik Osnabrück, Osnabrück. Praxis Dr. med. Christoph Schenk, Osnabrück. Klinikum Osnabrück GmbH, Neurologische Klinik, Osnabrück. Praxis Dr. Roth & Partner, Facharzt für Nervenheilkunde, Ostfildern. St. Vincenz-Krankenhaus GmbH Paderborn, Neurologische Klinik, Paderborn. Neurozentrum Peine, Peine. Facharztpraxis für Neurologie und Psychiatrie, Andreas Stockert und Dr. Claudia Rettenmayr, Pforzheim. St. Josefs-Krankenhaus Potsdam, Klinik für Neurologie, Potsdam. Praxis Dr. Altmann, Facharzt für Neurologie und Psychiatrie, Potsdam. VAMED Klinik Schloss Pulsnitz GmbH, Pulsnitz. Neurozentrum Ravensburg, Dres. Dieterle/Kunz, Ravensburg. Neurologische Praxis, Maier-Janson/Friedrich, Ravensburg. Knappschaftskrankenhaus Recklinghausen, Klinik für Neurologie und Klinische Neurophysiologie, Recklinghausen. Neurologische Praxis Dr. Wendelin Kyrill Blersch, Regensburg. Universitätsklinikum Regensburg, Klinik und Poliklinik für Neurologie am Bezirksklinikum Regensburg, Regensburg. Sächsisches Krankenhaus f. Psychiatrie u. Neurologie, Zentrum für Psychiatrie, Psychotherapie, Psychosomatik und Neurologie, Rodewisch. Universitätsmedizin Rostock, Klinik und Poliklinik für Neurologie, Rostock. Immanuel-Klinik-Rüdersdorf, MS-Ambulanz, Rüdersdorf. ZNS Südpfalz–Zentrum für Nervensystem & Seele, Rülzheim. Nordwest-Krankenhaus Sanderbusch, Neurologie, Sande. Medizinisches Versorgungszentrum Schlüchtern, Schlüchtern. Diakonie-Klinikum Schwäbisch Hall gGmbH, Neurologische Klinik, MS-Ambulanz, Schwäbisch Hall. Hephata-Klinik, Neurologie, Schwalmstadt. Fachklinik für Neurologie Dietenbronn GmbH, Akademisches Krankenhaus der Universität Ulm, Schwendi. HELIOS Kliniken Schwerin, Neurologische Klinik, Schwerin. Berufsausübungsgemeinschaft Prof. Wagner & Dr. Kaltenmaier, Schwetzingen. Asklepios Kliniken Schildautal Seesen, Klinik für Neurologische Rehabilitation, Seesen. Asklepios Kliniken Schildautal, Klinik für Neurologie, Seesen. Neurologisch-Psychiatrische Gemeinschaftspraxis, Dr. Schulz, Dr. Lindemuth, Hübner, Dr. Heimel, Albertus Magnus Zentrum, Siegen. Neurologische Praxis, MVZ Siegerlandzentrum, Siegen. E/M/S/A, Zentrum für Neurologie / Psychiatrie / Neuroradiologie, Singen. MediClin Klinikum Soltau, Soltau. Neurologische Praxisgemeinschaft, Sprockhövel. MediClin Bosenberg Kliniken, St. Wendel. Asklepios Fachklinikum Stadtroda, Klinik für Neurologie, Stadtroda. ZNS Straubing, Straubing. Klinikum Stuttgart Katharinenhospital, Neurologische Klinik, Neurozentrum, Stuttgart. Neurozentrum Sophienstrasse, Dr. Herbst, Dr. Wannenmacher, Dr. Hartmann, Stuttgart. Neurologisch-Psychiatrische Praxis Dr. Susanne Weber, Stuttgart. mind mvz GmbH, Stuttgart. Neurologische und psychiatrische Praxis Dr. Kowalik, Stuttgart. Dres. med. M. Appy, W. Moult, Prof. A. Melms & Kollegen, Berufsausübungsgemeinschaft, Stuttgart. Knappschaftskrankenhaus Sulzbach, Neurologische Klinik, Sulzbach/Saar. Sauerlandklinik Hachen, Neurologische Spezialklinik Multiple Sklerose, Sundern. Asklepios Fachklinikum Teupitz, Neurologische Klinik, Teupitz. Krankenhaus der Barmherzigen Brüder, Abteilung für Neurologie und Neurophysiologie, Trier. GFO Kliniken Troisdorf, Fachabteilung Neurologie, Troisdorf. Universitätsklinikum Tübingen, Zentrum für Neurologie, Tübingen. Helios Klinikum Uelzen GmbH, Uelzen. Universitätsklinikum Ulm, Klinik und Poliklinik für Neurologie, Ulm. Neuropraxis München Süd, Unterhaching. Westerwaldklinik Waldbreitbach gGmbH, Rehabilitationszentrum für Neurologie und Neurologische Psychosomatik, Waldbreitbach. Dr. med. Sabine Gschrey & Dr. med. Gerhard Gschrey, Ärzte für Neurologie und Psychiatrie, Wendlingen. Klinikum St. Georg/Fachkrankenhaus Hubertusburg, Klinik für Neurologie und Neurologische Intensivmedizin, Wermsdorf. Gemeinschaftspraxis Dr. Springub/Schwarz, Westerstede. Ammerland-Klinik GmbH, Klinik für Neurologie, Westerstede. DKD HELIOS Klinik Wiesbaden, Fachbereich Neurologie, Wiesbaden. MEDIAN Klinik Wilhelmshaven, Fachklinik für Orthopädie und Neurologie, Wilhelmshaven. Neurologisch-Psychiatrische Gemeinschaftspraxis Wolfenbüttel, Wolfenbüttel. Neurologische Praxis am Klieversberg, Wolfsburg. Praxis Dr. med. J. D. Seybold, Würzburg. Universitätsklinikum Würzburg, Neurologische Klinik und Poliklinik, Würzburg.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: The German MS Registry of the German MS Society was initiated and funded by the German MS Foundation and the German MS Society in 2001. The foundation and the society kept funding the operation since. It is operated by a non-profit company, the MSFP. In 2018, the MSFP received a grant from Merck and Novartis to support the extension of the registry and implementation of EMA requirements. In 2019, Biogen and Celgene joined the multi-stakeholder funding approach to support the registry’s operation and to allow the collection and reporting of data required as part of the EMA-minimal data set. Industry funding does not result in restriction to publish data, nor do the funders have access to raw data or influence in the scientific conduct of the registry. K.O.J. has nothing to disclose. D.E. has nothing to disclose. P.R. has received honoraria for lectures or consultancy from AbbVie, Alexion, Almirall, Biogen, Merck, Novartis, Sandoz, Sanofi Genzyme, Roche and Teva. He received research grants from Amicus, Biogen, Merck and Roche. A.S. has no personal pecuniary interests to disclose, other than being the lead of the German MS Registry, which receives funding from a range of public and corporate sponsors, recently including The German Innovation Fund (GBA), The German MS Trust, Biogen, German MS Society, Celgene (BMS), Merck and Novartis. U.Z. has received speaker fees, travel compensation and/or his section received research support from Alexion, Almirall, Bayer Health Care, Biogen, Celgene, Genzyme, Merck Serono, Novartis, Roche, Sanofi-Aventis, Teva and grants from German Ministry for Education and Research (BMBF), German Ministry for Economy (BMWi), Deutsche Forschungsgemeinschaft (DFG), European Union (EU), outside the submitted work. K.B. has received funding from the German Ministry of Education and Research, for a project within the Competence Net Multiple Sclerosis and from the German Innovation Fund (GBA) for the coordination of the VersiMs project (both to the University of Münster).
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Kris Oliver Jalusic  https://orcid.org/0000-0001-7715-089X
https://orcid.org/0000-0001-7715-089X
David Ellenberger  https://orcid.org/0000-0002-2274-5025
https://orcid.org/0000-0002-2274-5025
Paulus Rommer  https://orcid.org/0000-0001-5209-6647
https://orcid.org/0000-0001-5209-6647
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kris Oliver Jalusic, Institute of Epidemiology and Social Medicine, University of Muenster, Muenster, Germany.
David Ellenberger, MS Forschungs- und Projektentwicklungs-gGmbH, German MS Register, Hannover, Germany.
Paulus Rommer, Department of Neurology, University Medicine Rostock, Rostock, Germany/Department of Neurology, Medical University of Vienna, Vienna, Austria.
Alexander Stahmann, MS Forschungs- und Projektentwicklungs-gGmbH, German MS Register, Hannover, Germany.
Uwe Zettl, Department of Neurology, University Medicine Rostock, Rostock, Germany.
Klaus Berger, Institute of Epidemiology and Social Medicine, University of Muenster, Muenster, Germany.
References
- 1. Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med 2016; 16: s53–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leray E, Moreau T, Fromont A, et al. Epidemiology of multiple sclerosis. Rev Neurol 2016; 172: 3–13. [DOI] [PubMed] [Google Scholar]
- 3. Schmedt N, Khil L, Berger K, et al. Incidence of multiple sclerosis in Germany: A cohort study applying different case definitions based on claims data. Neuroepidemiology 2017; 49(3–-4): 91–98. [DOI] [PubMed] [Google Scholar]
- 4. Rommer PS, Zettl UK. Managing the side effects of multiple sclerosis therapy: Pharmacotherapy options for patients. Expert Opin Pharmacother 2018; 19(5): 483–498. [DOI] [PubMed] [Google Scholar]
- 5. Moiola L, Rommer PS, Zettl UK. Prevention and management of adverse effects of disease modifying treatments in multiple sclerosis. Curr Opin Neurol 2020; 33(3): 286–294. [DOI] [PubMed] [Google Scholar]
- 6. Wingerchuk DM, Carter JL. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc 2014; 89(2): 225–240. [DOI] [PubMed] [Google Scholar]
- 7. Tintore M, Vidal-Jordana A, Sastre-Garriga J. Treatment of multiple sclerosis – Success from bench to bedside. Nat Rev Neurol 2019; 15(1): 53–58. [DOI] [PubMed] [Google Scholar]
- 8. FDA. The Drug Development Process: Step 3: clinical research, https://www.fda.gov/patients/drug-development-process/step-3-clinical-research#collapse3 (2018, accessed 4 June 2020).
- 9. He Z, Tang X, Yang X, et al. Clinical trial generalizability assessment in the big data era: A review. Clin Transl Sci 2020; 13: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thiel S, Leypoldt F, Röpke L, et al. Neuroimmunological registries in Germany. Neurology Int Open 2018; 02: E25–E39. [Google Scholar]
- 11. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon Beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. [DOI] [PubMed] [Google Scholar]
- 12. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 2010; 362: 416–426. [DOI] [PubMed] [Google Scholar]
- 13. Kappos L, Wiendl H, Selmaj K, et al. Daclizumab HYP versus Interferon Beta-1a in relapsing multiple sclerosis. N Engl J Med 2015; 373: 1418–1428. [DOI] [PubMed] [Google Scholar]
- 14. Gold R, Giovannoni G, Selmaj K, et al. Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): A randomised, double-blind, placebo-controlled trial. Lancet 2013; 381: 2167–2175. [DOI] [PubMed] [Google Scholar]
- 15. Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 16. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 17. Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(3): 247–256. [DOI] [PubMed] [Google Scholar]
- 18. O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple Sclerosis. N Engl J Med 2011; 365: 1293–1303. [DOI] [PubMed] [Google Scholar]
- 19. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. The Lancet 2012; 380: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 20. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 21. Kappos L, Radue E-W, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362: 387–401. [DOI] [PubMed] [Google Scholar]
- 22. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 23. Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006; 354: 899–910. [DOI] [PubMed] [Google Scholar]
- 24. Hartung H-P, Gonsette R, Konig N, et al. Mitoxantrone in progressive multiple sclerosis: A placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002; 360: 2018–2025. [DOI] [PubMed] [Google Scholar]
- 25. Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. Ann Neurol 2001; 49(3): 290–297. [PubMed] [Google Scholar]
- 26. Johnsons KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: Results of a phase III multicenter, double-blind placebo-controlled trial. The copolymer 1 multiple sclerosis study group. Neurology 1995; 45: 1268–1276. [DOI] [PubMed] [Google Scholar]
- 27. Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon beta-1a for relapsing-remitting multiple sclerosis (ADVANCE): A randomised, phase 3, double-blind study. Lancet Neurology 2014; 13: 657–665. [DOI] [PubMed] [Google Scholar]
- 28. Ebers GC. Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet 1998; 352: 1498–1504. [PubMed] [Google Scholar]
- 29. Jacobs LD, Cookfair DL, Rudick RA, et al. A phase III trial of intramuscular recombinant interferon beta as treatment for exacerbating-remitting multiple sclerosis: Design and conduct of study and baseline characteristics of patients. Mult Scler 1995; 1(2): 118–135. [DOI] [PubMed] [Google Scholar]
- 30. The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993; 43(4): 655–661. [DOI] [PubMed] [Google Scholar]
- 31. Tavazzi E, Rovaris M, La Mantia L. Drug therapy for multiple sclerosis. CMAJ 2014; 186: 833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 2018; 24: 96–120. [DOI] [PubMed] [Google Scholar]
- 33. Sepúlveda M, Montejo C, Llufriu S, et al. Rebound of multiple sclerosis activity after fingolimod withdrawal due to planning pregnancy: Analysis of predisposing factors. Mult Scler Relat Disord 2020; 38: 101483. [DOI] [PubMed] [Google Scholar]
- 34. Schwartz JB. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 2007; 82(1): 87–96. [DOI] [PubMed] [Google Scholar]
- 35. Molyneux PD, Miller DH, Filippi M, et al. Visual analysis of serial T2-weighted MRI in multiple sclerosis: Intra- and interobserver reproducibility. Neuroradiology 1999; 41(12): 882–888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_1352458520985118 for Effect of applying inclusion and exclusion criteria of phase III clinical trials to multiple sclerosis patients in routine clinical care by Kris Oliver Jalusic, David Ellenberger, Paulus Rommer, Alexander Stahmann, Uwe Zettl and Klaus Berger in Multiple Sclerosis Journal