Abstract
Background and objective
Transcutaneous pulse oximetry saturation (SpO2) is widely used to diagnose severe hypoxaemia and to prescribe long-term oxygen therapy (LTOT) in COPD. This practice is not based on evidence. The primary objective of this study was to determine the accuracy (false positive and false negative rates) of oximetry for prescribing LTOT or for screening for severe hypoxaemia in patients with COPD.
Methods
In a cross-sectional study, we correlated arterial oxygen saturation (SaO2) and SpO2 in patients with COPD and moderate hypoxaemia (n=240) and calculated the false positive and false negative rates of SaO2 at the threshold of ≤88% to identify severe hypoxaemia (arterial oxygen tension (PaO2) ≤55 mmHg or PaO2 <60 mmHg) in 452 patients with COPD with moderate or severe hypoxaemia.
Results
The correlation between SaO2 and SpO2 was only moderate (intra-class coefficient of correlation: 0.43; 95% confidence interval: 0.32–0.53). LTOT would be denied in 40% of truly hypoxaemic patients on the basis of a SaO2 >88% (i.e., false negative result). Conversely, LTOT would be prescribed on the basis of a SaO2 ≤88% in 2% of patients who would not qualify for LTOT (i.e., false positive result). Using a screening threshold of ≤92%, 5% of severely hypoxaemic patients would not be referred for further evaluation.
Conclusions
Several patients who qualify for LTOT would be denied treatment using a prescription threshold of saturation ≤88% or a screening threshold of ≤92%. Prescription of LTOT should be based on PaO2 measurement.
Short abstract
Although transcutaneous pulse oximetry is widely used to diagnose severe hypoxaemia and prescribe long-term oxygen therapy in COPD, up to 40% of patients who qualify for this therapy would be denied treatment using the saturation threshold of ≤88% https://bit.ly/3jolWU5
Introduction
Two landmark trials conducted >40 years ago provided scientific evidence that long-term oxygen therapy (LTOT) may prolong life [1, 2]. These two trials targeted patients with COPD and severe daytime hypoxaemia documented by direct arterial blood gas (ABG) measurement. The inclusion criteria of both studies still serve as current indications for LTOT in COPD, with minor variations worldwide [3].
In several jurisdictions, LTOT is actually prescribed and reimbursed on the basis of the measurement of oxygen saturation by transcutaneous pulse oximetry (SpO2) alone. For instance, in the United States, clinicians may use either a partial pressure of oxygen in arterial blood (PaO2) ≤55 mmHg or a SpO2 ≤88% (or a PaO2 of 56 to 59 mmHg, or SpO2 of 89% with evidence of cor pulmonale or erythrocythaemia) at rest in establishing severe hypoxaemia [4]. The authors of the recently published American Thoracic Society clinical practice guidelines for home oxygen therapy in adults with chronic lung disease recognised the limitations of SpO2 for oxygen prescription. Nevertheless, the panel supported its use to “improve the usability of the guideline report in circumstances in which ABG measurements (are) not available” [5]. In Canada, the use of SpO2 as a criterion for funding is inconsistent among provinces [6]. Although convenient, the practice of using pulse oximetry to determine the need for LTOT is not based on clinical evidence. The most recent British Thoracic Society guidelines for home oxygen use in adults suggested that pulse oximetry may be used for screening patients who might be candidates for LTOT. The guidelines recommend that patients with a resting stable saturation measured by transcutaneous pulse oximetry (SpO2) ≤92% should be referred for ABG assessment in order to assess eligibility for LTOT [7]. A good practice is also to elevate this threshold to ≤94% if end-organ damage (cor pulmonale or erythrocythaemia) is noted [7].
SaO2 and PaO2 are predictably related from the oxygen–haemoglobin dissociation curve. Although clinicians often use SpO2 as a substitute for SaO2, several studies reported that SaO2 and SpO2 are only moderately correlated [8–10]. Consequently, SpO2 may not be as reliable as measuring PaO2 to establish the presence of hypoxaemia [11]. By using pulse oximetry alone for LTOT prescription, clinicians and patients should be aware of the potential for misclassification, that is denying LTOT in truly hypoxaemic patients on the basis of a SpO2 >88% (i.e., false negative result), or prescribing LTOT on the basis of a SpO2 ≤88% in patients who would not qualify for LTOT if ABG were actually measured (i.e., false positive result). Accordingly, our primary objectives were: 1) to demonstrate the correlation between SpO2 and SaO2; and 2) to determine the false positive and false negative rates of LTOT prescriptions in patients with COPD if it was based on SaO2 alone. A secondary objective was to determine the SaO2 thresholds at which the indication of LTOT can be ruled in or ruled out.
Material and methods
Design and patients
In a cross-sectional study, the results of ABG obtained from three separate groups of patients were analysed. First, we extracted the results of baseline ABGs of patients who participated in the International Nocturnal Oxygen (INOX) trial, a 4-year, multicentre, randomised, placebo-controlled trial of nocturnal oxygen therapy in patients with COPD [12]. To be included in the trial, patients had to have nocturnal oxygen desaturation without qualifying for LTOT. These patients usually have moderate hypoxaemia at rest (i.e., PaO2 approaching the threshold of LTOT prescription [13]). ABGs were available in 240 of 243 patients who were randomised in the INOX trial (Cohort 1). Arterial blood was drawn from patients in sitting position. As per protocol, SpO2 was also measured within 1 hour of arterial blood sampling using PalmSAT 2500™ oximeter only (Nonin Medical Inc., Plymouth, MN, USA).
Second, we obtained from the respiratory home care programme of the Quebec City area (province of Quebec, Canada) the result of the ABG of patients registered in the programme as of January 1, 2015 with a main diagnosis of COPD who were prescribed LTOT (Cohort 2; n=212). To be admitted to the programme, severe hypoxaemia (PaO2 ≤55 mmHg or PaO2 in the range of 56 to 59 mmHg with clinical evidence of cor pulmonale or erythrocythaemia [1]) must be strictly demonstrated in stable condition. Patients were not allowed in the programme on the basis of SpO2 alone, so that SpO2 was not recorded in this cohort.
We also retrieved from the laboratory of biochemistry of our institution the results of all consecutive ABGs measured between January 2009 and June 2017 in outpatients or inpatients while breathing room air (Cohort 3; n=848). ABG of patients in an intensive care unit or in the recovery room, or those receiving supplemental oxygen were therefore excluded. Each patient contributed only one sample of arterial blood. The underlying diagnoses and indication of ABG measurement were irrelevant to the objectives of this study.
Measurements
Modern blood gas analysers measure PaO2 using an amperometric electrode and SaO2 using spectrophotometry [14]. SaO2 is obtained by dividing the concentration of oxyhaemoglobin by the sum of the concentrations of oxyhaemoglobin and deoxyhaemoglobin in the sample. Patients in Cohorts 1 and 2 came from several locations, and ABGs were analysed using different blood gas analysers across institutions. In Cohort 3, all ABGs were analysed on an ABL 800 Flex blood gas analyser (Radiometer, Copenhagen, Denmark). Patient temperature was not noted; in all measurements it was assumed to be 37°C.
Patient and public involvement
Patients were not directly involved in this study, which is a secondary analysis of data obtained from the INOX trial and a retrospective analysis of data kept in files at the respiratory home care programme of the Quebec City area and at the laboratory of biochemistry of our institution. It received approval from the Research Ethics Committee of our institution (CER-IUCPQ-UL: 2021-3592, 22044).
Statistics
We used simple descriptive statistics (proportions, means and standard deviations, medians and interquartile ranges) throughout the study. Clinical characteristics of patients in the three cohorts were compared using chi-square tests for dichotomous variables and analyses of variance for continuous variables. We first correlated SaO2 and SpO2 in Cohort 1 using an intra-class coefficient of correlation (ICC) calculated from a two-way mixed effect model, with its 95% confidence interval. We also assessed graphically the agreement between the two measures using a Bland–Altman diagram [15]. In the three cohorts, we then plotted SaO2 against PaO2 to represent oxygen–haemoglobin dissociation curves. We also cross-tabulated the results of SaO2 and PaO2. In order to demonstrate the effect of arterial pH on the affinity of oxygen for haemoglobin [16], separate dissociation curves were also plotted after separating the cohorts into two groups at the median value of pH and summarised using local polynomial regression (locally estimated scatterplot smoothing [17]). By combining Cohort 1 and 2 (i.e., the two cohorts of patients with COPD), we calculated the false positive and false negative rates of SaO2 at the thresholds of ≤88% (i.e., the American prescription threshold) and ≤92% (i.e., the British screening threshold) to identify severe hypoxaemia defined according to: 1) the Nocturnal Oxygen Therapy Trial (NOTT [1]) criteria; 2) PaO2 ≤55 mmHg; or 3) PaO2 <60 mmHg regardless of oedema, haematocrit or ECG findings. From the same two cohorts, we constructed receiver operating characteristics (ROC) curves to determine the thresholds of saturation at which severe hypoxaemia is either ruled in or ruled out (i.e., false positive or negative results of 0%). Finally, we used Cohort 3 to validate the results obtained from patients with COPD (Cohorts 1 and 2). All the analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Patients
Patient characteristics are summarised in table 1. As expected, patients on LTOT (Cohort 2) had more severe hypoxaemia than those with nocturnal oxygen desaturation alone (Cohort 1). Although patients on LTOT were on average mildly hypercapnic, mean pH was normal (median: 7.42; interquartile range: 7.38–7.44), presumably indicating clinical stability when LTOT was initiated. Among the 240 patients who participated in the INOX trial (Cohort 1) and the 212 patients on LTOT (Cohort 2), 33 (14%) and 56 (26%) had a PaO2 in the range of 56 to 59 mmHg, respectively.
TABLE 1.
Baseline characteristics
| Cohort 1: patients with COPD and isolated nocturnal desaturation | Cohort 2: patients with COPD and severe hypoxaemia | Cohort 3: unselected patients | |
| Subjects n | 240 | 212 | 848 |
| Age years | 69±8 | 72±10 | 72±12 |
| Sex, male n (%) | 157 (65%) | 97 (46%) | 460 (54%) |
| PaO2 mmHg | 67±7 | 52±5 | 57±10 |
| SaO2 % | 93±2 | 87±4 | 89±5 |
| SpO2 % | 93±2 | N/A | N/A |
| PaCO2 mmHg | 42±6 | 47±8 | 44±8 |
| pH | 7.42±0.03 | 7.41±0.04 | 7.43±0.04 |
Data expressed as mean±sd unless otherwise stated. 1 mmHg=0.133 kPa; 1 kPa=7.50 mmHg. N/A: not available. PaO2: arterial oxygen tension; SaO2: arterial oxygen saturation; SpO2: pulse oximetry saturation; PaCO2: arterial carbon dioxide tension.
Correlation between SaO2 and SpO2
SaO2 and SpO2 were moderately correlated (ICC: 0.43; 95% CI: 0.32–0.53; n=240, all from Cohort 1; supplementary Figure 1S). The mean±sd difference between the two measures was 0.6±2.0%. The Bland–Altman diagram indicates that the difference did not vary in a systematic pattern over the range of measurements (figure 1).
FIGURE 1.
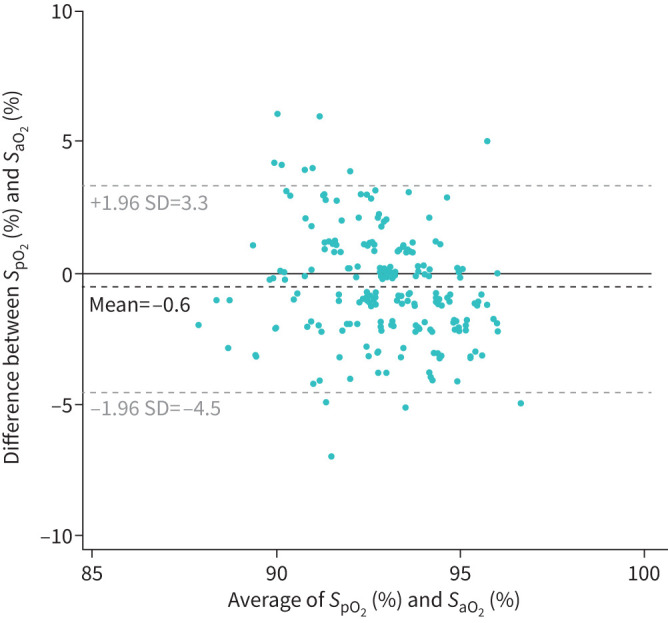
Bland–Altman diagram: agreement between pulse oximetry saturation (SpO2) and arterial oxygen saturation (SaO2) in the cohort of patients with isolated nocturnal oxygen desaturation (n=240).
SaO2 to predict PaO2
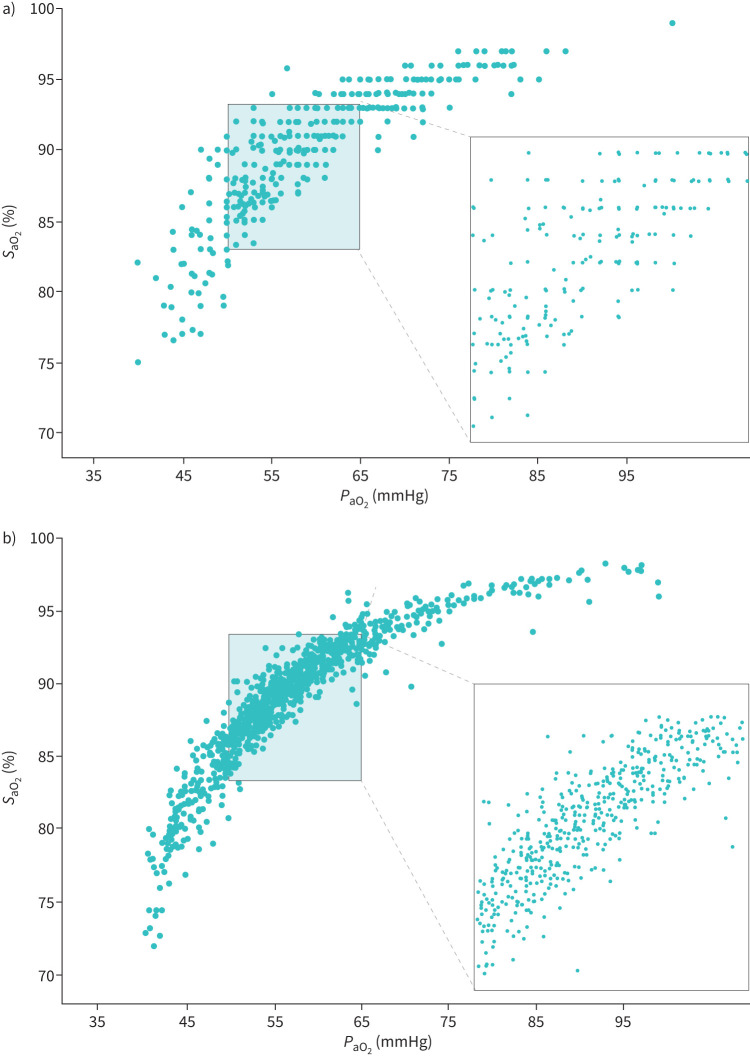
The relation between SaO2 and PaO2 in patients with COPD (Cohort 1 and Cohort 2) and in the validation cohort (Cohort 3) is shown in figure 2. They represent in effect oxygen–haemoglobin dissociation curves. Cross-tabulation of SaO2 and PaO2 is presented in supplementary Tables 1S and 2S. The dissociation curves and the cross-tabulation of SaO2 and PaO2 indicate the wide variability of PaO2 for a given SaO2, and conversely, the wide variability of SaO2 for a given PaO2. The significant effect of pH on the affinity of oxygen for haemoglobin is also demonstrated in supplementary Figure 2S.
FIGURE 2.
The relation between arterial oxygen saturation (SaO2) and arterial oxygen tension (PaO2). a) Patients with COPD: n=452. b) Validation cohort: n=848.
Saturation to prescribe LTOT (≤88%) or to screen for patient selection (≤92%)
Scatter plots of SaO2 values in patients with severe hypoxaemia and those with isolated nocturnal desaturation are presented in figure 3. Among the 240 patients fulfilling the indication for nocturnal oxygen alone, four had a SaO2 ≤88% (false positive rate: 1.7%). SaO2 was >88% in 84 of the 212 patients on LTOT (false negative rate for LTOT prescription: 39.6%) (table 2). Table 2 also includes the false positive and false negative rates of SaO2 at the threshold of ≤88% to detect severe hypoxaemia defined as PaO2 ≤55 mmHg or PaO2 <60 mmHg (regardless of oedema, haematocrit and ECG findings). At the SaO2 screening threshold of ≤92%, 4.7% (10 out of 212) of the truly hypoxaemic patients would not have been referred for further evaluation.
FIGURE 3.
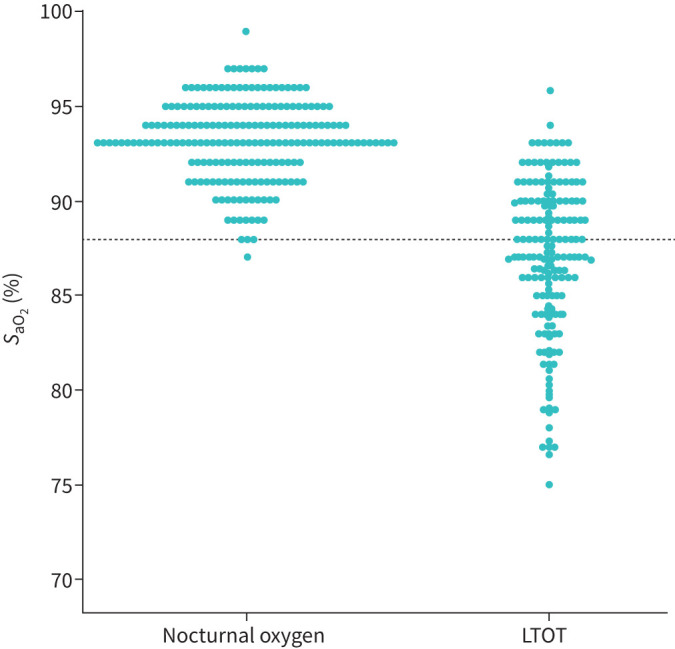
Scatter plot of the individual saturation values in patients on long-term oxygen therapy (LTOT) (n=212) and those with isolated nocturnal oxygen desaturation (n=240). The dashed line is located at the saturation threshold of 88%. SaO2: arterial oxygen saturation.
TABLE 2.
False positive and false negative rates of arterial oxygen saturation ( S aO2 ) at the threshold of ≤88% to identify severe hypoxaemia in COPD
| Severe hypoxaemia defined as | S aO2 ≤88% false positive rate | S aO2 ≤88% false negative rate | ||
| COPD (Cohort 1+2) | Validation (Cohort 3) | COPD (Cohort 1+2) | Validation (Cohort 3) | |
| NOTT criteria [1] % | 1.7# | 39.6# | ||
| PaO2 ≤55 mmHg % | 4.7¶ | 3.3+ | 21.9¶ | 15.7+ |
| PaO2 <60 mmHg % | 0.5¶ | 0+ | 45.4¶ | 40.3+ |
Saturation thresholds to rule in or rule out the indication of LTOT
From the ROC analysis, we determined in Cohorts 1 and 2 that a SaO2 threshold of ≤87% rules in the indication of LTOT according to the NOTT criteria (i.e., false positive rate=0%) while a SaO2 threshold of ≥96% rules out the indication of LTOT (i.e., false negative rate=0%) (figure 4 and supplementary Table 6S).
FIGURE 4.
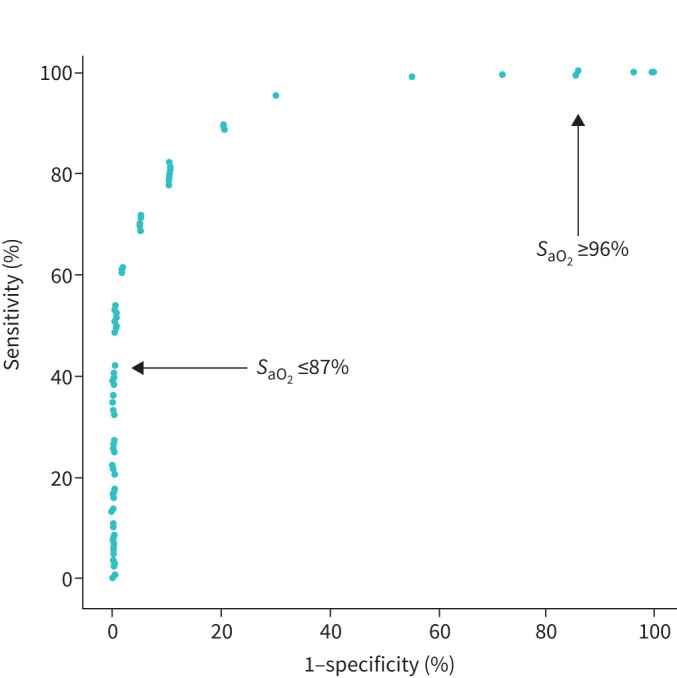
Receiver operating characteristics (ROC) curve: sensitivity and specificity of arterial oxygen saturation (SaO2) at varying thresholds to detect severe hypoxaemia (arterial oxygen tension (PaO2) ≤55 mmHg or PaO2 of 56 to 59 mmHg with evidence of cor pulmonale or erythrocythaemia) at the level to which long-term oxygen therapy (LTOT) is indicated according to the Nocturnal Oxygen Therapy Trial (NOTT) criteria. This analysis combined Cohorts 1 and 2 (i.e., the two cohorts of patients with COPD). The arrows indicate the saturation thresholds at which the false positive rate (SaO2 ≤87%) and false negative rate (SaO2 ≥96%) are null.
Validation study
The false positive and false negative rates in the determination of severe hypoxaemia (PaO2 ≤55 mmHg or PaO2 <60 mmHg) at a SaO2 threshold of ≤88% are shown in table 2. We determined, from the ROC analysis, that a SaO2 ≤82% rules in severe hypoxaemia defined as PaO2 ≤55 mmHg (i.e., false positive rate=0%), while a SaO2 ≥92% rules out severe hypoxaemia (i.e., false negative rate=0%) (supplementary Table 7S). Similarly, a SaO2 ≤88% rules in severe hypoxaemia defined as PaO2 <60 mmHg, while a SaO2 ≥94% rules out severe hypoxaemia (supplementary Table 8S).
Discussion
Our findings question the validity of using oxygen saturation alone for the prescription of LTOT in COPD. First, as others [8–10], we observed that SpO2 and SaO2 were only moderately correlated. Also, in a recent study [18], the mean±sd difference between SpO2 and SaO2 (SaO2 − SpO2) was remarkably similar to what we found (−0.6±2.6 in Ekström's study versus −0.6±2.0 in ours). Second, although not measured with pulse oximetry, pH may have, as predicted by physiology, a significant effect on the relation between SaO2 and PaO2 as demonstrated by the dissociation curves (figure 1 and supplementary Figure 1S). Third, by using saturation alone, patients are much more often denied LTOT (false negative rate: 40%) than they are prescribed LTOT when it is not indicated (false positive rate: 2%). Fourth, our data suggest that the thresholds of ≤82% or ≥96% to either rule in or rule out the indication of LTOT may be considered. Even at these thresholds, several factors limit the accuracy of cutaneous pulse oximetry to determine whether LTOT is indicated. These factors include: 1) the precision of the currently available pulse oximeters [19]; 2) the potential for technical errors with their use; 3) the shape of the oxygen–haemoglobin dissociation curve, which may vary due to several unknown or unmeasured variables such as arterial blood pH and temperature [20]; and 4) the imperfect correlation between SaO2 and SpO2.
The reasons why arterial puncture to determine the indication of LTOT has been abandoned in several jurisdictions are unclear since other reports have also underlined the limitations of pulse oximetry [11, 21, 22]. Yet, arterial puncture, most often of the radial artery, is a safe and simple procedure. With the exception of local pain, bruising and haematoma, clinically significant complications are rare [23, 24]. Local pain may be decreased by local anaesthetic infiltration or the application of ice prior to the puncture [25, 26]. Topical anaesthetics do not seem to be effective to reduce pain [27]. The technique can be performed by several health professionals after minimal training [28–30]. Successful punctures may be obtained in almost 90% of cases [8]. The use of ultrasonography to guide arterial puncture of the radial artery does not seem to improve success rate compared with the conventional technique [31]. Blood gas analysers are found in most hospitals. Portable blood gas analysers are also available, with performance similar to that of conventional laboratory blood gas analysers [32]. Cost of arterial puncture is minimal and hardly an issue when the outcome is LTOT, a major cost driver in the management of COPD [33].
ABG measurement has its own limitations. It does not provide continuous data. Measurement errors are also possible. Air bubbles in syringe increases PaO2, while elevated temperature and delayed analysis has the opposite effect [34, 35]. Transient hyperventilation occurring during arterial puncture may be sufficient to acutely increase PaO2, a situation that will not reflect the chronic state of hypoxaemia [36]. Acute respiratory alkalosis on ABG measurement must alert clinicians to this possibility. The analysis of capillary blood, either arterialised or not, has been proposed as an alternative to ABG measurement. At least two separate meta-analyses comparing capillary and ABG have been published [37, 38]. Richter et al. [38] developed regression models to predict ABG (including PaO2) from capillary blood gas values. The authors found excellent predictability of the models and emphasised the potential of capillary blood gases in the management of acute respiratory conditions, without mentioning chronic conditions. Zavorsky and colleagues [37] found that earlobe sampling better predicts PaO2 (adjusted r2=0.88, mean bias=3.8 mmHg compared to arterial) than fingertip sampling (adjusted r2=0.48, mean bias=11.5 mmHg compared to arterial). The authors concluded that, in most circumstances, sampling blood from earlobe but not from the fingertip may be, in some circumstances, appropriate as a replacement for PaO2, unless precision is required.
A limitation of our study is that we determined the false positive and negative rates of saturation to identify severe hypoxaemia as well as the saturation thresholds to rule in or rule out the indication of LTOT on the basis of SaO2 (not SpO2). Also, ABGs in our cohorts of patients with COPD were obtained from different analysers. In this regard, a study has found that differences in PaO2 values from measurements performed on different blood gas analysers in different laboratories are negligible [39]. Temperature, a significant factor affecting the binding of oxygen to haemoglobin, was not taken into account.
Conclusion
Our study confirmed that SaO2 and SpO2 are only loosely correlated. We found that several patients who qualify for LTOT would be denied treatment using a SaO2 prescription threshold ≤88% or a screening threshold ≤92%. We therefore conclude that oxygen saturation (SaO2, and a fortiori SpO2) is not an adequate replacement of direct PaO2 measurement for prescribing LTOT or for screening for further assessment of eligibility for LTOT. If chronic hypoxaemia is suspected, patient evaluation should rely on ABG measurement. This practice has its own limitations, since it represents a static and instantaneous measure that may not reflect patients’ long-term oxygenation status. However, it has the merit to be aligned with the current indications for LTOT that were defined by the NOTT and the British Medical Research Council trial.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00272-2021.SUPPLEMENT (595.8KB, pdf)
Supplementary material PULSEOXYMETRY_LACASSE ET AL_SUPPLEMENT - REVISED (139.2KB, docx)
Footnotes
Author contributions: Conception and design: Y. Lacasse, F. Sériès and F. Maltais; acquisition of data: S. Thériault, B. St-Pierre and S. Bernard; analysis: Y. Lacasse and H.J. Bernatchez; interpretation of data: Y. Lacasse, F. Sériès, H.J. Bernatchez and F. Maltais; writing: Y. Lacasse, S. Thériault, F. Sériès and F. Maltais.
Provenance: Submitted article, peer reviewed.
Editorial comment in ERJ Open Res 2021; 7: 00495-2021 [https://doi.org/10.1183/23120541.00495-2021].
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: Y. Lacasse has nothing to disclose.
Conflict of interest: S. Thériault has nothing to disclose.
Conflict of interest: B. St-Pierre has nothing to disclose.
Conflict of interest: S. Bernard has nothing to disclose.
Conflict of interest: F. Sériès has nothing to disclose.
Conflict of interest: H.J. Bernatchez has nothing to disclose.
Conflict of interest: F. Maltais has nothing to disclose.
Support statement: This study was supported by the Groupe de recherche en santé respiratoire de l'Université Laval. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Nocturnal Oxygen Therapy Trial Group . Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980; 93: 391–398. doi: 10.7326/0003-4819-93-3-391 [DOI] [PubMed] [Google Scholar]
- 2.Report of the Medical Research Council Working Party . Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981; 1: 681–686. [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global Strategy for the Diagnosis, Management and Prevention of COPD. 2018. http://goldcopd.org/ Date last accessed: 19 March 2020.
- 4.US Centers for Medicare and Medicaid Services . Medicare learning network. Home Oxygen Therapy. www.cms.gov/Research-Statistics-Data-and-Systems/Computer-Data-and-Systems/Electronic-Clinical-Templates/DMEPOS-Templates/DMEPOS-Home-Oxygen-Therapy Date last updated: 11 March 2021.
- 5.Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e121–ee41. doi: 10.1164/rccm.202009-3608ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacasse Y, Bernard S, Maltais F. Eligibility for home oxygen programs and funding across Canada. Can Respir J 2015; 22: 324–330. doi: 10.1155/2015/280604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardinge M, Annandale J, Bourne S, et al. British Thoracic Society guidelines for home oxygen use in adults. Thorax 2015; 70: Suppl. 1, i1–43. doi: 10.1136/thoraxjnl-2015-206865 [DOI] [PubMed] [Google Scholar]
- 8.Jensen LA, Onyskiw JE, Prasad NG. Meta-analysis of arterial oxygen saturation monitoring by pulse oximetry in adults. Heart Lung 1998; 27: 387–408. doi: 10.1016/S0147-9563(98)90086-3 [DOI] [PubMed] [Google Scholar]
- 9.Amalakanti S, Pentakota MR. Pulse oximetry overestimates oxygen saturation in COPD. Respir Care 2016; 61: 423–427. doi: 10.4187/respcare.04435 [DOI] [PubMed] [Google Scholar]
- 10.Lipnick MS, Feiner JR, Au P, et al. The accuracy of 6 inexpensive pulse oximeters not cleared by the Food and Drug Administration: the possible global public health implications. Anesth Analg 2016; 123: 338–345. doi: 10.1213/ANE.0000000000001300 [DOI] [PubMed] [Google Scholar]
- 11.Carlin BW, Clausen JL, Ries AL. The use of cutaneous oximetry in the prescription of long-term oxygen therapy. Chest 1988; 94: 239–241. doi: 10.1378/chest.94.2.239 [DOI] [PubMed] [Google Scholar]
- 12.Lacasse Y, Series F, Corbeil F, et al. Randomized trial of nocturnal oxygen in chronic obstructive pulmonary disease. N Engl J Med 2020; 383: 1129–1138. doi: 10.1056/NEJMoa2013219 [DOI] [PubMed] [Google Scholar]
- 13.Lacasse Y, Series F, Vujovic-Zotovic N, et al. Evaluating nocturnal oxygen desaturation in COPD – revised. Respir Med 2011; 105: 1331–1337. doi: 10.1016/j.rmed.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 14.Burtis C, Ashwood E, Bruns D. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 5th Edn. Philadelphia, PA, Saunders, 2012; pp. 825–826. [Google Scholar]
- 15.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–310. doi: 10.1016/S0140-6736(86)90837-8 [DOI] [PubMed] [Google Scholar]
- 16.Astrup P, Engel K, Severinghaus JW, et al. The influence of temperature and pH on the dissociation curve of oxyhemoglobin of human blood. Scand J Clin Lab Invest 1965; 17: 515–523. doi: 10.3109/00365516509083359 [DOI] [PubMed] [Google Scholar]
- 17.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Amer Statist Assoc 1988; 83: 596–610. doi: 10.1080/01621459.1988.10478639 [DOI] [Google Scholar]
- 18.Ekstrom M, Engblom A, Ilic A, et al. Calculated arterial blood gas values from a venous sample and pulse oximetry: clinical validation. PLoS One 2019; 14: e0215413. doi: 10.1371/journal.pone.0215413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitzan M, Romem A, Koppel R. Pulse oximetry: fundamentals and technology update. Med Devices (Auckl) 2014; 7: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffin J, Fisher J. Unknown in vivo factors influencing the oxygen dissociation curve? Respir Physiol Neurobiol 2013; 188: 81. doi: 10.1016/j.resp.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 21.Elborn JS, Finch MB, Stanford CF. Non-arterial assessment of blood gas status in patients with chronic pulmonary disease. Ulster Med J 1991; 60: 164–167. [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts CM, Bugler JR, Melchor R, et al. Value of pulse oximetry in screening for long-term oxygen therapy requirement. Eur Respir J 1993; 6: 559–562. [PubMed] [Google Scholar]
- 23.Giner J, Casan P, Belda J, et al. Pain during arterial puncture. Chest 1996; 110: 1443–1445. doi: 10.1378/chest.110.6.1443 [DOI] [PubMed] [Google Scholar]
- 24.Gillies ID, Morgan M, Sykes MK, et al. The nature and incidence of complications of peripheral arterial puncture. Anaesthesia 1979; 34: 506–509. doi: 10.1111/j.1365-2044.1979.tb06332.x [DOI] [PubMed] [Google Scholar]
- 25.Lightowler JV, Elliott MW. Local anaesthetic infiltration prior to arterial puncture for blood gas analysis: a survey of current practice and a randomised double blind placebo controlled trial. J R Coll Physicians Lond 1997; 31: 645–646. [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes JM. Randomized controlled trial of cryoanalgesia (ice bag) to reduce pain associated with arterial puncture. Respir Care 2015; 60: 1–5. doi: 10.4187/respcare.03312 [DOI] [PubMed] [Google Scholar]
- 27.Tran NQ, Pretto JJ, Worsnop CJ. A randomized controlled trial of the effectiveness of topical amethocaine in reducing pain during arterial puncture. Chest 2002; 122: 1357–1360. doi: 10.1378/chest.122.4.1357 [DOI] [PubMed] [Google Scholar]
- 28.Sackner MA, Avery WG, Sokolowski J. Arterial punctures by nurses. Chest 1971; 59: 97–98. doi: 10.1378/chest.59.1.97 [DOI] [PubMed] [Google Scholar]
- 29.Serbin AL. Arterial punctures by pulmonary function laboratory technicians. Ariz Med 1972; 29: 860–861. [PubMed] [Google Scholar]
- 30.Fagan MJ, Cece R. Using respiratory therapists to teach arterial puncture for blood gas procedures to third-year medical students. Acad Med 1999; 74: 594–595. doi: 10.1097/00001888-199905000-00067 [DOI] [PubMed] [Google Scholar]
- 31.Laursen CB, Pedersen RL, Lassen AT. Ultrasonographically guided puncture of the radial artery for blood gas analysis: a prospective, randomized controlled trial. Ann Emerg Med 2015; 65: 618–619. doi: 10.1016/j.annemergmed.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 32.Sediame S, Zerah-Lancner F, d'Ortho MP, et al. Accuracy of the i-STAT bedside blood gas analyser. Eur Respir J 1999; 14: 214–217. doi: 10.1034/j.1399-3003.1999.14a36.x [DOI] [PubMed] [Google Scholar]
- 33.Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One 2016; 11: e0152618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas CK, Ramos JM, Agroyannis B, et al. Blood gas analysis: effect of air bubbles in syringe and delay in estimation. Br Med J (Clin Res Ed) 1982; 284: 923–927. doi: 10.1136/bmj.284.6320.923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madiedo G, Sciacca R, Hause L. Air bubbles and temperature effect on blood gas analysis. J Clin Pathol 1980; 33: 864–867. doi: 10.1136/jcp.33.9.864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cinel D, Markwell K, Lee R, et al. Variability of the respiratory gas exchange ratio during arterial puncture. Am Rev Respir Dis 1991; 143: 217–218. doi: 10.1164/ajrccm/143.2.217 [DOI] [PubMed] [Google Scholar]
- 37.Zavorsky GS, Cao J, Mayo NE, et al. Arterial versus capillary blood gases: a meta-analysis. Respir Physiol Neurobiol 2007; 155: 268–279. doi: 10.1016/j.resp.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 38.Richter S, Kerry C, Hassan N, et al. Capillary blood gas as a substitute for arterial blood gas: a meta-analysis. Br J Hosp Med (Lond) 2014; 75: 136–142. doi: 10.12968/hmed.2014.75.3.136 [DOI] [PubMed] [Google Scholar]
- 39.Kampelmacher MJ, van Kesteren RG, Winckers EK. Instrumental variability of respiratory blood gases among different blood gas analysers in different laboratories. Eur Respir J 1997; 10: 1341–1344. doi: 10.1183/09031936.97.10061341 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00272-2021.SUPPLEMENT (595.8KB, pdf)
Supplementary material PULSEOXYMETRY_LACASSE ET AL_SUPPLEMENT - REVISED (139.2KB, docx)