Abstract
Background
The first potential focus for artemisinin resistance in South America was recently confirmed with the presence of the C580Y mutation in the Plasmodium falciparum kelch 13 gene (pfk13) in Guyana.
Objectives
This study aimed to strengthen pfk13 monitoring in the Amazon basin countries, to compile the available data and to evaluate the risk of spreading of mutations.
Methods
Sanger sequencing was done on 862 samples collected between 1998 and 2019, and a global map of pfk13 genotypes available for this region was constructed. Then, the risk of spreading of mutations based on P. falciparum case importation between 2015 and 2018 within countries of the Amazon basin was evaluated.
Results
No additional pfk13 C580Y foci were identified. Few mutations (0.5%, 95% CI = 0.3%–0.8%) in the propeller domain were observed in the general parasite population of this region despite a high proportion of K189T mutations (49.1%, 95% CI = 46.2%–52.0%) in the non-propeller domain. Case information revealed two patterns of intense human migration: Venezuela, Guyana and the Roraima State in Brazil; and French Guiana, Suriname and the Amapá State in Brazil.
Conclusions
There are few pfk13 mutant foci, but a high risk of dispersion in the Amazon basin, mainly from the Guiana Shield, proportionate to mining activities. Therefore, access to prompt diagnosis and treatment, and continuous molecular monitoring is essential in these geographical areas.
Introduction
Drug resistance of Plasmodium falciparum has become a major concern worldwide. To delay its propagation, artemisinin combination therapy (ACT) has been recommended since 2001. However, in 2008, resistance emerged in Cambodia and spread throughout south-east Asia, favoured by resistance to partner drugs, piperaquine and mefloquine.1,2 Studies from this region led to the identification of the P. falciparum kelch 13 gene (pfk13) as a molecular marker of artemisinin partial resistance.3 Currently, more than 100 mutations located in the propeller domain of pfk13 have been identified worldwide, but only 9 of them have been validated as responsible for partial artemisinin resistance.4 Currently, one mutation has quickly spread and has overcome the others in the eastern part of the Greater Mekong subregion, pfk13 C580Y.5
In the Amazon basin countries, where most of the malaria cases occur, the first-line treatment against P. falciparum is artemether/lumefantrine, except in Peru where artesunate/mefloquine is used.6 However, 6 years after its deployment, pfk13 C580Y mutant parasites were reported (5.1%, n = 5/98) in Guyana and have in time reached up to 25.0% prevalence in 2016–17, in Region 1.7,8 The selection of resistance occurred in parasites of South American genetic background and this mutation confers artemisinin resistance in vitro. Without threatening ACT efficacy if the partner drug is still efficient, the presence of this mutation exposes the partner drug to a quicker selection for resistant parasites. These findings also raise concerns about the risk of spread throughout the Amazon basin countries. In fact, mining, one of the main economic activities in the Guiana Shield, favours P. falciparum transmission and generates cross-border human migration, which could promote spreading of resistance in the subregion. Additionally, malaria transmission has increased in several countries of this continent, including Venezuela, Brazil, Colombia, Ecuador and Guyana.6
As WHO recommends pfk13 genotyping to monitor artemisinin resistance, this study aimed to strengthen it in the Amazon basin and to construct a global map of pfk13 genotypes by reviewing actual data available in published literature. It also assessed the risk of spreading of mutations within Guyana and between the different countries and subregions based on P. falciparum case migration patterns.
Methods
P. falciparum samples (n = 862) were collected in French Guiana (n = 475, 1998–2018), Brazil (Amazonas State, Manaus, n = 64, 2012–13), Guyana (n = 199, 2014–19) and Venezuela (Amazonas and Bolivar States, n = 124, 2016–18) from symptomatic patients infected by P. falciparum from only those who sought diagnosis and care.
The samples were collected as part of the malaria routine surveillance system implemented in French Guiana, Guyana and Venezuela. Healthcare facilities were in charge of collecting samples from anonymized P. falciparum-positive cases. Identification of individuals cannot be established. In accordance with WHO guidelines on ethical issues in public health surveillance, the sample collections were exempt from an ethical review since these interventions were part of routine monitoring of public health by the National Malaria Control Program of the Ministry of Public Health of each country (https://www.who.int/publications/i/item/who-guidelines-on-ethical-issues-in-public-health-surveillance). The analysis of the samples was also approved by the Environmental Protection Agency in the framework of the Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization. Samples were registered by the French Ministry for Research (declaration number DC-2010–1223). Regarding Brazilian samples, this study was approved by the Brazilian National Committee of Ethics (CONEP) (349.211/2013).
DNA extraction was performed from patient blood samples (filter paper or EDTA tube) using the QIAmp® DNA mini kit (Qiagen, Germany) and following the manufacturer’s protocol. The pfk13 gene was amplified using nested PCR methods with primers previously described.3 Double-strand sequencing of PCR-amplified products was performed by Eurofins (Paris, France) with the same primers used in nested PCR. Sequences were aligned with Geneious® v8.1.7 using the 3D7 pfk13 sequence as a reference. The pfk13 analysed sequences from this study are available in the GenBank repository (accession numbers MW037842–MW038738).
Some isolates were phenotyped using the ring-stage survival assay as previously described.9 The ring-stage survival assay was interpretable if the initial parasitaemia was greater than 0.25% and if the growth rate was greater than 2-fold per 48 h. Statistical significance between survival rates was calculated using Student’s t-test.
We performed a literature review on the PubMed database to identify studies reporting pfk13 genotyping in countries of the Amazon basin. Different searches with word combinations were used, including ‘k13’, ‘kelch 13’, ‘Plasmodium falciparum’, ‘artemisinin resistance’, ‘South America’, ‘Ecuador’, ‘Colombia’, ‘Peru’, ‘Bolivia’, ‘Brazil’, ‘Guyana’, ‘French Guiana’, ‘Suriname’ and ‘Venezuela’. When necessary, additional information (data collection, location etc.) were requested from the corresponding authors to properly localize the samples. A total of 3150 pfk13 genotyping results were found in 16 publications and were used to construct a general distribution map of pfk13 mutations in the Amazon basin (Table S1, available as Supplementary data at JAC Online).7,8,10–23
Cumulative numbers of P. falciparum mono or mixed infections from 2015 to 2018 reported by member states of PAHO/WHO were used to construct the malaria case migration maps. Countries report on the aggregate number of confirmed cases by species type, imported from other countries during a calendar year. Data were not available from Peru for 2015–17, Colombia for 2017, Ecuador for 2017 and 2018, and French Guiana for 2018. Data for cases imported from country A to country B were summed with those being imported from country B to country A, i.e. bidirectional movement of cases was calculated (Table S2). Aggregate data on the country from which cases were imported, reported at state level from Brazil, were sourced from a public database for the years 2015–18.24 Data here correspond to unidirectional movement of cases from other countries to Brazilian states. To construct the map of migration of P. falciparum cases within Guyana, information from the Malaria Surveillance System of Guyana Ministry of Public Health was used for the years 2015–18. Data on place of diagnosis of confirmed cases were cross-tabulated with the place the person was 2 weeks before the date of diagnosis, used as a proxy for the putative place of infection. Data were aggregated to bidirectional movement between a set of localities, i.e. both those that were imported from locality A to locality B, as well as those that were putatively infected in locality B and were diagnosed in locality A.
Results
Limited genetic diversity of the pfk13 gene in the Amazon basin countries
No mutation (non-synonymous or synonymous) was identified in the pfk13 propeller domain (codons 441–704) of the 862 newly analysed samples (Table 1).
Table 1.
pfk13 polymorphism of parasites from the Amazon basin
| French Guiana 1998–2018 | Brazil 2012–13 | Guyana 2014–19 | Venezuela 2016–18 | Total | |
|---|---|---|---|---|---|
| Total number of analysed isolates for non-propeller domain codons 50–440 | 274 | 63 | 199 | 112 | 648 |
| WT | 185 | 0 | 79 | 25 | 289 |
| K189T | 87 | 63 | 118 | 87 | 355 |
| K/T 189a | 0 | 0 | 2 | 0 | 2 |
| K189N | 1 | 0 | 0 | 0 | 1 |
| R/K 255a | 1 | 0 | 0 | 0 | 1 |
| number of mutants (%) | 88 (32.1)a | 63 (100.0) | 120 (60.3)a | 87 (77.7) | 359 (55.4)a |
| Total number of analysed isolates for propeller domain codons 441–704 | 475 | 64 | 199 | 124 | 862 |
| WT | 475 | 64 | 199 | 124 | 862 |
Mixed genotype, included for the calculation of mutants.
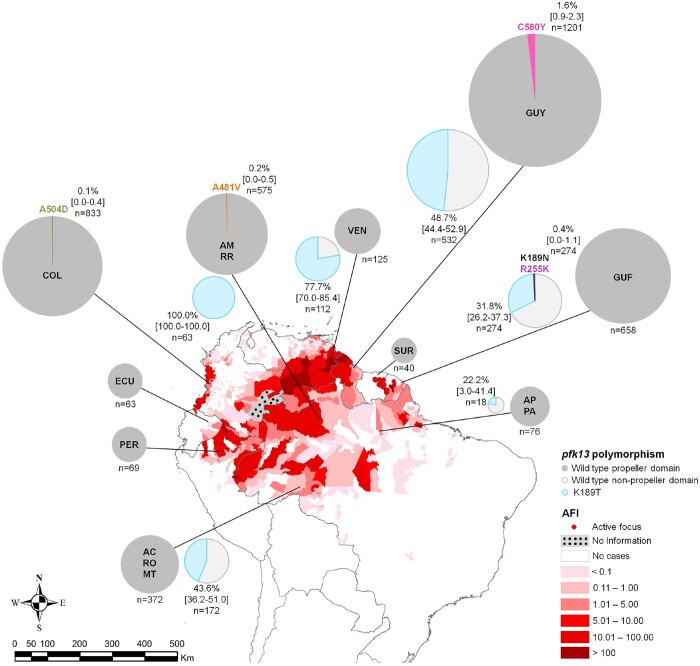
A map representing the distribution of pfk13 mutations in the Amazon basin was constructed using the genotyping of these 862 samples and 3150 from previously published data (Figure 1 and Table S1). Regarding the pfk13 propeller domain, a general mutant proportion of 0.5% (95% CI = 0.3%–0.8%) was observed in the Amazon basin countries. The mutation C580Y (TGT→TAT) was observed in 2010 and 2016–17 in Guyana (1.6%, 95% CI = 0.9%–2.3%), the mutation A481V (GCT→GTT) was observed in 2013 in Brazil (0.1%, 95% CI = 0.0%–0.3%) and the mutation A504D (GCT→GAT) was observed in 2018 in Colombia (0.1%, 95% CI = 0.0%–0.4%).
Figure 1.
Distribution of pfk13 mutations in the Amazon basin between 1983 and 2019. Pie charts represent pfk13 allele frequencies per country. The total number of analysed samples is shown with the percentage and the 95% CI (in brackets) when a mutation was identified. Results are shown in the map of the Annual Falciparum Incidence (AFI) in the Americas region in 2017 (Annual Country Reports to PAHO/CDENT/Malaria). For Brazil, results are regrouped by closely located states. Notably, most Colombian and Ecuadorian samples (mutant included) represented here were collected on the Pacific coast (99.8% and 93.7%, respectively) and not in the Amazon region of the country. Brazilian states: AC, Acre; AM, Amazonas; AP, Amapá; MT, Mato Grosso; PA, Pará; RO, Rondônia; and RR, Roraima. Countries: COL, Colombia; ECU, Ecuador; GUF, French Guiana; GUY, Guyana; PER, Peru; SUR, Suriname; and VEN, Venezuela.
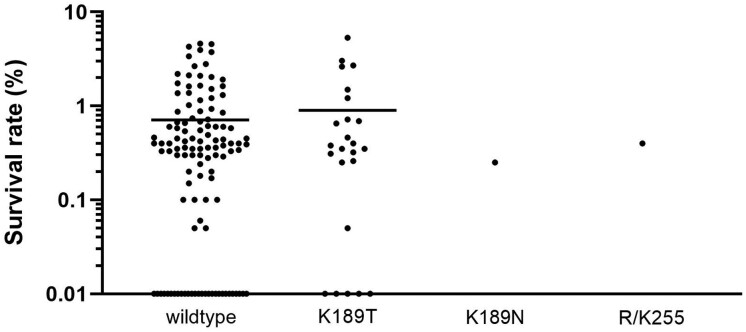
Among the 1171 pfk13 non-propeller genotypes available from this study (n = 648; Table 1) and the literature (n = 523), a mutant proportion of 49.3% (95% CI = 46.4%–52.1%) was found in this domain (codons 50–440; Table S1). K189T (AAA→ACA) was the most prevalent. This non-synonymous mutation has been fixed in the Amazonas State of Brazil since 2012 (Figure 1 and Table S1). Its minimal proportion was observed in the Amapá and Pará States of Brazil (22.2%, 95% CI = 3.0%–41.4%). No difference in the survival rates of parasites carrying (n = 24) or not carrying (n = 115) this mutation has been observed; 0.90% ± 0.26% versus 0.71%±0.09% (P = 0.43) (Figure 2). The K189N (AAA→AAT) mutation was found in 0.4% (95% CI = 0.0%–1.1%) of samples (n = 1) from French Guiana and R255K (AGA→AAA) as a mixed genotype was also found, both without impact on the survival rate (0.25%±0.25% and 0.40%±0.4%, respectively).
Figure 2.
Ring-stage survival assays for parasites from French Guiana. The survival rate of ring-stage parasites pfk13 WT (n = 115), pfk13 K189T (n = 24), pfk13 K189N (n = 1) and pfk13 R/K255 (mixed genotype, n = 1) after a 6 h pulse of 700 nM dihydroartemisinin as measured by microscopy 66 h later. Horizontal bars represent mean±SEM percentage survival.
Two major routes at risk of spreading pfk13 mutations
To assess the risk of spreading mutant parasites, we chose as a proxy the number of P. falciparum mono and mixed infections imported between the different Amazon basin countries at the time of observation of pfk13 C580Y in Guyana, i.e. the 2015–18 period.
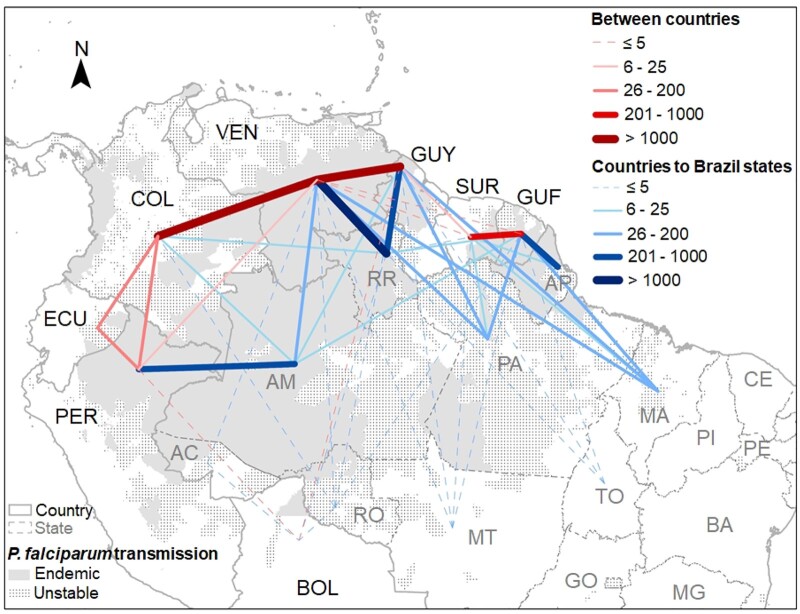
Two important migration patterns of malaria cases in South America were identified, both linked to the Guiana Shield, the area recording the highest P. falciparum transmission in South America (Figure 3). The Guiana Shield encompasses Amazon forest areas of Guyana, Suriname, French Guiana and parts of Brazil, Colombia and Venezuela.
Figure 3.
Map of P. falciparum infections imported between countries in the Amazon basin, 2015–18. Number of P. falciparum mono and mixed infections imported by country/state of diagnosis and putative country of infection. Each country/state is represented by a standardized location not linked with the putative contamination place. Lines in the map represent the cumulative numbers of infections recorded during the 2015–18 period. Lines between countries include bidirectional data, while lines from countries to Brazilian states are unidirectional (imported in Brazil). The width and colour of the lines are proportional to the number of imported cases. The grey P. falciparum transmission layer is based on the Annual Falciparum Incidence (AFI) reported during the 2015–18 period. Unstable corresponds to AFI <1 for ≥1 year and endemic (stable) corresponds to AFI >1 for all years. Countries: BOL, Bolivia; COL, Colombia; ECU, Ecuador; GUF, French Guiana; GUY, Guyana; PER, Peru; SUR, Suriname; and VEN, Venezuela. Brazilian states: AC, Acre; AM, Amazonas; AP, Amapá; BA, Bahia; CE, Ceará; GO, Goiás; MA, Maranhão; MG, Minas Gerais; MT, Mato Grosso; PA, Pará; PI, Piauí; PE, Pernambuco; RO, Rondônia; RR, Roraima; and TO, Tocantins.
The major migration pattern includes Venezuela, Guyana and the Roraima State of Brazil. Over the 2015–18 period, 3417 P. falciparum cases were imported between Venezuela and Guyana in a bidirectional manner (54.9%, 95% CI = 53.2%–56.6%, imported from Guyana to Venezuela; Table S2). Near these hotspots for malaria transmission, the Roraima State of Brazil recorded 3305 cases imported from Venezuela overwhelmingly in miners (2477; i.e. 75.0%, 95% CI = 73.5%–76.4%) (Table 2 and Figure 3). These movements were mainly unidirectional, from Venezuela to Brazil (97.4%, 95% CI = 96.9%–97.9%; Table S2). Moreover, the migration pattern into Brazil has been changing from being mostly in Brazilian miners diagnosed in Boa Vista municipality, which is far from the international border, in 2015 (59.6%, 95% CI = 54.7%–64.6%; n = 226/379 cases imported in that year in Brazil) to increasing in Venezuelan nationals working as miners being diagnosed in Pacaraima municipality right on the border with Venezuela in 2018 (44.4%, 95% CI = 41.9%–47.0%; n = 660/1485). Between Guyana and Brazil, 934 P. falciparum malaria cases were exchanged, 83.1% (95% CI = 80.7%–85.5%) being imported to Brazil (Table S2), mainly to the Roraima State (82.0%, 95% CI = 79.6%–84.5%; Table 2). These cases mainly occurred in Brazilian miners returning to Boa Vista municipality in Roraima (506 P. falciparum malaria cases).
Table 2.
Number of P. falciparum and mixed infections imported in Brazil (per state of diagnosis) by country of origin of infection, 2015–18
| State | Country |
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Bolivia | Colombia | Guyana | French Guiana | Peru | Suriname | Venezuela | ||
| Acre | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 4 |
| Amazonas | 0 | 15 | 13 | 9 | 743 | 0 | 94 | 874 |
| Amapá | 0 | 0 | 0 | 227 | 0 | 6 | 0 | 233 |
| Maranhão | 0 | 0 | 82 | 120 | 0 | 13 | 100 | 315 |
| Mato Grosso | 0 | 0 | 2 | 2 | 0 | 1 | 2 | 7 |
| Pará | 0 | 0 | 37 | 30 | 0 | 7 | 57 | 131 |
| Rondônia | 3 | 1 | 2 | 3 | 0 | 0 | 1 | 10 |
| Roraima | 1 | 6 | 628 | 6 | 0 | 3 | 3305 | 3949 |
| Tocantins | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 4 |
| Total | 7 | 22 | 766 | 398 | 743 | 30 | 3561 | 5527 |
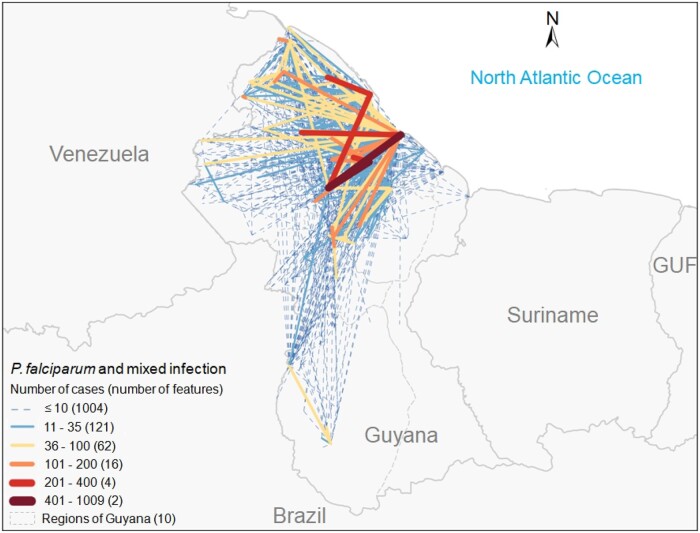
Within Guyana, intense movement of people exists. In fact, 40.8% of the P. falciparum cases were diagnosed in a region different from where the patient was possibly infected (Table S3). Additionally, 31.0% of the cases were diagnosed and associated with a putative place of infection within the same region, but in two different localities. These movements were mainly from the hinterlands of Regions 1, 7 and 8 to the capital Georgetown on the coast (Figure 4).
Figure 4.
Number of P. falciparum mono and mixed infection cases by place of diagnosis and putative place of infection 2 weeks prior to diagnosis in Guyana, 2015–18. Lines represent a set of localities wherein one is where a patient’s smear was taken for diagnosis and the second is the place where the patient self-reported they were 2 weeks prior to diagnosis. The width and colour of the lines represent the number of cases of P. falciparum mono and mixed infections. GUF, French Guiana.
The second major migration pattern, but proportionally much less important, involved Suriname, French Guiana and the northern states of Brazil (Figure 3). P. falciparum malaria case movements within this hotspot are generally unidirectional with Brazil and Suriname reporting imported cases from French Guiana. Over the 2015–18 period, 398 cases from French Guiana were reported imported in Brazil, mainly in Amapá (57.0%, 95% CI = 52.2%–61.9%), Maranhão (30.2%, 95% CI = 25.6%–34.7%) and Pará (7.5%, 95% CI = 4.9%–10.1%) States (Table 2 and Figure 3) with 70.9% (95% CI = 66.4%–75.3%) being in Brazilian miners (n = 282 cases). The last two states are farther removed geographically from French Guiana, but miners from these states work in the Guiana Shield area. In Suriname, cases from French Guiana represent 95.0% (95% CI = 92.9%–97.1%) (Table S2) of the parasite movements between those countries.
In the west of the Amazon basin, 510 cases from Venezuela (Table S2) have been reported imported in Colombia during this period, most likely in Colombian states bordering Venezuela, and 743 cases from Peru to the Amazonas State of Brazil (Figure 3 and Table 2). These later were mostly limited to municipalities sharing a border with the Loreto State of Peru and related to economic activities like agriculture and fishing in adjoining areas (51.5%, 95% CI = 48.0%–55.1%; n = 383 cases). Data from Peru were only available for 1 of the 4 years analysed, making it difficult to ascertain the level of migration, but migration of malaria cases was in both directions, as indicated by the fact that over 32.6% (95% CI = 29.2%–35.9%) (n = 242/743) of the infections imported in Brazil were in Brazilian nationals who had travelled to Peruvian territory. Of note, no cases were reported imported in Brazil from Ecuador within the same period.
In the same period, eight P. falciparum cases were reported imported in the Amazon basin countries from countries in south and south-east Asia. Six cases in Brazil were imported from the Philippines (n = 2), India (n = 2), Malaysia (n = 1) and Indonesia (n = 1), and two cases in Ecuador were imported from India.
Discussion
The selection of parasites bearing the pfk13 C580Y mutation and resistant in vitro to artemisinin derivatives in Guyana has recently been confirmed.8 This situation raised serious concerns about the risk of mutant parasites spreading or their presence in different unknown foci in the Amazon basin. No propeller mutation was observed in the analysed samples. A mutant parasite, A481V, was previously reported in Manaus/Brazil. This mutation was identified as being associated with delayed parasite clearance in south-east Asia.25 Unfortunately, no clinical data were associated with this sample. The A504D mutation observed in a Colombian sample was described once without associated phenotypic data.20
Globally, K189T was identified at a relatively high proportion in the Amazon basin (49.1%, 95% CI = 46.2%–52.0%). This mutation showed a similar prevalence in Africa, but was rarely described in south-east Asia.25–28 Some pfk13 K189T isolates have been associated with delayed parasite clearance time, but without clear correlation with artemisinin resistance.25,29,30 Survival rates observed in isolates carrying this mutation and collected in French Guiana suggested a natural polymorphism at this position. R255K, previously identified in Bangladesh, at the China–Myanmar border and also in Africa, was not linked to artemisinin resistance.25,30–32
Hosts of parasites disperse them; usually within a limited area by mosquitoes, especially in forests, but farther away by humans, who can move between endemic regions. Mobile populations greatly contribute to malaria dispersion and eventually to the spread of antimalarial drug resistance. Firstly, we excluded the risk of imported pfk13 mutated parasites from south-east Asia in our region, as over the 2015–18 period only eight P. falciparum cases have been imported and recorded in the Amazon basin countries and none of those was from the Greater Mekong subregion, the hotspot for artemisinin resistance. Regionally, based on an analysis of reported imported cases and identified endemic regions, we inferred two principal areas at risk of spreading of resistance in the Amazon basin: one between Venezuela, Guyana and the Roraima State in Brazil and the second between Suriname, French Guiana and the Amapá State in Brazil (Figure 3).
The current crisis in Venezuela contributed to a huge displacement of people and increase in malaria cases in the country.6 Several people have migrated to neighbouring countries and have consequently contributed to an increase in malaria cases there.33–35 Besides this political crisis, Venezuelan miners are involved in mining activities in Guyana and vice versa. It is likely that most of this movement has originated in the two major mining regions of Guyana, Regions 1 and 7 (where the mutation pfk13 C580Y was observed), and Sifontes municipality in Venezuela, which was the epicentre of malaria transmission during 2015–18.36 Linked to this bidirectional malaria case exchange, the Roraima State was the epicentre for malaria case importation and recorded 71.4% of the imported cases in Brazil, with specific routes of migration coming from Venezuela or Guyana.37,38
In the Amazon basin, the high prevalence of P. falciparum cases observed in mining areas is a concern.39,40 Miners represent a highly mobile population, as mines are generally located far from living areas and people move according to the quantity and the value of the gold they can find with limited trouble with police. During their life, miners generally move within two to three countries or different places within their own country.38,41 They move between Suriname, French Guiana and the Amapá State.41–43 However, the number of P. falciparum malaria cases moving within this area is lower (n = 651 for the analysed period) compared with the Venezuela–Guyana–Roraima triangle (n = 7350), as both malaria transmission and human migration intensity are lower.44,45 However, 12.3% of Brazilian imported cases occurred in Maranhão (n = 315), Amapá (n = 233) and Pará (n = 131). Those cases in the Amapá State (n = 227) and in the Maranhão State (n = 120) were mainly from French Guiana, because of the vicinity and the geographical origins of the miners, respectively.41 Maranhão is considered as a non-endemic region except for sporadic transmission or in small areas bordering Pará with 0.4% of the Brazilian Amazon P. falciparum cases (n = 72/16 987 malaria cases in 201946). Therefore, mutation spread is unlikely throughout this state.
In Guyana, the malaria surveillance system is effective and allows detailed tracing of patients and putative infection places within the country. Mining abounds on both a medium and a large scale, as well as on an artisanal scale, in Regions 1, 7 and 8. However, miners mostly live in the coastal area, which is non-endemic. They access health centres on the coast upon their return from 2–3 month stints in the mines. Therefore, they are frequently diagnosed in the Malaria Clinic in Georgetown or at other diagnostic posts en route in other regions. The situation is similar in Suriname and French Guiana, where miners work in remote areas and are generally diagnosed upon their return to Paramaribo or Cayenne, respectively. In French Guiana, miners could also be diagnosed at the closest health centre or cross the international borders into Suriname and Brazil, depending on the presence of the police in the area.
This analysis of the risk of spreading of mutations within the Amazon basin is based on malaria cases imported between countries/areas as a proxy of parasite movements. This approach is the result of the malaria risk encountered by the people exposed to the disease who have contracted and taken it with them to another country/areas. But it has limitations. First, the risk of spread and/or emergence of resistance is related to the number of people exposed to malaria, but also to their use of antimalarial drugs, as well as other variables, including drug quality, adherence, drug absorption etc. Second, the suggested maps represent the patterns but not precisely the path taken by people during their journey. These paths could include additional stops during the journey, in transmission areas, with a risk of mutant dispersal. Third, our approach is based on cases captured by surveillance systems, but in some areas access to malaria diagnosis is low and self-treatment rates are high. Therefore, cases are not identified and even less reported in this context, but selection for resistance and the risk of resistance spreading could be high.
With these additional pfk13 genotype results and this evaluation of the malaria case movements within the Amazon basin countries, we conclude that the pfk13 mutation associated with artemisinin resistance in vitro in South America appears to be limited to Guyana.
Between 2010 and 2016, pfk13 C580Y did not really increase in prevalence in Guyana and it was not observed in samples collected in 2018 and 2019, despite the fact that it confers in vitro artemisinin resistance.8 In western Cambodia it has displaced other mutations because of the KEL1/PLA1 lineage, which is resistant to artemisinins, but also piperaquine.30,47,48 The high level of efficacy of lumefantrine in the Guiana Shield associated with the negative impact on parasite growth could explain this low level of spread.8,20,21 However, as soon as resistance to the partner drug appears, the risk of artemisinin resistance spreading will be high, first within the country itself and then among the neighbouring countries or the areas linked by human migration, i.e. Venezuela and Roraima. This risk of spread will also be proportionate to the intensity of mining activities and inversely proportionate to the level of care offered to miners. With this in mind, countries will benefit by reinforcing malaria diagnosis and treatment availability within the mining sites deep in the forest. Practical deployment must be adapted according to the legal status of the mines and the miners and the regulation constraints of each country. When it exists, the private sector is an important partner and in the case of small-scale mining, which is often illegal, community health workers could be deployed, as is the case in Suriname, or a self-diagnosis and treatment approach could be considered, as in French Guiana.49,50 Moreover, regulation of sale of antimalarial drugs and use is key in these settings, as erratic use exists and commercialization of monotherapies of artemisinin is not strictly regulated in all countries of the region.8,51 Finally, molecular surveillance is crucial, particularly in Venezuela, which is experiencing a malaria outbreak with deficient control and because the analysed sample size in this study was limited [n = 124, i.e. 0.1% (95% CI = 0.1%–0.1%) of the 196 166 P. falciparum cases reported in 2016–186].
Supplementary Material
Acknowledgements
We thank all the patients whose samples were included in this study. In addition, we would like to thank all the partners of the French National Reference Centre for Malaria involved in monitoring malaria and associated resistance in French Guiana. We would also like to thank the Ministry of Health (MOH) personnel in the countries of the Americas for graciously sharing information on the malaria situation on an annual basis with PAHO/WHO, the Brazilian MOH for providing their malaria surveillance data under the open data policy and MOH Guyana for providing their malaria information. A considerable portion of the data discussed would not exist without their involvement.
Funding
This work was supported by Global Malaria Program (World Health Organization), Communicable Diseases and Environmental Determinants of Health Department (Pan American Health Organization), Guyamazon 3 Program, French Ministry for Research, European Commission Grant (Regional Fund for Development, Synergie GY0012082), Santé Publique France as National Reference Centre for Malaria and Investissement d’Avenir grant managed by Agence Nationale de la Recherche [CEBA (ref. ANR-10-LABX-25–01)]. L.C.M. received funding support from the Centre National d’Etudes Spatiales (CNES). The Manaus team was funded by Fundação de Amparo à Pesquisa do Estado do Amazonas-FAPEAM (PAPAC 005/2019, Posgrad and Pró-Estado). W.M.M. and M.V.G.L. are research fellows from CNPq.
Transparency declarations
M.-P.A., P.S., J.S.F.A. and A.M.A. are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy or views of the World Health Organization.
All other authors: none to declare.
Supplementary data
Tables S1 to S3 are available as Supplementary data at JAC Online.
References
- 1. Dondorp AM, Nosten F, Yi P. et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009; 361: 455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Imwong M, Suwannasin K, Kunasol C. et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis 2017; 17: 491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ariey F, Witkowski B, Amaratunga C. et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014; 505: 50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Status Report on Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy. August 2018. http://apps.who.int/iris/bitstream/handle/10665/274362/WHO-CDS-GMP-2018.18-eng.pdf?ua=1.
- 5. Anderson TJC, Nair S, McDew-White M. et al. Population parameters underlying an ongoing soft sweep in Southeast Asian malaria parasites. Mol Biol Evol 2017; 34: 131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. World Malaria Report. 2020. https://www.who.int/publications/i/item/9789240015791.
- 7. Chenet SM, Akinyi Okoth S, Huber CS. et al. Independent emergence of the Plasmodium falciparum kelch propeller domain mutant allele C580Y in Guyana. J Infect Dis 2016; 213: 1472–5. [DOI] [PubMed] [Google Scholar]
- 8. Mathieu LC, Cox H, Early AM. et al. Local emergence in Amazonia of Plasmodium falciparum k13 C580Y mutants associated with in vitro artemisinin resistance. eLife 2020; 9: e51015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Witkowski B, Amaratunga C, Khim N. et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in-vitro and ex-vivo drug-response studies. Lancet Infect Dis 2013; 13: 1043–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inoue J, Jovel I, Morris U. et al. Absence of Plasmodium falciparum K13 propeller domain polymorphisms among field isolates collected from the Brazilian Amazon basin between 1984 and 2011. Am J Trop Med Hyg 2018; 99: 1504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ménard D, Khim N, Beghain J. et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med 2016; 374: 2453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomes LR, Lavigne A, Peterka CL. et al. Absence of K13 polymorphism in Plasmodium falciparum from Brazilian areas where the parasite is endemic. Antimicrob Agents Chemother 2018; 62: e00354-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mita T, Culleton R, Takahashi N. et al. Little polymorphism at the K13 propeller locus in worldwide Plasmodium falciparum populations prior to the introduction of artemisinin combination therapies. Antimicrob Agents Chemother 2016; 60: 3340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chapadense F, Machado RLD, Ventura A. d S. et al. Plasmodium falciparum malarial parasites from Brazil lack artemisinin resistance-associated mutations in the kelch13 gene. Rev Soc Bras Med Trop 2019; 52: e20180225. [DOI] [PubMed] [Google Scholar]
- 15. Ladeia-Andrade S, de Melo GNP, de Souza-Lima R. d C. et al. No clinical or molecular evidence of Plasmodium falciparum resistance to artesunate–mefloquine in Northwestern Brazil. Am J Trop Med Hyg 2016; 95: 148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Itoh M, Negreiros do Valle S, Farias S. et al. Efficacy of artemether–lumefantrine for uncomplicated Plasmodium falciparum malaria in Cruzeiro do Sul, Brazil, 2016. Am J Trop Med Hyg 2018; 98: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lucchi NW, Abdallah R, Louzada J. et al. Molecular surveillance for polymorphisms associated with artemisinin-based combination therapy resistance in Plasmodium falciparum isolates collected in the State of Roraima, Brazil. Am J Trop Med Hyg 2020; 102: 310–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aponte S, Guerra ÁP, Álvarez-Larrotta C. et al. Baseline in vivo, ex vivo and molecular responses of Plasmodium falciparum to artemether and lumefantrine in three endemic zones for malaria in Colombia. Trans R Soc Trop Med Hyg 2017; 111: 71–80. [DOI] [PubMed] [Google Scholar]
- 19. Montenegro M, Neal AT, Posada M. et al. K13 propeller alleles, mdr1 polymorphism, and drug effectiveness at day 3 after artemether-lumefantrine treatment for Plasmodium falciparum malaria in Colombia, 2014-2015. Antimicrob Agents Chemother 2017; 61: e01036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olivera MJ, Guerra AP, Cortes LJ. et al. Artemether-lumefantrine efficacy for the treatment of uncomplicated Plasmodium falciparum malaria in Choco, Colombia after 8 years as first-line treatment. Am J Trop Med Hyg 2020; 102: 1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rahman R, Martin MJS, Persaud S. et al. Continued sensitivity of Plasmodium falciparum to artemisinin in Guyana, with absence of Kelch propeller domain mutant alleles. Open Forum Infect Dis 2016; 3: ofw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Valenzuela G, Castro LE, Valencia-Zamora J. et al. Genotypes and phenotypes of resistance in Ecuadorian Plasmodium falciparum. Malar J 2019; 18: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chenet SM, Akinyi Okoth S, Kelley J. et al. Molecular profile of malaria drug resistance markers of Plasmodium falciparum in Suriname. Antimicrob Agents Chemother 2017; 61: e02655-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ministério da saùde. Boletim malária nas fronteiras e importados de outros países_23.06.2020 - Malária - Brasil | Tableau Public. 2020. https://public.tableau.com/profile/mal.ria.brasil#!.
- 25.WWARN K13 Genotype-Phenotype Study Group. Association of mutations in the Plasmodium falciparum Kelch13 gene (Pf3D7_1343700) with parasite clearance rates after artemisinin-based treatments—a WWARN individual patient data meta-analysis. BMC Med 2019; 17: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boussaroque A, Fall B, Madamet M. et al. Emergence of mutations in the K13 propeller gene of Plasmodium falciparum isolates from Dakar, Senegal, in 2013-2014. Antimicrob Agents Chemother 2016; 60: 624–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conrad MD, Bigira V, Kapisi J. et al. Polymorphisms in K13 and falcipain-2 associated with artemisinin resistance are not prevalent in Plasmodium falciparum isolated from Ugandan children. PLoS One 2014; 9: e105690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torrentino-Madamet M, Fall B, Benoit N. et al. Limited polymorphisms in k13 gene in Plasmodium falciparum isolates from Dakar, Senegal in 2012–2013. Malar J 2014; 13: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashley EA, Dhorda M, Fairhurst RM. et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2014; 371: 411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takala-Harrison S, Jacob CG, Arze C. et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis 2015; 211: 670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Z, Wang Y, Cabrera M. et al. Artemisinin resistance at the China-Myanmar border and association with mutations in the K13 propeller gene. Antimicrob Agents Chemother 2015; 59: 6952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Z, Shrestha S, Li X. et al. Prevalence of K13-propeller polymorphisms in Plasmodium falciparum from China-Myanmar border in 2007–2012. Malar J 2015; 14: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaramillo-Ochoa R, Sippy R, Farrell DF. et al. Effects of political instability in Venezuela on malaria resurgence at Ecuador–Peru border, 2018. Emerg Infect Dis 2019; 25: 834–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Lima Junior MM, Rodrigues GA. et al. Evaluation of emerging infectious disease and the importance of SINAN for epidemiological surveillance of Venezuelans immigrants in Brazil. Braz J Infect Dis 2019; 23: 307–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodríguez-Morales AJ, Suárez JA, Risquez A. et al. Consequences of Venezuela’s massive migration crisis on imported malaria in Colombia, 2016–2018. Travel Med Infect Dis 2019; 28: 98–9. [DOI] [PubMed] [Google Scholar]
- 36. Grillet ME, Moreno JE, Hernández-Villena JV. et al. Malaria in Southern Venezuela: the hottest hotspot in Latin America. PLoS Negl Trop Dis 2021; 15: e0008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Louzada J, de Almeida NCV, de Araujo JLP. et al. The impact of imported malaria by gold miners in Roraima: characterizing the spatial dynamics of autochthonous and imported malaria in an urban region of Boa Vista. Mem Inst Oswaldo Cruz 2020; 115: e200043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arisco NJ, Peterka C, Castro MC.. Cross-border malaria in Northern Brazil. Malar J 2021; 20: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Douine M, Lambert Y, Musset L. et al. Malaria in gold miners in the Guianas and the Amazon: current knowledge and challenges. Curr Trop Med Rep 2020; 7: 37–47. [Google Scholar]
- 40. Recht J, Siqueira AM, Monteiro WM. et al. Malaria in Brazil, Colombia, Peru and Venezuela: current challenges in malaria control and elimination. Malar J 2017; 16: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Douine M, Mosnier E, Le Hingrat Q. et al. Illegal gold miners in French Guiana: a neglected population with poor health. BMC Public Health 2018; 18: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Douine M, Musset L, Corlin F. et al. Prevalence of Plasmodium spp. in illegal gold miners in French Guiana in 2015: a hidden but critical malaria reservoir. Malar J 2016; 15: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gomes M. d SM, Menezes R. d O, Vieira JLF. et al. Malaria in the borders between Brazil and French Guiana: social and environmental health determinants and their influence on the permanence of the disease. Saúde E Soc 2020; 29: e181046. [Google Scholar]
- 44. van Eer ED, Bretas G, Hiwat H.. Decreased endemic malaria in Suriname: moving towards elimination. Malar J 2018; 17: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mendes AM, Lima M. d S, Maciel AGP. et al. Malaria among indigenous peoples on the Brazil-French Guiana border, 2007-2016: a descriptive study. Epidemiol E Serviços Saúde 2020; 29: e2019056. [DOI] [PubMed] [Google Scholar]
- 46.Ministério da saùde. Boletim de casos de Malária da região Amazônica_26.05.2020 - Malária - Brasil | Tableau Public. 2020. https://public.tableau.com/profile/mal.ria.brasil#!.
- 47. Amato R, Pearson RD, Almagro-Garcia J. et al. Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: a retrospective genetic study. Lancet Infect Dis 2018; 18: 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hamilton WL, Amato R, van der Pluijm RW. et al. Evolution and expansion of multidrug-resistant malaria in southeast Asia: a genomic epidemiology study. Lancet Infect Dis 2019; 19: 943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hiwat H, Martinez-Lopez B, Cairo H. et al. Malaria epidemiology in Suriname from 2000 to 2016: trends, opportunities and challenges for elimination. Malar J 2018; 17: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Douine M, Sanna A, Galindo M. et al. Malakit: an innovative pilot project to self-diagnose and self-treat malaria among illegal gold miners in the Guiana Shield. Malar J 2018; 17: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Douine M, Lazrek Y, Blanchet D. et al. Predictors of antimalarial self-medication in illegal gold miners in French Guiana: a pathway towards artemisinin resistance. J Antimicrob Chemother 2018; 73: 231–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.