Abstract
Background:
With the advances in neuroimaging in amyotrophic lateral sclerosis (ALS), it has been speculated that multiparametric magnetic resonance imaging (MRI) is capable to contribute to early diagnosis. Machine learning (ML) can be regarded as the missing piece that allows for the useful integration of multiparametric MRI data into a diagnostic classifier. The major challenges in developing ML classifiers for ALS are limited data quantity and a suboptimal sample to feature ratio which can be addressed by sound feature selection.
Methods:
We conducted a systematic review to collect MRI biomarkers that could be used as features by searching the online database PubMed for entries in the recent 4 years that contained cross-sectional neuroimaging data of subjects with ALS and an adequate control group. In addition to the qualitative synthesis, a semi-quantitative analysis was conducted for each MRI modality that indicated which brain regions were most commonly reported.
Results:
Our search resulted in 151 studies with a total of 221 datasets. In summary, our findings highly resembled generally accepted neuropathological patterns of ALS, with degeneration of the motor cortex and the corticospinal tract, but also in frontal, temporal, and subcortical structures, consistent with the neuropathological four-stage model of the propagation of pTDP-43 in ALS.
Conclusions:
These insights are discussed with respect to their potential for MRI feature selection for future ML-based neuroimaging classifiers in ALS. The integration of multiparametric MRI including DTI, volumetric, and texture data using ML may be the best approach to generate a diagnostic neuroimaging tool for ALS.
Keywords: amyotrophic lateral sclerosis, machine learning, magnetic resonance imaging, motor neuron disease, neurodegeneration, neuroimaging, systematic review
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that affects several brain regions in a distinctive propagation pattern, with emphasis on the motor neurons. 1 To diagnose ALS as early as possible is a task of high clinical relevance for the optimized patients’ care and the opportunity to be enrolled in clinical trials. With advances in neuroimaging in neurodegenerative diseases like ALS,2,3 it has been speculated that cerebral magnetic resonance imaging (MRI) may be able to provide insights that support an early diagnosis. Multiparametric, quantitative MRI has been discussed as a way to achieve a composite neuroimaging index. 3 However, the amount of biomarkers, as well as their (non-linear) interactions, makes a straightforward approach likely unsuccessful. Machine learning (ML) might be the missing piece to integrate multiparametric MRI data into a useful classifier. 4 In a classification problem, the ML model is presented with a dataset and the correct outcomes (supervised learning). By model-specific rules, the model is fit to the data to predict the outcome. The model that is created in this process can then be used to predict the outcome of new data, that is, in a diagnostic set-up, a given patient’s data are used to predict the correct diagnosis. With advanced ML techniques, the promise for highly personalized medicine emerged. However, it was quickly realized that ML models in medicine could not outperform health professionals, even in favorable comparisons, 5 so a more realistic approach may be to restrict ML to very specific tasks. One of these tasks is quantitative neuroimaging, supporting (qualitative) human visual inspection.
In ALS, several studies have incorporated ML in a diagnostic pursuit (for a review, see Grollemund et al. 6 ). In an early approach using a linear support vector machine (SVM), independent component analysis (ICA) from resting-state functional MRI (rs-fMRI) data yielded 71% accurate classification. 7 With a similar approach, but using a multiple kernel SVM on rs-fMRI data, a 87% accurate classification was achieved. 8 However, there was no independent test sample, and overfitting might have occurred, that is, the model cannot be generalized because it fitted the training data too tightly. Using T1-weighted (T1w) and diffusion tensor imaging (DTI) data, various classification models achieved an accuracy of 78–90%,9–11 although high accuracy might have resulted from model overfitting, given that features were not defined a priori or samples were limited. By the use of ML classifiers on texture data gathered from the corticospinal tract, 70–80% accuracy was reported. 12 Interestingly, when re-training general image recognition models like ResNet or VCG-16, a 60–63% accuracy was still achieved.13,14 Although these results are promising, overfitting remains one of the main challenges in ML. One of the most important mechanisms behind overfitting is the sample to feature ratio (SFR) which is a general parameter that helps assessing how many features an ML model can process before overfitting or underfitting is likely to occur. 6 As a general rule, 10–15 samples per feature have been proposed; however, modern ML algorithms may even work with lower numbers. 15 To date, there are few databases that collect MR images from patients with ALS in a meaningful amount, and even those may not be able to establish a reasonable ML model without some preselection of MRI features. In 2016, Grolez et al. published a systematic review that provides a useful collection of ALS-specific neuroimaging biomarkers that could be used for feature selection. 16 The current review updates this previous work by specifically addressing ML models. To this end, we conducted a systematic review on neuroimaging in ALS, in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 17 To this end, we reviewed all available studies over the previous 4 years (i.e. from 2017 onward) that could contribute to disease classification. We compiled these data into a comprehensive list of findings that can be used as features in an ML model.
Methods
Search strategy and study selection
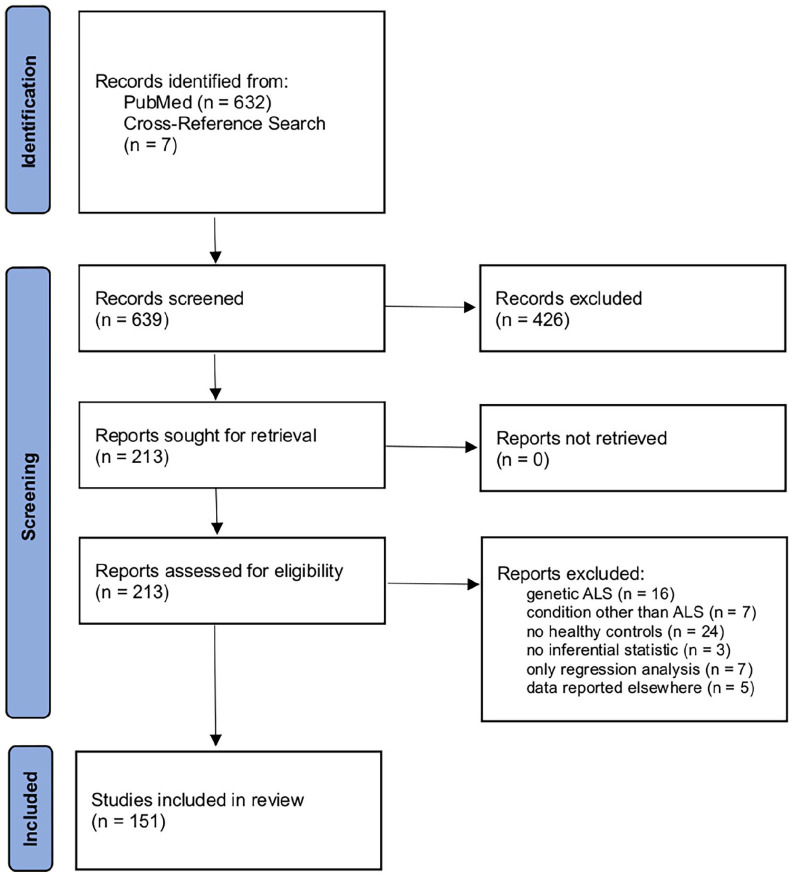
The literature review and study selection were conducted in accordance with the PRISMA guidelines. 17 In March, 2021, a systematic search was conducted on the online library PubMed (https://www.ncbi.nlm.nih.gov/pubmed/). The search queries were ALS AND MRI, ALS AND ‘magnetic resonance imaging’, ALS AND neuroimaging, ‘amyotrophic lateral sclerosis’ AND MRI, ‘amyotrophic lateral sclerosis’ AND ‘magnetic resonance imaging’, and ‘amyotrophic lateral sclerosis’ AND neuroimaging. Only publications that were listed after 1 January 2017 were considered for this review. Regarding prior publications, we refer to the systematic review by Grolez et al. 16 and to the meta-analyses by Shen et al. 18 and Gorges et al. 19 Our search yielded 632 database entries. In a cross-reference search, additional 7 records were found for a total of 639. These records were carefully checked by an experienced reviewer for the following criteria: the studies had to be published in a peer-reviewed journal in the English language; only human studies with in vivo cranial MRI were considered; and there had to be a group of healthy control subjects or mimic disorders and a group of subjects with sporadic ALS, according to common diagnostic guidelines. Primary lateral sclerosis (PLS), progressive bulbar palsy (PBP), and pure lower motor neuron disease (LMND) were considered as ALS spectrum disorders and thus also included.3,20,21 There had to be cross-sectional data, and inferential statistics had to be conducted on these data. Solely longitudinal data or regression analyses were not considered, as these would not help distinguishing ALS from healthy controls. However, if there were appropriate data in Supplementary Material, these studies could still be included. From the 639 entries, 213 reports were identified as eligible. In the full-text review, 62 reports were excluded due to the following exclusion criteria: genetic as opposed to sporadic ALS (n = 16), no healthy control group (n = 24), conditions other than ALS (n = 7), no inferential statistics (n = 3), only statistical regression (n = 7), or data that were already reported elsewhere (n = 5). In total, 151 studies were included in this systematic review. Figure 1 summarizes the literature review and study inclusion process according to the PRISMA guidelines. No automated tools were used in this systematic review. Ethics approval and informed consent were not required for this systematic review.
Figure 1.
PRISMA flow diagram illustrating the literature review and study selection process.
Semi-quantitative analysis
During the full-text review, all data that could be used for disease classification in an ML model were noted, both from the report itself and from its Supplementary Material if provided. In a semi-quantitative synthesis, it was analyzed which brain areas were most commonly reported. This was done by counting how many studies reported a brain region as a significant finding. We categorized these regions into ‘major’ and ‘minor’ findings, based upon the quantity of studies that reported each finding. This procedure was repeated for each MRI modality. The goal of this semi-quantitative analysis was to provide a comprehensive list of findings that could be used for disease classification in an ML model. As this approach relies solely on the quantity of reports, underreporting or overreporting of specific results can bias the analysis.
Results
There were several studies with data from different modalities; these data are reported separately for each of these modalities. From the 151 studies, a total of 221 datasets were analyzed. In addition, we provide a comprehensive summary of studies which included at least 50 subjects (ALS patients and controls combined) and statistical correction for multiple comparisons. A total of 129 datasets resulted, summarized in Tables 1–5.
Table 1.
Summary of main structural/morphometric findings from studies with T1-weighted imaging data in ALS.
| Publication | ALS | Controls | Main finding |
|---|---|---|---|
| Buhour et al. 22 | 37 | 37 | Gray matter atrophy in the right precentral gyrus, the left postcentral gyrus, the left paracentral lobule, the left inferior temporal gyrus, the left supramarginal gyrus, and the right putamen |
| Illán-Gala et al. 23 | 31 | 37 | Cortical thinning in the precentral gyrus, paracentral frontal regions, and the precuneus; in ALS with cognitive or behavioral symptoms: cortical thinning in frontoinsular and temporal regions |
| Cheng et al. 24 | 60 | 60 | White matter atrophy in the corticospinal tract |
| de Albuquerque et al. 25 | 63 | 64 | Gray matter atrophy in the precentral cortex and several frontal areas; cortical thinning in paracentral, precentral, and temporal areas |
| Schuster et al. 26 | 60 | 69 | Cortical thinning of the precentral and paracentral gyri contributes to survival prediction in ALS via binary logistic ridge regression |
| Shen et al. 27 | 55 | 20 | Gray matter atrophy in the frontal lobe, temporal lobe, precentral gyrus, cingulate gyrus, and the thalamus, most prominent in ALS-FTD |
| Hu et al. 28 | 42 | 21 | No significant difference in voxel-based morphometry between ALS with and without cognitive deficits |
| Dadar et al. 29 | 66 | 42 | Atrophy of the precentral gyrus, the corticospinal tract (including the internal capsule and brainstem), anterior cingulate cortex, and posterior parietal areas |
| Bede et al. 30 | 48 | 50 | Volume reduction in the basal ganglia; gray matter alterations of striatal subregions that project to rostral motor and executive cortical regions in ALS-FTD |
| Agosta et al. 31 | 67 | 22 | Cortical thinning in the precentral gyrus and prefrontal, parietal, temporal, and occipital cortices |
| Branco et al. 32 | 50 | 38 | Atrophy of the amygdala in ALS with cognitive impairment |
| Kim et al. 33 | 62 | 57 | Lower gray matter density mostly in the precentral gyrus and adjacent pre- and postcentral regions, with more widespread frontotemporal involvement in bulbar ALS |
| Bede et al. 10 | 75 | 75 | Precentral gyrus, thalamus, caudate, nucleus accumbens, hippocampus, and amygdala volume contribute to disease classification in a canonical discriminant analysis |
| Christidi et al. 34 | 56 | 25 | Gray matter atrophy in the pre- and postcentral gyri, frontal regions (especially in ALS with pathological laughing and crying), temporal regions, subcortical structures, and the left cerebellum |
| Acosta-Cabronero et al. 35 | 28 | 39 | No significant difference in subcortical volumetry, voxel-based morphometry, or cortical thickness analyses |
| Ogura et al. 36 | 71 | 69 | Gray matter volume reduction near the right parahippocampal gyrus and in the anterior part of the left temporal lobe, the latter related to semantic deficits in ALS |
| Trojsi et al. 37 | 54 | 22 | No significant difference in voxel-based morphometry |
| Trojsi et al. 38 | 32 | 21 | No significant difference in voxel-based morphometry |
| Qiu et al. 39 | 60 | 60 | Atrophy in the left precentral gyrus and increased gray matter volume in several cerebellar subregions, possibly as compensation |
| Gellersen et al. 40 | 60 | 1471 | Meta-analysis; cerebellar atrophy in the vermis (culmen and nodule), left posterior lobe, left inferior semi-lunar lobule, and bilateral anterior lobe |
| Qin et al. 41 | 28 | 28 | Atrophy in the precentral gyrus, more widespread in late-stage ALS |
| Ferraro et al. 11 | 123 | 78 | Cortical thinning of the precentral gyrus contributes to disease classification in a random forest analysis |
| Consonni et al. 42 | 48 | 26 | Cortical thinning in frontoparietal regions; widespread thinning in inferior frontal, temporal, cingular, and insular regions in ALS with cognitive impairment |
| Contarino et al. 43 | 42 | 23 | Cortical thinning in the precentral cortex and paracentral lobule |
| Bharti et al. 44 | 71 | 56 | No significant difference in gray matter volume in the cerebellum or its components |
| Chipika et al. 45 | 133 | 117 | Thalamic atrophy with the preferential involvement of nuclei mediating motor and cognitive functions |
| Machts et al. 46 | 111 | 85 | Progressive cortical thinning of the right parahippocampal gyrus; progressive hippocampal atrophy in ALS with memory impairment |
| Tu et al. 47 | 20 | 31 | Thalamic atrophy with deformation of the medial surface |
| Consonni et al. 48 | 36 | 26 | Cortical thinning in the right middle frontal sulcus and the right middle-posterior cingulate gyrus |
| Wirth et al. 49 | 20 | 30 | No significant difference in the cortical thickness of the pre- and postcentral gyrus |
| Chipika et al. 50 | 88 | 117 | Atrophy of the accessory basal nucleus and the cortical nucleus of the amygdala |
| Finegan et al. 51 | 133 | 117 | Thalamic, caudate, and hippocampal atrophy and shape alterations, both in ALS and in PLS |
| Jin et al. 52 | 108 | 90 | Cortical thinning of the precentral gyrus with focus on the head-face region in bulbar-onset and on the upper-limb region in cervical-onset |
| Machts et al. 53 | 31 | 29 | Hippocampal volume reduction with shape deformities in the right hippocampal head and body region in vertex analysis |
| Welton et al. 54 | 21 | 63 | Trend toward atrophy of the precentral gyrus, as part of a composite score for disease classification |
| Senda et al. 55 | 67 | 38 | Gray matter atrophy in the precentral gyrus, basal ganglia, and frontoremporal lobes, more pronounced in rapid progression |
| Placek et al. 56 | 109 | 113 | Cortical thinning within the frontal and temporal lobes |
| Steinbach et al. 57 | 85 | 62 | Gray and white matter density decreases in the frontal and temporal lobes, as well as disease phase–related spread to frontal, temporal, and occipital gray matter areas |
| Chenji et al. 58 | 53 | 43 | Decreased gray matter density in the precentral gyrus, and premotor and medial prefrontal cortex, associated with verbal fluency |
| Christidi et al. 59 | 50 | 40 | Hippocampal atrophy, most pronounced in the cornu ammonis 2/3 subfield and the hippocampus–amygdala transition area |
| Omer et al. 60 | 30 | 40 | Gray matter atrophy in the pre- and postcentral gyri, or bitofrontal cortex, Broca area, and the frontal/temporal lobes in ALS-FTD |
| Finegan et al. 61 | 33 | 100 | Gray matter atrophy and cortical thinning in the precentral gyrus and left pars opercularis region; cerebellar atrophy in PLS and cortical thinning in the postcentral gyrus in ALS |
| Finegan et al. 62 | 39 | 100 | Gray matter atrophy and cortical thinning in the precentral gyrus, more widespread in ‘definite‘ versus ‘probable‘ PLS |
| Tae et al. 63 | 32 | 43 | Regional shape contractions that suggest local atrophy in both pallida, the right putamen, and the right nucleus accumbens |
| Bede et al. 64 | 133 | 100 | Progressive, multisegmental brainstem atrophy with medullar predominance, both in ALS and in PLS |
| Cheng et al. 65 | 60 | 60 | Cortical thinning in right precentral gyrus, superior frontal gyrus, and superior temporal gyrus |
| Finegan et al. 66 | 40 | 100 | Widespread gray and white matter atrophy in PLS, most pronounced in the precentral gyrus, frontal lobe, thalamus, corpus callosum, and corticospinal tract |
| Ratti et al. 67 | 22 | 115 | Gray matter atrophy in the precentral gyrus, the dorsomedial and dorsolateral prefrontal cortex, and the orbitofrontal cortex, mainly driven by ALS-FTD |
| van der Burgh et al. 68 | 268 | 156 | Progressive cortical thinning in the precentral gyrus, frontal, and temporal regions; atrophy of the hippocampi, left amygdala, left accumbens nucleus, and right thalamus |
| Hensiek et al. 69 | 206 | 104 | Lower T1 intensity of the tongue in bulbar-onset compared with limb-onset AlS |
| Gorges et al. 70 | 251 | 112 | Atrophy of the hypothalamus is related to body mass index and unrelated to disease stage |
| Chen et al. 71 | 283 | 255 | White matter atrophy in the precentral gyrus, supplementary motor areas, left middle cerebellar peduncle, and right cerebellum, involving several fibers and tracts |
| Machts et al. 72 | 158 | 86 | Cortical thinning in the right precentral gyrus; trend toward cortical thinning in the left precentral gyrus |
ALS, amyotrophic lateral sclerosis; PLS, primary lateral sclerosis; FTD, frontotemporal dementia; PLS, primary lateral sclerosis.
Table 2.
Summary of microstructural/diffusion properties findings from studies with DTI data in ALS.
| Publication | ALS | Controls | Main finding |
|---|---|---|---|
| Illán-Gala et al. 23 | 31 | 37 | Increased cortical mean diffusivity in prefrontal regions in patients with cognitive or behavioral impairment |
| Baek et al. 73 | 96 | 47 | Altered diffusivity in the corticospinal tract at the brainstem and in the cerebellar peduncle area |
| Schuster et al. 26 | 60 | 69 | White matter degeneration of all segments of the corticospinal tract and the corpus callosum predicted survival via binary logistic regression |
| Bharti et al. 44 | 71 | 56 | Altered diffusivity in the superior, middle, and inferior cerebellar peduncle |
| Cheng et al. 24 | 60 | 60 | Reduced fiber density and fiber bundle cross-section in the corticospinal tract and the body of the corpus callosum |
| Qiu et al. 39 | 60 | 60 | Reduced FA in left corticospinal tract and the body of the corpus callosum |
| Christidi et al. 34 | 56 | 25 | Reduced FA in the corticospinal tract, the body and splenium of the corpus callosum, and major associative and limbic tracts |
| Rajagopalan et al. 74 | 45 | 14 | Loss of subcortical fibers and reduced FA in the corticospinal tract |
| Branco et al. 32 | 50 | 38 | Reduced FA in the fornix of ALS patients with behavioral impairment; no changes in the body of the corpus callosum |
| Tu et al. 47 | 20 | 31 | Increased diffusivity in thalamic parcellations associated with the left frontal lobe, bilateral premotor cortex, bilateral motor cortex, right somatosensory cortex, and bilateral parietal lobe |
| Weidman et al. 75 | 43 | 15 | Altered diffusivity in the corticospinal tract |
| Christidi et al. 59 | 50 | 40 | Altered diffusivity in the fornix and the right perforant pathway zone |
| Tu et al. 76 | 39 | 25 | Abnormal diffusivity in the rostral body, posterior midbody, and isthmus of the corpus callosum |
| Finegan et al. 62 | 39 | 100 | Altered diffusivity in the corticospinal tracts throughout their intracranial course and in the corpus callosum |
| Müller et al. 77 | 46 | 23 | FA reduction along the corticospinal, corticorubral, and corticopontine tracts in ALS and progressive bulbar palsy |
| Borsodi et al. 78 | 27 | 35 | Altered diffusivity in the corticospinal tract |
| Ferraro et al. 11 | 123 | 78 | Altered diffusivity in the corticospinal tract and in specific segments of the corpus callosum contribute to disease classification in a random forest analysis |
| Kassubek et al. 79 | 67 | 31 | Reduced FA in the corticospinal, corticopontine, and corticorubral tracts, the corticostriatal pathway, and the proximal portion of the perforant path |
| Müller et al. 80 | 100 | 50 | FA reduction in frontal and prefrontal brain areas, the corticospinal and corticopontine and corticorubral tracts, the corticostriatal pathway, and the proximal portion of the perforant path |
| Welton et al. 54 | 21 | 63 | Lower diffusion kurtosis measures in the motor cortex, as part of a composite score for disease classification |
| de Albuquerque et al. 25 | 53 | 64 | Widespread diffusivity abnormalities in the corticospinal tract and the corpus callosum, with longitudinal progression |
| Christidi et al. 81 | 50 | 25 | Altered diffusivity in the corticospinal tract, the body and genu of corpus callosum, and in several extramotor white matter tracts, more pronounced in patients with cognitive impairment |
| Senda et al. 55 | 67 | 38 | Decreased FA in the corticospinal tract (corona radiata and internal capsule), the frontotemporal lobes, and the basal ganglia, more pronounced in rapid progression |
| Omer et al. 60 | 30 | 40 | Widespread diffusivity changes in the corticospinal tract, in frontal and temporal regions and in the brainstem in ALS-FTD patients; no findings in ALS without behavioral or cognitive deficits |
| Bede et al. 10 | 75 | 75 | Altered diffusivity in the corticospinal tract and in the corpus callosum contributes to disease classification in a canonical discriminant analysis |
| Agosta et al. 31 | 67 | 22 | Diffusivity changes involving the motor and extramotor pathways |
| Trojsi et al. 37 | 54 | 22 | Altered diffusivity in the body of corpus callosum and the superior part of corticospinal tract, both in slow and in fast progressors |
| Steinbach et al. 82 | 145 | 69 | Altered diffusivity in the corticospinal tract, body and genu of the corpus callosum, and adjacent corona radiata, as well as brainstem and cerebellar pathways |
| Chenji et al. 58 | 53 | 43 | Altered diffusivity in the corticospinal tract; in ALS with impaired verbal fluency or executive dysfunction: altered diffusivity in the corpus callosum, cingulum, and superior longitudinal fasciculus |
| Bao et al. 83 | 33 | 32 | Increased diffusivity in premotor, primary motor, primary, and secondary somatosensory areas, along the corticospinal tract, and in the body of the corpus callosum |
| Finegan et al. 61 | 33 | 100 | Altered diffusivity in the corticospinal tract (centrum semiovale, corona radiata and internal capsule), body of the corpus callosum, splenium, brainstem, and cerebellum in PLS |
| Trojsi et al. 84 | 36 | 35 | Reduced FA in subcortical areas of the corticospinal tract and in the body of corpus callosum |
| Trojsi et al. 38 | 32 | 21 | Altered diffusivity in subcortical areas of the corticospinal tract, the body and genu of the corpus callosum, uncinate fasciculus, and the superior longitudinal fasciculus |
| Cheng et al. 65 | 60 | 60 | Decreased FA in the right corticospinal tract and the posterior body of the corpus callosum |
| Zhang et al. 85 | 396 | 360 | Reduced FA in the corticospinal tract, the corpus callosum, and the left superior longitudinal fasciculus |
| Gorges et al. 19 | 2064 | 1688 | Microstructural alterations along the corticospinal tract, frontal and midbrain regions, the corticorubral and corticopontine tracts, the corticostriatal pathway, and in hippocampal regions |
| Müller et al. 86 | 166 | 92 | Widespread FA reduction along the corticospinal tract, corticopontine and corticorubral tracts, corticostriatal pathway, proximal portion of the perforant path, and in frontal and prefrontal brain areas |
| Müller et al. 87 | 176 | 88 | FA reduction in the upper and lower corticospinal tract, corticopontine and corticorubral tract, corticostriatal pathway, and the frontal and temporal lobes, both in ALS and in PLS |
| Kalra et al. 88 | 66 | 43 | Progressive FA reduction in corticospinal tract and in the frontal lobes; reduced FA in corticopontine and corticorubral tracts, the corticostriatal pathway, and the midbrain |
ALS, amyotrophic lateral sclerosis; FTD, frontotemporal dementia; DTI, diffusion tensor imaging; FA, fractional anisotropy; PLS, primary lateral sclerosis.
Table 3.
Summary of main functional and structural connectivity findings from studies with rs-fMRI data in ALS.
| Publication | ALS | Controls | Main finding |
|---|---|---|---|
| Functional connectivity: resting-state functional magnetic resonance imaging | |||
| Ogura et al. 36 | 71 | 69 | Decreased intrinsic connectivity in the posterior right fusiform and the lingual gyrus, related to semantic deficit in ALS |
| Trojsi et al. 37 | 54 | 22 | Decreased functional connectivity in the sensorimotor network, default mode network, frontoparietal network, and salience network |
| Ma et al. 89 | 54 | 54 | Increased dynamic regional homogeneity in the left lingual gyrus; decreased dynamic regional homogeneity in the left rectus gyrus and left parahippocampal gyrus |
| Li et al. 90 | 38 | 35 | Decreased short-range functional connectivity density in the primary motor cortex; increased long-range functional connectivity density in the premotor cortex |
| Loewe et al. 91 | 64 | 38 | Decreased functional connectivity in motor-related areas; widespread functional connectivity changes across the temporo-occipital cortex |
| Hu et al. 28 | 42 | 21 | Decreased regional homogeneity in sensorimotor cortices; increased regional homogeneity in parietal and cerebellar areas, associated with cognitive impairment |
| Bharti et al. 44 | 71 | 56 | Decreased functional connectivity between the dentate nucleus and the right precentral gyrus/the supplementary motor area/frontal, parietal, temporal, and infratentorial regions |
| Qiu et al. 39 | 60 | 60 | Decreased functional connectivity between the precentral cortex and sensorimotor areas/parieto-occipital regions/the cerebellum |
| Trojsi et al. 38 | 32 | 21 | Decreased functional connectivity within the limbic system and between the limbic system and frontal/cerebellar areas |
| Agosta et al. 31 | 67 | 22 | Increased functional connectivity within the sensorimotor network and the dorsal attention network |
| Cheng et al. 65 | 60 | 60 | Decreased self-inhibitory influence in the precentral gyrus |
| Chen et al. 92 | 32 | 45 | Decreased temporal variability in functional network connectivity, with aberrant connectivity between the default mode network/cognitive control network and the sensorimotor network |
| Basaia et al. 93 | 173 | 79 | Increased functional connectivity in sensorimotor, basal ganglia, and frontal areas and to a lesser extent in temporal and parietal areas |
| Structural connectivity and connectomics | |||
| Meier et al. 94 | 60 | 120 | Connectome-based random walker aggregation levels can explain disease stages and contribute to survival prediction via deep learning |
| Serra et al. 95 | 39 | 15 | Strong-Weak Pruning for brain network identification reveals impaired structural connectivity between the frontal and temporal/parietal cortex and between the temporal and occipital cortex |
| van der Burgh et al. 68 | 268 | 156 | Reduced structural connectivity in the motor network |
| Basaia et al. 93 | 173 | 79 | Lower mean local efficiency as a global network property and regionally decreased structural connectivity in sensorimotor, basal ganglia, frontal, and parietal areas |
| Zhang et al. 96 | 60 | 60 | Increased path length, clustering coefficient, small-world index, and local efficiency; decreased global efficiency; altered nodal degree and betweenness in frontal lobe areas |
| Fortanier et al. 97 | 25 | 26 | Decreased global efficiency and decreased mean degree in the left postcentral gyrus and in the left interparietal and transverse parietal sulci |
ALS, amyotrophic lateral sclerosis; rs-fMRI, resting-state functional magnetic resonance imaging.
Table 4.
Summary of main susceptibility findings from studies with SWI or T2*-weighted data in ALS.
| Publication | ALS | Controls | Main finding |
|---|---|---|---|
| Acosta-Cabronero et al. 35 | 28 | 39 | Increased susceptibility in the motor cortex, premotor areas, substantia nigra, globus pallidus, and red nucleus |
| Lee et al. 98 | 26 | 26 | Increased susceptibility in the motor cortex and decreased susceptibility in subcortical regions |
| Weidman et al. 75 | 43 | 15 | Increased motor cortex susceptibility |
| Contarino et al. 43 | 42 | 23 | Significant susceptibility skewness and trend toward increased susceptibility in the prefrontal cortex |
| Conte et al. 99 | 48 | 28 | Increased motor cortex susceptibility |
| Conte et al. 100 | 47 | 38 | Increased susceptibility skewness in the precentral cortex, driven by upper motor neuron involvement |
| Borsodi et al. 78 | 24 | 33 | Increased susceptibility in the left corticospinal tract in bulbar ALS |
| Welton et al. 54 | 21 | 63 | Increased susceptibility/iron concentration in the motor cortex, as part of a composite score for disease classification |
ALS, amyotrophic lateral sclerosis; SWI, susceptibility weighted imaging.
Table 5.
Summary of main findings from studies with special MRI modalities or analyses in ALS.
| Publication | Method | ALS | Controls | Main finding |
|---|---|---|---|---|
| Reischauer et al. 101 | MRS | 24 | 27 | Altered diffusivity for several spectoscopic parameters in the precentral gyrus; reduced tNAA in the precentral gyrus |
| Grapperon et al. 102 | Sodium-MRI | 27 | 30 | Higher total sodium concentration in the right precentral gyrus and the corticospinal tract |
| Ishaque et al. 103 | Texture analysis | 83 | 74 | Texture analysis revealed alterations in the precentral gyrus, corticospinal tract, insula, basal ganglia, hippocampus, and frontal regions, including subcortical white matter |
| Müller et al. 104 | Texture analysis | 152 | 82 | Increased entropy in area II of the corpus callosum; increased inhomogeneity in areas I–III of the corpus callosum |
| Elahi et al. 12 | Texture analysis | 69 | 42 | Texture analysis of T1-slices of the corticospinal tract achieves disease classification in an ensemble stacking machine learning model |
| Wirth et al. 105 | T2-weighted FLAIR | 28 | 31 | Increased amount of FLAIR lesions in male patients, predominantly detected in the superior and posterior corona radiata, anterior capsula interna, and posterior thalamic radiation |
| Fabes et al. 106 | T2w FLAIR | 33 | 21 | Progressive FLAIR hyperintensity of the corticospinal tract |
| Shen et al. 27 | CBF | 55 | 20 | Reduced cerebral blood flow in the frontal lobe, insula, corpus callosum, and caudate in ALS-FTD |
| Welton et al. 54 | CBF | 21 | 63 | No significant difference in motor cortex perfusion |
ALS, amyotrophic lateral sclerosis; CBF, cerebral blood flow; FLAIR, fluid-attenuated inversion recovery; FTD, frontotemporal dementia; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy.
Structural alterations: T1w imaging
Seventy-eight datasets of T1w imaging–based studies were reviewed (Table 1). In accordance with the previous literature, 16 most studies reported loss of gray matter volume in the precentral gyrus, 72 either by cortical thickness assessment or by voxel-based morphometry. 33 In addition, further areas were reported that resembled the pattern of the neuropathological four-stage model of the propagation of pTDP43 in ALS. 107 These areas included the frontal cortex, the anterior cingulate cortex, as well as subcortical and temporal structures, especially the thalamus, the hippocampus, and the amygdala, summarized in a recent controlled study with 292 participants with ALS. 68 Single studies with high numbers of participants described significant atrophy in the hypothalamus in patients with ALS, irrespective of the disease stage 70 and abnormal T1 signal in the tongue, with further findings in area and shape of the tongue. 69 Few studies conducted white matter morphometry with mixed results, showing abnormalities in sensorimotor and cerebellar tracts. 71
Microstructural alterations: diffusion tensor imaging
Sixty-six datasets were reviewed for DTI data (Table 2). Overall, the analysis of fractional anisotropy (FA) revealed the most consistent results when comparing different studies. A meta-analysis with a total of 3752 subjects found the following four tracts to be the most important: the corticospinal tract, the corticorubral/corticopontine tract, the corticostriatal tract, and the proximal portion of the perforant path, which have previously been described as stage-defining tracts (stages 1–4).19,86,87,108 A recent multicenter study replicated these findings, with additional abnormalities found in the frontal lobe. 88 Within the corticospinal tract, the posterior limb of the internal capsule, the corona radiata, and the cerebral peduncle were shown to be the most affected. 85 Of note, the application of the analysis approach neurite orientation and dispersion density imaging (NODDI) to ALS demonstrated axonal loss in the corticospinal tract to be the major contribution to altered diffusivity and also identified dendritic alterations within the precentral gyrus. 109 In addition to these stage-defining tracts, the corpus callosum has consistently been found to exhibit reduced FA, especially in the motor and premotor segments.76,110
Functional alterations: resting-state functional magnetic resonance imaging
Twenty-three datasets were reviewed (Table 3). Overall, results were heterogeneous, with both increased and decreased functional connectivity in the pre- and postcentral gyri, the frontal and temporal lobes, the operculum, the insula, and the lingual gyrus.38,39 In a multicenter study with 173 patients with sporadic ALS and 79 healthy controls, increased functional connectivity in precentral, middle, and superior frontal areas in ALS and in the sensorimotor, basal ganglia, and temporal networks in PLS was reported. 93
Alterations in brain connectivity: connectomics
Eight datasets were reviewed (Table 3). As network parameters, only the global efficiency was consistently decreased in patients with ALS. 97 In addition, some studies found reduced nodal degree in the frontal lobe.75,97 In a multicenter study, decreased structural connectivity in sensorimotor, basal ganglia, frontal, and parietal areas was reported. 93 Of note, using a random walker model on connectivity data, a recent study simulated disease spreading that resembled propagation patterns in ALS, with additional survival prediction using deep learning. 94
Alterations in susceptibility-weighted imaging (SWI)
Twenty datasets were reviewed (Table 4). Increased susceptibility in the precentral gyrus was consistently reported.54,75,99 In addition, few studies reported increased susceptibility in subcortical structures and decreased susceptibility in the corticospinal tract. 35 Of note, the use of phase difference–enhanced (PADRE) MRI enabled to identify a characteristic low-signal intensity layer in the precentral cortex in 50% of ALS subjects, a finding which had been named the ‘zebra sign’ in an earlier publication due to the appearance of three- or four-layer organizations in the precentral cortex in ALS.111,112
Alterations in other MRI parameters or analyses
Twenty-six datasets were reviewed (Table 5). In a multicenter study, texture feature extraction [Modified Co-occurrence Histograms of Oriented Gradients (M-CoHOG)] from the corticospinal tract was able to differentiate patients with ALS from healthy controls. 12 Texture analysis of the corpus callosum showed significant differences in homogeneity and entropy in the motor segment. 104 By use of magnetic resonance spectroscopy (MRS), reduced N-acetylaspartate and increased myo-inositol levels were reported in the precentral gyrus. 101 In addition, sodium-MRS revealed higher total sodium concentration in the right precentral gyrus and the corticospinal tract. 102 Longitudinal fluid-attenuated inversion recovery (FLAIR) imaging demonstrated progressive hyperintensity of the corticospinal tract. 106 Cerebral blood flow (CBF) imaging showed hypoperfusion in several brain regions, 27 however, not confirmed in a recent study. 54
Semi-quantitative analysis
For the semi-quantitative analysis, it was counted how many of the included studies reported a specific area as a significant finding (note that this approach may have biased the analysis by underreporting or overreporting of specific results). The findings from the semi-quantitative analysis are summarized in Table 6. In morphometry studies, the precentral gyrus, the thalamus, the hippocampus, the amygdala, the insula, the anterior cingulate cortex (ACC), the orbitofrontal cortex, and the middle and inferior frontal gyri were most commonly reported. In DTI studies, the corticospinal tract, corticopontine/corticorubral tract, corticostriatal pathway, proximal portion of the perforant pathway, and the corpus callosum were most commonly reported. SWI and MRS studies showed abnormalities mostly in the motor cortex. In rs-fMRI studies, abnormalities were most commonly reported in the pre- and postcentral gyri, frontal and temporal lobe, the operculum, the insula and the lingual gyrus. In connectome studies, decreased global efficiency was the only consistent result.
Table 6.
Comprehensive list of candidate regions for each modality to be included as feature in ML models for disease classification from cranial MRI in ALS.
| Modality | Main regions | Further regions |
|---|---|---|
| T1 | Precentral gyrus, thalamus, hippocampus, amygdala, insula, ACC, orbitofrontal cortex, middle frontal gyrus, inferior frontal gyrus | Paracentral lobule, operculum, temporal pole, postcentral gyrus, posterior cingulate, superior temporal gyrus, medial frontal cortex, parahippocampal gyus, caudate, putamen, nucleus accumbens |
| DTI | Corticospinal tract (subcortical, superior corona radiata, posterior limb of the internal capsule, cerebral peduncle, brainstem), corticopontine/corticorubral tract, corticostriatal pathway, perforant pathway, corpus callosum (genu, body and splenium) | Frontal and temporal lobe (as a whole), corona radiata, superior longitudinal fascicle |
| rs-fMRI | Precentral gyrus, postcentral gyrus, superior and middle frontal gyrus, middle temporal gyrus, operculum, insula, lingual gyrus | Inferior frontal gyrus, superior parietal lobule, supramarginal gyrus, angular gyrus, precuneus, occipital fusiform gyrus, occipital pole |
| SWI | Precentral gyrus | Striatum (higher susceptibility) and corticospinal tract (lower susceptibility) |
| MRS | Precentral gyrus (NAA and myo-inositol) | Supplementary motor area, postcentral gyrus, brainstem/pontine region |
| connectomics | Global efficiency | Nodal degree in frontal lobe |
ACC, anterior cingulate cortex; ALS, amyotrophic lateral sclerosis; DTI, diffusion tensor imaging; ML, machine learning; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; NAA, n-acetylaspartate; SWI, susceptibility-weighted imaging.
Discussion
Summary
This systematic review provides a comprehensive overview of neuroimaging findings in ALS that can be used as features for disease classification in an ML model. In accordance with a previous systematic review and neuropathological findings,16,107,113,114 neuroimaging biomarkers were most commonly reported in the motor cortex and the corticospinal tract, together with frontal and temporal areas in later stages. Regarding feature selection, we provide a list of our findings in Table 6.
Brain structures in neuroimaging in ALS
The corticospinal tract, the corticopontine/corticorubral tract, the corticostriatal pathway, and the perforant pathway were among the most frequently reported regions that exhibited diffusivity changes. These tracts have previously been described as stage-dependent,3,79 which can be helpful in ML models when the presence of late-stage findings raises the confidence of the prediction.
The combination of structural and functional neuroimaging parameters in one ML model is very intriguing because it is expected that the complementary nature of these biomarkers as well as the low correlation between them theoretically might improve classification accuracy. Indeed, our review identified several candidate regions that could be included as features in such an ML model. However, both increased and decreased functional connectivity were reported with no clear directional effect. It can be argued that this inconsistency could ultimately introduce more noise than signal, especially when the theoretical background of this inconsistency remains ill-defined. It contrast, it may be of advantage to approach rs-fMRI feature extraction based on neuropathological concepts. 115 To incorporate functional connectivity measures as features in an ML model, a higher level of data preprocessing might be needed. Without preselection, the main challenge in analyzing rs-fMRI is the high amount of features that would have to be incorporated into the model. Early attempts have used spatial templates and principal component analysis for dimensionality reduction with some success.7,8 At any rate, implementing rs-fMRI data into ML models remains a challenge, and for such a combination of functional and structural data, multiple combined ML models may be needed.
In addition to the most commonly reported neuroimaging biomarkers in ALS, our analysis identified further promising candidates. T1w MRI of the tongue revealed decreased signal intensity in patients with ALS. 69 Extracting these data might be a makeshift method of measuring lower motor neuron (LMN) degeneration. With respect to ML, measuring LMN degeneration could increase classification accuracy, as little correlation with other [upper motor neuron (UMN)] features is expected. Another biomarker that caught our attention was the atrophy of the hypothalamus. Unlike other biomarkers, hypothalamic atrophy did not relate to disease stage and was detectable very early on, even in presymptomatic cases 70 ; this association has been investigated in orbitofrontal-hypothalamic projections in a murine ALS model and in human patients. 116
One of the more recent techniques in ML is texture analysis. While even general image recognition models like ResNet and VCG-16 are capable of detecting a distinct signature in the corticospinal tracts of patients with ALS, custom-tailored solutions are by far more powerful.13,14 Using M-CoHOG in an elaborated ML model, Elahi et al. achieved classification with up to 80% accuracy. 12 Müller et al. were able to differentiate patients with PLS and healthy controls with a sensitivity of 73% and a specificity of 84% using a single texture parameter (homogeneity) in the corpus callosum. 104 These types of analyses support the strength of ML and could substantially boost the accuracy of ML models.
SWI and MRS were found to only show abnormalities in the motor cortex. While this information per se can be very useful, there are other MRI modalities that can assess the integrity of the motor cortex without expanding the MR measuring time. Although it can be argued that having more features is preferable, redundancy might not increase diagnostic accuracy, but lower it due to worse SFR. With this in mind, adding these sequences to a standard protocol is hard to justify for ML classification. There were several MRI modalities with scarce data, like arterial spin labeling (ASL) for cerebral blood flow quantification. The contribution of these modalities to multiparametric classification models has to await a higher number of studies in the future. It has been concluded that the most important MRI sequences in this regard are T1w, DTI, and rs-fMRI, with MRS only being optional.117,118 Given that ML models usually need large datasets, multicenter initiatives are important for improving the methodological quality of future studies.
Limitations
This systematic review was confined to cranial MRI. However, there are plenty of studies that investigated spinal pathologies that can be considered to serve as biomarkers in ALS, for example, spinal diameter. 119 We decided against the inclusion of these studies in this review for two reasons. First, adding spinal MRI to an ML model would require a time-consuming MRI protocol that may not be suited for routine applications. Second, spinal MRI in ALS mostly measures pathologies of the corticospinal tract which can also be measured in the brain. Features extracted from spinal MRI might thus be redundant. Most of the studies compared patients with ALS with healthy controls. It can be argued that this is not useful in a diagnostic setting, where ALS has to be differentiated from its mimic disorders. However, many mimic disorders of ALS are peripheral neuropathies which are expected to be associated with a normal cranial MRI. When comparing patients with ALS with healthy controls and mimic disorders, Ferraro et al. found that the model actually performed better with the latter. 11 Although more studies with mimic disorders are needed, building an ML model with healthy control subjects (and fine-tuning it with mimic disorders) might suffice. In this review, we did not differentiate our findings between the clinical phenotypes of the ALS spectrum disorders (including the proportion of UMN/LMN involvement), that is, ‘classic’ ALS and primary lateral sclerosis, UMN-predominant (pyramidal), LMN-predominant (including flail arm and flail leg syndrome), and pure LMND (progressive muscular atrophy). Future studies should address fine phenotypic characterization given that there is a need for models discriminating not only classic ALS from mimic conditions but also classic ALS from pure/predominant UMN and pure/predominant LMN disease forms. Finally, our review focused on the neuroimaging domain, which means that the clinical domain, that is, a detailed assessment of the application of the ALS diagnostic criteria and their accuracy, was beyond the scope of this study. We used the inclusion criterion that the subjects with ALS had been diagnosed according to common diagnostic guidelines without further specification, while neuropathological confirmation was not a criterion due to the general lack of autopsies.
Conclusion
This review summarizes the most important findings that could be used as features in an ML model (Table 6). DTI and volumetric data can be considered to be the most robust features. Integrating functional or structural connectivity data might be challenging and may require dimensionality reduction techniques. Recently, texture analyses have demonstrated convincing results that may advance the field toward classification with higher accuracy. In the future, ML and multiparametric neuroimaging data might provide physicians with a powerful diagnostic tool.
Acknowledgments
The authors would like to thank the Ulm University Center for Translational Imaging MoMAN for its support.
Footnotes
Author contributions: TDK was involved in study concept and design, data analysis and interpretation of data, and drafting of manuscript. H-PM was involved in interpretation of data and critical revision of manuscript for intellectual content. ACL was involved in interpretation of data and critical revision of manuscript for intellectual content. JK was involved in study concept and design, interpretation of data, study supervision, and critical revision of manuscript for intellectual content.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors have no conflicts related to this study. The Associate Editor of Therapeutic Advances in Chronic Disease is an author of this paper; therefore, the peer review process was managed by alternative members of the Board, and the submitting Editor had no involvement in the decision-making process.
ORCID iD: Jan Kassubek  https://orcid.org/0000-0002-7106-9270
https://orcid.org/0000-0002-7106-9270
Contributor Information
Thomas D. Kocar, Department of Neurology, University of Ulm, Ulm, Germany
Hans-Peter Müller, Department of Neurology, University of Ulm, Ulm, Germany.
Albert C. Ludolph, Department of Neurology, University of Ulm, Ulm, Germany Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Ulm, Germany
Jan Kassubek, Department of Neurology, University of Ulm, Oberer Eselsberg 45, 89081 Ulm, Germany; Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE), Ulm, Germany.
References
- 1. van Es MA, Hardiman O, Chio A, et al. Amyotrophic lateral sclerosis. Lancet 2017; 390: 2084–2098. [DOI] [PubMed] [Google Scholar]
- 2. Kassubek J, Müller HP. Computer-based magnetic resonance imaging as a tool in clinical diagnosis in neurodegenerative diseases. Expert Rev Neurother 2016; 16: 295–306. [DOI] [PubMed] [Google Scholar]
- 3. Kassubek J, Müller HP. Advanced neuroimaging approaches in amyotrophic lateral sclerosis: refining the clinical diagnosis. Expert Rev Neurother 2020; 20: 237–249. [DOI] [PubMed] [Google Scholar]
- 4. Arbabshirani MR, Plis S, Sui J, et al. Single subject prediction of brain disorders in neuroimaging: promises and pitfalls. NeuroImage 2017; 145: 137–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilkinson J, Arnold KF, Murray EJ, et al. Time to reality check the promises of machine learning-powered precision medicine. Lancet Digit Health 2020; 2: e677–e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grollemund V, Pradat PF, Querin G, et al. Machine learning in amyotrophic lateral sclerosis: achievements, pitfalls, and future directions. Front Neurosci 2019; 13: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Welsh RC, Jelsone-Swain LM, Foerster BR. The utility of independent component analysis and machine learning in the identification of the amyotrophic lateral sclerosis diseased brain. Front Hum Neurosci 2013; 7: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fekete T, Zach N, Mujica-Parodi LR, et al. Multiple kernel learning captures a systems-level functional connectivity biomarker signature in amyotrophic lateral sclerosis. PLoS One 2013; 8: e85190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schuster C, Hardiman O, Bede P. Development of an automated MRI-based diagnostic protocol for amyotrophic lateral sclerosis using disease-specific pathognomonic features: a quantitative disease-state classification study. PLoS One 2016; 11: e0167331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bede P, Iyer PM, Finegan E, et al. Virtual brain biopsies in amyotrophic lateral sclerosis: diagnostic classification based on in vivo pathological patterns. Neuroimage Clin 2017; 15: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferraro PM, Agosta F, Riva N, et al. Multimodal structural MRI in the diagnosis of motor neuron diseases. Neuroimage Clin 2017; 16: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elahi GMME, Kalra S, Zinman L, et al. Texture classification of MR images of the brain in ALS using M-CoHOG: a multi-center study. Comput Med Imaging Graph 2020; 79: 101659. [DOI] [PubMed] [Google Scholar]
- 13. He K, Zhang X, Ren S, et al. Deep residual learning for image recognition. In: IEEE conference on computer vision and pattern recognition, Las Vegas, NV, 27–30 June 2016, pp. 770–778. New York: IEEE. [Google Scholar]
- 14. Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. In: International conference on learning representations, San Diego, CA, 7–9 May 2015. LaJolla: ICRL. [Google Scholar]
- 15. Raudys S. Statistical and neural classifiers: An integrated approach to design. London: Springer-Verlag, 2001. [Google Scholar]
- 16. Grolez G, Moreau C, Danel-Brunaud V, et al. The value of magnetic resonance imaging as a biomarker for amyotrophic lateral sclerosis: a systematic review. BMC Neurol 2016; 16: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen D, Cui L, Fang J, et al. Voxel-wise meta-analysis of gray matter changes in amyotrophic lateral sclerosis. Front Aging Neurosci 2016; 8: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gorges M, Del Tredici K, Dreyhaupt J, et al. Corticoefferent pathology distribution in amyotrophic lateral sclerosis: in vivo evidence from a meta-analysis of diffusion tensor imaging data. Sci Rep 2018; 8: 15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finegan E, Chipika RH, Shing SLH, et al. Primary lateral sclerosis: a distinct entity or part of the ALS spectrum? Amyotroph Lateral Scler Frontotemporal Degener 2019; 20: 133–145. [DOI] [PubMed] [Google Scholar]
- 21. Rosenbohm A, Müller HP, Hübers A, et al. Corticoefferent pathways in pure lower motor neuron disease: a diffusion tensor imaging study. J Neurol 2016; 263: 2430–2437. [DOI] [PubMed] [Google Scholar]
- 22. Buhour MS, Doidy F, Mondou A, et al. Voxel-based mapping of grey matter volume and glucose metabolism profiles in amyotrophic lateral sclerosis. EJNMMI Res 2017; 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Illán-Gala I, Montal V, Pegueroles J, et al. Cortical microstructure in the amyotrophic lateral sclerosis-frontotemporal dementia continuum. Neurology 2020; 95: e2565–e2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheng L, Tang X, Luo C, et al. Fiber-specific white matter reductions in amyotrophic lateral sclerosis. Neuroimage Clin 2020; 28: 102516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Albuquerque M, Branco LM, Rezende TJ, et al. Longitudinal evaluation of cerebral and spinal cord damage in amyotrophic lateral sclerosis. Neuroimage Clin 2017; 14: 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuster C, Hardiman O, Bede P. Survival prediction in amyotrophic lateral sclerosis based on MRI measures and clinical characteristics. BMC Neurol 2017; 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shen D, Hou B, Xu Y, et al. Brain structural and perfusion signature of amyotrophic lateral sclerosis with varying levels of cognitive deficit. Front Neurol 2018; 9: 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu T, Hou Y, Wei Q, et al. Patterns of brain regional functional coherence in cognitive impaired ALS. Int J Neurosci 2020; 130: 751–758. [DOI] [PubMed] [Google Scholar]
- 29. Dadar M, Manera AL, Zinman L, et al. Cerebral atrophy in amyotrophic lateral sclerosis parallels the pathological distribution of TDP43. Brain Commun 2020; 2: fcaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bede P, Omer T, Finegan E, et al. Connectivity-based characterisation of subcortical grey matter pathology in frontotemporal dementia and ALS: a multimodal neuroimaging study. Brain Imaging Behav 2018; 12: 1696–1707. [DOI] [PubMed] [Google Scholar]
- 31. Agosta F, Ferraro PM, Riva N, et al. Structural and functional brain signatures of C9orf72 in motor neuron disease. Neurobiol Aging 2017; 57: 206–219. [DOI] [PubMed] [Google Scholar]
- 32. Branco LMT, de Rezende TJR, Roversi CO, et al. Brain signature of mild stages of cognitive and behavioral impairment in amyotrophic lateral sclerosis Psychiatry. Res Neuroimaging 2018; 272: 58–64. [DOI] [PubMed] [Google Scholar]
- 33. Kim HJ, de Leon M, Wang X, et al. Relationship between clinical parameters and brain structure in sporadic amyotrophic lateral sclerosis patients according to onset type: a voxel-based morphometric study. PLoS One 2017; 12: e0168424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christidi F, Karavasilis E, Ferentinos P, et al. Investigating the neuroanatomical substrate of pathological laughing and crying in amyotrophic lateral sclerosis with multimodal neuroimaging techniques. Amyotroph Lateral Scler Frontotemporal Degener 2018; 19: 12–20. [DOI] [PubMed] [Google Scholar]
- 35. Acosta-Cabronero J, Machts J, Schreiber S, et al. Quantitative susceptibility MRI to detect brain iron in amyotrophic lateral sclerosis. Radiology 2018; 289: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ogura A, Watanabe H, Kawabata K, et al. Semantic deficits in ALS related to right lingual/fusiform gyrus network involvement. Ebiomedicine 2019; 47: 506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trojsi F, Di Nardo F, Siciliano M, et al. Resting state functional MRI brain signatures of fast disease progression in amyotrophic lateral sclerosis: a retrospective study. Amyotroph Lateral Scler Frontotemporal Degener 2020; 4: 1–10. [DOI] [PubMed] [Google Scholar]
- 38. Trojsi F, Di Nardo F, Caiazzo G, et al. Hippocampal connectivity in amyotrophic lateral sclerosis (ALS): more than Papez circuit impairment. Brain Imaging Behav 2021; 15: 2126–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qiu T, Zhang Y, Tang X, et al. Precentral degeneration and cerebellar compensation in amyotrophic lateral sclerosis: a multimodal MRI analysis. Hum Brain Mapp 2019; 40: 3464–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gellersen HM, O’Guo CC, O’Callaghan C, et al. Cerebellar atrophy in neurodegeneration—a meta-analysis. J Neurol Neurosurg Psychiatry 2017; 88: 780–788. [DOI] [PubMed] [Google Scholar]
- 41. Qin Y, Zhang S, Jiang R, et al. Region-specific atrophy of precentral gyrus in patients with amyotrophic lateral sclerosis. J Magn Reson Imaging 2018; 47: 115–122. [DOI] [PubMed] [Google Scholar]
- 42. Consonni M, Contarino VE, Catricalà E, et al. Cortical markers of cognitive syndromes in amyotrophic lateral sclerosis. Neuroimage Clin 2018; 19: 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Contarino VE, Conte G, Morelli C, et al. Toward a marker of upper motor neuron impairment in amyotrophic lateral sclerosis: a fully automatic investigation of the magnetic susceptibility in the precentral cortex. Eur J Radiol 2020; 124: 108815. [DOI] [PubMed] [Google Scholar]
- 44. Bharti K, Khan M, Beaulieu C, et al. Involvement of the dentate nucleus in the pathophysiology of amyotrophic lateral sclerosis: a multi-center and multi-modal neuroimaging study. Neuroimage Clin 2020; 28: 102385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chipika RH, Finegan E, Li Hi, Shing S, et al. “Switchboard” malfunction in motor neuron diseases: selective pathology of thalamic nuclei in amyotrophic lateral sclerosis and primary lateral sclerosis. Neuroimage Clin 2020; 27: 102300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Machts J, Keute M, Kaufmann J, et al. Longitudinal clinical and neuroanatomical correlates of memory impairment in motor neuron disease. Neuroimage Clin 2021; 29: 102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tu S, Menke RAL, Talbot K, et al. Regional thalamic MRI as a marker of widespread cortical pathology and progressive frontotemporal involvement in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2018; 89: 1250–1258. [DOI] [PubMed] [Google Scholar]
- 48. Consonni M, Dalla Bella E, Contarino VE, et al. Cortical thinning trajectories across disease stages and cognitive impairment in amyotrophic lateral sclerosis. Cortex 2020; 131: 284–294. [DOI] [PubMed] [Google Scholar]
- 49. Wirth AM, Khomenko A, Baldaranov D, et al. Combinatory biomarker use of cortical thickness, MUNIX, and ALSFRS-R at baseline and in longitudinal courses of individual patients with amyotrophic lateral sclerosis. Front Neurol 2018; 9: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chipika RH, Christidi F, Finegan E, et al. Amygdala pathology in amyotrophic lateral sclerosis and primary lateral sclerosis. J Neurol Sci 2020; 417: 117039. [DOI] [PubMed] [Google Scholar]
- 51. Finegan E, Li Hi, Shing S, Chipika RH, et al. Widespread subcortical grey matter degeneration in primary lateral sclerosis: a multimodal imaging study with genetic profiling. Neuroimage Clin 2019; 24: 102089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jin J, Hu F, Zhang Q, et al. Dominant heterogeneity of upper and lower motor neuron degeneration to motor manifestation of involved region in amyotrophic lateral sclerosis. Sci Rep 2019; 9: 20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Machts J, Vielhaber S, Kollewe K, et al. Global hippocampal volume reductions and local CA1 shape deformations in amyotrophic lateral sclerosis. Front Neurol 2018; 9: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Welton T, Maller JJ, Lebel RM, et al. Diffusion kurtosis and quantitative susceptibility mapping MRI are sensitive to structural abnormalities in amyotrophic lateral sclerosis. Neuroimage Clin 2019; 24: 101953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Senda J, Atsuta N, Watanabe H, et al. Structural MRI correlates of amyotrophic lateral sclerosis progression. J Neurol Neurosurg Psychiatry 2017; 88: 901–907. [DOI] [PubMed] [Google Scholar]
- 56. Placek K, Baer GM, Elman L, et al. UNC13A polymorphism contributes to frontotemporal disease in sporadic amyotrophic lateral sclerosis. Neurobiol Aging 2019; 73: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Steinbach R, Batyrbekova M, Gaur N, et al. Applying the D50 disease progression model to gray and white matter pathology in amyotrophic lateral sclerosis. Neuroimage Clin 2020; 25: 102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chenji S, Ishaque A, Mah D, et al. Neuroanatomical associations of the Edinburgh cognitive and behavioural ALS screen (ECAS). Brain Imaging Behav 2021; 15: 1641–1654. [DOI] [PubMed] [Google Scholar]
- 59. Christidi F, Karavasilis E, Rentzos M, et al. Hippocampal pathology in amyotrophic lateral sclerosis: selective vulnerability of subfields and their associated projections. Neurobiol Aging 2019; 84: 178–188. [DOI] [PubMed] [Google Scholar]
- 60. Omer T, Finegan E, Hutchinson S, et al. Neuroimaging patterns along the ALS-FTD spectrum: a multiparametric imaging study. Amyotroph Lateral Scler Frontotemporal Degener 2017; 18: 611–623. [DOI] [PubMed] [Google Scholar]
- 61. Finegan E, Chipika RH, Li Hi, Shing S, et al. The clinical and radiological profile of primary lateral sclerosis: a population-based study. J Neurol 2019; 266: 2718–2733. [DOI] [PubMed] [Google Scholar]
- 62. Finegan E, Li Hi Shing S, Siah WF, et al. Evolving diagnostic criteria in primary lateral sclerosis: the clinical and radiological basis of “probable PLS.” J Neurol Sci 2020; 417: 117052. [DOI] [PubMed] [Google Scholar]
- 63. Tae WS, Sung JH, Baek SH, et al. Shape analysis of the subcortical nuclei in amyotrophic lateral sclerosis without cognitive impairment. J Clin Neurol 2020; 16: 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bede P, Chipika RH, Finegan E, et al. Brainstem pathology in amyotrophic lateral sclerosis and primary lateral sclerosis: a longitudinal neuroimaging study. Neuroimage Clin 2019; 24: 102054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cheng L, Yuan Y, Tang X, et al. Structural and functional underpinnings of precentral abnormalities in amyotrophic lateral sclerosis. Eur J Neurol 2021; 28: 1528–1536. [DOI] [PubMed] [Google Scholar]
- 66. Finegan E, Shing SLH, Chipika RH, et al. Extra-motor cerebral changes and manifestations in primary lateral sclerosis. Brain Imaging Behav. Epub ahead of print 7 January 2021. DOI: 10.1007/s11682-020-00421-4. [DOI] [PubMed] [Google Scholar]
- 67. Ratti E, Domoto-Reilly K, Caso C, et al. Regional prefrontal cortical atrophy predicts specific cognitive-behavioral symptoms in ALS-FTD. Brain Imaging Behav. Epub ahead of print 15 Feburary 2021. DOI: 10.1007/s11682-021-00456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van der Burgh HK, Westeneng HJ, Walhout R, et al. Multimodal longitudinal study of structural brain involvement in amyotrophic lateral sclerosis. Neurology 2020; 94: e2592–e2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hensiek N, Schreiber F, Wimmer T, et al. Sonographic and 3T-MRI-based evaluation of the tongue in ALS. Neuroimage Clin 2020; 26: 102233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gorges M, Vercruysse P, Müller HP, et al. Hypothalamic atrophy is related to body mass index and age at onset in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2017; 88: 1033–1041. [DOI] [PubMed] [Google Scholar]
- 71. Chen G, Zhou B, Zhu H, et al. White matter volume loss in amyotrophic lateral sclerosis: a meta-analysis of voxel-based morphometry studies. Prog Neuropsychopharmacol Biol Psychiatry 2018; 83: 110–117. [DOI] [PubMed] [Google Scholar]
- 72. Machts J, Cardenas-Blanco A, Acosta-Cabronero J, et al. Prefrontal cortical thickness in motor neuron disease. Neuroimage Clin 2018; 18: 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Baek SH, Park J, Kim YH, et al. Usefulness of diffusion tensor imaging findings as biomarkers for amyotrophic lateral sclerosis. Sci Rep 2020; 10: 5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rajagopalan V, Pioro EP. Differential involvement of corticospinal tract (CST) fibers in UMN-predominant ALS patients with or without CST hyperintensity: a diffusion tensor tractography study. Neuroimage Clin 2017; 14: 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Weidman EK, Schweitzer AD, Niogi SN, et al. Diffusion tensor imaging and quantitative susceptibility mapping as diagnostic tools for motor neuron disorders. Clin Imaging 2019; 53: 6–11. [DOI] [PubMed] [Google Scholar]
- 76. Tu S, Wang C, Menke RAL, et al. Regional callosal integrity and bilaterality of limb weakness in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2020; 21: 396–402. [DOI] [PubMed] [Google Scholar]
- 77. Müller HP, Gorges M, Del Tredici K, et al. The same cortico-efferent tract involvement in progressive bulbar palsy and in “classical” ALS: a tract of interest-based MRI study. Neuroimage Clin 2019; 24: 101979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Borsodi F, Culea V, Langkammer C, et al. Multimodal assessment of white matter tracts in amyotrophic lateral sclerosis. PLoS One 2017; 12: e0178371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kassubek J, Müller HP, Del Tredici K, et al. Imaging the pathoanatomy of amyotrophic lateral sclerosis in vivo: targeting a propagation-based biological marker. J Neurol Neurosurg Psychiatry 2018; 89: 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Müller HP, Gorges M, Kassubek R, et al. Identical patterns of cortico-efferent tract involvement in primary lateral sclerosis and amyotrophic lateral sclerosis: a tract of interest-based MRI study. Neuroimage Clin 2018; 18: 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Christidi F, Karavasilis E, Riederer F, et al. Gray matter and white matter changes in non-demented amyotrophic lateral sclerosis patients with or without cognitive impairment: a combined voxel-based morphometry and tract-based spatial statistics whole-brain analysis. Brain Imaging Behav 2018; 12: 547–563. [DOI] [PubMed] [Google Scholar]
- 82. Steinbach R, Gaur N, Roediger A, et al. Disease aggressiveness signatures of amyotrophic lateral sclerosis in white matter tracts revealed by the D50 disease progression model. Hum Brain Mapp 2021; 42: 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bao Y, Yang L, Chen Y, et al. Radial diffusivity as an imaging biomarker for early diagnosis of non-demented amyotrophic lateral sclerosis. Eur Radiol 2018; 28: 4940–4948. [DOI] [PubMed] [Google Scholar]
- 84. Trojsi F, Caiazzo G, Siciliano M, et al. Microstructural correlates of Edinburgh Cognitive and Behavioural ALS Screen (ECAS) changes in amyotrophic lateral sclerosis. Psychiatry Res Neuroimaging 2019; 288: 67–75. [DOI] [PubMed] [Google Scholar]
- 85. Zhang F, Chen G, He M, et al. Altered white matter microarchitecture in amyotrophic lateral sclerosis: a voxel-based meta-analysis of diffusion tensor imaging. Neuroimage Clin 2018; 19: 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Müller HP, Agosta F, Riva N, et al. Fast progressive lower motor neuron disease is an ALS variant: a two-centre tract of interest-based MRI data analysis. Neuroimage Clin 2017; 17: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Müller HP, Agosta F, Gorges M, et al. Cortico-efferent tract involvement in primary lateral sclerosis and amyotrophic lateral sclerosis: a two-centre tract of interest-based DTI analysis. Neuroimage Clin 2018; 20: 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kalra S, Müller HP, Ishaque A, et al. A prospective harmonized multicenter DTI study of cerebral white matter degeneration in ALS. Neurology 2020; 95: e943–e952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ma X, Lu F, Hu C, et al. Dynamic alterations of spontaneous neural activity in patients with amyotrophic lateral sclerosis. Brain Imaging Behav 2021; 15: 2101–2108. [DOI] [PubMed] [Google Scholar]
- 90. Li W, Zhang J, Zhou C, et al. Abnormal functional connectivity density in amyotrophic lateral sclerosis. Front Aging Neurosci 2018; 10: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Loewe K, Machts J, Kaufmann J, et al. Widespread temporo-occipital lobe dysfunction in amyotrophic lateral sclerosis. Sci Rep 2017; 7: 40252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Chen HJ, Zou ZY, Zhang XH, et al. Dynamic changes in functional network connectivity involving amyotrophic lateral sclerosis and its correlation with disease severity. J Magn Reson Imaging 2021; 54: 239–248. [DOI] [PubMed] [Google Scholar]
- 93. Basaia S, Agosta F, Cividini C, et al. Structural and functional brain connectome in motor neuron diseases: a multicenter MRI study. Neurology 2020; 95: e2552–e2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Meier JM, van der Burgh HK, Nitert AD, et al. Connectome-based propagation model in amyotrophic lateral sclerosis. Ann Neurol 2020; 87: 725–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Serra A, Galdi P, Pesce E, et al. Strong-weak pruning for brain network identification in connectome-wide neuroimaging: application to amyotrophic lateral sclerosis disease stage characterization. Int J Neural Syst 2019; 29: 1950007. [DOI] [PubMed] [Google Scholar]
- 96. Zhang Y, Qiu T, Yuan X, et al. Abnormal topological organization of structural covariance networks in amyotrophic lateral sclerosis. Neuroimage Clin 2019; 21: 101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fortanier E, Grapperon AM, Le Troter A, et al. Structural connectivity alterations in amyotrophic lateral sclerosis: a graph theory based imaging study. Front Neurosci 2019; 13: 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lee JY, Lee YJ, Park DW, et al. Quantitative susceptibility mapping of the motor cortex: a comparison of susceptibility among patients with amyotrophic lateral sclerosis, cerebrovascular disease, and healthy controls. Neuroradiology 2017; 59: 1213–1222. [DOI] [PubMed] [Google Scholar]
- 99. Conte G, Sbaraini S, Morelli C, et al. A susceptibility-weighted imaging qualitative score of the motor cortex may be a useful tool for distinguishing clinical phenotypes in amyotrophic lateral sclerosis. Eur Radiol 2021; 31: 1281–1289. [DOI] [PubMed] [Google Scholar]
- 100. Conte G, Contarino VE, Casale S, et al. Amyotrophic lateral sclerosis phenotypes significantly differ in terms of magnetic susceptibility properties of the precentral cortex. Eur Radiol 2021; 31: 5272–5280. [DOI] [PubMed] [Google Scholar]
- 101. Reischauer C, Gutzeit A, Neuwirth C, et al. In-vivo evaluation of neuronal and glial changes in amyotrophic lateral sclerosis with diffusion tensor spectroscopy. Neuroimage Clin 2018; 20: 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Grapperon AM, Ridley B, Verschueren A, et al. Quantitative brain sodium MRI depicts corticospinal impairment in amyotrophic lateral sclerosis. Radiology 2019; 292: 422–428. [DOI] [PubMed] [Google Scholar]
- 103. Ishaque A, Mah D, Seres P, et al. Evaluating the cerebral correlates of survival in amyotrophic lateral sclerosis. Ann Clin Transl Neurol 2018; 5: 1350–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Müller HP, Dreyhaupt J, Roselli F, et al. Focal alterations of the callosal area III in primary lateral sclerosis: an MRI planimetry and texture analysis. Neuroimage Clin 2020; 26: 102223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wirth AM, Johannesen S, Khomenko A, et al. Value of fluid-attenuated inversion recovery MRI data analyzed by the lesion segmentation toolbox in amyotrophic lateral sclerosis. J Magn Reson Imaging 2019; 50: 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fabes J, Matthews L, Filippini N, et al. Quantitative FLAIR MRI in amyotrophic lateral sclerosis. Acad Radiol 2017; 24: 1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013; 74: 20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kassubek J, Müller HP, Del Tredici K, et al. Diffusion tensor imaging analysis of sequential spreading of disease in amyotrophic lateral sclerosis confirms patterns of TDP-43 pathology. Brain 2014; 137: 1733–1740. [DOI] [PubMed] [Google Scholar]
- 109. Broad RJ, Gabel MC, Dowell NG, et al. Neurite orientation and dispersion density imaging (NODDI) detects cortical and corticospinal tract degeneration in ALS. J Neurol Neurosurg Psychiatry 2019; 90: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hübers A, Böckler B, Abaei A, et al. Functional and structural impairment of transcallosal motor fibres in ALS: a study using transcranial magnetic stimulation, diffusion tensor imaging, and diffusion weighted spectroscopy. Brain Imaging Behav 2021; 15: 748–757. [DOI] [PubMed] [Google Scholar]
- 111. Sugiyama A, Sato N, Kimura Y, et al. Exploring the frequency and clinical background of the “zebra sign” in amyotrophic lateral sclerosis and multiple system atrophy. J Neurol Sci 2019; 401: 90–94. [DOI] [PubMed] [Google Scholar]
- 112. Kakeda S, Yoneda T, Ide S, et al. Zebra sign of precentral gyri in amyotrophic lateral sclerosis: a novel finding using phase difference enhanced (PADRE) imaging-initial results. Eur Radiol 2016; 26: 4173–4183. [DOI] [PubMed] [Google Scholar]
- 113. Braak H, Brettschneider J, Ludolph AC, et al. Amyotrophic lateral sclerosis–a model of corticofugal axonal spread. Nat Rev Neurol 2013; 9: 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Alzheimer’s disease. Adv Anat Embryol Cell Biol 2015; 215: 1–162. [PubMed] [Google Scholar]
- 115. Schulthess I, Gorges M, Müller HP, et al. Functional connectivity changes resemble patterns of pTDP-43 pathology in amyotrophic lateral sclerosis. Sci Rep 2016; 86: 38391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bayer D, Antonucci S, Müller HP, et al. Disruption of orbitofrontal-hypothalamic projections in a murine ALS model and in human patients. Transl Neurodegener 2021; 10: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Turner MR, Grosskreutz J, Kassubek J, et al. First Neuroimaging Symposium in ALS (NISALS). Towards a neuroimaging biomarker for amyotrophic lateral sclerosis. Lancet Neurol 2011; 10: 400–403. [DOI] [PubMed] [Google Scholar]
- 118. Filippi M, Agosta F, Grosskreutz J, et al. Neuroimaging Society in ALS (NiSALS). Progress towards a neuroimaging biomarker for amyotrophic lateral sclerosis. Lancet Neurol 2015; 14: 786–788. [DOI] [PubMed] [Google Scholar]
- 119. Querin G, El Mendili MM, Bede P, et al. Multimodal spinal cord MRI offers accurate diagnostic classification in ALS. J Neurol Neurosurg Psychiatry 2018; 89: 1220–1221. [DOI] [PubMed] [Google Scholar]