Abstract
Non-coding RNAs are emerging as critical molecules in the genesis, progression, and therapy resistance of cutaneous melanoma. This includes circular RNAs (circRNAs), a class of non-coding RNAs with distinct characteristics that forms through non-canonical back-splicing. In this review, we summarize the features and functions of circRNAs and introduce the current knowledge of the roles of circRNA in melanoma. We also highlight the various mechanisms of action of the well-studied circRNA CDR1as and how it acts as a melanoma tumor suppressor. We further discuss the utility of circRNAs as biomarkers, therapeutic targets, and therapeutic agents in melanoma and outline challenges that must be overcome to comprehensively characterize circRNA functions.
Keywords: circRNA, melanoma, CDR1as, ceRNA, miRNA, splicing
Melanoma
Melanoma of the skin, a cancer that arises from pigment-producing melanocytes, is highly invasive and metastasizes at an early clinical stage. While patients with localized melanoma are usually cured by surgical removal of the tumor, the 5-year survival rate of patients with metastatic melanoma is only 25%. Two distinguishing features of melanoma, a high mutational burden1 and near universal hyperactivation of the MAPK pathway2,3, prompted the development of immune checkpoint inhibitors and targeted therapies, respectively. While these treatments have improved the prognosis of malignant melanoma, inherent and acquired resistance continue to present significant clinical challenges. Accordingly, considerable effort has been invested into better understanding the alterations underlying melanoma progression and metastasis to identify and devise new treatment strategies. Pathologic somatic mutations in protein-coding genes have been extensively catalogued and primarily affect a limited number of pathways or processes: the MAPK pathway, the PI3K/AKT pathway, cell cycle regulation, the p53 pathway, and telomerase activity2–5. Interestingly, many of these mutations occur at an early clinical stage – for instance, over 80% of benign nevi already harbor a mutation in BRAF6 – and somatic mutations appear to not be drivers of metastatic progression3,7,8. Recent studies revealed a complex array of non-genetic alterations in melanoma progression, including genomic abnormalities8, epigenetic regulation of gene expression3, and transcriptional regulation9. Additionally, deregulation of non-coding RNAs (ncRNA) is emerging as a critical driver of melanoma progression10,11. ncRNAs are RNA molecules that do not encode proteins and that come in many flavors, from transfer RNA (tRNAs) and ribosomal RNAs (rRNAs) to microRNAs (miRNAs) and long-noncoding RNAs (lncRNAs). ncRNAs have important functions in most, if not all, biological processes and aberrant ncRNA expression or function contributes to the pathophysiology of various diseases. An example of a pro-tumorigenic ncRNA is SAMMSON, a lncRNA that neighbors the MITF melanocyte lineage gene. Copy number gains of the MITF locus and/or SAMMSON overexpression result in detectable levels of this lncRNA in 90% of human melanomas. SAMMSON promotes an aberrant p32/CARF protein complex, thereby enhancing nuclear and mitochondrial rRNA biogenesis and protein synthesis12,13. Furthermore, our group recently demonstrated in a melanoma mouse model that inactivation of the tumor suppressive miR-29 family accelerates tumor development, potentially through derepression of its targets MAFG and MYBL214.
circular RNAs (circRNAs) are a fascinating class of non-coding RNAs that has recently garnered attention in the RNA and cancer research fields. Their unique features and versatile functions make them attractive molecules to study from a basic biology perspective. While we are only beginning to understand the roles of circRNAs in cancer, we envision circRNAs as biomarkers, therapeutic targets, and maybe even therapeutic agents. In this review, we introduce the features and functions of circRNAs and discuss circRNAs that have been discovered to participate in melanomagenesis.
circRNA structure and biogenesis
circRNAs are characterized by a covalently closed circular structure, lacking 5’ and 3’ ends, a polyadenylated tail, and a 5’ CAP. circRNAs originate through a process termed back-splicing from mRNA precursors (pre-mRNA) that are otherwise processed to form mature linear mRNAs15,16. In contrast to canonical splicing where the 3’ end of an upstream exon joins the 5’ end of a downstream exon, back-splicing leads to the formation of a covalent bond between the 3’ end of a downstream exon and the 5’ end of an upstream exon. This results in a circular RNA fragment (i.e., the circRNA) and a linear RNA transcript lacking the circularized exons. It is hypothesized that back-splicing and canonical splicing occur simultaneously; however, it is not yet clear under which conditions canonical splicing or back-splicing is preferred. These two processes may happen either stochastically or even synergistically15,17.
circRNAs are exceptionally stable because their circular structure renders circRNAs more resistant to destabilization by miRNAs and exonucleases15,16. circRNAs accumulate due to their long half-lives and may even become the dominantly expressed isoforms. However, despite their stability, most circRNAs are less abundant than their corresponding linear mRNAs. This is probably because circRNAs are less efficiently generated than canonically spliced linear mRNAs15,16. Efficient back-splicing requires cis and trans factors to facilitate this process. Repeated Alu elements or complementary sequences located in the introns flanking the circularizing exons enhance the interaction of these introns, thereby promoting back-splicing. Trans factors typically are RNA binding proteins (RBP) that influence back-splicing by promoting the interaction of flanking introns or by regulating alternative splicing. Several RBPs that promote circRNA formation have been described, for instance MBNL118, QKI19, and FUS20. Moreover, A-to-I RNA editing by ADAR1 can impair the pairing of intronic repeats and thus reduce back-splicing21. ADAR1 is downregulated in metastatic melanoma22; however, whether a reduction in ADAR1-mediated RNA editing promotes melanoma via enhanced formation of circRNAs is unknown. In addition, the speed of RNA polymerase II-dependent transcription as well as the availability of spliceosome factors influences whether circular or linear splicing occurs18,23. It is therefore evident that circRNAs are not the product of splicing mistakes and that their formation is tightly regulated.
Roles of circRNAs in physiological and non-physiological processes
circRNAs were initially believed to be “junk” RNA or splicing artefacts and only the development of sophisticated genome and RNA sequencing approaches validated RNA circularization as a widespread physiological phenomenon. Indeed, circRNAs are evolutionarily conserved and display cell type- and differentiation state-specific expression patterns24–33. This strongly indicates that circRNAs must serve important functions, and diverse mechanisms underlying circRNA biology have already been identified. In this section, we describe the most commonly observed circRNA functions in physiology and cancer development.
circRNAs as natural miRNA sponges
Most studies describe circRNAs as natural miRNA sponges that bind to miRNAs to alter their activity, availability, or turnover. Several studies in 2013 sparked interest in this particular mechanism when they reported the existence of circRNAs that are peppered with miRNA binding sites. Specifically, the antisense transcript of Cerebellar Degeneration-Related Protein 1 (CDR1as), also called ciRS-7 (circular RNA sponge for miR-7) was reported to harbor over 70 binding sites for miR-726,34, while circSRY, a single-exon circRNA generated from the Sex-determining Region Y (SRY) gene contains 16 binding sites for miR-13834,35. The identification of these circRNAs prompted the hypothesis that circRNAs primarily act as competitive endogenous RNAs (ceRNA). The ceRNA hypothesis postulates that RNA molecules that share binding sites for the same miRNAs effectively compete for binding to these miRNAs. This posttranscriptional gene regulation mechanism is thus based on the sequestration of miRNAs, leading to impaired target mRNA repression. Cross-talk between ceRNAs through shared miRNAs represents a novel layer of gene regulation that plays important roles in physiological processes and diseases such as cancer36,37. circRNAs harboring multiple binding sites for the same miRNA could therefore represent very potent ceRNAs because they are resistant to miRNA-mediated degradation. However, their relatively low abundance and the lack of miRNA binding site enrichment argue against potent ceRNA activity for most circRNAs24. Nevertheless, given the appropriate circRNA:miRNA stoichiometry, deregulation of a circRNA harboring a single miRNA binding site could elicit biologically meaningful phenotypes. Indeed, most circRNAs with roles in melanoma are described as miRNA sponges (see below).
circRNA functions through protein binding
In addition to binding miRNAs, circRNAs interact with proteins to elicit diverse effects that largely depend on the function of the interacting proteins. For instance, circRNAs may promote or attenuate the formation of protein complexes, with circ-Foxo3 being an interesting example. This circRNA promotes the interaction of MDM2 and p53, thereby enhancing p53 ubiquitination by MDM2 and p53 degradation. circ-Foxo3 simultaneously increases the levels of the Foxo3 protein encoded by its cognate linear mRNA by impairing the interaction of MDM2 with Foxo3. Through these interactions, circ-Foxo3 has been implicated in promoting apoptosis in breast cancer cells38. In addition, circ-Foxo3 also promotes complex formation between CDK2 and p21, thereby impairing the interaction of CDK2 with CyclinA/E and preventing cell cycle progression39. Two circRNAs have been found to affect mRNA translation through their interactions with proteins. circANRIL binds to the 60S pre-ribosomal assembly factor PES1 to diminish pre-rRNA processing and ribosome biogenesis, resulting in reduced overall protein synthesis40. Additionally, circPABPN1 sequesters and inhibits HuR, an RNA-binding protein that promotes translation of mRNAs41. circRNAs can also work as protein shuttles. circ-Amotl1 binds c-Myc to stimulate its translocation to the nucleus, which prevents c-Myc degradation and induces multiple c-Myc target genes42. Curiously, circ-Amotl1 also binds to Stat3 to promote nuclear translocation and transcriptional activity43, indicating a common circRNA-dependent mechanism of c-Myc and Stat3 activation.
circRNA functions through transcription and splicing regulation
In addition to these shuttling circRNAs, some circRNAs are predominantly located in the nucleus where they can regulate gene transcription and mRNA splicing. Intron-retaining circRNAs, such as circEIF3J and circPAIP2, were shown to bind the U1 snRNPs (small nuclear ribonucleoproteins U1) through RNA:RNA interaction of the 5’ splice site of the retained intron and the U1 snRNA. This circRNA-U1 complex then interacts with RNA Polymerase II at the parental EIF3J and PAIP2 gene promoters, thereby enhancing transcription in cis44. Another example of a circRNA affecting RNA Polymerase II activity is the intronic circRNA ci-ANKRD52. This interaction promotes transcription elongation and thus increased expression of the parental ANKRD52 gene45. It is not yet clear how these circRNAs specifically regulate the transcription of their parental genes, but one possibility is that such circRNAs accumulate at their site of production. circRNAs can also affect splicing of linear pre-mRNAs. Splice sites likely compete during linear and circular splicing of a pre-mRNA molecule such that circRNAs are produced at the expense of linear mRNA18. This phenomenon is likely influenced by the splicing factors involved. As circularization occurs co-transcriptionally, this effect could be common to most circRNAs independent of their eventual subcellular localization. In addition to this passive process, splicing may be actively instructed by a circRNA binding to its cognate DNA host locus. Here, a circRNA as a single-stranded RNA may hybridize with DNA to form R-loops. R-loops classically form during transcription where DNA strand separation leads to the formation of an RNA:DNA hybrid when the nascent mRNA folds back onto its template. This leads to stalling of RNA Polymerase II or decompaction of nucleosomes, promoting exon skipping. An example of such a circRNA is the SEP3 exon 6 circRNA that can form an R-loop with the SEPALLATA3 gene46. Through this mechanisms, circ-SEP3 influences floral organ development in A. thaliana. However, whether this mechanism occurs only in plants or also in mammals has not been described yet.
circRNA functions through encoded polypeptides
While most circRNAs function through interactions with RNAs, proteins, or chromatin, there are some that defy their non-coding classification and produce proteins or polypeptides. When engineered to contain an internal ribosome entry site (IRES), circRNAs can be translated in vitro and in vivo47. However, until recently the only known naturally translated circRNA was found in Herpes Virus Delta. The past few years have witnessed the identification of a multitude of circRNAs in plants, flies, and mammals that contain ORFs, are associated with ribosomes, and have the potential to be translated48. What enables the natural, CAP-independent translation of these circRNAs is the backsplicing-mediated inclusion of host gene 5’UTRs that possess IRES-like properties. In addition, CAP-independent circRNA translation may also be initiated by a N6-methyladenosine modification49. circRNA-encoded proteins with putative functions in cancer have been identified for circSHPRH50,51, circβ-catenin52, circFBXW753, circPPP1R12A54, and circPINTexon255, and many more are likely going to be discovered. It will be important to not only elucidate the functions of these circRNA-encoded proteins, but also to carefully validate that they are endogenously expressed. Indeed, a protein encoded by circZNF609, a circRNA that promotes myoblast differentiation56, was recently shown to be an artefact of the circRNA overexpression system57.
circRNAs can influence cellular processes through their interactions with other RNAs and proteins, by regulating transcription and splicing, and by encoding proteins. It is conceivable that some circRNAs even have more than just one function. Given that circRNAs exert various functions and that the mode-of-action of a particular circRNA cannot be readily predicted based on its sequence, it will be necessary to empirically determine the function(s) of candidate circRNAs in melanoma as well as other contexts. Similar to many protein-coding linear transcripts, circRNA regulation and activity will be complicated and context-dependent and necessitates careful characterization. The most-studied circRNA, CDR1as, nicely illustrates the complex nature of circRNAs and how their characterization may take unexpected turns with implications also for melanoma.
The tale of CDR1as
CDR1as, also known as ciRS-7, was first identified by the group of Jørgen Kjems as a target of miR-67158 in HEK293 cells. CDR1as is a non-coding circular RNA that was thought to be a single-exon antisense RNA, formed from the antisense transcript of the Cerebellum Degeneration-Related antigen 1 (CDR1) gene. Based on the respective expression levels of CDR1as and CDR1, the circRNA appears to be the dominant transcript encoded by the locus58. Initially, hardly any linear transcript for CDR1as was detected26,58, suggesting a rare phenomenon where virtually all linear RNA molecules are back-spliced to form a circRNA. However, more recent analyses demonstrated that CDR1as arises from a long non-coding RNA, termed LINC00632, which is located upstream of CDR1as59,60. LINC00632 encodes three different transcripts, T1, T2 and T3, of which the T3 isoform may predominantly drive expression of CDR1as60,61. The gene locus of CDR1as lacks Alu elements that would mediate RNA circularization. Instead, CDR1as is flanked by mammalian-wide interspersed repeats (MIRs), long (>200 nt) inverted short interspersed nuclear elements (SINE) that have high complementarity and are conserved in human and mouse62. CDR1as is highly conserved in mammals, highly abundant in the mammalian brain, but absent or expressed at low levels in other tissues and organs26,34,58,63. Interestingly, neural crest-derived melanocytes, which share a common developmental lineage with the brain, express high levels of CDR1as. In fact, RNA sequencing showed that in three out of four human immortalized melanocyte cell lines (Hermes-1, −2, −3a, and −4b) CDR1as was the most abundant circRNA (NM, OV, FAK, unpublished observation). Thus, CDR1as may play a role in melanocyte biology and melanoma development.
Two studies published in Nature in 2013 sparked extensive interest in CDR1as when they reported that CDR1as harbors over 70 conserved binding sites for miR-726,34 (Figure 1). This finding suggested that CDR1as, and possibly circRNAs in general, could be a potent natural miRNA sponge and function as a ceRNA to regulate the expression of miR-7 targets. Many subsequent studies found correlations between the expression of CDR1as and miR-7 target genes in cancer development. miR-7 has primarily been described as a tumor suppressor miRNA, including in melanoma64,65, that represses proto-oncogenes such as EGFR, IGF1R, and PIK3CD66. Hence, putative oncogenic potential was ascribed to CDR1as as a negative regulator of miR-7 activity. CDR1as has indeed been proposed to inhibit miR-7 and activate miR-7 target genes in colorectal cancer67, hepatocellular carcinoma68, and nasopharyngeal carcinoma69.
Figure 1:
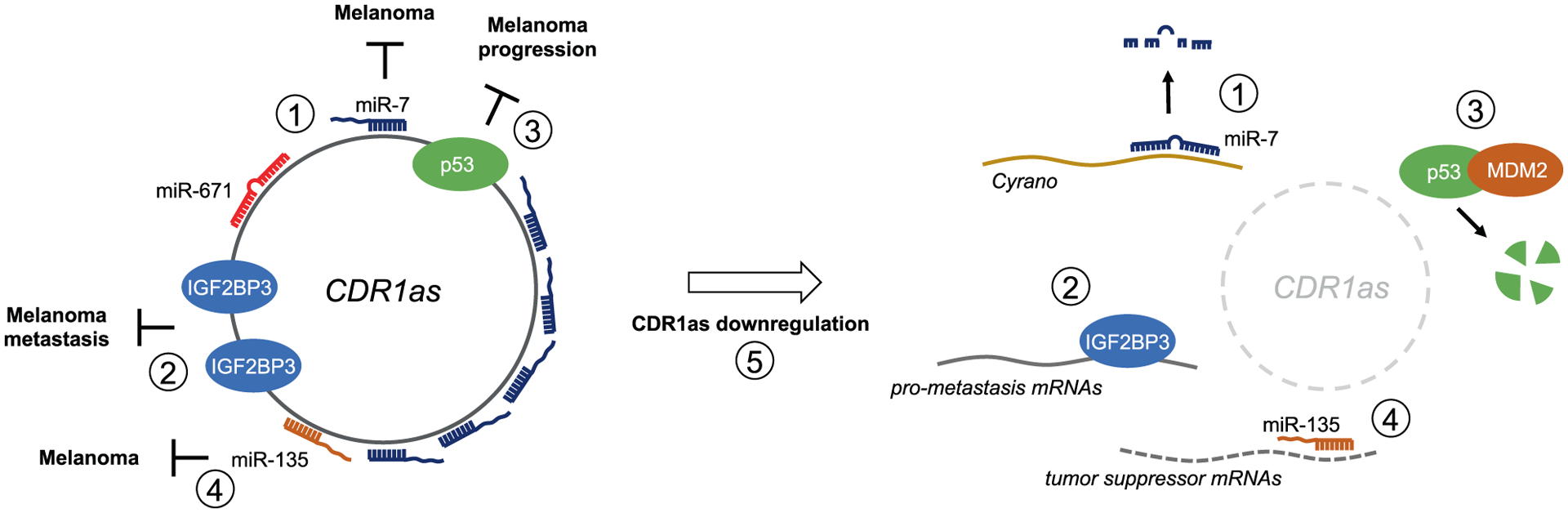
The many functions of CDR1as. (1) CDR1as harbors over 70 binding sites for miR-7, a miRNA with tumor suppressive functions in melanoma. CDR1as may serve as a reservoir, protector, or shuttle vector of miR-7. CDR1as harbors a near-perfect binding site for miR-671. Binding of miR-671 provokes CDR1as slicing, thereby releasing miR-7 or preventing the sponging of free miR-7. Free miR-7 binds to the lncRNA Cyrano via a near-perfect binding site, promoting miR-7 turnover via target RNA-directed microRNA degradation. (2) CDR1as binds the RNA-binding protein IGF2BP3. Downregulation of CDR1as in melanoma enables binding of IGF2BP3 to other mRNA targets, thereby provoking a pro-metastasis expression program. (3) CDR1as binds to p53 to enhance its stability. Downregulation of CDR1as in melanoma may promote p53 turnover, thereby promoting melanoma progression. (4) CDR1as binds to miR-135, which may possess tumor suppressive activity in melanoma. CDR1as may act as a ceRNA to inactivate miR-135. While IGF2BP3 has been experimentally validated as a CDR1as effector in melanoma, the roles of miR-7, miR-135, and p53 downstream of CDR1as need further investigation. (5) CDR1as downregulation in melanoma occurs through epigenetic silencing. Degradation of CDR1as by miR-671 binding may also contribute to its reduced levels in melanoma, but this remains to be experimentally confirmed.
A surprising twist, however, came from a study by Nikolaus Rajewsky and colleagues in 2017. The genetic knockout of CDR1as in mice led to downregulation of miR-7 and a simultaneous upregulation of miR-7 target genes in the brain63. If CDR1as functions as a ceRNA, then there should be a positive correlation between CDR1as and miR-7 targets, and CDR1as depletion would liberate miR-7 and result in repression of miR-7 targets. Further evidence against a ceRNA function of CDR1as was provided when the expression of CDR1as and miR-7 targets was analyzed in several different tumor types61. While CDR1as is expressed in the cancer cells of germinal and neuroendocrine carcinomas as well as melanomas, it is absent in adenocarcinoma cells. In these latter tumors, however, CDR1as is abundant in stromal cells. CDR1as expression is positively correlated with genes enriched in tumor stroma, while a negative correlation was observed for cancer cell-enriched genes. Any correlations observed between CDR1as and miR-7 targets are therefore likely explained by the extent of tumor stroma, arguing against CDR1as being an oncogene that sequesters miR-7 to regulate miR-7 targets.
What, then, is the molecular relationship between CDR1as and miR-7? Another piece of the puzzle came from the identification of Cyrano, a lncRNA harboring a highly conserved segment that contains a near-perfect binding site for miR-7 with a central 2-nucleotide bulge70. CDR1as and Cyrano are the two transcripts that most readily bind miR-7 in human and mouse brain63. However, while CDR1as appears to stabilize miR-763, Cyrano prompts the turnover of miR-7 through a process termed target RNA-directed microRNA degradation (TDMD)71. Thus, CDR1as protects miR-7 from the action of Cyrano rather than sequestering miR-7 to prevent repression of miR-7 targets (Figure 1). Nevertheless, engaging with either CDR1as or Cyrano would attenuate miR-7 activity, although through different mechanisms. This interaction is regulated by miR-671, which targets CDR1as through a near-perfect binding site that triggers AGO-mediated slicing58. miR-671 thus provokes CDR1as turnover and liberates miR-771. It is not yet clear if the role of miR-671 is to acutely degrade CDR1as to initiate bursts of miR-7 target repression or to initiate Cyrano-mediated miR-7 degradation. CDR1as may also serve as a shuttle that delivers the miR-7 cargo to the appropriate subcellular compartment, where miR-7 is released by the action of miR-671. These studies elegantly revealed that CDR1as is part of an elaborate regulatory circuit tightly controlling miR-7 activity, possibly in a spatiotemporal manner. Furthermore, given its role in stabilizing miR-7, CDR1as would be expected to function as a tumor suppressor in contexts where miR-7 has tumor suppressive properties.
In 2020, the group of Eva Hernando reported that CDR1as is downregulated in melanoma compared to melanocytes and demonstrated that CDR1as silencing promotes melanoma progression and metastasis60. Surprisingly, however, CDR1as elicited its effects in a miR-7-independent manner. Rather, CDR1as directly interacted with the RNA-binding protein IGF2BP3. IGF2BP3 targets were enriched among differentially expressed genes upon CDR1as depletion, indicating that CDR1as modulates expression of a pro-metastatic program through the interaction with IGF2BP3 (Figure 1). While this meticulous study revealed a miR-7-independent function for CDR1as, further research may be needed to unequivocally determine the role of miR-7 as a CDR1as effector in melanoma. If miR-7 is a potent melanoma suppressor, then it may be inactivated by CDR1as downregulation or by alternative mechanisms where high CDR1as expression is maintained. In either case, silencing of CDR1as, as performed by Hernando and colleagues, may not further impact miR-7 activity. Would CDR1as silencing in melanocytes, which express high levels of CDR1as, promote transformation in a miR-7-dependent manner? Similarly, would overexpression of CDR1as in CDR1as-low melanoma cells suppress melanoma by stabilizing miR-7? Do Cyrano and miR-671 play a role in suppressing melanoma? Addressing these questions will surely further resolve the mechanisms underlying CDR1as activity in melanoma and other cancers.
CDR1as may interact with other partners in addition to IGF2BP3, miR-7, and miR-671. It was recently discovered in glioma that CDR1as binds to and stabilizes p5372. CDR1as binds to the DNA binding domain of p53 (Figure 1), thereby disrupting MDM2/p53 complex formation and hindering p53 ubiquitination. CDR1as therefore maintains p53 expression and activity and inhibits gliomagenesis. p53 has been recognized as a tumor suppressor in melanoma that is inactivated either directly through mutation or deletion of the p53 gene or indirectly through MDM2 amplification or p14ARF loss-of-function. Downregulation of CDR1as could therefore represent another such indirect mechanisms that promotes the progression of melanoma via the degradation of p53. Additionally, CDR1as may have an anti-oncogenic effect in bladder and ovarian cancer by sponging miR-135a and miR-135b, respectively73,74. miR-135a is upregulated in malignant melanoma, which augments melanoma growth by targeting FOXO175. More work is needed to establish the role of miR-135b in melanoma – both pro-tumorigenic76 or suppressive77 functions have been suggested for miR-135b – but it is possible that CDR1as functions as a miR-135 sponge to oppose melanomagenesis (Figure 1). Further work is therefore needed to assess if the interaction of CDR1as with p53, miR-135a, or miR-135b impacts melanoma formation or biology.
The intriguing tale of CDR1as is sure to continue and other aspects of CDR1as regulation and activity are going to be uncovered. Not only will it be interesting to further elucidate the role of CDR1as in melanoma suppression, but also to identify other melanoma-associated circRNAs. With its numerous miR-7 binding sites and its role in a regulatory RNA circuit, CDR1as may be a unique circRNA. Whether there are other circRNAs that participate in complex interactions and how the expression of such circRNAs influences melanoma development remains to be seen. While not yet as comprehensibly understood as CDR1as, several circRNAs having potential roles in melanoma have been discovered.
Other circRNAs in Melanoma
Apart from CDR1as, the characterization of circRNAs in melanoma has been superficial and is still in its infancy. Additional studies are needed in patient samples and larger panels of cell lines to identify those circRNAs that are frequently deregulated and therefore likely drivers of melanoma formation, progression, and therapy resistance. To date, the most thorough RNA-sequencing study to identify melanoma circRNA identification was performed by Eva Hernando’s group where they compared four melanocyte and ten melanoma short-term cultures60. This revealed thousands of circRNAs, over 600 of which were differentially expressed in melanoma compared to melanocytes. While this study focused on the role of CDR1as in melanoma (see above), we expect that follow-up studies will molecularly and functionally characterize additional differentially expressed circRNAs and reveal their roles in melanoma biology. Another study analyzed circRNA expression by microarray in two melanoma cell lines, WM35 and 451Lu, and compared the expression to normal human melanocytes78. Interestingly, there were only nine differentially expressed circRNAs in common between the two cell lines, suggesting that circRNA expression is heterogeneous and may depend on the underlying genetics or tumor stage. Knock-down experiments in WM35 and 451Lu melanoma cells suggested that five differentially expressed circRNAs may have functions in melanoma biology78. Specifically, while circ_0023988, circ_0008157 and circ_0030388 appear to be tumor suppressive, circ_0000082 and circ_0016418 have oncogenic potential (Table 1, Table 2). Of these five circRNAs, circ_0000082, circ_0023988 and circ_0030388 were predicted to participate in ceRNA networks. However, the ceRNA predictions remain to be validated and further experimental proof in support of their tumor suppressive or oncogenic functions is needed. While not predicted as ceRNA in this study, circ_0016418 is capable of sequestering two miRNAs. Upregulation of circ_0016418 in melanoma increases the expression of GLS and YY1 by sponging miR-605-5p and miR-625, respectively, thereby enhancing melanoma cell proliferation and migration/invasion79,80.
Table 1:
Differentially expressed circRNAs in melanoma with validated or predicted miRNA sponging activity.
| circRNA | Reference | Expression change | Sponged miRNA | miRNA target | Effect |
|---|---|---|---|---|---|
| circ_0084043 | 82 | Up | miR-153-3p | Snail upregulation | Oncogenic |
| circ_0084043 | 81 | Up | miR-429 | TRIB2, β-catenin, c-Myc, cyclinD1 upregulation | Oncogenic |
| circ_0025039 | 83 | Up | miR-198 | CDK4 downregulation | Oncogenic |
| circ_0002770 | 85 | Up | miR-331-3p | DUSP5, TGFBR1 upregulation | Oncogenic |
| circ_0020710 | 86 | Up | miR-370-3p | CXCL12 upregulation | Oncogenic |
| circ_0016418 | 80 | Up | miR-625 | YYI upregulation | Oncogenic |
| circ_0016418 | 79 | Up | miR-605-5p | GLS upregulation | Oncogenic |
| circ-MYC | 89 | Up | miR-1236 | LDHA upregulation | Oncogenic |
| circ-FOXM1 | 84 | Up | miR-143-3p | FLOT2 upregulation | Oncogenic |
| circ_0079593 | 88 | Up | miR-516b | GRM3 upregulation | Oncogenic |
| circ_0001591 | 87 | Up | miR-431-5p | ROCK1, PI3K, AKT upregulation | Oncogenic |
Table 2:
Differentially expressed circRNAs in melanoma with unknown mechanisms or that operate through mechanisms unrelated to miRNA sponging.
| circRNA | Reference | Expression change | Mechanism | Target | Effect |
|---|---|---|---|---|---|
| circ-GLI1 | 90 | Up | Indirect interaction with Cyr61 | Cyr61 and β-catenin upregulation | Oncogenic |
| circ-Ccnb1 | 91 | Down | circCcnb1 translocate Ccnb1-Cdk1 in the nucleus | Ccnb1-Cdk1 complex disruption | Tumor suppressive |
| circ-ITCH | 9 | Down | Unknown | GLUT1 upregulation | Tumor suppressive |
Several other circRNAs that have so far been analyzed in cutaneous melanoma may act as miRNA sponges (Table 1). circ_0084043 is upregulated in melanoma and sponges miR-429 to de-repress TRIB2, resulting in enhanced WNT/β-catenin signaling and increased proliferation, migration, and invasion81. TRIB2 has been proposed as a regulator and transcriptional target of WNT/β -catenin, and the mechanism underlying TRIB2-mediated WNT/β-catenin signaling in melanoma remains to be elucidated. circ_0084043 may also promote melanoma proliferation and migration/invasion by sequestering miR-153-3p to increase the expression of Snail82, a driver of epithelial-to-mesenchymal transition. circ_0025039 is derived from the FOXM1 gene and promotes melanoma proliferation, invasion, and glucose uptake. This is accomplished by sponging miR-198 and miR-143-3p to increase the expression of CDK4 and FLOT283,84. The relative contribution of the different miRNAs and downstream effectors to the oncogenic effects of circ_0084043, circ_0016418, and circ_0025039 is not yet known.
Another five circRNAs, all of which are upregulated in melanoma, function as ceRNAs by sequestering single miRNAs, although additional miRNAs could be involved. These circRNAs generally promote proliferation, migration, and invasion in melanoma cell lines in vitro. circ_0002770 is encoded by the MDM2 gene and sponges miR-331-3p to enhance expression of DUSP5 and TGFBR1, regulators of the MAPK pathway85. circ_0020710 sponges miR-370-3p to increase expression of the chemokine CXCL1286, which is a known activator of the pro-tumorigenic PI3K/AKT and MAPK pathways downstream of the CXCR4 receptor. The PI3K/AKT pathway is also activated by circ_0001591, which sequesters miR-431-5p and to promote ROCK1 expression87. circ_0079593 sponges miR-516b, thereby increasing the expression of the metabotropic glutamate receptor 3 (GRM3)88. Finally, miR-1236 is sequestered by circ-MYC, resulting in derepression of LDHA and increased glycolysis89. Interestingly, none of the miRNAs impacted by the above circRNAs are well-known drivers of melanoma development. The limited information that is available on the function of these miRNAs in melanoma suggests that in most cases downregulated circRNAs interact with oncogenic miRNAs, while upregulated circRNAs interact with tumor suppressive miRNAs. This supports the notion that these circRNAs act as miRNA sponges that sequester and inhibit the function of the respective miRNAs. It further suggests that the elaborate regulatory CDR1as/miR-7 circuit may be unique and that sponging by other circRNAs is more compatible with the ceRNA hypothesis where sequestration inhibits miRNA activity.
In addition to these miRNA sponge circRNAs, a handful of other circRNAs that may not act as ceRNAs have been identified as differentially expressed in melanoma (Table 2). A recent study uncovered an elaborate pathway directed by circ-GLI1, a circRNA derived from the GLI1 gene whose upregulation in melanoma promotes metastasis and angiogenesis90. circ-GLI1 interacts with p70SK62 to facilitate the phosphorylation of GSK3β at Ser9. This results in inactivation of GSK3β and increased activity of the WNT/β-catenin and Hedgehog/GLI1 pathways. This, in turn, promotes MYC-mediated transcription of CYR61, a pro-angiogenic factor with putative oncogenic activity in several cancers90. Overexpression of a circRNA encoded by the CCNB1 gene, circ-Ccnb1, was shown to reduce tumor formation of murine B16 melanoma cells91. circ-Ccnb1 binds to Cyclin B1 and CDK1, thereby preventing formation and nuclear translocation of the Cyclin B1-CDK1 complex and inhibiting mitosis. However, this molecular mechanism was unraveled in breast cancer cells91. It therefore remains unknown whether circ-Ccnb1 affects melanomagenesis through a similar mechanism and if circ-Ccnb1 expression is altered in melanoma. Finally, a circular RNA encoded by the ITCH gene (circ-ITCH) is downregulated in melanoma. This results in enhanced glucose uptake via increased expression of the glucose transporter GLUT192. However, the mechanism underlying circ-ITCH-mediated GLUT1 regulation is not yet known.
Overall, the targets or mechanisms of action of most of circRNAs with putative roles in melanoma are still unknown or only very poorly characterized. Nevertheless, these studies indicate that circRNAs may be involved in mechanisms and pathways necessary for melanoma formation and progression. We believe that as our repertoire of tools and technologies to study circRNAs further expands, we will see a wealth of new studies revealing the functions of circRNAs as drivers of melanomagenesis.
Challenges and future directions
Technical limitations render the characterization of the molecular, biochemical, and cell biological functions of circRNAs challenging. How can circRNAs be analyzed more efficiently in basic, preclinical, and translational studies? One of the biggest hurdles in characterizing circRNAs is the lack of sophisticated overexpression tools, and stable, long-term circRNA overexpression in cells is not trivial. Indeed, back-splicing is not the favored process in cells, many cis and trans factors must contribute, and recapitulating all the splicing steps to ensure the production of a circular RNA without abundant unspliced linear transcript is difficult. circRNAs may be overexpressed using lentiviral constructs containing the human ZKSCAN1 introns or the D. melanogaster Laccase2 introns to mediate the circularization93. However, are these the most appropriate and effective introns to use for circRNA expression in any cell type or in cells from other species such as the mouse? Moreover, potent introns will promote circularization of the viral transcript in virus producing cells, thus leading to the formation of empty viral particles or virus containing a transcript lacking the circRNA94. Mammalian expression plasmids are a useful alternative but do not enable long-term ectopic expression. Our lab has developed a transposon-mediated delivery approach for stable circRNA overexpression in cells in vitro94. In addition, ectopic expression of candidate oncogenic circRNAs in genetically engineered mouse models (GEMMs) has not been reported. Using a split-GFP reporter construct, we showed that some of the circRNA expression tools could be used as ubiquitous and tissue-specific circRNA expression cassettes in melanoma GEMMs94. We anticipate that a further refinement of these in vitro and in vivo expression tools will enable the characterization of candidate driver circRNA functions in melanoma and other cancers.
Similarly, circRNA depletion studies, especially in an in vivo setting, are equally challenging. The CDR1as knockout mouse is so far the only mouse model in which a circRNA was genetically disrupted63. Importantly, however, the deletion of a circRNA exon would in most cases also affect the cognate linear transcript and the protein produced from it. The CDR1as knockout seemed straightforward as hardly any linear CDR1as transcript is detectable. However, the demonstration of CDR1as being embedded in LINC00632 begs the question whether the linear lncRNA is affected by the deletion of the CDR1as exon, thereby contributing to the observed phenotype. An alternative method of depleting circRNAs is by short hairpin RNA (shRNA) targeting the back-splicing junction. However, shRNAs may have off-target effects and a recent study showed discrepancies between circRNA knockdown efficiency and the biological effect on cell proliferation95. The CRISPR/Cas13d system may be a promising alternative to specifically silence circRNAs without affecting the cognate linear RNAs95. Indeed, this system employs arrayed and longer sgRNA against the backsplice junction of a circRNA. As Cas13d can be engineered to harbor a Nuclear Localization Sequences (NLS), nuclear circRNA depletion is possible with CRISPR/Cas13d unlike with shRNAs95. The optimization of these tools and the development of additional expression systems and mouse models will accelerate the characterization of circRNA functions in melanoma and other diseases.
The poor clinical prognosis of melanoma is mainly due its propensity to metastasize and the development of inherent or acquired resistance to current treatments. Both targeted and immune therapies have been FDA-approved in the last decade and are the current standard of care for melanoma. Only a subset of patients exhibits long-term responses to immune checkpoint inhibitor therapy and resistance mechanisms are beginning to emerge. Conversely, small molecule inhibitors, typically targeting the MAPK pathway, lead to remarkable but transient responses and tumors invariably recur. Therefore, novel treatment modalities are needed to further improve treatment outcomes for metastatic melanoma patients. The biology and pathobiology of circRNAs, which we summarized in this review, highlights the potential of circRNAs to be explored as diagnostic and prognostic biomarkers, potential therapeutic agents, and therapeutic targets.
One possible application of circRNAs in cancer is their use as liquid biopsy biomarkers. circRNAs are secreted via exosomes and found in the blood stream96. While the purpose for the release of circRNAs in extracellular vesicles is not entirely clear, secretion could be a mechanism of circRNA clearance from the cell97. Another possibility is that circRNAs may be involved in cell communication through exosomes. In such a scenario, the circRNA itself may be the regulatory molecule transferred from one cell to another. Alternatively, the circRNA may merely act as the shuttling vector that carries bound miRNAs or RBPs to a target cell. The utility of circRNAs as biomarkers for melanoma has so far not been explored and future studies are thus needed to identify and quantify exosomal circRNAs in the blood from melanoma patients and healthy individuals to determine their potential use as biomarkers.
Additionally, given the involvement of circRNAs in pathways and mechanisms underlying tumor development, could circRNAs be exploited as treatment targets or therapeutic agents? Nanoparticles have recently been used to deliver therapeutic non-coding RNAs such as siRNAs against c-Myc98 or the anti-tumorigenic miRNAs miR-204-5p and miR-199b-5p99 to inhibit melanoma cell growth. Thus, melanoma-targeted nanoparticles could be used to silence oncogenic circRNAs with RNAi approaches. Alternatively, tumor suppressive circRNAs, such as CDR1as, could serve as therapeutic agents and be delivered specifically to melanoma cells with nanoparticles to induce tumor suppression with limited off-target effects. Furthermore, a better understanding of the molecular pathways regulated by melanoma-associated circRNAs is likely to identify additional targets for therapy with small molecule inhibitors. However, additional oncogenic and tumor suppressive circRNAs remain to be identified and functionally characterized before therapeutic strategies can be explored. Even CDR1as, which has convincingly been shown to be a tumor suppressor in melanoma, requires further mechanistic insights to validate its potential as a therapeutic agent. circRNAs have come a long way since they were considered an experimental artefact, but much remains to be learned about this class of ncRNAs.
Acknowledgements:
This work was supported by grants from the NCI/NIH (R01 CA259046) and the Harry J. Lloyd Charitable Foundation to F.A.K.
Footnotes
Competing Interests: The authors have no competing financial interests to declare.
References
- 1.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SAJR, Behjati S, Biankin AV et al. Signatures of mutational processes in human cancer. Nature 2013; 500: 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017; 545: 175–180. [DOI] [PubMed] [Google Scholar]
- 3.Shain AH, Joseph NM, Yu R, Benhamida J, Liu S, Prow T et al. Genomic and Transcriptomic Analysis Reveals Incremental Disruption of Key Signaling Pathways during Melanoma Evolution. Cancer cell 2018; 34: 45–55.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A et al. The Genetic Evolution of Melanoma from Precursor Lesions. New Engl J Medicine 2015; 373: 1926–1936. [DOI] [PubMed] [Google Scholar]
- 5.The CGAN. Genomic Classification of Cutaneous Melanoma. Cell 2015; 161: 1681–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM et al. High frequency of BRAF mutations in nevi. Nature genetics 2003; 33: 19–20. [DOI] [PubMed] [Google Scholar]
- 7.Turner N, Ware O, Bosenberg M. Genetics of metastasis: melanoma and other cancers. Clinical & experimental metastasis 2018; 35: 379–391. [DOI] [PubMed] [Google Scholar]
- 8.Vergara IA, Mintoff CP, Sandhu S, McIntosh L, Young RJ, Wong SQ et al. Evolution of late-stage metastatic melanoma is dominated by aneuploidy and whole genome doubling. Nat Commun 2021; 12: 1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rambow F, Marine J-C, Goding CR. Melanoma plasticity and phenotypic diversity: therapeutic barriers and opportunities. Gene Dev 2019; 33: 1295–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkar D, Leung EY, Baguley BC, Finlay GJ, Askarian-Amiri ME. Epigenetic regulation in human melanoma: past and future. Epigenetics 2015; 10: 103–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vera O, Jasani N, Karreth FA. Long Non-Coding RNAs in Melanoma Development and Biology. Proc Singap National Acad Sci 2020; 14: 145–166. [Google Scholar]
- 12.Leucci E, Vendramin R, Spinazzi M, Laurette P, Fiers M, Wouters J et al. Melanoma addiction to the long non-coding RNA SAMMSON. Nature 2016; 531: 518–522. [DOI] [PubMed] [Google Scholar]
- 13.Vendramin R, Verheyden Y, Ishikawa H, Goedert L, Nicolas E, Saraf K et al. SAMMSON fosters cancer cell fitness by concertedly enhancing mitochondrial and cytosolic translation. Nat Struct Mol Biol 2018; 25: 1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vera O, Bok I, Jasani N, Nakamura K, Xu X, Mecozzi N et al. A MAPK/miR-29 Axis Suppresses Melanoma by Targeting MAFG and MYBL2. Cancers 2021; 13: 1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L-L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Bio 2020; 21: 475–490. [DOI] [PubMed] [Google Scholar]
- 16.Xiao M-S, Ai Y, Wilusz JE. Biogenesis and Functions of Circular RNAs Come into Focus. Trends Cell Biol 2020; 30: 226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L-L, Yang L. Regulation of circRNA biogenesis. Rna Biol 2015; 12: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M et al. circRNA biogenesis competes with pre-mRNA splicing. Molecular cell 2014; 56: 55–66. [DOI] [PubMed] [Google Scholar]
- 19.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA et al. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015; 160: 1125–1134. [DOI] [PubMed] [Google Scholar]
- 20.Errichelli L, Modigliani SD, Laneve P, Colantoni A, Legnini I, Capauto D et al. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat Commun 2017; 8: 14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR et al. Analysis of Intron Sequences Reveals Hallmarks of Circular RNA Biogenesis in Animals. Cell Reports 2015; 10: 170–177. [DOI] [PubMed] [Google Scholar]
- 22.Nemlich Y, Greenberg E, Ortenberg R, Besser MJ, Barshack I, Jacob-Hirsch J et al. MicroRNA-mediated loss of ADAR1 in metastatic melanoma promotes tumor growth. J Clin Invest 2013; 123: 2703–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang D, Tatomer DC, Luo Z, Wu H, Yang L, Chen L-L et al. The Output of Protein-Coding Genes Shifts to Circular RNAs When the Pre-mRNA Processing Machinery Is Limiting. Mol Cell 2017; 68: 940–954.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome biology 2014; 15: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA (New York, NY) 2013; 19: 141–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013; 495: 333–338. [DOI] [PubMed] [Google Scholar]
- 27.Rybak-Wolf A, Stottmeister C, Glažar P, Jens M, Pino N, Giusti S et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Molecular cell 2015; 58: 870–885. [DOI] [PubMed] [Google Scholar]
- 28.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PloS one 2012; 7: e30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salzman J, Chen RE, Olsen MN, Wang PL, Brown PO. Cell-type specific features of circular RNA expression. PLoS genetics 2013; 9: e1003777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo L, Morey R, Palpant NJ, Wang PL, Afari N, Jiang C et al. Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development. Genome biology 2015; 16: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venø MT, Hansen TB, Venø ST, Clausen BH, Grebing M, Finsen B et al. Spatio-temporal regulation of circular RNA expression during porcine embryonic brain development. Genome biology 2015; 16: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westholm JO, Miura P, Olson S, Shenker S, Joseph B, Sanfilippo P et al. Genome-wide analysis of drosophila circular RNAs reveals their structural and sequence properties and age-dependent neural accumulation. Cell reports 2014; 9: 1966–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nature neuroscience 2015; 18: 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK et al. Natural RNA circles function as efficient microRNA sponges. Nature 2013; 495: 384–388. [DOI] [PubMed] [Google Scholar]
- 35.Capel B, Swain A, Nicolis S, Hacker A, Walter M, Koopman P et al. Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 1993; 73: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 36.Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 2011; 146: 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karreth FA, Pandolfi PP. ceRNA cross-talk in cancer: when ce-bling rivalries go awry. Cancer discovery 2013; 3: 1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du WW, Fang L, Yang W, Wu N, Awan FM, Yang Z et al. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell death and differentiation 2017; 24: 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic acids research 2016; 44: 2846–2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holdt LM, Stahringer A, Sass K, Pichler G, Kulak NA, Wilfert W et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat Commun 2016; 7: 12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdelmohsen K, Panda AC, Munk R, Grammatikakis I, Dudekula DB, De S et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. Rna Biol 2017; 14: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Q, Du WW, Wu N, Yang W, Awan FM, Fang L et al. A circular RNA promotes tumorigenesis by inducing c-myc nuclear translocation. Cell death and differentiation 2017; 24: 1609–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Z-G, Awan FM, Du WW, Zeng Y, Lyu J, Wu D et al. The Circular RNA Interacts with STAT3, Increasing Its Nuclear Translocation and Wound Repair by Modulating Dnmt3a and miR-17 Function. Molecular therapy : the journal of the American Society of Gene Therapy 2017; 25: 2062–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z, Huang C, Bao C, Chen L, Lin M, Wang X et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol 2015; 22: 256–264. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Zhang X-O, Chen T, Xiang J-F, Yin Q-F, Xing Y-H et al. Circular Intronic Long Noncoding RNAs. Mol Cell 2013; 51: 792–806. [DOI] [PubMed] [Google Scholar]
- 46.Conn VM, Hugouvieux V, Nayak A, Conos SA, Capovilla G, Cildir G et al. A circRNA from SEPALLATA3 regulates splicing of its cognate mRNA through R-loop formation. Nat Plants 2017; 3: 17053. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. Rna 2015; 21: 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L et al. Translation of CircRNAs. Molecular cell 2017; 66: 9–21.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res 2017; 27: 626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Begum S, Yiu A, Stebbing J, Castellano L. Novel tumour suppressive protein encoded by circular RNA, circ-SHPRH, in glioblastomas. Oncogene 2018; 37: 4055–4057. [DOI] [PubMed] [Google Scholar]
- 51.Zhang M, Huang N, Yang X, Luo J, Yan S, Xiao F et al. A novel protein encoded by the circular form of the SHPRH gene suppresses glioma tumorigenesis. Oncogene 2018; 37: 1805–1814. [DOI] [PubMed] [Google Scholar]
- 52.Liang W-C, Wong C-W, Liang P-P, Shi M, Cao Y, Rao S-T et al. Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway. Genome Biol 2019; 20: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao F et al. Novel Role of FBXW7 Circular RNA in Repressing Glioma Tumorigenesis. Jnci J National Cancer Inst 2017; 110: 304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng X, Chen L, Zhou Y, Wang Q, Zheng Z, Xu B et al. A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer 2019; 18: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M, Zhao K, Xu X, Yang Y, Yan S, Wei P et al. A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun 2018; 9: 4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Legnini I, Timoteo GD, Rossi F, Morlando M, Briganti F, Sthandier O et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Molecular cell 2017; 66: 22–37.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho-Xuan H, Glažar P, Latini C, Heizler K, Haase J, Hett R et al. Comprehensive analysis of translation from overexpressed circular RNAs reveals pervasive translation from linear transcripts. Nucleic Acids Res 2020; 48: gkaa704-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hansen TB, Wiklund ED, Bramsen JB, Villadsen SB, Statham AL, Clark SJ et al. miRNA‐dependent gene silencing involving Ago2‐mediated cleavage of a circular antisense RNA. Embo J 2011; 30: 4414–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barrett SP, Parker KR, Horn C, Mata M, Salzman J. ciRS-7 exonic sequence is embedded in a long non-coding RNA locus. Plos Genet 2017; 13: e1007114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanniford D, Ulloa-Morales A, Karz A, Berzoti-Coelho MG, Moubarak RS, Sánchez-Sendra B et al. Epigenetic Silencing of CDR1as Drives IGF2BP3-Mediated Melanoma Invasion and Metastasis. Cancer cell 2020; 37: 55–70.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristensen LS, Ebbesen KK, Sokol M, Jakobsen T, Korsgaard U, Eriksen AC et al. Spatial expression analyses of the putative oncogene ciRS-7 in cancer reshape the microRNA sponge theory. Nat Commun 2020; 11: 4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshimoto R, Rahimi K, Hansen TB, Kjems J, Mayeda A. Biosynthesis of Circular RNA ciRS-7/CDR1as Is Mediated by Mammalian-wide Interspersed Repeats. Iscience 2020; 23: 101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piwecka M, Glažar P, Hernandez-Miranda LR, Memczak S, Wolf SA, Rybak-Wolf A et al. Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science (New York, NY) 2017; 8: eaam8526. [DOI] [PubMed] [Google Scholar]
- 64.Giles KM, Brown RAM, Ganda C, Podgorny MJ, Candy PA, Wintle LC et al. microRNA-7–5p inhibits melanoma cell proliferation and metastasis by suppressing RelA/NF-κB. Oncotarget 2016; 7: 31663–31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giles KM, Brown RAM, Epis MR, Kalinowski FC, Leedman PJ. miRNA-7–5p inhibits melanoma cell migration and invasion. Biochem Bioph Res Co 2013; 430: 706–710. [DOI] [PubMed] [Google Scholar]
- 66.Horsham JL, Kalinowski FC, Epis MR, Ganda C, Brown RAM, Leedman PJ. Clinical Potential of microRNA-7 in Cancer. J Clin Medicine 2015; 4: 1668–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang W, Ji M, He G, Yang L, Niu Z, Jian M et al. Silencing CDR1as inhibits colorectal cancer progression through regulating microRNA-7. Oncotargets Ther 2017; 10: 2045–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu L, Zhang M, Zheng X, Yi P, Lan C, Xu M. The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. J Cancer Res Clin 2017; 143: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong Q, Huang J, Wei J, Wu R. Circular RNA CDR1as sponges miR-7-5p to enhance E2F3 stability and promote the growth of nasopharyngeal carcinoma. Cancer Cell Int 2019; 19: 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ulitsky I, Shkumatava A, Jan CH, Sive H, Bartel DP. Conserved Function of lincRNAs in Vertebrate Embryonic Development despite Rapid Sequence Evolution. Cell 2012; 151: 684–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kleaveland B, Shi CY, Stefano J, Bartel DP. A Network of Noncoding Regulatory RNAs Acts in the Mammalian Brain. Cell 2018; 174: 350–362.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lou J, Hao Y, Lin K, Lyu Y, Chen M, Wang H et al. Circular RNA CDR1as disrupts the p53/MDM2 complex to inhibit Gliomagenesis. Mol Cancer 2020; 19: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li P, Yang X, Yuan W, Yang C, Zhang X, Han J et al. CircRNA-Cdr1as Exerts Anti-Oncogenic Functions in Bladder Cancer by Sponging MicroRNA-135a. Cell Physiol Biochem 2018; 46: 1606–1616. [DOI] [PubMed] [Google Scholar]
- 74.Chen H, Mao M, Jiang J, Zhu D, Li P. Circular RNA CDR1as acts as a sponge of miR-135b-5p to suppress ovarian cancer progression. Oncotargets Ther 2019; 12: 3869–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ren J-W, Li Z-J, Tu C. MiR-135 post-transcriptionally regulates FOXO1 expression and promotes cell proliferation in human malignant melanoma cells. Int J Clin Exp Patho 2015; 8: 6356–66. [PMC free article] [PubMed] [Google Scholar]
- 76.Hu Y, Wang Q, Zhu X. MiR-135b is a novel oncogenic factor in cutaneous melanoma by targeting LATS2. Melanoma Res 2018; 29: 119–125. [DOI] [PubMed] [Google Scholar]
- 77.Zhang X-H, Xin Z-M. MiR-135b-5p inhibits the progression of malignant melanoma cells by targeting RBX1. Eur Rev Med Pharmaco 2020; 24: 1309–1315. [DOI] [PubMed] [Google Scholar]
- 78.Wang Q, Chen J, Wang A, Sun L, Qian L, Zhou X et al. Differentially expressed circRNAs in melanocytes and melanoma cells and their effect on cell proliferation and invasion. Oncol Rep 2018; 39: 1813–1824. [DOI] [PubMed] [Google Scholar]
- 79.Lu R, Zhang X, Li X, Wan X. Circ_0016418 promotes melanoma development and glutamine catabolism by regulating the miR-605-5p/GLS axis. Int J Clin Exp Patho 2020; 13: 1791–1801. [PMC free article] [PubMed] [Google Scholar]
- 80.Zou Y, Wang S-S, Wang J, Su H-L, Xu J-H. CircRNA_0016418 expedites the progression of human skin melanoma via miR-625/YY1 axis. Eur Rev Med Pharmaco 2019; 23: 10918–10930. [DOI] [PubMed] [Google Scholar]
- 81.Chen Z, Chen J, Wa Q, He M, Wang X, Zhou J et al. Knockdown of circ_0084043 suppresses the development of human melanoma cells through miR-429/tribbles homolog 2 axis and Wnt/β-catenin pathway. Life Sci 2020; 243: 117323. [DOI] [PubMed] [Google Scholar]
- 82.Luan W, Shi Y, Zhou Z, Xia Y, Wang J. circRNA_0084043 promote malignant melanoma progression via miR-153-3p/Snail axis. Biochem Bioph Res Co 2018; 502: 22–29. [DOI] [PubMed] [Google Scholar]
- 83.Bian D, Wu Y, Song G. Novel circular RNA, hsa_circ_0025039 promotes cell growth, invasion and glucose metabolism in malignant melanoma via the miR-198/CDK4 axis. Biomed Pharmacother 2018; 108: 165–176. [DOI] [PubMed] [Google Scholar]
- 84.Tian S, Han G, Lu L, Meng X. Circ-FOXM1 contributes to cell proliferation, invasion, and glycolysis and represses apoptosis in melanoma by regulating miR-143-3p/FLOT2 axis. World J Surg Oncol 2020; 18: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qian P, Linbo L, Xiaomei Z, Hui P. Circ_0002770, acting as a competitive endogenous RNA, promotes proliferation and invasion by targeting miR-331-3p in melanoma. Cell Death Dis 2020; 11: 264. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Wei C-Y, Zhu M-X, Lu N-H, Liu J-Q, Yang Y-W, Zhang Y et al. Circular RNA circ_0020710 drives tumor progression and immune evasion by regulating the miR-370-3p/CXCL12 axis in melanoma. Mol Cancer 2020; 19: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin D, Wei G, Yang F, Sun X. Circular RNA has circ 0001591 promoted cell proliferation and metastasis of human melanoma via ROCK1/PI3K/AKT by targeting miR-431-5p. Hum Exp Toxicol 2021; 40: 310–324. [DOI] [PubMed] [Google Scholar]
- 88.Lu J, Li Y. Circ_0079593 facilitates proliferation, metastasis, glucose metabolism and inhibits apoptosis in melanoma by regulating the miR-516b/GRM3 axis. Mol Cell Biochem 2020; 475: 227–237. [DOI] [PubMed] [Google Scholar]
- 89.Jin C, Dong D, Yang Z, Xia R, Tao S, Piao M. CircMYC Regulates Glycolysis and Cell Proliferation in Melanoma. Cell Biochem Biophys 2020; 78: 77–88. [DOI] [PubMed] [Google Scholar]
- 90.Chen J, Zhou X, Yang J, Sun Q, Liu Y, Li N et al. Circ-GLI1 promotes metastasis in melanoma through interacting with p70S6K2 to activate Hedgehog/GLI1 and Wnt/β-catenin pathways and upregulate Cyr61. Cell Death Dis 2020; 11: 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fang L, Du WW, Awan FM, Dong J, Yang BB. The circular RNA circ-Ccnb1 dissociates Ccnb1/Cdk1 complex suppressing cell invasion and tumorigenesis. Cancer Lett 2019; 459: 216–226. [DOI] [PubMed] [Google Scholar]
- 92.Lin Q, Jiang H, Lin D. Circular RNA ITCH downregulates GLUT1 and suppresses glucose uptake in melanoma to inhibit cancer cell proliferation. J Dermatol Treat 2019; 32: 1–16. [DOI] [PubMed] [Google Scholar]
- 93.Kramer MC, Liang D, Tatomer DC, Gold B, March ZM, Cherry S et al. Combinatorial control of Drosophila circular RNA expression by intronic repeats, hnRNPs, and SR proteins. Genes & development 2015; 29: 2168–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mecozzi N, Nenci A, Vera O, Falzone A, DeNicola GM, Karreth FA. Genetic tools for the stable overexpression of circular RNAs. Biorxiv 2021; : 2021.05.27.446018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Nguyen TM, Zhang X-O, Wang L, Phan T, Clohessy JG et al. Optimized RNA-targeting CRISPR/Cas13d technology outperforms shRNA in identifying functional circRNAs. Genome Biol 2021; 22: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res 2015; 25: 981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lasda E, Parker R. Circular RNAs Co-Precipitate with Extracellular Vesicles: A Possible Mechanism for circRNA Clearance. Plos One 2016; 11: e0148407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen Y, Bathula SR, Yang Q, Huang L. Targeted Nanoparticles Deliver siRNA to Melanoma. J Invest Dermatol 2010; 130: 2790–2798. [DOI] [PubMed] [Google Scholar]
- 99.Fattore L, Campani V, Ruggiero CF, Salvati V, Liguoro D, Scotti L et al. In Vitro Biophysical and Biological Characterization of Lipid Nanoparticles Co-Encapsulating Oncosuppressors miR-199b-5p and miR-204-5p as Potentiators of Target Therapy in Metastatic Melanoma. Int J Mol Sci 2020; 21: 1930. [DOI] [PMC free article] [PubMed] [Google Scholar]


