Abstract
A collection of six commercially available, 3D printer filaments were analyzed with respect to their gasphase emissions, specifically volatile organic compounds (VOCs), during simulated fused filament fabrication (FFF). Filaments were chosen because they were advertised to contain metal particles or carbon nanotubes. During experimentation, some were found to contain other non-advertised additives that greatly influenced gas-phase emissions. Three polylactic acid (PLA) filaments containing either copper, bronze, or stainless steel particles were studied along in addition to three carbon nanotube (CNT) filaments made from PLA, acrylonitrile-butadiene-styrene (ABS), and polycarbonate (PC). The metal-additive PLA filaments were found to emit primarily lactide, acetaldehyde, and 1-chlorododecane. The presence of metal particles in the PLA is a possible cause of the increased total emissions, which were higher than any other PLA filament reported in the literature. In addition, the filament with stainless steel particles had a threefold increase in total VOCs compared to the copper and bronze particles. Two of three CNT-containing filaments emitted compounds that have not been reported before for PLA and PC. A comparison between certain emitted VOCs and their suggested maximum inhalation limits shows that printing as little as 20 g of certain filaments in a small, unventilated room can subject the user to hazardous concentrations of multiple toxic VOCs with carcinogenic properties (e.g., acetaldehyde, 1,4-dioxane, and bis(2-ethylhexyl) phthalate). The use of certain additives, whether advertised or not, should be reevaluated due to their effects on VOC emissions during 3D printing.
Keywords: Additive manufacturing, Indoor air quality, 3D printing, Polymer degradation, Volatile organic compounds, VOCs
1. Introduction
As 3D printers become increasingly utilized in educational, industrial, and household settings, the concern over hazardous emission from polymer filaments is growing (Thomas 2016; Ngo et al., 2018). The temperatures required to print objects from polymer filament are sufficient to thermally degrade the polymers and produce compounds that pose an inhalation risk. Emissions from filaments used in fused filament fabrication (FFF), the most common form of 3D printing, can be organized into two distinct categories: aerosolized particulate matter (PM) and gas-phase compounds. PM from 3D printers has been detected across a wide range of sizes (Stephens et al., 2013; Azimi et al., 2016; Steinle 2016; Stabile et al., 2017; Vance et al., 2017; Zhang et al., 2019), including nano-sized emissions (less than 100 nm) (Kim et al., 2015; Deng et al., 2016; Kwon et al., 2017; Zhang et al., 2017). PM poses a health risk based on both its size and ability to adsorb organic compounds. Studies involving gas-phase compounds typically include volatile organic compounds (VOCs) and semivolatile organic compounds (SVOCs) (Stefaniak et al., 2017; Davis et al., 2019; Ding et al., 2019; Potter et al., 2019). Fully characterizing and quantifying VOC emissions is necessary to properly assess the health risk posed by 3D printer filaments.
Polylactic acid (PLA) and acrylonitrile-butadiene-styrene (ABS) are the primary polymer filament types used in FFF. Other polymers used include polyethylene terephthalate (PET), polycarbonate (PC), high impact polystyrene (HIPS), and polyvinyl acetate (PVA). Filaments often contain additives to adjust their physicochemical properties. Common additives include dyes, plasticizers, stabilizers, wood, metal, and carbon allotropes. Although not strictly defined as “additives,” many filaments contain unreacted monomer or other reactants from the polymerization process. Many of these additives have the ability to degrade into VOCs or influence VOC formation (Potter et al., 2019).
Carbon nanotubes (CNTs) are added to 3D printer filaments to increase their conductivity, and therefore, make printed objects less susceptible to electrostatic discharge. They are also added to confer structural stability to certain objects. We have previously shown that carbon nanotubes have the ability to influence VOC formation in ABS filaments (Potter et al., 2019). CNTs can be added to many polymer types, including: ABS, PLA, and PC. The effects of CNTs on gas-phase emissions from PLA and PC have not been investigated prior to this work.
Metal particles are included in 3D printer filaments for aesthetic and practical purposes. Objects printed with these filaments can have a metallic color, and some can be sintered to remove the polymer matrix and produce an entirely metallic object with structural advantages over a polymer object. Metal-containing filaments typically use PLA as their polymer matrix, although ABS/metal filaments have been sporadically seen on the market. Transition metals, particularly copper and iron, can catalyze the thermal degradation of organic compounds at the temperatures typically found in a 3D printer. Although particulate emissions from metal-containing filaments have been investigated (Vance et al., 2017; Alberts et al., 2021; Stabile et al., 2017), there have been no studies involving VOC emissions from metal-containing filaments.
In our previous work, we found that not all emitted compounds can be traced directly to degradation of the polymer backbone. Some VOCs result from the volatilization or degradation of polymer additives such as plasticizers and stabilizers (Potter et al., 2019). The presence of non-advertised additives in other polymer filaments is highly likely and their effect on VOC emissions is almost entirely unknown and unexplored.
Six filaments were selected based on their advertised ingredients: stainless steel particles, bronze particles, copper particles, and carbon nanotubes. Some metal-containing filaments have been investigated. The original focus of this research was to quantify VOC emissions from unique filament types containing metal particles or carbon nanotubes during simulated 3D printing. During experimentation, additional additives were discovered that greatly influenced VOC emissions. The scope of this project was widened to include all known detected and identified additives in the studied filaments.
2. Materials and methods
The 3D printer filaments were purchased from four separate manufacturers with the three metal-particle filaments being purchased from the same manufacturer. Pure reference standards used for quantification (Sigma-Aldrich Corporation) were dissolved in HPLC-grade dichloromethane (Fisher Scientific International, Inc.) to create calibration curves. Quartz sample baskets were made in the LSU Chemistry Department glass-blowing shop.
The 3D printing process was simulated using the System for Thermal Diagnostic Studies (STDS) (Rubey and Grant, 1988). In a prior publication, we have established that the STDS is capable of simulating 3D printer conditions (Potter et al., 2019). VOC emissions from ABS filament in the STDS exhibited a similar compound profile and relative concentrations compared to studies that sampled directly from 3D printers (Davis et al., 2019). The STDS is a customized, modular instrument that has been previously used to simulate incinerator conditions to study incineration of hazardous waste components (Nganai et al. 2012; Guan et al., 2019). The general composition of the STDS can be seen in Fig. S1 in the Supporting Information. The STDS used in this work consisted of a ceramic furnace and quartz tube reactor placed within a gas chromatograph (GC) oven. A heated transfer line is used to move gas-phase compounds into the injection port of a second GC, where they are separated and detected by a mass spectrometer (MS). The advantages of the STDS include being a closed system with direct transfer of products into the detection module, which minimizes sample loss and increases detection sensitivity. For each experiment, approximately 100 mg of filament was placed in a quartz sample holder (15 mm × 4 mm i. d.) and held above the heated reactor. For pyrolysis experiments, the sample was purged with nitrogen (N2) at room temperature for 3 min prior to thermal exposure. The sample was then lowered into the preheated reactor. During thermal degradation, the second GC oven was kept at −60 °C to condense and collect all compounds as they passed through the 280 °C transfer line. The gas flow rate was selected for each individual set of experimental conditions to maintain a vapor residence time in the reactor of approximately 0.2 s. After reaction completion, the transfer line was removed from the injection port of the second GC. Analysis of reaction products was conducted using an Agilent 6890 N GC equipped with a DB5-MS column (30 m × 0.25 mm × 0.25 μm) and an Agilent 5973 N MS with an electron impact (EI) ion source set at 70 eV. The temperature program was as follows: −60 °C initial temperature for 0.5 min, heating 15 °C/min to reach −30 °C, 10 °C/min to 0 °C, 5 °C/min to 50 °C, 15 °C/min to 130 °C, held 1 min, 25 °C/min to 225 °C, and finally 10 °C/min to 300 °C and held 7 min for a total runtime of 40.13 min. The GC inlet and MS quadrupole temperatures were 280 and 150 °C, respectively. Identification of pyrolysis products was performed using the Wiley and NIST libraries, requiring a >90% score from the spectral analysis software, and by comparison of retention times with those of purchased standard compounds. VOC emissions were calculated by dividing the emitted mass of each VOC by the mass of filament used in the experiment and reported as μg/g.
Reaction conditions were selected to simulate standard 3D printing parameters. The printing temperatures were chosen based on the manufacturer recommendation and are shown in Table 1, although users often change printing temperatures to alter printed objects. Additionally, although the STDS provides a consistent temperature throughout the furnace, some extruder nozzles are unlikely to heat uniformly. The residence time in the heated area of the printer is based on the feed rate, width of the filament, and the length of the extruder nozzle. Feed rate can be selected by the user to control the quality of the printed object. A filament residence time of 3 min was selected. While this is higher than typical filament feed rates, which vary but are generally on the order of seconds, we showed in Potter et al., (2019) that comparable results can be achieved under this residence time. The design of 3D printer nozzles can lead to oxygen-depleted conditions inside the nozzle, where melted filament is exposed to the highest temperatures. Experiments were carried out in both pyrolytic (N2) and oxidative (4% O2/N2) conditions to investigate the role of oxygen in the thermal degradation of 3D printer filament. Conditions in a FFF 3D printer are likely similar to our pyrolytic experiments due to the lack of airflow in the extruder.
Table 1.
Relevant information for all studied filaments.
| Filament | polymer | Manufacturer | recommended print temp. (°C) | selected print temp. (°C) | manufacturer advertised additives |
|---|---|---|---|---|---|
| PLA-S.Steel | PLA | Manufacturer A | 210 | 210 | stainless steel particles (80% w/w) |
| PLA-Bronze | bronze particles (87% w/w) | ||||
| PLA-Copper | copper particles (90% w/w) | ||||
| PLA-CNT | Manufacturer B | 210–230 | 220 | carbon nanotubes | |
| ABS-CNT | ABS | Manufacturer C | 235–260 | 250 | carbon nanotubes |
| PC-CNT | PC | Manufacturer D | 260–300 | 280 | carbon nanotubes |
3. Results and discussion
Metal-containing PLA.
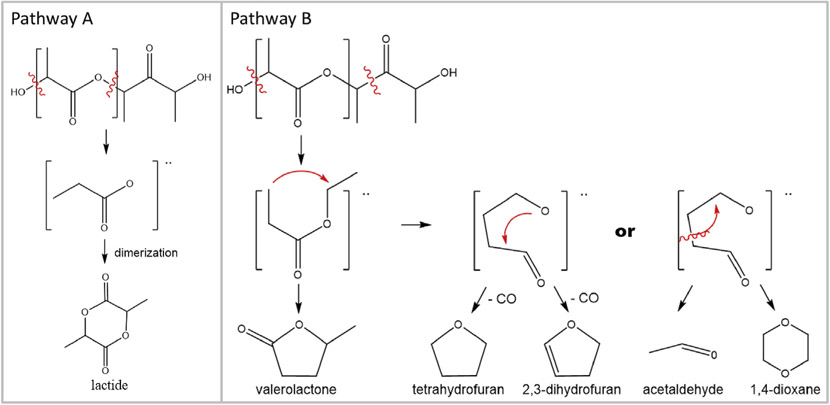
The primary gas-phase emission from the three metal-containing PLA filaments was lactide. Two studies involving multiple PLA filaments have also identified lactide as the primary VOC emitted during printing (Azimi et al., 2016; Davis et al., 2019). Lactide is a common monomer utilized in ring-opening polymerization synthesis of PLA, and as such, could be a residual compound trapped in the polymer matrix and released during melting of the polymer. However, lactide can be also formed due to thermal depolymerization at ester linkages, followed by dimerization of the intermediate products (Fig. 1, Pathway A). Bond scission at the α and β bonds produces other observed structure-related products (Fig. 1, Pathway B), with intermediate condensation and methyl group elimination producing 2,3-dihydrofuran, which has never been reported in PLA printing emissions.
Fig. 1.
PLA degradation mechanism leading to detected VOC emissions. Pathway A shows degradation pathway to lactide. Pathway B shows general pathway to other PLA structure-related products.
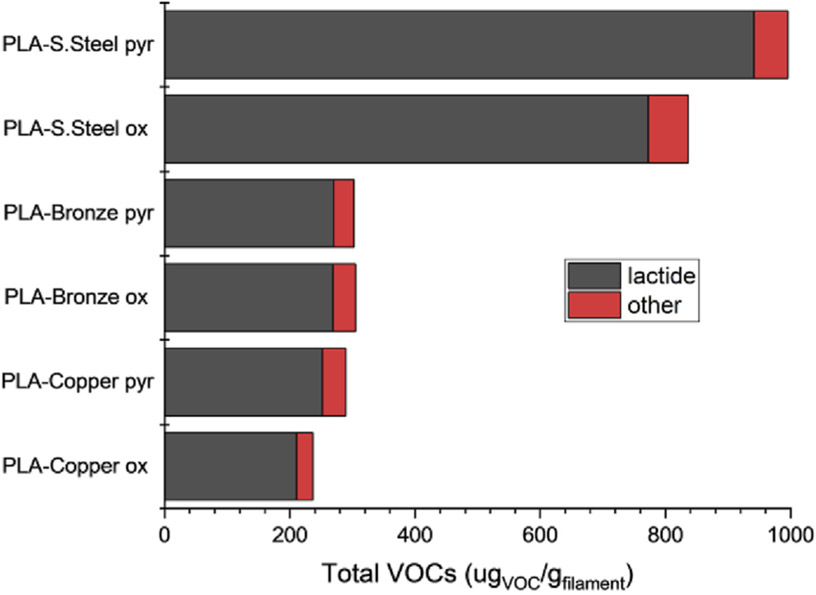
According to the manufacturer, the metal content (w/w) of the three filaments is approximately: 90% copper, 87% bronze, and 80% stainless steel, respectively. The VOC emissions from the PLA-S. Steel are expected to be higher, due to approximately twice the polymer content (20% PLA) compared to the PLA-Copper and PLA-Bronze filaments (10% and 13% PLA, respectively). The polymer content alone does not fully explain the fourfold increase in lactide emission from the PLA-S. Steel filament seen in Fig. 2. It is likely, that catalytic activity of iron and its ability to bind the organic oxygen contributes to low temperature degradation and the formation of lactide, likely from the bond scission shown in Fig. 1, Pathway A. While some of the lactide emission is likely due to volatilized polymerization intermediate, a significant portion is produced during thermal degradation and influenced by the metals that are present. The size and shape of the metal particulates was not measured, but surface area of the particulate could also influence the degree of interaction with polymeric matrix and formation of VOCs. A recent study by Alberts et al. showed that filaments containing copper and tungsten exhibited particle emissions rates up to one order of magnitude higher than their neat polymer counterparts, further confirming the role of metals in the thermal degradation of polymers (Alberts et al., 2021).
Fig. 2.
Total VOC emissions from PLA-Copper, PLA-Bronze, and PLA-S. Steel filaments. Pyr and Ox refer to reaction in N2 and 4% O2/N2, respectively.
As seen in Table 2, the second most prominent VOC from the metal-containing PLAs is acetaldehyde. Acetaldehyde is often detected emitting from PLA and ABS filaments (Davis et al., 2019; Stefaniak et al., 2017). Acetaldehyde is a respiratory irritant and probable carcinogen with EPA recommended inhalation risk reference doses. The PLA-S. Steel emitted approximately three times higher acetaldehyde than the PLA-Copper filament and two times higher than the PLA-Bronze filament. Although the increase is partially explained by the higher polymer content of the PLA-S. Steel, the catalytic activity of iron could also affect acetaldehyde formation. As an oxidation product, acetaldehyde formation also increased during degradation in 4% O2, most significantly in the stainless steel filament. Under oxygenated conditions, total VOC emissions decreased but certain oxygen-containing VOCs increased. This result matched our previous study of CNT-containing ABS filament (Potter et al., 2019). Under high temperatures, oxygen can increase the likelihood of complete oxidation of polymer breakdown intermediates, thereby decreasing formation of VOCs.
Table 2.
VOC emissions from three metal-containing PLA filaments. All data in μgVOC/gfilament. Pyr and Ox refer to reaction in N2 and 4% O2/N2, respectively.
| PLA-Copper |
PLA-Bronze |
PLA-S. Steel |
||||
|---|---|---|---|---|---|---|
| Pyr | Ox | Pyr | Ox | Pyr | Ox | |
| Lactide | 251.65 | 210.69 | 269.90 | 269.05 | 941.68 | 772.90 |
| Acetaldehyde | 11.32 | 11.96 | 16.37 | 20.17 | 30.44 | 39.99 |
| 1-chlorododecane | 20.12 | 10.22 | 5.87 | 5.11 | 17.15 | 17.92 |
| 2,3-dihydrofuran | 4.99 | 3.36 | 10.05 | 10.14 | 5.55 | 4.64 |
| 1-chlorotetradecane | 0.95 | 0.27 | 0.31 | 0.28 | 0.71 | 0.71 |
| DEHP | 0.018 | 0.012 | 0.056 | 0.023 | 0.20 | 0.030 |
| Naphthalene | 0.0072 | 0.0023 | 0.0065 | 0.0054 | 0.011 | 0.0086 |
| 1,3-di-tert-butylbenzene | 0.0015 | 0.0010 | 0.0023 | 0.0017 | 0.0027 | 0.0019 |
As a non-advertised additive influencing VOC emission, the plasticizer bis(2-ethylhexyl) phthalate (DEHP) was emitted by all three metal PLA filaments. Small amounts of naphthalene, which has never been reported emitting from 3D printer filaments, were also detected. The naphthalene could result from carbonization of the polymer material or degradation of an azo dye.
Ideally, a PLA filament without metal additives from the same manufacturer would have been tested as a control, but Manufacturer A does not sell a ‘plain’ PLA filament. These results suggest that the presence of the metal additives is one possible explanation for the differences between our measured VOC emissions and those found in similar studies. In addition, the top two VOC emissions from our filaments, lactide and acetaldehyde, are also the top two VOCs emitted from all PLA filaments in a study involving filaments from various manufacturers (Davis et al., 2019).
Carbon Nanotubes.
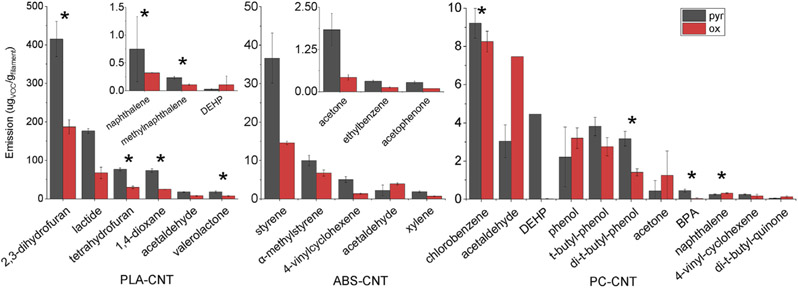
The VOC emissions for the PLA-CNT, ABS-CNT, and PC-CNT filaments are shown in Fig. 3. The typical hierarchy of reported total VOC emissions between filaments is PC, PLA, and ABS, with ABS producing the highest emissions (Azimi et al., 2016; Davis et al., 2019). However, the PLA-CNT filament in our work had significantly higher total VOC emissions compared to the ABS-CNT and PC-CNT, primarily due to emission of three compounds reported here for the first time: 2,3-dihydrofuran, tetrahydrofuran, and 1,4-dioxane. The formation pathways of all three compounds are shown in Fig. 1, Pathway B. In addition, both naphthalene and methylnaphthalene, which had never been detected from any filament prior to this work, were emitted by PLA-CNT. We have previously shown that the presence of CNTs in a filament can influence degradation pathways during printing (Potter et al., 2019). CNT presence changes the primary emitted product pathway for PLA-CNT filament from Pathway A, where intermediates dimerize into lactide, to Pathway B, where intermediates condense to form 2,3-dihydrofuran.
Fig. 3.
VOC emissions from three CNT-containing nanocomposite filaments. Left: PLA-CNT (inset: low concentration emissions), middle: ABS-CNT (inset: low concentration emissions), right: PC-CNT. * = compound not reported in literature for similar filaments without CNTs. Pyr and Ox refer to reaction in N2 and 4% O2/N2, respectively.
The ABS-CNT filament exhibited a similar compound profile to our previously studied ABS-CNT filament from a different manufacturer. The total emissions for the current ABS-CNT filament are similar to other studied ABS filaments. The total emissions were lower than PLA-CNT, which is abnormal compared to typical ABS filaments. The mechanism of the effect of CNTs on VOC emissions is still not clear but could be influenced by adsorption of aromatic species onto the surface of CNTs. The ABS filament emits primarily aromatic species, while PLA emits very few.
Polycarbonate has not been extensively studied with regard to its 3D printing emissions. Therefore, it is not unexpected that we detected several compounds that have not been previously reported. Both bisphenol A (BPA), a reactant in polycarbonate synthesis, and phenol are products of polymer degradation, while chlorobenzene, DEHP, and di-tert-butylphenol are present as polymerization reactant, plasticizer, and UV stabilizer, respectively. Both tert-butylphenol and 2,6-di-tert-butylbenzoquinone are degradation products of di-tert-butylphenol.
Silanol Co-polymers.
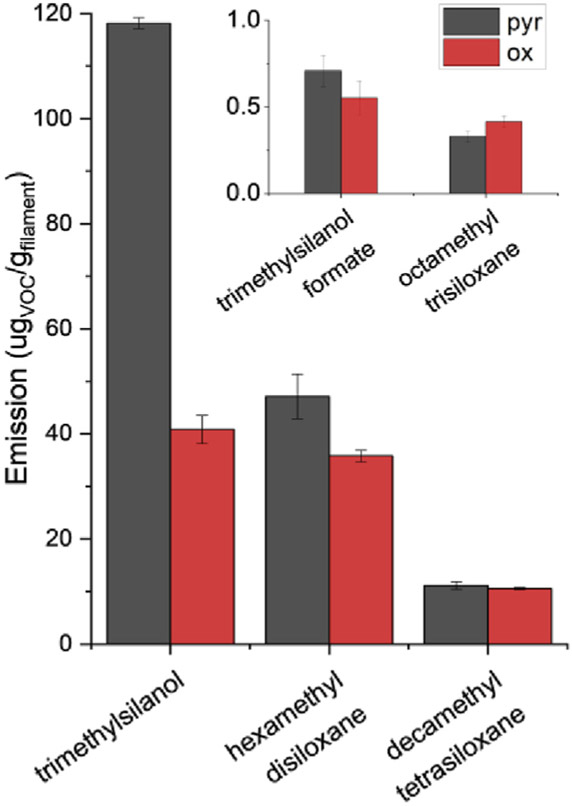
During experimentation, some filaments were found to emit compounds with silanol and siloxane groups. Polymerization of polylactic acid and dimethyl siloxane is an established method for controlling the thermal stability of the resulting copolymer (Zhang et al. 1996). The PLA-CNT filament emitted a total of five compounds with silanol or siloxane groups, including: trimethylsilanol, trimethylsilanol formate, hexamethyl disiloxane, decamethyl tetrasiloxane, and octamethyl trisiloxane (Fig. 4). In Davis et al. hexamethyl cyclotrisiloxane was detected in three of nine studied PLA filaments, nine of twelve studied ABS filaments, one high impact polystyrene (HIPS) filament, and in one polyvinyl alcohol (PVA) filament. The average emission rate of hexamethyl cyclotrisiloxane in the three PLA filaments was more than 100 times less than the lactide emission rate. In our PLA filament, the sum of silanols and siloxanes makes them the second most prevalent gas-phase emission product. The amount of silanol co-polymer likely varies between filaments and could explain the difference in silanol emissions. A second explanation for the increased silanol emissions could be due to the presence of CNTs in this PLA filament. We propose three pathways through which CNTs can affect VOC emissions: (1) acting as adsorption sites for certain volatile species, and thereby, shielding them from gas-phase detection methods, (2) residual metal content from CNT synthesis providing catalytic power for thermal decomposition, and (3) adsorbed oxygen on the CNT surface increasing oxidation of degradation products. A third explanation may be increased silanol co-polymer content to account for structural deficiencies caused by CNT content, such as brittleness.
Fig. 4.
Emission of silanol and siloxane compounds from a PLA-CNT filament. Pyr and Ox refer to reaction in N2 and 4% O2/N2, respectively.
The inhalation toxicity of compounds with silanol and siloxane groups has not been extensively studied, but their presence in landfill gas, where their incineration leads to silica nanoparticle formation, has been shown (Tansel and Surita 2014). Silica inhalation is linked to numerous adverse respiratory effects (Merget et al., 2002). Although the temperatures used to incinerate landfill gas are much higher than typical 3D printer extruder temperatures, there is a potential for formation of lower amounts of silica at lower temperatures.
Chlorinated VOCs.
During experimentation, two filaments were found to emit chlorinated compounds. The PC-CNT filament emitted chlorobenzene as its most prevalent VOC (Fig. 3, right). There are two PC synthesis methods that would explain the presence of chlorine in the filament: one in which chlorobenzene is used as a polymerization solvent (Fox 1964), and one that involves reaction between bisphenol A (BPA) and carbonyl chloride, also known as phosgene (Serini 2000). Other PC synthesis methods are available that do not include chlorinated substances; use of these alternative methods would remove an unnecessary exposure of users to inhalation of chlorinated aromatics when printing with polycarbonate. The three metal-containing PLA filaments all emitted 1-chlorododecane, as one of the primary VOCs, and 1-chlorotetradecane, in trace amounts. The source of chlorine in the PLA filaments is currently unknown. The presence of halogenated hydrocarbons is another example of a non-advertised additive that greatly influences the total amount and toxicity of VOC emissions from the 3D printing process and presents an inhalation risk, of which even informed users are likely unaware.
4. Conclusions
While many of the gas-phase emissions reported in this work have not been evaluated with respect to their inhalation toxicity, some of the VOCs have and their recommended maximum exposure limits can be correlated with typical 3D printer usage. The CompTox Chemicals Dashboard is a database designed by the Environmental Protection Agency (EPA) to provide toxicity information for assessing the threat posed by various compounds to human health and the environment. The CompTox database draws toxicity assessments from various other databases, including the Integrated Risk Information System (IRIS). IRIS is an EPA-created tool for categorizing chemicals and their associated health risks through various methods of exposure (EPA 1999). Table 3 shows recommended maximum inhalation limits for VOCs detected in this work, along with the amount of filament needed to achieve the maximum limit. Our filament residence time of 3 min is longer than that of a typical FFF 3D printer. While this is necessary due to the construction of our reactor, we acknowledge that this could lead to elevated VOC emission rates. Despite this limitation, we believe that the values presented under the ‘printed mass to achieve limit (g)’ column of Table 3 are so concerning that even an order of magnitude decrease would still be a cause for concern.
Table 3.
Detected VOCs, their recommended maximum inhalation limits, and the associated filament mass necessary to achieve the recommended maximum inhalation limit. Calculated “printed mass to achieve limit” values based on highest emission of individual VOC in a 37.5 m3 unventilated room.
| Compound | filament | measurement type | Limit | source database | comment | printed mass to achieve limit (g) |
|---|---|---|---|---|---|---|
| Acetaldehyde | all filaments | RfC (mg/m3) | 9e-3 | EPA IRIS | probable carcinogen (IRIS) | 8.44 |
| 1,4-dioxane | PLA-CNT | RfC (mg/m3) | 3e-2 | EPA IRIS | likely carcinogen (IRIS) |
15.37 |
| DEHP | all except ABS-CNT | cancer unit risk (μg/m3) | 2.4 | Cal OEHHA | probable carcinogen (IRIS) | 20.24 |
| Naphthalene | all except ABS-CNT | RfC (mg/m3) | 3e-3 | EPA IRIS | carcinogenic potential (IRIS) | 150.25 |
| Chlorobenzene | PC-CNT | RfC (mg/m3) | 0.05 | EPA NCEA | – | 203.56 |
| Methylnaphthalene | PLA-CNT | RfC (mg/m3) | 4e-3 | EPA IRIS | – | 639.47 |
| Tetrahydrofuran | PLA-CNT | RfC (mg/m3) | 2 | EPA IRIS | carcinogenic potential (IRIS) | 978.35 |
| Styrene | ABS-CNT | RfC (mg/m3) | 1 | EPA IRIS | – | 1023.76 |
| Xylene | ABS-CNT | RfC (mg/m3) | 1e-1 | EPA IRIS | – | 2006.04 |
| Trimethylsilanol | PLA-CNT | MEG (mg/m3) | 7.5 | TG230 DOD | – | 2379.50 |
Cal OEHHA = California Office of Environmental Health Hazard Assessment, NCEA = National Center of Environmental Assessment, MEG = Military Exposure Guidelines, TG230 DOD = Technical Guide 230 – Department of Defense.
Acetaldehyde, 1,4-dioxane, and DEHP emissions are particularly alarming, due to their known carcinogenic properties and low required printed mass to surpass the recommended maximum inhalation limit. For PLA-CNT filaments, printing just over 20 g (approximately a 3 cm cube) could expose the user to hazardous concentrations of all three compounds. As the required printed mass of the other entries in Table 3 increases, there is a lesser chance that the maximum inhalation limit would be surpassed in practical printing applications. The persistence of these compounds in indoor environments is not well studied, and their accumulation is possible over multiple printing sessions.
Many of the non-advertised additives in 3D printer filaments have EPA-recommended inhalation limits that are easily achievable in small rooms during normal printer operation. The presence of known hazardous additives, such as chlorobenzene and DEHP, is of concern. The toxicity of other emissions, such as silanols and siloxanes, is not well studied. Significant changes in VOC emissions were detected from filaments containing metals and CNTs. The role of these additives in the thermal polymer degradation mechanism should be researched further. The presence of CNTs reduces overall VOC emissions, but shifts the emitted product profiles to more toxic species. There are numerous research gaps in the study of hazardous 3D printer emissions. The primary gap is in methodology. The variance in detected compound profiles from similar filaments across different laboratories suggests the need for standardized sample collection methods. There is also a lack of exposure data involving actual 3D printer emissions that could be used to develop risk assessment.
Supplementary Material
HIGHLIGHTS.
Additives are used to alter properties of 3D printed objects.
Filament additives can also influence hazardous emissions during printing.
Carbon nanotubes increase hazardous compound emission from certain polymers.
Metals such as steel and copper can also increase these emissions.
Non-advertised additives are present in many filaments and influence emissions.
Acknowledgments
This research was funded and conducted by the National Risk Management Research Laboratory of the U.S. Environmental Protection Agency (EPA), Cincinnati, OH. This project was supported, in part, by appointments in the Research Participation Program at the Office of Research and Development (ORD), EPA, administered by the Oak Ridge Institute for Science and Education (92431601) through an interagency agreement between the DOE and the EPA, and in part, with funding from the CPSC through interagency agreement #61320618H0021. This manuscript was subjected to EPA internal reviews and quality assurance approval. The research results presented in this paper do not necessarily reflect the views of the Environmental Protection Agency or its policy. Mention of trade names or products does not constitute endorsement or recommendation for use. This manuscript was also prepared by EOA and CPSC staff; it has not been reviewed or approved by, and may not necessarily reflect the views of the Commission.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2021.130543.
References
- Alberts E, Ballentine M, Barnes E, Kennedy A, 2021. ’Impact of Metal Additives on Particle Emission Profiles from a Fused Filament Fabrication 3D Printer. Atmospheric Environment, p. 244. [Google Scholar]
- Azimi P, Zhao D, Pouzet C, Crain NE, Stephens B, 2016. ’Emissions of ultrafine particles and volatile organic compounds from commercially available desktop three-dimensional printers with multiple filaments. Environ. Sci. Technol 50, 1260–1268. [DOI] [PubMed] [Google Scholar]
- Davis Aika Y., Zhang Qian, Wong Jenny PS., Weber Rodney J., Black Marilyn S., 2019. Characterization of Volatile Organic Compound Emissions from Consumer Level Material Extrusion 3D Printers. Building and Environment, p. 106209. [Google Scholar]
- Deng YL, Cao SJ, Chen AL, Guo YS, 2016. The impact of manufacturing parameters on submicron particle emissions from a desktop 3D printer in the perspective of emission reduction. Build. Environ 104, 311–319. [Google Scholar]
- Ding S, Ng BF, Shang X, Liu H, Lu X, Wan MP, 2019. ’The characteristics and formation mechanisms of emissions from thermal decomposition of 3D printer polymer filaments. Sci. Total Environ 692, 984–994. [DOI] [PubMed] [Google Scholar]
- EPA, US, 1999. Integrated Risk Information System (IRIS). EPA, Washington, DC. [Google Scholar]
- Fox DW, 1964. Polycarbonate polymerization process. [Google Scholar]
- Guan Xia, Ghimire Ajit, Potter Phillip M., Lomnicki Slawomir M., 2019. ’Role of Fe2O3 in fly ash surrogate on PCDD/FS formation from 2-monochlorophenol. Chemosphere 226, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Yoon C, Ham S, Park J, Kim S, Kwon O, Tsai PJ, 2015. ’Emissions of nanoparticles and gaseous material from 3D printer operation. Environ. Sci. Technol 49, 12044–12053. [DOI] [PubMed] [Google Scholar]
- Kwon O, Yoon C, Ham S, Park J, Lee J, Yoo D, Kim Y, 2017. ’Characterization and control of nanoparticle emission during 3D printing. Environ. Sci. Technol 51,10357–10368. [DOI] [PubMed] [Google Scholar]
- Merget R, Bauer T, Kupper HU, Philippou S, Bauer HD, Breitstadt R, Bruening T, 2002. ’Health hazards due to the inhalation of amorphous silica. Arch. Toxicol 75, 625–634. [DOI] [PubMed] [Google Scholar]
- Nganai S, Lomnicki S, Dellinger B, 2012. ’Formation of PCDD/Fs from oxidation of 2-monochlorophenol over an Fe2O3/silica surface. Chemosphere 88, 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo Tuan D., Kashani Alireza, Imbalzano Gabriele, Nguyen Kate TQ., Hui David, 2018. ’Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos. B Eng 143, 172–196. [Google Scholar]
- Potter PM, Al-Abed SR, Lay D, Lomnicki SM, 2019. ’VOC emissions and formation mechanisms from carbon nanotube composites during 3D printing. Environ. Sci. Technol 53, 4364–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubey WA, Grant RA, 1988. ’Design aspects of a modular instrumentation system for thermal diagnostic studies. Rev. Sci. Instrum 59, 265–269. [Google Scholar]
- Serini Volker, 2000. Polycarbonates. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley. [Google Scholar]
- Stabile L, Scungio M, Buonanno G, Arpino F, Ficco G, 2017. ’Airborne particle emission of a commercial 3D printer: the effect of filament material and printing temperature. Indoor Air 27, 398–408. [DOI] [PubMed] [Google Scholar]
- Stefaniak AB, LeBouf RF, Yi JH, Ham J, Nurkewicz T, Schwegler-Berry DE, Chen BT, Wells JR, Duling MG, Lawrence RB, Martin SB, Johnson AR, Virji MA, 2017. ’Characterization of chemical contaminants generated by a desktop fused deposition modeling 3-dimensional printer. J. Occup. Environ. Hyg 14, 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinle P, 2016. ’Characterization of emissions from a desktop 3D printer and indoor air measurements in office settings. J. Occup. Environ. Hyg 13, 121–132. [DOI] [PubMed] [Google Scholar]
- Stephens B, Azimi P, El Orch Z, Ramos T, 2013. Ultrafine particle emissions from desktop 3D printers. Atmos. Environ 79, 334–339. [Google Scholar]
- Tansel B, Surita SC, 2014. ’Oxidation of siloxanes during biogas combustion and nanotoxicity of Si-based particles released to the atmosphere. Environ. Toxicol. Pharmacol 37, 166–173. [DOI] [PubMed] [Google Scholar]
- Thomas Douglas, 2016. ’Costs, benefits, and adoption of additive manufacturing: a supply chain perspective. Int. J. Adv. Manuf. Technol 85, 1857–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance ME, Pegues V, Van Montfrans S, Leng W, Marr LC, 2017. ’Aerosol emissions from fuse-deposition modeling 3D printers in a chamber and in real indoor environments. Environ. Sci. Technol 51, 9516–9523. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Pardo M, Rudich Y, Kaplan-Ashiri I, Wong JPS, Davis AY, Black MS, Weber RJ, 2019. ’Chemical composition and toxicity of particles emitted from a consumer-level 3D printer using various materials. Environ. Sci. Technol 53, 12054–12061. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wong JPS, Davis AY, Black MS, Weber RJ, 2017. ’Characterization of particle emissions from consumer fused deposition modeling 3D printers. Aerosol. Sci. Technol 51,1275–1286. [Google Scholar]
- Zhang S, Hou Z, Gonsalves KE, 1996. ’Copolymer synthesis of poly (L-lactide-b-DMS-L-lactide) via the ring opening polymerization of L-lactide in the presence of α, ω-hydroxylpropyl-terminated PDMS macroinitiator. J. Polym. Sci. Polym. Chem 34, 2737–2742. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.