Abstract
Circulating autoantibodies (auto-Abs) neutralizing high concentrations (10 ng/mL, in plasma diluted 1 to 10) of IFN-α and/or -ω are found in about 10% of patients with critical COVID-19 pneumonia, but not in subjects with asymptomatic infections. We detect auto-Abs neutralizing 100-fold lower, more physiological, concentrations of IFN-α and/or -ω (100 pg/mL, in 1/10 dilutions of plasma) in 13.6% of 3,595 patients with critical COVID-19, including 21% of 374 patients > 80 years, and 6.5% of 522 patients with severe COVID-19. These antibodies are also detected in 18% of the 1,124 deceased patients (aged 20 days-99 years; mean: 70 years). Moreover, another 1.3% of patients with critical COVID-19 and 0.9% of the deceased patients have auto-Abs neutralizing high concentrations of IFN-β. We also show, in a sample of 34,159 uninfected subjects from the general population, that auto-Abs neutralizing high concentrations of IFN-α and/or -ω are present in 0.18% of individuals between 18 and 69 years, 1.1% between 70 and 79 years, and 3.4% >80 years. Moreover, the proportion of subjects carrying auto-Abs neutralizing lower concentrations is greater in a subsample of 10,778 uninfected individuals: 1% of individuals <70 years, 2.3% between 70 and 80 years, and 6.3% >80 years. By contrast, auto-Abs neutralizing IFN-β do not become more frequent with age. Auto-Abs neutralizing type I IFNs predate SARS-CoV-2 infection and sharply increase in prevalence after the age of 70 years. They account for about 20% of both critical COVID-19 cases in the over-80s, and total fatal COVID-19 cases.
Autoantibodies neutralizing type I IFNs increase in prevalence over 60 years of age and underlie about 20% of all fatal COVID-19 cases.
INTRODUCTION
Since the start of the COVID-19 pandemic in December 2019, more than 200 million people have been infected with SARS-CoV-2, resulting in at least 4 million deaths, and probably closer to 7 to 9 million deaths worldwide. Interindividual clinical variability in the course of acute infection is vast, extending from silent or mild infection in about 90% of subjects to pneumonia and respiratory failure, both requiring hospitalization, in less than 10% and 2% of cases, respectively. Age is the major epidemiological risk factor for hospitalization or death from pneumonia, the risk doubling with every five years of age (1, 2). The frequencies of critical disease and death from COVID-19 are higher in men than in women (3–5). With the COVID Human Genetic Effort (6), we previously reported that inborn errors of TLR3- and IRF7-dependent type I IFN induction and amplification can underlie life-threatening COVID-19 pneumonia in a small subset of patients (7, 8). Autosomal dominant disorders were found in 19 patients, but our cohort also included four previously healthy unrelated adults aged 25 to 50 years with autosomal recessive, complete IRF7 (N=2) or IFNAR1 (N=2) deficiency. These findings indicated that type I IFN immunity is essential for protective immunity to respiratory infection with SARS-CoV-2 but surprisingly redundant otherwise. We also reported that an autoimmune phenocopy of inborn errors of type I IFN-dependent immunity can underlie critical COVID-19 pneumonia (9). Indeed, autoantibodies (auto-Abs) neutralizing 10 ng/mL IFN-α2 and/or -ω were found in the blood of at least 10% of an international cohort of patients with life-threatening COVID-19 pneumonia, but in none of the tested individuals with asymptomatic or paucisymptomatic infection (9). These auto-Abs were detected in serum or plasma diluted 1/10. The auto-Abs in the patients’ undiluted blood can therefore probably neutralize as much as 100 ng/mL IFN-α2 and/or -ω. The 17 subtypes of type I IFNs, including 13 IFN-α subtypes, IFN-ω, IFN-β, IFN-ε, and IFN-κ, bind to the same heterodimeric receptor (IFNAR1 and IFNAR2). (10). The 13 IFN-α subtypes and IFN-ω are closely related phylogenetically, while IFN-β, IFN-ε, and IFN-κ are more distant (9). The auto-Abs to IFN-α2 and/or -ω were mostly found in men (95%) and in the elderly (half the patients with antibodies being over the age of 65 years) (9). These findings were later replicated in independent cohorts from Amsterdam, Lyon, Madrid, New Haven, and San Francisco (11–16).
These auto-Abs against type I IFNs were found in about 0.3% of a general population sample of 1,227 subjects collected before the pandemic and aged 20 to 69 years, suggesting that they predated SARS-CoV-2 infection and caused critical COVID-19 rather than being triggered by it (9). Moreover, production of these antibodies can be genetically driven, and can begin during early childhood, as attested by their presence in almost all patients with autoimmune polyendocrine syndrome type-1 (APS-1) due to germline mutations of AIRE (17–19). APS-1 patients are, indeed, at very high risk of developing severe or critical COVID-19 pneumonia (20, 21). These auto-Abs are also found in patients with combined immunodeficiency and hypomorphic mutations of RAG1 or RAG2 (22), in men with immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome and mutations of FOXP3 (23), and in women with incontinentia pigmenti and heterozygous null mutations of X-linked NEMO (9). They are also seen in patients treated with IFN-α or IFN-β (24, 25), in patients with systemic lupus erythematosus (26, 27), thymoma (28), or with myasthenia gravis (29, 30). Finally, they underlie a third of adverse reactions to the 17D live attenuated vaccine against yellow fever virus (YFV), further suggesting that they were present in these patients, as in patients with critical COVID-19, before viral infection (31). Remarkably, for all patients tested, the auto-Abs neutralized the protective effect of ~400 pg/mL IFN-α2 against SARS-CoV-2 or YFV-17D in vitro, even when the plasma was diluted by >1/1,000 (9). As blood IFN-α concentrations during acute asymptomatic or paucisymptomatic SARS-CoV-2 infection typically range from 1 to 100 pg/mL (32, 33), and IFN-α levels in the respiratory tract might be even lower yet protective, we hypothesized that auto-Abs neutralizing concentrations of type I IFNs below 10 ng/mL may underlie life-threatening COVID-19 pneumonia in more than 10% of cases. We also hypothesized that the prevalence of auto-Abs against type I IFNs in the general, uninfected, population may increase with age and that these antibodies may be more common in men than in women.
RESULTS
High and intermediate levels of IgG auto-Abs against IFN-α2 and/or IFN-ω in ~20% of patients with critical COVID-19
We recruited a cohort of 3,595 patients hospitalized with critical COVID-19 pneumonia (hereafter referred to as “critical patients”, and defined as pneumonia in patients with critical disease, including (i) pulmonary, with high-flow oxygen (> 6 L/min) or mechanical ventilation (continuous positive airway pressure, bilevel positive airway pressure, intubation), (ii) cardiovascular shock, or (iii) any other organ failure requiring admission to an intensive care unit), including 566 patients of our previously described cohort of 987 patients with critical COVID-19 pneumonia for whom residual samples were available (9), 623 individuals with severe COVID-19 pneumonia (with less than 6 L/min of oxygen supplementation, hereafter referred to as “severe patients”), and 1,639 individuals with asymptomatic or paucisymptomatic (mild) upper respiratory tract SARS-CoV-2 infection (the “controls”, infected with SARS-CoV-2 (as demonstrated by a positive PCR and/or serological test and/or displaying typical manifestations, such as anosmia/ageusia after exposure to a confirmed COVID-19 case) who remained asymptomatic or developed mild, self-healing, ambulatory disease with no evidence of pneumonia), including 427 samples from the initial control cohort of 663 individuals (9). The patients originated from 38 different countries, across all continents. We did not include patients with moderate pneumonia, who did not receive oxygen therapy (7, 9). We searched for auto-Abs against IFN-α2 and -ω, by establishing novel, sensitive, and robust assays for the detection of circulating IgG auto-Abs. We used Gyros technology (34), a high-throughput automated enzyme-linked immunosorbent assay (ELISA)-like assay capable of detecting a large range of auto-Ab levels (Fig. S1A). We confirmed that the Gyros technique was as sensitive as the techniques previously used (ELISA and Luminex), and that all tested patients with high levels of anti-IFN-α2 and/or anti-IFN-ω auto-Abs on ELISA, as reported in our previous studies (defined as an optical density > 0.5) had high levels of auto-Abs when assessed with Gyros (defined as levels >100) (Fig. S1B). We then screened newly recruited critical or severe patients and controls from our COVID-19 cohort (Fig. 1A). We found high levels of anti-IFN-α2 and/or anti-IFN-ω auto-Abs in 6.9% of critical patients, 3.4% of patients with severe COVID-19, and only 0.6% of the asymptomatic or paucisymptomatic controls (Fig. 1A). We also found that another 12.7% of patients with critical COVID-19 had intermediate levels of anti-IFN-α2 and/or IFN-ω auto-Abs in Gyros assays (defined as levels >30 and <100, based on the distribution observed in healthy controls), whereas this was the case for 8.6% of patients with severe COVID-19 and 11% of the individuals in our control cohort. Collectively, these findings replicate and extend our previous results and those of other groups (9, 11–15, 35), while suggesting that intermediate levels of auto-Abs against type I IFNs might be neutralizing and underlie critical disease.
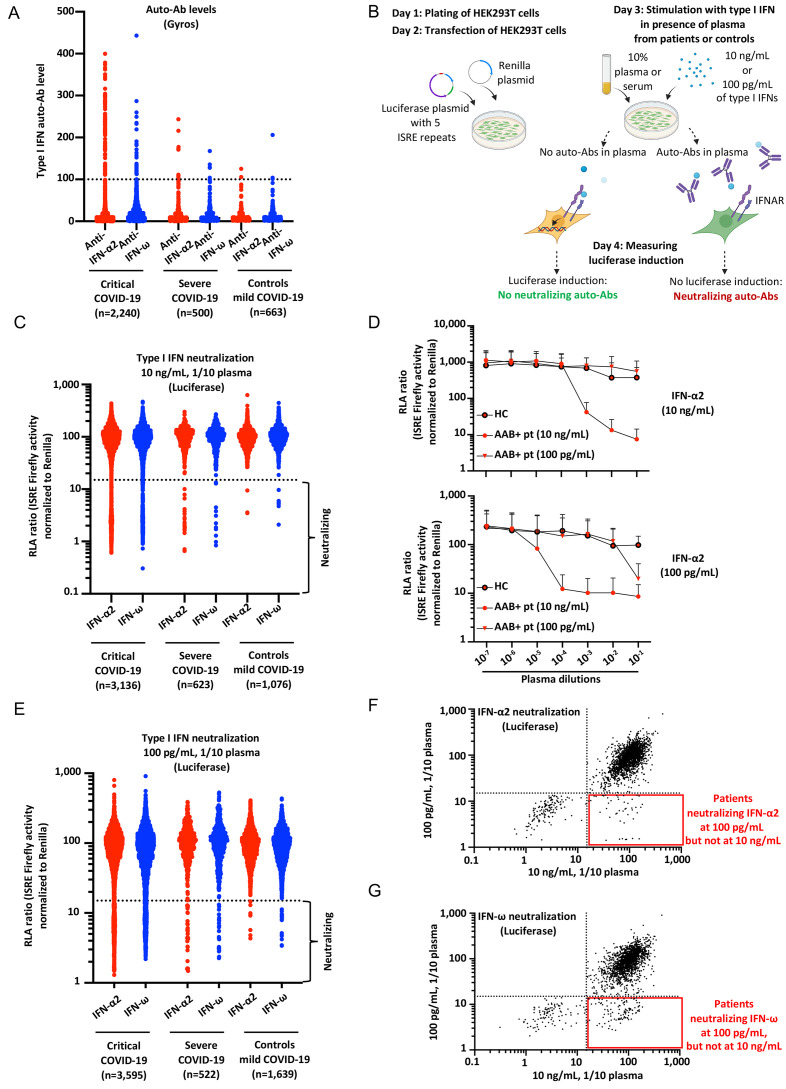
Fig. 1.
Neutralizing auto-Abs against IFN-α2 and/or IFN-ω in patients with life-threatening COVID-19. (A) Gyros (high-throughput automated ELISA) results for auto-Abs against IFN-α2 and/or IFN-ω in patients with critical COVID-19 (N=2,240), severe COVID-19 (N=500), or asymptomatic/mild SARS-CoV-2 infection (N=663). (B) Schematic representation of the neutralization assay developed in HEK293T cells, using a luciferase system. ISRE: interferon-sensitive response elements. (C) Results for the neutralization of 10 ng/mL IFN-α2 or IFN-ω in the presence of plasma 1/10 from patients with critical COVID-19 (N=3,136), severe COVID-19 (N=623), or controls with mild/asymptomatic infection (N=1,076). Relative luciferase activity is shown (ISRE dual luciferase activity, with normalization against Renilla luciferase activity) after stimulation with 10 ng/mL IFN-α2 or IFN-ω in the presence of plasma 1/10. RLA: relative luciferase activity. (D) RLA after stimulation with IFN-α2 at a concentration of 10 ng/mL or 100 pg/mL, with various dilutions of plasma from a positive control (from 1/10 to 1/107) neutralizing 10 ng/mL of type I IFNs (AAB+ pt, 10 ng/mL), a patient neutralizing 100 pg/mL of type I IFNs but not 10 ng/mL (AAB+ pt, 100 pg/mL), and a healthy control (HC). AAB: auto-Ab. Pt: patient. (E) Neutralization of 100 pg/mL IFN-α2 or IFN-ω in the presence of plasma 1/10 from patients with critical COVID-19 (N=3,595), severe COVID-19 (N=522), or controls with asymptomatic/mild infection (N=1,639). (F) Plot showing luciferase induction after stimulation with 10 ng/mL or 100 pg/mL IFN-α2, in the presence of plasma from patients with critical COVID-19. Dotted lines indicate neutralizing levels, defined as induction levels below 15% of the mean value for controls tested the same day. Patients with antibodies neutralizing both 10 ng/mL and 100 pg/mL IFN-α2 are shown in the bottom left corner, whereas the patients in the bottom right corner had antibodies capable of neutralizing only 100 pg/mL IFN-α2. (G) Plot showing luciferase induction after stimulation with 10 ng/mL or 100 pg/mL IFN-ω, for patients with critical COVID-19.
Auto-Abs neutralizing 10 ng/mL IFN-α2 and/or -ω in almost 10% of the critical patients
We investigated the ability of these auto-Abs to neutralize high concentrations of type I IFNs, as defined in our previous reports (10 ng/mL IFN-α2 or IFN-ω in medium containing 1/10 plasma or serum, the equivalent of 100 ng/mL IFN-α2 or IFN-ω in undiluted plasma). We tested not only the patients with high levels of auto-Abs, as in our previous study (9), but all the available patients with critical COVID-19 (N=3,136), or severe COVID-19 (N=623), and controls (N=1,076) from our expanded cohort. We designed a high-throughput luciferase assay in which we transfected human embryonic kidney (HEK)293T cells with (i) a plasmid containing five IFN-stimulated response element (ISRE) repeats and a firefly luciferase reporter, and (ii) a plasmid encoding the Renilla luciferase. We stimulated these cells with an individual recombinant type I IFN (IFN-α2 or IFN-ω), in the presence of plasma diluted 1/10 (plasma 1/10) from patients or controls. We then measured firefly luciferase induction, normalized against Renilla luciferase activity (Fig. 1B). We confirmed the robustness of this assay by comparing the results with our previous pSTAT1 flow cytometry data (9). Consistent results were obtained for all 50 patients tested with both techniques (Fig. S1C, D). We then tested all patients and controls. Most plasma samples with high auto-Ab levels (>100) against IFN-α2 according to the Gyros assay were neutralizing (Fig. S1E). We found that 9.8% (307 of 3,136) of the critical patients tested and 3.53% (22 of 623) of the severe patients had auto-Abs neutralizing IFN-α2 and/or IFN-ω, versus only 0.37% (4 of 1,076) controls (Fig. 1C) (Table 1 and Table S1). In the patients with neutralizing auto-Abs, these auto-Abs were able to neutralize both IFN-α2 and IFN-ω in 175 of the 307 critical patients (57%), 6 of the severe patients (27%), and none of the controls; IFN-α2 alone in 106 critical patients (34.5%), 11 severe patients (50%), and only one of the controls (25%); IFN-ω alone in 26 of critical patients (8.5%), 5 severe patients (22%), and 3 controls (75%) (Table S1). None of the patients with these auto-Abs had inborn errors of TLR3- or TLR7-dependent type I IFN immunity (7, 36).
Table 1. Risk of critical COVID-19 pneumonia for subjects carrying auto-Abs to specific sets of type I IFNs, when compared with that of asymptomatic/mild infection, adjusted on age and sex.
Odds ratios (OR) and P-values were estimated by means of Firth’s bias-corrected logistic regression. The numbers and proportions of subjects with critical COVID-19 pneumonia (patients) and asymptomatic or mild infection (controls) are shown in Figs. 1 to 3. Two combinations are not shown due to insufficient number of individuals: anti-IFN-β (10 ng/mL) and anti-IFN-α2 (100 pg/mL) auto-Abs only; anti-IFN-β (10 ng/mL) and anti-IFN-ω (100 pg/mL) auto-Abs only.
|
Anti-type I IFN auto-Ab positive
(amount of type I IFN neutralized, in plasma diluted 1/10) |
Proportion of critical patients with neutralizing auto-Abs | OR [95% CI] | P-value |
| anti-IFN-α2 and anti-IFN-ω auto-Abs (10 ng/mL) | 5.6% | 67 [4-1109] | 7.8x10−13 |
| anti-IFN-α2 and/or anti-IFN-ω auto-Abs (10 ng/mL) | 9.8% | 17 [7-45] | < 10−13 |
| anti-IFN-α2 auto-Abs (10 ng/mL) | 9% | 45 [9-225] | < 10−13 |
| anti-IFN-α2 auto-Abs only (10 ng/mL) | 3.4% | 21 [4-107] | 1.8x10−09 |
| anti-IFN-ω auto-Abs (10 ng/mL) | 6.4% | 13 [4-38] | 1.4x10−12 |
| anti-IFN-ω auto-Abs only (10 ng/mL) | 0.8% | 3 [0.9-10] | 0.057 |
| anti-IFN-α2 and anti-IFN-ω auto-Abs (100 pg/mL) | 7.1% | 54 [11-275] | < 10−13 |
| anti-IFN-α2 and/or anti-IFN-ω auto-Abs (100 pg/mL) | 13.6% | 13 [8-21] | < 10−13 |
| anti-IFN-α2 auto-Abs (100 pg/mL) | 10% | 23 [10-55] | < 10−13 |
| anti-IFN-α2 auto-Abs only (100 pg/mL) | 2.9% | 10 [3-26] | 2.8x10−09 |
| anti-IFN-ω auto-Abs (100 pg/mL) | 10.7% | 13 [7-23] | < 10−13 |
| anti-IFN-ω auto-Abs only (100 pg/mL) | 3.6% | 6 [3-12] | 3.9x10−10 |
| anti-IFN-β auto-Abs (10 ng/mL) | 1.3% | 8 [2-36] | 1.7x10−3 |
| anti-IFN-β auto-Abs only (10 ng/mL) | 0.96% | 5 [1-25] | 0.043 |
| anti-IFN-β auto-Abs (10 ng/mL) and, anti-IFN-α2 and/or anti-IFN-ω auto-Abs (100 pg/mL) | 0.34% | 16 [0.5-497] | 0.018 |
| anti-IFN-β (10 ng/mL) and, anti-IFN-α2 and anti-IFN-ω auto-Abs (100 pg/mL) | 0.28% | 16 [0.5-502] | 0.019 |
Auto-Abs neutralizing 100 pg/mL IFN-α2 and/or -ω in at least 13.6% of critical patients and 6.8% of severe patients
As the amounts of circulating type I IFNs in infected individuals are 100 to 1,000 times lower than the amounts tested previously (32, 33), we investigated the neutralization of more physiological concentrations of type I IFNs, by performing assays with 100 pg/mL type I IFN. We observed a robust response in our luciferase system, in the presence of 1/10 dilutions of control plasma (Fig. S1F). The plasma or serum was diluted 1/10, so the concentration neutralized corresponds to 1 ng/mL IFN in circulating whole blood. With diluted plasma samples from a positive control, we gained at least two orders of magnitude of sensitivity in terms of neutralizing activity, providing proof-of-concept that these auto-Abs can neutralize lower, more physiological, amounts of type I IFNs (Fig. 1D, Fig. S1G), lower than the concentrations previously tested by a factor of 100 (9). We then retested all available samples from our extended cohort. Overall, 13.6% of all critical patients tested (N=489 of 3,595), 6.5% (N=34 of 522) of the severe patients, and 1% of the controls (N=17 of 1,639) had circulating auto-Abs that neutralized 100 pg/mL IFN-α2 and/or IFN-ω in plasma 1/10 (Fig. 1E-G) (Table 1 and Table S1). In the patients with neutralizing auto-Abs, these auto-Abs were able to neutralize both IFN-α2 and IFN-ω in 256 of the 489 positive critical patients (52%), 18 of the 34 severe patients (53%), and 1 of the 17 controls (6%); IFN-α2 alone in 104 critical patients (21%), 14 severe patients (41%), and 4 of the controls (23.5%); IFN-ω alone in 129 critical patients (26%), 2 severe patients (6%), and 12 controls (70%) (Table S1). Further dilution of a plasma sample from one patient neutralizing 100 pg/mL of type I IFNs led to a loss of neutralizing activity (Fig. 1D, Fig. S1G). Importantly, for four unrelated patients, all of whom suffered from critical COVID-19, including one who died, samples collected before COVID-19 were available and tested positive for neutralizing auto-Abs against type I IFNs. One neutralized IFN-α2 and IFN-ω at a concentration of 10 ng/mL, two neutralized both cytokines at 100 pg/mL and one IFN-ω only at 100 pg/mL (Fig. S1H). The four patients tested therefore had auto-Abs neutralizing 10 ng/mL or 100 pg/mL IFN-α2 and/or -ω before infection with SARS-CoV-2. These four patients, and another two reported in our previous study (9) all, therefore, had auto-Abs neutralizing type I IFNs before infection with SARS-CoV-2. We then assessed the risk, adjusted for age and sex, of having critical or severe disease for subjects carrying auto-Abs against each individual IFN and the different possible combinations. We found that all auto-Abs, except those neutralizing only IFN-ω at a concentration of 10 ng/mL, were highly significant risk factors in comparisons of patients with critical or severe COVID-19 with controls (Table 1 and Table S2). The strongest association was with auto-Abs against both IFN-α2 and IFN-ω neutralizing concentrations of 10 ng/mL (OR=67, P=8x10−13) and 100 pg/mL (OR=54, P<10−13), followed by those against IFN-α2 +/− IFN-ω neutralizing 10 ng/mL (OR=45, P<10−13) and 100 pg/mL (OR=23, P<10−13) (Table 1). As the serum/plasma samples were diluted 1/10 in these assays, these findings suggest that more than 13.6% of patients with life-threatening COVID-19 have circulating auto-Abs neutralizing 1 ng/mL IFN-α2 and/or IFN-ω in vivo, a greater proportion than the 10% of patients with auto-Abs neutralizing 100 ng/mL reported in previous studies (9, 11–15, 35).
Auto-Abs neutralize low concentrations of IFN-α2 protective against SARS-CoV-2
We previously reported that plasma diluted 1/100 from patients with auto-Abs against type I IFNs neutralized the ability of IFN-α2 (at a concentration of 20 pM, approximately 400 pg/mL) to block SARS-CoV-2 and YFV-17D replication in Huh-7.5 cells (9, 31). Strikingly, this neutralization was seen in all patients tested, even for a 1,000-fold dilution, and, in most patients, it was more potent than the neutralizing effect of a commercially available neutralizing monoclonal Ab (mAb) against IFN-α2. These auto-Abs against type I IFNs were, therefore, able to neutralize IFN-α2 at concentrations well beyond physiological levels. We therefore hypothesized that patients with lower titers of auto-Abs against type I IFNs, which can neutralize 100 pg/mL but not 10 ng/mL in plasma diluted 1/10, would also neutralize the protective effect of IFN-α2 against SARS-CoV-2. We therefore performed our SARS-CoV-2 assay with 5 pM (~100 pg/mL) or 20 pM (~400 pg/mL) IFN-α2, on five samples from patients with life-threatening COVID-19 and two samples from uninfected elderly individuals with auto-Abs neutralizing 100 pg/mL but not 10 ng/mL IFN-α2. As controls, we tested a commercial mAb against IFN-α2, a sample from a patient with auto-Abs neutralizing 10 ng/mL IFN-α2, and samples from three patients with life-threatening COVID-19 and three healthy controls without detectable auto-Abs against type I IFNs. We found that the 1/100 dilutions of plasma from four of the five critical COVID-19 patients and one of the two elderly individuals with auto-Abs neutralizing 100 pg/mL IFN-α2 were able to neutralize the protective effect of ~400 pg/mL IFN-α2 against SARS-CoV-2, whereas samples from all these individuals fully or partially neutralized ~100 pg/mL IFN-α2 (Fig. 2A). No such neutralizing effect was observed for any of the auto-Ab-negative controls. Overall, our findings indicate that auto-Abs against type I IFNs capable of neutralizing 100 pg/mL IFN in 1% plasma can block the protective effect of ~100 pg/mL or ~400 pg/mL IFN-α2 against SARS-CoV-2. These findings raise the possibility that even 100-fold lower levels of auto-Abs against type I IFNs, capable of neutralizing lower, physiological concentrations of 10 pg/mL IFN-α2, may be present in an even larger proportion of patients. The testing of this hypothesis will require the development of new, more sensitive methods to screen for neutralization.
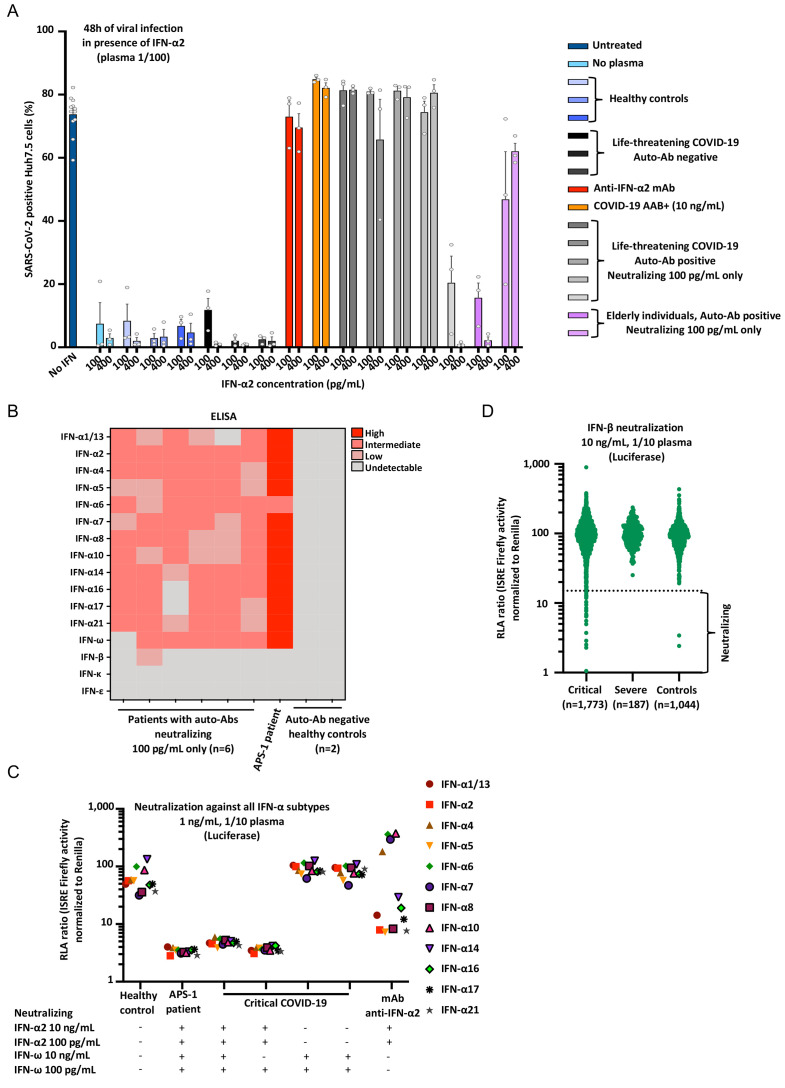
Fig. 2.
Enhanced SARS-CoV-2 replication, despite the presence of IFN-α2, in the presence of plasma from patients with auto-Abs neutralizing 100 pg/mL IFN-α2. (A) SARS-CoV-2 replication in Huh-7.5 cells untreated (in dark blue), or treated with ~100 pg/mL or ~400 pg/mL IFN-α2 in the presence of 1/100 plasma from healthy controls without auto-Abs (N=3, in blue), from patients with life-threatening COVID-19 but without auto-Abs against IFN-α2 (N=3, in black), a commercial anti–IFN-α2 antibody (mAb, in red); from a patient with life-threatening COVID-19 and auto-Abs neutralizing 10 ng/mL IFN-α2 in plasma 1/100 (COVID-19 AAB+, N=1, in orange), from patients with life-threatening COVID-19 and auto-Abs neutralizing 100 pg/mL IFN-α2 in plasma 1/100 (N=5, in grey); elderly individuals with auto-Abs neutralizing 100 pg/mL IFN-α2 in plasma 1/100 (N=2, in purple). Each dot represents a technical replicate. All experiments were done in triplicate. (B) ELISA (enzyme-linked immunosorbent assay) for auto-Abs against the 13 IFN-α forms, IFN-ω, IFN-β, IFN-ε, and IFN-κ in patients with life-threatening COVID-19 and auto-Abs neutralizing 100 pg/mL IFN-α2 (N=6), APS-1 patient with life-threatening COVID-19 and auto-Abs neutralizing 10 ng/mL IFN-α2 and IFN-ω (N=1), and healthy controls (N=2). (C) RLA after stimulation with the all individual IFN-α at a concentration of 1ng/mL, with 1/10 plasma from a healthy control (negative control), an APS-1 patient (positive control), patients with life-threatening COVID-19 and neutralizing IFN-α2 and/or IFN-ω, or a monoclonal antibody anti-IFN-α2. (D) Neutralization of 10 ng/mL IFN-β in the presence of plasma 1/10 from patients with critical COVID-19 (N=1,773), severe COVID-19 (N=187), or asymptomatic/mild controls (N=1,044).
Neutralization of type I IFNs in the absence of detectable auto-Abs against IFN-α2 or -ω
The neutralization assays performed on all patients and controls revealed that some patients with neutralizing activity against 10 ng/mL IFN-α2 and/or IFN-ω, as shown in luciferase assays, did not have high, or even intermediate levels of IgG auto-Abs in Gyros assays (Fig. S1E). We also observed that some patients with neutralizing auto-Abs had low or undetectable levels of auto-Abs in Luminex assays (Fig. S1I). For these individuals, we assessed the prevalence of IgA and IgM auto-Abs against type I IFNs; we found that none of the patients tested (N=12) had detectable titers of IgA or IgM auto-Abs (Fig. S1J). We then tested the alternative hypothesis that these auto-Abs were directed against the IFNAR1 or IFNAR2 chain of type I IFN receptors, assessing the ability of plasma samples from these patients to neutralize IFN-β. None of the samples from these patients neutralized IFN-β, suggesting that the auto-Abs in these patients were not directed against IFNAR1 or IFNAR2 (Fig. S1K). An alternative plausible hypothesis is that the epitope recognized by the auto-Abs might be concealed by the binding of the cytokine to the plate (ELISA), biotinylation of the cytokine (Gyros), or covalent coupling of the cytokine to magnetic beads at lysine residues (Luminex) (19). This observation has important clinical implications, suggesting that a lack of detection of auto-Abs against type I IFNs does not rule out the possibility of such antibodies being present and having neutralization capacity.
Auto-Abs typically neutralize the 13 IFN-α subtypes and/or IFN-ω
In six patients with auto-Abs neutralizing 100 pg/mL but not 10 ng/mL IFN-α2 and/or IFN-ω, we tested the reactivity of the antibodies against the 17 type I IFNs (the 13 IFN-α forms, IFN-ω, IFN-β, IFN-ε, and IFN-κ). Like patients with auto-Abs neutralizing 10 ng/mL type I IFNs (9), those capable of neutralizing only 100 pg/mL had detectable auto-Abs against most of the 13 IFN-α forms and/or IFN-ω, albeit at lower levels (Fig. 2B). Of the six patients with auto-Abs against IFN-α and/or IFN-ω tested, only one also had auto-Abs against IFN-β and none had detectable auto-Abs against IFN-ε or IFN-κ. Overall, the patients with auto-Abs against IFN-α2 and/or IFN-ω capable of neutralizing 100 pg/mL IFN displayed patterns of reactivity to the 17 type I IFNs similar to those reported in previously described patients with auto-Abs neutralizing 10 ng/mL (9). We then set up an assay for assessing neutralization of the 13 IFN-α forms, using our luciferase-based assay. We tested two patients with auto-Abs neutralizing IFN-α2 and IFN-ω, two patients with auto-Abs neutralizing only IFN-α2, and two patients with auto-Abs neutralizing only IFN-ω. Interestingly, we found that the APS-1 patient, and the two patients with auto-Abs neutralizing 10 ng/mL IFN-α2 and IFN-ω were able to neutralize all 13 IFN-α subtypes, as were the two patients with neutralizing auto-Abs against IFN-α2. Conversely, in the conditions tested, the two patients with auto-Abs neutralizing IFN-ω only, but not IFN-α2, were not able to neutralize any of the 13 IFN-α subtypes (Fig. 2C). In addition, to confirm that the IgG auto-Abs detected were indeed the cause of the neutralization activity observed, we performed an IgG depletion experiment and found that the removal of the IgG fraction abolished the neutralizing activity, whereas the purified IgG fraction had full neutralizing activity (Fig. S2A). Thus, patients with neutralizing auto-Abs against only IFN-ω do not seem to neutralize any of the 13 IFN-α subtypes, whereas patients with auto-Abs neutralizing IFN-α2 neutralize all these subtypes.
Auto-Abs neutralizing IFN-β in 1.3% of critical patients
We previously reported that auto-Abs neutralizing IFN-β were detected in only two of 101 critical patients with auto-Abs neutralizing 10 ng/mL IFN-α2 and/or IFN-ω (9). Given the potential therapeutic use of IFN-β (37, 38), and the absence of IFN-β-neutralization data for COVID-19 patients, we tested a larger number of patients and controls, including patients without auto-Abs against IFN-α or IFN-ω, for auto-Abs against IFN-β, assessing the levels and neutralizing activity of auto-Abs against 10 ng/mL IFN-β. We screened 1,773 patients with critical COVID-19 pneumonia, and found that 1.3% (N=23) had neutralizing auto-Abs against IFN-β; by contrast, such antibodies were present in none of the 187 severe patients tested and in only two of the 1,044 controls tested (0.18%) (Fig. 2D, S2B and Table S3). Interestingly, only six of the 23 (21.7%) critical patients also had auto-Abs neutralizing IFN-α2 and/or IFN-ω at 100 pg/mL, and none of the controls had such antibodies. Of note, five of these six patients had auto-Abs neutralizing all three cytokines. All the other critical patients and controls had only neutralizing auto-Abs against IFN-β. The presence of neutralizing auto-Abs against IFN-β was significantly associated with critical, but not severe, disease relative to the controls (Table 1, Tables S2-3). Interestingly, Gyros did not appear to be able to detect auto-Abs against IFN-β, perhaps because of the biotinylation of the cytokine hiding the epitope recognized by the auto-Abs. As most (78.3%) of the patients with neutralizing auto-Abs against IFN-β did not have neutralizing auto-Abs against IFN-α2 or IFN-ω, this suggests that auto-Abs against IFN-β alone may also underlie life-threatening COVID-19 (Table 1).
Neutralizing auto-Abs against type I IFNs in at least 20% of critical patients over 80 years of age
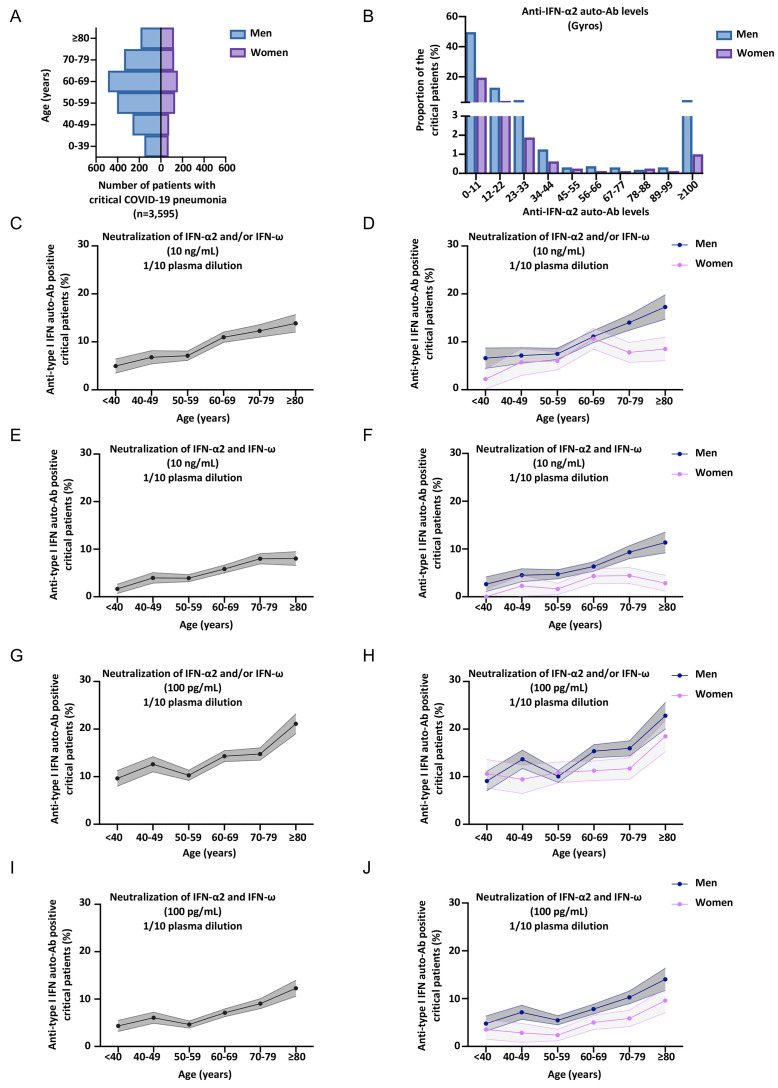
We further assessed the percentage of critical COVID-19 patients positive for neutralizing auto-Abs per decade of life and by sex (Fig. 3A-J, S3A-W) (Tables S1-4). In our previous report, we found that critical COVID-19 patients with auto-Abs neutralizing IFN-α2 or IFN-ω at 10 ng/mL were older (more than half the patients with auto-Abs were over the age of 65 years) and more likely to be male (95% of the antibody carriers were men) (9). These results have been confirmed by other groups, albeit with a smaller proportion of men (11–14, 35). In our expanded cohort of patients with critical COVID-19 pneumonia (N=3,595), the mean age was 61 years and 73% of the patients were men (Fig. 3A, Table S4). We confirmed that critical patients with auto-Abs neutralizing IFN-α and/or IFN-ω at 10 ng/ml were significantly older than those not carrying auto-Abs (mean age [SD] 65.8 years [14.1] versus 61.6 years [15.5], Firth’s multivariable logistic regression, P=3x10−6) and more likely to be male (78.5% versus 71%, Firth’s multivariable logistic regression, P=0.003). The proportion of critical COVID-19 patients with auto-Abs neutralizing 10 ng/mL IFN-α2 and/or IFN-ω increased continuously, with auto-Abs detected in 5% of patients under the age of 40 years, 6.8% of those between 40 and 49 years of age, 7.1% of those between 50 and 59 years of age, 10.7% of those between 60 and 69 years of age, 12.3% of those between 70 and 79 years, and almost 14% in those over 80 (Fig. 3C-F, S3B-I). In severe patients, the proportion of auto-Abs was much more stable with age (Fig. S3T-W, Firth’s multivariable logistic regression P=0.16) and sex (Firth’s multivariable logistic regression P=0.44). Similar results were obtained for critical COVID-19 patients with auto-Abs neutralizing 100 pg/mL IFN-α2 and/or IFN-ω, but with even higher proportions (Fig. 3G-J, S3L-S) (Table S1). Indeed, the proportion of patients with auto-Abs ranged from 9.6% of patients below the age of 40 years, to more than 21% of those over 80 (Fig. 3G-J, S3L-S). In men, the proportion of critical COVID-19 patients carrying auto-Abs neutralizing 100 pg/mL IFN-α2 and/or IFN-ω increased to up to 23% over 80 years of age. A very different pattern was seen for auto-Abs neutralizing 10 ng/mL IFN-β, with a more stable proportion of auto-Abs carriers according to age (Fig. S3J, K, Firth’s multivariable logistic regression, P=0.68) (Table S3). Overall, the prevalence of auto-Abs neutralizing 10 ng/mL and/or 100 pg/mL IFN-α2 and/or IFN-ω increased sharply with age in critical patients. A striking enrichment in patients with neutralizing auto-Abs against IFN-α2 and/or IFN-ω was observed in the elderly, with more than 20% of patients, and 23% of men, over the age of 80 years with critical COVID-19 having neutralizing auto-Abs against these type I IFNs.
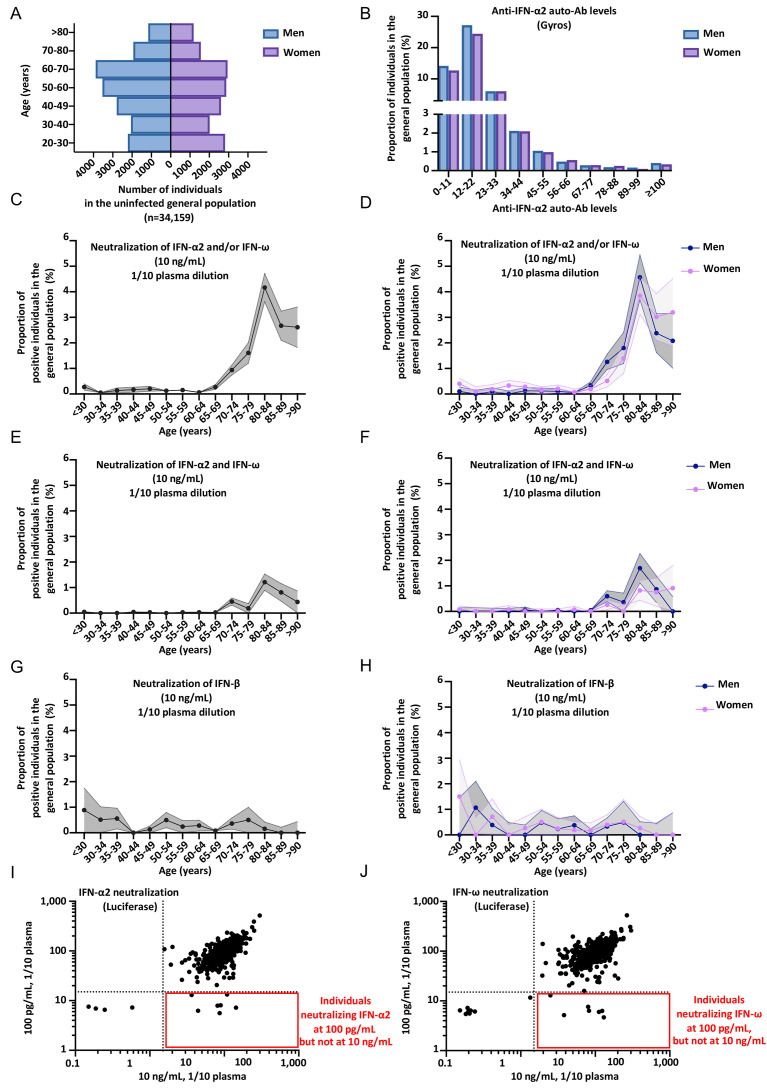
Fig. 3.
Higher prevalence of neutralizing auto-Abs against type I IFNs in elderly patients with critical COVID-19. (A) Bar plot of the age and sex distribution of the patients with life-threatening COVID-19 included in our expanded cohort (N=3,595). (B) Graph showing the anti-IFN-α2 auto-Ab levels, assessed by Gyros, in patients with life-threatening COVID-19. Men and women are shown separately. The upper section of the Y-axis starts at 3%. (C-J) Proportion by decade of patients with critical COVID-19, and positive for neutralizing auto-Abs (in plasma 1/10) against (C) IFN-α2 and/or IFN-ω, at 10 ng/mL, for both sexes. (D) IFN-α2 and/or IFN-ω, at 10 ng/mL, for men or women. (E) IFN-α2 and IFN-ω, at 10 ng/mL, for both sexes. (F) IFN-α2 and IFN-ω, at 10 ng/mL, for men or women. (G) IFN-α2 and/or IFN-ω, at 100 pg/mL, for both sexes. (H) IFN-α2 and/or IFN-ω, at 100 pg/mL, for men or women. (I) IFN-α2 and IFN-ω, at 100 pg/mL, for both sexes. (J) IFN-α2 and IFN-ω, at 100 pg/mL, for men or women.
Neutralizing auto-Abs against type I IFNs in at least 18% of deceased patients
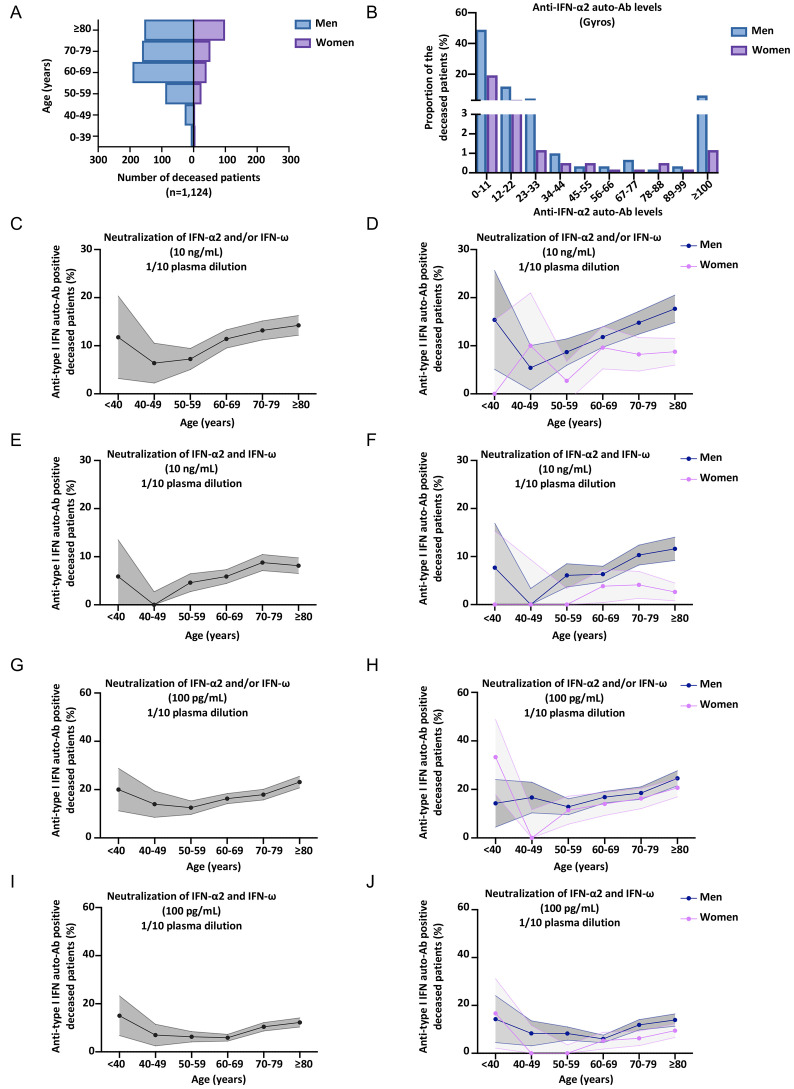
The prevalence of auto-Abs against type I IFNs in patients dying from COVID-19 pneumonia is unknown. For the 3,595 patients with critical COVID-19, we analyzed data for the 1,124 who died. These patients were aged 20 days to 99 years (mean age: 71 years), 73% were male, and all had confirmed SARS-CoV-2 infection and critical COVID-19 pneumonia before death (Fig. 4A). In these patients, we analyzed the presence of neutralizing auto-Abs against type I IFNs at concentrations of 10 ng/mL and 100 pg/mL for IFN-α2 and IFN-ω, and at 10 ng/mL for IFN-β (Fig. 4B-J, S4A-K). We found that 13.3% of the deceased patients carried auto-Abs neutralizing 10 ng/mL IFN-α2 and/or IFN-ω (Fig. 4B-F, S4A-E). Strikingly, 18.5% carried auto-Abs neutralizing 100 pg/mL of either or both cytokines (Fig. 4G-J, S4F-I). In addition, 0.9% had auto-Abs neutralizing IFN-β (Fig. S4J-K). An analysis of the prevalence of neutralizing auto-Abs against type I IFNs in these patients who died of COVID-19 by decade of age revealed a moderate increase with age for auto-Abs neutralizing 10 ng/mL (Firth’s multivariable logistic regression P=0.03) or 100 pg/mL (Firth’s multivariable logistic regression P=0.01) (Table S1-2). For a type I IFN concentration of 100 pg/mL, the prevalence of auto-Abs neutralizing IFN-α2 and/or IFN-ω was 20% below the age of 40 years, 14% for individuals between 40 and 49 years old, 12.5% for those between 50 and 60 years old, 16.3% for those between 60 and 69 years old, 17.9% for those between 70 and 79 years old, and greater than 23% for those over the age of 80 years. Overall, at least 18% of patients dying from COVID-19 pneumonia have auto-Abs capable of neutralizing 100 pg/mL type I IFNs in plasma 1/10.
Fig. 4.
Higher prevalence of neutralizing auto-Abs against type I IFNs in patients who died of COVID-19. (A) Bar plot of the age and sex distribution of the patients who died of COVID-19 included in our cohort (N=1,124). (B) Graph showing the anti-IFN-α2 auto-Ab levels, assessed by Gyros, in patients who died of COVID-19. Men or women are shown separately. The upper section of the Y-axis starts at 3%. (C-J) Proportion by decade of patients who died of COVID-19, and positive for neutralizing auto-Abs (in plasma 1/10) against (C) IFN-α2 and/or IFN-ω, at 10 ng/mL, for both sexes. (D) IFN-α2 and/or IFN-ω, at 10 ng/mL, for men or women. (E) IFN-α2 and IFN-ω, at 10 ng/mL, for both sexes. (F) IFN-α2 and IFN-ω, at 10 ng/mL, for men or women. (G) IFN-α2 and/or IFN-ω, at 100 pg/mL, for both sexes. (H) IFN-α2 and/or IFN-ω, at 100 pg/mL, for men or women. (I) IFN-α2 and IFN-ω, at 100 pg/mL, for both sexes. (J) IFN-α2 and IFN-ω, at 100 pg/mL, for men or women.
Auto-Abs capable of neutralizing IFN-α2 and/or IFN-ω at 10 ng/mL in 0.53%, and at 100 pg/mL in 2.3% of individuals from the general population
We previously tested a sample of 1,227 individuals aged 20 to 65 years from the general population collected in 2015-2017. This sample had an equal sex distribution, and we identified four individuals with auto-Abs against type I IFNs among the 1,227 tested (0.3%), suggesting that the auto-Abs pre-dated COVID-19 (9). These findings were replicated at the University of California San Francisco (UCSF) in a sample of 4,041 subjects aged 4 to 90 years (0.32%) (16). In the current study, we tested a much larger cohort of 34,159 individuals aged 20 to 100 years from the general population, with an equal distribution between the sexes (Fig. 5A). Samples were collected before 2018 for blood donors at the French blood bank (19,966 individuals), the 3C cohort (801) and in 2019 for participants in the French CONSTANCES cohort (8,850) and Cerba HealthCare (4,542). We performed serological tests for SARS-CoV-2 on the samples collected in 2019, and included only the individuals who had not been infected with SARS-CoV-2 in the sample. We used Gyros to screen this whole cohort for IgG auto-Abs against IFN-α2 and IFN-ω (Fig. 5B, S5A). We did not measure auto-Abs against IFN-β by Gyros. We found that only 0.05% and 4.2% had anti-IFN-α2 and/or anti-IFN-ω auto-Abs above the thresholds of 100 and 30, respectively (Fig. 5B, S5A). We then assessed the ability of these antibodies to neutralize 10 ng/mL IFN-α2 or IFN-ω, for all individuals with a high or intermediate level of IgG auto-Abs against IFN-α2 or IFN-ω. We found 181 individuals with neutralizing auto-Abs, for whom 1/10 dilutions of plasma neutralized 10 ng/mL IFN-α2 and/or IFN-ω, giving an overall prevalence of 0.53% (Fig. 5C-F, S5B-I) (Table S5-6), consistent with our two previous reports (9, 16). We may have slightly underestimated the number of positive individuals, as some may have had neutralizing auto-Abs at too low a titer for detection. Next, we assessed the prevalence of auto-Abs neutralizing 10 ng/mL of IFN-β in 9,583 individuals, and found an overall prevalence of 0.26% (Fig. 5G-H) (Table S5-6). Finally, for a subset of 10,778 samples, we further assessed the ability of plasma/serum samples (diluted 1/10) to neutralize 100 pg/mL IFN-α2 and/or IFN-ω in the luciferase assay (Fig. 5I-J, 6A-H). The prevalence of auto-Abs neutralizing 100 pg/mL IFN-α2 and/or IFN-ω was 2.3% (Table S1).
Fig. 5.
Neutralizing auto-Abs against IFN-α2 and/or IFN-ω at 10 ng/mL are more prevalent in the elderly, in the general population. (A) Bar plot of the age and sex distribution of individuals from the general population (N=34,159). (B) Graph showing the IFN-α2 auto-Ab levels, assessed by Gyros, in individuals from the general population. Men or women are shown separately. The upper section of the Y-axis starts at 3%. (C-H) Proportion by 5 years of individuals from the general population, and positive for neutralizing auto-Abs (in plasma 1/10) against (C) IFN-α2 and/or IFN-ω, at 10 ng/mL, for both sexes. (D) IFN-α2 and/or IFN-ω, at 10 ng/mL, for men or women. (E) IFN-α2 and IFN-ω, at 10 ng/mL, for both sexes. (F) IFN-α2 and IFN-ω, at 10 ng/mL, for men or women. (G) IFN-β, at 10 ng/mL, for both sexes. (H) IFN-β, at 10 ng/mL, for men or women. (I) Plot showing luciferase induction after stimulation with 10 ng/mL or 100 pg/mL IFN-α2, in the presence of plasma from individuals from the general population. Dotted lines indicate neutralizing levels, defined as induction levels below 15% of the mean value for controls tested the same day. Individuals with antibodies neutralizing both 10 ng/mL and 100 pg/mL IFN-α2 are shown in the bottom left corner, whereas the individuals in the bottom right corner had antibodies capable of neutralizing only 100 pg/mL IFN-α2. (J) Plot showing luciferase induction after stimulation with 10 ng/mL or 100 pg/mL IFN-ω, for individuals from the general population.
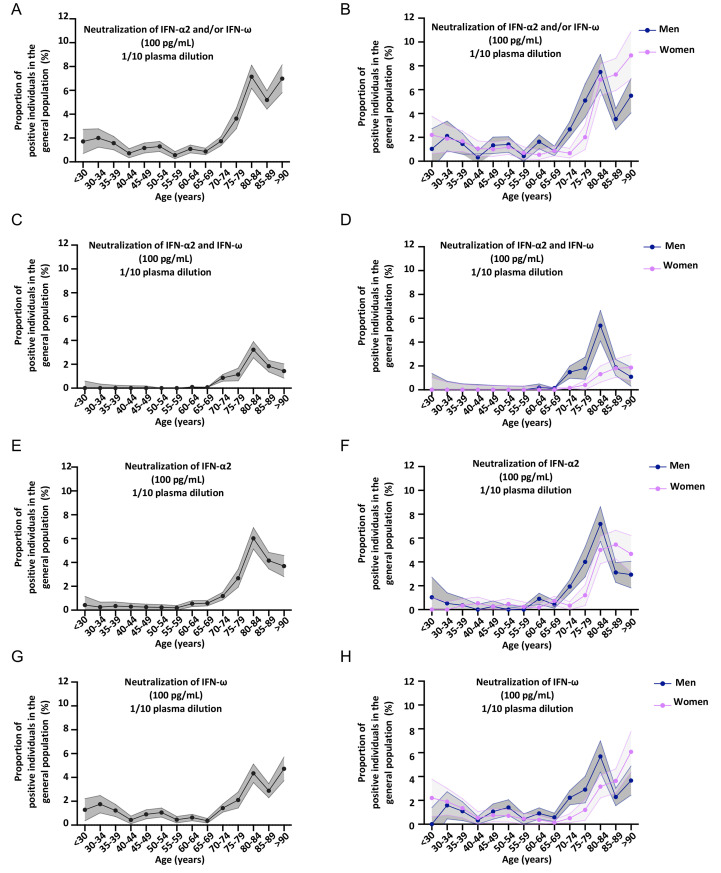
Fig. 6.
Neutralizing auto-Abs against IFN-α2 and/or IFN-ω at 100 pg/mL are more prevalent in the elderly, in the general population. (A-H) Proportion, binned every 5 years, of individuals from the general population, and positive for neutralizing auto-Abs (in plasma 1/10) against (A) IFN-α2 and/or IFN-ω, at 100 pg/mL, for both sexes. (B) IFN-α2 and/or IFN-ω, at 100 pg/mL, for men or women. (C) IFN-α2 and IFN-ω, at 100 pg/mL, for both sexes. (D) IFN-α2 and IFN-ω, at 100 pg/mL, for men or women. (E) IFN-α2, at 100 pg/mL, for both sexes. (F) IFN-α2, at 100 pg/mL, for men or women. (G) IFN-ω, at 100 pg/mL, for both sexes. (H) IFN-ω, at 100 pg/mL, for men or women.
Sharp increase in the prevalence of auto-Abs against IFN-α2 and/or IFN-ω after the age of 70 years in the general population
We then assessed the percentage of individuals from the general population positive for neutralizing auto-Abs per decade of life and by sex. Strikingly, we noted that the prevalence of auto-Abs neutralizing 10 ng/mL type I IFN was more than 10 times higher in individuals over the age of 70 years than in those below this age (Firth’s multivariable logistic regression, P<10−13) (Fig. 5C-F, S5B-I) (Table S5-6). The prevalence of auto-Abs capable of neutralizing 10 ng/mL IFN-α2 and/or IFN-ω was 0.17% in individuals below 70 years of age, 0.9% in individuals between 70 and 75 years of age, 1.6% between the ages of 75 and 80 years and more than 4% between the ages of 80 and 85 years. Intriguingly, after 85 years, the prevalence of these antibodies decreased to about 2.6%. These findings were replicated independently in two cohorts of 703 and 376 elderly individuals from Estonia and Japan, tested with Luciferase-based immunoprecipitation assay (LIPS) and ELISA assays, respectively (Fig. S5J, K). A strong increase in the prevalence of auto-Abs neutralizing 100 pg/mL IFN-α2 and/or IFN-ω was observed with age (Fig. 6A-H, S6A-D), with the prevalence almost doubling with every five years from 65 to 85 years of age. Indeed, 0.87% of individuals between the ages of 65 and 70 years, 1.73% of those between 70 and 75 years, and 7.1% of those between 75 and 80 years were positive for auto-Abs. Interestingly, there was an overall decrease in the prevalence of auto-Abs after 85 years of age, especially in men. By contrast, the prevalence of auto-Abs neutralizing IFN-β did not vary significantly with age (Fig. 5G, H) (Table S4). We then assessed the risk, adjusted for age and sex, of having critical or severe disease, for subjects carrying auto-Abs against each individual IFN and the different possible combinations, relative to the general population. We also found that all auto-Abs were highly significant risk factors in comparisons of patients with critical or severe COVID-19 with the general population (Table 1 and Table S2). The strongest association was again that for auto-Abs neutralizing both IFN-α2 and IFN-ω at 10 ng/mL (OR=30, P<1 x10−13), followed by those neutralizing IFN-α2 +/− IFN-ω at 10 ng/mL (OR=20, P<10−13), and IFN-ω +/− IFN-α2 at 10 ng/mL (OR =15, P<10−13) (Table 1). Auto-Abs neutralizing both IFN-α2 and IFN-ω at 100 pg/mL were also highly significant risk factors (OR [95% CI]=12 [9-16], P<10−13) (Table 1). Overall, these findings indicate that there is a sharp increase in the prevalence of auto-Abs neutralizing type I IFNs with age in elderly uninfected individuals, with at least 4% of those over the age of 70 years positive for auto-Abs against IFN-α2 and/or IFN-ω, and that these auto-Abs pre-date COVID-19.
DISCUSSION
We report that at least 20% of patients over 80 years of age with life-threatening COVID-19 pneumonia carry circulating auto-Abs neutralizing 100 pg/mL IFN-α2 and/or IFN-ω, and that such antibodies are present in more than 13.6% of patients of all ages with this condition. Some of these auto-Abs are not identified by immunoassays and are only detectable by a neutralization assay. In addition, at least 18% of deceased individuals in most age groups were found to have such auto-Abs. We also report that auto-Abs against IFN-β are found in about 1.3% and 0.9% of critical and deceased patients, most of whom do not have auto-Abs against IFN-α2 and/or IFN- ω. In all four patients tested for whom pre-COVID-19 samples were also available, the auto-Abs against IFN-α2 and/or IFN-ω were clearly present before SARS-CoV-2 infection, as in patients with APS-1 (9, 20), and in two other previously described patients (9). Importantly, auto-Abs capable of neutralizing high concentrations of type I IFNs have been found in patients without inborn errors of TLR3- or TLR7-dependent type I IFN immunity (7, 36), suggesting that both inborn errors and auto-Abs are independently causal of critical disease. It is also striking that inborn errors are more common in patients under the age of 60 years, whereas auto-Abs are more common in patients over the age of 70 years. We also report that the prevalence of auto-Abs neutralizing 10 ng/mL (and 100 pg/mL) type I IFNs, except for IFN-β, increases significantly with age in the general population, with 0.17% (1.1%) of individuals positive for these antibodies before the age of 70 years, and more than 1.4% (4.4%) positive after the age of 70 years, with a prevalence of 4.2% (7.1%) between the ages of 80 and 85 years.
These auto-Abs provide an explanation for the major increase in the risk of critical COVID-19 in the elderly. This increase with age is consistent with studies of various auto-Abs since the 1960s (39–43). These auto-Abs appear to have remained clinically silent in these individuals until SARS-CoV-2 infection. Our results also suggest that the neutralization of only one type I IFN (IFN-α2, IFN-ω, or IFN-β) can underlie life-threatening COVID-19 (Table 1, Tables S1-S3). Auto-Abs neutralizing 10 ng/mL IFN-β have a frequency only about one tenth that of auto-Abs neutralizing the same concentrations of IFN-α2 and/or IFN-ω (Table 1, Table S3). We have shown that auto-Abs neutralizing 100 pg/mL type I IFN in plasma diluted 1/10, corresponding to the neutralization of 1 ng/mL IFN in vivo, can account for at least 18% of deaths and more than 20% of critical cases in the elderly >80 years of age. It is tempting to speculate that an even greater proportion of life-threatening COVID-19 cases are due to auto-Abs neutralizing lower, physiological concentrations of type I IFNs. In vitro, concentrations of type I IFN as low as 100 pg/mL can impair SARS-CoV-2 replication in epithelial cells (Fig. 2A). Moreover, the levels of type I IFN detected in the blood of patients with acute and benign SARS-CoV-2 infections are in the range of 1 to 100 pg/mL (32, 33).
Our findings have immediate clinical applications. First, it is quick and easy to test for auto-Abs against type I IFNs in patients infected with SARS-CoV-2. Screening for these antibodies is even possible in the general population before infection. The type I IFN-neutralizing activity of these antibodies is a better read-out than their mere detection, which can be falsely negative. Tests should be performed for auto-Abs against at least three individual IFNs: IFN-α2, IFN-ω, and IFN-β. Particular attention should be paid to elderly individuals, and patients with known autoimmune or genetic conditions associated with auto-Abs against type I IFNs (17–20, 22, 23, 26–29). Second, patients with auto-Abs against type I IFN should be vaccinated against COVID-19 as a priority. Third, live attenuated vaccines, including YFV-17D and vaccines using the YFV-17D backbone against SARS-CoV-2, should not be given to patients with auto-Abs (31, 44). Fourth, these patients appeared to be healthy before SARS-CoV-2 infection, but they should also be carefully followed for other viral illnesses, as exemplified by adverse reactions to YFV-17D (31). Fifth, in cases of SARS-CoV-2 infection in unvaccinated individuals with auto-Abs against type I IFNs, the patients should be hospitalized for prompt management. Early treatment with monoclonal antibodies (45, 46) can be administered in patients without symptoms of severe COVID-19 pneumonia, and IFN-β can be administered in the absence of both pneumonia and auto-Abs against IFN-β (37, 38). Rescue treatment by plasma exchange is another therapeutic option in patients who already have pneumonia (47).
Sixth, blood products, especially plasma, should be screened for anti-IFN auto-Abs and any products containing such antibodies should be excluded from donation (13). Plasma from donors convalescing from COVID-19 should be tested for such auto-Abs (13). Seventh, given the documented innocuity and potential efficacy of a single injection, early therapy with IFN-β may be considered for the contacts of contagious subjects or during the first week after infection, even in the absence of, or before the documentation of auto-Abs against type I IFNs, in elderly patients, who have a higher risk of critical pneumonia and auto-Abs against IFN-α2 and IFN-ω, but not IFN-β (48). Another possibility would be the administration of monoclonal antibodies that can neutralize SARS-CoV-2 (45, 46). Finally, it will be important to decipher the mechanism underlying the development of these auto-Abs, which may differ in patients over and under 65 years of age. Overall, our findings show that auto-Abs neutralizing concentrations of type I IFN lower than previously reported (9, 11–16), but still higher than physiological concentrations, are common in the elderly population. Their prevalence increases with age in the uninfected general population, reaching more than 4% of individuals after the age of 70 years. They underlie about 20% of cases of critical COVID-19 pneumonia in patients over the age of 80 years, and about 20% of total COVID-19 deaths. We previously reported that they can underlie severe adverse reactions to the yellow fever live attenuated virus (31). It is tempting to speculate that they may also underlie other severe viral diseases, especially in the elderly.
MATERIALS AND METHODS
Study design
We enrolled, from 38 countries across all continents, 3,595 patients with proven critical COVID-19, 623 patients with severe COVID-19, 1,639 asymptomatic or paucisymptomatic individuals with proven COVID-19, and 34,159 healthy controls in this study. We collected plasma or serum samples for all these individuals to test by immunoassay for the presence of IgG auto-Abs to type I IFNs. All subjects were recruited according to protocols approved by local institutional review boards (IRBs).
COVID-19 classification
The severity of COVID-19 was assessed for each patient as follows (7, 9). “Critical COVID-19 pneumonia” was defined as pneumonia developing in patients with critical disease, whether pulmonary, with high-flow oxygen, mechanical ventilation (Continuous positive airway pressure, bilevel positive airway pressure, intubation), septic shock, or with damage to any other organ requiring admission to the intensive care unit. “Severe COVID-19” was defined as pneumonia developing in patients requiring low-flow oxygen (<6 L/min). The controls were individuals infected with SARS-CoV-2 (as demonstrated by a positive PCR and/or serological test and/or displaying typical symptoms, such as anosmia/ageusia after exposure to a confirmed COVID-19 case) who remained asymptomatic or developed mild, self-healing, ambulatory disease with no evidence of pneumonia.
Detection of anti-cytokine autoantibodies
Gyros
Cytokines, recombinant human (rh)IFN-α2 (Miltenyi Biotec, ref. number 130-108-984) or rhIFN-ω (Merck, ref. number SRP3061), were first biotinylated with EZ-Link Sulfo-NHS-LC-Biotin (Thermo Fisher Scientific, cat. number A39257), according to the manufacturer’s instructions, with a biotin-to-protein molar ratio of 1:12. The detection reagent contained a secondary antibody (Alexa Fluor 647 goat anti-human IgG (Thermo Fisher Scientific, ref. number A21445) diluted in Rexxip F (Gyros Protein Technologies, ref. number P0004825; 1/500 dilution of the 2 mg/mL stock to yield a final concentration of 4 μg/mL). Buffer PBS-T 0.01% and Gyros Wash buffer (Gyros Protein Technologies, ref. number P0020087) were prepared according to the manufacturer’s instructions. Plasma or serum samples were then diluted 1/100 in PBS-T 0.01% and tested with the Bioaffy 1000 CD (Gyros Protein Technologies, ref. number P0004253), and the Gyrolab xPand (Gyros Protein Technologies, ref. number P0020520). Cleaning cycles were performed in 20% ethanol.
Multiplex particle-based assay
Serum/plasma samples were screened for autoantibodies (auto-Abs) against IFN-α2 and IFN-ω in a multiplex particle-based assay, in which magnetic beads with differential fluorescence were covalently coupled to recombinant human proteins (2.5 μg/reaction). Beads were combined and incubated with 1/100-diluted serum/plasma samples for 30 min. Each sample was tested once. The beads were then washed and incubated with PE-labeled goat anti-human IgG (1 μg/mL) for an additional 30 min. They were then washed again and used for a multiplex assay on a Bio-Plex X200 instrument.
Enzyme-linked immunosorbent assays (ELISA)
ELISA was performed as previously described. In brief, 96-well ELISA plates (MaxiSorp; Thermo Fisher Scientific) were coated by incubation overnight at 4°C with 2 μg/mL rhIFN-α2 (Miltenyi Biotec, ref. number 130-108-984), and rhIFN-ω (Merck, ref. number SRP3061). Plates were then washed (PBS 0.005% Tween), blocked by incubation with 5% nonfat milk powder in the same buffer, washed, and incubated with 1:50 dilutions of plasma from the patients or controls for 2 hours at room temperature (or with specific mAbs as positive controls). Each sample was tested once. Plates were thoroughly washed. Horseradish peroxidase (HRP)–conjugated Fc-specific IgG fractions from polyclonal goat antiserum against human IgG, IgM or IgA (Nordic Immunological Laboratories) were added to a final concentration of 2 μg/mL. Plates were incubated for 1 hour at room temperature and washed. Substrate was added and the optical density (OD) was measured. A similar protocol was used to test for antibodies against 12 subtypes of IFN-α, except that the plates were coated with cytokines from PBL Assay Science (catalog #11002-1), or IFN-β (Miltenyi Biotech, ref. number: 130-107-888).
Functional evaluation of anti-cytokine autoantibodies
Luciferase reporter assays
The blocking activity of anti-IFN-α2 and anti-IFN-ω auto-Abs was determined with a reporter luciferase activity. Briefly, HEK293T cells were transfected with a plasmid containing the firefly luciferase gene under the control of the human ISRE promoter in the pGL4.45 backbone, and a plasmid constitutively expressing Renilla luciferase for normalization (pRL-SV40). Cells were transfected in the presence of the X-tremeGene9 transfection reagent (Sigma-Aldrich, ref. number 6365779001) for 24 hours. Cells in Dulbecco’s modified Eagle medium (DMEM, Thermo Fisher Scientific) supplemented with 2% fetal calf serum (FCS) and 10% healthy control or patient serum/plasma (after inactivation at 56°C, for 20 min) were either left unstimulated or were stimulated with IFN-α2 (Miltenyi Biotec, ref. number 130-108-984), IFN-ω (Merck, ref. number SRP3061), at 10 ng/mL or 100 pg/mL, or IFN-β (Miltenyi Biotech, ref. number: 130-107-888) at 10 ng/mL, for 16 hours at 37°C. Each sample was tested once for each cytokine and dose. Finally, cells were lysed for 20 min at room temperature and luciferase levels were measured with the Dual-Luciferase® Reporter 1000 assay system (Promega, ref. number E1980), according to the manufacturer’s protocol. Luminescence intensity was measured with a VICTOR-X Multilabel Plate Reader (PerkinElmer Life Sciences, USA). Firefly luciferase activity values were normalized against Renilla luciferase activity values. These values were then normalized against the median induction level for non-neutralizing samples, and expressed as a percentage. Samples were considered neutralizing if luciferase induction, normalized against Renilla luciferase activity, was below 15% of the median values for controls tested the same day. A similar protocol was used to test for auto-Abs against 12 subtypes of IFN-α, except that we used cytokines from PBL Assay Science (catalog #11002-1) at 1 ng/mL for stimulation.
pSTAT1 induction in PBMC
The blocking activity of anti-IFN-α2 and anti-IFN-ω auto-Abs was determined by assessing STAT1 phosphorylation in healthy control cells following stimulation with the appropriate cytokines in the presence of 10% healthy control or patient serum/plasma. Surface-stained healthy control PBMCs (350,000/reaction) were cultured in serum-free RPMI medium with 10% healthy control or patient serum/plasma and were either left unstimulated or were stimulated with IFN-α2 or IFN-ω (10 ng/mL) for 15 min at 37°C. Each sample was tested once. Cells were fixed, permeabilized, and stained for intranuclear phospho-STAT1 (Y701). Cells were acquired on a BD LSRFortessa cytometer with gating on CD14+ monocytes and the data were analyzed with FlowJo software.
Luciferase-based immunoprecipitation assay (LIPS)
Levels of autoantibodies against IFN-α subtypes were measured in luciferase-based immunoprecipitation assay (LIPS), as previously described (9). IFNA1, IFNA2, IFNA8, and IFNA21 sequences were inserted into a modified pPK-CMV-F4 fusion vector (PromoCell GmbH, Germany), in which the firefly luciferase replaced the NanoLuc luciferase (Promega, USA). The resulting constructs were used to transfect HEK293 cells and the IFNA-luciferase fusion proteins were collected in the tissue culture supernatant. For autoantibody screening, we combined 2x106 luminescence units (LU) of IFNA1, IFNA2, IFNA8 and IFNA21 in a single IP reaction mixture (pool 1), and IFNA4, IFNA5, IFNA6 and IFNA7 in another IP reaction mixture (pool 2). Serum samples were incubated with Protein G agarose beads (Exalpha Biologicals, USA) at room temperature for 1 hour in a 96-well microfilter plate (Merck Millipore, Germany), and we then added 2x106 luminescence units (LU) of antigen and incubated for another hour. Each sample was tested once. The plate was washed with a vacuum system and Nano-Glo® Luciferase Assay Reagent (Promega, USA) was added. Luminescence intensity was measured with a VICTOR X Multilabel Plate Reader (PerkinElmer Life Sciences, USA). The results are expressed in arbitrary units (AU), as a fold-difference relative to the mean of the negative control samples.
IgG purification
We demonstrated that the IFN-α2 or IFN-ω neutralizing activity observed was due to auto-Abs and not another plasma factor, by depleting IgG from the plasma with a protein G buffer (Pierce Protein G IgG Binding Buffer, 21011) and column (NAb Protein G Spin Columns, 89953). All buffers were homemade: glycine 0.1 M pH=2.7, Tris 1.5 M pH = 8. Total plasma was loaded onto the column. Each sample was tested once. Purified IgG were then concentrated (Pierce Protein Concentrators PES, 50K MWCO, 88504). Without eluting the IgG, the flow-through fraction (IgG-depleted) was then collected and compared to total plasma in the luciferase neutralization assay.
Statistical analysis
Odds ratios (OR) and P-values for the effect of auto-Abs neutralizing each type I IFN on critical or severe COVID-19, using asymptomatic/mild patients or the general population as controls and adjusted on age in years and sex, were estimated by means of Firth’s bias-corrected logistic regression (49, 50) as implemented in the “logistf” R package (https://rdrr.io/cran/logistf/). Effect of age (quantitative in years or binary +/− 65 years) and sex on the presence of neutralizing auto-Abs in each cohort (critical, severe, deceased and general population) was tested by multivariable Firth’s bias-corrected logistic regression. The standard error of the prevalence of neutralizing auto-Abs to each type I IFN per age groups and sex were estimated using the Agresti-Coull approximation (51).
Schematic representation
Schematic representations (Fig. 1B) were created with BioRender.com.
SARS-CoV-2 experiment
SARS-CoV-2 strain USA-WA1/2020 was obtained from BEI Resources and amplified in Caco-2 cells at 37°C. Viral titers were measured on Huh-7.5 hepatoma cells in a standard plaque assay. Caco-2 (H. sapiens, sex: male, colon epithelial) and Huh-7.5 cells (H. sapiens, sex: male, liver epithelial) were cultured in DMEM supplemented with 1% nonessential amino acids (NEAA) and 10% fetal bovine serum (FBS) at 37°C, under an atmosphere containing 5% CO2. Both cell lines have been tested negative for contamination with mycoplasma. SARS-CoV-2 experiments were performed as follows. Huh-7.5 cells were used to seed 96-well plates at a density of 7.5x103 cells/well. The following day, plasma samples or a commercial anti-IFN-α2 antibody (catalog number 21100-1; R&D Systems) were diluted to 1% and incubated with 5 pM (~100 pg/mL) or 20 pM (~400 pg/mL) recombinant IFN-α2 (catalog number 11101-2; R&D systems) for 1 hour at 37°C (dilutions: plasma samples = 1/100 and anti-IFN-α2 antibody = 1/1,000). Molar ratio was calculated according to the manufacturer’s datasheet and with http://molbiol.ru/eng/scripts/01_04.html. Following this incubation period, the cell culture medium was removed from the 96-well plates by aspiration and replaced with the plasma/anti-IFN-α2 antibody and IFN-α2 mixture. Each sample was tested once, in triplicate. The plates were incubated overnight and the plasma/anti-IFN-α2 antibody plus IFN-α2 mixture was removed by aspiration. The cells were washed once with PBS to remove potential anti-SARS-CoV-2-neutralizing antibodies and fresh medium was then added. Cells were then infected with SARS-CoV-2 by directly adding the virus to the wells. Cells infected at a MOI of 0.05 PFU/cell and incubated at 33°C for 48 hours. The cells were fixed with 7% formaldehyde, stained for SARS-CoV-2 with an anti-N antibody (catalog no. GTX135357; GeneTex), imaged and analyzed as previously described (9).
Acknowledgments
We thank the patients and their families for placing their trust in us. We warmly thank the members of both branches of the Laboratory of Human Genetics of Infectious Diseases. We warmly thank Y. Nemirovskaya, M. Woollett, D. Liu, S. Boucherit, C. Rivalain, M. Chrabieh and L. Lorenzo for administrative assistance. We also thank the staff of the Imagine facilities: C. Bureau, L. Colonna, S. Paillet, N. Ghouas, M. Sy. We are also grateful to the legal team and technology transfer staff of the Imagine Institute: M. Pilorges, R. Marlanges, E. Rubino, W. Loewen, D. Beudin, N. Wuylens. We thank all the staff of the Imagine Institute, Necker Hospital and Necker sorting center for their help. We warmly thank S. Nagashima (Department of Epidemiology, Infectious Disease Control and Prevention, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan). Funding: The Laboratory of Human Genetics of Infectious Diseases is supported by the Howard Hughes Medical Institute, the Rockefeller University, the St. Giles Foundation, the National Institutes of Health (NIH) (R01AI088364), the National Center for Advancing Translational Sciences (NCATS), NIH Clinical and Translational Science Award (CTSA) program (UL1 TR001866), a Fast Grant from Emergent Ventures, Mercatus Center at George Mason University, the Yale Center for Mendelian Genomics and the GSP Coordinating Center funded by the National Human Genome Research Institute (NHGRI) (UM1HG006504 and U24HG008956), the Yale High Performance Computing Center (S10OD018521), the Fisher Center for Alzheimer’s Research Foundation, the Meyer Foundation, the JPB Foundation, the French National Research Agency (ANR) under the “Investments for the Future” program (ANR-10-IAHU-01), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French Foundation for Medical Research (FRM) (EQU201903007798), the FRM and ANR GENCOVID project (ANR-20-COVI-0003), ANRS Nord-Sud (ANRS-COV05), ANR GENVIR (ANR-20-CE93-003) and ANR AABIFNCOV (ANR-20-CO11-0001) projects, the European Union’s Horizon 2020 research and innovation program under grant agreement No 824110 (EASI-genomics), the Square Foundation, Grandir - Fonds de solidarité pour l’enfance, the Fondation du Souffle, the SCOR Corporate Foundation for Science, Institut National de la Santé et de la Recherche Médicale (INSERM) and the University of Paris. P.B. was supported by the French Foundation for Medical Research (FRM, EA20170638020). P.B., J.R. and T.L.V. were supported by the MD-PhD program of the Imagine Institute (with the support of the Fondation Bettencourt Schueller). Work in the Laboratory of Virology and Infectious Disease was supported by the NIH (P01AI138398-S1, 2U19AI111825, and R01AI091707-10S1), a George Mason University Fast Grant, and the G. Harold and Leila Y. Mathers Charitable Foundation. The French COVID Cohort study group was sponsored by INSERM and supported by the REACTing consortium and by a grant from the French Ministry of Health (PHRC 20-0424). The Cov-Contact Cohort was supported by the REACTing consortium, the French Ministry of Health, and the European Commission (RECOVER WP 6). This work was also partly supported by the Intramural Research Program of the NIAID and NIDCR, NIH (grants ZIA AI001270 to LDN and 1ZIAAI001265 to HCS). This program is supported by the Agence Nationale de la Recherche, reference ANR-10-LABX-69-01. K. Kisand’s group was supported by the Estonian Research Council grant PRG117 and PRG377. R. Halwani was supported by an Al Jalila Foundation Seed Grant (AJF202019), Dubai, UAE, and a COVID-19 research grant (CoV19-0307) from University of Sharjah, UAE. L. Imberti reported funding from Regione Lombardia, Italy (project “Risposta immune in pazienti con COVID-19 e co-morbidità”). L. Imberti and G.L. Marseglia reported funding from Regione Lombardia, Italy (project “Risposta immune in pazienti con COVID-19 e co-morbidità”). This research was partially supported by the Instituto de Salud Carlos III (COV20/0968). J. R. Heath reported funding from Biomedical Advanced Research and Development Authority HHSO10201600031C. S. Okada reports funding Research Program on Emerging and Re-emerging Infectious Diseases from Japan Agency for Medical Research and development, AMED (Grant Number: JP20fk0108531). G. Gorochov was supported by ANR Flash COVID-19 program and SARS-CoV-2 Program of the Faculty of Medicine from Sorbonne University iCOVID programs. The Three-City (3C) Study was conducted under a partnership agreement among the INSERM, the Victor Segalen–Bordeaux II University, and Sanofi-Aventis. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The 3C Study was also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Mutuelle Générale de l’Education Nationale (MGEN), Institut de la Longévité, Conseils Régionaux of Aquitaine and Bourgogne, Fondation de France, and Ministry of Research–INSERM Programme “Cohortes et collections de données biologiques”. S. Debette was supported by the University of Bordeaux Initiative of Excellence. P. K. Gregersen reports funding from the National Cancer Institute, NIH, under Contract No. 75N91019D00024, Task Order No. 75N91021F00001. J.W. is supported by an FWO Fundamental Clinical Mandate (1833317N). Sample processing at IrsiCaixa was possible thanks to the crowdfunding initiative YoMeCorono. Work at Vall d’Hebron was also partly supported by research funding from Instituto de Salud Carlos III grant PI17/00660 cofinanced by the European Regional Development Fund (ERDF). C.R.G. and colleagues of the Canarian Health System Sequencing Hub were supported by the Instituto de Salud Carlos III (COV20_01333 and COV20_01334, Spanish Ministry of Science and Innovation RTC-2017-6471-1; AEI/FEDER, UE); from Grupo DISA (OA18/017 and OA20/024); and Cabildo Insular de Tenerife (CGIEU0000219140 and “Apuestas científicas del ITER para colaborar en la lucha contra la COVID-19”). C.M.B. is supported by a MSFHR Health Professional-Investigator Award. P.Q.H. and L.H. were funded by the European Union’s Horizon 2020 research and innovation program (ATAC, 101003650). Work at Y.-L. Lau’s laboratory in the University of Hong Kong (HKU) was supported by the Society for the Relief of Disabled Children. MBBS/PhD study of D.L. Leung in HKU was supported by the Croucher Foundation. J.L.F. was supported in part by the Coopération Scientifique France-Colciencias (ECOS-Nord/COLCIENCIAS/MEN/ICETEX (806-2018) and Colciencias contract 713-2016 (code 111574455633). A.K. was in part supported by grants NU20-05-00282 and NV18-05-00162 issued by the Czech Health Research Council and Ministry of Health, Czech Republic. L.P. was funded by Program Project COVID-19 OSR-UniSR and Ministero della Salute (COVID-2020-12371617). I.M. is a Senior Clinical Investigator at the Research Foundation – Flanders, and is supported by the CSL Behring Chair of Primary Immunodeficiencies, by the KU Leuven C1 Grant C16/18/007, by a VIB GC PID Grant, by the FWO Grants G0C8517N, G0B5120N and G0E8420N and by the Jeffrey Model Foundation. I.M. has received funding under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 948959). E.A. received funding from the Hellenic Foundation for Research and Innovation (INTERFLU, no. 1574). M.V. received funding from the Sao Paulo Research Foundation (FAPESP) [grant number 2020/09702-1] and JBS S.A [grant number 69004]. The NH-COVAIR study group consortium was supported by a grant from the Meath Foundation. Author contributions: P.B., A.G., T.L.V., J.R., Q.P., E.M., H.-H.H., S.E., L.H., M.G.P., L.B., A.P.M., R.Y., M.M., P.P., K.S., J.M., S.T.-A., A.B., K.S., E.S., L.B.R., M.M., A.A., B.C. A.F., S.M.H., O.M.D., Y.Z., B.B., V.B., S.-Y.Z., L.D.N., H.C.S., K.K., S.O., A.P., E.J., C.M.R. and Q.Z. performed or supervised experiments, generated and analyzed data, and contributed to the manuscript by providing figures and tables. J.M., A.C., and L.A. performed computational analyses of data. P.B., N.D.-P., Y.T.L., C.-E.L., B.A.-B., A.G., J.P., P.M., P.R., F.C., J.T., J.R., L.L., J.-C.L., S.G., S.T.-A., A.B., K.S., P.G., D.D., P.-L.T., D.S., A.S., B.M., V.T., J.R.H., J.C.F., J.-M.A., A.C.-N., L.I., A.B., R.C., P.Bo., A.B., A.L.S., A.M.P., F.H., S.D., R.L.N., T.M., A.A.B., T.O., S.K., C.R., S.P., P.Q.H., L.H., A.D., A.K., C.N.M., A.A., G.C., V.L.L, F.C., L.A.B., E.D.-G. L.V., D.V.D.B., S.G.T., S.B., D.D., L.Q.-M., M.C.N., R.A., D.A., I.,B., H.B.-F., J.W., I.M. D.H., N.S.S.-A., R.H., K.D., J.S., S.M.S., L.G., A.K., F.M., Y.N., J.S.-V., A.H.D., S.P.K, N.M.B., S.A.A.A.-K, Y.S., J.T., O.B., N.Y.K., Y.-L.-L, D.L., M.C., J.M., R.K., L.F.R., C.B., M.S.A., R.R.-B., R.M., M.V., M.Z., A.C.G., F.V., G.M., D.C.V., L.R., S.R.O., A.S., E.A., S.S., I.T., J.F., S.L., K.B., R.P.L., S.M., S.B., V.V., O.H., A.P.O., T.H.M., L.R., J.M., S.D., X.D.L., X.D., F.M., M.Z., P.S.-P., R.C., G.G., X.S.M., S.S., J.M.-P., D.R., M.V., P.K.G., L.P., C.R.-G., L.D.N., H.C.S., P.T., Q.Z., and J.-L.C. evaluated and recruited patients to COVID and/or control cohorts of patients, and/or cohorts of individuals from the general population. P.B. and J.-L.C. wrote the manuscript. J.-L.C. supervised the project. All the authors edited the manuscript. Competing interests: Jean-Laurent Casanova is an inventor on patent application PCT/US2021/042741, filed July 22, 2021, submitted by The Rockefeller University, that covers diagnosis of, susceptibility to, and treatment of, viral disease and viral vaccines, including COVID-19 and vaccine-associated diseases. M. C. Nussenzweig is an inventor on patent application PCT/US2021/070472 submitted by The Rockefeller University that covers neutralizing anti-SARS-CoV-2 antibodies and methods of use thereof. M. C. Nussenzweig reports being on the Scientific Advisory Board of Celldex and Frontier Biotechnologies. Richard P. Lifton reports being a non-executive director of Roche. France Mentré receives fees for consulting from IPSEN and Da Volterra. Her research group receives research grants from Roche, Sanofi and Da Volterra. Data and materials availability: All the data are available in the manuscript or in the supplementary materials. Plasma, cells, and genomic DNA are available from J.-L.C. under a material transfer agreement with The Rockefeller University or the Imagine Institute. Huh-7.5 cells are available on request from C.M.R. under a material transfer agreement with The Rockefeller University and Apath, LLC. The materials and reagents used are almost exclusively commercially available and nonproprietary. Materials derived from human samples may be made available on request, subject to any underlying restrictions concerning such samples. This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license, permitting unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/. This license does not apply to figures/photos/artwork or other content included in the article that is credited to a third party; authorization from the rights holder before using such material. For patients enrolled in the Italian cohort, patient specimens may be available from Monza, subject to approval by their local IRB, through an MTA.
Members of consortium groups with co-author status:
HGID Lab
Benedetta Bigio1, Soraya Boucherit2,3, Aliénor de la Chapelle2, Jie Chen1, Maya Chrabieh2,3, Boubacar Coulibaly2,3, Dana Liu1, Yelena Nemirowskaya1, Inés Marín Cruz2, Marie Materna2,3, Sophie Pelet2, Yoann Seeleuthner2,3, Chloé Thibault2,3, Zhiyong Liu1.
1St. Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, NY, USA. 2Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U1163, Necker Hospital for Sick Children, Paris, France. 3University of Paris, Imagine Institute, Paris, France.
COVID Clinicians
Jorge Abad1, Giulia Accordino2, Cristian Achille3, Sergio Aguilera-Albesa4, Aina Aguiló-Cucurull5, Alessandro AIUTI6, Esra Akyüz Özkan7, Ilad Alavi Darazam8, Jonathan Antonio Roblero Albisures9, Juan C Aldave10, Miquel Alfonso Ramos11, Taj Ali Khan12, Anna Aliberti13, Seyed Alireza Nadji14, Gulsum Alkan15, Suzan A. AlKhater16, Jerome Allardet-Servent17, Luis M Allende18, Rebeca ALONSO-ARIAS19, Mohammed S Alshahrani20, Laia Alsina21, Marie-Alexandra Alyanakian22, Blanca Amador Borrero23, Zahir Amoura24, Arnau Antolí25, Romain Arrestier26, Mélodie Aubart27, Teresa Auguet28, Iryna Avramenko29, Gökhan Aytekin30, Axelle Azot31, Seiamak Bahram32, Fanny Bajolle33, Fausto Baldanti34, Aurélie Baldolli35, Maite Ballester36, Hagit Baris Feldman37, Benoit Barrou38, Federica BARZAGH6, Sabrina Basso39, Gulsum Iclal BAYHAN40, Alexandre Belot41, Liliana BEZRODNIK42, Agurtzane Bilbao43, Geraldine Blanchard-Rohner44, Ignacio Blanco45, Adeline Blandinières46, Daniel Blázquez-Gamero47, Alexandre Bleibtreu48, Marketa Bloomfield49, Mireia Bolivar-Prados50, Anastasiia BONDARENKO51, Alessandro Borghesi3, Raphael Borie52, Elisabeth Botdhlo-Nevers53, Ahmed A Bousfiha54, Aurore Bousquet55, David Boutolleau56, Claire Bouvattier57, Oksana Boyarchuk58, Juliette Bravais59, M. Luisa Briones60, Marie-Eve Brunner61, Raffaele Bruno62, Maria Rita P Bueno63, Huda Bukhari64, Jacinta Bustamante33, Juan José Cáceres Agra65, Ruggero Capra66, Raphael Carapito67, Maria Carrabba68, Giorgio CASARI6, Carlos Casasnovas69, Marion Caseris70, Irene Cassaniti34, Martin Castelle71, Francesco Castelli72, Martín Castillo de Vera73, Mateus V Castro63, Emilie Catherinot74, Jale Bengi Celik75, Alessandro Ceschi76, Martin Chalumeau77, Bruno Charbit78, Matthew P. Cheng79, Père Clavé50, Bonaventura Clotet80, Anna Codina81, Yves Cohen82, Roger Colobran83, Cloé Comarmond84, Alain Combes85, Patrizia Comoli39, Angelo G Corsico2, Taner Coşkuner86, Aleksandar Cvetkovski87, Cyril Cyrus88, David Dalmau89, François Danion90, David Ross Darley91, Vincent Das92, Nicolas Dauby93, Stéphane Dauger94, Paul De Munter95, Loic de Pontual96, Amin Dehban97, Geoffroy Delplancq98, Alexandre Demoule99, Isabelle Desguerre100, Antonio Di Sabatino101, Jean-Luc Diehl102, Stephanie Dobbelaere103, Elena Domínguez-Garrido104, Clément Dubost105, Olov EKWALL106, Şefika Elmas Bozdemir107, Marwa H Elnagdy108, Melike Emiroglu15, Akifumi Endo109, Emine Hafize Erdeniz110, Selma Erol Aytekin111, Maria Pilar ETXART LASA112, Romain Euvrard113, Giovanna Fabio68, Laurence Faivre114, Antonin Falck115, Muriel Fartoukh116, Morgane Faure117, Miguel Fernandez Arquero118, Ricard Ferrer119, Jose Ferreres120, Carlos Flores121, Bruno Francois122, Victoria Fumadó123, Kitty S C Fung124, Francesca Fusco125, Alenka Gagro126, Blanca Garcia Solis127, Pascale Gaussem128, Zeynep GAYRETLI129, Juana Gil-Herrera130, Laurent Gilardin131, Audrey Giraud Gatineau132, Mònica Girona-Alarcón133, Karen Alejandra Cifuentes Godínez134, Jean-Christophe Goffard135, Nacho GONZALES136, Luis I Gonzalez-Granado137, Rafaela González-Montelongo138, Antoine Guerder139, Belgin Gülhan140, Victor Daniel Gumucio141, Leif Gunnar Hanitsch142, Jan Gunst143, Marta Gut144, Jérôme Hadjadj145, Filomeen Haerynck146, Rabih Halwani147, Lennart Hammarström148, Selda HANCERLI149, Tetyana Hariyan150, Nevin Hatipoglu151, Deniz Heppekcan152, Elisa Hernandez-Brito153, Po-ki Ho154, María Soledad Holanda-Peña155, Juan P Horcajada156, Sami Hraiech157, Linda Humbert158, Ivan F N Hung159, Alejandro D. Iglesias160, Antonio Íñigo-Campos138, Matthieu Jamme161, María Jesús Arranz89, Marie-Thérèse Jimeno162, Iolanda Jordan133, Saliha Kanık Yüksek163, Yalcin Burak Kara164, Aydın Karahan165, Adem KARBUZ166, Kadriye Kart Yasar167, Ozgur Kasapcopur168, Kenichi Kashimada169, Sevgi Keles111, Yasemin Kendir Demirkol170, Yasutoshi Kido171, Can KIZIL172, Ahmet Osman Kılıç173, Adam Klocperk174, Antonia Koutsoukou175, Zbigniew J. Król176, Hatem Ksouri177, Paul Kuentz178, Arthur M C Kwan179, Yat Wah M Kwan180, Janette S Y Kwok181, Jean-Christophe Lagier182, David S Y Lam183, Vicky Lampropoulou184, Fanny Lanternier185, Yu-Lung LAU186, Fleur Le Bourgeois94, Yee-Sin Leo187, Rafael Leon Lopez188, Daniel Leung186, Michael Levin189, Michael Levy94, Romain Lévy33, Zhi Li78, Daniele Lilleri34, Edson Jose Adrian Bolanos Lima190, Agnes Linglart191, Eduardo López-Collazo192, José M. Lorenzo-Salazar138, Céline Louapre193, Catherine Lubetzki193, Kwok-Cheung Lung194, Charles-Edouard Luyt195, David C Lye196, Cinthia MAGNONE197, Davood Mansouri198, Enrico Marchioni199, Carola Marioli2, Majid Marjani200, Laura MARQUES201, Jesus Marquez Pereira202, Andrea Martín-Nalda203, David Martínez Pueyo204, Javier Martinez-Picado205, Iciar Marzana206, Carmen Mata-Martínez207, Alexis Mathian24, Larissa RB Matos63, Gail V Matthews208, Julien Mayaux209, Raquel McLaughlin-Garcia210, Philippe Meersseman211, Jean-Louis Mège212, Armand Mekontso-Dessap213, Isabelle Melki115, Federica Meloni2, Jean-François Meritet214, Paolo Merlani215, Özge METIN AKCAN216, Isabelle Meyts217, Mehdi Mezidi218, Isabelle Migeotte219, Maude Millereux220, Matthieu Million221, Tristan Mirault222, Clotilde Mircher223, Mehdi Mirsaeidi224, Yoko Mizoguchi225, Bhavi P Modi226, Francesco Mojoli13, Elsa MONCOMBLE227, Abián Montesdeoca Melián228, Antonio Morales Martinez229, Francisco Morandeira230, Pierre-Emmanuel Morange231, Clémence Mordacq158, Guillaume Morelle232, Stéphane J Mouly233, Adrián Muñoz-Barrera138, Cyril Nafati234, Shintaro Nagashima235, Yu Nakagama171, Bénédicte Neven236, João Farela Neves237, Lisa FP Ng238, Yuk-Yung Ng239, hubert Nielly105, Yeray Novoa Medina210, Esmeralda Nuñez Cuadros240, J. Gonzalo Ocejo-Vinyals241, Keisuke Okamoto109, Mehdi Oualha33, Amani Ouedrani22, Tayfun Özçelik242, Aslinur Ozkaya-Parlakay140, Michele Pagani13, Qiang Pan-Hammarström148, Maria Papadaki243, Christophe Parizot209, Philippe Parola244, Tiffany Pascreau245, Stéphane Paul246, Estela Paz-Artal247, Sigifredo Pedraza248, Nancy Carolina González Pellecer134, Silvia Pellegrini249, Rebeca Pérez de Diego127, Xosé Luis Pérez-Fernández141, Aurélien Philippe250, Quentin Philippot116, Adrien Picod251, Marc Pineton de Chambrun85, Antonio Piralla34, Laura Planas-Serra252, Dominique Ploin253, Julien Poissy254, Géraldine Poncelet70, Garyphallia Poulakou175, Marie S Pouletty255, Persia Pourshahnazari256, Jia Li Qiu-Chen257, Paul Quentric209, Thomas Rambaud258, Didier Raoult212, Violette RAOULT259, Anne-Sophie Rebillat223, Claire Redin260, Léa Resmini261, Pilar Ricart262, Jean-Christophe Richard263, Raúl Rigo-Bonnin264, Nadia rivet46, Jacques G Rivière265, Gemma Rocamora-Blanch25, Mathieu P RODERO266, Carlos Rodrigo267, Luis Antonio Rodriguez190, Carlos Rodriguez-Gallego268, Agustí Rodriguez-Palmero269, Carolina Soledad Romero270, Anya Rothenbuhler271, Damien Roux272, Nikoletta Rovina175, Flore Rozenberg273, Yvon Ruch90, Montse Ruiz274, Maria Yolanda Ruiz del Prado275, Juan Carlos Ruiz-Rodriguez119, Joan Sabater-Riera141, Kai Saks276, Maria Salagianni184, Oliver Sanchez277, Adrián Sánchez-Montalvá278, Silvia Sánchez-Ramón279, Laire Schidlowski280, Agatha Schluter252, Julien Schmidt281, Matthieu Schmidt282, Catharina Schuetz283, Cyril E Schweitzer284, Francesco Scolari285, Anna Sediva286, Luis Seijo287, Analia Gisela Seminario42, Damien Sene23, Piseth Seng221, Sevtap Senoglu167, Mikko Seppänen288, Alex Serra Llovich289, Mohammad Shahrooei97, Anna Shcherbina290, Virginie Siguret291, Eleni Siouti292, David M Smadja293, Nikaia Smith78, Ali Sobh294, Xavier Solanich25, Jordi Solé-Violán295, Catherine Soler296, Pere Soler-Palacín297, Betül Sözeri86, Giulia Maria Stella2, Yuriy Stepanovskiy298, Annabelle Stoclin299, Fabio Taccone219, Yacine Tandjaoui-Lambiotte300, Jean-Luc Taupin301, Simon J Tavernier302, Loreto Vidaur Tello112, Benjamin Terrier303, Guillaume Thiery304, Christian Thorball260, Karolina THORN305, Caroline Thumerelle158, Imran Tipu306, Martin Tolstrup307, Gabriele Tomasoni308, Julie Toubiana77, Josep Trenado Alvarez309, Vasiliki TRIANTAFYLLIA310, Sophie TROUILLET-ASSANT311, Jesús Troya312, Owen T Y Tsang313, Liina Tserel314, Eugene Y K Tso315, Alessandra Tucci316, Şadiye Kübra Tüter Öz15, Matilde Valeria Ursini125, Takanori Utsumi225, Yurdagul Uzunhan317, Pierre Vabres318, Juan Valencia-Ramos319, Ana Maria Van Den Rym127, Isabelle Vandernoot320, Valentina Velez-Santamaria321, Silvia Patricia Zuniga Veliz134, Mateus C Vidigal322, Sébastien Viel253, Cédric Vilain323, Marie E Vilaire-Meunier223, Judit Villar-García324, Audrey Vincent57, Guillaume Vogt325, Guillaume Voiriot326, Alla Volokha327, Fanny Vuotto158, Els Wauters328, Joost Wauters329, Alan K L Wu330, Tak-Chiu Wu331, Aysun Yahşi332, Osman YESILBAS333, Mehmet Yildiz168, Barnaby E Young187, Ufuk Yükselmiş334, Mayana Zatz63, Marco Zecca39, Valentina Zuccaro62, Van Praet Jens335, Lambrecht Bart N.336, Van Braeckel Eva336, Bosteels Cédric336, Hoste Levi337, Hoste Eric338, Fré Bauters336, Jozefien De Clercq336, Heijmans Cathérine339, Slabbynck Hans340, Naesens Leslie341, Benoit Florkin342, Cécile Boulanger343, Dimitri Vanderlinden344
1Germans Trias i Pujol University Hospital and Research Institute, Badalona, Barcelona, Spain. 2Respiratory Diseases Division, IRCCS Policlinico San Matteo Foundation, University of Pavia, Pavia, Italy. 3Neonatal Intensive Care Unit, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. 4Navarra Health Service Hospital, Pamplona, Spain. 5Jeffrey Model Diagnostic and Research Center for Primary Immunodeficiencies, Barcelona, Catalonia, Spain, Immunology Division, Genetics Department, Vall d’Hebron University Hospital (HUVH), Vall d’Hebron Research Institute (VHIR), Vall d’Hebron Barcelona Hospital Campus, Universitat Autònoma de Barcelona (UAB), Barcelona, Catalonia, Spain. Catalonia, Barcelona, Spain. 6Immunohematology Unit, San Raffaele Hospital, Milan, Italy. 7Ondokuz Mayıs University Medical Faculty Pediatrics, Samsun, Turkey. 8Department of Infectious Diseases, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 9Hospital Regional de Huehuetenango, “Dr. Jorge Vides de Molina”, Guatemala. 10Hospital Nacional Edgardo Rebagliati Martins, Lima, Peru. 11Parc Sanitari Sant Joan de Déu, Sant Boi de Llobregat Spain. 12Khyber Medical University, Khyber Pakhtunkhwa, Pakistan. 13Anesthesia and Intensive Care, Rianimazione I, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. 14Virology Research Center, National institutes of Tuberculosis and Lung diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 15Department of Pediatrics, Division of Pediatric Infectious Diseases, Selcuk University Faculty of Medicine, Konya, Turkey. 16College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; Department of Pediatrics, King Fahad Hospital of the University, Al-Khobar, Saudi Arabia. 17Intensive care unit, Hôpital Européen, Marseille, France. 18Immunology Department, Hospital 12 de Octubre, Research Institute imas12, Complutense University, Madrid, Spain. 19Immunology Department, Asturias Central University Hospital, Biosanitary Research Institute of the Principality of Asturias (ISPA), Oviedo, Spain. 20Emergency and Critical Care Medicine Departments, College of Medicine, Imam AbdulRahman Ben Faisal University, Dammam, Saudi Arabia. 21Clinical Immunology and Primary Immunodeficiencias Unit, Hospital Sant Joan de Déu, Institut de Recerca Sant Joan de Déu, Barcelona; Universitat de Barcelona, Barcelona, Spain. 22Department of Biological Immunology, Necker Hospital for Sick Children, APHP and INEM, Paris, France. 23Internal medicine department, Hôpital Lariboisière, APHP; Université de Paris, Paris, France. 24Internal medicine department, Pitié-Salpétrière Hospital, Paris, France. 25Department of Internal Medicine, Hospital Universitari de Bellvitge, IDIBELL, Barcelona, Spain. 26Service de Médecine Intensive Réanimation, Hôpitaux Universitaires Henri Mondor, AP-HP; Groupe de Recherche Clinique CARMAS, Faculté de Santé de Créteil, Université Paris Est Créteil, Créteil, France. 27INSERM U1163, University of Paris, Imagine Institute, Paris, France & Pediatric Neurology Department, Necker-Enfants malades Hospital, APHP, Paris, France. 28Hospital U. de Tarragona Joan XXIII. Universitat Rovira i Virgili (URV). IISPV, Tarragona, Spain. 29Department of Propedeutics of Pediatrics and Medical Genetics, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine. 30Department of Immunology and Allergy, Konya City Hospital, Konya, Turkey. 31Private practice, Paris, France. 32INSERM U1109, University of Strasbourg, Strasbourg, France. 33Necker Hospital for Sick Children, AP-HP, Paris, France. 34Molecular Virology Unit, Microbiology and Virology Department, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. 35Department of Infectious Diseases, CHU de Caen, Caen, France. 36Consorcio Hospital General Universitario, Valencia, Spain. 37The Genetics Institute, Tel Aviv Sourasky Medical Center and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. 38Dept Urology, Nephrology, Transplantation, APHP-SU, Sorbonne Université, INSERM U 1082, Paris, France. 39Cell Factory and Pediatric Hematology-Oncology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. 40Yildirim Beyazit University, Faculty of Medicine, Ankara City Hospital, Children's Hospital, Ankara, Turkey. 41University of Lyon, CIRI, INSERM U1111, National referee center RAISE, Pediatric Rheumatology, HFME, Hospices Civils de Lyon, Lyon, France. 42Center for Clinical Immunology, CABA, Buenos Aires, Argentina. 43Cruces University Hospital, Bizkaia, Spain. 44Pediatric Immunology and Vaccinology Unit, Geneva University Hospitals and Faculty of Medicine, Geneva, Switzerland. 45University Hospital and Research Institute “Germans Trias i Pujol”, Badalona, Spain. 46Hematology, Georges Pompidou Hospital, APHP, Paris, France. 47Pediatric Infectious Diseases Unit, Instituto de Investigación Hospital 12 de Octubre (imas12), Hospital Universitario 12 de Octubre, Universidad Complutense, Madrid, Spain. 48Infectious disease Unit, Pitié-Salpêtrière Hospital, AP-AP, Paris, France. 49Department of Pediatrics, Thomayer’s Hospital, 1st Faculty of Medicine, Charles University, Prague, Czech Republic; Department of Immunology, Motol University Hospital, 2nd Faculty of Medicine, Charles University, Prague, Czech Republic. 50Centro de Investigación Biomédica en Red de Enfermedades Hepàticas y Digestivas (Ciberehd). Hospital de Mataró, Consorci Sanitari del Maresme, Mataró, Spain. 51Shupyk National Healthcare University of Ukraine, Kyiv, Ukraine. 52Service de Pneumologie, Hopital Bichat, APHP, Paris, France. 53Department of infectious diseases, CIC1408, GIMAP CIRI INSERM U1111, University Hospital of Saint-Etienne, Saint-Etienne, France. 54Clinical immunology unit, pediatric infectious disease departement, Faculty of Medicine and Pharmacy, Averroes University Hospital. LICIA Laboratoire d'immunologie clinique, d'inflammation et d'allergie, Hassann Ii University., Casablanca, Morocco. 55Bégin Military Hospital, St Mandé, France. 56Sorbonne Université, INSERM, Institut Pierre Louis d'Epidémiologie et de Santé Publique (iPLESP), AP-HP, Hôpital Pitié Salpêtrière, Service de Virologie, Paris, France. 57Endocrinology unit, APHP Hôpitaux Universitaires Paris-Sud, Le Kremlin-Bicêtre, France. 58Department of Children's Diseases and Pediatric Surgery, I.Horbachevsky Ternopil National Medical University, Ternopil, Ukraine. 59Pneumology Unit, Tenon Hospital, AP-HP, Paris, France. 60Department of Respiratory Diseases, Hospital Clínico y Universitario de Valencia, Valencia, Spain. 61Intensive care unit, Réseau Hospitalier Neuchâtelois, Neuchâtel, Switzerland. 62Infectious Diseases Unit, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy. 63Human Genome and stem-cell research center- University of São Paulo, São Paulo, Brazil. 64Department of Internal Medicine, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia. 65Hospital Insular, Las Palmas de Gran Canaria, Spain. 66MS Center, Spedali Civili, Brescia, Italy. 67Laboratoire d'ImmunoRhumatologie Moléculaire, plateforme GENOMAX, INSERM UMR_S 1109, Faculté de Médecine, ITI TRANSPLANTEX NG, Université de Strasbourg, Strasbourg, France. 68Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy. 69Neuromuscular Unit. Neurology Department. Hospital Universitari de Bellvitge - IDIBELL and CIBERER, Barcelona, Spain. 70Hopital Robert Debré, Paris, France. 71Pediatric Immuno-hematology Unit, Necker Enfants Malades Hospital, AP-HP, Paris, France. 72Department of Infectious and Tropical Diseases, University of Brescia, ASST Spedali Civili di Brescia, Brescia, Italy. 73Doctoral Health Care Center, Canarian Health System, Las Palmas de Gran Canaria, Spain. 74Hôpital Foch, Suresnes, France. 75Selcuk University Faculty of Medicine, Department of Anesthesiology and Reanimation, Intensive Care Medicine Unit, Konya, Turkey. 76Division of Clinical Pharmacology and Toxicology, Institute of Pharmacological Sciences of Southern Switzerland, Ente Ospedaliero Cantonale & Faculty of Biomedical Sciences, Università della Svizzera italiana, Lugano, Switzerland. 77Necker Hospital for Sick Children, Paris University, AP-HP, Paris, France. 78Pasteur Institute, Paris, France. 79McGill University Health Centre, Montreal, Canada. 80University Hospital and Research Institute “Germans Trias i Pujol”, IrsiCaixa AIDS Research Institute, UVic-UCC, Badalona, Spain. 81Clinical Biochemistry, Pathology, Pediatric Neurology and Molecular Medicine Departments and Biobank, Institut de Recerca Sant Joan de Déu and CIBERER-ISCIII, Esplugues, Spain. 82AP-HP, Avicenne Hospital, Intensive Care Unit, Bobigny, France; University Sorbonne Paris Nord, Bobigny, France; INSERM, U942, F-75010, Paris, France. 83Hospital Universitari Vall d’Hebron, Barcelona, Spain. 84Pitié-Salpêtrière Hospital, Paris, France. 85Service de médecine Intensive Réanimation, Groupe Hospitalier Pitié-Salpêtrière, Sorbonne Université, France. 86Umraniye Training and Research Hospital, Istanbul, Turkey. 87Faculty of Medical Sciences at University “Goce Delcev”, Shtip, North Macedonia. 88Department of Biochemistry, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia. 89Fundació Docencia i Recerca Mutua Terrassa, Barcelona, Spain. 90Maladies Infectieuses et Tropicales, Nouvel Hôpital Civil, CHU Strasbourg, Strasbourg, France. 91UNSW Medicine, St Vincent's Clinical School; Department of Thoracic Medicine, St Vincent's Hospital Darlinghurst, Sidney, Australia. 92Intensive Care unit, Montreuil hospital, Montreuil, France. 93CHU Saint-Pierre, Université Libre de Bruxelles (ULB), Brussels, Belgium. 94Pediatric Intensive Care Unit, Robert-Debré University Hospital, APHP, Paris, France. 95General Internal Medicine, University Hospitals Leuven, Belgium. 96Hôpital Jean Verdier, APHP, Bondy, France. 97Specialized Immunology Laboratory of Dr. Shahrooei, Sina Medical Complex, Ahvaz, Iran. 98Centre de génétique humaine, CHU Besançon, Besançon, France. 99Sorbonne Université médecine and APHP Sorbonne université site Pitié-Salpêtrière, Paris, France. 100Pediatric Neurology Department, Necker-Enfants malades hospital, APHP, Paris, France. 101Department of Internal Medicine, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy. 102Intensive Care unit, Georges Pompidou Hospital, APHP, Paris, France. 103Department of Pneumology, AZ Delta, Roeselare, Belgium. 104Molecular Diagnostic Unit, Fundación Rioja Salud, Logroño, La Rioja, Spain. 105Bégin military Hospital, Saint Mandé, France. 106Department of Pediatrics, Institute of Clinical Sciences, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden; Department of Rheumatology and Inflammation Research, Institute of Medicine, The Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden. 107Bursa City Hospital, Bursa, Turkey. 108Department of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Mansoura University, Mansoura, Egypt. 109Tokyo Medical and Dental University, Tokyo, Japan. 110Ondokuz Mayıs University Faculty of Medicine, Samsun, Turkey. 111Necmettin Erbakan University, Meram Medical Faculty, Division of Pediatric Allergy and Immunology, Konya, Turkey. 112University Donostia Hospital, Gipuzkoa, Spain. 113Internal Medicine, University Hospital Edouard Herriot, Hospices Civils de Lyon, Lyon, France. 114Centre de Génétique, CHU Dijon, Dijon, France. 115Robert Debré Hospital, Paris, France. 116APHP Tenon Hospital, Paris, France. 117Sorbonne Universités, UPMC University of Paris, Paris, France. 118Department of Clinical Immunology, Hospital Clínico San Carlos, Madrid, Spain. 119Intensive Care Department, Vall d’Hebron University Hospital (HUVH), Vall d’Hebron Barcelona Hospital Campus, Barcelona, Catalonia, Spain, Shock, Organ Dysfunction and Resuscitation Research Group. Vall d’Hebron Research Institute (VHIR), Vall d’Hebron Barcelona Hospital Campus, Barcelona, Catalonia, Spain. 120Intensive Care Unit, Hospital Clínico y Universitario de Valencia, Valencia, Spain. 121Genomics Division, Instituto Tecnológico y de Energías Renovables (ITER), Santa Cruz de Tenerife, Spain; CIBER de Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain; Research Unit, Hospital Universitario N.S. de Candelaria, Santa Cruz de Tenerife, Spain; Instituto de Tecnologías Biomédicas (ITB), Universidad de La Laguna, San Cristóbal de La Laguna, Spain, Santa Cruz de Tenerife, Spain. 122CHU Limoges and INSERM CIC 1435 & UMR 1092, Limoges, France. 123Infectious Diseases Unit, Department of Pediatrics, Hospital Sant Joan de Déu, Barcelona, Spain; Institut de Recerca Sant Joan de Déu, Spain; Universitat de Barcelona (UB), Barcelona, Spain. 124Department of Pathology, United Christian Hospital, Hong Kong. 125Institute of Genetics and Biophysics ‘Adriano Buzzati-Traverso’, IGB-CNR, Naples, Italy. 126Department of Pediatrics, Children's Hospital Zagreb, University of Zagreb School of Medicine, Zagreb, Josip Juraj Strossmayer University of Osijek, Medical Faculty Osijek, Osijek, Croatia. 127Laboratory of Immunogenetics of Human Diseases, IdiPAZ Institute for Health Research, La Paz Hospital, Madrid, Spain. 128Hematology, APHP, Hopital Européen Georges Pompidou and INSERM UMR-S1140, Paris, France. 129Faculty of Medicine, Department of Pediatrics, Division of Pediatric Infectious Diseases, Karadeniz Technical University, Trabzon, Turkey. 130Division of Immunology, Hospital General Universitario and Instituto de Investigación Sanitaria “Gregorio Marañón”, Madrid, Spain. 131Bégin military Hospital, Bégin, France. 132Aix Marseille Univ, IRD, AP-HM, SSA, VITROME, IHU Méditerranée Infection, Marseille, France, French Armed Forces Center for Epidemiology and Public Health (CESPA), Marseille, France. 133Pediatric Intensive Care Unit, Hospital Sant Joan de Déu, Barcelona, Spain. 134Guatemala. 135Department of Internal Medicine, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium. 136Immunodeficiencies Unit, Research Institute Hospital, madrid, Spain. 137Primary Immunodeficiencies Unit, Pediatrics, University Hospital 12 octubre, Madrid, Spain; School of Medicine Complutense University of Madrid, Madrid, Spain. 138Genomics Division, Instituto Tecnológico y de Energías Renovables (ITER), Santa Cruz de Tenerife, Spain. 139Assistance Publique Hôpitaux de Paris, Paris, France. 140Ankara City Hospital, Ankara, Turkey. 141Department of Intensive Care, Hospital Universitari de Bellvitge, IDIBELL, Barcelona, Spain. 142Immunodeficiency Outpatient Clinic, Institute for Medical Immunology, FOCIS Center of Excellence, Charité Universitätsmedizin Berlin, Germany. 143Surgical Intensive Care Unit, University Hospitals Leuven, Leuven, Belgium. 144CNAG-CRG, Barcelona Institute of Science and Technology, Barcelona, Spain. 145Department of Internal Medicine, National Reference Center for Rare Systemic Autoimmune Diseases, AP-HP, APHP-CUP, Hôpital Cochin, Paris, France. 146Department of Pediatric Immunology and Pulmonology, Center for Primary Immunodeficiency Ghent, Jeffrey Model Diagnosis and Research Center, PID research lab, Ghent University Hospital, Ghent, Belgium. 147Sharjah Institute of Medical Research, College of Medicine, University of Sharjah, Sharjah, UAE, Sharjah, UAE. 148Department of Biosciences and Nutrition, SE14183, Huddinge, Karolinska Institutet, Stockholm, Sweden. 149Department of Pediatrics (Infectious Diseases), Istanbul Faculty of Medicine, Istanbul University, Istanbul, Turkey. 150I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine. 151Pediatric Infectious Diseases Unit, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, University of Health Sciences, Istanbul, Turkey. 152Health Sciences University, Darıca Farabi Education and Research Hospital, Kocaeli, Turkey. 153Department of Immunology, Hospital Universitario de Gran Canaria Dr. Negrín, Canarian Health System, Las Palmas de Gran Canaria, Spain. 154Department of Pediatrics, Queen Elizabeth Hospital, Hong Kong. 155IntensivenCare Unit. Marqués de Valdecilla Hospital, Santander, Spain. 156Hospital del Mar, Institut Hospital del Mar d'Investigacions Mèdiques (IMIM), UAB, UPF, Barcelona. 157Intensive care unit, APHM, Marseille, France. 158 CHU Lille, unité de pneumologie et al.lergologie pédiatriques, Lille, France. 159Department of Medicine, The University of Hong Kong, Hong Kong. 160Department of Pediatrics, Columbia University, New York, NY, USA. 161Centre hospitalier intercommunal Poissy Saint Germain en Laye, Poissy, France. 162IHU Méditerranée Infection, Service de l'Information Médicale, Hôpital de la Timone, Marseille, France. 163Health Science University Ankara City Hospital, Ankara, Turkey. 164School of Medicine, General Surgery Department Fevzi Çakmak Mah, Marmara University, Istanbul, Turkey. 165Mersin City Education and Research Hospital, Mersin, Turkey. 166Division of Pediatric Infectious Diseases, Prof. Dr. Cemil Tascıoglu City Hospital, Istanbul, Turkey. 167Departments of Infectious Diseases and Clinical Microbiology, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, University of Health Sciences, Istanbul, Turkey. 168Department of Pediatric Rheumatology, Istanbul University-Cerrahpasa, Istanbul, Turkey. 169Department of Pediatrics, Tokyo Medical and Dental University, Tokyo, Japan. 170Health Sciences University, Umraniye Education and Research Hospital, Istanbul, Turkey. 171Department of Parasitology and Research Center for Infectious Disease Sciences, Graduate School of Medicine, Osaka City University, Osaka, Japan. 172Pediatric Infectious Diseases Unit of Osman Gazi University Medical School in Eskişehir, Turkey. 173Meram Medical Faculty, Necmettin Erbakan University, Konya, Turkey. 174Department of Immunology, 2nd Faculty of Medicine, Charles University and University Hospital in Motol, Prague, Czech Republic. 175ICU, 1st Department of Respiratory Medicine, National and Kapodistrian University of Athens, Medical School, 'Sotiria' General Hospital of Chest Diseases, Athens, Greece. 176Central Clinical Hospital of the Ministry of Interior and Administration, Warsaw, Poland. 177Clinique des soins intensifs, HFR Fribourg, Fribourg, Switzerland. 178Oncobiologie Génétique Bioinformatique, PC Bio, CHU Besançon, Besançon, France. 179Department of Intensive Care, Tuen Mun Hospital, Hong Kong. 180Pediatric Infectious Disease Unit, Hospital Authority Infectious Disease Center, Princess Margaret Hospital, Hong Kong (Special Administrative Region), China. 181Department of Pathology, Queen Mary Hospital, Hong Kong. 182Aix Marseille Univ, IRD, MEPHI, IHU Méditerranée Infection, Marseille, France. 183Department of Pediatrics, Tuen Mun Hospital, Hong Kong. 184Biomedical Research Foundation of the Academy of Athens, Athens, Greece. 185Necker hospital, Paris, France. 186Department of Pediatrics and Adolescent Medicine, The University of Hong Kong, Hong Kong, China. 187National Centre for Infectious Diseases, Singapore. 188Hospital Universitario Reina Sofía, Cordoba, Spain. 189Imperial College, London, England. 190Hospital General San Juan de Dios, Ciudad de Guatemala, Guatemala. 191Endocrinology and diabetes for children, AP-HP, Bicêtre Paris-Saclay hospital, Le Kremlin-Bicêtre, France. 192Innate Immunity group, IdiPAZ Institute for Health Research, La Paz Hospital, Madrid, Spain. 193Neurology unit, APHP Pitié-Salpêtrière Hospital, Paris University, Paris, France. 194Department of Medicine, Pamela Youde Nethersole Eastern Hospital, Hong Kong. 195Intensive care unit, APHP Pitié-Salpêtrière Hospital, Paris University, Paris, France. 196National Centre for Infectious Diseases; Tan Tock Seng Hospital; Yong Loo Lin School of Medicine; Lee Kong Chian School of Medicine, Singapore. 197Hospital de Niños Dr Ricardo Gutierrez, Buenos Aires, Argentina. 198Department of Clinical Immunology and Infectious Diseases, National Research Institute of Tuberculosis and Lung Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 199Neurooncology and Neuroinflammation Unit, IRCCS Mondino Foundation, Pavia, Italy. 200Clinical Tuberculosis and Epidemiology Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran. 201Coordenadora da Unidade de Infeciologia e Imunodeficiências do Serviço de Pediatria, Centro Materno-Infantil do Norte, Porto, Portugal. 202Hospital Sant Joan de Déu and University of Barcelona, Barcelona, Spain. 203Pediatric Infectious Diseases and Immunodeficiencies Unit, Hospital Universitari Vall d’Hebron, Vall d’Hebron Research Institute, Vall d’Hebron Barcelona Hospital Campus, Universitat Autònoma de Barcelona (UAB), Barcelona, Catalonia, Spain. 204Hospital Universitari Mutua de Terrassa, Universitat de Barcelona, Barcelona, Spain. 205IrsiCaixa AIDS Research Institute, ICREA, UVic-UCC, Research Institute “Germans Trias i Pujol”, Badalona, Spain. 206Department of Laboratory, Cruces University Hospital, Barakaldo, Bizkaia, Spain, Bizkaia, Spain. 207Intensive Care Unit, Hospital General Universitario “Gregorio Marañón”, Madrid, Spain. 208University of New South Wales, Australia. 209APHP Pitié-Salpêtrière Hospital, Paris, France. 210Department of Pediatrics, Complejo Hospitalario Universitario Insular-Materno Infantil, Canarian Health System, Las Palmas de Gran Canaria, Spain. 211Medical Intensive Care Unit, University Hospitals Leuven, Leuven, Belgium. 212Aix-Marseille University, APHM, Marseille, France. 213Service de Médecine Intensive Réanimation, Hôpitaux Universitaires Henri Mondor, Assistance Publique - Hôpitaux de Paris (AP-HP). Groupe de Recherche Clinique CARMAS, Faculté de Santé de Créteil, Université Paris Est Créteil, France. 214APHP Cohin Hospital, Paris, France. 215Department of Critical Care Medicine, Ente Ospedaliero Cantonale, Bellinzona, Switzerland. 216Necmettin Erbakan University, Meram Medical Faculty, Division of Pediatric Infectious Diseases, Konya, Turkey. 217Department of Pediatrics, University Hospitals Leuven; KU Leuven, Department of Microbiology, Immunology and Transplantation; Laboratory for Inborn Errors of Immunity, KU Leuven, Leuven, Belgium. 218Hospices Civils de Lyon, Hôpital de la Croix-Rousse, Lyon, France. 219Hôpital Erasme, Brussels, Belgium. 220Centre hospitalier de Gonesse, Gonesse, France. 221Aix Marseille Univ, IRD, AP-HM, MEPHI, IHU Méditerranée Infection, Marseille, France. 222Vascular Medicine, Georges Pompidou Hospital, APHP, Paris, France. 223Institut Jérôme Lejeune, Paris, France. 224Division of Pulmonary and Critical Care, College of Medicine-Jacksonville, University of Florida, Jacksonville, FL, USA. 225Department of Pediatrics, Hiroshima University Graduate School of Biomedical and Health Sciences, Hiroshima, Japan. 226BC Children's Hospital Research Institute, University of British Columbia, Vancouver, Canada. 227Médecine Intensive Réanimation, Hôpitaux Universitaires Henri Mondor, Assistance Publique – Hôpitaux de Paris (AP-HP), Créteil, France. 228Guanarteme Health Care Center, Canarian Health System, Las Palmas de Gran Canaria, Spain. 229Regional University Hospital of Malaga, Malaga, Spain. 230Department of Immunology, Hospital Universitari de Bellvitge, IDIBELL, Barcelona, Spain. 231Aix Marseille Univ, INSERM, INRAE, C2VN, Marseille, France. 232Department of General Pediatrics, Hôpital Bicêtre, AP-HP, University of Paris Saclay, Le Kremlin-Bicêtre, France. 233INSERM U1144, Université de Paris, DMU INVICTUS, APHP-Nord, Département de Médecine Interne, Lariboisière Hospital, Paris, France. 234CHU de La Timone, Marseille, France. 235Department of Epidemiology, Infectious Disease Control and Prevention, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan. 236Pediatric Immunology and rhumatology Department,Necker Hospital, AP-HP, Paris, France. 237Centro Hospitalar Universitário de Lisboa Central, Lisbon, Portugal. 238Infectious Diseases Horizontal Technlogy Centre, A*STAR; Singapore Immunology Network, A*STAR, Singapore. 239Department of Medicine and Geriatrics, Tuen Mun Hospital, Hong Kong. 240Regional Universitary Hospital of Malaga, Málaga, Spain. 241Department of Immunology, Hospital Universitario Marqués de Valdecilla, Santander, Spain. 242Bilkent University, Department of Molecular Biology and Genetics, Ankara, Turkey. 243BRFAA, Athens, Greece. 244IHU Méditerranée Infection, Aix Marseille Univ, IRD, AP-HM, SSA, VITROME, IHU Méditerranée Infection, Marseille, France. 245L'Hôpital Foch, Suresnes, France. 246Department of Immunology, CIC1408, GIMAP CIRI INSERM U1111, University Hospital of Saint-Etienne, St Etienne, France. 247Department of Immunology, Hospital Universitario 12 de Octubre, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain. 248Mexico. 249Diabetes Research Institute, IRCCS San Raffaele Hospital, Milan, Italy. 250APHP Hôpitaux Universitaires Paris-Sud, Le Kremlin-Bicêtre, France. 251AP-HP, Avicenne Hospital, Intensive Care Unit, Bobigny, France; INSERM UMR-S 942, Cardiovascular Markers in Stress Conditions (MASCOT), University of Paris, Paris, France. 252Neurometabolic Diseases Laboratory, IDIBELL-Hospital Duran i Reynals, Barcelona; CIBERER U759, ISCiii Madrid, Spain. 253Hospices Civils de Lyon, Lyon, France. 254Univ. Lille, INSERM U1285, CHU Lille, Pôle de médecine intensive-réanimation, CNRS, UMR 8576 - Unité de Glycobiologie Structurale et Fonctionnelle, Lille, France. 255Department of General pediatrics, Robert Debre Hospital, Paris, France. 256University of British Columbia, Vancouver, Canada. 257Jeffrey Model Diagnostic and Research Center for Primary Immunodeficiencies, Barcelona, Catalonia, Spain, Diagnostic Immunology Research Group, Vall d’Hebron Research Institute (VHIR), Vall d’Hebron University Hospital (HUVH), Vall d’Hebron Barcelona Hospital Campus, Barcelona, Catalonia, Spain. 258AP-HP, Avicenne Hospital, Intensive Care Unit, Bobigny, France; University Sorbonne Paris Nord, Bobigny, France. 259Centre Hospitalier de Saint-Denis, St Denis, France. 260Precision Medicine Unit, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland. 261Paris Cardiovascular Center, PARCC, INSERM, Université de Paris, Paris, France. 262Germans Trias i Pujol Hospital, Badalona, Spain. 263Medical intensive care unit. Hopital de la Croix-Rousse. Hospices Civils de Lyon, Lyon, France. 264Department of Clinical Laboratory, Hospital Universitari de Bellvitge, IDIBELL, Barcelona, Spain. 265Pediatric Infectious Diseases and Immunodeficiencies Unit, Hospital Universitari Vall d’Hebron, Vall d'Hebron Research Institute, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain. 266Université de Paris, CNRS UMR-8601; Team Chemistry & Biology, Modeling & Immunology for Therapy, CBMIT, Paris, France. 267Germans Trias i Pujol University Hospital and Research Institute. Badalona, Badalona, Spain. 268Department of Immunology, University Hospital of Gran Canaria Dr. Negrín, Canarian Health System, Las Palmas de Gran Canaria, Spain; Department of Clinical Sciences, University Fernando Pessoa Canarias, Las Palmas de Gran Canaria, Spain. 269Neurometabolic Diseases Laboratory, Bellvitge Biomedical Research Institute (IDIBELL), 08908 L'Hospitalet de Llobregat; University Hospital Germans Trias i Pujol, Badalona, Barcelona, Catalonia, Spain. 270Consorcio Hospital General Universitario, Valencia, Spain. 271APHP Hôpitaux Universitaires Paris-Sud, Paris, France. 272Intensive Care Unit, Louis-Mourier Hospital, Colombes, France. 273Virology unit, Université de Paris, Cohin Hospital, APHP, Paris, France. 274Neurometabolic Diseases Laboratory and CIBERER U759, Barcelona, Spain. 275Hospital San Pedro, Logroño, Spain. 276University of Tartu, Institute of Biomedicine and Translational Medicine, Tartu, Estonia. 277Respiratory medicine, Georges Pompidou Hospital, APHP, Paris, France. 278Infectious Diseases Department, International Health Program of the Catalan Insitute of Health (PROSICS), Vall d’Hebron University Hospital (HUVH), Vall d’Hebron Barcelona Hospital Campus, Universitat Autónoma de Barcelona, Barcelona, Spain. 279Hospital Clínico San Carlos and IdSSC, Madrid, Spain. 280Faculdades Pequeno Príncipe, Instituto de Pesquisa Pelé Pequeno Príncipe, Curitiba, Brazil. 281AP-HP, Avicenne Hospital, Intensive Care Unit, Bobigny, France. 282Service de Médecine Intensive Réanimation, Institut de Cardiologie, Hopital Pitié-Salpêtrière, Paris, France. 283Department of Pediatrics, Medizinische Fakultät Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany. 284CHRU de Nancy, Hôpital d'Enfants, Vandoeuvre, France. 285Chair of Nephrology, University of Brescia, Brescia, Italy. 286Department of Immunology, 2nd Faculty of Medicine, Charles University and Motol University Hospital, Prague, Czech Republic. 287Clínica Universidad de Navarra and Ciberes, Madrid, Spain. 288HUS Helsinki University Hospital, Children and Adolescents, Rare Disease Center, and Inflammation Center, Adult Immunodeficiency Unit, Majakka, Helsinki, Finland. 289Fundació Docencia i Recerca Mutua Terrassa, Terrassa, Spain. 290D. Rogachev National Medical and Research Center of Pediatric Hematology, Oncology, Immunoogy, Moscow, Russia. 291Haematology Laboratory, Lariboisière Hospital, University of Paris, Paris, France. 292Biomedical Research Foundation of the Academy of Athens. 293INSERM U1140, University of Paris, European Georges Pompidou Hospital, Paris, France. 294Department of Pediatrics, Faculty of Medicine, Mansoura University, Mansoura, Egypt. 295Critical Care Unit, Hospital Universitario de Gran Canaria Dr. Negrín, Canarian Health System, Las Palmas de Gran Canaria, Spain. 296CHU de Saint Etienne, Saint-Priest-en-Jarez, France. 297Pediatric Infectious Diseases and Immunodeficiencies Unit, Hospital Universitari Vall d’Hebron, Vall d'Hebron Research Institute, Vall d’Hebron Barcelona Hospital Campus. Universitat Autònoma de Barcelona (UAB). Barcelona, Catalonia, Spain, EU., Barcelona, Spain. 298Department of pediatric infectious diseases and pediatric immunology, Shupyk National Healthcare University of Ukraine, Kyiv, Ukraine. 299Gustave Roussy Cancer Campus, Villejuif, France. 300Intensive Care Unit, Avicenne Hospital, APHP, Bobigny, France. 301Laboratory of Immunology and Histocompatibility, Saint-Louis Hospital, Paris University, Paris, France. 302 Center for Inflammation Research, Laboratory of Molecular Signal Transduction in Inflammation, VIB, Ghent, Belgium. 303Department of Internal Medicine, Université de Paris, INSERM, U970, PARCC, F-75015, Paris, France. 304Service de médecine intensive réanimation, CHU de Saint-Etienne, France. 305Dept of Rheumatology and Inflammation Research, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden. 306University of Management and Technology, Lahore, Pakistan. 307Department of Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark. 308First Division of Anesthesiology and Critical Care Medicine, University of Brescia, ASST Spedali Civili di Brescia, Brescia, Italy. 309Intensive Care Department, Hospital Universitari MutuaTerrassa, Universitat Barcelona, Terrassa, Spain. 310Laboratory of Immunobiology, Center for Clinical, Experimental Surgery and Translational Research, Biomedical Research Foundation of the Academy of Athens, Athens, Greece. 311International Center of Research in Infectiology, Lyon University, INSERM U1111, CNRS UMR 5308, ENS, UCBL, Lyon, France; Hospices Civils de Lyon, Lyon Sud Hospital, Pierre-Bénite, France. 312Infanta Leonor University Hospital, Madrid, Spain. 313Department of Medicine and Geriatrics, Princess Margaret Hospital, Hong Kong. 314University of Tartu, Institute of Clinical Medicine, Tartu, Estonia. 315Department of Medicine, United Christian Hospital, Hong Kong. 316Hematology Department, ASST Spedali Civili di Brescia, Brescia, Italy. 317Pneumologie, Hôpital Avicenne, APHP, INSERM U1272, Université Sorbonne Paris Nord, Bobigny, France. 318Dermatology unit, Laboratoire GAD, INSERM UMR1231 LNC, université de Bourgogne, Dijon, France. 319University Hospital of Burgos, Burgos, Spain. 320Center of Human Genetics, Hôpital Erasme, Université Libre de Bruxelles, Brussels, Belgium. 321Bellvitge University Hospital, L'Hospitalet de Llobregat, Barcelona, Spain. 322University of São Paulo, São Paulo, Brazil. 323CHU de Caen, Caen, France. 324Hospital del Mar - IMIM Biomedical Research Institute, Barcelona, Catalonia, Spain. 325Neglected Human Genetics Laboratory, INSERM, University of Paris, Paris, France. 326Sorbonne Université, Service de Médecine Intensive Réanimation, Hôpital Tenon, Assistance Publique-Hôpitaux de Paris, Paris, France. 327Pediatric Infectious Disease and Pediatric Immunology Department, Shupyk National Healthcare University of Ukraine, Kyiv, Ukraine. 328Department of Pneumology, University Hospitals Leuven, Leuven, Belgium. 329Laboratory for Clinical Infectious and Inflammatory Disorders, Departement of Microbiology, Immunology and Transplantation, Leuven, Belgium. 330Department of Clinical Pathology, Pamela Youde Nethersole Eastern Hospital, Hong Kong. 331Department of Medicine, Queen Elizabeth Hospital, Hong Kong. 332Ankara City Hospital, Children's Hospital, Ankara, Turkey. 333Division of Pediatric Infectious Disease, Department of Pediatrics, Faculty of Medicine, Karadeniz Technical University, Trabzon, Turkey. 334Health Sciences University, Lütfi Kırdar Kartal Education and Research Hospital, İstanbul, Turkey. 335Department of Nephrology and Infectiology, AZ Sint-Jan, Bruges, Belgium. 336Department of Pulmonology, Ghent University Hospital, Belgium. 337Department of Pediatric pulmonology and immunology, Ghent University Hospital, Belgium. 338Department of Intensive Care Unit, Ghent University Hospital, Belgium. 339Department of Pediatric hemato-oncology, Jolimont Hospital; Department of Pediatric hemato-oncology, HUDERF, Brussels, Belgium. 340Department of Pulmonology, ZNA Middelheim, Antwerp, Belgium. 341Department of Internal Medicine, Ghent University Hospital, Belgium. 342Department of Pediatric immuno-hémato-rhumatology, CHR Citadelle, Liége, Belgium. 343Department of Pediatric hemato-oncology, UCL Louvain, Belgium. 344Department of Pediatrics, Saint Luc, UCL Louvain, Belgium.
COVID-STORM Clinicians
Giuseppe Foti1, Giacomo Bellani1, Giuseppe Citerio1, Ernesto Contro1, Alberto Pesci2, Maria Grazia Valsecchi3, Marina Cazzaniga4
1Department of Emergency, Anesthesia and Intensive Care, School of Medicine and Surgery, University of Milano-Bicocca, San Gerardo Hospital, Monza, Italy. 2Department of Pneumology, School of Medicine and Surgery, University of Milano-Bicocca, San Gerardo Hospital, Monza, Italy. 3Center of Bioinformatics and Biostatistics, School of Medicine and Surgery, University of Milano-Bicocca, San Gerardo Hospital, Monza, Italy. 4Phase I Research Center, School of Medicine and Surgery, University of Milano-Bicocca, San Gerardo Hospital, Monza, Italy.
NIAID Immune Response to COVID Group
Jeffrey J. Danielson1, Kerry Dobbs1, Anuj Kashyap1, Li Ding1, Clifton L. Dalgard2, Alessandra Sottini3, Virginia Quaresima3, Eugenia Quiros-Roldan4, Camillo Rossi5, Laura Rachele Bettini6, Mariella D’Angio’6, Ilaria Beretta7, Daniela Montagna8, Amelia Licari9, Gian Luigi Marseglia10
1Laboratory of Clinical Immunology and Microbiology, Division of Intramural Research, NIAID, NIH, Bethesda, MD, USA. 2Department of Anatomy, Physiology & Genetics, Uniformed Services University of the Health Sciences; The American Genome Center, Uniformed Services University of the Health Sciences, Bethesda, MD, USA. 3CREA Laboratory, Diagnostic Department, ASST Spedali Civili di Brescia, Brescia, Italy. 4Department of Infectious and Tropical Diseases, University of Brescia and ASST Spedali Civili di Brescia, Brescia, Italy. 5Chief Medical Officer, ASST Spedali Civili di Brescia, Brescia, Italy. 6Pediatric Departement and Centro Tettamanti-European Reference Network PaedCan, EuroBloodNet, MetabERN-University of Milano-Bicocca-Fondazione MBBM-Ospedale, San Gerardo, Monza, Italy. “7Department of Infectious Diseases, University of Milano-Bicocca, San Gerardo Hospital, Monza, Italy. ” 8Laboratory of Immunology and Transplantation, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy; Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy. 9Department of Pediatrics, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy. 10Department of Pediatrics, Fondazione IRCCS Policlinico San Matteo, University of Pavia, Pavia, Italy; Department of Clinical, Surgical, Diagnostic and Pediatric Sciences, University of Pavia, Pavia, Italy.
NH-COVAIR Study Group
Isabella Batten1, Conor Reddy1, Matt McElheron1, Claire Noonan1, Emma Connolly1, Aoife Fallon1
1Department of Age-Related Healthcare, Tallaght University Hospital & Department of Medical Gerontology, School of Medicine, Trinity College Dublin
Danish CHGE
Merete Storgaard1, Sofie Jørgensen1, Martin Tolstrup1
1Dept. Infectious Diseases, Aarhus University Hospital, Aarhus, Denmark.
The Danish Blood Donor Study (DBDS)
Christian Erikstrup1, Ole Birger Pedersen2, Erik Sørensen3, Susan Mikkelsen1, Khoa Manh Dinh1, Margit Anita Hørup Larsen3, Isabella Worlewenut Paulsen2, Jakob Hjorth Von Stemann3, Morten Bagge Hansen3, Sisse Rye Ostrowski3
1Dept. Clinical Immunology, Aarhus University Hospital, Aarhus, Denmark. 2Dept. Clinical Immunology, Zeeland University Hospital, Køge, Denmark, 3Dept. of Clinical Immunology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark
St. James's Hospital, SARS CoV2 Interest group
Liam Townsend1, Cliona Ni Cheallaigh1, Colm Bergin1, Ignacio Martin-Loeches2, Jean Dunne3, Niall Conlon3, Nollaig Bourke4, Cliona O'Farrelly5
1Department of Infectious Diseases, St. James's Hospital; Department of Clinical Medicine, School of Medicine, Trinity Translational Medicine Institute, Trinity College Dublin, Dublin, Ireland. 2Department of Intensive Care Medicine, St James's Hospital, Dublin, Ireland. 3Department of Immunology, St. James's Hospital; Department of Immunology, School of Medicine, Trinity College Dublin, Ireland. 4Department of Medical Gerontology, School of Medicine, Trinity Translational Medicine Institute, Trinity College Dublin, Dublin, Ireland. 5School of Biochemistry and Immunology, Trinity Biomedical Sciences Institute, Trinity College Dublin; School of Medicine, Trinity College Dublin, Dublin, Ireland.
French COVID Cohort Study Group
Laurent ABEL1, Clotilde ALLAVENA2, Claire ANDREJAK3, François ANGOULVANT4, Cecile AZOULAY5, Delphine BACHELET6, Marie BARTOLI7, Romain BASMACI8, Sylvie BEHILILL9, Marine BELUZE10, Nicolas BENECH11, Dehbia BENKERROU12, Krishna BHAVSAR6, Laurent BITKER11, Lila BOUADMA6, Maude BOUSCAMBERT-DUCHAMP13, Pauline CARAUX PAZ14, Minerva CERVANTES-GONZALEZ6, Anissa CHAIR6, Catherine CHIROUZE15, Alexandra COELHO16, Hugues CORDEL17, Camille COUFFIGNAL6, Sandrine COUFFIN-CADIERGUES18, Eric d’ORTENZIO7, Etienne DE MONTMOLLIN6, Alexa DEBARD19, Marie-Pierre DEBRAY6, Dominique DEPLANQUE20, Diane DESCAMPS6, Mathilde DESVALLÉE21, Alpha DIALLO7, Jean-Luc DIEHL22, Alphonsine DIOUF16, Céline DORIVAL12, François DUBOS23, Xavier DUVAL6, Philippine ELOY6, Vincent ENOUF9, Olivier EPAULARD24, Hélène ESPEROU18, Marina ESPOSITO-FARESE6, Manuel ETIENNE25, Denis GAROT26, Nathalie GAULT6, Alexandre GAYMARD13, Jade GHOSN6, Tristan GIGANTE27, Morgane GILG27, François GOEHRINGER28, Jérémie GUEDJ29, Alexandre HOCTIN16, Isabelle HOFFMANN6, Ikram HOUAS18, Jean-Sébastien HULOT22, Salma JAAFOURA18, Ouifiya KAFIF6, Florentia KAGUELIDOU30, Sabrina KALI6, Younes KERROUMI31, Antoine KHALIL6, Coralie KHAN21, Antoine KIMMOUN32, Fabrice LAINE33, Cédric LAOUÉNAN6, Samira LARIBI6, Minh LE6, Cyril LE BRIS34, Sylvie LE GAC6, Quentin LE HINGRAT6, Soizic LE MESTRE7, Hervé LE NAGARD35, Adrien LEMAIGNEN26, Véronique LEMEE25, François-Xavier LESCURE6, Sophie LETROU6, Yves LEVY36, Bruno LINA13, Guillaume LINGAS35, Jean Christophe LUCET6, Moïse MACHADO37, Denis MALVY38, Marina MAMBERT16, Aldric MANUEL39, France MENTRÉ6, Amina MEZIANE12, Hugo MOUQUET9, Jimmy Mullaert6, Nadège NEANT35, Duc NGUYEN38, Marion NORET40, Aurélie PAPADOPOULOS18, Christelle PAUL7, Nathan PEIFFER-SMADJA6, Vincent PEIGNE41, Ventzislava PETROV-SANCHEZ7, Gilles PEYTAVIN6, Huong PHAM6, Olivier PICONE8, Valentine PIQUARD6, Julien POISSY23, Oriane PUÉCHAL42, Manuel ROSA-CALATRAVA13, Bénédicte ROSSIGNOL27, Patrick ROSSIGNOL28, Carine ROY6, Marion SCHNEIDER6, Richa SU6, Coralie TARDIVON6, Marie-Capucine TELLIER6, François TÉOULÉ12, Olivier TERRIER13, Jean-François TIMSIT6, Christelle TUAL43, Sarah TUBIANA6, Sylvie VAN DER WERF9, Noémie VANEL44, Aurélie VEISLINGER43, Benoit VISSEAUX6, Aurélie WIEDEMANN45, Yazdan YAZDANPANAH6
1INSERM UMR 1163, Paris, France. 2CHU Nantes, France. 3CHU Amiens, France. 4Hôpital Necker, Paris, France. 5Hopitâl Cochin, Paris, France. 6Hôpital Bichat, Paris, France. 7ANRS, Paris, France. 8Hôpital Louis Mourier, Colombes, France. 9Pasteur Institute, Paris, France. 10F-CRIN Partners Platform, Paris, France. 11CHU Lyon, France. 12INSERM UMR 1136, Paris, France. 13INSERM UMR 1111, Lyon, France. 14CH Villeneuve Saint Georges, France. 15CHRU Jean Minjoz, Besançon, France. 16INSERM UMR 1018, Paris, France. 17Hôpital Avicenne, Bobigny, France. 18INSERM sponsor, Paris, France. 19CHU Toulouse, France. 20Hôpital Calmette, Lille, France. 21INSERM UMR 1219, Bordeaux, France. 22Hôpital Européen Georges Pompidou, Paris, France. 23CHU Lille, France. 24CHU Grenoble, France. 25CHU Rouen, France. 26CHU Tours, France. 27F-CRIN INI-CRCT, Nancy, France. 28CHU Nancy, France. 29Université de Paris, INSERM, IAME, F-75018 Paris, France. 30Hôpital Robert Debré, Paris, France. 31GH Diaconesses, Paris, France. 32Université de Lorraine, CHRU de Nancy, Service de Médecine Intensive et Réanimation Brabois, INSERM U116, Nancy, France. 33CHU Rennes, France. 34CH Beziers, France. 35INSERM UMR 1137, Paris, France. 36Vaccine Research Insitute (VRI), INSERM U955, Créteil, France. 37Grand Hôpital de l’Est Francilien, Marne-la-Vallée, France. 38CHU Bordeaux, France. 39CH Annecy, France. 40RENARCI, Annecy, France. 41CH Métropole Savoie, Cambery, France. 42REACTing, Paris, France. 43INSERM CIC-1414, Rennes, France. 44Hôpital la Timone, Marseille, France. 45Vaccine Research Insitute (VRI), INSERM UMR 955, Créteil, France.
Imagine COVID-Group
Jean-Philippe Annereau1, Luis Briseño-Roa1, Olivier Gribouval2, Anna Pelet2
1Medetia Pharmaceuticals, Paris, France. 2Imagine Institute, Université de Paris, INSERM UMR 1163, Paris, France.
The Milieu Intérieur Consortium
Laurent Abel1, Andres Alcover2, Hugues Aschard2, Philippe Bousso2, Nollaig Bourke3, Petter Brodin4, Pierre Bruhns2, Nadine Cerf-Bensussan5, Ana Cumano2, Christophe D’Enfert2, Ludovic Deriano2, Marie-Agnès Dillies2, James Di Santo2, Françoise Dromer2, Gérard Eberl2, Jost Enninga2, Jacques Fellay6, Ivo Gomperts-Boneca2, Milena Hasan2, Gunilla Karlsson Hedestam4, Serge Hercberg7, Molly A Ingersoll2, Olivier Lantz8, Rose Anne Kenny3, Mickaël Ménager5, Frédérique Michel2, Hugo Mouquet2, Cliona O'Farrelly3, Etienne Patin2, Sandra Pellegrini2, Antonio Rausell5, Frédéric Rieux-Laucat5, Lars Rogge2, Magnus Fontes9, Anavaj Sakuntabhai2, Olivier Schwartz2, Benno Schwikowski2, Spencer Shorte2, Frédéric Tangy2, Antoine Toubert10, Mathilde Touvier12, Marie-Noëlle Ungeheuer2, Christophe Zimmer2, Matthew L. Albert11, Darragh Duffy2, Lluis Quintana-Murci2
1Hôpital Necker, Paris, France. 2Institut Pasteur, Paris, France. 3Trinity College, Dublin, Ireland. 4Karolinska Institutet, Stockholm, Sweden. 5INSERM U1163, Institut Imagine, Paris, France. 6EPFL, Lausanne, Switzerland. 7Université Paris 13, Paris, France. 8Institut Curie, Paris, France. 9Institut Roche, Paris, France. 10Hôpital Saint-Louis, Paris, France. 11In Sitro, San Francisco, USA. 12Sorbonne Paris Nord University, INSERM U1153, INRAE U1125, CNAM, Nutritional Epidemiology Research Team (EREN), Epidemiology and Statistics Research Center – University of Paris (CRESS), Bobigny, France.
CoV-Contact Cohort
Loubna Alavoine1, Sylvie Behillil2, Charles Burdet3, Charlotte Charpentier3,4, Aline Dechanet5, Diane Descamps3,6, Xavier Duval1,3, Jean-Luc Ecobichon1, Vincent Enouf8, Wahiba Frezouls1, Nadhira Houhou5, Ouifiya Kafif5, Jonathan Lehacaut1, Sophie Letrou1, Bruno Lina9, Jean-Christophe Lucet10, Pauline Manchon5, Mariama Nouroudine1, Valentine Piquard5, Caroline Quintin1, Michael Thy11, Sarah Tubiana1, Sylvie van der Werf8, Valérie Vignali1, Benoit Visseaux3,10, Yazdan Yazdanpanah3,10, Abir CHAHINE12, Nawal WAUCQUIER12, Maria-Claire MIGAUD12, Dominique DEPLANQUE12, Félix DJOSSOU13, Mayka Mergeay-Fabre14, Aude LUCARELLI15, Magalie DEMAR13, Léa Bruneau16, Patrick Gérardin17, Adrien Maillot16, Christine Payet18, Bruno Laviolle19, Fabrice Laine19, Christophe Paris19, Mireille Desille-Dugast19, Julie Fouchard19, Denis MALVY20, Duc NGUYEN20, Thierry PISTONE20, Pauline PERREAU20, Valérie GISSOT21, Carole LE GOAS21, Samatha Montagne22, Lucie Richard23, Catherine Chirouze24, Kévin Bouiller24, Maxime Desmarets25, Alexandre Meunier26, Benjamin Lefévre27, Hélène Jeulin28, Karine Legrand29, Sandra Lomazzi30, Bernard Tardy31, Amandine Gagneux-Brunon32, Frédérique Bertholon33, Elisabeth Botelho-Nevers32, KOUAKAM Christelle KOUAKAM Christelle34, LETURQUE Nicolas LETURQUE Nicolas34, Layidé Roufai34, Karine Amat35, Sandrine Couffin-Cadiergues34, Hélène Espérou36, Samia Hendou34
1Centre d'Investigation Clinique, INSERM CIC 1425, Hôpital Bichat Claude Bernard, APHP, Paris, France. 2Institut Pasteur, Paris, France. 3Université de Paris, IAME, INSERM U1137, Paris, France, Hôpital Bichat Claude Bernard, APHP, Paris, France. 4 Service de Virologie, Université de Paris, INSERM, IAME, UMR 1137, AP-HP, Hôpital Bichat-Claude Bernard, F-75018 Paris, France. 6IAME INSERM U1140, Hôpital Bichat Claude Bernard, APHP, Paris, France. 7Centre d'Investigation Clinique, INSERM CIC 1425, APHP, IAME, Paris University, Paris, France. 8Institut Pasteur, U3569 CNRS, Université de Paris, Paris, France. 9Virpath Laboratory, International Center of Research in Infectiology, Lyon University, INSERM U1111, CNRS U5308, ENS, UCBL, Lyon, France. 10IAME INSERM U1138, Hôpital Bichat Claude Bernard, APHP, Paris, France. 11Center for Clinical Investigation, Assistance Publique-Hôpitaux de Paris, Bichat-Claude Bernard University Hospital, Paris, France. 12Centre d'Investigation Clinique, INSERM CIC 1403, Centre Hospitalo universitaire de Lille, Lille, France. 13Service des maladies infectieuses, Centre Hospitalo universitaire de Cayenne, Guyane, France. 14Centre d'Investigation Clinique, INSERM CIC 1424, Centre Hospitalier de Cayenne, Cayenne, Guyane Française. 15Service Hôpital de jour Adulte, Centre Hospitalier de Cayenne, Guyane, France. 16Centre d'Investigation Clinique, INSERM CIC 1410, Centre Hospitalo universitaire de la Réunion, La Réunion, France. 17Centre d'Investigation Clinique, INSERM CIC 1410, CHU Reunion, Saint-Pierre, Reunion island. 18Centre d'Investigation Clinique, INSERM CIC 1410, Centre de Ressources Biologiques, Centre Hospitalo universitaire de la Réunion, La Réunion, France. 19Centre d'Investigation Clinique, INSERM CIC 1414, Centre Hospitalo universitaire de Rennes, Rennes, France. 20Service des maladies infectieuses, Centre Hospitalo universitaire de Bordeaux, Bordeaux, France. 21Centre d'Investigation Clinique, INSERM CIC 1415, CHRU Tours, Tours, France. 22CRBT, Centre Hospitalo universitaire de Tours, Tours, France. 23Pole de Biologie Médicale, Centre Hospitalo universitaire de Tours, Tours, France. 24Service des maladies infectieuses, Centre Hospitalo universitaire de Besançon, Besançon, France. 25Service des maladies infectieuses, Centre d'investigation clinique, INSERM CIC1431, Centre Hospitalier Universitaire de Besançon, Besançon, France. 26Centre de Ressources Biologiques - Filière Microbiologique de Besançon, Centre Hospitalier Universitaire, Besançon, France. 27Université de Lorraine, CHRU-Nancy and APEMAC, Infectious and tropical diseases, Nancy, France. 28Laboratoire de Virologie, CHRU de Nancy Brabois, Vandoeuvre-lès-Nancy, France. 29INSERM CIC-EC 1433, Centre Hospitalo universitaire de Nancy, Nancy, France. 30Centre de ressources Biologiques, Centre Hospitalo universitaire de Nancy, Nancy, France. 31Centre d'Investigation Clinique, INSERM CIC 1408, Centre Hospitalo universitaire de Saint Etienne, Saint Etienne, France. 32Service des maladies infectieuses, Centre Hospitalo universitaire de Saint Etienne, Saint Etienne, France. 33Service des maladies infectieuses, CRB42-BTK, Centre Hospitalo Universitaire de Saint Etienne, Saint Etienne, France. 34Pole Recherche Clinique, INSERM, Paris France. 35IMEA Fondation Léon M'Ba, Paris, France. 36INSERM Pôle Recherche Clinique, Paris, France.
Amsterdam UMC Covid-19 Biobank Investigators
Michiel van Agtmael2, Anne Geke Algera1, Brent Appelman2, Frank van Baarle1, Diane Bax3, Martijn Beudel4, Harm Jan Bogaard5, Marije Bomers2, Peter Bonta5, Lieuwe Bos1, Michela Botta1, Justin de Brabander2, Godelieve de Bree2, Sanne de Bruin1, David T.P. Buis1, Marianna Bugiani5, Esther Bulle1, Osoul Chouchane2 Alex Cloherty3, Mirjam Dijkstra12, Dave A. Dongelmans1, Romein W.G. Dujardin1, Paul Elbers1, Lucas Fleuren1, Suzanne Geerlings2 Theo Geijtenbeek3, Armand Girbes1, Bram Goorhuis2, Martin P. Grobusch2, Florianne Hafkamp3, Laura Hagens1, Jorg Hamann7, Vanessa Harris2, Robert Hemke8, Sabine M. Hermans2 Leo Heunks1, Markus Hollmann6, Janneke Horn1, Joppe W. Hovius2, Menno D. de Jong9, Rutger Koning4, Endry H.T. Lim1, Niels van Mourik1, Jeaninne Nellen2, Esther J. Nossent5, Frederique Paulus1, Edgar Peters2, Dan A.I. Pina-Fuentes4, Tom van der Poll2, Bennedikt Preckel6, Jan M. Prins2, Jorinde Raasveld1, Tom Reijnders2, Maurits C.F.J. de Rotte12, Michiel Schinkel2, Marcus J. Schultz1, Femke A.P. Schrauwen12, Alex Schuurmans10, Jaap Schuurmans1, Kim Sigaloff1, Marleen A. Slim1,2, Patrick Smeele5, Marry Smit1, Cornelis S. Stijnis2, Willemke Stilma1, Charlotte Teunissen11, Patrick Thoral1, Anissa M Tsonas1, Pieter R. Tuinman2, Marc van der Valk2, Denise Veelo6, Carolien Volleman1, Heder de Vries1, Lonneke A. Vught1,2, Michèle van Vugt2, Dorien Wouters12, A. H (Koos) Zwinderman13, Matthijs C. Brouwer4, W. Joost Wiersinga2, Alexander P.J. Vlaar1, Diederik van de Beek4.
1Department of Intensive Care, Amsterdam UMC, Amsterdam, The Netherlands; 2Department of Infectious Diseases, Amsterdam UMC, Amsterdam, The Netherlands; 3Experimental Immunology, Amsterdam UMC, Amsterdam, The Netherlands; 4Department of Neurology, Amsterdam UMC, Amsterdam Neuroscience, Amsterdam, The Netherlands; 5Department of Pulmonology, Amsterdam UMC, Amsterdam, The Netherlands; 6Department of Anesthesiology, Amsterdam UMC, Amsterdam, The Netherlands; 7Amsterdam UMC Biobank Core Facility, Amsterdam UMC, Amsterdam, The Netherlands; 8Department of Radiology, Amsterdam UMC, Amsterdam, The Netherlands; 9Department of Medical Microbiology, Amsterdam UMC, Amsterdam, The Netherlands; 10Department of Internal Medicine, Amsterdam UMC, Amsterdam, The Netherlands; 11Neurochemical Laboratory, Amsterdam UMC, Amsterdam, The Netherlands; 12Department of Clinical Chemistry, Amsterdam UMC, Amsterdam, The Netherlands; 13Department of Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam UMC, Amsterdam, The Netherlands.
COVID Human Genetic Effort
Laurent Abel1, Alessandro Aiuti2, Saleh Al-Muhsen3, Fahd Al-Mulla4, Mark S. Anderson5, Evangelos Andreakos6, Andrés A. Arias7, Hagit Baris Feldman8, Alexandre Belot9, Catherine M. Biggs10, Dusan Bogunovic11, Alexandre Bolze12, Anastasiia Bondarenko13, Ahmed A. Bousfiha14, Petter Brodin15, Yenan Bryceson16, Carlos D. Bustamante17, Manish J. Butte18, Giorgio Casari19, Samya Chakravorty20, John Christodoulou21, Antonio Condino-Neto22, Stefan N. Constantinescu23, Megan A. Cooper24, Clifton L. Dalgard25, Murkesh Desai26, Beth A. Drolet27, Jamila El Baghdadi28, Sara Espinosa-Padilla29, Jacques Fellay30, Carlos Flores31, José Luis Franco7, Antoine Froidure32, Peter K. Gregersen33, Filomeen Haerynck34, David Hagin35, Rabih Halwani36, Lennart Hammarström37, James R. Heath38, Sarah E. Henrickson39, Elena W.Y. Hsieh40, Eystein S. Husebye41, Kohsuke Imai42, Yuval Itan43, Erich D. Jarvis44, Timokratis Karamitros45, Kai Kisand46, Cheng-Lung Ku47, Yu-Lung Lau48, Yun Ling49, Carrie L. Lucas50, Tom Maniatis51, Davood Mansouri52, László Maródi53, Isabelle Meyts54, Joshua D. Milner55, Kristina Mironska56, Trine H. Mogensen57, Tomohiro Morio58, Lisa F.P. Ng59, Luigi D. Notarangelo60, Antonio Novelli61, Giuseppe Novelli62, Cliona O'Farrelly63, Satoshi Okada64, Tayfun Ozcelik65, Qiang Pan-Hammarström37, Rebeca Perez de Diego66, Anna M. Planas67, Carolina Prando68, Aurora Pujol69, Lluis Quintana-Murci70, Laurent Renia59, Igor Resnick71, Carlos Rodríguez-Gallego72, Vanessa Sancho-Shimizu73, Anna Sediva74, Mikko R.J. Seppänen75, Mohammed Shahrooei76, Anna Shcherbina77, Ondrej Slaby78, Andrew L. Snow79, Pere Soler-Palacín80, András N. Spaan81, Ivan Tancevski82, Stuart G. Tangye83, Ahmad Abou Tayoun84, Sathishkumar Ramaswamy84, Stuart E Turvey85, K M Furkan Uddin86, Mohammed J. Uddin87, Diederik van de Beek88, Donald C. Vinh89, Horst von Bernuth90, Mayana Zatz91, Pawel Zawadzki92, Helen C. Su60, Jean-Laurent Casanova93
1INSERM U1163, University of Paris, Imagine Institute, Paris, France. 2San Raffaele Telethon Institute for Gene Therapy, IRCCS Ospedale San Raffaele, and Vita Salute San Raffaele University, Milan, Italy. 3Immunology Research Laboratory, Department of Pediatrics, College of Medicine and King Saud University Medical City, King Saud University, Riyadh, Saudi Arabia. 4Dasman Diabetes Institute, Department of Genetics and Bioinformatics, Dasman, Kuwait. 5Diabetes Center, University of California San Francisco, San Francisco, CA, USA. 6Laboratory of Immunobiology, Center for Clinical, Experimental Surgery and Translational Research, Biomedical Research Foundation of the Academy of Athens, Athens, Greece. 7Group of Primary Immunodeficiencies, University of Antioquia UDEA, Medellin, Colombia. 8The Genetics Institute, Tel Aviv Sourasky Medical Center and Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. 9Pediatric Nephrology, Rheumatology, Dermatology, HFME, Hospices Civils de Lyon, National Referee Centre RAISE, and INSERM U1111, Université de Lyon, Lyon, France. 10Department of Pediatrics, British Columbia Children’s Hospital, The University of British Columbia, Vancouver, BC, Canada 11Icahn School of Medicine at Mount Sinai, New York, NY, USA. 12Helix, San Mateo, CA, USA. 13Shupyk National Medical Academy for Postgraduate Education, Kiev, Ukraine. 14Clinical Immunology Unit, Department of Pediatric Infectious Disease, CHU Ibn Rushd and LICIA, Laboratoire d'Immunologie Clinique, Inflammation et Allergie, Faculty of Medicine and Pharmacy, Hassan II University, Casablanca, Morocco. 15SciLifeLab, Department Of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden 16Department of Medicine, Center for Hematology and Regenerative Medicine, Karolinska Institutet, Stockholm, Sweden. 17Stanford University, Stanford, CA, USA. 18Division of Immunology, Allergy, and Rheumatology, Department of Pediatrics and the Department of Microbiology, Immunology, and Molecular Genetics, University of California, Los Angeles, CA, USA. 19Clinical Genomics, IRCCS San Raffaele Scientific Institute and Vita-Salute San Raffaele University, Milan, Italy 20Department of Pediatrics and Children’s Healthcare of Atlanta, Emory University, Atlanta, GA, USA. 21Murdoch Children's Research Institute and Department of Pediatrics, University of Melbourne, Australia 22Department of Immunology, Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil. 23de Duve Institute and Ludwig Cancer Research, Brussels, Belgium 24Washington University School of Medicine, St. Louis, MO, USA. 25Department of Anatomy, Physiology & Genetics, Uniformed Services University of the Health Sciences, Bethesda, MD, USA. 26Bai Jerbai Wadia Hospital for Children, Mumbai, India. 27School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA. 28Genetics Unit, Military Hospital Mohamed V, Rabat, Morocco. 29Instituto Nacional de Pediatria (National Institute of Pediatrics), Mexico City, Mexico. 30School of Life Sciences, Ecole Polytechnique Fédérale de Lausanne, Lausanne, Switzerland; Precision Medicine Unit, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland. 31Genomics Division, Instituto Tecnológico y de Energías Renovables (ITER), Santa Cruz de Tenerife, Spain; Research Unit, Hospital Universitario N.S. de Candelaria, Santa Cruz de Tenerife, Spain; Instituto de Tecnologías Biomédicas (ITB), Universidad de La Laguna, San Cristóbal de La Laguna, Spain; CIBER de Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain. 32Pulmonology Department, Cliniques Universitaires Saint-Luc ; Institut de Recherche Expérimentale et Clinique (IREC), Université Catholique de Louvain, Brussels, Belgium. 33Feinstein Institute for Medical Research, Northwell Health USA, Manhasset, NY, USA. 34Department of Pediatric Immunology and Pulmonology, Centre for Primary Immunodeficiency Ghent (CPIG), PID Research Laboratory, Jeffrey Model Diagnosis and Research Centre, Ghent University Hospital, Ghent, Belgium. 35The Genetics Institute Tel Aviv Sourasky Medical Center, Tel Aviv, Israel. 36Sharjah Institute of Medical Research, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates. 37Department of Biosciences and Nutrition, Karolinska Institutet, Stockholm, Sweden. 38Institute for Systems Biology, Seattle, WA, USA. 39Department of Pediatrics, Division of Allergy Immunology, Children’s Hospital of Philadelphia, Philadelphia, PA, USA; Department of Microbiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA 40Departments of Pediatrics, Immunology and Microbiology, University of Colorado, School of Medicine, Aurora, Colorado, USA 41 Department of Clinical Science and K.G. Jebsen Center for Auoimmune Diseases, University of Bergen, Bergen, Norway. 42Department of Community Pediatrics, Perinatal and Maternal Medicine, Tokyo Medical and Dental University (TMDU) 43Institute for Personalized Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA; Department of Genetics and Genomic Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA. 44Laboratory of Neurogenetics of Language and Howard Hughes Medical Institute, The Rockefeller University, New York, NY, USA. 45Bioinformatics and Applied Genomics Unit, Hellenic Pasteur Institute, Athens, Greece 46Molecular Pathology, Department of Biomedicine, Institute of Biomedicine and Translational Medicine, University of Tartu, Tartu Estonia. 47Chang Gung University, Taoyuan County, Taiwan. 48Department of Pediatrics & Adolescent Medicine, The University of Hong Kong, Hong Kong, China. 49Shanghai Public Health Clinical Center, Fudan University, Shanghai, China. 50Department of Immunobiology, Yale University School of Medicine, New Haven, CT, USA. 51Columbia University Zuckerman Institute, New York, NY 52Department of Clinical Immunology and Infectious Diseases, National Research Institute of Tuberculosis and Lung Diseases, The Clinical Tuberculosis and Epidemiology Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Masih Daneshvari Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. 53Primary Immunodeficiency Clinical Unit and Laboratory, Department of Dermatology, Venereology and Dermatooncology, Semmelweis University, Budapest, Hungary. 54Department of Pediatrics, University Hospitals Leuven; KU Leuven, Department of Microbiology, Immunology and Transplantation; Laboratory for Inborn Errors of Immunity, KU Leuven, Leuven, Belgium. 55Department of Pediatrics, Columbia University Irving Medical Center, New York, NY, USA. 56University Clinic for Children's Diseases, Department of Pediatric Immunology, Medical Faculty, University “St.Cyril and Methodij” Skopje, North Macedonia. 57Department of Biomedicine, Aarhus University, Aarhus, Denmark 58Tokyo Medical & Dental University Hospital, Tokyo, Japan. 59A*STAR Infectious Disease Labs, Agency for Science, Technology and Research, Singapore; Lee Kong Chian School of Medicine, Nanyang Technology University, Singapore. 60National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA. 61Laboratory of Medical Genetics, IRCCS Bambino Gesù Children’s Hospital, Rome, Italy. 62Department of Biomedicine and Prevention, Tor Vergata University of Rome, Rome, Italy. 63Comparative Immunology Group, School of Biochemistry and Immunology, Trinity Biomedical Sciences Institute, Trinity College Dublin, Ireland. 64Department of Pediatrics, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan. 65Department of Molecular Biology and Genetics, Bilkent University, Bilkent, Ankara, Turkey. 66Laboratory of Immunogenetics of Human Diseases, Innate Immunity Group, IdiPAZ Institute for Health Research, La Paz Hospital, Madrid, Spain. 67IIBB-CSIC, IDIBAPS, Barcelona, Spain. 68Faculdades Pequeno Príncipe, Instituto de Pesquisa Pelé Pequeno Príncipe, Curitiba, Brazil. 69Neurometabolic Diseases Laboratory, Bellvitge Biomedical Research Institute (IDIBELL), L'Hospitalet de Llobregat, Barcelona, Spain; Catalan Institution of Research and Advanced Studies (ICREA), Barcelona, Spain; Center for Biomedical Research on Rare Diseases (CIBERER), ISCIII, Barcelona, Spain. 70Human Evolutionary Genetics Unit, CNRS U2000, Institut Pasteur, Paris, France; Human Genomics and Evolution, Collège de France, Paris, France. 71University Hospital St. Marina, Varna, Bulgaria. 72Department of Immunology, University Hospital of Gran Canaria Dr. Negrín, Canarian Health System, Las Palmas de Gran Canaria, Spain; Department of Clinical Sciences, University Fernando Pessoa Canarias, Las Palmas de Gran Canaria, Spain 73Department of Pediatric Infectious Diseases and Virology, Imperial College London, London, UK; Centre for Pediatrics and Child Health, Faculty of Medicine, Imperial College London, London, UK. 74Department of Immunology, Second Faculty of Medicine Charles University, V Uvalu, University Hospital in Motol, Prague, Czech Republic. 75Adult Immunodeficiency Unit, Infectious Diseases, Inflammation Center, University of Helsinki and Helsinki University Hospital, Helsinki, Finland; Rare Diseases Center and Pediatric Research Center, Children's Hospital, University of Helsinki and Helsinki University Hospital, Helsinki, Finland 76Saeed Pathobiology and Genetics Lab, Tehran, Iran; Department of Microbiology and Immunology, Clinical and Diagnostic Immunology, KU Leuven, Leuven, Belgium. 77Department of Immunology, Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, Moscow, Russia. 78Central European Institute of Technology & Department of Biology, Faculty of Medicine, Masaryk University, Brno, Czech Republic. 79Department of Pharmacology & Molecular Therapeutics, Uniformed Services University of the Health Sciences, Bethesda, MD, USA. 80Pediatric Infectious Diseases and Immunodeficiencies Unit, Vall d’Hebron Barcelona Hospital Campus, Barcelona, Spain. 81St. Giles Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, NY, USA.; Department of Medical Microbiology, University Medical Center Utrecht, Utrecht, Netherlands 82Department of Internal Medicine II, Medical University of Innsbruck, Innsbruck, Austria. 83Garvan Institute of Medical Research, Darlinghurst, NSW, Australia; St Vincent’s Clinical School, Faculty of Medicine, UNSW Sydney, NSW, Australia. 84Al Jalila Children's Hospital, Dubai, UAE 85BC Children's Hospital, The University of British Columbia, Vancouver, Canada 86Centre for Precision Therapeutics, Genetic and Genomic Medicine Centre, NeuroGen Children Healthcare, Dhaka, Bangladesh; Holy Family Red Crescent Medical College, Dhaka, Bangladesh 87College of Medicine, Mohammed Bin Rashid University of Medicine and Health Sciences, Dubai, UAE; Cellular Intelligence (Ci) Lab, GenomeArc Inc., Toronto, ON, Canada 88Department of Neurology, Amsterdam Neuroscience, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, The Netherlands. 89Department of Medicine, Division of Infectious Diseases, McGill University Health Centre, Montréal, Québec, Canada; Infectious Disease Susceptibility Program, Research Institute, McGill University Health Centre, Montréal, Québec, Canada. 90Department of Pediatric Pneumology, Immunology and Intensive Care, Charité Universitätsmedizin, Berlin University Hospital Center, Berlin, Germany; Labor Berlin GmbH, Department of Immunology, Berlin, Germany; Berlin Institutes of Health (BIH), Berlin-Brandenburg Center for Regenerative Therapies, Berlin, Germany. 91Biosciences Institute, University of São Paulo, São Paulo, Brazil. 92Molecular Biophysics Division, Faculty of Physics, A. Mickiewicz University, Poznań, Poland. 93The Rockefeller University & Howard Hughes Medical Institute, New York, NY, USA; Necker Hospital for Sick Children & INSERM, Paris, France.
CONSTANCES cohort
Rachel Nadif1, Marcel Goldberg2, Anna Ozguler2, Joseph Henny2, Sylvie Lemonnier2, Mireille Coeuret-Pellicer3, Stéphane Le Got2, Marie Zins2
1Université de Paris-Saclay, UVSQ, Université Paris-Sud, Inserm, Equipe d'Epidémiologie Respiratoire Intégrative, Inserm CESP, Villejuif, France. 2Université de Paris, Université Paris-Saclay, UVSQ, Inserm UMS11, Villejuif, France. 3Inserm U011 Constances cohort, Villejuif, France.
3C-Dijon Study
Christophe Tzourio1, Stéphanie Debette2, Carole Dufouil1, Aïcha Soumaré1, Morgane Lachaize2, Nathalie Fievet3, Amandine Flaig3
1University of Bordeaux; Bordeaux Population Health Center, INSERM U1219, Bordeaux, France. 2University of Bordeaux; Bordeaux Population Health Center, INSERM U1219; Bordeaux University Hospital, Department of Neurology, Institute of Neurodegenerative Diseases, Bordeaux, France. 3Laboratoire d'Analyses Génomiques - Centre de Ressources Biologiques; Institut Pasteur de Lille, Lille, France.
Cerba Health-Care
Fernando Martin1
1Cerba Health Care, Issy-les-Moulineaux, France.
Etablissement du Sang study group
Brigitte Bonneaudeau1, Dorothée Cannet2, Pierre Gallian3, Michel Jeanne4, Magali Perroquin4, Hind Hamzeh-Cognasse5, 6
1La Plaine St-Denis, France. 2Dijon, France. 3Marseille, France. 4Bordeaux, France. 5Saint-Etienne, France. 6 SAINBIOSE, INSERM, U1059, University of Lyon, Université Jean-Monnet-Saint-Etienne.
Supplementary Materials
immunology.sciencemag.org/cgi/content/full/6/62/eabl4340/DC1
Supplementary Materials and Methods
Figures S1 to S6
Tables S1 to S6; these tables are found on the tabs of a single Excel spreadsheet that contains the raw data
Contributor Information
Collaborators: Benedetta Bigio, Soraya Boucherit, Aliénor de la Chapelle, Jie Chen, Maya Chrabieh, Boubacar Coulibaly, Dana Liu, Yelena Nemirowskaya, Inés Marín Cruz, Marie Materna, Sophie Pelet, Yoann Seeleuthner, Chloé Thibault, Zhiyong Liu, Jorge Abad, Giulia Accordino, Cristian Achille, Sergio Aguilera-Albesa, Aina Aguiló-Cucurull, Alessandro Aiuti, Esra Akyüz Özkan, Ilad Alavi Darazam, Jonathan Antonio Roblero Albisures, Juan C Aldave, Miquel Alfonso Ramos, Taj Ali Khan, Anna Aliberti, Seyed Alireza Nadji, Gulsum Alkan, Suzan A. Alkhater, Jerome Allardet-Servent, Luis M Allende, Rebeca Alonso-Arias, Mohammed S Alshahrani, Laia Alsina, Marie-Alexandra Alyanakian, Blanca Amador Borrero, Zahir Amoura, Arnau Antolí, Romain Arrestier, Mélodie Aubart, Teresa Auguet, Iryna Avramenko, Gökhan Aytekin, Axelle Azot, Seiamak Bahram, Fanny Bajolle, Fausto Baldanti, Aurélie Baldolli, Maite Ballester, Hagit Baris Feldman, Benoit Barrou, Federica Barzagh, Sabrina Basso, Gulsum Iclal Bayhan, Alexandre Belot, Liliana Bezrodnik, Agurtzane Bilbao, Geraldine Blanchard-Rohner, Ignacio Blanco, Adeline Blandinières, Daniel Blázquez-Gamero, Alexandre Bleibtreu, Marketa Bloomfield, Mireia Bolivar-Prados, Anastasiia Bondarenko, Alessandro Borghesi, Raphael Borie, Elisabeth Botdhlo-Nevers, Ahmed A Bousfiha, Aurore Bousquet, David Boutolleau, Claire Bouvattier, Oksana Boyarchuk, Juliette Bravais, M. Luisa Briones, Marie-Eve Brunner, Raffaele Bruno, Maria Rita P Bueno, Huda Bukhari, Jacinta Bustamante, Juan José Cáceres Agra, Ruggero Capra, Raphael Carapito, Maria Carrabba, Giorgio Casari, Carlos Casasnovas, Marion Caseris, Irene Cassaniti, Martin Castelle, Francesco Castelli, Martín Castillo de Vera, Mateus V Castro, Emilie Catherinot, Jale Bengi Celik, Alessandro Ceschi, Martin Chalumeau, Bruno Charbit, Matthew P. Cheng, Père Clavé, Bonaventura Clotet, Anna Codina, Yves Cohen, Roger Colobran, Cloé Comarmond, Alain Combes, Patrizia Comoli, Angelo G Corsico, Taner Coşkuner, Aleksandar Cvetkovski, Cyril Cyrus, David Dalmau, François Danion, David Ross Darley, Vincent Das, Nicolas Dauby, Stéphane Dauger, Paul De Munter, Loic de Pontual, Amin Dehban, Geoffroy Delplancq, Alexandre Demoule, Isabelle Desguerre, Antonio Di Sabatino, Jean-Luc Diehl, Stephanie Dobbelaere, Elena Domínguez-Garrido, Clément Dubost, Olov Ekwall, Şefika Elmas Bozdemir, Marwa H Elnagdy, Melike Emiroglu, Akifumi Endo, Emine Hafize Erdeniz, Selma Erol Aytekin, Maria Pilar Etxart Lasa, Romain Euvrard, Giovanna Fabio, Laurence Faivre, Antonin Falck, Muriel Fartoukh, Morgane Faure, Miguel Fernandez Arquero, Ricard Ferrer, Jose Ferreres, Carlos Flores, Bruno Francois, Victoria Fumadó, Kitty S C Fung, Francesca Fusco, Alenka Gagro, Blanca Garcia Solis, Pascale Gaussem, Zeynep Gayretli, Juana Gil-Herrera, Laurent Gilardin, Audrey Giraud Gatineau, Mònica Girona-Alarcón, Karen Alejandra Cifuentes Godínez, Jean-Christophe Goffard, Nacho Gonzales, Luis I Gonzalez-Granado, Rafaela González-Montelongo, Antoine Guerder, Belgin Gülhan, Victor Daniel Gumucio, Leif Gunnar Hanitsch, Jan Gunst, Marta Gut, Jérôme Hadjadj, Filomeen Haerynck, Rabih Halwani, Lennart Hammarström, Selda Hancerli, Tetyana Hariyan, Nevin Hatipoglu, Deniz Heppekcan, Elisa Hernandez-Brito, Po-ki Ho, María Soledad Holanda-Peña, Juan P Horcajada, Sami Hraiech, Linda Humbert, Ivan F N Hung, Alejandro D. Iglesias, Antonio Íñigo-Campos, Matthieu Jamme, María Jesús Arranz, Marie-Thérèse Jimeno, Iolanda Jordan, Saliha Kanık Yüksek, Yalcin Burak Kara, Aydın Karahan, Adem Karbuz, Kadriye Kart Yasar, Ozgur Kasapcopur, Kenichi Kashimada, Sevgi Keles, Yasemin Kendir Demirkol, Yasutoshi Kido, Can Kizil, Ahmet Osman Kılıç, Adam Klocperk, Antonia Koutsoukou, Zbigniew J. Król, Hatem Ksouri, Paul Kuentz, Arthur M C Kwan, Yat Wah M Kwan, Janette S Y Kwok, Jean-Christophe Lagier, David S Y Lam, Vicky Lampropoulou, Fanny Lanternier, Yu-Lung Lau, Fleur Le Bourgeois, Yee-Sin Leo, Rafael Leon Lopez, Daniel Leung, Michael Levin, Michael Levy, Romain Lévy, Zhi Li, Daniele Lilleri, Edson Jose Adrian Bolanos Lima, Agnes Linglart, Eduardo López-Collazo, José M. Lorenzo-Salazar, Céline Louapre, Catherine Lubetzki, Kwok-Cheung Lung, Charles-Edouard Luyt, David C Lye, Cinthia Magnone, Davood Mansouri, Enrico Marchioni, Carola Marioli, Majid Marjani, Laura Marques, Jesus Marquez Pereira, Andrea Martín-Nalda, David Martínez Pueyo, Javier Martinez-Picado, Iciar Marzana, Carmen Mata-Martínez, Alexis Mathian, Larissa RB Matos, Gail V Matthews, Julien Mayaux, Raquel McLaughlin-Garcia, Philippe Meersseman, Jean-Louis Mège, Armand Mekontso-Dessap, Isabelle Melki, Federica Meloni, Jean-François Meritet, Paolo Merlani, Özge Metin Akcan, Isabelle Meyts, Mehdi Mezidi, Isabelle Migeotte, Maude Millereux, Matthieu Million, Tristan Mirault, Clotilde Mircher, Mehdi Mirsaeidi, Yoko Mizoguchi, Bhavi P Modi, Francesco Mojoli, Elsa Moncomble, Abián Montesdeoca Melián, Antonio Morales Martinez, Francisco Morandeira, Pierre-Emmanuel Morange, Clémence Mordacq, Guillaume Morelle, Stéphane J Mouly, Adrián Muñoz-Barrera, Cyril Nafati, Shintaro Nagashima, Yu Nakagama, Bénédicte Neven, João Farela Neves, Lisa FP Ng, Yuk-Yung Ng, Hubert Nielly, Yeray Novoa Medina, Esmeralda Nuñez Cuadros, J. Gonzalo Ocejo-Vinyals, Keisuke Okamoto, Mehdi Oualha, Amani Ouedrani, Tayfun Özçelik, Aslinur Ozkaya-Parlakay, Michele Pagani, Qiang Pan-Hammarström, Maria Papadaki, Christophe Parizot, Philippe Parola, Tiffany Pascreau, Stéphane Paul, Estela Paz-Artal, Sigifredo Pedraza, Nancy Carolina González Pellecer, Silvia Pellegrini, Rebeca Pérez de Diego, Xosé Luis Pérez-Fernández, Aurélien Philippe, Quentin Philippot, Adrien Picod, Marc Pineton de Chambrun, Antonio Piralla, Laura Planas-Serra, Dominique Ploin, Julien Poissy, Géraldine Poncelet, Garyphallia Poulakou, Marie S Pouletty, Persia Pourshahnazari, Jia Li Qiu-Chen, Paul Quentric, Thomas Rambaud, Didier Raoult, Violette Raoult, Anne-Sophie Rebillat, Claire Redin, Léa Resmini, Pilar Ricart, Jean-Christophe Richard, Raúl Rigo-Bonnin, Nadia Rivet, Jacques G Rivière, Gemma Rocamora-Blanch, Mathieu P Rodero, Carlos Rodrigo, Luis Antonio Rodriguez, Carlos Rodriguez-Gallego, Agustí Rodriguez-Palmero, Carolina Soledad Romero, Anya Rothenbuhler, Damien Roux, Nikoletta Rovina, Flore Rozenberg, Yvon Ruch, Montse Ruiz, Maria Yolanda Ruiz del Prado, Juan Carlos Ruiz-Rodriguez, Joan Sabater-Riera, Kai Saks, Maria Salagianni, Oliver Sanchez, Adrián Sánchez-Montalvá, Silvia Sánchez-Ramón, Laire Schidlowski, Agatha Schluter, Julien Schmidt, Matthieu Schmidt, Catharina Schuetz, Cyril E Schweitzer, Francesco Scolari, Anna Sediva, Luis Seijo, Analia Gisela Seminario, Damien Sene, Piseth Seng, Sevtap Senoglu, Mikko Seppänen, Alex Serra Llovich, Mohammad Shahrooei, Anna Shcherbina, Virginie Siguret, Eleni Siouti, David M Smadja, Nikaia Smith, Ali Sobh, Xavier Solanich, Jordi Solé-Violán, Catherine Soler, Pere Soler-Palacín, Betül Sözeri, Giulia Maria Stella, Yuriy Stepanovskiy, Annabelle Stoclin, Fabio Taccone, Yacine Tandjaoui-Lambiotte, Jean-Luc Taupin, Simon J Tavernier, Loreto Vidaur Tello, Benjamin Terrier, Guillaume Thiery, Christian Thorball, Karolina THORN, Caroline Thumerelle, Imran Tipu, Martin Tolstrup, Gabriele Tomasoni, Julie Toubiana, Josep Trenado Alvarez, Vasiliki Triantafyllia, Sophie Trouillet-Assant, Jesús Troya, Owen T Y Tsang, Liina Tserel, Eugene Y K Tso, Alessandra Tucci, Şadiye Kübra Tüter Öz, Matilde Valeria Ursini, Takanori Utsumi, Yurdagul Uzunhan, Pierre Vabres, Juan Valencia-Ramos, Ana Maria Van Den Rym, Isabelle Vandernoot, Valentina Velez-Santamaria, Silvia Patricia Zuniga Veliz, Mateus C Vidigal, Sébastien Viel, Cédric Vilain, Marie E Vilaire-Meunier, Judit Villar-García, Audrey Vincent, Guillaume Vogt, Guillaume Voiriot, Alla Volokha, Fanny Vuotto, Els Wauters, Joost Wauters, Alan K L Wu, Tak-Chiu Wu, Aysun Yahşi, Osman Yesilbas, Mehmet Yildiz, Barnaby E Young, Ufuk Yükselmiş, Mayana Zatz, Marco Zecca, Valentina Zuccaro, Van Praet Jens, Bart N. Lambrecht, Van Braeckel Eva, Bosteels Cédric, Hoste Levi, Hoste Eric, Fré Bauters, Jozefien De Clercq, Heijmans Cathérine, Slabbynck Hans, Naesens Leslie, Benoit Florkin, Cécile Boulanger, Dimitri Vanderlinden, Giuseppe Foti, Giacomo Bellani, Giuseppe Citerio, Ernesto Contro, Alberto Pesci, Maria Grazia Valsecchi, Marina Cazzaniga, Jeffrey J. Danielson, Kerry Dobbs, Anuj Kashyap, Li Ding, Clifton L. Dalgard, Alessandra Sottini, Virginia Quaresima, Eugenia Quiros-Roldan, Camillo Rossi, Laura Rachele Bettini, Mariella D’Angio’, Ilaria Beretta, Daniela Montagna, Amelia Licari, Gian Luigi Marseglia, Isabella Batten, Conor Reddy, Matt McElheron, Claire Noonan, Emma Connolly, Aoife Fallon, Merete Storgaard, Sofie Jørgensen, Martin Tolstrup, Christian Erikstrup, Ole Birger Pedersen, Erik Sørensen, Susan Mikkelsen, Khoa Manh Dinh, Margit Anita Hørup Larsen, Isabella Worlewenut Paulsen, Jakob Hjorth Von Stemann, Morten Bagge Hansen, Sisse Rye Ostrowski, Liam Townsend, Cliona Ni Cheallaigh, Colm Bergin, Ignacio Martin-Loeches, Jean Dunne, Niall Conlon, Nollaig Bourke, Cliona O'Farrelly, Laurent Abel, Clotilde Allavena, Claire Andrejak, François Angoulvant, Cecile Azoulay, Delphine Bachelet, Marie Bartoli, Romain Basmaci, Sylvie Behilill, Marine Beluze, Nicolas Benech, Dehbia Benkerrou, Krishna Bhavsar, Laurent Bitker, Lila Bouadma, Maude Bouscambert-Duchamp, Pauline Caraux Paz, Minerva Cervantes-Gonzalez, Anissa Chair, Catherine Chirouze, Alexandra Coelho, Hugues Cordel, Camille Couffignal, Sandrine Couffin-Cadiergues, Eric d’Ortenzio, Etienne De Montmollin, Alexa Debard, Marie-Pierre Debray, Dominique Deplanque, Diane Descamps, Mathilde Desvallée, Alpha Diallo, Jean-Luc Diehl, Alphonsine Diouf, Céline Dorival, François Dubos, Xavier Duval, Philippine Eloy, Vincent Enouf, Olivier Epaulard, Hélène Esperou, Marina Esposito-Farese, Manuel Etienne, Denis Garot, Nathalie Gault, Alexandre Gaymard, Jade Ghosn, Tristan Gigante, Morgane Gilg, François Goehringer, Jérémie Guedj, Alexandre Hoctin, Isabelle Hoffmann, Ikram Houas, Jean-Sébastien Hulot, Salma Jaafoura, Ouifiya Kafif, Florentia Kaguelidou, Sabrina Kali, Younes Kerroumi, Antoine Khalil, Coralie Khan, Antoine Kimmoun, Fabrice Laine, Cédric Laouénan, Samira Laribi, Minh Le, Cyril Le Bris, Sylvie Le Gac, Quentin Le Hingrat, Soizic Le Mestre, Hervé Le Nagard, Adrien Lemaignen, Véronique Lemee, François-Xavier Lescure, Sophie Letrou, Yves Levy, Bruno Lina, Guillaume Lingas, Jean Christophe Lucet, Moïse Machado, Denis Malvy, Marina Mambert, Aldric Manuel, France Mentré, Amina Meziane, Hugo Mouquet, Jimmy Mullaert, Nadège Neant, Duc Nguyen, Marion Noret, Aurélie Papadopoulos, Christelle Paul, Nathan Peiffer-Smadja, Vincent Peigne, Ventzislava Petrov-Sanchez, Gilles Peytavin, Huong Pham, Olivier Picone, Valentine Piquard, Julien Poissy, Oriane Puéchal, Manuel Rosa-Calatrava, Bénédicte Rossignol, Patrick Rossignol, Carine Roy, Marion Schneider, Richa Su, Coralie Tardivon, Marie-Capucine Tellier, François Téoulé, Olivier Terrier, Jean-François Timsit, Christelle Tual, Sarah Tubiana, Sylvie Van Der Werf, Noémie Vanel, Aurélie Veislinger, Benoit Visseaux, Aurélie Wiedemann, Yazdan Yazdanpanah, Jean-Philippe Annereau, Luis Briseño-Roa, Olivier Gribouval, Anna Pelet, Laurent Abel, Andres Alcover, Hugues Aschard, Philippe Bousso, Nollaig Bourke, Petter Brodin, Pierre Bruhns, Nadine Cerf-Bensussan, Ana Cumano, Christophe D’Enfert, Ludovic Deriano, Marie-Agnès Dillies, James Di Santo, Françoise Dromer, Gérard Eberl, Jost Enninga, Jacques Fellay, Ivo Gomperts-Boneca, Milena Hasan, Gunilla Karlsson Hedestam, Serge Hercberg, Molly A Ingersoll, Olivier Lantz, Rose Anne Kenny, Mickaël Ménager, Frédérique Michel, Hugo Mouquet, Cliona O'Farrelly, Etienne Patin, Sandra Pellegrini, Antonio Rausell, Frédéric Rieux-Laucat, Lars Rogge, Magnus Fontes, Anavaj Sakuntabhai, Olivier Schwartz, Benno Schwikowski, Spencer Shorte, Frédéric Tangy, Antoine Toubert, Mathilde Touvier, Marie-Noëlle Ungeheuer, Christophe Zimmer, Matthew L. Albert, Darragh Duffy, Lluis Quintana-Murci, Loubna Alavoine, Sylvie Behillil, Charles Burdet, Charlotte Charpentier, Aline Dechanet, Diane Descamps, Xavier Duval, Jean-Luc Ecobichon, Vincent Enouf, Wahiba Frezouls, Nadhira Houhou, Ouifiya Kafif, Jonathan Lehacaut, Sophie Letrou, Bruno Lina, Jean-Christophe Lucet, Pauline Manchon, Mariama Nouroudine, Valentine Piquard, Caroline Quintin, Michael Thy, Sarah Tubiana, Sylvie van der Werf, Valérie Vignali, Benoit Visseaux, Yazdan Yazdanpanah, Abir Chahine, Nawal Waucquier, Maria-Claire Migaud, Dominique Deplanque, Félix Djossou, Mayka Mergeay-Fabre, Aude Lucarelli, Magalie Demar, Léa Bruneau, Patrick Gérardin, Adrien Maillot, Christine Payet, Bruno Laviolle, Fabrice Laine, Christophe Paris, Mireille Desille-Dugast, Julie Fouchard, Denis Malvy, Duc Nguyen, Thierry Pistone, Pauline Perreau, Valérie Gissot, Carole Le Goas, Samatha Montagne, Lucie Richard, Catherine Chirouze, Kévin Bouiller, Maxime Desmarets, Alexandre Meunier, Benjamin Lefévre, Hélène Jeulin, Karine Legrand, Sandra Lomazzi, Bernard Tardy, Amandine Gagneux-Brunon, Frédérique Bertholon, Elisabeth Botelho-Nevers, Christelle Kouakam, Nicolas Leturque, Layidé Roufai, Karine Amat, Sandrine Couffin-Cadiergues, Hélène Espérou, Samia Hendou, Michiel van Agtmael, Anne Geke Algera, Brent Appelman, Frank van Baarle, Diane Bax, Martijn Beudel, Harm Jan Bogaard, Marije Bomers, Peter Bonta, Lieuwe Bos, Michela Botta, Justin de Brabander, Godelieve de Bree, Sanne de Bruin, David T.P. Buis, Marianna Bugiani, Esther Bulle, Osoul Chouchane, Alex Cloherty, Mirjam Dijkstra, Dave A. Dongelmans, Romein W.G. Dujardin, Paul Elbers, Lucas Fleuren, Suzanne Geerlings Theo Geijtenbeek, Armand Girbes, Bram Goorhuis, Martin P. Grobusch, Florianne Hafkamp, Laura Hagens, Jorg Hamann, Vanessa Harris, Robert Hemke, Sabine M. Hermans, Leo Heunks, Markus Hollmann, Janneke Horn, Joppe W. Hovius, Menno D. de Jong, Rutger Koning, Endry H.T. Lim, Niels van Mourik, Jeaninne Nellen, Esther J. Nossent, Frederique Paulus, Edgar Peters, Dan A.I. Pina-Fuentes, Tom van der Poll, Bennedikt Preckel, Jan M. Prins, Jorinde Raasveld, Tom Reijnders, Maurits C.F.J. de Rotte, Michiel Schinkel, Marcus J. Schultz, Femke A.P. Schrauwen, Alex Schuurmans, Jaap Schuurmans, Kim Sigaloff, Marleen A. Slim, Patrick Smeele, Marry Smit, Cornelis S. Stijnis, Willemke Stilma, Charlotte Teunissen, Patrick Thoral, Anissa M Tsonas, Pieter R. Tuinman, Marc van der Valk, Denise Veelo, Carolien Volleman, Heder de Vries, Lonneke A. Vught, Michèle van Vugt, Dorien Wouters, A. H (Koos Zwinderman, Matthijs C. Brouwer, W. Joost Wiersinga, Alexander P.J. Vlaar, Diederik van de Beek, Laurent Abel, Alessandro Aiuti, Saleh Al-Muhsen, Fahd Al-Mulla, Mark S. Anderson, Evangelos Andreakos, Andrés A. Arias, Hagit Baris Feldman, Alexandre Belot, Catherine M. Biggs, Dusan Bogunovic, Alexandre Bolze, Anastasiia Bondarenko, Ahmed A. Bousfiha, Petter Brodin, Yenan Bryceson, Carlos D. Bustamante, Manish J. Butte, Giorgio Casari, Samya Chakravorty, John Christodoulou, Antonio Condino-Neto, Stefan N. Constantinescu, Megan A. Cooper, Clifton L. Dalgard, Murkesh Desai, Beth A. Drolet, Jamila El Baghdadi, Sara Espinosa-Padilla, Jacques Fellay, Carlos Flores, José Luis Franco, Antoine Froidure, Peter K. Gregersen, Filomeen Haerynck, David Hagin, Rabih Halwani, Lennart Hammarström, James R. Heath, Sarah E. Henrickson, Elena W.Y. Hsieh, Eystein S. Husebye, Kohsuke Imai, Yuval Itan, Erich D. Jarvis, Timokratis Karamitros, Kai Kisand, Cheng-Lung Ku, Yu-Lung Lau, Yun Ling, Carrie L. Lucas, Tom Maniatis, Davood Mansouri, László Maródi, Isabelle Meyts, Joshua D. Milner, Kristina Mironska, Trine H. Mogensen, Tomohiro Morio, Lisa F.P. Ng, Luigi D. Notarangelo, Antonio Novelli, Giuseppe Novelli, Cliona O'Farrelly, Satoshi Okada, Tayfun Ozcelik, Qiang Pan-Hammarström, Rebeca Perez de Diego, Anna M. Planas, Carolina Prando, Aurora Pujol, Lluis Quintana-Murci, Laurent Renia, Igor Resnick, Carlos Rodríguez-Gallego, Vanessa Sancho-Shimizu, Anna Sediva, Mikko R.J. Seppänen, Mohammed Shahrooei, Anna Shcherbina, Ondrej Slaby, Andrew L. Snow, Pere Soler-Palacín, András N. Spaan, Ivan Tancevski, Stuart G. Tangye, Ahmad Abou Tayoun, Sathishkumar Ramaswamy, Stuart E Turvey, K M Furkan Uddin, Mohammed J. Uddin, Diederik van de Beek, Donald C. Vinh, Horst von Bernuth, Mayana Zatz, Pawel Zawadzki, Helen C. Su, Jean-Laurent Casanova, Rachel Nadif, Marcel Goldberg, Anna Ozguler, Joseph Henny, Sylvie Lemonnier, Mireille Coeuret-Pellicer, Stéphane Le Got, Marie Zins, Christophe Tzourio, Stéphanie Debette, Carole Dufouil, Aïcha Soumaré, Morgane Lachaize, Nathalie Fievet, Amandine Flaig, Fernando Martin, Brigitte Bonneaudeau, Dorothée Cannet, Pierre Gallian, Michel Jeanne, Magali Perroquin, and Hind Hamzeh-Cognasse
References and Notes
- 1.Levin A. T., Hanage W. P., Owusu-Boaitey N., Cochran K. B., Walsh S. P., Meyerowitz-Katz G., Assessing the age specificity of infection fatality rates for COVID-19: Systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 35, 1123–1138 (2020). 10.1007/s10654-020-00698-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Driscoll M., Ribeiro Dos Santos G., Wang L., Cummings D. A. T., Azman A. S., Paireau J., Fontanet A., Cauchemez S., Salje H., Age-specific mortality and immunity patterns of SARS-CoV-2. Nature 590, 140–145 (2021). 10.1038/s41586-020-2918-0 [DOI] [PubMed] [Google Scholar]
- 3.Williamson E. J., Walker A. J., Bhaskaran K., Bacon S., Bates C., Morton C. E., Curtis H. J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H. I., MacKenna B., Tomlinson L., Douglas I. J., Rentsch C. T., Mathur R., Wong A. Y. S., Grieve R., Harrison D., Forbes H., Schultze A., Croker R., Parry J., Hester F., Harper S., Perera R., Evans S. J. W., Smeeth L., Goldacre B., Factors associated with COVID-19-related death using OpenSAFELY. Nature 584, 430–436 (2020). 10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodin P., Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 27, 28–33 (2021). 10.1038/s41591-020-01202-8 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Q., Bastard P., Bolze A., Jouanguy E., Zhang S. Y., Cobat A., Notarangelo L. D., Su H. C., Abel L., Casanova J. L.; COVID Human Genetic Effort , Life-Threatening COVID-19: Defective Interferons Unleash Excessive Inflammation. Med (N Y) 1, 14–20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casanova J. L., Su H. C.; COVID Human Genetic Effort , A Global Effort to Define the Human Genetics of Protective Immunity to SARS-CoV-2 Infection. Cell 181, 1194–1199 (2020). 10.1016/j.cell.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I. K. D., Hodeib S., Korol C., Rosain J., Bilguvar K., Ye J., Bolze A., Bigio B., Yang R., Arias A. A., Zhou Q., Zhang Y., Onodi F., Korniotis S., Karpf L., Philippot Q., Chbihi M., Bonnet-Madin L., Dorgham K., Smith N., Schneider W. M., Razooky B. S., Hoffmann H.-H., Michailidis E., Moens L., Han J. E., Lorenzo L., Bizien L., Meade P., Neehus A.-L., Ugurbil A. C., Corneau A., Kerner G., Zhang P., Rapaport F., Seeleuthner Y., Manry J., Masson C., Schmitt Y., Schlüter A., Le Voyer T., Khan T., Li J., Fellay J., Roussel L., Shahrooei M., Alosaimi M. F., Mansouri D., Al-Saud H., Al-Mulla F., Almourfi F., Al-Muhsen S. Z., Alsohime F., Al Turki S., Hasanato R., van de Beek D., Biondi A., Bettini L. R., D’Angio’ M., Bonfanti P., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Oler A. J., Tompkins M. F., Alba C., Vandernoot I., Goffard J.-C., Smits G., Migeotte I., Haerynck F., Soler-Palacin P., Martin-Nalda A., Colobran R., Morange P.-E., Keles S., Çölkesen F., Ozcelik T., Yasar K. K., Senoglu S., Karabela Ş. N., Rodríguez-Gallego C., Novelli G., Hraiech S., Tandjaoui-Lambiotte Y., Duval X., Laouénan C., Snow A. L., Dalgard C. L., Milner J. D., Vinh D. C., Mogensen T. H., Marr N., Spaan A. N., Boisson B., Boisson-Dupuis S., Bustamante J., Puel A., Ciancanelli M. J., Meyts I., Maniatis T., Soumelis V., Amara A., Nussenzweig M., García-Sastre A., Krammer F., Pujol A., Duffy D., Lifton R. P., Zhang S.-Y., Gorochov G., Béziat V., Jouanguy E., Sancho-Shimizu V., Rice C. M., Abel L., Notarangelo L. D., Cobat A., Su H. C., Casanova J.-L.; COVID-STORM Clinicians; COVID Clinicians; Imagine COVID Group; French COVID Cohort Study Group; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort; NIAID-USUHS/TAGC COVID Immunity Group , Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 370, eabd4570 (2020). 10.1126/science.abd4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Novelli G., Biancolella M., Mehrian-Shai R., Colona V. L., Brito A. F., Grubaugh N. D., Vasiliou V., Luzzatto L., Reichardt J. K. V., COVID-19 one year into the pandemic: From genetics and genomics to therapy, vaccination, and policy. Hum. Genomics 15, 27 (2021). 10.1186/s40246-021-00326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastard P., Rosen L. B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., Manry J., Shaw E., Haljasmägi L., Peterson P., Lorenzo L., Bizien L., Trouillet-Assant S., Dobbs K., de Jesus A. A., Belot A., Kallaste A., Catherinot E., Tandjaoui-Lambiotte Y., Le Pen J., Kerner G., Bigio B., Seeleuthner Y., Yang R., Bolze A., Spaan A. N., Delmonte O. M., Abers M. S., Aiuti A., Casari G., Lampasona V., Piemonti L., Ciceri F., Bilguvar K., Lifton R. P., Vasse M., Smadja D. M., Migaud M., Hadjadj J., Terrier B., Duffy D., Quintana-Murci L., van de Beek D., Roussel L., Vinh D. C., Tangye S. G., Haerynck F., Dalmau D., Martinez-Picado J., Brodin P., Nussenzweig M. C., Boisson-Dupuis S., Rodríguez-Gallego C., Vogt G., Mogensen T. H., Oler A. J., Gu J., Burbelo P. D., Cohen J. I., Biondi A., Bettini L. R., D’Angio M., Bonfanti P., Rossignol P., Mayaux J., Rieux-Laucat F., Husebye E. S., Fusco F., Ursini M. V., Imberti L., Sottini A., Paghera S., Quiros-Roldan E., Rossi C., Castagnoli R., Montagna D., Licari A., Marseglia G. L., Duval X., Ghosn J., Tsang J. S., Goldbach-Mansky R., Kisand K., Lionakis M. S., Puel A., Zhang S.-Y., Holland S. M., Gorochov G., Jouanguy E., Rice C. M., Cobat A., Notarangelo L. D., Abel L., Su H. C., Casanova J.-L.; HGID Lab; NIAID-USUHS Immune Response to COVID Group; COVID Clinicians; COVID-STORM Clinicians; Imagine COVID Group; French COVID Cohort Study Group; Milieu Intérieur Consortium; CoV-Contact Cohort; Amsterdam UMC Covid-19 Biobank; COVID Human Genetic Effort , Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 370, eabd4585 (2020). 10.1126/science.abd4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazear H. M., Schoggins J. W., Diamond M. S., Shared and Distinct Functions of Type I and Type III Interferons. Immunity 50, 907–923 (2019). 10.1016/j.immuni.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koning R., Bastard P., Casanova J.-L., Brouwer M. C., van de Beek D.; with the Amsterdam U.M.C. COVID-19 Biobank Investigators , Autoantibodies against type I interferons are associated with multi-organ failure in COVID-19 patients. Intensive Care Med. 47, 704–706 (2021). 10.1007/s00134-021-06392-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troya J., Bastard P., Planas-Serra L., Ryan P., Ruiz M., de Carranza M., Torres J., Martínez A., Abel L., Casanova J.-L., Pujol A., Neutralizing Autoantibodies to Type I IFNs in >10% of Patients with Severe COVID-19 Pneumonia Hospitalized in Madrid, Spain. J. Clin. Immunol. 41, 914–922 (2021). 10.1007/s10875-021-01036-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vazquez S. E., Bastard P., Kelly K., Gervais A., Norris P. J., Dumont L. J., Casanova J.-L., Anderson M. S., DeRisi J. L., Neutralizing Autoantibodies to Type I Interferons in COVID-19 Convalescent Donor Plasma. J. Clin. Immunol. 41, 1169–1171 (2021). 10.1007/s10875-021-01060-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goncalves D., et al., Antibodies against type-I Interferon: detection and association with severe clinical outcome in COVID-19 patients. medRxiv, (2021). [DOI] [PMC free article] [PubMed]
- 15.Wang E. Y., Mao T., Klein J., Dai Y., Huck J. D., Jaycox J. R., Liu F., Zhou T., Israelow B., Wong P., Coppi A., Lucas C., Silva J., Oh J. E., Song E., Perotti E. S., Zheng N. S., Fischer S., Campbell M., Fournier J. B., Wyllie A. L., Vogels C. B. F., Ott I. M., Kalinich C. C., Petrone M. E., Watkins A. E., Dela Cruz C., Farhadian S. F., Schulz W. L., Ma S., Grubaugh N. D., Ko A. I., Iwasaki A., Ring A. M.; Yale IMPACT Team , Diverse functional autoantibodies in patients with COVID-19. Nature 595, 283–288 (2021). 10.1038/s41586-021-03631-y [DOI] [PubMed] [Google Scholar]
- 16.van der Wijst M. G. P., et al., Longitudinal single-cell epitope and RNA-sequencing reveals the immunological impact of type 1 interferon autoantibodies in critical COVID-19. bioRxiv, (2021).
- 17.Levin M., Anti-interferon auto-antibodies in autoimmune polyendocrinopathy syndrome type 1. PLOS Med. 3, e292 (2006). 10.1371/journal.pmed.0030292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meager A., Visvalingam K., Peterson P., Möll K., Murumägi A., Krohn K., Eskelin P., Perheentupa J., Husebye E., Kadota Y., Willcox N., Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLOS Med. 3, e289 (2006). 10.1371/journal.pmed.0030289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer S., Woodward M., Hertel C., Vlaicu P., Haque Y., Kärner J., Macagno A., Onuoha S. C., Fishman D., Peterson H., Metsküla K., Uibo R., Jäntti K., Hokynar K., Wolff A. S. B., Krohn K., Ranki A., Peterson P., Kisand K., Hayday A.; APECED patient collaborative , AIRE-Deficient Patients Harbor Unique High-Affinity Disease-Ameliorating Autoantibodies. Cell 166, 582–595 (2016). 10.1016/j.cell.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastard P., Orlova E., Sozaeva L., Lévy R., James A., Schmitt M. M., Ochoa S., Kareva M., Rodina Y., Gervais A., Le Voyer T., Rosain J., Philippot Q., Neehus A.-L., Shaw E., Migaud M., Bizien L., Ekwall O., Berg S., Beccuti G., Ghizzoni L., Thiriez G., Pavot A., Goujard C., Frémond M.-L., Carter E., Rothenbuhler A., Linglart A., Mignot B., Comte A., Cheikh N., Hermine O., Breivik L., Husebye E. S., Humbert S., Rohrlich P., Coaquette A., Vuoto F., Faure K., Mahlaoui N., Kotnik P., Battelino T., Trebušak Podkrajšek K., Kisand K., Ferré E. M. N., DiMaggio T., Rosen L. B., Burbelo P. D., McIntyre M., Kann N. Y., Shcherbina A., Pavlova M., Kolodkina A., Holland S. M., Zhang S.-Y., Crow Y. J., Notarangelo L. D., Su H. C., Abel L., Anderson M. S., Jouanguy E., Neven B., Puel A., Casanova J.-L., Lionakis M. S., Preexisting autoantibodies to type I IFNs underlie critical COVID-19 pneumonia in patients with APS-1. J. Exp. Med. 218, e20210554 (2021). 10.1084/jem.20210554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meisel C., Akbil B., Meyer T., Lankes E., Corman V. M., Staudacher O., Unterwalder N., Kölsch U., Drosten C., Mall M. A., Kallinich T., Schnabel D., Goffinet C., von Bernuth H., Mild COVID-19 despite autoantibodies against type I IFNs in autoimmune polyendocrine syndrome type 1. J. Clin. Invest. 131, e150867 (2021). 10.1172/JCI150867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter J. E., Rosen L. B., Csomos K., Rosenberg J. M., Mathew D., Keszei M., Ujhazi B., Chen K., Lee Y. N., Tirosh I., Dobbs K., Al-Herz W., Cowan M. J., Puck J., Bleesing J. J., Grimley M. S., Malech H., De Ravin S. S., Gennery A. R., Abraham R. S., Joshi A. Y., Boyce T. G., Butte M. J., Nadeau K. C., Balboni I., Sullivan K. E., Akhter J., Adeli M., El-Feky R. A., El-Ghoneimy D. H., Dbaibo G., Wakim R., Azzari C., Palma P., Cancrini C., Capuder K., Condino-Neto A., Costa-Carvalho B. T., Oliveira J. B., Roifman C., Buchbinder D., Kumanovics A., Franco J. L., Niehues T., Schuetz C., Kuijpers T., Yee C., Chou J., Masaad M. J., Geha R., Uzel G., Gelman R., Holland S. M., Recher M., Utz P. J., Browne S. K., Notarangelo L. D., Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J. Clin. Invest. 125, 4135–4148 (2015). 10.1172/JCI80477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg J. M., Maccari M. E., Barzaghi F., Allenspach E. J., Pignata C., Weber G., Torgerson T. R., Utz P. J., Bacchetta R., Neutralizing Anti-Cytokine Autoantibodies Against Interferon-α in Immunodysregulation Polyendocrinopathy Enteropathy X-Linked. Front. Immunol. 9, 544 (2018). 10.3389/fimmu.2018.00544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallbracht A., Treuner J., Flehmig B., Joester K. E., Niethammer D., Interferon-neutralizing antibodies in a patient treated with human fibroblast interferon. Nature 289, 496–497 (1981). 10.1038/289496a0 [DOI] [PubMed] [Google Scholar]
- 25.Rudick R. A., Simonian N. A., Alam J. A., Campion M., Scaramucci J. O., Jones W., Coats M. E., Goodkin D. E., Weinstock-Guttman B., Herndon R. M., Mass M. K., Richert J. R., Salazar A. M., Munschauer F. E. 3rd, Cookfair D. L., Simon J. H., Jacobs L. D.; Multiple Sclerosis Collaborative Research Group (MSCRG) , Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis. Neurology 50, 1266–1272 (1998). 10.1212/WNL.50.5.1266 [DOI] [PubMed] [Google Scholar]
- 26.Panem S., Check I. J., Henriksen D., Vilcek J., Antibodies to alpha-interferon in a patient with systemic lupus erythematosus. J. Immunol. 129, 1–3 (1982). [PubMed] [Google Scholar]
- 27.Gupta S., Tatouli I. P., Rosen L. B., Hasni S., Alevizos I., Manna Z. G., Rivera J., Jiang C., Siegel R. M., Holland S. M., Moutsopoulos H. M., Browne S. K., Distinct Functions of Autoantibodies Against Interferon in Systemic Lupus Erythematosus: A Comprehensive Analysis of Anticytokine Autoantibodies in Common Rheumatic Diseases. Arthritis Rheumatol. 68, 1677–1687 (2016). 10.1002/art.39607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiono H., Wong Y. L., Matthews I., Liu J. L., Zhang W., Sims G., Meager A., Beeson D., Vincent A., Willcox N., Spontaneous production of anti-IFN-alpha and anti-IL-12 autoantibodies by thymoma cells from myasthenia gravis patients suggests autoimmunization in the tumor. Int. Immunol. 15, 903–913 (2003). 10.1093/intimm/dxg088 [DOI] [PubMed] [Google Scholar]
- 29.Bello-Rivero I., Cervantes M., Torres Y., Ferrero J., Rodríguez E., Pérez J., García I., Díaz G., López-Saura P., Characterization of the immunoreactivity of anti-interferon alpha antibodies in myasthenia gravis patients. Epitope mapping. J. Autoimmun. 23, 63–73 (2004). 10.1016/j.jaut.2004.03.013 [DOI] [PubMed] [Google Scholar]
- 30.Meager A., Wadhwa M., Dilger P., Bird C., Thorpe R., Newsom-Davis J., Willcox N., Anti-cytokine autoantibodies in autoimmunity: Preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clin. Exp. Immunol. 132, 128–136 (2003). 10.1046/j.1365-2249.2003.02113.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bastard P., Michailidis E., Hoffmann H.-H., Chbihi M., Le Voyer T., Rosain J., Philippot Q., Seeleuthner Y., Gervais A., Materna M., de Oliveira P. M. N., Maia M. L. S., Dinis Ano Bom A. P., Azamor T., Araújo da Conceição D., Goudouris E., Homma A., Slesak G., Schäfer J., Pulendran B., Miller J. D., Huits R., Yang R., Rosen L. B., Bizien L., Lorenzo L., Chrabieh M., Erazo L. V., Rozenberg F., Jeljeli M. M., Béziat V., Holland S. M., Cobat A., Notarangelo L. D., Su H. C., Ahmed R., Puel A., Zhang S.-Y., Abel L., Seligman S. J., Zhang Q., MacDonald M. R., Jouanguy E., Rice C. M., Casanova J.-L., Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 218, e20202486 (2021). 10.1084/jem.20202486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadjadj J., Yatim N., Barnabei L., Corneau A., Boussier J., Smith N., Péré H., Charbit B., Bondet V., Chenevier-Gobeaux C., Breillat P., Carlier N., Gauzit R., Morbieu C., Pène F., Marin N., Roche N., Szwebel T.-A., Merkling S. H., Treluyer J.-M., Veyer D., Mouthon L., Blanc C., Tharaux P.-L., Rozenberg F., Fischer A., Duffy D., Rieux-Laucat F., Kernéis S., Terrier B., Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724 (2020). 10.1126/science.abc6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trouillet-Assant S., Viel S., Gaymard A., Pons S., Richard J.-C., Perret M., Villard M., Brengel-Pesce K., Lina B., Mezidi M., Bitker L., Belot A.; COVID HCL Study group , Type I IFN immunoprofiling in COVID-19 patients. J. Allergy Clin. Immunol. 146, 206–208.e2 (2020). 10.1016/j.jaci.2020.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honda N., Lindberg U., Andersson P., Hoffmann S., Takei H., Simultaneous multiple immunoassays in a compact disc-shaped microfluidic device based on centrifugal force. Clin. Chem. 51, 1955–1961 (2005). 10.1373/clinchem.2005.053348 [DOI] [PubMed] [Google Scholar]
- 35.M. G. P. v. d. Wijst et al., Longitudinal single-cell epitope and RNA-sequencing reveals the immunological impact of type 1 interferon autoantibodies in critical COVID-19. Submitted (2021).
- 36.Asano T., et al., X-linked recessive TLR7 deficiency in 1% of men under 60 years with life-threatening COVID-19. Sci. Immunol. 6, eabl4348 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bastard P., Lévy R., Henriquez S., Bodemer C., Szwebel T.-A., Casanova J.-L., Interferon-β Therapy in a Patient with Incontinentia Pigmenti and Autoantibodies against Type I IFNs Infected with SARS-CoV-2. J. Clin. Immunol. 41, 931–933 (2021). 10.1007/s10875-021-01023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monk P. D., Marsden R. J., Tear V. J., Brookes J., Batten T. N., Mankowski M., Gabbay F. J., Davies D. E., Holgate S. T., Ho L.-P., Clark T., Djukanovic R., Wilkinson T. M. A.; Inhaled Interferon Beta COVID-19 Study Group , Safety and efficacy of inhaled nebulised interferon beta-1a (SNG001) for treatment of SARS-CoV-2 infection: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Respir. Med. 9, 196–206 (2021). 10.1016/S2213-2600(20)30511-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hooper B., Whittingham S., Mathews J. D., Mackay I. R., Curnow D. H., Autoimmunity in a rural community. Clin. Exp. Immunol. 12, 79–87 (1972). [PMC free article] [PubMed] [Google Scholar]
- 40.Shu S., Nisengard R. J., Hale W. L., Beutner E. H., Incidence and titers of antinuclear, antismooth muscle, and other autoantibodies in blood donors. J. Lab. Clin. Med. 86, 259–265 (1975). [PubMed] [Google Scholar]
- 41.Potocka-Płazak K., Pituch-Noworolska A., Kocemba J., [Prevalence of autoantibodies in serum of healthy persons over 85 years of age]. Przegl. Lek. 52, 544–546 (1995) [Prevalence of autoantibodies in serum of healthy persons over 85 years of age]. [PubMed] [Google Scholar]
- 42.Parks C. G., Miller F. W., Satoh M., Chan E. K. L., Andrushchenko Z., Birnbaum L. S., Jusko T. A., Kissling G. E., Patel M. D., Rose K. M., Weinberg C., Zeldin D. C., Sandler D. P., Reproductive and hormonal risk factors for antinuclear antibodies (ANA) in a representative sample of U.S. women. Cancer Epidemiol. Biomarkers Prev. 23, 2492–2502 (2014). 10.1158/1055-9965.EPI-14-0429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myasoedova E., Davis J., Matteson E. L., Crowson C. S., Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Ann. Rheum. Dis. 79, 440–444 (2020). 10.1136/annrheumdis-2019-216694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Felipe L., Vercruysse T., Sharma S., Ma J., Lemmens V., Van Looveren D., Arkalagud Javarappa M. P., Boudewijns R., Malengier-Devlies B., Liesenborghs L., Kaptein S. J. F., De Keyzer C., Bervoets L., Debaveye S., Rasulova M., Seldeslachts L., Li L.-H., Jansen S., Yakass M. B., Verstrepen B. E., Böszörményi K. P., Kiemenyi-Kayere G., van Driel N., Quaye O., Zhang X., Ter Horst S., Mishra N., Deboutte W., Matthijnssens J., Coelmont L., Vandermeulen C., Heylen E., Vergote V., Schols D., Wang Z., Bogers W., Kuiken T., Verschoor E., Cawthorne C., Van Laere K., Opdenakker G., Vande Velde G., Weynand B., Teuwen D. E., Matthys P., Neyts J., Jan Thibaut H., Dallmeier K., A single-dose live-attenuated YF17D-vectored SARS-CoV-2 vaccine candidate. Nature 590, 320–325 (2021). 10.1038/s41586-020-3035-9 [DOI] [PubMed] [Google Scholar]
- 45.Chen P., Nirula A., Heller B., Gottlieb R. L., Boscia J., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., Shawa I., Adams A. C., Van Naarden J., Custer K. L., Shen L., Durante M., Oakley G., Schade A. E., Sabo J., Patel D. R., Klekotka P., Skovronsky D. M.; BLAZE-1 Investigators , SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N. Engl. J. Med. 384, 229–237 (2021). 10.1056/NEJMoa2029849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinreich D. M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., Musser B. J., Soo Y., Rofail D., Im J., Perry C., Pan C., Hosain R., Mahmood A., Davis J. D., Turner K. C., Hooper A. T., Hamilton J. D., Baum A., Kyratsous C. A., Kim Y., Cook A., Kampman W., Kohli A., Sachdeva Y., Graber X., Kowal B., DiCioccio T., Stahl N., Lipsich L., Braunstein N., Herman G., Yancopoulos G. D.; Trial Investigators , REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 384, 238–251 (2021). 10.1056/NEJMoa2035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Prost N., Bastard P., Arrestier R., Fourati S., Mahévas M., Burrel S., Dorgham K., Gorochov G., Tandjaoui-Lambiotte Y., Azzaoui I., Fernandes I., Combes A., Casanova J.-L., Mekontso-Dessap A., Luyt C.-E., Plasma Exchange to Rescue Patients with Autoantibodies Against Type I Interferons and Life-Threatening COVID-19 Pneumonia. J. Clin. Immunol. 41, 536–544 (2021). 10.1007/s10875-021-00994-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vinh D. C., Abel L., Bastard P., Cheng M. P., Condino-Neto A., Gregersen P. K., Haerynck F., Cicalese M. P., Hagin D., Soler-Palacín P., Planas A. M., Pujol A., Notarangelo L. D., Zhang Q., Su H. C., Casanova J. L., Meyts I.; COVID Human Genetic Effort , Harnessing type I IFN immunity against SARS-CoV-2 with early administration of IFN-beta. JoCI, in press (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Firth D., Bias reduction of maximum likelihood estimates. Biometrika 80, 27–38 (1993). 10.1093/biomet/80.1.27 [DOI] [Google Scholar]
- 50.Heinze G., Schemper M., A solution to the problem of separation in logistic regression. Stat. Med. 21, 2409–2419 (2002). 10.1002/sim.1047 [DOI] [PubMed] [Google Scholar]
- 51.Agresti A., Coull B. A., Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 52, 119–126 (1998). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
immunology.sciencemag.org/cgi/content/full/6/62/eabl4340/DC1
Supplementary Materials and Methods
Figures S1 to S6
Tables S1 to S6; these tables are found on the tabs of a single Excel spreadsheet that contains the raw data