Abstract
Background
Stroke can affect people’s ability to swallow, resulting in passage of some food and drink into the airway. This can cause choking, chest infection, malnutrition and dehydration, reduced rehabilitation, increased risk of anxiety and depression, longer hospital stay, increased likelihood of discharge to a care home, and increased risk of death. Early identification and management of disordered swallowing reduces risk of these difficulties.
Objectives
Primary objective
• To determine the diagnostic accuracy and the sensitivity and specificity of bedside screening tests for detecting risk of aspiration associated with dysphagia in people with acute stroke
Secondary objectives
• To assess the influence of the following sources of heterogeneity on the diagnostic accuracy of bedside screening tools for dysphagia
‐ Patient demographics (e.g. age, gender)
‐ Time post stroke that the study was conducted (from admission to 48 hours) to ensure only hyperacute and acute stroke swallow screening tools are identified
‐ Definition of dysphagia used by the study
‐ Level of training of nursing staff (both grade and training in the screening tool)
‐ Low‐quality studies identified from the methodological quality checklist
‐ Type and threshold of index test
‐ Type of reference test
Search methods
In June 2017 and December 2019, we searched CENTRAL, MEDLINE, Embase, CINAHL, and the Health Technology Assessment (HTA) database via the Centre for Reviews and Dissemination; the reference lists of included studies; and grey literature sources. We contacted experts in the field to identify any ongoing studies and those potentially missed by the search strategy.
Selection criteria
We included studies that were single‐gate or two‐gate studies comparing a bedside screening tool administered by nurses or other healthcare professionals (HCPs) with expert or instrumental assessment for detection of aspiration associated with dysphagia in adults with acute stroke admitted to hospital.
Data collection and analysis
Two review authors independently screened each study using the eligibility criteria and then extracted data, including the sensitivity and specificity of each index test against the reference test. A third review author was available at each stage to settle disagreements. The methodological quality of each study was assessed using the Quality Assessment of Studies of Diagnostic Accuracy (QUADAS‐2) tool. We identified insufficient studies for each index test, so we performed no meta‐analysis. Diagnostic accuracy data were presented as sensitivities and specificities for the index tests.
Main results
Overall, we included 25 studies in the review, four of which we included as narratives (with no accuracy statistics reported). The included studies involved 3953 participants and 37 screening tests. Of these, 24 screening tests used water only, six used water and other consistencies, and seven used other methods. For index tests using water only, sensitivity and specificity ranged from 46% to 100% and from 43% to 100%, respectively; for those using water and other consistencies, sensitivity and specificity ranged from 75% to 100% and from 69% to 90%, respectively; and for those using other methods, sensitivity and specificity ranged from 29% to 100% and from 39% to 86%, respectively. Twenty screening tests used expert assessment or the Mann Assessment of Swallowing Ability (MASA) as the reference, six used fibreoptic endoscopic evaluation of swallowing (FEES), and 11 used videofluoroscopy (VF). Fifteen screening tools had an outcome of aspiration risk, 20 screening tools had an outcome of dysphagia, and two narrative papers did not report the outcome. Twenty‐one screening tests were carried out by nurses, and 16 were carried out by other HCPs (not including speech and language therapists (SLTs)).
We assessed a total of six studies as low risk across all four QUADAS‐2 risk of bias domains, and we rated 15 studies as low concern across all three applicability domains.
No single study demonstrated 100% sensitivity and specificity with low risk of bias for all domains. The best performing combined water swallow and instrumental tool was the Bedside Aspiration test (n = 50), the best performing water plus other consistencies tool was the Gugging Swallowing Screen (GUSS; n = 30), and the best water only swallow screening tool was the Toronto Bedside Swallowing Screening Test (TOR‐BSST; n = 24). All tools demonstrated combined highest sensitivity and specificity and low risk of bias for all domains. However, clinicians should be cautious in their interpretation of these findings, as these tests are based on single studies with small sample sizes, which limits the estimates of reliability of screening tests.
Authors' conclusions
We were unable to identify a single swallow screening tool with high and precisely estimated sensitivity and specificity based on at least one trial with low risk of bias. However, we were able to offer recommendations for further high‐quality studies that are needed to improve the accuracy and clinical utility of bedside screening tools.
Plain language summary
Screening for aspiration risk associated with dysphagia in acute stroke
Question
How accurate are swallow screening tools for detecting when food and drink enter the airway in people with acute stroke?
Background
Stroke often affects a person’s ability to swallow, allowing food and drink into the airway. This can cause choking, chest infection, malnutrition, dehydration, and reduced rehabilitation, with increased risk of anxiety, depression, discharge to a care home, and death. Early identification and management of disordered swallowing through the most accurate testing reduces these risks. If the test fails to identify swallowing difficulties, the person will continue oral intake and may experience the difficulties identified above. If the test incorrectly identifies swallowing difficulties, the person may not be given anything to eat or drink, significantly impacting quality of life, until a more detailed assessment is undertaken (usually the next day).
Study characteristics
We identified 25 studies that used a total of 37 tools. Seven tools did not use water or other consistencies, 24 used only water, and six considered water and other consistencies.
Key results
We were unable to identify a tool that could accurately identify everyone with food and drink entering their airway, as well as detect all those who definitely did not. Many studies involved different healthcare professionals, food and fluid testing consistencies, and time between stroke onset and the screening test, so it is unclear which tool is best. We were unable to directly compare the different tools because most studies used different methods.
We were able to identify the tools most able to detect people with and without risk of swallowing difficulties from studies with good quality evidence. The best combined water swallow and instrumental test was the Bedside Aspiration test, the best water plus other consistencies tool was the Gugging Swallowing Screen, and the best water only tool was the Toronto Bedside Swallowing Screening Test. However, clinicians should be cautious in their interpretation of these findings, as these tests are based on single studies with small sample sizes.
Quality of the evidence
Most included studies were poorly conducted or were unclear in reporting what they did (i.e. unclear or high risk of bias).
Conclusion
We were unable to identify a single tool with combined high levels of accuracy and good quality evidence. However, we are able to offer recommendations for further high‐quality studies that are needed to improve the accuracy and clinical utility of swallow screening tools.
Summary of findings
Summary of findings 1. Review criteria and findings.
| Review question | What is the diagnostic accuracy of screening tools for aspiration risk associated with dysphagia in acute stroke? |
| Importance | A simple and reliable screening tool for identification of aspiration associated with dysphagia would reduce diagnostic delay and the risk of developing pneumonia in acute stroke patients |
| Patients/population | Patients (over the age of 18) who have been admitted to an acute hospital setting, where there is a clinical diagnosis of stroke. Patients with subarachnoid haemorrhage were excluded from the study |
| Settings | Stroke units or hospital wards where acute stroke patients are admitted |
| Index tests | Bedside swallowing screening tools carried out by healthcare professionals (excluding SLTs) |
| Reference standards | Expert assessment, including the MASA VF FEES |
| Studies | 25 studies were included, 4 of which are presented as narratives (with no accuracy statistics reported). The included studies reported 37 screening tests |
| Risk of bias | Overall judgement: poor quality for the majority of studies – only 6 studies were at low risk across all 4 risk of bias domains, and 2 studies were at low risk of bias for 3 domains |
| Patient selection bias: high risk = 12 studies; unclear risk = 11 studies; low risk = 2 studies | |
| Index test interpretation bias: high risk = 14 studies; unclear risk = 10 studies; low risk = 1 study | |
| Reference standard interpretation bias: high risk = 16 studies; unclear risk = 8 studies; low risk = 1 study | |
| Flow and timing selection bias: high risk = 13 studies; unclear risk = 6 studies; low risk = 6 studies | |
| Applicability concern | Concerns regarding patient selection: high risk = 16 studies; unclear risk = 4 studies; low risk = 5 studies |
| Concerns regarding index test: high risk = 20 studies; unclear risk = 4 studies; low risk = 1 study | |
| Concerns regarding reference standard: high risk = 22 studies; unclear risk = 3 studies; low risk = 0 studies | |
| Overall findings | The best performing swallow screening tools were the Combined WST and Oxygen Saturation Test (Lim 2001a), GUSS (Trapl 2007b), and TOR‐BSST (Martino 2009). The best water only swallow screening test was the TOR‐BSST (Martino 2009). All demonstrate a combined highest sensitivity and specificity and low risk of bias across all domains. These tools may be considered useful in clinical practice |
| The water plus various consistencies (n = 6) performed better than the water‐only tools in terms of sensitivity and specificity | |
| Only a few studies (e.g. GUSS) (Trapl 2007b) gave direction on what food and drink consistencies should be given to an individual following the screen | |
| The quality of evidence varied. Studies often failed to distinguish between dysphagia and aspiration as the primary outcome. Some did not identify the time interval between stroke onset or admission to hospital and the screen, or the time interval between index test and reference test. Training required by different healthcare professionals to implement the screening tool was not reported well. This has implications for future research |
FEES: fibreoptic endoscopic evaluation of swallowing. MASA: Mann Assessment of Swallowing Ability. SLT: speech and language therapist. VF: videofluoroscopy. WST: water‐swallowing test.
Summary of findings 2. Screening tests.
| Screening test | N participants (studies) | Reference standard | Diagnostic estimates (95% CI) | Implications |
| Water‐only tests | 2914 (13) | |||
| Toronto Bedside Swallowing Screening Test (TOR‐BSST): Martino 2009 Study 1 | 24 (1) | VFSS | Sens = 1.00 (0.75 to 1.00) spec = 0.64 (0.31 to 0.89) | Insufficient evidence to allow firm conclusions |
| Acute Stroke Dysphagia Screening (ASDS) Aspiration: Edmiaston 2010 | 300 (1) | Expert assessment and MASA | Sens = 0.95 (0.87 to 0.99) spec = 0.69 (0.62 to 0.75) | Large study with unclear risk of bias and low applicability concerns |
| Barnes‐Jewish Hospital‐Stroke Dysphagia Screen (BJH‐SDS) Aspiration: Edmiaston 2014 | 223 (1) | VFSS | Sens = 0.95 (0.86 to 0.99) spec = 0.50 (0.42 to 0.58) | Large study with unclear/low risk of bias and low applicability concerns |
| Edith‐Huhn‐Matesic Bedside Aspiration Screen (EHMBAS) followed by simple water swallow test: Huhn‐Matesic 2015 | 52 (1) | Expert assessment and MASA | Sens = 0.94 (0.71 to 1.00) spec = 0.77 (0.60 to 0.90) | Insufficient evidence to allow firm conclusions |
| Barnes‐Jewish Hospital‐Stroke Dysphagia Screen (BJH‐SDS) Dysphagia: Edmiaston 2014 | 225 (1) | VFSS | Sens = 0.94 (0.88 to 0.98) spec = 0.66 (0.57 to 0.75) | Large study with low risk of bias and low applicability concerns. |
| Rapid Aspiration Screening for Suspected Stroke (RAS3): Daniels 2016 | 250 (1) | VFSS | Sens = 0.93 (0.77 to 0.99) spec = 0.43 (0.36 to 0.50) | Large study with low risk of bias and low applicability concerns. |
| Clinical examination: Daniels 1997 | 59 (1) | VFSS | Sens = 0.92 (0.75 to 0.99) spec = 0.64 (0.45 to 0.80) | Insufficient evidence to allow firm conclusions |
| Acute Stroke Dysphagia Screening (ASDS) Dysphagia: Edmiaston 2010 | 300 (1) | Expert assessment and MASA | Sens = 0.91 (0.83 to 0.96) spec = 0.75 (0.68 to 0.80) | Large study with unclear risk of bias and low applicability concerns |
| Nurse Dysphagia Screen: Cummings 2015 | 49 (1) | Expert assessment and MASA | Sens = 0.89 (0.65 to 0.99) spec = 0.90 (0.74 to 0.98) | Insufficient evidence to allow firm conclusions |
| Bedside swallow test ‐ WST only: Lim 2001 | 50 (1) | FEES | Sens = 0.85 (0.65 to 0.96) spec = 0.75 (0.53 to 0.90) | Insufficient evidence to allow firm conclusions |
| Chinese version of the modified SSA – original 8 items: Jiang 2019 | 127 (1) | Expert assessment and MASA | Sens = 0.83 (0.71 to 0.91) spec = 0.59 (0.46 to 0.71) | Insufficient evidence to allow firm conclusions |
| Chinese version of the modified SSA – reduced 6 items: Jiang 2019 | 127 (1) | Expert assessment and MASA | Sens = 0.81 (0.69 to 0.90) spec = 0.64 (0.51 to 0.76) | Insufficient evidence to allow firm conclusions |
| 2‐step swallowing provocation test (SPT) ‐ step 1 ‐ 0.4 mL: Warnecke 2008 | 100 (1) | FEES | Sens = 0.74 (0.63 to 0.83) spec = 1.00 (0.82 to 1.00) | Insufficient evidence to allow firm conclusions |
| Rapid Aspiration Screening for Suspected Stroke (RAS3) ‐ WST only: Daniels 2016 | 250 (1) | VFSS | Sens = 0.72 (0.53 to 0.87) spec = 0.60 (0.53 to 0.66) | Large study with low risk of bias and low applicability concerns. |
| Gag function ‐ Test3: Perry 2001 Study 1 | 22 (1) | Expert assessment and MASA | Sens = 0.71 (0.42 to 0.92) spec = 0.63 (0.24 to 0.91) | Insufficient evidence to allow firm conclusions |
| DePaul Hospital Swallow Screener (DHSS) Aspiration: Behera 2018 | 226 (1) | Expert assessment and MASA | Sens = 0.70 (0.55 to 0.83) spec = 0.90 (0.85 to 0.94) | Large study with unclear risk of bias and unclear applicability concerns |
| DePaul Hospital Swallow Screener (DHSS) Dysphagia: Behera 2018 | 225 (1) | Expert assessment and MASA | Sens = 0.68 (0.55 to 0.80) spec = 0.93 (0.88 to 0.96) | Large study with unclear risk of bias and unclear applicability concerns |
| Gag function ‐ Test4: Perry 2001 Study 1 | 157 (1) | Expert assessment and MASA | Sens = 0.67 (0.57 to 0.76) spec = 0.78 (0.65 to 0.88) | Insufficient evidence to allow firm conclusions |
| 2‐step swallowing provocation test (SPT) ‐ step 2 ‐ 2.0 mL: Warnecke 2008 | 100 (1) | FEES | Sens = 0.49 (0.38 to 0.61) spec = 1.00 (0.82 to 1.00) | Insufficient evidence to allow firm conclusions |
| Johns Hopkins Hospital Brain Rescue Unit Modified 3 oz Swallow Screen: Mulheren 2019 | 48 (1) | VFSS | Sens = 0.46 (0.28 to 0.66) spec = 1.00 (0.83 to 1.00) | Insufficient evidence to allow firm conclusions |
| Water plus consistencies tests | 412 (5) | |||
| Registered Dietitian (RD) Dysphagia Screening tool: Huhmann 2004 | 32 (1) | Expert assessment and MASA | Sens = 1.00 (0.69 to 1.00) spec = 0.86 (0.65 to 0.97) | Insufficient evidence to allow firm conclusions |
| Gugging Swallowing Screen (GUSS) ‐ Group2: Trapl 2007 | 30 (1) | FEES | Sens = 1.00 (0.77 to 1.00) spec = 0.69 (0.41 to 0.89) | Insufficient evidence to allow firm conclusions |
| Standardized Swallowing Assessment tool (SSA) – Test2: Perry 2001 Study 1 | 68 (1) | Expert assessment and MASA | Sens = 0.97 (0.86 to 1.00) spec = 0.90 (0.74 to 0.98) | Insufficient evidence to allow firm conclusions |
| Nursing Bedside Dysphagia Screen (NBDS): Campbell 2016 | 75 (1) | Expert assessment and MASA | Sens = 0.97 (0.90 to 1.00) spec = 0.75 (0.35 to 0.97) | Insufficient evidence to allow firm conclusions |
| Standardized Swallowing Assessment tool (SSA) ‐ Test1: Perry 2001 Study 1 | 161 (1) | Expert assessment and MASA | Sens = 0.94 (0.87 to 0.98) spec = 0.75 (0.63 to 0.84) | Insufficient evidence to allow firm conclusions |
| Nursing Bedside Swallowing Screen (NBSS): Ellis 2013 | 46 (1) | Expert assessment and MASA | Sens = 0.75 (0.35 to 0.97) spec = 0.89 (0.75 to 0.97) | Insufficient evidence to allow firm conclusions |
| Other tests | 627 (5) | |||
| Bedside aspiration ‐ Combined WST & Oxygen Saturation: Lim 2001 | 50 (1) | FEES | Sens = 1.00 (0.87 to 1.00) spec = 0.71 (0.49 to 0.87) | Insufficient evidence to allow firm conclusions |
| Emergency Department (ED) dysphagia screen: Turner‐Lawrence 2009 | 84 (1) | Expert assessment and MASA | Sens = 0.96 (0.86 to 0.99) spec = 0.56 (0.38 to 0.72) | Insufficient evidence to allow firm conclusions |
| Modified MASA (MMASA) Neurologist 1: Antonios 2010 | 150 (1) | Expert assessment and MASA | Sens = 0.93 (0.82 to 0.98) spec = 0.86 (0.78 to 0.93) | Insufficient evidence to allow firm conclusions |
| Modified MASA (MMASA) Neurologist 2: Antonios 2010 | 150 (1) | Expert assessment and MASA | Sens = 0.87 (0.75 to 0.95) spec = 0.84 (0.76 to 0.91) | Insufficient evidence to allow firm conclusions |
| Oxygen saturation ≥ 2% ‐ Test2 for Aspiration: Smith 2000 | 53 (1) | VFSS | Sens = 0.87 (0.60 to 0.98) spec = 0.39 (0.24 to 0.57) | Insufficient evidence to allow firm conclusions |
| Stroke Severity using National Institutes of Health Stroke Scale (NIHSS): Bravata 2009 | 101 (1) | Expert assessment and MASA | Sens = 0.79 (0.63 to 0.90) spec = 0.68 (0.55 to 0.79) | Insufficient evidence to allow firm conclusions |
| Nursing Screening Tool: Bravata 2009 | 39 (1) | Expert assessment and MASA | Sens = 0.29 (0.08 to 0.58) spec = 0.84 (0.64 to 0.95) | Insufficient evidence to allow firm conclusions |
FEES: fibreoptic endoscopic evaluation of swallowing. MASA: Mann Assessment of Swallowing Ability. sens: sensitivity. spec: specificity. VFSS: videofluoroscopic swallowing study. WST: water‐swallowing test.
Background
Stroke is the second most common cause of death worldwide (Katan 2018), and it is the largest cause of complex preventable disability in adults (WHF 2016). Over 13.7 million new strokes are reported globally each year (Johnson 2019), with a projected increase in incidence due to an ageing population (Avan 2019).
The reported incidence of dysphagia (swallowing difficulties) following acute stroke varies between 37% and 78% (Martino 2005); this is related to type and severity of stroke and individual characteristics (e.g. comorbidities). It is also related to the type of diagnostic assessment used to detect dysphagia and time from admission to that assessment. Dysphagia may lead to aspiration, defined as food or fluid entering the airway below the level of the vocal cords (Blitzer 1988), then into the trachea. People with dysphagia are three times, and those with aspiration 11 times, more likely to develop pneumonia (Kumar 2010; Rofes 2011), which is a significant cause of further morbidity leading to increased hospital stay and risk of death (Intercollegiate Stroke Working Party 2012).
Stroke‐associated pneumonia is more common if identification of dysphagia is delayed because of aspiration of food, drink, medications, and oral secretions (Bray 2016). Therefore, early screening for dysphagia may be an important avenue for reducing deaths from acute stroke (Bray 2016; Donovan 2013).
Internationally, different clinical guidelines are available for detecting swallowing difficulties in acute stroke. Identification of dysphagia is a criterion that is considered for obtaining Stroke Unit accreditation by the European Stroke Organisation. Within the UK, Europe, Canada, the USA, and Australia, guidelines state that on admission to hospital, people with acute stroke should have their swallowing screened promptly following admission (European Stroke Organisation 2015; Intercollegiate Stroke Working Party 2016; National Stroke Foundation 2010; Powers 2019; Teasell 2019).
Diagnostic methods such as videofluoroscopy (VF) and fibreoptic endoscopic evaluation of swallowing (FEES) are available. However, these methods have significant limitations when used for early identification of dysphagia in acute stages of stroke. Limitations may be patient specific (patients may be unable to comply with instructions due to poor posture, cognition, medical state, or fluctuating swallowing ability); organisational (speech and language therapists (SLTs) may not be available in the acute setting, and in most hospitals, instrumental examinations such as VF and FEES can be requested only after SLT evaluation; furthermore, not all staff are trained to interpret the results of these reference tests); or procedural (specialist equipment is not available in all acute settings) (Boaden 2011). Therefore, screening tools are needed to identify those likely to have dysphagia, who might be at greater risk of aspiration, so that interventions can be put in place to reduce morbidity until access to specialist equipment and staff is available for assessment and/or until the patient is fit enough to undergo the procedures. These screening tools are called bedside swallow screening tools.
Bedside swallow screening tools must be quick and easy to administer. To be clinically useful, screening tools must accurately identify those with dysphagia with its associated risk of aspiration (sensitivity), without leading to unnecessary restrictions (e.g. withholding food and drinks) for those who do not have dysphagia (specificity). The outcome of a screening test is binary (present or not), although it is acknowledged there may be different levels of severity of swallowing difficulty and subsequent management. A positive test should lead to a referral for more definitive assessment to confirm the presence of dysphagia or not, when this is available. Screening tools also need to be acceptable and feasible for use in people with a range of sequelae following stroke (e.g. different levels of consciousness, cognitive levels, postural difficulties). Screening tools must be undertaken prior to any oral intake and therefore need to be administered by staff members who are at the bedside throughout a 24‐hour period.
There is wide variation in available screening tools. Some swallow screening tools rely on questionnaires, whilst others use food and drink as testing materials. Swallow screening tools vary in the types of foods and fluids tested. Water swallow tools, such as the Standardised Swallow Assessment (Perry 2001 Study 1), or the Massey Bedside Swallow Screen (Massey 2002), are very similar in that they offer people different quantities of fluids from assorted utensils; some studies use water plus a range of consistencies to prescribe food and drink consistency management plans. This group of swallow screening tests are more dissimilar from each other as they offer a variety of food and drink, give different consistencies, trial different volumes, and alter the order in which foods and drinks are offered. One concern is that some of these tools have been developed and assessed with various reference tests and different professional groups not routinely available at the bedside, which limits their clinical utility.
There is no universally accepted screening tool for identification and management of aspiration associated with dysphagia, and a wide range of screening tests are used in clinical practice throughout the world. A false‐negative result is much worse than a false‐positive result. If the swallow screening test fails to identify swallowing difficulties, the person will continue oral intake and may experience the difficulties identified above. If the swallow screening test erroneously identifies swallowing difficulties, the patient is placed 'nil by mouth', with significant impact on quality of life, until the SLT undertakes a more detailed assessment (usually the next day). So a bedside swallow screening tool should be chosen while taking into account this simple clinical reality. Hence, there is a need to identify the most clinically useful screening test or tools that most accurately identify the presence or absence of aspiration associated with dysphagia, and to prescribe a food and drink consistency management plan for individuals with aspiration to improve their medical, social, and psychological outcomes.
Target condition being diagnosed
Aspiration risk associated with dysphagia in people who have had an acute stroke.
Index test(s)
In this review, bedside swallowing screening tests not administered by SLTs are the index tests. A wide range of index tests (swallow screening tests) are used at the bedside by healthcare professionals for recognition or determination of whether the patient is at risk of aspirating food and fluids.
Clinical pathway
Bedside swallow screening tests are commonly used in the typical care pathway in compliance with clinical guidelines for stroke. However, there are varying degrees of compliance. Usually these tests are implemented by allied healthcare professionals (mainly nurses) following admission to the acute care sector. Patients who are identified as at risk of swallowing difficulties are often placed 'nil by mouth' while awaiting an expert assessment or a further reference test. The care pathway for patients who have a negative test result usually allows patients to continue to eat and drink orally.
Prior test(s)
No tests to assess swallowing are conducted on patients before hospital admission.
Role of index test(s)
Index tests, that is, bedside screening tests not administered by SLTs, have the potential to improve the identification of people with risk of aspiration associated with dysphagia following stroke. Therefore, these tests may reduce the need for SLT evaluation and for more complex, invasive, and more expensive imaging methods, such as VF, FEES, or scintigraphy, which may not be available in some healthcare settings.
Alternative test(s)
Other index tests include questionnaires that rely on self‐reported dysphagia symptoms. However, these tools, for example, the Sydney Swallow Questionnaire (SSQ) and the Swallowing Disturbance Questionnaire (SDQ), were used or designed and validated for use in patient populations such as those with head and neck cancer or Parkinson’s disease. These are not considered in this review.
Rationale
We reviewed the diagnostic accuracy of currently available swallow screening tests. A systematic review of published evaluations of these screening tests will assist practitioners to identify those that have undergone rigorous development and testing. This review will also identify gaps in evidence for further research.
Healthcare professionals within acute stroke care settings are responsible for deciding which bedside swallow screening test they will use to detect people at risk of aspiration associated with dysphagia in adult acute stroke patients. When a bedside swallow screening test is considered, a test with high sensitivity and specificity is paramount. False‐negative results may lead to serious consequences within the clinical context, as continued oral intake may precipitate aspiration pneumonia and death. High specificity is also beneficial, as false‐positive results may result in patients being designated ‘nil by mouth’ and provided with clinically assisted nutrition and hydration unnecessarily. This adversely affects the well‐being of the patient and incurs unnecessary costs. However, false‐positive results are less dangerous, in that usually an SLT will see the patient within 24 hours.
A recent systematic review of bedside swallow screening tests provided a descriptive analysis of the different elements within screening tests but did not compare these with a reference standard (Almeida 2015). Other reviews have not specifically focused on a stroke population (Brodsky 2016; O'Horo 2015), have included studies in which screening was not undertaken in a timely manner (Schepp 2012), have focused on individual clinical determinants or behaviours associated with aspiration (Daniels 2012), have reviewed only multi‐consistency tests (Benfield 2020), or have accepted delays longer than 24 hours between the index test and the reference standard (Daniels 2012). The results of this review will help guide policy makers and healthcare workers in acute hospital settings on selection of the most appropriate bedside swallow screening test from those currently available and might also identify the research agenda going forward.
Objectives
Primary objective
To determine the diagnostic accuracy and the sensitivity and specificity of bedside screening tests for detecting risk of aspiration associated with dysphagia in people with acute stroke
Secondary objectives
-
To assess the influence of the following sources of heterogeneity on the diagnostic accuracy of bedside screening tools for dysphagia
Patient demographics (e.g. age, gender)
Time post stroke that the study was conducted (from admission to 48 hours) to ensure only hyperacute and acute stroke swallow screening tools are identified
Definition of dysphagia used by the study
Level of training of nursing staff (both grade and training in the screening tool)
Low‐quality studies identified from the methodological quality checklist
Type and threshold of index test
Type of reference test
Methods
Criteria for considering studies for this review
Types of studies
We considered single‐gate and two‐gate studies if they compared the accuracy of a bedside screening tool administered by nursing staff or other healthcare professionals (excluding SLTs) with identified reference tests (VF, FEES, scintigraphy, expert assessment). We applied no restrictions in terms of language of publication.
Participants
We included studies if they involved adults (aged 18 and over) who had been admitted to an acute hospital setting with a clinical diagnosis of stroke. We considered studies that were inclusive of people with subarachnoid haemorrhage and we excluded this sample subgroup from the analysis, when possible. We excluded studies that included only patients with subarachnoid haemorrhage. We also excluded studies that admitted patients with trauma.
Index tests
We included studies if they evaluated a bedside swallow screening test, used by nursing staff or other healthcare professionals (excluding SLTs who have expert knowledge in this area), for recognition or determination of whether patients were at risk of aspiration associated with dysphagia.
Target conditions
We included studies if they reported the accuracy of the bedside screening tool for identification of the risk of aspiration owing to dysphagia post stroke.
Reference standards
We included studies if a bedside swallow screening test, carried out by nursing staff or other healthcare professionals, was compared with VF, FEES, scintigraphy, or an expert assessment. Videofluoroscopy is an X‐ray video of swallowing, allowing the swallow to be analysed in real time. FEES involves insertion of a fibreoptic flexible endoscope that is passed through the nasal passages to view the throat pre‐swallows and post‐swallows for secretion management, residue, and aspirated material. Scintigraphy uses radioisotopes that are swallowed, and the emitted radiation is captured by external detectors (gamma cameras) to form images of swallowed material. Expert assessments included assessments conducted by dysphagia‐trained professionals such as SLTs. All reference standards are not equally valid and available (the SLT is not usually available in the acute setting and is not available 24 hours each day and seven days a week), and some procedures are more invasive than others. Expert assessment was included as a reference standard owing to the lack of immediate access to imaging or instrumental assessment in many centres, and to the inability of some patients to co‐operate with these assessments. However, expert assessments have limitations in their ability to identify at bedside those patients who have swallowing difficulties owing to silent aspiration when the patient demonstrates no clinical signs of aspiration.
Search methods for identification of studies
Electronic searches
The search strategy used was developed with the help of the Cochrane Stroke Group Information Specialist. We searched relevant electronic databases for eligible diagnostic studies from inception until 9 December 2019: the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 12), in the Cochrane Library; MEDLINE Ovid (from 1946); Embase Ovid (from 1980); the Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO (from 1937 onward), and the Health Technology Assessment (HTA) Database via the Centre for Reviews and Dissemination (University of York). The search strategy for MEDLINE is presented in Appendix 1 and was adapted for CENTRAL (Appendix 2), Embase (Appendix 3), CINAHL (Appendix 4), and HTA (Appendix 5).
Searching other resources
We checked the reference lists of all included studies, and we performed a cited reference search using Science Citation Index, to identify additional relevant studies and systematic reviews. We also contacted experts in the field to identify ongoing studies and those potentially missed by the search strategy.
We searched targeted grey literature sources, which we identified from the the Canadian Agency for Drugs and Technologies in Health (CADTH) Grey Matters document (CADTH 2018). A full list of the grey literature sources searched is displayed in Appendix 6.
Data collection and analysis
Selection of studies
Two review authors from the team (EB, JB, HR, DD) independently screened all titles and abstracts identified by the electronic database searches and excluded duplications and irrelevant records. Conflicts were resolved by involvement of a third review author (PD, AA). We then obtained full‐text articles for the remaining studies, which were independently assessed for inclusion by two review authors (EB, ML, HR, PD) using the eligibility criteria described above. Disagreements were resolved by consensus meetings, with arbitration provided by a third review author (AA) who had not initially reviewed the paper. When multiple papers used the same cohort of patients, we used the paper with the most complete and up‐to‐date data. When we identified relevant conference abstracts, we looked for the corresponding full‐text article. If we found no full‐text article, we contacted the authors of the conference abstract. We excluded conference abstracts with insufficient data to calculate the 2 × 2 table. The selection process is detailed in the PRISMA flow diagram in Figure 1.
1.

PRISMA flow diagram.
Data extraction and management
Two review authors (JB, EM) designed a bespoke data extraction form to collect details from the included studies. We piloted the form on other diagnostic accuracy studies related to acute stroke management that are beyond the scope of this review. For each study, we extracted information related to characteristics of the study, the patient population, index tests, reference standards, and any relevant outcomes.
Among a group of review authors (JB, AC, LH, CEL, ML, CW), two independently extracted the data to ensure adequate reliability and quality. These two review authors then met to agree on final data extractions for each included study. When there was disagreement between the two review authors, a third review author from the group acted as an arbitrator. Once data extraction was complete, we entered the information into RevMan 5 (Review Manager 2014).
Assessment of methodological quality
Two review authors from the group (JB, AC, LH, CEL, ML, CW) independently reviewed the methodological quality of each included study, using criteria from the Quality Assessment of Studies of Diagnostic Accuracy (QUADAS‐2) tool, as recommended by Cochrane (Appendix 7). As with previous diagnostic test accuracy reviews (Gupta 2016), we changed the QUADAS‐2 question "Was a case‐control design avoided?" to "Was a two‐gate design avoided?".
We piloted the QUADAS‐2 to promote agreement in interpretation within the team. We resolved disagreements with a third review author. We rated the QUADAS‐2 criteria as ’yes’, ’no’, or ’unclear’ for each included study. As stated in our protocol, guidance indicates that criteria 1, 2, 3, and 9 should receive particular attention regarding definitions used as the basis for decisions. The other criteria are more self‐explanatory with regards to their interpretation.
Statistical analysis and data synthesis
From each index test, the parameters of interest were sensitivity and specificity. For a majority of index tests, these were calculated from the 2 × 2 tables. For other index tests, information from reported parameters (sensitivity, specificity, or predictive values) was used to inform the 2 × 2 table, yielding true‐positive, false‐positive, true‐negative, and false‐negative values. From available information, sensitivities, specificities, and their 95% confidence intervals were calculated by RevMan 5 and plotted in forest plots and summary receiver operating characteristic (SROC) plots. In the absence of sufficient data, to calculate the 2 × 2 tables, we contacted study authors for further information. If this information remained unavailable, we included the study in the review as a narrative study.
Data from a single study were used only once in each analysis. We checked all studies to see if all patients were included, or if the number of patients was fixed, a priori in the design, from a condition (from the reference test) or from the index test. If the numbers of patients were fixed, the formulae for sensitivity and specificity would be different. No included studies used such numbers fixed a priori.
Pooling of sensitivity and specificity results was intended for each index test. However, due to the small number of studies using the same index test (three or fewer), we did not perform any meta‐analyses of diagnostic accuracy data. Pooling of screening tests by general categories was performed as part of the descriptive analysis, presented along with sensitivity and specificity.
Investigations of heterogeneity
Possible co‐variates of interest were patient demographics (e.g. age, gender), time post stroke that the study was conducted, and level of training of nursing staff. However, the potential influence of these co‐variates could not be investigated due to the small number of studies for each index test. The definitions of dysphagia used by the study could have been grouped, and the type and quality of the reference test, as well as the type of index test, investigated as sources of heterogeneity. For the same reason, no subgroup analyses were performed involving age, gender, time post stroke of the index test, or index test type, and whether there had been a significant change in the participant’s condition between performance of index and reference tests. Index tests were pooled by broad categories (by index test type, by healthcare professional (HCP), by outcome, and by reference test), and graphical comparisons were made. We therefore performed only a narrative review running exploratory analysis in RevMan 5 to show graphically if the co‐variates of interest are likely to be related to test accuracy, displayed using forest plots and SROC plots.
Sensitivity analyses
We did not carry out any sensitivity analyses due to the small number of studies for each index test. We had proposed to exclude low‐quality studies and possibly reporting a delay in time between index and reference tests due to potential changes in patient consciousness and cognitive state.
Assessment of reporting bias
Since no methods are available to quantify publication bias in diagnostic test accuracy reviews, we did not conduct an assessment of reporting bias.
Results
Results of the search
We conducted two searches ‐ the first in June 2017 and the same search in December 2019. We identified 26,701 papers through database searches, and we found two additional studies by handsearching the references of existing systematic reviews, for a total of 20,567 articles once duplicates were removed. After titles and abstracts of articles had been screened, we assessed the full texts of 233 articles against the eligibility criteria. Of these, we excluded 208 articles ‐ 35 because they did not compare appropriate index and reference tests, 24 because they did not involve consecutive acute stroke patients, 16 because they did not relate to screening for dysphagia, eight because index tests were carried out by experts, three because they involved only patients with dysphagia or aspiration and so sensitivity and specificity could not be calculated, two because they were not single‐gate or two‐gate studies, and one because not all patients received the same reference test (see Characteristics of excluded studies). Additionally, we excluded 57 references that were conference abstracts, 38 that were not primary research studies, 12 that were duplicate papers, six that had been withdrawn or were unavailable, three that were research protocols or trial registrations, two that were Master's or PhD theses, and one that was not relevant. Twenty‐five articles went through to data extraction and were included in the final review. Four of the included articles did not include enough detail for calculating the diagnostic accuracy of the index test, thus we included these in narrative descriptions only. More detail is shown in the PRISMA diagram (Figure 1).
We checked the reference lists of included studies, but this did not reveal additional eligible papers. Details on participants, index tests, reference tests, and sensitivity and specificity of each index test are displayed in the Characteristics of included studies table.
The 25 included studies investigated a total of 37 screening tools, most of which were different tools. All 25 studies are single‐gate studies. Of 37 screening tools, 21 were used by nurses and 16 by other HCPs. The other HCPs were doctor or physician (n = 6), dietician (n = 1), neurologist (n = 2), multiple HCPs (n = 4), and HCP not recorded (n = 3). Of the 37 screening tools, four could not be included in an available quantitative analysis as 2 × 2 tables could not be constructed to estimate sensitivity or specificity (Eren 2019;Martino 2014;Nishiwaki 2005;Zhou 2011). Training was given for screening tools used by 17 (81%) nurses and by four (25%) other HCPs.
Demographic information and characteristics of all 37 screening tests (including the four narrative tests) are given in Table 3. When reported, mean age was 58.6 years to 76.8 years, the percentage of males in the study was 35% to 100%, and the start date of study recruitment was between 1995 and 2016. Twenty‐two (59%) tests were conducted in the USA and Canada, eight (22%) in the UK and Europe, and six (16%) in other countries; in one (3%) study, the country was not recorded. Only two (5%) studies used an established classification system to record the location of the stroke. The median sample size was 100, with an interquartile range (IQR) of 50 to 161; 16 (48%) screening tools had a sample size less than 100.
1. Description of screening tests.
| Tests (percentage) (n = 37 tests) |
||
| Complete or narrative | Complete ‐ 2 × 2 table data extracted | 33 (89%) |
| Narrative ‐ 2 × 2 table data not available | 4 (11%) | |
| Participants | ||
| Na | Median (IQR) 100 (50 to 161) | Min = 22, max = 300 |
| N with dysphagia/aspirationa | Median (IQR) 38 (17 to 63) | Min = 8, max = 106 |
| Mean age | Median (IQR) 67.6 (64.75 to 71.4) | Min = 58.6, max = 76.8 |
| Sex % male | Median (IQR) 54.7 (49 to 65.6) | Min = 35, max = 100 |
| Year recruitment started | Min = 1995, max = 2016 | |
| Country | UK and Europe | 8 (22%) |
| USA and Canada | 22 (59%) | |
| Other | 6 (16%) | |
| Not recorded | 1 (3%) | |
| Study design | ||
| Admission to index test | ≤ 24 hours | 16 (43%) |
| > 24 hours and < 72 hours | 6 (16%) | |
| ≥ 72 hours | 2 (5%) | |
| Not recorded | 13 (35%) | |
| Order tests applied | Index then reference | 28 (76%) |
| Reference then index | 1 (3%) | |
| Mixed or not specified | 5 (14%) | |
| Not recorded | 3 (8%) | |
| Index/reference time interval | ≤ 24 hours | 18 (49%) |
| > 24 hours | 10 (27%) | |
| Not recorded | 9 (24%) | |
| Index test training given | Yes | 21 (57%) |
| Not recorded | 16 (43%) | |
| Index test type | Water only | 24 (65%) |
| Water plus other consistencies | 6 (16%) | |
| Other | 7 (19%) | |
| Index test HCP | Nurse | 21 (57%) |
| Other | 16 (43%) | |
| Outcome | Aspiration | 15 (41%) |
| Dysphagia | 20 (54%) | |
| NR | 2 (5%) | |
| Reference test | Expert assessment and MASA | 20 (54%) |
| FEES | 6 (16%) | |
| VF | 11 (30%) | |
aUsing complete studies only. FEES: fibreoptic endoscopic evaluation of swallowing. HCP: healthcare professional. IQR: interquartile range. MASA: Mann Assessment of Swallowing Ability. VF: videofluoroscopy.
For 16 (43%) tests, admission to index test time was ≤ 24 hours, for six (16%) between 24 hours and 72 hours, and for two (5%) ≥ 72 hours; for 13 (35%), this information was not recorded. For 28 (76%) tests the index test was applied before the reference test, for one (3%) the reference test was applied before the index test, for five (14%) the order was not specified or mixed, and for three (8%) this was not recorded. The time interval between index and reference tests was ≤ 24 hours for 18 (49%) tests, was > 24 hours for 10 (27%) tests, and was not recorded for nine (24%) tests.
Twenty‐four (64.9%) screening tools used water only (Behera 2018a; Behera 2018b; Cummings 2015; Daniels 1997; Daniels 2016a; Daniels 2016b; Edmiaston 2010a; Edmiaston 2010b; Edmiaston 2014a; Edmiaston 2014b; Eren 2019; Huhn‐Matesic 2015; Jiang 2019a; Jiang 2019b; Lim 2001b; Martino 2009 Study 1; Martino 2014; Mulheren 2019; Nishiwaki 2005; Perry 2001 Study 1a; Perry 2001 Study 1b; Warnecke 2008a; Warnecke 2008b; Zhou 2011), six (16.2%) used water and other consistencies (Campbell 2016; Ellis 2013; Huhmann 2004; Perry 2001 Study 1c; Perry 2001 Study 1d; Trapl 2007b), and seven (18.9%) used other methods. The other methods were evaluation of patient characteristics only (Antonios 2010a; Antonios 2010b; Bravata 2009a), notes review (Bravata 2009b), combined water swallow test and oxygen saturation (Lim 2001a), oxygen saturation (Smith 2000), and evaluation of patient characteristics followed by water swallow test and oxygen saturation (Turner‐Lawrence 2009). Several references reported use of more than one screening tool as detailed in Table 4.
2. Index test reference IDs.
| Test ID | Test name |
| Antonios 2010a | Modified MASA (MMASA) Neurologist 1: Antonios 2010 |
| Antonios 2010b | Modified MASA (MMASA) Neurologist 2: Antonios 2010 |
| Behera 2018a | DePaul Hospital Swallow Screener (DHSS) Aspiration: Behera 2018 |
| Behera 2018b | DePaul Hospital Swallow Screener (DHSS) Dysphagia: Behera 2018 |
| Bravata 2009a | Nursing Screening Tool: Bravata 2009 |
| Bravata 2009b | Stroke Severity using National Institutes of Health Stroke Scale (NIHSS): Bravata 2009 |
| Campbell 2016 | Nursing Bedside Dysphagia Screen (NBDS): Campbell 2016 |
| Cummings 2015 | Nurse Dysphagia Screen: Cummings 2015 |
| Daniels 1997 | Clinical examination: Daniels 1997 |
| Daniels 2016a | Rapid Aspiration Screening for Suspected Stroke (RAS3): Daniels 2016 |
| Daniels 2016b | Rapid Aspiration Screening for Suspected Stroke (RAS3) ‐ WST only: Daniels 2016 |
| Edmiaston 2010a | Acute Stroke Dysphagia Screening (ASDS) Aspiration: Edmiaston 2010 |
| Edmiaston 2010b | Acute Stroke Dysphagia Screening (ASDS) Dysphagia: Edmiaston 2010 |
| Edmiaston 2014a | Barnes‐Jewish Hospital‐Stroke Dysphagia Screen (BJH‐SDS) Aspiration: Edmiaston 2014 |
| Edmiaston 2014b | Barnes‐Jewish Hospital‐Stroke Dysphagia Screen (BJH‐SDS) Dysphagia: Edmiaston 2014 |
| Ellis 2013 | Nursing Bedside Swallowing Screen (NBSS): Ellis 2013 |
| Eren 2019 | Barnes‐Jewish Hospital Stroke Dysphagia Screen – Turkish version (BJH‐SDS) or (T‐BJH): Eren 2019 |
| Huhmann 2004 | Registered Dietitian (RD) Dysphagia Screening tool: Huhmann 2004 |
| Huhn‐Matesic 2015 | Edith‐Huhn‐Matesic Bedside Aspiration Screen (EHMBAS) followed by simple water swallow test: Huhn‐Matesic 2015 |
| Jiang 2019a | Chinese version of the modified SSA – original 8 items: Jiang 2019 |
| Jiang 2019b | Chinese version of the modified SSA – reduced 6 items: Jiang 2019 |
| Lim 2001a | Bedside aspiration ‐ Combined WST and Oxygen Saturation: Lim 2001 |
| Lim 2001b | Bedside swallow test ‐ WST only: Lim 2001 |
| Martino 2009 | Toronto Bedside Swallowing Screening Test (TOR‐BSST): Martino 2009 Study 1 |
| Martino 2014 | TOR‐BSST water swallow item: Martino 2014 |
| Mulheren 2019 | Johns Hopkins Hospital Brain Rescue Unit Modified 3 oz Swallow Screen: Mulheren 2019 |
| Nishiwaki 2005 | Clinical swallowing tests: Nishiwaki 2005 |
| Perry 2001a | Gag function ‐ Test3: Perry 2001 Study 1 |
| Perry 2001b | Gag function ‐ Test4: Perry 2001 Study 1 |
| Perry 2001c | Standardized Swallowing Assessment tool (SSA) ‐ Test1: Perry 2001 Study 1 |
| Perry 2001d | Standardized Swallowing Assessment tool (SSA) – Test2: Perry 2001 Study 1 |
| Smith 2000 | Oxygen saturation ≥ 2% ‐ Test2 for Aspiration: Smith 2000 |
| Trapl 2007b | Gugging Swallowing Screen (GUSS) ‐ Group2: Trapl 2007 |
| Turner‐Lawrence 2009 | Emergency Department (ED) dysphagia screen: Turner‐Lawrence 2009 |
| Warnecke 2008a | 2‐step swallowing provocation test (SPT) ‐ step 1 ‐ 0.4 mL: Warnecke 2008 |
| Warnecke 2008b | 2‐step swallowing provocation test (SPT) ‐ step 2 ‐ 2.0 mL: Warnecke 2008 |
| Zhou 2011 | Clinical predicative scale of aspiration (CPSA): Zhou 2011 |
Expert assessment or the Mann Assessment of Swallowing Ability (MASA) was utilised for 20 (54.1%) screening tools as the reference test (Antonios 2010a; Antonios 2010b; Behera 2018a; Behera 2018b; Bravata 2009a; Bravata 2009b; Campbell 2016; Cummings 2015; Edmiaston 2010a; Edmiaston 2010b; Ellis 2013; Huhmann 2004; Huhn‐Matesic 2015; Jiang 2019a; Jiang 2019b; Perry 2001 Study 1a; Perry 2001 Study 1b; Perry 2001 Study 1c; Perry 2001 Study 1d; Turner‐Lawrence 2009); six (16.2%) used FEES (Eren 2019; Lim 2001a; Lim 2001b; Trapl 2007b; Warnecke 2008a; Warnecke 2008b), and 11 (29.7%) used VF (Daniels 1997; Daniels 2016a; Daniels 2016b; Edmiaston 2014a; Edmiaston 2014b; Martino 2009 Study 1; Martino 2014; Mulheren 2019; Nishiwaki 2005; Smith 2000; Zhou 2011).
We identified 15 (40.5%) screening tools that had an outcome of aspiration risk (Behera 2018a; Daniels 1997; Daniels 2016a; Daniels 2016b; Edmiaston 2010a; Edmiaston 2014a; Huhn‐Matesic 2015; Lim 2001a; Lim 2001b; Nishiwaki 2005; Smith 2000; Trapl 2007b; Turner‐Lawrence 2009; Warnecke 2008a; Warnecke 2008b), and we found 20 (54.1%) that had an outcome of dysphagia (Antonios 2010a; Antonios 2010b; Behera 2018b; Bravata 2009a; Bravata 2009b; Campbell 2016; Cummings 2015; Edmiaston 2010b; Edmiaston 2014b; Ellis 2013; Eren 2019; Huhmann 2004; Jiang 2019a; Jiang 2019b; Martino 2009 Study 1; Mulheren 2019; Perry 2001 Study 1a; Perry 2001 Study 1b; Perry 2001 Study 1c; Perry 2001 Study 1d). Only two (5.4%) narrative papers did not record the outcome (Martino 2014; Zhou 2011).
Methodological quality of included studies
Risk of bias
Results of the methodological quality assessment for each of the 25 included studies are shown in Figure 2. We considered six studies to be at low risk across all four risk of bias domains: patient selection, index test, reference standard, and flow and timing (Daniels 2016; Lim 2001; Martino 2009 Study 1; Martino 2014; Trapl 2007; Warnecke 2008). Two studies were at low risk of bias for three domains (index test, reference standard, and flow and timing) but at uncertain risk for patient selection (Edmiaston 2014;Mulheren 2019).
2.

Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
A major concern for risk of bias across the other included studies was that they had not enrolled consecutive patients. We identified 12 studies that had enrolled consecutive patients (Antonios 2010; Daniels 1997; Daniels 2016; Jiang 2019; Lim 2001; Martino 2009 Study 1; Martino 2014; Nishiwaki 2005; Perry 2001 Study 1; Smith 2000; Trapl 2007; Warnecke 2008), but for 12 studies this was unclear (Behera 2018; Bravata 2009; Campbell 2016; Cummings 2015; Edmiaston 2010; Edmiaston 2014; Ellis 2013; Eren 2019; Huhmann 2004; Huhn‐Matesic 2015; Mulheren 2019; Zhou 2011), and one study employed a convenience sampling method (Turner‐Lawrence 2009). For 11 studies, it is unclear whether inappropriate exclusions had been avoided when participants were recruited (Behera 2018; Bravata 2009; Cummings 2015; Edmiaston 2010; Ellis 2013; Huhmann 2004; Huhn‐Matesic 2015; Martino 2014; Perry 2001 Study 1; Turner‐Lawrence 2009; Zhou 2011). Finally, it is unclear in eight studies whether there had been an appropriate time interval between the index test and the reference standard (Behera 2018; Bravata 2009; Edmiaston 2010; Ellis 2013; Huhmann 2004; Huhn‐Matesic 2015; Perry 2001 Study 1; Zhou 2011), and it was reported that the time interval was not appropriate in two studies (Daniels 1997; Nishiwaki 2005).
Applicability concerns
There were no applicability concerns for 15 studies across the three applicability domains: patient selection, index test, and reference standard (Antonios 2010; Bravata 2009; Daniels 2016; Edmiaston 2010; Edmiaston 2014; Eren 2019; Jiang 2019; Lim 2001; Martino 2009 Study 1; Martino 2014; Mulheren 2019; Perry 2001 Study 1; Smith 2000; Trapl 2007; Warnecke 2008). However, concern was high in five studies (Campbell 2016; Cummings 2015; Huhmann 2004; Nishiwaki 2005; Turner‐Lawrence 2009), and concern was unclear in four other studies (Behera 2018; Ellis 2013; Huhn‐Matesic 2015; Zhou 2011), which included patients that did not match the review question. Concern that the index test, its conduct, or its interpretation differs from the review question was high in Huhmann 2004 and unclear in four studies (Daniels 1997; Huhn‐Matesic 2015; Nishiwaki 2005; Zhou 2011).
Findings
Healthcare professionals’ level of training across studies
The level of training for healthcare professionals undertaking the index test in dysphagia and/or use of the index test was underreported, with only 21 of 37 screening tests stating that training was offered. Of those that detailed the training, there was variation in both length and content. Some index tests required 10 minutes of training (n = 4), some took several hours (n = 3), and two detailed two days of training. The content of training varied between training to use the test plus element of competence (n = 5), instructions on how to use the index test (n = 4), instructions with a practice component (n = 2), training in anatomy and physiology of swallowing, identification and management of dysfunction and five successful practice assessments (n = 2), on‐line training in swallowing anatomy and physiology (n = 1), clinical signs of dysphagia and aspiration (n = 1), and digitised examples of five stroke patients with a review of basic anatomy and physiology of swallowing (n = 1). Some index tests reported that training was offered but did not detail the contents (n = 5).
Synthesis of results
The high level of heterogeneity between studies as identified by the review means that statistical pooling of diagnostic accuracy data was not possible. We performed a descriptive analysis from extracted data (2 × 2 tables) and sensitivity and specificity for all but the four screening tests for which this data could not be extracted (Eren 2019;Martino 2014;Nishiwaki 2005;Zhou 2011); these four studies are not included from this point onwards.
Values for sensitivity and specificity presented here include the point estimate (95% confidence interval (CI)). The criteria of both high sensitivity and high specificity indicate that the best performing test overall was the Standardized Swallowing Assessment tool (SSA) (Perry 2001 Study 1d) (n = 68), which had sensitivity of 0.97 (95% CI 0.86 to 1.00) and specificity of 0.90 (95% CI 0.74 to 0.98). However, Perry 2001 Study 1 performed poorly on the risk of bias assessment, showing, specifically, high risk of bias in the flow and timing domain and unclear risk of bias in both index test and reference standard domains, so this result should be interpreted with caution. Several tests performed better on sensitivity but less well on specificity: the Registered Dietician (RD) Dysphagia Screening tool (n = 32) had sensitivity of 1.00 (95% CI 0.69 to 1.00) with specificity of 0.86 (95% CI 0.65 to 0.97) (Huhmann 2004); the Bedside Aspiration test (n = 50) had sensitivity of 1.00 (95% CI 0.87 to 1.00) with specificity of 0.71 (95% CI 0.49 to 0.87) (Lim 2001a); the Gugging Swallowing Screen (GUSS) (n = 30) had sensitivity of 1.00 (95% CI 0.77 to 1.00) with specificity of 0.69 (95% CI 0.41 to 0.89) (Trapl 2007b); and the Toronto Bedside Swallowing Screening Test (TOR‐BSST) (n = 24) had sensitivity of 1.00 (95% CI 0.75 to 1.00) with specificity of 0.64 (95% CI 0.31 to 0.89) (Martino 2009 Study 1).
Of the best performing screening tests, we have the most confidence in the following studies: Bedside Aspiration test (Lim 2001a), GUSS (Trapl 2007b), and TOR‐BSST (Martino 2009 Study 1), which we rated as having low risk of bias across all four QUADAS‐2 risk of bias domains. Of those with low risk of bias, the best performing screening test using only water was TOR‐BSST (Martino 2009 Study 1), and the best performing screening test using water plus other consistencies was GUSS (Trapl 2007b). A description of these tests is provided in Appendix 8. However, all of these screening tools had small sample sizes (n < 100), which limits interpretation of the estimates of reliability of these tests.
Screening tests were grouped into general categories according to taxonomy (water only tests, water plus other consistencies tests, and other tests), healthcare professionals who administered the test (nurses versus other healthcare professionals), types of outcomes (aspiration versus dysphagia), and types of reference standards (expert assessment or MASA versus VF versus FEES), and we generated related summary receiver operating characteristic (ROC) plots using RevMan 5 (see Figure 3; Figure 4; Figure 5; Figure 6). This informed the descriptive analysis, but no meta‐analysis was done because of the small number of studies identified for each individual screening test.
3.
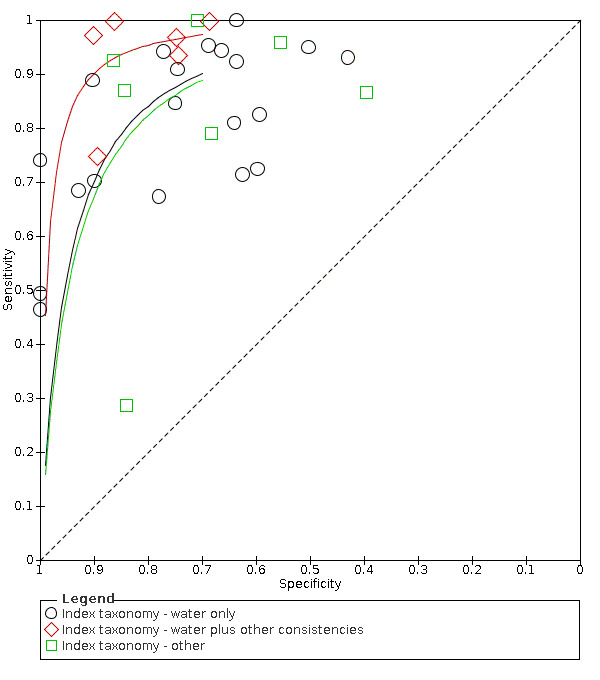
Summary ROC plot of tests: Grouped by Index taxonomy ‐ water only, water plus bolus and other.
4.
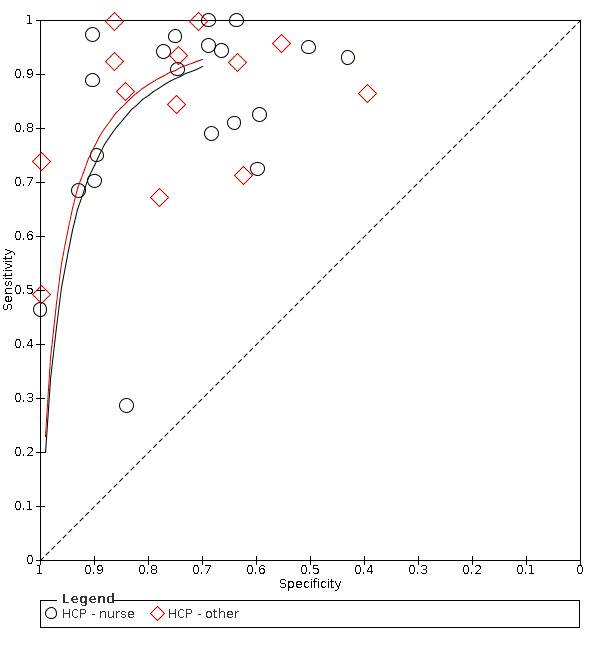
Summary ROC plot of tests grouped by index test healthcare professional ‐ nurse and other.
5.
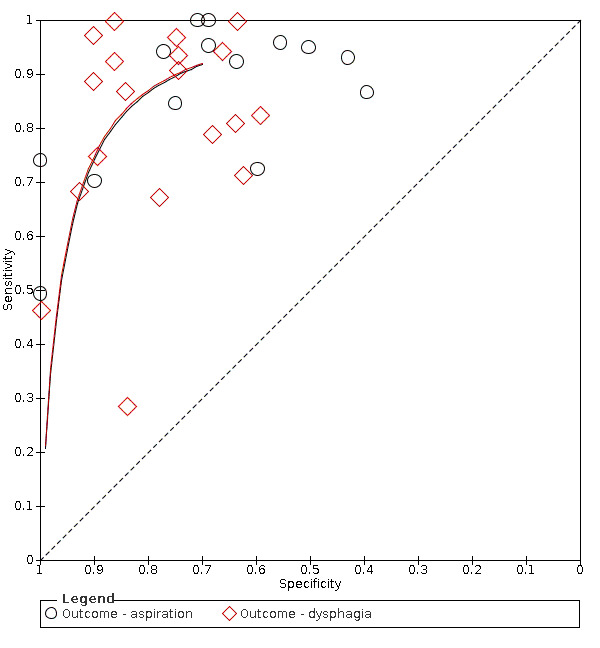
Summary ROC plot of tests: grouping index tests by outcome ‐ aspiration and dysphagia.
6.
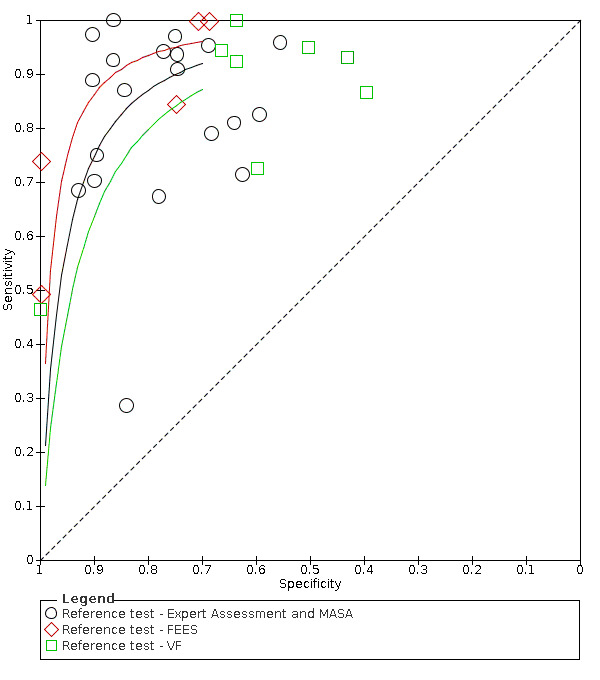
Summary ROC plot of tests: index tests grouped by reference test used ‐ Expert Assessment, FEES and VS.
Type of test (water only tests versus water plus other consistencies tests versus other tests)
Screening tools that used water and other consistencies (accuracy ranged from sensitivity of 0.75 (95% CI 0.35 to 0.97) with specificity of 0.89 (95% CI 0.75 to 0.97), to sensitivity of 1.00 (95% CI 0.69 to 1.00) with specificity of 0.86 (95% CI 0.65 to 0.97)) generally were more accurate than screening tests that used only water (accuracy ranged from sensitivity of 0.46 (95% CI 0.28 to 0.66) with specificity of 1.00 (95% CI 0.83 to 1.00) to sensitivity of 1.00 (95% CI 0.75 to 1.00) with specificity of 0.64 (95% CI 0.31 to 0.89)). Those that used methods other than water only and water and other consistencies had mixed results; some performed as well as the water‐only tests, with the most accurate scoring sensitivity of 1.00 (95% CI 0.87 to 1.00) with specificity of 0.71 (95% CI 0.49 to 0.87)) (Figure 7). Others performed worse, with the least accurate scoring having sensitivity of 0.29 (95% CI 0.08 to 0.58) with specificity of 0.84 (95% CI 0.64 to 0.95)). Pooling of data by the screening test taxonomy confirmed that screening tests that used water plus other consistencies (participants n = 412) may be more sensitive with similar or better specificity than screening tests that used water only (participants n = 2914) or other methods (participants n = 627) (Figure 3). However, only six tests used this method. Screening tests that used only water had similar accuracy (in terms of sensitivity and specificity) compared with screening tests using other methods.
7.
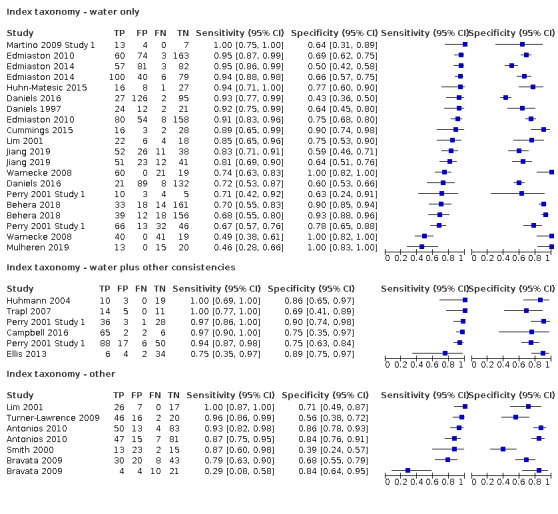
Forest plot of tests grouped by Index Test Taxonomy ‐ Water only, Water plus other consistencies, and Other tests.
Type of reference standard (expert assessment or MASA versus VF versus FEES)
Screening tools showed better agreement with expert assessment or the MASA (accuracy ranged from sensitivity of 0.29 (95% CI 0.08 to 0.58) with specificity of 0.84 (95% CI 0.64 to 0.95) to sensitivity of 1.00 (95% CI 0.69 to 1.00) with specificity of 0.86 (95% CI 0.65 to 0.97)) than they did with the instrument‐based reference test of FEES (accuracy ranged from sensitivity of 0.49 (95% CI 0.38 to 0.61) with specificity of 1.00 (95% CI 0.82 to 1.00) to sensitivity of 1.00 (95% CI 0.87 to 1.00) with specificity of 0.71 (95% CI 0.49 to 0.87)) or VF (accuracy ranged from sensitivity of 0.46 (95% CI 0.28 to 0.66) with specificity of 1.00 (95% CI 0.83 to 1.00) to sensitivity of 1.00 (95% CI 0.75 to 1.00) with specificity of 0.64 (95% CI 0.31 to 0.89) (Figure 8). Pooling of screening tests by reference test (Figure 6) confirmed that screening tests that used expert assessment or the MASA (participants n = 2491) had the highest accuracy. This may be expected, as the screening tests will be more closely aligned to expert assessment methods than to instrumental assessment. Screening tests that used VF as a reference test had high sensitivity but lower specificity than those using expert assessment or the MASA. However, the number of index tests compared to instrument‐based reference tests was low: five were compared to FEES (participants n = 330) and eight were compared to VF (participants n = 1132).
8.
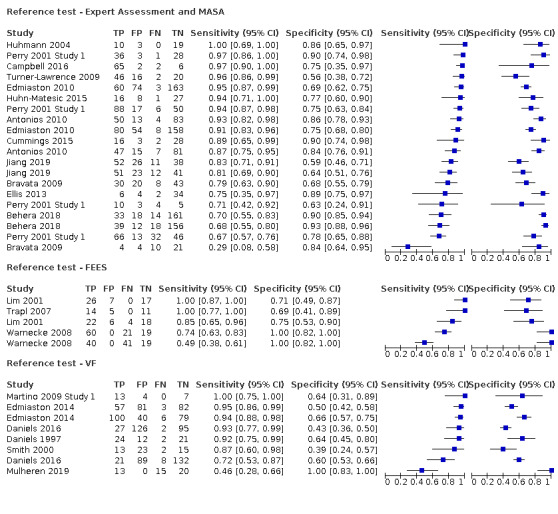
Forest plot of tests grouped by Reference Test ‐ Expert Assessment and MASA, FEES, and VF.
Type of primary outcome (aspiration versus dysphagia)
Screening tests with dysphagia as the primary outcome (accuracy ranged from sensitivity of 0.29 (95% CI 0.08 to 0.58) with specificity of 0.84 (95% CI 0.64 to 0.95) to sensitivity of 1.00 (95% CI 0.69 to 1.00) with specificity of 0.86 (95% CI 0.65 to 0.97)) generally performed better than screening tests for which the primary outcome was aspiration (accuracy ranged from sensitivity of 0.49 (95% CI 0.38 to 0.61) with specificity of 1.00 (95% CI 0.82 to 1.00) to sensitivity of 1.00 (95% CI 0.87 to 1.00) with specificity of 0.71 (95% CI 0.49 to 0.87)) (Figure 9). Pooling of screening tests by outcome (Figure 5) showed that screening tests with the greatest accuracy, with both high sensitivity and high specificity, had an outcome of dysphagia (participants n = 2126). This was to be expected, as dysphagia is more easily observed than aspiration. Several tests that detected aspiration risk (participants n = 1827) had high sensitivity but slightly lower specificity.
9.
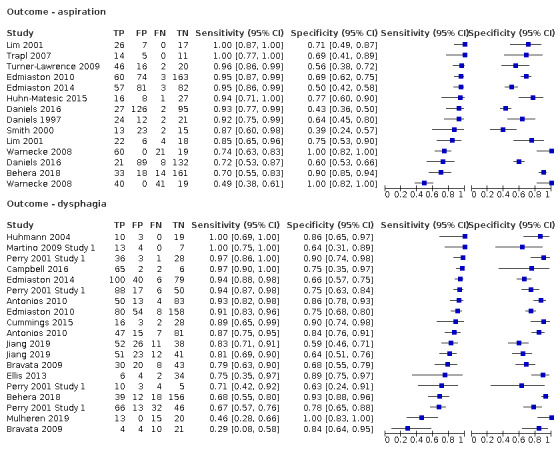
Forest plot of tests grouped by Index Test Outcome ‐ aspiration and dysphagia.
Tests designed for nurses compared to tests designed for other healthcare professionals
Screening tools carried out by nurses (accuracy ranged from sensitivity of 0.29 (95% CI 0.08 to 0.58) with specificity of 0.84 (95% CI 0.64 to 0.95) to sensitivity of 1.00 (95% CI 0.77 to 1.00) with specificity of 0.69 (95% CI 0.41 to 0.89)) performed consistently better than those carried out by other HCPs (excluding SLTs) (accuracy ranged from sensitivity of 0.49 (95% CI 0.38 to 0.61) with specificity of 1.00 (95% CI 0.82 to 1.00) to sensitivity of 1.00 (95% CI 0.69 to 1.00) with specificity of 0.86 (95% CI 0.65 to 0.97)) (Figure 10). Generally, screening tests designed for use by nurses (participants n = 2785) performed equally well as those designed for use by other HCPs (excluding SLTs) (participants n = 1168) in terms of sensitivity and specificity (Figure 4).
10.
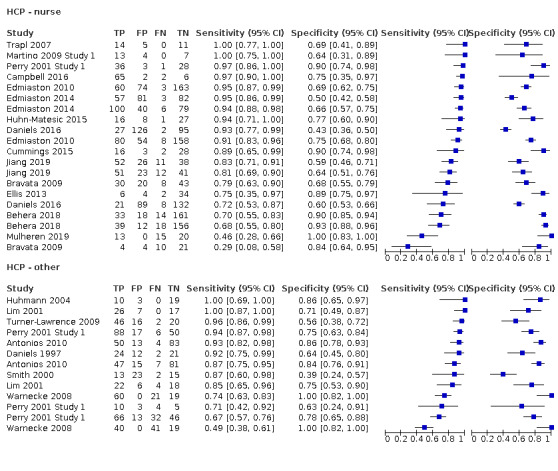
Forest plot of tests grouped by HCP ‐ nurse and other.
Other considerations
Screening tests with larger sample sizes (n > 200) were Behera 2018a, Behera 2018b, Daniels 2016a, Daniels 2016b, Edmiaston 2010a, Edmiaston 2010b, Edmiaston 2014a, and Edmiaston 2014b. Of these, screening tests with the greatest accuracy (both high sensitivity and high specificity) were Edmiaston 2010a, Edmiaston 2010b, and Edmiaston 2014b. All of these had narrow 95% CIs for estimates of sensitivity and specificity. Their lower 95% CIs for sensitivity had a higher value than for some of the overall best performing tests ‐ Lim 2001a, Trapl 2007b, and Martino 2009 Study 1. None of the tests with a larger sample size were assessed as low risk for all four QUADAS‐2 risk of bias (ROB) domains. Edmiaston 2010a and Edmiaston 2010b were unclear for three domains in the ROB and were low for one domain. Edmiaston 2014b was unclear for one domain in the ROB and was low for the other three domains. In summary, even though 95% CIs for estimates of sensitivity and specificity are narrower for the best performing screening tests, which used a larger sample size, their QUADAS‐2 risk of bias is not as low as that for the overall best performing tests.
Discussion
Numerous variables need to be systematically reported regarding training required by the healthcare professional undertaking the swallow screening tool, consistencies offered as part of the tool, and food and drink consistency management options available to the person undertaking the test post screen. From a clinical perspective, some bedside swallow screening tests are criticised for making recommendations for consistencies that have not been assessed. For example, water‐only tests allow normal dietary intake without assessment. Similarly water and other consistency screening tests show a discrepancy between diet and fluid consistencies tested and those recommended. Future studies should take these concerns into consideration.
Summary of main results
This review aimed to summarise the evidence regarding accuracy of swallow screening tests to detect aspiration risk associated with dysphagia in acute stroke. Studies did not always report sufficient information regarding study characteristics; therefore, it is not possible to explore the influence of sources of heterogeneity. Results show that a number of screening tools offered high sensitivity. However, we were unable to identify a swallow screening tool with concomitant high specificity. Furthermore, we were unable to identify a diagnostically accurate swallow screening tool that tests a variety of consistencies and offers immediate management advice for healthcare settings that do not have the facility to offer expert comprehensive assessments.
Population and setting
Although this review focused on acute stroke patients, some studies did not define the time period (from stroke onset or from admission) in which use of swallow screening tool was undertaken. Owing to fluctuation in swallow function within a 24‐hour period, it is incumbent on researchers to specify the time period to direct clinical practice (i.e. use of different screening tools at different time periods post stroke) and to allow for comparisons of sensitivity versus other studies. Screening tools that were used in the outpatient setting or in the rehabilitation setting were excluded from the review.
Index tests
The ideal balance of sensitivity and specificity may vary according to the care setting. For example, a very sensitive screening test may be appropriate for untrained staff who have professional backup to rapidly identify false‐positives. No screening test identified or excluded swallowing difficulties with perfect accuracy. We were able to identify tests with the best ability to detect people with and without risk of aspiration from studies providing good quality evidence. The best performing swallow screening tools were the Bedside Aspiration test (Lim 2001a), the Gugging Swallowing Screen (GUSS) (Trapl 2007b), and the Toronto Bedside Swallowing Screening Test (TOR‐BSST) (Martino 2009 Study 1). Although screening tests with larger sample sizes (n > 200) had smaller confidence intervals (CIs) in estimates of sensitivity and specificity, they had higher risk of bias than the best performing tests so are not among the recommended screening tests. For clinicians with a variety of consistencies available, the best performing water plus other consistencies test was GUSS (Trapl 2007b). Although GUSS offers different consistencies as part of the assessment and offers a consistency management plan for clinicians to implement, the consistencies used are limited to puree, water, and solids, and the test does not include all the consistencies identified in the International Dysphagia Diet Standardised Initiative (International Dysphagia Diet Std Initiative 2017). Therefore, this test may not be suitable for implementation across all healthcare settings. The best water‐only swallow screening test was TOR‐BSST (Martino 2009 Study 1). Although all tests demonstrate a combined highest sensitivity and specificity and low risk of bias across all domains, clinicians should be cautious in their interpretation of these findings, as these tests are based on single studies with small sample sizes, which limits the interpretation of estimates of reliability for these screening tests.
Sensitivity is similar for tools with a primary outcome of dysphagia or aspiration, but tools with dysphagia as their outcome perform better in terms of specificity. This may be due to the greater number of clinical signs of difficulty (e.g. chewing function) that may be observed in dysphagia compared to aspiration, making it more easily detected.
There is general agreement between index tests that some degree of behavioural assessment (e.g. alertness), together with oromotor assessment, was fundamental before progression to a test of oral intake (either various sizes of water bolus or water plus various consistencies). Generally, water plus various consistencies (n = 6) performed better than water‐only tools in terms of sensitivity and specificity. The small number of index tests that did not use direct patient assessment (e.g. used national stroke scales to identify risk of swallowing difficulty) did not consistently perform well.
Only a few studies gave direction on what food and drink consistencies should be given to an individual following the screen (e.g. GUSS; Trapl 2007b). These studies will be useful in healthcare settings, nationally and internationally, when an expert assessment or an instrumental assessment is not available.
The best performing index tests are included in the group carried out by nurses. Index tests carried out by other HCPs are less consistent. This may be due to confounding factors regarding the amount of training received by nursing staff within studies rather than a reflection on the type of index test or professional group undertaking the test. Of the 21 index tests undertaken by nurses, 17 (81.0%) recorded some form of training; for other HCPs, only four (25.0%) papers reported a training element. Furthermore, papers that reported training was required often failed to present the elements included in the training. In future studies, the type of training delivered should be reported.
Reference standard
Reference tests were defined as either expert clinical assessments (usually undertaken by speech and language therapists (SLTs)) or instrumental assessments. A variety of reference tests were included to capture patients who could access different instrumental assessments. However, the reliability of instrumental assessments used to diagnostically identify aspiration is of clinical concern.
Following the SLT opinion, we considered the Mann Assessment of Swallowing Ability (MASA) as equivalent to an expert clinical assessment and included the seven studies that used MASA as a reference test. Tools that used the MASA or expert assessment for reference testing had a better combined sensitivity and specificity than tools that used instrumental tests of fibreoptic endoscopic evaluation of swallowing (FEES) or videofluoroscopy (VF). This may be due to the similarity between index tests and reference tests of MASA/expert assessment in that both rely on clinical observations rather than on instrumental assessment.
From summary receiver operating characteristic (SROC) plots, which plot sensitivity versus (1‐specificity), index tests that used FEES as the reference test generally had higher sensitivity but similar specificity than index tests that used VF or MASA as the reference test combined with expert assessment. Most of the studies that used FEES or VF report sensitivity greater than 85% but poorer specificity than those that used MASA or expert assessments as the reference test. No studies were compared to scintigraphy, which remains mainly a research tool.
Quality of the evidence
The quality of evidence varied across included studies. The most common reasons for reduced quality was considerable heterogeneity between studies that affected our ability to combine and directly compare results.
Studies often failed to distinguish between dysphagia and aspiration as the primary outcome. Some did not routinely identify time between stroke onset or admission to hospital and time the screen was undertaken. Similarly, some studies failed to report time between index test and reference test, and the swallow may have improved or deteriorated in the interval between tests. Many studies failed to report the training required by different healthcare professionals to implement the screening tool.
Strengths and weaknesses of the review
Strengths
This DTA undertook the literature search from an International perspective and included all relevant studies irrespective of language of publication. Therefore we can be assured that no relevant publications were excluded from our searches. We were able to identify the best performing tools within each category of swallow screening tools that used water alone, water plus other bolus consistencies, and other methods. We included as narrative studies trials that did not report statistical results.
Weaknesses
Apart from one screening tool that was assessed by two studies, all remaining screening tools were assessed by single studies. This hampered the possibility to perform any meaningful meta‐analysis. Only descriptive analyses grouped by general categories were presented. Studies often failed to distinguish between dysphagia and aspiration as the primary outcome. Most studies had small sample sizes, which limits interpretation of the estimates of reliability of the screening tests. Some did not routinely identify time between stroke onset or admission to hospital and time the screen was undertaken. Similarly, some studies failed to report time between the index test and the reference test, and the swallow may have improved or deteriorated in the interval between tests. Many studies failed to report the training required by different healthcare professionals to implement the screening tool. This has had an impact on the quality of this review. We performed no formal assessment of the overall quality of the evidence, for example, by using GRADE. We have not included any ongoing studies.
Applicability of findings to the review question
This review demonstrates that currently, high‐quality evidence from research is insufficient to provide conclusive results regarding the accuracy of bedside swallow screening tools in identifying aspiration associated with dysphagia in the acute stage of stroke.
Applicability of evidence
The review authors are aware of ongoing international studies that are currently unpublished and are not included as part of this review. However, this review does identify currently available swallow screening tools that screen with water and those that screen with a variety of consistencies to offer an ongoing food and drink consistency management plan that may be implemented in the clinical setting.
Implications for research
High‐quality, appropriately statistically powered studies are needed to evaluate the accuracy of a swallow screening tool in identifying aspiration associated with dysphagia in acute stages of stroke.
Future research studies should clearly define their primary outcomes. They should provide more detail regarding participant inclusion and exclusion criteria, and when a study includes individuals with differing underlying aetiologies that precipitate swallowing difficulties, these data should be reported separately.
Future studies should provide greater detail on participant location and timing of the swallow screening tool used post stroke or post admission. This, together with a description of participant characteristics and swallow severity, would allow researchers to identify the accuracy of a screening tool within different time frames, revealing alterations in swallow function post stroke onset.
Studies should clarify the reference tests used, and when several tests are undertaken, these should be reported separately.
The level of training provided to healthcare professionals undertaking the index test in dysphagia and/or using the index test has been underreported, and studies that detailed the training show variation in both length and content of training. Future research should detail the amount and content of training offered and should examine the impact of training on study fidelity and outcomes.
In addition to technical performance in accurately identifying patients with dysphagia with its associated risk of aspiration, bedside swallow screening tools should demonstrate clinical utility. Evaluation of both short‐term and long‐term participant outcomes at defined periods would facilitate assessment of whether incidence of aspiration pneumonia, quality of life, and participant mortality were influenced by timing of the screening test post stroke or post admission.
Bedside swallow screening test cost‐effectiveness data should be reported to support healthcare services with clinical implementation.
Future studies should apply the better performing screening tests that we have identified, making minor changes in their design to ensure that they have low Quality Assessment of Studies of Diagnostic Accuracy (QUADAS‐2) risk of bias and using larger sample sizes, rather than continuing to develop local screening tests. This would build up a body of evidence, would reduce heterogeneity between screening tests, and ultimately would allow a meta‐analysis of different tests to be conducted.
Researchers should be encouraged to consider inclusion of these issues to when performing future comparative studies.
Agreements and disagreements with other studies or reviews
This is the largest, most inclusive international and current review on identification of risk of aspiration associated with dysphagia as the main symptom of concern in the acute phase of stroke. Several recent systematic reviews have explored the accuracy of screening tools. However, these studies have limited the scope of their review owing to the restricted range of languages accepted by the search (Brodsky 2016; Daniels 2012; Jiang 2016; Oliveira 2001; Schepp 2012), the heterogeneity of included disorders (Brodsky 2016; Jiang 2016; O'Horo 2015), the paucity of information available regarding the healthcare professional undertaking the study (Brodsky 2016; Chen 2016; Daniels 2012; O'Horo 2015), comparison of only water swallow tools (Brodsky 2016; Chen 2016), exclusion of instrumental assessments from reference tests (Jiang 2016), consideration of only dysphagia as the primary outcome (Jiang 2016; Oliveira 2001; Schepp 2012), and specification that no training in dysphagia should be required (Schepp 2012). However, this current review had no language restrictions, focuses on acute stages of stroke, identifies the range of healthcare professionals undertaking the test, explores the diagnostic accuracy of tools including water and water with other consistencies, and includes instrumental and expert assessments as reference tests with aspiration associated with dysphagia as the focus of the assessment. Therefore, direct comparisons are difficult to make.
Authors' conclusions
Implications for practice.
This review demonstrates that current high‐quality evidence from research is insufficient to provide conclusive results regarding the accuracy of bedside swallow screening tools to identify aspiration associated with dysphagia in the acute stage of stroke.
Implications for research.
We have identified the need for high‐quality research. Study authors should be encouraged to include information regarding patient demographics (including comorbidities that may impact outcome and stroke location or classification) and definitions regarding dysphagia and aspiration and time intervals (admission to index test and time from index test to reference test). We recommend that future studies should apply better performing screening tests, enhanced to ensure they have low QUADAS‐2 risk of bias domains, and should use larger sample sizes.
What's new
| Date | Event | Description |
|---|---|---|
| 13 July 2021 | Amended | Paper submitted with amendments in response to reviewers' comments |
History
Protocol first published: Issue 6, 2017
| Date | Event | Description |
|---|---|---|
| 26 February 2021 | Amended | Paper submitted with amendments in response to reviewers' comments |
| 30 September 2020 | Amended | Paper submitted |
| 30 January 2017 | Amended | Protocol amended in response to reviewers' comments |
Acknowledgements
The review authors acknowledge the contributions of Brigit Chesworth, Kerry Hannah, Dawn Doran, Janet Reed, Hazel Fraser, and Josh Cheyne.
Appendices
Appendix 1. MEDLINE search strategy
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h? ematoma$ or bleed$)).tw.
5. hemiplegia/ or exp paresis/ or exp Gait Disorders, Neurologic/
6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw.
7. or/1‐6
8. Deglutition/
9. exp Deglutition Disorders/
10. ((swallow$ or deglutit$ or dysphag$) adj3 (disturbance$ or disorder$ or difficult$ or dysfunction$ or impair$ or condition$ or abnormal$ or damage$ or injur$)).tw.
11. ((swallow$ or deglutit$ or dysphag$) adj3 (scale$ or screen$ or checklist$ or assess$ or exam$ or identif$ or recogni$ or evaluat$ or diagnos$ or detect$ or hazard or risk or test)).tw.
12. exp Respiratory Aspiration/ or exp Pneumonia, Aspiration/
13. ((inhal$ or aspirat$ or ingest$) adj3 (scale$ or screen$ or checklist$ or assess$ or exam$ or identif$ or recogni$ or evaluat$ or diagnos$ or detect$ or hazard or risk or test)).tw.
14. Pharynx/ or pharyngeal muscles/ or esophageal sphincter, upper/ or exp Esophagus/
15. ((throat or oesophag$ or esophag$ or pharyn$ or oropharyn$) adj3 (disturbance$ or disorder$ or difficult$ or dysfunction$ or impair$ or condition$ or abnormal$ or damage$ or injur$)).tw.
16. exp Fluoroscopy/ or bronchoscopy/ or laryngoscopy/ or endoscopy/ or manometry/
17. Fiber Optic Technology/
18. (fluoroscop$ or videofluroscop$ or fluorescence radiation or fluorescent scan$ or fluorophotograph$ or photofluoroscop$ or radiofluoroscop$ or roentgenfluoroscop$ or bronchoscop$ or bronchial endoscop$ or laryngotracheobronchoscop$ or tracheobronchoscop$).tw.
19. or/8‐18
20. 7 and 19
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL)
June 2017 search – was applied to CENTRAL and HTA
December 2019 search –applied separately to CENTRAL and HTA. HTA was only available until March 2018.
ID Search Hits
#1 MeSH descriptor: [Cerebrovascular Disorders] this term only
#2 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] this term only
#3 MeSH descriptor: [Brain Ischemia] explode all trees
#4 MeSH descriptor: [Carotid Artery Diseases] explode all trees
#5 MeSH descriptor: [Cerebral Small Vessel Diseases] explode all trees
#6 MeSH descriptor: [Intracranial Arterial Diseases] explode all trees
#7 MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees
#8 MeSH descriptor: [Intracranial Hemorrhages] explode all trees
#9 MeSH descriptor: [Stroke] explode all trees
#10 MeSH descriptor: [Vasospasm, Intracranial] this term only
#11 MeSH descriptor: [Vertebral Artery Dissection] this term only
#12 (stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH):ti,ab,kw (Word variations have been searched)
#13 ((brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab,kw (Word variations have been searched)
#14 ((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) near/5 (h?emorrhag* or h?ematoma* or bleed*)):ti,ab,kw (Word variations have been searched)
#15 MeSH descriptor: [Hemiplegia] this term only
#16 MeSH descriptor: [Paresis] explode all trees
#17 MeSH descriptor: [Gait Disorders, Neurologic] explode all trees
#18 (hemipleg* or hemipar* or paresis or paraparesis or paretic):ti,ab,kw (Word variations have been searched)
#19 {or #1‐#18}
#20 MeSH descriptor: [Deglutition] this term only
#21 MeSH descriptor: [Deglutition Disorders] explode all trees
#22 ((swallow* or deglutit* or dysphag*) near/3 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*)):ti,ab,kw (Word variations have been searched)
#23 ((swallow* or deglutit* or dysphag*) near/3 (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test)):ti,ab,kw (Word variations have been searched)
#24 MeSH descriptor: [Respiratory Aspiration] explode all trees
#25 MeSH descriptor: [Pneumonia, Aspiration] explode all trees
#26 ((inhal* or aspirat* or ingest*) near/3 (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test)):ti,ab,kw (Word variations have been searched)
#27 MeSH descriptor: [Pharynx] this term only
#28 MeSH descriptor: [Pharyngeal Muscles] this term only
#29 MeSH descriptor: [Esophageal Sphincter, Upper] this term only
#30 MeSH descriptor: [Esophagus] explode all trees
#31 ((throat or oesophag* or esophag* or pharyn* or oropharyn*) near/3 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*)):ti,ab,kw (Word variations have been searched)
#32 MeSH descriptor: [Fluoroscopy] explode all trees
#33 MeSH descriptor: [Bronchoscopy] this term only
#34 MeSH descriptor: [Laryngoscopy] this term only
#35 MeSH descriptor: [Endoscopy] this term only
#36 MeSH descriptor: [Manometry] this term only
#37 MeSH descriptor: [Fiber Optic Technology] this term only
#38 (fluoroscop* or videofluroscop* or fluorescence radiation or fluorescent scan* or fluorophotograph* or photofluoroscop* or radiofluoroscop* or roentgenfluoroscop* or bronchoscop* or bronchial endoscop* or laryngotracheobronchoscop* or tracheobronchoscop*):ti,ab,kw (Word variations have been searched)
#39 {or #20‐#38}
#40 #19 and #39
Appendix 3. Embase search strategy
1. cerebrovascular disease/ or brain disease/ or exp basal ganglion hemorrhage/ or exp brain hemangioma/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or exp cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp vertebrobasilar insufficiency/
2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw.
3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw.
5. exp hemiplegia/ or exp paresis/ or neurologic gait disorder/
6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw.
7. or/1‐6
8. dysphagia/
9. swallowing/
10. ((swallow$ or deglutit$ or dysphag$) adj3 (disturbance$ or disorder$ or difficult$ or dysfunction$ or impair$ or condition$ or abnormal$ or damage$ or injur$)).tw.
11. ((swallow$ or deglutit$ or dysphag$) adj3 (scale$ or screen$ or checklist$ or assess$ or exam$ or identif$ or recogni$ or evaluat$ or diagnos$ or detect$ or hazard or risk or test)).tw.
12. aspiration pneumonia/ or aspiration/
13. acid aspiration/
14. ((inhal$ or aspirat$ or ingest$) adj3 (scale$ or screen$ or checklist$ or assess$ or exam$ or identif$ or recogni$ or evaluat$ or diagnos$ or detect$ or hazard or risk or test)).tw.
15. exp pharynx/
16. exp esophagus/ or esophagus muscle/ or upper esophagus sphincter/
17. ((throat or oesophag$ or esophag$ or pharyn$ or oropharyn$) adj3 (disturbance$ or disorder$ or difficult$ or dysfunction$ or impair$ or condition$ or abnormal$ or damage$ or injur$)).tw.
18. fluoroscopy/ or fluoroscopy system/
19. exp bronchoscopy/ or bronchus examination/
20. exp laryngoscope/ or exp laryngoscopy/
21. endoscopy/ or fiberscope endoscopy/ or videoendoscopy/
22. manometry/
23. (fluoroscop$ or videofluroscop$ or fluorescence radiation or fluorescent scan$ or fluorophotograph$ or photofluoroscop$ or radiofluoroscop$ or roentgenfluoroscop$ or bronchoscop$ or bronchial endoscop$ or laryngotracheobronchoscop$ or tracheobronchoscop$).tw.
24. or/8‐23
25. 7 and 24
Appendix 4. CINAHL search strategy
S1 (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR ( (MH "Intracranial Embolism and Thrombosis") ) OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Stroke Patients") OR (MH "Stroke Units")
S2 TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH)
S3 TI ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)
S4 AB ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*)
S5 TI ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S6 AB ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
S7 (MH "Hemiplegia") or (MH "Gait Disorders, Neurologic+")
S8 TI ( (hemipleg* or hemipar* or paresis or paretic) ) OR AB ( (hemipleg* or hemipar* or paresis or paretic) )
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8
S10 (MH "Deglutition") OR (MH "Gagging")
S11 (MH "Deglutition Disorders")
S12 TI ( (swallow* or deglutit* or dysphag*) N5 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*) ) OR AB ( (swallow* or deglutit* or dysphag*) N5 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*) )
S13 TI ((swallow* or deglutit* or dysphag*) N5 (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test)) OR AB ((swallow* or deglutit* or dysphag*) N5 (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test))
S14 (MH "Aspiration")
S15 (MH "Pneumonia, Aspiration")
S16 (MH "Risk for Aspiration (NANDA)")
S17 TI ((inhal* or aspirat* or ingest*) N5 (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test)) OR AB ((inhal* or aspirat* or ingest*) N5 (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test))
S18 (MH "Pharynx+")
S19 (MH "Esophagus")
S20 TI ((throat or oesophag* or esophag* or pharyn* or oropharyn*) N5 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*)) OR AB ((throat or oesophag* or esophag* or pharyn* or oropharyn*) N5 (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*))
S21 (MH "Fiber Optics")
S22 TI ( (fluoroscop* or videofluroscop* or fluorescence radiation or fluorescent scan* or fluorophotograph* or photofluoroscop* or radiofluoroscop* or roentgenfluoroscop* or bronchoscop* or bronchial endoscop* or laryngotracheobronchoscop* or tracheobronchoscop*) ) OR AB ( (fluoroscop* or videofluroscop* or fluorescence radiation or fluorescent scan* or fluorophotograph* or photofluoroscop* or radiofluoroscop* or roentgenfluoroscop* or bronchoscop* or bronchial endoscop* or laryngotracheobronchoscop* or tracheobronchoscop*) )
S23 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
S24 S9 AND S23
Appendix 5. HTA search strategy
1. cerebrovascular disorders/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/
2. (stroke or strokes or poststroke or cerebral vasc* or brain vasc$ or cerebrovasc* or cva or SAH) any field
3. ((brain or cerebr* or cerebell* or vertebrobasil* or hemisphere* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) and (ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)) any field
4. ((brain or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or posterior fossa or hemisphere* or subarachnoid) and (haemorrhag* or hemorrhag* or hematoma* or haematoma* or bleed*)) any field
5. exp paresis/ or exp Gait Disorders, Neurologic/
6. (hemipleg* or paresis or paraparesis or paretic) any field
7. or/1‐6
8. Deglutition/
9. exp Deglutition Disorders/
10. ((swallow* or deglutit* or dysphag*) and (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*)) any field
11. ((swallow* or deglutit* or dysphag*) and (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test)) any field
12. exp Pneumonia, Aspiration/
13. ((inhal* or aspirat* or ingest*) and (scale* or screen* or checklist* or assess* or exam* or identif* or recogni* or evaluat* or diagnos* or detect* or hazard or risk or test)) any field
14. Pharynx/ or pharyngeal muscles/ or exp Esophagus/
15. ((throat or oesophag* or esophag* or pharyn* or oropharynx*) and (disturbance* or disorder* or difficult* or dysfunction* or impair* or condition* or abnormal* or damage* or injur*)) any field
16. exp Fluoroscopy/ or bronchoscopy/ or laryngoscopy/ or endoscopy/ or manometry/
17. (fluoroscop* or videofluoroscop* or fluorescence radiation or fluorescent scan* or fluorophotograph* or photofluoroscop* or radiofluoroscop* or roentgenfluoroscop* or bronchoscop* or bronchial endoscop* or laryngotracheobronchoscop* or tracheobronchoscop*) any field
18. or/8‐17
29. 7 and 18
Appendix 6. List of targeted grey literature sources (CADTH, 2018)
Health Quality Council of Alberta (HQCA) http://hqca.ca/studies-and-reviews/completed-reviews/
Institut national d’excellence en santé et en services sociaux (INESSS) [formerly AETMIS] http://www.inesss.qc.ca/en/publications/publications.html
Manitoba Centre for Health Policy (MCHP) http://mchp-appserv.cpe.umanitoba.ca/deliverablesList.html
NLCAHR : Newfoundland and Labrador Centre for Applied Health Research. Contextualized Health Research Synthesis Program (CHRSP) http://www.nlcahr.mun.ca/CHRSP/CompletedCHRSP.php
Ottawa Hospital Research Institute (OHRI) http://www.ohri.ca/ksgroup/publications.asp
Therapeutics Initiative. Therapeutics Letter http://www.ti.ubc.ca/TherapeuticsLetter
University of British Columbia. Centre for Health Services and Policy Research http://chspr.ubc.ca/publications/
World Health Organization Regional Office for Europe. Health Evidence Network (WHO HEN) http://www.euro.who.int/en/data-and-evidence/evidence-informed-policy-making/publications/by-keyword
Australian Government. Department of Health and Ageing. Australia and New Zealand Horizon Scanning Network (ANZHSN). http://www.horizonscanning.gov.au/internet/horizon/publishing.nsf/Content/technologies-assessed-lp-2
Australian Government Department of Health and Ageing. Medical Services Advisory Committee (MSAC). http://www.msac.gov.au/internet/msac/publishing.nsf/Content/completed-assessments
Joanna Briggs Institute (JBI). The Joanna Briggs Institute EBP Database http://connect.jbiconnectplus.org/Search.aspx
Monash Health. Centre for Clinical Effectiveness (CCE). Centre for Clinical Effectiveness – Publications http://monashhealth.org/health-professionals/cce/cce-publications/
Queensland Government (Australia). Health Technology Reference Group. Health Technologies Evaluated‐Reports and Briefs (COAG Health Council). https://www.coaghealthcouncil.gov.au/AHMAC/Health-Technology-Reference-Group/Reports-and-Briefs
Kenniscentrum voor de Gezondheidszorg / Le Centre d'expertise des soins de santé. Belgian Health Care Knowledge Centre (KCE) https://kce.fgov.be/en/all-reports
Sundhedsstyrelsen. Danish Health and Medicines Authority (DHMA). http://sundhedsstyrelsen.dk/en/publications
Haute Autorité de santé/ French National Authority for Health (HAS). Haute Autorité de santé http://www.has-sante.fr/portail/jcms/c_946986/en/english-toutes-nos-publications-ligne-principale?portal=r_1457306
Health Service Executive. Irish Health Repository (Lenus) http://www.lenus.ie/hse/
De Gezondheidsraad (GR). Health Council of the Netherlands http://www.gezondheidsraad.nl/en/publications
Zorginstituut Nederland. National Health Care Institute Netherlands https://english.zorginstituutnederland.nl/publications
Folkehelseinstituttet. Norwegian Institute of Public Health. Publications https://www.fhi.no/en/publ/
Agència de Qualitat i Avaluació Sanitàries de Catalunya (AQuAS). Agency for Health Quality and Assessment of Catalonia http://aquas.gencat.cat/ca/publicacions/
Sahlgrenska Universitetssjukhuset. Sahlgrenska University Hospital. Regional activity‐based HTA. https://www.sahlgrenska.se/forskning/htacentrum/
Healthcare Improvement Scotland. http://www.healthcareimprovementscotland.org/
NHS National Institute for Health and Care Excellence (NICE). http://www.nice.org.uk/
UK Department of Health (NHS). International Resource for Infection Control (INRIC) https://www.nric.org.uk/resources
Agency for Healthcare Research and Quality (AHRQ). Technology Assessments https://www.ahrq.gov/research/findings/ta/index.html
Institute for Clinical and Economic Review (ICER). https://icer-review.org/materials/
Appendix 7. QUADAS‐2 tool: risk of bias and applicability judgements
| Domain 1. Patient selection | |
|
|
| Describe methods of patient selection: | |
| · Was a consecutive or random sample of patients enrolled? | Yes/No/Unclear |
|
Yes/No/Unclear |
|
Yes/No/Unclear |
| · Could the selection of patients have introduced bias? | RISK: LOW/HIGH/UNCLEAR |
|
|
| Describe included patients (prior testing, presentation, intended use of index test and setting): | |
| Is there concern that the included patients do not match the review question? | CONCERN: LOW/HIGH/UNCLEAR |
| Domain 2. Index test(s) (if more than 1 index test was used, please complete for each test) | |
|
|
| Describe the index test and how it was conducted and interpreted: | |
|
Yes/No/Unclear |
|
Yes/No/Unclear |
| · Could conduct or interpretation of the index test have introduced bias? | RISK: LOW/HIGH/UNCLEAR |
|
|
| Is there concern that the index test, its conduct, or its interpretation differ from the review question? | CONCERN: LOW/HIGH/UNCLEAR |
| Domain 3. Reference standard | |
|
|
| Describe the reference standard and how it was conducted and interpreted: | |
|
Yes/No/Unclear |
|
Yes/No/Unclear |
| · Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: LOW/HIGH/UNCLEAR |
|
|
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW/HIGH/UNCLEAR |
| Domain 4. Flow and timing | |
|
|
|
Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2 ×2 table (refer to flow diagram): Describe the time interval and any interventions between index test(s) and reference standard: | |
|
Yes/No/Unclear |
|
Yes/No/Unclear |
|
Yes/No/Unclear |
|
Yes/No/Unclear |
| · Could the patient flow have introduced bias? | RISK: LOW/HIGH/UNCLEAR |
Appendix 8. Description of screening tests
Bedside Aspiration Test (Lim 2001a)
Stage 1: the Water Swallow Test assessed patient’s ability to swallow 50 mL of water in 10‐mL aliquots. The patient is seated upright and is asked to drink all of the 50 mL. Patients were deemed to be clinically aspirating if they coughed or choked during the water drinking test or had a change in their voice quality following the swallow. Patients were monitored for these signs of possible aspiration for up to 5 minutes after swallowing.
Stage 2: after a rest period of 10 minutes, the Oxygen Saturation Test was conducted. The finger probe of a Novametrix Oxypleth pulse oximeter (model 520A) was placed on the index finger of the unaffected hand of the patient, with the patient in an upright position. After equilibrating for 5 minutes, a baseline oxygen saturation reading was taken. A drop > 2% in arterial oxygen saturation was considered clinically significant (Collins and Bakheit 1997). Maximum and minimum oxygen saturation readings during and for up to 2 minutes after swallowing 10 mL of water were noted 3 times, and highest and lowest readings were taken. If oxygen saturation fell by 5% or more, the test was stopped immediately to stop an additional decline in arterial blood oxygenation levels, especially in patients who may have compromised pulmonary function. Aspiration was deemed present if overt signs of aspiration and/or oxygen desaturation of 2% or more was noted.
Gugging Swallowing Screen (Trapl 2007b)
The Bedside Gugging Swallow Screen (GUSS) consists of two parts. Part 1, the preliminary assessment, involved a simple saliva swallow test. Patients who were unable to produce enough saliva because of dry mouth were given saliva spray as a substitute. Vigilance, voluntary cough, throat clearing, and saliva swallowing were assessed. Part 2 consisted of 3 sequentially performed subtests, starting with semisolid (thickened distilled water), then liquid (increasing amounts of 3, 5, 10, 20, and 50 mL), and finally solid textures (5 tests with dry bread). Ten seconds is the time limit for a small solid bolus, including the oral preparatory phase. The test was stopped if 1 of the 4 aspiration signs (deglutition, cough, drooling, and voice change) was positive. Patients must successfully complete all repetitions in the subtest to achieve the full score of 5 points. If a subtest results in < 5 points, the examination is stopped and a special oral diet and/or further investigation by videofluoroscopy or fibreoptic endoscopy is recommended. Diet recommendations are then offered, aligned to the functional oral intake scale, and are given according to points reached in the GUSS.
The Toronto Bedside Swallow Screening Test (Martino 2009 Study 1)
The Toronto Bedside Swallowing Screening Test (TOR‐BSST) included the following tests: the Kidd 50 mL water swallow test, pharyngeal sensation, tongue movement, and general dysphonia split into 2 items ‐ 'voice before' and 'voice after'. A pass/fail response was assigned to each item, so that failure on any item constitutes a positive screen result and therefore increased risk for dysphagia. Administration continues only until the first TOR‐BSST item is failed.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.

Registered Dietitian (RD) Dysphagia Screening tool ‐ Huhmann (2004)
2. Test.

Bedside aspiration ‐ Combined WST & Oxygen Saturation ‐ Lim (2001)
3. Test.

Gugging Swallowing Screen (GUSS) ‐ Group2 ‐ Trappl (2007)
4. Test.

Toronto Bedside Swallowing Screening Test (TOR‐BSST) ‐ Martino (2009)
5. Test.

Standardized Swallowing Assessment tool (SSA) ‐ Test2 ‐ Perry (2001) Test2
6. Test.

Nursing Bedside Dysphagia Screen (NBDS) ‐ Campbell (2016)
7. Test.

Emergency Department (ED) dysphagia screen ‐ Turner‐Lawrence (2009)
8. Test.

Acute Stroke Dysphagia Screening (ASDS) ‐ Aspiration ‐ Edmiaston (2010)
9. Test.

Barnes‐Jewish Hospital‐Stroke Dysphagia Screen (BJH‐SDS) Aspiration ‐ Edmiaston (2014)
10. Test.

Edith‐Huhn‐Matesic Bedside Aspiration Screen (EHMBAS) plus water swallow test ‐ Huhn‐Matesic (2015)
11. Test.

Standardized Swallowing Assessment tool (SSA) ‐ Test1 ‐ Perry (2001)
12. Test.

Barnes‐Jewish Hospital‐Stroke Dysphagia Screen (BJH‐SDS) Dysphagia ‐ Edmiaston (2014)
13. Test.

Modified MASA (MMASA) Neurologist 1 ‐ Antonios (2010)
14. Test.

Rapid Aspiration Screening for Suspected Stroke (RAS3) ‐ Daniels (2016)
15. Test.

Clinical examination ‐ Daniels (1997)
16. Test.

Acute Stroke Dysphagia Screening (ASDS) Dysphagia ‐ Edmiaston (2010)
17. Test.

Nurse Dysphagia Screen ‐ Cummings (2015)
18. Test.

Modified MASA (MMASA) Neurologist 2 ‐ Antonios (2010)
19. Test.

Oxygen saturation ≥ 2% ‐ Test2 for Aspiration ‐ Smith (2000)
20. Test.

Bedside swallow test ‐ WST only ‐ Lim (2001)
21. Test.

Chinese version of the modified SSA original 8 items ‐ Jiang (2019)
22. Test.

Chinese version of the modified SSA reduced 6 items ‐ Jiang (2019)
23. Test.

Stroke Severity using National Institutes of Health Stroke Scale (NIHSS) ‐ Bravata (2009)
24. Test.

Nursing Bedside Swallowing Screen (NBSS) ‐ Ellis (2013)
25. Test.

2‐step Swallowing Provocation Test (SPT) ‐ step 1 ‐ 0.4 mL ‐ Warneke (2008)
26. Test.

Rapid Aspiration Screening for Suspected Stroke (RAS3) ‐ WST only ‐ Daniels (2016)
27. Test.

Gag function ‐ Test3 ‐ Perry (2001)
28. Test.

DePaul Hospital Swallow Screener (DHSS) for Aspiration Risk ‐ Behera (2018)
29. Test.

DePaul Hospital Swallow Screener (DHSS) for Dysphagia ‐ Behera (2018)
30. Test.

Gag function ‐ Test4 ‐ Perry (2001)
31. Test.

2‐step swallowing provocation test (SPT) step 2 ‐ 2.0 mL ‐ Warneke (2008)
32. Test.

Johns Hopkins Hospital Brain Rescue Unit Modified 3 oz Swallow Screen ‐ Mulheren (2019)
33. Test.

Nursing Screening Tool ‐ Bravata (2009)
34. Test.

Barnes‐Jewish Hospital Stroke Dysphagia Screen (BJH‐SDS) – Turkish version (T‐BJH) ‐ Eren (2019)
35. Test.

Clinical Predicative Scale of Aspiration (CPSA) ‐ Zhou (2011)
36. Test.

TOR‐BSST water swallow item ‐ Martino (2014)
37. Test.

Clinical swallowing tests ‐ 6 oromotor examinations ‐ Nishiwaki (2005)
38. Test.
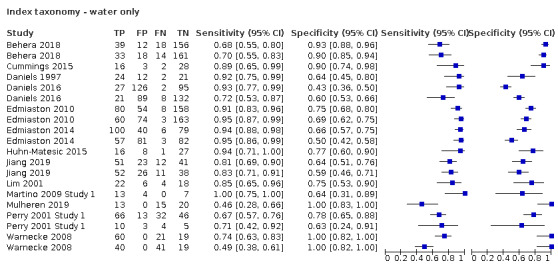
Index taxonomy ‐ water only
39. Test.
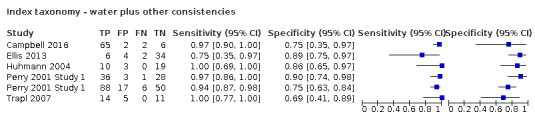
Index taxonomy ‐ water plus other consistencies
40. Test.

Index taxonomy ‐ other
41. Test.
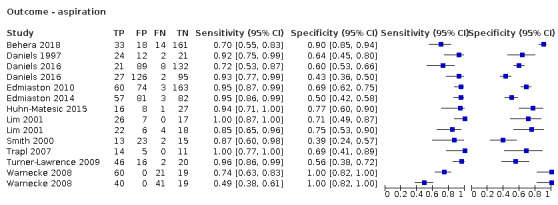
Outcome ‐ aspiration
42. Test.
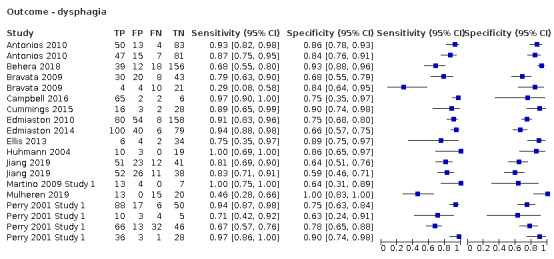
Outcome ‐ dysphagia
43. Test.
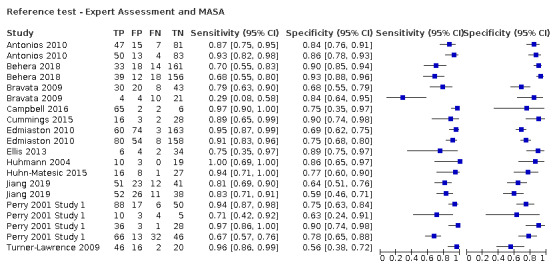
Reference test ‐ Expert Assessment and MASA
44. Test.

Reference test ‐ FEES
45. Test.
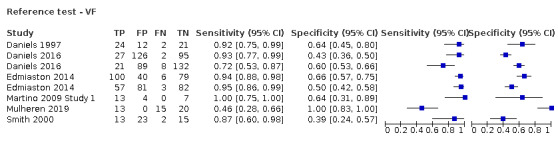
Reference test ‐ VF
46. Test.
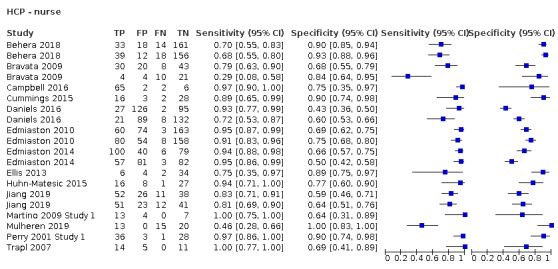
HCP ‐ nurse
47. Test.
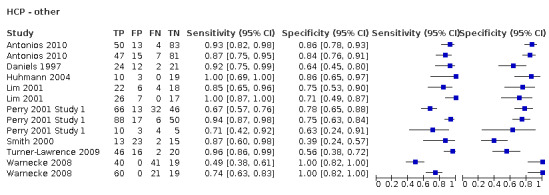
HCP ‐ other
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antonios 2010.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: AIS, diagnosed clinically by a neurologist; radiographic (CT or MRI) confirmation of AIS Exclusion criteria: unresolved dysphagia before stroke; history of head/neck surgery or trauma that may impact swallowing ability; any other concomitant neurological disorder that could impact oropharyngeal swallowing ability Setting: 1 × tertiary care academic medical centre with a certified comprehensive stroke centre |
||
| Index tests | Modified MASA (MMASA) | ||
| Target condition and reference standard(s) | Dysphagia MASA |
||
| Flow and timing | All patients received the index test(s) and reference standard. All patients were included in the 2 × 2 table Interventions between index/reference tests: not reported Interval between tests ≤ 4 hours |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | High risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Behera 2018.
| Study characteristics | |||
| Patient Sampling | All patients were admitted under a standard stroke protocol | ||
| Patient characteristics and setting | Inclusion criteria: patients admitted to a comprehensive stroke centre Exclusion criteria: unclear Setting: 1 × hospital designated comprehensive stroke centre | ||
| Index tests | The DePaul Hospital Swallow Screening tool includes a combination of non‐swallow and swallow items. The 8 non‐swallow items (including tests of alertness, presence of a feeding tube, presence of a tracheostomy tube, presence of drooling, facial asymmetry, abnormal tongue movement, abnormal vocal quality, and abnormal voluntary cough) are scored, and if patients pass (score ≤ 5), they progresses onto the 3 oz WST. If patients fail the non‐swallow items (score ≥ 6), or if they fail the WST (clinical signs of aspiration), they are kept nil by mouth | ||
| Target condition and reference standard(s) | Two analyses: dysphagia and aspiration risk MASA |
||
| Flow and timing | 374 were admitted to the stroke unit; 225 received the DHSS Index test. The reason for the drop in numbers is not reported. 224 received at least 1 MASA assessment 2 × 2 table is reported for 224 patients Interventions between index/reference tests: not reported Interval between index and reference tests: not reported | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Bravata 2009.
| Study characteristics | |||
| Patient Sampling | Patients who had received at least 1 Index test plus the reference test | ||
| Patient characteristics and setting | Inclusion criteria: discharge diagnosis with ischaemic stroke (ICD9 434.X and 436) Exclusion criteria: none recorded Setting: 1 × tertiary care ‐ Department of Veterans Affairs Medical Centre | ||
| Index tests | Nurse Screening Tool applied during admission process. NIHSS applied retrospectively | ||
| Target condition and reference standard(s) | Dysphagia SLP: expert assessment |
||
| Flow and timing | Flow is not possible to report. Study reported only on those who had at least 1 Index test + the reference test Timing not reported. In fact, study authors list possible time differences as a limitation |
||
| Comparative | |||
| Notes | QUADAS‐2 is different for each index test. Stroke Severity NIHSS (test2) reported here, as it is the 'worst', compared to the Nurse Screening Tool (test1) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Campbell 2016.
| Study characteristics | |||
| Patient Sampling | Convenience sample | ||
| Patient characteristics and setting | Inclusion criteria: diagnosis of stroke or TIA; admitted between 8:30am and 4:30pm; no change in initial NIHSS score between screening by the nurse and evaluation by the speech pathologist Exclusion criteria: non‐stroke and non‐TIA admissions; stroke and TIA admissions after 4:30pm; NIHSS score change from initial screening by the nurse, and subsequent evaluation performed by speech pathologist Setting: 1 × neuroscience unit ‐ part of a Joint Commission Disease Specific certified stroke centre | ||
| Index tests | NBDS | ||
| Target condition and reference standard(s) | Dysphagia SLP: expert assessment |
||
| Flow and timing | 3 patients not screened by Nurse 2
Interventions between index/reference tests: not reported Interval between index and reference tests: < 1 hour |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Cummings 2015.
| Study characteristics | |||
| Patient Sampling | Convenience sample | ||
| Patient characteristics and setting | Inclusion criteria: medical diagnosis of stroke; age 18 or older; ability to follow commands Exclusion criteria: individuals who were NPO for any reason other than swallowing problems; history of previous swallowing problems; mechanical ventilation and/or intubation longer than 24 hours during current admission; inability to follow commands Setting: 2 × hospital ‐ 20‐bed medical neurology unit and 9‐bed neurology intermediate care unit | ||
| Index tests | Nurse dysphagia screen | ||
| Target condition and reference standard(s) | Dysphagia Dysphagia evaluation by SLP |
||
| Flow and timing | All patients received index test(s) and reference standard. All patients were included in 2 × 2 table Interventions between index/reference tests: not reported Index to reference tests interval: ≤ 2 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Daniels 1997.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: new neurological deficit; stroke confirmed by documentation of acute infarct by CT or MRI scan Exclusion criteria: obtunded and agitated patients; those with prior history of oropharyngeal dysphagia, oropharyngeal structural damage, or neurological disease other than stroke that may produce dysphagia Setting: 1 × Veterans Affairs Medical Center in New Orleans | ||
| Index tests | Oropharyngeal assessment to include clinical swallowing assessment | ||
| Target condition and reference standard(s) | Dysphagia severity or risk of aspiration VSS |
||
| Flow and timing | All patients received index test(s) and reference standard. All patients included in 2 × 2 table apparently, but numbers do not really add up Interventions between index/reference tests: not reported Average time between test: 48 hours | ||
| Comparative | |||
| Notes | Flow and timing high risk due to 48‐hour average time gap | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Daniels 2016.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: suspected stroke Exclusion criteria: non‐stroke neurological disease; head/neck structural changes; dysphagia unrelated to stroke; TIA/stroke ruled out; not competent/no legally authorised representative; medically unstable; repeat stroke; previously participated in the study; > 3 weeks from symptom onset; > 5 days from admission; childbearing potential female; deceased Setting: 1 × Michael E DeBakey Veterans Affairs Medical Center – comprehensive stroke centre | ||
| Index tests | RAS3 and WST from RAS3 | ||
| Target condition and reference standard(s) | Aspiration in patients with suspected stroke VFSS |
||
| Flow and timing | 8 patients who received the index test were not reported on. Reasons for no VFSS included C‐spine surgery evident on X‐ray (n = 1) and no oral intake before surgery (n = 1) Reasons for exclusion from VFSS analyses included dysphagia history prior to stroke (n = 2), equipment failure (n = 1), and C‐spine damage (n = 3) Interventions between index/reference tests: not reported Index to reference test time interval within 2 hours of screening; mean (SD) 0.49 (0.24) hours |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Edmiaston 2010.
| Study characteristics | |||
| Patient Sampling | Not recorded | ||
| Patient characteristics and setting | Inclusion criteria: admitted to stroke service Exclusion criteria: none recorded Setting: 1 × Stroke Service at Barnes‐Jewish Hospital, Washington University Medical Centre | ||
| Index tests | ASDS | ||
| Target condition and reference standard(s) | Aspiration risk and dysphagia MASA |
||
| Flow and timing | All patients received index test(s) and reference standard. All patients included in 2 × 2 table Interventions between index/reference tests: not reported Interval between tests: approximately 24 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Edmiaston 2014.
| Study characteristics | |||
| Patient Sampling | Acute stroke patients prospectively enrolled from Barnes‐Jewish Hospital inpatient stroke service (p2) | ||
| Patient characteristics and setting | Inclusion criteria: clinical diagnosis of stroke (ischaemic or haemorrhagic); age ≥ 18 years Exclusion criteria: decreased level of alertness preventing participation in VFSS (defined as a score of 2 on the 'Alertness' component of the MASA); physical limitations preventing ability to sit upright (excluding intubation, or if treating physician had ordered the patient to have the head of the bed flat); confirmed or suspected pregnancy Setting: 1 × Barnes‐Jewish Hospital inpatient stroke service (urban, tertiary care referral centre admitting 1300 stroke patients annually) | ||
| Index tests | BJH‐SDS | ||
| Target condition and reference standard(s) | Dysphagia and aspiration VFSS |
||
| Flow and timing | Study included 2 tests: 1 for dysphagia and 1 for aspiration. For the dysphagia test, all patients received the reference test; for the aspiration test, 2 patients were excluded and did not receive the reference test Interventions between index/reference tests: not reported Interval between index and reference test: 0 to 8 hours (mean interval 2 hours) |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Low risk | ||
Ellis 2013.
| Study characteristics | |||
| Patient Sampling | Not recorded | ||
| Patient characteristics and setting | Inclusion criteria: patient suffered stroke or TIA Exclusion criteria: not recorded Setting: 1 × small community hospital ‐ certified primary stroke centre | ||
| Index tests | Ellis Nursing Bedside Swallowing Screen | ||
| Target condition and reference standard(s) | Dysphagia Complete dysphagia evaluation by SLT |
||
| Flow and timing | 53 participants were included, baseline data for 49 participants are given, and 46 participants received both screening tests. Outline data are provided for patients who were excluded or withdrawn; however numbers do not necessarily specify the difference between original sample and those receiving the tests Interventions between index/reference tests: not reported Interval between tests: not reported |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | High risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Eren 2019.
| Study characteristics | |||
| Patient Sampling | Unclear | ||
| Patient characteristics and setting | Inclusion criteria: diagnosis of acute stroke based on clinical and MRI results; aged > 18 years; normal cognitive function (Mini‐Mental Test Score > 24 points) Exclusion criteria: presence of previous stroke; neurodegenerative or muscular disease history potentially associated with swallowing disorder; malignancy; history of surgery in the head and neck region; bilateral cranial infarction; psychiatric disorder; presence of infectious disease such as HIV, hepatitis B or C, decompensated heart failure, and nasal obstruction (exclusion criteria for FEES) Setting: 1 × neurology department | ||
| Index tests | Turkish version of BJH‐SDS consists of 5 items, each with 2 choices (i.e. present = yes, absent = no). The first 4 items assess consciousness and asymmetry or weakness in facial, tongue, and palatal muscles. Level of consciousness is assessed by the Glasgow Coma Scale, and presence of dysarthria is identified together with the use of other items. The fifth item consists of the 3‐oz water test, and the abnormality was defined as coughing, choking, or breathlessness while swallowing, or wet/gurgled voice after swallowing. The screening tool is a pass/fail | ||
| Target condition and reference standard(s) | Dysphagia FEES |
||
| Flow and timing | It is not clear whether the same number of patients received index and reference tests 2 × 2 table: not reported Interventions between index/reference test: not reported Interval between tests: ≤ 24 hours |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Huhmann 2004.
| Study characteristics | |||
| Patient Sampling | Convenience sample | ||
| Patient characteristics and setting | Inclusion criteria: none recorded Exclusion criteria: none recorded Setting: 1 × admitted to the stroke team | ||
| Index tests | Registerd Dietician Dysphagia Screening Tool | ||
| Target condition and reference standard(s) | Dysphagia risk SLT bedside swallow assessment |
||
| Flow and timing | All patients received index test(s) and reference standard. All patients were included in the 2 × 2 table Interventions between index/reference tests: not reported Interval between tests: not reported | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Huhn‐Matesic 2015.
| Study characteristics | |||
| Patient Sampling | Unclear | ||
| Patient characteristics and setting | Regional non‐critical care stroke unit. Patient inclusion and exclusion criteria not reported | ||
| Index tests | EHMBAS followed by simple WST; interpretation not reported | ||
| Target condition and reference standard(s) | MASA | ||
| Flow and timing | All patients received the index test(s) and reference standard. All patients were included in the 2 × 2 table Interventions between index/reference tests: not reported Interval between tests: not reported |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
Jiang 2019.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: diagnosis of stroke, clinical diagnosis from a neurologist, radiographic (CT or MRI) confirmation of stroke Exclusion criteria: history of head/neck surgery, trauma; brain tumour that could influence swallowing abilities; any other concomitant neurological disorder that could influence oropharyngeal swallowing ability Setting: 1 × neurology unit | ||
| Index tests | The 8‐item Chinese version of the modified Standardized Swallowing Assessment instrument is divided into 2 parts. In part 1, patients must (1) voluntarily cough, (2) control saliva, (3) lick top and bottom lips, (4) breathe freely, and (5) cannot have a wet or hoarse voice. In part 2 (items 6 to 8), patients are first given 1 mL of water. Problems are identified if no attempts are made to swallow or water leaks straight out of the mouth, if coughing, choking, or breathlessness is observed, or if a wet/gurgly voice develops. In the absence of any problems, the process is repeated with second and third tests with 1 mL of water. Gradually, increasing volumes ranging from 1 to 10 mL sequential swallowing are used based on patient tolerance. If no problems are evident, half a glass of water (75 mL) is administered. The screening instrument is a pass vs fail procedure The 6‐item Chinese version of the modified Standardized Swallowing Assessment includes the following items from the 8‐item instrument: items 1, 3, and 5 of the oromotor examinations, and items 6, 7, and 8 of the WST |
||
| Target condition and reference standard(s) | Dysphagia SLP expert assessment |
||
| Flow and timing | All patients received both index and reference tests. All patients were included in the analysis Interventions between index/reference tests: none reported Interval between tests: ≤ 48 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Lim 2001.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: acute stroke patients admitted to stroke unit of Tan Tock Seng Hospital Exclusion criteria: severe peripheral vascular disease; consciousness level not sufficient to give informed consent; no CT evidence of stroke or no significant neurological deficit (e.g. new hemiplegia) lasting > 24 hours; insufficient lip seal to retain 10 mL of water in the mouth Setting: 1 × stroke unit | ||
| Index tests | WST and bedside aspiration (combined WST and oxygen saturation test) | ||
| Target condition and reference standard(s) | Aspiration FEES |
||
| Flow and timing | Patient flow is unclear ‐ 58 patients were eligible, 50 patients received reference and index tests, 8 patients subsequently declined FEES
Interventions between index/reference tests: not reported Time interval between tests: < 24 hours for 45 patients; < 48 hours for 5 people due to 'technical issues' |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Martino 2009 Study 1.
| Study characteristics | |||
| Patient Sampling | Consecutive. However 1 in 5 patients receiving a negative screen and all receiving a positive screen on the index test received the reference test | ||
| Patient characteristics and setting | Inclusion criteria: newly admitted to hospital; confirmed diagnosis of brain stem stroke or cerebellar stroke and all other stroke types with NIHSS score ≥ 4; stroke confirmed from physician’s clinical note, CT scan or MRI Exclusion criteria: non‐brain stem and non‐cerebellar stroke patients with low NIHSS scores; patients with current respiratory compromise; a non‐oral feeding regime; history of non‐stroke neurological disorder; surgery to the head or neck; history of previous oropharyngeal dysphagia, dementia, or decreased level of consciousness; not alert; cannot be supported to sit upright; cannot follow simple instructions Setting: 2 acute settings will be used for analysis, originally 4. Inpatient stroke units at 2 acute (used in data extract) and 2 rehabilitation tertiary care hospitals (not used in data extract) in southern Ontario | ||
| Index tests | TOR‐BSST | ||
| Target condition and reference standard(s) | Dysphagia risk Videofluoroscopic assessment of swallowing |
||
| Flow and timing | Of the 311 patients recruited, only 103 were from an acute setting, and of these only 24 received both tests. Remaining patients were therefore excluded from the analysis Interventions between index/reference tests: not reported Interval between tests ≤ 24 hours |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Martino 2014.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: eligible and consented stroke patients in the original study
Exclusion criteria: not reported Setting: 2 × acute and 2 × rehabilitation facilities |
||
| Index tests | TOR‐BSST | ||
| Target condition and reference standard(s) | Dysphagia VFSS |
||
| Flow and timing | All patients recruited received both index and reference tests. All patients were included in the analysis, but no 2 × 2 table was reported Interventions between index/reference tests: none reported Interval between tests < 24 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Mulheren 2019.
| Study characteristics | |||
| Patient Sampling | Acute stroke patients admitted to the unit | ||
| Patient characteristics and setting | Inclusion criteria: acute stroke patients on the units Exclusion criteria: history of swallowing dysfunction or neurological disease that might lead to swallowing dysfunction; medical compromise barring transport to fluoroscopy; insufficient level of alertness to follow directions during VFSS as determined by the care team Setting: 1 × neuroscience critical and acute care units of a tertiary care facility | ||
| Index tests | The Johns Hopkins Hospital Brain Rescue Unit Modified 3 oz Swallow Screen is based on the 3‐oz water swallow test by DePippo 1992. The screen consists of 3 steps: (1) medical history and status; (2) 1 tsp water swallow with palpation for hyolaryngeal excursion and observation for signs of aspiration; (3) 3 oz water swallow with palpation for hyolaryngeal excursion and observation for signs of aspiration. Observation for signs of aspiration is continuous during and up to 1 minute after each water trial. Wet vocal quality is assessed objectively during the vocalisation trial by trained nurses. Each step is administered sequentially, and failure of 1 step results in discontinuation and failure of the entire screen (positive aspiration risk) with nil‐by‐mouth status and referral to speech and language pathology for bedside evaluation | ||
| Target condition and reference standard(s) | Dysphagia VFSS |
||
| Flow and timing | All patients recruited received both index and reference tests. All patients were included in the analysis Interventions between index/reference tests: none reported Interval between tests < 72 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Nishiwaki 2005.
| Study characteristics | |||
| Patient Sampling | Consecutive patients | ||
| Patient characteristics and setting | Inclusion criteria: stroke was diagnosed with CT or MRI. Patients presented with ≥ 1 of the following: (1) bilateral or brain stem stroke; (2) history of aspiration pneumonia or increased sputum secretion; (3) cough associated with feeding and/or drinking; (4) weight loss, decreased oral intake, or prolonged feeding times; (5) complaint of difficulty in swallowing; (6) need for a therapeutic diet or non‐oral feeding Exclusion criteria: patients who could not follow commands, who had a tracheostomy, who had a prior history of oropharyngeal impairment, or who had active respiratory infection Setting: 5 × university hospital and affiliated hospitals | ||
| Index tests | Clinical swallowing tests: 6 oromotor examinations | ||
| Target condition and reference standard(s) | Aspiration VFSS |
||
| Flow and timing | All patients recruited received both index and reference tests. All patients were included in the analysis Interventions between index/reference tests: none reported Interval between tests < 7 days, but < 24 hours for acute patients | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | High risk | ||
Perry 2001 Study 1.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: admissions with a clinical diagnosis of acute stroke (ICD 10 codes 160‐164, with or without CT confirmation) Exclusion criteria: none recorded Setting: 1 × study wards of Mayday University Hospital (all wards that normally care for stroke patients with the exception of 2 female care of the elderly wards where another project was in progress) | ||
| Index tests | Standardized Swallowing Assessment tool (test 1) Standardized Swallowing Assessment tool (test 2) (subset of test 1) Gag reflex (test 3) (subset of test 4) Gag reflex data from the 20‐month audit period used (test 4) |
||
| Target condition and reference standard(s) | Dysphagia Summative clinical judgement ‐ used VFS when requested by SLT to clarify decision |
||
| Flow and timing | Flow of patients is unclear from the paper. Although participants are discussed in the paper, results are presented as episodes. The results also include repeat screenings for participants; participants are excluded after recruitment and are not included in the analysis Interventions between index/reference tests: not reported Interval between tests: not reported |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | High risk | ||
Smith 2000.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: acute haemorrhagic or infarctive stroke; stroke confirmed by CT scan; age 18 to 90 years Exclusion criteria: impaired consciousness level; cognitive impairment or receptive dysphasia sufficient to prevent informed consent; inability to sit upright without minimal support; current lower respiratory infection; additional neurological condition; terminal illness; any medical condition that precluded VF Setting: 2 × university teaching hospitals in Manchester | ||
| Index tests | Smith Bedside Swallowing, pulse oximetry (Minolta Pulsox 7), or both | ||
| Target condition and reference standard(s) | Aspiration and penetration VF |
||
| Flow and timing | All patients received the index test(s) and the reference standard. All patients were included in the 2 × 2 table, but for tests 1 and 5, the 2 × 2 was recovered in the RevMan calculator. This gave test 5 with N = 52, 1 patient missing Interventions between index/reference tests: not reported Interval between tests ≤ 24 hours (all tests carried out on the same day) |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Trapl 2007.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: patients with first‐ever acute stroke and suspected dysphagia; admitted to the acute stroke unit on weekdays between Monday and Thursday Exclusion criteria: multiple infarcts visible on CT or MRI scan; dysphagia of other known cause; somnolence or coma within 24 hours Setting: 1 × acute stroke unit | ||
| Index tests | GUSS | ||
| Target condition and reference standard(s) | Aspiration FEES |
||
| Flow and timing | In group 1, 1 patient refused FEES (reference test) and so was not included in the 2 × 2 table In group 2, all patients received the index test(s) and the reference standard. All patients were included in the 2 × 2 table Interventions between index/reference tests: not reported Interval between tests < 24 hours |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Could the patient flow have introduced bias? | Low risk | ||
Turner‐Lawrence 2009.
| Study characteristics | |||
| Patient Sampling | Convenience sample | ||
| Patient characteristics and setting | Inclusion criteria: presumptive stroke diagnosis; age ≥ 18 years; presenting to the emergency department within 24 hours of symptom onset; Glasgow Coma Scale score > 12; hospital admission Exclusion criteria: non‐stroke diagnosis (based on admitting or consultant physician evaluation); primary brain lesion as cause of stroke; intubation before completion of ED screening or SLP evaluation; history of dysphagia or modified feeding route; pre‐existing neuromuscular disorder; history of head and neck cancer or radiation; preexisting pneumonia on ED chest radiography; pregnancy Setting: 1 × 850‐bed, tertiary‐care, TJC‐certified, primary stroke centre located in the southeast | ||
| Index tests | ED dysphagia screen (2‐tiered approach) | ||
| Target condition and reference standard(s) | Aspiration SLP dysphagia assessment |
||
| Flow and timing | All patients received the index test(s) and the reference standard. All patients were included in the 2 × 2 table Interventions between index/reference tests: not reported Interval between tests ≤ 24 hours | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | High risk | ||
| Are there concerns that the included patients and setting do not match the review question? | High | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Warnecke 2008.
| Study characteristics | |||
| Patient Sampling | Consecutive | ||
| Patient characteristics and setting | Inclusion criteria: first‐ever stroke; admitted within 24 hours of onset of symptoms; patients eligible for advanced dysphagia assessment as defined by NIHSS score ≥ 3 points and/or who had to present with facial palsy and/or dysarthria Exclusion criteria: patients with history of a pre‐existing dysphagia or disease probably causing dysphagia; severely reduced state of consciousness (i.e. stupor or coma) Setting: not recorded | ||
| Index tests | 2‐step Swallowing Provocation Test | ||
| Target condition and reference standard(s) | Aspiration FEES |
||
| Flow and timing | All patients received the index test(s) and the reference standard. All patients were included in the 2 × 2 table Interventions between index/reference tests: not reported Interval between tests ≤ 24 hours, reported as immediately following | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Low risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Low concern | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Could the conduct or interpretation of the index test have introduced bias? | Low risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Low concern | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Could the patient flow have introduced bias? | Low risk | ||
Zhou 2011.
| Study characteristics | |||
| Patient Sampling | Unclear | ||
| Patient characteristics and setting | Inclusion criteria: patients suffering a first‐ever hemispheric stroke; symptoms lasting at least 24 hours; objective lesions on cerebral imaging (CT and/or MRI) confirmed Exclusion criteria: patients with subarachnoid haemorrhage, subtentorial stroke or TIA, patients with sufficient loss of consciousness to prevent oral feeding; patients with history of otolaryngological cancer; < 18 years of age; those who did not expressly agree to participate in the study 1 × Neurology Department at university hospital. 1 × Department of Physical Medicine and Readaptation |
||
| Index tests | Clinical Predicative Scale of Aspiration; Practical Aspiration Screening Scheme | ||
| Target condition and reference standard(s) | Reference standard: videofluoroscopic examination | ||
| Flow and timing | |||
| Comparative | |||
| Notes | This paper is classified as narrative | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Was a two‐gate design avoided? | Yes | ||
| Could the selection of patients have introduced bias? | Unclear risk | ||
| Are there concerns that the included patients and setting do not match the review question? | Unclear | ||
| DOMAIN 2: Index Test (All tests) | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Could the conduct or interpretation of the index test have introduced bias? | Unclear risk | ||
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Unclear risk | ||
| Are there concerns that the target condition as defined by the reference standard does not match the question? | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Could the patient flow have introduced bias? | Unclear risk | ||
AIS: acute ischaemic stroke. ASDS: Acute Stroke Dysphagia Screening. BJH‐SDS: Barnes‐Jewish Hospital‐Stroke Dysphagia Screen. CT: computed tomography. ED: emergency department. EHMBAS: Edith‐Huhn‐Matesic Bedside Aspiration Screen. FEES: fibreoptic endoscopic evaluation of swallowing. GUSS: Gugging Swallowing Screen. MASA: Mann Assessment of Swallowing Ability. MRI: magnetic resonance imaging. NBDS: Nursing Bedside Dysphagia Screen. NIHSS: National Institutes of Health Stroke Scale. NPO: nil by mouth. RAS3: Rapid Aspiration Screening for Suspected Stroke. SLP: speech and language pathologist. SLT: speech and language therapist. TIA: transient ischaemic attack. TOR‐BSST: Toronto Bedside Swallowing Screening Test. VF: videofluoroscopy. VFSS: videofluoroscopic swallowing study. WST: water‐swallowing test.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Addington 1999 Study 1 | No acute stroke |
| Addington 1999 Study 2 | No acute stroke |
| Alsibai 2014 | No outcome of interest |
| Anderson 2016 | Does not compare tests |
| Archer 2015 Study 1 | No index or reference test of interest |
| Archer 2015 Study 2 | No index or reference test of interest |
| Armesto 2015 | No outcome of interest |
| Aviv 1997 | No outcome of interest |
| Aydogdu 2015 | Not limited to stroke patients |
| Babi 2014 | Does not compare tests |
| Bahia 2014 | Does not compare tests |
| Bahia 2016 | Not an acute setting |
| Bax 2013 | Does not compare tests |
| Bax 2014 | No index or reference test of interest |
| Benjamin 2015 | Not a single‐gate or 2‐gate study |
| Burks 2011 | No screening for dysphagia |
| Carnaby 2014 | No index or reference test of interest |
| Carrington 2013 | Does not compare tests |
| Chavarria 2015 | No screening for dysphagia |
| Choi 2010 | No index or reference test of interest |
| Chong 2003 | Not an acute setting |
| Cichero 2009 | Not limited to stroke patients |
| Clayton 2006 | No index or reference test of interest |
| Collins 1997 | Not an acute setting |
| Crary 2013 Study 1 | No index or reference test of interest |
| Crary 2013 Study 2 | No index or reference test of interest |
| Crary 2014 Study 1 | No index or reference test of interest |
| Crary 2014 Study 2 | No index or reference test of interest |
| Daniels 2009 | Does not compare tests |
| Davis‐De Geus 2009 | No outcome of interest |
| DePippo 1992 | Not an acute setting |
| Farneti 2015 | Index test undertaken by experts |
| Giraldo‐Cadavid 2015 | No outcome of interest |
| Guerra 2011 | Does not compare tests |
| Guillén‐Solà 2011 | No screening for dysphagia |
| Guillén‐Solà 2013 | Not an acute setting |
| Gurcay 2018 | Sample not restricted to acute stages of stroke |
| Higo 2003 | No index or reference test of interest |
| Joundi 2017 | Not a single‐gate or 2‐gate study |
| Karaca 2018 | Reference test MASA considered an SLT dysphagia assessment |
| Logemann 1999 | Not limited to stroke patients |
| Mandysova 2015 | Not limited to stroke patients |
| Marques 2008 | Does not compare tests |
| Martino 2009 Study 2 | No index or reference test of interest |
| Massey 2002 | Not all patients received the same reference test |
| McCullough 2001 | SLT dysphagia assessment, not screening |
| McCullough 2005 | No acute stroke |
| Moalli 2016 | Does not compare tests |
| Moon 2010 | No consecutive stroke patients |
| Mozzanica 2017 | Heterogeneous population. Stroke not analysed separately |
| Murray 2011 | No outcome of interest |
| NCT00306501 | No accuracy of screening |
| NCT00580138 | No index or reference test of interest |
| NCT01765673 | No accuracy of screening |
| NCT02080988 | No outcome of interest |
| NCT02848664 | Not limited to stroke patients |
| NCT03167892 | No accuracy of screening |
| Nomura 2014 | Does not compare tests |
| Oh 2016 | All patients had dysphagia or aspiration; therefore sensitivity/specificity cannot be calculated |
| Ohira 2013 | Not limited to stroke patients |
| Ohira 2017 | Not an acute setting |
| Osawa 2013 | No consecutive stroke patients |
| Ostrofsky 2016 | Index tests carried out by SLT/SLP |
| Palli 2017 | No reference test performed on same patients who had nurse swallow screen – the comparison was with case note data from historic SLT assessment |
| Pennsylvania Patient Safety Authority 2009 | Not limited to stroke patients |
| Perry 2000 | Does not compare tests |
| Perry 2001 Study 2 | Does not compare tests |
| Radhakrishnan 2013 | No screening for dysphagia |
| Rosenbek 2004 | SLT dysphagia assessment, not screening |
| Sato 2012 | Not an acute setting |
| Schrock 2011 | No index or reference test of interest |
| Sellars 1998 | All patients had dysphagia or aspiration; therefore sensitivity/specificity cannot be calculated |
| Smith Hammond 2009 | Index tests carried out by SLT/SLP |
| Sohn 2018 | Reference test is a retrospective evaluation of case records |
| Soria 2013 | Not limited to stroke patients |
| Stroke and Chewing 2012 | No outcome of interest |
| Sung 2018 | Does not compare tests |
| Sørensen 2013 | Does not compare tests |
| Tanuma 2002 | Not screening for dysphagia |
| Teramoto 2000 | Not an acute setting |
| Toscano 2019 | Index test undertaken by experts |
| Tuncay 2011 | Not index or reference test of interest |
| Umay 2013 | No acute stroke |
| Virvidaki 2019 | Index test undertaken by experts |
| Warnecke 2017 | Index tests carried out by SLT/SLP |
| Weinhardt 2008 | Does not compare tests |
| Ye 2018 | No reference test was included |
| Yeh 2011 | Does not compare tests |
| Zhang 2004 | All patients had dysphagia or aspiration; therefore sensitivity/specificity cannot be calculated |
MASA: Mann Assessment of Swallowing Ability. SLP: speech and language pathologist. SLT: speech and language therapist.
Differences between protocol and review
We did not perform meta‐analyses, as the number of studies for each individual index test was insufficient, that is, did not include at least four per group. We have presented only descriptive analyses grouped by general categories. Hence we performed no analysis of the influence of sources of heterogeneity, no subgroup analysis, and no sensitivity analysis. None of the included studies used scintigraphy as the reference test. We included studies in which the index test was carried out by any healthcare professionals with the exception of speech and language therapists (SLTs).
Contributions of authors
EB, JB, and LH drafted the review and developed the background section with support from CW. PD, AC, MH, and CW, who also provided advice regarding the methodological perspective and content. EB, JB, LH, PD, LL, ML, HR, EM, AA, and CW provided support for data collection and analysis, and MH provided statistical expertise. All review authors commented on all sections of the review and reviewed the final version prior to submission.
Sources of support
Internal sources
No sources of support provided
External sources
No sources of support provided
Declarations of interest
Elizabeth Boaden: none known. Jane Burnell: none known. Lucy Hives: none known. Paola Dey: "my institution has received funding for consultancies and grants in aid from NHS, government and research charities. I have also been in receipt of a travel bursary from the organisers of the United European Gastroenterology Week in Berlin in 2006" Andrew Clegg: none known. Mary W Lyons: none known. C Elizabeth Lightbody: none known. Margaret A Hurley: none known. Hazel Roddam: none known. Elizabeth McInnes: none known. Anne Alexandrov: none known. Caroline L Watkins: none known.
New
References
References to studies included in this review
Antonios 2010 {published data only}
- Antonios N, Carnaby-Mann G, Crary M, Miller L, Hubbard H, Hood K, et al. Analysis of a physician tool for evaluating dysphagia on an inpatient stroke unit: the Modified Mann Assessment of Swallowing Ability. Journal of Stroke and Cerebrovascular Diseases 2010;19(1):49-57. [DOI] [PubMed] [Google Scholar]
Behera 2018 {published data only}
- Behera A, Read D, Jackson N, Saour B, Alshekhlee D, Mosier AK. A validated swallow screener for dysphagia and aspiration in patients with stroke. Journal of Stroke and Cerebrovascular Diseases 2018;27(7):1897-904. [DOI: 10.1016/j.jstrokecerebrovasdis.2018.02.038] [DOI] [PubMed] [Google Scholar]
Bravata 2009 {published data only}
- Bravata DM, Daggett VS, Woodward-Hagg H, Damush T, Plue L, Russell S, et al. Comparison of two approaches to screen for dysphagia among acute ischemic stroke patients: nursing admission screening tool versus National Institutes of Health Stroke Scale. Journal of Rehabilitation Research and Development 2009;46(9):1127-33. [DOI] [PubMed] [Google Scholar]
Campbell 2016 {published data only}
- Campbell GB, Carter T, Kring D, Martinez C. Nursing bedside dysphagia screen: is it valid? Journal of Neuroscience Nursing 2016;48(2):75-9. [DOI] [PubMed] [Google Scholar]
Cummings 2015 {published data only}
- Cummings J, Soomans D, O'Laughlin J, Snapp V, Jodoin A, Proco H, et al. Sensitivity and specificity of a nurse dysphagia screen in stroke patients. MEDSURG Nursing 2015;24(4):219-63. [PubMed] [Google Scholar]
Daniels 1997 {published data only}
- Daniels SK, McAdam CP, Brailey K, Foundas AL. Clinical assessment of swallowing and prediction of dysphagia severity. American Journal of Speech-Language Pathology 1997;6(4):17-24. [Google Scholar]
Daniels 2016 {published data only}
- Daniels SK, Pathak S, Rosenbek JC, Morgan RO, Anderson JA. Rapid aspiration screening for suspected stroke: part 1: development and validation. Archives of Physical Medicine and Rehabilitation 2016;97(9):1440-8. [DOI] [PubMed] [Google Scholar]
Edmiaston 2010 {published data only}
- Edmiaston J, Connor LT, Loehr L, Nassief A. Validation of a dysphagia screening tool in acute stroke patients. American Journal of Critical Care 2010;19(4):357-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edmiaston 2014 {published data only}
- Edmiaston J, Connor LT, Steger-May K, Ford AL. A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. Journal of Stroke and Cerebrovascular Diseases 2014;23(4):712-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis 2013 {published data only}
- Ellis AL, Hannibal RR. Nursing swallow screens: why is testing water only not enough? Journal of Neuroscience Nursing 2013;45(5):244-53. [DOI] [PubMed] [Google Scholar]
Eren 2019 {published data only}
- Eren Y, Karaca EU, Saylam G, Yaylaci A, Alicura S, Çomoğlu SS. Validity and reliability of the Turkish version of the Barnes-Jewish Hospital stroke dysphagia screen test in patients with acute stroke. Neurological Sciences and Neurophysiology 2019;36(2):78-83. [DOI: 10.5152/NSN.2019.10815] [DOI] [Google Scholar]
Huhmann 2004 {published data only}
- Huhmann M, Decker RT, Byham-Gray L, Maillet JO, VonHagen S. Comparison of dysphagia screening by a registered dietitian in acute stroke patients to speech language pathologist's evaluation. Topics in Clinical Nutrition 2004;19(3):239-49. [Google Scholar]
Huhn‐Matesic 2015 {published data only}
- Huhn-Matesic EK. Developing and testing a bedside aspiration screen to protect stroke patients from aspiration and mortality. Clinical Scholars Review 2015;8(1):117-24. [DOI: 10.1891/1939-2095.8.1.117] [DOI] [Google Scholar]
Jiang 2019 {published data only}
- Jiang J, Yu J, Wang J, Wang Y, Wang W. Evaluation of the Chinese version of the swallowing screen in stroke patients with dysphagia. Tzu Chi Medical Journal 2019;31(4):270-5. [DOI: 10.4103/tcmj.tcmj_158_18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2001 {published data only}
- Lim SHB, Lieu PK, Phua SY, Seshadri R, Venketasubramanian N, Lee SH, et al. Accuracy of bedside clinical methods compared with fiberoptic endoscopic examination of swallowing (FEES) in determining the risk of aspiration in acute stroke patients. Dysphagia 2001;16(1):1-6. [DOI] [PubMed] [Google Scholar]
Martino 2009 Study 1 {published data only}
- Martino R, Silver F, Teasell R, Bayley M, Nicholson G, Streiner DL, et al. The Toronto Bedside Swallowing Screening Test (TOR-BSST) development and validation of a dysphagia screening tool for patients with stroke. Stroke 2009;40(2):555-61. [DOI] [PubMed] [Google Scholar]
Martino 2014 {published data only}
- Martino R, Maki E, Diamant N. Identification of dysphagia using the Toronto Bedside Swallowing Screening Test (TOR-BSST®): are 10 teaspoons of water necessary? International Journal of Speech-Language Pathology 2014;16(3):193-8. [DOI] [PubMed] [Google Scholar]
Mulheren 2019 {published data only}
- Mulheren RW, González-Fernández M. Swallow screen associated with airway protection and dysphagia after acute stroke. Archives of Physical Medicine and Rehabilitation 2019;100(7):1289-93. [DOI: 10.1016/j.apmr.2018.12.032] [DOI] [PubMed] [Google Scholar]
Nishiwaki 2005 {published data only}
- Nishiwaki K, Tsuji T, Liu M, Hase K, Tanaka N, Fujiwara T. Identification of a simple screening tool for dysphagia in patients with stroke using factor analysis of multiple dysphagia. Journal of Rehabilitation Medicine 2005;37:247-51. [DOI] [PubMed] [Google Scholar]
Perry 2001 Study 1 {published data only}
- Perry L. Screening swallowing function of patients with acute stroke. Part one: identification, implementation and initial evaluation of a screening tool for use by nurses. Journal of Clinical Nursing 2001;10(4):463-73. [DOI] [PubMed] [Google Scholar]
Smith 2000 {published data only}
- Smith HA, Lee SH, O'Neill PA, Connolly MJ. The combination of bedside swallowing assessment and oxygen saturation monitoring of swallowing in acute stroke: a safe and humane screening tool. Age and Ageing 2000;29(6):495-9. [DOI] [PubMed] [Google Scholar]
Trapl 2007 {published data only}
- Trapl M, Enderle P, Nowotny M, Teuschl Y, Matz K, Dachenhausen A, et al. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke 2007;38(11):2948-52. [DOI] [PubMed] [Google Scholar]
Turner‐Lawrence 2009 {published data only}
- Turner-Lawrence DE, Peebles M, Price MF, Singh SJ, Asimos AW. A feasibility study of the Sensitivity of Emergency Physician Dysphagia Screening in acute stroke patients. Annals of Emergency Medicine 2009;54(3):344-8.e1. [DOI] [PubMed] [Google Scholar]
Warnecke 2008 {published data only}
- Warnecke T, Teismann I, Meimann W, Olenberg S, Zimmermann J, Kramer C, et al. Assessment of aspiration risk in acute ischaemic stroke - evaluation of the simple swallowing provocation test. Journal of Neurology, Neurosurgery and Psychiatry 2008;79(3):312-4. [DOI] [PubMed] [Google Scholar]
Zhou 2011 {published data only}
- Zhou Z, Salle JY, Daviet JC, Stuit A, Nguyen CL. Combined approach in bedside assessment of aspiration risk post stroke: PASS. European Journal of Physical and Rehabilitation Medicine 2011;47(3):441-6. [PubMed] [Google Scholar]
References to studies excluded from this review
Addington 1999 Study 1 {published data only}
- Addington WR, Stephens RE, Gilliland K, Rodriguez M. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke. Archives of Physical Medicine and Rehabilitation 1999;80(2):150-4. [DOI] [PubMed] [Google Scholar]
Addington 1999 Study 2 {published data only}
- Addington WR, Stephens RE, Gilliland KA. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke: an interhospital comparison. Stroke 1999;30(6):1203-7. [DOI] [PubMed] [Google Scholar]
Alsibai 2014 {published data only}
- Alsibai A, Voytas J. Use of electrical stimulation in treatment of dysphagia, a nursing home experience. Journal of the American Geriatrics Society 2014;62:S37. [Google Scholar]
Anderson 2016 {published data only}
- Anderson JA, Pathak S, Rosenbek JC, Morgan RO, Daniels SK. Rapid aspiration screening for suspected stroke: Part 2: initial and sustained nurse accuracy and reliability. Archives of Physical Medicine and Rehabilitation 2016;97(9):1449-55. [DOI] [PubMed] [Google Scholar]
Archer 2015 Study 1 {published data only}
- Archer S, Smith C, Newham D. Surface electromyography biofeedback in dysphagia therapy: do patients think it helps? International Journal of Stroke 2015;10:63. [Google Scholar]
Archer 2015 Study 2 {published data only}
- Archer S, Smith C, Newham D. Surface electromyographic biofeedback and the effortful swallow exercise for stroke-related dysphagia. International Journal of Stroke 2015;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armesto 2015 {published data only}
- Armesto A, Howald R, Roseman E, Kitchen-Clark T, Convery C, Boyle K, et al. Swallowing screening in the emergency department: a collaborative effort to improve patient care. International Journal of Stroke 2015;10:59-60. [Google Scholar]
Aviv 1997 {published data only}
- Aviv JE, Sacco RL, Mohr JP, Thompson JLP, Levin B, Sunshine S, et al. Laryngopharyngeal sensory testing with modified barium swallow as predictors of aspiration pneumonia after stroke. Laryngoscope 1997;107(9):1254-60. [DOI] [PubMed] [Google Scholar]
Aydogdu 2015 {published data only}
- Aydogdu I, Kiylioglu N, Tarlaci S, Tanriverdi Z, Alpaydin S, Acarer A, et al. Diagnostic value of "dysphagia limit" for neurogenic dysphagia: 17 years of experience in 1278 adults. Clinical Neurophysiology 2015;126(3):634-43. [DOI] [PubMed] [Google Scholar]
Babi 2014 {published data only}
- Babi MA, Gorman M, Commichau C, Kenney S, Boyle L, Comeau M, et al. Failed bedside dysphagia screen predicts pneumonia treatment in acute stroke: a resident quality initiative project in a JC-certified primary stroke center. Neurology 2014;82(10 Suppl 1).
Bahia 2014 {published data only}
- Bahia MM, Mourao LF, Lima FO, Li LM. Clinical predictors of oropharyngeal dysphagia following acute stroke. Dysphagia 2014;29(6):774. [Google Scholar]
Bahia 2016 {published data only}
- Bahia MM, Mourao LF, Chun RYS. Dysarthria as a predictor of dysphagia following stroke. NeuroRehabilitation 2016;38(2):155-62. [DOI] [PubMed] [Google Scholar]
Bax 2013 {published data only}
- Bax L, McFarlane M, Green E, Miles A. Introducing a speech and language therapy (SLT) led fibre optic endoscopic evaluation of swallowing (FEES): functional outcomes for acute stroke patients. International Journal of Stroke 2013;8:40. [Google Scholar]
Bax 2014 {published data only}
- Bax L, McFarlane M, Green E, Miles A. Speech-language pathologist-led fiberoptic endoscopic evaluation of swallowing: functional outcomes for patients after stroke. Journal of Stroke and Cerebrovascular Diseases 2014;23(3):e195-e200. [DOI] [PubMed] [Google Scholar]
Benjamin 2015 {published data only}
- Bax L, McFarlane M, Green E, Miles A. Introducing a speech and language therapy (SLT) led fibre optic endoscopic evaluation of swallowing (FEES): functional outcomes for acute stroke patients. International Journal of Stroke 2013;8:40. [Google Scholar]
Burks 2011 {published data only}
- Burks C, Meyer DM. Validation of the TOR-BSST swallow screening tool. Stroke 2011;42(3):e129-e130. [Google Scholar]
Carnaby 2014 {published data only}
- Carnaby GD. Relationships among videofluoroscopic scales and stroke outcome. Dysphagia 2014;29(6):751. [Google Scholar]
Carrington 2013 {published data only}
- Carrington S, Archer S, Smith C. Evaluating the impact of fibreoptic endoscopic (FEES) evaluation of swallowing with dysphagic patients on a stroke unit. International Journal of Stroke 2013;8:27. [Google Scholar]
Chavarria 2015 {published data only}
- Chavarria M, Arauz A, Barboza M, Patino M, Becerril M. Scale for severe dysphagia in lateral medullary stroke. Journal of the Neurological Sciences 2015;357:e108. [Google Scholar]
Choi 2010 {published data only}
- Choi MG, Sung HY, Kim JS, Cho YK, Park JM, Lee IS, et al. Usefulness of dysphagia screening protocol including high resolution manometry in acute cerebrovascular accident. Gastroenterology 2010;138(5 Suppl 1):S346. [Google Scholar]
Chong 2003 {published data only}
- Chong MS, Lieu PK, Sitoh YY, Meng YY, Leow LP. Bedside clinical methods useful as screening test for aspiration in elderly patients with recent and previous strokes. Annals of the Academy of Medicine Singapore 2003;32(6):790-4. [PubMed] [Google Scholar]
Cichero 2009 {published data only}
- Cichero JA, Heaton S, Bassett L. Triaging dysphagia: nurse screening for dysphagia in an acute hospital. Journal of Clinical Nursing 2009;18(11):1649-59. [DOI] [PubMed] [Google Scholar]
Clayton 2006 {published data only}
- Clayton J, Jack CIA, Ryall C, Tran J, Hilal E, Gosney M. Tracheal pH monitoring and aspiration in acute stroke. Age and Ageing 2006;35(1):47-53. [DOI] [PubMed] [Google Scholar]
Collins 1997 {published data only}
- Collins MJ, Bakheit AMO. Does pulse oximetry reliably detect aspiration in dysphagic stroke patients? Stroke 1997;28(9):1773-5. [DOI] [PubMed] [Google Scholar]
Crary 2013 Study 1 {published data only}
- Crary M, Carnaby G, Sura L, Sia I, Rolon S, Levien E, et al. Spontaneous swallow frequency identifies dysphagia in acute stroke. Dysphagia 2013;28(4):612. [Google Scholar]
Crary 2013 Study 2 {published data only}
- Crary M, Carnaby-Mann G, Sura L, Sia I. Spontaneous swallow frequency as a screening protocol for dysphagia in acute stroke. Neurology 2013;80(1 Meeting Abstracts).
Crary 2014 Study 1 {published data only}
- Crary M, Carnaby G. Multidisciplinary clinical rehabilitation spontaneous swallow frequency and dysphagia/stroke related outcomes in acute stroke. International Journal of Stroke 2014;9:220. [Google Scholar]
Crary 2014 Study 2 {published data only}
- Crary MA, Carnaby GD, Sia I. Spontaneous swallow frequency compared with clinical screening in the identification of dysphagia in acute stroke. Journal of Stroke and Cerebrovascular Diseases 2014;23(8):2047-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Daniels 2009 {published data only}
- Daniels SK, Schroeder MF, DeGeorge PC, Corey DM, Foundas AL, Rosenbek JC. Defining and measuring dysphagia following stroke. American Journal of Speech-Language Pathology 2009;18(1):74-81. [DOI] [PubMed] [Google Scholar]
Davis‐De Geus 2009 {published data only}
- Davis-De Geus M. Using an evidence-based bedside swallow screen for all stroke patients: how health care providers can improve stroke patients' outcomes. Dysphagia 2009;24(4):463. [Google Scholar]
DePippo 1992 {published data only}
- DePippo KL, Holas MA, Reding MJ. Validation of the 3-oz water swallow test for aspiration following stroke. Archives of Neurology 1992;49(12):1259-61. [DOI] [PubMed] [Google Scholar]
Farneti 2015 {published data only}
- Farneti D, Prencipe RS, Montano L, Nicolini L, Genovese E. Reliability of screening and bedside procedures in the management of acute stroke patients in a community hospital. Dysphagia 2015;30(5):626. [Google Scholar]
Giraldo‐Cadavid 2015 {published data only}
- Giraldo-Cadavid LF, Burguete J, Rueda F, Galvis AM, Castaneda N, Arbulu M, et al. Accuracy of the laryngeal adductor reflex explored by a new laryngo-pharyngeal esthesiometer during the fiberoptic endoscopic evaluation of swallowing. American Journal of Respiratory and Critical Care Medicine 2015;191:A2123. [Google Scholar]
Guerra 2011 {published data only}
- Guerra L, Ottaviani C, Grazia Scucchi M, Amendola L, Macchiaolo S, Capecchi D, et al. Assessment of dysphagia in acute stroke: analysis of degree of agreement between nurses. L'Infermiere 2011;48(2):e9-13. [Google Scholar]
Guillén‐Solà 2011 {published data only}
- Guillén-Solà A, Martínez-Orfila J, Gómez RB, Castelló SM, Marco E. Screening of swallowing disorders in stroke: utility of clinical signs and clinical examination method of volume-viscosity in comparison with videofluoroscopy. Rehabilitacion 2011;45(4):293-300. [Google Scholar]
Guillén‐Solà 2013 {published data only}
- Guillen-Sola A, Marco E, Martinez-Orfila J, Donaire Mejias MF, Depolo Passalacqua M, Duarte E, et al. Usefulness of the volume-viscosity swallow test for screening dysphagia in subacute stroke patients in rehabilitation income. NeuroRehabilitation 2013;33(4):631-8. [DOI] [PubMed] [Google Scholar]
Gurcay 2018 {published data only}
- Gurcay E, Bahceci K, Ozturk E, Yilmaz V, Gundogdu I, Ceylan T, et al. Validity and reliability of Turkish version of the gugging swallowing screen test in the early period of hemispheric stroke. Neurological Sciences and Neurophysiology 2018;35(1):6-13. [Google Scholar]
Higo 2003 {published data only}
- Higo R, Tayama N, Watanabe T, Nito T. Pulse oximetry monitoring for the evaluation of swallowing function. European Archives of Oto-Rhino-Laryngology 2003;260(3):124-7. [DOI] [PubMed] [Google Scholar]
Joundi 2017 {published data only}
- Joundi RA, Martino R, Saposnik G, Giannakeas V, Jiming F, Kapral MK, et al. Predictors and outcomes of dysphagia screening after acute ischemic stroke. Stroke 2017;48(4):900-6. [DOI] [PubMed] [Google Scholar]
Karaca 2018 {published data only}
- Karaca Umay E, Gundogdu I, Gurcay E, Ozturk E, Yilmaz V, Karaahmet O, et al. The psychometric evaluation of the Turkish version of the Mann assessment of swallowing ability in patients in the early period after stroke. Turkish Journal of Medical Sciences 2018;48(6):1153-61. [DOI] [PubMed] [Google Scholar]
Logemann 1999 {published data only}
- Logemann JA, Veis S, Colangelo L. A screening procedure for oropharyngeal dysphagia. Dysphagia 1999;14(1):44-51. [DOI] [PubMed] [Google Scholar]
Mandysova 2015 {published data only}
- Mandysova P, Ehler E, Skvrnakova J, Cerny M, Bartova I, Pellant A. Development of the Brief Bedside Dysphagia Screening Test - Revised: a cross-sectional Czech study. Acta Medica (Hradec Kralove) 2015;58:49-55. [DOI] [PubMed] [Google Scholar]
Marques 2008 {published data only}
- Marques CHD, De Rosso ALZ, Andre C. Bedside assessment of swallowing in stroke: water tests are not enough. Topics in Stroke Rehabilitation 2008;15(4):378-83. [DOI] [PubMed] [Google Scholar]
Martino 2009 Study 2 {published data only}
- Martino R, Streiner D, Maki E, Diamant N. A sensitivity analysis to determine whether ten teaspoons of water are really necessary. Dysphagia 2009;24(4):473. [Google Scholar]
Massey 2002 {published data only}
- Massey R, Jedlicka D. The Massey Bedside Swallowing Screen. Journal of Neuroscience Nursing 2002;34(5):252-60. [DOI] [PubMed] [Google Scholar]
McCullough 2001 {published data only}
- McCullough GH, Wertz RT, Rosenbek JC. Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. Journal of Communication Disorders 2001;34(1-2):55-72. [DOI] [PubMed] [Google Scholar]
McCullough 2005 {published data only}
- McCullough GH, Rosenbek JC, Wertz RT, McCoy S, Mann G, McCullough K. Utility of clinical swallowing examination measures for detecting aspiration post-stroke. Journal of Speech, Language, and Hearing Research 2005;48(6):1280-93. [DOI] [PubMed] [Google Scholar]
Moalli 2016 {published data only}
- Moalli D, Dell'Angelo D, Bedard L. CVAS: a rapid dysphagia evaluation tool. Connecticut Medicine 2016;80(4):205-7. [PubMed] [Google Scholar]
Moon 2010 {published data only}
- Moon KH, Sohn HS, Lee ES, Paek EK, Kang EJ, Lee SH, et al. Comparison for risk estimate of aspiration between the revised dysphagia assessment tool and videofluoroscopy in post-stroke patients. Journal of Korean Academy of Nursing 2010;40(3):359-66. [DOI] [PubMed] [Google Scholar]
Mozzanica 2017 {published data only}
- Mozzanica F, Scarponi L, Pedrali S, Pizzorni N, Pinotti C, Foieni F, et al. Dysphagia screening in subacute care settings using the Italian version of the Royal Brisbane and Women's Hospital (I-RBWH) dysphagia screening tool [Screening della disfagia nelle unita di cure sub-acute utilizzando la versione italiana del Royal Brisbane and Women's Hospital (I-RBWH) dysphagia screening tool]. Acta Otorhinolaryngologica Italica 2017;37(1):25-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2011 {published data only}
- Murray J, Milich A, Ormerod D. Screening for dysphagia. Australian Nursing Journal 2011;18(11):44-6. [PubMed] [Google Scholar]
NCT00306501 {published data only}
- NCT00306501. Volitional swallowing in stroke patients with chronic dysphagia. https://clinicaltrials.gov/ct2/show/record/NCT00306501 (first received 23 March 2006).
NCT00580138 {published data only}
- NCT00580138. Real-time Internet evaluation of swallowing. https://clinicaltrials.gov/ct2/show/NCT00580138 (first received 24 December 2007).
NCT01765673 {published data only}
- NCT01765673. The Passy Muir Swallowing Self Training Device (PMSST). https://clinicaltrials.gov/ct2/show/NCT01765673 (first received 10 January 2013).
NCT02080988 {published data only}
- NCT02080988. Reflexive coughing force in severe aspirators. https://clinicaltrials.gov/ct2/show/NCT02080988 (first received 7 March 2014).
NCT02848664 {published data only}
- NCT02848664. Use of the Passy Muir Swallowing Self Trainer. https://clinicaltrials.gov/ct2/show/NCT02848664 (first received 28 July 2016).
NCT03167892 {published data only}
- NCT03167892. Oral Screens in Post Stroke Training. https://clinicaltrials.gov/ct2/show/NCT03167892 (first received 30 May 2017).
Nomura 2014 {published data only}
- Nomura JT, Poole CL, Boyer BW, Papas MA, Thomas J, Gregore DR, et al. Customized swallow screening tool to exclude aspiration pneumonia risk in acute stroke patients. Academic Emergency Medicine 2014;21(5 Suppl 1):S257. [Google Scholar]
Oh 2016 {published data only}
- Oh JC, Park JH, Jung MY, Yoo EY, Chang KY, Lee TY. Relationship between quantified instrumental swallowing examination and comprehensive clinical swallowing examination. Occupational Therapy International 2016;23(1):3-10. [DOI] [PubMed] [Google Scholar]
Ohira 2013 {published data only}
- Ohira M, Ishida R, Maki Y, Okubo M, Sugiyama T, Sakayori T, et al. Evaluation of swallowing ability using Mann assessment of swallowing ability (MASA) of dependent elderly in Japan. Dysphagia 2013;28(4):635. [DOI] [PubMed] [Google Scholar]
Ohira 2017 {published data only}
- Ohira M, Ishida R, Maki Y, Ohkubo M, Sugiyama T, Sakayori T, Sato T. Evaluation of a dysphagia screening system based on the Mann Assessment of Swallowing Ability for use in dependent older adults. Geriatrics & Gerontology International 2017;17(4):561-7. [DOI] [PubMed] [Google Scholar]
Osawa 2013 {published data only}
- Osawa A, Maeshima S, Tanahashi N. Water-swallowing test: screening for aspiration in stroke patients. Cerebrovascular Diseases 2013;35(3):276-81. [DOI] [PubMed] [Google Scholar]
Ostrofsky 2016 {published data only}
- Ostrofsky C, Seedat J. The South African dysphagia screening tool (SADS): a screening tool for a developing context. South African Journal of Communication Disorders 2016;63(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palli 2017 {published data only}
- Palli C, Fandler S, Doppelhofer K, Niederkorn K, Enzinger C, Vetta C, et al. Early dysphagia screening by trained nurses reduces pneumonia rate in stroke patients: a clinical intervention study. Stroke 2017;48(9):2583-5. [DOI] [PubMed] [Google Scholar]
Pennsylvania Patient Safety Authority 2009 {published data only}
- Pennsylvania Patient Safety Authority. Does your admission screening adequately predict aspiration risk? Pennsylvania Patient Safety Authority 2009;6(4):115-21. [http://patientsafety.pa.gov/ADVISORIES/Pages/200912_115.aspx] [Google Scholar]
Perry 2000 {published data only}
- Perry L, McLaren SM. Dysphagia screening, assessment and outcomes in stroke patients: Phase 1 of an evidence-based practice project. Journal of Clinical Excellence 2000;1(4):201-8. [Google Scholar]
Perry 2001 Study 2 {published data only}
- Perry L. Screening swallowing function of patients with acute stroke. Part two: detailed evaluation of the tool used by nurses. Journal of Clinical Nursing 2001;10(4):474-81. [DOI] [PubMed] [Google Scholar]
Radhakrishnan 2013 {published data only}
- Radhakrishnan S, Menon UK, Anandakuttan A. A combined approach of bedside clinical examination and flexible endoscopic evaluation of swallowing in poststroke dysphagia: a pilot study. Annals of Indian Academy of Neurology 2013;16(3):388-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenbek 2004 {published data only}
- Rosenbek JC, McCullough GH, Wertz RT. Is the information about a test important? Applying the methods of evidence-based medicine to the clinical examination of swallowing. Journal of Communication Disorders 2004;37(5):437-50. [DOI] [PubMed] [Google Scholar]
Sato 2012 {published data only}
- Sato M, Tohara H, Iida T, Wada S, Inoue M, Ueda K. Simplified cough test for screening silent aspiration. Archives of Physical Medicine and Rehabilitation 2012;93(11):1982-6. [DOI] [PubMed] [Google Scholar]
Schrock 2011 {published data only}
- Schrock JW, Bernstein J, Glasenapp M, Drogell K, Hanna J. A novel emergency department dysphagia screen for patients presenting with acute stroke. Academic Emergency Medicine 2011;18(6):584-9. [DOI] [PubMed] [Google Scholar]
Sellars 1998 {published data only}
- Sellars C, Dunnet C, Carter R. A preliminary comparison of videofluoroscopy of swallow and pulse oximetry in the identification of aspiration in dysphagic patients. Dysphagia 1998;13(2):82-6. [DOI] [PubMed] [Google Scholar]
Smith Hammond 2009 {published data only}
- Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest 2009;135(3):769-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sohn 2018 {published data only}
- Sohn D, Park G-Y, Koo HJ, Jang YJ, Han Y, Im S. Determining peak cough flow cutoff values to predict aspiration pneumonia among patients with dysphagia using the Citric Acid Reflexive Cough Test. Archives of Physical Medicine & Rehabilitation 2018;99(12):2532-9. [DOI] [PubMed] [Google Scholar]
Soria 2013 {published data only}
- Soria F, Furkim AM, Nunes MC, Pinto G, Lange M, Zetola V. Identification risk of aspiration pneumonia in two groups of patients with stroke. Dysphagia 2013;28(2):325-6. [Google Scholar]
Stroke and Chewing 2012 {published data only}
- Stroke and chewing. Dental Abstracts 2012;57(3):165.
Sung 2018 {published data only}
- Sung HY, Lee KS, Choi MG, Kim JS. Dysphagia screening using high-resolution impedance manometry in acute stroke. International Journal of Gerontology 2018;12(2):110-5. [Google Scholar]
Sørensen 2013 {published data only}
- Sørensen RT, Rasmussen RS, Overgaard K, Lerche A, Johansen AM, Lindhardt T. Dysphagia screening and intensified oral hygiene reduce pneumonia after stroke. Journal of Neuroscience Nursing 2013;45(3):139-46. [DOI] [PubMed] [Google Scholar]
Tanuma 2002 {published data only}
- Tanuma S, Sudo E, Takahashi Y, Yoshida A, Kobayashi C, Ohama Y. The usefulness of the water swallowing test and videofluorography in swallowing rehabilitation in patients with cerebrovascular disease. Nippon Ronen Igakkai Zasshi Japanese Journal of Geriatrics 2002;39(4):427-32. [DOI] [PubMed] [Google Scholar]
Teramoto 2000 {published data only}
- Teramoto S, Fukuchi Y. Detection of aspiration and swallowing disorder in older stroke patients: simple swallowing provocation test versus water swallowing test. Archives of Physical Medicine and Rehabilitation 2000;81(11):1517-9. [DOI] [PubMed] [Google Scholar]
Toscano 2019 {published data only}
- Toscano M, Vigano A, Rea A, Verzina A, Sasso D'Elia T, Puledda F, et al. Sapienza Global Bedside Evaluation of Swallowing after Stroke: the GLOBE-3S study. European Journal of Neurology 2019;26(4):596-602. [DOI] [PubMed] [Google Scholar]
Tuncay 2011 {published data only}
- Tuncay F, Tasbas Ö, Borman P, Geçene M, Coskun Ö. The bedside clinical evaluation of swallowing function of stroke patients in acute stage. Journal of Physical Medicine & Rehabilitation Sciences 2011;14(2):33-8. [Google Scholar]
Umay 2013 {published data only}
- Umay E, Unlu E, Kilic G, Cakci A, Korkmaz H. Evaluation of dysphagia in early period stroke patients by bedside screening test, endoscopic and electrophysiological methods. Dysphagia 2013;28(3):395-403. [DOI] [PubMed] [Google Scholar]
Virvidaki 2019 {published data only}
- Virvidaki IE, Giannopoulos S, Nasios G, Dimakopoulos G, Michou E, Milionis H. Predictive value of a novel pragmatic tool for post-stroke aspiration risk: the Functional Bedside Aspiration Screen. Neurogastroenterology and Motility 2019;31(10):e13683. [DOI] [PubMed] [Google Scholar]
Warnecke 2017 {published data only}
- Warnecke T, Im S, Kaiser C, Hamacher C, Oelenberg S, Dziewas R. Aspiration and dysphagia screening in acute stroke - the Gugging Swallowing Screen revisited. European Journal of Neurology 2017;24(4):594-601. [DOI] [PubMed] [Google Scholar]
Weinhardt 2008 {published data only}
- Weinhardt J, Hazelett S, Barrett D, Lada R, Enos T, Keleman R. Accuracy of a bedside dysphagia screening: a comparison of registered nurses and speech therapists. Rehabilitation Nursing 2008;33(6):247-52. [DOI] [PubMed] [Google Scholar]
Ye 2018 {published data only}
- Ye T, Huang SY, Dong Y, Dong Q. Bedside modified volume-viscosity swallow test might be reliably to predict risk of aspiration pneumonia after stroke. Stroke 2019;50 Suppl 1:AWP479. [Google Scholar]
Yeh 2011 {published data only}
- Yeh SJ, Huang KY, Wang TG, Chen YC, Chen CH, Tang SC, et al. Dysphagia screening decreases pneumonia in acute stroke patients admitted to the stroke intensive care unit. Journal of the Neurological Sciences 2011;306(1-2):38-41. [DOI] [PubMed] [Google Scholar]
Zhang 2004 {published data only}
- Zhang J, Wang YJ, Cui T. Reliability and validity of nine rating scales for dysphagia following stroke. Chinese Journal of Clinical Rehabilitation 2004;8(7):1201-3. [Google Scholar]
Additional references
Almeida 2015
- Almeida TM, Cola PC, Pernambuco LA, Magalhaes HV Jr, Silva RG. Screening tools for oropharyngeal dysphagia in stroke. Audiology - Communication Research 2015;20(4):361-70. [Google Scholar]
Avan 2019
- Avan A, Digaleh H, Di Napoli M, Stranges S, Behrouz R, Shojaeianbabaei G, et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study. BMC Medicine 2019;17(191). [DOI: 10.1186/s12916-019-1397-3] [DOI] [PMC free article] [PubMed]
Benfield 2020
- Benfield JK, Everton LF, Bath PM, England TJ. Accuracy and clinical utility of comprehensive dysphagia screening assessments in acute stroke: a systematic review and meta‐analysis. Journal of Clinical Nursing 2020;29(9-10):1527-38. [DOI: 10.1111/jocn.15192] [DOI] [PubMed] [Google Scholar]
Blitzer 1988
- Blitzer A, Krespi YP, Oppenheimer RW, Levine TM. Surgical management of aspiration. Otolaryngologic Clinics of North America 1988;4:743–50. [PubMed] [Google Scholar]
Boaden 2011
- Boaden EE. Improving the identification and management of aspiration after stroke [Doctoral thesis]. University of Central Lancashire 2011.
Bray 2016
- Bray BD, Smith CJ, Cloud GC, Enderby P, James M, Paley L, et al. The association between delays in screening for and assessing dysphagia after acute stroke, and the risk of stroke-associated pneumonia. Journal of Neurology, Neurosurgery and Psychiatry 2017;88(1):25-30. [DOI] [PubMed] [Google Scholar]
Brodsky 2016
- Brodsky MB, Suiter DM, Gonzalez-Fernandez M, Michtalik HJ, Frymark TB, Venediktov R, et al. Screening accuracy for aspiration using bedside water swallow tests: a systematic review and meta-analysis. Chest 2016;150(1):148-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
CADTH 2018
- CADTH. Grey matters: a practical tool for searching health-related grey literature [Internet]. Available from https://www.cadth.ca/resources/finding-evidence 2018.
Chen 2016
- Chen PC, Chuang CH, Leong CP, Guo SE, Hsin YJ. Systematic review and meta-analysis of the diagnostic accuracy of the water swallow test for screening aspiration in stroke patients. Journal of Advanced Nursing 2016;72(11):2575-86. [DOI: 10.1111/jan.13013] [DOI] [PubMed] [Google Scholar]
Daniels 2012
- Daniels SK, Anderson JA, Willson PC. Valid items for screening dysphagia risk in patients with stroke. Stroke 2012;43(3):892-7. [DOI] [PubMed] [Google Scholar]
Donovan 2013
- Donovan NJ, Daniels SK, Edmiaston J, Weinhardt J, Summers D, Mitchell PH. Dysphagia screening: state of the art: invitational conference proceeding from the State-of-the-Art Nursing Symposium, International Stroke Conference 2012. Stroke 2013;44(4):e24-31. [DOI] [PubMed] [Google Scholar]
European Stroke Organisation 2015
- European Stroke Organisation. The European Stroke Organisation Guidelines: a standard operating procedure. International Journal of Stroke 2015;10(SA100):128-35. [DOI: 10.1111/ijs.12583] [DOI] [PubMed] [Google Scholar]
Gupta 2016
- Gupta D, Hull ML, Fraser I, Miller L, Bossuyt PMM, Johnson N, et al. Endometrial biomarkers for the non-invasive diagnosis ofendometriosis. Cochrane Database of Systematic Reviews 2016, Issue 4. Art. No: CD012165. [DOI: 10.1002/14651858.CD012165] [DOI] [PMC free article] [PubMed] [Google Scholar]
Intercollegiate Stroke Working Party 2012
- Intercollegiate Stroke Working Party. National Sentinel Stroke Clinical Audit 2010. Royal College of Physicians, 2012.
Intercollegiate Stroke Working Party 2016
- Intercollegiate Stroke Working Party. Intercollegiate Stroke Working Party National Clinical Guideline for Stroke. 5th edition. London: Royal College of Physicians, 2016. [Google Scholar]
International Dysphagia Diet Std Initiative 2017
- International Dysphagia Diet Standardisation Initiative. Complete IDDSI Framework Detailed definitions. 2017. https://iddsi.org/framework/. [http://iddsi.org/framework/]
Jiang 2016
- Jiang JL, Fu SY, Wang WH, Ma YC. Validity and reliability of swallowing screening tools used by nurses for dysphagia: a systematic review. Ci Ji Yi Xue Za Zhi 2016;28(2):41-8. [DOI: 10.1016/j.tcmj.2016.04.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2019
- Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Abate D, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology 2019;18(5):439-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katan 2018
- Katan M, Luft A. Global burden of stroke. Seminars in Neurology 2018;38(2):208-11. [DOI] [PubMed] [Google Scholar]
Kumar 2010
- Kumar S, Selim MH, Caplan LR. Medical complications after stroke. Lancet Neurology 2010;9:105-18. [DOI] [PubMed] [Google Scholar]
Martino 2005
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke 2005;36(12):2756-63. [DOI] [PubMed] [Google Scholar]
National Stroke Foundation 2010
- National Stroke Foundation. Clinical guidelines for stroke management, 2010. https://extranet.who.int/ncdccs/Data/AUS_D1_Clinical%20Guidelines%20for%20Stroke%20Management.pdf.
O'Horo 2015
- O’Horo JC, Rogus-Pulia N, Garcia-Arguello L, Robbins J, Safdar N. Bedside diagnosis of dysphagia: a systematic review. Journal of Hospital Medicine 2015;10(4):256-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliveira 2001
- Oliveira I, Mota L, Freitas S, Ferreira P. Dysphagia screening tools for acute stroke patients available for nurses: a systematic review. Nursing Practice Today 2019;6(3):103-15. [DOI: 10.18502/npt.v6i3.1253] [DOI] [Google Scholar]
Powers 2019
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al, on behalf of the American Heart Association Stroke Council. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2019;50:e344–e418. [DOI: 10.1161/STR.0000000000000211] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, the Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014.
Rofes 2011
- Rofes L, Arreola V, Almirall J. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterology Research and Practice 2011;2011:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schepp 2012
- Schepp K, Tirschwell DL, Miller RM, Longstreth WT Jr. Swallowing screens after acute stroke: a systematic review. Stroke 2012;43(3):869-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teasell 2019
- Teasell R, Salbach NM, Acerra N, Bastasi D, Carter SL, Fung J, et al, on behalf of the Rehabilitation and Recovery following Stroke Writing Group. Assessment and Management of Dysphagia and Malnutrition following Stroke. In Lindsay MP, Mountain A, Gubitz G, Dowlatshahi D, Casaubon L, de Jong A, Smith EE (Editors); on behalf of the Canadian Stroke Best Practices Advisory Committee. Canadian Stroke Best Practice Recommendations 2019:71-5.
WHF 2016
- World Heart Federation. The global burden of stroke, 2016. www.world-heart-federation.org/cardiovascular-health/stroke/.
References to other published versions of this review
Boaden 2017
- Boaden E, Doran D, Burnell J, Clegg A, Dey P, Hurley M, et al. Screening for aspiration risk associated with dysphagia in acute stroke. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD012679. [DOI: 10.1002/14651858.CD012679] [DOI] [PMC free article] [PubMed] [Google Scholar]


