Summary
As companion animals, dogs and cats live in close contact with humans, generating the possibility of interspecies pathogen transmission events. Equine origin H3N8 and avian origin H5N1 influenza virus have been reported in dogs and cats respectively since 2004 with outbreaks associated with different strains recorded for both species in Asia and North America. To date, there have been no reports of influenza viruses from companion animals in South America. To fill this gap in knowledge, we performed active epidemiological surveillance in shelters that received abandoned animals, backyard production systems and veterinary clinics between May 2017 and January 2019 to estimate the burden of influenza infection in cats and dogs in the central region of Chile. Blood samples, oropharyngeal swabs or both were collected for influenza A virus detection by RT-qPCR, NP-ELISA, and hemagglutination inhibition assay. Logistic regression models were performed to assess the association between NP-ELISA-positivity and variables including sex and animal origin. The percentage of ELISA-positive samples was 43.5% (95% CI: 37.0 to 50.1) and 23.3% (95% CI: 10.6 to 42.7) for dogs and cats, respectively. No association was found between NP-ELISA results and sex or animal origin for either dogs or cats. Two ELISA positive samples showed hemagglutination inhibition titers against pandemic H1N1 influenza. One dog sample tested positive by RT-qPCR, indicating an overall RT-qPCR positivity in dogs of 1.1% (95% CI: 0.05 to 6.7). None of the tested cat samples were positive by this assay.
Keywords: cats, Chile, dogs, Influenza A, serology, ELISA, RT-qPCR
Introduction:
Influenza A viruses (IAV) can infect a wide range of species, including humans, pigs, horses, seals, birds, cats, and dogs (Kawaoka et al., 1989; Beeler, 2009; Anthony et al., 2012; Yoon et al., 2014). Dogs and cats live in close contact with humans, generating the possibility of zoonotic and reverse-zoonotic transmission events (Chomel, 2014). Initial reports of influenza in dogs came from racing greyhounds infected with equine H3N8 influenza virus in 2004. The disease rapidly spread to companion dogs and has been considered endemic in the United States the late 2000’s (Payungporn et al., 2008). Moreover, an avian origin H3N2 influenza virus affecting dogs in 2007 infected and transmitted between dogs and has since become endemic in China and Korea (Song et al., 2008; Song et al., 2009) and in the US since 2015 (Voorhees et al., 2017). While other subtypes have also been detected in dogs, including H5N1 (Songserm et al., 2006b; Dundon et al., 2010), pandemic H1N1 Influenza (pdmH1N1) (Dundon et al., 2010) and human-canine reassortant H3N2 (Moon et al., 2015), none of these strains have established themselves in dog populations, nor have caused major zoonotic events. While dogs are relatively permissive to different strains of Influenza virus, cats seem less prone to infection. To date, only avian origin H5N1, H5N6, H7N2 and pdmH1N1 have been identified in cats. While pdmH1N1 (Sponseller et al., 2010; Fiorentini et al., 2011) and avian origin H5Nx viral infections (Songserm et al., 2006a; Yu et al., 2015) is sporadic and has not lead to sustained transmission in cats, low pathogenic avian influenza H7N2 was identified as the causative agent of an outbreak of zoonotic influenza in a cat shelter in the US that also infected the treating veterinarian and subsequently spread to other areas of the country (Belser et al., 2017; Hatta et al., 2018). Although avian and equine origin IAV appear to play a major role in the genesis of canine and feline IAV infections, the potential for companion animals to serve as reservoirs of zoonotic infections has not been established to date. This could be due to specific host barrier that preclude natural transmission of human-origin Influenza viruses in dogs (Cauldwell et al., 2014) or could simply be due to a lack of systemic Influenza surveillance in dogs that could detect these inter-species transmission events, similarly to a situation that could be occurring in cats (Borland et al., 2020). While mounting literature highlights infections in dogs and cats in Asia and North America, little information is available in Latin America, where only Mexico and Argentina have reported surveillance results. In Mexico, one study detected antibodies to pdmH1N1 and H3N8 influenza in samples obtained from 113 household dogs by hemagglutination inhibition assay (HAI) but did not detect viral RNA in swabs (Ramírez-Martínez et al., 2013). In Argentina, HAI performed on sera obtained from 161 dogs belonging to both households and shelters did not detect any antibodies against equine H3N8 IAV (Bratanich et al., 2011). Thus, the aim of this study was to identify evidence of IAV infection in dogs and cats through active surveillance in central Chile.
Material and Methods:
Ethics statement:
All sampling activities and animal experiments were approved by the Institutional Animal Care and Use Committee (CICUA) of the University of Chile.
Sampling:
A cross-sectional study was performed using convenience sampling method from dogs and cats at veterinary clinics located in the city of Santiago and animal shelters and small rural backyard production farms (BPS) across the Metropolitan and Valparaíso regions of central Chile between May 2017 and January 2019. Samples were obtained by trained veterinarians and included blood samples, oropharyngeal swabs, or both. Oropharyngeal swabs (OP) were stored in 1 mL universal transport media (Copan, Italy) and transported to the laboratory at 4 °C and stored at −80 °C until analysis. For serological analysis, up to 1 mL (cats) and 5ml (dogs) blood was obtained from the cephalic or jugular vein and centrifuged at 4000g for 15 minutes for sera separation and stored at −20 °C until analysis.
Serology
A competitive Influenza A nucleoprotein ELISA (NP-ELISA) was performed to detect overall anti-NP antibodies in sera (Virusys Corporation, USA) following manufacturer’s instructions. Sensibility and specificity information of the ELISA test were not available from the vendor due to the “for research only” characteristic of the kit. ELISA plates were read at 450 nm using a Sunrise absorbance microplate reader (TECAN, Switzerland). ELISA positive samples were further tested by HAI to screen for the presence of antibodies against the following Influenza A strains: A/California/7/09 (pdmH1N1), A/Indiana/Can/1177-17-1/2017 (avian origin H3N2) and A/canine/Florida /14/2006 (equine origin H3N8). Briefly, 25 μL of each serum sample was treated with a receptor destroying enzyme (RDE) (Denka Seiken, Co., Japan) and then serially diluted 2-fold in 25 μL of PBS in duplicate in a 96-well v-bottom plate. Twenty-five μL of a solution containing 4 hemagglutinin units of each virus were added and the plate was incubated at room temperature for 15 min before adding 50 μL of 0.5% chicken red blood cells diluted in PBS. The plate was stored at 4°C for 30 min before analysis.
Molecular analysis:
To detect the presence of Influenza A in OP swabs, viral RNA was extracted using the MagMax viral RNA isolation kit (Thermofisher, USA), following manufacturer’s instructions. Viral RNA was screened by real time RT-PCR (RT-qPCR) using an Mx3000p Thermocycler (Agilent, USA) with the TaqMan Fast virus Master Mix (Thermofisher, USA) using primers and probes to specifically targeting the M influenza A matrix gene, following WHO guidelines (Control and Prevention, 2009). Samples with a cycle threshold value below 38 were considered positives (Shu et al., 2011).
Data Analysis
A logistic regression model was built for each species to assess the association between NP-ELISA test results as the outcome variable and sex and animal origin (veterinary clinics, animal shelters or BPS) as independent variables. Significance level was set at 5% and InfoStat statistical software was used (Di Rienzo et al., 2011).
Results:
Serologic and molecular analysis:
A total of 282 animals (248 dogs and 34 cats) were screened. One hundred and seven samples were obtained from BPS’s (101 dogs and 6 cats), 126 from animal shelters (111 dogs and 15 cats) and 49 from veterinary clinics (36 dogs and 13 cats). From 282 blood samples collected, 260 samples (230 dogs and 30 cats) yielded sufficient sera for ELISA analysis. Forty-three point five percent (95% CI: 37.0 to 50.1) (n = 100 positive samples) dogs and 23.3% (95% CI: 10.6 to 42.7) (n = 7 positive samples) cats were positive, respectively (Figure 1). No association was found between ELISA-positivity and sex or animal origin for both dogs and cats (Table 1).
Figure 1:
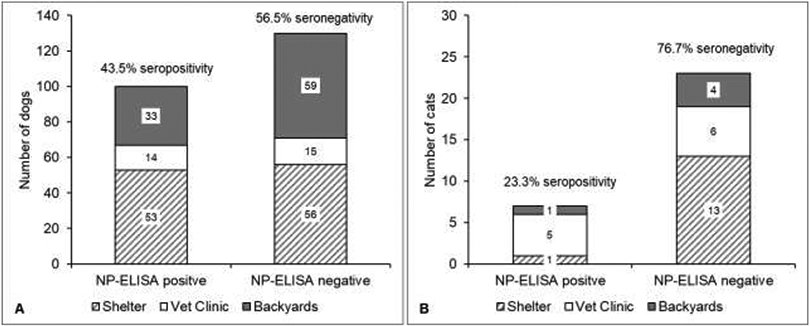
NP-ELISA results for dogs (A) and cats (B) by animal origin. Samples obtained from shelter animals in gray/white parallel lines; samples obtained at veterinary clinics in white and samples obtained at backyard production systems in gray. Overall seropositivity/seronegativity indicated for each specie.
Table 1:
Logistic regression models for the association between NP-ELISA test results and independent variables (sex and animal origin) for dogs and cats.
| Predictor | Estimate | SE | OR | 95% CI | P-value |
|---|---|---|---|---|---|
| Logistic model for dogs | |||||
| Intercept | −0.80 | 0.27 | |||
| Sex (Ref: female) | |||||
| Male | 0.39 | 0.28 | 1.47 | 0.85-2.56 | 0.171 |
| Animal origin (Ref: BPSa) | |||||
| Shelter | 0.48 | 0.29 | 1.61 | 0.91-2.86 | 0.102 |
| Vet Clinic | 0.47 | 0.43 | 1.61 | 0.69-3.75 | 0.274 |
| Logistic model for cats | |||||
| Intercept | −1.04 | 1.18 | |||
| Sex (Ref: female) | |||||
| Male | −1.08 | 1.27 | 0.34 | 0.03-4.10 | 0.394 |
| Animal origin (Ref: BPSa) | |||||
| Shelter | −1.31 | 1.55 | 0.27 | 0.01-5.65 | 0.399 |
| Vet Clinic | 1.74 | 1.49 | 5.72 | 0.31-106.71 | 0.243 |
Backyard production system; SE: Standard Error; OR: Odd Ratio; CI: Confidence interval
None of the NP-ELISA positive sera reacted against the canine H3Nx viruses by HAI; however, sera of a 7-year old male shelter dog and a 1-year old male cat sampled at a veterinary clinic showed 1:20 inhibition titers against pdmH1N1. The HAI test showed that specific influenza antibodies against pdmH1H1 were present in 0.43% (95% CI: 0.02 to 2,8) (1 in 230) and 3.3% (95% CI: 0.2 to 19.1) (1 in 30) of sera obtained from dogs and cats, respectively.
Finally, a total of 131 nasal swabs were collected from dogs (n = 93) and cats (n = 38). One dog sample tested positive by RT-qPCR, with a Ct value of 36.1, indicating an overall RT-qPCR positivity in dogs of 1.1% (95% CI: 0.05 to 6.7). The sample belonged to a 4-year-old backyard farm dog without clinical signs. None of the tested cat samples were positive. Regrettably, attempts to isolate the RT-qPCR positive sample by blind-passaging through embryonated eggs or MDCK cells were unsuccessful, nor could the sample be subtyped.
Discussion:
Our results show that IAV is circulating in dogs and cats in both urban and rural settings in Central Chile and are similar to studies in pet cats in North America in 2009, where the seroprevalence was 21.8% and 25.6% against pdmH1N1 and H3N2 viruses, respectively (McCullers et al., 2011). We were unable to establish any significant association between seropositivity and sex or animal origin. This is similar to other studies showing only circumstantial or no association to possible risk factors associated with seropositive dog samples (Barrell et al., 2010; Ramírez-Martínez et al., 2013). Surprisingly, overall seropositivity for dogs as measured by NP-ELISA was up to two times higher than farmed dogs and eight times higher than pet dogs in a study performed in China in 2011 (Su et al., 2013). The high seropositivity as measured by NP-ELISA coupled with the absence of equally robust HAI results in our study, could therefore be due to strain mismatch in the HAI assay to a not yet identified influenza strain circulating in dogs and cats in Chile.
Ideally, subtype and sequence information would have provided valuable information for both phylogenetic analysis and for the implementation of subtype specific neutralization or ELISA assays. Regrettably, we were not able to grow the PCR positive sample in neither cells nor eggs. This was most certainly due to the high cycle threshold value (36.1) of the RT-qPCR positive sample, thus making subsequent subtyping and sequencing efforts fruitless. Another drawback of this study is the lack of both specificity and sensibility information of the NP-ELISA assay. While this information is available for many commercial ELISA test kits, we are confident of the quality of the assay mainly due to years of successful quality assurance and control inspections at our laboratory using this kit as well as peer-reviewed publications that back the use of this assay (Karlsson et al., 2012; Richt et al., 2012; Ahmed et al., 2015; Jang et al., 2018).
Our results indicate that past infections in dogs and cats by influenza viruses in Chile are common, and that influenza viruses are actively circulating, at least, in dogs. While little attention has been given to companion animals as hosts of the disease in the region, these results indicate that perhaps symptomatic animals have been to date misdiagnosed due to a false perception of absence of this disease in the country or due to the lack of available diagnostic tools for clinicians. Further prospective studies should therefore be conducted in dogs and cats to determine the true prevalence of this disease in these species in the region.
Impacts:
Influenza A virus is circulating in dogs and cats in Chile.
The lack of data on influenza in dogs and cats in Chile could be due to a lack of diagnostic tools available to clinicians.
Surveillance of Influenza A virus infections in dogs and cats is important given the potential for emergence of novel influenza viruses with pandemic potential, particularly in an understudied region like South America.
Acknowledgments:
We would like to thank pet owners, animal shelter supervisors and veterinary clinics who voluntarily agreed to have their animals sampled.
Funding:
This work was supported by National Institute of Allergy and Infectious Diseases, contract HHSN272201400006C.
Footnotes
Conflict of interest
the authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SS, Volkmuth W, Duca J, Corti L, Pallaoro M, Pezzicoli A, Karle A, Rigat F, Rappuoli R, Narasimhan V, 2015. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Science translational medicine 7, 294ra105–294ra105. [DOI] [PubMed] [Google Scholar]
- Anthony S, Leger JS, Pugliares K, Ip HS, Chan J, Carpenter Z, Navarrete-Macias I, Sanchez-Leon M, Saliki J, Pedersen J, 2012. Emergence of fatal avian influenza in New England harbor seals. mBio 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell E, Pecoraro H, Torres-Henderson C, Morley P, Lunn K, Landolt G, 2010. Seroprevalence and risk factors for canine H3N8 influenza virus exposure in household dogs in Colorado. Journal of veterinary internal medicine 24, 1524–1527. [DOI] [PubMed] [Google Scholar]
- Beeler E, 2009. Influenza in dogs and cats. Veterinary Clinics of North America: Small Animal Practice 39, 251–264. [DOI] [PubMed] [Google Scholar]
- Belser JA, Pulit-Penaloza JA, Sun X, Brock N, Pappas C, Creager HM, Zeng H, Tumpey TM, Maines TR, 2017. A novel A (H7N2) influenza virus isolated from a veterinarian caring for cats in a New York City animal shelter causes mild disease and transmits poorly in the ferret model. Journal of virology 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland S, Gracieux P, Jones M, Mallet F, Yugueros-Marcos J, 2020. Influenza A Virus Infection in Cats and Dogs: A Literature Review in the Light of the “One Health” Concept. Frontiers in public health 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratanich A, Gómez N, Aznar M, Correas I, Baumberger C, Loiza M, Bokenhans R, 2011. Estudio preliminar sobre la circulación del virus de influenza canina en Buenos Aires. [Google Scholar]
- Cauldwell AV, Long JS, Moncorgé O, Barclay WS, 2014. Viral determinants of influenza A virus host range. Journal of General Virology 95, 1193–1210. [DOI] [PubMed] [Google Scholar]
- Chomel BB, 2014. Emerging and re-emerging zoonoses of dogs and cats, animals 4, 434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Control, C.f.D., Prevention, 2009. CDC protocol of realtime RTPCR for influenza A (H1N1). Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW, 2011. InfoStat. [Google Scholar]
- Dundon WG, De Benedictis P, Viale E, Capua I, 2010. Serologic evidence of pandemic (H1N1) 2009 infection in dogs, Italy. Emerging infectious diseases 16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentini L, Taddei R, Moreno A, Gelmetti D, Barbieri I, De Marco M, Tosi G, Cordioli P, Massi P, 2011. Influenza A pandemic (H1N1) 2009 virus outbreak in a cat colony in Italy. Zoonoses Public Hlth 58, 573–581. [DOI] [PubMed] [Google Scholar]
- Hatta M, Zhong G, Gao Y, Nakajima N, Fan S, Chiba S, Deering KM, Ito M, Imai M, Kiso M, 2018. Characterization of a feline influenza A (H7N2) virus. Emerging infectious diseases 24, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H, Elaish M, Kc M, Abundo MC, Ghorbani A, Ngunjiri JM, Lee C-W, 2018. Efficacy and synergy of live-attenuated and inactivated influenza vaccines in young chickens. PloS one 13, e0195285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EA, Engel GA, Feeroz M, San S, Rompis A, Lee BP-H, Shaw E, Oh G, Schillaci MA, Grant R, 2012. Influenza virus infection in nonhuman primates. Emerging infectious diseases 18, 1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaoka Y, Bean WJ, Webster RG, 1989. Evolution of the hemagglutinin of equine H3 influenza viruses. Virology 169, 283–292. [DOI] [PubMed] [Google Scholar]
- McCullers JA, Van De Velde L-A, Schultz RD, Mitchell CG, Halford C, Boyd KL, Schultz-Cherry S, 2011. Seroprevalence of seasonal and pandemic influenza A viruses in domestic cats. Archives of virology 156, 117–120. [DOI] [PubMed] [Google Scholar]
- Moon H, Hong M, Kim J, Seon B, Na W, Park S-J, An D, Jeoung H, Kim D, Kim J, 2015. H3N2 canine influenza virus with the matrix gene from the pandemic A/H1N1 virus: infection dynamics in dogs and ferrets. Epidemiology & Infection 143, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payungporn S, Crawford PC, Kouo TS, Chen L.-m., Pompey J, Castleman WL, Dubovi EJ, Katz JM, Donis RO, 2008. Influenza A virus (H3N8) in dogs with respiratory disease, Florida. Emerging infectious diseases 14, 902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Martínez LA, Contreras-Luna M, De la Luz J, Manjarrez ME, Rosete DP, Rivera-Benitez JF, Saavedra-Montañez M, Ramírez-Mendoza H, 2013. Evidence of transmission and risk factors for influenza A virus in household dogs and their owners. Influenza and other respiratory viruses 7, 1292–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richt JA, Rockx B, Ma W, Feldmann F, Safronetz D, Marzi A, Kobasa D, Strong JE, Kercher L, Long D, 2012. Recently emerged swine influenza A virus (H2N3) causes severe pneumonia in Cynomolgus macaques. PloS one 7, e39990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu B, Wu K-H, Emery S, Villanueva J, Johnson R, Guthrie E, Berman L, Warnes C, Barnes N, Klimov A, 2011. Design and performance of the CDC real-time reverse transcriptase PCR swine flu panel for detection of 2009 A (H1N1) pandemic influenza virus. Journal of clinical microbiology 49, 2614–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Kang B, Lee C, Jung K, Ha G, Kang D, Park S, Park B, Oh J, 2008. Transmission of avian influenza virus (H3N2) to dogs. Emerging infectious diseases 14, 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Lee C, Kang B, Jung K, Oh T, Kim H, Park B, Oh J, 2009. Experimental infection of dogs with avian-origin canine influenza A virus (H3N2). Emerging infectious diseases 15, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N, Payungporn S, Theamboonlers A, Poovorawan Y, 2006a. Avian influenza H5N1 in naturally infected domestic cat. Emerging infectious diseases 12, 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Pariyothorn N, Payungporn S, Theamboonlers A, Chutinimitkul S, Thanawongnuwech R, Poovorawan Y, 2006b. Fatal avian influenza A H5N1 in a dog. Emerging infectious diseases 12, 1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponseller BA, Strait E, Jergens A, Trujillo J, Harmon K, Koster L, Jenkins-Moore M, Killian M, Swenson S, Bender H, 2010. Influenza A pandemic (H1N1) 2009 virus infection in domestic cat. Emerging infectious diseases 16, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Li H-T, Zhao F-R, Chen J-D, Xie J.-x., Chen Z-M, Huang Z, Hu Y-M, Zhang M-Z, Tan L-K, 2013. Avian-origin H3N2 canine influenza virus circulating in farmed dogs in Guangdong, China. Infection, genetics and evolution 14, 444–449. [DOI] [PubMed] [Google Scholar]
- Voorhees IE, Glaser AL, Toohey-Kurth K, Newbury S, Dalziel BD, Dubovi EJ, Poulsen K, Leutenegger C, Willgert KJ, Brisbane-Cohen L, 2017. Spread of canine influenza A (H3N2) virus, United States. Emerging infectious diseases 23, 1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S-W, Webby RJ, Webster RG, 2014. Evolution and ecology of influenza A viruses. Influenza Pathogenesis and Control-Volume I. Springer, 359–375. [DOI] [PubMed] [Google Scholar]
- Yu Z, Gao X, Wang T, Li Y, Li Y, Xu Y, Chu D, Sun H, Wu C, Li S, 2015. Fatal H5N6 avian influenza virus infection in a domestic cat and wild birds in China. Scientific reports 5, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


