Abstract
The risk of foodborne illnesses caused by pathogens could be increased in fresh-cut fruit products owing to contamination during processing. Therefore, this study was conducted to investigate the microbiological quality and safety of commercial fresh-cut fruit products in Korea. Additionally, the growth of Listeria monocytogenes in selected fresh-cut fruits was evaluated, and their growth curves were analyzed using predictive growth modeling. The mean count of total aerobic bacteria, coliforms, and yeast/mold was 3.67±1.73 log10 CFU/g, 1.54±1.01 log10 CFU/g, and 3.81±1.51 log10 CFU/g, respectively. Escherichia coli, Staphylococcus aureus, Escherichia coli O157:H7, L. monocytogenes, Salmonella spp., and Cyclospora spp. were not detected in any of the tested samples. Only Bacillus cereus was detected in a few samples at the mean level of 1.72±0.13 log10 CFU/g. The growth of L. monocytogenes varied depending on the type of fruit; they grew well in non-acidic fresh-cut fruit products during storage at 10 °C.
Keywords: Fresh-cut fruit products, Microbiological quality, Microbial safety, Low temperature storage, Predictive growth modeling
Introduction
Consumption of fruits has been recommended by various organizations such as the World Health Organization (WHO), as they contain abundant dietary fiber, vitamins, minerals, and phytochemicals and are low in calories (Singla et al., 2020). According to the Ministry of Agriculture, Food and Rural Affairs (2020), the per capita fruit consumption in Korea was 56.6 kg in 2019 and has been steadily over 55 kg since the 2000s. Specifically, the consumption of fresh-cut fruit products has recently increased significantly owing to the development of processing technology, a busy lifestyle, and the increase in single-person households. According to the Korea Rural Economic Institute (Kim, 2019), the sales of domestic fresh-cut foods in 2018 reached 181.7 billion—an annual increase of 22.9% compared to 2008 and a 7.9-fold increase in 10 years. Between 2016 and 2017, it increased by 48.3%. The consumption of fresh-cut fruit products will continue to increase owing to the expansion of online markets, the availability of early morning delivery services, an increase in fresh produce at convenience stores, and the appearance of customized fresh-fruit products.
Pathogenic microbial contamination of fresh produce, including fruits, may occur from farm to table (Paramithiotis et al., 2017). From 2010 to 2017, 1,797 food-borne outbreaks were caused by pathogenic microorganisms in the USA, of which 228 (12.7%) were attributed to fresh produce (CDC, 2018). Based on the incidence of food-borne outbreaks in fresh-produce historically, the pathogens of concern are Salmonella spp., Escherichia coli O157:H7, Shigella spp., Listeria monocytogenes, Norovirus, and pathogenic protozoa (Juneja and Sofos, 2009). In the USA, foodborne outbreaks associated with fresh produce were listeriosis from contaminated cantaloupe in 2011 and salmonellosis from cantaloupe, mango, tomato, and papaya in 2011–2017 (Carstens et al., 2019); Salmonella enterica serovar Enteritidis infection from peaches (CDC, 2020); and E. coli infection from berries (strawberries and raspberries) and apple juice in 2004–2012 (Callejón et al., 2015). Additionally, in the USA, during 2013–2017, intestinal infection caused by the parasite Cyclospora cayetanensis was linked to a salad mix that contained lettuce, romaine lettuce, red cabbage, carrot, and coleslaw (Buss et al., 2016; Fox, 2017).
In particular, the risk of foodborne illnesses caused by pathogens could be increased in fresh-cut fruit products because they could be contaminated with pathogens during processing, such as peeling, slicing, and packaging (Gombas et al., 2017). Additionally, the growth of pathogens in fresh-cut fruit products can be enhanced by nutrients in the fruit cells exposed during the peeling and cutting processes (Qadri et al., 2015). Contamination of foodborne pathogens in fresh-cut fruit products has been recently reported; for example, L. monocytogenes in cut apples and melons in Canada (Zhang et al., 2020) and Bacillus cereus in cut apples in Korea (Tango et al., 2018). Nevertheless, studies on the microbial contamination of fresh-cut fruit products are limited. Furthermore, information on microbial contamination assessment of related products should be provided to ensure the microbial safety of fresh-cut fruit products distributed in retail. Therefore, in this study, microbial contamination in a total of 100 fresh-cut fruit products in Korea was evaluated to investigate the microbiological quality and safety (general microbial contamination, pathogenic bacteria, and Cyclospora spp.) of these products. Further, the growth of L. monocytogenes as a psychrotrophic pathogen in four fresh-cut fruit—products (watermelon, orange, green kiwi, and melon) during—storage at 10 °C was investigated, and the growth curves were analyzed via predictive growth modeling using the modified Gompertz equation to determine its growth rate and lag time on fresh-cut fruits.
Materials and methods
Sampling of fresh-cut fruit products
A total 25 different types of mixed or single-packaged fresh-cut fruit product were evaluated in this study. Each product was evaluated in 5 repetitions therefore a total of 100 samples were analyzed by collecting 25 samples of 5 varieties for each season. The samples tested are shown in Table 1. All samples were purchased online as commercial products and analyzed on the day of purchase. The collected fruit samples for each season were influenced by their availability in the Korean market at the time of sampling.
Table 1.
Quantitative (log10 CFU/g) of bacterial count isolated from seasonal fresh-cut fruit products
| Season | Sample | Raw material | Type of microorganism1 | |||||
|---|---|---|---|---|---|---|---|---|
| TAB | Coliform | E. coli | Y/M | S. aureus | B. cereus | |||
| Winter (February-March) | 1 | Cherry tomatoes, grapes, pear | 3.42±1.062ef3 | 0.76±0.13e | ND4 | 3.15±0.64cd | ND | NDb |
| 2 | Apple, cherry tomatoes, grapes | 3.82±1.12def | 3.51±0.61a | ND | 1.56±1.92e | ND | NDb | |
| 3 | Pineapple | NDg | NDe | ND | 3.73±0.25bcd | ND | NDb | |
| 4 | Apple, grapes, pear | 4.67±0.92abcd | NDe | ND | 1.72±0.59e | ND | NDb | |
| 5 | Apple, dragon fruit, grapes, green grapes, green kiwi, orange, pineapple, tomatoes | 1.24±0.45g | 0.70±0.00e | ND | 3.16±0.42cd | ND | 1.70±0.00b | |
| Spring (April-May) | 6 | Pineapple | 0.82±0.27g | NDe | ND | 6.77±0.33a | ND | NDb |
| 7 | Apple, dragon fruit, grapes, green kiwi, orange, pineapple | 4.74±0.70abcd | 0.91±0.47e | ND | 6.22±0.76a | ND | NDb | |
| 8 | Apple, cherry tomatoes, grapes, pineapple | 4.06±0.23cdef | 1.96±1.57c | ND | 6.31±0.77a | ND | NDb | |
| 9 | Cherry tomatoes, grapes, green kiwi, pineapples, sweet persimmons | 2.95±2.33f | 1.06±0.37de | ND | 3.38±0.28bcd | ND | NDb | |
| 10 | Apples, cherry tomatoes, grapes, green kiwi, oranges, pineapples, sweet persimmons | 3.07±0.56ef | 1.72±0.55cd | ND | 4.23±0.16bc | ND | NDb | |
| Summer (June-July) | 11 | Apple, citrus, dragon fruit, grapes, green kiwi, orange, pineapple, tomatoes | 3.52±0.82def | 1.13±0.40de | ND | 3.68±0.36bcd | ND | 2.08±0.47a |
| 12 | Pineapple | 1.24±0.48g | 0.70±0.00e | ND | 4.49±0.43b | ND | 1.70±0.00b | |
| 13 | Melon | 5.05±0.97abc | 2.89±1.06ab | ND | 3.25±1.11cd | ND | NDb | |
| 14 | Melon | 5.78±0.64a | 2.10±0.33bc | ND | 4.00±0.35bcd | ND | NDb | |
| 15 | Watermelon | 5.46±0.82ab | 2.54±0.89bc | ND | 3.28±0.28cd | ND | NDb | |
| Fall (August-October) | 16 | Orange, pear | 4.28±0.30bcde | 0.98±0.17de | ND | 2.89±0.96d | ND | NDb |
| 17 | Grapes, pear | 3.70±0.52def | NDe | ND | 2.91±0.47d | ND | NDb | |
| 18 | Melon, tomatoes | 5.50±0.61a | 2.36±0.41bc | ND | 2.85±1.23d | ND | NDb | |
| 19 | Melon, watermelon | 4.26±0.44bcde | 2.13±0.62bc | ND | 4.48±0.54b | ND | NDb | |
| 20 | Dragon fruit, fig, watermelon | 5.13±0.68abc | 2.51±0.49bc | ND | 3.85±0.94bcd | ND | NDb | |
| Total | 3.67±1.73 | 1.54±1.01 | ND | 3.80±1.51 | ND | 1.72±0.13 | ||
1 Detection limit was 0.7 log10 CFU/g for TAB (total aerobic bacteria), coliforms, E. coli, and Y/M (yeast/mold) and 1.70 log10 CFU/g for S. aureus, and B. cereus, respectively.
2Means ± standard deviation (n = 5).
3Means with no significant difference among the same lowercase letters in the same column (P > 0.05).
4ND: not detected
Quantitative microbial risk assessments
To evaluate the microbial prevalence in fresh-cut fruit products, 25 g of fresh-cut fruit product samples were homogenized in sterile filter stomacher bags (Difco Laboratories, Detroit, MI, USA) containing 100 mL of Butterfield's phosphate-buffered dilution water (BPDW, Difco Laboratories), and each sample bag was stomachered (BagMixer 400, Interscience, Breteche, France) for 90 s.
Total aerobic bacteria (TAB), E. coli/coliforms (EC), and yeast/mold (YM) were analyzed using a petrifilm aerobic count plate, petrifilm EC count plate, and petrifilm YM count plate (3M, Seoul, Korea), respectively. The sample suspensions were diluted with 0.2% sterile peptone water (PW, Difco), and 1.0 mL of the aliquots was placed on three different petrifilms. The petrifilms were incubated at 37 °C overnight for TAB and EC and at 30 °C for 48 h for YM. For B. cereus and S. aureus, 0.1 mL of the aliquots was inoculated on mannitol egg yolk polymyxin agar (MYP, Difco) and Baird-Parker agar (BPA, Difco) and incubated at 30 °C for 24 h and 37 °C for 48 h, respectively. When presumptive colonies with typical shapes for each pathogen were determined, colonies were confirmed by 16S rRNA sequencing (SolGent Co., Daejeon, Korea) (Ponsawat et al., 2012).
Qualitative microbial risk assessments and identifications
Foodborne pathogenic bacteria
The microbial prevalence of B. cereus, E. coli O157:H7, L. monocytogenes, Salmonella spp., and S. aureus was investigated qualitatively using the methods described in the Korean Food Code with some modifications (MFDS, 2021a). Briefly, 25 g of samples were enriched in 225 mL tryptic soy broth (TSB, Difco) supplemented with 10% NaCl, modified tryptone soy broth (mTSB, Microgiene Co., Gunpo-si, Korea), BPDW, and buffered peptone water (BPW, Difco) at 37 °C for 24 h, and Listeria enrichment broth (LEB, Difco) at 30 °C for 24 h. For isolation of B. cereus and L. monocytogenes, pre-enrichment samples were inoculated on MYP and Oxford agar base (OAB, Difco), respectively, and incubated at 30 °C for 24 h. For E. coli and S. aureus, the samples were placed on Tellurite Cefixime-Sorbitol MacConkey Agar (TC-SMAC, Difco) and BPA, respectively, and incubated at 37 °C for 24 h. For Salmonella spp., 0.1 mL of BPDW enrichment was transferred to 10 mL of Rappaport-Vassiliadis R10 (RV, Difco) and incubated for an additional 24 h at 42 °C. Another 1.0 mL of the sample was added to 10 mL of tetrathionate broth (TB, Difco) and incubated at 37 °C for 24 h. Then, the enriched samples of RV and TB were streaked on xylose lysine deoxycholate (XLD, Difco) and incubated at 37 °C for 24 h. For S. aureus and B. cereus, presumptive colonies with typical shapes for each pathogen were confirmed by 16S rRNA sequencing (SolGent Co., Daejeon, Korea) (Ponsawat et al., 2012).
Cyclospora
The presence of Cyclospora was determined by real-time PCR (RT-PCR, GeneMate, Bioexpress, Kaysville, UT, USA) using the method of the Korean Food Code (MFDS, 2017, 2019, 2021a). Fifty grams of fresh-cut fruit products was placed in zipper bags (S. C. Johnson & Son, Inc., Seoul, Korea), and 100 mL of 0.1% liquinoxTM (Alconox Inc., White Plains, NY, USA) and 0.01 M phosphate buffered saline (PBS, pH 7.4) were added. The samples were shaken at 160 rpm for 15 min using an orbital shaker (OFD 300, Best of Lab Equipment, Seoul, Korea) and passed through a 200–250 μm mesh sieve. Subsequently, the supernatant was discarded by centrifugation at 8,000 rpm for 20 min, and this process was repeated twice. The pellet was resuspended in 40 mL PBS and centrifuged at 2,500 rpm for 20 min and then at 5000 rpm for 10 min. The final pellet was resuspended in 200 μL PBS, and DNA was extracted according to the manufacturer’s instructions using the QIAamp DNA Mini Kit 50 (Qiagen, Hilden, Germany). The method used for sample preparation and DNA extraction for detecting Cyclospora in this study has been tested in previous studies (Murphy et al., 2017; Shelds et al., 2013). The extracted DNA served as a template for RT-PCR, and positive DNA control of Cyclospora (PRA-3000SD) was purchased from ATCC (The Global Bioresource Center, Manassas, VA, USA). PCR reaction mixtures included 12.5 μL TaqMan Universal Master Mix II with UNG (2×, Applied Biosystems, Carlsbad, CA, USA), 1 μL primer (F), 1 μL primer (R), 5 μL distilled water, and 5 μL template DNA. The primer sequences were as follows: primer (F) sequence to 5′-GCA GCA TGG AAT AAT AAG ATA GGA CC-3′ and primer (R) sequence to 5′-CGC AGT AGT TCG TCT TTA ACA AAT CTA AG-3′. The RT-PCR conditions consisted of 2 min at 50 °C for UNG incubation, 10 min at 95 °C for denaturation, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min, and extension at 72 °C for 5 min. The PCR products were confirmed by 2% agarose gel electrophoresis (Mupid-exU, Advance, Tokyo, Japan).
Growth kinetics of L. monocytogenes on fresh-cut fruits
Preparation of bacterial strains
L. monocytogenes was tested in this study because it is a psychrophilic pathogen therefore suitable for microbial growth prediction at cold temperature as a major foodborne pathogen although L. monocytogenes was not detected in any tested samples. L. monocytogenes has been used as the target bacteria for the growth prediction models in various foods including fruits at cold temperature in previous studies (Hong et al. 2014; Marik et al. 2020). L. monocytogenes ATCC 7644, 19114, and 19115 were obtained from the bacterial culture collection of Chung-Ang University (Anseong-si, Korea). All stock cultures were maintained at – 80 °C in 20% glycerol and activated by cultivation in TSB supplemented with 0.6% yeast extract (TSBY) at 37 °C for 24 h. To prepare cocktails, each culture of L. monocytogenes was mixed equally and harvested by centrifugation at 10,000 rpm for 10 min. The pellet was washed with 0.2% PW and diluted to a final concentration of 2–3 log CFU/mL.
Microbial growth on fresh-cut fruits
To evaluate the effect of pH values on the growth of L. monocytogenes at cold temperature, four types of fruits, watermelon, navel orange, green kiwi, and melon were selected and tested in this study. Watermelon (Citrullus lanatus), navel orange (Citrus sinensis), green kiwi (Actinidia deliciosa), and melon (Cucumis melo var. cantalupensis) were purchased from a local grocery store (Anseong-si, Korea) and stored at 4 °C until further use. All fresh fruits were washed with tap water for 30 s, dried in a laminar flow biosafety hood, and cut into similar sizes. Each fresh-cut fruit was inoculated with 0.1 mL of L. monocytogenes cocktail suspension and dried in a laminar flow biosafety hood for 30 min for attachment of the tested bacteria. Inoculated fresh-cut fruits were stored in polyethylene terephthalate (PET) containers individually at 10 °C for 8 days to observe the growth kinetics of the tested microorganism. After storage, samples were diluted (1:2) in 0.2% PW and homogenized for 90 s in a stomacher. Then, 0.1 mL of the aliquots was placed on OAB and incubated at 30 °C for 24 h.
Predictive modeling
Reliable estimates of the growth rate (GR) and lag time (LT) of L. monocytogenes on fresh-cut fruits were obtained using the primary model (Prism, version 4.0, GraphPad Software, San Diego, CA, USA). The growth kinetics were fitted using the modified Gompertz equation (Gibson et al., 1998):
where Y is the log cell number (log10 CFU/g), N0 is the initial cell number (log CFU/g), C is the difference between initial and final cell numbers, X is the storage time (h), μ is the maximum rate of GR (log CFU/h), and lag is the delay before growth (LT, h).
Statistical analysis
The experiments were repeated thrice with duplicate plates, and the data were analyzed using the Statistical Analysis System (Version 9.1; SAS Institute, Cary, NC, USA) or Excel 2010 (Microsoft Office XP; Microsoft, Redmond, WA, USA). Significant differences (P ≤ 0.05) among the mean values of the treatment groups were determined by analysis of variance (ANOVA) and Duncan’s multiple range tests or Student’s t-test.
Results and discussion
Quantitative microbial risk assessments
A total of 100 packaged fresh-cut fruit products were collected for bacterial analysis. Of these, 30% contained a single kind of fruit, mainly grapes, pineapples, melons, and watermelons; 40% of the samples contained less than three kinds of fruits; and 30% of the samples consisted of four or more kinds of fruits. The most commonly used fruits were grapes, pineapples, apples, and cherry tomatoes. Samples containing a single fruit were most common in summer, whereas samples containing more than four kinds of fruits were common in winter and spring. Further, samples containing melons and watermelons were produced only during summer and fall. All fresh-cut fruit products were labeled according to their country of origin; 70% of the products contained imported fruits, which included grapes from Chile, Peru, the USA, and the Philippines; oranges from Australia and the USA; green kiwi from New Zealand; and dragon fruit from Vietnam. All samples were taken before the expiration date, and no visible signs of decay were observed. Nevertheless, consumption of produce with pathogens, in the absence of any signs of decay, leads to food-borne illness (Qadri et al., 2015).
Table 1 and Fig. 1 show the mean levels of TAB, coliform, E. coli, YM, S. aureus, and B. cereus in fresh-cut fruit products by season. The mean TAB count of the 100 samples was 3.67±1.73 log10 CFU/g. The mean TAB in winter samples was 2.77±1.74 log10 CFU/g. Similar numbers (3.13±1.70 log10 CFU/g) were also found in spring samples. The mean TAB counts in summer and fall samples were 4.21±1.85 log10 CFU/g and 4.58 ± 0.82 log10 CFU/g, respectively; therefore, the mean TAB in summer and fall samples was relatively higher than that in winter and spring samples but was not significantly different (P > 0.05). The expiration date of fresh produce is generally considered unsuitable when the TAB population reaches 7 log10 CFU/g (López et al., 2008). In this study, the TAB levels in all fresh-cut fruit products tested were less than 7 log10 CFU/g. Nevertheless, the results are not completely satisfactory, as TAB levels above 6 log10 CFU/g were detected in six samples (8%). In the case of sample 9, TAB was higher than 6 log10 CFU/g in only one out of the five replicates. Conversely, for samples 13, 14, 15, 18, and 20, high levels of TAB were detected in all five repetitions. This may be due to the presence of contaminated watermelons and melons. According to Kim et al. (2017), the levels of TAB in watermelons and melons were 6.0 ± 1.2 and 4.8 ± 1.0 log10 CFU/g, respectively, which are similar to the results of this study. Further, relatively high levels of TAB in watermelons and melons have also been reported in other studies (Johnston et al, 2005; Kim et al., 2014). The mean coliform count for the 100 fresh-cut fruit products tested was 1.54±1.01 log10 CFU/g, and there was no significant difference among seasonal products (P > 0.05). The mean coliform counts in winter, spring, summer, and fall samples were 1.27±1.17, 1.27±0.87, 1.87±1.04, and 1.73±0.85 log10 CFU/g, respectively. Among the samples, sample 2, containing apple, cherry tomatoes, and grapes, showed the highest mean coliform counts at 3.51±0.61 log10 CFU/g. This may be due to microbial cross-contamination or microbial growth during post-harvest processes. Samples 13, 14, 15, 18, 19, and 20, containing watermelons and melons, showed relatively higher levels of coliform contamination than the other samples. The mean YM count for the 100 samples was 3.50±1.51 log10 CFU/g. The mean YM count in spring samples was 5.38±1.44 log10 CFU/g with a range of 2.92 to 7.11 log10 CFU/g, which was relatively higher than that in samples from other seasons, although significantly difference was not found (P > 0.05). Spring samples contained pineapples; thus, these relatively high levels of YM contamination in spring samples may be related to pineapples. Deak et al. (1993) reported that several yeasts, including Clavispora lusitaniae, Cryptococcus laurentii, Hanseniaspora guilliamondii, and Saccharomyces cerevisiae, contaminated pineapples. According to another study, strawberries, pineapples, and mango showed high levels of YM contamination at 5.2, 5.1, and 4.7 log10 CFU/g, respectively (Graça et al., 2017). Lloyd et al. (2005) investigated fundal infections of fresh-cut fruit using the gas chromatography-mass spectrometric method and detected various fungal contamination in fresh-cut fruit including apples, melons, oranges, and pineapples. Also, Manthou et al (2021) reported that various fungi including Candida argentea, Candida sake, and Fusarium cirinatum were found in freshly cut pineapple. In summary, the microbiological quality of fresh-cut watermelon, melon, and pineapples is more concerning than that of other fresh-cut fruits examined, especially in terms of TAB, coliform, and YM. Fresh-cut watermelon, melon, and pineapples are vulnerable to microbial contamination because, unlike the whole fruits, they are not protected by hard shells and contain more moisture than other fruits. From the results of quantitative analysis for pathogens, B. cereus was only detected in a few samples (5%) at a mean level of 1.72±0.13 log10 CFU/g. However, the levels of B. cereus in positive samples (5, 11, and 12) were suitable for the microbial standard of the Korea Food Code (MFDS, 2021a). Notably, E. coli and S. aureus were not detected in any of the tested samples.
Fig. 1.
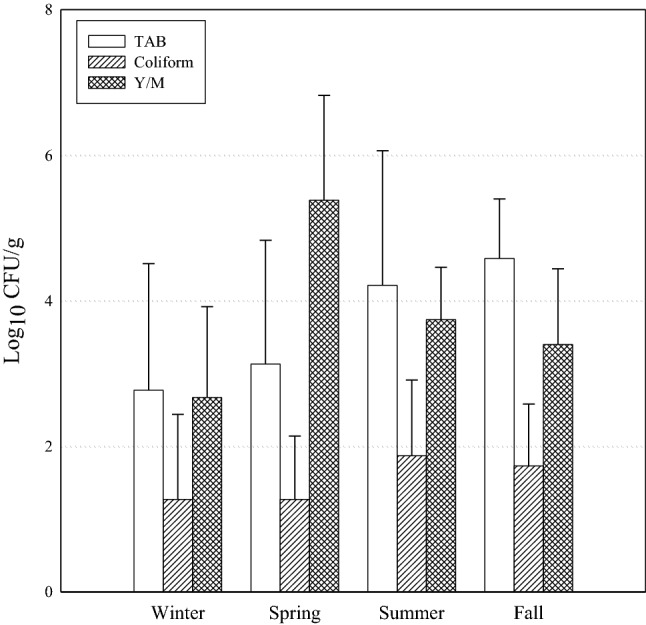
Mean (Log10 CFU/g) of microbial count isolated from seasonal fresh-cut fruit products. TAB (□), Coliform (▨), and Y/M (▩)
Qualitative microbial risk assessments
Table 2 shows the prevalence of pathogenic bacteria for microbial quality evaluation. S. aureus was isolated from four samples from two different brands: one of sample 13 (only melon) and three of sample 15 (only watermelon). While colonized food handlers are the main source of S. aureus dissemination, equipment and environmental surfaces can also be implicated in outbreaks (Juneja and Sofos, 2009). Therefore, the presence of S. aureus might be due to cross-contamination during processing. B. cereus was isolated from five samples from three different brands: one of sample 5 (containing: apple, dragon fruit, grapes, green grapes, green kiwi, orange, pineapple, and tomato), three of sample 11 (containing: apple, citrus, dragon fruit, grape, green kiwi, orange, pineapple, and tomato), and one of sample 12 (only pineapple). B. cereus not only survives at extreme temperatures but can also form biofilms and spores. Therefore, it is difficult to estimate their origin. One possibility might be soil contamination because contains fruits that grow on the ground. L. monocytogenes, Salmonella spp., E. coli O157:H7, and Cyclospora (Fig. 2) were not detected in any fresh-cut fruit products analyzed in this study. According to the Korea Food Code, the microbiological standards of fresh-cut fruits are that E. coli O157:H7 and Salmonella spp. should be negative, S. aureus should be 100 CFU/g or less, and B. cereus should be 1,000 CFU/g or less. The results of our study indicate that all samples are in accordance with the microbiological standards of the Korea Food Code (MFDS, 2021b).
Table 2.
Prevalence (positive sample no./total sample no. tested) of pathogenic contamination of seasonal fresh-cut fruit products
| Season | S. aureus | B. cereus | L. monocytogenes | Salmonella spp. | E. coli O157:H7 |
|---|---|---|---|---|---|
| Winter | ND1 | 4% (1/25) | ND | ND | ND |
| Spring | ND | ND | ND | ND | ND |
| Summer | 16% (4/25) | 16% (4/25) | ND | ND | ND |
| Fall | ND | ND | ND | ND | ND |
| Total | 4% (4/100) | 5% (5/100) | ND | ND | ND |
1 ND: not detected
Fig. 2.
PCR result of Cyclospora in winter (A), spring (B), summer (C), and fall (D) fresh-cut fruit products, respectively. Numbers from 1 to 20 indicate the number of samples
Growth kinetics of L. monocytogenes on fresh-cut fruit
For quantitative microbial risk assessment, a microbial growth prediction model that can predict microbial growth changes according to various environmental factors such as temperature and pH is essential. Figure 3 shows the growth curves of L. monocytogenes in watermelon, navel orange, green kiwi, and melon during storage at 10 °C for 8 days. Levels of L. monocytogenes on fresh-cut watermelon and melon with an initial load of 4.41±0.28 and 4.37±0.27 log CFU/g reached over 7.11±0.47 and 8.00±0.61 log CFU/g, respectively, after storage. Therefore, it was confirmed that L. monocytogenes could grow quickly and reach high concentrations without visual signs of decay when fresh-cut watermelon and melon were stored at 10 °C. Conversely, the levels of L. monocytogenes were maintained or slightly increased on fresh-cut oranges and kiwis after storage. L. monocytogenes has a wide growth range of pH 4.4 to 9.6 (Juneja and Sofos, 2009). The pH of watermelon, orange, kiwi, and melon used in this study was 5.54±0.21, 3.72±0.33, 3.14±0.09, and 6.19±0.18, respectively. Therefore, it appears that the growth of L. monocytogenes on oranges and kiwis was inhibited because of their low pH.
Fig. 3.
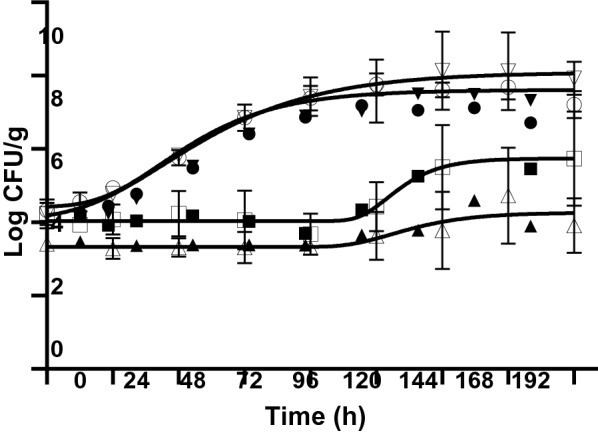
Growth of L. monocytogenes on watermelon (●), orange (■), green kiwi (▲), and melon (▼) at 10 °C for 8 days
The growth parameters determined by predictive growth modeling using the modified Gompertz model are listed in Table 3. The GR value of both watermelon and melon was 0.05 CFU/g/h, and the LT values of melon and watermelon were 11.35 and 18.39 h, respectively. According to another study using the predictive growth modeling with the modified Gompertz equation for L. monocytogenes growth on fresh-cut melon during storage at 10 °C, the GR and LT values were 2.22 CFU/g/h and 3.57 h, respectively (Hong et al., 2014). However, another study showed a GR value of 0.047 CFU/g/h at 10 °C for L. innocua growth on fresh-cut melon by predictive modeling using the Gompertz model (Guzel and Mustafa, 2015), which is similar to the results of the current study. The GR and LT levels could differ depending on the bacterial strains and testing conditions. The growth of L. monocytogenes on fresh-cut oranges and kiwi at 10 °C was hampered and failed to develop into full growth curves. Hence, despite storage at 10 °C, non-acidic fresh-cut fruit products such as watermelons and melons were susceptible to L. monocytogenes contamination and growth.
Table 3.
Parameters obtained from the modified Gompertz equation for the growth of L. monocytogenes on various fresh-cut fruit products stored at 10 °C
| Fresh-cut fruit products | Parameters of modified Gompertz equation1 | ||
|---|---|---|---|
| GR | LT | R2 | |
| Watermelon | 0.05 | 18.39 | 0.9828 |
| Orange | NA2 | NA | NA |
| Green kiwi | 0.02 | 102.30 | 0.6945 |
| Melon | 0.05 | 11.35 | 0.9951 |
1 GR: the maximum growth rate (1/h); LT, the lag time before growth (h)
2 NA, not available
In conclusion, this study investigated the microbiological quality of fresh-cut fruit products. TAB was detected in all seasons, but samples from summer and fall had relatively higher TAB levels than those from winter and spring. Samples containing watermelon and melon had higher TAB levels than the other samples. However, the samples containing pineapple had a relatively higher level of YM than others products. Among pathogens, only B. cereus and S. aureus were detected in a few samples; E. coli, E. coli O157:H7, L. monocytogenes, Salmonella spp., and Cyclospora spp. were not detected in this study. L. monocytogenes inoculated in non-acidic fresh-cut fruit products, including watermelon and melon, grew well during storage at 10 °C. Although the risk of food illnesses in fresh-cut fruit products were not confirmed in this study, relatively high levels of microbial contamination have been observed in some fresh-cut fruit products. Therefore, it is necessary to continuously monitor microbiological quality and develop food safety management technologies for related products.
Acknowledgements
This research was supported by grant (20162MFDS012) from the Ministry of Food and Drug Safety in 2020.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
A-Ra Jang, Email: jar9766@naver.com.
Areum Han, Email: chzh_9303@naver.com.
Soyul Lee, Email: xeonge@naver.com.
Suyoung Jo, Email: suyoung3223@naver.com.
Hana Song, Email: gksk2512@naver.com.
Danbi Kim, Email: rlaeksql135@naver.com.
Sun-Young Lee, Email: nina6026@cau.ac.kr.
References
- Buss BF, Joshi MV, O’Keefe AL, Allensworth CD, Garvey A, Obbink K, Mandernach S, Safranek TJ. Regional investigation of a cyclosporiasis outbreak linked to imported romaine lettuce—Nebraska and Iowa, June–August 2013. Epidemiology and Infection. 2016;144:1807–1817. doi: 10.1017/S0950268815002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejón RM, Rodríguez-Naranjo MI, Ubeda C, Hornedo-Ortega R, Garcia-Parrilla MC, Troncoso AM. Reported foodborne outbreaks due to fresh produce in the United States and European Union: trends and causes. Foodborne Pathogens and Disease. 2015;12:32–38. doi: 10.1089/fpd.2014.1821. [DOI] [PubMed] [Google Scholar]
- Carstens CK, Salazar JK, Darkoh C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Frontiers in Microbiology. 2019;10:2667. doi: 10.3389/fmicb.2019.02667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2018). National Outbreak Reporting System (NORS). Available from: https: //wwwn.cdc.gov/norsdashboard/. Accessed Jan. 27, 2021
- CDC (2020). Foodborne Outbreaks List. Available from: https: //wwwn.cdc.gov/norsdashboard/. Accessed Feb. 10, 2021
- Deak T, Beuchat LR. Yeasts associated with fruit juice concentrates. Journal of Food Protection. 56: 777-782 (1993) [DOI] [PubMed]
- Fox L (2017) Restaurant-associated outbreak of Cyclosporiasis in Austin, Texas 2016. In: Proceedings of the 2017 CSTE Annual Conference, 4–8 June 2017, Austin, TX, USA. Available from: https://cste.confex.com/cste/2017/webprogram/Paper8569.html. Accessed Feb. 24, 2021
- Gibson AM, Bratchell N, Roberts TA. Predicting microbial growth: growth responses of salmonellae in a laboratory medium as affected by pH, sodium chloride and storage temperature. International Journal of Food Microbiology. 1988;6(2):155–178. doi: 10.1016/0168-1605(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Gombas D, Luo Y, Brennan J, Shergill G, Petran R, Walsh R, Hau H, Khurana K, Zomorodi B, Rosen J, Varley R, Deng K. Guidelines to validate control of cross-contamination during washing of fresh-cut leafy vegetables. Journal of Food Protection. 2017;80:312–330. doi: 10.4315/0362-028X.JFP-16-258. [DOI] [PubMed] [Google Scholar]
- Gómez-López VM, Ragaert P, Jeyachchandran V, Debevere J, Devlieghere F. Shelf-life of minimally processed lettuce and cabbage treated with gaseous chlorine dioxide and cysteine. International Journal of Food Microbiology. 2008;121:74–83. doi: 10.1016/j.ijfoodmicro.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Graça A, Esteves E, Nunes C, Abadias M, Quintas C. Microbiological quality and safety of minimally processed fruits in the marketplace of southern Portugal. Food Control. 2017;73:775–783. doi: 10.1016/j.foodcont.2016.09.046. [DOI] [Google Scholar]
- Guzel M Quantitative microbial risk assessment of Listeria monocytogenes on fresh-cut lettuce and fresh-cut cantaloupe. PhD thesis, Texas A&M University, College station, TX, USA (2015)
- Hong YK, Yoon WB, Huang L, Yuk HG. Predictive modeling for growth of non- and cold-adapted Listeria monocytogenes on fresh-cut cantaloupe at different storage temperatures. Journal of Food Science. 2014;79:M1168–M1174. doi: 10.1111/1750-3841.12468. [DOI] [PubMed] [Google Scholar]
- Johnston LM, Jaykus LA, Moll D, Martinez MC, Anciso J, Mora B, Moe CL. A field study of the microbiological quality of fresh produce. Journal of Food Protection. 2005;68:1840–1847. doi: 10.4315/0362-028X-68.9.1840. [DOI] [PubMed] [Google Scholar]
- Juneja VK, Sofos JN. Pathogens and toxins in foods: challenges and interventions. DC, USA: ASM Press. Washington; 2009. [Google Scholar]
- Kim JW, Yu YM, Na WS, Baljii E, Choi IW, Youn YN, Lee YH. Monitoring of biosafety of agricultural products from urban community gardens and roof gardens in Korea. Horticultural Science & Technology. 2014;32:400–407. doi: 10.7235/hort.2014.13124. [DOI] [Google Scholar]
- Kim WI, Gwak MG, Jo AR, Ryu SD, Kim SR, Ryu SH, Kim HY, Ryu JG. Investigation of microbiological safety of on-farm produce in Korea. Journal of Food Hygiene and Safety. 2017;32:20–26. doi: 10.13103/JFHS.2017.32.1.20. [DOI] [Google Scholar]
- Kim S, Lee KI, Heo SY, Lee W. Research on fresh-cut fruit products and vegetables. Korea Rural Economic Institute (KREI) Basic Research Report. 1-307 (2019)
- Lloyd SW, Grimm CC, Klich MA, Beltz SB. Fungal infections of fresh-cut fruit can be detected by the gas chromatography–mass spectrometric identification of microbial volatile organic compounds. Journal of Food Protection. 2005;68(6):1211–1216. doi: 10.4315/0362-028X-68.6.1211. [DOI] [PubMed] [Google Scholar]
- Manthou E, Coeuret G, Chaillou S, Nychas GJE. Evolution of fungal community associated with ready-to-eat pineapple during storage under different temperature conditions. Food Microbiology. 97: 103736 (2021) [DOI] [PubMed]
- Marik CM, Zuchel J, Schaffner DW, Strawn LK. Growth and survival of Listeria monocytogenes on intact fruit and vegetable surfaces during postharvest handing: a systematic literature review. Journal of Food Protection. 2020;83:108–128. doi: 10.4315/0362-028X.JFP-19-283. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). Major Statistics of Ministry of Agriculture, Food and Rural Affairs. 11-1543000-000128-10, pp. 352 (2020)
- Ministry of Food and Drug Safety in Korea (MFDS). A manual for detecting pathogenic bacteria in food. Available from: https://www.foodsafetykorea.go.kr/foodcode/01_02.jsp?idx=263. Accessed Feb. 20, 2021a
- Ministry of Food and Drug Safety in Korea (MFDS). Fresh convenience food standards and specifications. Available from: www.foodsafetykorea.go.kr/foodcode/01_03.jsp?idx=63 Accessed Feb. 26, 2021b
- Ministry of Food and Drug Safety in Korea (MFDS). National Institute of Food and Drug Safety Evaluation, 2019 Test method for identifying causes of foodborne illness. pp. 288-302 (2019)
- Ministry of Food and Drug Safety in Korea (MFDS). National Institute of Food and Drug Safety Evaluation, Research on the distribution of noroviruses and parasites in underground water. p. 144 (2017)
- Murphy HR, Lee S, de Silva AJ. Evaluation of an improved U.S. Food and Drug Administration method for the detection of Cyclospora cayetanensis in produce using real-time RCR. Journal of Food Proction. 80: 1133-1144 (2017) [DOI] [PubMed]
- Paramithiotis S, Drosinos EH, Skandamis PN. Food recalls and warnings due to the presence of foodborne pathogens—A focus on fresh fruits, vegetables, dairy and eggs. Current Opinion in Food Science. 2017;18:71–75. doi: 10.1016/j.cofs.2017.11.007. [DOI] [Google Scholar]
- Ponsawat S, Latiful BM, Isobe S, Inatsu Y. Prevalence of foodborne pathogens in retailed foods in Thailand. Foodborne Pathogens and Disease. 2012;9:835–840. doi: 10.1089/fpd.2012.1169. [DOI] [PubMed] [Google Scholar]
- Qadri OS, Yousuf B, Srivastava AK. Fresh-cut fruit products and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food & Agriculture. 2015;1(1):1121606. doi: 10.1080/23311932.2015.1121606. [DOI] [Google Scholar]
- Shields JM, Joo J, Kim R. Murphy HR. Assessment of three commercial DNA extraction kits and a laboratory-developed method for detecting Cryptosporidium and Cyclospora in raspberry wash, basil wash and pesto. Journal of Microbiological Method. 92: 51-58 (2013) [DOI] [PubMed]
- Singla G, Chaturvedi K, Sandhu PP. Status and recent trends in fresh-cut fruit products and vegetables. pp. 17-49. In: Fresh-cut fruit products and vegetables. Siddiqui MW (ed). Academic Press, Cambridge, MA, USA (2020)
- Tango CN, Wei S, Khan I, Hussain MS, Kounkeu PFN, Park JH, Kim SH, Oh DH. Microbiological quality and safety of fresh fruits and vegetables at retail levels in Korea. Journal of Food Science. 2018;83:386–392. doi: 10.1111/1750-3841.13992. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yamamoto E, Murphy J, Locas A. Microbiological safety of ready-to-eat fresh-cut fruit products and vegetables sold on the Canadian retail market. International Journal of Food Microbiology. 335: 108855 (2020) [DOI] [PubMed]