Abstract
Introduction
Over the past decades prevalence of diabetes has increased in Iran and other countries. This study aimed to update the prevalence of diabetes and prediabetes in Iran and to determine associated sociodemographic risk factors, as well as diabetes awareness and control.
Methods
This is a nationally representative cross-sectional survey that included 163,770 Iranian adults aged 35–70 years, from different ethnic backgrounds, between 2014 and 2020. Diabetes was diagnosed at fasting blood sugar of ≥ 6.99 mmol/L (126 mg/dL), or receiving blood glucose-lowering treatment. Multivariable logistic regression was applied to detect determinants associated with prevalence of diabetes and prediabetes, as well as predictors of diabetes awareness and glycemic control.
Results
Sex- and age-standardized prevalence of diabetes and prediabetes was 15.0% (95% CI 12.6–17.3) and 25.4% (18.6–32.1), respectively. Among patients with diabetes, 79.6% (76.2–82.9) were aware of their diabetes. Glycemic control was achieved in 41.2% (37.5–44.8) of patients who received treatment. Older age, obesity, high waist to hip ratio (WHR), and specific ethnic background were associated with a significant risk of diabetes and prediabetes. Higher awareness of diabetes was observed in older patients, married individuals, those with high WHR, and individuals with high wealth score. Moreover, glycemic control was significantly better in women, obese individuals, those with high physical activity, educational attainment, and specific ethnic background.
Conclusions
The prevalence of diabetes and prediabetes is increasing at an alarming rate in Iranian adults. High proportion of uncontrolled patients require particular initiatives to be integrated in the health care system.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-021-01152-5.
Keywords: Diabetes, Glycemic control, Awareness, Ethnic background, Household wealth, Education
Key Summary Points
| Why carry out this study? |
| Previous national reports highlight the high prevalence of diabetes in Iran. Considering the increasing life expectancy of Iranian people, it will inevitably impose a high burden on the Iranian health care system. |
| Determining the exact sex- and age-standardized prevalence of diabetes and prediabetes and the associated risk factors is essential to improve health care models for management of this chronic disease. |
| What was learned from the study? |
| Sex- and age-standardized prevalence of diabetes and prediabetes among Iranian adults aged 35–70 years was 15.0% (95% CI 12.6–17.3) and 25.4% (18.6–32.1), respectively. |
| Although 79.6% (76.2–82.9) of participants were aware of their diabetes, only 41.2% (37.5–44.8) of patients who received treatment got their diabetes under control. |
| Since the study conducted in 2011, the prevalence of diabetes and prediabetes has been rising in Iran, emphasizing the urgent need for developing and implementing prevention strategies. |
Introduction
Diabetes is a serious chronic and considerably preventable disease. It is known as one of the four major types of non-communicable diseases (NCD) [1]. The latest information and projections provided by the International Diabetes Federation (IDF) indicated that approximately 463 million adults (9.3%) were diagnosed with diabetes in 2019; the prevalence will rise to 700 million (10.9%) by 2045. Moreover, the global prevalence of impaired glucose tolerance (IGT) was estimated to be 374 million (7.5%) and projected to reach 548 million (8.6%) by 2045, showing that a large number of people are at increased risk of developing type 2 diabetes (T2D) [2].
In Iran the national prevalence of diabetes mellitus (DM) and impaired fasting glucose (IFG) was estimated to be 11.4% and 14.6%, respectively, in 2011; this represents a 35% increase in the prevalence of DM since 2005 [3]. However, no significant change was reported in the prevalence of IFG [3]. Moreover, the National Program for Prevention and Control of Diabetes (NPPCD) investigating the proportions of different types of diabetes demonstrated that approximately 85.5% of people with diabetes who were registered in the Iranian university-affiliated adult diabetes outpatient clinics were diagnosed with T2D, followed by 11.4% with type 1 diabetes (T1D), and 1.3% with other types of diabetes [4].
Updating information on the prevalence of diabetes and prediabetes and determining the trends are essential for priority setting and planning of health care services to achieve the goals of the World Health Organization Global Action Plan for the Prevention and Control of NCDs in 2025 [5]. On the other hand, there is now growing evidence suggesting the role of awareness of diabetes in implementing prevention strategies [6, 7]. The IDF also emphasized “prevention through awareness” [8]. It is crucial to determine and raise public awareness of diabetes and its complications [8]. Once awareness is improved, people would be more willing to participate in prevention and control activities [9]. A recent study investigating disease awareness in the UK demonstrated that 59.4% of the cohort had adequate awareness of diabetes symptoms and 60.6% were able to identify the diabetes risk factors [10]. However, there are still gaps in knowledge and awareness about diabetes [10, 11].
Thus, we aimed to explore the prevalence of diabetes and prediabetes by sex, age, the region of residence (rural/urban), ethnic background, and socioeconomic status (SES) measured by educational attainment and household wealth, among Iranian adults. We further intended to provide comprehensive information on the level and determinants of diabetes awareness and glycemic control.
Methods
Study Design and Participants
Prospective Epidemiological Research Studies in IrAN (PERSIAN) is a nationwide prospective study launched in 2014 [12]. This study was designed to evaluate the epidemiology of NCDs and the associated risk and protective factors. This study included all of the major ethnic groups living in different geographical areas of Iran, enabling it to capture a wide range of environmental diversity, lifestyle, and socioeconomic differences, as well as many other exposures influencing disease patterns that may be very unique to one area. The study includes 163,770 Iranians aged 35–70 years from 18 geographically distinct areas of Iran between 2014 and 2020. The PERSIAN cohort includes major ethnic groups within Iran and constitutes both rural and urban areas with low migration rates in order to limit loss to follow-up. Adults of Iranian descent residing in one of the designated areas for at least the past 9 months were recruited in this study. Individuals with physical or psychological disabilities who could not participate in the interview and complete the enrollment process were excluded from the study. A total of 163,770 participants were recruited in this study. Details on sampling design, quality assurance, and quality control program were previously reported [12]. The design of the PERSIAN Cohort Study was approved by the ethics committees of the Ministry of Health and Medical Education, the Digestive Diseases Research Institute (Tehran University of Medical Sciences), and participating universities and performed in accordance with the Helsinki Declaration and its later amendments. At the time of enrollment, a written informed consent was obtained from all individuals.
Exposures of Interest
A comprehensive questionnaire including 482 items was administered by trained interviewers. Demographic characteristics included sex, age, area of residence (rural, urban), and marital status (married versus non-married). Socioeconomic status was defined on the basis of education and wealth index. Education was defined in five levels: no schooling (< 1 year of primary school), primary school (1–5 years), middle school (6–8 years), high school (9–12 years), and university (> 12 years). Wealth index was calculated using multiple correspondence analyses (MCA) on household assets and divided into five quintiles. For physical activity, metabolic equivalents of tasks (METs) were calculated and divided into tertiles. Anthropometric indices including height (in centimeters), weight (in kilograms), waist, and hip circumferences (in centimeters) were measured using US National Institutes of Health protocols [13]. Body mass index (BMI) was calculated and divided into four groups: underweight (< 18.5 kg/m2), normal (≥ 18.5 and < 25 kg/m2), overweight (≥ 25 and < 30 kg/m2), and obese (≥ 30 kg/m2). A high waist to hip ratio (WHR) was defined as ≥ 0.9 in men or ≥ 0.85 in women. Data on ethnicity of the participants was self-reported. If the ethnicity of both parents was the same, the same ethnicity was reported for the participant. If parents had different ethnicities, the individual was considered to have a “Mixed” ethnicity.
Blood samples were collected after an overnight fasting of at least 12 h, and stored at − 70 °C.
Outcomes
Diabetes was diagnosed at fasting blood sugar (FBS) of greater than or equal to 6.99 mmol/L (126 mg/dL), according to the American Diabetes Association (ADA) 2020 criteria [14]. In addition, individuals who were under treatment for physician-diagnosed diabetes were considered to have diabetes. Prediabetes was defined as FBS level of 5.55–6.94 mmol/L (100–125 mg/dL) in individuals without diabetes. Awareness of diabetes was defined as the proportion of individuals with diabetes who were aware of their diabetes. Glycemic control was defined as the proportion of individuals with FBS level of 4.44–7.22 mmol/L (80–130 mg/dL) among patients with diabetes who were under treatment [14].
Statistical Analysis
We calculated the sex- and age-standardized prevalence of diabetes and prediabetes, the proportion of diabetes awareness, and the proportion of glycemic control. Given the cluster sampling, we used a complex survey design to obtain summary measures. We used sampling weights defined as the inverse probability of being selected in the survey based on data of the national census in 2016. For all estimates, 95% confidence intervals were reported. Data were analyzed using Stata software (version 14.1) (Stata Corp, College Station, TX, USA).
Results
Prevalence of Diabetes and Prediabetes and Associated Factors
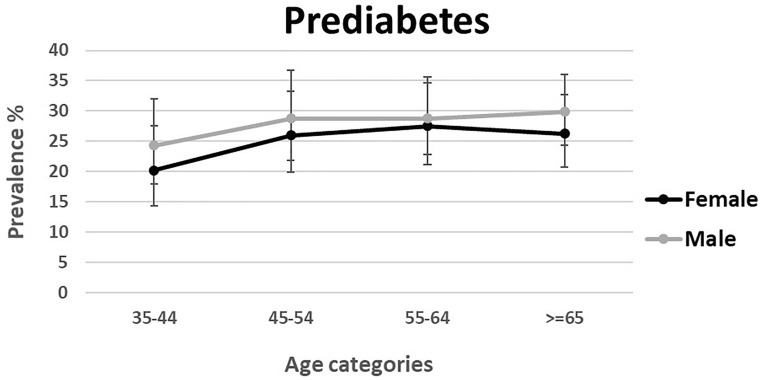
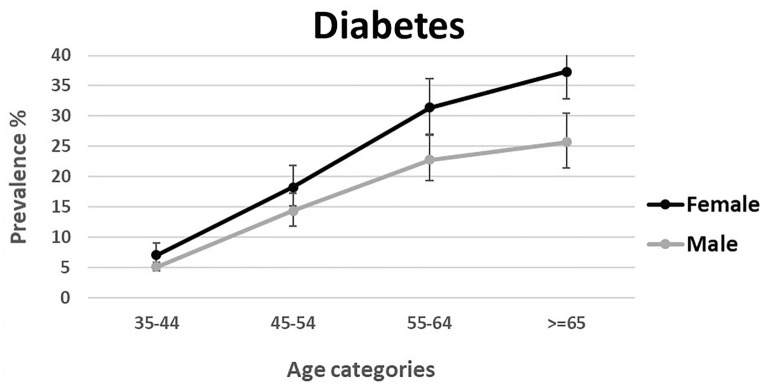
Data were available for 163,770 individuals aged 35–70 years. Data on FBS was missing in 2018 participants. Sex- and age-standardized prevalence of diabetes and prediabetes was 15.0% (95% CI 12.6–17.3) and 25.4% (18.6–32.1), respectively. Age-standardized prevalence of diabetes and prediabetes was 12.7% (10.7–14.7) and 26.9% (19.9–33.8) in men, and 17.3% (14.5–20.2) and 23.8% (17.3–30.3) in women. Moreover, 20.4% (17.3–24.0) of the total patients with diabetes were undiagnosed. Prevalence of prediabetes and diabetes stratified by age and sex is shown in Figs. 1 and 2. Furthermore, the weighted prevalence of diabetes and prediabetes by different age groups, sex, ethnicity, residential area, marital status, educational level, wealth index, physical activity, BMI, WHR, smoking status, and opium use is demonstrated in Supplementary Table 1.
Fig. 1.
Weighted prevalence of prediabetes among Iranian adults aged 35–70 years by sex and age
Fig. 2.
Weighted prevalence of diabetes among Iranian adults aged 35–70 years by sex and age
Table 1 illustrates the results of logistic regression to explore the factors associated with diabetes and prediabetes. The odds of diabetes and prediabetes increased steadily with age. Overweight and obese adults were more likely to develop diabetes compared to those with normal BMI (adjusted OR 1.43, 1.33–1.52 and adjusted OR 1.83, 1.70–1.97, respectively). High WHR was associated with significantly higher odds of diabetes compared to normal WHR (adjusted OR 2.20, 2.03–2.39). Moreover, rural residents were less likely to develop diabetes compared to the urban population (adjusted OR 0.79, 0.73–0.85). Participants who received education for ≤ 5, 6–8, 9–12, and > 12 years were less likely to develop diabetes compared to the illiterate counterparts. Moderate and high physical activity were associated with lower odds of diabetes compared to low physical activity (adjusted OR 0.79, 0.74–0.84 and adjusted OR 0.64, 0.61–0.69, respectively). Current smokers were less likely to develop diabetes compared to never-smokers (adjusted OR 0.82, 0.72–0.92). Regarding the prevalence of prediabetes, the results show that the odds of developing prediabetes increased with age. Overweight and obese adults were more likely to develop prediabetes compared to those with normal BMI (adjusted OR 1.41, 1.31–1.51 and adjusted OR 1.96, 1.73–2.23, respectively). High WHR was associated with significantly higher odds of prediabetes compared to normal WHR (adjusted OR 1.39, 1.29–1.49). Moreover, women were less likely to develop prediabetes compared to men (adjusted OR 0.69, 0.63–0.74). Married participants were less likely to develop prediabetes compared to non-married participants (adjusted OR 0.89, 0.85–0.92). Compared to the illiterate participants, those with higher education were less likely to develop prediabetes. High physical activity was associated with lower odds of prediabetes compared to low physical activity (adjusted OR 0.89, 0.81–0.99). Current smokers were less likely to develop prediabetes compared to the never-smokers (adjusted OR 0.80, 0.75–0.85).
Table 1.
Factors associated with prevalence of diabetes and prediabetes among Iranian adults between 2014 and 2020
| Diabetes | Prediabetes | |||
|---|---|---|---|---|
| Adjusted OR* | p value | Adjusted OR* | p value | |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 1.10 (1, 1.21) | 0.058 | 0.69 (0.63, 0.74) | < 0.001 |
| Age categories | ||||
| 35–44 | 1 | 1 | ||
| 45–54 | 2.71 (2.57, 2.87) | < 0.001 | 1.49 (1.36, 1.63) | < 0.001 |
| 55–64 | 4.86 (4.57, 5.17) | < 0.001 | 1.94 (1.74, 2.16) | < 0.001 |
| ≥ 65 | 5.73 (5.04, 6.53) | < 0.001 | 2.15 (1.80, 2.56) | < 0.001 |
| Residence | ||||
| Urban | 1 | 1 | ||
| Rural | 0.79 (0.73, 0.85) | < 0.001 | 0.81 (0.63, 1.06) | 0.116 |
| Marital status | ||||
| Non-married | 1 | 1 | ||
| Married | 1.02 (0.97, 1.08) | 0.467 | 0.89 (0.85, 0.92) | < 0.001 |
| Education | ||||
| Illiterate (no schooling) | 1 | 1 | ||
| ≤ 5 years (primary) | 0.87 (0.82, 0.93) | < 0.001 | 0.98 (0.91, 1.05) | 0.503 |
| 6–8 years (middle) | 0.84 (0.78, 0.9) | < 0.001 | 0.9 (0.83, 0.97) | 0.011 |
| 9–12 years (secondary) | 0.77 (0.73, 0.81) | < 0.001 | 0.88 (0.81, 0.96) | 0.004 |
| > 12 years (university) | 0.71 (0.68, 0.75) | < 0.001 | 0.84 (0.75, 0.94) | 0.004 |
| Wealth index | ||||
| Quintile 1 (poorest) | 1 | 1 | ||
| Quintile 2 | 1.06 (0.99, 1.14) | 0.112 | 1 (0.95, 1.05) | 0.953 |
| Quintile 3 | 1.06 (0.98, 1.15) | 0.144 | 1.02 (0.95, 1.11) | 0.537 |
| Quintile 4 | 1 (0.9, 1.1) | 0.949 | 1.01 (0.91, 1.12) | 0.795 |
| Quintile 5 (richest) | 1.02 (0.94, 1.1) | 0.704 | 0.97 (0.87, 1.07) | 0.491 |
| Body mass index | ||||
| Normal | 1 | 1 | ||
| Underweight | 0.39 (0.26, 0.57) | < 0.001 | 0.75 (0.55, 1.01) | 0.054 |
| Overweight | 1.43 (1.33, 1.52) | < 0.001 | 1.41 (1.31, 1.51) | < 0.001 |
| Obese | 1.83 (1.7, 1.97) | < 0.001 | 1.96 (1.73, 2.23) | < 0.001 |
| Physical activity | ||||
| Low | 1 | 1 | ||
| Moderate | 0.79 (0.74, 0.84) | < 0.001 | 0.95 (0.89, 1.02) | 0.155 |
| High | 0.64 (0.61, 0.69) | < 0.001 | 0.89 (0.81, 0.99) | 0.037 |
| Waist to hip ratio | ||||
| Normal | 1 | 1 | ||
| High | 2.2 (2.03, 2.39) | < 0.001 | 1.39 (1.29, 1.49) | < 0.001 |
| Smoking | ||||
| Never | 1 | 1 | ||
| Former | 1 (0.87, 1.16) | 0.997 | 0.92 (0.83, 1.04) | 0.167 |
| Current | 0.82 (0.72, 0.92) | 0.002 | 0.80 (0.75, 0.85) | < 0.001 |
| Ever opium use | ||||
| No | 1 | 1 | ||
| Yes | 1.00 (0.92, 1.08) | 0.979 | 1.05 (0.94, 1.17) | 0.393 |
| Ethnicity | ||||
| Fars (ref) | 1 | 1 | ||
| Azari | 0.98 (0.86, 1.13) | 0.826 | 0.97 (0.87, 1.09) | 0.628 |
| Balouch | 1.26 (1.21, 1.31) | < 0.001 | 1.03 (0.97, 1.08) | 0.297 |
| Kurd | 1.08 (0.8, 1.46) | 0.589 | 0.98 (0.87, 1.09) | 0.688 |
| Lor | 0.94 (0.77, 1.14) | 0.503 | 0.66 (0.38, 1.15) | 0.135 |
| Arab | 0.82 (0.56, 1.18) | 0.271 | 0.83 (0.71, 0.97) | 0.019 |
| Zaboli | 1.11 (1.08, 1.14) | < 0.001 | 0.81 (0.76, 0.87) | < 0.001 |
| Gilak | 1.04 (0.87, 1.25) | 0.652 | 0.94 (0.75, 1.18) | 0.584 |
| Turk nomad | 0.78 (0.7, 0.88) | < 0.001 | 1.01 (0.78, 1.31) | 0.937 |
| Arab nomad | 0.59 (0.32, 1.09) | 0.089 | 1.35 (1.04, 1.74) | 0.024 |
| Mazani | 0.93 (0.86, 1.01) | 0.072 | 1.00 (0.84, 1.19) | 0.991 |
| Mixed | 1.04 (0.92, 1.18) | 0.477 | 0.88 (0.74, 1.05) | 0.140 |
*All variables included in the adjusted model
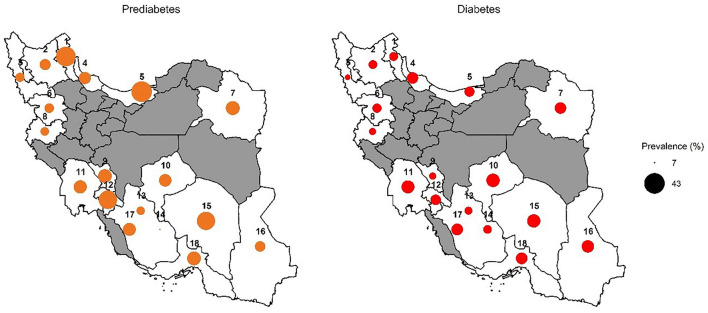
We further examined the prevalence of diabetes and prediabetes across the study centers (Fig. 3). The highest and lowest prevalence of diabetes was observed in Yazd (20.8%, 20–21.5) and Urmia Lake (8.3%, 7.6–9.00), respectively. The highest and lowest prevalence of prediabetes was observed in Sari (42.8%, 41.8–43.9) and Fasa (6.5%, 6.00–6.90), respectively.
Fig. 3.
Prevalence of diabetes and prediabetes across the study centers (Iran map)
Diabetes Awareness, Glycemic Control, and Associated Factors
The overall proportion of patients who were aware of their diabetes was 79.6% (76.2–82.9). The proportion of women with diabetes awareness was 81.9% (78.7–85.0). For men, it was 76.5% (72.5–80.5). Among patients who were under treatment for diabetes, 41.2% (37.5–44.8) had controlled FBS. Among treated women and men, the proportion of those with controlled FBS was 43.8% (40.0–47.7) and 37.2% (33.3–41.1), respectively. The proportion of awareness and glycemic control by age, sex, ethnicity, residential area, marital status, education level, wealth index, physical activity, BMI, WHR, smoking status, and opium use is demonstrated in Supplementary Table 2.
Table 2 shows the results of the multivariable logistic regression model to explore the factors associated with awareness and glycemic diabetes. Women were more likely to be aware of their diabetes compared to men (adjusted OR 1.58, 1.32–1.90). Awareness increased with age and was more prevalent among individuals with higher wealth scores. Married individuals were more likely to be aware of their diabetes compared to the non-married (adjusted OR 1.21, 1.01–1.45). Participants with high WHR were more likely to be aware of their diabetes compared to those with normal WHR (adjusted OR 1.33, 1.06–1.67). Participants who were overweight and obese were less likely to be aware of their diabetes compared to those with normal BMI (adjusted OR 0.66, 0.53–0.81 and adjusted OR 0.46, 0.39–0.55, respectively). Compared to the patients with low physical activity, those with moderate and high physical activity were less likely to be aware of their diabetes. Moreover, never-smokers were less likely to be aware of their diabetes (adjusted OR 0.86, 0.75–0.99). Regarding glycemic control, the following groups were more likely to have controlled FBS: women (adjusted OR 1.39, 1.19–1.63), obese individuals, (adjusted OR 1.31, 1.15–1.48), and participants with high physical activity (adjusted OR 1.09, 1.00–1.18). Glycemic control decreased with age. Moreover, participants with high WHR were less likely to have controlled FBS (adjusted OR 0.55, 95% CI 0.50–0.60).
Table 2.
Factors associated with awareness and control of diabetes among Iranian adults between 2014 and 2020
| Awareness | Control | |||
|---|---|---|---|---|
| Adjusted OR* | p value | Adjusted OR* | p value | |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 1.58 (1.32, 1.9) | < 0.001 | 1.39 (1.19, 1.63) | < 0.001 |
| Age categories | ||||
| 35–44 | 1 | 1 | ||
| 45–54 | 1.71 (1.44, 2.04) | < 0.001 | 0.66 (0.57, 0.76) | < 0.001 |
| 55–64 | 2.37 (2, 2.81) | < 0.001 | 0.70 (0.61, 0.8) | < 0.001 |
| ≥ 65 | 2.58 (1.98, 3.37) | < 0.001 | 0.79 (0.66, 0.93) | 0.007 |
| Residence | ||||
| Urban | 1 | 1 | ||
| Rural | 0.92 (0.73, 1.16) | 0.481 | 1.06 (0.87, 1.29) | 0.557 |
| Marital status | ||||
| Non-married | 1 | 1 | ||
| Married | 1.21 (1.01, 1.45) | 0.042 | 1.04 (0.92, 1.17) | 0.515 |
| Education | ||||
| Illiterate (no schooling) | 1 | 1 | ||
| ≤ 5 years (primary) | 0.89 (0.79, 1) | 0.058 | 1.15 (1, 1.32) | 0.054 |
| 6–8 years (middle) | 0.92 (0.76, 1.12) | 0.396 | 1.2 (1.01, 1.44) | 0.040 |
| 9–12 years (secondary) | 0.87 (0.68, 1.1) | 0.230 | 1.18 (0.91, 1.54) | 0.205 |
| > 12 years (university) | 0.79 (0.64, 0.98) | 0.030 | 1.48 (1.14, 1.92) | 0.004 |
| Wealth index | ||||
| Quintile 1 (poorest) | 1 | 1 | ||
| Quintile 2 | 1.22 (1.08, 1.37) | 0.003 | 0.9 (0.77, 1.06) | 0.200 |
| Quintile 3 | 1.12 (1.01, 1.25) | 0.034 | 0.97 (0.82, 1.14) | 0.677 |
| Quintile 4 | 1.25 (1.12, 1.4) | < 0.001 | 0.94 (0.78, 1.13) | 0.495 |
| Quintile 5 (richest) | 1.15 (1.01, 1.31) | 0.034 | 1.03 (0.87, 1.23) | 0.695 |
| Body mass index | ||||
| Normal | 1 | 1 | ||
| Underweight | 1.22 (0.64, 2.32) | 0.533 | 1.82 (0.85, 3.92) | 0.119 |
| Overweight | 0.66 (0.53, 0.81) | < 0.001 | 1.15 (0.98, 1.35) | 0.081 |
| Obese | 0.46 (0.39, 0.55) | < 0.001 | 1.31 (1.15, 1.48) | < 0.001 |
| Physical activity | ||||
| Low | 1 | 1 | ||
| Moderate | 0.81 (0.72, 0.91) | 0.001 | 1.06 (0.95, 1.19) | 0.284 |
| High | 0.73 (0.62, 0.86) | < 0.001 | 1.09 (1, 1.18) | 0.049 |
| Waist to hip ratio | ||||
| Normal | 1 | 1 | ||
| High | 1.33 (1.06, 1.67) | 0.014 | 0.55 (0.5, 0.6) | < 0.001 |
| Smoking | ||||
| Never | 1 | 1 | ||
| Former | 0.99 (0.82, 1.2) | 0.953 | 1.09 (0.91, 1.3) | 0.345 |
| Current | 0.86 (0.75, 0.99) | 0.034 | 1.01 (0.86, 1.19) | 0.867 |
| Ever opium use | ||||
| No | 1 | 1 | ||
| Yes | 1.01 (0.78, 1.3) | 0.936 | 1.08 (0.92, 1.28) | 0.325 |
| Ethnicity | ||||
| Fars | 1 | 1 | ||
| Azari | 1.02 (0.82, 1.27) | 0.846 | 1.17 (0.71, 1.93) | 0.521 |
| Balouch | 1.71 (1.47, 1.98) | < 0.001 | 0.85 (0.76, 0.94) | 0.003 |
| Kurd | 1.14 (0.56, 2.34) | 0.709 | 1.28 (0.53, 3.08) | 0.567 |
| Lor | 1.54 (0.93, 2.57) | 0.092 | 1.43 (0.99, 2.06) | 0.055 |
| Arab | 1.74 (1.4, 2.15) | < 0.001 | 0.83 (0.5, 1.4) | 0.473 |
| Zaboli | 1.30 (1.13, 1.49) | < 0.001 | 1.16 (1.1, 1.21) | < 0.001 |
| Gilak | 0.96 (0.8, 1.15) | 0.654 | 0.84 (0.55, 1.3) | 0.418 |
| Turk nomad | 1.02 (0.55, 1.89) | 0.951 | 1.23 (0.84, 1.79) | 0.283 |
| Arab nomad | 0.85 (0.72, 1.01) | 0.069 | 1.07 (0.75, 1.53) | 0.683 |
| Mazani | 1.31 (0.82, 2.07) | 0.246 | 1.22 (0.97, 1.54) | 0.084 |
| Mixed | 1.30 (1.03, 1.63) | 0.027 | 1.09 (0.78, 1.51) | 0.599 |
*All variables included in the adjusted model
Proportion of awareness and glycemic control across the study centers is illustrated in Supplementary Table 3. The highest and lowest proportion of awareness of diabetes was observed among inhabitants of Fasa (89.1%, 86.6–91.6) and Sari (59.9%, 56.2–63.6), respectively. The highest and lowest proportion of controlled FBS was observed among inhabitants of Fasa (69.8%, 66.5–73.1) and Hoveizeh (26.3%, 23.2–29.3), respectively.
Ethnic Variations
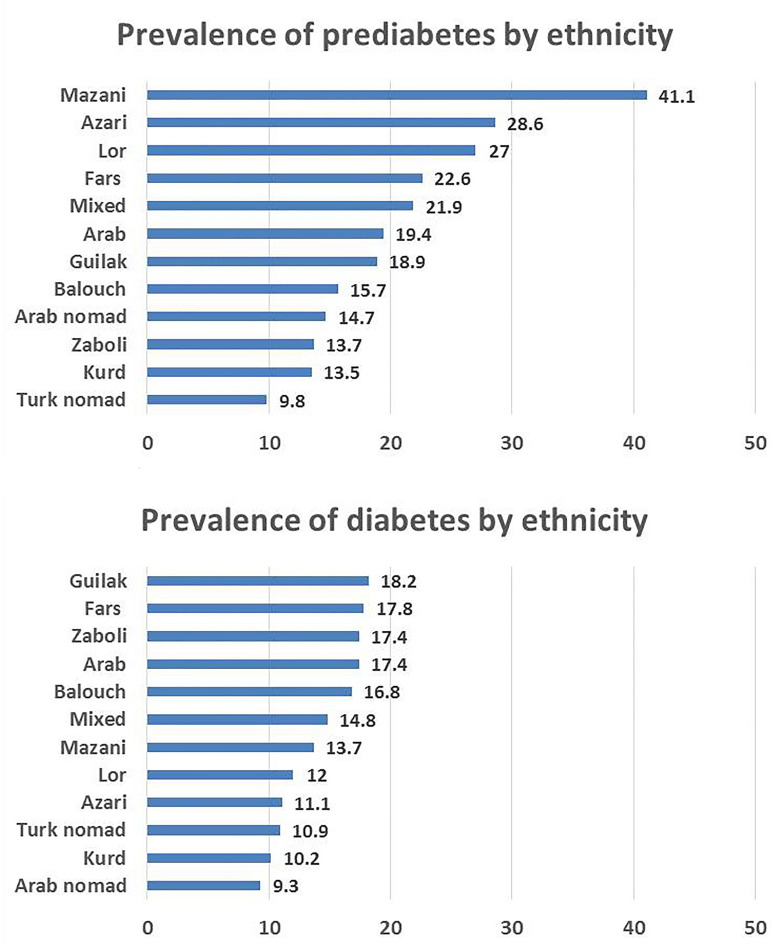
Prevalence of diabetes and prediabetes in different ethnic groups is illustrated in Fig. 4. Multivariable logistic regression was used to determine the role of ethnicity on prevalence of diabetes and prediabetes. Compared with Fars ethnic background, as the largest ethnic group in this cohort, Balouch and Zaboli were more likely to develop diabetes (adjusted OR 1.26, 1.21–1.31) and (adjusted OR 1.11, 1.08–1.14), respectively. However, Turk nomad was associated with significantly lower odds of diabetes (adjusted OR 0.78, 0.70–0.88). On the other hand, Arab nomad showed higher odds of prediabetes (adjusted OR 1.35, 1.04–1.74). However, Arab and Zaboli were associated with significantly lower odds of prediabetes (adjusted OR 0.83, 0.71–0.97 and adjusted OR 0.81, 0.76–0.87, respectively) (Table 1). Compared with Fars ethnic background, Balouch, Arab, and Zaboli, and those with mixed ethnicity were more likely to be aware of their diabetes.
Fig. 4.
Prevalence of diabetes and prediabetes among different ethnic groups
Regarding glycemic control, Zaboli ethnic background was more likely to have controlled FBS (adjusted OR 1.16, 1.10–1.21), while Balouch ethnic background was less likely to have good glycemic control (adjusted OR 0.85, 0.76–0.94) (Table 2).
Discussion
In this large national study representative of major ethnic groups living in different geographical areas of Iran, it was estimated that 15.0% and 25.4% of adults, aged 35–70 years, had diabetes and prediabetes, respectively. Furthermore, it includes updates on the prevalence of diabetes among different ethnic groups.
The prevalence of diabetes and prediabetes in this study was higher than those reported in 2011: 15.0% vs. 11.37% in 2011 and 25.4% vs. 14.6% in 2011, respectively [3]. Given the similar age groups and definitions applied in these two national surveys, the increase in the prevalence of diabetes and prediabetes highlights the urgent need for developing and implementing prevention strategies.
The global prevalence of diabetes reported by the IDF was 8.5% in 2014. It rose to 8.8% in 2017, and 9.3% in 2019 [2, 15, 16]. Moreover, based on data from National Health and Nutrition Examination Survey (NHANES) 2013 to 2016, the estimated prevalence of diabetes and prediabetes among US adults was 13.5% and 37.6%, respectively [17]. Our results showed that the prevalence of diabetes is considerably higher than the global estimations during the past decades. Although the higher estimation of diabetes prevalence in this study might be to some extent due to the age range of the participants, the results are alarming.
Consistent with prior studies, we also found that age, BMI, and WHR are incrementally associated with a significant increase in the risk of diabetes and prediabetes [18, 19], while higher physical activity is protective [18, 20]. These modifiable risk factors are the main target of diabetes prevention programs [21, 22].
Moreover, the association between cigarette smoking and diabetes and prediabetes is challenging. Although some studies, similar to ours, found protective effects of cigarette smoking on prediabetes and diabetes [23–25], a large body of evidence demonstrated a dose–response association between cigarette smoking and diabetes [26, 27] as well as prediabetes [28, 29]. Moreover, the effect of quitting smoking on diabetes is also conflicting. A meta-analysis of 22 prospective studies in Japan indicated that the risk of diabetes steadily decreased after smoking cessation to a risk level comparable to that of never-smokers [26]. On the contrary, some studies showed that quitting may increase the risk of diabetes [30, 31], possibly due to the weight gain and increase in the waist circumference that occur after quitting [32]. Since the relationship between smoking and diabetes is controversial, a cause–effect link between cigarette smoking and diabetes needs to be confirmed through well-designed studies controlling all possible confounders. Nevertheless, the value of smoking cessation could not be overlooked. Although some studies reported the effect of opium on control of hyperglycemia in patients with diabetes, no study explored the association of opium use and the risk of developing diabetes. We found no association between opium use and diabetes risk as well as diabetes control. The effect of opium use on glycemic control is controversial [33–35]. Further research is required to get a robust conclusion on association of opium use and risk of diabetes or its control.
Upon controlling all confounders, we found that educational attainment was associated with a lower risk of diabetes and prediabetes. However, we found no significant relationship between household wealth and the presence of diabetes or prediabetes. Unlike well-characterized traditional risk factors for diabetes, such as older age, higher BMI, and lower physical activity, the relationship between SES and diabetes is complex and may differ across countries at varying levels of economic development. A study that investigated the prevalence of diabetes and its relationship with education and wealth in low- and middle-income countries (LMIC) found that educational attainment and wealth are associated with the increased prevalence of diabetes in these countries [36]. It has been proposed that the rapid economic progression of LMIC is associated with large shifts in dietary and physical activity patterns [37, 38]. Thus, in high-income countries, diabetes tends to be more prevalent among populations with lower SES [39, 40]. Some studies demonstrated an inverse association between the prevalence of diabetes and educational attainment as well as household wealth, although this inverse relationship was not observed in some racial/ethnic groups [41, 42]. However, the relationship between educational level and diabetes is complex and is mediated by lifestyle, behavior, BMI, access to health services, and knowledge of health promotion [43]. Thus, improvement of the educational level of the general population could reduce the risk of diabetes through changes in these well-established risk factors. Moreover, evaluation of the association between SES and diabetes risk in the context of a dynamic socioeconomic gradient can help to better identify high-risk individuals.
Iran is a multiethnic nation with different ethnic groups including Fars, Kurds, Lors, Arabs, Balouch, Turkmen, Azari, Mazani, Gilak, Zaboli, Turk Nomad, and Arab Nomad. There are substantial differences in genetic background, culture, socioeconomic status, climate and geographic characteristics, lifestyle, and dietary patterns among various ethnic groups. Upon adjustment for all possible risk factors, Balouch and Zaboli groups were more likely to have diabetes while the Turk Nomad group was less likely to have diabetes. The large sample size of this study provides a comprehensive comparison of diabetes prevalence among different ethnic groups in Iran and helps us to identify the high-risk populations. The reason why Turk Nomads were less likely to develop diabetes might be explained by the healthy lifestyle they have. They are physically active and usually consume natural and fresh food. However, more studies are needed to identify the risk or protective factors associated with diabetes in this specific population.
Our findings revealed that 79.6% of the total population was aware of their diabetes. However, only 41.2% had controlled FBS as it is defined by the ADA [14]. The rate of awareness of diabetes is to some extent higher than that reported in 2011 in Iran (76.14%) [3]. Moreover, glycemic control was considerably higher than what was reported previously in Iran (30.1%) [4]. Although the former study was conducted among Iranian clinically registered adult patients with different types of diabetes, the higher rate of glycemic control in the present study represents an improvement in the surveillance programs. From 2013 to 2016, 10.9% of US adult men and 8.9% of US adult women with diabetes reported being told that they had diabetes by a health care professional [44]. Moreover, based on data from NHANES 2013 to 2016, 20.9% of US adults had their diabetes controlled [17].
To better understand the barriers to diabetes awareness and control, we further investigated the possible associated factors. The results showed that age and WHR as well as specific ethnic backgrounds were significantly associated with a higher rate of awareness but not better glycemic control. Women were more likely to be aware of their diabetes and had controlled FBS. Wealth index and being married were also associated with a higher rate of diabetes awareness, with no effect on diabetes control.
Although the participants with more than 12 years of education and those with high physical activity were less likely to be aware of diabetes, they better control their diabetes.
There are controversial reports on the association between demographic characteristics as well as some aspects of SES and control of diabetes [45–47]. Results from a national population-based survey in Iran in 2005 found that control of FBS level was better among relatively younger patients with diabetes and in rural areas [45]. We found that older patients with diabetes are more likely to be aware of their diabetes, although they are less likely to control their diabetes. This might be due to the presence of multiple comorbidities in the elderly which leads to more medical attention and makes detection of diabetes more likely and control of FBS level less likely. Contrary to the previous study in Iran, we found no association between residential region and diabetes awareness and glycemic control. The reason that the study in 2005 found better control of diabetes among rural residents might be due to the successful primary health care (PHC) and effective management of NCD by community health workers in rural areas in Iran [48]. Thus, policymakers should reform PHC programs through the improvement of the performance and quality of services. The results emphasized that revision at PHC programs seems to be essential. Although some previous studies concluded that literacy level has no significant effect on the control of diabetes [45, 49], we found that educational attainment is associated with better control of diabetes. The education level may affect glycemic control by influencing self-care behaviors. Educated individuals are more likely to attend diabetes education programs, follow the rules of a healthy diet, and diabetes self-management. A possible explanation for the controversy observed in different studies might be the various definitions used for glycemic control and educational level. We did not find any significant association between wealth index and awareness or control of diabetes. However, a previous study demonstrated that patients with the highest household income were less likely to reach HbA1c < 7.0% [47]. Frequent attendance of high-income individuals at dinner parties was expressed as a possible reason [47]. On the contrary, some studies concluded that individuals with low SES have worse glycemic control than those with high SES [50, 51]. It is suggested that the effects of SES on glycemic control are mediated by perception of the disease, coping with diabetes-related stress, depressive symptoms, and diet regimen [52]. Moreover, the effect of SES on glycemic control might be different across various ethnic groups [51, 53]. However, the association between socioeconomic inequality and diabetes control is complex and affected by various factors that have not been taken into account in all studies. Although there is no one-size-fits-all solution, the reduction of social inequality is crucial for better glycemic control in the deprived population.
Strengths and Limitations
This study was conducted in a large population-based sample in Iran following a strict quality assurance and control program. To the best of our knowledge, this is the first study that included a variety of major ethnic groups living in different geographical areas of Iran and provides a direct comparison of diabetes prevalence among different ethnicities. This study also has some limitations. Diagnosis of diabetes and prediabetes as well as assessment of achieving glycemic control were based on measurement of FBS. Moreover, we did not distinguish between type 1 and type 2 diabetes.
Conclusion
The estimated overall prevalence of diabetes was 15% and that of prediabetes was 25.4% in Iran during 2014–2020. Moreover, the proportion of individuals with controlled diabetes is relatively low (41.2%). We suggest that the health care system should put more emphasis on controlling modifiable sociodemographic risk factors of diabetes, especially in high-risk ethnic groups.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study used the data obtained from the PERSIAN (Prospective Epidemiological Research Studies in IrAN) Cohort study in Iran. The Iranian Ministry of Health and Medical Education has contributed to the funding used in the PERSIAN Cohort through Grant no. 700/534.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
MEK designed the project, interpreted results, and critically revised the manuscript. SGS analyzed and cleaned the data, and revised the manuscript. NHM wrote and revised the manuscript. FJ, AHM, EF, HOA, ZR, AR, RH, FM, NV, MK, EZ, AM, AA, BH, JH, FP, MRA, ARS, and NA collected data and revised the manuscript. ZM and SE collected and cleaned data. HP designed the project and critically revised the manuscript. RM took responsibility for the project. All authors had full access to the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosures
Mohammad. E Khamseh, Sadaf G. Sepanlou, Nahid Hashemi-Madani, Farahnaz Joukar, Amir Houshang Mehrparvar, Elnaz Faramarzi, Hssan Okati-Aliabad, Zahra Rahimi, Abbas Rezaianzadeh, Reza Homayounfar, Farhad Moradpour, Neda Valizadeh, Masoumeh Kheirandish, Ehsan Zaboli, Alireza Moslem, Ali Ahmadi, Behrooz Hamzeh, Javad Harooni, Farhad Pourfarzi, Mohammad Reza Abolghasemi, Ali Reza Safarpour, Nayyereh Aminisani, Zahra Mohammadi, Sareh Eghtesad, Hossein Poustchi and Reza Malekzadeh have nothing to disclose.
Compliance with Ethics Guidelines
PERSIAN was approved by the ethics committees of the Digestive Disease Research Institute in Tehran University of Medical Sciences and the Medical Sciences Universities supervising each cohort in local study centers. The study was performed in accordance with the Helsinki Declaration and its later amendments. Informed consent was obtained from all individual participants included in the study.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Mohammad E. Khamseh and Sadaf G. Sepanlou have contributed equally to this manuscript as co-first authors.
Contributor Information
Hossein Poustchi, Email: h.poustchi@gmail.com.
Reza Malekzadeh, Email: malek@tums.ac.ir, Email: dr.reza.malekzadeh@gmail.com.
References
- 1.Miranda S, Marques A. Pilates in noncommunicable diseases: a systematic review of its effects. Complement Ther Med. 2018;39:114–130. doi: 10.1016/j.ctim.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.Esteghamati A, Etemad K, Koohpayehzadeh J, et al. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005–2011. Diabetes Res Clin Pract. 2014;103(2):319–327. doi: 10.1016/j.diabres.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 4.Esteghamati A, Larijani B, Aghajani MH, et al. Diabetes in Iran: prospective analysis from first nationwide diabetes report of National Program for Prevention and Control of Diabetes (NPPCD-2016) Sci Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-13379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Geneva: WHO; 2013.
- 6.Alomar MJ, Al-Ansari KR, Hassan NA. Comparison of awareness of diabetes mellitus type II with treatment’s outcome in term of direct cost in a hospital in Saudi Arabia. World J Diabetes. 2019;10(8):463. doi: 10.4239/wjd.v10.i8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somannavar S, Lanthorn H, Deepa M, Pradeepa R, Rema M, Mohan V. Increased awareness about diabetes and its complications in a whole city: effectiveness of the “prevention, awareness, counselling and evaluation” [PACE] Diabetes Project [PACE-6] J Assoc Physicians India. 2008;56:497–502. [PubMed] [Google Scholar]
- 8.Lefebre P, Pierson A. Prevention through awareness-raising global awareness of diabetes and its complications. Eur Endocrinol. 2006;2:24–28. doi: 10.17925/EE.2006.00.02.24. [DOI] [Google Scholar]
- 9.Petty RE, Cacioppo JT. Communication and persuasion: central and peripheral routes to attitude change. Berlin: Springer; 2012. pp. 231–242. [Google Scholar]
- 10.Kayyali R, Slater N, Sahi A, Mepani D, Lalji K, Abdallah A. Type 2 diabetes: how informed are the general public? A cross-sectional study investigating disease awareness and barriers to communicating knowledge in high-risk populations in London. BMC Public Health. 2019;19(1):138. doi: 10.1186/s12889-019-6460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deepa M, Bhansali A, Anjana RM, et al. Knowledge and awareness of diabetes in urban and rural India: the Indian Council of Medical Research India Diabetes Study (Phase I): Indian Council of Medical Research India Diabetes 4. Indian J Endocrinol Metab. 2014;18(3):379–385. doi: 10.4103/2230-8210.131191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poustchi H, Eghtesad S, Kamangar F, et al. Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): rationale, objectives, and design. Am J Epidemiol. 2018;187(4):647–655. doi: 10.1093/aje/kwx314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. Atlanta, Ga, USA, 2009. https://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/lab.pdf. Accessed 20 June 2021.
- 14.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Supplement 1):S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Global report on diabetes: executive summary. Geneva, Switzerland. 2016. https://www.who.int/publications/i/item/9789241565257. Accessed 18 June 2021.
- 16.Cho NH, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. National diabetes statistics report. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services. 2020:12-5. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Accessed 19 June 2021.
- 18.Joseph JJ, Echouffo-Tcheugui JB, Carnethon MR, et al. The association of ideal cardiovascular health with incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis. Diabetologia. 2016;59(9):1893–1903. doi: 10.1007/s00125-016-4003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Lacy ME, Jankowich M, Correa A, Wu W-C. Association between obesity phenotypes of insulin resistance and risk of type 2 diabetes in African Americans: the Jackson Heart Study. J Clin Transl Endocrinol. 2020;19:100210. doi: 10.1016/j.jcte.2019.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyu HH, Bachman VF, Alexander LT, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ Clin Res Ed. 2016;354:i3857. doi: 10.1136/bmj.i3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman WH, Pan Q, Edelstein SL, et al. Impact of lifestyle and metformin interventions on the risk of progression to diabetes and regression to normal glucose regulation in overweight or obese people with impaired glucose regulation. Diabetes Care. 2017;40(12):1668–1677. doi: 10.2337/dc17-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegel KR, Bullard KM, Imperatore G, et al. Prevalence of major behavioral risk factors for type 2 diabetes. Diabetes Care. 2018;41(5):1032–1039. doi: 10.2337/dc17-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Chen J, Wang Y, et al. Cigarette smoking is negatively associated with the prevalence of type 2 diabetes in middle-aged men with normal weight but positively associated with stroke in men. J Diabetes Res. 2019;2019:1853018. doi: 10.1155/2019/1853018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onat A, Ozhan H, Esen AM, et al. Prospective epidemiologic evidence of a “protective” effect of smoking on metabolic syndrome and diabetes among Turkish women–without associated overall health benefit. Atherosclerosis. 2007;193(2):380–388. doi: 10.1016/j.atherosclerosis.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Hou X, Qiu J, Chen P, et al. Cigarette smoking is associated with a lower prevalence of newly diagnosed diabetes screened by OGTT than non-smoking in Chinese men with normal weight. PLoS One. 2016;11(3):e0149234. doi: 10.1371/journal.pone.0149234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akter S, Goto A, Mizoue T. Smoking and the risk of type 2 diabetes in Japan: a systematic review and meta-analysis. J Epidemiol. 2017;27(12):553–561. doi: 10.1016/j.je.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298(22):2654–2664. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 28.Aeschbacher S, Schoen T, Clair C, et al. Association of smoking and nicotine dependence with pre-diabetes in young and healthy adults. Swiss Med Wkly. 2014;144:w14019. doi: 10.4414/smw.2014.14019. [DOI] [PubMed] [Google Scholar]
- 29.Bucheli JR, Manshad A, Ehrhart MD, Camacho J, Burge MR. Association of passive and active smoking with pre-diabetes risk in a predominantly Hispanic population. J Investig Med. 2017;65(2):328–332. doi: 10.1136/jim-2016-000246. [DOI] [PubMed] [Google Scholar]
- 30.Akter S, Okazaki H, Kuwahara K, et al. Smoking, smoking cessation, and the risk of type 2 diabetes among Japanese adults: Japan Epidemiology Collaboration on Occupational Health Study. PLoS One. 2015;10(7):e0132166. doi: 10.1371/journal.pone.0132166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung YT, Hsiao CT, Chang IJ, Lin YC, Yueh CY. Smoking cessation carries a short-term rising risk for newly diagnosed diabetes mellitus independently of weight gain: a 6-year retrospective cohort study. J Diabetes Res. 2016;2016:3961756. doi: 10.1155/2016/3961756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y, Zong G, Liu G, et al. Smoking cessation, weight change, type 2 diabetes, and mortality. New Engl J Med. 2018;379(7):623–632. doi: 10.1056/NEJMoa1803626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Najafi M, Sheikhvatan M. Plausible impact of dietary habits on reduced blood sugar in diabetic opium addicts with coronary artery disease. Cardiovasc Res. 2012;6(3):75–78. [PMC free article] [PubMed] [Google Scholar]
- 34.Gozashti MH, Yazdi F, Salajegheh P, Dehesh MM, Divsalar K. Fasting blood glucose and insulin level in opium addict versus non-addict individuals. Addiction Health J. 2015;7(1–2):54. [PMC free article] [PubMed] [Google Scholar]
- 35.Azod L, Rashidi M, Afkhami-Ardekani M, Kiani G, Khoshkam F. Effect of opium addiction on diabetes. Am J Drug Alcohol Abuse. 2008;34(4):383–388. doi: 10.1080/00952990802122580. [DOI] [PubMed] [Google Scholar]
- 36.Gurung MS, Guwatudde D, Msaidié M, et al. Diabetes prevalence and its relationship with education, wealth, and BMI in twenty-nine low-and middle-income countries. Diabetes Care. 2020;43:1. doi: 10.2337/dc19-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popkin BM. An overview on the nutrition transition and its health implications: the Bellagio meeting. Public Health Nutr. 2002;5(1a):93–103. doi: 10.1079/phn2001280. [DOI] [PubMed] [Google Scholar]
- 38.Deepa M, Anjana RM, Manjula D, Narayan KM, Mohan V. Convergence of prevalence rates of diabetes and cardiometabolic risk factors in middle and low income groups in urban India: 10-year follow-up of the Chennai Urban Population Study. J Diabetes Sci Technol. 2011;5(4):918–927. doi: 10.1177/193229681100500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espelt A, Borrell C, Roskam AJ, et al. Socioeconomic inequalities in diabetes mellitus across Europe at the beginning of the 21st century. Diabetologia. 2008;51(11):1971–1979. doi: 10.1007/s00125-008-1146-1. [DOI] [PubMed] [Google Scholar]
- 40.Clark ML, Utz SW. Social determinants of type 2 diabetes and health in the United States. World J Diabetes. 2014;5(3):296–304. doi: 10.4239/wjd.v5.i3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borrell LN, Dallo FJ, White K. Education and diabetes in a racially and ethnically diverse population. Am J Public Health. 2006;96(9):1637–1642. doi: 10.2105/AJPH.2005.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacerdote C, Ricceri F, Rolandsson O, et al. Lower educational level is a predictor of incident type 2 diabetes in European countries: the EPIC-InterAct study. Int J Epidemiol. 2012;41(4):1162–1173. doi: 10.1093/ije/dys091. [DOI] [PubMed] [Google Scholar]
- 43.Winkleby MA, Jatulis DE, Frank E, Fortmann SP. Socioeconomic status and health: how education, income, and occupation contribute to risk factors for cardiovascular disease. Am J Public Health. 1992;82(6):816–820. doi: 10.2105/ajph.82.6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag. 2017;20(1):6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirzazadeh A, Baradaran HR, Haghdoost AA, Salari P. Related factors to disparity of diabetes care in Iran. Med Sci Mon Int Med J Exp Clin Res. 2009;15(5):Ph32–Ph36. [PubMed] [Google Scholar]
- 46.Badedi M, Solan Y, Darraj H, et al. Factors associated with long-term control of type 2 diabetes mellitus. J Diabetes Res. 2016;2016:2109542. doi: 10.1155/2019/8756138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tao X, Li J, Zhu X, et al. Association between socioeconomic status and metabolic control and diabetes complications: a cross-sectional nationwide study in Chinese adults with type 2 diabetes mellitus. Cardiovasc Diabetol. 2016;15:61. doi: 10.1186/s12933-016-0376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tabrizi JS, Pourasghar F, Nikjoo RG. Status of Iran’s primary health care system in terms of health systems control knobs: a review article. Iran J Public Health. 2017;46(9):1156. [PMC free article] [PubMed] [Google Scholar]
- 49.Jahanlou AS, Alishan KN. The effect of literacy level on health related-quality of life, self-efficacy and self-management behaviors in diabetic patients. Acta Med Iran. 2011;49(3):153–158. [PubMed] [Google Scholar]
- 50.Jotkowitz AB, Rabinowitz G, Raskin Segal A, Weitzman R, Epstein L, Porath A. Do patients with diabetes and low socioeconomic status receive less care and have worse outcomes? A national study. Am J Med. 2006;119(8):665–669. doi: 10.1016/j.amjmed.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 51.James GD, Baker P, Badrick E, Mathur R, Hull S, Robson J. Ethnic and social disparity in glycaemic control in type 2 diabetes; cohort study in general practice 2004–9. J R Soc Med. 2012;105(7):300–308. doi: 10.1258/jrsm.2012.110289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houle J, Lauzier-Jobin F, Beaulieu MD, et al. Socioeconomic status and glycemic control in adult patients with type 2 diabetes: a mediation analysis. BMJ Open Diabetes Res Care. 2016;4(1):e000184. doi: 10.1136/bmjdrc-2015-000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Assari S, Moghani Lankarani M, Piette JD, Aikens JE. Socioeconomic status and glycemic control in type 2 diabetes; race by gender differences. Healthcare. 2017 doi: 10.3390/healthcare5040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.