Abstract
The differences of interaction between interphase microbial communities were evaluated caused by two kinds of Daqu, including conventional Daqu (CDQ) and fortified Daqu (FDQ). The community diversity, functional genera and metabolites in pit mud (PM) and Zaopei (ZP) were investigated by polyphasic detecting approaches. FDQ evolved the core microbial community fitting Baijiu brewing faster than CDQ. Compared with CPM, the abundance of Aspergillus, Hyphopichia, and Penicillium in FPM were 1.54, 14.75, and 1.68 times, while that of Lactobacillus, Bacillus, Methanobrevibacter, and Methanosaeta were 2.13, 1.85, 6.35, and 3.36 times, respectively. Furthermore, the content of key flavor components was increased in ZP using FDQ. These results suggested the interaction between interphase microbial communities in various phases of Baijiu fermentation niches was significant influenced by Daqu. It can not only enhance the key volatiles in ZP but also evolve the community to fit Baijiu fermentation by introducing functional genera to Daqu.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-021-00975-z.
Keywords: Exogenous microbiota, Strong-flavor Baijiu, Artificial pit mud, Community diversity, Metabolites
Introduction
As one of the famous distilled spirits in the world, Baijiu has been attracted much interest for its unique aroma, good taste, and production technique. Strong-flavor Baijiu, one of three kinds of typical Baijiu, accounted for the majority market share in the annual output of traditional Baijiu (Tao et al., 2017). The quality and yield of fresh Baijiu are closely related to the bioactive of pit mud (PM) and pit ages. PM, a kind of special clay perched microbial community, is involved in various functional strains for Baijiu fermentation. The community is formed throughout a long-time process of natural domestication unremittingly or by an artificial cultivation technology (Zhang et al., 2014) (Ding et al., 2014; Zhang et al., 2014). Zaopei (ZP) is a mixture, which was composed of streamed grain, Daqu powder, rice husk, fermented grain, etc., the main biochemical reactions, such as the conversion of starch into alcohol and other components, occurred here (Shi et al., 2011). As the essential starter and unique raw materials, a large number of microbes of Baijiu fermentation were originated from Daqu (Wang et al., 2017). Therefore, the microbial community diversity, as well as the quality of Daqu was closely related to the quality and yield of fresh Baijiu (Gou et al., 2015). For example, the quality of fresh Baijiu was enhanced by fortified Daqu (FDQ), meanwhile, the abundance of some functional genera also increased in both ZP and PM, such as Caproiciproducens, Clostridium, Methanobacterium, Methanosarcina, etc. (He et al., 2020). However, Baijiu fermentation was performed by the synergistic effect of microbes originated from three-phase, including PM, ZP, and Huang Shui (Ding et al., 2015; Li et al., 2017). These functional genera and species were enriched by niches selecting, biogeochemical properties, and the interspecific interactions among the microbial communities during the artificial pit mud (APM) manufacturing (Zhang et al., 2015). The shift of eubacterial and archaeal communities in APM used from one year to four years was investigated, these results indicated that the abundance of functional genera and species for bacteria became dominant, accounted for 46.20% in the first two years, and then tended to be stable (Ding et al., 2014). How to change the functional microbes in the APM was unclear so far.
Two kinds of Daqu were added aiming to know how to influence the community composition in PMs by ZPs. Of them, CDQ was added to one group and FDQ was added to another group in the simulated pits. The difference in the community composition was characterized by combing fluorescence in situ hybridization (FISH) with the Illumina Miseq platform. The volatile metabolites were examined by HS-HPME-GC–MS. To the best of our knowledge, this is the first report that reveals the influence of the microbial composition of Daqu on that of ZP and PM comprehensively. It will lay a foundation that accelerating the functional microbial communities evolving by applying FDQ.
Materials and methods
Materials
APM, FDQ, conventional Daqu (CDQ), sorghum, and ZP were supplied by Luzhou Laojiao Co. Ltd (Luzhou, China). APM was cultured for six months according to the process described in previous literature (Zhang et al., 2015). Daqu was produced according to the manufacturing process described by He et al. (He et al., 2019), the main difference between the two kinds of Daqu was that the fortified Daqu (FDQ) was inoculated Bacillus velezensis and B. subtilis.
Experiment equipment and procedure
Simulated pits: gauze was hung on the wall and bottom of the 5 L plastic box, the thickness of 2–3 cm and 5–5.5 cm APM was spread evenly on the wall and bottom of the gauze, respectively. The container was used as simulated pits.
Fermentation procedure: the procedure was carried out according to DB510500/T36-2016 with some modification. Fresh ZP is a mixture composed of sorghum, fermented grains, and pre-cooked rice husk with a proportion of 1: 4: 0.2. After cooked and cooled to 25 °C–28 °C, Daqu powder was added into the ZP by the ratio of 20% corresponding to the weight of sorghum. Soon afterward, ZP was embedded into the simulated pits, and sealed by 3–5 cm of a thickness APM, fermented under ambient temperature for 60 days. Corresponding PM and ZP were abbreviated as FPM and FZP, respectively, when FDQ powder was used. Similarly, those samples were abbreviated as CPM and CZP when CDQ powder was used.
The whole process of simulated pit fermentation was based on the fermentation process of Chinese Luzhou-flavor liquor, as shown in Fig. 1.
Fig. 1.
Manufacture process of Chinese strong-flavor liquor
Analyzing methods
Physicochemical properties analyzing
The content of acidity, starch, reducing sugar, and moisture was detected according to the previous literature (Jacinto et al., 2003).
Metabolites determining
Before analysis, 5.00 g of each ZP and PM were properly diluted with 9.00 mM of sulfuric acid (H2SO4 in ultrapure water). The suspensions were eddied for 5 min and treated with ultrasonication for 1 h at 100 w, 40 kHz. During ultrasonication, they were eddied for 1 min per 15 min to extract the organic acid sufficiently. Subsequently, they were centrifuged (10,000 × g, 4 °C) for 5 min, and the supernatant was purified by SPE column (Swell scientific instruments Co., Ltd. Chengdu, China), filtered through a 0.22 µm filter (Micron Separation Inc., Westborough, MA, USA). The filtered samples were injected into the Agilent 1260 HPLC (Agilent Technologies, Santa Clara, CA, USA) system equipped with an Alltech OA-1000 organic acid column (300 mm × 6.5 mm, Grace, Columbia, USA) maintained at 75 °C according to the operation condition described by Liang et al. (Liang et al., 2019). Organic acids were quantified by external standards.
Volatile components determining
Volatiles were extracted using a DVB/CAR/PDMS fiber (Supelco, Inc., Bellefonte, PA, USA) according to the method described by Zheng et al. (Zheng et al., 2014). Then, the adsorbed volatiles were analyzed by GC–MS-MS (Trace GC Ultra-DSQ II GC–MS, Thermo Electron Corporation, Waltham, MA, USA), equipped with HP-Innowax (30 m × 0.25 mm × 0.25 μm, Agilent J&W). The detection protocol was the same as described by Zhang et al. (Zhang et al., 2016). Each volatile compound was identified by comparing its mass spectrum with those in the NIST library database (Finnigan Co., Silicon Valley, CA, USA), based on the following criterion: similarity (SI) > 800 (the highest value is 1000). The analytical data were processed with X-Caliber software, the contents of each volatile component were calculated by peak area normalization utilizing the concentration ratio of internal standard to compounds. The concentration of compounds was finally described as mean ± standard deviation.
Microbial community diversity
Fluorescence in situ hybridization analysis: Samples were pretreated before the examination. 5.00 g of each sample was suspended in 30 mL of phosphate buffer solution (10 mmol/L, pH 7.2). The mixture eddied-well was centrifuged (800 × g, 4 °C) for 10 min, and the precipitates were washed thrice with the same buffer, and then the supernatants were pooled and centrifuged again (12,000 × g, 4 °C) for 10 min. The resulting pellets were washed thrice with the same buffer and stored at − 20 °C. The number of microbes in the pellet was quantitatively analyzed by FISH technology described by Ding et al. (Ding et al., 2014). Fluorescently labeled oligo-probes were synthesized as previous documents (Crocetti et al., 2006; Ficker et al., 1999; Friedrich et al., 1999; Hu et al., 2018; Raskin et al., 1994). All oligonucleotide probes we used have been listed in Table S1 and were synthesized with the dye Cy3 at the 5’ end by Sangon (Shanghai, China).
Community diversity characterization based on the sequencing results: Genomic DNA was extracted according to the operating instructions of the Fast DNA SPIN extraction kit (MP Biomedicals Santa Ana, CA. USA), and the extracted DNA was tested for its purity and concentration by 1% agarose gel electrophoresis and ultraviolet spectrophotometer. The primers listed in Table S2 were used to amplify, respectively, referenced the methods described by Li et al. (Li et al., 2014). PCR amplicons were purified with Agencourt AMPure Beads (Beckman Coulter, Indianapolis, IN, USA) and quantified using the PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, USA). After quantifying with NanoDrop ND-1000 spectrophotometer, homo-molecular mixing was carried out, and pair-end 2 × 300 bp sequencing was performed with MiSeq Reagent Kit v3 by the Illumina MiSeq platform (NovaSeq Sequenator, Shanghai Personal Biotechnology Co., Ltd., Shanghai, China). The Quantitative Insights Into Microbial Ecology (QIIME, v1.8.0) pipeline was employed to process the low-quality sequences (length below 150 bp, average Phred scores less than 20, mononucleotide repeats over 8 bp, and with ambiguous bases) (Chen and Jiang, 2014; Steven et al., 2006) as previously described (Caporaso et al., 2010). After chimera detection, the remaining high-quality sequences were clustered into operational taxonomic units (OTUs) at 97% sequence identity by UCLUST (Edgar, 2010).
Metabolic pathway predicting.
The metabolic pathway was predicted by PICRUST2 described by Gavin et al. (Gavin et al., 2020). The known 16S rRNA/18S rRNA/ITS gene sequences were aligned to construct the evolutionary tree and infer the gene function spectrum. Align the 16S rRNA/18S rRNA/ITS feature sequence with the reference sequence to construct a new evolutionary tree. Hidden-state prediction algorithms from Castor (Louca and Doebeli, 2018) was used to infer gene family copy number corresponding to the reference sequence in the evolutionary tree. Calculate the nearest sequence species index (NTSI) and exclude the sequences of NTSI > 2. The copy number of gene families per sample was calculated combined with the abundance of characteristic sequences. Hierarchical processing was used here, which means the functional units of different feature sequences are not combined for processing. Gene families were mapped to various databases. MinPath (Ye and Thomas, 2009) was used to infer the existence of metabolic pathways to obtain the abundance data.
Statistical analysis
Mothur (v1.31.2) and QIIME (V. 8.0, ttp:/org/) are used to assess the α-diversity and β-diversity with the OTUs abundance > 0.5%. R software was applied to draw heat map realizing visualization and clustering and to perform redundancy analysis as well. The gene function was enriched to obtain the bacterial metabolic pathway in combination with KEGG or COG database. All the data has three parallels, so the results are expressed as data plus standard deviation.
Accession number of nucleotide sequences
The raw sequencing data sets have been deposited in the NCBI Sequence Read Archive (SRA, http://www.ncbi.nlm.nih.gov/Traces/sra) with accession number PRJNA701113.
Results and discussion
Difference of microbial community profiles based on the results of FISH
As shown in Table 1, the quantity of total bacteria in both PM and ZP were higher based on the results detected by the DAPI probe when fermented. The quantity of archaea in FPM was higher compared with CPM. While the quantity of archaea in CPM, as well as that of eubacteria in both FZP and CZP, were decreased. As the major functional genus and species, the quantity of Clostridium, and Clostridium kluyveri increased by 70.75% and 46.43% in CPM and FPM, respectively. However, the quantity of Clostridium only enhanced 13.08% in CZP and decreased 32.15% in FZP, as well as the quantity of Clostridium kluvyer decreased by 12.50% and 34.63% in CZP and FZP, respectively. The ratios of archaea and eubacteria to total bacteria in both PMs and ZPs tended to be similar, and the ratio of Clostridium and C. kluyveri to eubacteria in PM increased compared with that in the initial phase, while those ratios in ZP was the opposite (Figure S1).
Table 1.
Concentration of different microorganisms detected by FISH in two kinds of PMs and ZPs (*109cells/g)
| Probe | 0 day | 60 days | 0 day | 60 days | ||||
|---|---|---|---|---|---|---|---|---|
| CPM | FPM | CPM | FPM | CZP | FZP | CZP | FZP | |
| DAPI | 60.54 ± 1.63 | 66.54 ± 3.40 | 96.54 ± 1.63 | 111.27 ± 3.27 | 51.81 ± 0.94 | 43.09 ± 1.88 | 60.00 ± 1.88 | 64.36 ± 1.88 |
| ARCH915 | 33.27 ± 0.94 | 32.18 ± 0.94 | 51.81 ± 0.94 | 62.18 ± 1.63 | 24.54 ± 1.63 | 25.63 ± 0.94 | 37.09 ± 0.94 | 30.54 ± 1.88 |
| EUB338 | 22.36 ± 0.94 | 30.54 ± 0.94 | 38.18 ± 1.88 | 44.72 ± 1.88 | 20.72 ± 0.94 | 22.90 ± 1.63 | 29.45 ± 0.00 | 26.18 ± 0.00 |
| MG1200b | 7.63 ± 0.08 | 9.27 ± 0.24 | 13.63 ± 0.94 | 18.54 ± 0.94 | 7.63 ± 0.24 | 8.18 ± 0.00 | 4.36 ± 0.94 | 7.63 ± 0.24 |
| MB311 | 7.63 ± 0.08 | 8.72 ± 0.08 | 19.09 ± 0.94 | 23.45 ± 0.94 | 9.81 ± 0.00 | 12.00 ± 0.94 | 9.27 ± 0.24 | 11.45 ± 0.63 |
| MSMX860 | 18.54 ± 0.88 | 10.36 ± 0.94 | 16.90 ± 0.94 | 20.72 ± 1.88 | 8.72 ± 0.94 | 7.09 ± 0.24 | 9.27 ± 0.24 | 10.90 ± 0.24 |
| CLZ | 15.1 ± 0.88 | 20.72 ± 0.94 | 27.27 ± 0.94 | 29.45 ± 1.63 | 12.54 ± 0.94 | 15.27 ± 0.94 | 14.18 ± 0.94 | 10.36 ± 0.88 |
| KCLZ | 6.00 ± 0.08 | 13.63 ± 0.94 | 19.63 ± 1.63 | 24.00 ± 1.88 | 8.72 ± 0.24 | 14.18 ± 0.94 | 7.63 ± 0.18 | 9.27 ± 0.44 |
The quantity of Clostridium and C. kluyveri in ZP decreased since these species and genus were antagonistic with Lactobacillus and Acetobacter, which predominated in Daqu. Moreover, as the important functional archaea, the amplification of Methanobacteriales, Methanomicrobiales, and Methanosarcinales was higher in FPM. It suggested that the ability to utilize H2, CO2, format, and acetic acid, was enhanced in FPM compared with CPM (Wu et al., 2015) and beneficial to maintain the stability of acidity in Baijiu fermentation niches.
Effect on the microbial community diversity
Alpha and beta diversity were used to estimate species diversity within and between habitats, respectively, in order to evaluate the overall diversity comprehensively (Whittaker, 1960, 1972). Chao1/Observed species and Shannon/Simpson indices were used to characterize the species richness and individual abundance accounting for α-diversity, which refers to the species diversity within a community or habitat (Chao and Yang, 1993). It was conducted with normalized sequenced and the effect of FDQ on α-diversity of PM and ZP were more prominent than that of CDQ. The amplification of bacterial species richness was higher in contrast to that in CPM and CZP, while that of fungi was the opposite. The archaeal species richness in both PMs and ZPs decreased. Changes between Simpson and Shannon indices of FPM and CPM were not consistent (Table S3). For ZP, the richness indices amplitude of bacteria and fungi, as well as damp of archaea was steeper in CZP. There was no significant difference in bacterial and fungal diversity indices between CZP and FZP, while that of archaea in CZP was higher than that of FZP.
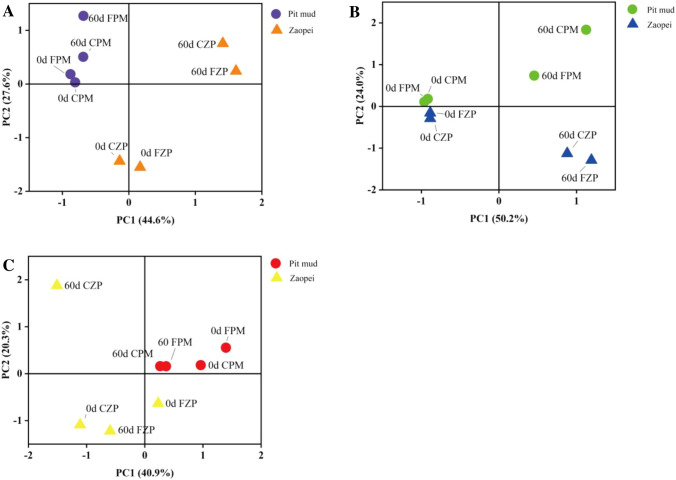
Principal component analysis (PCA) indicated that the community types could be identified using a genus-level abundance profile (Fig. 2). The bacterial community constituents among ZPs were different, while those in PM were similar. The archaeal community among samples was also alike except that in 60-day CZP, which was located at the second quadrant. For the fungal community, there is a significant difference among both PMs and ZPs when fermented for 60 days. These results indicated that the bacterial- and fungal- community diversity of ZP was affected by FDQ, significantly.
Fig. 2.
Principal components analysis (PCA) of microbial community structure in ZPs and PMs. 0d and 60d was the prophase and anaphase of fermentation. A is bacterial community, B is fungal community, C is archaeal community
Difference of microbial community structure in PM and ZP
As shown in Fig. 3A, the archaea included Crenarchaeota, Euryarchaeota, and Thaumarchaeota, and their abundance is similar in the initial CPM and FPM. Of them, the abundance of Bathyarchaeia belonging to Crenarchaeota was higher than 61.27% and increased after fermentation. After fermentation, the abundance of Methanobacteriales, Methanomicrobiales, and Methanosarcinales was significantly decreased in PMs and ZPs except for Methanobacteriales in ZPs. The three orders described above belonged to Euryarchaeota, whose abundance increased from 0.25 ± 0.02 to 0.82 ± 0.04 in CZP since that of Methanobacteriales enhanced significantly. While the abundance of Thaumarchaeota decreased significantly in both PMs and ZPs. Methanobacteriales, Methanomicrobiales, and Methanosarcinales played important roles in keeping the stable of acidity stable in the niches.
Fig. 3.
Community structure of dominant microbes in phylum and genus level (OUT > 0.50%). A is the community structure of dominant archaeal phylum, B is the community structure of dominant bacterial phylum, C is the community structure of dominant fungal phylum. A, B, C are investigated at phylum level with OUT > 0.50%. D is the community structure of dominant bacterial genus, E is the community structure of dominant fungal genus, F is the community structure of dominant archaeal genus. D, E, F are investigated at genus level with OUT > 0.50%
The eubacterial community was composed of four phyla, Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria as shown in Fig. 3B. The abundance of Proteobacteria increased from 0.01 ± 0.00 to 0.16 ± 0.00 in FPM and increased from 0.01 ± 0.00 to 0.26 ± 0.01 in CPM. Similarly, the abundance of Firmicutes was 0.90 ± 0.01 and 0.95 ± 0.02 in initial FZP and CZP, respectively. The abundance of Proteobacteria was 0.70 ± 0.03 and 0.78 ± 0.03 in FZP and CZP, respectively, and turned into dominant eubacteria flora after fermentation. Of them, the abundance of Actinobacteria increased by 3.51-fold, and that of Bacterioidetes enhanced by 13.40% in FZP. And the abundance of Actinobacteria and Bacterioidetes increased by 13.43-fold and 5.32-fold in CZP, respectively. The abundance of Clostridiales is one of the important functional microbes, whose abundance increased significantly in PMs and slightly in CZP. Besides, the abundance of Clostridium_senu_stricto was slightly increased in FPM and decreased in CPM, but not detected in ZP. The abundance of Caproiciproducens also increased in PMs. Butyric acid and hexanoic acid produced by Clostridium and Caproiciproducens are important precursors of skeleton composition ethyl hexanoate in Chinese strong-flavor liquor (Shi et al., 2011; Zhang et al., 2007). So the abundance of Clostridium and Caproiciproducens was considered as the indicator of PM activity (Liu et al., 2015). Besides, Clostridium and Caproiciproducens maintain the stability of the PM niches through interspecific interactions (Hu et al., 2015), and play important roles in improving the PM quality and their ability (Masayuki et al., 2008) of interspecific hydrogen transfer between Methanogens and Clostridium (Hahnke et al., 2014).
As shown in Fig. 3C, the fungal community structure in initial PMs was similar, mainly composed of Ascomycota, Basidiomycota, and Mucoromycota with similar abundance. The abundance of Ascomycota was > 0.99. And the abundance of Candida in Ascomycota was 0.92 ± 0.04 and 0.96 ± 0.02 in initial FPM and CPM, respectively, but decreased to 0.15 ± 0.02 and 0.10 ± 0.04 when fermented. Basidiomycota and Mucoromycota slightly increased. Besides, Pichia barely changed before and after fermentation, and Issatchenkia increased from 0.02 ± 0.00 and 0.04 ± 0.00 to 0.38 ± 0.02 and 0.22 ± 0.02 in FPM and CPM, respectively. The abundance of four different fungal genera (Figure S2), including Penicillium, Aspergillus, and Rhizopus increased slightly.
The composition of fungi in ZP was similar to PM. The abundance of Ascomycota decreased when fermented, but Basidiomycota increased to 0.13 ± 0.01 in CZP and was slightly higher than that of FZP. Issatchenkia decreased from 0.97 ± 0.04 and 0.98 ± 0.04 to 0.50 ± 0.02 and 0.47 ± 0.02 in FZP and CZP, respectively. Except for Hyphyopichia, the abundance of 20 different fungal genera increased slightly. It was noteworthy that Candida, the dominant fungi in PM, was replaced by Issatchenki.
Difference on physicochemical properties
As shown in Table S4, the utilization ability of acidity in FZP was stronger than that in CZP with the lower acidity in FZP, and the content of residual sugar was lower as well. It suggested that the hydrolysis and fermentation capacity of FDQ was higher than that of CDQ (He et al., 2019), which resulted in the difference on physiochemical indices between FZP and CZP. Meanwhile, the content of total acids and esters in initial PMs were similar, while in anaphase fermentation, these content in FPM (0.96 ± 0.02 g/100 g, 1.74 ± 0.02 g/100 g) were higher than that in CPM (0.85 ± 0.02 g/100 g, 1.06 ± 0.02 g/100 g), respectively.
Difference on metabolites shift caused by FDQ and CDQ
In the beginning, the content of eleven organic acids (OA) identified in FPM (14.36%) and FZP (13.13%) were slightly higher compared with CPM and CZP (Figure S2). The content of OAs increased to 2.10 ± 0.12 g/100 g and 3.35 ± 0.25 g/100 g in FPM and FZP when fermented, respectively. Among them, the content of six organic acids (lactic acid, acetic acid, propionic acid, isobutyric acid, octanoic acid, isovaleric acid) increased significantly.
It is very meaningful to characterize the difference in volatiles in PM and ZP disturbed by FDQ compared with CDQ. In our present research, the species of the constituents identified in the initial FPM and CPM enhanced from 73 and 75 to 107 and 88, respectively. The total content of these species was increased by 6.68 times and 4.76 times (Chen et al., 2020) as well. Similarly, the species identified were 101 and 99 in initial FZP and CZP but decreased to 82 and 79 after fermentation, respectively. However, the total content of these species described above was increased by 4.72 times and 4.43 times. Among them, the volatiles that increased significantly were major esters and acids whether in PM or ZP, except for 60-days ZP, in which aldehydes and others were also dominant volatiles (Figure S3 and Figure S4).
The acid content of fermented FZP and CZP was almost the same, which were 279.64 ± 18.24 mg/kg and 235.99 ± 6.98 mg/kg, respectively. The content of hexanoic acid was significant enhanced in FZP. Although there was no significant discrepancy for esters content in initial ZPs, their content increased to 864.32 ± 34.56 mg/kg (in FZP) and 252.11 ± 9.98 mg/kg (in CZP) after fermentation. Various esters were significantly increased in FZP compared to CZP, except butyl valerate. In particular, the content of ethyl hexanoate in FZP was 3.84 times higher than that of CZP. However, the flavor components showed different variation rules in PMs. Acetic acid, ethyl heptanoate, amyl hexanoate, and ethyl benzeneacetate were only detected in FPM, while isopentyl hexanoate was only examined in CPM. The content of acids in FPM and CPM enhanced from 74.91 ± 5.65 mg/kg and 50.33 ± 2.50 mg/kg to 336.62 ± 13.78 mg/kg and 208.84 ± 15.42 mg/kg, respectively. In these constituents, acetic acid, hexanoic acid, heptanoic acid, and octanoic acid in FPM was significantly higher than that of CPM. In addition, the contents of esters increased from 19.87 ± 0.42 mg/kg and 4.29 ± 0.02 mg/kg to 282.01 ± 12.86 mg/kg and 47.96 ± 4.20 mg/kg, respectively. Among them, the content of the thirteen dominant esters identified in FPM were significantly higher than CPM. For example, the main constituent ethyl butyrate and ethyl hexanoate were higher 0.74-fold and 0.98-fold than that of CZP as the skeleton flavor components in strong flavor Baijiu, respectively.
In the traditional fermentation process, ethyl caproate was mainly produced by Saccharomyces, bacteria, and molds of PM in the anaphase of fermentation (Chen et al., 2016). The introduction of Bacillus by adding FDQ was aimed to intervene microbial community of ZP, increased the abundance of yeast in FZP (0.58 ± 0.02), and promoted the conversion of acids and alcohols into esters. The abundance of yeast in FPM (0.65 ± 0.03) was also higher than that in CPM (0.46 ± 0.04), which also promoted the synthesis of esters in PM.
Correlation of dominant microbes with flavor compounds by redundancy analysis
As shown in Fig. 4, most flavor compounds (most esters, acids, and alkanes) were positively correlated with Penicillium, Aspergillus, Methanobacterium, Methanobrevibacterium, Pichia, Thermoascus, etc. Besides, Issatchenkia and Bacillus were also positively correlated with ketones. Candida and Caproiciproducens were positively correlated with aldehydes and aromatics, respectively. Acids, including hexanoic acid, pentanoic acid, acetic acid, etc., can not only maintain the acidity of the pit niche and avoid the living contaminants (Tao et al., 2014), but also be the precursors of ester biosynthesis. Ethyl hexanoate, ethyl hexanoate, ethyl octanoate, etc., were the core aromatic components of Luzhou-flavor Baijiu. Especially the ethyl hexanoate, as one of the four major esters, gave a special aroma of pineapple to liquor (Pino and Queris, 2011). Penicillium and Aspergillus are enriched with amylase and glucoamylase, which are correlated with the decomposition and utilization of starch. Caproiciproducens are related to the production of organic acids, such as acetic acid and hexanoic acid, which are contributors to aromatics. Methanbacterium, Methanbrevibacterium, etc., played important roles in interspecific hydrogen transfer to maintain pH or acidity stability of pit niche and facilitate the conversion of both hydrogen/CO2 and acetate into methane (De Vrieze et al., 2012; Tao et al., 2014). CO2 can also be used as energy by other bacteria. Besides, interspecific hydrogen transfer makes the formation of hexanoic acid more thermodynamically favorable under anaerobic conditions (Tao et al., 2017). The results indicated that the FDQ made functional microbial communities in PM and ZP evolve in favor of the liquor fermentation.
Fig. 4.

The correlation of microbes with flavor compounds in fermentation system by redundancy analysis. The dominant microbes consist of the dominant fungi (Candida, Issatchenkia, Pencillium, etc.), bacteria (Lactobacillus, Clostridium, Bacillus, Caproiciprodecens, etc.), and archaea (Methanocella, Methanosarcina, Methanobacterium, etc.). The flavor compounds consist of classical esters (ethyl hexanoate, ethyl hexadecanoate, etc.), acids (hexanoic acid, acetic acid, pentanoic acid, etc.) and other flavor compounds, such as ketones, aldehydes and aromatics and so on
Change of metabolite pathway by FDQ for pit niches
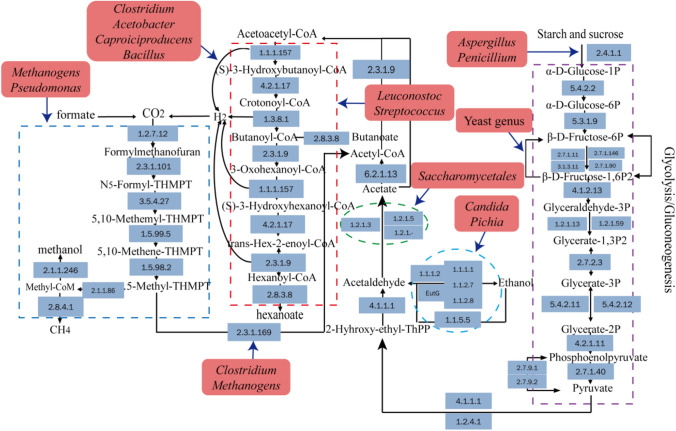
As shown in Fig. 5, the quality of fermented grains was enhanced by adding FDQ since the content of esters increased and the content of acids decreased, which may be caused by the regulation of metabolic pathways. As the abundance of Aspergillus and Penicillium increased in FZP, the expression of key enzymes in glycolysis pathways were up-regulated since the two kinds of fungi genera were major producers of various polysaccharides hydrolase. These increased key enzymes involved EC: 3.1.3.11, EC: 4.1.2.13, EC: 5.3.1.9. Besides, it was an important reason for the down-regulation of EC: 5.4.2.11, EC: 5.4.2.12, and other enzymes. The results showed that the ability of energy supply was enhanced in ZP. The expression of pyruvate dehydrogenase (EC: 1.2.4.1) was up-regulated in FZP and increased the accumulation of 2-Hyhroxy-ethyl-ThPP. Acetaldehyde was generated by the dehydrogenation of ethanol and dehydrogenated to acetate in two pathways. Aldehyde reductase (NADPH2 +) (EC: 1.1.1.2), as the key enzyme in the alcohol-producing pathway, was up-regulated in FZP and conducive to acetaldehyde accumulating. And the content of acetoacetyl-CoA was enhanced because of the up-regulation of EC: 6.2.1.13 and EC: 2.3.1.9 in FZP. Similarly, the expression of EC: 1.1.1.157 and EC: 4.2.1.17 in CZP were up-regulated, while that of EC: 1.3.8.1 and EC: 2.3.1.9 were inhibited. The regulation was conducive to the biosynthesis of butanoate and hexanoate in FZP. On the other hand, the up-regulated expression of EC: 2.3.1.101, EC: 1.2.7.12, EC: 1.5.98.2, and EC: 2.1.1.86 in FZP enhanced the decomposition and utilization of carbon dioxide. In the pathway of methane biosynthesis, the higher expression of EC: 1.2.7.1, EC: 1.5.98.2 and EC: 2.1.1.86 decided the more production of methanol and methane in FZP, which explained by the higher quantity of hydrogenotrophic methanogens (Methanomicrobiales and Methanobacteriales) and acetotrophic methanogens (Methanosarcinales) as well. The results indicated that FDQ had a positive effect on the microbial community, which was beneficial to the composition of unique aromatic compounds, such as hexanoate and butyrate increasing but acetate decreasing.
Fig. 5.
The main reactions in the microbial metabolic pathway, include glycolysis, ethanol synthesis, production of acetate, bytanoate, hexanoate, and production of methanol and methane by carbon dioxide
Interaction of microbial community between PMs and ZPs
There were significant differences in community diversity and constitutes between FPM and CMP, which were evoked by ZP with FDQ and CDQ intervention, respectively. It was suggested that the exogenous functional strains in FDQ not only influenced the microbial community diversity in ZP but also affected the bioactivity of PM. It has a stronger effect on the community evolving to Baijiu fermentation niche than CDQ as well. The abundance of functional genera in Baijiu brewing was enhanced significantly, such as methanogens, Caproiciproducens, Clostridium, and Clostridium kluyver. Most of the identified flavor compounds, especially ethyl hexanoate, were positively correlated with the species and genera described above. It was contributed to enhancing interspecific hydrogen transfer rates that the abundance of Clostridium and methanogens increased, which was also evidenced by the prediction based on PICRUSt2. These results lay an important understructure for the development of new approaches to accelerate the evolution of the functional microbial community by using FDQ.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was financially supported by National Research Center of Solid-State Brewing (17H1038, 17H1040), Sichuan University—Luzhou City Cooperation (2017CDLZ-S20), and Sichuan Province Science and Technology Support Program (2015KJT022-2011SZ).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics statement
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Suqi Chen, Email: 827517094@qq.com.
Jun Huang, Email: Huangjun@scu.edu.cn.
Hui Qin, Email: qinhui@lzlj.com.
Rongqing Zhou, Email: zhourqing@scu.edu.cn.
Yan Yang, Email: yangyan1@lzlj.com.
Chuanfeng Qiu, Email: qiucf@lzlj.com.
Suyi Zhang, Email: zhangsy@lzlj.com.
References
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 7(5): 335-336 (2010) [DOI] [PMC free article] [PubMed]
- Chao A, Yang MCK. Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika. 1993;80(1):193. doi: 10.1093/biomet/80.1.193. [DOI] [Google Scholar]
- Chen H, Jiang W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Frontiers in Microbiology. 5: 508 (2014) [DOI] [PMC free article] [PubMed]
- Chen Y, Luo W, Gong R, Xue X, Guan X, Song L, Guo X, Xiao D. Improved ethyl caproate production of Chinese liquor yeast by overexpressing fatty acid synthesis genes with OPI1 deletion. Journal of Industrial Microbiology & Biotechnology. 43(9): 1261-1270 (2016) [DOI] [PubMed]
- Chen S, Huang J, Qin H, He G, Zhou RQ, Yang Y, Qiu C, Zhang SY. Evolving the core microbial community in pit mud based on bioturbation of fortified Daqu. Canadian Journal of Microbiology. 67(5): 396-405 (2020) [DOI] [PubMed]
- Crocetti G, Murto M, Björnsson L. An update and optimisation of oligonucleotide probes targeting methanogenic archaea for use in fluorescence in situ hybridisation (FISH). Journal of Microbiological Methods. 65(1): 194-201 (2006) [DOI] [PubMed]
- De Vrieze J, Hennebel T, Boon N, Verstraete W. Methanosarcina: the rediscovered methanogen for heavy duty biomethanation. Bioresource Technology. 112: (1-9) (2012) [DOI] [PubMed]
- Ding X, Wu C, Zhang L, Zheng J, Zhou R. Characterization of eubacterial and archaeal community diversity in the pit mud of Chinese Luzhou-flavor liquor by nested PCR-DGGE. World Journal of Microbiology and Biotechnology. 30(2): 605-612 (2014) [DOI] [PubMed]
- Ding X, Wu C, Huang J, Li H, Zhou R, Jin Y. Eubacterial and archaeal community characteristics in the man-made pit mud revealed by combined PCR-DGGE and FISH analyses. Food Research International. 62: 1047-1053 (2014)
- Ding X, Wu C, Huang J, Zhou R. Interphase microbial community characteristics in the fermentation cellar of Chinese Luzhou -flavor liquor determined by PLFA and DGGE profiles. Food Research International. 72: 16-24 (2015)
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Ficker M, Krastel K, Orlicky S, Edwards E. Molecular characterization of a toluene-degrading methanogenic consortium. Applied and Environmental Microbiology. 65(12): 5576-5585 (1999) [DOI] [PMC free article] [PubMed]
- Friedrich AB, Merkert H, Fendert T, Hacker J, Proksch P, Hentschel U. Microbial diversity in the marine sponge Aplysina cavernicola (formerly Verongia cavernicola) analyzed by fluorescence in situ hybridization (FISH). Marine Biology. 134(3): 461-470 (1999)
- Gavin MD, Vincent JM, Jesse RZ, Svetlana NY, James RB, Christopher MT, Curtis H, Morgan GIL. PICRUSt2 for prediction of metagenome functions. Nature Biotechnology: The Science and Business of Biotechnology. 38(D1): 685-688 (2020) [DOI] [PMC free article] [PubMed]
- Gou M, Wang H, Yuan H, Zhang W, Tang Y, Kida K. Characterization of the microbial community in three types of fermentation starters used for Chinese liquor production. Journal of the Institute of Brewing. 121(4): 620-627 (2015)
- Hahnke S, Striesow J, Elvert M, Xavier PM, Michael K. Clostridium bornimense sp. nov., isolated from a mesophilic, two-phase, laboratory-scale biogas reactor. International Journal of Systematic and Evolutionary Microbiology. 64(Pt_8): 2792-2797 (2014) [DOI] [PubMed]
- He G, Dong Y, Huang J, Wang X, Zhang S, Wu C, Jin Y, Zhou R. Alteration of microbial community for improving flavor character of Daqu by inoculation with Bacillus velezensis and Bacillus subtilis. LWT. 2019;111:1–8. doi: 10.1016/j.lwt.2019.04.098. [DOI] [Google Scholar]
- He G, Huang J, Wu C, Jin Y, Zhou R. Bioturbation effect of fortified Daqu on microbial community and flavor metabolite in Chinese strong-flavor liquor brewing microecosystem. Food research international (Ottawa, Ont.). 129(8): 108851 (2020) [DOI] [PubMed]
- Hu X, Du H, Xu Y. Identification and quantification of the caproic acid-producing bacterium Clostridium kluyveri in the fermentation of pit mud used for Chinese strong-aroma type liquor production. International Journal of Food Microbiology. 214: 116-122 (2015) [DOI] [PubMed]
- Hu L, Huang J, Li H, Jin Y, Wu C, Zhou R. Design, optimization and verification of 16S rRNA oligonucleotide probes of fluorescence in-situ hybridization for targeting Clostridium spp. and Clostridium kluyveri. Journal of Microbiology and Biotechnology. 2018;28(11):1823–1833. doi: 10.4014/jmb.1805.04057. [DOI] [PubMed] [Google Scholar]
- Jacinto DM, Antonio SH, Carlos DR, Eugenio DD. Comparative study of methods for determination of titrable acidity in wine. Journal of Food Composition and Analysis. 16(5): 555-562 (2003)
- Li Z, Bai Z, Wang D, Zhang W, Zhang M, Lin F, Gao L, Hui B, Zhang H. Cultivable bacterial diversity and amylase production in three typical Daqus of Chinese spirits. International Journal of Food Science & Technology. 2014;49(3):776–786. doi: 10.1111/ijfs.12365. [DOI] [Google Scholar]
- Li H, Huang J, Liu X, Zhou R, Ding X, Xiang Q, Zhang L, Wu C. Characterization of interphase microbial community in Luzhou-flavored liquor manufacturing pits of various ages by polyphasic detection methods. Journal of Microbiology and Biotechnology. 2017;27(1):130–140. doi: 10.4014/jmb.1605.05036. [DOI] [PubMed] [Google Scholar]
- Liang R, Huang J, Wu X, Xu Y, Fan J, Wu C, Jin Y, Zhou R. Characterizing the metabolites and the microbial communities of the soy sauce mash affected by temperature and hydrostatic pressure. Food Research International. 123: 801-808 (2019) [DOI] [PubMed]
- Liu M, Zhao K, Tang Y, Ren D, Yao W, Tian X, Zhang X, Yi B, Deng B. Analysis of Clostridium cluster I community diversity in pit mud used in manufacture of Chinese Luzhou-flavor liquor. Food Science and Biotechnology. 24(3): 995-1000 (2015)
- Louca S, Doebeli M. Efficient comparative phylogenetics on large trees. Bioinformatics (Oxford, England). 34(6): 1053-1055 (2018) [DOI] [PubMed]
- Masayuki M, Osamu Y, Katsuya G. Genomics of Aspergillus oryzae : Learning from the history of Koji mold and exploration of its future. DNA Research. 15(4): 173-183 (2008) [DOI] [PMC free article] [PubMed]
- Pino JA, Queris O. Analysis of volatile compounds of mango wine. Food Chemistry. 2011;125(4):1141–1146. doi: 10.1016/j.foodchem.2010.09.056. [DOI] [Google Scholar]
- Raskin L, Poulsen LK, Noguera DR, Rittmann BE, Stahl DA. Quantification of methanogenic groups in anaerobic biological reactors by oligonucleotide probe hybridization. Applied and Environmental Microbiology. 60(4): 1241-1248 (1994) [DOI] [PMC free article] [PubMed]
- Shi S, Zhang L, Wu Z, Zhang W, Deng Y, Zhong F, Li J. Analysis of the fungi community in multiple- and single-grains Zaopei from a Luzhou - flavor liquor distillery in western China. World Journal of Microbiology and Biotechnology. 27(8): 1869-1874 (2011)
- Steven RG, Mihai P, Robert TD, Paul BE, Peter JT, Buck SS, Jeffrey IG, David AR, Claire MF, Karen EN. Metagenomic analysis of the human distal gut microbiome. Science. 312(5778): 1355-1359 (2006) [DOI] [PMC free article] [PubMed]
- Tao Y, Li J, Rui J, Xu Z, Zhou Y, Hu X, Wang X, Liu M, Li D, Li X. Prokaryotic communities in pit mud from different-aged cellars used for the production of Chinese strong-flavored liquor. Applied and Environmental Microbiology. 80(7):2254-2260 (2014) [DOI] [PMC free article] [PubMed]
- Tao Y, Wang X, Li X, Wei N, Jin H, Xu Z, Tang Q, Zhu X. The functional potential and active populations of the pit mud microbiome for the production of Chinese strong-flavour liquor. Microbial Biotechnology. 2017;10(6):1603–1615. doi: 10.1111/1751-7915.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Du H, Xu Y. Source tracking of prokaryotic communities in fermented grain of Chinese strong-flavor liquor. International Journal of Food Microbiology. 2017;244:27–35. doi: 10.1016/j.ijfoodmicro.2016.12.018. [DOI] [PubMed] [Google Scholar]
- Wu C, Ding X, Huang J, Zhou R. Characterization of archaeal community in Luzhou-flavour pit mud. Journal of the Institute of Brewing. 121(4): 597-602 (2015)
- Ye Y, Thomas GD. A parsimony approach to biological pathway reconstruction/inference for genomes and metagenomes. PLOS Computational Biology. 5(8): e1000465 (2009) [DOI] [PMC free article] [PubMed]
- Zhang WX, Qiao ZW, Tang YQ, Hu C, Sun Q, Morimura S, Kida K. Analysis of the fungal community in Zaopei during the production of Chinese Luzhou-flavour liquor. Journal of the Institute of Brewing. 2007;113(1):21–27. doi: 10.1002/j.2050-0416.2007.tb00251.x. [DOI] [Google Scholar]
- Zhang L, Wu C, Ding X, Zheng J, Zhou R. Characterisation of microbial communities in Chinese liquor fermentation starters Daqu using nested PCR-DGGE. World Journal of Microbiology & Biotechnology. 30(12): 3055-3063 (2014) [DOI] [PubMed]
- Zhang L, Zhou R, Niu M, Zheng J, Wu C. Difference of microbial community stressed in artificial pit muds for Luzhou-flavour liquor brewing revealed by multiphase culture-independent technology. Journal of Applied Microbiology. 2015;119(5):1345–1356. doi: 10.1111/jam.12943. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou R, Cui R, Huang J, Wu C. Characterizing soy sauce moromi manufactured by high-salt dilute-state and low-salt solid-state fermentation using multiphase analyzing methods. Journal of food science. 81(11): C2639-C2646 (2016) [DOI] [PubMed]
- Zheng J, Liang R, Wu C, Zhou R, Liao X. Discrimination of different kinds of Luzhou-flavor raw liquors based on their volatile features. Food Research International. 56: 77-84 (2014)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.