Abstract
Background:
National-level data of cancer patients’ readmissions after ST-segment elevation myocardial infarction (STEMI) are lacking.
Objectives:
The primary aim of this study was to compare the rates and causes of 30-day readmissions in patients with and without cancer.
Methods:
Among patients admitted with STEMI in the United States National Readmission Database (NRD) from October 2015–December 2017, we identified patients with the diagnosis of active breast, colorectal, lung, or prostate cancer. The primary endpoint was the 30-day unplanned readmission rate. Secondary endpoints included in-hospital outcomes during the index admission and causes of readmissions. A propensity score model was used to compare the outcomes of patients with and without cancer.
Results:
A total of 385,522 patients were included in the analysis: 5956 with cancer and 379,566 without cancer. After propensity score matching, 23,880 patients were compared (Cancer = 5949, No Cancer = 17,931). Patients with cancer had higher 30-day readmission rates (19% vs. 14%, p < 0.01). The most common causes for readmission among patients with cancer were cardiac (31%), infectious (21%), oncologic (17%), respiratory (4%), stroke (4%), and renal (3%). During the first readmission, patients with cancer had higher adjusted rates of in-hospital mortality (15% vs. 7%; p < 0.01) and bleeding complications (31% vs. 21%; p < 0.01), compared to the non-cancer group. In addition, cancer (OR 1.5, 95% CI 1.2–1.6, p < 0.01) was an independent predictor for 30-day readmission.
Conclusions:
About one in five cancer patients presenting with STEMI will be readmitted within 30 days. Cardiac causes predominated the reason for 30-day readmissions in patients with cancer.
Keywords: Cancer, ST-segment elevation myocardial infarction, In-hospital outcomes, 30-day outcomes
1. Introduction
Cardiovascular disease and cancer account for up to 50% of mortality in the United States (U.S.) [1]. The interaction between cancer diagnoses and coronary artery disease is multifaceted. Several cancer treatments, including radiation therapy and some chemotherapeutic and biologic agents, are known to have harmful effects on the heart and are associated with the accelerated development of coronary artery disease [2–4]. Moreover, while cancer can be related to a pro-thrombotic state that can promote acute coronary syndrome (ACS), cancer can also be paradoxically coupled with a high bleeding risk due to cancer-related coagulopathies [5–7]. Furthermore, the two diseases share some common risk factors, including smoking, obesity, sedentary lifestyle, age, and diabetes mellitus [8,9].
ST-segment elevation myocardial infarction (STEMI) presents significant management challenges among patients with cancer. While the main goals of STEMI management are immediate reperfusion and timely administration of antithrombotic and antiplatelet therapy, challenges are often encountered due to the higher risk of bleeding among patients with cancer [10]. Furthermore, randomized clinical trials of STEMI management have excluded patients with cancer due to the potential risk of bleeding and complications, and a perceived limited life expectancy [11,12]. Observational studies have shed some light on the in-hospital outcomes of STEMI in patients with cancer and suggested lower utilization of percutaneous coronary intervention (PCI) and higher mortality and complications rates [6,13–15]. There is limited data on post-discharge outcomes among patients with cancer following hospitalization for STEMI. We aimed to compare the rate and main causes of 30-day readmissions among patients with and without cancer presenting with STEMI using the U.S. National Readmission Database (NRD).
2. Methods
2.1. Data source
The study was derived from the NRD, from October 2015 to December 2017. The NRD is part of the Healthcare Cost and Utilization Project (HCUP) databases and is sponsored by the Agency for Healthcare Research and Quality (AHRQ) [16]. In its most recent release, the NRD reported data collected from 28 states, accounting for 60% of the total U.S. population and 58.2% of all U.S. hospitalizations. Unweighted, each year of the NRD provides data on about 17 million discharges and weighted, it estimates more than 36 million discharges. Due to the public availability of the database and the anonymous nature of the data, institutional review board approval was not required. The NRD has been utilized extensively in studying different diseases and cardiovascular procedures [17–19].
2.2. Study population
The NRD was queried using the International Classification of Diseases, 10th Revision (ICD-10), clinical modification (CM) codes (Online Supplement). We included patients who were treated for STEMI and had a diagnosis of active cancer during the study period. We only included patients with active breast cancer, colorectal cancer, lung cancer, or prostate cancer in the analysis, as these are the most common cancers worldwide [20]. We excluded: (a) patients <18 years old, (b) patients who have other types of cancers apart from the previously specified ones or more than one type of primary cancer, (c) transfers between hospitals or same-day admissions, and (d) patients with missing data for age, sex or mortality. Additionally, patients who died during the index admission were excluded from the readmission analysis. The NRD provides data on an annual basis. Patients discharged in December will not have a 30-day follow-up; therefore, we excluded patients discharged in December from the readmission analysis.
2.3. Study outcomes
The primary endpoint was the 30-day unplanned readmission rate. Secondary endpoints included causes for first unplanned readmission after an index admission with STEMI, in-hospital mortality, and bleeding complications (a composite of post-procedural bleeding, bleeding from other sites, and need for blood transfusion) during the first unplanned readmission. Causes of readmission were captured by identifying the principal diagnosis of the admission (ICD-10 diagnosis at the number one position in the database). Other secondary endpoints included vascular complications, bleeding complications, surrogates of severe disability (mechanical ventilation and non-home discharge), and hospitalization costs. Moreover, we reported the PCI utilization rates among the different types of cancers included in the current study.
2.4. Statistical analysis
Descriptive statistics of baseline and outcome measures were reported and stratified by cancer status. All variables are expressed as weighted national estimates. This was done following the survey analysis method by incorporating the (DISWT) variable as a weight variable (HOSP_NRD) as a clustering variable and accounting for the different strata in the design of the NRD using the (NRD_STRATUM) as recommended in the AHRQ methods series [21]. Categorical variables were expressed as count (percentage) and compared using the Scott-Rao Chi-square test. Continuous variables were expressed as median (interquartile range) and compared using the Wilcoxon rank-sum test.
Binary logistic regression was performed to estimate odds ratios (O.R.s) with 95% confidence intervals (C.I.s) to determine predictors for 30-day readmission. Moreover, to potentially match covariates related to cancer, a propensity score matching model was calculated using a logistic regression model to derive two matched groups for comparative outcomes analysis (cancer versus no cancer). The variables included in the propensity score matching are shown in the eTable 1. A nearest-neighbor 1:3 variable ratio, parallel, balanced propensity-matching model was made using a caliper width of 0.01 for the primary analysis. For the secondary analyses comparing the outcomes among cancer patients (Metastasis vs. no metastasis and PCI vs. no PCI), a nearest neighbor 1:1 variable ratio, parallel, balanced propensity-matching model was made using a caliper width of 0.1. We included the discharge weight provided by the HCUP in the propensity score analysis to minimize the differences between the matched cohorts. We used the cost-to-charge ratio files provided by the HCUP to convert the hospital charged to more accurate hospital costs for cost calculation. Furthermore, we performed a sensitivity analysis to report the main outcomes among individual cancers in the current study. A p-value of <0.05 was considered statistically significant. For the calculation of the readmission rates, we followed the methods recommended by the HCUP [16]. All statistical analyses were performed using statistical package for social science (SPSS) version 26 (IBM Corp), R, version 3.5 for propensity matching, and STATA version 15 (Stata Corp. 2017).
3. Results
3.1. Baseline characteristics
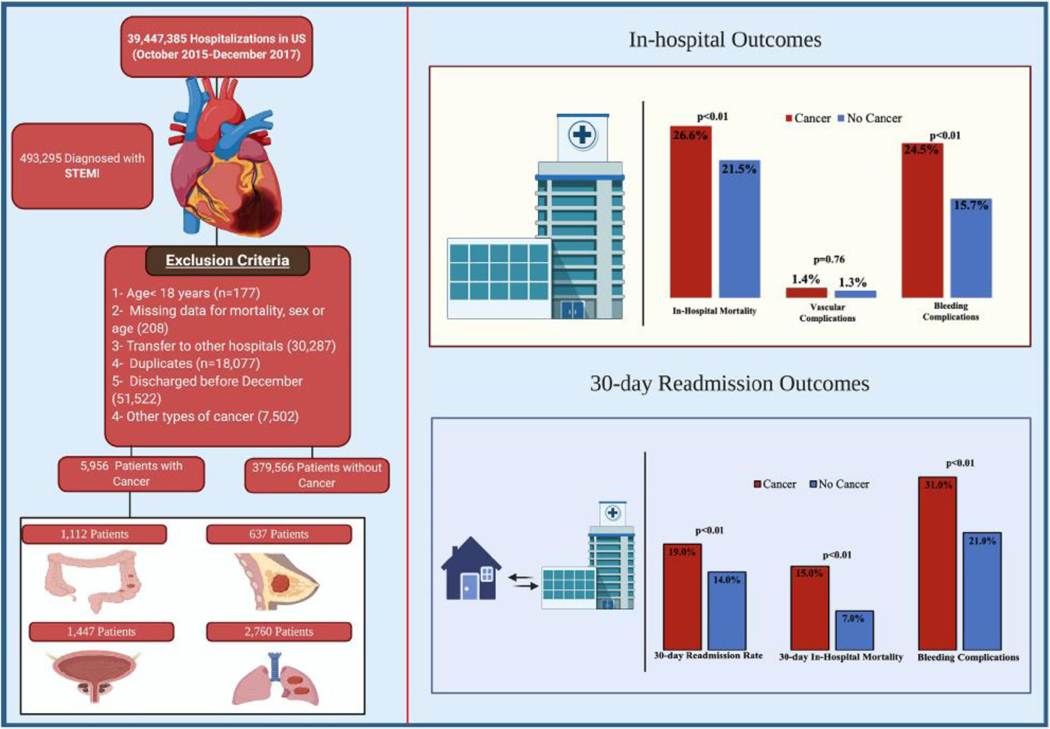
A total of 493,295 weighted patients were treated for STEMI between October 2015–December 2017. After applying the inclusion and exclusion criteria, 385,522 patients were included in the analysis (cancer = 5956; no cancer = 379,566). A flow chart of the study is shown in Fig. 1. Comparing patients with a cancer diagnosis to those without cancer, patients with cancer were older (72 years [IQR 64–80] vs 64 years [IQR55–74], p < 0.01), had more females (37.1% vs 32.9%, p < 0.01), and had a lower prevalence of diabetes mellitus (28.2% vs 31.7%, p < 0.01), obesity (8.2% vs 16.4%, p < 0.01), tobacco smoking (18.8% vs 29.3%, p < 0.01) and renal failure (18.3% vs 21.2%, p < 0.01). Additionally, at the time of index admissions, patients with cancer had lower utilization of PCI (43.5% vs. 72.1%, p < 0.01), coronary artery by-pass grafting (1.9% vs. 5%, p < 0.01), and mechanical circulatory support (6% vs. 8.2%, p < 0.01). The baseline characteristics of the two groups are shown in Table 1.
Fig. 1.
Study design and summary of outcomes.
Table 1.
Baseline characteristic of the patients included in the analysis before & after the propensity score matching. Abbreviations: NE; Weighted National Estimates.
| Variables | Unmatched cohorts | Matched cohorts | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| Cancer | No cancer | Total | p-value | SMD | Cancer | No cancer | Total | p-value | SMD | |
| (n = 5956) | (n = 379,566) | (n = 385,522) | (n = 5949) | (n = 17,931) | (n =23,880) | |||||
|
| ||||||||||
| Age, median (25th-75th IQR) | 72 (64–80) | 64(55–74) | 64 (55–74) | <0.01 | 0.58 | 72 (64–80) | 72 (64–82) | 72 (64–81) | 0.06 | −0.02 |
| Female | 37.1 | 32.9 | 32.9 | <0.01 | 0.08 | 37 | 36.2 | 36.4 | 0.36 | 0.01 |
| Diabetes mellitus | 28.2 | 31.7 | 31.6 | <0.01 | −0.07 | 28.3 | 28.9 | 28.8 | 0.50 | −0.01 |
| Hypertension | 72.5 | 72.1 | 72.1 | 0.62 | −0.007 | 72.5 | 72.6 | 72.6 | 0.93 | −0.006 |
| Cardiogenic shock | 12.7 | 11.9 | 11.9 | 0.17 | 0.02 | 12.7 | 13.3 | 13.2 | 0.41 | −0.02 |
| Peripheral vascular disease | 12.2 | 8.8 | 8.8 | <0.01 | 0.1 | 12.2 | 12.8 | 12.6 | 0.43 | −0.02 |
| Obesity | 8.2 | 16.4 | 16.2 | <0.01 | −0.3 | 8.2 | 8.5 | 8.4 | 0.54 | 0.002 |
| Tobacco smoking | 18.8 | 29.3 | 29.2 | <0.01 | −0.3 | 18.8 | 18.6 | 18.7 | 0.84 | 0.01 |
| Coagulopathy | 11.8 | 5.8 | 5.9 | <0.01 | 0.2 | 11.7 | 11 | 11.2 | 0.38 | 0.01 |
| Chronic lung disease | 34 | 16.7 | 17 | <0.01 | 0.35 | 33.9 | 31.9 | 32.4 | 0.06 | 0.04 |
| Chronic liver disease | 2.8 | 2 | 2 | <0.01 | 0.05 | 2.8 | 2.9 | 2.9 | 0.62 | −0.009 |
| PCI | 43.5 | 72.1 | 71.7 | <0.01 | −0.5 | 43.5 | 43.1 | 43.2 | 0.69 | 0.003 |
| Multivessel PCI | 7 | 13 | 12.9 | <0.01 | −0.23 | 7 | 7.3 | 72 | 0.63 | −0.01 |
| Drug eluting stent | 25.3 | 59.9 | 59.3 | <0.01 | −0.8 | 25.3 | 24.4 | 24.6 | 0.34 | 0.008 |
| Bare metal stent | 14.2 | 8.6 | 8.7 | <0.01 | −0.2 | 142 | 14.7 | 14.6 | 0.58 | −0.001 |
| CABG | 1.9 | 5 | 4.9 | <0.01 | −0.2 | 1.9 | 1.8 | 1.8 | 0.92 | 0.004 |
| Mechanical circulatory support | 6 | 8.2 | 8.2 | <0.01 | −0.11 | 6.0 | 6.2 | 6.1 | 0.32 | −0.01 |
| Hospital characteristics | ||||||||||
| Hospital bed size | ||||||||||
| Small | 14.1 | 12.7 | 12.8 | 0.14 | 14.1 | 15 | 14.8 | 0.53 | 0.009 | |
| Medium | 28 | 27.9 | 27.9 | −0.05 | 28 | 27.7 | 27.8 | |||
| Large | 57.9 | 59.4 | 59.4 | 57.9 | 57.3 | 57.4 | ||||
| Hospital teaching status | ||||||||||
| Metropolitan non-teaching | 24 | 25.9 | 25.8 | <0.01 | 0.06 | 24 | 23.2 | 23.4 | 0.34 | −0.02 |
| Metropolitan teaching | 66.9 | 66.8 | 66.8 | 66.9 | 66.8 | 66.8 | ||||
| Non-metropolitan hospital | 9.1 | 7.3 | 7.4 | 9 | 10 | 9.8 | ||||
4. Index admission outcomes
4.1. Unmatched cohort
Patients with cancer had higher rates of in-hospital mortality (26.6% vs 11.3%; OR 2.8, 95% CI 2.6–3.1, p < 0.01) and more bleeding complications (24.5% vs 12.1%, p < 0.01) with similar rate of vascular complications (1.4% vs 1.5%; p = 0.5). Surrogates of severe disability [mechanical ventilation (19.5% vs 12.7%; p < 0.01) and non-home discharges (17.5% vs 10.4%; p < 0.01)] were more frequent among patients with cancer. Additionally, patients with cancer had a longer median length of hospital stay (4 [IQR 2–8] vs 3 [IQR 2–5] days; p < 0.01) and lower median cost of hospitalization ($18,479 vs. $19,416, p < 0.01) (Table 2).
Table 2.
In-hospital outcomes of the patients included in the analysis before & after the propensity score matching.
| Variables no.(%) | Unmatched cohorts | Matched cohorts | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Cancer | No cancer | Total | p-value | Cancer | No cancer | Total | p-value | |
| (n = 5956) | (n = 379,566) | (n = 385,522) | (n = 5949) | (n = 17,931) | (n = 23,880) | |||
|
| ||||||||
| In-hospital outcomes Death | 26.6 | 11.3 | 11.6 | <0.01 | 26.6 | 21.5 | 22.8 | <0.01 |
| Vascular complications | 1.4 | 1.5 | 1.5 | 0.57 | 1.4 | 1.3 | 1.3 | 0.76 |
| Bleeding complications | 24.5 | 12.1 | 12.3 | <0.01 | 24.5 | 15.7 | 17.9 | <0.01 |
| Post-procedural bleeding | 10.1 | 6.4 | 6.4 | <0.01 | 10.1 | 6.8 | 7.6 | <0.01 |
| Gastrointestinal bleeding | 7.8 | 3.0 | 3.1 | <0.01 | 7.8 | 5.0 | 5.7 | <0.01 |
| Intracranial hemorrhage | 1.0 | 0.4 | 0.4 | <0.01 | 1.0 | 0.7 | 0.7 | 0.055 |
| Hematuria | 2.4 | 1.2 | 1.2 | <0.01 | 2.4 | 1.8 | 1.9 | 0.09 |
| Blood transfusion | 10.8 | 3.9 | 4 | <0.01 | 10.8 | 6.2 | 7.4 | <0.01 |
| Severe disability surrogates | ||||||||
| Non-home discharge | 17.5 | 10.4 | 10.5 | <0.01 | 17.5 | 17.7 | 17.6 | 0.79 |
| Mechanical ventilation | 19.5 | 12.7 | 12.8 | <0.01 | 19.4 | 18.8 | 18.9 | 0.42 |
| Resources utilization | ||||||||
| Length of hospitalization median days (25th–75th IQR) | 4 (2–8) | 3 (2–5) | 3 (2–5) | <0.01 | 4 (2–8) | 3 (2–6) | 3 (2–6) | <0.01 |
| Total cost of hospitalization median $ (25th–75th IQR) | 18,479 (10,960–30,241) |
19,416 (13,830–28,758) |
19,405 (13,797–28,783) |
<0.01 | 18,540 (10,936–30,721) |
16,821 (9,741–27,259) |
17,299 (10,046–28,059) |
<0.01 |
4.2. Matched cohort
After propensity matching, two cohorts of well-matched patients with and without cancer were compared (cancer = 5949; no cancer = 17,931) (Table 2). After propensity score matching, standardized mean differences were reduced to <10% for all variables indicating an adequately balanced population (Table 1 and eFigs. 1 & 2). The two groups were well balanced in terms of the baseline demographic, hospital, and morbidity characteristics.
In the matched analysis, patients with cancer still had higher rates of in-hospital mortality (26.6% vs 21.5%; p < 0.01) and bleeding complications (24.5% vs 15.7%, p < 0.01) with similar rates of vascular complications (1.4% vs 1.3%, p = 0.76) (Fig. 1). In the sensitivity analysis, the highest in-hospital mortality was seen among patients with lung cancer (32.9%), followed by colorectal cancer (24.6%), with similar mortality among patients with breast cancer and prostate cancer (19% for both). Patients with cancer had a longer length of hospital stay (4 [IQR 2–8] vs 3 [IQR 2–6] days; p < 0.01) and a higher median cost of hospitalization ($18,540 vs $16,821, p < 0.01) with similar rates of non-home discharges (17.5% vs 17.7%; p < 0.79) (Table 2).
PCI use among patients with cancer was explored, and after applying propensity score matching, a total of 4097 were included in this analysis (PCI = 2055; no PCI = 2043). After propensity score matching, the two cohorts were balanced apart from age; patients who received PCI were younger (71 years [IQR64–78] vs. 73 years [IQR64–82], p < 0.01). The use of PCI was associated with lower in-hospital mortality (12.3% vs 35.3%, OR 0.22, 95% CI 0.18–0.27, p < 0.01), fewer non-home discharges (12.5% vs 19.7%, p < 0.01) and less use of mechanical ventilation (11.6% vs 24%; p < 0.01) (eTables 2 & 3).
Among patients with cancer, patients with distant metastases had worse outcomes. After applying propensity score matching, a total of 4019 were included in this analysis (with metastases = 2000; without metastasis = 2019). Patients with metastases had higher rates of in-hospital mortality (34.2% vs 27.4%, p < 0.01) and intracranial bleeding (2% vs 0.6%, p < 0.01) (eTable 4).
4.3. Readmission outcomes
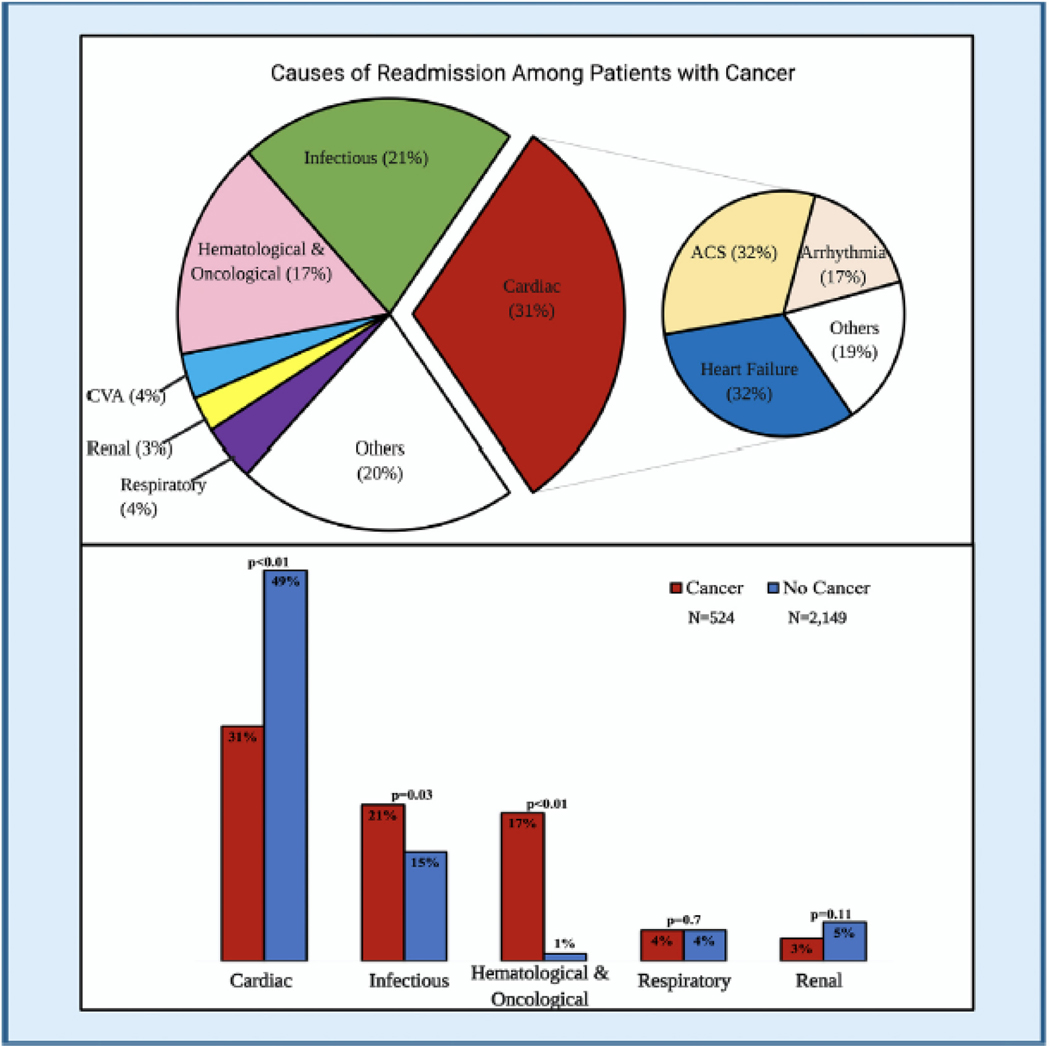
At 30 days of follow-up in those patients who survived the index admission, an unplanned readmission rate of 15% was observed among the overall population. Patients with cancer had a higher 30-day unplanned readmission rate than those without cancer (19% vs. 14%, p < 0.01). However, there was no difference between the two groups in the median time to first readmission (8 days vs. 9 days; p = 0.81) (eFig. 3). The most common causes for readmission among patients with cancer were cardiac-related (31%), followed by infectious (21%), oncologic (17%), respiratory (4%), stroke (4%), and renal (3%). Among cardiac causes, 32% of the patients were readmitted due to ACS and heart failure, respectively, and 17% secondary to arrhythmias. Cardiac causes of readmissions (49% vs. 31%, p < 0.01) were more common in the group without cancer. In comparison, infectious etiologies and hematological and oncological causes were more common in the cancer group (21% vs. 15%, p = 0.021) and (17% vs. 1%; p < 0.01) (Fig. 2).
Fig. 2.
Causes of 30-day readmission among cancer patients presenting with ST-segment elevation myocardial infarction.
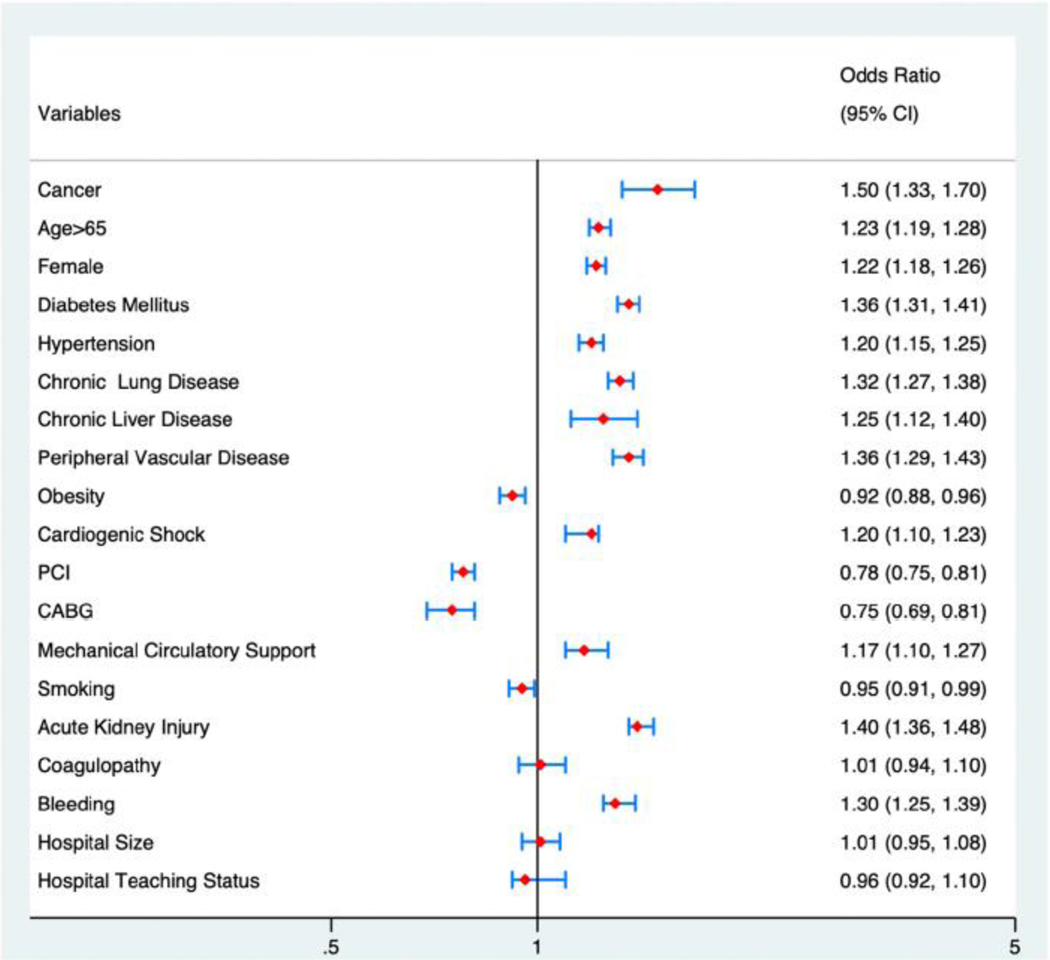
During the first readmission, patients with cancer had higher rates of in-hospital mortality (15% vs. 7%; p < 0.01) and bleeding complications (31% vs. 21%; p < 0.01) (Fig. 1). Furthermore, we observed higher venous thromboembolism rates in the cancer population during the first readmission (10% vs. 5%; p < 0.01). In logistic regression analysis, and after adjusting for multiple comorbidities, a cancer diagnosis was an independent predictor for 30-day readmission (OR 1.5, 95% CI 1.2–1.6, p < 0.01) (Fig. 3). Details of the bleeding and venous thromboembolism rates by cancer type are shown in e-Fig. 4.
Fig. 3.
Logistic regression for the predictors of 30-day readmission in patients with cancer patients after index admission with ST-segment elevation myocardial infarction.
5. Discussion
In the current study using a national readmission database to compare the outcomes among patients with and without cancer presenting with STEMI, we made several observations. First, 19% of patients with cancer admitted for STEMI had readmissions within 30 days, with cancer being an independent predictor of 30-day readmission. Second, the most common causes for readmission among patients with cancer were cardiac; however, readmission for infections and hematological and oncological issues were significantly higher among patients with cancer than those without cancer. Third, during the first readmission following STEMI, patients with cancer had notably higher rates of in-hospital mortality, bleeding complications, and venous thromboembolism. Finally, compared to the general population, patients with cancer had higher in-hospital morbidity and mortality during the index admission with STEMI.
Cancer has been previously shown to portend a worse in-hospital course among patients admitted with STEMI, including higher all-cause and cardiovascular mortality [6,13,22]. In a previous study from the Nationwide Inpatient Sample data between 2001 and 2011, patients with breast, lung, and colon cancer who were admitted for STEMI had higher in-hospital mortality (57 vs. 26%) and lower utilization of PCI (31%, 20%, and 17%) than patients without cancer (50%) [22].
To our knowledge, this is the first study to report national-level data on readmissions of patients with a diagnosis of cancer following admission for STEMI. In our study, 69% of the readmissions for patients with cancer following a STEMI admission were for non-cardiac-related, and approximately one in five patients with cancer were readmitted for a hematologic oncologic diagnosis. Due to the acute nature of the disease, interventionists have to make rapid decisions regarding PCI use. While urgent oncologic consultation might not be practical at the time of STEMI presentation, early oncology service involvement can facilitate further management, prognostication, and follow up. Furthermore, cardio-oncologists’ participation with knowledge in both the fields of cardiology and oncology may help further refine management strategies balancing thrombotic and hemorrhagic risks versus benefits. Future studies should address whether such a multidisciplinary team approach would mitigate the readmission rate among cancer patients.
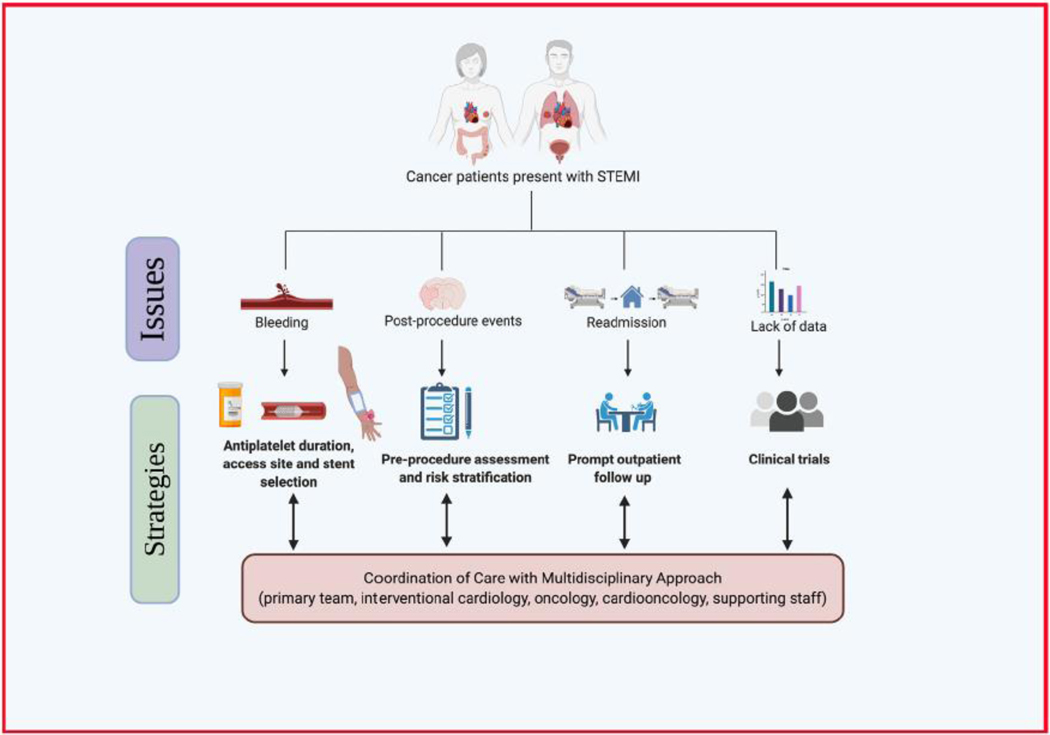
Bleeding and the need for blood transfusion are well known to be associated with higher mortality among ACS patients [23,24]. In the current analysis, patients with cancer had higher rates of bleeding complications, including the need for blood transfusion during the index admission, and continued to have higher rates of bleeding complications in the first readmission after discharge. These findings are consistent with previous studies and current guidelines [4,25,26]. Several considerations should be taken into account when managing patients with cancer who develop a STEMI. One of the findings was that patients with cancer are less likely to receive drug-eluting stents, which have been shown to be superior to bare-metal stents. This observation’s potential reason is that the bare metal stents would allow clinicians to usually shorter duration of dual antiplatelet therapy. Access site selection during the procedure plays a crucial role in bleeding outcomes. Studies have shown that radial access in the context of STEMI is associated with favorable bleeding outcomes compared to femoral access. Hence, it should be considered as the access strategy in patients with cancer whenever expertise is available [27]. The 2015 European Society of Cardiology Guidelines for the management of ACS recommended using radial access as the preferred access strategy for strategy in patients with ACS (Class 1A) [28]. Moreover, physicians should consider the higher bleeding risk among patients with cancer when selecting the type and duration of antiplatelet therapy. While dual antiplatelet therapy is the cornerstone of the post revascularization phase of STEMI management, multiple studies have supported shorter duration of dual antiplatelet therapy or an aspirin-free strategy in which aspirin is eliminated early in the course of the therapy to reduce the bleeding risk [29]. Furthermore, proper coordination of care beyond index admission is of utmost importance. Further studies are needed to risk stratify bleeding and thromboembolic risk in patients with cancer, considering the impact of platelet count, other coagulopathy indices, and the specific cancer type and stage (Fig. 4).
Fig. 4.
Issues facing the care of cancer patients presenting with STEMI and suggested strategies.
Our study has highlighted important findings in STEMI care in patients with cancer. With advances in cardiovascular care in general and STEMI care specifically, data on the optimal strategy, including types of stents and antiplatelet therapy, and antiplatelet therapy duration are lacking among the cancer population [11,12]. The inclusion of patients with cancer in broader trials or dedicated prospective studies is needed for further guidance.
6. Study limitations
The current study has several limitations that need to be acknowledged. First, the NRD is constructed in a format that precludes linkage between years so that the same patient may appear in more than 1 year, and it is not possible to follow patients across years. Second, the NRD is not designed to determine regional variations within the dataset, and our results may be generalizable only to the U.S. health care system. Third, The NRD is an administrative data that comes with uncertainty about some diagnoses as we rely on appropriate capture of ICD 10 codes. Fourth, the NRD does not provide data regarding medications (including chemotherapy, biologic agents that may predispose to thromboses and antithrombotic agents), immunosuppression status, the timing of cancer treatment (e.g., recent vs. distant), laboratory data, ejection fraction. Consequently, we could not adjust these crucial factors in the current analysis. The present dataset is also further limited because it does not include outpatient PCI procedures and represents only hospital admissions. Moreover, as with any observational data, it would be incorrect to make causal inferences with these data, and there are limitations related to unmeasured confounders. Finally, an important limitation to the current study is the competing risk of mortality for cancer patients. As the NRD data does not track the patients out of the hospital setting, it is difficult to ascertain if the patients who did not get readmitted are still alive or have expired at home. The competing risk of mortality is higher for some patients with cancer. Therefore, readmission rates are likely to be under-estimated in the cancer cohort compared to the real world.
6. Conclusions
About one in five patients with cancer presenting with STEMI will be readmitted within 30 days. Among patients with cancer, 30-day readmissions are still predominantly cardiac-related, but there are higher rates of admissions for infectious, hematological and oncological, bleeding, and venous thromboembolic causes than patients without cancer. Inclusion of patients with cancer in broader trials or dedicated prospective studies is needed for further guidance.
Supplementary Material
Acknowledgments
Funding sources
None.
Abbreviations:
- ACS
Acute Coronary Syndrome
- STEMI
ST-Segment Elevation Myocardial Infarction
- NRD
National Readmission Database
- HCUP
Healthcare Cost and Utilization Project
- AHRQ
Agency for Healthcare Research and Quality
Footnotes
CRediT authorship contribution statement
Conception and design: M.Osman, B. Patel, M. Benjamin, M. Mamas. Analysis and interpretation of the data: M. Osman, B. Patel, M. Benjamin, S. Balla, C. Bianco, M.Mamas.
Drafting of the article: M.Osman, B. Patel, M. Benjamin, S. Balla, C. Bianco, P.Sengupta. R. Daggubati, M. Mamas, S. Liu.
Critical revision of the article for important intellectual content: All authors
Final approval of the article: All authors
Statistical expertise: M. Osman, B. Patel, B. Kheiri.
Administrative, technical, or logistic support: B. Patel, M. Mamas, P. Sengupta, R. Daggubati, C. Bianco, S. Balla.
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.carrev.2021.04.015.
References
- [1].Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021:Cir0000000000000950. [DOI] [PubMed] [Google Scholar]
- [2].Desai MY, Jellis CL, Kotecha R, Johnston DR, Griffin BP. Radiation-associated cardiac disease: a practical approach to diagnosis and management. JACC Cardiovasc Imaging 2018;11(8):1132–49. [DOI] [PubMed] [Google Scholar]
- [3].Chang HM, Okwuosa TM, Scarabelli T, Moudgil R, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J Am Coll Cardiol 2017;70(20):2552–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol 2017;70(20):2536–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Campia U, Moslehi JJ, Amiri-Kordestani L, Barac A, Beckman JA, Chism DD, et al. Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. 2019;139(13):e579–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Potts JE, Iliescu CA, Lopez Mattei JC, Martinez SC, Holmvang L, Ludman P, et al. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J 2019;40(22):1790–800. [DOI] [PubMed] [Google Scholar]
- [7].Hess CN, Roe MT, Clare RM, Chiswell K, Kelly J, Tcheng JE, et al. Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention. J Am Heart Assoc. 2015;4(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hayek SS, Ganatra S, Lenneman C, Scherrer-Crosbie M, Leja M, Lenihan DJ, et al. Preparing the cardiovascular workforce to care for oncology patients: JACC review topic of the week J Am Coll Cardiol 2019;73(17):2226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133(11):1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140(3):240–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O’Keefe J, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The primary angioplasty in Myocardial Infarction Study Group N Engl J Med 1993; 328(10):673–9. [DOI] [PubMed] [Google Scholar]
- [12].Suryapranata H, van ‘t Hof AW, Hoorntje JC, de Boer MJ, Zijlstra F. Randomized comparison of coronary stenting with balloon angioplasty in selected patients with acute myocardial infarction. Circulation. 1998;97(25):2502–5. [DOI] [PubMed] [Google Scholar]
- [13].Velders MA, Boden H, Hofma SH, Osanto S, van der Hoeven BL, Heestermans AA, et al. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol 2013;112 (12):1867–72. [DOI] [PubMed] [Google Scholar]
- [14].Wang F, Gulati R, Lennon RJ, Lewis BR, Park J, Sandhu GS, et al. Cancer history portends worse acute and long-term noncardiac (but not cardiac) mortality after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction Mayo Clin Proc 2016;91(12):1680–92. [DOI] [PubMed] [Google Scholar]
- [15].Bharadwaj A, Potts J, Mohamed MO, Parwani P, Swamy P, Lopez-Mattei JC, et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. 2020;41(23): 2183–93. [DOI] [PubMed] [Google Scholar]
- [16].HCUP National Readmission Database (NRD). Healthcare cost and utilization project (HCUP). 2015–2017.Agency for Healthcare Research and Quality; R, MD. https://www.hcup-us.ahrq.gov/nrdoverview.jsp. [PubMed] [Google Scholar]
- [17].Osman M, Al-Hijji MA, Kawsara A, Patel B, Alkhouli M. Comparative outcomes of mitral valve in valve implantation versus redo mitral valve replacement for degenerated bioprotheses. Am J Cardiol 2020;132:175–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Osman M, Patel B, Munir MB, Kawsara A, Kheiri B, Balla S, et al. Sex-stratified analysis of the safety of percutaneous left atrial appendage occlusion. Catheter Cardiovasc Interv. 2020;97. [DOI] [PubMed] [Google Scholar]
- [19].Osman M, Syed M, Abdul Ghaffar Y, Patel B, Abugroun A, Kheiri B, et al. Gender-based outcomes of impeller pumps percutaneous ventricular assist devices. Catheter Cardiovasc Interv. 2020;97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- [21].Yoon F SM, Jiang HJ, Steiner CA, Barrett ML. Calculating Nationwide Readmissions Database (NRD) Variances. HCUP methods. Series Report # 2017–01 ONLINE. January 24, 2017. U.S. Agency for Healthcare Research & Quality. [Google Scholar]
- [22].Pothineni NV, Shah NN, Rochlani Y, Saad M, Kovelamudi S, Marmagkiolis K, et al. Temporal trends and outcomes of acute myocardial infarction in patients with cancer. Ann Transl Med. 2017;5(24):482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126(7):875–910. [DOI] [PubMed] [Google Scholar]
- [24].Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. Jama. 2004;292(13):1555–62. [DOI] [PubMed] [Google Scholar]
- [25].Iliescu C, Durand JB, Kroll M. Cardiovascular interventions in thrombocytopenic cancer patients. Tex Heart Inst J 2011;38(3):259–60. [PMC free article] [PubMed] [Google Scholar]
- [26].Iliescu C, Grines CL, Herrmann J, Yang EH, Cilingiroglu M, Charitakis K, et al. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista). Catheter Cardiovasc Interv 2016;87(5):895–9. [DOI] [PubMed] [Google Scholar]
- [27].Osman M, Saleem M, Osman K, Kheiri B, Regner S, Radaideh Q, et al. Radial versus femoral access for percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction: trial sequential analysis. Am Heart J. 2020;224:98–104. [DOI] [PubMed] [Google Scholar]
- [28].Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37(3):267–315. [DOI] [PubMed] [Google Scholar]
- [29].Osman M, Farjo PD, Osman K, Radaideh Q, Munir MB, Kheiri B, et al. The dawn of aspirin free strategy after short term dual antiplatelet for percutaneous coronary intervention: meta-analysis of randomized controlled trials. J Thromb Thrombolysis 2020;49(2):184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.