Abstract
Cutaneous malignant melanoma is a rare but fatal disease in East Asia. Despite its increasing incidence, a general lack of awareness about the disease was noted. This study aims to provide population-based prognostic analysis of melanoma with sentinel lymph node biopsy (SLNB) in Taiwan. We conducted this retrospective cohort study using the data from Taiwan National Health Insurance Research Database during 1997–2013. The study cohort contains 3284 patients. The 5-year survival rates of patients undergoing SLNB and not undergoing SLNB were 45.5% and 33.6%. In multivariate analysis, age ≥ 80 years [adjusted hazard ratio (aHR) = 2.15] and male (aHR = 1.19) were associated with a poorer prognosis, while high social economic status (SES) (aHR = 0.69) and undergoing SLNB (aHR = 0.84) were good prognostic factors. Old age and low SES were associated with lower percentages of patients undergoing SLNB (P < 0.001). E-value analysis suggested robustness to unmeasured confounding. In conclusion, undergoing SLNB was associated with a better prognosis. The poor prognosis of old age and low SES may be due to decreased percentages of patients undergoing SLNB. Therefore, we recommend that SLNB should be performed on patients, especially in old age or low SES, who are candidates for SLNB according to current guidelines to achieve maximal survival.
Subject terms: Melanoma, Cancer epidemiology, Surgical oncology, Prognosis
Introduction
Cutaneous malignant melanoma is a rare but fatal disease in East Asia, with increasing incidence in Taiwan according to Cancer Registry Annual Report published by Ministry of Health and Welfare, Taiwan in 20181. In spite of the increasing incidence of melanoma, a general lack of awareness about the disease and the fact that melanoma remains overlooked and undertreated in Asian populations were noted in recent studies2,3. It’s worth mentioning that the two major subtypes in white people are superficial spreading melanoma and nodular melanoma, whereas acral lentiginous melanoma, which is the most common subtype in Asians, is actually rare in the Western countries4,5. Therefore, to better understand this disease, statistical data regarding demographic characteristics of melanoma in Asian populations seems to be basic and indispensable. However, studies focusing on this area appears to be limited. To our knowledge, no large-scale, population-based study was performed to provide demographic characteristics of melanoma patients in Taiwan in the past.
Sentinel lymph node biopsy (SLNB) is the most accurate staging tool and offers useful prognostic information for melanoma patients. SLNB has become the standard of care in the treatment of clinically node-negative melanoma. According to current National Comprehensive Cancer Network (NCCN) guideline6, SLNB should be discussed with all patients with clinical stage IB or II melanoma. However, controversies of SLNB exist because of the lack of clear evidence regarding the survival advantage SLNB provides7–10. One randomized study11 in 2014 has been designed to resolved this issue and showed that SLNB leads to an improved survival in node-positive patients, but debate remains as most melanoma patients have negative nodes12. In Asia, few studies have been performed to analyze the difference in prognosis between melanoma patients undergoing SLNB or not undergoing SLNB13,14.
As a result, this study aims to provide prognostic factors and population-based analysis of melanoma with SLNB in Taiwan.
Materials and methods
Data sources
The Taiwan National Health Insurance (NHI) program was initiated in March 1995 and covers approximately 99% of the Taiwanese population. The NHI Research Database (NHIRD), derived from the payment system of the NHI Administration (NHIA) and managed by the National Health Research Institute (NHRI), possesses abundant information regarding nearly all kinds of medical services, such as outpatient visits, inpatient records, and medical illness diagnosis codes according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding system. Confidentiality is maintained according to the directives of the NHIA in Taiwan. Details on the generation, monitoring and maintenance of the NHIRD are published online by the NHRI. Previous studies have described the high accuracy and validity of ICD-9-CM diagnoses in the NHIRD15,16. All data from the NHIRD are anonymous and encrypted to protect participants’ privacy; therefore, no informed consent was required from the study population. Additionally, this study was approved by the Institutional Review Board of Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan (VGHKS15-EM4-01).
Study design
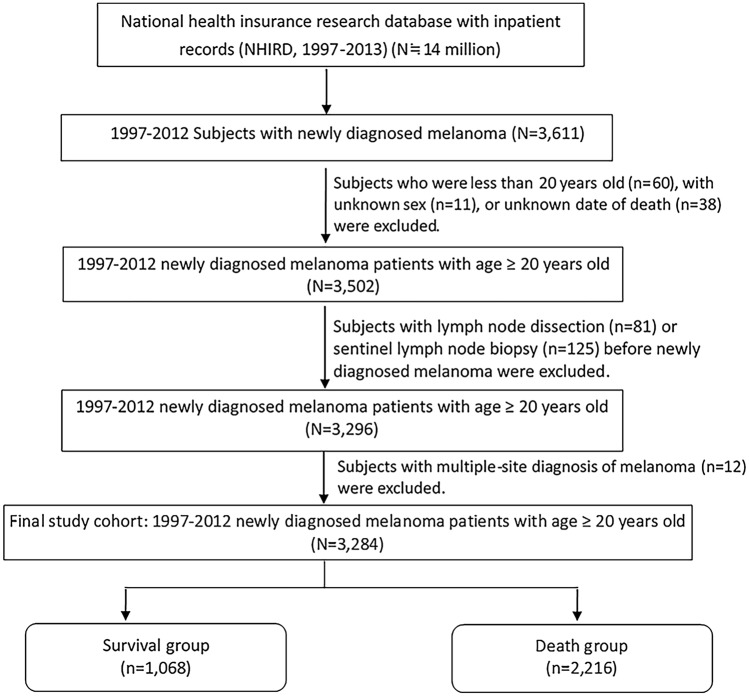
We conducted this retrospective cohort study using the data extracted from the inpatient records of NHIRD during 1997–2013, with a total patient number of approximately 14 million. Adults with newly diagnosed malignant melanoma of skin (ICD-9-CM Code 172) were identified from the inpatient claims as the cutaneous malignant melanoma cohort. Exclusion criteria included follow-up time less than 1 year, unknown sex, unknown date of death, age below 20 years, undergoing SLNB or lymph node dissection (LND) before the diagnosis of melanoma, and multiple-site diagnosis of melanoma. The final study cohort contains 3284 patients. Figure 1 shows detailed information on the enrollment process. Overall, 3284 melanoma patients were followed up until death or the end of 2013, with 1068 patients in the survival group and 2216 patients in the death group. According to Cancer Registry Annual Report published by Ministry of Health and Welfare, Taiwan in 20181, the total number of patients diagnosed with melanoma during 1997–2012 was 3057, which was close to the cohort number of this study. The Taiwan Cancer Registry Annual Report was based on the Taiwan Cancer Registry database, which provides complete core information for cancer cases in Taiwan17,18.
Figure 1.
Design and flowchart of patient selection.
Demographic data were derived from NHIRD, including age at diagnosis, sex, social economic status (SES) and residential area. SES was classified as non-income (no income), low (income ranges from 1 to 583 US$ per month), middle (income ranges from 584 to 833 US$ per month) and high (income ≥ 834 US$ per month) categories. The baseline comorbidity history for each participant was also determined from the inpatient claims data. Several possibly relevant diseases, according to previous studies19–22, including diabetes (ICD-9-CM Code 250), coronary artery disease (ICD-9-CM Codes 410–414), cerebral vascular disease (ICD-9-CM Codes 430–438), heart failure (ICD-9-CM Code 428), Parkinson disease (ICD-9-CM Code 332) and all cancers (ICD-9-CM Codes 140–208), were considered. As for clinical characteristics, primary site of cutaneous melanoma was assessed by ICD-9-CM Codes, with 172.0–172.4 for head and neck, 172.5 for trunk, 172.6 for upper limbs and 172.7 for lower limbs. ICD-9-CM Codes 172.8–172.9 or simply 172 were defined as unspecific group, in addition. Patients undergoing SLNB (procedure codes 4011, 4021, 4023, 4024 and 4029) and LND (procedure codes 403, 4041, 4051, 4053, 4054 and 4059) were also identified.
Sentinel lymph node biopsy
Current NCCN guideline indicates that SLNB should be discussed with all patients with clinical stage IB or II melanoma6. In this study, on account of the lack of consistent guidelines throughout the relatively long time frame, SLNB was performed mainly based on the clinical consideration of clinicians. In Taiwan, generally, the procedure of SLNB included subdermal injection of 1 mL of technetium-99 sulfur colloid and vital dye followed by lymphoscintigraphy and surgical harvest of sentinel lymph nodes23. Dissected sentinel lymph nodes were further stained with hematoxylin and eosin or HMB-45.
Statistical analysis
In this study, survival was analyzed by the Kaplan–Meier method. Univariate analysis of the association between survival and prognostic factors including demographic variables, comorbidities and clinical characteristics was performed using Chi-square test. Multivariate analysis was further performed via the Cox proportional hazard model. As for SLNB, univariate analysis of the association between demographic characteristics as predictive factors and SLNB was also performed using Chi-square test. In addition, subgroup analysis of age, sex, and SES for SLNB by prognosis was further performed using Chi-square test. A P value of 0.05 or less was regarded to be statistically significant. SPSS statistical software, version 19.0 for Windows, was used for all data analysis.
Sensitivity analysis
Due to the limitation of NHIRD, the data of several important clinical characteristics such as Breslow thickness, stage and histologic subtype of melanoma was not analyzed in this study. To assess the robustness of our findings, multiple sensitivity analyses were performed. First, we modified the patient enrollment process by adding another exclusion criterion of follow-up time less than 3 years (see Supplementary Fig. 1 online). The association between SLNB and the end point of 1-year or 3-year overall survival was assessed using crude analysis, multivariable analysis and propensity-score analyses. Second, we explored the potential for unmeasured confounding between SLNB and 1-year or 3-year overall survival by calculating E-values24. The E-value is the minimum strength that an unmeasured confounder would need to have to negate the observed association between SLNB and 1-year or 3-year overall survival.
Results
Demographic characteristics
Of all 3284 patients, the mean age (± standard deviation) was 65.2 (± 16.1) years. The most common age of diagnosis was between 70 and 79 years. Old age was associated with a significantly increased risk of death (P < 0.001). There were 1791 males and 1493 females. The male-to-female ratio was 1.20:1. Male gender was associated with a worse survival (P < 0.001) than female gender. As for SES, there were 1485, 711, 666 and 373 patients in middle, low, high and non-income groups, respectively. High SES was associated with a better survival (P < 0.001) than other groups. Most of the patients resided in northern Taiwan, while there’s no significant association between residential area and survival. Details of the demographic characteristics of the melanoma patients are described in Table 1.
Table 1.
Demographic characteristics, comorbidity and clinical characteristics of first-event melanoma patients in survival and death groups (n = 3284).
| Variables | Total | Survival (n = 1,068) |
Death (n = 2,216) |
p Valuea |
|---|---|---|---|---|
| Demographics | ||||
| Age, years, mean ± SD (%) | 65.2 ± 16.1 | 59.0 ± 16.1 | 68.2 ± 15.2 | < 0.001 |
| < 50 | 584 (17.8) | 299 (51.2) | 285 (48.8) | < 0.001 |
| 50–59 | 495 (15.1) | 219 (44.2) | 276 (55.8) | |
| 60–69 | 670 (20.4) | 228 (34.0) | 442 (66.0) | |
| 70–79 | 903 (27.5) | 218 (24.1) | 685 (75.9) | |
| ≥ 80 | 632 (19.2) | 104 (16.5) | 528 (83.5) | |
| Sex (%) | < 0.001 | |||
| Female | 1493 (45.5) | 565 (37.8) | 928 (62.2) | |
| Male | 1791 (54.5) | 503 (28.1) | 1288 (71.9) | |
| Social economic status (%)bc | < 0.001 | |||
| Non-income | 373 (11.5) | 80 (21.4) | 293 (78.6) | |
| Low | 711 (22.0) | 205 (28.8) | 506 (71.2) | |
| Middle | 1485 (45.9) | 432 (29.1) | 1053 (70.9) | |
| High | 666 (20.6) | 333 (50.0) | 333 (50.0) | |
| Residential area (%)d | 0.130 | |||
| Northern | 1304 (40.7) | 450 (34.5) | 854 (65.5) | |
| Central | 814 (25.4) | 251 (30.8) | 563 (69.2) | |
| Southern | 952 (29.7) | 301 (31.6) | 651 (68.4) | |
| Other | 137 (4.3) | 37 (27.0) | 100 (73.0) | |
| Comorbidity | ||||
| Diabetes (%) | < 0.001 | |||
| No | 2725 (83.0) | 926 (34.0) | 1799 (66.0) | |
| Yes | 559 (17.0) | 142 (25.4) | 417 (74.6) | |
| Coronary artery disease (%) | < 0.001 | |||
| No | 2262 (68.9) | 787 (34.8) | 1475 (65.2) | |
| Yes | 1022 (31.1) | 281 (27.5) | 741 (72.5) | |
| Cerebral vascular disease (%) | < 0.001 | |||
| No | 2907 (88.5) | 1004 (34.5) | 1903 (65.5) | |
| Yes | 377 (11.5) | 64 (17.0) | 313 (83.0) | |
| Heart failure (%) | < 0.001 | |||
| No | 3087 (94.0) | 1036 (33.6) | 2051 (66.4) | |
| Yes | 197 (6.0) | 32 (16.2) | 165 (83.8) | |
| Parkinson disease (%) | 0.240 | |||
| No | 3225 (98.2) | 1053 (32.7) | 2172 (67.3) | |
| Yes | 59 (1.8) | 15 (25.4) | 44 (74.6) | |
| Malignancy (%) | < 0.001 | |||
| No | 2041 (62.1) | 860 (42.1) | 1181 (57.9) | |
| Yes | 1243 (37.9) | 208 (16.7) | 1035 (83.3) | |
| Melanoma site (%) | < 0.001 | |||
| Head and neck | 488 (14.9) | 141 (28.9) | 347 (71.1) | |
| Trunk | 310 (9.4) | 107 (34.5) | 203 (65.5) | |
| Upper limbs | 271 (8.3) | 123 (45.4) | 148 (54.6) | |
| Lower limbs | 1,622 (49.4) | 585 (36.1) | 1,037 (63.9) | |
| Unspecific | 593 (18.1) | 112 (18.9) | 481 (81.1) | |
| Sentinel lymph node biopsy (%) | < 0.001 | |||
| No | 2,351 (71.6) | 667 (28.4) | 1,684 (71.6) | |
| Yes | 933 (28.4) | 401 (43.0) | 532 (57.0) | |
| Lymph node dissection (%) | 0.002 | |||
| No | 2,813 (85.7) | 945 (33.6) | 1,868 (66.4) | |
| Yes | 471 (14.3) | 123 (26.1) | 3483.9) | |
aUsing Chi-square test. Bold type indicates statistical significantly (P < 0.05).
bSocial economic status was classified as non-income (no income), low (income ranges from 1 to 583 US$ per month), middle (income ranges from 584 to 833 US$ per month) and high (income ≥ 834 US$ per month) categories.
c49 patients were unknown.
d77 patients were unknown.
Comorbidity
Among all the comorbidities considered, the most common comorbidity in these 3284 melanoma patients was malignancy (37.9%), followed by coronary artery disease (31.1%) and diabetes (17.0%). On the other hand, the least common comorbidity was Parkinson disease (1.8%), followed by heart failure (6.0%) and cerebral vascular disease (11.5%). In the univariate analysis, the diagnosis of malignancy, coronary artery disease, diabetes, cerebral vascular disease and heart failure were associated with an increased risk of death (P < 0.001). Details of the prevalence of comorbidity in survival and death groups are also shown in Table 1.
Clinical characteristics
According to the ICD-9-CM Codes, the most commonly diagnosed site was on lower limbs (49.4%), followed by unspecific sites (18.1%), head and neck (14.9%), trunk (9.4%) and upper limbs (8.3%). Of the 3284 cases, we identified 933 (28.4%) patients undergoing SLNB, which was associated with a better prognosis (P < 0.001). In addition, 471 (14.3%) patients underwent LND, which was associated with an increased risk of death (P = 0.002). Detailed clinical characteristics of melanoma patients in survival and death groups are also shown in Table 1.
Overall survival
The overall survival curve for these 3284 melanoma patients made by the Kaplan–Meier method is shown in Fig. 2. The 1-year, 3-year, 5-year and 10-year overall survival rate were 71.7%, 47.3%, 37.0% and 26.3%, respectively. High SES and undergoing SLNB were associated with a significantly better prognosis. The overall survival curves of patients with different SES and patients undergoing SLNB or not are shown in Fig. 2. The 5-year survival rates of patients with high, middle, low and non-income SES were 51.4%, 33.3%, 36.1% and 29.6%, respectively. The 5-year survival rates of patients undergoing SLNB and not undergoing SLNB were 45.5% and 33.6%.
Figure 2.
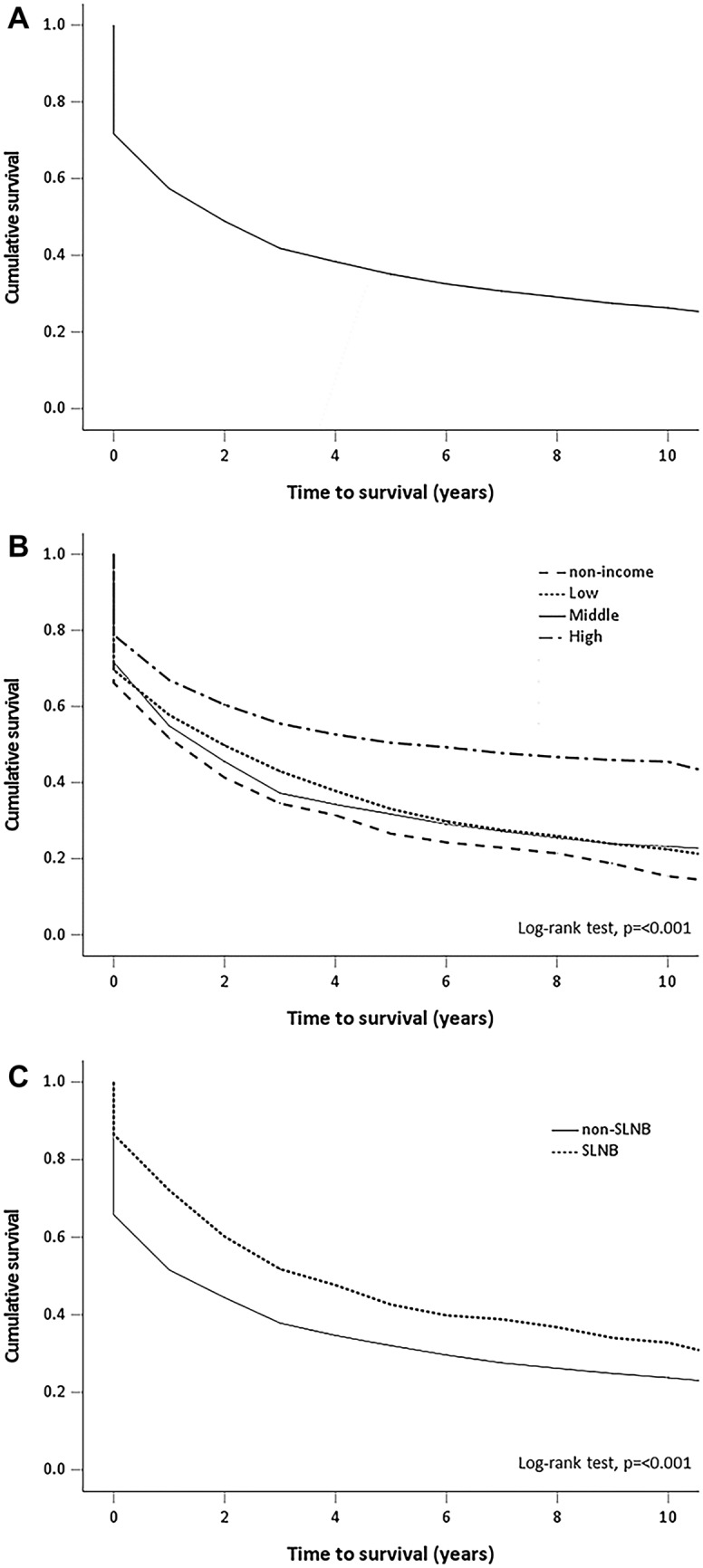
Overall survival of patients with malignant melanoma in Taiwan. (A) Cumulative proportion of the 3284 patients expected to survive. (B) Patients in different categories of social economic status. (C) Patients undergoing sentinel lymph node biopsy or not.
Multivariate analysis
In the univariate analysis, age, sex, SES, comorbidity, site of melanoma, undergoing SLNB and LND were significantly associated with prognosis. To avoid confounding and to better reflect independent prognostic factors, Table 2 provides the multivariate analysis of these factors. Patients aged ≥ 80 years [adjusted hazard ratio (aHR) = 2.15], between 70 and 79 years of age (aHR = 1.63), between 60 and 69 years of age (aHR = 1.38) or between 50 and 59 years of age (aHR = 1.22) had a worse prognosis than patients aged less than 50 years. Male gender was associated with a worse survival (aHR = 1.19) than female gender. The aHRs for low, middle and high SES were 0.84 (95% CI 0.72–0.97), 0.90 (95% CI 0.78–1.02) and 0.69 (95% CI 0.58–0.83), respectively, compared with non-income group. Comorbidities including diabetes, coronary artery disease and heart failure were significantly poor prognostic factors in univariate analysis, but was not significant in multivariate analysis. Cerebral vascular disease (aHR = 1.22, P = 0.002) and malignancy (aHR = 2.13, P < 0.001) remained significant poor prognostic factors in multivariate analysis. Undergoing SLNB (aHR = 0.84, P = 0.001) and LND (aHR = 1.15, P = 0.022) were also significantly prognostic factors in multivariate analysis.
Table 2.
Demographic and clinical characteristics of patients in the mortality by Cox-proportional hazard regression analysis with stepwise model (n = 3284).
| Variables | aHRb | 95% C.I.b | p Valuea |
|---|---|---|---|
| Demographics | |||
| Age, years (ref. = < 50) | |||
| 50–59 | 1.22 | 1.03–1.45 | 0.021 |
| 60–69 | 1.38 | 1.19–1.62 | < 0.001 |
| 70–79 | 1.63 | 1.40–1.89 | < 0.001 |
| ≥ 80 | 2.15 | 1.83–2.54 | < 0.001 |
| Sex (ref. = female) | |||
| Male | 1.19 | 1.09–1.30 | < 0.001 |
| Social economic status (ref. = Non-income)c | |||
| Low | 0.84 | 0.72–0.97 | 0.020 |
| Middle | 0.90 | 0.78–1.02 | 0.105 |
| High | 0.69 | 0.58–0.83 | < 0.001 |
| Melanoma site (ref. = Head and neck) | |||
| Trunk | 1.10 | 0.92–1.32 | 0.277 |
| Upper limb | 0.92 | 0.76–1.12 | 0.426 |
| Lower limb | 0.97 | 0.85–1.10 | 0.636 |
| Unspecific | 1.27 | 1.10–1.46 | 0.001 |
| Comorbidity (ref. = No) | |||
| Cerebral vascular disease | 1.22 | 1.07–1.38 | 0.002 |
| Malignancy | 2.13 | 1.95–2.33 | < 0.001 |
| Clinical surgery (ref. = No) | |||
| Sentinel lymph node biopsy | 0.84 | 0.76–0.93 | 0.001 |
| Lymph node dissection | 1.15 | 1.02–1.30 | 0.022 |
aUsing Cox proportional hazard model. Bold type indicates statistical significantly (P < 0.05).
bAbbreviations: aHR adjusted hazard ratio, C.I. confidence interval.
cSocial economic status was classified as non-income (no income), low (income ranges from 1 to 583 US$ per month), middle (income ranges from 584 to 833 US$ per month) and high (income ≥ 834 US$ per month) categories.
Sentinel lymph node biopsy
Of all 3284 melanoma patients, 933 (28.4%) cases underwent SLNB whereas 2351 (71.6%) cases didn’t undergo SLNB. Sex was not associated with the percentage of patients undergoing SLNB, while old age and low SES were associated with a lower percentage of patients undergoing SLNB (P < 0.001). Details of percentage of patients undergoing SLNB in different age, sex and SES categories are shown in Table 3. To further investigate the relationship between SLNB and prognosis, we conducted the subgroup analysis of age, sex and SES for SLNB by prognosis (Table 4). Among patients in different age, sex and SES subgroups, SLNB was associated with a significantly better prognosis, except for patients aged less than 50 years (P = 0.105) and the high SES subgroup (P = 0.175).
Table 3.
Percentage of patients undergoing SLNB in different age, sex, SES categories (n = 3284).
| Variables | Sentinel lymph node biopsy | p Valuea | |
|---|---|---|---|
| No (n = 2351) |
Yes (n = 933) |
||
| Age, years (%) | < 0.001 | ||
| < 50 | 370 (63.4) | 214 (36.6) | |
| 50–59 | 304 (61.4) | 191 (38.6) | |
| 60–69 | 460 (68.7) | 210 (31.3) | |
| 70–79 | 679 (75.2) | 224 (24.8) | |
| ≥ 80 | 538 (85.1) | 94 (14.9) | |
| Sex (%) | 0.826 | ||
| Female | 1066 (71.4) | 427 (28.6) | |
| Male | 285 (71.7) | 506 (28.3) | |
| Social economic status (%)bc | < 0.001 | ||
| Non-income | 286 (76.7) | 87 (23.3) | |
| Low | 545 (76.7) | 166 (23.3) | |
| Middle | 1066 (71.8) | 419 (28.2) | |
| High | 411 (61.7) | 255 (38.3) | |
aUsing Chi-square test. Bold type indicates statistical significantly (P < 0.05).
bSocial economic status was classified as non-income (no income), low (income ranges from 1 ot 583 US$ per month), middle (income ranges from 584 to 833 US$ per month) and high (income ≥ 834 US$ per month) categories.
c49 patients were unknown.
Table 4.
Subgroup analysis of age, sex and social economic status for SLNB by prognosis (n = 3284).
| Variables | Survival (n = 1068) |
Death (n = 2216) |
p Valuea |
|---|---|---|---|
| Age | |||
| < 50 years (%) | 0.105 | ||
| Non-SLNB | 180 (48.6) | 190 (51.4) | |
| SLNB | 119 (55.6) | 95 (44.4) | |
| 50–59 years (%) | 0.012 | ||
| Non-SLNB | 121 (39.8) | 183 (60.2) | |
| SLNB | 98 (51.3) | 93 (48.7) | |
| 60–69 years (%) | 0.043 | ||
| Non-SLNB | 145 (31.5) | 315 (68.5) | |
| SLNB | 83 (39.5) | 127 (60.5) | |
| 70–79 years (%) | 0.001 | ||
| Non-SLNB | 146 (21.5) | 533 (78.5) | |
| SLNB | 72 (32.1) | 152 (67.9) | |
| ≥ 80 years (%) | < 0.001 | ||
| Non-SLNB | 75 (13.9) | 463 (86.1) | |
| SLNB | 29 (30.9) | 65 (69.1) | |
| Sex | |||
| Female (%) | < 0.001 | ||
| Non-SLNB | 341 (32.0) | 725 (68.0) | |
| SLNB | 224 (52.5) | 203 (47.5) | |
| Male (%) | < 0.001 | ||
| Non-SLNB | 326 (25.4) | 959 (74.6) | |
| SLNB | 177 (35.0) | 329 (65.0) | |
| Social economic statusbc | |||
| Non-income (%) | < 0.001 | ||
| Non-SLNB | 48 (16.8) | 238 (83.2) | |
| SLNB | 32 (36.8) | 55 (63.2) | |
| Low (%) | 0.001 | ||
| Non-SLNB | 140 (25.7) | 405 (74.3) | |
| SLNB | 65 (39.2) | 101 (60.8) | |
| Middle (%) | < 0.001 | ||
| Non-SLNB | 270 (25.3) | 796 (74.7) | |
| SLNB | 162 (38.7) | 257 (61.3) | |
| High (%) | 0.175 | ||
| Non-SLNB | 197 (47.9) | 214 (52.1) | |
| SLNB | 136 (53.3) | 119 (46.7) | |
aUsing Chi-square test. Bold type indicates statistical significantly (P < 0.05).
bSocial economic status was classified as non-income (no income), low (income ranges from 1 to 583 US$ per month), middle (income ranges from 584 to 833 US$ per month) and high (income ≥ 834 US$ per month) categories.
c49 patients were unknown.
Sensitivity analysis
There are 2823 patients in our sensitivity analysis. The 1-year and 3-year overall survival rate were 70.7% and 47.5%, respectively (see Supplementary Fig. 1 online). The association between SLNB and 1-year or 3-year overall survival in the crude analysis, multivariable analysis and propensity-score analyses was demonstrated in Table 5, which showed consistently better survival in the SLNB group. Moreover, the results of E-value analysis were shown in Table 6. In propensity-score analysis with matching, undergoing SLNB was associated with a better 1-year overall survival that had a hazard ratio of 0.40 (95% CI 0.31–0.50). The E-value for this was 3.16, meaning that residual confounding could explain the observed association if there exists an unmeasured covariate having a relative risk association at least as large as 3.16 with both 1-year overall survival and with undergoing SLNB. The baseline characteristics before and after propensity score matching can be seen as Supplementary Table 1 online, while univariate analysis with survival outcomes can be found as Supplementary Table 2 online.
Table 5.
Association between SLNB and the End Point of 1-year or 3-year Overall Survival in the Crude Analysis, Multivariable Analysis and Propensity-Score Analyses.
| Analysis | 1-Year overall survival | 3-Year overall survival |
|---|---|---|
| No. of events/no. of patients at risk (%) | ||
| No SLNB | 717/2044 | 1156/2044 |
| SLNB | 109/779 | 326/779 |
| Crude analysis—hazard ratio (95% CI) | 0.34 (0.28, 0.42) | 0.59 (0.52, 0.67) |
| Multivariable analysis—hazard ratio (95% CI)* | 0.45 (0.36, 0.57) | 0.71 (0.62, 0.82) |
| Propensity-score analyses—hazard ratio (95% CI) | ||
| With inverse probability weighting† | 0.41 (0.30, 0.54) | 0.81 (0.65, 0.99) |
| With matching‡ | 0.40 (0.31, 0.50) | 0.70 (0.60, 0.81) |
*Shown is the hazard ratio from the multivariable Cox proportional-hazards model, with adjustment for age, sex, social economic status, cancer sites, lymph node dissection, diabetes, hypertension, coronary artery disease, stroke, congestive heart failure, cirrhosis, Parkinson disease, COPD, malignancy. The analysis included all 2823 patients.
†Shown is the primary analysis with a hazard ratio from the multivariable Cox proportional-hazards model with covariates with inverse probability weighting according to the propensity score. The analysis included 2823 patients.
‡Shown is the hazard ratio from a multivariable Cox proportional-hazards model with covariates with matching according to the propensity score. The analysis included 2049 patients (1366 without SLNB and 683 undergoing SLNB).
Table 6.
Evaluation for unmeasured confounding between SLNB groups by E-value analysis.
| Analysis | 1-Year overall survival | 3-Year overall survival |
|---|---|---|
| Crude analysis—E value | 3.60 | 2.24 |
| Multivariable analysis—E value | 2.86 | 1.85 |
| Propensity-score analyses | ||
| With inverse probability weighting—E value | 3.09 | 1.58 |
| With matching—E value | 3.16 | 1.88 |
The larger the E-value, the lower the probability that an unmeasured confounder was to explain the entirety of the treatment effect.
Discussion
Old age was associated with a worse prognosis in previous studies in Taiwan4 and Western countries25–27. Age has been proved to be a very strong and independent predictor of survival outcome after accounting for all the dominant prognostic factors27. One reason that old age may be associated with a poor prognosis is that melanomas in older patients were more frequently late diagnosed26, which may be related to less attention to changes on their skin, low awareness of the early signs and symptoms of melanoma, increase of seborrheic keratoses (with which melanoma can be easily confused) or development of a higher proportion of melanomas in hard-to-see anatomical sites28. Another possible reason for old age being a poor prognostic factor is that melanomas in older patients were generally thicker, more likely to be ulcerated and with higher mitotic rates, which are thought to be prognostically unfavorable features26,27. One review article in 2018 has discussed the relationship between age and sentinel lymph node status29 and indicated decreased sentinel lymph node positivity but increased mortality with age. This may be due to the decreased possibility of senescent melanocytes reaching sentinel nodes and the inability of local immune system to control melanocytes when they reach sentinel nodes. In this study, old age was associated with a significantly increased risk of death (P < 0.001), which was consistent with former studies4,25–27.
According to cancer registries in Taiwan, the age‑adjusted incidence rates for invasive melanoma in 2018 were 0.77/100,000 for males and 0.64/100,000 for females, with the age‑adjusted male‑to‑female ratio of 1.20:11. One Taiwanese study of 181 melanoma cases in 2004 reported a male‑to‑female ratio of 0.88:14, while another study of 56 cases in 2019 reported a male‑to‑female ratio of 3.67:130. In our study, the male-to-female ratio of 3284 melanoma cases was 1.20:1, which was between the data of the two above-mentioned studies and was closer to the data in 2018 cancer registries in Taiwan. This ratio difference may be result from the larger case number and the population-based case source in our study. Many studies have demonstrated that female gender confers a better prognosis than male gender31,32, which was also shown in this study. The underlying protective effect of female gender is not well understood. Possible explanations include a greater incidence of unfavorable primary tumor characteristics32 and an older age at which melanoma were presented with in male patients31,33.
Although few comorbidities were thought to be closely related to melanoma, previous studies have suggested that cardiovascular disease was a significant risk factor for melanoma development while diabetes was a non-significant protective factor for melanoma development19,20. In addition, it’s also reported that there appears to be an association between melanoma and Parkinson disease21. In this study, these possibly relevant comorbidities were taken as prognostic factors and analyzed using a total of 3284 melanoma cases. Multivariate analysis showed that cerebral vascular disease (aHR = 1.22, P = 0.002) and malignancy (aHR = 2.13, P < 0.001) were significantly poor prognostic factors. However, the relationships between melanoma and these diseases and the clinical significance of our results remained unclear due to limited relevant studies at present. Further studies regarding this topic would be needed to unveil more details.
Whether anatomic site of melanoma is a significant predictor of survival remains controversial due to inconsistent statistical data among previous studies. According to a review article in 200934, many studies have suggested that extremity lesions offer a better prognosis than truncal lesions, while other studies have reported a completely opposite result. There’re still other studies which have failed to demonstrate the prognostic significance of anatomic location, particularly when the studies controlled for thickness. The discrepancy between these studies has yet to be explained, but may be possibly caused by diverse sample sizes, inconsistent study design and different confounding factors considered in previous studies. The fact that the most common anatomic sites differ in different melanoma subtypes, and that the prevalence of each melanoma subtype differs in western and eastern countries, may also contribute to this discrepancy. In this study, anatomic site of cutaneous melanoma, identified using ICD-9-CM Codes, was also analyzed as a prognostic factor. Our data suggested that the most commonly diagnosed site was on lower limbs (49.4%), followed by unspecific sites (18.1%), head and neck (14.9%), trunk (9.4%) and upper limbs (8.3%). In multivariate analysis, the only significant prognostic factor was unspecific site, with an aHR of 1.27 (95% CI 1.10–1.46, P = 0.001), compared with head and neck.
Issues surrounding health inequality have gained increasing attention over the past decades. The association between SES and melanoma was discussed in many Western studies previously35–39, yet relevant studies among Asian populations were rare. Several Western studies have demonstrated that melanoma is more common in high SES than in low SES populations35–38. Potential explanations for this finding are the more frequent exposure to recreational UV radiation, typically intermittent and high-intensity, in high SES individuals and the possible underreporting of melanoma in low SES individuals35–37,40. In contrast, lower SES was shown to be associated with a worse survival in previous studies, which may be a consequence of the relative lack of awareness of the severity of melanoma, delayed diagnosis and less management received after diagnosis35,41. Another possible reason may be the fact that acral lentiginous melanoma, which was of a worse prognosis compared to other subtypes, seemed to be associated with farmers, who were generally categorized as low SES group4,42. In our study, there were 1485, 711, 666 and 373 patients in middle, low, high and non-income groups, respectively. High SES was associated with a better survival (P < 0.001) than other groups in both univariate and multivariate analysis, which was compatible with former studies. Our finding indicated that similar disadvantages including lack of awareness of the disease or lack of access to medical resources among low SES populations may exist in Taiwan.
In the past, LND was usually recommended for patients with positive SLNB results. However, the two recent randomized controlled trials, German Dermatologic Cooperative Oncology Group-Selective Lymphadenectomy Trial (DeCOG-SLT)43 and Multicenter Selective Lymphadenectomy Trial II (MSLT-II)44, have shown that immediate LND provides no survival benefits. In DeCOG-SLT, there was no significant difference in 3-year distant-metastases-free survival, 3-year overall survival, 3-year recurrence-free survival and regional recurrence rate between patients treated with LND or nodal observation following a positive SLNB. In MSLT-II, no significant difference in melanoma-specific survival was seen between the LND arm and the observation arm. Therefore, LND is likely to be performed with diminishing frequency. In our study, undergoing LND was analyzed as a prognostic factor in melanoma cohort, and was associated with an increased risk of death in univariate (P = 0.002) and multivariate analysis (aHR = 1.15, P = 0.022). We attributed this result to the fact that patients who received LND were generally in advanced stage of disease and thus had a worse prognosis.
As the most effective method of lymphatic mapping and regional lymph node staging currently, SLNB provides useful prognostic information for clinicians to make ongoing treatment plan45. A meta-analysis conducted in 2016 reported that the melanoma-specific survival of patients undergoing SLNB plus wide local excision is better than that of patients undergoing wide local excision alone46. To date, the only randomized controlled trial evaluating SLNB is the Multicenter Selective Lymphadenectomy Trial (MSLT-1)11, which showed a significantly improved disease-free survival in the biopsy group compared to the observation group, among melanoma patients with lesions ≥ 1.2 mm in thickness. However, controversies exist due to the lack of clear evidence regarding the survival advantage SLNB provides7–10. In MSLT-1, no significant treatment-related difference in the 10-year melanoma-specific survival rate was seen in the overall study population. Other concerns included the cost to health system and the risk of complications such as lymphedema10,47,48. Despite all the controversies, SLNB for accurate staging has become the standard work-up for patients with intermediate-thickness or thick primary melanomas according to a number of relatively consistent guidelines49–51.
Histologic subtype of melanoma has been shown to be an independent prognostic factor in several studies52,53. It’s worth mentioning that the two major subtypes in white people are superficial spreading melanoma and nodular melanoma, whereas acral lentiginous melanoma, which is the most common subtype in Asians, is actually rare in the Western countries4,5. Therefore, studies evaluating the survival benefit of SLNB among Asian populations seem to be important. However, few studies regarding this area have been conducted so far. A China study comprising 47,351 patients reported that patients who underwent SLNB had significantly longer 5-year rates for overall survival and melanoma-specific survival compared with patients who did not undergo SLNB54, and another Taiwan study comprising 209 cases had similar result14. In our study of 3284 melanoma patients, undergoing SLNB was a statistically significant good prognostic factor in both univariate and multivariate analysis. In addition, old age and low SES were associated with a decreased percentage of patients undergoing SLNB. This may be due to clinical consideration that older patients are likely to have a higher rate of comorbidities contraindicating SLNB55. This may be also due to the lack of willingness or access to medical resources in old age and low SES patients. For example, patients with low SES who are required to work a lot simply to maintain their daily expenditures may prefer conservative management rather than undergoing further survey such as SLNB. Moreover, in subgroup analysis of age, sex and SES for SLNB by prognosis, SLNB cohorts in each subgroup had a better prognosis compared to non-SLNB cohorts. The association between undergoing SLNB and a better prognosis was observed consistently in our statistic data. As a result, we recommend that SLNB should be performed on patients, especially in old age or low SES, who are candidates for SLNB according to current guidelines to achieve maximal survival.
However, this study still has a number of limitations. First, SLNB was not randomly assigned but was examined retrospectively in this study. Second, the data of several important clinical characteristics such as Breslow thickness, stage and histologic subtype of melanoma was unavailable from NHIRD, and thus we could not adjust for these important factors in our analysis. Third, outcomes were evaluated using overall survival rather than melanoma-specific survival due to the limitation of NHIRD. Based on the aforementioned reasons, the result of this study should be interpreted with caution.
To address the potential bias caused by missing several important prognostic factors in this study, we further performed propensity score-based sensitivity analysis and E-value sensitivity analysis. The results of crude analysis, multivariable analysis and propensity-score analysis showed consistently better survival in the SLNB group. In E-value sensitivity analysis, potential implications of unmeasured confounders were quantified and we found that an unmeasured confounder was unlikely to explain the entirety of the treatment effect. Therefore, the robustness of our finding that undergoing SLNB was associated with a better prognosis was ensured by sensitivity analyses.
Conclusions
Undergoing SLNB was associated with a better prognosis. The poor prognosis of old age and low SES may be due to decreased percentages of patients undergoing SLNB. Therefore, we recommend that SLNB should be performed on patients, especially in old age or low SES, who are candidates for SLNB according to current guidelines to achieve maximal survival. Further studies are needed to provide better evidential support for the survival benefit of SLNB.
Supplementary Information
Acknowledgements
This study was supported by the Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, Grant No. VGHKS103-G02, 104-133.
Author contributions
P.C. Wu, Y.C. Chen, H.M. Chen and L.W. Chen all contributed to the conceptualization of this study. P.C. Wu wrote the main manuscript text. H.M. Chen was responsible for data collection and statistical analysis. Y.C. Chen and L.W. Chen contributed to critical revision of the article. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-99950-1.
References
- 1.Health Promotion Administration Ministry of Health and Welfare, T. Cancer Registry Annual Report, 2018 Taiwan https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=~/File/Attach/13498/File_15611.pdf (2020).
- 2.Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. PeerJ. 2016;4:e2809. doi: 10.7717/peerj.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang JW, et al. Sunrise in melanoma management: Time to focus on melanoma burden in Asia. Asia Pac. J. Clin. Oncol. 2017;13:423–427. doi: 10.1111/ajco.12670. [DOI] [PubMed] [Google Scholar]
- 4.Chang, J. W.-C. et al. Malignant melanoma in Taiwan: A prognostic study of 181 cases. Melanoma Res.14, 537-541 (2004). [DOI] [PubMed]
- 5.Luk NM, et al. Clinicopathological features and prognostic factors of cutaneous melanoma among Hong Kong Chinese. Clin. Exp. Dermatol. 2004;29:600–604. doi: 10.1111/j.1365-2230.2004.01644.x. [DOI] [PubMed] [Google Scholar]
- 6.Cutaneous Melanoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1492 Accessed 30 Apr 2021.
- 7.Kyrgidis, A. et al. Sentinel lymph node biopsy followed by lymph node dissection for localised primary cutaneous melanoma. Cochrane Database Syst. Rev. 10.1002/14651858.CD010307.pub2 (2015). [DOI] [PMC free article] [PubMed]
- 8.Morton DL, et al. Sentinel-node biopsy or nodal observation in melanoma. N. Engl. J. Med. 2006;355:1307–1317. doi: 10.1056/NEJMoa060992. [DOI] [PubMed] [Google Scholar]
- 9.Printz C. Physicians differ on the use of sentinel lymph node biopsy for melanoma: Published data receive various interpretations. Cancer. 2013;119:2515–2516. doi: 10.1002/cncr.28229. [DOI] [PubMed] [Google Scholar]
- 10.Torjesen, I. Sentinel node biopsy for melanoma: unnecessary treatment? BMJ (Clin. Res. Ed.)346, e8645. 10.1136/bmj.e8645 (2013). [DOI] [PubMed]
- 11.Morton DL, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014;370:599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bello DM, Faries MB. The landmark series: MSLT-1, MSLT-2 and DeCOG (Management of Lymph Nodes) Ann. Surg. Oncol. 2020;27:15–21. doi: 10.1245/s10434-019-07830-w. [DOI] [PubMed] [Google Scholar]
- 13.Wu CE, et al. Prognostic factors for Taiwanese patients with cutaneous melanoma undergoing sentinel lymph node biopsy. J. Formos. Med. Assoc. 2015;114:415–421. doi: 10.1016/j.jfma.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Chang JW, et al. Sentinel lymph node biopsy was associated with favorable survival outcomes for patients with clinically node-negative asian melanoma. Cancer Manag. Res. 2019;11:9655–9664. doi: 10.2147/cmar.s227837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011;20:236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 16.Chung WS, et al. Rheumatoid arthritis and risk of acute myocardial infarction—A nationwide retrospective cohort study. Int. J. Cardiol. 2013;168:4750–4754. doi: 10.1016/j.ijcard.2013.07.233. [DOI] [PubMed] [Google Scholar]
- 17.Chiang C-J, et al. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015;45:291–296. doi: 10.1093/jjco/hyu211. [DOI] [PubMed] [Google Scholar]
- 18.Cheng C-Y, Chiang C-J, Hsieh C-H, Chang Y-K, Lai M-S. Is quality of registry treatment data related to registrar experience and workload? A study of Taiwan cancer registry data. J. Formos. Med. Assoc. 2018;117:1093–1100. doi: 10.1016/j.jfma.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Tseng, H. W. et al. Risk of skin cancer in patients with diabetes mellitus: A nationwide retrospective cohort study in Taiwan. Medicine (Baltimore)95, e4070. 10.1097/MD.0000000000004070 (2016). [DOI] [PMC free article] [PubMed]
- 20.Tseng H-W, et al. Risk of melanoma in patients with diabetes mellitus: A nationwide retrospective cohort study in Taiwan. Diabetes Res. Clin. Pract. 2016;120:S206. doi: 10.1016/s0168-8227(16)31483-8. [DOI] [Google Scholar]
- 21.Dalvin LA, et al. Parkinson disease and melanoma: Confirming and reexamining an association. Mayo Clin. Proc. 2017;92:1070–1079. doi: 10.1016/j.mayocp.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradford PT, Freedman DM, Goldstein AM, Tucker MA. Increased risk of second primary cancers after a diagnosis of melanoma. Arch. Dermatol. 2010;146:265–272. doi: 10.1001/archdermatol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu S-H, et al. Lymphoscintigraphy and intraoperative gamma probe-directed sentinel lymph node mapping in patients with malignant melanoma. J. Formos. Med. Assoc. 2004;103:41–46. [PubMed] [Google Scholar]
- 24.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321:602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 25.Caraco C, et al. Age as predictor in patients with cutaneous melanoma submitted to sentinel lymph node biopsy. Eur. J. Surg. Oncol. 2006;32:970–973. doi: 10.1016/j.ejso.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Lasithiotakis K, et al. Age and gender are significant independent predictors of survival in primary cutaneous melanoma. Cancer. 2008;112:1795–1804. doi: 10.1002/cncr.23359. [DOI] [PubMed] [Google Scholar]
- 27.Balch CM, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann. Surg. Oncol. 2013;20:3961–3968. doi: 10.1245/s10434-013-3100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lasithiotakis KG, Petrakis IE, Garbe C. Cutaneous melanoma in the elderly: Epidemiology, prognosis and treatment. Melanoma Res. 2010;20:163–170. doi: 10.1097/CMR.0b013e328335a8dd. [DOI] [PubMed] [Google Scholar]
- 29.Ribero S, et al. Effect of age on melanoma risk, prognosis and treatment response. Acta Derm. Venereol. 2018;98:624–629. doi: 10.2340/00015555-2944. [DOI] [PubMed] [Google Scholar]
- 30.Tsai K-C, et al. Cutaneous malignant melanoma in Eastern Taiwan: Clinicopathologic analysis of 56 cases. Dermatol. Sin. 2019;37:187–193. doi: 10.4103/ds.ds_13_19. [DOI] [Google Scholar]
- 31.Lindholm, C. et al. Invasive cutaneous malignant melanoma in Sweden, 1990–1999. A prospective, population-based study of survival and prognostic factors. Cancer101, 2067–2078. 10.1002/cncr.20602 (2004). [DOI] [PubMed]
- 32.Scoggins, C. R. et al. Gender-related differences in outcome for melanoma patients. Ann. Surg.243, 693–698; discussion 698–700.10.1097/01.sla.0000216771.81362.6b (2006). [DOI] [PMC free article] [PubMed]
- 33.Chao C, et al. Correlation between prognostic factors and increasing age in melanoma. Ann. Surg. Oncol. 2004;11:259–264. doi: 10.1245/aso.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Payette MJ, Katz M, 3rd, Grant-Kels JM. Melanoma prognostic factors found in the dermatopathology report. Clin. Dermatol. 2009;27:53–74. doi: 10.1016/j.clindermatol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Ortiz, C. A., Goodwin, J. S. & Freeman, J. L. The effect of socioeconomic factors on incidence, stage at diagnosis and survival of cutaneous melanoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res.11, Ra163–172 (2005). [PubMed]
- 36.Clarke CA, Moy LM, Swetter SM, Zadnick J, Cockburn MG. Interaction of area-level socioeconomic status and UV radiation on melanoma occurrence in California. Cancer Epidemiol. Biomark. Prev. 2010;19:2727–2733. doi: 10.1158/1055-9965.EPI-10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birch-Johansen F, Hvilsom G, Kjaer T, Storm H. Social inequality and incidence of and survival from malignant melanoma in a population-based study in Denmark, 1994–2003. Eur. J. Cancer. 2008;44:2043–2049. doi: 10.1016/j.ejca.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 38.van der Aa MA, de Vries E, Hoekstra HJ, Coebergh JW, Siesling S. Sociodemographic factors and incidence of melanoma in the Netherlands, 1994–2005. Eur. J. Cancer. 2011;47:1056–1060. doi: 10.1016/j.ejca.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 39.Borghi A, et al. Impact of socioeconomic status and district of residence on cutaneous malignant melanoma prognosis: A survival study on incident cases between 1991 and 2011 in the province of Ferrara, northern Italy. Melanoma Res. 2017;27:619–624. doi: 10.1097/CMR.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 40.Pearce J, Barnett R, Kingham S. Slip! Slap! Slop! Cutaneous malignant melanoma incidence and social status in New Zealand, 1995–2000. Health Place. 2006;12:239–252. doi: 10.1016/j.healthplace.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Eide MJ, Weinstock MA, Clark MA. Demographic and socioeconomic predictors of melanoma prognosis in the United States. J. Health Care Poor Underserved. 2009;20:227–245. doi: 10.1353/hpu.0.0113. [DOI] [PubMed] [Google Scholar]
- 42.Chang HY, Feng HL, Wang L, Chou P, Wang PF. The incidence, prevalence, and survival of malignant melanoma in Taiwan. Value Health. 2014;17:A740. doi: 10.1016/j.jval.2014.08.135. [DOI] [PubMed] [Google Scholar]
- 43.Leiter U, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:757–767. doi: 10.1016/s1470-2045(16)00141-8. [DOI] [PubMed] [Google Scholar]
- 44.Faries MB, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N. Engl. J. Med. 2017;376:2211–2222. doi: 10.1056/NEJMoa1613210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pay, A. Malignant melanoma (non-metastatic): Sentinel lymph node biopsy. BMJ Clin. Evid.2016 (2016). [PMC free article] [PubMed]
- 46.Santos-Juanes, J., Fernandez-Vega, I., Galache Osuna, C., Coto-Segura, P. & Martinez-Camblor, P. Sentinel lymph node biopsy plus wide local excision vs. wide location excision alone for primary cutaneous melanoma: a systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. JEADV31, 241–246. 10.1111/jdv.13824 (2017). [DOI] [PubMed]
- 47.Moody JA, Ali RF, Carbone AC, Singh S, Hardwicke JT. Complications of sentinel lymph node biopsy for melanoma—A systematic review of the literature. Eur. J. Surg. Oncol. 2017;43:270–277. doi: 10.1016/j.ejso.2016.06.407. [DOI] [PubMed] [Google Scholar]
- 48.Campanholi LL, Duprat JP, Fregnani J. Incidence of LE due to treating cutaneous melanoma. J. Lymphoedema. 2011;6:30–34. [Google Scholar]
- 49.Wong SL, Kennedy EB, Lyman GH. Sentinel lymph node biopsy and management of regional lymph nodes in melanoma: American Society of Clinical Oncology and Society of Surgical Oncology Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2018;14:242–245. doi: 10.1200/jop.2017.028241. [DOI] [PubMed] [Google Scholar]
- 50.Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019;30:1884–1901. doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 51.Coit, D. G. et al. Cutaneous Melanoma, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN17, 367–402. 10.6004/jnccn.2019.0018 (2019). [DOI] [PubMed]
- 52.Lattanzi M, et al. Primary melanoma histologic subtype: Impact on survival and response to therapy. J. Natl. Cancer Inst. 2019;111:180–188. doi: 10.1093/jnci/djy086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carrera C, et al. Prognostic role of the histological subtype of melanoma on the hands and feet in Caucasians. Melanoma Res. 2017;27:315–320. doi: 10.1097/cmr.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 54.Chen J, et al. Prognostic role of sentinel lymph node biopsy for patients with cutaneous melanoma: A retrospective study of surveillance, epidemiology, and end-result population-based data. Oncotarget. 2016;7:45671–45677. doi: 10.18632/oncotarget.10140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boada A, et al. Sentinel lymph node biopsy versus observation in thick melanoma: A multicenter propensity score matching study. Int. J. Cancer. 2018;142:641–648. doi: 10.1002/ijc.31078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.