Important Compound Classes
Title
Novel PRMT5 Inhibitors
Patent Publication Number
WO 2021/163344 A1
Publication Date
August 19, 2021
Priority Application
US 62/975,258
Priority Date
February 12, 2020
Inventors
Allen, J. R.; Amegadzie, A.; Beylkin, D. J.; Booker, S.; Bourbeau, M. P.; Butler, J. R.; Frohn, M. J.; Glad, S. O. S.; Husemoen, B. W.; Kaller, M. R.; Kohn, T. J.; Lanman, B. A.; Li, K.; Liu, Q.; Lopez, P.; Ma, V. V.; Manoni, F.; Medina, J.; Minatti, A. E.; Peiro Cadahia, J.; Pettus, L.; Pickrell, A. J.; Sarvary, I.; Tamayo, N. A.; Vestergaard, M.
Assignee Company
Amgen, Inc., USA
Disease Area
Cancer
Biological Target
PRMT5
Summary
Epigenetic regulation of gene expression is an important biological determinant of protein production, cellular differentiation and plays a significant pathogenic role in several human diseases. Epigenetic regulation involves heritable modification of genetic material without changing its nucleotide sequence. Typically, epigenetic regulation is mediated by selective and reversible modification. (e.g., methylation) of DNA and proteins (e.g., histones) that control the conformational transition between transcriptionally active and inactive states of chromatin. These covalent modifications can be controlled by enzymes such as methyltransferases (e.g., protein arginine N-methyltransferase 5 (PRMT5)), many of which are associated with specific genetic alterations that can cause human disease. PRMT5 plays a role in diseases such as proliferative disorders, metabolic disorders, and blood disorders.
The homozygous deletion of tumor suppressor genes is a key driver of cancer, frequently resulting in the collateral loss of passenger genes located in close genomic proximity to the tumor suppressor. Deletion of these passenger genes can create therapeutically tractable vulnerabilities that are specific to tumor cells. Deletion of MTAP (methylthioadenosine phosphorylase), a key enzyme in the methionine and adenine salvage pathways, results in accumulation of its substrate, methylthioadenosine (MTA). MTA shares close structural similarity to S-adenosylmethionine (SAM), the substrate methyl donor for the type II methyltransferase PRMT5. Targeting PRMT5 with an MTA-cooperative small molecule inhibitor could preferentially target the MTA bound state of PRMT5, enriched in MTAP null tumor cells, while providing an improved therapeutic index over normal cells where MTAP is intact and MTA levels are low.
The present application describes a series of novel PRMT5 inhibitors for the treatment of cancer. Further, the application discloses compounds, their preparation, use, pharmaceutical composition, and treatment.
Definitions
 represents a single or double bond;
represents a single or double bond;
X1 and X2 = N or C, wherein if X1 is C, it can be optionally substituted with halo or C1–6alkyl;
Ar = six membered aromatic ring having 0–2 N atoms, wherein each Ar is independently substituted with 0–2 Ra groups;
R = H or methyl;
R1 and R2 = H, optionally substituted with C1–6alkyl, C1–6alkynyl, -C(ORc), single and double cyclyl having 0–3 N, S or O atoms, wherein substituents are selected from halo, optionally substituted with C1–6alkyl, −C(O)NRfRg, OH and 5-membered ring having 0–3 N atoms; and
R3 and R4 = H, halogen, alkynyl, cyano and C1–6alkyl, optionally substituted with halo or deuterium.
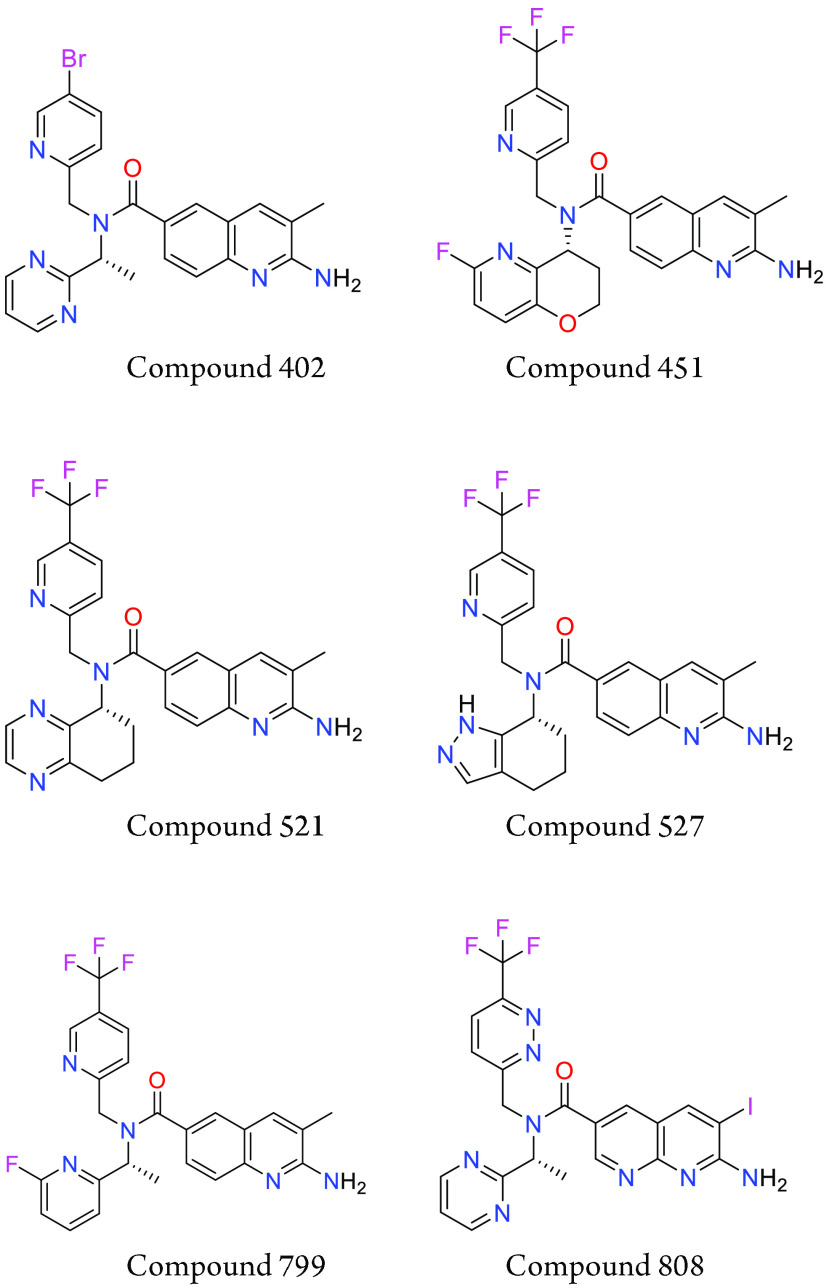
Key Structures
Biological Assay
The HTC116 MTAP null proliferation assay was performed. The compounds described in this application were tested for their ability to inhibit PRMT5. The PRMT5 IC50 (μM) are shown in the following table.
Biological Data
The table below shows representative
compounds were tested for PRMT5 inhibition. The biological data obtained
from testing representative examples are listed in the following table.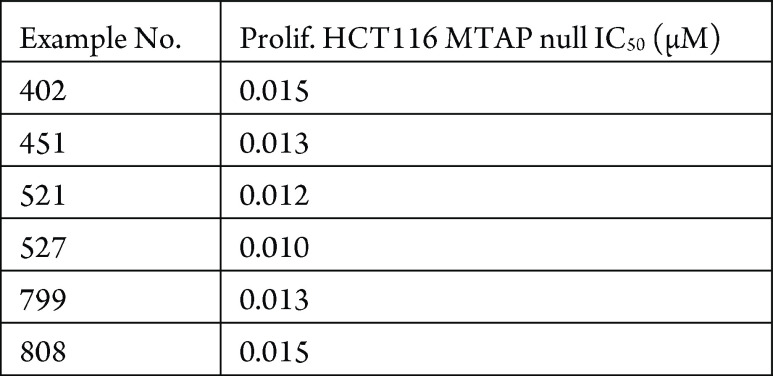
Claims
Total claims: 25
Compound claims: 19
Pharmaceutical composition claims: 2
Method of treatment claims: 4
Recent Review Articles
-
1.
Guccione E.; Schwarz M.; Di Tullio F.; Mzoughi S.. Curr. Opin. Pharmacol. 2021, 59, 33.
-
2.
Lei Y.; Han P.; Tian D.. Transl. Oncol. 2021, 14, 101194.
-
3.
Tang Z.; Xu Z.; Zhu X.; Zhang J.. Cancer Commun. 2021, 41, 16.
-
4.
Pellarin I.; Belletti B.; Baldassarre G.. Med. Res. Rev. 2021, 41, 586.
-
5.
Verheul T. C. J.; Trinh V. T.; Vazquez O.; Philipsen S.. ChemMedChem. 2020, 15, 2436.
-
6.
Sengupta S.; Kennemer A.; Patrick K.; Tsichlis P.; Guerau-de-Arellano M.. Trends Immunol. 2020, 41, 918.
The author declares no competing financial interest.
Special Issue
Published as part of the ACS Medicinal Chemistry Letters special issue “Epigenetics 2022”.