Abstract
An indazole/aza-indazole scaffold was developed as a novel chemotype for JNK3 inhibition. Extensive structure activity relationship (SAR) studies utilizing various in vitro and in vivo assays led to potent and highly selective JNK3 inhibitors with good oral bioavailability and high brain penetration. One lead compound, 29, was a potent and selective JNK3 inhibitor (IC50 = 0.005 μM) that had significant inhibition (>80% at 1 μM) to only JNK3 and JNK2 in a panel profiling of 374 wild-type kinases, had high potency in functional cell-based assays, had high stability in the human liver microsome (t1/2 = 92 min), and was orally bioavailable and brain penetrant (brain/plasma ratio: 56%). The cocrystal structure of 29 in human JNK3 at a 2.1 Å resolution showed that indazole or aza-indazole-based JNK3 inhibitors demonstrated a type I kinase inhibition/binding.
Keywords: MAPK10, JNK3, kinase inhibitor, Alzheimer’s disease (AD), indazole, aza-indazole, neurodegeneration
The c-jun N-terminal kinase 3 (JNK3), also known as MAPK10 (mitogen-activated protein kinase 10),1 is mainly expressed in the brain,2 whereas its two isoforms JNK1 and JNK2 are present ubiquitously.2,3 JNK3 has been discovered to be in the pathways related to many central nervous (CNS) diseases, such as Parkinson’s disease (PD),4−8 spinal muscular atrophy (SMA),9 Alzheimer’s disease (AD),10−16 epilepsy,17 etc. Therefore, brain-penetrant JNK3-selective inhibitors are highly sought after as tools to investigate and as treatments for these CNS diseases.18 Many JNK3 inhibitors have been identified and developed18−34 but so far none of them have been advanced to clinical trials for treating CNS diseases. The reason might be due to either inadequate ADMET properties (including brain-penetration capability) of existing JNK3 inhibitors or mechanism-based issues related to the JNK3 pathways. Therefore, new proprietary JNK3 inhibitors with desirable properties suitable for CNS applications are still needed for investigations.
We have previously identified highly selective and potent JNK3 inhibitors with good oral bioavailability and/or brain penetration.18,22,33,35 Some of these inhibitors are based on a pyrazole-urea scaffold with an N-aromatic ring substitution on the pyrazole moiety, as exampled by inhibitors A(22) and B(18) in Figure 1. Inhibitors A and B were basically JNK3 specific, had high selectivity against both JNK1 and JNK2, and kinase p38α. Profiling studies in a panel of ∼370 wild-type kinases, showed that B, at 5 μM, had strong inhibition (>80% inh.) only for JNK3.18 Both inhibitors A and B had low P450 inhibitions and B also provided good oral bioavailability and excellent brain exposure in mice.18 However, for this pyrazole-urea chemotype, good brain penetration could be obtained from only a few analogs (internal data, not published) due to the intrinsically unfavorable properties of the urea moiety (presence of two NH counts and high PSA values). Therefore, novel scaffolds (for JNK3 inhibitions) with higher intrinsic brain exposures are still desirable.
Figure 1.
Two JNK3-selective inhibitors from the pyrazole-urea scaffold. The carbonylamino and the pyrazole moieties are coplanar in the inhibitor binding modes based on crystal structures.
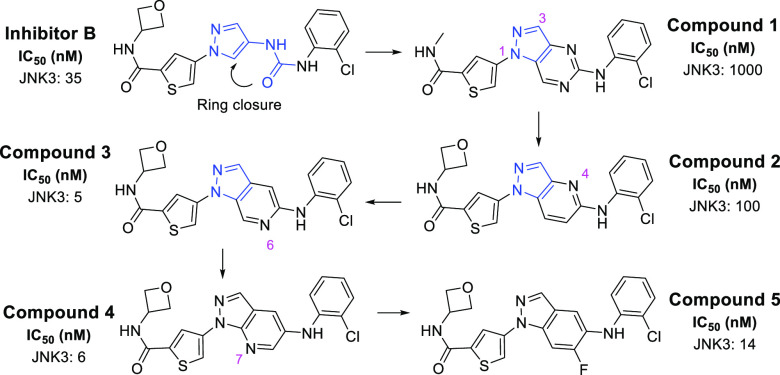
In the crystal structures of inhibitors A and B with JNK3 (PDB IDs: 4WHZ and 7KSK),18,22 the pyrazole nitrogen is binding to the hinge of the ATP pocket. Remarkably, we noticed that the urea moiety and the pyrazole moiety are almost coplanar.18,22 We reasoned that derivatives based on a ring-closure from the urea carbonyl group to the pyrazole 5-position (Figure 2) might still give a high JNK3 affinity. In addition, the newly formed indazole/aza-indazole ring is expected to have a higher intrinsic brain penetration than the pyrazole-urea combination in inhibitors A and B, mainly due to the more rigid structures and lower PSA values.
Figure 2.
New scaffold design and the evolution to a high JNK3 inhibition.
As shown in Figure 2, the closest analog to inhibitor B by a simple ring closure is compound 1, a pyrimidine-indazole chemotype. However, 1 had a low JNK3 inhibition with an IC50 of only ∼1 μM (replacing the oxetane amide by a methyl amide will not make much difference in JNK3 inhibition based on SAR described below). We reasoned that the aminopyrimidine moiety in 1 could compete with the pyrazole (or indazole) nitrogen atom for hinge-binding, thus had disturbed the optimal binding conformation for a high JNK3 affinity. Therefore, three pyridine-based analogs (compounds 2, 3, and 4) were prepared and evaluated. Indeed, better JNK3 inhibitors were obtained (Figure 2). The 4-pyridine analog 2 was 10-times better than compound 1 (IC50: 100 nM for 2 vs 1000 nM for 1). Remarkably, the 6-pyridine analog 3 was a highly potent JNK3 inhibitor with an IC50 of 5 nM. Moving the nitrogen atom from the 6-position to 7-position on the aza-indazole ring (compound 4) also gave a high JNK3 inhibition. It is speculated that the 4-position nitrogen atom in 2 might be H-bound to surrounding protein residues thus might have disturbed the optimal JNK3 binding, while the 6- and 7-position nitrogen atoms in 3 and 4 are exposed to solvents (referring to the crystal structures) and thus have minimal effects on ligand binding. In addition to the aza-indazole chemotypes in 3 and 4, the JNK3 IC50 value (14 nM) for compound 5 indicated that the parent indazole chemotype was also a good scaffold of JNK3 inhibitions.
Compounds 3, 4, and 5 had high JNK3 inhibitions, and all three could be leads for further optimizations. Because of the lower tPSA value (higher potentials for brain exposure) of compound 5 (tPSA: 66 vs 78 in 3 and 4), the simple indazole chemotype (in 5) was selected to carry out further SAR studies for the amide moiety. JNK1, JNK2, and p38α inhibitions were also measured as the counter screens to evaluate the compound selectivity. Data in Table 1 demonstrated that compound 5 was selective against JNK1/2, whereas its selectivity against p38α (29-fold) was lower than that of the corresponding pyrazole-urea-based inhibitor B (>570-fold).
Table 1. SAR Studies for the Amide Moiety.
The average of ≥2 experiments with errors within 30% of the average.
Not determined.
R: ratio of IC50 values for JNK1, JNK2, and p38α over JNK3.
Several amides that contained a heterocyclic ring were prepared to optimize the JNK3 inhibitory potency and selectivity. Thus, replacement of the 4-membered O-containing ring in compound 5 by 5- and 6-membered O-containing rings (compounds 6 and 7) both led to higher JNK3 inhibitions but similar selectivity against JNK1, whereas it reduced selectivity against p38α. Replacement by N-containing rings (compounds 8 and 9) also gave high JNK3 inhibition and good selectivity against JNK1. Interestingly, the amide compound 9 had a low selectivity against p38α while that for compound 8 was fair (60-fold, Table 1). Introduction of a nitrogen-containing heterocycle in compounds 8, 9, 15, and 16 (see below) was aimed to improve the aqueous solubility and/or to enhance the plasma stability (the -CD3 substitution).
Similar results were also obtained for amides containing simple alkyl chains (compounds 10–14). These compounds all had high inhibitions to JNK3 and a good selectivity against JNK1. Most of them had a low selectivity against p38α (<20-fold). The only exception was compound 10, which had a 62-fold selectivity against p38α (Table 1). Interestingly, although it had only a marginal selectivity against p38α (20-fold), the simple methyl amide inhibitor 14 was a highly potent JNK3 inhibitor (IC50 = 5 nM), much higher than its corresponding analog in the pyrazole-urea series (IC50 = 118 nM, inhibitor 7 in ref (18)). SAR studies were also performed for the 6-position of the indazole. Replacing 6-F by a 6-H (15) slightly increased the JNK3 inhibition (IC50 = 1 nM vs 5 nM for compound 8) and it enhanced the selectivity against p38α (226-fold). Change of the heterocyclic ring chirality or introduction of a bulky ring N-substitution, or a combination of both were detrimental to JNK3 binding (compound 16). Replacement by a methoxy group (compound 17) also deteriorated the JNK3 inhibition and decreased the p38α selectivity (8-fold).
An overall consideration of JNK3 inhibitory potency and selectivity against JNK1/p38α demonstrated that compounds 5, 8, 14, and 15 are the best JNK3 inhibitors in Table 1. However, data from cell-based qPatch hERG assays indicated that the tertiary amine-containing analogs, such as 8 and 15, had strong hERG inhibition with IC50 values of <1 μM. On the other hand, those analogs containing no tertiary amines in their amide moiety, such as 14, were relatively clean in hERG inhibition with much higher IC50 values. The qPatch hERG inhibition curves for compounds 14 and 15 are shown in Figure 3, which clearly demonstrated the high hERG inhibition for the tertiary amine-containing inhibitor 15. Therefore, further optimizations will all be based on leads with a methyl amide (14) and/or an oxetane amide (5).
Figure 3.

qPatch hERG cell-based assays for JNK3 inhibitors 14 and 15. The cell line used: CHO hERG DUO.
We then turned to the SAR studies for the aniline moiety and the central indazole ring. As shown in Table 2, introduction of a 6-F substitution to 14 yielded compound 18 which had a slight increase in JNK3 inhibition. Replacement of the 2-Cl by a methoxy group (19) slightly decreased the JNK3 inhibitory potency, whereas the p38α selectivity remained the same. Interestingly, replacing the aniline moiety by a phenoxy group (compound 20, compared to 14) simultaneously reduced the JNK3 inhibitory potency and the selectivity against p38α, which is probably due to a less favorable orientation of an O-ether linkage compared to that of an amino linkage. The selectivity against p38α for all 4 compounds (14, 18, 19, and 20) was only fair.
Table 2. SAR Studies for Other Parts of Lead Compounds.
| biochemical
inhibition IC50 (μM)a |
|||||
|---|---|---|---|---|---|
| compd | R | JNK3 | JNK1 (R)b | JNK2 (R)b | p38α (R)b |
| 18 | 0.002 | 0.258 (129) | 0.029 (15) | 0.024 (12) | |
| 19 | 0.005 | 1.24 (248) | ndc | 0.065 (13) | |
| 20 | 0.03 | 2.42 (80) | 1.02 (33) | 0.286 (9) | |
| 21 | 0.024 | 4.73 (197) | 0.414 (17) | 0.481 (20) | |
| 3 | 0.005 | 0.69 (139) | ndc | 0.086 (17) | |
| 22 | 0.002 | 0.26 (130) | 0.074 (37) | 0.057 (28) | |
| 4 | 0.006 | 0.94 (157) | 0.256 (43) | 0.26 (43) | |
| 23 | 0.002 | 0.213 (106) | 0.052 (26) | 0.106 (50) | |
| 24 | 0.012 | 0.872 (73) | 0.14 (11) | 0.38 (31) | |
| 25 | >10 | >10 | ndc | >10 | |
| 26 | 2-Cl-3-F | 0.169 | >10 (>59) | ndc | 1.42 (8) |
| 27 | 2-Cl-4-F | 0.079 | 6.0 (76) | ndc | 0.49 (51) |
| 28 | 2-Cl-5-F | 1.07 | >10 | ndc | 2.89 |
| 29 | 2-Cl-6-F | 0.005 | 0.387 (77) | 0.074 (15) | 0.106 (21) |
The average of ≥2 experiments with errors within 30% of the average.
R: ratio of IC50 values for JNK1, JNK2, and p38α over JNK3.
Not determined.
Remarkably, the SAR is different in the aza-indazole chemotype. For example, an oxetane amide led to increased JNK3 inhibitory potency (4 vs 21), whereas it decreased the JNK3 inhibitory activity in the indazole series (5 vs 14 in Table 1). Introduction of a 6-F substitution to the aniline moiety in 21 led to an increase of JNK3 inhibition (compound 23), while no big differences in the indazole chemotype (14 vs 18). The SAR in p38α selectivity is also different between the indazole and aza-indazole chemotypes. Compound 18 (6-F substitution) had lower p38α selectivity than compound 14 (no 6-F substitution), whereas inhibitor 23 (6-F substitution) had higher p38α selectivity than inhibitor 21 (no 6-F substitution).
It is interesting to point out that the 7′-aza analog 23 had the same JNK3 inhibitory activity but an increased p38α selectivity compared to the 6′-aza inhibitor 22 (50-fold vs 28-fold). Adding a methyl group to the indazole 6′-position of 23 (compound 24) led to a slightly deteriorated JNK3 inhibition. Data for compounds 25 and 26 indicated that simultaneous substitutions to both the 2- and 3-positions of the aniline ring was not favorable for JNK3 inhibition, and a larger substitution (3-methoxy in compound 26, larger than F) could even lead to inactivity against JNK3 (no any inhibition until 10 μM). Data for compounds 26 to 29 demonstrated that an additional F-substitution on the aniline ring was only well tolerated at the 6-position. Compound 29 had similar properties to lead compound 14 (JNK3 inhibitory potency, and selectivity against JNK1/2 and p38α).
In vitro DMPK and cytotoxicity assays were carried out to assess selected compounds that had p38α IC50 values of >100 nM. Data in Table 3 showed that these JNK3 inhibitors had low inhibitions against four selected P450 isozymes with the exception of a few compounds against 2C9. The most significant inhibitions came from inhibitor 29 against CYP-2C9 (90%) and CYP-3A4 (71%). Titration experiments gave an IC50 against CYP-2C9 at 1.1 μM, and that for CYP-3A4 was 1.7 μM. These IC50 values are still much higher than the JNK3 biochemical IC50 value (5 nM) and the cell-based inhibition IC50 value (see Figure 5). Titration experiments were also carried out for those inhibitors (4, 21, 23) which had high inhibitions against CYP-2C9 at 10 μM. The IC50 values were all between 4 and 6 μM (see the Supporting Information).
Table 3. Data for P450 Inhibition, Microsomal Stability, MTT Cytotoxicity, and qPatch hERG Assays for Selected JNK3 Inhibitors.
| CYP-450% inhibitiona | microsomal
stability t1/2 (min) |
||||
|---|---|---|---|---|---|
| compd | 1A2/2C9/2D6/3A4 | human | mouse | MTTb (%) | qPatch hERG IC50 μM) |
| 4 | 19/76/15/14 | 21 | 9 | 103 | n.d.c |
| 5 | 4/41/–16/–56 | 92 | 20 | 98 | n.d. |
| 14 | 21/39/–4/–13 | 37 | 42 | 83 | >30 |
| 20 | 64/68/25/–14 | 72 | 15 | 94 | 15.7 |
| 21 | 30/66/30/57 | 15 | 14 | 119 | >100 |
| 23 | 33/65/24/34 | 30 | 22 | 104 | 9.0 |
| 24 | 43/61/19/–2 | 26 | 17 | 109 | >30 |
| 29 | 33/90/40/71 | 92 | 77 | 116 | 10 |
| Inh-B | 6/8/–15/–34 | 66 | 24 | 104 | >30 |
% inh. at 10 μM.
MTT cell viability at 10 μM, data are the average of ≥2 experiments, in SHSY5Y cells.
Not determined.
Figure 5.
Cell-based inhibition of APPT668 phosphorylation in the presence or absence of JNK3 inhibitors.
All compounds had good stabilities in the human microsome. In mice, the stability of most compounds was fair to good, and only compound 4 had a half-life of <10 min. The cytotoxicity for most compounds in SHSY5Y cells was low, and except compound 14 which showed some toxicity with a 83% viability at 10 μM. In order to evaluate other potential side effects/toxicity, more optimized JNK3 inhibitors were subjected to qPatch hERG assays. As shown in Table 3, the cell-based hERG inhibition IC50 values for most selected compounds were excellent except for compounds 23 and 29, which had only a fair/reasonable IC50 values of ∼10 μM.
The general kinase selectivity for JNK3 inhibitors derived from this indazole/aza-indazole chemotype was assessed against the wild-type 374-kinase panel of the Reaction Biology Corporation (www.reactionbiology.com). The compound was profiled at a concentration of >100-fold of its JNK3 inhibition IC50. Thus, inhibitor 29 was evaluated at 1 μM (200-fold of its IC50 = 5 nM, Table 2). Data (see the Supporting Information) showed 29 had significant inhibition (>80%) against only JNK3 (89%) and JNK2 (81%). In addition, 29 at 1 μM had also some inhibition for CK1e (78%, IC50 = 100 nM), DDR1 (73%, IC50 = 247 nM), p38α (75%, IC50 = 110 nM), BRK (55%), and JNK1 (30%). The profiling data demonstrated that compounds derived from this indazole/aza-indazole scaffold had isoform selectivity of JNK3 vs JNK1 and the broad kinase panel selectivity was also high (a hit ratio of 1.6%) counting in all those hits with >50% inhibitions at 1 μM.
Compared to the pyrazole-urea scaffold of JNK3 inhibitors we previously published,18,22 where optimized JNK3 inhibitors hit only JNK3 in the same kinase panel, this indazole/aza-indazole scaffold had lower general kinase selectivity. The reason is probably due to the extra hydrophobic interactions between the indazole/aza-indazole ring and protein residues around it under the P-loop (see crystal structure below) in the ATP pocket, which has low differentiation among various kinases.
The crystal structure of 29 in JNK3 (human) was pursued to elucidate the binding mode for JNK3 inhibitors based on this indazole/aza-indazole chemotype. The 2.1 Å crystal structure (Figure 4, top) showed that 29 had a typical type I binding mode: the indazole nitrogen atom and the amide NH moiety formed H-bonds to the hinge residue M149 of JNK3; water-bridged H-bonds were also observed between aniline NH and the side chain amino group of catalytic residue K93. The amide chain on the thiophene ring pointed to the outside of the binding pocket, and toward the solvent. The aniline aromatic ring was sitting in hydrophobic pocket I (the selectivity pocket) and was twisted with the aza-indazole ring (∼45°). As described previously, this binding in the selectivity pocket is responsible for the isoform selectivity of JNK3 against JNK1.22,36 The thiophenyl moiety stood around hydrophobic pocket II and its aromatic ring was also twisted (∼30°) relative to the aza-indazole ring.
Figure 4.

X-ray crystal structure of inhibitor 29 in human JNK3 (2.1 Å), PDB ID 7S1N. The inhibitor is shown as a stick model with the protein in a transparent surface model (top), and the JNK3:29 is overlaid with JNK3:A (PDB ID: 4WHZ) (bottom).
Hydrophobic interactions in hydrophobic pocket I and hydrophobic pocket II, and that around the aza-indazole ring under the P-loop all contributed to the high JNK3 affinity. Compared to the pyrazole-urea scaffold, the extra hydrophobic interactions induced by the indazole 6-membered aromatic moiety was responsible for both the higher JNK3 inhibitory potency and the relatively lower general kinase selectivity of 29 (compared to pyrazole-urea based inhibitor B. See the overlaying display of 29 and inhibitor B in JNK3. Figure 4, bottom).
The phosphorylation of APP at T668 by JNK3 is critical for APP processing through enhanced endocytosis of APP.10 We thus assessed the extent to which our newly developed JNK3 inhibitors’ block of APPT668 phosphorylation by the endogenous JNK3 in primary neuronal cultures. As shown in Figure 5, both JNK3 inhibitors 14 and 29 had significant inhibitions for the phosphorylation of residue T668 of APP, at a concentration of even as low as 1 nM.
In vivo PK studies were carrieded out in mice to evaluate the oral bioavailability and brain-penetration capability of selected JNK3 inhibitors based on this indazole/aza-indazole scaffold. These experiments were performed for several of our lead JNK3 inhibitors. Data for a representative compound, 29, are shown in Table 4, which lists the plasma and brain compound concentration at 2 h after po dosing (30 mg/kg). These data demonstrated that inhibitor 29 is orally bioavailable and has significant brain exposure.
Table 4. Brain and Plasma Concentration of Inhibitor 29 in Micea.
| compd | plasma concentration (2 h) | brain concentration (2 h) |
|---|---|---|
| 29 (po, 30 mg/kg) | 5.9 μM | 3.3 μM |
Data were generated from four determinations, and samples were collected 2 h after dosing. Formulation: 1.75% DMSO, 2% Tween80, 18% of the lipid mixes in PBS (pH 6.0). The lipid mixture: 3:1 mixture of CapMul MCM:Captex 355 was mixed with PEG35 at 15:85. Brain/plasma ratio: 56%.
In summary, we have identified, through a ring-closure structure manipulation from our previously discovered pyrazole-urea scaffold, a new class of indazole- and/or aza-indazole-based scaffold as JNK3-selective inhibitors. In addition, by SAR studies, we have developed several highly selective (including isoform selectivity against JNK1) and potent JNK3 inhibitors from this chemotype. Compared to the pyrazole-urea-based reference Inhibitor-B (Table 3 and Figure 1), the new lead JNK3 inhibitors developed from the indazole/aza-indazole chemotypes had similar or better potency and in vitro DMPK properties. More importantly, lead inhibitors from the new chemotypes had good brain-penetration capability as demonstrated by compound 29.
Inhibitor 29 had strong inhibition (>80% inhibition at 1 μM) for only JNK3 and JNK2 in a panel of 374 wild-type kinases and had a general kinase hit ratio of only 1.6%. Inhibitors 14 and 29 had potent activities in inhibiting the phosphorylation of APPT668 of primary cortical and hippocampal neurons. In addition, the newly optimized JNK3 inhibitors (such as 29) had good in vivo DMPK properties, good oral bioavailability, and high brain penetration, which are all indicative of good utility for these JNK3 inhibitors in CNS applications. Several lead JNK3 inhibitors from this scaffold are currently under investigations in animal models against neurodegenerative diseases and will be reported in due course.
Acknowledgments
This work was supported by NIH/NIA grant RO1AG055059 (S.O.Y. and Y.F.) and ADDF (S.O.Y.). We thank Ms. Jiaming Hu of the University of Miami Miller School of Medicine for the preparation of graphs.
Glossary
Abbreviations
- JNK
c-jun N-terminal kinases
- ATP
adenosine triphosphate
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- SAR
structure–activity relationship
- DMPK
drug metabolism and pharmacokinetics
Supporting Information Available
This material is available free of charge via the Internet at http://pubs.acs.org/. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00334.
Details for the synthesis of 1–29; H NMR data; LC-MS data; HRMS data, procedures for bioassays, and DMPK studies; kinase panel profiling data for 29; cocrystal structure of 29 (2.1 Å) in human JNK3; coordinates of the JNK3:29 complex PDB ID 7S1N (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Gupta S.; Barrett T.; Whitmarsh A. J.; Cavanagh J.; Sluss H. K.; Derijard B.; Davis R. J. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996, 15, 2760–2770. 10.1002/j.1460-2075.1996.tb00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derijard B.; Hibi M.; Wu I. H.; Barrett T.; Su B.; Deng T. L.; Karin M.; Davis R. J. Jnk1 - a Protein-Kinase Stimulated by Uv-Light and Ha-Ras That Binds and Phosphorylates the C-Jun Activation Domain. Cell 1994, 76, 1025–1037. 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Shi X. Q.; Zhang Q. J.; Hampong M.; Paddon H.; Wahyuningsih D.; Pelech S. Nocodazole-induced p53-dependent c-Jun N-terminal kinase activation reduces apoptosis in human colon carcinoma HCT116 cells. J. Biol. Chem. 2002, 277, 43648–43658. 10.1074/jbc.M203214200. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Zheng X.; Wang Y.; Song J. Effect of PI3K/Akt/mTOR signaling pathway on JNK3 in Parkinsonian rats. Exp. Ther. Med. 2018, 17, 1771–1775. 10.3892/etm.2018.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J.; Li H.; Zhang B.; Xiong R.; Zhang Y.; Kang W. Y.; Chen W.; Zhao Z. B.; Chen S. D. Small peptide inhibitor of JNK3 protects dopaminergic neurons from MPTP induced injury via inhibiting the ASK1-JNK3 signaling pathway. PLoS One 2015, 10, e0119204. 10.1371/journal.pone.0119204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi D. S.; Tao J. H.; Zhang L. Q.; Li M.; Wang M.; Qu R.; Zhang S. C.; Liu P.; Liu F.; Miu J. C.; Ma J. Y.; Mei X. Y.; Zhang F. Neuroprotection of Cilostazol against ischemia/reperfusion-induced cognitive deficits through inhibiting JNK3/caspase-3 by enhancing Akt1. Brain Res. 2016, 1653, 67–74. 10.1016/j.brainres.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Ries V.; Silva R. M.; Oo T. F.; Cheng H. C.; Rzhetskaya M.; Kholodilov N.; Flavell R. A.; Kuan C. Y.; Rakic P.; Burke R. E. JNK2 and JNK3 combined are essential for apoptosis in dopamine neurons of the substantia nigra, but are not required for axon degeneration. J. Neurochem. 2008, 107, 1578–88. 10.1111/j.1471-4159.2008.05713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. W.; Pachori A.; Howard S.; Ganno M.; Hansen D. Jr; Kamenecka T.; Song X.; Duckett D.; Chen W.; Ling Y. Y.; Cherry L.; Cameron M. D.; Lin L.; Ruiz C. H.; Lograsso P. Small Molecule c-jun-N-terminal Kinase (JNK) Inhibitors Protect Dopaminergic Neurons in a Model of Parkinson’s Disease. ACS Chem. Neurosci. 2011, 2, 198–206. 10.1021/cn100109k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genabai N. K.; Ahmad S.; Zhang Z.; Jiang X.; Gabaldon C. A.; Gangwani L. Genetic inhibition of JNK3 ameliorates spinal muscular atrophy. Hum. Mol. Genet. 2015, 24, 6986–7004. 10.1093/hmg/ddv401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S. O.; Park D. J.; Ryu J. C.; Ozer H. G.; Tep C.; Shin Y. J.; Lim T. H.; Pastorino L.; Kunwar A. J.; Walton J. C.; Nagahara A. H.; Lu K. P.; Nelson R. J.; Tuszynski M. H.; Huang K. JNK3 perpetuates metabolic stress induced by Abeta peptides. Neuron 2012, 75, 824–37. 10.1016/j.neuron.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y.; Chen J.; Jin Y.; Wang C.; Zheng M.; He L. GW9508 ameliorates cognitive impairment via the cAMP-CREB and JNK pathways in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neuropharmacology 2020, 164, 107899. 10.1016/j.neuropharm.2019.107899. [DOI] [PubMed] [Google Scholar]
- He B.; Chen W.; Zeng J.; Tong W.; Zheng P. MicroRNA-326 decreases tau phosphorylation and neuron apoptosis through inhibition of the JNK signaling pathway by targeting VAV1 in Alzheimer’s disease. J. Cell. Physiol. 2020, 235, 480–493. 10.1002/jcp.28988. [DOI] [PubMed] [Google Scholar]
- Kwon O. Y.; Lee S. H. Ameliorating Activity of Ishige okamurae on the Amyloid Beta-Induced Cognitive Deficits and Neurotoxicity through Regulating ERK, p38 MAPK, and JNK Signaling in Alzheimer’s Disease-Like Mice Model. Mol. Nutr. Food Res. 2020, 64, e1901220. 10.1002/mnfr.201901220. [DOI] [PubMed] [Google Scholar]
- Liu J.; Li H.; Gong T.; Chen W.; Mao S.; Kong Y.; Yu J.; Sun J. Anti-neuroinflammatory Effect of Short-Chain Fatty Acid Acetate against Alzheimer’s Disease via Upregulating GPR41 and Inhibiting ERK/JNK/NF-kappaB. J. Agric. Food Chem. 2020, 68, 7152–7161. 10.1021/acs.jafc.0c02807. [DOI] [PubMed] [Google Scholar]
- Saha P.; Guha S.; Biswas S. C. P38K and JNK pathways are induced by amyloid-beta in astrocyte: Implication of MAPK pathways in astrogliosis in Alzheimer’s disease. Mol. Cell. Neurosci. 2020, 108, 103551. 10.1016/j.mcn.2020.103551. [DOI] [PubMed] [Google Scholar]
- Gourmaud S.; Paquet C.; Dumurgier J.; Pace C.; Bouras C.; Gray F.; Laplanche J. L.; Meurs E. F.; Mouton-Liger F.; Hugon J. Increased levels of cerebrospinal fluid JNK3 associated with amyloid pathology: links to cognitive decline. J. Psychiatry Neurosci 2015, 40, 151–61. 10.1503/jpn.140062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos L.; Junyent F.; Verdaguer E.; Folch J.; Romero R.; Pallas M.; Ferrer I.; Auladell C.; Camins A. Differences in activation of ERK1/2 and p38 kinase in Jnk3 null mice following KA treatment. J. Neurochem. 2010, 114, 1315–1322. 10.1111/j.1471-4159.2010.06853.x. [DOI] [PubMed] [Google Scholar]
- Feng Y.; Park H.; Bauer L.; Ryu J. C.; Yoon S. O. Thiophene-Pyrazolourea Derivatives as Potent, Orally Bioavailable, and Isoform-Selective JNK3 Inhibitors. ACS Med. Chem. Lett. 2021, 12, 24–29. 10.1021/acsmedchemlett.0c00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk P. P.; Khan A.; Bhatia G. S.; Palmer V.; Medland D.; Numata H.; Oinuma H.; Catchick J.; Dunne A.; Ellis M.; Smales C.; Whitfield J.; Neame S. J.; Shah B.; Wilton D.; Morgan L.; Patel T.; Chung R.; Desmond H.; Staddon J. M.; Sato N.; Inoue A. The neuroprotective action of JNK3 inhibitors based on the 6,7-dihydro-5H-pyrrolo[1,2-a]imidazole scaffold. Bioorg. Med. Chem. Lett. 2005, 15, 4666–70. 10.1016/j.bmcl.2005.07.076. [DOI] [PubMed] [Google Scholar]
- Jung H.; Aman W.; Hah J. M. Novel scaffold evolution through combinatorial 3D-QSAR model studies of two types of JNK3 inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2139–2143. 10.1016/j.bmcl.2017.03.063. [DOI] [PubMed] [Google Scholar]
- Tuffaha G. O.; Hatmal M. M.; Taha M. O. Discovery of new JNK3 inhibitory chemotypes via QSAR-Guided selection of docking-based pharmacophores and comparison with other structure-based pharmacophore modeling methods. J. Mol. Graphics Modell. 2019, 91, 30–51. 10.1016/j.jmgm.2019.05.015. [DOI] [PubMed] [Google Scholar]
- Zheng K.; Iqbal S.; Hernandez P.; Park H.; LoGrasso P. V.; Feng Y. Design and synthesis of highly potent and isoform selective JNK3 inhibitors: SAR studies on aminopyrazole derivatives. J. Med. Chem. 2014, 57, 10013–30. 10.1021/jm501256y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K.; Iqbal S.; Hernandez P.; Park H.; LoGrasso P. V.; Feng Y. Correction to Design and Synthesis of Highly Potent and Isoform Selective JNK3 Inhibitors: SAR Studies on Aminopyrazole Derivatives. J. Med. Chem. 2016, 59, 9276. 10.1021/acs.jmedchem.6b01369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T.; Inesta-Vaquera F.; Niepel M.; Zhang J.; Ficarro S. B.; Machleidt T.; Xie T.; Marto J. A.; Kim N.; Sim T.; Laughlin J. D.; Park H.; LoGrasso P. V.; Patricelli M.; Nomanbhoy T. K.; Sorger P. K.; Alessi D. R.; Gray N. S. Discovery of potent and selective covalent inhibitors of JNK. Chem. Biol. 2012, 19, 140–154. 10.1016/j.chembiol.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou X.; Huang H.; Jiang L.; Zhu G.; Jin H.; Jiao N.; Zhang L.; Liu Z.; Zhang L. Rational modification, synthesis and biological evaluation of 3,4-dihydroquinoxalin-2(1H)-one derivatives as potent and selective c-Jun N-terminal kinase 3 (JNK3) inhibitors. Eur. J. Med. Chem. 2020, 201, 112445. 10.1016/j.ejmech.2020.112445. [DOI] [PubMed] [Google Scholar]
- Dou X.; Huang H.; Li Y.; Jiang L.; Wang Y.; Jin H.; Jiao N.; Zhang L.; Zhang L.; Liu Z. Multistage Screening Reveals 3-Substituted Indolin-2-one Derivatives as Novel and Isoform-Selective c-Jun N-terminal Kinase 3 (JNK3) Inhibitors: Implications to Drug Discovery for Potential Treatment of Neurodegenerative Diseases. J. Med. Chem. 2019, 62, 6645–6664. 10.1021/acs.jmedchem.9b00537. [DOI] [PubMed] [Google Scholar]
- Oh Y.; Jang M.; Cho H.; Yang S.; Im D.; Moon H.; Hah J. M. Discovery of 3-alkyl-5-aryl-1-pyrimidyl-1H-pyrazole derivatives as a novel selective inhibitor scaffold of JNK3. J. Enzyme Inhib. Med. Chem. 2020, 35, 372–376. 10.1080/14756366.2019.1705294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantevin Krenitsky V.; Nadolny L.; Delgado M.; Ayala L.; Clareen S. S.; Hilgraf R.; Albers R.; Hegde S.; D’Sidocky N.; Sapienza J.; Wright J.; McCarrick M.; Bahmanyar S.; Chamberlain P.; Delker S. L.; Muir J.; Giegel D.; Xu L.; Celeridad M.; Lachowitzer J.; Bennett B.; Moghaddam M.; Khatsenko O.; Katz J.; Fan R.; Bai A.; Tang Y.; Shirley M. A.; Benish B.; Bodine T.; Blease K.; Raymon H.; Cathers B. E.; Satoh Y. Discovery of CC-930, an orally active anti-fibrotic JNK inhibitor. Bioorg. Med. Chem. Lett. 2012, 22, 1433–8. 10.1016/j.bmcl.2011.12.027. [DOI] [PubMed] [Google Scholar]
- He Y.; Duckett D.; Chen W.; Ling Y. Y.; Cameron M. D.; Lin L.; Ruiz C. H.; Lograsso P. V.; Kamenecka T. M.; Koenig M. Synthesis and SAR of novel isoxazoles as potent c-jun N-terminal kinase (JNK) inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 161–4. 10.1016/j.bmcl.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. J.; Kamenecka T. M.; Shin Y. S.; Song X. Y.; Jiang R.; Noel R.; Duckett D.; Chen W. M.; Ling Y. Y.; Cameron M. D.; Lin L.; Khan S.; Koenig M.; LoGrasso P. V. Synthesis and SAR of novel quinazolines as potent and brain-penetrant c-jun N-terminal kinase (JNK) Inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 1719–1723. 10.1016/j.bmcl.2011.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenecka T.; Habel J.; Duckett D.; Chen W. M.; Ling Y. Y.; Frackowiak B.; Jiang R.; Shin Y. S.; Song X. Y.; LoGrasso P. Structure-Activity Relationships and X-ray Structures Describing the Selectivity of Aminopyrazole Inhibitors for c-Jun N-terminal Kinase 3 (JNK3) over p38. J. Biol. Chem. 2009, 284, 12853–12861. 10.1074/jbc.M809430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. S.; Chen W. M.; Habel J.; Duckett D.; Ling Y. Y.; Koenig M.; He Y. J.; Vojkovsky T.; LoGrasso P.; Kamenecka T. M. Synthesis and SAR of piperazine amides as novel c-jun N-terminal kinase (JNK) inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 3344–3347. 10.1016/j.bmcl.2009.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y.; Chambers J. W.; Iqbal S.; Koenig M.; Park H.; Cherry L.; Hernandez P.; Figuera-Losada M.; LoGrasso P. V. A small molecule bidentate-binding dual inhibitor probe of the LRRK2 and JNK kinases. ACS Chem. Biol. 2013, 8, 1747–1754. 10.1021/cb3006165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynders M.; Chaikuad A.; Berger B. T.; Bauer K.; Koch P.; Laufer S.; Knapp S.; Trauner D. Controlling the Covalent Reactivity of a Kinase Inhibitor with Light. Angew. Chem. 2021, 133, 20340. 10.1002/ange.202103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K.; Park C. M.; Iqbal S.; Hernandez P.; Park H.; LoGrasso P. V.; Feng Y. Pyridopyrimidinone Derivatives as Potent and Selective c-Jun N-Terminal Kinase (JNK) Inhibitors. ACS Med. Chem. Lett. 2015, 6, 413–8. 10.1021/ml500474d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.; Iqbal S.; Hernandez P.; Mora R.; Zheng K.; Feng Y.; LoGrasso P. Structural Basis for JNK2/3 Isoform Selective Aminopyrazoles. Sci. Rep. 2015, 5, 8047. 10.1038/srep08047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.