Calcific aortic valve disease (CAVD) represents an enormous public health problem in terms of adverse health consequences and economic burden. Approximately 5-10% of older adults have aortic stenosis (AS) and over 25% have aortic sclerosis, the precursor to AS; clinically significant valve disease is anticipated to double by 2050.1, 2 To date, there are no drugs approved to slow progression of CAVD. Anticipation regarding statins turned into disappointment after several trials showed no benefit, which may have dampened enthusiasm for CAVD therapy. Although it attracts comparatively less attention and is less mature than mechanistic research in atherosclerosis, numerous studies have elucidated the molecular biology underlying CAVD and point to several potentially promising drug targets (reviewed previously3, 4). For example, a causal role for Lp(a) has been demonstrated, which along with oxidized phospholipids provides a potential link between atherosclerotic disease and CAVD and offers rationale for testing drugs that lower Lp(a) (e.g. anti-sense oligonucleotides, PCSK9 inhibitors).5 However, despite numerous promising targets and drugs available directed at those targets, as of December 2, 2020 on clinicaltrials.gov, there are currently no actively enrolling clinical trials testing medical therapy to slow progression of CAVD; one trial testing both denosumab and bisphosphonates is completed and undergoing analysis. This dearth of active trials is sobering. We offer several potential reasons for this along with suggestions for addressing barriers (Figure).
Figure.
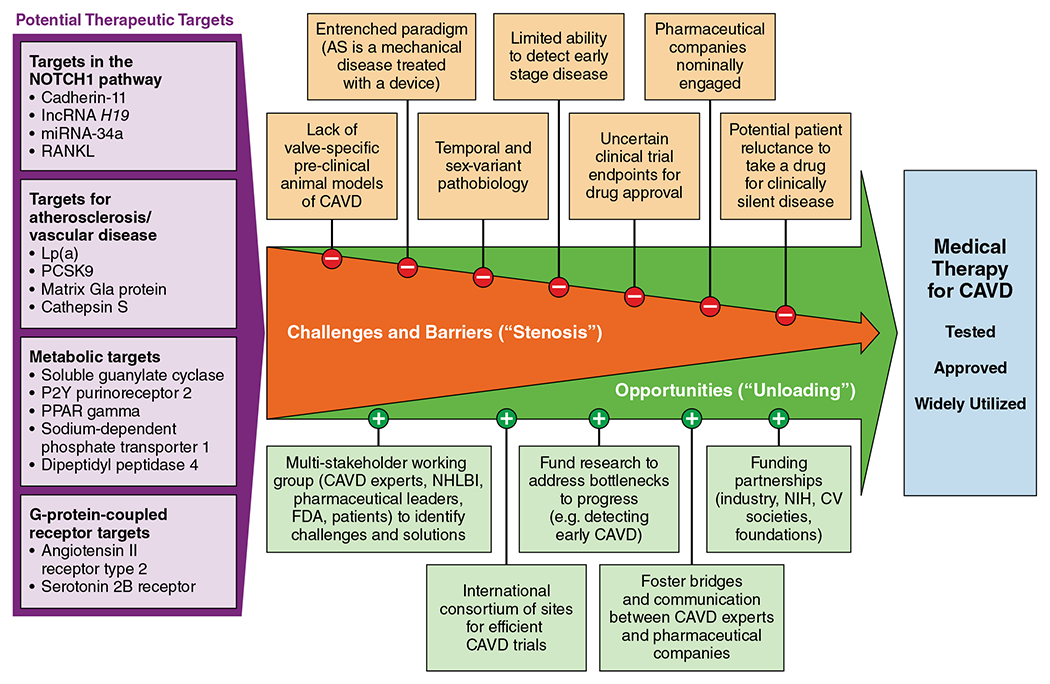
Unloading the Stenotic Path Toward Medical Therapy for Calcific Aortic Valve Disease (CAVD).
Paradigm shift needed.
AS is conceptualized as a mechanical problem to be treated with a mechanical solution and, historically, has been viewed as a degenerative disease suggestive of passive “wear and tear” rather than the product of active biological processes. For over 50 years, aortic valve replacement (AVR) has been the treatment for AS. The transformative innovation of transcatheter AVR (TAVR) has garnered significant attention as a less invasive approach. Its low morbidity has hastened studies testing whether AVR earlier in the disease course may be beneficial. Accordingly, TAVR has engendered optimism regarding therapy for AS and reinforced the notion that AS is a mechanical disease treated with a mechanical solution not medical therapy.
Pathobiology of CAVD evolves as the disease progresses.
Because the molecular biology underlying CAVD evolves from early (inflammation/fibrosis) to later (osteogenesis) stage disease, the most promising targets for medical therapy to slow or reverse CAVD may likely differ depending on disease stage. Additionally, females exhibit a more fibrotic phenotype of AS whereas calcification is greater in males. This temporal and sex-variant pathobiology yields complexity when considering clinical trials testing potential therapies.
Lack of effective tools to identify patients with early stage CAVD.
Patients with earlier stage CAVD are the ones likely to benefit most from medical therapy targeting the valve pathology. Currently, detection of CAVD relies on auscultation, symptom onset, or an echocardiogram done for other reasons fortuitously revealing CAVD. Given the poor sensitivity and specificity of auscultation for detecting AS and the absence of AS-related symptoms until obstruction is severe, there is a pressing need for innovative ways to screen for and detect early stage CAVD if trials of medical therapy are to be efficiently enrolled and therapies shown to be effective broadly applied.
Drug delivery, adherence, and clinical trial endpoints.
Given that CAVD is generally asymptomatic until it is severe and that disease progression takes years, will individuals adhere to a medication potentially for many years for a condition that is clinically silent until it is end-stage? Because of the characteristics of our healthcare system and population, will individuals prefer a bail-out strategy (AVR) more than prevention (medication)? Will adherence be substantially influenced by the delivery of the drug (i.e. pill versus infusion versus injection), which has implications for therapeutic development and real-world implementation. Additionally, while drug approval is typically based on trials with hard endpoints (e.g. death, hospitalization), imaging-based endpoints that characterize disease progression seem more fitting when evaluating drugs to slow CAVD. While AS hemodynamic indices on echocardiography have been used in statin trials, gated CT scans to measure valve calcification may provide more precise and accurate measurements of disease progression allowing for a smaller trial sample size. Engagement with FDA to identify appropriate endpoints for drug labeling will be critical.
Engagement and commitment from drug companies.
Given the absence of any medical therapy for CAVD—thus no need to improve upon existing medical therapy (as would occur for drugs targeting heart failure or coronary disease)—and the large number of individuals with CAVD, the apparent lack of an aggressive pursuit of drug development for CAVD is puzzling. Opportunities for experts in CAVD and pharmaceutical company leaders to dialogue, collaborate, and mutually educate are needed to ensure the magnitude of the unmet clinical need is recognized, the challenges of the drug discovery and approval pipeline understood, and a productive path forward charted.
Despite numerous barriers, we remain hopeful that progress can be made in the quest to identify, approve, and disseminate effective medical therapy for CAVD and offer these initial suggestions:
Convene a multi-stakeholder working group including: basic, translational, and clinical investigators with expertise in CAVD, cardiac imaging, biomarkers, clinical trials, and drug development and delivery; pharmaceutical leaders; NHLBI; FDA; and patient advocacy organizations to identify key challenges and potential solutions. The recently launched Heart Valve Collaboratory (already chartered, developed, and FDA-registered) could be an ideal forum for this initiative.
Develop an international consortium of sites with clinical and research expertise in CAVD who can establish clinical trial pathways, screening and enrollment processes, and refine imaging protocols needed to efficiently and reliably test several candidate drugs.
Direct attention and funding to key bottlenecks to progress (e.g. innovative ways to reliably detect CAVD at earlier stages of disease).
Funding partnerships – given mutual interests, funding for work on medical therapy for CAVD could be combined from multiple stakeholders, including NIH/government, foundations, cardiovascular societies, and industry.
While our options for replacing the aortic valve have improved and expanded over the years, medical therapy to prevent or delay such a need would introduce a seismic shift in the management of individuals with CAVD with important public health implications.
Funding:
Dr. Merryman is supported by the National Institutes of Health (R35-HL135790) and Fondation Leducq.
Conflicts of interest:
Dr. Lindman has served on the scientific advisory board for Roche Diagnostics, has received research grants from Edwards Lifesciences and Roche Diagnostics, and has consulted for Medtronic.
References
- 1.Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, Bogers AJ, Piazza N and Kappetein AP. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–12. [DOI] [PubMed] [Google Scholar]
- 2.Coffey S, Cox B and Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. J Am Coll Cardiol. 2014;63:2852–61. [DOI] [PubMed] [Google Scholar]
- 3.Hutcheson JD, Aikawa E and Merryman WD. Potential drug targets for calcific aortic valve disease. Nat Rev Cardiol. 2014;11:218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquis-Gravel G, Redfors B, Leon MB and Genereux P. Medical Treatment of Aortic Stenosis. Circulation. 2016;134:1766–1784. [DOI] [PubMed] [Google Scholar]
- 5.Boffa MB and Koschinsky ML. Oxidized phospholipids as a unifying theory for lipoprotein(a) and cardiovascular disease. Nat Rev Cardiol. 2019;16:305–318. [DOI] [PubMed] [Google Scholar]


