Abstract
Growth, division, and development of healthy cells relies on efficient response to environmental survival cues. The conserved mitogen-activated protein kinase (MAPK) family of pathways interface extracellular stimuli to intracellular processes for this purpose. Within these pathways, the MEK family has been identified as a target of interest due to its clinical relevance. Particularly, MEK4 has drawn recent attention for its indications in pancreatic and prostate cancers. Here, we report two potent MEK4 inhibitors demonstrating significant reduction of phospho-JNK and antiproliferative properties against pancreatic cancer cell lines. Furthermore, molecular inhibition of MEK4 pathway activates the MEK1/2 pathway, with the combination of MEK1/2 and MEK4 inhibitors demonstrating synergistic effects against pancreatic cancer cells. Our inhibitors provided insight into the crosstalk between MAPK pathways and new tools for elucidating the roles of MEK4 in disease states, findings which will pave the way for better understanding of the MAPK pathways and development of additional probes.
Keywords: mitogen-activated protein kinases, drug discovery, small molecule inhibitors, lead optimization, pancreatic cancer
The mitogen-activated protein kinase (MAPK) signal cascades are ubiquitous across most organisms and responsible for essential cellular processes such as cell growth, division, and death.1−3 Within the MAPK signaling pathways, the MAPK/ERK (MEK or MAP2K) family of kinases are intermediary proteins that directly activate the MAP kinases responsible for cellular response (Figure 1A).4 Due to the far-reaching biological effects of the MEK family, these kinases have become an enduring area of study. Of the seven kinases in this family, MEK1 and MEK2 were the first to be discovered and thoroughly studied, ultimately giving rise to four FDA-approved MEK1/2 inhibitors for single-agent or combination therapies for various melanomas and neurofibromatosis type 1.5−7 However, there has been little investigation into the rest of the MEK family to date, despite the high clinical relevance.8 In particular, MEK4 plays a role in both essential and detrimental capacities. There is evidence that in early stages of development, MEK4 is vital for proper maturation of the liver and the immune system.9 However, overexpression of MEK4 has been observed in cancers of the pancreas and prostate, which have some of the lowest survival rates among the various cancers.10 Additionally, MEK4 has been implicated in neurological conditions including stroke, Parkinson’s, Huntington’s, and Alzheimer’s disease as well as cardiac hypertrophy.11
Figure 1.

(A) Simplified MAPK signaling cascade. (B) Structures of MEK4 probes to date, distinguishing targeted MEK probe development campaigns. (C) The evolution of MEK4 inhibitors from previous lead compound 7 discussed in this work.
While the roles of MEK4 in the progression of these diseases are now being more heavily studied, many of the molecular mechanisms remain unknown. To further understand the role of MEK4 in disease states, new selective and potent molecular probes are needed. Currently, there are seven reported unique MEK4 inhibitors (Figure 1B). Three inhibitors (1–3) were discovered through a top-down approach, in which they were found to positively affect disease models, and their mechanism of action was later determined to be MEK4 inhibition.12−14 As these compounds are mostly derived from natural products, the use and further development of these compounds can be challenging. Additionally, the potency and selectivity profile of these compounds are usually not optimal, and many compounds are either not tested for selectivity or found to have significant off-target effects. “Targeted” campaigns are comparatively more effective in developing tool compounds for studying specific enzymes, as this tactic allows for precise molecular tuning of the scaffold. Most of these tool compounds are highly modular, derived from commercially available building blocks, and synthetically tractable in a few chemical transformations. HWY336 (4), developed by Song and coworkers in 2014, was the first report of a “targeted” MEK4 inhibitor campaign.15 However, HWY336 displayed only modest potency and poor selectivity. Since then, only three additional reports have been published on new MEK4 inhibitors (compounds 5–7).16−18 Each probe compound faced different challenges: 5 did not have great selectivity for MEK4 over MEK7, 6 was not evaluated for activity or in cellular models, and 7 showed poor cellular potency. Given the gap in knowledge regarding the molecular mechanisms surrounding MEK4 and the small number of MEK4 probes available, there is a clear need for continued development of potent and selective MEK4 inhibitors.
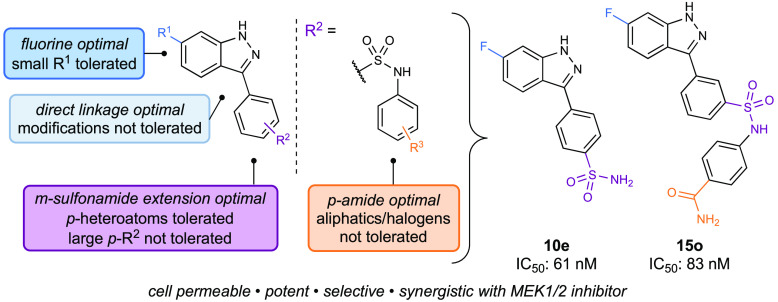
This report describes the development of novel indazole-derived inhibitors as an addition to the existing tools available for studying MEK4. Leveraging our existing MEK profiling platform,19 we performed extensive SAR studies around initial lead compound 7(16) in an effort to improve cellular permeability while maintaining potency. Through these studies and bioisosteric replacement of the carboxylic acid on our previous lead, we discovered para-substituted sulfonamide 10e as a new lead. Based on this lead, we developed a viable new class of potent MEK4 inhibitors (Figure 1C, 13–15). Further optimization yielded our second lead compound 15o that was capable of clearing the cell membrane in cellular studies. Additional studies provided preliminary evidence of the synergistic effects of codosing with MEK1/2 inhibitors in addition to revealing crosstalk relationships across the two MAPK pathways.
For our first designs, we turned to our previously reported refined MEK4 model to approximate the active site.20 We also used our model to rationalize experimentally observed trends in potency throughout our studies. While several crystal structures have been reported for MEK4 with resolutions up to 2.3 Å,21,22 the active sites in these crystal structures are disordered, and thus offer limited assistance in the development of new structure-based inhibitors. This model also informed us of how the compounds could be positioned in the binding pocket. In lieu of a crystal structure, we continued to use this model to rationally design our newest series of MEK4 inhibitors.
To improve the cellular potency of 7, we proposed the synthesis of covalent inhibitors, heavily guided by computational modeling. Covalent inhibitors inherently have an advantage over reversible inhibitors as covalent binding can prevent dissociation from the protein and maintain potency even if the binding site is more shallow.23 Additionally, by targeting cysteines that are unique to the protein, selectivity can also be improved. Our proposed covalent inhibitor (Figure 2A, inset) added an electrophilic warhead onto the ortho position of the aryl ring of a compound we previously published (Figure S-1, compound S1).16 The generated computational pose suggested that the analogue would be able to react covalently with Cys246 (Figure 2A).
Figure 2.
Initial steps to improve compound 7 led to the development of the covalent and extended linkage series, which did not yield substantial improvements. (A) Proposed covalent inhibitor docked pose with Cys246 (inset: structure). (B) Docked pose of 7 (gray) overlaid with 9c (magenta). (C) SAR studies of covalent inhibitors and an extended linker between the indazole and aryl ring.
To evaluate whether covalent inhibitors would be more potent, we synthesized model compound 8a for comparison with our covalent inhibitors. All compounds were evaluated using the ADP-Glo assay, an assay with readouts that utilizes ATP to measure the functional inhibition of activity, following protocols developed in our MEK family survey report (See Supporting Information for details).19,24,258a had an IC50 value of 0.31 μM, notably about 10-fold less potent than the original compound. Through an amine in the ortho position on the phenyl ring, we installed three variants of conjugate acceptors with varying reactivity, as well as a noncovalent control. Unfortunately, compounds 8c–8f, in addition to the unsubstituted amine precursor 8b, were inactive (Figure 2C), indicating that nucleophilic addition was not occurring. We hypothesize that the hydrophilic substituents at the ortho position experience repulsion from hydrophobic residues in that area of the pocket, resulting in a loss of potency. Therefore, we avoided substitution at that position moving forward. However, future covalent inhibitors may be able to leverage this cysteine residue once a more refined picture of this binding site emerges, either through new crystallography activities or more sophisticated computational models.
We next turned our attention to investigating noncovalent inhibitors and proposed the synthesis of compounds with an elongated linkage between the key indazole and the aryl substituent. We predicted that this type of compound would be able to reach more residues or strengthen existing interactions with residue contacts that we discovered with 7 (gray compound, Figure 2B). Following this strategy, we installed various benzylamine derivatives on the indazole (compounds 9a–9d). However, this modification resulted in compounds with micromolar potency, a large decrease in potency compared to 7 (IC50 = 0.041 μM).16 Based on the computationally docked pose of 8d (magenta compound, Figure 2B), the phenyl rings of these new compounds were interacting negatively with Lys187, which was previously interacting productively with the carboxylic acid of 7. Observations of the model indicated to us that elongation of the linkage between the indazole and the aryl ring created compounds that potentially did not fit in the binding site, and further designs would not improve potency. Additional substitutions at the indazole nitrogen (Figure S-1, compounds S2–3) led to inactive compounds. Our model indicated that these substitutions could prevent the indazole nitrogen from interacting with key residue Glu179 at the back of the binding pocket. This interaction is present in the docked poses of all of our most potent compounds.
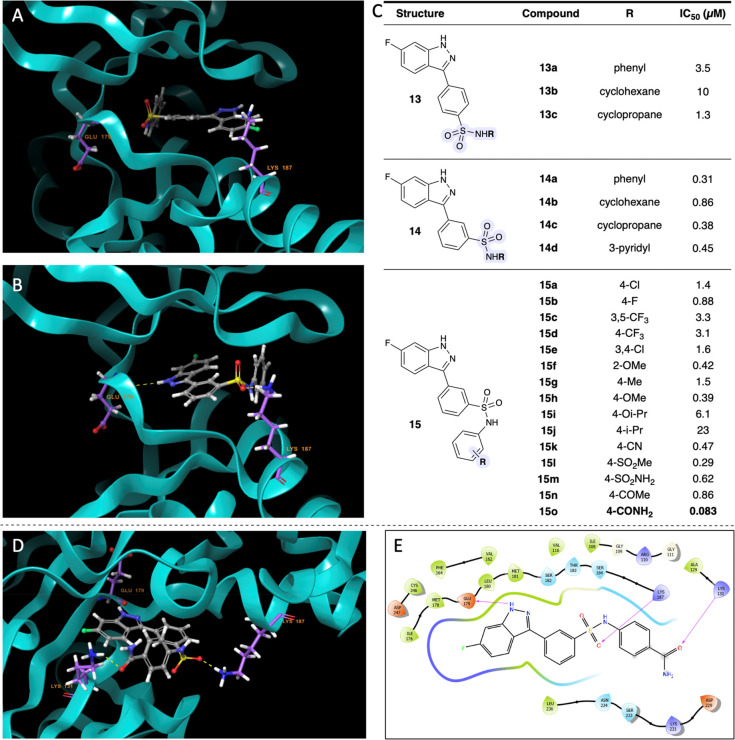
Restoring the original indazole-aryl linkage, we looked for other alterations that we could make. We chose to focus on the carboxylic acid on 7, not only to further explore the effects of different functional groups, but to potentially improve membrane permeability.26 Therefore, we reasoned that the next step was to synthesize bioisosteres of 7 (10, Figure 3B). We focused on more lipophilic bioisostere designs with similar ratios of hydrogen bond donors and acceptors in efforts to retain contact with Lys187 (compounds 10a–e, Figure 3B). Most analogues of 10 had submicromolar IC50 values, but the primary sulfonamide 10e stood out with an IC50 value of 0.061 μM. The modeled pose of 10e showed the indazole still interacting with the back of the binding pocket, and the terminal primary sulfonamide interacting with a polar residue. However, instead of interacting with residues Glu179 and Lys187 like 7 does, 10e appears to interacts with the backbone of Met181 and residue Ser233 in our model, creating a pose that sits shallower in the pocket (Figure 3A). However, the binding mode is similar to 7, and we attribute the excellent potency of 10e to the similarity in the computationally observed binding pose. Given that the carboxylic acid was modified to the sulfonamide, we revisited substitution at the 6-position on the indazole. Modification of the fluoro group on the indazole of 10e did not yield improved compounds (Figure S-1, compounds S4–5), so we started to introduce further modifications by adding substituents to the sulfonamide of 10e. Analogues 11–12 were the first compounds synthesized with the sulfonamide handle. Notably, these compounds were first made with the nitrogen of the sulfonamide closer to the aryl ring, followed by the sulfonyl group. This synthetic route was designed based on the commercially available borylated anilines and wide variety of sulfonyl chlorides that would allow facile access to a diverse library of compounds. Unfortunately, none of the analogues performed better than 10e (Figure 3B). Therefore, our next alteration installed the sulfonyl closer to the aryl ring, mimicking the regiochemistry of the primary sulfonamide (compounds 13–14, Figure 4C).
Figure 3.

Bioisostere and sulfonamide series compounds captured similar residues to 7, leading to increased potency and the identification of 10e. (A) Docked pose of 10e, showing interactions with Met 181 and Ser 233, compared to the Glu 179 and Lys 187 with which 7 interacts. (B) SAR studies of bioisosteres and sulfonamides.
Figure 4.
Expansion of the sulfonamide series to the “reverse” sulfonamide series led to identification of 15o. (A) Docked pose of 13a. (B) Docked pose of 14a. (C) SAR studies of “reverse” sulfonamides. (D) Docked pose of 15o. (E) 2D view of 15o.
Since we based these compounds on the structure of 10e, we expected the para-substituted analogues would exhibit higher potencies than their meta-substituted counterparts. To our surprise, the para-substituted analogues (13) were outperformed by the meta-substituted analogues (14). When representative analogues 13a and 14a were docked into our model, we found that the para-substituted compound poses did not include the key indazole ring interaction with the back of the binding pocket or Glu179 (Figure 4A). Instead, the computationally generated pose featured the aryl ring toward the back of the binding pocket. Additionally, the pose of 13a did not appear to have any other residue interactions with the binding pocket. In comparison, the binding pose of 14a showed the sulfonyl group interacting with Lys187, which we attribute to the higher potency (Figure 4B). We inferred that the major differences in interactions with the binding pocket were the source of the ∼10-fold difference in potency. Encouraged by the binding pose of 14a, we decided to expand this scaffold into a series of new analogues. Among the early compounds of this series (Figure 4C, 14a–d,), the phenyl derivative performed the best and was the most synthetically tractable derivative to expand from. With the phenyl ring as the scaffold, we diversified our compound library to include functional groups such as halogens, alkyl groups, and heteroatom-containing functional groups at various positions on the ring (compounds 15a–o,Figure 4C). The heteroatom-containing functional groups (compounds 15f, h, i, k–o) performed the best, and the para-primary amide, 15o was the most potent of the series with an IC50 value of 0.083 μM. We attribute this to the new interaction between the primary amide of 15o and Lys131, in addition to the previously identified interactions in our MEK4 model between Glu179 and the indazole and between Lys187 and the meta-sulfonamide (Figure 4D and E).
With 10e and 15o in hand, we focused on evaluating our best compounds both in vitro and in a cellular system. When studying kinase inhibitors, there are a multitude of ways to evaluate selectivity across kinases. As a first point of evaluation, we tested a focused library of 10 compounds, with analogues from each series of compounds, against MEK7 in the ADP-Glo functional assay (Figure 5A).19,24,25 Ratios ranged from slim margins to almost 10-fold selectivity for MEK4. In general, the selectivity decreased as larger substituents were added to the aryl ring.
Figure 5.
Selectivity studies showed good to modest selectivity across the MEK family in addition to modest selectivity across the kinome. (A) Selectivity against MEK7 for selected compounds. (B) 10e and 15o selectivity against the MEK family at 1 and 10 μM. (C) DiscoverX TREEspot kinome-wide selectivity analysis of 15o with a cutoff of over 80% inhibition (see Supporting Information for specifics).
To compare our compounds to the rest of the MEK inhibitors discovered thus far, we turned to the DiscoverX platform (KINOMEscan, Eurofins DiscoverX), a widely used assay for comparing selectivity across the kinome. The DiscoverX platform reports the thermodynamic Kd to facilitate direct comparison of inhibitor affinity across the kinome, independent of ATP concentration. To test for selectivity across the MEK family, we submitted 10e and 15o for testing using the scanELECT DiscoverX platform. We found that with the addition of the extra phenyl ring in 15o compared to 10e, the overall selectivity against the MEK family decreased. Nevertheless, 15o maintained a slim margin of selectivity toward MEK4, and 10e showed larger margins of selectivity toward MEK4 (Figure 5B). We then subjected 15o to a kinome-wide scan using the scanEDGE DiscoverX platform. To ensure we observed all potential interactions with other kinases across the kinome and matched our cellular studies, we tested 15o against 97 kinases at a concentration of 10 μM. 15o hit 28 out of 97 kinases at 80% or higher inhibition (Figure 5C). The main classes of kinases affected were tyrosine-like kinases (TK), tyrosine kinase-like kinases (TKL), serine/threonine kinases (STE), cyclin-dependent kinases, mitogen-activated protein kinases, glycogen synthase kinases and CDK-like kinases (all encompassed within “CMGC”). Notably, it did not inhibit related kinases ERK1, BRAF, or RAF, though it did inhibit downstream kinases JNK1/2/3.
Additionally, we sought to evaluate some standard pharmacokinetic properties of our lead compounds, preceding their potential use as probe compounds in in vivo studies (Figure S-4). At a pH of 7.4, the logD of 15o was determined to be 3.22, while the logD of 10e was determined to be 2.80 (Figure S-4A). Both compounds exhibited good stability in the microsome assay, with 15o having a half-life of greater than 185 min, and 10e having a half-life of 114 min (Figure S-4B). In the PAMPA assay, 15o exhibited low permeability, while 10e performed slightly better (Figure S-4C). The Caco-2 permeability assay revealed that while both compounds showed medium to high permeability, only 15o was likely to be a Pgp substrate (Figure S-4D). Lastly, 10e showed a better selectivity profile than 15o in relation to CYP inhibition (Figure S-5E). While both compounds did not inhibit CYP isoforms 3A4/5, 10e also avoided inhibiting both 2D6 and 2C9. 15o exhibited low inhibition of 2D6 while inhibiting 2C9 to a higher degree. Both compounds inhibited CYP isoforms 1A2 and 2C19 to varying degrees.
To evaluate the cellular efficacy of our two MEK4 inhibitors, we examined the effects of 15o and 10e on JNK phosphorylation. HEK293T cells were pretreated with anisomycin, a potent JNK agonist,27,28 before treatment with increasing doses of 15o and 10e. Treatment with 15o and 10e attenuated anisomycin-induced JNK phosphorylation in a dose-dependent manner (Figure 6A). Additionally, our compounds decreased HEK293T cell proliferation in a dose-dependent manner (Figure 6D). We also examined the effects of these inhibitors on JNK phosphorylation and proliferation of two pancreatic cancer cell lines (CD18 and MiaPaCa2). As with the HEK293T cells, our inhibitors attenuated anisomycin-induced JNK phosphorylation and decreased proliferation (Figures 6B, C, E, and F).
Figure 6.
15o and 10e inhibit JNK phosphorylation and exhibit antiproliferative effects against pancreatic cancer cells. MEK1/2 inhibitors enhance the antiproliferative effects of 15o and 10e in pancreatic cancer cells. (A) HEK293T cells were pretreated with anisomycin (3 μg/mL) for 45 min prior to the addition of 15o and 10e for 5 h. The effects of 15o and 10e on JNK phosphorylation and total JNK expression levels were determined by Western blotting. HSP90 was used as loading control. The blots are representative of at least three biological replicates. (B, C) Human pancreatic cancer cells (CD18, MiaPaCa2) were pretreated with anisomycin (3 μg/mL) for 45 min prior to the addition of 15o and 10e for 5 h. The effects of 15o and 10e on JNK phosphorylation and total JNK expression levels were determined by Western blotting. HSP90 was used as loading control. The blots are representative of at least three biological replicates. (D) HEK293T (∼30,000 cells) cells were treated with either DMSO or varying doses of 15o and 10e. After 72 h, cells were trypsinized and counted, and the cell numbers were normalized against the DMSO treatment. Mean ± SD. Graphs are representative of three biological replicates. One-way ANOVA; ns, nonsignificant **, p < 0.01 ***, p < 0.001. (E, F) Human pancreatic cancer cells (∼30,000 cells) were treated with either DMSO or varying doses of 15o and 10e. After 72 h, cells were trypsinized, counted, and the cell numbers were normalized against the DMSO treatment. Mean ± SD. Graphs are representative of three biological replicates. One-way ANOVA; ns, nonsignificant ***, p < 0.001 ****, p < 0.0001. (G) HEK293T cells were pretreated with anisomycin (3 μg/mL) for 45 min prior to the addition of 15o (100 μM) and 10e (100 μM) for 5 h. The effects of 15o and 10e on MEK7, phospho-p42/44 MAPK, total p42/44 MAPK, phospho-p38 MAPK, and total p38 MAPK expression levels were evaluated by Western blotting. HSP90 was used as loading control. The blots are representative of at least three biological replicates. (H, K) Human pancreatic cancer cells CD18 and MiaPaCa2 were pretreated with U0126 (1 or 5 μM) for 1 h prior to the addition of MEK4 inhibitors (10 μM) for 5 h. The effects of different treatments on phospho-p42/44 MAPK and total p42/44 MAPK expression levels were determined by Western blotting. HSP90 was used as loading control. The blots are representative of at least three biological replicates. (I, L) Human pancreatic cancer cells (∼30,000 cells) were treated with either DMSO, MEK1/2 inhibitor (U0126, 1 or 5 μM), MEK4 inhibitors (10 μM), or the combination of MEK1/2 and MEK4 inhibitors. After 72 h, cells were trypsinized and counted, and the cell numbers were normalized against the DMSO treatment. Mean ± SD. Graphs are representative of three biological replicates. Two-way ANOVA. *, p < 0.05 **, p < 0.01 ****, p < 0.0001. (J, M) Coefficient of drug interaction (CDI) calculations. Cell numbers from different treatments were normalized against the DMSO treatment, and CDI is calculated as follows: CDI = AB/(A × B), with AB being the ratio of the combination groups to control group and A or B being the ratio of the single agent group to the DMSO group. Mean ± SEM from three biological replicates.
We next evaluated the effects of our inhibitors on the phosphorylation activity and expression of MEK1/2, MEK3/6, and MEK7. HEK293T cells were pretreated with anisomycin and then treated with either 15o or 10e. There was no effect on the expression of MEK7 (Figure 6G). However, as shown previously,27,28 treatment with anisomycin did induce phosphorylation of the MEK3/6 effector p38 MAPK, which was not affected by either 15o or 10e (Figure 6G). We also evaluated the effects on p42/44 MAPK (ERK1/2), which functions downstream of MEK1/2. Consistent with previously published reports,29−31 we observed that anisomycin decreases p42/44 MAPK phosphorylation in HEK293T cells (Figure 6G). Notably, treatment with 15o and 10e reversed the effects of anisomycin on p42/44 MAPK phosphorylation. Though we could not discount other off-target effects playing a role in decreased cellular proliferation, we found these results to be encouraging in that they revealed some degree of MEK family selectivity in a cellular system. Additionally, these results lay the groundwork for additional compound development, and we aim to develop tool probes that will be able to rule out off-target engagement in the future.
In additional experiments, we observed the effects of 15o and 10e on p42/44 MAPK phosphorylation in CD18 and MiaPaCa2 cells. Treatment with 15o and 10e increased p42/44 MAPK phosphorylation in CD18 cells (Figure 6H), which was suppressed by cotreating with the MEK1/2 inhibitor U0126. While 15o and 10e did not induce p42/44 MAPK phosphorylation in MiaPaCa2 cells, treatment with U0126 nonetheless decreased p42/44 MAPK phosphorylation (Figure 6K). Finally, we evaluated the effects of combining 15o or 10e with U0126 on pancreatic cancer cell proliferation. The combination of U0126 and MEK4 inhibitors suppressed the proliferation of CD18 (Figures 6H, I) and MiaPaCa2 (Figures 6K, L) cell lines, with the combination more effective in CD18 cells (Figures 6J, M). Our findings underscore crosstalk between the MEK1/2 and the MEK4 signaling pathways. Notably, others have also shown this connection between these two MEK pathways: Bernards and coworkers showed that treatment with MEK1/2 inhibitors could activate the MEK4 signaling pathway, with cotargeting of these two pathways resulting in synergistic antitumor effects.32 Our results indicate that inhibiting the MEK4 pathway activates the MEK1/2 pathway, with the combination of the MEK1/2 and MEK4 inhibitors demonstrating antiproliferative effects in pancreatic cancer cells.
We have developed novel cell-permeable, potent, and selective MEK4 inhibitors. Our lead optimization was guided by an iterative cycle of computational modeling, experimental synthesis, and in vitro evaluation of our designed compounds. Additionally, we evaluated the selectivity of our lead analogues across the MEK family as well as across the selected kinome, in addition to various pharmacokinetic properties. Future work will focus on improving the inhibitor selectivity for MEK4 over other kinases while retaining potency and cell permeability. Our lead compounds 10e and 15o successfully inhibited the phosphorylation of JNK and displayed antiproliferative properties against pancreatic cancer lines CD18 and MiaPaCa2. Finally, the promising data from the cotreatment with MEK1/2 inhibitors serves as a proof-of-concept for potential combination therapy using MEK1/2 and MEK4 inhibitors against pancreatic cancer, especially in tumors that exhibit activation of MEK1/2 following treatment with MEK4 inhibitors.
Acknowledgments
This research was supported by the Lurie Research Innovation Challenge Award (to K.A.S. and H.G.M.) and grants R01CA217907 (to H.G.M.) and R21CA255291 (to H.G.M.). A.J.K. was supported by the NIH under award numbers T32GM105538 and F31CA250353. T.N.D.P. was supported by the NIH/NCI under award number T32CA070085. H.E.O. acknowledges support from the Northwestern Undergraduate Research Grant. This work made use of the High Throughput Analysis Laboratory at Northwestern University and the IMSERC NMR and MS facilities at Northwestern University, which has received support from the Soft and Hybrid Nanotechnology Experiment (SHyNE) Resource (NSF ECCS-2025633). We also thank Matt Clutter (NU) and Michael Gullette (NU) for valuable discussion, and Saman Shafaie (NU) for assistance with high-resolution mass spectrometry. Kinome Tree image was generated using the TREEspot Software Tool and reprinted with permission from KINOMEscan, a division of DiscoveRx Corporation, DISCOVERX CORPORATION 2010.
Glossary
Abbreviations
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- MEK/MAP2K
mitogen-activated protein kinase kinase
- MAPK
mitogen-activated protein kinase
- HEK293
human embryonic kidney 293 cells
- Cys
cysteine
- Lys
lysine
- Glu
glutamate
- Met
methionine
- Ser
serine
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- IC50
half-maximal inhibitory concentration
- Kd
dissociation constant
- TK
tyrosine kinases
- TKL
tyrosine kinase-like kinases
- STE
serine/threonine kinases
- CDK
cyclic dependent kinase
- CMGC
cyclin-dependent kinases, mitogen-activated protein kinases, glycogen synthase kinases and CDK-like kinases
- BRAF
v-raf murine sarcoma viral oncogene homologue B1
- RAF
rapidly accelerated fibrosarcoma
- JNK
c-Jun N-terminal kinase
- Pgp
P-glycoprotein
- CYP
cytochrome P450
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00376.
Synthesis and characterization of compounds; general protocols for synthesis, modeling, and cell culture; raw data for kinome screens and ADMET studies (PDF)
Author Contributions
A.J.K., H.G.M., and K.A.S. conceived and directed the project. A.J.K. and H.E.O. conducted all organic synthesis and characterization. A.J.K. carried out all ADP-Glo assays and molecular docking for compound design. T.N.D.P. conducted all cell-based assays. The manuscript was written through contributions of all authors, who have given approval to the final version of the manuscript.
The authors declare the following competing financial interest(s): Patent applications have been filed for the compounds reported in this manuscript.
Supplementary Material
References
- Zhang W.; Liu H. T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9. 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- Raman M.; Chen W.; Cobb M. H. Differential regulation and properties of MAPKs. Oncogene 2007, 26, 3100. 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Chang L.; Karin M. Mammalian MAP kinase signalling cascades. Nature 2001, 410, 37. 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Cargnello M.; Roux P. P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50. 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinleye A.; Furqan M.; Mukhi N.; Ravella P.; Liu D. MEK and the inhibitors: from bench to bedside. J. Hematol. Oncol. 2013, 6, 27. 10.1186/1756-8722-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.; Tian H. Current Development Status of MEK Inhibitors. Molecules 2017, 22, 1551. 10.3390/molecules22101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.; Wang H.; Zheng C.; Liu Z.; Gao X.; Xu F.; Niu Y.; Zhang L.; Xu P. Research progress of MEK1/2 inhibitors and degraders in the treatment of cancer. Eur. J. Med. Chem. 2021, 218, 113386. 10.1016/j.ejmech.2021.113386. [DOI] [PubMed] [Google Scholar]
- Kwong A. J.; Scheidt K. A. Non-‘classical’ MEKs: A review of MEK3–7 inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127203. 10.1016/j.bmcl.2020.127203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Destrument A.; Tournier C. Physiological roles of MKK4 and MKK7: Insights from animal models. Biochim. Biophys. Acta, Mol. Cell Res. 2007, 1773, 1349. 10.1016/j.bbamcr.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Dhillon A. S.; Hagan S.; Rath O.; Kolch W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279. 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Bozyczko-Coyne D.; Saporito M. S.; Hudkins R. L. Targeting the JNK Pathway for Therapeutic Benefit in CNS Disease. Curr. Drug Targets: CNS Neurol. Disord. 2002, 1, 31. 10.2174/1568007023339472. [DOI] [PubMed] [Google Scholar]
- Chang T.-S. Isolation, Bioactivity, and Production of ortho-Hydroxydaidzein and ortho-Hydroxygenistein. Int. J. Mol. Sci. 2014, 15, 5699. 10.3390/ijms15045699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong C.; Kluwe L.; Li S.; Liu X.; Liu Y.; Liu H.; Gui W.; Liu T.; Xu L. Paeoniflorin inhibits tributyltin chloride-induced apoptosis in hypothalamic neurons via inhibition of MKK4-JNK signaling pathway. J. Ethnopharmacol. 2019, 237, 1. 10.1016/j.jep.2019.03.030. [DOI] [PubMed] [Google Scholar]
- Ogura M.; Kikuchi H.; Shakespear N.; Suzuki T.; Yamaki J.; Homma M. K.; Oshima Y.; Homma Y. Prenylated quinolinecarboxylic acid derivative prevents neuronal cell death through inhibition of MKK4. Biochem. Pharmacol. 2019, 162, 109. 10.1016/j.bcp.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Kim N.; Park J.; Gadhe C. G.; Cho S. J.; Oh Y.; Kim D.; Song K. A Protoberberine Derivative HWY336 Selectively Inhibits MKK4 and MKK7 in Mammalian Cells: The Importance of Activation Loop on Selectivity. PLoS One 2014, 9, e91037. 10.1371/journal.pone.0091037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibler K. K.; Schiltz G. E.; Clutter M. R.; Mishra R. K.; Vagadia P. P.; O’Connor M.; George M. D.; Gordon R.; Fowler G.; Bergan R.; Scheidt K. A. Synthesis and Biological Evaluation of 3-Arylindazoles as Selective MEK4 Inhibitors. ChemMedChem 2019, 14, 615. 10.1002/cmdc.201900019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klövekorn P.; Pfaffenrot B.; Juchum M.; Selig R.; Albrecht W.; Zender L.; Laufer S. A. From off-to on-target: New BRAF-inhibitor-template-derived compounds selectively targeting mitogen activated protein kinase kinase 4 (MKK4). Eur.. Eur. J. Med. Chem. 2021, 210, 112963. 10.1016/j.ejmech.2020.112963. [DOI] [PubMed] [Google Scholar]
- Jiang J.; Jiang B.; He Z.; Ficarro S. B.; Che J.; Marto J. A.; Gao Y.; Zhang T.; Gray N. S. Discovery of Covalent MKK4/7 Dual Inhibitor. Cell Chem. Biol. 2020, 27, 1553. 10.1016/j.chembiol.2020.08.014. [DOI] [PubMed] [Google Scholar]
- Deibler K. K.; Mishra R. K.; Clutter M. R.; Antanasijevic A.; Bergan R.; Caffrey M.; Scheidt K. A. A Chemical Probe Strategy for Interrogating Inhibitor Selectivity Across the MEK Kinase Family. ACS Chem. Biol. 2017, 12, 1245. 10.1021/acschembio.6b01060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R. K.; Deibler K. K.; Clutter M. R.; Vagadia P. P.; O’Connor M.; Schiltz G. E.; Bergan R.; Scheidt K. A. Modeling MEK4 Kinase Inhibitors through Perturbed Electrostatic Potential Charges. J. Chem. Inf. Model. 2019, 59, 4460. 10.1021/acs.jcim.9b00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T.; Kinoshita T.; Kirii Y.; Tada T.; Yamano A. Crystal and solution structures disclose a putative transient state of mitogen-activated protein kinase kinase 4. Biochem. Biophys. Res. Commun. 2012, 425, 195. 10.1016/j.bbrc.2012.07.066. [DOI] [PubMed] [Google Scholar]
- Matsumoto T.; Kinoshita T.; Kirii Y.; Yokota K.; Hamada K.; Tada T. Crystal structures of MKK4 kinase domain reveal that substrate peptide binds to an allosteric site and induces an auto-inhibition state. Biochem. Biophys. Res. Commun. 2010, 400, 369. 10.1016/j.bbrc.2010.08.071. [DOI] [PubMed] [Google Scholar]
- Sutanto F.; Konstantinidou M.; Dömling A. Covalent inhibitors: a rational approach to drug discovery. RSC Med. Chem. 2020, 11, 876. 10.1039/D0MD00154F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegzouti H.; Zdanovskaia M.; Hsiao K.; Goueli S. A. ADP-Glo: A Bioluminescent and Homogeneous ADP Monitoring Assay for Kinases. Assay Drug Dev. Technol. 2009, 7, 560. 10.1089/adt.2009.0222. [DOI] [PubMed] [Google Scholar]
- Tanega C.; Shen M.; Mott B. T.; Thomas C. J.; MacArthur R.; Inglese J.; Auld D. S. Comparison of bioluminescent kinase assays using substrate depletion and product formation. Assay Drug Dev. Technol. 2009, 7, 606. 10.1089/adt.2009.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C.; Huryn D. M.; Smith A. B. Carboxylic Acid (Bio)Isosteres in Drug Design. ChemMedChem 2013, 8, 385. 10.1002/cmdc.201200585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazzalin C. A.; Cano E.; Cuenda A.; Barratt M. J.; Cohen P.; Mahadevan L. C. p38/RK is essential for stress-induced nuclear responses: JNK/SAPKs and c-Jun/ATF-2 phosphorylation are insufficient. Curr. Biol. 1996, 6, 1028. 10.1016/S0960-9822(02)00649-8. [DOI] [PubMed] [Google Scholar]
- Hazzalin C. A.; Le Panse R.; Cano E.; Mahadevan L. C. Anisomycin selectively desensitizes signalling components involved in stress kinase activation and fos and jun induction. Mol. Cell. Biol. 1998, 18, 1844. 10.1128/MCB.18.4.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Shi X.; Hampong M.; Blanis L.; Pelech S. Stress-induced Inhibition of ERK1 and ERK2 by Direct Interaction with p38 MAP Kinase. J. Biol. Chem. 2001, 276, 6905. 10.1074/jbc.C000917200. [DOI] [PubMed] [Google Scholar]
- Croons V.; Martinet W.; Herman A. G.; Timmermans J.-P.; De Meyer G. R. Y. The Protein Synthesis Inhibitor Anisomycin Induces Macrophage Apoptosis in Rabbit Atherosclerotic Plaques through p38 Mitogen-Activated Protein Kinase. J. Pharmacol. Exp. Ther. 2009, 329, 856. 10.1124/jpet.108.149948. [DOI] [PubMed] [Google Scholar]
- Li J.-y.; Huang J.-y.; Li M.; Zhang H.; Xing B.; Chen G.; Wei D.; Gu P.-y.; Hu W.-x. Anisomycin induces glioma cell death via down-regulation of PP2A catalytic subunit in vitro. Acta Pharmacol. Sin. 2012, 33, 935. 10.1038/aps.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z.; Vis D. J.; Bruna A.; Sustic T.; van Wageningen S.; Batra A. S.; Rueda O. M.; Bosdriesz E.; Caldas C.; Wessels L. F. A.; Bernards R. MAP3K1 and MAP2K4 mutations are associated with sensitivity to MEK inhibitors in multiple cancer models. Cell Res. 2018, 28, 719. 10.1038/s41422-018-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.