Abstract
Cervical insufficiency generally refers to a condition in which there is mid-trimester cervical dilatation or protruding chorioamniotic membranes in the absence of uterine contractions. Such condition is a risk factor for spontaneous mid-trimester abortion or early preterm birth, and is associated with adverse neonatal outcomes. Both intra-amniotic infection and inflammation ascertained by amniocentesis have been identified in patients with cervical insufficiency, and are poor prognostic factors. A subset of patients with intra-amniotic inflammation will have no demonstrable microorganisms detected via cultivation or molecular methods, and therefore represent cases of sterile intra-amniotic inflammation. Amniotic fluid sludge (free-floating hyperechogenic material within the amniotic fluid in close proximity to the uterine cervix) identified on sonography is a biomarker for intra-amniotic infection and inflammation. Recent evidence suggests that intra-amniotic infection, as well as sterile intra-amniotic inflammation can be treated successfully using antimicrobial agents. We report a unique case in which administration of antibiotics in the presence of mid-trimester cervical insufficiency, sterile intra-amniotic inflammation, and amniotic fluid sludge was associated with resolution of the cervical findings, as demonstrated on both sonographic and speculum examination. The patient successfully underwent elective cesarean delivery at 36 2/7 weeks of gestation. This case illustrates that antibiotic therapy may be effective despite the presence of several high-risk pregnancy conditions, and that successful outcome is possible.
Keywords: amniotic fluid analysis, biomarkers, ceftriaxone, cerclage, clarithromycin, CRP, interleukin-6, maternal inflammation, metronidazole, short cervix, sterile intra-amniotic inflammation, white blood cell count
Introduction
Cervical insufficiency generally refers to a condition in which there is mid-trimester cervical dilatation or protruding chorioamniotic membranes in the absence of uterine contractions. It is thought that cervical sufficiency/insufficiency is a continuum [1], and is syndromic in nature caused by various factors [2]. Unfortunately, cervical insufficiency is a risk factor for spontaneous mid-trimester abortion or early preterm birth [3]. Intra-amniotic infection has been reported in 8-52% [4–11], and intra-amniotic inflammation in 81% [7] of such patients when ascertained by amniocentesis. Moreover, intra-amniotic inflammation, regardless of amniotic fluid culture results, is a risk factor for impending preterm delivery and adverse outcomes [7]. Indeed, Lee et al. reported that when cervical insufficiency and intra-amniotic inflammation are present, 50% will deliver preterm within 7 days [7]. A subset of patients with intra-amniotic inflammation will have no demonstrable microorganisms detected via cultivation or molecular methods, and therefore represent cases of sterile intra-amniotic inflammation [12–17].
Amniotic fluid sludge describes free-floating hyperechogenic or particulate material within the amniotic fluid in close proximity to the uterine cervix, which is demonstrable on transvaginal sonography. Sludge is a biomarker for intra-amniotic infection and inflammation [18], and is an independent risk factor for preterm prelabor rupture of membranes (PROM), as well as spontaneous preterm delivery [19–21].
The conventional view has been that in the setting of cervical insufficiency, intra-amniotic infection/inflammation cannot be successfully treated [10]. Yet, recent evidence suggests that intra-amniotic infection, as well as sterile intra-amniotic inflammation can be treated successfully with antimicrobial agents [10,13,22–23]. We report herein a case in which administration of antibiotics in the presence of mid-trimester cervical insufficiency, sterile intra-amniotic inflammation, and amniotic fluid sludge was associated with resolution of cervical findings as demonstrated on both sonographic and speculum examination. When the antibiotic regimen was initiated, the maternal C-reactive protein concentration was elevated, but normalized six days later during treatment. The patient successfully underwent elective cesarean delivery at 36 2/7 weeks of gestation due to a history of prior myomectomy. Histologic examination of the placenta demonstrated no acute inflammatory lesions or funisitis. This unique case illustrates that antibiotic therapy may be effective despite the presence of several high-risk pregnancy conditions, and that successful outcome is possible.
Case Description
A 39-year-old woman, gravida 2, para 0010 was referred to our institution for a fetal anatomy survey at 20 3/7 weeks of gestation due to advanced maternal age, multiple fibroids, and an echogenic intracardiac focus noted on the office ultrasound exam. The gestation was dated by a first trimester ultrasound examination at 5 weeks. The patient’s past obstetrical and medical history was significant for a prior ectopic pregnancy, anemia, mild asthma, and partial thyroidectomy, as well as a history of myomectomy with entry into the endometrial cavity and a vertical uterine incision. At 7 6/7 weeks of gestation, she received vaginal metronidazole for bacterial vaginosis.
For the Case presented herein, she was examined at Detroit Medical Center/Wayne State University, and the Perinatology Research Branch of NICHD, NIH, DHHS. The patient was enrolled in a research protocol approved by the Institutional Review Board of NICHD, NIH, and by the Human Investigation Committee of Wayne State University. She provided written informed consent for the use of sonographic images for research purposes.
On transabdominal sonographic examination, the uterine cervix appeared short; therefore, a transvaginal examination was performed, confirming a short cervical length (7.3 mm) with funneling and amniotic fluid sludge present (Figure 1). The funnel width and length were 13.8 mm and 29.2 mm, respectively. In addition, more than 15 uterine leiomyomas were identified (largest measured 4.4 x 4.9 x 5.5 cm).
Figure 1: Sonographic cervical evaluation at 20 3/7 gestational weeks.
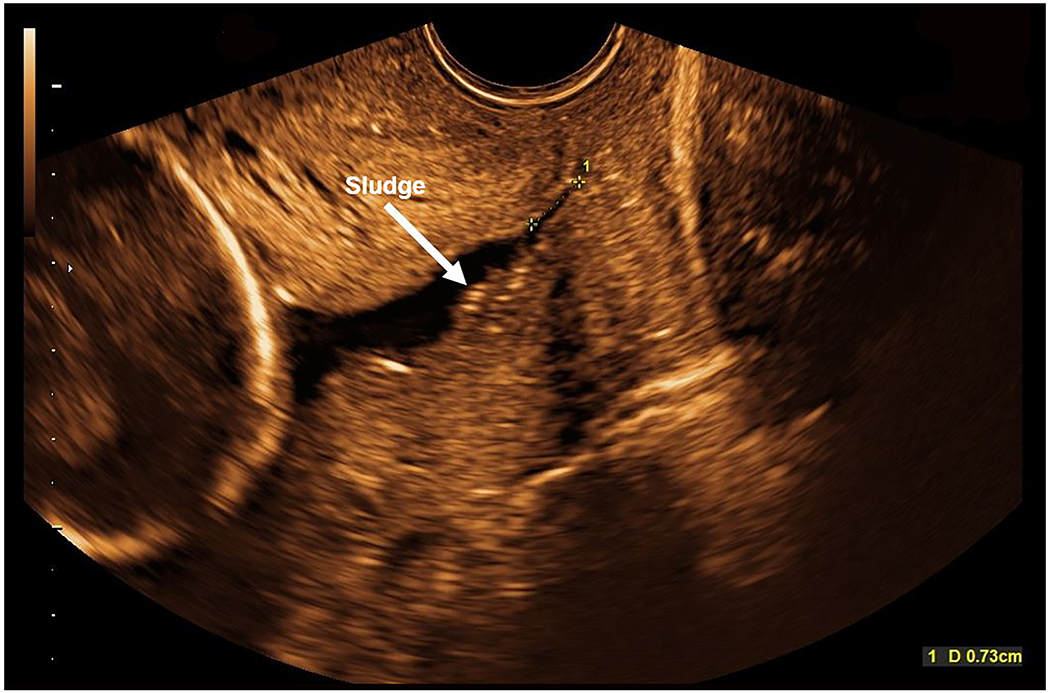
On transabdominal ultrasound, the cervix appeared short, therefore a transvaginal sonographic examination was performed. The cervical length is 7.3 mm with funneling and amniotic fluid sludge noted (white arrow).
Due to the sonographic findings, the patient was sent to the obstetrical triage unit. She gave a history of occasional cramping, but denied bleeding or leaking of fluid. Digital exam revealed the uterine cervix to be closed and 70% effaced. Speculum exam showed a small amount of white discharge in the vaginal vault, but there was absent bleeding or pooling of fluid. No contractions were evident on the tocodynamometer. Urine culture obtained that day eventually returned as positive for group B streptococcus (10-100,000 CFU/mL). The patient was counseled on the availability of amniocentesis testing to determine the microbial status of the amniotic cavity. She was discharged home with instructions to take vaginal progesterone nightly, and returned four days later as an outpatient at 21 weeks of gestation for transabdominal amniocentesis. Fetal karyotype and microarray analysis were normal. Amniotic fluid analysis showed a negative Gram stain, white blood cell count of 2 cells/mm3, and glucose of 24 mg/dl. Amniotic fluid cultures for aerobic and anaerobic bacteria, as well as Ureaplasma urealyticum and Mycoplasma hominis were negative. However, the amniotic fluid interleukin-6 (IL-6), a pro-inflammatory cytokine, was elevated at 3.055 ng/ml (normal <2.6 ng/ml), consistent with sterile intra-amniotic inflammation [15]. It is noteworthy that at a later time, the amniotic fluid supernatant was also analyzed by PCR for Candida spp., and returned negative.
Hospital Course
Due to the amniocentesis results, the patient was admitted to the hospital at 22 weeks of gestation. She complained of vaginal discharge, but denied leaking of fluid, bleeding, or contractions (also confirmed on tocodynamometer). However, speculum exam showed the cervix to be 3 cm dilated with bulging membranes, and amniotic fluid sludge was visibly “passing by the membranes”, consistent with cervical insufficiency. The patient was counseled that in the presence of cervical insufficiency with intra-amniotic inflammation, the prognosis is poor, with 50% of women experiencing a preterm delivery within 7 days [7]. She was also counseled about the study by Oh et al, in which the authors reported that in patients with cervical insufficiency and intra-amniotic infection/inflammation, administration of antibiotics was followed by resolution of the intra-amniotic inflammatory process or intra-amniotic infection in 75% of patients, and was associated with treatment success in 59% of cases [10]. After discussion, the patient agreed to receive triple antibiotic therapy: 1) ceftriaxone 1 gram IV every 24 hours; 2) clarithromycin 500 mg oral every 12 hours; and 3) metronidazole 500 mg IV every 8 hours [10]. She had been taking vaginal progesterone since 20 3/7 weeks of gestation, and this medication was continued in-house. The patient was placed in Trendelenburg position, although this was not followed consistently. To evaluate for the presence of maternal systemic inflammation or infection, the following labs were ordered at admission (before antibiotic administration) and serially during the hospital stay: 1) C-reactive protein (Figure 2); 2) procalcitonin; 3) complete blood count with differential; 4) ferritin; and 5) coagulation panel (fibrinogen, D-dimer, prothrombin time, and partial thromboplastin time). Genital cultures on admission were negative for yeast, but later yielded numerous group B streptococcus. In addition, the urine culture on admission showed group B streptococcus (>100,000 CFU/ml).
Figure 2: Maternal C-reactive protein concentrations during hospital stay (22 to 24 1/7 gestational weeks).
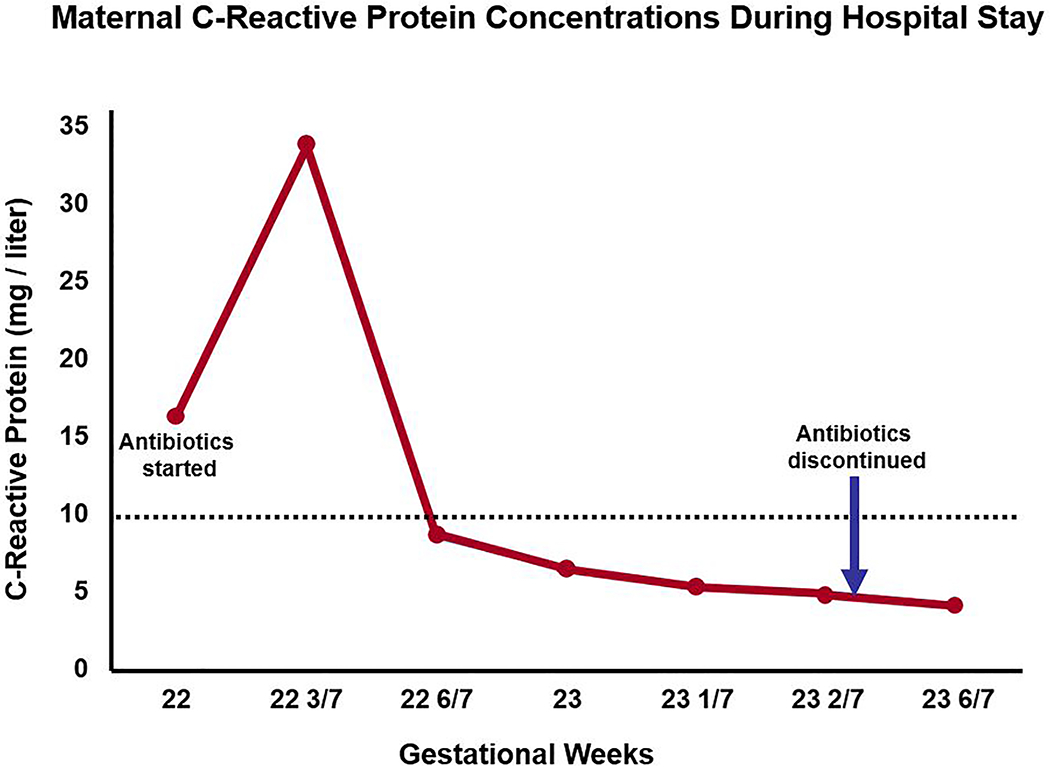
Just before antibiotics were started, the initial C-reactive protein concentration was elevated (16.48 mg/l) at 22 weeks, and increased further (33.24 mg/l) at 22 3/7 weeks. However, subsequent C-reactive protein concentrations became normal six days later during antibiotic treatment, and remained as such even after antibiotics were discontinued (normal < 10 mg/l; dotted line).
Based on recommendations by neonatology, antenatal corticosteroids were administered at 22 2/7 – 22 3/7 weeks. At 22 3/7 weeks of gestation (two weeks after the initial cervical sonographic examination), the patient underwent a repeat transvaginal ultrasound examination, and this now demonstrated no measurable cervical length. The chorioamniotic membranes were bulging past the external cervical os into the vagina (Figure 3, Supplementary Movies 1 and 2). Moreover, a large quantity of amniotic fluid sludge was visualized within the bulging membranes (Figure 3, Supplementary Movies 1 and 2). Due to such findings, vaginal progesterone was discontinued; altogether, the patient received this only between 20 3/7 and 22 5/7 weeks of gestation.
Figure 3 (A and B): Sonographic cervical evaluation at 22 3/7 gestational weeks.
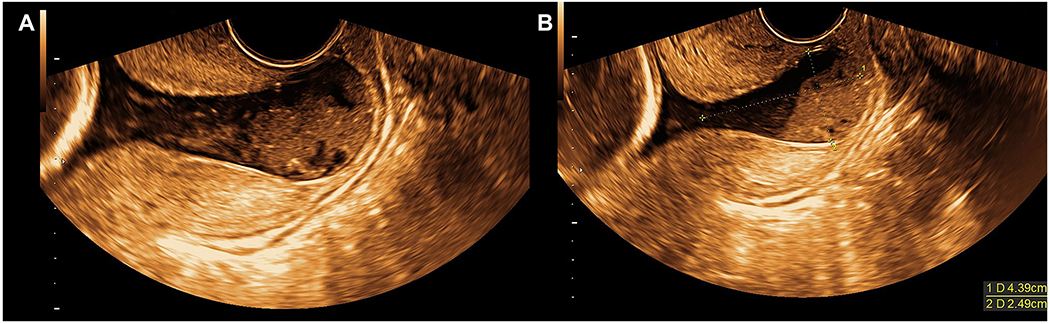
A) Transvaginal sonographic examination revealed no measurable cervical length with chorioamniotic membranes bulging past the external cervical os into the vagina. A large quantity of amniotic fluid sludge is present within the bulging membranes (also see Supplementary Movies 1 and 2). B) Same findings as A; in addition, the anterior-posterior diameter of the bulging membranes measured 24.9 mm.
During the hospital course, the patient received triple antibiotics for 11 days total (i.e. 22 to 23 3/7 weeks of gestation). As seen in Figure 2, maternal C-reactive protein concentrations were elevated on the same day antibiotics were initiated, but became normal six days later during treatment, and remained as such during the hospital stay, even after antibiotics were discontinued. The other laboratory results obtained serially were not consistent with maternal systemic inflammation or infection. The patient was counseled about repeat amniocentesis testing to monitor the microbiologic and inflammatory status of the amniotic cavity. The plan was to use this information (as well as sonographic examination results) to determine the duration of antibiotic therapy. However, the patient complained of several side effects (i.e. nausea, dyspepsia, night sweats) and requested discontinuation of all antibiotics. Repeat amniocentesis was not performed at the discretion of her providers. Three days after antibiotics were discontinued, transvaginal sonogram at 23 6/7 weeks of gestation still demonstrated sludge; however, the cervical length was surprisingly longer in length (21.5 mm) with mild funneling present (Figure 4, Supplementary Movie 3). Moreover, on ultrasound, the membranes were no longer visualized bulging through the external cervical os into the vagina. Continued in-patient management was recommended until 32 weeks; however, the patient decided to leave the hospital against medical advice at 24 1/7 weeks of gestation.
Figure 4: Sonographic cervical evaluation at 23 6/7 gestational weeks.
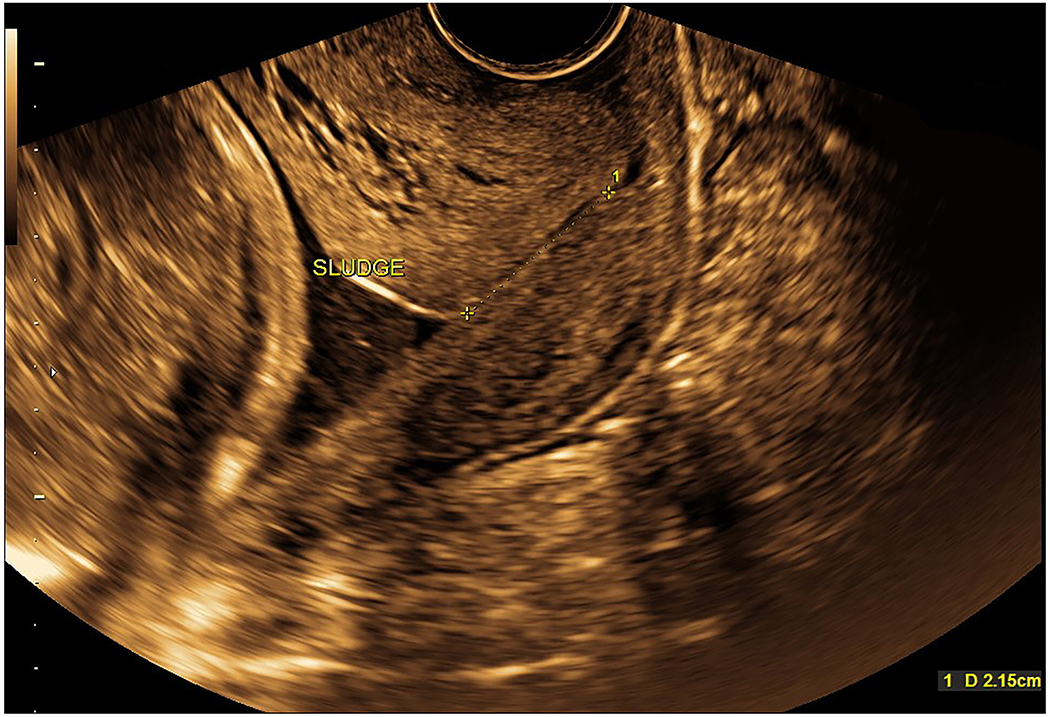
Transvaginal cervical length was 21.5 mm with mild funneling present. Amniotic fluid sludge was still identified (also see Supplementary Movie 3). It is noteworthy that bulging membranes past the external cervical os into the vagina are no longer present.
Outpatient Course
Approximately one week later, the patient presented to the hospital at 25 weeks of gestation complaining of pelvic pressure. Speculum exam revealed white discharge in the vaginal vault and the cervix appeared 1 to 2 cm dilated, but no bulging membranes were visualized. Light microscopy showed clue cells and there was a positive whiff test, consistent with bacterial vaginosis. The patient was discharged home with oral metronidazole for 7 days.
At 25 3/7 weeks of gestation, the estimated fetal weight was noted to be at the 11th percentile, and amniotic fluid volume was normal. Transvaginal cervical length was 12.7 mm with funneling and sludge again seen; however, the quantity of sludge appeared much less than previously (Figure 5, Supplementary Movie 4).
Figure 5: Sonographic cervical evaluation at 25 3/7 gestational weeks.
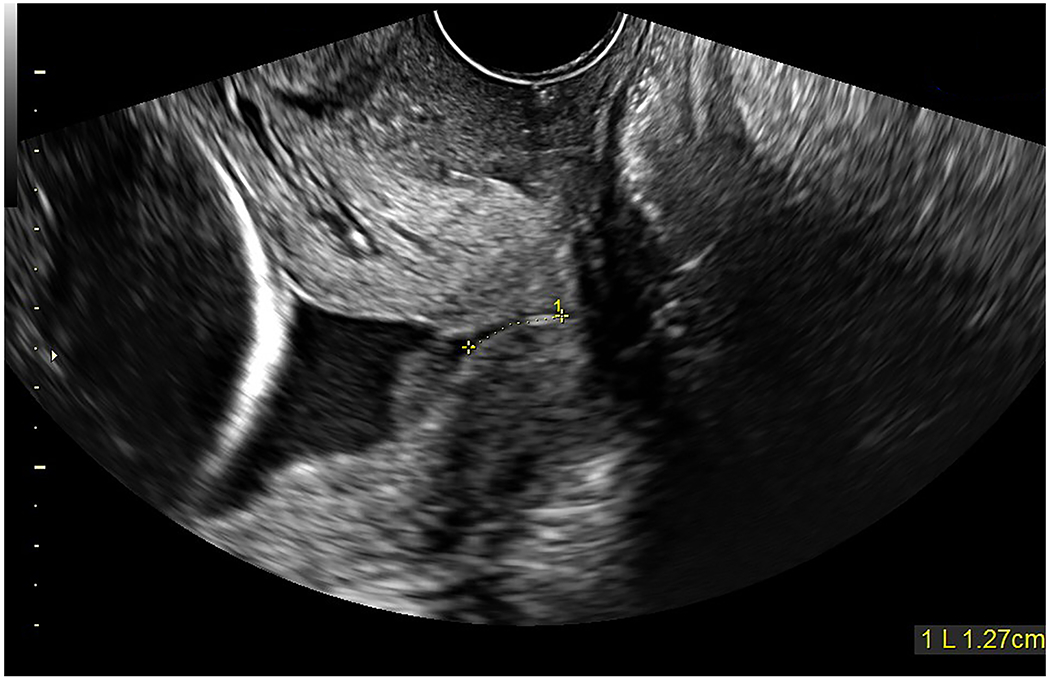
Transvaginal cervical length was 12.7 mm with funneling and the presence of amniotic fluid sludge (collection just proximal to the internal cervical os); however, the quantity of sludge appeared much less than seen previously (also see Supplementary Movie 4).
The patient continued obstetrical care with her provider, and she underwent an ultrasound examination at 33 2/7 weeks of gestation. The estimated fetal weight was noted to be at the 5th percentile, and the abdominal circumference measured 3 weeks behind dates. However, the amniotic fluid volume and fetal Doppler velocimetry (umbilical artery, umbilical vein, middle cerebral artery, ductus venosus) were within normal limits. The transvaginal cervical length was 20.9 mm, and there was no evidence of funneling or dynamic changes (Figure 6, Supplementary Movie 5). In addition, no amniotic fluid sludge could be identified below the presenting vertex. The patient was completely asymptomatic and was doing well.
Figure 6: Sonographic cervical evaluation at 33 2/7 gestational weeks.
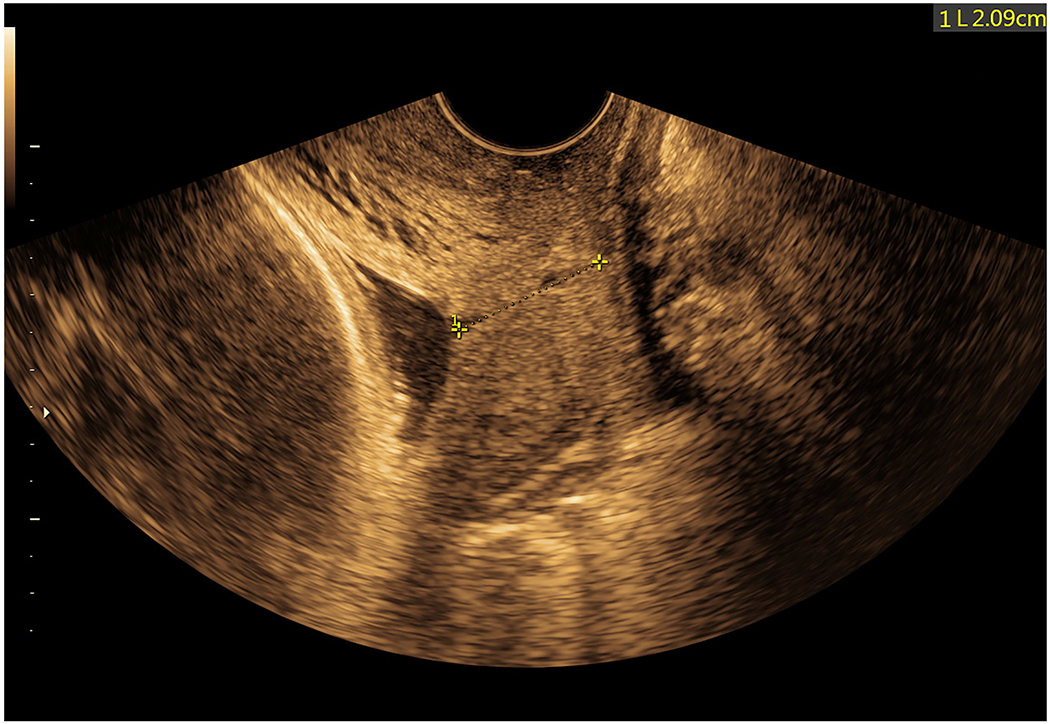
Transvaginal cervical length was 20.9 mm and there was no evidence of funneling or dynamic changes. No amniotic fluid sludge could be identified below the presenting vertex (also see Supplementary Movie 5).
Delivery
Due to the history of prior myomectomy, the patient underwent an elective caesarean delivery at 36 2/7 weeks of gestation. Just prior to the delivery, a transvaginal ultrasound was performed, and the cervical length was 19.7 mm with mild funneling distal to the chorioamniotic membranes (Figure 7, Supplementary Movie 6). No amniotic fluid sludge was identified below the presenting vertex. The patient had received one prophylactic IV dose of azithromycin and cefazolin prior to delivery. A viable female neonate weighing 2560 grams was born, with Apgar scores of 8 and 9 (1 and 5 minutes, respectively). Umbilical cord arterial and venous blood gases were within normal limits. During the cesarean section, an attempt was made to collect amniotic fluid from the exposed amniotic sac to evaluate the microbial status of the amniotic cavity. However, due to decreased amniotic fluid, this was not successful. The amniotic fluid was noted to be clear. The placenta was manually extracted and noted to be adherent to the right posterior uterine wall; therefore, curettage was performed.
Figure 7: Sonographic cervical evaluation at 36 2/7 gestational weeks on the day of delivery.
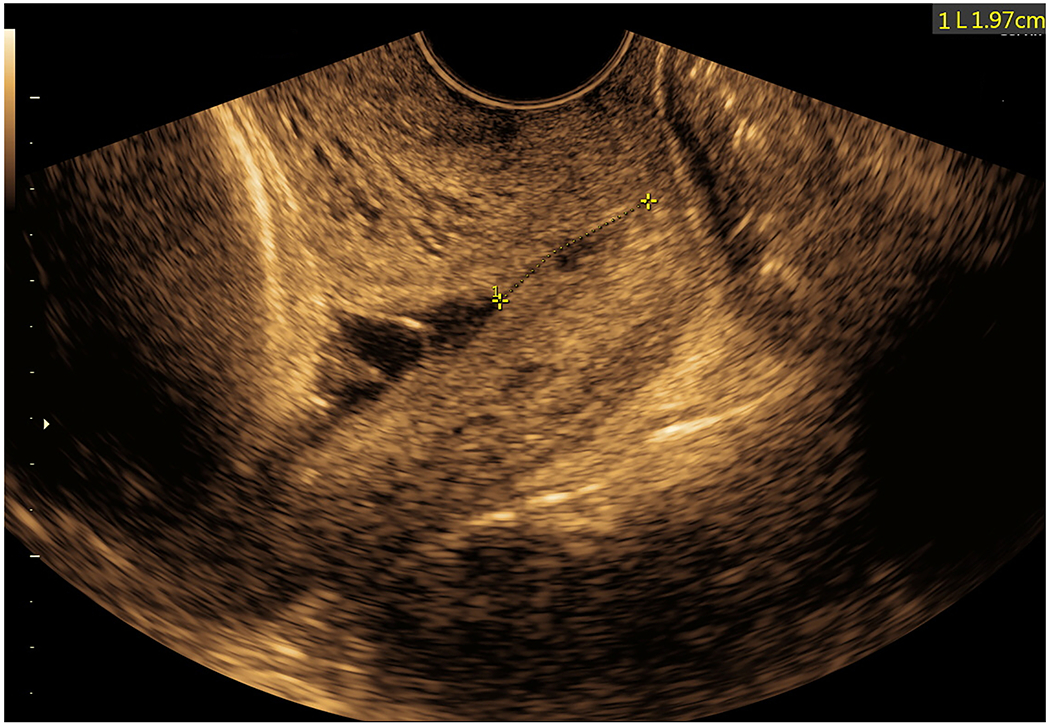
Transvaginal cervical length was 19.7 mm with mild funneling distal to the chorioamniotic membranes. No amniotic fluid sludge could be identified below the presenting vertex (also see Supplementary Movie 6).
Histologic examination of the placenta showed no acute inflammatory lesions or funisitis, no lesions associated with maternal vascular or fetal vascular malperfusion, and marginal intervillous fibrin deposition was present. However, chronic lymphoplasmacytic deciduitis was identified. Gross examination revealed the fetal surface to be blue-gray in color, and extraplacental membranes were translucent (Figure 8). The maternal surface was unremarkable. Serial sections of the placenta revealed diffuse fibrosis around the periphery, collectively occupying less than 5% of the total cut surfaces.
Figure 8: Gross examination of the placenta.
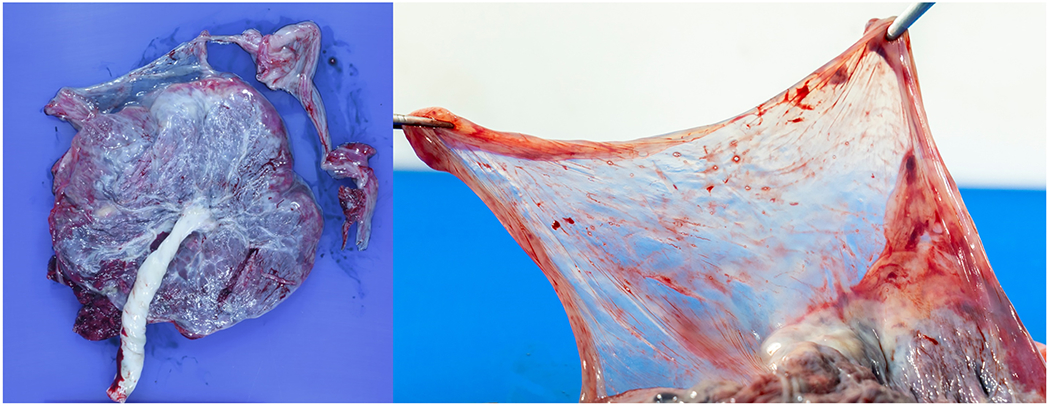
The fetal surface was noted to be blue-gray in color (left image). Extraplacental membranes were translucent (right image).
Neonatal Course
At delivery, the neonate had a temperature of 36.5°C, and in the nursery, the temperature was unstable, ranging from 36 to 36.3°C. Due to the history of positive maternal urine and genital cultures for group B streptococcus, neonatal temperature instability, and concern for possible neonatal infection, ampicillin and gentamicin were initiated. However, labs (i.e. complete blood count, blood cultures, and C-reactive protein) obtained from the neonate on the day of delivery (before the first dose of antibiotics) were within normal limits. Repeat complete blood count and C-reactive protein labs were also normal. The neonatal temperature stabilized, antibiotics were discontinued after a 3-day course, and the neonate was discharged home on day of life four. As of this writing (two months after delivery), the patient and neonate are doing well.
Discussion
The main message of this report is that administration of antibiotics in the presence of mid-trimester cervical insufficiency, sterile intra-amniotic inflammation, and amniotic fluid sludge was associated with resolution of the cervical findings, as demonstrated on both sonographic and speculum examination. Although the patient was asymptomatic, we offered an amniocentesis when a sonographic short cervix and sludge were identified at 21 weeks of gestation, in order to determine the microbial status of the amniotic cavity. Prior observations have suggested an association between short cervix and intra-amniotic infection [24–25]. Moreover, investigators have also reported that a mid-trimester sonographic short cervix (≤ 25 mm) is associated with intra-amniotic inflammation, and such patients are at risk for adverse pregnancy outcomes [26–29]. For example, in asymptomatic women with a mid-trimester sonographic short cervix (≤ 15 mm), the prevalence of intra-amniotic inflammation and negative amniotic fluid culture was 22.2% [27]. In such patients, there was a higher rate of preterm delivery within 7 days compared to those without intra-amniotic inflammation (40% vs. 5.7%; p=0.016) [27].
Cervical insufficiency and intra-amniotic infection / inflammation
Cervical insufficiency generally refers to a condition in which there is mid-trimester cervical dilatation or protruding chorioamniotic membranes in the absence of uterine contractions. Unfortunately, such condition is a risk factor for spontaneous mid-trimester abortion or early preterm birth [10]. Studies have reported an 8-52% prevalence of intra-amniotic infection (ascertained by amniocentesis) in patients with cervical insufficiency [4–11]. Moreover, intra-amniotic inflammation has been identified in patients with cervical insufficiency [7,30–31], and has been associated with preterm delivery, as well as adverse pregnancy and neonatal outcomes [7,30].
A subset of patients with intra-amniotic inflammation will have no demonstrable microorganisms detected via cultivation or molecular methods, and therefore represent cases of sterile intra-amniotic inflammation, which applies to the case herein [12–17]. The inflammatory response detected in amniotic fluid may be due to a sterile inflammatory response activated by non-microbial endogenous danger signals, such as cellular alarm signals from distressed and injured cells or tissues [15–16,32–35]. Indeed, sterile intra-amniotic inflammation is more common than intra-amniotic infection in asymptomatic patients with a sonographic short cervix [15], and the same has been reported for patients with cervical insufficiency [7]. Lee et al. reported that a very high percentage (81%) of patients with acute cervical insufficiency have intra-amniotic inflammation demonstrated by amniocentesis (regardless of amniotic fluid culture results), and that the prognosis is poor, with 50% delivering preterm within 7 days [7]. The same authors also reported that in patients with intra-amniotic inflammation but with negative amniotic fluid cultures, 95% of patients had a preterm birth, and the perinatal mortality rate was 63% [7]. Therefore, based on such data, our patient underwent amniocentesis and the results were consistent with sterile intra-amniotic inflammation.
Amniotic fluid sludge is an indicator of microbial invasion of the amniotic cavity and intra-amniotic inflammation
Amniotic fluid sludge (free-floating hyperechogenic or particulate material within the amniotic fluid in close proximity to the uterine cervix) identified on transvaginal sonography represents a sign that microbial invasion of the amniotic cavity and an inflammatory process are occurring [18]. It is feasible that progressive infection induces an intense inflammatory response, and that the combination of inflammatory cells with microorganisms leads to sludge formation. The observation that the shorter a cervical length is, the greater the likelihood of sludge has been interpreted to indicate that intra-amniotic infection/inflammation will eventually lead to a short cervix [19]. Moreover, sludge is an independent risk factor for preterm PROM, as well as spontaneous preterm delivery [19–21].
For our case, antibiotic therapy was initiated at 22 weeks of gestation and sonographic examination 3 days later showed a large quantity of sludge. Antibiotics were administered for a duration of 11 days until 23 3/7 weeks of gestation. Yet, it is noteworthy that at 25 3/7 weeks of gestation, the quantity of sludge had diminished greatly. Moreover, by 33 2/7 and 33 6/7 weeks of gestation, no sludge could be visualized below the presenting vertex. It is unclear if such finding was a false-negative (see below) or if there was true resolution of sludge by the third trimester. Although the diminished quantity and then disappearance of sludge cannot be attributed directly to antibiotic therapy (i.e. causation), it is possible, since no other treatment was administered except for vaginal progesterone. The patient received such medication when the cervical length was 7.3 mm; however, during the course of vaginal progesterone therapy, the cervix progressed to 3 cm dilatation on speculum exam, and transvaginal sonogram demonstrated no measurable cervical length with membranes bulging past the external cervical os into the vagina. Therefore, vaginal progesterone was not effective in this case. Such observations are not surprising. Fonseca et al. noted that vaginal progesterone reduced the rate of spontaneous preterm delivery <34 weeks of gestation by only 25% in patients with a cervical length of 6-10 mm, but the effect size was 75% in those with a cervical length between 11-15 mm [36]. A possible explanation for these results is that women with a very short cervix are more likely to have intra-amniotic inflammation and may be less responsive to progesterone, as was the case herein [26–27,37].
Several points regarding amniotic fluid sludge are noteworthy. First, the “resolution” of sludge observed in the current case may not occur all the time and in all patients, such as those who have intra-amniotic infection. Microbial biofilms have been identified in the presence of intra-amniotic infection with sludge [38]. Such biofilms are communities of sessile microorganisms formed by cells that are attached irreversibly to a substratum or interface, or to each other and embedded in a hydrated matrix of extracellular polymeric substances [38]. This is relevant, because bacteria in these biofilms are more resistant to antimicrobial agents and to the host response to infection [39–41].
Second, we theorize that sometimes the “disappearance” of sludge is a false-negative finding. Based upon a breadth of experience in our unit evaluating sludge via transvaginal ultrasound throughout gestation, there may be several reasons for a false-negative result: 1) the fetal vertex, particularly with advancing gestational age in the third trimester, may prohibit the collection of sludge in close proximity to the internal os (i.e. the vertex acts as a “plug”); 2) it is possible that maternal positional changes may influence the likelihood that sludge settles into the lower uterine segment. For example, patients who have been lying supine (e.g. in the hospital bed) may not demonstrate sludge in the lower uterine segment on transvaginal sonography, because they simply have not been upright; 3) an active fetus (and/or perhaps an active mother) can disperse the free-floating hyperechogenic material throughout the amniotic cavity, such that it does not collect in the lower uterine segment; and 4) sonographic examinations capture only a moment in time; sludge may still be present, but for various reasons just described is not evident at the time of exam. To confirm the presence or absence of sludge, we always tap gently on the maternal abdomen in the area of the lower uterine segment to observe dispersion of any hyperechogenic or particulate material (see Supplementary Movies 2, 5, 6).
Antibiotics are effective in treating patients with intra-amniotic infection / inflammation and cervical insufficiency
The conventional view is that intra-amniotic infection and/or intra-amniotic inflammation in patients with acute cervical insufficiency cannot be treated successfully, and that delivery is inevitable. For our patient, the sonographic findings (i.e. membranes bulging past the external cervical os into the vagina) at 22 3/7 weeks of gestation were very concerning, especially in the presence of sludge. Yet, Lee et al. observed unexpectedly that some patients with bulging membranes and intra-amniotic inflammation [defined as an elevated amniotic fluid concentration of matrix metalloproteinase-8 (MMP-8) > 23 ng/ml] who were treated with parenteral antibiotics for more than 7 days ultimately delivered after 34 weeks of gestation [7]. Of the women who underwent repeat amniocentesis, in all cases the MMP-8 concentration was lower in the second amniotic fluid sample, suggesting that either antibiotic therapy eradicated a microbial stimulus for inflammation, or that the natural history of this condition includes a reversible phase [7].
Subsequently, Oh et al. described a series of women with cervical insufficiency (16 to 27.9 weeks of gestation) and intra-amniotic infection/inflammation who were treated with three antimicrobial agents (ceftriaxone, clarithromycin, and metronidazole) [10]. Cervical dilatation was determined by visual examination during sterile speculum examination (vs. transvaginal sonography), and intra-amniotic inflammation was defined as IL-6 > 2.6 ng/ml. After antibiotic therapy, follow-up amniocentesis was routinely offered to monitor the microbiologic and inflammatory status of the amniotic cavity. The authors reported that: 1) 75% of women had objective evidence of resolution of the intra-amniotic infectious/inflammatory process after treatment; and 2) there was overall treatment success (i.e. resolution of intra-amniotic infection/inflammation or delivery ≥34 weeks) in 59% of cases [10]. Therefore, this report provided strong evidence that antibiotics can be effective in treating intra-amniotic infection/inflammation in patients with cervical insufficiency. It is especially remarkable that for three patients with bulging membranes who received antibiotics (but not cervical cerclage placement), there was biochemical evidence of successful treatment of intra-amniotic inflammation, as well as spontaneous reduction of membranes back into the uterine cavity while receiving antibiotic therapy. Indeed, the authors reported that this was an unexpected observation made during the course of their study. We also surprisingly observed resolution of cervical insufficiency in our patient, as demonstrated on sonographic examination at 23 6/7 weeks of gestation (Figure 4, Supplementary Movie 3), as well as speculum examination at 25 weeks of gestation.
Our patient received triple antibiotic therapy for 11 days only, because she experienced symptomatic side effects. Yet, she also received oral metronidazole to treat bacterial vaginosis for an additional 7 days (25 – 26 weeks of gestation) when the cervix appeared 1 – 2 cm dilated on speculum exam. In Oh et al’s study, the use and discontinuation of antibiotics were left to the discretion of the treating clinician. For the patients who had confirmed resolution of intra-amniotic inflammation and delivered ≥ 34 weeks, the median (range) duration of the antibiotic regimen was 27 (7-66) days [10]. Metronidazole was administered for a maximum of 4 weeks. Therefore, in contrast to Oh’s study, our patient received a much shorter course of antibiotic treatment. In addition, the amniotic fluid IL-6 concentration was 3.055 ng/ml (normal < 2.6 ng/ml). Oh et al. reported that patients with cervical insufficiency who received antimicrobial agents and who delivered within 1 week of amniocentesis had a significantly higher median (range) amniotic fluid IL-6 concentration than those who remained undelivered for at least 1 week after amniocentesis [47.7 (5.7 – 49.3) vs. 9.4 (2.8 – 35.4) ng/mL; p = 0.005] [10]. Therefore, one can speculate that despite the shorter duration of antibiotics that our patient received, perhaps there was a successful outcome because the amniotic fluid IL-6 concentration was slightly above the 2.6 ng/ml cut-off. Ideally, the duration of antibiotic treatment can be directed clinically via a follow-up amniocentesis to determine the microbial and inflammatory status of the amniotic cavity.
Why did we choose the specific antibiotic regimen for our case? Erythromycin or azithromycin has been shown to be frequently inadequate in eradicating intra-amniotic infection with Ureaplasma spp., perhaps due to limited transplacental passage leading to inadequate antimicrobial activity in the amniotic fluid [42]. In contrast, clarithromycin is not only effective for treating intra-amniotic infection by Ureasplasma spp. [13], but this agent also has a much higher rate of transplacental passage [42]. Moreover, in patients with preterm PROM and either intra-amniotic infection or sterile intra-amniotic inflammation, IV clarithromycin is associated with attenuation in the intensity of the intra-amniotic inflammatory response [23]. Ceftriaxone was administered due to its enhanced coverage of aerobic bacteria and high rate of transplacental passage [43–44], while metronidazole was utilized because it has a powerful effect against anaerobic bacteria. Finally, our group has previously described the successful eradication of intra-amniotic inflammation and/or infection in at least 33% of patients with preterm PROM after administration of this specific antibiotic regimen [22].
Another interesting question is the mechanisms by which antibiotics can treat intra-amniotic inflammation [10]. This is not entirely clear. One possibility is that clarithromycin suppresses intra-amniotic inflammation caused by danger signals by down-regulating the expression of pro-inflammatory transcription factors (e.g. activator protein-1 and nuclear factor-ĸB), which can induce the production of pro-inflammatory cytokines [10]. Another possibility is that antibiotics are effective against microorganisms that were not detected via cultivation techniques [15,17,45–47].
For the case herein, we do not know if intra-amniotic inflammation was truly eradicated, because a repeat analysis of amniotic fluid was not performed after antibiotic treatment. However, several points are notable which may provide indirect support that this was the case. First, on the day antibiotic therapy was initiated, the maternal C-reactive protein concentration was elevated, but became normal six days later during treatment and remained as such, even after antibiotics were discontinued. Second, neonatal labs (i.e. complete blood count, blood cultures and C-reactive protein) obtained on the day of delivery were within normal limits. Finally, placental histologic examination did not show evidence of acute inflammatory lesions such as acute chorioamnionitis, funisitis, or chorionic vasculitis. Indeed, sterile intra-amniotic inflammation is known to be associated with acute histologic chorioamnionitis in 40-60% of cases [15–17,48–49]. Instead, chronic lymphoplasmacytic deciduitis was identified, and this may not be surprising. Chronic deciduitis is characterized by the presence of lymphocytes and plasma cells in the basal plate of the placenta, and chronic microbial infection or immune mechanisms have been implicated in its etiology [48,50]. Yet, chronic lymphoplasmacytic deciduitis has also been reported in 9% of placentas delivered from women with term pregnancies and a normal outcome [51].
Conclusions
In conclusion, intra-amniotic inflammation is a poor prognostic factor in the presence of cervical insufficiency. Therefore, we propose that for patients presenting with acute cervical insufficiency, the presence of intra-amniotic inflammation (and/or infection) should be considered, and amniocentesis should be offered to determine the microbial and inflammatory status of the amniotic cavity, particularly when amniotic fluid sludge is present. Recent evidence suggests that antibiotics can be effective in treating intra-amniotic infection/inflammation in the setting of cervical insufficiency. We report herein a case in which administration of antibiotic therapy in the presence of mid-trimester cervical insufficiency, sterile intra-amniotic inflammation, and amniotic fluid sludge was associated with resolution of the cervical findings. Therefore, based on scientific evidence and the outcome of our case, a viable option is to offer antibiotic therapy such as the regimen described herein to such patients.
Supplementary Material
Acknowledgments
This report was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Iams JD, Johnson FF, Sonek J, et al. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–1103. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oh KJ, Romero R, Park JY, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol. 2019;221:140.e1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R, Gonzalez R, Sepulveda W, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–1091. [DOI] [PubMed] [Google Scholar]
- 5.Goodlin RC. Cervical incompetence, hour-glass membranes, and amniocentesis. Obstet Gynecol.1979;54:748–750. [PubMed] [Google Scholar]
- 6.Mays JK, Figueroa R, Shah J, et al. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652–655. [DOI] [PubMed] [Google Scholar]
- 7.Lee SE, Romero R, Park CW, et al. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633.e1–8. [DOI] [PubMed] [Google Scholar]
- 8.Bujold E, Morency AM, Rallu F, et al. Bacteriology of amniotic fluid in women with suspected cervical insufficiency. J Obstet Gynaecol Can. 2008;30:882–887. [DOI] [PubMed] [Google Scholar]
- 9.Airoldi J, Pereira L, Cotter A, et al. Amniocentesis prior to physical exam-indicated cerclage in women with midtrimester cervical dilation: results from the expectant management compared to Physical Exam-indicated Cerclage international cohort study. Am J Perinatol. 2009;26:63–68. [DOI] [PubMed] [Google Scholar]
- 10.Oh KJ, Lee SE, Jung H, et al. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lisonkova S, Sabr Y, Joseph KS. Diagnosis of subclinical amniotic fluid infection prior to rescue cerclage using gram stain and glucose tests: an individual patient meta-analysis. J Obstet Gynaecol Can. 2014;36:116–122. [DOI] [PubMed] [Google Scholar]
- 12.Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339–1345. [DOI] [PubMed] [Google Scholar]
- 13.Yoon BH, Romero R, Park JY, et al. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2019;221:142.e1–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2015;28:1343–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intraamniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R, Kusanovic JP, Espinoza J, et al. What is amniotic fluid ‘sludge’? Ultrasound Obstet Gynecol. 2007;30:793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adanir I, Ozyuncu O, Gokmen Karasu AF, et al. Amniotic fluid “sludge”; prevalence and clinical significance of it in asymptomatic patients at high risk for spontaneous preterm delivery. J Matern Fetal Neonatal Med. 2018;31:135–140. [DOI] [PubMed] [Google Scholar]
- 21.Tsunoda Y, Fukami T, Yoneyama K, et al. The presence of amniotic fluid sludge in pregnant women with a short cervix: an independent risk of preterm delivery. J Matern Fetal Neonatal Med. 2020;33:920–923. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Romero R, Kim SM, et al. A new antibiotic regimen treats and prevents intra-amniotic inflammation/infection in patients with preterm PROM. J Matern Fetal Neonatal Med. 2016;29:2727–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kacerovsky M, Romero R, Stepan M, et al. Antibiotic administration reduces the rate of intraamniotic inflammation in preterm prelabor rupture of the membranes. Am J Obstet Gynecol. 2020;223:114.e1–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rust OA, Atlas RO, Reed J, et al. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098–1105. [DOI] [PubMed] [Google Scholar]
- 26.Kiefer DG, Keeler SM, Rust OA, et al. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200:374.e1–5. [DOI] [PubMed] [Google Scholar]
- 27.Vaisbuch E, Hassan SS, Mazaki-Tovi S, et al. Patients with an asymptomatic short cervix (< or = 15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counselling. Am J Obstet Gynecol. 2010;202:433.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keeler SM, Kiefer DG, Rust OA, et al. Comprehensive amniotic fluid cytokine profile evaluation in women with a short cervix: which cytokine(s) correlates best with outcome? Am J Obstet Gynecol. 2009;201:276.e.1–e6. [DOI] [PubMed] [Google Scholar]
- 29.Kiefer DG, Keeler SM, Rust O, et al. Amniotic fluid inflammatory score is associated with pregnancy outcome in patients with mid trimester short cervix. Am J Obstet Gynecol 2012. 206:68.e1–e6. [DOI] [PubMed] [Google Scholar]
- 30.Jung EY, Park KH, Lee SY, et al. Non-invasive prediction of intra-amniotic infection and/or inflammation in patients with cervical insufficiency or an asymptomatic short cervix (</=15 mm). Arch Gynecol Obstet. 2015.292:579–587. [DOI] [PubMed] [Google Scholar]
- 31.Diago Almela VJ, Martinez-Varea A, Perales-Puchalt A, et al. Good prognosis of cerclage in cases of cervical insufficiency when intra-amniotic inflammation/infection is ruled out. J Matern Fetal Neonatal Med. 2015;28:1563–1568. [DOI] [PubMed] [Google Scholar]
- 32.Matzinger P The danger model: a renewed sense of self. Science. 2002;296:301–305. [DOI] [PubMed] [Google Scholar]
- 33.Ogura Y, Sutterwala FS, Flavell RA. The inflammasome: first line of the immune response to cell stress. Cell. 2006;126:659–662. [DOI] [PubMed] [Google Scholar]
- 34.Pedra JH, Cassel SL, Sutterwala FS. Sensing pathogens and danger signals by the inflammasome. Curr Opin Immunol. 2009;21:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fonseca EB, Celik E, Parra M, et al. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124.e1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Schaudinn C, Kusanovic JP, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135.e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J. 2000;46:S47–S52. [DOI] [PubMed] [Google Scholar]
- 40.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. [DOI] [PubMed] [Google Scholar]
- 41.Davies D Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–122. [DOI] [PubMed] [Google Scholar]
- 42.Witt A, Sommer EM, Cichna M, et al. Placental passage of clarithromycin surpasses other macrolide antibiotics. Am J Obstet Gynecol. 2003;188:816–819. [DOI] [PubMed] [Google Scholar]
- 43.Kafetzis DA, Brater DC, Fanourgakis JE, et al. Ceftriaxone distribution between maternal blood and fetal blood and tissues at parturition and between blood and milk postpartum. Antimicrob Agents Chemother. 1983;23:870–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamb HM, Ormrod D, Scott LJ, et al. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs. 2002;62:1041–1089. [DOI] [PubMed] [Google Scholar]
- 45.DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowlands S, Danielewski JA, Tabrizi SN, et al. Microbial invasion of the amniotic cavity in midtrimester pregnancies using molecular microbiology. Am J Obstet Gynecol. 2017;217:71.e1–e5. [DOI] [PubMed] [Google Scholar]
- 48.Kim CJ, Romero R, Chaemsaithong P, et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213 (4 Suppl):S29–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khong TY, Bendon RW, Qureshi F, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol. 2000;31:292–295. [DOI] [PubMed] [Google Scholar]
- 51.Romero R, Kim YM, Pacora P, et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinat Med. 2018;46:613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


