Abstract
Purpose:
We examined the associations between retinal microvascular density, cognition, and physical fitness in healthy older adults with no reported cognitive decline.
Methods:
Twenty cognitively normal older adults (age: 70.3 ± 4.6 years) were recruited. Both eyes of each subject were imaged using optical coherence tomography angiography. The vessel densities of the retinal vascular network (RVN), superficial vascular plexus (SVP), and deep vascular plexus (DVP) were measured. Cognitive function was assessed using the Mini-mental state examination (MMSE) and Montreal Cognitive Assessment (MoCA), while physical performance was evaluated using the total work during the YMCA cycle ergometer test (TW-YMCA). Spearman correlations (rs) were computed between measures of retinal microvascular density, cognitive function, and physical performance.
Results:
The MoCA was significantly correlated to vessel density of SVD (rs = 0.53, P = 0.02) but not RVN (rs = 0.39, P = 0.09) and DVP (rs = 0.02, P = 0.93). MoCA was not correlated with TW-YMCA (rs = 0.05, P = 0.83). Retinal microvascular densities were not related to TW-YMCA (rs = −0.05 ~ 0.18, P > 0.05). Additionally, MMSE was not related the retinal vessel densities (rs = −0.10 ~ 0.21, P > 0.05) and TW-YMCA (rs = − 0.19, P = 0.41).
Conclusions:
This is the first study to reveal the association between retinal vessel density and cognition as measured with MoCA in healthy older adults with no reported cognitive decline.
Keywords: retinal microvasculature, cognition, older adults, fitness
INTRODUCTION
There are more than 50 million people with dementia worldwide, and there are approximately 10 million new cases every year.1 Dementia predominantly affects older adults and is one of the major causes of disability, leading to a substantial burden on society and high healthcare costs.1 Accumulating evidence indicates that microvascular alterations in the brain, especially at the capillary level,2–4 are one of the major pathogenic contributors to cognitive impairment and dementia in older adults.5,6 These cerebral small vessel alterations have been reported to correlate with cognitive decline.7 The ability to detect cerebral vascular alterations at a micro-vessel level in patients with varying degrees of cognitive impairment would allow monitoring of the disease progression and intervention efficacy.8 However, it is difficult and decidedly expensive to visualize and assess the cerebral microvasculature in vivo directly. Current technologies such as MRI are costly, may not be readily available in all communities, and thus may not be practical for routine screening and early detection of cognitive impairment.9 Furthermore, typical MRI signs of cerebral small vessel disease, such as white matter hyperintensity (WMH) volume, are often nonspecific and present at later stages of small vessel damage.10 Identification of a valid and reliable biomarker for alterations in the cerebral microvasculature would certainly be valuable in early detection, diagnosis, and monitoring disease progression in at-risk older adults and individuals with cognitive impairment.11
The brain and retinal vasculature share similar anatomic and physiologic features.12,13 Alterations of retinal microstructure and microvasculature have been suggested to reflect similar changes occurring in the brain.14 The retinal microvasculature has been used as a proxy in studying cerebral vascular abnormalities such as Alzheimer’s disease.14 With the recent advance of optical coherence tomography angiography (OCTA), direct visualization and further qualification of the retinal microvasculature is possible,15 which provides a window to study the cerebral microvasculature. Wei et al. demonstrated the age-related decline of retinal blood flow velocity and microvessel density as well as intraretinal thickness in a group of the cognitively normal population.15 The age-related thickness changes of the inner retina were also found to relate to the changes in retinal microvessel density during normal aging.15 Similarly, Yu et al. reported decreased retinal vessel density and flow index with aging.16
There is accumulating evidence that cardiovascular fitness can reduce the impact of aging on cognitive function,17–19 brain morphology20,21 and cerebrovascular function.22,23 Additionally, higher levels of cardiovascular fitness are associated with reduced cardiovascular risk, and therefore, increased cerebrovascular density.24,25
To our knowledge, however, no studies have examined the relationships among retinal vessel density, cardiovascular fitness, and cognitive function in presumed cognitively normal older adults. Therefore, the aim of this pilot study was to evaluate the relationships between retinal vessel density, physical performance, and cognitive function in older adults with no known cognitive function decline. We hypothesized that cognitive performance would be associated with (a) physical performance and (b) retinal vessel density.
METHODS
The Institutional Review Board for Human Research at the University of Miami approved the study. After a detailed description of the study was provided to each participant, they read and signed the approved written informed consent form. Healthy older subjects (age > 65 years) were recruited at the Department of Kinesiology and Sports Sciences at the University of Miami.
An ophthalmic examination was conducted by an ophthalmologist (HJ) to confirm eligibility. These examinations included best-corrected visual acuity, intraocular pressure (IOP), and a slit-lamp examination of anterior and posterior segments. Exclusion criteria included the presence of diagnosed dementia or MCI, refractive error greater than ± 6 diopters (D), obvious ocular media opacity, macular degeneration, and glaucoma. Other exclusion criteria were cardiovascular diseases or systemic diseases such as a history of stroke, coagulopathy, and uncontrolled hypertension and diabetes. A total of 20 eligible subjects were enrolled.
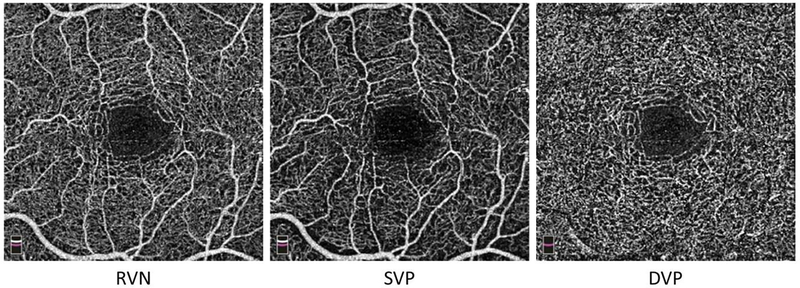
Both eyes of each subject were imaged using an OCTA device (AngioVue, Optovue, Inc., Fremont, CA, USA). The OCTA system is a spectral-domain optical coherence tomography system with a scan speed of 70,000 Å per second and an axial resolution of 5 μm. Retinal angiography and the corresponding tissue volume were obtained using the retinal 3 × 3 mm angiography scan protocol. The macula, centered on the fovea, was imaged. The image quality was set as ≥ 7/10 to ensure the acquisition of high-quality angiography. Angiographic images (i.e., en face view images) of the vascular slabs, including total retinal vascular network (RVN), superficial vascular plexus (SVP), and deep vascular plexus (DVP), were created (Fig. 1).26,27 The SVP includes the ganglion cell and inner plexiform layers, whereas the DVP consists of the inner nuclear and outer plexiform layers. RVN includes both SVP and DVP.
Figure 1. OCTA enface images.
A study subject was scanned at the macula centered on the fovea (i.e., central area with no vessels) with a field of view 3 × 3 mm. Angiographic images (i.e., enface view images) of the vascular slabs, including total retinal vascular network (RVN), superficial vascular plexus (SVP), and deep vascular plexus (DVP), show capillary network around the avascular fovea. Note, there is only the capillary network in the DVP slab, in contrast to RVN and SVP, which mixed capillary network with small vessels (i.e., arterioles and venules).
Image processing to measure the vessel density of these retinal vascular slabs has been reported in detail previously.15 Briefly, the angiographs of the images were analyzed using fractal analysis. The box-counting method was used to yield the fractal dimension (Dbox), representing vessel density. The measurements included vessel density in RVN (RVD), in SVP (SVD), and in DVP (DVD).
Two commonly used brief screening tests were used to assess cognitive function: The mini-mental state examination (MMSE)28 and Montreal Cognitive Assessment (MoCA).29 The MMSE and MoCA both capture cognitive domains of memory, orientation, and construction, but the MoCA has additional visuospatial, executive, and language items and is more sensitive to mild cognitive impairments and reductions in cognitive function resulting from cerebrovascular abnormalities.30 However, the MoCA may be more subject to educational biases, and scores are typically lower than MMSE when both are administered.31,32 To account for this, an adjustment of 1 point is added to the overall score for individuals with ≤12 years of education.33 Both tests were administered in a controlled environment immediately following completion of the health screening and informed consent documents. Participants were recruited based on self-reports of being cognitively normal with no prior diagnosis of mild cognitive impairment or dementia. The MMSE and MoCA were collected at baseline and used as continuous variables in analyses.
The YMCA submaximal cycle ergometer test was used to determine estimated peak oxygen consumption (VO2peak).34 This graded exercise test is designed with three-minute stages, with only very fit individuals completing three stages. Prior to the onset of testing, the test was explained to the subject, and seat and handlebar heights were adjusted to optimize each subject’s mechanical advantage. Each participant began pedaling on a Monark cycle ergometer (Model 839E, Vansbro, Sweden) at 0.5 kg and 50 rpm (150 kpm-min−1). Cadence was maintained by matching sound cues from a metronome. The resistance added at the beginning of each stage was dependent upon the subject’s heart rate response during the first stage. Each stage of the test was performed at 50 rpm. If the participant’s heart rate varied > 5 beats per minute between the last two minutes of each stage, an additional minute was added to that stage. This process was continued until a steady state was reached. If the subject was unable to maintain cadence, or if they were unable to reach a steady state, the test was concluded. Additionally, the test was terminated upon the participant’s request. Two completed stages were required to calculate estimated VO2peak; however, in the current study, eight participants were unable to complete two stages. Therefore, total work (TW-YMCA) was calculated and used to quantify the performance.35 Total work was computed as the product of the distance traveled per pedal cycle (6 m), the cadence (50rpm), and the external load multiplied by the duration for each completed phase. The result was then converted to joules (J) by multiplying by 9.807 J·kg-m−1 and multiplied by 0.001 to convert J to kJ.
Descriptive statistics were obtained, and data analyses were conducted using an SPSS statistical software package (SPSS for Windows 25.0; SPSS Inc, Chicago, Illinois, USA). Shapiro-Wilk test of normality was used to test whether the data are normally distributed. As the MMSE was not normally distributed, we chose to use nonparametric Spearman correlations (rs) to determine the relationships between MMSE, MoCA, TW-YMCA, and ocular variables. Exploratory analysis using partial correlations controlling for age, sex, education, and BMI was also performed to determine the relationships among vessel density, cognition tests, and cardiovascular performance. Significance was set a priori at P < 0.05.
RESULTS
Characteristics of the participants are presented in Table 1. There were no significant differences in any variable between female and male participants (P > 0.05), except for the height (159.7 cm vs. 173.9 cm, P < 0.001) and TW-YMCA (11.7 KJ vs. 22.8 KJ, P = 0.04). Tests of normality showed vessel densities and MoCA were normally distributed, while MMSE was not normally distributed.
Table 1:
Sample Characteristics
| Variable | Mean (SD) or % | Range |
|---|---|---|
| Age, y | 71.2 (5.3) | 63–83 |
| Education, y | 16.5 (2.0) | 12–22 |
| Sex, %F | 55.0 | |
| Body Mass Index (kg/m2) | 29.0 (6.3) | 20.6–43.5 |
| Hypertension, % | 50.0 | |
| Dyslipidemia, % | 55.0 | |
| Heart Disease, % | 20.0 | |
| Alcohol Use, % | 15.0 | |
| SBP (mmHg) | 134 (20) | 101–171 |
| DBP (mmHg) | 79 (10) | 55–97 |
| HR (beat / min) | 63 (12) | 43–84 |
| IOP (mmHg) | 16.6 (2.8) | 10.5–20.5 |
| MAP (mmHg) | 97 (11) | 70.3–116.3 |
| MOPP (mmHg) | 54 (6) | 38.6–64.2 |
| TW-YMCA (KJ) | 16.6 (9.2) | 4.4–35.3 |
| MoCA | 27.6 (1.9) | 23–30 |
| MMSE | 29.7 (0.6) | 28–30 |
| Mean RVD | 1.79 (0.01) | 1.78–1.81 |
| Mean SVD | 1.78 (0.19) | 1.73–1.81 |
| Mean DVD | 1.80 (0.01) | 1.79–1.81 |
KEY: SBP = systolic blood pressure; DBP = diastolic blood pressure; HR = heart rate; IOP = intraocular pressure; MAP = mean arterial pressure; MOPP = mean ocular perfusion pressure; TW-YMCA = Total Work on the YMCA Ergometer; MoCA = Montreal Cognitive Assessment; MMSE = Mini Mental State Examination; RVD = vessel density in retinal vascular network; SVD = vessel density in superficial vascular plexus; DVD = vessel density in deep vascular plexus.
The MoCA was significantly correlated to vessel density of SVD (rs = 0.53, P = 0.02, Table 2) but not RVN (rs = 0.39, P = 0.09) and DVP (rs = 0.02, P = 0.93). MoCA was not correlated with TW-YMCA (rs = 0.05, P = 0.83). Retinal microvascular densities were not related to TW-YMCA (rs = −0.05 ~ 0.18, P > 0.05). Additionally, MMSE was not related the retinal vessel densities (rs = −0.10 ~ 0.21, P > 0.05) and TW-YMCA (rs = - 0.19, P = 0.41).
Table 2:
Correlation and Partial Correlations Between Cognitive and Physical Performance and OCTA Measures
| Spearman Correlation | |||
| Variable | MoCA | MMSE | TW-YMCA |
| TW-YMCA | 0.05 (0.83) | −0.19 (0.41) | 1 |
| Mean RVD | 0.39 (0.09) | 0.21 (0.37) | 0.18 (0.49) |
| Mean SVD | 0.53 (0.02) | −0.10 (0.67) | −0.15 (0.52) |
| Mean DVD | 0.02 (0.93) | 0.12 (0.63) | −0.05 (0.84) |
| Partial Correlations (adjusted for age, sex, education, BMI) | |||
| Variable | MoCA | MMSE | TW-YMCA |
| TW-YMCA | 0.49 (0.06) | −0.30 (0.27) | 1 |
| Mean RVD | 0.75 (0.001) | 0.21 (0.43) | 0.47 (0.07) |
| Mean SVD | 0.73 (0.001) | 0.00 (0.99) | 0.49 (0.05) |
| Mean DVD | 0.11 (0.67) | −0.15 (0.58) | 0.04 (0.89) |
Correlation coefficient (p-value); Bold signifies significance after correction for multiple comparisons. KEY: TW-YMCA = Total Work on the YMCA Ergometer; MoCA=Montreal Cognitive Assessment; MMSE = Mini Mental State Examination; RVD = vessel density in retinal vascular network; SVD = vessel density in superficial vascular plexus; DVD = vessel density in deep vascular plexus.
Analyses using partial correlations (rpartial) after controlling age, sex, education and BMI, revealed that the MoCA was significantly correlated to vessel density of RVN (rpartial = 0.75, P = 0.001) and SVP (rpartial = 0.73, P = 0.001), but not DVP (rpartial = 0.11, P = 0.67). MoCA was not related with TW–YMCA (rpartial = 0.49, P = 0.06). Retinal microvascular densities was not related to TW–YMCA (rpartial= 0.04 ~ 0.49, P > 0.05). Additionally, MMSE was not related to retinal vessel densities (rpartial = −0.15 ~ 0.21, P > 0.05) and TW-YMCA (rpartial = −0.30, P = 0.27).
DISCUSSION
To the best of our knowledge, this is the first study to reveal the positive correlation between retinal microvascular density and MoCA score in cognitively normal older adults without a diagnosis of mild cognitive impairment (MCI) or dementia. This result suggests that retinal microvascular changes could be further developed into a simple and cost-effective marker for evaluating the vascular contributions to cognitive impairment and dementia (VCID). Previous studies have explored the relations between retinal microvasculature and cognition in patients with Alzheimer’s disease (AD), and MCI (Table 3),36–40 and retinal vessel density were found to be positively related to MoCA in patients with AD and MCI,40 and MMSE in patients with AD.36 However, other studies did not support the correlations between retinal vessel density and MMSE in patients with AD38,39,41 or in patients with MCI.37 Few studies have looked solely at cognitively normal older adults. Dissimilarities in study cohorts, imaging techniques, and sample sizes may have contributed to the differences in findings among studies.36–40 The present study adds new information to the field by establishing the relationship between retinal microvascular network density and cognitive performance in cognitively normal older adults.
Table 3.
Retinal microvasculature and cognition in previous studies
| Study | Sample | Age (year) | Cognition | OCTA parameters | Correlation (r) |
|---|---|---|---|---|---|
|
| |||||
| Present Study | 20 cognitively normal older adults | 71.0 ± 5.4 | MoCA | RVD SVD DVD |
RVD, rs = 0.37, P = 0.02 SVD, rs = 0.60, P < 0.001 |
| Criscuolo et al. 201837 | 27 aMCI | 73 ± 6 | MMSE | SCP, DCP, RPC, FAZ | No association |
| Zabel et al. 201939 | 27 AD | 74.1 ± 5.9 | MMSE | DVD, SVD, FAZ | No correlation |
| Lahme et al. 201838 | 36 AD | 68.0 ± 9.3 | MMSE | FD | No correlation |
| Zhang et al. 201940 | 3 eAD 13 aMCI |
73.0 ± 8.2 | MoCA | SVD, peripapillary RPC VLD | SVD, r = 0.36, P = 0.043, PRC VLD, r = 0.46, P = 0.01 |
| Bulut et al. 201836 | 26 ATD | 74.2 ± 7.6 | MMSE | VD FAZ |
VD, r = 0.438, P = 0.001 FAZ, r = −0.531, P < 0.001 |
| Wang et al. 202141 | 62 AD, 47 MCI, and 49 HC | 74.2 ± 7.6 | MMSE Fazekas score | VD | No correlation |
MoCA: Montreal cognitive assessment; RVD: vessel density in the retinal vascular network; SVD: vessel density in the superficial vessel plexus; aMCI: Amnestic Mild Cognitive Impairment; MMSE: the Mini-Mental State Examination test; SCP: superficial capillary plexus; DCP: deep capillary plexus; RPC: radial peripapillary capillary; FAZ fovea avascular zone; AD: Alzheimer’s Disease; MCI: mild cognitive impairment; HC: healthy control; DVD: vessel density in deep retinal vascular plexus; FD: flow density; eAD: early Alzheimer’s Disease; RPC VLD: radial peripapillary capillary vessel length density; ATD: Alzheimer’s type dementia; VD: vessel density.
Both MMSE and MoCA were used in the present study, but only MoCA showed correlations with retinal microvascular network density. This could be attributed to the greater range of cognitive domains testing in the MoCA, including executive, visuospatial, language, and attention tasks,42,43 while the MMSE has an over-representation of orientation questions (i.e., 10 of 30 points) that may not reflect early cognitive changes. We chose to use the MMSE and MoCA in this study because both are often used in clinical practice as quick screening tools for patients with memory complaints.44 The MoCA may provide higher classification accuracy than the MMSE in differentiating MCI from normal age-related changes.44 Moreover, the MoCA has been used to reliably detect cognitive changes in MCI over a 3.5 years period, whereas MMSE could not.45 The MoCA has also been reported to be impacted by age, education, and physical activity in healthy older adults.46
The TW-YMCA was used to evaluate the individual fitness levels in this group of cognitively normal older adults.35 The results showed a trend toward a correlation between MoCA and TW-YMCA but did not reach a significant level. In addition, the TW-YMCA did not correlate with retinal vessel density, although in other studies, fitness levels (measured as running speed) were found to correlate to retinal vessel density determined by the size of the foveal avascular zone in young, healthy adults.47 The lack of correlations between TW-YMCA and retinal vessel densities in the participants enrolled in the current study may be due to the small sample size and the non-homogeneous nature of the sample with respect to physical fitness levels. Several of the participants engaged in daily exercise, whereas others had a sedentary lifestyle. Future large sample studies may reveal the relationships between physical performance and retinal vessel densities in older adults, and especially when using less challenging cardiovascular assessments, like the six-minute walk test, which has previously been used in subjects with MCI.48 Alternatively, baseline activity levels could be measured with a patient-reported outcome such as the Quick Physical Activity Rating to be used as a covariate.49
The correlation between retinal microvasculature and the MoCA scores found in the present study may suggest a potential link between the microvasculature of the retina and brain. Decreased retinal microvascular network density has been reported to be correlated with the Fazekas scale of brain white matter.50 Moreover, Lee et al. recently reported that the density of peripapillary microvascular network was positively correlated with brain cerebral small vessel disease (CSVD) score assessed using 3D brain magnetic resonance imaging (MRI) in patients with subcortical vascular-related cognitive impairment (SCVI).51 CSVD markers such as lacunas, white matter hyperintensity (WMH), and microbleeds are the hallmarks of vascular dementia.52 Hence, the loss of the retinal microvascular network appears to reflect cerebral hypoperfusion in patients with both AD and MCI,53–55 which may also be due to aging and other vascular risk factors.7,8,56,57 Wei et al. demonstrated the decline of retinal vasculature in aged compared to young people.15 Further, similar rates of decline were seen in RVN, SVP, and DVP vessel density (−0.03% to −0.08%) in a subgroup with the age of 35 years or older.58 It has also been shown that the decline rate of retinal vessel density (−0.44% per year in DVP, normalized by tissue volume)58 was about the same as the decline rate in cerebral blood flow (−0.38 to −0.47% per year).59,60 It is worth noting that the annular rate was analyzed in a cohort with a wide age range (≥ 35 years), and the comparison of the comparative rates in the retina and brain were derived from different studies with dissimilar cohorts.58–60 Future studies incorporating simultaneous measures of cerebral and retinal microvascular alterations with aging may provide more accurate information on this topic. Nevertheless, it can be speculated that the decreased retinal microvascular density due to normal aging in the older adult cohort in the present study may reflect cerebral vascular changes. Additionally, the lack of association between retinal vessel density in DVP and cognition in the present study may have been due to the narrow age range and the small size of our sample. Further studies with large sample sizes and a wider age range may validate the position.
There are several limitations to this study. First, the sample size was small, which limited the ability to detect small effect sizes. As a pilot study, this work permits hypothesis generation for a larger study.24,28 Second, we did not study cerebral vasculature. Future studies should simultaneously study both the retinal and cerebral microvascular differences due to aging or fitness levels or changes due to specific interventions such as exercise to help establish the retinal microvascular network as a proxy in monitoring cognitive changes in older adults. Third, we focused on cognitively normal older people and did not include patients with diagnoses of cognitive impairment, although previous studies reported the retinal microvascular alterations in such patients. Three individuals had MoCA scores below the recommended cut-off of 26, suggesting they could have MCI. However, these individuals self-reported no cognitive impairment, had no diagnosis of MCI or dementia, were functionally independent, had normal MMSE, and although they had the lowest MoCA scores, also had the lowest educational attainment in the cohort (12, 14, and 16 years). These factors support the classification of these individuals as cognitively normal. Fourth, our cognitive assessment in this pilot was limited to brief, commonly used screening tests (MoCA and MMSE). Future studies should include a more detailed cognitive assessment. Fourth, when assessing correlations between physical fitness, cognitive performance, and retinal microvasculature, long-term training studies would produce a clearer picture of these relationships. Last, we employed the TW-YMCA as a marker of cardiovascular fitness rather than employing peak oxygen consumption tests (VO2peak).
In summary, this pilot study provides some of the first evidence that retinal microvasculature, measured as the retinal microvascular network density, was positively related to cognition, as determined by the MoCA. This relationship provides evidence that evaluation of microvasculature in the retina could be further developed into a viable and cost-effective tool for examining the contribution of vascular changes to age-related cognitive deficits.
Highlights.
The densities of retinal vessels were measured in older people.
Cognitive function was also measured.
The vessel density was correlated with the cognitive function measured with MoCA.
ACKNOWLEDGMENTS
Financial disclosure: This study was supported by NIH Center Grant P30 EY014801, NINDS 1R01NS111115-01 (Wang), the Ed and Ethel Moor Alzheimer’s Disease Research Program (Florida Health, 20A05, to Jiang) and a grant from Research to Prevent Blindness (RPB). Scholarly activities of Dr. Juan Zhang were supported by a grant from the Research to Prevent Blindness (G20190023).
Sponsor’s Role: The funding sources had no involvement in the design, analysis, or reporting of the results.
Footnotes
Conflict of interest: The authors have no competing interests of conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dementia. World Health Organization; https://www.who.int/news-room/fact-sheets/detail/dementia; accessed 17 August. 2020. [Google Scholar]
- 2.Hunter JM, Kwan J, Malek-Ahmadi M, et al. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PLoS One. 2012;7:e36893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat NR. Vasculoprotection as a Convergent, Multi-Targeted Mechanism of Anti-AD Therapeutics and Interventions. J Alzheimers Dis. 2015;46:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostergaard L, Jespersen SN, Engedahl T, et al. Capillary dysfunction: its detection and causative role in dementias and stroke. Curr Neurol Neurosci Rep. 2015;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11:710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corriveau RA, Bosetti F, Emr M, et al. The Science of Vascular Contributions to Cognitive Impairment and Dementia (VCID): A Framework for Advancing Research Priorities in the Cerebrovascular Biology of Cognitive Decline. Cell Mol Neurobiol. 2016;36:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorelick PB. Prevention of cognitive impairment: scientific guidance and windows of opportunity. J Neurochem. 2018;144:609–616. [DOI] [PubMed] [Google Scholar]
- 9.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma N, Singh AN. Exploring Biomarkers for Alzheimer’s Disease. J Clin Diagn Res. 2016;10:KE01–KE06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patton N, Aslam T, Macgillivray T, et al. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53. [DOI] [PubMed] [Google Scholar]
- 14.Jiang H, Liu Y, Wei Y, et al. Impaired retinal microcirculation in patients withAlzheimer’s disease. PLoS One. 2018;13:e0192154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Jiang H, Shi Y, et al. Age-Related Alterations in the Retinal Microvasculature, Microcirculation, and Microstructure. Invest Ophthalmol Vis Sci. 2017;58:3804–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J, Jiang C, Wang X, et al. Macular perfusion in healthy Chinese: an optical coherencetomography angiogram study. Invest Ophthalmol Vis Sci. 2015;56:3212–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng G, Xia R, Zhou W, Tao J, Chen L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2016;50:1443–1450. [DOI] [PubMed] [Google Scholar]
- 18.Voelcker-Rehage C, Godde B, Staudinger UM. Physical and motor fitness are both related to cognition in old age. Eur J Neurosci. 2010;31:167–176. [DOI] [PubMed] [Google Scholar]
- 19.Mekari S, Dupuy O, Martins R, et al. The effects of cardiorespiratory fitness on executivefunction and prefrontal oxygenation in older adults. Geroscience. 2019;41:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franchetti MK, Bharadwaj PK, Nguyen LA, et al. Interaction of Age and Self-reported Physical Sports Activity on White Matter Hyperintensity Volume in Healthy Older Adults. Front Aging Neurosci. 2020;12:576025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding K, Tarumi T, Zhu DC, et al. Cardiorespiratory Fitness and White Matter Neuronal Fiber Integrity in Mild Cognitive Impairment. J Alzheimers Dis. 2018;61:729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ainslie PN, Cotter JD, George KP, et al. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol. 2008;586:4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maass A, Duzel S, Goerke M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20:585–593. [DOI] [PubMed] [Google Scholar]
- 24.Tarumi T, Zhang R. Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem. 2018;144:595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williamson W, Lewandowski AJ, Forkert ND, et al. Association of Cardiovascular Risk Factors With MRI Indices of Cerebrovascular Structure and Function and White Matter Hyperintensities in Young Adults. JAMA. 2018;320:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toto L, Borrelli E, Di AL, Carpineto P, Mastropasqua R. Retinal vascular plexuses’ changes in dry age-related macular degeneration evaluated by means of optical coherence tomography. Retina. 2016;36:1566–1572. [DOI] [PubMed] [Google Scholar]
- 27.Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am J Ophthalmol. 2015;160:35–44. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method forgrading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 29.Damian AM, Jacobson SA, Hentz JG, et al. The Montreal Cognitive Assessment and the mini-mental state examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dement Geriatr Cogn Disord. 2011;31:126–131. [DOI] [PubMed] [Google Scholar]
- 30.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron D, Flynn K, Verret L, et al. Multicenter Validation of an MMSE-MoCA Conversion Table. J Am Geriatr Soc. 2017;65:1067–1072. [DOI] [PubMed] [Google Scholar]
- 32.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malek-Ahmadi M, Powell JJ, Belden CM, et al. Age- and education-adjusted normative data for the Montreal Cognitive Assessment (MoCA) in older adults age 70–99. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015;22:755–761. [DOI] [PubMed] [Google Scholar]
- 34.Golding LA, Myers CR, Sinning WE: Y’s way to physical fitness. Champaign, IL, Human Kinetics, 1989. [Google Scholar]
- 35.Gordon RS, Franklin KL, Baker JS, Davies B. Determination of aerobic work and power on a rope-braked cycle ergometer by direct measurement. Appl Physiol Nutr Metab. 2006;31:392–397. [DOI] [PubMed] [Google Scholar]
- 36.Bulut M, Kurtulus F, Gozkaya O, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol. 2018;102:233–237. [DOI] [PubMed] [Google Scholar]
- 37.Criscuolo C, Cennamo G, Montorio D, et al. Assessment of retinal vascular network in amnestic mild cognitive impairment by optical coherence tomography angiography. PLoS One. 2020;15:e0233975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lahme L, Esser EL, Mihailovic N, et al. Evaluation of Ocular Perfusion in Alzheimer’s Disease Using Optical Coherence Tomography Angiography. J Alzheimers Dis. 2018;66:1745–1752. [DOI] [PubMed] [Google Scholar]
- 39.Zabel P, Kaluzny JJ, Wilkosc-Debczynska M, et al. Comparison of Retinal Microvasculature in Patients With Alzheimer’s Disease and Primary Open-Angle Glaucoma by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2019;60:3447–3455. [DOI] [PubMed] [Google Scholar]
- 40.Zhang YS, Zhou N, Knoll BM, et al. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer’s Disease on optical coherence tomography angiography. PLoS One. 2019;14:e0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Zhao Q, Tao R, et al. Decreased Retinal Vascular Density in Alzheimer’s Disease (AD) and Mild Cognitive Impairment (MCI): An Optical Coherence Tomography Angiography (OCTA) Study. Front Aging Neurosci. 2020;12:572484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trzepacz PT, Hochstetler H, Wang S, Walker B, Saykin AJ. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. BMC Geriatr. 2015;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam B, Middleton LE, Masellis M, et al. Criterion and convergent validity of the Montreal cognitive assessment with screening and standardized neuropsychological testing. J Am Geriatr Soc. 2013;61:2181–2185. [DOI] [PubMed] [Google Scholar]
- 44.Roalf DR, Moberg PJ, Xie SX, et al. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013;9:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnan K, Rossetti H, Hynan LS, et al. Changes in Montreal Cognitive Assessment Scores Over Time. Assessment. 2017;24:772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Innocenti A, Cammisuli DM, Sgromo D, et al. Lifestyle, Physical Activity and Cognitive Functions: the impact on the scores of Montreal Cognitive Assessment (MoCa). Arch Ital Biol. 2017;155:25–32. [DOI] [PubMed] [Google Scholar]
- 47.Nelis P, Schmitz B, Klose A, et al. Correlation analysis of physical fitness and retinal microvasculature by OCT angiography in healthy adults. PLoS One. 2019;14:e0225769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Makizako H, Shimada H, Doi T, et al. Six-minute walking distance correlated with memory and brain volume in older adults with mild cognitive impairment: a voxel-based morphometry study. Dement Geriatr Cogn Dis Extra. 2013;3:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galvin JE, Tolea MI, Rosenfeld A, Chrisphonte S. The Quick Physical Activity Rating (QPAR) scale: A brief assessment of physical activity in older adults with and without cognitive impairment. PLoS One. 2020;15:e0241641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351356. [DOI] [PubMed] [Google Scholar]
- 51.Lee JY, Kim JP, Jang H, et al. Optical coherence tomography angiography as a potential screening tool for cerebral small vessel diseases. Alzheimers Res Ther. 2020;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2018;134:226–239. [DOI] [PubMed] [Google Scholar]
- 53.Hays CC, Zlatar ZZ, Wierenga CE. The Utility of Cerebral Blood Flow as a Biomarker of Preclinical Alzheimer’s Disease. Cell Mol Neurobiol. 2016;36:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruitenberg A, den HT, Bakker SL, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. [DOI] [PubMed] [Google Scholar]
- 55.Austin BP, Nair VA, Meier TB, et al. Effects of hypoperfusion in Alzheimer’s disease. J Alzheimers Dis. 2011;26 Suppl 3:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Staals J, Booth T, Morris Z, et al. Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol Aging. 2015;36:2806–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gorelick PB. World Stroke Day Proclamation 2015: Call to Preserve Cognitive Vitality. Stroke. 2015;46:3037–3038. [DOI] [PubMed] [Google Scholar]
- 58.Lin Y, Jiang H, Liu Y, et al. Age-Related Alterations in Retinal Tissue Perfusion and Volumetric Vessel Density. Invest Ophthalmol Vis Sci. 2019;60:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen JJ, Rosas HD, Salat DH. Age-associated reductions in cerebral blood flow are independent from regional atrophy. Neuroimage. 2011;55:468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume andoxygen utilization. Normal values and effect of age. Brain. 1990;113 ( Pt 1):27–47. [DOI] [PubMed] [Google Scholar]