Abstract

We developed a highly potent anticancer agent, dolastatinol, which is a methylene hydroxyl derivative of dolastatin 10. Dolastatinol is a synthetic analog of dolastatin 10, synthesized by a solid-phase peptide Fmoc chemistry protocol on 2-chlorotrityl chloride resin utilizing a pH-triggering self-immolative monosuccinate linker. The introduction of the C-terminus hydroxyl methylene functionality preserves the anticancer properties of the parent dolastatin 10, including strong suppression of the cell proliferation, migration, high cytotoxicity. Our research establishes a new facile route toward the further development of C-terminus-modified dolastatin-10-based microtubule inhibitors for anticancer treatment.
Keywords: Dolastatin 10, self-immolative, solid phase peptide synthesis, Fmoc chemistry, inhibition of tubulin polymerization
Dolastatin 10, a linear pentapeptide, is a highly cytotoxic natural product that has been isolated from the marine sea hare, Dolabella Auricularia.1 Its mechanism of action includes tubulin polymerization inhibition, tubulin-dependent guanosine triphosphate hydrolysis, and noncompetitive inhibition of vincristine binding to tubulin.2 Dolastatin 10 exhibits cytotoxicity (subnanomolar range) that is more than two orders of magnitude higher than that of other tubulin-binding drugs from the Taxol and Vinka alkaloid families and remains in the cell longer due to much slower efflux.3,4 Promising preclinical data led to the clinical evaluation of dolastatin 10,5 although it failed in human clinical trials exhibiting severe off-target cytotoxicity.6 Nevertheless, it served as the parent compound for a number of structurally related clinical candidates conjugated to antibodies (antibody drug conjugates (ADCs)): monomethyl auristatin E (MMAE),7,8 monomethyl auristatin F (MMAF),9 auristatin PE (soblidotin),10 and N-terminus 2-aminoisobutyric acid (Aib) acestatin D.11 Still, dolastatin 10 is considered one of the most cytotoxic peptides among the dolastatin family.12
Dolastatins contain unusual amino acids, dolaisoleucine (Dil) and dolaproine (Dap), assembled to valine and dimethyl valine amino acids (Figure 1). For instance, MMAE contains the carboxy-terminal amine norephedrine and the four amino acids monomethylvaline (MeVal), valine (Val), Dil, and Dap. In addition, MMAF differs from MMAE by the C-terminal substitution of norephedrine with phenylalanine (Phe), soblidotin lacks the C-terminus carboxylic group, and dolastatin 10 possesses a thiazole ring at the C-terminus instead of carboxyl.
Figure 1.

Structures of dolastatin 10, MMAE, MMAF, acestatin D, soblidotin, and dolastatinol.
Evidently, dolastatin 10 is a natural product and therefore suffers from the common disadvantages of drugs with a natural origin such as the low synthetic feasibility associated with structure activity relationship (SAR)-related structural modifications.13 The idea of facile synthesis is to circumvent some of the inherent disadvantages of this biologically active natural product.
Here we report the discovery and solid-phase peptide synthesis (SPPS) of a novel highly cytotoxic dolastatin 10 derivative, dolastatinol, that structurally differs from its parent dolastatin 10 by a methylene hydroxyl tether on a thiazole ring at the C-terminus. Such a modification minimally modifies the structure of dolastatin 10, preserving the medicinally important dimethylated N-terminus.
The synthesis began with the preparation of Boc-protected unusual building units (BUs), dolaproine N-Boc-Dap 6a and dolaisoleucine N-Boc-Dil 6b (Scheme 1). Several reported synthetic routes were applied for the synthesis of 6a,b,14−16 but the most successful procedure was based on Wang et al.’s work17 employing as a key step crotylation of N-Boc-l-prolinal with potassium (Z)-2-butenyltrifluoroborate and allylation of N-Boc-l-isoleucinal with potassium allyltrifluoroborate, respectively. The obtained intermediates were first subjected to the dihydroxylation of olefins in the presence of a catalytic amount of OsO4 followed by the addition of NaIO4 to cleave the vicinal diols, finally furnishing Boc-protected 6a and 6b BUs, respectively, with correct stereochemistry.
Scheme 1. Synthesis of Fmoc Building Units 2a,b.
TFA/DCM (1:1), 30 min, 0 °C.
Fmoc-Cl, DIPEA, DCM, 0 °C to rt, 10 h.
Furthermore, 6a,b were simply transformed into the related Fmoc counterparts 2a,b in good yields by two sequential steps: the removal of Boc in acidic media (trifluoroacetic acid (TFA)/dichloromethane (DCM) 1:1) and the subsequent Fmoc protection of the residue by Fmoc chloride (Fmoc-Cl, DIPEA, DCM). The 1H and 13C nuclear magnetic resonance (NMR) spectra of 2a,b18 indicate that each compound exists as one stereoisomer with two conformers in a 3:2 ratio. (See the Supporting Information.) The full analysis was carried out based on 2D experiments, 1H-1H correlation (COSY) and 1H-13C correlation, heteronuclear multiple quantum correlation (HMQC) (through one bond), and heteronuclear multiple bond correlation (HMBC) (through two to three bonds) at 280 (2a) and 295 K (2b). The nuclear Overhauser enhancement spectroscopy (NOESY) of both conformers of Fmoc-protected 2b shows an NOE correlation between the β proton to the carboxylic group (major: δ 3.93; minor: δ 3.73) and the methyl group in position δ (major: δ 0.96; minor δ 0.75). In addition, there was an NOE correlation between the methyl group on the nitrogen (major: δ 2.74; minor δ 2.71) and the δ proton to the carboxylic group (major: δ 1.93; minor δ 1.73). In the NOESY experiment of compound 2a at 280 K, there were positive correlations between the two conformers, which clearly indicate a dynamic process of at least two conformers of one stereoisomer. Remarkably, it was recently reported that in solution, half of the MMAF molecules are locked in an inactive conformation, severely decreasing their efficiency and potentially increasing the risk of side effects.18 It was noted that a predominant cis conformer (55%) was due to a partially hindered rotation around the dolaproline–dolaisoleucine amide bond. We found by NMR studies (Supporting Information) that Fmoc-dolaproine exists as one stereoisomer with two cis/trans conformers related to the Fmoc carbamate group. The defined ratio of 3:2 was in agreement with the reported results for the dolaproine–dolaisoleuine amide bond in MMAF. Such a finding may contribute to the design of optimized dolastatin-related AA building blocks and peptide derivatives.
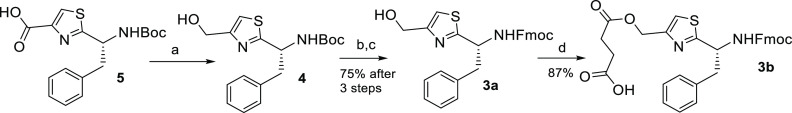
In the next step, we prepared two thiazole-containing building blocks 3a and 3b from the reported 5 (Scheme 2).19 The idea was to compare the loading capacity onto the 2-chlorotrityl resin of 3a through HO versus 3b through CO2H and the resulting impact on the synthetic yield of dolastatinol. We assumed that dolastatinol monosuccinate 1 (derived from 3b) containing a self-immolative monosuccinate linker would display the cascade mechanism of release of dolastatinol after a basic pH stimulus. The direct preparation of peptidols on Barlos’ 2-chlorotrityl resin was previously reported.20 Moreover, trityl ethers derived from alcohols or Fmoc amino acid alcohols can be easily formed in solution under mild reaction conditions.24 However, the esterification of the 2-chlorotrityl chloride (Cl-Trt) resin with Fmoc amino acids proceeds quickly without byproduct formation.21−23 Thus 5 was subjected to reduction of the carboxylic group using an asymmetrical anhydride method with ethyl chloroformate and NaBH4 as the reducing reagent. This reaction proceeded smoothly (thin layer chromatography (TLC) monitoring) to afford 4, and after the subsequent removal of Boc (TFA/DCM) and following Fmoc protection (Fmoc-OSu), the BU 3a was obtained in good yield (75% after three steps). The reaction of 3a with succinic anhydride in tetrahydrofuran (THF) and N,N-diisopropylethylamine (DIEA) in the presence of catalytic 4-dimethylaminopyridine (DMAP) finally yielded 3b in plausible yield (87%).
Scheme 2. Synthesis of Fmoc-Protected Thiazole Containing Building Units 3a and 3b.
NaBH4, TEA, ethyl chloroformate, THF, 30 min.
TFA/DCM (1:1), 0 °C, 30 min.
Fmoc-OSu, 1N Na2CO3/MeOH (1:2), 0 °C; 1 h.
Succinic anhydride, DIEA, DMAP (cat), THF, rt, 3 h.
Having all necessary Fmoc-protected BUs in hand, we moved to the SPPS of dolastatinol and 1 (Scheme 3) on 2-chlorotrityl resin (substitution level, 1.0 to 1.8 mmol/g, 100 mg). In our study, a commercial 2-chlorotrityl resin leads to a somewhat lower loading with Fmoc-protected amino acid alcohol 3a even after using a binary mixture of DCM and dimethylformamide (DMF) with pyridine as a base.25 Loading of ∼0.40 mmol/g was obtained after 48 h; however, the monosuccinate building unit 3b bearing the superior nucleophile carboxylate reacted smoothly with 2-chlorotrityl resin under standard loading conditions (Scheme 3). The Fmoc chemistry protocol was applied to synthesize both peptides utilizing 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b] pyridinium 3-oxide hexafluorophosphate (HATU) as a coupling reagent of choice in DMF with the assistance of DIEA as a base. The N-terminal Fmoc group was removed using 20% piperidine in DMF (5 mL), releasing the primary amine toward the consequent assembly of peptides. The synthesis of dolastatinol and 1 was accomplished by the coupling of the final amino acid (Me)2Val followed by cleavage under mild acidic conditions from the solid support (cold TFA/triisopropylsilane (TIS)/DCM, 2.5:2.5:95), affording, after purification (semipreparative high-performance liquid chromatography (HPLC), Supporting Information) and high-resolution mass spectrometry (HRMS) detection, the final dolastatinol and 1, respectively. As anticipated, 1 was obtained in a higher yield (46%) than dolastatinol (26%) due to the utilization of more of the nucleophilic carboxylate group in 3b instead of the hydroxyl in 3a upon loading onto the resin. Furthermore, the conversion of 1 to dolastatinol under mild basic conditions (1 N Na2CO3/methanol (MeOH) 1:4) at room temperature was studied (Figure 2A). The results show the full degradation of 1 and the release of dolastatinol in high purity (>98%) within 70 h (Figure 2B) with an estimated t1/2 = 36 h. The experiment was monitored by liquid chromatography–mass spectrometry (LC-MS). The kinetics of the dolastatinol release process is demonstrated by pick conversion (Figure 2C). After completion, the basic reaction mixture was acidified to pH 6.8 (cold 0.5 N HCl), lyophilized, and redissolved in THF. The precipitated salt was filtered out, and the solvent was evaporated, affording pure dolastatinol that was used for biological studies without any further purification. The optimization of the synthetic yields is currently under investigation in our lab.
Scheme 3. Synthesis of Dolastatinol and Its Monosuccinate Acid Derivative 1.

(i) 3a, Py/DMF (1:1), rt; 48 h; (ii) 20% piperidine in DMF.
(i) 2a, HATU, DIEA, DMF, rt; (ii) 20% piperidine in DMF.
(i) 2b, HATU, DIEA, DMF, rt; (ii) 20% piperidine in DMF.
(i) Fmoc-Val-OH, HATU, DIEA, DMF, rt; (ii) 20% piperidine in DMF.
(Me)2Val-OH, HATU, DIEA, DMF, rt.
2.5:2.5:95 TFA/TIS/DCM, 0 °C then rt, 30 min.
3b, DIEA, DCM, rt, 1 h.
1 N Na2CO3 in MeOH (1:4) rt.
Figure 2.
Degradation of 1 and release of dolastatinol (DOLA). (A) Mechanism of release of DOLA. (B) Degradation of 1/release of DOLA plots. (C) LC-MS measurements of DOLA release kinetics. The reaction mixture was prepared by dissolving 10 mg of 1 in 2.5 mL of 1 N Na2CO3/MeOH (ratio 1:4) and incubated at room temperature (25 °C). Throughout the incubation period, aliquots were taken at the desired time points and analyzed by LC-MS. The experiment was repeated twice.
The microtubule inhibition assay was performed on triple-negative breast cancer MDA MB 231 cells to assess the anticancer activities of dolastatinol.26,27 We compared the efficiency of dolastatinol in the destabilization of microtubule dynamics vis-à-vis another approved microtubule destabilizer MMAF. These microtubule-targeting drugs alter the “dynamic instability” of microtubule networks in the interphase and nucleation of tubulin dimers into spindle fibers necessary for mitosis, and hence they are widely used as anticancer drugs.28 The immunofluorescence analysis of cells treated with these drugs showed that the microtubule networks in interphase cells diminish in a concentration-dependent manner, as shown in Figure 3. In MMAF treatment, the disruption of microtubules started at 125 nM and can be clearly observed at 625 nM concentration. However, dolastatinol treatment disrupted the microtubules even at 5 nM, and the damage can be clearly seen at 25 nM concentration. Hence, dolastatinol is a significantly more potent inhibitor of microtubule “dynamic instability” compared with MMAF.
Figure 3.
(A) Microtubule inhibition assay: Immunofluorescence image of the MDA MB 231 cells treated with drugs, monomethyl auristatin F (MMAF) dolastatinol (DOLA), at 5, 25, 125, and 625 nM concentration. The green fibrous structure depicts the microtubule fiber inside the cell. The nucleus was stained blue with 4′,6-diamidino-2-phenylindole (DAPI). Treatment with drugs was carried out for 24 h. The scale bar depicts a length of 12.5 μm. (B) Quantification of data presented in Figure 3A. The two-tailed P value was calculated by the unpaired t test.
In interphase cells, mitochondria are transported toward the positive end of the microtubule by molecular motor kinesin-129,30 and toward the negative end by dynein.31 The association of mitochondria with intact microtubules is necessary to maintain the mitochondrial dynamics, and the disruption of microtubule networks leads to mitochondrial fission.32 Accordingly, we stained the mitochondria with MitoTracker and followed them using fluorescence microscopy. Treatment with dolastatinol or MMAF led to the distribution of mitochondria, so that they lost their filamentous shape and disintegrated into smaller mitochondrial bodies (Figure 4). Dolastatinol was significantly more potent in mitochondrial disintegration compared with MMAF. Indeed, the full disruption of mitochondria was seen at 25 nM dolastatinol, whereas with MMAF, we did not observe full disruption, even at 625 nM (Figure 4). Therefore, the effective disruption of microtubules with dolastatinol affects vital cell functions even in the interphase.
Figure 4.
Fluorescence image (40×) shows the distribution of mitochondria within the cytoplasm of MDA MB 231 cells treated with MMAF or dolastatinol (DOLA) for 24 h. Small mitochondria following fission appear in the cells treated with drugs (red arrow). The scale bar depicts a length of 12.5 μm.
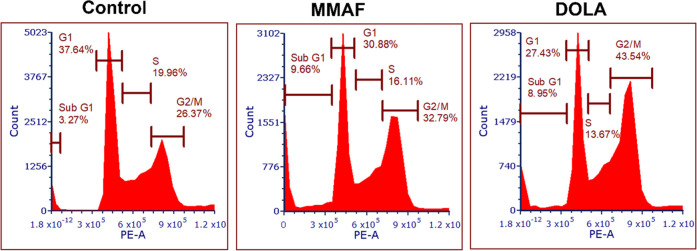
The inhibition of microtubules also impedes the progression of cells through the cell cycle. The effects of dolastatinol and MMAF on the cell-cycle progression were compared in MDA MB 231 cells using fluorescence-activated cell sorting (FACS) analysis. As expected, treatment with dolastatinol and MMAF increased the population of cells halted at the G2/M phase, as the drug inhibited the formation of spindle fibers and the disjunction of chromosomes. These data were in agreement with our fluorescence microscopy study, which showed that the 125 nM dolastatinol destabilizes the microtubule filaments to a greater extent than MMAF, and hence the percentage of observed population halted at the G2/M phase was higher in cells treated with dolastatinol (43.5%) compared with that of cells treated with MMAF (32.7%) (Figure 5).
Figure 5.
Cell-cycle stage analysis shows the increase in the population of MDA MB 231 cells in the G2/M phase following treatment with 125 nM MMAF and dolastatinol (DOLA). Cells were synchronized through serum starvation for 48 h and then treated for 24 h with the drugs before the analysis.
Microtubules along with actin filaments help the cell attachment, polarization, motility, and invasion, all of which are involved in cancer metastasis.33,34 Hence we compared the effects of these drugs on the migration of cells using an in vitro “wound healing” assay. The cells were grown overnight and then treated with 5 nM of either MMAF or dolastatinol under serum-starved conditions. Then scratch was done, and the cells were allowed to migrate for 24 h in the absence of drugs in complete medium. Drug treatments slowed down the migration of cells and the filling of the wound area (Figure 6A,B). As with other assays, dolastatinol inhibited the migration to a comparatively greater extent than MMAF (Figure 6B).
Figure 6.
(A) Microscope image (10×) of MDA MB 231 cells treated with 5 nM of MMAF or dolastatinol (DOLA) for 48 h in a serum-starved Dulbecco’s modified Eagle’s medium (DMEM) culture medium followed by migration into the wound. (B) Quantification of data presented in Figure 6A. The two-tailed P value was calculated by unpaired t test.
Next, we assessed the in vitro cytotoxicity of dolastatinol versus MMAF. Cells were treated with increasing concentrations of dolastatinol or MMAF, and their viability was monitored using the XTT assay to determine the half-maximal inhibitory concentration (IC50). The viability assay was carried out with three human breast cancer cell lines: MDA MB 231 (triple-negative), BT474 (HER2-positive), and SKBR3 (HER2-positive).8,26
The metabolic activities of the cells were measured after incubation for 96 h. The treatment of all three cell lines with dolastatinol showed a significantly reduced viability compared with that with MMAF, with the IC50 ranging between 0.95 and 2.3 nM for dolastatinol (Figure 7A–C). Importantly, dolastatinol exhibited single-digit nanomolar potency on the triple-negative MDA MB 231 cell line (IC50 = 1.54 nM), whereas MMAF was not active (Figure 7A).
Figure 7.

Dose-dependent cytotoxicity of dolastatinol (DOLA) and MMAF in (A) MDA MB 231, (B) BT474, and (C) SKBR3 cell lines. IC50 values were obtained by using a four-parameter variable slope and best-fit values. Experiments were conducted in technical replicates (n = 4). The data shown are representative of three independent experiments. All measurements were normalized to the 1% dimethyl sulfoxide (DMSO) controls. For each cell assay, 5000 cells/well were seeded, allowed to adhere overnight, and incubated with the compounds for 96 h in complete medium.
In summary, we report the development of dolastatinol, a highly potent synthetic microtubule-inhibiting dolastatin 10 analog. It was synthesized by a facile SPPS protocol on standard Cl-Trt resin utilizing a self-immolative monosuccinate linker in higher yields than using a direct method through the formation of trityl ether. Upon mild basic stimulation in solution, the obtained dolastatinol monosuccinate releases dolastatinol quantitatively in high purity. The addition of the methylene hydroxyl extension to the C-terminus did not affect the potency of dolastatinol. Our study suggests that dolastatinol induced the disruption of microtubules even at extremely low concentrations, and the potency is significantly higher than that of the commercially available MMAF. Moreover, dolastatinol also induced the mitotic arrest of MDA MB 231 cells at G2/M and increased the mitochondria disintegration. Dolastatinol prevented the migration of cells into the wound area, which indicates its antimetastatic potential. Being highly potent and cytotoxic to both HER2-positive and triple-negative breast cancer cells in the low nanomolar range makes dolastatinol a highly potent drug candidate for the treatment of cancer. Notably, dolastatinol, unlike dolastatin 10, contains a C-terminus methylene hydroxyl group that can potentially be used for conjugation to various carriers for targeted drug delivery.
Experimental Procedures
The amino acids Fmoc-Val-OH and (Me)2Val-OH were purchased from Sigma-Aldrich. Other amino acids, the coupling reagent HATU, and the Cl-Trt resin were purchased from Bachem (Israel). MMAF (CAS: 745017-94-1) was purchased from TBD-Biodiscovery (Estonia). The reagents MeOH, DCM, DMF, acetonitrile (ACN), and anhydrous ethyl ether were obtained from BioLab (Israel). TFA, DMSO, TIS, piperidine, and DIEA were purchased from Sigma-Aldrich (Israel). All reagents and chemicals were ACS grade or better and were used without further purification. Chemical reactions were monitored by TLC (silica gel 60 F-254, Merck) and LC-MS. LC-MS analyses were performed on an Agilent Technologies 1260 Infinity (LC) 6120 quadruple (MS) apparatus with an Inertsil ODS-4 column (2 μm, 3.0 × 100 mm) with the following conditions: column temperature 50 °C, eluent: water—ACN containing 0.1% of formic acid, UV detection at 270, 254, and 214 nm. HRMS was performed in electrospray ionization (ESI) positive mode by using an Agilent 6550 iFunnel quadrupole time-of-flight (Q-TOF) LC-MS instrument. HPLC purifications were carried out on an ECOM preparative system.
Peptide Synthesis
Peptides were manually synthesized on a Cl-Trt resin (100–200 mesh, 1.0 to 1.8 mmol/g loading) using the standard Fmoc chemistry protocol.27 The syntheses involved two repeated cycles: (1) deprotection of the Fmoc group using 20% piperidine in DMF (2 × 15 min, 5 mL each) and subsequent washing (2× CH2Cl2, 2× DMF, 5 mL each) and (2) coupling of the protected amino acid or last dimethyl valine (2.5 equiv each) using HATU (2.5 equiv) and DIEA (5.1 equiv) in DMF (5 mL) for 1.5 h. The coupling and deprotection reactions were monitored using the ninhydrin colorimetric assay or LC-MS (sample cleavage), and the reactions were repeated if necessary. The peptides were cleaved from the resin using a cold solution of TFA/TIP/DCM (2.5:2.5:95) (4 mL) for 1 h. The peptides were assayed as TFA salts and not amended to the peptide content. The yields of pure peptides were calculated according to the loading determined by the substitution of the first Fmoc-AA. Crude peptides were purified using ECOM preparative system, with dual UV detection at 254 and 214 nm. The Phenomenex Gemini 10 μm RP18 110 Å, LC 250 × 21.2 mm column was used. The column was kept at 25 °C. Eluents A (0.1% formic acid in water) and B (0.1% formic acid in ACN) were used. A typical elution was a gradient from 100% A to 100% B over 35 min at a flow rate of 25 mL/min.
Microtubule Inhibition Assay
MDA MB 231 cells were seeded in a 96-well plate with a density of 10 000 cells/well and allowed to grow for 24 h to get 70–80% confluency. Thereafter, wells were decanted, and cells were treated with 5, 25, 125, and 625 nM of paclitaxel (PTX), MMAF, and dolastatinol in DMEM culture medium for 24 h. After treatment with these drugs, the cells were washed with Dulbecco’s phosphate-buffered saline (DPBS) twice and fixed with 4% paraformaldehyde in DPBS for 10 min. After 10 min of fixation, the cells were washed with phosphate-buffered saline (PBS) and permeabilized with 0.2% Triton X-100 solution in DPBS for 10 min at room temperature. Again, cells were washed with PBS and blocked with 5% bovine serum albumin (BSA) in PBST (0.05% Tween 20 in PBS) solution at room temperature for 1 h. Then, cells were washed again twice with PBST. The cells were incubated with 1:800 diluted (in 5% BSA in PBST) primary antibody α-tubulin (DM1A, mouse, Cell Signaling) at 40 °C for 16 h. Thereafter, cells were washed five times with PBST and treated with 1:800 diluted (in 5% BSA in PBST) secondary antibody labeled with Alexa Fluor 488 (donkey antimouse, H+L, Invitrogen) for 1 h. The cells were washed eight times with PBST to remove the unbound secondary antibody. The cells were treated with 1:10 000 diluted (in PBS) DAPI for 10 min to visualize the nuclei and then washed three times with PBST to remove the excess of DAPI. One percent sodium azide solution in PBS was added to the wells, and the cells were imaged through a fluorescence microscope (WiScan Hermes) at 40× objective.
MitoTracker Assay
The MDA MB 231 cells were seeded in a 96-well culture plate in a number of 7000 cells/well. The cells were allowed to grow overnight in a DMEM culture medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic solution. The next day, the wells were decanted and filled with culture medium containing 5, 25, 125, and 625 nM MMAF and dolastatinol. The cells were treated with drugs for 24 h; thereafter, cells were washed with DMEM complete medium without phenol red or serum. Then, the cells were treated with 75 nM MitoTracker (MitoTracker Green FM, Abcam) dissolved in DMEM without phenol red or serum for 30 min. The excess MitoTracker was removed, and the medium was replaced with 1000× diluted DAPI (stock: 1 mg/mL) in a DMEM culture medium without phenol red or serum. The cells were incubated for 15 min. after which the medium was replaced by blank DMEM without phenol red or serum for live-cell imaging using a fluorescence microscope (WiScan Hermes) at 40× objective.
Cell-Cycle Stage Analysis
The MDA MB 231 cells were grown in a T25 flask in a number of 1 × 106 cells/flask for 24 h; then, the cells were synchronized to the G1 phase through serum starvation for 48 h in DMEM culture medium without fetal bovine serum. Thereafter, the cells were treated with 125 nM MMAF and dolastatinol for 24 h. Then, the cells were washed with molecular-biology-grade PBS and isolated through trypsinization (0.025% trypsin). Thereafter, the cells were washed once with ice-cold PBS and fixed with 70% ethanol with continuous vortexing to get a single cell suspension and kept at −20 °C overnight. The next day, the fixed cells were centrifuged, and a pellet was resuspended in 0.5 mL of staining solution containing 0.1% (v/v) Triton X-100 with 0.2 mg/mL DNase-free RNase A and 0.02 mg/mL propidium iodide. The cells were incubated for 20 min at room temperature then analyzed by flow cytometry for the cell-cycle stages.
Migration Test (Well Scratch Method)
The MDA MB 231 cells were seeded in 3 × 104 cells/well in a 96-well culture plate and kept overnight for the attachment. The next day, cells were treated with 5 nM MMAF and dolastatinol dissolved in DMEM culture medium without serum for 48 h. Thereafter, the wells were decanted and scratched with 10 μL of sterile microtip. The cellular debris generated due to scratching was washed with PBS, and the wells were filled with a complete culture medium. The cells were imaged at this time point (0 h) using a light microscope (Olympus microscope) at 10× objective. After imaging, the cells were incubated for another 24 h, and the images were recorded again to analyze the percentage covering of the wound.
Cytotoxicity Test (XTT)
The cytotoxicity of the dolastatinol and MMAF was determined by measuring the mitochondrial enzyme activity using a commercial XTT assay kit. All samples contained DMSO at a final concentration of <0.05%. The cells were cultured in micro wells at 5 × 104 cells/mL and incubated for 96 h with different concentrations of the tested substances. After the incubation period, the cultures were washed and then provided with fresh medium. Then, 50 μL of freshly prepared XTT reagent (XTT Cell Viability Assay, Cell Signaling, Beverly, MA) was added, and the cells were reincubated for an additional 3 h. During that time, the absorbance of each well was measured at 450 nm using an Infinite 200 PRO Tecan apparatus (TECAN, Switzerland). The difference between these measurements was used to calculate the percent growth inhibition (GI) in test wells compared with that in untreated cells (control). All of the tests were done in tetraplicate; each experiment was conducted three times. Statistical Analysis: The results of the in vitro drug efficacy studies were analyzed using GraphPad Prism. For the XTT (viability) assay, the mean and standard error of the mean were determined, with each assay completed in triplicate. The IC50 was calculated using the log (inhibitor) versus normalized response—variable slope function in GraphPad Prism.
Acknowledgments
This work was supported by the Israel Science Foundation (ISF), project 810/18 and Ariel University. The authors thank Helena Tuchinsky, Ariel University for technical support.
Glossary
Abbreviations
- DOLA
dolastatinol
- MMAF
monomethyl auristatin F
- HER2
human epidermal growth factor receptor 2
- TIS
triisopropylsilane
- HRMS
high-resolution mass spectrometry
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.1c00432.
Synthetic procedures and selected MS, 1H, and 13C NMR spectra (PDF)
Author Contributions
H.G. and O.D. were involved in the study design and synthesis and performed analytical experiments, data analysis, interpretation, and drafting of the manuscript. A.B. and V.M. designed, performed, and analyzed all spectroscopical and fluorescent experiments and were involved in drafting the manuscript. C.P. designed, performed, and interpreted all foci experiments and drafted the manuscript. A.H. helped to design, perform, and interpret cell-based studies. L.P. and G.G. designed the study, reviewed experiments, and drafted, edited, and reviewed the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Pettit G. R.; Kamano Y.; Herald C. L.; Tuinman A. A.; Boettner F. E.; Kizu H.; Schmidt J. M.; Baczynskyj L.; Tomer K. B.; Bontems R. J. The isolation and structure of a remarkable marine animal antineoplastic constituent: dolastatin 10. J. Am. Chem. Soc. 1987, 109 (22), 6883–6885. 10.1021/ja00256a070. [DOI] [Google Scholar]
- Bai R.; Pettit G. R.; Hamel E. Structure-activity studies with chiral isomers and with segments of the antimitotic marine peptide dolastatin 10. Biochem. Pharmacol. 1990, 40, 1859–1864. 10.1016/0006-2952(90)90367-T. [DOI] [PubMed] [Google Scholar]
- Verdier-Pinard P.; Kepler J. A.; Pettit G. R.; Hamel E. Sustained intracellular retention of dolastatin 10 causes its potent antimitotic activity. Mol. Pharmacol. 2000, 57, 180–187. [PubMed] [Google Scholar]
- Aherne G. W.; Hardcastle A.; Valenti M.; Bryant A.; Rogers P.; Pettit G. R.; Srirangam J. K.; Kelland L. R. Antitumour evaluation of dolastatins 10 and 15 and their measurement in plasma by radioimmunoassay. Cancer Chemother. Pharmacol. 1996, 38 (3), 225–232. 10.1007/s002800050475. [DOI] [PubMed] [Google Scholar]
- Beckwith M.; Urba W. J.; Longo D. L. Growth Inhibition of Human Lymphoma Cell Lines by the Marine Products, Dolastatins 10 and 15. JNCI J. Nat. Canc. Inst. 1993, 85 (6), 483–488. 10.1093/jnci/85.6.483. [DOI] [PubMed] [Google Scholar]
- Vaishampayan U.; Glode M.; Du W.; Kraft A.; Hudes G.; Wright J.; Hussain M. Phase II. Study of dolastatin-10 in patients with hormone-refractory metastatic prostate adenocarcinoma. Clin. Canc. Res. 2000, 6, 4205–4208. [PubMed] [Google Scholar]
- Francisco J. A.; Cerveny C. G.; Meyer D. L.; Mixan B. J.; Klussman K.; Chace D. F.; Rejniak S. X.; et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 2003, 102 (4), 1458–1465. 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- Li W. Q.; Guo H. F.; Li L. Y.; Zhang Y. F.; Cui J. W. The promising role of antibody drug conjugate in cancer therapy: Combining targeting ability with cytotoxicity effectively. Cancer Med. 2021, 10 (14), 4677–4696. 10.1002/cam4.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doronina S. O.; Mendelsohn B. A.; Bovee T. D.; Cerveny C. G.; Alley S. C.; Meyer D. L.; Oflazoglu E.; Toki B. E.; Sanderson R. J.; Zabinski R. F.; Wahl A. F.; Senter P. D. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjugate Chem. 2006, 17, 114–124. 10.1021/bc0502917. [DOI] [PubMed] [Google Scholar]
- Miyazaki K.; Kobayashi M.; Natsume T.; Gondo M.; Mikami T.; Sakakibara K.; Tsukagoshi S. Synthesis and antitumor activity of novel dolastatin 10 analogs. Chem. Pharm. Bull. 1995, 43, 1706–1718. 10.1248/cpb.43.1706. [DOI] [PubMed] [Google Scholar]
- ACES Pharma. http://www.acespharma.com/ADC_services_Technology_products.html (accessed 21-07-2021)
- Maderna A.; Doroski M.; Subramanyam C.; Porte A.; Leverett C. A.; Vetelino B. C.; Chen Z.; Risley H.; Parris K.; Pandit J.; Varghese A. H.; Shanker S.; Song C.; Sukuru S. C. K.; Farley K. A.; Wagenaar M. M.; Shapiro M. J.; Musto S.; Lam M.-H.; Loganzo F.; O’Donnell C. J. Discovery of Cytotoxic Dolastatin 10 Analogues with N-Terminal Modifications. J. Med. Chem. 2014, 57 (24), 10527–10543. 10.1021/jm501649k. [DOI] [PubMed] [Google Scholar]
- Maier M. E. Design and synthesis of analogues of natural products. Org. Biomol. Chem. 2015, 13 (19), 5302–5343. 10.1039/C5OB00169B. [DOI] [PubMed] [Google Scholar]
- Dugal-Tessier J.; Barnscher S. D.; Kanai A.; Mendelsohn B. A. Synthesis and Evaluation of Dolastatin 10 Analogues Containing Heteroatoms on the Amino Acid Side Chains. J. Nat. Prod. 2017, 80 (9), 2484–2491. 10.1021/acs.jnatprod.7b00359. [DOI] [PubMed] [Google Scholar]
- Cella R.; Venturoso R. C.; Stefani H. A. Stereoselective synthesis of the dolastatin units by organotrifluoroborates additions to a-amino aldehydes. Tetrahedron Lett. 2008, 49, 16–19. 10.1016/j.tetlet.2007.11.031. [DOI] [Google Scholar]
- Pettit G. R.; Grealish M. P. A cobalt-phosphine complex directed Reformatsky approach to a stereospecific synthesis of the dolastatin 10 unit dolaproine (Dap). J. Org. Chem. 2001, 66, 8640–8642. 10.1021/jo010530t. [DOI] [PubMed] [Google Scholar]
- Wang X.; Dong S.; Feng D.; Chen Y.; Ma M.; Hu W. Synthesis and biological activity evaluation of dolastatin 10 analogues with N-terminal modifications. Tetrahedron 2017, 73, 2255–2266. 10.1016/j.tet.2017.03.006. [DOI] [Google Scholar]
- Johansson M. P.; Maaheimo H.; Ekholm F. S. New insight on the structural features of the cytotoxic auristatins MMAE and MMAF revealed by combined NMR spectroscopy and quantum chemical modelling. Sci. Rep. 2017, 7, 15920–15930. 10.1038/s41598-017-15674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North M.; Pattenden G. Synthetic studies towards cyclic peptides. Concise synthesis of thiazoline and thiazole containing amino acids. Tetrahedron 1990, 46 (24), 8267–8290. 10.1016/S0040-4020(01)81482-4. [DOI] [Google Scholar]
- Wenschuh H.; Beyermann M.; Haber H.; Seydel J. K.; Krause E.; Bienert M.; Carpino L. A.; El-Faham A.; Albericio F. Stepwise automated solid phase synthesis of naturally occurring peptaibols using FMOC amino Acid fluorides. J. Org. Chem. 1995, 60 (2), 405–410. 10.1021/jo00107a020. [DOI] [Google Scholar]
- Ragozin E.; Redko B.; Tuchinsky E.; Rozovsky A.; Albeck A.; Grynszpan F.; Gellerman G. Biolabilepeptidyl delivery systems toward sequential drug release. Biopolymers 2016, 106 (1), 119–132. 10.1002/bip.22794. [DOI] [PubMed] [Google Scholar]
- Gilad Y.; Noy E.; Senderowitz H.; Albeck A.; Firer M. A.; Gellerman G. Dual-drug RGD conjugates provide enhanced cytotoxicity to melanoma and non-small lung cancer cells. Biopolymers 2016, 106 (2), 160–171. 10.1002/bip.22800. [DOI] [PubMed] [Google Scholar]
- Redko B.; Tuchinsky H.; Segal T.; Tobi D.; Luboshits G.; Ashur-Fabian O.; Pinhasov A.; Gerlitz G.; Gellerman G. Toward the development of a novel non-RGD cyclic peptide drug conjugate for treatment of human metastatic melanoma. Oncotarget 2017, 8 (1), 757–768. 3 10.18632/oncotarget.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubey S. A.; Mishra J. S.; Mishra R. Efficient Approach for the Tritylation of Alcohols Using Recyclable Lewis Acid-Based Ionic Liquid (EMIM·AlCl4). ACS omega 2018, 3 (8), 9607–9612. 10.1021/acsomega.8b00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin-Messager S.; Girard J.-P.; Rossi J. X. Convenient method for the preparation of trityl ethers from secondary alcohols. Tetrahedron Lett. 1992, 33, 2689–2692. 10.1016/S0040-4039(00)79058-7. [DOI] [Google Scholar]
- Burns K. E.; Robinson M. K.; Thévenin D. Inhibition of cancer cell proliferation and breast tumor targeting of pHLIP-monomethylauristatin E conjugates. Mol. Pharmaceutics 2015, 12 (4), 1250–1258. 10.1021/mp500779k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z.; Yu P.; Tang J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. OncoTargets Ther. 2020, 13, 5395–5405. 10.2147/OTT.S249756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontet C.; Jordan M. A. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat. Rev. Drug Discovery 2010, 9, 790–803. 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlan K.; Gelfand V. I. Microtubule-based transport and the distribution, tethering, and organization of organelles. Cold Spring Harbor Perspect. Biol. 2017, 9 (5), a025817. 10.1101/cshperspect.a025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichs V.; Grycova L.; Barinka C.; Nahacka Z.; Neuzil J.; Diez S.; Rohlena J.; Braun M.; Lansky Z. Mitochondria-adaptor TRAK1 promotes kinesin-1 driven transport in crowded environments. Nat. Commun. 2020, 11 (1), 1–13. 10.1038/s41467-020-16972-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardanho-Ramos C.; Faria-Pereira A.; Morais V. A. Orchestrating mitochondria in neurons: Cytoskeleton as the conductor. Cytoskeleton 2020, 77, 65–75. 10.1002/cm.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K.; Chacko L. A.; Chug M. K.; Jhunjhunwala S.; Ananthanarayanan V. Association of mitochondria with microtubules inhibits mitochondrial fission by precluding assembly of the fission protein Dnm1. J. Biol. Chem. 2019, 294 (10), 3385–3396. 10.1074/jbc.RA118.006799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijman M. N. A.; van NieuwAmerongen G. P.; Laurens N.; van Hinsbergh V. W. M.; Boven E. Microtubule-targeting agents inhibit angiogenesis at subtoxic concentrations, a process associated with inhibition of Rac1 and Cdc42 activity and changes in the endothelial cytoskeleton. Mol. Cancer Ther. 2006, 5 (9), 2348–2357. 10.1158/1535-7163.MCT-06-0242. [DOI] [PubMed] [Google Scholar]
- Su M.; Huang J.; Liu S.; Xiao Y.; Qin X.; Liu J.; Pi C.; Luo T.; Li J.; Chen X.; Luo Z. The anti-angiogenic effect and novel mechanisms of action of Combretastatin A-4. Sci. Rep. 2016, 6 (June), 1–11. 10.1038/srep28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.