Significance
Declines of wild megafauna are expected to transform ecosystems and are known to influence tree–grass balance in African savannas, but the effects of large herbivores on lianas are unknown. Using diet analysis, long-term exclosure experiments, and smaller-scale manipulations, we show that liana infestation occurred rapidly after the loss of large herbivores and suppressed tree growth and reproduction. We show theoretically that extended absence of herbivores could potentially generate a liana-dominated alternative state—but both experimental and theoretical results indicate that herbivore reintroduction can reverse endemic infestation, even after decades. We found low functional redundancy among wild herbivore species and between wildlife and livestock. Our results suggest that diverse large-herbivore assemblages promote resilience and robustness of savannas to liana encroachment.
Keywords: competition and facilitation, DNA metabarcoding, defaunation, ecological regime shifts, trophic rewilding
Abstract
African savannas are the last stronghold of diverse large-mammal communities, and a major focus of savanna ecology is to understand how these animals affect the relative abundance of trees and grasses. However, savannas support diverse plant life-forms, and human-induced changes in large-herbivore assemblages—declining wildlife populations and their displacement by livestock—may cause unexpected shifts in plant community composition. We investigated how herbivory affects the prevalence of lianas (woody vines) and their impact on trees in an East African savanna. Although scarce (<2% of tree canopy area) and defended by toxic latex, the dominant liana, Cynanchum viminale (Apocynaceae), was eaten by 15 wild large-herbivore species and was consumed in bulk by native browsers during experimental cafeteria trials. In contrast, domesticated ungulates rarely ate lianas. When we experimentally excluded all large herbivores for periods of 8 to 17 y (simulating extirpation), liana abundance increased dramatically, with up to 75% of trees infested. Piecewise exclusion of different-sized herbivores revealed functional complementarity among size classes in suppressing lianas. Liana infestation reduced tree growth and reproduction, but herbivores quickly cleared lianas from trees after the removal of 18-y-old exclosure fences (simulating rewilding). A simple model of liana contagion showed that, without herbivores, the long-term equilibrium could be either endemic (liana–tree coexistence) or an all-liana alternative stable state. We conclude that ongoing declines of wild large-herbivore populations will disrupt the structure and functioning of many African savannas in ways that have received little attention and that may not be mitigated by replacing wildlife with livestock.
Tropical savannas, which are defined by coexistence of grasses and trees, cover ∼13% of global land surface and support large populations of mammalian wildlife and livestock (1). The distribution of savannas is strongly influenced by fire and rainfall (2–4), which interact to maintain savannas in an intermediate state between forest and grassland. In Africa, large herbivores also influence savanna vegetation at local-to-continental scales, and shifts in herbivory regime influence the balance of trees and grasses (5, 6) with ramifications for biodiversity and ecosystem functions (4, 7). Accordingly, competitive interactions between trees and grasses, and the effects of abiotic and biotic disturbances on the relative abundance of these plant types, are major themes in savanna ecology (8, 9). By contrast, little research has addressed the potential effects of direct and indirect interactions involving other plant life-forms, such as forbs, succulents, and vines (10).
In tropical forests, lianas (woody vines) play a key role in regulating tree abundance, diversity, growth, and survival. In the Neotropics, liana abundance has increased over the last 40 y in response to intensifying anthropogenic disturbance, rising atmospheric CO2, and hydrological changes (11, 12). Such perturbations are thought to benefit lianas relative to trees, in part because lianas have greater capacity for growth during drought (13, 14), invest less in structural tissues (15), and can colonize rapidly via clonal reproduction (16, 17). In light-rich treefall gaps, lianas often proliferate and can competitively suppress forest regeneration for decades (18, 19).
The limited available evidence suggests that lianas are widespread but locally rare in savannas (20, 21). On the one hand, this scarcity may reflect fundamental differences between biomes: the discontinuous tree cover that defines savannas, for example, may restrict the establishment and spread of a plant life-form that uses woody canopies for structural support (18, 22). On the other hand, lianas may be a potentially significant presence in savannas that have simply been overlooked owing to strong top-down control by herbivores combined with a bias toward studying systems with intact wildlife assemblages (23). If savanna lianas are regulated by mammalian browsers, then the decline of large herbivores in Africa—especially threatened megaherbivores such as elephant and giraffe (24)—may lead to rapid increases in liana abundance analogous to those in Neotropical forests. The determinants of liana cover in savannas and the impacts of lianas on savanna trees are essentially unstudied, and lianas are often omitted from savanna plant censuses (25).
We combined small- and large-scale field experiments with diet analyses to understand the effects of large herbivores on lianas and liana–tree interactions in a semiarid Kenyan savanna. We hypothesized that browsers control liana abundance, thereby mitigating the negative effects of lianas on trees. Specifically, we predicted that native herbivores frequently eat lianas but that livestock rarely do; that experimental exclusion of large herbivores would increase the abundance and size of lianas; and that liana infestation would reduce tree growth and reproduction. We also experimentally assessed the resilience of this system to nearly two decades of large-herbivore exclusion, predicting that liana cover would decrease rapidly when large herbivores were “reintroduced” (by removing exclosure fences) after 18 y. Last, for insight into the potential dynamics of lianas and trees on even longer timescales, we developed a simple differential equations model analogous to the SIR (susceptible-infected-recovered) class of models commonly used in epidemiology.
Results
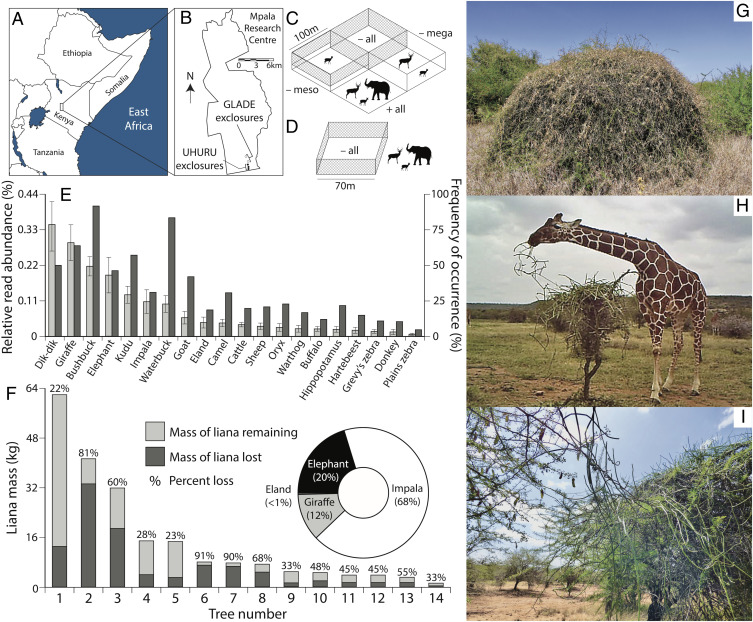
We collected data at the Mpala Research Centre and Conservancy in the Laikipia highlands of central Kenya (0°20´ N, 36°53´ E, mean annual rainfall ∼630 mm). The tree community is dominated by several species of Acacia sensu lato (25, 26). Caustic creeper (Cynanchum viminale [syn. Sarcostemma viminale], Apocynaceae, henceforth “Cynanchum;” ref. 27), a wind-dispersed, succulent vine with a woody trunk, is the dominant climber and accounts for >85% of vine infestations in tree canopies. The branching stems of Cynanchum produce white latex containing pregnane glycosides that can cause convulsions and paralysis in livestock (28). The large-herbivore (≥5 kg) community includes 22 wild species, along with cattle, camel, sheep, goat, and donkey. We worked in two long-term large-herbivore–exclusion experiments (Fig. 1 A–D): UHURU (Ungulate Herbivory Under Rainfall Uncertainty, established 2008) comprises four size-selective treatments applied to 1-ha plots in randomized blocks; GLADE (Glade Legacies and Defaunation Experiment, established 1999) comprises 0.5-ha total-exclusion plots (29). Here, we focused on the southernmost replicates of UHURU (n = 3 blocks, 12 plots) and GLADE (n = 2 plots), all of which are ≤2.5 km apart. The two GLADE exclosures were removed in 2017, enabling us to measure effects of herbivore reintroduction after nearly two decades of exclusion. The three unfenced control plots in UHURU, where herbivores have unfettered access, served as our reference point for the effects of exclusion.
Fig. 1.
Liana–herbivore–tree interactions at Mpala Research Centre, Kenya. (A) Location of Mpala and (B) southern GLADE and UHURU herbivore exclosures. Schematics of (C) UHURU and (D) GLADE experiments. (E) Relative read abundance (mean percent Cynanchum DNA per fecal sample ± SEM; light-gray bars, Left y-axis) and frequency of occurrence (percentage of samples with ≥0.1% Cynanchum DNA; dark-gray bars, Right y-axis) of Cynanchum in the diets of 20 large-herbivore species at Mpala (SI Appendix, Tables S1 and S2). (F) Consumption of transplanted lianas in 14 trials lasting 4 to 20 d (mean 9.8 d, mean percent weight loss per day across replicates 7.9 ± 1.9% SEM). Bar height is total mass transplanted, light gray is mass reweighed after the trial, and dark gray is the difference (mass lost); numbers above bars show percent of initial wet mass lost for each liana. Inset shows frequency of herbivory by different browsers in n = 247 recorded herbivory events. (G) Heavily infested A. etbaica in a total-exclusion (−all) plot in UHURU. (H) Giraffe eating transplanted liana (Movies S1–S4 show illustrative camera-trap footage). (I) Lateral spread of Cynanchum between adjacent tree canopies.
Consumption of Lianas by Large Herbivores.
We extracted publicly available diet data produced by DNA metabarcoding fecal samples collected at Mpala over multiple seasons and years (30–32). We analyzed data for the 20 most common herbivore species to assess the frequency and relative intensity of consumption of Cynanchum. For seven native species, Cynanchum accounted for 0.10 to 0.35% of diet (i.e., mean proportion of dietary sequence reads per sample) and occurred in 31 to 92% (median 57%) of samples (Fig. 1E). Some individuals of the four dominant browsers (dik-dik, impala, giraffe, and elephant) ate larger proportions of Cynanchum (up to 5.2, 4.5, 2.5, and 3.7%, respectively). By contrast, among the five livestock species, Cynanchum accounted for only 0.01 to 0.06% of diet, occurred in just 10 to 42% (median 21%) of samples, and never accounted for >0.5% of sequence reads in any individual sample (Fig. 1E and SI Appendix, Tables S1 and S2).
Although Cynanchum was frequently eaten by multiple species, it was not abundant in any species’ average diet, which could reflect either low availability or low herbivore preference. We therefore performed a cafeteria-style experiment in which we transplanted large Cynanchum (mean ± SEM, 14.9 ± 4.8 kg) from trees in exclosures onto trees accessible to herbivores. Transplanted lianas were rapidly consumed, losing 51.5 ± 6.4% of initial wet weight in trials lasting an average of 10 d (Fig. 1F). Most of this loss was attributable to consumption rather than desiccation: lianas transplanted onto trees in a fenced exclosure lost only 16.5 ± 2.1% of initial wet weight over 10 d, suggesting that ∼35% of transplanted liana biomass was eaten by herbivores. Camera-trap footage showed that impala, elephant, and giraffe were the main consumers of transplanted lianas (Fig. 1 F and H and Movies S1–S4). Thus, native browsers ate substantial quantities of Cynanchum when it was available, suggesting that its low relative abundance in herbivore diets is a function of its limited abundance in the landscape.
Effects of Herbivore Exclusion on Liana Infestation.
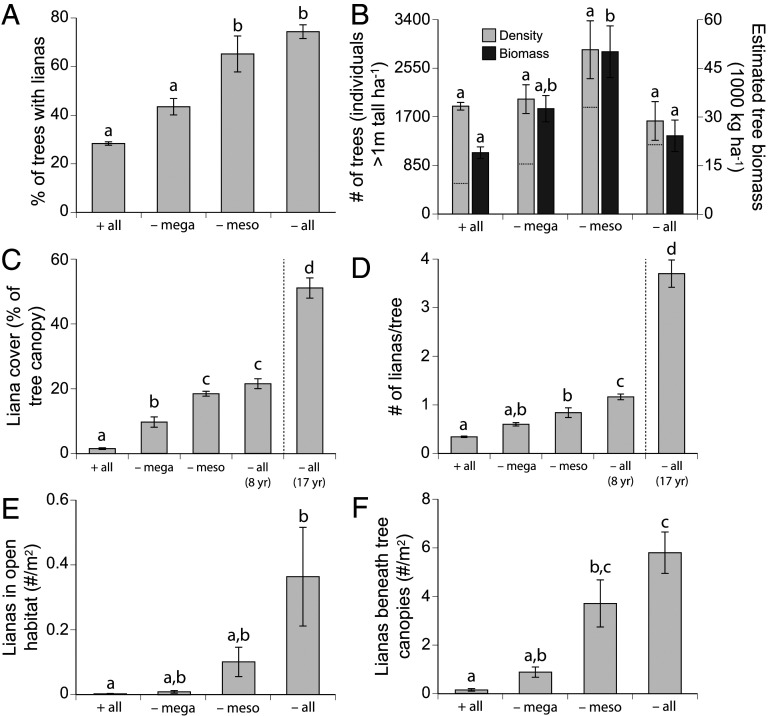
All measures of liana abundance and infestation severity were lowest in unfenced (“+all”) plots and increased monotonically with the successive exclusion of elephant and giraffe (“−mega”), mesoherbivores (predominantly impala, “−meso”), and dik-dik (“−all”), although not all treatments differed significantly in pairwise contrasts (Fig. 2 and SI Appendix, Fig. S1). Mean proportion of trees infested (Fig. 2A) and percent cover of lianas on tree canopies (Fig. 2C) were suppressed most strongly by mega- and mesoherbivores, with limited additional effect of dik-dik, whereas herbivores of all sizes contributed similarly to reducing the mean number of individual lianas per tree (Fig. 2D). The subset of responses measured in the 17-y GLADE −all exclosures were two- to threefold stronger than those in the 8-y UHURU −all plots (Fig. 2 C and D). These results were reproduced in a separate survey in November 2018 (10 y into UHURU), when we measured plant species composition by passing a 6-m pin from the ground through the canopy and recording all vegetation touching the pin. Cynanchum abundance increased monotonically from +all to −all treatments, accounting for nearly 10% of the vegetation in some exclosure plots (SI Appendix, Fig. S1A). Moreover, mean height of Cynanchum pin hits decreased from −mega, to −meso, to −all plots (SI Appendix, Fig. S1B), as expected if browsers in each size class ate the lianas within reach. These data show that herbivores exert strong top-down control on lianas, that effects of different-sized herbivores are additive and complementary (not redundant), and that infestation severity increases over time in the absence of herbivores.
Fig. 2.
Responses of lianas and trees to experimental herbivory regimes. (A) Percentage of trees infested by C. viminale in each treatment (F3,8 = 23.22, P = 0.0003). (B) Light bars, Left y-axis: density of all trees >1-m tall (F3,8 = 2.67, P = 0.12); dotted lines show estimated mean number of liana-infested trees, inferred by multiplying the proportion of trees infested by tree density. Dark bars, Right y-axis: estimated biomass density of trees >1-m tall (F3,8 = 6.95, P = 0.013). (C) Percent cover of lianas on individual tree canopies (F4,9 = 333.96, P < 0.0001). (D) Number of individual lianas per tree (F4,9 = 139.70, P < 0.0001). Number of juvenile lianas rooted in (E) open habitat between tree canopies (F3,8 = 4.54, P = 0.039) and (F) beneath tree canopies (F3,8 = 15.92, P = 0.001). All data are from the UHURU experiment except for the 17-y −all treatment shown at right of the vertical dotted lines in C and D, which are from GLADE exclosures prior to fence removal. Data are plot-level means ±1 SEM (n = 3 plots per treatment in UHURU, 2 plots in GLADE). Statistical tests are from one-way ANOVA by treatment; letters denote significant differences between treatments in post hoc contrasts (Tukey’s HSD).
In contrast to lianas, mean density and estimated biomass of trees (>1-m tall) in UHURU were greatest in −meso exclosures and least in +all and −all plots (although the density response was not statistically significant; Fig. 2B). The discrepant responses of trees and lianas reinforce our conclusion that herbivores’ effects on lianas were direct and consumptive, as opposed to purely indirect effects mediated by tree density.
Liana recruitment was greatest in exclosures and beneath tree canopies (Fig. 2 E and F), suggesting strong influences of both herbivores and the number and proximity of large adult lianas. Juvenile lianas in open habitat (i.e., not beneath trees) were almost nonexistent in +all plots and most abundant in −all plots, showing that dik-dik play a major role in suppressing recruitment outside tree canopies (Fig. 2E). Across all treatments, however, juvenile liana density was more than an order-of-magnitude greater beneath tree canopies than in the open, indicating that trees provide associational refuges from even the smallest ungulates (26). The annual growth rate of individually tagged liana stems did not differ significantly across exclosure treatments, but the fastest growing ∼2% of stems grew 43 to 593 cm/y, showing that liana growth and tree-to-tree spread can be rapid.
Using data from semiannual (wet and dry season) surveys in UHURU, we found that Cynanchum also constituted an appreciable fraction of the understory after 10 y—but only in −all plots, where they accounted for an average of 3% (max 18%) and 2.5% (max 12%) of understory pin hits per plot in October 2018 (wet) and February 2019 (dry), respectively (SI Appendix, Fig. S1C). In contrast, Cynanchum was essentially undetected in the understory of the other three treatments (e.g., one solitary pin hit in a single −meso plot in 2019), further underscoring the role of dik-dik in suppressing recruitment. Mean prevalence of Cynanchum in the understory of −all plots increased exponentially across 20 surveys from 2008 to 2019 (R2 = 0.87; SI Appendix, Fig. S1D).
Observations in March 2021 (12.5 y into UHURU) indicated that Cynanchum prevalence has continued to increase in −all plots, where it dominated large swaths of understory and overstory vegetation (SI Appendix, Fig. S2). Importantly, many free-standing lianas (understory individuals unsupported by trees) had reproduced, suggesting that Cynanchum does not need trees to persist (SI Appendix, Fig. S2 A and E).
Effects of Herbivore Reintroduction on Liana Cover.
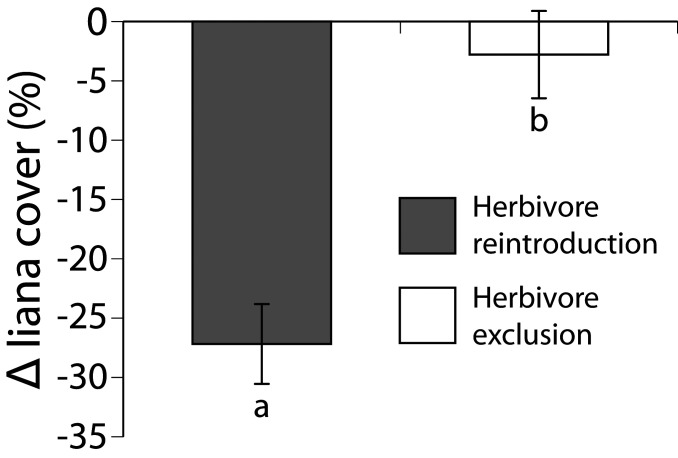
Removal of the GLADE exclosures in 2017 provided an unusual opportunity to test the resilience of this system after 18 y without large herbivores. Shortly before fence removal, liana prevalence in these exclosures was the highest we measured anywhere (Fig. 2 C and D), with lianas covering >50% of the average tree canopy. Within 2 mo of fence removal, mean liana cover decreased by more than half, to 23.9 ± 3.0% (Fig. 3), corroborating our transplant experiment showing that lianas are consumed in large quantities when available. Over roughly the same period, liana cover was stable in UHURU −all plots (−2.8 ± 3.7%; Fig. 3), indicating that the change in the deconstructed exclosures was the result of herbivory.
Fig. 3.
Effect of herbivore reintroduction on liana cover. Change in liana cover on 50 infested trees (gray bar) following removal of the GLADE −all exclosure fences after 18 y. Control trees (n = 50; white bar) were monitored in the nearby UHURU −all exclosures over the same period. Comparison of plot-level changes in liana cover (n = 2 plots for GLADE; n = 3 plots for UHURU) revealed a significant reduction in liana cover just 2 mo after herbivore reintroduction (ANOVA, F1,3 = 20.48, P = 0.020). Data are means ±1 SEM.
Effects of Lianas on Trees.
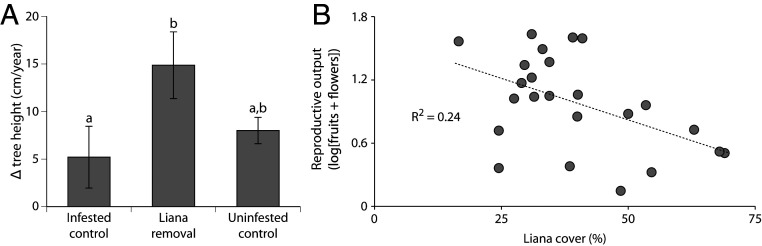
Trees infested with lianas grew 5.2 ± 3.2 cm per year. Trees from which we experimentally removed lianas grew nearly three times more over the same period (14.9 ± 3.5 cm). Uninfested, unmanipulated control trees grew intermediately (8.0 ± 1.4 cm; Fig. 4A). Tree reproductive output also declined as a function of infestation severity, with heavily infested Acacia etbaica producing up to 85% fewer fruits and flowers than lightly infested conspecifics (Fig. 4B). In 2021, we found that multiple heavily infested trees in −all plots had been top killed (SI Appendix, Fig. S2G), and we have seen trees broken under heavy masses of lianas (which can exceed 100 kg; reference Fig. 1F). Thus, lianas competitively reduce tree fitness, but individual trees can tolerate infestation up to a point and recover if lianas are removed.
Fig. 4.
Effects of liana cover on tree growth and reproductive output. (A) Change in tree (A. etbaica and A. drepanolobium) height following liana removal (n = 48 trees, 16 per treatment; linear mixed-effects model, treatment χ2 = 6.06, degrees of freedom = 2, P = 0.048). Data are means ±1 SEM; lowercase letters denote significant differences between treatments in post hoc contrasts (Tukey’s HSD). (B) Relationship between liana cover and reproductive output (fruits + flowers) for A. etbaica (n = 24 trees) within UHURU −all exclosures (F1,22 = 6.90, P = 0.015); note log scale on y-axis.
Theoretical Model.
We built a simple, spatially implicit ordinary differential equations model to qualitatively explore the long-term dynamics of trees and lianas (SI Appendix, Supplementary Information Text and Fig. S3). This model is analogous to epidemiological models used to study disease transmission, which have recently been adapted to study the dynamics of Neotropical lianas (33). Trees and lianas compete for space and resources. Trees can be either healthy (S) or infested (I), and lianas can be either free-standing (L) or growing on trees (henceforth, climbers). We modeled infestation as a contagion process in which both free-standing and climbing lianas can spread to and infest healthy trees. Free-standing lianas and climbers both produce seeds that are wind dispersed and therefore assumed to be randomly scattered across the landscape (SI Appendix, Fig. S2). Germinating seeds give rise to free-standing lianas; we assume that these (like Cynanchum: ref. 34) can grow adventitious roots and thus vegetatively grow laterally until they encounter a tree that they can climb (regardless of distance). Thus, we assume that infestation by free-standing lianas is a density-dependent process. Climbers can cause new infestations via lateral spread to neighboring, possibly healthy trees. Thus, we assume that infestation by climbing lianas is a frequency-dependent process. We further assume that both seed production and vegetative growth are higher for climbers than for free-standing lianas; conversely, consistent with our empirical observations, infested trees have lower reproduction and higher mortality than healthy trees. Herbivores increase mortality of both trees and lianas. We incorporated the herbivory regime by including separate herbivore-induced mortality terms for healthy trees, infested trees, and lianas in addition to their natural death rates (SI Appendix, Supplementary Information Text); thus, the effects of herbivores (collectively) are either present (i.e., herbivore-induced mortality terms are positive, albeit not necessarily equivalent) or absent (i.e., herbivore-induced mortality terms are zero). Although the model is agnostic as to the “type” of herbivore exerting these effects (e.g., wild versus domestic), different intensities and regimes of herbivory can be simulated by varying the absolute and/or relative magnitudes of the herbivore-induced mortality terms for the different categories of plant (e.g., SI Appendix, Fig. S4 C and D). We realistically assume that lianas have a positive growth rate at least in the absence of herbivores, while trees have a positive growth rate even in the presence of herbivores (SI Appendix, Supplementary Information Text).
The model has three nontrivial equilibria: no lianas (All-S); no trees (All-L); and a mixed, endemic equilibrium in which both trees and lianas persist. Thus, as long as trees persist, some fraction will be healthy (i.e., infestation never reaches 100%) because new saplings always start healthy. However, either lianas or trees can be completely absent from the system. This simple model allows analytical treatment of equilibrium stability (SI Appendix, Supplementary Information Text). We find that in the absence of herbivory, the endemic equilibrium (illustrated in SI Appendix, Fig. S4A) is the only stable one under the following conditions: 1) free-standing lianas have lower per capita fitness than healthy trees; 2) climbers have fast lateral spread; and either 3) free-standing lianas also have lower per capita fitness than infested trees, or 4) free-standing lianas are slow in their lateral vegetative spread (or both 3 and 4). Conversely, the pure-liana equilibrium All-L (illustrated in SI Appendix, Fig. S4B) is the only stable one provided either that free-standing lianas have higher per capita fitness than healthy trees or, otherwise, that free-standing lianas have higher per capita fitness than infested trees and both free-standing lianas and climbers have rapid lateral spread (SI Appendix, Supplementary Information Text). The All-L scenario seems biologically less likely, yet simulations show that the long-term outcome may not be predictable in the short term (SI Appendix, Fig. S4 B, Inset).
When the long-term equilibrium is endemic, reintroduction of herbivores can quickly return the system to the preextirpation healthy-tree/no-liana equilibrium if the reintroduced herbivores eat lianas at a similar rate as before. Alternatively, the system can be maintained in an endemic equilibrium with possibly lower liana abundance if the reintroduced herbivores feed less (or less effectively) on lianas (SI Appendix, Fig. S4 C and D). We emphasize that the simulations shown in SI Appendix, Fig. S4 are for illustration only and that the conclusions of our analytical stability analysis do not depend on parameterization.
Discussion
Previous work on herbivore–plant interactions in African savannas has focused almost exclusively on trees and grasses. Our results show that although lianas are scarce in an East African landscape with a largely intact native herbivore community, they can proliferate rapidly in the absence of large herbivores, with deleterious effects on tree growth and reproduction. Native browsers spanning a wide range of sizes and foraging modes—chiefly elephant (up to 5,000 kg), giraffe (∼1,000 kg), impala (∼50 kg), and dik-dik (∼5 kg)—ate Cynanchum and contributed in different ways to suppressing its abundance: whereas megaherbivores could consume considerable biomass and clear large lianas from treetops (Figs. 1 F and H and 2 A and C and SI Appendix, Fig. S1B), smaller-bodied species were effective at limiting juvenile establishment and prevalence of free-standing lianas in the understory (Fig. 2 D–F and SI Appendix, Fig. S1C). Our simple, qualitative model of liana spread predicts that in the sustained absence of large herbivores, liana abundance should continue to increase (consistent with experimental results; Fig. 2 C and D and SI Appendix, Fig. S1D) and that endemic liana infestation or even an all-liana state are possible (SI Appendix, Fig. S4 A and B). Importantly, however, the ultimate outcome may not be predictable for the first decades (or longer) after the extirpation of herbivores; during the transient period, the system will be in an endemic state (SI Appendix, Fig. S4 B, Inset). Thus, the model not only predicts that an all-liana alternative stable state is possible but also suggests that it might not be preceded by any detectable early warning signals.
However, both theoretical (SI Appendix, Fig. S4 C and D) and experimental results (Figs. 3 and 4A) also indicate that savannas retain the capacity to recover from severe endemic liana infestation for decades after the loss of large herbivores. These findings are noteworthy in the context of trophic rewilding, which aims to restore ecological processes by reintroducing extirpated megafauna (35). The crucial but uncertain premise of this approach is that such processes are indeed recoverable and that defaunation does not rapidly lead to recalcitrant alternative states. The dramatic recovery observed in our fence-removal experiment—where an already diverse and abundant large-herbivore community halved liana cover in just 2 mo—probably overestimates the rate at which lianas would be removed in a system rebounding from large-scale defaunation. Nonetheless, work at larger scales is consistent with our inference that defaunation-induced savanna plant encroachment can be reversed even after decades. In Mozambique’s Gorongosa National Park, for example, ungulate populations recovering from near-extirpation have reasserted biotic control over the invasive shrub Mimosa pigra (36).
Our results also highlight the lack of functional redundancy among wild browsers of different sizes (Fig. 2 and SI Appendix, Fig. S1 A–C), as well as between wild and domesticated browsers: even goats and camels ate Cynanchum rarely relative to the most abundant wild browsers (Fig. 1 E and F), and previous studies have noted “the dislike of livestock for [C.] viminale” (34, p. 121). This pattern underscores the importance of conserving functionally diverse native assemblages and is consistent with a previous study at Mpala showing that the diversity of native large herbivores was important in reducing encroachment by the shrub Solanum campylacanthum (37). Notably, the toxicity of secondary metabolites in both of these plants appears to be greater for domesticated than wild ungulates (28, 38). The glycosides in Cynanchum cause a potentially lethal poisoning syndrome (cynanchosis) that affects farmed cattle, goats, sheep, horses, and ostriches in southern Africa (39–41) and may be a particular risk to naïve livestock (e.g., ref. 42). Although livestock may be functional proxies for wildlife in certain respects (7), there is growing evidence that replacement of native herbivore guilds with (invariably less species-rich and functionally diverse) livestock has altered plant communities and disrupted ecosystem processes in African savannas (43, 44). Cynanchum’s toxicity to domesticated ungulates, its competitive effects on trees (Fig. 4) and perhaps grasses (SI Appendix, Figs. S1 and S2), and its ability to form dense mats that reduce access to forage plants and increase the odds of accidental ingestion all suggest that removing native browsers might limit the productivity and profitability of both grazing and browsing livestock. Explicit investigation of this possibility would be useful.
Our simple theoretical model omits several fundamental savanna processes (e.g., tree–grass interactions, rainfall, fire), as well as nuances such as potential effects of tree and herbivore species composition. Accordingly, and because we lack empirically validated parameter estimates, we do not use the model for quantitative prediction. Rather, the model provides an analytically tractable heuristic tool for qualitative insight into possible outcomes of liana–tree interactions on long timescales and the conditions associated with alternative stable states. The all-liana state minimally requires high per capita fitness of free-standing lianas, and although free-standing lianas do reproduce, we consider this condition relatively unlikely (perhaps explaining why we are unaware of any savanna dominated by vines to the exclusion of trees). The more likely endemic equilibrium matches the scenario in −all exclosures maintained for up to 17 y, and the predicted reversibility of this scenario back to an essentially all-tree state accords with our data—yet our data also show that liana prevalence in −all exclosures was increasing exponentially after more than a decade (SI Appendix, Fig. S1D), indicating that no stable state has been reached. We hope that our model will stimulate further research on the long-term consequences of liana encroachment in megafauna-free savannas.
The marginal effect of herbivore exclusion on total tree density in the 12 southern UHURU plots (Fig. 2B) was useful in showing that liana abundance did not simply track tree density across treatments but is surprising in light of previous exclosure studies documenting stronger effects over comparable timespans. In the GLADE experiment, for example, tree density was roughly threefold greater in exclosures than unfenced plots after 10 y (45, 46). The treatment effect on tree biomass in UHURU was more pronounced than that on overall density, reflecting the greater number of large trees in (especially −meso) exclosures, yet the persistent lack of difference between −all and +all plots remains puzzling. Although it is tempting to speculate that competition with Cynanchum in −all plots might have something to do with this unexpected null result, the previous findings from GLADE (where liana prevalence was also high: Fig. 2 C and D) seem at odds with this interpretation. A fuller investigation of tree dynamics in UHURU is needed.
In several respects, our results are broadly consistent with patterns reported from Neotropical forests, where the majority of liana research has occurred to date (but see ref. 47). For example, we found evidence that liana infestation reduces tree fitness and that this effect is correlated with infestation severity (Fig. 4), mirroring findings from Panama and Bolivia (48, 49). In both forest and savanna, the distribution and abundance of lianas is also linked to the availability of trellises, and the rate of spread of lianas between tree canopies is strongly influenced by the proximity of neighboring trees (Fig. 2 E and F; ref. 50). There are also similarities between the all-liana state that our model suggests is possible in savannas and the “stalled gap” phenomenon in tropical forests, where dense liana tangles arrest succession in treefall gaps (18). These similarities notwithstanding, the major differences between Neotropical forests and African savannas (climate, tree diversity and structure, liana traits, disturbance regimes) dictate caution in attempting cross-biome comparisons; deeper insights will require a better understanding of the drivers of liana abundance in savannas and savanna–forest mosaics. Chief among the unknowns are the susceptibility of lianas to fire (which is infrequent in many dry savannas, including Mpala, but is a dominant force in the savanna biome more broadly), the role of water-limitation in liana recruitment and survival, and the vulnerability of dominant liana species to herbivory.
Predicting how savanna plant communities are likely to change over the next century—and how those changes will affect ecologically and economically important ecosystem processes—is an important goal at the nexus of ecology and conservation (3). Presently, the integration of lianas into savanna vegetation models is precluded by a paucity of information about the diversity, distribution, and ecology of this life-form, even at the coarsest spatial and temporal scales. A first step in this regard is to catalog the distribution and abundance of lianas across the continent (21), with particular attention to forest–savanna transitions where significant shifts in floristic composition have already occurred—and where, intriguingly, liana encroachment into grasslands has been documented in conjunction with reductions in herbivore density (51). The concerted effort to understand how liana abundance and diversity have changed over recent decades in Neotropical forests provides a roadmap for similar efforts in savannas. A comparative, multibiome approach to liana ecology has the potential to deepen our understanding of forests and savannas while extending our ability to effectively manage and conserve them.
Materials and Methods
Study Site and Long-Term Herbivore Exclosure Experiments.
Mpala encompasses 20,000 ha of semiarid thorn-scrub savanna (Fig. 1). The study area is underlain by infertile red sandy loams. The woody-plant community is dominated by spinescent Acacia s.l. (including Senegalia and Vachellia spp.) trees and shrubs—predominantly A. (S.) brevispica, A. (V.) etbaica, and A. (S.) mellifera, with several other species occurring more patchily. The understory comprises several hundred species of grasses, forbs, and subshrubs (26, 52, 53). Elephant (Loxodonta africana), impala (Aepyceros melampus), dik-dik (Madoqua cf. M. guentheri), plains zebra (Equus quagga), and giraffe (Giraffa camelopardalis) account for the majority of native large-herbivore biomass (54). Controlled burns are not used for management, and unintentional fires are infrequent, in part because understory biomass is low and interspersed with patches of bare soil (25), in part because the trimodal annual rainfall pattern with a short (∼3 mo) dry season limits fuel accumulation, and in part because property managers practice preventative measures.
Although African lianas are comparatively little studied (55), >2,200 species of lianas and vines have been cataloged continent-wide; diversity is highest in forested parts of West and Central Africa, but most savanna-dominated stretches of East Africa also support at least 5 to 10 liana species (21). Cynanchum viminale is the most abundant scandent plant native to Mpala (others include a closely related but much rarer congener, Cynanchum gerrardii, along with herbaceous Plectranthus and Kleinia spp.) and is widespread throughout Africa, Asia, and Australia. In this range, C. viminale occurs in habitats ranging from dry scrub to forest; in savannas, it is common in thickets, where it adopts a climbing habit (56), and in the absence of support it forms a short shrub (39). Like many succulent lianas, C. viminale reproduces sexually by wind-dispersed seeds and clonally from root and stem fragments, with laterally growing stems forming adventitious roots where they touch the ground—a suite of traits that facilitates rapid expansion (34).
The GLADE experiment (25) comprised six paired 70 × 70 m total exclosures and unfenced control plots (Fig. 1D), three each in bushy habitat and anthropogenic clearings (glades). Exclosures consisted of wire-mesh fencing from 0 to 50 cm and 11 strands of electrified wires up to 3 m, excluding all mammalian herbivores ≥5 kg (i.e., dik-dik and everything larger). Fences in the three bushy replicates were maintained from 1999 until mid-2017, when they were removed. The UHURU experiment comprises nine replicate blocks of four treatments (Fig. 1C), three blocks each in southern, central, and northern Mpala (57). Total exclosures (−all), directly analogous to the GLADE exclosures, are surrounded by 1-m tall mesh fences and electrified wires up to 2 m, excluding all herbivores ≥5 kg. Mesoherbivore exclosures (−meso) consist only of electrified wires and lack mesh, allowing access to herbivores <50 cm tall; thus, the difference between −all and −meso is effectively the presence of dik-dik, the smallest and most abundant ungulate at Mpala. Megaherbivore exclosures (−mega) consist of electrified wires at 2 m, allowing access to all herbivores except elephant and giraffe; thus, −meso and −mega differ in the presence of multiple ungulate species, but of these, impala are ∼15-fold more abundant than any other and account for more biomass than all others combined (54). Unfenced open plots (+all) are marked with wooden posts and are freely accessible to all species. The impact of each herbivore size class can thus be assessed by comparing UHURU treatment pairs, while the net impact of successively larger-bodied herbivore groups can be assessed by comparing each treatment to +all (57).
Consumption of Lianas by Large Herbivores.
We used DNA metabarcoding data from 1,322 fecal samples of 33 herbivore species collected at Mpala from 2013 to 2016, which we made publicly available with previous studies (30, 32). Detailed methods are in the original sources. Briefly, for each of the 20 most abundant large-herbivore species, we analyzed data from 6 to 163 fecal samples per species (total n = 1,176; SI Appendix, Tables S1 and S2). DNA metabarcoding used the trnL-P6 chloroplast marker. Samples were rarefied to an even sequencing depth, and taxonomic assignments were based on a comprehensive reference library of trnL-P6 sequences from locally collected and taxonomically verified specimens (53). These data could not differentiate the two Cynanchum species at Mpala, C. viminale and C. gerrardii, which share the same barcode (along with similar ecological habits); plant surveys in UHURU prior to 2014 likewise lumped these taxa, but subsequent data show that C. viminale is ∼100-fold more abundant than C. gerrardii. We calculated two complementary metrics of interaction intensity. Relative read abundance is the mean percentage of plant DNA sequence reads per sample that matched Cynanchum and is considered a reasonable proxy for proportional consumption in analyses of herbivore diets based on the trnL-P6 marker. Frequency of occurrence is the percentage of samples that contained Cynanchum—a presence–absence metric where relative read abundance ≥0.1% was interpreted as evidence of presence.
To test which herbivores might eat C. viminale if it were more available, we transplanted entire lianas from 14 trees in −all plots onto size-matched conspecific trees outside exclosures. We weighed lianas immediately before transplanting and reweighed the remaining unconsumed biomass again between 4 and 20 d later, after some noticeable fraction of the liana had disappeared (mean trial duration: 9.8 d). We used Bushnell TrophyCams to determine which herbivore species ate transplanted lianas. To estimate weight loss attributable to desiccation alone, we transplanted five lianas from their host trees onto other trees inside a fenced exclosure, weighing before transplanting and again after 10 d to match the mean duration of the herbivory trials.
Effects of Herbivore Exclusion on Liana Infestation.
To test the hypothesis that severity and frequency of liana infestation increases as browsing pressure decreases, we conducted several surveys in UHURU in January 2017. First, we haphazardly identified 1,150 trees (∼100 per plot; mean height 3.1 m) and recorded the number of individual (separately rooted) Cynanchum on each, as well as the areal percentage of each canopy covered. We used the same data to estimate the proportion of trees infested by at least one liana in each plot. These data were averaged within each plot and analyzed with separate one-factor ANOVA, with exclosure treatment as the factor, in R [version 3.3.2 (58)]. We surveyed juvenile (<1-m tall) C. viminale along four 50 × 4 m transects per plot to test the hypothesis that liana recruitment varies as a function of herbivore-exclusion treatment. Transects were aligned with a permanent grid of 49 metal stakes in the central 60 × 60 m of each plot (57). Because trees have previously been shown to provide associational refuges from herbivores at Mpala (26), we separately compared juvenile Cynanchum growing 1) beneath tree canopies and 2) in open habitat between trees; data were again averaged at the plot level and analyzed with one-factor ANOVA as functions of treatment.
To measure liana growth rates, we marked the terminal 10 cm on each of 10 haphazardly selected branches on 10 C. viminale in each UHURU plot in August 2016 (n = 10 stems/plant × 10 plants/plot × 12 plots). We remeasured and calculated mean growth rate per plant in January 2017 (n = 117 of the original 120 plants); we then averaged plant-level data per plot and compared annualized growth rates across treatments with ANOVA.
To determine tree density in UHURU, we recorded the number and identity of all trees (binned into height classes) in 10 × 10 m subsections of each plot in each year from 2016 to 2018 (up to 36 subplots per plot, although not all subplots were surveyed in each year). To obtain a single time-integrated plot-level value of tree density (>1-m tall) for analysis, we first averaged across subplots in each plot in each year and then averaged these means across the 3 y. To estimate the total number of infested trees in each plot, we multiplied plot-level tree densities by the proportion of trees infested per plot. To estimate tree biomass per plot, we used data from 3,281 permanently tagged trees in UHURU that were measured at least once between 2009 to 2018 (57). We calculated biomass as a function of crown diameter (CD, average of the widest canopy axis and its perpendicular, in m) using the following equation (59):
This equation has been used in previous studies from Mpala (25, 45, 46). We then regressed these biomass estimates as a function of height (H, in m) of the same tagged trees, yielding the following linear regression (R2 = 0.69, F1,3279 = 7224, P < < 0.0001):
For this same set of permanently tagged trees (57), we calculated the mean height (in 2015) for each of the size classes used in the annual censuses of tree density described above: 1 to 2 m (1.61 m), 2 to 3 m (2.52 m), 3 to 4 m (3.48 m), and >4 m (4.69 m). We plugged these mean height values into the regression of biomass as a function of H, multiplied by the mean density of trees in each size class per plot (calculated as described above for total density), and summed these products across height classes to obtain biomass per plot. We note that the series of conversions and approximations used to estimate biomass inevitably introduces error; thus, although we are confident in the relative comparison of biomass among treatments in UHURU, the absolute values should be regarded with caution. We analyzed tree density and biomass using one-factor ANOVA.
To quantify relative abundance of Cynanchum in the plant community and test for differences in height across treatments, we used data from a canopy-intercept survey (60) in November 2018. At each of the 49 grid stakes in each plot, we placed a telescoping pole on the ground and extended it upwards. We recorded the number of contacts between plants and pole and the species and height of each hit (0 to 600 cm, encompassing the full range of heights accessible to browsers from dik-dik to giraffe). We analyzed Cynanchum pin hits (total and as percent of all species) and mean Cynanchum height using one-factor ANOVA by treatment on square-root-transformed data (excluding the +all treatment from the height analysis, as Cynanchum accounted for just three total pin hits in just two +all plots). To assess Cynanchum in the understory, we used a similar canopy-intercept approach, but with a 10-pin frame at each grid stake (n = 490 pin placements per plot) in each of 20 surveys from 2008 to 2019 (57). We fit an exponential-growth model to mean Cynanchum prevalence in the understory of −all plots across surveys. We conducted these canopy-intercept surveys in all 36 UHURU plots (as opposed to just the 12 southern plots used for the other surveys in this study) and lumped C. viminale and C. gerrardii (which we did not distinguish in understory surveys prior to 2014), but C. viminale accounted for 99% of pin hits in both survey types such that C. gerrardii did not influence the overall pattern.
Effects of Herbivore Reintroduction on Liana Cover.
We measured percent cover of lianas on 50 haphazardly selected trees within the GLADE −all exclosures in August 2016 (before fence removal) and again in July 2017 (∼2 mo after fence removal). For comparison, we selected 50 trees from the UHURU −all plots and measured change in liana cover from June 2016 to June 2017. We averaged data within each plot (n = 2 plots for GLADE, n = 3 plots for UHURU) and compared the change in liana cover between deconstructed and intact exclosure experiments with one-factor ANOVA.
Effects of Liana Infestation on Trees.
In June 2016, we identified 30 liana-infested and 15 uninfested Acacia trees within the UHURU −all and −meso exclosure plots and divided them into 15 triplets of nearby individuals matched by species (A. etbaica or Acacia drepanolobium) and height (as closely as possible; mean height disparity 0.46 m). In each triplet, we randomly assigned one infested tree to a liana-removal treatment, which involved manually removing all lianas from the canopy and trunk; the average weight of lianas removed from each tree was 14.1 ± 4.2 kg. We left the other two trees in each triplet as infested and uninfested controls. We measured tree heights after the manipulation (to account for any immediate physical rebound) and again in June 2017. We analyzed change in tree height using a linear mixed-effects model, with treatment as the main effect and triplet identity as a random effect; we estimated the P value using a likelihood-ratio test and used Tukey’s honestly significant difference (HSD) to contrast each pair of treatments.
In January 2017, we assessed the impact of liana infestation on tree reproduction by surveying liana loads on 24 reproductive A. etbaica in UHURU −all plots (to control for the effect of herbivory on reproductive output; ref. 61). For each tree, we haphazardly placed 10 quadrats (50 × 50 cm) on the canopy and recorded the mean number of tree reproductive units (fruits and flowers) and mean percent liana cover. We used linear regression to assess the correlation between liana cover and reproductive output per tree (after log-transforming reproductive output to meet the assumption of normality).
Supplementary Material
Acknowledgments
We thank the Government of Kenya (NACOSTI/P/14/8746/1626, NACOSTI/P/20/6262), D. Martins, and Mpala Research Centre for permission to conduct this study. N. Carlson, M. Crosley, C. Coverdale, E. Coverdale, M. Dyck, J. Echekan, P. Echekan, A. Hassan, S. Kurukura, S. Mwinzi, A. Marsh, I. McGeary, and C. Muskin assisted with field work, data collection, and analyses. A. Agrawal, J. Daskin, A. Davies, and C.E.T.’s research group provided valuable feedback on the manuscript. We received support from the US NSF (DEB-1601538, DEB-1355122, DEB-1457697, DEB-1930820, IOS-1656527, DEB-1556728, DEB-1149980), the American Philosophical Society, the Garden Club of America, Princeton University and the High Meadows Environmental Institute, the Universities of Florida and Wyoming, and a NatureNet Science Fellowship from The Nature Conservancy. The UHURU experiment was built with a Natural Sciences and Engineering Research Council Tools and Instruments grant. The GLADE exclosure plots were built with funding from the National Geographic Society and NSF (DEB-9813050).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2101676118/-/DCSupplemental.
Data Availability
Data from this study are available in Dryad Digital Repository, https://doi.org/10.5061/dryad.gmsbcc2np (62). Original DNA-metabarcoding data are also available in Dryad, https://doi.org/10.5061/dryad.c119gm5 (63).
References
- 1.Scholes R., Archer S., Tree-grass interactions in savannas. Annu. Rev. Ecol. Syst. 28, 517–544 (1997). [Google Scholar]
- 2.Staver A. C., Archibald S., Levin S., Tree cover in sub-Saharan Africa: Rainfall and fire constrain forest and savanna as alternative stable states. Ecology 92, 1063–1072 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Sankaran M., et al., Determinants of woody cover in African savannas. Nature 438, 846–849 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Lehmann C. E. R., et al., Savanna vegetation-fire-climate relationships differ among continents. Science 343, 548–552 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Daskin J. H., Stalmans M., Pringle R. M., Ecological legacies of civil war: 35-year increase in savanna tree cover following wholesale large-mammal declines. J. Ecol. 104, 79–89 (2016). [Google Scholar]
- 6.van Langevelde F., et al., Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 84, 337–350 (2003). [Google Scholar]
- 7.Veblen K. E., Porensky L. M., Riginos C., Young T. P., Are cattle surrogate wildlife? Savanna plant community composition explained by total herbivory more than herbivore type. Ecol. Appl. 26, 1610–1623 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Sankaran M., Ratnam J., Hanan N., Woody cover in African savannas: The role of resources, fire and herbivory. Glob. Ecol. Biogeogr. 17, 236–245 (2008). [Google Scholar]
- 9.Bond W. J., What limits trees in C4 grasslands and savannas? Annu. Rev. Ecol. Evol. Syst. 39, 641–659 (2008). [Google Scholar]
- 10.Siebert F., Dreber N., Forb ecology research in dry African savannas: Knowledge, gaps, and future perspectives. Ecol. Evol. 9, 7875–7891 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnitzer S. A., A mechanistic explanation for global patterns of liana abundance and distribution. Am. Nat. 166, 262–276 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Wright S., Calderón O., Hernandéz A., Paton S., Are lianas increasing in importance in tropical forests? A 17-year record from Panama. Ecology 85, 484–489 (2004). [Google Scholar]
- 13.Chen Y. J., et al., Water-use advantage for lianas over trees in tropical seasonal forests. New Phytol. 205, 128–136 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Cai Z. Q., Schnitzer S. A., Bongers F., Seasonal differences in leaf-level physiology give lianas a competitive advantage over trees in a tropical seasonal forest. Oecologia 161, 25–33 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Heijden G. M. F., Powers J. S., Schnitzer S. A., Lianas reduce carbon accumulation and storage in tropical forests. Proc. Natl. Acad. Sci. U.S.A. 112, 13267–13271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledo A., Schnitzer S. A., Disturbance and clonal reproduction determine liana distribution and maintain liana diversity in a tropical forest. Ecology 95, 2169–2178 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Yorke S. R., Schnitzer S. A., Mascaro J., Letcher S. G., Carson W. P., Increasing liana abundance and basal area in a tropical forest: The contribution of long-distance clonal colonization. Biotropica 45, 317–324 (2013). [Google Scholar]
- 18.Schnitzer S. A., Dalling J. W., Carson W. P., The impact of lianas on tree regeneration in tropical forest canopy gaps: Evidence for an alternative pathway of gap-phase regeneration. J. Ecol. 88, 655–666 (2000). [Google Scholar]
- 19.Tymen B., et al., Evidence for arrested succession in a liana-infested Amazonian forest. J. Ecol. 104, 149–159 (2016). [Google Scholar]
- 20.Zhang S., Zhang J., Cao K., Differences in the photosynthetic efficiency and photorespiration of co-occurring Euphorbiaceae liana and tree in a Chinese savanna. Photosynthetica 54, 438–445 (2016). [Google Scholar]
- 21.Sosef M. S. M., et al., Exploring the floristic diversity of tropical Africa. BMC Biol. 15, 15 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putz F., How trees avoid and shed lianas. Biotropica 16, 19–23 (1984). [Google Scholar]
- 23.Daskin J. H., Pringle R. M., Warfare and wildlife declines in Africa’s protected areas. Nature 553, 328–332 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Ripple W. J., et al., Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Augustine D. J., McNaughton S. J., Regulation of shrub dynamics by native browsing ungulates on East African rangeland. J. Appl. Ecol. 41, 45–58 (2004). [Google Scholar]
- 26.Coverdale T. C., et al., Elephants in the understory: Opposing direct and indirect effects of consumption and ecosystem engineering by megaherbivores. Ecology 97, 3219–3230 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Goyder D. J., Nomenclatural changes resulting from the transfer of tropical African Sarcostemma to Cynanchum (Apocynaceae: Asclepiadoideae). Kew Bull. 63, 471–472 (2008). [Google Scholar]
- 28.Botha C. J., Penrith M. L., Poisonous plants of veterinary and human importance in southern Africa. J. Ethnopharmacol. 119, 549–558 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Goheen J., et al., Conservation and management lessons from large-mammal manipulations in East African rangelands: KLEE, GLADE, and UHURU experiments. Ann. N. Y. Acad. Sci. 1, 1–19 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Kartzinel T. R., Hsing J. C., Musili P. M., Brown B. R. P., Pringle R. M., Covariation of diet and gut microbiome in African megafauna. Proc. Natl. Acad. Sci. U.S.A. 116, 23588–23593 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kartzinel T. R., et al., DNA metabarcoding illuminates dietary niche partitioning by African large herbivores. Proc. Natl. Acad. Sci. U.S.A. 112, 8019–8024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kartzinel T. R., Pringle R. M., Multiple dimensions of dietary diversity in large mammalian herbivores. J. Anim. Ecol. 89, 1482–1496 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Visser M. D., et al., A host-parasite model explains variation in liana infestation among co-occurring tree species. J. Ecol. 106, 2435–2445 (2018). [Google Scholar]
- 34.Liede S., Whitehead V., Studies in the pollination biology of Sarcostemma viminale R. BR. sensu lato. S. Afr. J. Bot. 57, 115–122 (1991). [Google Scholar]
- 35.Svenning J. C., Rewilding should be central to global restoration efforts. One Earth 3, 657–660 (2020). [Google Scholar]
- 36.Guyton J. A., et al., Trophic rewilding revives biotic resistance to shrub invasion. Nat. Ecol. Evol. 4, 712–724 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Pringle R. M., et al., Low functional redundancy among mammalian browsers in regulating an encroaching shrub (Solanum campylacanthum) in African savannah. Proc. Biol. Sci. 281, 20140390 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thaiyah A., et al., Acute, sub-chronic and chronic toxicity of Solanum incanum L in sheep in Kenya. Kenya Vet. 35, 1–8 (2011). [Google Scholar]
- 39.Kellerman T., Coetzer J., Naude T., Botha C., Plant Poisonings & Mycotoxicoses of Livestock in South Africa (Oxford University Press Southern Africa, ed. 1, 1988). [Google Scholar]
- 40.Van Wyk B. E., Van Heerden F., Van Oudtshoorn B., Poisonous Plants of South Africa (Briza Publications, ed. 1, 2002). [Google Scholar]
- 41.Vleggaar R., Van Heerden F. R., Anderson L. A. P., Erasmus G. L., Toxic constituents of the Asclepiadaceae. Structure elucidation of sarcovimiside A-C, pregnane glycosides of Sarcostemma viminale. J. Chem. Soc., Perkin Trans. 1 483–487 (1993). [Google Scholar]
- 42.Basson P., Norval A., Hofmyer J., Ebedes H., Schultz R., Antelope and poisonous plants: 1. Gifblaar Dichapetalum cymosum (Hooker) Engler & Prantl containing monofluoroacetate. Madoqua 13, 59–70 (1982). [Google Scholar]
- 43.Du Toit J. T., Cumming D. H. M., Functional significance of ungulate diversity in African savannas and the ecological implications of the spread of pastoralism. Biodivers. Conserv. 8, 1643–1661 (1999). [Google Scholar]
- 44.Hempson G. P., Archibald S., Bond W. J., The consequences of replacing wildlife with livestock in Africa. Sci. Rep. 7, 17196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sankaran M., Augustine D. J., Ratnam J., Native ungulates of diverse body sizes collectively regulate long-term woody plant demography and structure of a semi-arid savanna. J. Ecol. 101, 1389–1399 (2013). [Google Scholar]
- 46.Wigley B. J., Augustine D. J., Coetsee C., Ratnam J., Sankaran M., Grasses continue to trump trees at soil carbon sequestration following herbivore exclusion in a semiarid African savanna. Ecology 101, e03008 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Poulsen J. R., et al., Forest structure determines the abundance and distribution of large lianas in Gabon. Glob. Ecol. Biogeogr. 26, 472–485 (2017). [Google Scholar]
- 48.Wright S. J., et al., Reproductive size thresholds in tropical trees: Variation among individuals, species and forests. J. Trop. Ecol. 21, 307–315 (2005). [Google Scholar]
- 49.Nabe-Nielsen J., Kollmann J., Peña-Claros M., Effects of liana load, tree diameter and distances between conspecifics on seed production in tropical timber trees. For. Ecol. Manage. 257, 987–993 (2009). [Google Scholar]
- 50.Putz F. E., The natural history of lianas on Barro Colorado Island, Panama. Ecology 65, 1713–1724 (1984). [Google Scholar]
- 51.Stewart T., Scogings P. F., Baijnath H., Dispersal of a forest liana into grasslands and post-establishment stand expansion. S. Afr. J. Bot. 131, 51–55 (2020). [Google Scholar]
- 52.Kartzinel T., et al., Plant and small-mammal responses to large-herbivore exclusion in an African savanna: Five years of the UHURU experiment. Ecology 95, 787 (2014). [Google Scholar]
- 53.Gill B. A., et al., Plant DNA-barcode library and community phylogeny for a semi-arid East African savanna. Mol. Ecol. Resour. 19, 838–846 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Augustine D. J., Response of native ungulates to drought in semi-arid Kenyan rangeland. Afr. J. Ecol. 48, 1009–1020 (2010). [Google Scholar]
- 55.Schnitzer S. A., Bongers F., Increasing liana abundance and biomass in tropical forests: Emerging patterns and putative mechanisms. Ecol. Lett. 14, 397–406 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Moolman H. J., Cowling R. M., The impact of elephant and goat grazing on the endemic flora of South African succulent thicket. Biol. Conserv. 68, 53–61 (1994). [Google Scholar]
- 57.Goheen J. R., et al., Piecewise disassembly of a large-herbivore community across a rainfall gradient: The UHURU experiment. PLoS One 8, e55192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.R Core Development Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, Austria, 2015). [Google Scholar]
- 59.Epp H., Herlocker D., Peden D., The Use of Large-Scale Aerial Photography to Determine Wood Biomass in the Arid and Semi-Arid Areas of Kenya (Kenya Rangeland Ecological Monitoring Unit, Nairobi, Kenya, 1982). [Google Scholar]
- 60.Frank D. A., Mcnaughton S. J., Aboveground biomass estimation with the canopy intercept method: A plant growth form caveat. Oikos 57, 57–60 (1990). [Google Scholar]
- 61.Young T. P., Augustine D. J., Interspecific variation in the reproductive response of Acacia species to protection from large mammalian herbivores. Biotropica 39, 559–561 (2007). [Google Scholar]
- 62.Coverdale T. C., et al., Data from: Large herbivores suppress liana infestation in an African savanna. Dryad Digital Repository. 10.5061/dryad.gmsbcc2np. Deposited 27 July 2021. [DOI] [PMC free article] [PubMed]
- 63.Kartzinel T. R., Hsing J. C., Musili P. M., Pringle R. M., Data from: Covariation of diet and gut microbiome in African megafauna. Dryad Digital Repository. 10.5061/dryad.c119gm5. Deposited 5 November 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study are available in Dryad Digital Repository, https://doi.org/10.5061/dryad.gmsbcc2np (62). Original DNA-metabarcoding data are also available in Dryad, https://doi.org/10.5061/dryad.c119gm5 (63).