Significance
Insulin is a major self-antigen in type 1 diabetes (T1D), and as such, insulin-based immunotherapies have been trialed to treat the underlying autoimmunity but with minimal clinical benefit. Here, we comprehensively assessed reactivity to insulin and its precursor, preproinsulin, by CD8 T cells obtained from the pancreatic islets of organ donors with and without T1D. CD8 T cells highly reactive to peptides throughout the entire preproinsulin protein were only found in T1D donors at varying frequencies. Our results suggest considering the use of preproinsulin rather than just insulin for intervention immunotherapies and have important implications for identifying individuals that may respond to antigen-specific therapies designed to treat autoimmune disorders.
Keywords: type 1 diabetes, CD8 T cells, antigens, epitopes, insulin
Abstract
Cytotoxic CD8 T lymphocytes play a central role in the tissue destruction of many autoimmune disorders. In type 1 diabetes (T1D), insulin and its precursor preproinsulin are major self-antigens targeted by T cells. We comprehensively examined preproinsulin specificity of CD8 T cells obtained from pancreatic islets of organ donors with and without T1D and identified epitopes throughout the entire preproinsulin protein and defective ribosomal products derived from preproinsulin messenger RNA. The frequency of preproinsulin-reactive T cells was significantly higher in T1D donors than nondiabetic donors and also differed by individual T1D donor, ranging from 3 to over 40%, with higher frequencies in T1D organ donors with HLA-A*02:01. Only T cells reactive to preproinsulin-related peptides isolated from T1D donors demonstrated potent autoreactivity. Reactivity to similar regions of preproinsulin was also observed in peripheral blood of a separate cohort of new-onset T1D patients. These findings have important implications for designing antigen-specific immunotherapies and identifying individuals that may benefit from such interventions.
Type 1 diabetes (T1D) results from the chronic immune-mediated targeting of insulin-producing beta cells within pancreatic islets with T cells playing a central role in disease pathogenesis (1, 2). Tissue specificity and a strong genetic association with the human leukocyte antigen (HLA) locus suggest that antigen specificity is necessary for T cells to attack beta cells and induce T1D (3). Therefore, considerable efforts have been undertaken to identify antigens for disease-associated T cells in order to understand the disorder’s pathogenesis and develop therapies to prevent T1D (4–8). As such, many large well-controlled clinical trials evaluating antigen-specific immunotherapies have been conducted (e.g., oral, intranasal, subcutaneous, and DNA vaccines), especially with a focus on insulin- or preproinsulin-related epitopes. Unfortunately, none have achieved favorable clinical outcomes to date (9–14). However, several trials have identified subsets of responders who may potentially benefit from antigen-specific immunotherapy (13–18), thus suggesting the potential for heterogeneity of antigen specificity targeted by the adaptive immune system in individual T1D patients. Indeed, a recent report from the Environmental Determinants of Diabetes in the Young study demonstrated a trend of first appearing islet autoantibodies classified by HLA haplotypes (19), implicating that individual patients may have different antigen specificities that initiate and drive T1D development. To improve prevention efforts and direct tissue- and autoantigen-specific immunotherapy, an improved understanding of epitopes that activate T cells within inflamed pancreatic islets and subsequent reactivity in the peripheral blood is needed to optimally select patients for these therapies.
While the contribution of both CD4 and CD8 T cells to T1D development is evident, several lines of evidence have highlighted the importance of CD8 T cells as mediators of disease pathogenesis. First, CD8 T cells predominate within the immune infiltrates of inflamed pancreatic islets from T1D organ donors (7, 20). Second, as HLA class I molecules present peptides to activate CD8 T cells, beta cells within inflamed T1D islets up-regulate HLA class I molecules, which has the potential to enhance CD8 T cell−beta cell–specific interactions (20, 21). Finally, islet-specific CD8 T cells have been measured in the peripheral blood of T1D patients using fluorescent peptide/major histocompatibility complex (MHC) multimers (22, 23), with some of these specificities correlating to the functional loss of insulin secretion following the clinical onset of T1D (24, 25).
Fluorescent peptide/MHC multimer reagents have previously been used to identify self-reactive CD8 T cells within human T1D islets by in situ staining frozen pancreas sections (20, 26–28). These studies focused on islet peptides presented by the HLA-A2 variant, as it is most frequent in both T1D and the general population, with estimated frequencies of 50 to 70% and 20 to 40%, respectively (29). While these assays using peptide/MHC reagents efficiently detect antigen-specific T cells, they are biased toward limited epitopes and HLA usage and sometimes cannot discriminate T1D patients from nondiabetic controls, especially when staining T cells from the peripheral blood (26, 30–32). Thus, a compelling need exists to comprehensively examine CD8 T cell antigen specificity across various HLA class I molecules within human pancreatic islets. Therefore, in this study, we analyzed the antigen specificity from hundreds of CD8 T cells obtained from the islets of both T1D and nondiabetic organ donors without bias for HLA types and with minimal ex vivo amplification. Herein, we provide evidence that islet-infiltrating CD8 T cells reactive to a major self-antigen, insulin and its precursor preproinsulin, are abundant in three of seven T1D organ donors but not in nondiabetic donors. Preproinsulin-reactive CD8 T cell epitopes are presented by various HLA class I molecules, including epitopes spread throughout the entire preproinsulin protein with three hot spots, and elicit reactivity from the peripheral blood in a separate cohort of newly diagnosed T1D patients.
Results
T Cells within Pancreatic Islets.
We analyzed islet samples obtained from seven T1D and seven nondiabetic organ donors for T cell infiltration and T cell receptor (TCR) sequence repertoires (Table 1). The T1D donors were children, adolescents, or young adults and had T1D for relatively short periods of time, months to a few years, except one donor who had had diabetes for 15 y. Of note, three T1D donors who had a shorter diabetes duration than the other study subjects had low but detectable levels of C-peptide as measured within 24 h prior to or at death, indicating some residual beta cell function in these donors.
Table 1.
Donor information
| Donor ID | Donor reference | Sex | Age (years) | Duration of diabetes (years) | Islet-AAbs* | C-peptide (ng/mL) | HLA-DRB1 | HLA-A | HLA-B | HLA-C |
| T1D-1 | nPOD 69 | F | 6 | 3 | IAA | <0.05 | 04:01/07:01 | 02:01/26:01 | 35:01/50:01 | 04:01/06:02 |
| T1D-2 | nPOD 6323 | F | 22 | 6 | GADA IA2A | <0.02 | 03:01/04:02 | 01:01/25:01 | 08:01/18:01 | 07:01/12:03 |
| T1D-3 | nPOD 6342 | F | 14 | 2 | IAA IA-2A | 0.26 | 01:01/04:01 | 02:01/68:01 | 40:01 | 03:04 |
| T1D-4 | nPOD 6367 | M | 24 | 2 | None | 0.39 | 04:01/07:01 | 02:01/29:02 | 18:01/44:03 | 07:01/16:01 |
| T1D-5 | nPOD 6414 | M | 23 | 0.4 | IAA GADA ZnT8A | 0.16 | 03:01/09:01 | 01:01/23:01 | 07:02/08:01 | 07:01/07:02 |
| T1D-6 | nPOD 6472 | F | 10 | 4 | IAA | 0.02 | 03:01/04:04 | 02:01/32:01 | 08:01/18:01 | 05:01/07:01 |
| T1D-7 | VUMC/ Pittsburgh | F | 28 | 15 | IAA | 0.03 | 08:04/09:01 | 02:05/23:01 | 35:01/41:02 | 04:01/17:01 |
| ND-1 | IIDP 9657 | F | 34 | No diabetes | Not tested | Not tested | 03:01/15:01 | 01:01/02:01 | 08:01/44:02 | 05:01/07:01 |
| ND-2 | IIDP 1726 | M | 21 | No diabetes | Not tested | Not tested | 07:01 | 02:01/29:02 | 27:05/44:03 | 02:02/16:01 |
| ND-3 | R300 | F | 30 | No diabetes | Not tested | Not tested | 03:01 | 01:01/30:02 | 08:01/18:01 | 05:01/07:01 |
| ND-4 | R301 | M | 18 | No diabetes | Not tested | Not tested | 11:01/13:02 | 02:01 | 14:02/49:01 | 07:04/08:02 |
| ND-5 | IIDP 5642 | M | 30 | No diabetes | Not tested | Not tested | 10:01/13:02 | 02:01/24:02 | 40:01/45:01 | 03:04/16:01 |
| ND-6 | IIDP 7400 | F | 30 | No diabetes | Not tested | Not tested | 03:01/15:01 | 03:01/30:02 | 07:02/18:01 | 05:01/07:02 |
| ND-7 | R283 | M | 22 | No diabetes | Not tested | Not tested | 1,13 | 1,2 | 51 (Bw4) | Cw15 |
Islet-autoantibodies: IAA = insulin antibodies; GADA = glutamic acid decarboxylase antibodies; IA-2A = insulinoma antigen-2 antibodies; ZnT8A = zinc transporter 8 antibodies
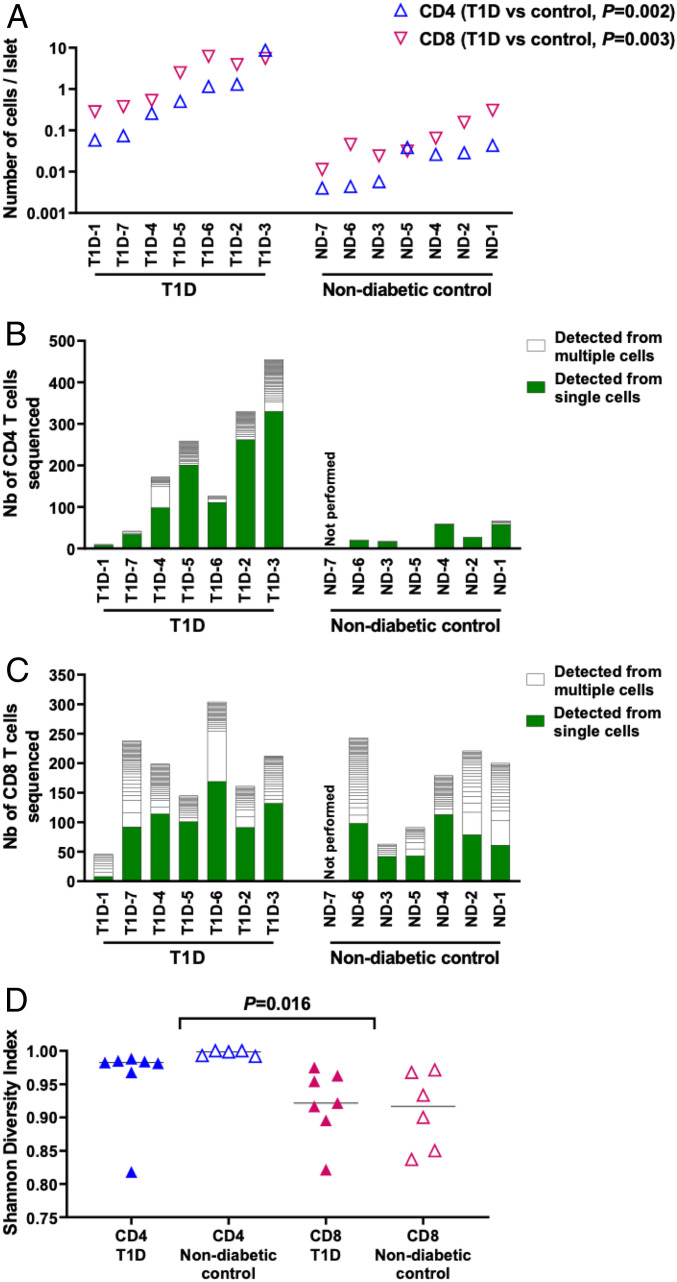
We first assessed numbers of CD4 and CD8 T cells in islet samples within a few days after islets were isolated and cultured in media containing interleukin-2, interleukin-15, and interleukin-7; one exception was the sample from T1D-1, from which islets had been cultured over 10 d. Thus, the presence of T cells in islets were determined relatively soon after their isolation, when repertoire changes from in vitro culture could be minimized. We dispersed the isolated islets into single cells and analyzed them for the presence of CD4 and CD8 T cells by flow cytometry (SI Appendix, Fig. 1). While individual donors displayed a broad range of T cell numbers in the islets, T1D donors had significantly higher numbers of both CD4 and CD8 T cells compared to nondiabetic donors (Fig. 1A, P = 0.002 [CD4], P = 0.003 [CD8], T1D versus nondiabetic donors), and CD8 T cells tended to be more prevalent than CD4 T cells in both T1D and the nondiabetic donor groups (Fig. 1A, P = 0.22 [T1D donors], P = 0.03 [nondiabetic donors], CD4 versus CD8).
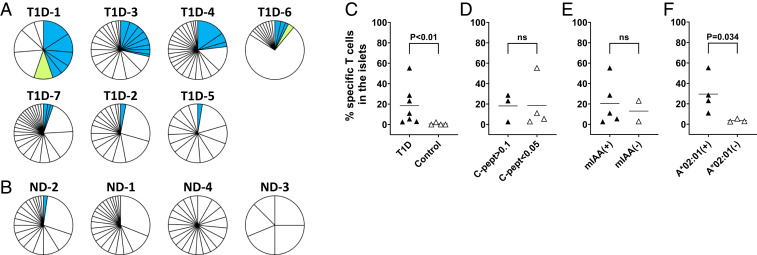
Fig. 1.
T cells in the islets of organ donors with and without T1D. (A) Numbers of CD4 (blue triangles) and CD8 (magenta inverse triangles) T cells detected per islet in each donor are shown. (B and C) Frequencies of TCR sequences detected from single and multiple cells are shown in green and white bars, respectively. White rectangles represent each unique TCR clonotype. Panels (B and C) show TCR sequencing results from CD4 and CD8 T cells, respectively. (D) Individual symbols (blue: CD4, magenta: CD8, filled triangles: T1D donors, and open triangles: nondiabetic donors) indicate Shannon diversity indexes of TCR repertoires, in which 1 represents each T cell expresses a different TCR clonotype from all other T cells in a sample, and 0 represents all T cells in a sample express an identical TCR clonotype. Statistical significance for numbers of CD4 and CD8 T cells per islet and Shannon Diversity Index of TCR repertoires were assessed by the Wilcoxon rank sum test.
To further evaluate diversity and clonality, we examined TCR sequences expressed by tens to hundreds of T cells isolated from the islet samples. Certain proportions of TCR clonotypes were detected from multiple cells in both CD4 and CD8 subsets in the islets of T1D and nondiabetic donors, and some samples such as the CD4 subset of T1D-4 and CD8 subset of T1D-6 contained clonotypes that were expressed by over 20% of all T cells, suggesting expansion of specific islet-infiltrating T cells (Fig. 1 B and C). When analyzing TCR repertoire diversity in each sample by calculating Shannon’s diversity index, all subsets were relatively diverse as index values of all samples were over 0.8, and there was no apparent difference between T1D and nondiabetic controls (Fig. 1D). However, CD4 repertoires were more diverse than CD8 repertoires (Fig. 1D, P = 0.016, CD4 versus CD8). This suggests that CD8 repertoires contained a higher proportion of clonally expanding cells than the CD4 repertoires, although it should be noted that the cell expansion may have occurred during in vitro culture for several samples such as T1D-1. Taken together, these results indicate that the pancreatic islets in T1D organ donors contain larger numbers of T cells than those in nondiabetic donors, and TCR repertoires in the islets of both T1D and nondiabetic donors were relatively diverse, yet there were clonally identical T cells especially within the CD8 subset.
Screening Islet-Residing CD8 T Cells for Reactivity to Preproinsulin Peptides.
Next, we addressed the issue of which antigens are targeted by the T cells in the islets. To evaluate this question, we began by examining reactivity to preproinsulin, since this molecule is an abundant protein that is exclusively produced by pancreatic beta cells. We previously analyzed specificity to preproinsulin by CD4 T cells and identified several TCR clonotypes that responded to an insulin B chain, A chain, or C-peptide, a fragment that is cleaved from the A and B chains of insulin during production of the mature insulin molecule (8, 33). In the current study, we focused on CD8 T cells and sought to determine their specificity to preproinsulin-related peptides. To test functional autoreactivity of islet-residing CD8 T cells, we expressed specific TCR clonotypes determined from the islet samples in a 5KC T-hybridoma cell line, which is devoid of endogenous TCR expression, and used these TCR transductants for specificity analysis. TCR clonotypes were selected from those that were expressed by two or more CD8 T cells in the islets of the seven T1D donors, resulting in a total of 151 TCR clonotypes expressed by 136 unique T cells (SI Appendix, Table 1). Only seven TCRs failed to be expressed on the cell surface. These seven were derived from T cells that had two in-frame alpha chain clonotypes and may be intrinsically nonfunctional, leaving 144 TCR clonotypes that were subject to functional autoreactivity analysis.
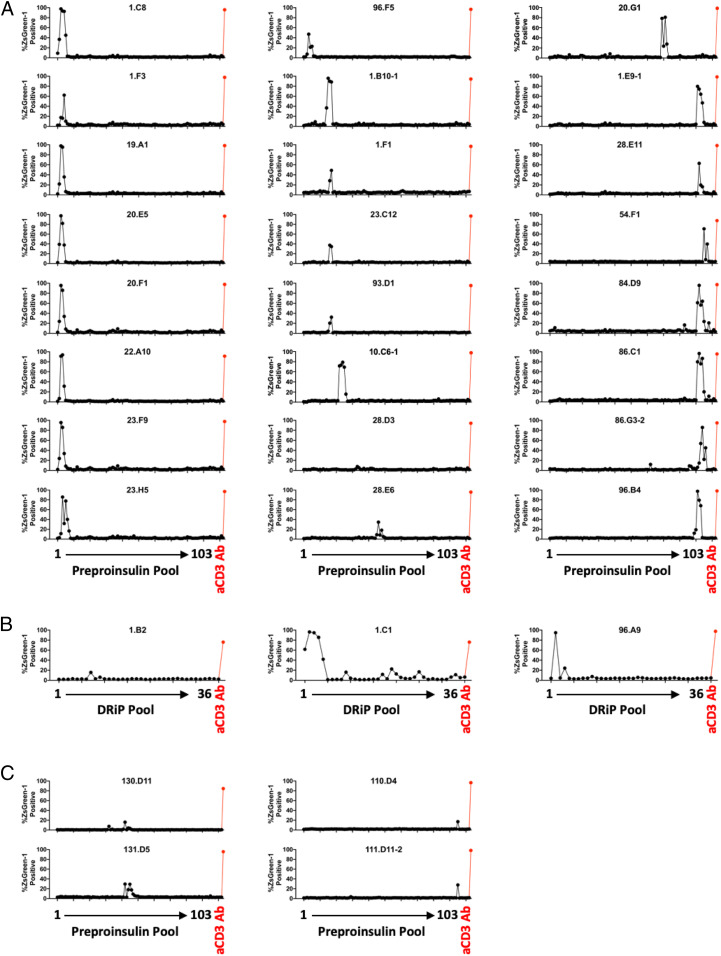
To screen for antigen specificity, we adopted a multiplex assay system, in which 5KC T-hybridoma cells expressing each TCR clonotype and marked with unique fluorescent proteins were engineered to express a ZsGreen-1 fluorescent protein upon activation, thereby being capable of discretely assessing activation of multiple TCR transductants in a single culture well by flow cytometry (34). Using this assay system, we first tested responses to 103 preproinsulin-derived peptide pools in the presence of Epstein–Barr virus (EBV)-transformed autologous B cells. Each peptide pool consists of 8 to 11 mers of peptides ending at the same position of preproinsulin (SI Appendix, Table 2) and thus all peptide sequences that could be generated from preproinsulin were examined. After excluding two TCR transductants that showed nonspecific responses without antigen stimulation, 24 out of 142 TCR clonotypes (17%) reacted with one or more preproinsulin peptide pools (SI Appendix, Fig. 2A). As expected, many of these TCR transductants responded to peptide pools containing consecutive sequences (Fig. 2A).
Fig. 2.
TCR clonotypes with positive responses in the screening with preproinsulin and insulin DRiP truncated peptide pools. TCR transductant cell lines with an NFAT reporter were cultured with 103 preproinsulin (A and C) and 36 insulin DRiP (B) truncated peptide pools or an anti-CD3 antibody in the presence of autologous EBV-transformed B cells. TCR transductants express a ZsGreen-1 fluorescent protein when activated. Percentages of TCR transductant cells expressing ZsGreen-1 in response to each peptide pool or anti-CD3 antibody are plotted. Individual panels show screening results of each TCR transductant responding to one or more peptide pools derived from T1D organ donors (A and B) or nondiabetic control donors (C).
In addition to preproinsulin peptides, it has been demonstrated that a defective ribosomal product of preproinsulin messenger RNA (mRNA) (insulin DRiP) can be created. Insulin DRiP is formed from an alternate start codon in the INS gene and expressed by pancreatic beta cells, in particular under conditions of stress (35). CD8 T cells reactive to this product can be detected in peripheral blood of T1D patients and proved diabetogenic ex vivo (35, 36). Therefore, we further tested for insulin DRiP–reactive T cells in insulitic islets by surveying the same set of TCR transductants for responses to 36 peptide pools containing insulin DRiP–derived truncated peptides (SI Appendix, Table 3). Three TCR clonotypes derived from two T1D donors responded to insulin DRiP peptide pools in the presence of autologous EBV transformed B cells (Fig. 2B and SI Appendix, Fig. 2B). Additionally, we tested responses to 11 other islet protein–derived peptides that are known to be autoantigens for T1D (SI Appendix, Table 4); however, none of the 142 studied TCR transductants responded to those peptides, including the zinc-transporter 8 (ZnT-8) 186-194 peptide and islet-specific glucose-6-phosphatase catalytic subunit–related protein 265-273 peptide (SI Appendix, Fig. 2C). CD8 T cells in the islets could be specific to proteins derived from cells within islets other than beta cells. Therefore, we screened for a response to peptide pools derived from glucagon, a major protein expressed by pancreatic alpha cells (SI Appendix, Table 5), and detected no responses to 173 peptide pools ranging from the first through the last amino acid of glucagon (SI Appendix, Fig. 3). In sum, screening of 142 TCR clonotypes derived from CD8 T cells in the residual pancreatic islets of T1D donors identified 27 TCR clonotypes that are potentially reactive to beta cell protein products, preproinsulin or insulin DRiP.
HLA Restriction for Preproinsulin-Responsive CD8 T Cells.
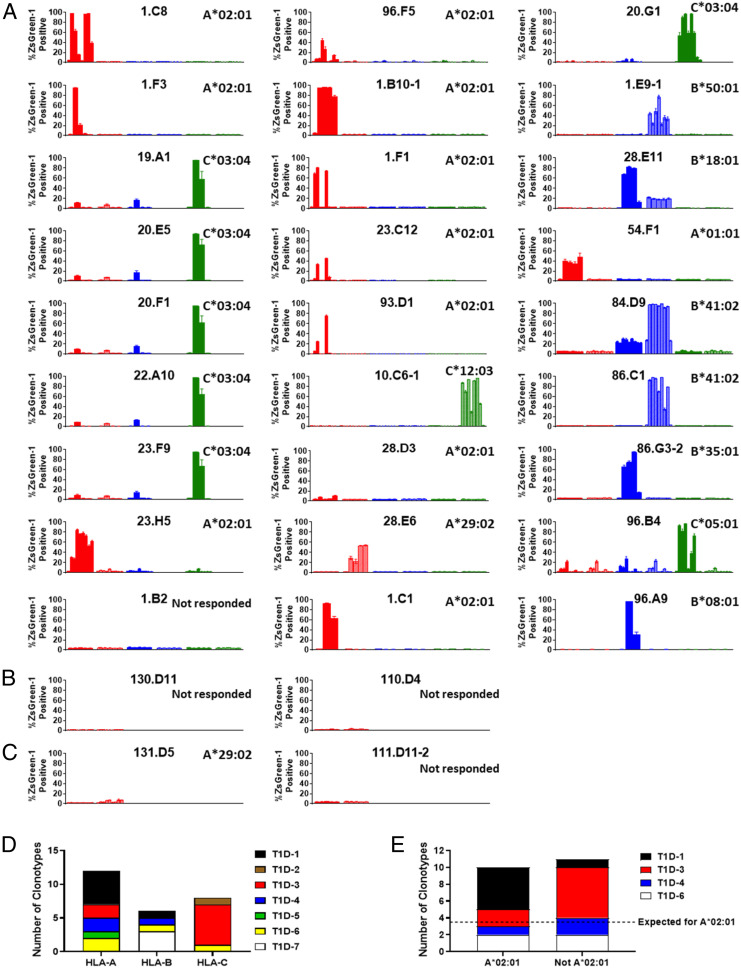
To confirm that the 27 TCR clonotypes truly respond to peptides identified by screening with peptide pools, individual peptides contained in each pool that provided positive responses were newly synthesized and used to stimulate cognate TCR transductants. Simultaneously, we sought to identify HLA molecules that present peptides to the TCRs by testing reactivity in the presence of antigen-presenting cell lines transduced with each HLA class I allele of the donors. We introduced each HLA class I gene into myelogenic K562 cells, which are devoid of endogenous HLA expression, and used them as antigen-presenting cells. TCR transductants were individually cultured with or without each single peptide in the presence of each HLA transductant line (Fig. 3 A and B and SI Appendix, Table 6). Overall, responsiveness to the newly synthesized peptides was confirmed in all TCR transductants except 1.B2, which responded to an insulin DRiP–truncated peptide pool only weakly in the screening. The remaining 26 TCR transductants reacted with one or more peptides presented by a particular HLA molecule, thus allowing identification of the HLA restriction molecules that present peptides to individual TCR clonotypes. All types of class I molecules, HLA-A, -B, and -C, presented preproinsulin peptides to the tested TCR clonotypes (Fig. 3D). However, among donors having the HLA-A*02:01 allele, which is known to confer genetic risk for T1D (37, 38), 10 out of 21 TCR clonotypes (48%) recognized their cognate peptide presented by HLA-A*02:01. This is significantly more than those restricted by all other available class I molecules assuming equal use among a total of six possible presenting molecules (Fig. 3E, P < 0.001). Thus, polymorphic variants of HLA-A, -B, and -C class I molecules are capable of presenting preproinsulin or DRiP peptides to islet-derived CD8 TCRs from T1D organ donors, with an enrichment for preproinsulin reactivity in T cells restricted by HLA-A*02:01.
Fig. 3.
HLA restrictions of preproinsulin and insulin DRiP–reactive CD8 T cells. (A–C) TCR transductants were cultured with or without peptides in the presence of K562 antigen–presenting cells expressing each HLA class I molecule. Peptides contained in one or two peptide pools that induced the strongest response in the screening test were used as stimulus, and responses to each peptide are shown in the order listed in SI Appendix, Table 6a. Peptide sequences are shown in SI Appendix, Table 6b. Responses in the presence of antigen-presenting cells expressing each HLA-A, HLA-B, and HLA-C molecule are shown in red, blue, and green bars, respectively, and HLA alleles tested for each TCR transductant are indicated in SI Appendix, Table 6a. Results for first and second HLA alleles listed in the table are shown in dark and light colors in the figures, respectively. ZsGreen-1 expression levels in wells without peptides are indicated in the most Left bars for each HLA allele. Results for preproinsulin-reactive TCR clonotypes derived from T1D donors, insulin DRiP–reactive TCRs derived from T1D donors, and preproinsulin-reactive TCR clonotypes derived from nondiabetic control donors are shown in panels A, B, and C, respectively. Mean values ± SEM obtained from three independent experiments are plotted, and a determined HLA allele is indicated in the Upper Right corner of each panel. (D) The numbers of clonotypes restricted by HLA-A, -B, and -C molecules are indicated. Individual colors represent TCR clonotypes derived from different donors. (E) Among 21 clonotypes derived from donors having HLA-A*02:01, the numbers of clonotypes restricted by A*02:01 and those restricted by alleles other than A*02:01 are shown. The dashed line indicates an expected probability of restriction by each allele (i.e., 21 divided by 6 possible alleles in each individual). An exact binomial test was used to test whether the proportion of TCR clonotypes restricted with HLA-A*02:01 was equal to 1/6, and this was not the case as 10/21 (48%) restricted to HLA-A*02:01 (P < 0.001).
Defining Optimal Epitopes and Measuring Intensity of T Cell Response.
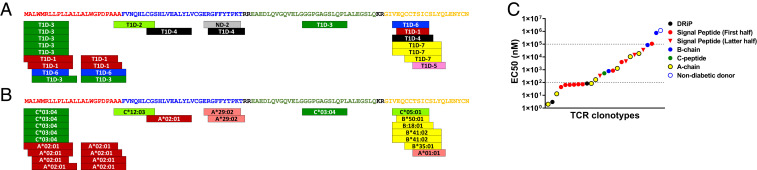
The avidity between a TCR and a peptide-MHC complex is one factor that determines phenotype and cytotoxic activity of CD8 T cells (39, 40). To determine the preferred epitopes and intensity of responses to those peptides, we titrated peptide concentrations with each TCR transductant in the presence of K562 antigen–presenting cells expressing cognate HLA molecules (SI Appendix, Fig. 4 A and B). Epitopes were found throughout all regions of preproinsulin: signal peptide, B-chain, C-peptide, and A-chain; but there were three regions that were preferentially recognized by a number of TCR clonotypes derived from different T1D donors, two distinct regions in signal peptide and one in A-chain (Fig. 4A). Among TCR clonotypes recognizing peptides from the same region, the preferred peptide was identical when TCRs were restricted by the same HLA-I molecule. However, epitope sequences diverged between TCRs restricted by different HLA-I molecules within the same region of preproinsulin (Fig. 4B). For example, five TCR clonotypes that preferably responded to preproinsulin 1 to 11 were restricted by HLA-C*03:04, whereas those restricted by HLA-A*02:01 recognized peptides shifted toward the C terminus of preproinsulin. These results suggest that peptides from certain regions of preproinsulin are discriminatorily generated in either pancreatic beta cells or antigen-presenting cells and presented in a particular register by HLA class I molecules to activate T cells.
Fig. 4.
Preproinsulin epitopes inducing CD8 T cell reactivity. Positions of epitopes within preproinsulin targeted by 25 TCR clonotypes are indicated with donor ID (A) and HLA restriction (B). Amino acid sequences in the signal peptide, B-chain, C-peptide, and A-chain regions are shown in red, blue, green, and yellow, respectively. (C) Intensity of responses by individual TCRs was evaluated by EC50 values. EC50 values of TCR transductants responding to peptides within insulin-DRiP, signal peptide, B-chain, C-peptide, and A-chain regions are shown in black, red, blue, green, and yellow. TCR clonotypes reactive to peptides in the first half (positions 1 to 13) and the latter half (positions 15 to 26) of signal peptide region are indicated by circles and inverse triangles, respectively. The TCR clonotype derived from a nondiabetic control donor (ND-2) is shown in blue open circle.
Intensity of response by individual TCR transductants was diverse, as represented by half-maximal effective concentration (EC50) values ranging from low nM to over 100 µM (Fig. 4C and Table 2). Some A-chain–reactive TCR transductants reacted strongly, and responsiveness to the first half of the signal peptide region was very intense as well. Intriguingly, EC50 values of insulin DRiP–reactive TCR transductants are in the low nanomolar range, ranked among the highest avidities. This observation could relate to DRiP being a neoantigen expressed in periphery only (stressed islets) and unlikely to contribute to thymic education, clonal deletion, and central tolerance (35). On the other hand, TCR transductants reactive to the latter half of signal peptide or B-chain regions need a higher concentration of peptide for activation. These TCRs that were weakly responsive to peptide may require a posttranslational modification to become antigenic, as has been described for C-peptide–reactive CD4 T cells in the pancreatic islets of T1D organ donors (41, 42). Since the cell surface expression level of HLA molecules on antigen-presenting cells can contribute to TCR responsiveness, we examined HLA expression on our K562 antigen–presenting cells and the majority of the HLA class I variants are expressed at similar levels (SI Appendix, Fig. 5). Taken together, optimal epitopes recognized by the 26 CD8 TCR clonotypes spanned various regions of preproinsulin, and the intensity of response to these peptides differed by individual TCRs.
Table 2.
Preproinsulin (PPI) and insulin DRiP–reactive T cell receptors
| Donor ID | TCR ID | Freq | HLA | Epitope | EC50 (nM) | TRAV | TRAJ | CDR3a | TRBV | TRBJ | CDR3b |
| T1D-1 | 1.C8 | 5.3% | A0201 | PPI: 1-11/2-12/2-10 | 68 | TRAV24 | TRAJ58 | CAFKRETSGSRLTF | TRBV13 | TRBJ2-1 | CASSTRLAGDEQFF |
| T1D-1 | 1.F3 | 15.8% | A0201 | PPI: 2-12 | 848 | TRAV39 | TRAJ39 | CAVENAGNMLTF | TRBV10-2 | TRBJ2-1 | CASWTVSYNEQFF |
| T1D-1 | 1.B10-1 | 7.9% | A0201 | PPI: 15-23 | 332 | TRAV8-3 | TRAJ33 | CAVVADSNYQLIW | TRBV4-2/4-3 | TRBJ2-2 | CASSQTKGTGELFF |
| T1D-1 | 1.F1 | 5.3% | A0201 | PPI: 15-24 | 14,418 | TRAV39 | TRAJ41 | CAVSNSGYALNF | TRBV29-1 | TRBJ2-5 | CSVFHRGETQYF |
| T1D-1 | 1.E9-1 | 10.5% | B5001 | PPI: 92-99 | 18,470 | TRAV12-2 | TRAJ34 | CAVNIRYNTDKLIF | TRBV6-2/6-3 | TRBJ1-5 | CASSSIQGSGSGQPQHF |
| T1D-1 | 1.C1 | 10.5% | A0201 | DRiP: 1-9 | 3 | TRAV12-1 | TRAJ39 | CGENNAGNMLTF | TRBV27 | TRBJ2-5 | CASSLQPPGTSTETQYF |
| T1D-2 | 10.C6-1 | 2.9% | C1203 | PPI: 23-32 | 784 | TRAV19 | TRAJ39 | CALSGALNNAGNMLTF | TRBV27 | TRBJ2-5 | CASSLFGYRQETQYF |
| T1D-3 | 23.F9 | 3.9% | C0304 | PPI: 1-11 | 73 | TRAV12-3 | TRAJ48 | CAMSALGNFGNEKLTF | TRBV19 | TRBJ2-1 | CASSIAGGNEQFF |
| T1D-3 | 19.A1 | 2.6% | C0304 | PPI: 1-11 | 64 | TRAV8-4 | TRAJ11 | CAVSDQGSGYSTLTF | TRBV28 | TRBJ1-5 | CASSWTANQPQHF |
| T1D-3 | 20.E5 | 3.9% | C0304 | PPI: 1-11 | 66 | TRAV14/DV4 | TRAJ52 | CAMSNAGGTSYGKLTF | TRBV28 | TRBJ1-4 | CASSLARYNEKLFF |
| T1D-3 | 20.F1 | 3.9% | C0304 | PPI: 1-11 | 44 | TRAV14/DV4 | TRAJ43 | CAMRLHNNNDMRF | TRBV28 | TRBJ1-5 | CASIASRYNQPQHF |
| T1D-3 | 22.A10 | 1.3% | C0304 | PPI: 1-11 | 73 | TRAV8-1 | TRAJ13 | CAVNAAGGYQKVTF | TRBV28 | TRBJ2-1 | CASIPDRYNEQFF |
| T1D-3 | 23.H5 | 5.2% | A0201 | PPI: 3-13 | 3,992 | TRAV38-2/DV8 | TRAJ22 | CAYRSPARQLTF | TRBV6-1 | TRBJ2-7 | CASSEGWGVPSYEQYF |
| T1D-3 | 23.C12 | 2.6% | A0201 | PPI: 15-24/15-25 | 35,347 | TRAV41 | TRAJ42 | CAVSGGSQGNLIF | TRBV28 | TRBJ1-2 | CASSPPTGWGGYTF |
| T1D-3 | 20.G1 | 5.2% | C0304 | PPI: 69-77/69-79 | 525 | TRAV1-2 | TRAJ8 | CAVRMNTGFQKLVF | TRBV9 | TRBJ2-1 | CASSVGMDPGLGYNEQFF |
| T1D-4 | 28.D3 | 15.4% | A0201 | PPI: 31-41/34-41 | 753,461 | TRAV26-2 | TRAJ26 | CILTDNYGQNFVF | TRBV27 | TRBJ1-1 | CASSLIGLNTEAFF |
| T1D-4 | 28.E6 | 2.6% | A2902 | PPI: 46-54/47-54 | 85,759 | TRAV19 | TRAJ28 | CALSEAGAGSYQLTF | TRBV2 | TRBJ2-5 | CASSPSGTSSQETQYF |
| T1D-4 | 28.E11 | 5.1% | B1801 | PPI: 91-100 | 1,278 | TRAV12-2 | TRAJ49 | CAVSMNTGNQFYF | TRBV29-1 | TRBJ2-1 | CSVQVYNEQFF |
| T1D-5 | 54.F1 | 2.6% | A0101 | PPI: 96-103 | 10,519 | TRAV3 | TRAJ26 | CAVPDNYGQNFVF | TRBV7-2 | TRBJ2-2 | CASSLVVELFF |
| T1D-6 | 96.A9 | 3.4% | B0801 | DRiP: 1-9 | 83 | TRAV12-2 | TRAJ39 | CAVNVYNAGNMLTF | TRBV30 | TRBJ1-1 | CAWSVRGGSYMNTEAFF |
| T1D-6 | 96.F5 | 1.7% | A0201 | PPI: 3-11 | 108,241 | TRAV8-6 | TRAJ48 | CAVSDISNFGNEKLTF | TRBV9 | TRBJ2-3 | CASSVVGLGTDTQYF |
| T1D-6 | 93.D1 | 2.5% | A0201 | PPI: 15-25 | 4,487 | TRAV5 | TRAJ8 | CAVTKDTGFQKLVF | TRBV20-1 | TRBJ2-1 | CSARDHFGGSGYEQFF |
| T1D-6 | 96.B4 | 3.4% | C0501 | PPI: 91-99 | 168 | TRAV12-2 | TRAJ31 | CAVNNARLMF | TRBV6-5 | TRBJ2-1 | CASRPTSGGYNEQFF |
| T1D-7 | 86.C1 | 2.3% | B4102 | PPI: 91-100/92-100/92-102 | 13 | TRAV19 | TRAJ16 | CALSEAGFSDGQKLLF | TRBV19 | TRBJ2-1 | CASSIQFSYNEQFF |
| T1D-7 | 84.D9 | 1.6% | B4102 | PPI: 91-100/92-100/92-102 | 2 | TRAV29/DV5 | TRAJ43 | CAASNSNDMRF | TRBV7-9 | TRBJ2-1 | CASSLAQREQFF |
| T1D-7 | 86.G3-2 | 1.6% | B3501 | PPI: 94-102 | 84 | TRAV8-6 | TRAJ33 | CAVSDGYQLIW | TRBV6-1 | TRBJ2-7 | CASSGREAPYEQYF |
| ND-2 | 131.D5 | 2.2% | A2902 | PPI: 45-53 | 1,185,142 | TRAV12-2 | TRAJ35 | CAVNIGFGNVLHC | TRBV7-9 | TRBJ2-2 | CASSGGNTGELFF |
Islet-Residing Preproinsulin–Reactive CD8 T Cells Are Disease Specific.
Some T cells were detectable in the islets of nondiabetic donors by flow cytometry, although the number of T cells in nondiabetic islets was significantly lower than those from T1D donors (Fig. 1A). To address whether these islet-residing T cells in nondiabetic donors were specific to preproinsulin or insulin DRiP, TCR clonotypes that were detected from two or more CD8 T cells in nondiabetic islets were expressed on 5KC T hybridoma cell lines using the same method used to analyze TCR clonotypes found from T1D organ donors. We then tested them for response to the preproinsulin peptide pools, insulin DRiP pools, and 11 known islet peptides. Since peripheral immune tissues, such as spleen, were unavailable to generate autologous B cell lines from nondiabetic donors, responses were examined in the presence of K562 antigen–presenting cell lines transduced with each HLA allele present in a given donor. TCR transductants typically respond to antigens more strongly in the presence of K562 cells expressing a cognate HLA molecule than autologous EBV-transformed B cells. Therefore, assay sensitivity may be higher, but more false-positive responses would be detected in the screening of TCRs from nondiabetic donors compared to those from T1D donors. Among 71 TCR transductants that were successfully expressed and did not show nonspecific responses independent of peptide (SI Appendix, Table 1), four TCR transductants weakly responded to preproinsulin peptide pools, two derived from donor ND-1 and the other two from ND-2 (Fig. 2C and SI Appendix, Fig. 6). However, three of the four TCR transductants did not respond when tested with newly synthesized individual peptides. Only one TCR transductant from a nondiabetic organ donor (131.D5) responded to preproinsulin peptides presented by HLA-A*29:02 (Fig. 3C and SI Appendix, Table 6 and Fig. 4C). Notably, responsiveness of this TCR transductant was extremely weak, with an EC50 value of about 1,000 μM (Fig. 4C and Table 2). Thus, the presence of CD8 T cells having strong reactivity to preproinsulin or insulin DRiP appears specific to the islets of T1D organ donors (26/142 TCRs [T1D donors] versus 1/71 [nondiabetic donors], P < 0.001).
Heterogeneity in the Presence of Preproinsulin-Reactive CD8 T Cells.
As demonstrated in Islet-Residing Preproinsulin–Reactive CD8 T Cells Are Disease Specific, the presence of preproinsulin and insulin DRiP–specific TCR clonotypes in pancreatic islets is a unique feature of T1D organ donors. We further evaluated the frequency of CD8 T cells with these specificities within the pancreatic islets of the studied organ donors. As expected, the prevalence of preproinsulin or insulin DRiP–reactive T cells in T1D donors were significantly higher than those in nondiabetic donors (Fig. 5 A–C, P < 0.01). However, there were also differences in the frequencies among donors with T1D, ranging from only 3 (T1D-5 and T1D-2) to 55% (T1D-1). To explore determinants that influenced the frequency of preproinsulin or insulin DRiP–specific T cells in the islets, we analyzed the association with serum C-peptide values (C-peptide is cleaved 1:1 from insulin when secreted from beta cells) and the presence of insulin autoantibodies. There was no association with either of these factors (Fig. 5 D and E, P > 0.5 in both analyses). Thus, there was no evidence that the presence of functional beta cells or the presence of humoral immunity to insulin are associated with the presence of preproinsulin or insulin DRiP–reactive CD8 T cells in islets. Since preproinsulin or insulin DRiP–reactive TCR clonotypes were preferentially restricted by HLA-A*02:01 (Fig. 3E), we further investigated whether donors with the HLA-A*02:01 allele have a high frequency of preproinsulin or insulin DRiP–specific CD8 T cells in the islets. As shown in Fig. 5F, donors having HLA-A*02:01 had significantly higher proportions of preproinsulin or insulin DRiP–specific T cells in the islets compared to those not having A*02:01 (P = 0.034). These data provide a basis for particular HLA alleles being a determinant for individuals developing autoimmune responses directed against preproinsulin or insulin DRiP peptides.
Fig. 5.
Frequency of preproinsulin and insulin DRiP–reactive CD8 T cells. (A and B) Individual pie-charts indicate frequencies of CD8 T cells responding to preproinsulin (blue) and insulin DRiP (lime) peptides in CD8 T cells represented more than once in the islets of each donor. T cells expressing TCR clonotypes for which preproinsulin and insulin DRiP reactivity screening was performed were included in analysis. Each slice represents T cells expressing an identical unique TCR clonotypes. Panels A and B contain T1D and nondiabetic control donors, respectively. (C–F) Individual symbols represent frequencies of preproinsulin and insulin DRiP–reactive CD8 T cells among CD8 T cells studied for response to preproinsulin and insulin DRiP. Statistical significances for frequencies of preproinsulin and insulin DRiP–reactive CD8 T cells by donors with and without T1D (C), levels of C-peptide (D), the presence of insulin autoantibodies (E), and donors with and without HLA-A*02:01 (F) were assessed by the Mann–Whitney U test.
Preproinsulin Immune Responses in the Peripheral Blood of New-Onset T1D Patients.
We next sought to determine whether new-onset T1D patients have CD8 T cell reactivity to preproinsulin peptides similar to that observed in the islets of T1D organ donors. As the islet CD8 TCR clonotypes had optimal epitopes across regions of preproinsulin and 10 separate HLA class I molecules presented these epitopes, we elected to screen patients for peripheral blood responses using a cytokine enzyme-linked immunosorbent spot (ELISPOT) assay for IFN-γ, as opposed to using a tetramer-based assay. Similar to our studies screening islet TCR transductants, we used preproinsulin peptide pools consisting of 8 to 11 mers and grouped the pools into 10 separate conditions that spanned the entire protein. Peripheral blood mononuclear cells (PBMCs) from new-onset T1D patients and nondiabetic controls (Table 3 and Dataset S1) were tested for response to these pools. The groups were well matched in terms of age, sex, and the presence of HLA-A*02:01. All of the T1D patients were within less than 3 wk of diagnosis, with an average of only 3.5 d, such that exogenous insulin administration would not induce T cell responses.
Table 3.
Demographic and immunologic characteristics of study participants with preproinsulin ELISPOT assays
| New-onset T1D [n = 30] | Non-diabetic [n = 18] | P value* | |
| Age, years | 0.269 | ||
| Mean (SD) | 15.0 (6.1) | 17.5 (9.0) | |
| Median | 13.4 | 14.5 | |
| Range | 5.1 to 30.9 | 4.8 to 31.5 | |
| Gender | 0.127 | ||
| Female % [number] | 27% [n = 8] | 50% [n = 9] | |
| Race/Ethnicity | 0.293 | ||
| Non-Hispanic White | 80% [n = 27] | 88% [n = 16] | |
| Non-Hispanic Black | 10% [n = 3] | 0% [n = 0] | |
| Hispanic | 10% [n = 3] | 6% [n = 1] | |
| Asian | 0% [n = 0] | 6% [n = 1] | |
| Diabetes duration, days | |||
| Mean (SD) | 3.5 (4.0) | NA | |
| Median | 3.0 | NA | |
| Range | 0 to 21 | NA | |
| Islet autoantibodies, % [number] | |||
| Insulin | 47% [n = 14] | NA | |
| GAD65 | 70% [n = 21] | NA | |
| IA-2 | 67% [n = 20] | NA | |
| ZnT8 | 63% [n = 19] | NA | |
| Positive for ≥1, % [number] | 87% [n = 26] | NA | |
| HLA-A*02:01, % [number] | 0.546 | ||
| Present | 62% [n = 18] | 50% [n = 9] | |
| Absent | 38% [n = 11] | 50% [n = 9] |
SD = SD, NA = not applicable, GAD65 = glutamic acid decarboxylase 65, IA-2 = insulinoma antigen-2, ZnT8 = zinc transporter 8
P values for continuous variables use Student’s t test, categorical variables use Fisher’s exact test.
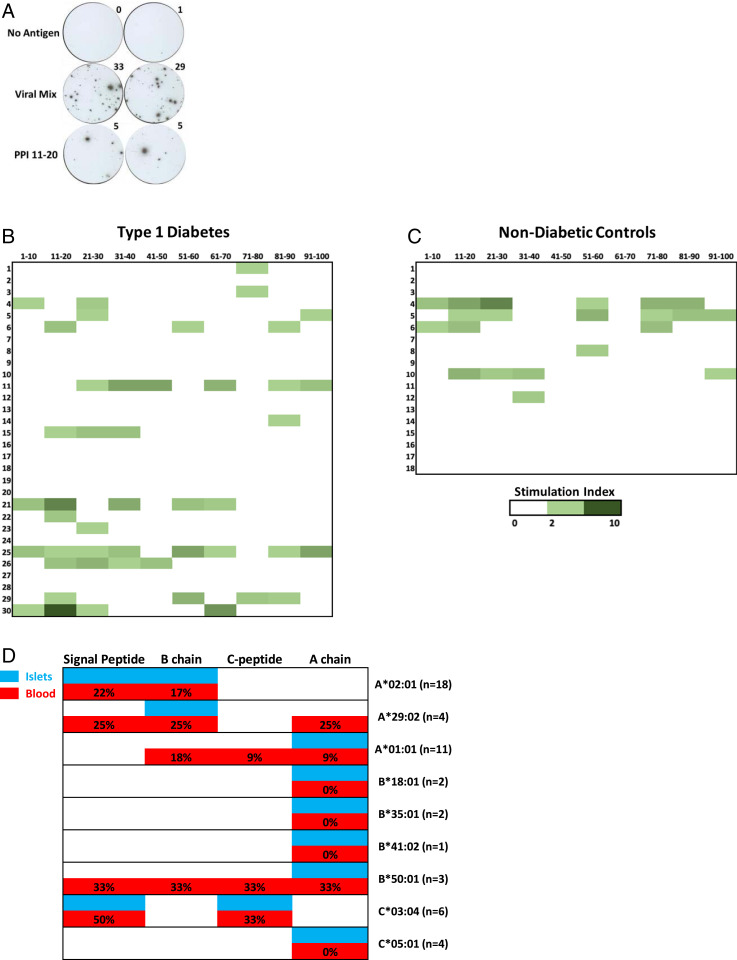
IFN-γ ELISPOTs could be clearly delineated (Fig. 6A), and the majority of patients had a response to pooled viral peptides that are known to be CD8 T cell epitopes from cytomegalovirus, EBV, and measles (22) (SI Appendix, Fig. 7). To compare responses between subjects and groups, a stimulation index was calculated by dividing the number of ELISPOTs for a given preproinsulin peptide pool by those without any in vitro added antigen. A subset of new-onset T1D patients had responses to multiple preproinsulin peptide pools, 33% (10/30, Fig. 6B). Similar to our findings from islet-derived CD8 TCRs, the IFN-γ responses spanned all regions of the preproinsulin protein. Nondiabetic controls also had some responses with 22% (4/18) responding to two or more pools (Fig. 6C), which is a comparable frequency to one out of four nondiabetic organ donors having a preproinsulin reactive CD8 TCR (Fig. 5B). When comparing islet CD8 TCR reactivity from T1D organ donors to the blood of new-onset patients (Fig. 6D), there was remarkable similarity in the region of preproinsulin targeted when accounting for HLA associations. These data indicate that peripheral blood T cell responses to preproinsulin epitopes in new-onset T1D patients mirror islet-derived CD8 T cell reactivity from T1D organ donors.
Fig. 6.
Peripheral blood immune responses to preproinsulin (PPI) peptide pools. In each case, cryopreserved PBMCs were cultured in the presence or absence of peptides for 48 h, washed, and then cells transferred to an IFN-γ monoclonal antibody–coated plate for overnight culture followed by development and enumeration of ELISPOTs. (A) Representative IFN-γ ELISPOT images from PBMCs stimulated with no antigen, a viral mix of peptides, and preproinsulin peptide pools containing amino acids 11 to 20. Heat maps showing the response of PPI pools in (B) new-onset T1D patients (n = 30) and (C) nondiabetic subjects (n = 18) as a stimulation index (SI) (No. ELISPOTs in a PPI pool / No. ELISPOTs no antigen). (D) Comparison of islet-derived TCR clonotypes responding to PPI epitopes from T1D organ donors to those from the peripheral blood of new-onset T1D subjects by HLA class I association. Percentages depict the number of new-onset T1D subjects with a given HLA allele that had a SI ≥ 3 for peptide pools within a region of preproinsulin; n = number of new-onset T1D patients with a given HLA allele. White indicates no reactivity found in islets from T1D organ donors or peripheral blood of new-onset subjects.
Discussion
Cytotoxic CD8 T cells are implicated in tissue destruction of many autoimmune disorders through self-antigen presentation by HLA class I molecules. Here, we studied CD8 T cells in the pancreatic islets of organ donors with autoimmune T1D and those without diabetes without HLA bias and characterized the antigen receptors from these T cells for autoreactivity to insulin, its precursor preproinsulin, a major self-antigen in T1D, and the alternative preproinsulin mRNA peptide DRiP that is an islet neoantigen. Our findings indicate that 1) there are higher numbers of T cells within the residual islets of donors with T1D compared to those without diabetes, 2) CD8 T cells highly specific to preproinsulin are enriched within the islets of a subset of T1D organ donors, and (3) a number of different HLA class I molecules present epitopes throughout the entire preproinsulin protein (signal peptide, B-chain, C-peptide, and A-chain) to these islet-derived CD8 T cells.
Our data indicate a remarkable difference in T cell numbers and preproinsulin specificity between T1D and nondiabetic donors. The finding of CD8 T cell infiltration within the islets of T1D donors being greater than that for CD4 is consistent with previous studies analyzing pancreas histology sections (20, 26, 31, 43), several of these studies also identified islet reactive CD8 T cells using fluorescent peptide/MHC multimers (20). We also found CD8 T cells present in non-T1D islets, albeit in low numbers, with a highly significant difference between preproinsulin reactive TCRs in T1D (26/142 TCR) versus non-T1D (1/71 TCR) islets. Multiple studies have demonstrated that islet-specific T cells are present in peripheral blood of individuals without T1D in a similar frequency to those with T1D, but such antigen-specific T cells are enriched in the pancreas of only those with T1D (27, 31). More specifically, von Herrath and colleagues recently showed that preproinsulin-specific T cells reside in the pancreatic exocrine compartment of nondiabetic organ donors, but over the course of disease development, they accumulate into insulin-containing islets (27). Our results analyzing preproinsulin specificity of T cells within the islets are consistent with these findings, and we further demonstrate that CD8 T cells in the islets of T1D donors exhibit high reactivity to preproinsulin. Important questions remain: Are T cells specific to insulin DRiP, which are not considered to be expressed in thymic epithelial cells, present in the exocrine tissue? Do pancreatic exocrine T cells move into the islets and what factors drives these T cells into the islets?
Tissue-resident CD8 T cells may not be sufficient to cause diabetes and may play a role in maintaining homeostatic tolerance (44, 45). In addition to preproinsulin-reactive CD8 T cells being infrequent in nondiabetic organ donor islets, we observed that the TCR transductants from those without diabetes required large concentrations of epitopes for stimulation compared to T1D organ donors. Whether or not these T cells reside in the pancreas due to coincidental tissue specificity, the fact that the majority of preproinsulin-reactive TCR transductants from T1D donor islets potently react with antigen at nanomolar concentrations implies that CD8 T cells in a diseased condition are clonally different from those without diabetes. It will be important to elucidate the timing within disease in which clonally different T cells that are highly preproinsulin reactive infiltrate the islets. Pancreas tissues isolated from prediabetic organ donors (i.e., autoantibody positive but not yet develop clinical diabetes) will aid in answering this question.
We also found heterogeneity in the frequency of preproinsulin-reactive CD8 T cells in the islets among T1D organ donors. Despite all seven donors having at least one TCR transductant responding to a preproinsulin epitope, there was a range of frequencies from 3 to over 40% of T cells responding to this self-antigen with three of seven donors having an abundant response. These frequencies were calculated within CD8 T cells for which antigen specificity analysis was conducted, and therefore, absolute frequencies could be over- or underestimated depending on the prevalence of preproinsulin or insulin DRiP–reactive T cells that were not analyzed for functional specificity. Nevertheless, it is likely that individual patients have different frequencies of CD8 T cells specific to preproinsulin or insulin DRiP in the islets. A key question remains how to identify individuals with frequent preproinsulin-reactive T cells in the pancreatic islets. HLA-A2+ T1D islet donors tended to have higher frequencies of preproinsulin-reactive CD8 T cells in the islets compared to those without this allele. This finding is comparable to those from a recent report showing a high frequency of T cells from pancreatic biopsies of recent onset T1D patients that were stained with a preproinsulin:15-24/HLA-A2 fluorescent multimer (28). Further studies analyzing the association between HLA haplotypes and preferred antigen specificity are warranted to dissect the heterogeneity of T cell responses in T1D.
Understanding antigen specificity of T cells within pancreatic islets has important implications for designing and testing antigen-specific immunotherapy. Many clinical trials have used proinsulin- or insulin-based therapies in an attempt to prevent T1D onset and induce tolerance (16, 17). These approaches have included parenteral insulin, oral insulin, nasal insulin, CD4 T cell epitopes within proinsulin, and a DNA proinsulin vaccine (9–14, 18). Randomized-placebo–controlled trials using these therapies proved safe and showed heterogeneous efficacy with subsets of responders, and specific immune effects are apparent with the DNA proinsulin vaccine reducing the frequency of CD8 T cells reactive to proinsulin (12). Unfortunately, none of these insulin-based antigen-specific therapies demonstrated sufficient clinical benefit to date (46). The diversity of preproinsulin reactivity within the islets, in terms of individual patient response and epitopes utilized for CD8 T cells, may account for some of these heterogeneous results. Therapies that utilize insulin (parenteral, oral, and nasal) are composed of just the A and B chains of the protein, and from our studies, it is clear that there are CD8 T cell responses in the islets and blood to C-peptide and the signal peptide in addition to the A and B chains, highlighting consideration of using the whole preproinsulin protein rather than just insulin. In addition, clinical trials pursuing the relationship between HLA genotypes and responders to insulin-based antigen-specific therapies may allow us to classify individuals having clinical benefits from these immunotherapies.
A large number of T cell epitopes have been identified within preproinsulin as previously reviewed (4, 47, 48). Our assay system allowed us to screen reactivity to preproinsulin in a comprehensive manner and confirmed several known epitopes including preproinsulin:15-24 as a target for CD8 T cells in the islets (4, 48). We also identified additional epitopes presented by HLA class I molecules other than HLA-A*02:01. Our results indicate that several HLA class I molecules across HLA-A, -B, and -C can present epitopes of preproinsulin to activate islet-derived CD8 T cells. This has important implications for pathogenesis as well as biomarker development, as focusing on a single HLA class I allele will likely underestimate the total number of self-antigen–specific CD8 T cells. Importance of broad HLA coverage has been raised by a recent comprehensive review article about T cell epitopes for T1D (48), as several reports shed light on epitopes presented by various HLA class I molecules (49, 50). Additionally, we identified “hot spots” within the preproinsulin protein that were epitopes for islet-derived CD8 Tcells. Assays such as cytokine ELIPSOT and T cell proliferation assays with antigen are able to make use of all available HLA class I molecules in a patient (51, 52) and can be directed toward peptide sequences within “hot spots” of a self-antigen.
A proportion of nondiabetic individuals also have islet-reactive T cells in their blood (26, 30, 31), and we confirmed this through testing preproinsulin peptide pools in ELISPOT assays. We elected to use an IFN-γ ELISPOT assay to screen for reactivity in peripheral blood as there were many preproinsulin epitopes presented by 10 different HLA class I molecules in the islets. Although preproinsulin reactivity in PBMCs mirrored islet-derived CD8 T cell reactivity from T1D organ donors, we propose that additional T cell–based assays, markers, and signatures are likely required to determine disease specificity and possibly disease heterogeneity (36, 53). While it is still unresolved whether phenotypes of islet antigen–specific T cells have different features in various stages of T1D development in terms of advancing from genetic risk to islet autoantibody seroconversion to clinical T1D onset, a recent study demonstrated exhausted self-reactive CD8 T cell phenotypes predict loss of beta cell function following clinical T1D onset (54). Thus, it will be important to characterize CD8 T cell phenotypes and fitness, in addition to TCR sequences and antigen specificity, in the islets across the stages of T1D development.
In addition to preproinsulin, other self-antigen specificities within T1D are recognized by CD8 T cells, including those to glutamic acid decarboxylase (GAD), insulinoma antigen 2 (IA-2), and zinc transporter 8 (23, 26, 48, 49, 55, 56). Translationally modified antigens include insulin DRiP peptides, with two such islet-derived TCRs responding to this antigen, and posttranslationally modified islet antigens such as fusion peptides within islet amyloid polypeptide (26). Our current study analyzed responses to a limited number of peptides derived from islet proteins other than preproinsulin. Future studies are warranted to define the antigen specificity of the remaining TCR transductants to native and posttranslationally modified islet antigens as well as those unrelated to islet proteins but possibly involved in the T1D pathogenesis such as virus-derived peptides.
In conclusion, our findings demonstrate the presence of CD8 T cells strongly responding to preproinsulin is specific to islets of individuals with T1D. The frequency of such preproinsulin-specific CD8 T cells in the islets varies by individual and associates with certain HLA alleles. Understanding the self-antigens recognized by CD8 T cells within the target organ for autoimmune diabetes holds promise for improving therapies to prevent disease onset and identify those individuals that may benefit from immune intervention therapies.
Materials and Methods
Study Subjects and Study Approvals.
Organ donors for islet isolation were identified from the Network for Pancreatic Organ Donors with Diabetes program (nPOD; Research Resource Identifiers [RRID]:SCR_014641) (57), a protocol at Vanderbilt University Medical Center/University of Pittsburgh (A.C.P. and Rita Bottino) (58), the Integrated Islet Distribution program (IIDP; RRID:SCR_014387), and the Alberta Diabetes Institute IsletCore. Pancreatic islets were isolated from pancreata (all except T1D-6), or pancreas slices were generated (T1D-6) by each organization. For pancreas slice samples obtained from a donor T1D-6, pancreatic islets were further isolated by treating with Liberase DL (Roche). Measurement of T1D-associated autoantibodies and HLA typing were performed through the nPOD Standard Operating Procedures Protocol (https://www.jdrfnpod.org/for-investigators/standard-operating-procedures/) for donors identified by nPOD. The Colorado Multiple Institutional Review Board reviewed the use these postmortem tissues and approved the study.
Study subjects for the T cell assays using peripheral blood were recruited from the Barbara Davis Center for Diabetes Clinics. Peripheral blood was obtained for islet autoantibody measurements, HLA typing, and cytokine ELISPOT assays. Written informed consent was obtained from each participant and guardian when the participant was less than 18 y of age, and the Colorado Multiple Institutional Review Board approved the study.
T Cell Isolation and TCR Sequencing.
Isolation of T cells from islet tissues were performed using a protocol as described previously (8), and the details of methods were included in SI Appendix, Supplementary Methods and Materials.
Generation of TCR and HLA Transductants.
Transductants expressing TCR or HLA molecules were generated using a recently published protocol (34, 59), and the details of methods were included in SI Appendix, Supplementary Methods and Materials. TCR and HLA transductants were generated using murine T cell–derived 5KC T-hybridoma reporter cells and human myeloma–derived K562 cells, respectively. Expression of HLA class I molecules was confirmed by staining with anti-human beta-2 microglobulin antibody (Clone W6/32, Biolegend) and being analyzed on a Cytoflex flow cytometer (Beckman Coulter) (SI Appendix, Fig. 5).
Screening for Reactivity to Preproinsulin, Insulin DRiP, Glucagon, and Other Islet Antigen Peptides.
We used a multiplex T cell stimulation assay system to test reactions to peptides and truncated peptide pools using a recently published protocol (34, 59). The details of methods were included in SI Appendix, Supplementary Methods and Materials.
Determining HLA Restriction and Potency of Responses to Optimal Epitopes.
TCR transductants that responded to one or more truncated peptide pools were analyzed using peptides newly synthesized by Genemed Synthesis as described in SI Appendix, Supplementary Methods and Materials. Peptides and HLA class I alleles tested for each TCR clonotype are shown in SI Appendix, Tables 6a and 6b.
Cytokine ELISPOT Assays.
Assays were conducted as previously described using the human IFN-γ ELISPOT kits (UCyTech Biosciences) (51). The details of methods were included in SI Appendix, Supplementary Methods and Materials.
Islet Autoantibody Measurements and Molecular HLA Typing.
Serum obtained from peripheral blood was used to measure islet autoantibodies to insulin, GAD65, IA-2, and ZnT8 by fluid-phase radio-binding assays as previously described (60, 61). DNA obtained from peripheral blood or organ donor tissue was used to type HLA-DRB1, -A, -B, and -C alleles using oligonucleotide probes as previously described (62).
Statistical Analyses.
Statistical analyses were performed using R software (R Core Team), GraphPad Prism 8 software (GraphPad Software), and SAS 9.4 (SAS Institute). The statistical tests used for each experiment are indicated in the corresponding table or figure legend. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank the organ donors and their families. This research was performed with the support of nPOD (RRID: SCR_014641), a collaborative T1D research project. The content and views expressed are the responsibility of the authors and do not necessarily reflect the official view of nPOD. Organ Procurement Organizations partnering with nPOD to provide research resources are listed at https://www.jdrfnpod.org/for-partners/npod-partners/. Several human pancreatic islets were provided by The National Institute of Diabetes and Digestive and Kidney Diseases-funded IIDP (RRID:SCR_014387) at City of Hope. We thank Taylor Armstrong and Lester Acosta for technical assistance (University of Colorado School of Medicine). We thank Rita Bottino at the Institute of Cellular Therapeutics, Allegheny-Singer Research Institute, Allegheny Health Network, in Pittsburgh, PA, for isolating islets from T1D-7 donor. This work was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases (Grants R01DK099317 [M.N. and A.W.M.], R01DK032083 [M.N. and A.W.M.], R01DK108868 [M.N. and A.W.M.], DP3DK110845 [M.N. and A.W.M.], UC4DK104223 [M.N. and A.W.M.], P30 DK116073-01A1 [University of Colorado Diabetes Research Center], UG3 DK122638-01 [C.E.M.], P01 AI042288 [M.A.A. and C.E.M.], UC4 DK104194-01 [C.E.M.], DK104211 [A.C.P.], DK106755 [A.C.P.], DK20593 [Vanderbilt Diabetes Research and Training Center], and 2UC4DK098085 [IIDP]), Department of Veterans Affairs (Grant BX000666 [A.C.P.]), JDRF (Grants 2-SRA-2018-480-S-B [M.N. and A.W.M.], 1-SRA-2020-911-A-N [M.N.], and 5-SRA-2018-557-Q-R [nPOD], the Leona M. and Harry B. Helmsley Charitable Trust (Grant 2018PG-T1D053 [M.A.A.]), and the Culshaw Family Junior Investigator Award [M.N.].
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107208118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or supporting information.
References
- 1.Atkinson M. A., Eisenbarth G. S., Michels A. W., Type 1 diabetes. Lancet 383, 69–82 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluestone J. A., Herold K., Eisenbarth G., Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464, 1293–1300 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Concannon P., Rich S. S., Nepom G. T., Genetics of type 1A diabetes. N. Engl. J. Med. 360, 1646–1654 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Di Lorenzo T. P., Peakman M., Roep B. O., Translational mini-review series on type 1 diabetes: Systematic analysis of T cell epitopes in autoimmune diabetes. Clin. Exp. Immunol. 148, 1–16 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roep B. O., Peakman M., Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb. Perspect. Med. 2, a007781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pathiraja V., et al., Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes 64, 172–182 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Babon J. A., et al., Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat. Med. 22, 1482–1487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michels A. W., et al., Islet-derived CD4 T cells targeting proinsulin in human autoimmune diabetes. Diabetes 66, 722–734 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anonymous; Diabetes Prevention Trial—Type 1 Diabetes Study Group , Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med. 346, 1685–1691 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Bonifacio E.et al.; Pre-POINT Study Group , Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: The Pre-POINT randomized clinical trial. JAMA 313, 1541–1549 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Näntö-Salonen K., et al., Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: A double-blind, randomised controlled trial. Lancet 372, 1746–1755 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Roep B. O.et al.; BHT-3021 Investigators , Plasmid-encoded proinsulin preserves C-peptide while specifically reducing proinsulin-specific CD8+ T cells in type 1 diabetes. Sci. Transl. Med. 5, 191ra82 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krischer J. P., Schatz D. A., Bundy B., Skyler J. S., Greenbaum C. J.; Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group , Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: A randomized clinical trial. JAMA 318, 1891–1902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alhadj Ali M., et al., Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci. Transl. Med. 9, eaaf7779 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Skyler J. S., et al., Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial—Type 1. Diabetes Care 28, 1068–1076 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Atkinson M. A., Roep B. O., Posgai A., Wheeler D. C. S., Peakman M., The challenge of modulating β-cell autoimmunity in type 1 diabetes. Lancet Diabetes Endocrinol. 7, 52–64 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roep B. O., Wheeler D. C. S., Peakman M., Antigen-based immune modulation therapy for type 1 diabetes: The era of precision medicine. Lancet Diabetes Endocrinol. 7, 65–74 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Nikolic T., et al., Safety and feasibility of intradermal injection with tolerogenic dendritic cells pulsed with proinsulin peptide-for type 1 diabetes. Lancet Diabetes Endocrinol. 8, 470–472 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Krischer J. P.et al.; TEDDY Study Group , Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: The TEDDY study. Diabetes Care 40, 1194–1202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppieters K. T., et al., Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 209, 51–60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson S. J., et al., Islet cell hyperexpression of HLA class I antigens: A defining feature in type 1 diabetes. Diabetologia 59, 2448–2458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velthuis J. H., et al., Simultaneous detection of circulating autoreactive CD8+ T-cells specific for different islet cell-associated epitopes using combinatorial MHC multimers. Diabetes 59, 1721–1730 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James E. A.et al.; Immunology of Diabetes Society T Cell Workshop Committee , Combinatorial detection of autoreactive CD8+ T cells with HLA-A2 multimers: A multi-centre study by the Immunology of Diabetes Society T Cell Workshop. Diabetologia 61, 658–670 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Pinkse G. G., et al., Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc. Natl. Acad. Sci. U.S.A. 102, 18425–18430 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeo L., et al., Autoreactive T effector memory differentiation mirrors β cell function in type 1 diabetes. J. Clin. Invest. 128, 3460–3474 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez-Duque S., et al., Conventional and neo-antigenic peptides presented by β cells are targeted by circulating naïve CD8+ T cells in type 1 diabetic and healthy donors. Cell Metab. 28, 946–960.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Bender C., Rodriguez-Calvo T., Amirian N., Coppieters K. T., von Herrath M. G., The healthy exocrine pancreas contains preproinsulin-specific CD8 T cells that attack islets in type 1 diabetes. Sci. Adv. 6, eabc5586 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Calvo T., Krogvold L., Amirian N., Dahl-Jørgensen K., von Herrath M., One in ten CD8+ cells in the pancreas of living individuals with recent-onset type 1 diabetes recognizes the preproinsulin epitope PPI15-24. Diabetes 70, 752–758 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis J. M., et al., Frequencies of HLA-A2 alleles in five U.S. population groups. Predominance Of A*02011 and identification of HLA-A*0231. Hum. Immunol. 61, 334–340 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Skowera A., et al., β-cell-specific CD8 T cell phenotype in type 1 diabetes reflects chronic autoantigen exposure. Diabetes 64, 916–925 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Culina S.et al.; ImMaDiab Study Group , Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci. Immunol. 3, eaao4013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seay H. R., et al., Tissue distribution and clonal diversity of the T and B cell repertoire in type 1 diabetes. JCI Insight 1, e88242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landry L. G., et al., Proinsulin-reactive CD4 T cells in the islets of type 1 diabetes organ donors. Front. Endocrinol. (Lausanne) 12, 622647 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mann S. E., et al., Multiplex T cell stimulation assay utilizing a T cell activation reporter-based detection system. Front. Immunol. 11, 633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kracht M. J., et al., Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat. Med. 23, 501–507 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Claessens L. A., et al., Clinical and genetic correlates of islet-autoimmune signatures in juvenile-onset type 1 diabetes. Diabetologia 63, 351–361 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble J. A.et al.; Type 1 Diabetes Genetics Consortium , HLA class I and genetic susceptibility to type 1 diabetes: Results from the Type 1 Diabetes Genetics Consortium. Diabetes 59, 2972–2979 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noble J. A., Valdes A. M., Genetics of the HLA region in the prediction of type 1 diabetes. Curr. Diab. Rep. 11, 533–542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stone J. D., Chervin A. S., Kranz D. M., T-cell receptor binding affinities and kinetics: Impact on T-cell activity and specificity. Immunology 126, 165–176 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakraborty A. K., Weiss A., Insights into the initiation of TCR signaling. Nat. Immunol. 15, 798–807 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delong T., et al., Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351, 711–714 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baker R. L., et al., Hybrid insulin peptides are autoantigens in type 1 diabetes. Diabetes 68, 1830–1840 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodriguez-Calvo T., Ekwall O., Amirian N., Zapardiel-Gonzalo J., von Herrath M. G., Increased immune cell infiltration of the exocrine pancreas: A possible contribution to the pathogenesis of type 1 diabetes. Diabetes 63, 3880–3890 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radenkovic M., et al., Characterization of resident lymphocytes in human pancreatic islets. Clin. Exp. Immunol. 187, 418–427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisberg S. P., et al., Tissue-resident memory T cells mediate immune homeostasis in the human pancreas through the PD-1/PD-L1 pathway. Cell Rep. 29, 3916–3932.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dayan C. M., Korah M., Tatovic D., Bundy B. N., Herold K. C., Changing the landscape for type 1 diabetes: The first step to prevention. Lancet 394, 1286–1296 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Purcell A. W., Sechi S., DiLorenzo T. P., The Evolving landscape of autoantigen discovery and characterization in type 1 diabetes. Diabetes 68, 879–886 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James E. A., Mallone R., Kent S. C., DiLorenzo T. P., T-cell epitopes and neo-epitopes in type 1 diabetes: A comprehensive update and reappraisal. Diabetes 69, 1311–1335 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scotto M., et al., HLA-B7-restricted islet epitopes are differentially recognized in type 1 diabetic children and adults and form weak peptide-HLA complexes. Diabetes 61, 2546–2555 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kronenberg D., et al., Circulating preproinsulin signal peptide-specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill β-cells. Diabetes 61, 1752–1759 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagata M., et al., Detection of autoreactive T cells in type 1 diabetes using coded autoantigens and an immunoglobulin-free cytokine ELISPOT assay: Report from the fourth immunology of diabetes society T cell workshop. Ann. N. Y. Acad. Sci. 1037, 10–15 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Mannering S. I., et al., A sensitive method for detecting proliferation of rare autoantigen-specific human T cells. J. Immunol. Methods 283, 173–183 (2003). [DOI] [PubMed] [Google Scholar]
- 53.Battaglia M., et al., Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43, 5–12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiedeman A. E., et al., Autoreactive CD8+ T cell exhaustion distinguishes subjects with slow type 1 diabetes progression. J. Clin. Invest. 130, 480–490 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi K., Honeyman M. C., Harrison L. C., Cytotoxic T cells to an epitope in the islet autoantigen IA-2 are not disease-specific. Clin. Immunol. 99, 360–364 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Scotto M., et al., Zinc transporter (ZnT)8(186-194) is an immunodominant CD8+ T cell epitope in HLA-A2+ type 1 diabetic patients. Diabetologia 55, 2026–2031 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pugliese A., et al., The Juvenile Diabetes Research Foundation Network for Pancreatic Organ Donors with Diabetes (nPOD) Program: Goals, operational model and emerging findings. Pediatr. Diabetes 15, 1–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brissova M., et al., α Cell function and gene expression are compromised in type 1 diabetes. Cell Rep. 22, 2667–2676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Landry L. G., Mann S. E., Anderson A. M., Nakayama M., Multiplex T-cell stimulation assay utilizing a T-cell activation reporter-based detection system. Bio Protoc. 11, e3883 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu L., et al., Antiislet autoantibodies usually develop sequentially rather than simultaneously. J. Clin. Endocrinol. Metab. 81, 4264–4267 (1996). [DOI] [PubMed] [Google Scholar]
- 61.Wenzlau J. M., et al., The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl. Acad. Sci. U.S.A. 104, 17040–17045 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rewers A., et al., Ethnic differences in the associations between the HLA-DRB1*04 subtypes and type 1 diabetes. Ann. N. Y. Acad. Sci. 1005, 301–309 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.