Abstract
Rationale: Untreated obstructive sleep apnea (OSA) is associated with adverse outcomes in patients with coronary artery disease (CAD). Continuous positive airway pressure (CPAP) is the most common treatment, but despite interventions addressing established adherence determinants, CPAP use remains poor.
Objectives: To determine whether physiological traits that cause OSA are associated with long-term CPAP adherence in patients with CAD.
Methods: Participants in the RICCADSA (Randomized Intervention with CPAP in CAD and OSA) trial with objective CPAP adherence (h/night) over 2 years and analyzable raw polysomnography data were included (N = 249). The physiological traits—loop gain, arousal threshold (ArTH), pharyngeal collapsibility (Vpassive), and pharyngeal muscle compensation (Vcomp)—were measured by using polysomnography. Linear mixed models were used to assess the relationship between the traits and adherence. We also compared actual CPAP adherence between those with physiologically predicted “poor” adherence (lowest quartile of predicted adherence) and those with physiologically predicted “good” adherence (all others).
Measurements and Main Results: The median (interquartile range) CPAP use declined from 3.2 (1.0–5.8) h/night to 3.0 (0.0–5.6) h/night over 24 months (P < 0.001). In analyses adjusted for demographics, anthropometrics, OSA characteristics, and clinical comorbidities, a lower ArTH was associated with worse CPAP adherence (0.7 h/SD of the ArTH; P = 0.021). Both high and low Vcomp were associated with lower adherence (P = 0.008). Those with predicted poor adherence exhibited markedly lower CPAP use than those with predicted good adherence for up to 2 years of follow-up (group differences of 2.0–3.2 h/night; P < 0.003 for all).
Conclusions: A low ArTH, as well as a very low and high Vcomp, are associated with worse long-term CPAP adherence in patients with CAD and OSA. Physiological traits—alongside established determinants—may help predict and improve CPAP adherence. Clinical trial registered with www.clinicaltrials.gov (NCT 00519597).
Keywords: obstructive sleep apnea, coronary artery disease, adherence, physiologic traits, arousal threshold
At a Glance Commentary
Scientific Knowledge on the Subject
In patients with coronary artery disease, untreated obstructive sleep apnea (OSA) is associated with adverse outcomes. The most common therapy for OSA is continuous positive airway pressure (CPAP). However, adherence to CPAP is poor, despite interventions addressing established social and psychological determinants of CPAP use. A better understanding of the factors influencing CPAP adherence in this vulnerable population is needed.
What This Study Adds to the Field
Here, we show that the physiological traits that cause OSA also influence long-term CPAP adherence among those with OSA and coronary artery disease. Specifically, we find that a lower arousal threshold (propensity to easily awaken from a respiratory stimulus) is associated with a marked reduction in CPAP use over a 2-year follow-up. Moreover, we find that both high and low pharyngeal muscle compensation are linked to poor CPAP adherence. Our findings suggest that a priori knowledge of an individual’s OSA pathophysiology may aid with the identification of patients who are at risk of poor CPAP adherence and may improve OSA therapy in a more precise way.
Obstructive sleep apnea (OSA) is a common disorder among patients with coronary artery disease (CAD). Observational studies in this population reveal that untreated OSA is associated with an increased risk of recurrent myocardial infarction, revascularization, and cardiovascular death (1, 2). In contrast, randomized controlled clinical trials of OSA treatment with continuous positive airway pressure (CPAP) show no benefit for cardiovascular outcomes in intention-to-treat analyses (3–5). A key factor postulated to explain the null findings in these trials is poor adherence to CPAP therapy (average CPAP use of 2.8–3.5 h/night) (3–6).
Adherence to CPAP is a complex behavior that is influenced by biomedical (e.g., OSA severity), social (e.g., partner support), and psychological factors (e.g., claustrophobia) (7). Despite interventions to address these factors, adherence to CPAP has remained largely unchanged over the past 20 years (8).
Recent reports suggest that new biomedical factors, namely the physiological traits that cause OSA, may also influence CPAP adherence. Specifically, a low arousal threshold (ArTH) is associated with reduced CPAP use rates, particularly among OSA subgroups that include individuals without obesity (9–11). In addition, increased pharyngeal muscle compensation (Vcomp) is associated with decreased adherence in patients with stroke (9). However, these studies 1) relied on surrogate metrics of a single trait and did not account for potential interactions between the traits (12); 2) were performed in small samples of patients with stroke, limiting the findings’ generalizability (9); or 3) evaluated CPAP adherence at a single time point, despite evidence that adherence changes with time (13, 14). A better understanding of how—and in which patient subgroups—the physiological traits influence longitudinal CPAP adherence may help predict who is at risk of nonadherence. This can inform the design of more rigorous clinical trials in patients with CAD and OSA. Moreover, because the physiological traits are modifiable, this understanding may also offer novel approaches to improving CPAP use.
Our primary aim was to determine whether physiological OSA traits are associated with longitudinal CPAP adherence in patients with CAD and OSA. Our secondary aims were to assess 1) the influence of OSA on CPAP adherence independently of other biomedical and psychological factors; 2) the associations between the traits and adherence within specific clinical OSA subgroups (e.g., men vs. women, REM vs. non-REM–related OSA) (15, 16); and 3) whether the traits’ association with adherence is mediated by baseline sleep characteristics (e.g., sleep time, arousals), psychological factors (e.g., anxiety, depression), and factors reflecting patient experience with CPAP (e.g., residual respiratory events, change in sleepiness) (7, 17–19). Some of the results of this study have been previously reported in the form of an abstract (20, 21).
Methods
For a detailed description of the methods, see the online supplement.
Study Design, Participants, and Analytic Sample
We performed a secondary data analysis of the RICCADSA (Randomized Intervention with CPAP in CAD and OSA) study (3). In brief, we included revascularized participants with angiographically verified CAD and OSA (apnea–hypopnea index [AHI] ⩾ 15/h) who were allocated to autotitrating CPAP with 2-year follow-up (n = 280). See the online supplement for details of the parent study (3) and selection criteria for our final analytic sample (N = 249; see Figure E1 in the online supplement).
Outcome
Adherence was defined as the average CPAP use (h/night) at each follow-up point (1, 3, 6, 12, and 24 mo) as measured by using data card downloads.
Physiological Traits and Their Measurement
Physiological OSA traits (loop gain [LG], ArTH, pharyngeal collapsibility [Vpassive], and Vcomp) differentially contribute to the causation of OSA in each individual (22). LG reflects ventilatory control stability (elevated LG signifies an exaggerated ventilatory drive response to reduced airflow and the associated hypoxia and hypercapnia). ArTH reflects the amount of ventilatory drive required to cause an arousal from sleep (a small rise in the ventilatory drive to terminate sleep represents a low ArTH). Vpassive reflects the ventilation at eupneic ventilatory drive (higher values reflect lower pharyngeal collapsibility ). Finally, the Vcomp was determined by calculating the difference between the ventilation at the maximum and eupneic ventilatory drives (higher values reflect better pharyngeal muscle compensation ).
The traits were measured from baseline polysomnography (see Table E1 in the online supplement for measurement description and interpretation of trait values) (23). Median trait values across non-REM sleep were used for each individual. For consistency with prior studies, the arousal duration was rescored by four scorers accredited by the American Academy of Sleep Medicine, whose scoring exhibited excellent trait measurement reliability (intraclass correlation coefficient, 0.91–0.96) (24).
Covariates and Mediating Factors
Factors that could influence CPAP use (see Table E2 for list) were categorized into the following domains: demographics and anthropometrics (e.g., age), OSA characteristics (e.g., AHI, Epworth Sleepiness Scale [ESS] score), medical comorbidities (e.g., atrial fibrillation), and psychiatric (e.g., anxiety) comorbidities. Specific covariates were selected on the basis of prior reports suggesting associations with CPAP adherence (see Statistical Analysis, Adjustment for covariates section below). Factors reflecting participant experience with CPAP (e.g., residual AHI, change in the ESS score) at each follow-up (Table E3)—as well as total sleep time and anxiety (18)—were used for mediation analyses.
Statistical Analysis
Primary aim
We assessed the association between the traits and CPAP adherence by using a linear mixed model with time (mo) as both a fixed and random effect, accounting for the correlation of repeated measures within an individual over time (25). Because trait-by-trait interactions (26) and quadratic terms (26) were previously reported as being relevant to OSA treatment efficacy and CPAP adherence, we also evaluated these terms. The final physiological CPAP adherence model always included the four main traits and only the interaction or squared terms with P values of <0.20 to avoid overfitting (27). To determine whether the addition of traits significantly improved the fit of the CPAP adherence model that included time, we used a likelihood ratio test (9). See the online supplement for a power assessment and exploratory analyses.
Adjustment for adherence covariates
The physiological adherence model was sequentially adjusted for 1) age, sex, and body mass index; 2) OSA characteristics (AHI and ESS score); and 3) the medical and psychological domains (atrial fibrillation, prior myocardial infarction, anxiety, depression). We also evaluated additional potential confounders in the domains of OSA characteristics and comorbidities (Table E2), as described in the online supplement.
OSA subgroups
Because CPAP adherence differs among subgroups of patients with OSA in whom physiological traits may vary, we performed analyses in a priori–defined subgroups: men versus women (16), participants who were sleepy versus participants who were nonsleepy (13, 28), patients with REM-predominant OSA versus those without (15, 29), and long-term CPAP users (any use at 2-yr follow-up; see online supplement) (25). Because traits are not measured in REM sleep and there were a small number of women in our sample, we also assessed a subgroup of “men without REM-predominant OSA.”
Mediation analyses
To evaluate potential mechanisms by which the traits may influence CPAP adherence, we first built a model for each potential mediator (e.g., residual AHI; Table E3) with CPAP adherence as an outcome. Factors with a P value of <0.05 were then added to the physiological adherence model to determine the mediation effect. The Monte Carlo method was used to test the significance of the mediation effect (30).
Prediction modeling
To assess for the potential clinical utility of physiological OSA traits in adherence, we performed prediction modeling to identify those at risk of poor adherence (lowest quartile of CPAP use) at the 1-month follow-up. The primary test of prediction analysis was the comparison in actual adherence between those with predicted “poor” adherence (⩽lowest quartile) and those with predicted “good” adherence (all others) at each time point, and a Wilcoxon rank sum test was used to assess the role of physiological traits in the predefined subgroups (31). For a more conservative prediction analysis, we also performed leave-one-out cross-validation.
The mean ± SD and median (interquartile range [IQR]) are reported for normal and nonnormal distributions, respectively. Values were accepted as significant at a P value of <0.05. Statistical analyses were performed by using R (R Foundation for Statistical Computing).
Results
Cohort Characteristics and CPAP Adherence
The majority of participants were men without obesity with a mean age of 63.9 ± 7.9 years and prior revascularization (53.4%) (Table 1). Over half were sleepy (ESS score ⩾10), demonstrating a median AHI of 35.2 (22.6–57.0) events/h and 1.9% (0.3–6.6%) of sleep time at an SaO2 below 90%. Median CPAP adherence at 1 month was 3.2 (1.0–5.8) h/night, and this decreased to 3.0 (0.0–5.6) h/night by 24 months (P < 0.001; Figure 1, Table E5).
Table 1.
Demographic, Clinical, and Polysomnographic Characteristics of the Analytic Sample (N = 249)
| Characteristic | Mean ± SD, Median (IQR), or n (%) |
|---|---|
| Demographics and anthropometrics | |
| Age, yr | 63.9 ± 7.9 |
| Men | 213 (85.5) |
| BMI, kg/m2 | 29.2 ± 4.2 |
| Obesity | 90 (36.1) |
| Comorbidities | |
| Anxiety | 49 (19.7) |
| Depression | 13 (5.2) |
| Former or current smoker | 160 (64.3) |
| Diabetes | 62 (24.9) |
| Hypertension | 154 (61.8) |
| History of atrial fibrillation | 36 (14.5) |
| Stroke | 16 (6.4) |
| Lung disease | 17 (6.8) |
| Prior AMI | 133 (53.4) |
| Prior PCI | 28 (11.2) |
| Prior CABG | 27 (10.8) |
| Prior revascularization | 52 (20.9) |
| NT-pro-BNP, pg/ml | 194.5 (100.0–464.5) |
| LVEF | 57.6 ± 8.2 |
| Sleep characteristics | |
| ESS score | 9.2 ± 4.1 |
| Sleepy (ESS score >10) | 138 (55.4) |
| TST, min | 386 ± 90 |
| Sleep efficiency, % | 77.9 ± 13 |
| Stage 1 sleep, % | 34.5 ± 16.5 |
| Slow-wave sleep, % | 7.6 ± 10.6 |
| REM sleep, % | 12.8 ± 6.2 |
| Total arousal index, events/h | 47.6 (35.4–65.6) |
| Respiratory arousal index, events/h | 29.9 (19.2–50) |
| AHI, events/h | 35.2 (22.6–57) |
| Hypopnea index, events/h | 19.9 (13–30.3) |
| REM/non-REM AHI ratio | 1.1 (0.7–2.1) |
| Supine/nonsupine AHI ratio | 1.8 (1.1–3.8) |
| ODI of 3%, events/h | 19.2 (8.9–32.2) |
| Lowest O2 saturation, % | 81.3 ± 6.9 |
| Time at O2 saturation of <90%, % of TST | 1.9 (0.3–6.6) |
| Physiological traits | |
| Loop gain (unitless) | 0.66 ± 0.18 |
| Arousal threshold, %Ve | 116 (104.8–134.7) |
| Pharyngeal collapsibility−1, %Ve | 90.7 (83.6–94.9) |
| Pharyngeal muscle compensation, %Ve | 3.8 (0.0–8.8) |
| Arousal threshold transformed, %Ve | 140.0 (121.9–158.9) |
| Pharyngeal collapsibility transformed−1, %Ve | 69.5 (59.5–77.4) |
Definition of abbreviations: AHI = apnea–hypopnea index; AMI = acute myocardial infarction; BMI = body mass index; CABG = coronary artery bypass graft; ESS = Epworth Sleepiness Scale; IQR = interquartile range; LVEF = left ventricular ejection fraction; ODI = oxygen desaturation index; PCI = percutaneous coronary intervention; TST = total sleep time; Ve = eupneic ventilation.
Figure 1.
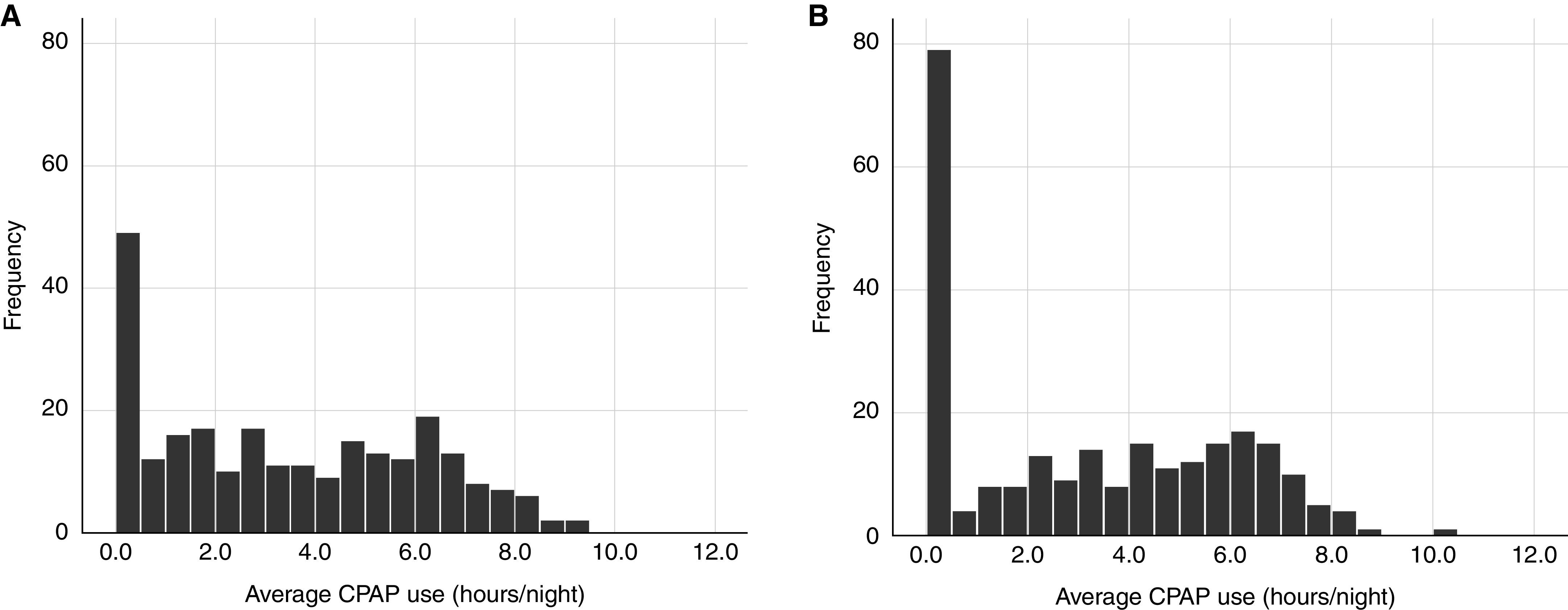
Distribution of average continuous positive airway pressure (CPAP; h/night) use at the (A) 1-month follow-up and (B) 24-month follow-up.
Physiological OSA Traits and CPAP Adherence among Patients with CAD
On bivariate analyses, only the squared Vcomp term and Vcomp–Vpassive interaction term were significantly associated with adherence (P = 0.034 and P = 0.041, respectively).
In the multivariable physiological adherence model (Table 2), a lower ArTH was associated with reduced adherence (P = 0.008), with a decline of 49 min/night (95% confidence interval, 12–87 min/night) per each 1-SD reduction in the ArTH. The relationship between Vcomp and adherence was an inverted U shape (P = 0.005). Specifically, at high and low Vcomp, adherence was reduced compared with 50th percentile of Vcomp (3.5 [IQR, 2.0–4.9] h/night, 3.7 [IQR, 2.2–5.0] h/night, and 3.3 [IQR, 1.9–4.7] h/night at 6 months for the 10th, 50th, and 90th centiles of Vcomp, respectively). The addition of physiological traits significantly improved the adherence model that used time alone (χ2 = 38.8, P = 7.8 × 10−7). The associations of ArTH and Vcomp with adherence remained significant after adjustment for all covariates (Table 2).
Table 2.
Physiological Trait Model of CPAP Adherence with Sequential Adjustment for Potential Confounders among Participants with CAD Treated with CPAP during 24 Months of Follow-up
| Variable | Model 1 (Unadjusted) |
Model 2 (Model 1 + Age, Sex, and BMI) |
Model 3 (Model 2 + Sleep Factors)* |
Model 4 (Model 3 + Comorbidities)† |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | β SD | P Value | β (SE) | β SD | P Value | β (SE) | β SD | P Value | β (SE) | β SD | P Value | |
| Time, mo | −0.012 (0.005) | −0.037 | 0.019 | −0.012 (0.005) | −0.037 | 0.019 | −0.012 (0.005) | −0.037 | 0.019 | −0.014 (0.005) | −0.043 | 0.007 |
| ArTH, %Ve | 0.026 (0.010) | 0.299 | 0.008 | 0.026 (0.010) | 0.300 | 0.007 | 0.025 (0.010) | 0.291 | 0.010 | 0.023 (0.010) | 0.263 | 0.021 |
| Loop gain | −0.496 (1.098) | −0.033 | 0.652 | −0.521 (1.141) | −0.035 | 0.649 | −0.078 (1.197) | −0.005 | 0.948 | −0.212 (1.191) | −0.014 | 0.859 |
| Vpassive, %Ve | 0.019 (0.011) | 0.167 | 0.079 | 0.021 (0.011) | 0.184 | 0.056 | 0.019 (0.012) | 0.167 | 0.109 | 0.020 (0.012) | 0.169 | 0.105 |
| Vcomp, %Ve | −0.007 (0.009) | −0.059 | 0.430 | −0.007 (0.009) | −0.060 | 0.425 | −0.008 (0.019) | −0.063 | 0.399 | −0.009 (0.009) | −0.076 | 0.312 |
| Vcomp2 | −4.75 × 10−4 (1.67 × 10−4) | −0.223 | 0.005 | −4.71 × 10−4 (1.70 × 10−4) | −0.221 | 0.006 | −4.58 × 10−4 (1.69 × 10−4) | −0.215 | 0.007 | −4.49 × 10−4 (1.68 × 10−4) | −0.214 | 0.008 |
Definition of abbreviations: AHI = apnea–hypopnea index; ArTH = arousal threshold; BMI = body mass index; CAD = coronary artery disease; CPAP = continuous positive airway pressure; ESS = Epworth Sleepiness Scale; Vcomp = pharyngeal muscle compensation (higher value represents higher compensation); Ve = eupneic ventilation; Vpassive = 1/pharyngeal collapsibility (higher value represents lower collapsibility). The model contains four main trait effects and interaction terms with a P value of <0.20 in exploratory analyses. Trait values were subtracted from the mean before terms were generated and applied to the model (see Measurement of Physiological Traits in the online supplement). “β SD” describes the number of SDs of change in CPAP adherence per each SD increase in each term. This enables the comparison of effect sizes among physiological traits. The mean values of the physiological traits before mean subtraction are as follows: Vpassive = 80.9 ± 26.2%, Vpassive transformed = 63.0 ± 23.4%, loop gain = 0.67 ± 0.18, Vcomp = 0.43 ± 23.8%, ArTH = 128.5 ± 37.5%, and ArTH transformed = 143.5 ± 31.6%. Bold indicates P < 0.05.
Sleep factors: ESS score and AHI.
Comorbidities: anxiety, depression, history of atrial fibrillation, and history of acute myocardial infarction (present vs. absent).
Physiological Traits and Adherence in OSA Subgroups
Findings for the subgroups of men and of patients without REM-predominant OSA were analogous to those for the entire cohort (data not shown). Among all subgroups, the ArTH exhibited the strongest association with adherence (β SD, 0.44; P = 0.001) in the subgroup of men without REM-predominant OSA (Table 3, fully adjusted model). Vpassive was inversely associated with adherence (β SD, 0.30; P = 0.015). In participants who were sleepy, associations were similar but were of a lower magnitude. No significant associations for traits or their interactions were identified among the nonsleepy, female, or REM-predominant OSA groups (data not shown).
Table 3.
Physiological Models of CPAP Adherence (Adjusted) among Subgroups of Participants with CAD Treated with CPAP during 24 Months of Follow-up
| Variable | Men without REM-Predominant OSA (n = 182)* |
Sleepy OSA (n = 138)* |
Long-term CPAP Users (n = 174) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β (SE) | β SD | P Value | β (SE) | β SD | P Value | β (SE) | β SD | P Value | |
| Time, mo | −0.013 (0.006) | −0.040 | 0.036 | −0.016 (0.007) | −0.050 | 0.024 | −0.004 (0.006) | −0.015 | 0.529 |
| ArTH, %Ve | 0.037 (0.011) | 0.439 | 0.001 | 0.027 (0.011) | 0.325 | 0.021 | −0.001 (0.009) | −0.024 | 0.875 |
| Loop gain | −1.617 (1.389) | −0.108 | 0.246 | −1.024 (1.473) | −0.072 | 0.489 | −0.355 (1.136) | −0.020 | 0.755 |
| Vpassive, %Ve | 0.033 (0.013) | 0.297 | 0.015 | 0.028 (0.016) | 0.258 | 0.072 | 0.009 (0.012) | 0.100 | 0.446 |
| Vcomp, %Ve | −0.006 (0.009) | −0.060 | 0.483 | −0.010 (0.011) | −0.084 | 0.268 | −0.005 (0.009) | −0.046 | 0.570 |
| Vcomp2 | −5.03 × 10−4 (1.69 × 10−4) | −0.271 | 0.003 | −4.87 × 10−4 (1.93 × 10−4) | −0.257 | 0.013 | — | — | — |
| Vcomp × Vpassive | — | — | — | — | — | — | 0.001 (5.59 × 10−4) | 0.226 | 0.061 |
| ArTH × loop gain | — | — | — | — | — | — | 0.065 (0.030) | 0.154 | 0.030 |
| Vcomp × loop gain | — | — | — | — | — | — | 0.107 (0.049) | 0.174 | 0.031 |
Definition of abbreviations: ArTH = arousal threshold; CAD = coronary artery disease; CPAP = continuous positive airway pressure; ESS = Epworth Sleepiness Scale; OSA = obstructive sleep apnea; Vcomp = pharyngeal muscle compensation (higher value represents higher compensation); Ve = eupneic ventilation; Vpassive = 1/pharyngeal collapsibility (higher value represents lower collapsibility).
The models contain four main trait effects and interaction and squared terms with a P value of <0.20. Trait values were subtracted from the mean before terms were generated and applied to the model (see Measurement of Physiological Traits in the online supplement). “β SD” describes the number of SDs of change in CPAP adherence per each SD increase in each term. This enables the comparison of effect size among physiological traits. Bold indicates P < 0.05.
Adjustments are the same as those noted in Table 2 with the following exceptions. In the subgroup of men without REM-predominant OSA, there was no adjustment for sex. In the subgroup of patients with sleepy OSA, there was no adjustment for the ESS score.
Among individuals with ongoing CPAP use at 2 years, ArTH was no longer associated with adherence (per main effect). Instead, high LG in the setting of a low ArTH was related to poor CPAP use (Table 3, Figure E2). The association of LG with adherence was also modified by Vcomp. Specifically, at a high LG, adherence declined with worsening Vcomp (Figure E3).
Mediators of Association between Physiological Traits and Adherence
Anxiety and decreased total sleep time at baseline were associated with less CPAP use (β = 1.03 and β = 0.30; P = 0.001 and P = 0.003, respectively) in the entire sample. Anxiety was not a significant mediator (P = 0.132), whereas sleep time accounted for 23.6% (P = 0.010) of the relationship between the ArTH and CPAP adherence (Figure 2). There was no association between adherence and an ESS score change, mask leak, or residual AHI (Table E7). Higher CPAP pressures were associated with better adherence (Table E8; β = 0.11, P < 0.001) in the entire sample. The device pressure explained 7.1% and 8.3% of the change in adherence driven by the ArTH and Vcomp, respectively (P = 0.018 and P = 0.012).
Figure 2.
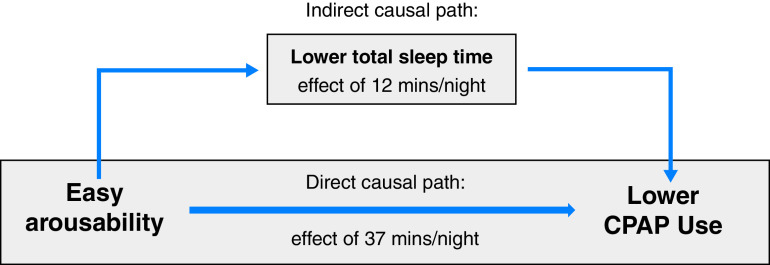
Relationship among a low arousal threshold (ArTH), the sleep duration at baseline, and the longitudinal continuous positive airway pressure (CPAP) adherence. A directed acyclic graph and mediation analysis model for the change in CPAP adherence caused by a change in the ArTH (see also Table 2) as mediated by the total sleep time at baseline (P = 0.010 for mediation) is shown. A lower total sleep time at baseline accounted for 23.5% (interquartile range, 2.3–100.0%) of the relationship between the ArTH and CPAP adherence (12 min of the 49-min decrease in CPAP use were attributed to each 1-SD decrease in the ArTH).
Prediction Modeling
The predictive value of the physiological multivariable adherence model (Table 2, model 1) was assessed by using a cutoff for the lowest quartile of adherence (estimated adherence = 3.2 h/night at 1 mo). Resultant measures (area under the curve, 0.65; accuracy = 70.3%, sensitivity = 40.3%, specificity = 80.2%, positive predictive value = 40.3%, and negative predictive value = 80.2%) were not meaningfully reduced with cross-validation (accuracy = 69.8%, sensitivity = 38.7%, specificity = 80.2%, positive predictive value = 39.3%, and negative predictive value = 79.8%).
The actual adherence rates of those with predicted poor adherence and those with predicated good adherence were substantially different (difference in medians = 2.0–3.2 h/night; P < 0.003 for all) across 24 months of follow-up, and the difference persisted after cross-validation (Figure 3, Table E11). Differences between subgroups 1) were not meaningfully diminished with adjustment for clinical predictors (see Prediction Modeling in the online supplement) and 2) were contingent on the inclusion of ArTH in the physiological prediction model (Table E12).
Figure 3.
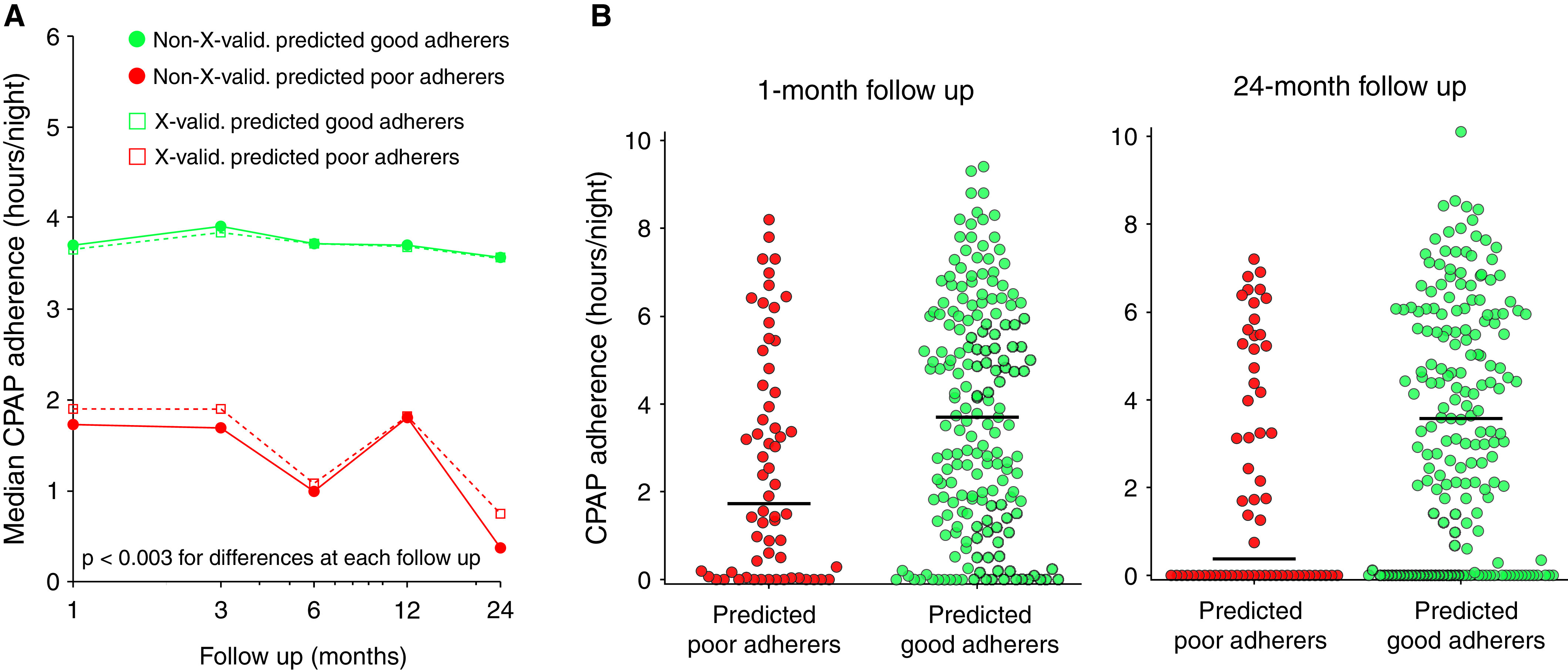
Comparison of continuous positive airway pressure (CPAP) adherence in those with predicted “good” adherence and those with predicted “poor” adherence. (A) Comparison made by using the physiological adherence model between the median actual CPAP adherence (h/night) of those with predicted good adherence (green) and the median actual CPAP adherence (h/night) of those with predicted poor adherence (red). Solid circles represent results before leave-one-out cross-validation, and open squares represent results after leave-one-out cross-validation. Differences between groups are statistically different across each time point (P < 0.003). (B) Distributions and medians (bars) of actual CPAP adherence among those with predicted good adherence (green, n = 187) and those with predicted poor adherence (red, n = 62) at the 1-month follow-up (change in median, 2.0 h/night; P = 0.003) and 24-month follow-up (change in median, 3.2 h/night; P < 0.001).
Discussion
The novel finding of our study is that among patients with CAD, the physiological traits that cause OSA are also important for long-term adherence to CPAP, OSA’s first-line therapy. Specifically, we show that a decrease in the ArTH by 32% (1 SD) is associated with a clinically meaningful, 49-minute decline in long-term CPAP use that is independent of other biomedical and psychological factors. We also identified a novel relationship between Vcomp and adherence whereby both high and low Vcomp are linked to poor adherence. Notably, we found that traits may be more relevant for adherence in certain patient subgroups. For example, in men without REM-predominant OSA (73% of our participants with CAD and OSA), the association of the ArTH with adherence is even stronger (a 71-min decline per each SD of the ArTH). Finally, those with poor adherence as predicted by the physiological adherence model exhibited an actual adherence that was >2 hours lower than that of those with predicted good adherence. Taken together, our findings suggest that a priori knowledge of an individual’s OSA pathophysiology and clinical characteristics may aid with the identification of patients at risk of poor CPAP adherence. This knowledge may help us develop precision medicine interventions to improve CPAP use in patients with OSA and CAD.
ArTH and CPAP Adherence
In patients with OSA, the propensity to easily awaken from sleep because of a respiratory stimulus such as a hypopnea is also known as a low ArTH. It is found in nearly 40% of those with OSA (11, 22). A low ArTH results in lighter and less continuous sleep (32), and it may thus affect a patient’s ability to tolerate and adhere to CPAP. Indeed, we and others have shown that an estimated low ArTH, as derived from the AHI, the frequency of hypopneas, and the nadir O2 saturation, is associated with less regular CPAP use (11, 19). However, this derived ArTH metric has not been consistently associated with CPAP adherence (33, 34), challenging the validity of this association. The discrepant findings in prior work may be due to several factors. First, the effects of time on CPAP adherence, known to change over time (13, 14), were not considered. Second, the varied inclusion of adherence factors such as sleep duration, CPAP experience characteristics, and psychological and medical comorbidities (35, 36) in adherence models may result in discrepant results. Finally, other OSA traits such as Vpassive or LG—which are known to affect the relationship of the traits with treatment response (12, 26)—were not included in prior analyses.
In the current study, we accounted for the effects of time on CPAP adherence; important sleep characteristics such as sleep duration; relevant CPAP factors (e.g., CPAP pressure, mask leak); and comorbidities such as depression, anxiety, and atrial fibrillation. Importantly, we assessed the role of ArTH together with other physiological traits. We found that the ArTH was not associated with adherence in bivariate analyses but that a clinically meaningful relationship with CPAP use (49 min/SD of the ArTH) became apparent after incorporating Vpassive, LG, and Vcomp. This suggests that, akin to the traits’ role in the causation of OSA, the interplay among the traits is important in understanding an individual’s response to OSA therapies. The value of considering the ArTH in the context of other traits is highlighted by studies assessing responses to other therapies (e.g., oral appliances, oxygen) (26, 37). Intuitively, a low ArTH may have a different impact on therapy response in an individual with severe versus mild pharyngeal compromise. Approaches such as cluster analysis (29) may be one way to increase understanding of how different patterns of traits combine to form a subtype of OSA and whether such subtypes have implications for adherence and other outcomes.
Mechanisms by which a low ArTH impairs CPAP adherence have not been investigated. Plausible pathways include a decreased ability to reach effective CPAP pressures without arousals, ongoing residual respiratory events, and a lack of a perceived benefit of treatment due to impaired sleep quality or daytime symptoms (38). Indeed, in our study, the higher CPAP pressures were associated with longer CPAP use, but they mediated a small fraction (7.1%) of the ArTH’s relationship with adherence. We also found no association between adherence and the residual AHI or changes in sleepiness (P = 0.463 and P = 0.808, respectively). Thus, current metrics of experience with CPAP may not adequately capture what is important for adherence. In contrast, study participants with a lower ArTH exhibited a shorter sleep duration at baseline, which was, in turn, associated with lower CPAP use. Sleep duration accounted for nearly 25% of the ArTH’s association with CPAP use. A better understanding of the relationship between sleep duration and the ArTH is needed, especially in terms of whether short sleep is a manifestation of insomnia, a known predictor of lower CPAP adherence (39). Knowing whether the ArTH is associated with adherence independently of—or in combination with—insomnia is important before trialing therapies to reduce arousability.
Vcomp and CPAP Adherence
In our study, both high and low Vcomp were associated with a reduction in CPAP use, independent of other factors, including OSA severity and the ArTH. The association of high Vcomp with lower adherence was expected because higher-than-normal Vcomp is commonly accompanied by a lower ArTH and milder OSA (40) (P < 0.001 and P < 0.044, respectively, in our study). Intuitively, individuals with vigorous Vcomp rely less on CPAP to maintain nocturnal airflow and may therefore not experience as much benefit from CPAP. The association between very poor Vcomp and lower adherence was, however, unexpected. It is possible that, among those with poor Vcomp, the increased ventilatory drive at event termination results in a higher symptomatic burden, which may not be fully addressed with CPAP therapy. Notably, such individuals can exhibit “negative effort dependence” (worsening pharyngeal compromise with rising ventilatory drive). This phenomenon is a marker of the obstruction of non–tongue-based structures (such as the epiglottis) (41) that is associated with worsening collapse on CPAP (42), which could impact the perceived benefit of therapy. Further work is needed to reproduce our findings and assess the aforementioned hypotheses.
Physiological Traits in Clinical OSA Subgroups
The strongest association between the ArTH and CPAP adherence occurred among men without REM-predominant OSA (1.2 h/night per each SD of the ArTH; P = 0.003), a group composing 73% of our participants with CAD. These results align with recent findings that non-REM OSA is more common among men and that the ArTH accounts for much of this sex difference (43). Our results may also reflect that the traits were measured during non-REM sleep. Because ArTH increases with worsening Vpassive, we anticipated that adherence would be increased with worsening Vpassive. Our finding that adherence improved with milder Vpassive after accounting for the ArTH suggests that adherence to CPAP may be easier for those with a mildly compromised upper airway, so long as it is not accompanied by a low ArTH. The physiological traits were not related to adherence among women (n = 36) or among patients with REM-predominant OSA (n = 47). These findings likely reflect low analytic power. Studies focused on each subgroup are needed to understand the trait’s role in CPAP use.
Clinical Implications
Sleep apnea affects over 6 million U.S. adults with CAD (44, 45). Together with the work of others, our prior work shows that CPAP adherence is poor in this population (3, 4) and that cardiovascular risk is lowest in those who adhere to CPAP (3). Predicting who will adhere to CPAP over the long term, however, is challenging. Most factors are nonmodifiable (e.g., age, myocardial infarction history) or occur after experience with CPAP (e.g., 1-mo adherence) (13, 14). We show that physiological traits, including a low ArTH, may aid with the identification of patients who are at risk of poor adherence. In our study, those with predicted poor adherence (based on the traits) exhibited markedly lower CPAP use (by 2.0–3.2 h/night) than those with predicted good adherence. This suggests that physiological traits might be useful for identifying a subgroup of patients with poor CPAP use (median of <2 h/night) who may benefit from an early adherence intervention.
This is important because proven behavioral interventions, such as motivational enhancement (46), may be targeted to those at risk of nonadherence. Moreover, because the ArTH is modifiable by sedative hypnotics, these agents may be used at the outset of OSA therapy to improve adherence to CPAP. In unselected sleep clinic patients, eszopiclone improved adherence at 6 months (47). Selecting those with a low ArTH may help maximize the effects of this intervention (19), especially in men without REM-predominant OSA. Such patients exhibit lower amounts of hypoxemia, and recent data show that zopiclone and zolpidem (without CPAP) can increase the ArTH without significantly impacting nocturnal hypoxemia or daytime alertness (48, 49). Although validation in clinical trials is needed, this suggests a novel avenue for improving OSA treatment with CPAP in the CAD population.
The role of physiological traits in CPAP adherence may differ according to the time course of therapy. We observed that the ArTH was no longer associated with adherence (per main effect) among long-term (2-yr) CPAP users. Because CPAP therapy lowers the ArTH (50), the predictive value of a low ArTH may be greatest in early adherence and weaker among those who continue to use CPAP over the long term. Instead, among long-term CPAP users, we found that increasing LG, especially in those with a low ArTH, was associated with lower adherence. Similarly, high LG was associated with low adherence in the setting of poor Vcomp. Thus, our work highlights that opportunities to improve early adherence may differ from those for long-term CPAP use. It is plausible that addressing the ArTH with sedative hypnotics may be important in the early phase of therapy. In contrast, focusing on other traits—such as LG—by using oxygen (37) or acetazolamide (51) may help those with poor Vcomp who struggle with CPAP over the longer term.
Methodological Considerations
This study’s large sample size, together with longitudinal, objective, CPAP use data over a period of 2 years, enabled us to examine relationships in clinical participant subgroups with different rates of CPAP adherence. The use of validated measures of each of the key pathophysiological traits enabled the evaluation of interactions between the traits, which revealed new relationships, such as that between Vcomp and LG, in long-term adherence. Our trait assessments were robust, with the use of scorers accredited by the American Academy of Sleep Medicine resulting in excellent reliability in the trait measurements (intraclass correlation coefficient, 0.91–0.96). Detailed polysomnographic and CPAP therapy experience data (e.g., CPAP pressure, ESS score changes) enabled us to examine the mechanisms by which traits may influence adherence, including the influence of sleep duration and CPAP pressure.
There are several limitations. Our trait measurements were made during non-REM sleep only. OSA physiology during REM sleep is different (52), and the relationship between traits and adherence may be unique in patients with REM-predominant OSA. For a comprehensive assessment of the relationship between physiology and CPAP across sleep states, future studies should measure traits in both REM and non-REM sleep. In addition, because physiological trait values may vary depending on the scoring criteria used (53), special care is needed to ensure that event scoring is harmonized in studies validating relationships between the traits and outcomes.
In our analyses, the trait values were aggregated across all body positions. Although the baseline position has a substantial impact on Vpassive, other traits are not affected (54). In addition, neither the ratio of supine-to-nonsupine AHI nor the percentage of time spent supine were associated with adherence (P = 0.7 and P = 0.4, respectively). Thus, we believe that separating traits by position is unlikely to improve the relationships between traits and adherence.
Our secondary analytic sample included predominantly white men (86%). This limits our ability to assess racial, ethnic, and sex differences in adherence and physiological traits. Although our data showed no difference in adherence between men and women (P = 0.8), prior data are conflicting (7), and addressing sex differences is a key gap in the OSA research. Insomnia is more common among women and is known to affect CPAP adherence (39), and the ArTH differs between men and women (43). Understanding the relationships among insomnia symptoms, OSA physiology, and CPAP adherence is needed to personalize OSA management.
Lastly, our analyses included a large number of potential biological, medical, and psychological factors that may affect CPAP use (Table E2). However, our secondary analysis design did not allow for the inclusion of previously established key factors in adherence, such as self-efficacy, readiness for change, and partner support (7). Such unaccounted-for confounders may impact the observed relationships. This highlights the urgent need to measure and account for such factors in future prospective studies examining the physiological determinants of CPAP adherence. Additional considerations of applying physiological trait measurements to predict and improve CPAP adherence in the future are noted in the Methodologic Considerations section of the online supplement.
Conclusions
Among patients with CAD and OSA, reduced long-term CPAP adherence is associated with a low ArTH as well as very high and very low Vcomp. This finding is independent of other biomedical (e.g., age, AHI) and psychological (e.g., anxiety) determinants of adherence and may be mediated by a reduced sleep duration. Moreover, adherence in certain clinical subgroups, such as men without REM-predominant OSA, may be particularly affected by the ArTH. Understanding the physiological contributors to CPAP adherence may be a key to predicting CPAP use and improving OSA therapy in a personalized way.
Acknowledgments
Acknowledgment
The authors thank all of those involved in the data collection of the parent study in Sweden for their essential role, especially sleep technologist Lena Andersson, who precisely conducted and coordinated the polysomnography recordings. The authors thank John Denos, Lauren Hess, and Erik Smales for their meticulous scoring of polysomnograms and thank James Kim for assistance with data exports.
Footnotes
Supported by NIH grants R01 HL102321, R01 HL128658, and P01 HL149630 (A.W.); the Parker B. Francis Fellowship award (A.V.Z.); NIH grants K24 HL132093 (H.K.Y.) and R01HL146697 (S.A.S.); the American Heart Association (15SDG25890059); and the American Academy of Sleep Medicine Foundation (228SR20). The parent RICCADSA (Randomized Intervention with Continuous Positive Airway Pressure in Coronary Artery Disease and Obstructive Sleep Apnea) trial was supported by Swedish Research Council grants 521-2011-537 and 521-2013-3439 (Y.P.); Swedish Heart‐Lung Foundation grants 20080592, 20090708, and 20100664; “Agreement Concerning Research and Education of Doctors” of Vastra Gotalandsregionen grants ALFGBG-11538 and ALFGBG-150801; Research Fund at Skaraborg Hospital grants VGSKAS-4731, VGSKAS-5908, VGSKAS-9134, VGSKAS-14781, VGSKAS-40271, and VGSKAS-116431; Skaraborg Research and Development Council grant VGFOUSKB-46371; the Heart Foundation of Kärnsjukhuset; and the ResMed Foundation.
Author Contributions: All authors contributed substantially to the conception, design, analysis, or interpretation of the data in this study. Conception and design of the work: A.V.Z., H.K.Y., Y.P., and S.A.S. Data collection/management, trait analysis, and statistical analysis: A.V.Z., Y.P., S.A.S., J.C., and J.L. Data interpretation: all co-authors. Drafting of the manuscript: A.V.Z. and J.C. Critical revision of the manuscript: all co-authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202101-0055OC on June 22, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Cassar A, Morgenthaler TI, Lennon RJ, Rihal CS, Lerman A. Treatment of obstructive sleep apnea is associated with decreased cardiac death after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:1310–1314. doi: 10.1016/j.jacc.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 2. Lee CH, Sethi R, Li R, Ho HH, Hein T, Jim MH, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133:2008–2017. doi: 10.1161/CIRCULATIONAHA.115.019392. [DOI] [PubMed] [Google Scholar]
- 3. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med. 2016;194:613–620. doi: 10.1164/rccm.201601-0088OC. [DOI] [PubMed] [Google Scholar]
- 4. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. SAVE Investigators and Coordinators. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 5. Sánchez-de-la-Torre M, Sánchez-de-la-Torre A, Bertran S, Abad J, Duran-Cantolla J, Cabriada V, et al. Spanish Sleep Network. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): a randomised controlled trial. Lancet Respir Med. 2020;8:359–367. doi: 10.1016/S2213-2600(19)30271-1. [DOI] [PubMed] [Google Scholar]
- 6. Peker Y, Thunström E, Glantz H, Eulenburg C. Effect of obstructive sleep apnea and CPAP treatment on cardiovascular outcomes in acute coronary syndrome in the RICCADSA trial. J Clin Med. 2020;9:4051. doi: 10.3390/jcm9124051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crawford MR, Espie CA, Bartlett DJ, Grunstein RR. Integrating psychology and medicine in CPAP adherence—new concepts? Sleep Med Rev. 2014;18:123–139. doi: 10.1016/j.smrv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 8. Bakker JP, Weaver TE, Parthasarathy S, Aloia MS. Adherence to CPAP: what should we be aiming for, and how can we get there? Chest. 2019;155:1272–1287. doi: 10.1016/j.chest.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 9. Zinchuk AV, Redeker NS, Chu JH, Liang J, Stepnowsky C, Brandt CA, et al. Physiological traits and adherence to obstructive sleep apnea treatment in patients with stroke. Am J Respir Crit Care Med. 2020;201:1568–1572. doi: 10.1164/rccm.201911-2203LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray EL, McKenzie DK, Eckert DJ. Obstructive sleep apnea without obesity is common and difficult to treat: evidence for a distinct pathophysiological phenotype. J Clin Sleep Med. 2017;13:81–88. doi: 10.5664/jcsm.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zinchuk A, Edwards BA, Jeon S, Koo BB, Concato J, Sands S, et al. Prevalence, associated clinical features, and impact on continuous positive airway pressure use of a low respiratory arousal threshold among male United States Veterans with obstructive sleep apnea. J Clin Sleep Med. 2018;14:809–817. doi: 10.5664/jcsm.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194:1413–1422. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luyster FS, Strollo PJ, Jr, Thunström E, Peker Y. Long-term use of continuous positive airway pressure therapy in coronary artery disease patients with nonsleepy obstructive sleep apnea. Clin Cardiol. 2017;40:1297–1302. doi: 10.1002/clc.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chai-Coetzer CL, Luo YM, Antic NA, Zhang XL, Chen BY, He QY, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep. 2013;36:1929–1937. doi: 10.5665/sleep.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almeneessier AS, Almousa Y, Hammad O, Olaish AH, ALAnbay ET, BaHammam AS. Long-term adherence to continuous positive airway pressure in patients with rapid eye movement-only obstructive sleep apnea: a prospective cohort study. J Thorac Dis. 2017;9:3755–3765. doi: 10.21037/jtd.2017.09.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121:430–435. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 17. Balcan B, Thunström E, Strollo PJ, Jr, Peker Y. Determinants of depressive mood in coronary artery disease patients with obstructive sleep apnea and response to continuous positive airway pressure treatment in non-sleepy and sleepy phenotypes in the RICCADSA cohort. J Sleep Res. 2019;28:e12818. doi: 10.1111/jsr.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Celik Y, Thunström E, Strollo PJ, Jr, Peker Y. Continuous positive airway pressure treatment and anxiety in adults with coronary artery disease and nonsleepy obstructive sleep apnea in the RICCADSA trial. Sleep Med. 2021;77:96–103. doi: 10.1016/j.sleep.2020.11.034. [DOI] [PubMed] [Google Scholar]
- 19. Schmickl CN, Lettieri CJ, Orr JE, DeYoung P, Edwards BA, Owens RL, et al. The arousal threshold as a drug-target to improve CPAP adherence: secondary analysis of a randomized trial. Am J Respir Crit Care Med. 2020;202:1592–1595. doi: 10.1164/rccm.202003-0502LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zinchuk A, Yaggi H, Liang J, Chu J, Op De Beeck S, Stepnowski C, et al. 0568 Physiologic OSA traits and CPAP adherence among patients with coronary artery disease and OSA. Sleep. 2020;43:A218–A218. [Google Scholar]
- 21. Zinchuk AV, Yaggi HK, Liang J, Chu J-H, Op De Beeck S, Stepnowsky C, et al. Physiologic traits predict therapeutic pressure requirements and residual respiratory events among patients with coronary artery disease and obstructive sleep apnea [abstract] Am J Respir Crit Care Med. 2020;201:A6439. [Google Scholar]
- 22. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea: identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sands SA, Edwards BA, Terrill PI, Taranto-Montemurro L, Azarbarzin A, Marques M, et al. Phenotyping pharyngeal pathophysiology using polysomnography in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2018;197:1187–1197. doi: 10.1164/rccm.201707-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zinchuk AV, Yaggi HK, Concato J, Wellman DA, Smales E, Hess L, et al. Effect of arousal scoring on reliability of automated, non-invasive phenotyping of physiologic sleep apnea traits from polysomnography [abstract] Am J Respir Crit Care Med. 2019;199:A7078. [Google Scholar]
- 25. Babbin SF, Velicer WF, Aloia MS, Kushida CA. Identifying longitudinal patterns for individuals and subgroups: an example with adherence to treatment for obstructive sleep apnea. Multivariate Behav Res. 2015;50:91–108. doi: 10.1080/00273171.2014.958211. [DOI] [PubMed] [Google Scholar]
- 26. Bamagoos AA, Cistulli PA, Sutherland K, Madronio M, Eckert DJ, Hess L, et al. Polysomnographic endotyping to select patients with obstructive sleep apnea for oral appliances. Ann Am Thorac Soc. 2019;16:1422–1431. doi: 10.1513/AnnalsATS.201903-190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosmer DW, Lemeshow S, Sturdivant RX. Applied logistic regression. Hoboken, NJ: Wiley; 2013. [Google Scholar]
- 28. Pien GW, Ye L, Keenan BT, Maislin G, Björnsdóttir E, Arnardottir ES, et al. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic Sleep Apnea Cohort. Sleep. 2018;41:zsx201. doi: 10.1093/sleep/zsx201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zinchuk AV, Jeon S, Koo BB, Yan X, Bravata DM, Qin L, et al. Polysomnographic phenotypes and their cardiovascular implications in obstructive sleep apnoea. Thorax. 2018;73:472–480. doi: 10.1136/thoraxjnl-2017-210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods. 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 31. Op de Beeck S, Wellman A, Dieltjens M, Strohl KP, Willemen M, Van de Heyning PH, et al. STAR Trial Investigators. Endotypic mechanisms of successful hypoglossal nerve stimulation for obstructive sleep apnea. Am J Respir Crit Care Med. 2021;203:746–755. doi: 10.1164/rccm.202006-2176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985) 2014;116:302–313. doi: 10.1152/japplphysiol.00649.2013. [DOI] [PubMed] [Google Scholar]
- 33. Holley AB, Londeree WA, Sheikh KL, Andrada TF, Powell TA, Khramtsov A, et al. Zolpidem and eszopiclone pre-medication for PSG: effects on staging, titration, and adherence. Mil Med. 2018;183:e251–e256. doi: 10.1093/milmed/usx038. [DOI] [PubMed] [Google Scholar]
- 34. El-Solh AA, Lawson Y, Wilding GE. Impact of low arousal threshold on treatment of obstructive sleep apnea in patients with post-traumatic stress disorder. Sleep Breath. 2020;25:597–604. doi: 10.1007/s11325-020-02106-0. [DOI] [PubMed] [Google Scholar]
- 35. Law M, Naughton M, Ho S, Roebuck T, Dabscheck E. Depression may reduce adherence during CPAP titration trial. J Clin Sleep Med. 2014;10:163–169. doi: 10.5664/jcsm.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Budhiraja R, Kushida CA, Nichols DA, Walsh JK, Simon RD, Gottlieb DJ, et al. Impact of randomization, clinic visits, and medical and psychiatric cormorbidities on continuous positive airway pressure adherence in obstructive sleep apnea. J Clin Sleep Med. 2016;12:333–341. doi: 10.5664/jcsm.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sands SA, Edwards BA, Terrill PI, Butler JP, Owens RL, Taranto-Montemurro L, et al. Identifying obstructive sleep apnoea patients responsive to supplemental oxygen therapy. Eur Respir J. 2018;52:1800674. doi: 10.1183/13993003.00674-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malhotra A, Jordan A. The importance of arousal in obstructive sleep apnea-updates from the American Thoracic Society 2016. J Thorac Dis. 2016;8:S542–S544. doi: 10.21037/jtd.2016.06.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sweetman A, Lack L, Bastien C. Co-morbid Insomnia and Sleep Apnea (COMISA): prevalence, consequences, methodological considerations, and recent randomized controlled trials. Brain Sci. 2019;9:371. doi: 10.3390/brainsci9120371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol (1985) 2008;105:197–205. doi: 10.1152/japplphysiol.01214.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Genta PR, Sands SA, Butler JP, Loring SH, Katz ES, Demko BG, et al. Airflow shape is associated with the pharyngeal structure causing OSA. Chest. 2017;152:537–546. doi: 10.1016/j.chest.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Torre C, Camacho M, Liu SY, Huon LK, Capasso R. Epiglottis collapse in adult obstructive sleep apnea: a systematic review. Laryngoscope. 2016;126:515–523. doi: 10.1002/lary.25589. [DOI] [PubMed] [Google Scholar]
- 43. Won CHJ, Reid M, Sofer T, Azarbarzin A, Purcell S, White D, et al. Sex differences in obstructive sleep apnea phenotypes, the multi-ethnic study of atherosclerosis. Sleep. 2020;43:zsz274. doi: 10.1093/sleep/zsz274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109:659–663. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 45. Peker Y, Kraiczi H, Hedner J, Löth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–184. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 46. Bakker JP, Wang R, Weng J, Aloia MS, Toth C, Morrical MG, et al. Motivational enhancement for increasing adherence to CPAP: a randomized controlled trial. Chest. 2016;150:337–345. doi: 10.1016/j.chest.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. CPAP Promotion and Prognosis-The Army Sleep Apnea Program Trial. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151:696–702. doi: 10.7326/0003-4819-151-10-200911170-00006. [DOI] [PubMed] [Google Scholar]
- 48. Carter SG, Carberry JC, Cho G, Fisher LP, Rollo CM, Stevens DJ, et al. Effect of 1 month of zopiclone on obstructive sleep apnoea severity and symptoms: a randomised controlled trial. Eur Respir J. 2018;52:1800149. doi: 10.1183/13993003.00149-2018. [DOI] [PubMed] [Google Scholar]
- 49. Messineo L, Eckert DJ, Lim R, Chiang A, Azarbarzin A, Carter SG, et al. Zolpidem increases sleep efficiency and the respiratory arousal threshold without changing sleep apnoea severity and pharyngeal muscle activity. J Physiol. 2020;598:4681–4692. doi: 10.1113/JP280173. [DOI] [PubMed] [Google Scholar]
- 50. Loewen A, Ostrowski M, Laprairie J, Atkar R, Gnitecki J, Hanly P, et al. Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired? Sleep. 2009;32:1355–1365. doi: 10.1093/sleep/32.10.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590: 1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Joosten SA, Landry SA, Wong AM, Mann DL, Terrill PI, Sands SA, et al. Assessing the physiological endotypes responsible for REM- and NREM-based OSA. Chest. 2021;159: 1998–2007. doi: 10.1016/j.chest.2020.10.080. [DOI] [PubMed] [Google Scholar]
- 53. Landry SA, Joosten SA, Thomson LDJ, Turton A, Wong A-M, Leong P, et al. Effect of hypopnea scoring criteria on noninvasive assessment of loop gain and surgical outcome prediction. Ann Am Thorac Soc. 2020;17:484–491. doi: 10.1513/AnnalsATS.201906-436OC. [DOI] [PubMed] [Google Scholar]
- 54. Joosten SA, Edwards BA, Wellman A, Turton A, Skuza EM, Berger PJ, et al. The effect of body position on physiological factors that contribute to obstructive sleep apnea. Sleep. 2015;38: 1469–1478. doi: 10.5665/sleep.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]