To the Editor:
A great debate has started as to whether acute respiratory failure (ARF) induced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (coronavirus disease [COVID-19]) should be classified as a classic form of acute respiratory distress syndrome (ARDS) or a subtype of lung injury with different pathophysiological characteristics (1) and mechanisms of progression (2). The magnitude of inspiratory effort correlated with the need to switch to invasive ventilation in patients without COVID-19 suggest that self-inflicted lung injury could play a role (3).
We aimed to describe and compare the inspiratory effort (primary outcome) and the breathing pattern of spontaneously breathing patients with ARF due to COVID-19 and historically matched patients without COVID-19, either type a candidate for noninvasive mechanical ventilation (NIV).
Patients with COVID-19 were treated at the Respiratory Intensive Care Unit and the Intensive Care Unit of the University Hospital of Modena (from August 1, 2020, to March 15, 2021) and 1:1 propensity matched (by PaO2/FiO2 ratio, age, body mass index, and Sequential Organ Failure Assessment score) with non–COVID-19 patients extracted from our dataset (period 2016 to 2021). The logit of the score was taken with a caliper of 0.2 to maximize the number of patients without compromising the match. All patients were in a similar phase from onset of ARF, unable to maintain SaO2 > 92% despite optimized high-flow oxygen, and thus were candidates to receive NIV according to local protocol. The study was conducted in accordance with the local “AVEN Ethics Committee” approval (protocol 4485/CE) and represents a report of ancillary data of a registered protocol (www.clinicaltrials.gov [NCT03826797]).
On admission, demographics, clinical characteristics and severity, respiratory function, and peripheral blood lactate and D-dimer concentrations were recorded.
A nasogastric tube with pressure transducer and monitoring system (NutriVent and OptiVentTM, SIDAM, Mirandola-I) to record swings in esophageal pressure (Pes) and dynamic transpulmonary pressure (Pl) was placed as previously described (3). For recording, we always referred to ΔPes and ΔPl from the end-expiratory level, calculated as recommended (4). Expiratory tidal volume (Vte) was obtained by numerical integration of the flow signal and adjusted to the predicted body weight (in kilograms). The Vte/ΔPl ratio was measured as a surrogate for lung compliance (“dynamic compliance”). A simplified surrogate of mechanical power (“dynamic mechanical power”) was calculated as 0.098 × RR × Vte × (ΔPl + positive end-expiratory pressure), where RR represents respiratory rate (5).
Measures were recorded for patients of both groups under standardized conditions with unassisted breathing (5 min long) and then 2 hours after starting NIV (Engström Carestation [GE Healthcare]). Pressure support was started at 10 cm H2O and then adjusted according to Carteaux and colleagues (6) to target a peripheral oxygen saturation as measured by pulse oximetry >92% with a delivered FiO2 < 0.7. Endotracheal intubation rate and 28-day death rate were recorded.
The Student’s t test assessed the between-group difference when data were distributed normally; otherwise, the Mann-Whitney U test was used. A comparison between dichotomous variables was performed with the χ2 test or Fisher exact test. ANOVA was used to test as an interaction for whether the change in physiological variables 2 hours after NIV was different between groups.
Table 1 shows the characteristics of patients at admission and the clinical outcomes. Compared with the non–COVID-19 group, patients with COVID-19 before NIV showed lower values of inspiratory effort as assessed by ΔPes (Figure 1). Shown are baseline values of lung mechanics, RR, V˙e, Vte, and dynamic mechanical power at baseline and 2 hours after NIV are resumed (Table 1). The COVID-19 group showed lower values of RR (P < 0.001), Vte (P = 0.003), V˙e (P < 0.001), and dynamic mechanical power (P < 0.001) and higher dynamic compliance (P < 0.001) compared with the non–COVID-19 group. After NIV, a reduction in ΔPes and RR and an increase in dynamic mechanical power was reported for both groups, whereas no change was noted in either dynamic compliance or Vte. Despite there being no group interactions or changes in the physiological variables after NIV, ΔPl showed a significant increase in the COVID-19 group. In this group, the baseline value of dynamic mechanical power was considerably lower than in the non–COVID-19 group (27 vs. 95 J/min, P < 0.0001) and was significantly increased following NIV. This may suggest an unfavorable interaction between kinetic energy transferred from the respiratory muscles and the NIV to the lungs of these patients, at least early. Nevertheless, the absolute value of dynamic mechanical power was lower in patients with COVID-19 than in non–COVID-19 patients.
Table 1.
Characteristics of the Study Groups at Inclusion, Ventilatory Settings, and Clinical Outcome and Mechanical Variables before and after NIV
| Clinical Variables | COVID-19 (n = 30) | Non–COVID-19 (n = 30) | P Value | ||||
|---|---|---|---|---|---|---|---|
| Age, yr | 68 (57–77) | 68 (57–78) | 0.9 | ||||
| Sex, M | 23 (77) | 22 (73) | 0.9 | ||||
| BMI, kg/m2 | 24 (21–27) | 24 (20–26) | 0.9 | ||||
| Charlson index score | 3 (2–4) | 4 (2–5) | 0.1 | ||||
| Kelly scale score | 1 (1–1) | 1 (1–1) | 0.9 | ||||
| SOFA score | 3 (3–4) | 3 (3–4) | 0.9 | ||||
| PaO2/FiO2, mm Hg* | 127 (100–138) | 124 (100–133) | 0.9 | ||||
| PaO2/FiO2 2 h after NIV, mm Hg | 133 (118–155) | 139 (119–158) | 0.4 | ||||
| pH | 7.48 (7.46–7.5) | 7.48 (7.44–7.5) | 0.7 | ||||
| pH after NIV | 7.45 (7.44–7.46) | 7.46 (7.43–7.48) | 0.5 | ||||
| PaCO2, mm Hg | 33 (30–38) | 34 (30–40) | 0.7 | ||||
| PaCO2 after NIV | 35 (32–37) | 35 (32–36) | 0.7 | ||||
| Blood lactate, mmol/L | 1 (0.7–1.2) | 1.7 (1–2.2) | 0.001 | ||||
| Serum creatinine, mg/dl | 0.7 (0.6–0.9) | 0.7 (0.5–0.9) | 0.8 | ||||
| D-dimer, mg/dl | 1,310 (862–9,400) | 815 (540–1,233) | 0.01 | ||||
| PEEP, cm H2O† | 10 (8–10) | 10 (8–11) | 0.7 | ||||
| PSV, cm H2O† | 12 (10–12) | 12 (10–16) | 0.2 | ||||
| ETI | 9 (30) | 11 (37) | 0.8 | ||||
| 28-d mortality | 6 (20) | 5 (17) | 0.9 | ||||
| Mechanical Variables | Baseline | 2 Hours after NIV | P Value | Baseline | 2 Hours after NIV | P Value | Interaction Test (ANOVA) |
|---|---|---|---|---|---|---|---|
| ΔPes, cm H2O | 12.5 (11.8–17.3) | 7.6 (6–10) | <0.0001 | 32 (25–38) | 18 (12–34) | <0.0001 | 0.1 |
| ΔPl, cm H2O | 12.5 (11.8–17.3) | 18.4 (16.9–21.3) | <0.0001 | 32 (25–38) | 32 (26–43) | 0.1 | 0.1 |
| RR, breaths/min | 28 (25–30) | 24 (21–26) | <0.0001 | 35 (30–41) | 31 (24–38) | 0.02 | 0.1 |
| V˙e, L/min | 20 (17–23) | 18 (15–22) | 0.1 | 27 (23–32) | 24 (20–28) | 0.004 | 0.6 |
| Vte, ml/kg of PBW | 9.2 (8.1–10.3) | 10.1 (8.7–11.2) | 0.3 | 10.9 (9.3–12) | 10.2 (9.9–12) | 0.8 | 0.6 |
| Dynamic compliance, ml/cm H2O | 55 (40–69) | 41 (31–52) | 0.1 | 25 (19–31) | 21 (16–34) | 0.7 | 0.2 |
| Dynamic mechanical power, J/min | 27 (19–40) | 56 (41–60) | <0.0001 | 95 (68–107) | 102 (66–130) | 0.1 | 0.2 |
Definition of abbreviations: BMI = body mass index; COVD-19 = coronavirus disease; ETI = endotracheal intubation; IQR = interquartile range; NIV = noninvasive mechanical ventilation; PBW = predicted body weight; PEEP = positive end-expiratory pressure; Pes = esophageal pressure; Pl = dynamic transpulmonary pressure; PSV = pressure support value; RR = respiratory rate; SOFA = Sequential Organ Failure Assessment; Vte = expiratory tidal volume.
Data are presented as number (percentage) for dichotomous values or median (IQR) for continuous values.
The values of the PaO2/FiO2 ratio used for matching these groups as well as pH and Pco2 values were those measured during high-flow nasal oxygen (see inclusion criteria earlier) immediately before starting NIV.
PEEP and PSV values reported were those measured during the first 2 hours of NIV.
Figure 1.
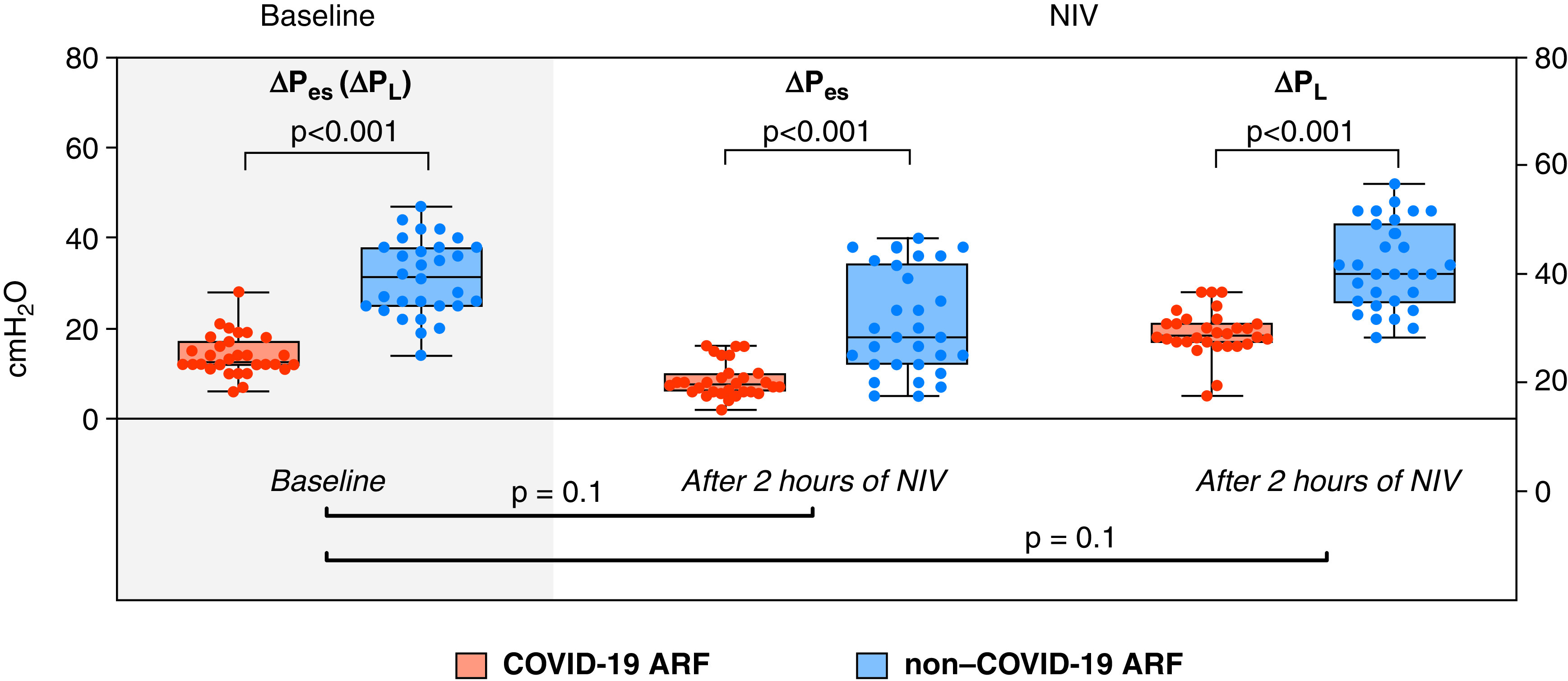
Measured individual values of change in esophageal pressure (ΔPes) and change in dynamic transpulmonary pressure (ΔPl) in the matched study groups at baseline and 2 hours after initiating noninvasive mechanical ventilation (NIV). No statistical difference was found when testing as an interaction for whether the change of ΔPes and ΔPl 2 hours after starting NIV was different between coronavirus disease (COVID-19) and non–COVID-19 ARF (P = 0.1 and P = 0.1, respectively). ARF = acute respiratory failure.
To our knowledge, this is the first report assessing inspiratory effort and respiratory mechanics in spontaneously breathing patients with COVID-19 developing ARF. At their early onset of ARF, these patients showed different mechanical characteristics and breathing patterns when compared with non–COVID-19 patients.
It has been speculated that the progression across COVID-19 may be triggered by excessive inspiratory drive activation (7). We have previously found that patients with moderate to severe ARDS exhibited very high inspiratory effort—even early during NIV—which was associated with unfavorable outcomes (3). Because the mean arterial pH was 7.48 in both groups, patients with COVID-19 also appeared to have a (relatively) high respiratory drive. However, the inspiratory effort of patients with COVID-19 was lower than in non–COVID-19 patients, thus suggesting a mismatch between lung mechanics and hypoxia, at variance with the typical form of ARDS. Indeed, a different lung morphology, as compared with ARDS, might explain this: the higher presence of areas with a low V˙/Q˙ ratio (i.e., ground glass = poorly aerated tissue with higher compliance) as compared with true shunted areas (atelectasis = consolidations with lower compliance) may be the basis of these findings. This study showed that, when spontaneous breathing is preserved, the dynamic compliance of the respiratory system is twice as high in patients with COVID-19 compared with the matched non–COVID-19 patients; furthermore, the abnormally elevated mean D-dimer value found in patients with COVID-19 (Table 1) suggests microvasculature involvement in the mechanism of ARF.
Nevertheless, previous reports (8, 9) led to the recommendation of similar ventilatory strategies in patients with hypoxemic ARF, without any regard as to whether they follow COVID-19 or non–COVID-19 underlying conditions (10). At least in the early phase of ARF, the magnitude of inspiratory effort in patients with COVID-19 is lower than in non–COVID-19, probably limiting the likely significance of self-inflicted lung injury. Indeed, a simple surrogate marker of mechanical power was considerably lower in patients with COVID-19. Although the application of NIV resulted in a significant reduction of inspiratory effort and RR (Table 1), ΔPl increased, thus calling into question the optimal time to start ventilation.
Our study suffers from several limitations. First, it represents an exploratory analysis with no sample size assessment, a limited number of patients, and a monocentric design. Second, the higher baseline blood lactate value in the non–COVID-19 group may reflect a difference in terms of balance from oxygen delivery to tissue metabolic needs, with a potential influence on the breathing pattern. An elevated value of lactic anions as a nonmechanical factor, even in the absence of blood acidosis, might have boosted the ventilatory response, influencing the different behavior between groups. Third, the observational nature of the study design does not allow us to draw firm conclusions about respiratory response to NIV application. Nevertheless, we believe that this preliminary observation supports the implementation of continuous monitoring of the patient’s inspiratory effort during episodes of hypoxemic ARF. In contrast with non–COVID-19 patients, the findings indicate a limited inspiratory effort in the early phase of ARF in patients with COVID-19, thus suggesting that caution should be taken when considering early endotracheal intubation to anticipate protective mechanical ventilation in these individuals.
Acknowledgments
Acknowledgment
The authors thank professional editor Colin Woodham (Alpha Science Editors) for language editing and Prof. Roberto D’Amico (Statistics Unit, Department of Diagnostics, Clinical and Public Health Medicine, University of Modena and Reggio Emilia, Modena, Italy) for statistical assistance.
Footnotes
Author Contributions: R.T., S.B., and A.M. designed the study, enrolled the patients, analyzed data, and wrote the paper. L.T., R.F., and I.C. made substantial contributions to the literature review, data collection, and paper writing. E.B. and C.M. reviewed and edited the manuscript. M.G. and E.C. designed the study and reviewed and edited the manuscript. All authors have read and approved the final version of the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202104-1029LE on July 2, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. 2021;6:622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tonelli R, Marchioni A, Tabbì L, Fantini R, Busani S, Castaniere I, et al. Spontaneous breathing and evolving phenotypes of lung damage in patients with COVID-19: review of current evidence and forecast of a new scenario. J Clin Med. 2021;10:975. doi: 10.3390/jcm10050975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tonelli R, Fantini R, Tabbì L, Castaniere I, Pisani L, Pellegrino MR, et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in de novo respiratory failure: a pilot study. Am J Respir Crit Care Med. 2020;202:558–567. doi: 10.1164/rccm.201912-2512OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellani G, Grasselli G, Teggia-Droghi M, Mauri T, Coppadoro A, Brochard L, et al. Do spontaneous and mechanical breathing have similar effects on average transpulmonary and alveolar pressure? A clinical crossover study. Crit Care. 2016;20:142. doi: 10.1186/s13054-016-1290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becher T, van der Staay M, Schädler D, Frerichs I, Weiler N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019;45:1321–1323. doi: 10.1007/s00134-019-05636-8. [DOI] [PubMed] [Google Scholar]
- 6. Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med. 2016;44:282–290. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 7. Gattinoni L, Marini JJ, Busana M, Chiumello D, Camporota L. Spontaneous breathing, transpulmonary pressure and mathematical trickery. Ann Intensive Care. 2020;10:88. doi: 10.1186/s13613-020-00708-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mezidi M, Daviet F, Chabert P, Hraiech S, Bitker L, Forel JM, et al. Transpulmonary pressures in obese and non-obese COVID-19 ARDS. Ann Intensive Care. 2020;10:129. doi: 10.1186/s13613-020-00745-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baedorf Kassis E, Schaefer MS, Maley JH, Hoenig B, Loo Y, Hayes MM, et al. Transpulmonary pressure measurements and lung mechanics in patients with early ARDS and SARS-CoV-2. J Crit Care. 2021;63:106–112. doi: 10.1016/j.jcrc.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49:e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]


