Abstract
Oxygen supplementation is one of the most common interventions in critically ill patients. Despite over a century of data suggesting both beneficial and detrimental effects of supplemental oxygen, optimal arterial oxygenation targets in adult patients remain unclear. Laboratory animal studies have consistently showed that exposure to a high FiO2 causes respiratory failure and early death. Human autopsy studies from the 1960s purported to provide histologic evidence of pulmonary oxygen toxicity in the form of diffuse alveolar damage. However, concomitant ventilator-induced lung injury and/or other causes of acute lung injury may explain these findings. Although some observational studies in general populations of critically adults showed higher mortality in association with higher oxygen exposures, this finding has not been consistent. For some specific populations, such as those with cardiac arrest, studies have suggested harm from targeting supraphysiologic PaO2 levels. More recently, randomized clinical trials of arterial oxygenation targets in narrower physiologic ranges were conducted in critically ill adult patients. Although two smaller trials came to opposite conclusions, the two largest of these trials showed no differences in clinical outcomes in study groups that received conservative versus liberal oxygen targets, suggesting that either strategy is reasonable. It is possible that some strategies are of benefit in some subpopulations, and this remains an important ongoing area of research. Because of the ubiquity of oxygen supplementation in critically ill adults, even small treatment effects could have a large impact on a global scale.
Keywords: oxygen inhalational therapy, ICUs, hyperoxia
Since the discovery of oxygen in the 18th century, its life-giving properties have been known to coexist with the potential to damage and destroy (1). Although essential for cellular respiration, excess oxygen can lead to the production of reactive oxygen species (ROS), causing oxidative damage to cellular structures and activating cell-death pathways (2). Although there have been concerns about potential oxygen toxicity since its introduction into clinical medicine in the 1920s (3), optimal dosing of oxygen in critically ill patients remains unclear. Any effective strategy would need to balance the deleterious effects of hypoxemia with the potential consequences of direct pulmonary toxicity from high concentrations of inspired oxygen and systemic toxicity from high concentrations of oxygen in the blood (hyperoxemia) and tissues (hyperoxia). Given the prevalence of oxygen therapy in ICUs, an oxygen dosing strategy that optimized patient outcomes could be beneficial on a large scale. In this review, we outline the experimental and observational evidence for oxygen toxicity in critically ill patients. We then review the emerging data from clinical trials in critically ill patients with respiratory failure and discuss future research priorities.
Animal Models
Numerous studies in laboratory animals demonstrated that exposure to an FiO2 greater than 0.7 over 3–6 days can cause death from progressive respiratory failure (4, 5). Histopathologic examination of these animals revealed diffuse alveolar damage comparable to acute respiratory distress syndrome (ARDS) (6). The species and age of the laboratory animals influenced susceptibility. For example, some studies showed that lower-order primates succumb to oxygen toxicity later in the course of high FiO2 exposure than do rabbits or rodents (7–9). This apparent increased oxygen tolerance in primates is of unclear significance regarding oxygen’s potential toxicity to humans. Although these studies suggest that cumulative exposure to high levels of FiO2 is an important determinant of toxicity, other preclinical studies demonstrated the occurrence of biological changes with exposure to FiO2 levels of <0.6 and at durations of ⩽3 hours (10, 11).
Preclinical Studies in Humans
In one of the earliest systematic studies of oxygen tolerance in humans, young healthy volunteers breathed different concentrations of oxygen for up to 24 hours (12). Between Hours 12 and 16, most subjects receiving 100% oxygen developed cough, substernal chest discomfort, and a decrease in VC. These findings were also frequent in subjects breathing 75% oxygen but not in those breathing 50% oxygen. Subsequent studies of high oxygen exposure in humans demonstrated similar results (13–16). Substernal discomfort in this setting may be from local effects of absorptive atelectasis induced by nitrogen washout rather than from tissue damage due to oxygen toxicity. Atelectasis as a prominent mechanism is supported by a study in which resolution of substernal discomfort occurred with scheduled coughs and sighs (14). In another study, subjects breathed a mixture of 50% oxygen and 50% nitrogen at 2 atmospheres of pressure for 3 hours. In this design, high alveolar oxygen concentrations were maintained without nitrogen washout, and subjects did not experience substernal discomfort (14). High-FiO2 breathing in both healthy and critically ill adults also affects gas exchange and can lead to increased intrapulmonary shunt and / mismatch (17, 18). This shunt may be reversed by the application of positive end-expiratory pressure (PEEP), again supporting atelectasis as the prominent mechanism (19). Other studies in healthy humans suggested direct pulmonary toxic effects. Bronchoscopic examination of research subjects exposed to a high FiO2 showed erythematous airways, histologic evidence of tracheal inflammation (15, 20), and suppressed mucociliary clearance, a marker of tracheal epithelial dysfunction (20, 21). At the level of the alveolus, bronchoalveolar fluid from subjects breathing 50–100% oxygen for 17–45 hours contained increased concentrations of albumin and profibrotic mediators, suggesting early alveolar injury and increased vascular and epithelial permeability (15, 22). Although these studies suggest both local physiologic and direct tissue toxicity effects, most were conducted in healthy volunteers. Whether or not a high amount of inspired oxygen causes the same effects in critically patients and how these affect clinical outcomes is uncertain.
Context for Human Clinical Studies of Oxygen Toxicity
Autopsy studies in patients who had died of critical illness in the 1960s and 1970s provided the first human histopathologic evidence of lung injury potentially attributable to oxygen toxicity (23–26). Lung tissue from 70 patients who died after prolonged mechanical ventilation (MV) was compared with lung tissue from control patients who died but had not received MV (23). In the MV group, lung histopathologic analysis showed early exudative and late proliferative stages of what would later be recognized as diffuse alveolar damage (27). These findings were most frequent in patients with >10 days of MV with an FiO2 ⩾0.9. In another autopsy study, diffuse histopathologic lung injury was only found in those exposed to an FiO2 ⩾40% (26). However, these studies should be interpreted with caution. The practices of MV during this period used a larger t and lower PEEP than are used in current MV strategies. It is not clear whether the demonstrated lung injury was due to oxygen toxicity, ventilator-induced lung injury, or other causes of ARDS.
Several aspects of critical illness may influence susceptibility to oxygen toxicity. During critical illness, concomitant lung injury, systemic inflammation, increased metabolism, and preexisting tissue hypoxia may potentiate or modify the risks of oxygen toxicity (5). In animal experiments, injured lungs were more vulnerable to high-FiO2 lung injury, although the timing of oxygen delivery in relation to initial injury affected this vulnerability (11, 28–32). In addition, hyperoxia may act synergistically with ventilator-induced lung injury, as shown in multiple animal models (33–35). Recent work also demonstrates that in both a mouse model and a cohort of critically ill humans, exposure to a high FiO2 was associated with alterations in the lung microbiota (the community of microorganisms residing in the lung) (36). This change precedes the development of lung injury and leads to selection of oxygen-tolerant microbes such as Staphylococcus aureus. In this group of studies, a germ-free mouse model or preceding antibiotic treatment eliminated/prevented altered lung microbiota and attenuated subsequent development of lung injury. This suggests a causal mechanism of oxygen-induced injury that may be particularly relevant to infection-prone critically ill patients.
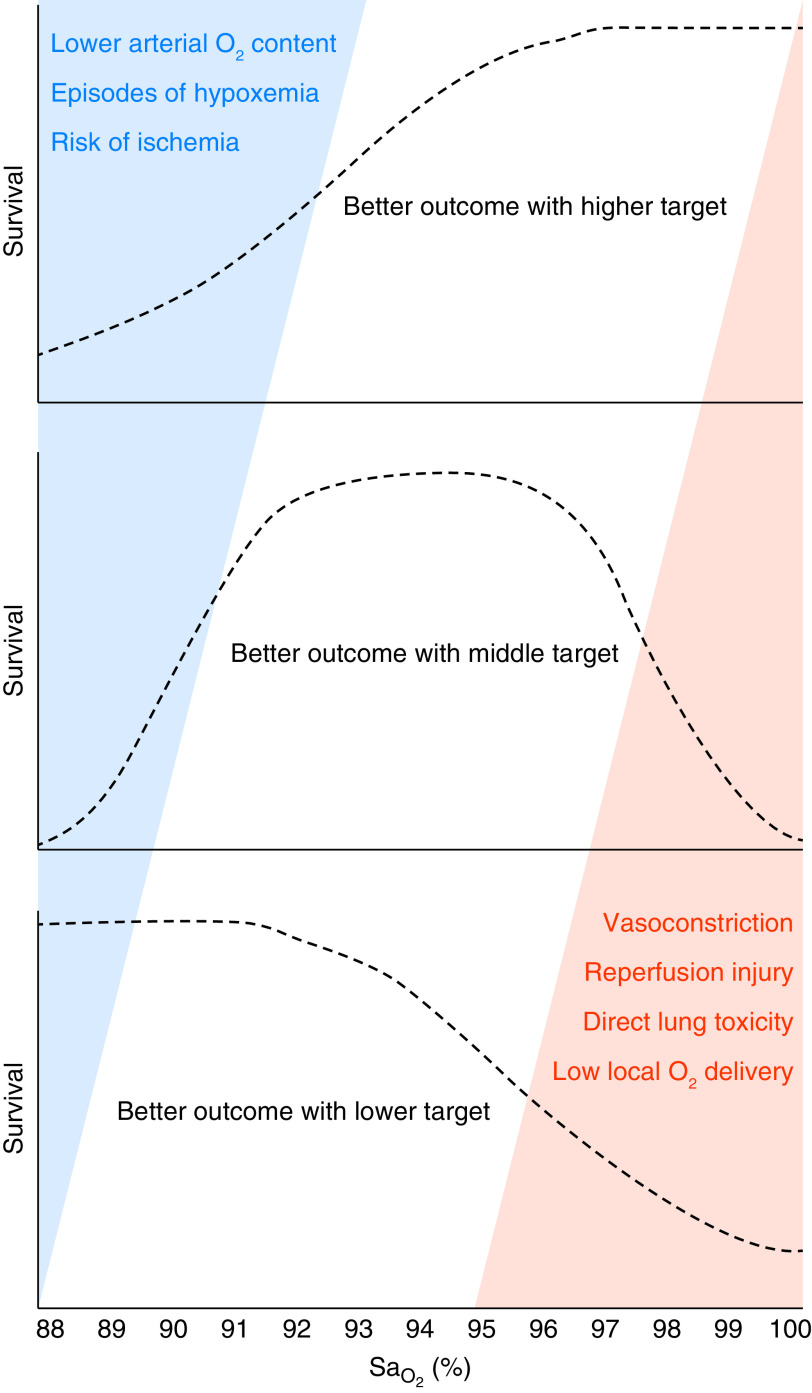
Damage from excessive oxygen can arise from both direct pulmonary toxicity from a high FiO2 and from systemic effects of high PaO2 in blood and tissues (5, 37). Each pathway has shared and distinct mechanisms and may pose risks to different patient populations. Direct pulmonary toxicity from a high FiO2 may occur from the elaboration of ROS. This can cause oxidative cellular damage and propagate a further inflammatory response (2). Systemic oxygen toxicity from tissue exposure to high PaO2 also involves ROS but can also lead to hyperoxemic vasoconstriction starting at PaO2 levels at or above 150 mm Hg (37). This vasoconstrictive effect differs across tissue beds and is prominent in brain, retinal, and cardiac tissue. Because of this, arterial oxygen content can increase while oxygen delivery decreases (37). These effects likely have differential impacts across populations of critically ill patients and need to be considered when developing oxygen-targeting schemes (Figure 1). For example, in a patient with severe underlying lung injury and impaired gas exchange, exposure to a high FiO2 could increase the risk of pulmonary toxicity without increasing the risk of systemic toxicity, given an inability to obtain high PaO2. On the other hand, patients without impaired gas exchange may have supraphysiologic PaO2 despite having an only modestly elevated FiO2. In such patients, particularly in those with cardiac or brain ischemia, there may be an increased risk of reperfusion injury or decreased oxygen delivery from vasoconstriction in vulnerable tissue beds.
Figure 1.
The risks of hypoxemia and hyperoxemia and the impact on higher versus lower arterial oxygenation targets for critically ill adults. The SaO2 values (x-axis) at which the potential detrimental effects of hypoxemia (blue triangle) or hyperoxemia (orange triangle) occur remain uncertain—and may differ for patients with different acute illnesses and comorbidities. If detrimental effects of hyperoxemia occur only at very high PaO2 values and detrimental effects from hypoxemia occur even with modestly low oxygen saturation as measured by pulse oximetry (SpO2)/PaO2 values, then using a higher SpO2 target might improve outcomes (dashed line in upper panel). Conversely, if even modestly supraphysiological PaO2 values incur the detrimental effects of hyperoxemia and only severely low SaO2/PaO2 values incur the detrimental effects of hypoxemia, then using a lower SpO2 target might improve outcomes (dashed line in lower panel). Physiologically, patients with impaired oxygen delivery (e.g., anemia) or increased oxygen consumption (e.g., sepsis) might be hypothesized to experience better outcomes with a higher SpO2 target (upper panel), whereas patients adapted to chronic hypoxemia (e.g., chronic obstructive pulmonary disease) or certain types of brain injury (e.g., after cardiac arrest) might be hypothesized to experience better outcomes with a lower SpO2 target (lower panel).
On the other hand, there also may be beneficial effects of hyperoxemia (38). Although dissolved oxygen contributes little to arterial oxygen content when the oxygen-carrying capacity is normal, even small increases in oxygen content may be helpful when there is an ongoing supply–demand mismatch. In some of these conditions, such as critical anemia or hemorrhagic shock, animal models have suggested that hyperoxemia may be beneficial (39). In addition, the surge in ROS that occurs with hyperoxemia has been proposed to be potentially beneficial for helping the immune system fight off infection (40, 41). These tradeoffs between the potential harms and benefits of hyperoxemia help to frame clinical studies of hyperoxia and hyperoxemia in critically ill patients.
Observational Studies in Critically Ill Adults
A number of observational studies have examined the associations between hyperoxemia and/or hyperoxia and clinical outcomes in populations of critically ill adults (42–55). Although there have been important studies involving high oxygen exposure in specific populations, such as in those with stroke, traumatic brain injury, perioperative settings, and trauma, we will focus on studies examining general populations of critically ill patients more commonly encountered in medical ICUs. Comparing these studies is complicated by varying definitions and durations of oxygen exposure (Table 1). However, a consistent finding is a U-shaped relationship between PaO2 and mortality in unadjusted analyses: that is, higher mortality in both hypoxemic and hyperoxemic patients (42, 43, 45, 47, 52, 53, 55). However, when the effect of exposure to the FiO2 is carefully accounted for, studies have yielded conflicting results regarding the high PaO2–mortality association (42, 43). Moreover, this biphasic relationship generally occurs in unadjusted analyses but is not always demonstrated in adjusted models, suggesting residual or unmeasured confounding and casting doubt on oxygen toxicity as a causal effect (43, 48, 51). In cohorts of patients who have suffered cardiac arrest, both retrospective (56, 57) and recent high-quality prospective observational data (58) showed increased mortality and worse neurologic outcomes in patients exposed to a PaO2 >300 mm Hg early in the course after cardiac arrest. Although there is inconsistency in these data, they raise concern for targeting supraphysiologic PaO2 levels and support a narrowing of oxygen targets to physiologic ranges.
Table 1.
Observational Studies of Oxygenation in Critically Ill Adult Patients
| Study | Study Population* (n) | Oxygen Exposure Metric | Association of Hyperoxia and Mortality in Adjusted Models? |
|---|---|---|---|
| de Jonge et al. (2008) (42) | Receiving IMV (36,307) | PaO2 and FiO2 in first 24 h† | Yes |
| Eastwood et al. (2012) (43) | Receiving IMV (152,680) | PaO2 in first 24 h† | No |
| Rachmale et al. (2012) (44) | Patients with ALI receiving IMV for ⩾48 h (210) | Excessive FiO2 (FiO2 >0.5 with SpO2 >92%) | No |
| Helmerhorst et al. (2017) (45) | Patients with ⩾1 ABG (14,441) | Various‡ (i.e., mean/median PaO2 from ICU stay) | Yes |
| Kraft et al. (2018) (48) | IMV for ⩾7 consecutive d (20,889) | Time-weighted PaO2 over 7 d | No |
| Aggarwal et al. (2018) (46) | ARDS Network trials (all received IMV) (2,994) | Excessive FiO2 (FiO2 >0.5 with PaO2 >80 mm Hg) | Yes |
| Ramanan and Fisher (2018) (47) | Receiving IMV (219,732) | PaO2 in first 24 h§; analysis stratified by Hb | No |
| Ruggiu et al. (2018) (49) | ICU patients (130) | Any PaO2 >100 mm Hg during ICU admission | Yes |
| Palmer et al. (2019) (50) | ICU stay >24 h (45,188) | Time-weighted AUC for PaO2 >100 mm Hg | Yes |
| Harvey et al. (2020) (54) | Receiving IMV with ⩾3 ABGs (7,452) | Time-weighted CaO2 over ICU admission | Yes |
| Madotto et al. (2020) (51) | ARDS within 2 d of ICU admission (2,005) | PaO2 >100 mm Hg on ICU Day 1 | No |
| Schjørring et al. (2020) (52) | Receiving IMV (4,998) | Time-weighted AUC PaO2 >13.7 kPa (103 mm Hg) | Yes |
| van den Boom et al. (2020) (53) | All ICU admissions (124,984/46,476)‖ | Median SpO2 | Yes |
| Zhou et al. (2020) (55) | IMV in first 24 h of ICU (25,669) | Percentage of time spent at SpO2 of 100% | Yes |
Definition of abbreviations: a–a = alveolar–arterial; ABG = arterial blood gas; ALI = acute lung injury; APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; AUC = area under the curve; CaO2 = arterial oxygen concentration; IMV = invasive mechanical ventilation; SpO2 = oxygen saturation as measured by pulse oximetry.
All study samples include ICU patients only.
The PaO2 value was taken from blood gas with worst PaO2/FiO2 ratio in Reference 42 or from highest a–a gradient in Reference 43.
The purpose of the study was to evaluate multiple metrics; the mean/median PaO2 across the ICU stay had the strongest association with mortality.
Taken from the PaO2 value associated with highest APACHE score.
Sample sizes from replicate analyses of two retrospective cohorts.
Several studies have examined the effects of “excessive oxygen exposure,” defined as the administration of an FiO2 greater than required to maintain normal arterial oxygenation (42, 45, 46). In a small, single-center study, excessive oxygen exposure was defined as an FiO2 >0.5 with an oxygen saturation as measured by pulse oximetry (SpO2) >92% (44). A greater duration of exposure in the first 48 hours of ICU admission was associated with subsequent worsening of arterial oxygenation and longer durations of ICU admission and MV (44). Using a similar approach, a post hoc analysis of ARDS Network trial participants in the first 5 days of ICU admission showed an association of increased mortality with increasing days of excessive oxygen delivery, defined as PaO2 >80 mm Hg at an FiO2 >0.5 (46). Although these studies suggest that cumulative measures of excessive oxygen exposure influence mortality, a similar analysis of the LUNG SAFE (Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure) observational study did not find an association between excessive oxygen exposure on ICU Day 1 and mortality in either adjusted models or a propensity score analysis (51).
Other investigators have used different methods to account for cumulative oxygen exposure. In a large observational study of critically ill patients, a PaO2 >100 mm Hg was considered a surrogate for excessive oxygen exposure (50). The “hyperoxemia dose” was modeled by using a time-weighted area under the curve measure of PaO2 >100 mm Hg, with higher values indicating a higher dose exposure. The occurrence of hyperoxemia examined over several different exposure windows was significantly associated with ICU mortality, but the hyperoxemia dose was not. To explain these results, it is possible that the occurrence of a high FiO2 or high PaO2 during an ICU admission is a marker of increased mortality risk but is not a causal factor. For example, a transfer of a patient to an invasive procedure may occur on a 100% FiO2. In addition, some clinicians may attempt to increase oxygen delivery in the sickest patients, and high PaO2 could hence potentially be a marker of illness severity. Finally, a higher than necessary FiO2 or high PaO2 levels might show a lack of attention to FiO2 titration by an ICU team and could indicate other differences in ICU care.
Some studies have suggested a dose–response relationship between cumulative measures of oxygen exposure and mortality (52, 53). In one study, a dose–response relationship between a PaO2 area under the curve measure and mortality was shown and persisted regardless of whether the FiO2 was ⩾0.4 or ⩽0.4 (52). If the results of this study are valid, reducing oxygen exposure even at a relatively lower FiO2 could be beneficial. Another study in ICU patients examined the association of various SpO2 ranges throughout an ICU stay and mortality (53). A median SpO2 of 96% was associated with the lowest ICU mortality. Patients with more than 80% of their ICU time with SpO2 levels of 94–98% had lower mortality than those with less than 40% of their time in this range. Furthermore, those with more than 80% versus less than 40% of time with an SpO2 greater than 98% had increased odds of mortality, suggesting a possible contribution from hyperoxia. Given concern for the time-dependency of oxygen exposure, the investigators conducted sensitivity analyses limited to the first 24, 48, and 72 hours of oxygen therapy and found similar results.
A concern that is raised when targeting lower SpO2 and PaO2 targets is the occurrence of detected or undetected episodes of hypoxia. Although some immediate effects of hypoxia may be clinically apparent at the bedside, subtle long-term deficits related to hypoxia exposure may not occur until later. In one of the first studies to describe long-term cognitive outcomes in ARDS survivors, the amount of time spent with hypoxia was significantly correlated with worse neurocognitive performance at 1 year (59). In another study examining cognitive outcomes, each 10–mm Hg decrease in PaO2 was associated with an ∼50% increased odds of cognitive impairment at 1 year (60). Whether worse long-term cognitive function is attributable to hypoxia itself or whether hypoxia is associated with other factors that impair cognition remains unclear. Ongoing randomized trials of oxygen targets are collecting outcome data on long-term cognitive function to inform this issue.
Randomized Trials of Arterial Oxygen Targets for ICU Patients
Recent randomized controlled trials (RCTs) have begun to provide important data to inform our understanding of arterial oxygenation targets in general populations of critically ill patients (Table 2) (61–65). In the single-center OX-ICU (Oxygen–ICU trial), patients anticipated to need at least 72 hours of ICU care were randomized to conservative versus usual-care oxygenation targets. This population included, but was not exclusive to, patients receiving invasive MV (291/434 [67%]) (62). In the conservative group, PaO2 and SpO2 targets were 70–100 mm Hg and 94–98%. In the usual-care group, PaO2 could range up to 150 mm Hg, and the SpO2 goal was 97–100%. Patients with acute exacerbations of chronic obstructive pulmonary disease and moderate-to-severe ARDS were excluded. The conservative and usual-care groups had median PaO2 values of 87 mm Hg and 102 mm Hg and mean FiO2 values of 0.36 and 0.39, respectively. In the conservative oxygen arm, there was an 8.6% absolute risk reduction in ICU mortality (95% confidence interval [CI], 1.7–15.0%). This trial was stopped early for slow recruitment.
Table 2.
Randomized Trials of Conservative vs. Liberal Oxygen Targets in Critically Ill Patients
| Study | Population | Sample Size | Target (Conservative vs. Liberal) | Primary Outcome | Results (Conservative vs. Liberal) |
|---|---|---|---|---|---|
| Panwar et al. (2016) (61) | Adult ICU patients requiring IMV | 103 | SpO2 of 88–92% vs. ⩾96% | Mean AUC for SpO2, SaO2, PaO2, and FiO2 | Feasibility study; good separation in study groups; no adverse safety signals |
| Girardis et al. (2016) (62): OX-ICU | Adult ICU admission for >72 h anticipated (IMV and no IMV) | 434 | PaO2 of 70–100 mm Hg (SpO2 of 94– 98%) vs. PaO2 of up to 150 mm Hg and SpO2 of 97–100% | ICU mortality | Decreased mortality; ARR, 8.6% (95% CI, 1.7–15.0%) |
| Mackle et al. (2020) (64): ICU-ROX | Adult ICU patients on IMV | 1,000 | SpO2 >90% with alarm set at 97%, “usual-care group”; no upper-limit alarm (FiO2 of <0.3 discouraged) | VFD | No differences in VFD or 90- or 180-d mortality |
| Barrot et al. (2020) (63): LOCO2 | Adult ARDS patients on IMV | 205 | PaO2 of 55–70 mm Hg/SpO2 of 88–92% vs. PaO2 of 90–105 mm Hg/SpO2 of >96% for first 7 d of MV | 28-d mortality | No difference in 28-d mortality; higher 90-d mortality (absolute risk increase of 7.8% [95% CI, 0.7–27.2%]). Trial stopped early for five events of mesenteric ischemia in conservative arm |
| Schjørring et al. (2021) (65): HOT-ICU | Adults with acute hypoxemic respiratory failure (FiO2 ⩾0.5 on IMV or O2 ⩾10 L/min in open system) | 2,928 | PaO2 of 60 mm Hg vs. PaO2 of 90 mm Hg | 90-d mortality | No difference in 90-d mortality or other secondary outcomes; no difference in serious adverse events |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; ARR = absolute risk reduction; AUC = area under the curve; CI = confidence interval; HOT-ICU = Handling Oxygen Targets in the ICU; ICU-ROX = ICU Randomized Trial Comparing Two Approaches to Oxygen Therapy; IMV = invasive MV; LOCO2 = Liberal Oxygenation versus Conservative Oxygenation in ARDS; MV = mechanical ventilation; OX-ICU = Oxygen–ICU; RCT = randomized controlled trial; SpO2 = oxygen saturation as measured by pulse oximetry; TBI = traumatic brain injury; VFD = ventilator-free days.
Note this table does not include RCTs of patients with cardiac arrest, TBI, or stroke.
In the ICU-ROX (ICU Randomized Trial Comparing Two Approaches to Oxygen Therapy) trial, 1,000 invasively mechanically ventilated patients were randomized to conservative versus usual-care oxygenation strategies titrated to SpO2 (64). In the conservative oxygen study group, the SpO2 target range was 91–96%, and FiO2 was adjusted to the lowest level that achieved this range (including a FiO2 of 0.21). In the usual-care arm, an SpO2 ⩾91% was also targeted, but there was no SpO2 upper limit, and in contrast to the conservative group, lowering the FiO2 to <0.3 was discouraged. The conservative oxygenation group spent significantly more time with an FiO2 of 0.21 (29 h vs. 1 h) and less time with a SpO2 >97% (27 h vs. 49 h) without increased time with hypoxia (SpO2 < 88%). There were no differences in the primary outcome of ventilator-free days or secondary outcomes of 90- or 180-day mortality. In a subgroup analysis, patients with hypoxemic ischemic encephalopathy treated in the conservative oxygen arm had more ventilator-free days and decreased mortality compared with the usual-care arm. No differences in cognitive function at 180 days were detected, but a significantly higher proportion of patients in the usual-care oxygen group reported severe problems with mobility and personal care. This hypothesis-generating secondary outcome suggests that exposure to higher oxygen targets and presumably hyperoxia and/or hyperoxemia could influence long-term functional outcomes and warrant further study. Importantly, there were no concerning safety issues in the conservative oxygen group, suggesting that a strategy that weans oxygen to obtain an SpO2 at or below a maximum of 96% is safe.
In the LOCO2 (Liberal Oxygenation vs. Conservative Oxygenation in ARDS) trial, patients with ARDS were randomized to a conservative arterial oxygenation target (PaO2 of 55–70 mm Hg [SpO2 of 88–92%]) or a liberal target (PaO2 of 90–105 mm Hg [SpO2 ⩾ 96%]) (63). In the conservative group (n = 99), time-adjusted differences in the FiO2 (−0.15), PaO2 (−28.1 mm Hg), and SaO2 (−3.8%) were all significantly lower than those of the liberal oxygenation group (n = 102). This trial was stopped early after enrollment of 205 patients because 5 patients in the conservative oxygen arm developed mesenteric ischemia. In addition, 90-day mortality, a secondary outcome, was higher in the conservative oxygenation group (44.4% vs. 30.4%; risk difference of 14.0%; 95% CI, 0.7–27.2%). As with the other trials discussed above, there was no masking of participants or clinicians to the intervention. As such, there is concern that the detection of mesenteric ischemia in the lower oxygenation target group could be biased by the lack of masking, and this is a major limitation of this trial. Early termination of this trial yielded an imprecise 90-day mortality risk difference effect estimate and casts doubt on this being a valid effect.
The recent HOT-ICU (Handling Oxygen Targets in the ICU) trial is the largest RCT of oxygen targets in critically ill patients to date (65). In this trial, 2,928 patients with hypoxemic respiratory failure receiving an FiO2 of ⩾0.5 via invasive MV or receiving ⩾10 L/min oxygen in an open system were randomized to PaO2 targets of 60 mm Hg in the conservative arm versus 90 mm Hg in the liberal arm. Median values of daily mean PaO2 were 70.8 mm Hg versus 93.3 mm Hg at median FiO2 levels of 0.43 and 0.56 in the conservative and liberal arms, respectively. At 90 days, 42.9% versus 42.4% of participants in the conservative versus liberal arms had met the primary outcome of 90-day mortality (risk ratio, 1.02; 95% CI, 0.94–1.11). There were no differences in secondary outcomes or serious adverse events, including conditions related to ischemia (myocardial infarction [MI], ischemic stroke, or intestinal ischemia.) Follow-up of long-term cognitive and physical function is ongoing.
The results of HOT-ICU and ICU-ROX both suggest that the choice of oxygen targets within narrow physiologic ranges (e.g., PaO2 ⩽ 100) does not significantly affect mortality in critically ill patients with respiratory failure. In contrast to the findings of the LOCO2 trial, these trials support the relative safety of conservative oxygen targets. Although the conservative arm of LOCO2 had a slightly lower PaO2 lower bound than HOT-ICU, both studies achieved similar PaO2 levels in the conservative group. Thus, it is unlikely that differences in the studied oxygen targets drove the difference in findings. The patient populations for each trial differed. Although all patients in the LOCO2 trial had ARDS, only 12.8% patients in HOT-ICU had ARDS, and in ICU-ROX, only 65% of patients met PaO2/FiO2 criteria for ARDS. HOT-ICU included patients with chronic obstructive pulmonary disease (19.3%) and cardiac arrest (11.5%), and ICU-ROX included many patients with acute brain disease (40%)—all groups for whom the potential benefit of conservative oxygen targets is believed to be greater. Lastly, both HOT-ICU and ICU-ROX may have had lower proportions of patients with sepsis than LOCO2. Hyperoxemia has been proposed to be potentially beneficial in sepsis, in part because of systemic vasoconstrictive effects, although a trial of 100%-FiO2 delivery to septic patients suggested harm from this strategy (66). In addition, a post hoc analysis of ICU-ROX participants with sepsis did not demonstrate statistically significant differences in 90-day mortality (67). Finally, with the small number of adverse events in LOCO2 and the early cessation of trial enrollment, the observed between-group differences may have occurred by chance.
None of these trials explicitly tested targets more extreme than a PaO2 of ⩾150 mm Hg, and the largest of the trials did not include a PaO2 target above a physiologic range (PaO2 > 100 mm Hg). However, the small, single-center OX-ICU trial suggested that a strategy that allowed the PaO2 to range up to 150 mm Hg was inferior to PaO2 targets of 70–100 mm Hg. Randomized trials in other populations have tested oxygen supplementation strategies that have achieve supranormal targets. The HYPERS2S (Hyperoxia and Hypertonic Saline in Patients with Septic Shock) trial compared administering 100% oxygen for 24 hours with targeting normoxia (SpO2 of 88–95%) in patients with sepsis (66). Patients exposed to 100% oxygen (independent of oxygen needs) had numerically higher mortality and increased rates of ICU-acquired weakness and atelectasis compared with the normoxia group. In a post hoc analysis of this trial, hyperoxemia was only associated with harm in the subgroup of patients with persistently elevated lactate and hypotension (meeting Sepsis 3.0 criteria) (68). This suggests that harm may be limited to those with impaired oxygen use or delivery in the setting of septic shock. However, the post hoc nature of the analysis limits causal conclusions. Supraphysiologic oxygen delivery has also been studied in RCTs of normoxemic patients with acute ST-elevation MI, in which 6–8 L/min oxygen versus room air led to larger infarct size (69). In larger RCTs of patients with suspected acute MI, no differences in short- or long-term mortality were shown (70, 71). In a surgical population, the PROXI (Perioperative Oxygen Fraction) trial tested an 80% FiO2 versus a 30% FiO2 given for 2 hours to patients immediately postsurgery with a goal of decreasing the rates of wound infection. There was no improvement in the rates of wound infection, 30-day mortality was numerically higher in the high FiO2 group (72), and 1-year mortality was significantly higher in the group that received a high FiO2 (73). These randomized trials provide no support for supplementing oxygen to target supraphysiologic PaO2 levels. In addition, in those with cardiac arrest, meta-analyses provide evidence for increased mortality with this strategy (74). Although there is a small, single-center RCT and some observational studies in the traumatic brain injury literature that suggest improved functional outcomes and/or improved mortality with exposure to supraphysiologic PaO2 levels (75, 76), these findings are not consistent and are limited by methodological concerns.
Trials in critically ill adult patients also do not support or directly test a strategy of permissive hypoxemia. However, the results of randomized trials in neonates can help inform our understanding of this strategy. BOOST II (Benefits of Oxygen Saturation Targeting) and SUPPORT (Surfactant Positive Airway Pressure and Pulse Oximetry Trial) were designed to test whether or not a lower SpO2 goal (85–89% or 85–91%, respectively, vs. 91–95%) decreased the risk of retinopathy in neonatal ICU patients (77, 78). Although rates of retinopathy were indeed decreased, mortality was higher in the lower-oxygen arms. Despite being exposed to hypoxic conditions throughout gestation and being born with higher concentrations of fetal Hb with higher oxygen affinity, neonates remain vulnerable to these levels of hypoxemia. Although the implications of these trials for critically ill adults are uncertain, there is no evidence to support the use of permissive hypoxemia in adult patients.
Meta-analyses of Randomized Trials
Meta-analyses examining evidence for optimal oxygenation targets contain studies with more heterogenous populations, including patients with sepsis, stroke, cardiac arrest, or MI and patients undergoing emergency surgery. The IOTA (Improving Oxygen Therapy in Acute Illness) meta-analysis suggested that, across the included trials, conservative oxygenation targets were beneficial and decreased mortality in comparison with liberal targets (79). Furthermore, metaregression demonstrated a dose-dependent increase in mortality with a rising SpO2. This study spurred the inclusion of an upper SpO2 limit in a subsequent guideline on use of supplemental oxygen in critically ill patients (80). A more recent meta-analysis that included the ICU-ROX and LOCO2 trials showed no evidence of mortality difference or adverse effects with conservative versus liberal oxygenation strategies. However, effect sizes of a <15% relative decrease in mortality, which could still be clinically meaningful, were not ruled out (81).
Research Priorities
Several ongoing RCTs of oxygenation targets in critically ill adults may provide further guidance for oxygen dosing (NCT03537937: PILOT [Pragmatic Investigation of Optimal Oxygen Targets Trial]; NCT03287466: TOXYC [Targeted Oxygen Therapy in Critical Illness]; and ACTRN12620000391976: MEGA-ROX [The Mega Randomised Registry Trial Comparing Conservative vs. Liberal Oxygenation Targets] [82]). MEGA-ROX is a multinational trial targeting recruitment of 40,000 patients. If completed, it will be the largest trial of oxygenation targets to date and is powered to detect a 1.5% absolute difference in mortality (82). However, even if the ongoing trials establish optimal ranges of SpO2 for large groups of critically ill patients, important unanswered questions will remain. For example, regardless of the “average treatment effect” of oxygen targets across a population of critically ill adults, some patients may experience better outcomes with a lower SpO2 target (e.g., a young person with a normal Hb concentration admitted for hypoxemic ischemic encephalopathy after cardiac arrest), and some patients may experience better outcomes with a higher SpO2 target (e.g., an older person with anemia, coronary artery disease, and sepsis-induced ARDS). MEGA-ROX will have statistical power to evaluate several subgroups of interest (hypoxemic ischemic encephalopathy and sepsis, for example), although it is likely that targeted trials in other populations will be needed. For example, if a lower SpO2 range is validated as superior by MEGA-ROX, further analysis of this study and other targeted trials may need to be completed in populations such as those with trauma or critical anemia in which a lower oxygen-carrying capacity may favor higher PaO2 targets. Data from these studies could help to derive and validate estimates of the optimal SpO2 target for individual patients (“individual treatment effects”) and ultimately guide clinicians toward a personalized approach to oxygen therapy in the ICU.
In addition, the effect of oxygen targets on long-term patient-important outcomes such as cognitive and physical function should be evaluated carefully. As previously mentioned, the HOT-ICU trial has ongoing follow-up to examine physical and cognitive functioning. Given no differences in short-term outcomes in the two largest trials (HOT-ICU and ICU-ROX), a trial designed and powered specifically to assess long-term outcomes would be warranted at this time. Lastly, although trials have shown that a separation in oxygenation between groups can be maintained with different targets, arterial oxygenation frequently exceeds stated goals. This probably results from healthcare workers’ tendency to react quickly when escalating therapy (FiO2) but to react slowly when deescalating therapy (42, 43, 50). The development of safe, closed-loop systems of adjusting the delivery of supplemental oxygen may help ameliorate this problem (83), particularly once safe and optimal oxygenation targets are better defined.
Conclusions
Despite the knowledge that administering oxygen to acutely ill patients may confer both benefits and harms, we are just beginning to understand the nuances of oxygen therapy in critical illness. Although observational and trial data are inconsistent, there is accumulating evidence that targeting supranormal PaO2 levels can lead to harm. In contrast, targeting oxygen therapy to maximum PaO2 and SpO2 targets within a physiologic range appears to be safe. As one of the most widespread interventions in medicine worldwide, further optimizing oxygen therapy could have large global effects on important patient outcomes. We look forward to results of ongoing RCTs to help further guide our understanding of how to best use oxygen therapy to improve outcomes for critically ill patients.
Acknowledgments
Acknowledgment
The authors thank Wesley H. Self of Vanderbilt University Medical Center for his intellectual contributions to framing the potential risks and benefits of oxygen therapy during acute illness and for helping to develop the figure.
Footnotes
Supported by NIH grant T32HL007534.
Author Contributions: C.H.H., M.W.S., and R.G.B. each contributed to the literature review and writing of this manuscript.
CME will be available for this article at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202102-0417CI on June 4, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. West JB. Joseph Priestley, oxygen, and the enlightenment. Am J Physiol Lung Cell Mol Physiol. 2014;306:L111–L119. doi: 10.1152/ajplung.00310.2013. [DOI] [PubMed] [Google Scholar]
- 2. Rogers LK, Cismowski MJ. Oxidative stress in the lung: the essential paradox. Curr Opin Toxicol. 2018;7:37–43. doi: 10.1016/j.cotox.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warren CPW. The introduction of oxygen for pneumonia as seen through the writings of two McGill University professors, William Osler and Jonathan Meakins. Can Respir J. 2005;12:81–85. doi: 10.1155/2005/146951. [DOI] [PubMed] [Google Scholar]
- 4. Clark JM, Lambertsen CJ. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971;23:37–133. [PubMed] [Google Scholar]
- 5. Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58:123–141. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frank L, Bucher JR, Roberts RJ. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol. 1978;45:699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- 7. Friedrich M, Grayzelw DM. High resistance of rhesus monkeys to 90 plus per gent oxygen. Proc Soc Exp Biol Med. 1944;56:204–205. [Google Scholar]
- 8. Robinson FR, Casey HW, Weibel ER. Animal model: oxygen toxicity in nonhuman primates. Am J Pathol. 1974;76:175–178. [PMC free article] [PubMed] [Google Scholar]
- 9. de los Santos R, Seidenfeld JJ, Anzueto A, Collins JF, Coalson JJ, Johanson WG, Jr, et al. One hundred percent oxygen lung injury in adult baboons. Am Rev Respir Dis. 1987;136:657–661. doi: 10.1164/ajrccm/136.3.657. [DOI] [PubMed] [Google Scholar]
- 10. Fessel JP, Flynn CR, Robinson LJ, Penner NL, Gladson S, Kang CJ, et al. Hyperoxia synergizes with mutant bone morphogenic protein receptor 2 to cause metabolic stress, oxidant injury, and pulmonary hypertension. Am J Respir Cell Mol Biol. 2013;49:778–787. doi: 10.1165/rcmb.2012-0463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knight PR, Kurek C, Davidson BA, Nader ND, Patel A, Sokolowski J, et al. Acid aspiration increases sensitivity to increased ambient oxygen concentrations. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1240–L1247. doi: 10.1152/ajplung.2000.278.6.L1240. [DOI] [PubMed] [Google Scholar]
- 12. Comroe JH, Dripps RD, Dumke PR, Deming M. Oxygen toxicity: the effect of inhalation of high concentrations of oxygen for twenty-four hours on normal men at sea level and at a simulated altitude of 18,000 feet. J Am Med Assoc. 1945;128:710–717. [Google Scholar]
- 13. Caldwell PR, Lee WL, Jr, Schildkraut HS, Archibald ER. Changes in lung volume, diffusing capacity, and blood gases in men breathing oxygen. J Appl Physiol. 1966;21:1477–1483. doi: 10.1152/jappl.1966.21.5.1477. [DOI] [PubMed] [Google Scholar]
- 14. Burger EJ, Jr, Mead J. Static properties of lungs after oxygen exposure. J Appl Physiol. 1969;27:191–197. doi: 10.1152/jappl.1969.27.2.191. [DOI] [PubMed] [Google Scholar]
- 15. Davis WB, Rennard SI, Bitterman PB, Crystal RG. Pulmonary oxygen toxicity: early reversible changes in human alveolar structures induced by hyperoxia. N Engl J Med. 1983;309:878–883. doi: 10.1056/NEJM198310133091502. [DOI] [PubMed] [Google Scholar]
- 16. Montgomery AB, Luce JM, Murray JF. Retrosternal pain is an early indicator of oxygen toxicity. Am Rev Respir Dis. 1989;139:1548–1550. doi: 10.1164/ajrccm/139.6.1548. [DOI] [PubMed] [Google Scholar]
- 17. Suter PM, Fairley HB, Schlobohm RM. Shunt, lung volume and perfusion during short periods of ventilation with oxygen. Anesthesiology. 1975;43:617–627. doi: 10.1097/00000542-197512000-00003. [DOI] [PubMed] [Google Scholar]
- 18. Santos C, Ferrer M, Roca J, Torres A, Hernández C, Rodriguez-Roisin R. Pulmonary gas exchange response to oxygen breathing in acute lung injury. Am J Respir Crit Care Med. 2000;161:26–31. doi: 10.1164/ajrccm.161.1.9902084. [DOI] [PubMed] [Google Scholar]
- 19. Aboab J, Jonson B, Kouatchet A, Taille S, Niklason L, Brochard L. Effect of inspired oxygen fraction on alveolar derecruitment in acute respiratory distress syndrome. Intensive Care Med. 2006;32:1979–1986. doi: 10.1007/s00134-006-0382-4. [DOI] [PubMed] [Google Scholar]
- 20. Sackner MA, Hirsch JA, Epstein S, Rywlin AM. Effect of oxygen in graded concentrations upon tracheal mucous velocity: a study in anesthetized dogs. Chest. 1976;69:164–167. doi: 10.1378/chest.69.2.164. [DOI] [PubMed] [Google Scholar]
- 21. Sackner MA, Landa J, Hirsch J, Zapata A. Pulmonary effects of oxygen breathing: a 6-hour study in normal men. Ann Intern Med. 1975;82:40–43. doi: 10.7326/0003-4819-82-1-40. [DOI] [PubMed] [Google Scholar]
- 22. Griffith DE, Holden WE, Morris JF, Min LK, Krishnamurthy GT. Effects of common therapeutic concentrations of oxygen on lung clearance of 99mTc DTPA and bronchoalveolar lavage albumin concentration. Am Rev Respir Dis. 1986;134:233–237. doi: 10.1164/arrd.1986.134.2.233. [DOI] [PubMed] [Google Scholar]
- 23. Nash G, Blennerhassett JB, Pontoppidan H. Pulmonary lesions associated with oxygen therapy and artificial ventilation. N Engl J Med. 1967;276:368–374. doi: 10.1056/NEJM196702162760702. [DOI] [PubMed] [Google Scholar]
- 24. Barber RE, Hamilton WK. Oxygen toxicity in man: a prospective study in patients with irreversible brain damage. N Engl J Med. 1970;283:1478–1484. doi: 10.1056/NEJM197012312832702. [DOI] [PubMed] [Google Scholar]
- 25. Kapanci Y, Tosco R, Eggermann J, Gould VE. Oxygen pneumonitis in man: light- and electron-microscopic morphometric studies. Chest. 1972;62:162–169. doi: 10.1378/chest.62.2.162. [DOI] [PubMed] [Google Scholar]
- 26. Sevitt S. Diffuse and focal oxygen pneumonitis: a preliminary report on the threshold of pulmonary oxygen toxicity in man. J Clin Pathol. 1974;27:21–30. doi: 10.1136/jcp.27.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage: the role of oxygen, shock, and related factors. A review. Am J Pathol. 1976;85:209–228. [PMC free article] [PubMed] [Google Scholar]
- 28. Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aggarwal NR, D’Alessio FR, Tsushima K, Files DC, Damarla M, Sidhaye VK, et al. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L371–L381. doi: 10.1152/ajplung.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Smith G, Winter PM, Wheelis RF. Increased normobaric oxygen tolerance of rabbits following oleic acid-induced lung damage. J Appl Physiol. 1973;35:395–400. doi: 10.1152/jappl.1973.35.3.395. [DOI] [PubMed] [Google Scholar]
- 31. Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980;122:123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- 32. Crapo JD, Sjostrom K, Drew RT. Tolerance and cross-tolerance using NO2 and O2: I. toxicology and biochemistry. J Appl Physiol. 1978;44:364–369. doi: 10.1152/jappl.1978.44.3.364. [DOI] [PubMed] [Google Scholar]
- 33. Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. 2004;32:2496–2501. doi: 10.1097/01.ccm.0000148231.04642.8d. [DOI] [PubMed] [Google Scholar]
- 34. Li L-F, Liao S-K, Ko YS, Lee CH, Quinn DA. Hyperoxia increases ventilator-induced lung injury via mitogen-activated protein kinases: a prospective, controlled animal experiment. Crit Care. 2007;11:R25. doi: 10.1186/cc5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bailey TC, Martin EL, Zhao L, Veldhuizen RAW. High oxygen concentrations predispose mouse lungs to the deleterious effects of high stretch ventilation. J Appl Physiol (1985) 2003;94:975–982. doi: 10.1152/japplphysiol.00619.2002. [DOI] [PubMed] [Google Scholar]
- 36. Ashley SL, Sjoding MW, Popova AP, Cui TX, Hoostal MJ, Schmidt TM, et al. Lung and gut microbiota are altered by hyperoxia and contribute to oxygen-induced lung injury in mice. Sci Transl Med. 2020;12:eaau9959. doi: 10.1126/scitranslmed.aau9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brugniaux JV, Coombs GB, Barak OF, Dujic Z, Sekhon MS, Ainslie PN. Highs and lows of hyperoxia: physiological, performance, and clinical aspects. Am J Physiol Regul Integr Comp Physiol. 2018;315:R1–R27. doi: 10.1152/ajpregu.00165.2017. [DOI] [PubMed] [Google Scholar]
- 38. Calzia E, Asfar P, Hauser B, Matejovic M, Ballestra C, Radermacher P, et al. Hyperoxia may be beneficial. Crit Care Med. 2010;38:S559–S568. doi: 10.1097/CCM.0b013e3181f1fe70. [DOI] [PubMed] [Google Scholar]
- 39. Knöller E, Stenzel T, Broeskamp F, Hornung R, Scheuerle A, McCook O, et al. Effects of hyperoxia and mild therapeutic hypothermia during resuscitation from porcine hemorrhagic shock. Crit Care Med. 2016;44:e264–e277. doi: 10.1097/CCM.0000000000001412. [DOI] [PubMed] [Google Scholar]
- 40. Knighton DR, Halliday B, Hunt TK. Oxygen as an antibiotic: the effect of inspired oxygen on infection. Arch Surg. 1984;119:199–204. doi: 10.1001/archsurg.1984.01390140057010. [DOI] [PubMed] [Google Scholar]
- 41. Kotani N, Hashimoto H, Sessler DI, Muraoka M, Hashiba E, Kubota T, et al. Supplemental intraoperative oxygen augments antimicrobial and proinflammatory responses of alveolar macrophages. Anesthesiology. 2000;93:15–25. doi: 10.1097/00000542-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 42. de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12:R156. doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eastwood G, Bellomo R, Bailey M, Taori G, Pilcher D, Young P, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. 2012;38:91–98. doi: 10.1007/s00134-011-2419-6. [DOI] [PubMed] [Google Scholar]
- 44. Rachmale S, Li G, Wilson G, Malinchoc M, Gajic O. Practice of excessive FiO2 and effect on pulmonary outcomes in mechanically ventilated patients with acute lung injury. Respir Care. 2012;57:1887–1893. doi: 10.4187/respcare.01696. [DOI] [PubMed] [Google Scholar]
- 45. Helmerhorst HJF, Arts DL, Schultz MJ, van der Voort PHJ, Abu-Hanna A, de Jonge E, et al. Metrics of arterial hyperoxia and associated outcomes in critical care. Crit Care Med. 2017;45:187–195. doi: 10.1097/CCM.0000000000002084. [DOI] [PubMed] [Google Scholar]
- 46. Aggarwal NR, Brower RG, Hager DN, Thompson BT, Netzer G, Shanholtz C, et al. National Institutes of Health Acute Respiratory Distress Syndrome Network Investigators. Oxygen exposure resulting in arterial oxygen tensions above the protocol goal was associated with worse clinical outcomes in acute respiratory distress syndrome. Crit Care Med. 2018;46:517–524. doi: 10.1097/CCM.0000000000002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ramanan M, Fisher N. The Association between arterial oxygen tension, hemoglobin concentration, and mortality in mechanically ventilated critically ill patients. Indian J Crit Care Med. 2018;22:477–484. doi: 10.4103/ijccm.IJCCM_66_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kraft F, Andel H, Gamper J, Markstaller K, Ullrich R, Klein KU. Incidence of hyperoxia and related in-hospital mortality in critically ill patients: a retrospective data analysis. Acta Anaesthesiol Scand. 2018;62:347–356. doi: 10.1111/aas.13047. [DOI] [PubMed] [Google Scholar]
- 49. Ruggiu M, Aissaoui N, Nael J, Haw-Berlemont C, Herrmann B, Augy J-L, et al. Hyperoxia effects on intensive care unit mortality: a retrospective pragmatic cohort study. Crit Care. 2018;22:218. doi: 10.1186/s13054-018-2142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Palmer E, Post B, Klapaukh R, Marra G, MacCallum NS, Brealey D, et al. The association between supraphysiologic arterial oxygen levels and mortality in critically ill patients: a multicenter observational cohort study. Am J Respir Crit Care Med. 2019;200:1373–1380. doi: 10.1164/rccm.201904-0849OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Madotto F, Rezoagli E, Pham T, Schmidt M, McNicholas B, Protti A, et al. LUNG SAFE Investigators; ESICM Trials Group. Hyperoxemia and excess oxygen use in early acute respiratory distress syndrome: insights from the LUNG SAFE study. Crit Care. 2020;24:125. doi: 10.1186/s13054-020-2826-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schjørring OL, Jensen AKG, Nielsen CG, Ciubotariu A, Perner A, Wetterslev J, et al. Arterial oxygen tensions in mechanically ventilated ICU patients and mortality: a retrospective, multicentre, observational cohort study. Br J Anaesth. 2020;124:420–429. doi: 10.1016/j.bja.2019.12.039. [DOI] [PubMed] [Google Scholar]
- 53. van den Boom W, Hoy M, Sankaran J, Liu M, Chahed H, Feng M, et al. The search for optimal oxygen saturation targets in critically ill patients: observational data from large ICU databases. Chest. 2020;157:566–573. doi: 10.1016/j.chest.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 54. Harvey JP, Jayawardena DG, Ramanan M. Oxygen exposure as quantified by time-weighted area under curve for arterial oxygen content is associated with mortality in mechanically ventilated critically ill patients. Res Opin Anesth Intensive Care. 2020;7:197–204. [Google Scholar]
- 55. Zhou D, Li Z, Shi G, Zhou J. Time spent in oxygen saturation 95-99% is associated with reduced mortality in critically ill patients with mechanical ventilation. Crit Care. 2020;24:414. doi: 10.1186/s13054-020-03126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Emergency Medicine Shock Research Network (EMShockNet) Investigators. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 57. Helmerhorst HJF, Roos-Blom M-J, van Westerloo DJ, Abu-Hanna A, de Keizer NF, de Jonge E. Associations of arterial carbon dioxide and arterial oxygen concentrations with hospital mortality after resuscitation from cardiac arrest. Crit Care. 2015;19:348. doi: 10.1186/s13054-015-1067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Roberts BW, Kilgannon JH, Hunter BR, Puskarich MA, Pierce L, Donnino M, et al. Association between early hyperoxia exposure after resuscitation from cardiac arrest and neurological disability: prospective multicenter protocol-directed cohort study. Circulation. 2018;137:2114–2124. doi: 10.1161/CIRCULATIONAHA.117.032054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-LOHR V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 60. Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185:1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Panwar R, Hardie M, Bellomo R, Barrot L, Eastwood GM, Young PJ, et al. CLOSE Study Investigators; ANZICS Clinical Trials Group. Conservative versus liberal oxygenation targets for mechanically ventilated patients: a pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016;193:43–51. doi: 10.1164/rccm.201505-1019OC. [DOI] [PubMed] [Google Scholar]
- 62. Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the Oxygen-ICU randomized clinical trial. JAMA. 2016;316:1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 63. Barrot L, Asfar P, Mauny F, Winiszewski H, Montini F, Badie J, et al. LOCO2 Investigators; REVA Research Network. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Engl J Med. 2020;382:999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 64. Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, Eastwood G, et al. ICU-ROX Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group. Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2020;382:989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 65. Schjørring OL, Klitgaard TL, Perner A, Wetterslev J, Lange T, Siegemund M, et al. Lower or higher oxygenation targets for acute hypoxemic respiratory failure. N Engl J Med. 2021;384:1301–1311. doi: 10.1056/NEJMoa2032510. [DOI] [PubMed] [Google Scholar]
- 66. Asfar P, Schortgen F, Boisramé-Helms J, Charpentier J, Guérot E, Megarbane B, et al. HYPERS2S Investigators; REVA Research Network. Hyperoxia and Hypertonic Saline in Patients with Septic Shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017;5:180–190. doi: 10.1016/S2213-2600(17)30046-2. [DOI] [PubMed] [Google Scholar]
- 67. Young P, Mackle D, Bellomo R, Bailey M, Beasley R, Deane A, et al. ICU-ROX Investigators; Australian New Zealand Intensive Care Society Clinical Trials Group. Conservative oxygen therapy for mechanically ventilated adults with sepsis: a post hoc analysis of data from the intensive care unit randomized trial comparing two approaches to oxygen therapy (ICU-ROX) Intensive Care Med. 2020;46:17–26. doi: 10.1007/s00134-019-05857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Demiselle J, Wepler M, Hartmann C, Radermacher P, Schortgen F, Meziani F, et al. HYPER2S investigators. Hyperoxia toxicity in septic shock patients according to the Sepsis-3 criteria: a post hoc analysis of the HYPER2S trial. Ann Intensive Care. 2018;8:90. doi: 10.1186/s13613-018-0435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stub D, Smith K, Bernard S, Nehme Z, Stephenson M, Bray JE, et al. AVOID Investigators. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131:2143–2150. doi: 10.1161/CIRCULATIONAHA.114.014494. [DOI] [PubMed] [Google Scholar]
- 70. Hofmann R, James SK, Jernberg T, Lindahl B, Erlinge D, Witt N, et al. DETO2X–SWEDEHEART Investigators. Oxygen therapy in suspected acute myocardial infarction. N Engl J Med. 2017;377:1240–1249. doi: 10.1056/NEJMoa1706222. [DOI] [PubMed] [Google Scholar]
- 71. Stewart RAH, Jones P, Dicker B, Jiang Y, Smith T, Swain A, et al. High flow oxygen and risk of mortality in patients with a suspected acute coronary syndrome: pragmatic, cluster randomised, crossover trial. BMJ. 2021;372:n355. doi: 10.1136/bmj.n355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Meyhoff CS, Wetterslev J, Jorgensen LN, Henneberg SW, Høgdall C, Lundvall L, et al. PROXI Trial Group. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543–1550. doi: 10.1001/jama.2009.1452. [DOI] [PubMed] [Google Scholar]
- 73. Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS. PROXI Trial Group. Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomized clinical trial. Anesth Analg. 2012;115:849–854. doi: 10.1213/ANE.0b013e3182652a51. [DOI] [PubMed] [Google Scholar]
- 74. Young PJ, Bailey M, Bellomo R, Bernard S, Bray J, Jakkula P, et al. Conservative or liberal oxygen therapy in adults after cardiac arrest: an individual-level patient data meta-analysis of randomised controlled trials. Resuscitation. 2020;157:15–22. doi: 10.1016/j.resuscitation.2020.09.036. [DOI] [PubMed] [Google Scholar]
- 75. Alali AS, Temkin N, Vavilala MS, Lele AV, Barber J, Dikmen S, et al. Matching early arterial oxygenation to long-term outcome in severe traumatic brain injury: target values. J Neurosurg. 2019;132:537–544. doi: 10.3171/2018.10.JNS18964. [DOI] [PubMed] [Google Scholar]
- 76. Taher A, Pilehvari Z, Poorolajal J, Aghajanloo M. Effects of normobaric hyperoxia in traumatic brain injury: a randomized controlled clinical trial. Trauma Mon. 2016;21:e26772. doi: 10.5812/traumamon.26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stenson BJ, Tarnow-Mordi WO, Darlow BA, Simes J, Juszczak E, Askie L, et al. BOOST II United Kingdom Collaborative Group; BOOST II Australia Collaborative Group; BOOST II New Zealand Collaborative Group. Oxygen saturation and outcomes in preterm infants. N Engl J Med. 2013;368:2094–2104. doi: 10.1056/NEJMoa1302298. [DOI] [PubMed] [Google Scholar]
- 78. Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362: 1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chu DK, Kim LH-Y, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391:1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 80. Siemieniuk RAC, Chu DK, Kim LH-Y, Güell-Rous M-R, Alhazzani W, Soccal PM, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. doi: 10.1136/bmj.k4169. [DOI] [PubMed] [Google Scholar]
- 81. Barbateskovic M, Schjørring OL, Krauss SR, Meyhoff CS, Jakobsen JJ, Rasmussen BS, et al. Higher vs lower oxygenation strategies in acutely ill adults: a systematic review with meta-analysis and trial sequential analysis. Chest. 2021;159:154–173. doi: 10.1016/j.chest.2020.07.015. [DOI] [PubMed] [Google Scholar]
- 82.Young P. Camberwell, VIC, Australia: Australian and New Zealand Intensive Care Society; 2021. https://www.anzics.com.au/current-active-endorsed-research/mega-rox/ [Google Scholar]
- 83. Brogi E, Cyr S, Kazan R, Giunta F, Hemmerling TM. Clinical performance and safety of closed-loop systems: a systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2017;124:446–455. doi: 10.1213/ANE.0000000000001372. [DOI] [PubMed] [Google Scholar]