Abstract
Pheromonal communication plays a key role in the sociosexual behavior of rodents. The coadaptation between pheromones and chemosensory systems has been well illustrated in insects but poorly investigated in rodents and other mammals. We aimed to investigate whether coadaptation between male pheromones and female reception might have occurred in brown rats Rattus norvegicus. We recently reported that major urinary protein (MUP) pheromones are associated with male mating success in a brown rat subspecies, R. n. humiliatus (Rnh). Here, we discovered that MUPs were less polymorphic and occurred at much lower concentrations in males of a parapatric subspecies, R. n. caraco (Rnc), than in Rnh males, and found no association between pheromones and paternity success. Moreover, the observation of Rnc males that experienced chronic dyadic encounters and established dominance–submission relationships revealed that the dominant males achieved greater mating success than the subordinate males, but their MUP levels did not differ by social status. These findings suggest that male mating success in Rnc rats is related to social rank rather than to pheromone levels and that low concentration of MUPs might not be a reliable signal for mate choice in Rnc rats, which is different from the findings obtained in Rnh rats. In addition, compared with Rnh females, Rnc females exhibited reduced expression of pheromone receptor genes, and a lower number of vomeronasal receptor neurons were activated by MUP pheromones, which imply that the female chemosensory reception of pheromones might be structurally and functionally coadapted with male pheromone signals in brown rats.
Keywords: coevolution, major urinary protein, social rank, subspecies divergence, vomeronasal reception
In many animal taxa, females often invest much more in each individual offspring than males do and cannot produce as many offspring as males can; thus, careful choice of mates is very important for female reproductive success and fitness (Andersson and Simmons 2006). A female’s mate choice often relies on the male’s sexual traits, which include cues or signals and male courtship behavior, and these sexual traits are determined by both genetic and environmental factors, which reflect the quality, social status, immune status, and other attributes of the bearers (Qvarnstrom and Forsgren 1998; Andersson and Simmons 2006; Janotova and Stopka 2011; Nelson et al. 2015; Fang et al. 2016; Lee et al. 2017). Due to female mate choice, males with exaggerated traits often exhibit enhanced reproductive success, and the traits, favorable alleles, and genetic benefits of these males are passed to their offspring (Birkhead and Møller 1993; Møller et al. 1998; Møller and Ninni 1998; Qvarnstrom and Forsgren 1998; Candolin 2003; Andersson and Simmons 2006; Demary and Lewis 2007; Taylor et al. 2007; Hosken et al. 2008; Zhang et al. 2019b). Another factor that influences male traits is male–male competition, which often facilitates the establishment of a social hierarchy and the differentiation of sexual traits, and as a result, dominant males have priority access to females and present exaggerated sexual traits to attract females (Candolin 2000; Meagher et al. 2000; Fang et al. 2016; Tinghitella et al. 2018; Wright et al. 2019).
As suggested by the Fisherian runaway or sexy son hypothesis, the association between sexually selected signals and mating preferences or reproductive success can be attributed to the genetic coupling and coevolution of signal production by males and reception by females (Andersson and Simmons 2006; Brennan and Zufall 2006; Bousquet et al. 2012; Charlton et al. 2019). The sexually selected signals utilized by the choosy sex for mate assessment should be sufficiently strong to honestly reflect the individual’s quality and satisfy the key criteria necessary for their evolution, heritability, and maintenance (Møller et al. 1998; Johansson and Jones 2007). The higher the intensity of a sexual signal, the more effective the signal is in terms of the receiver response (Searcy and Nowicki 2005). For example, in the pied flycatcher Ficedula hypoleuca, the white forehead patch of males is a sexually selected trait in Spanish and Swedish populations with large white forehead patches but not in a Norwegian population with a small patch (Dale et al. 1999). The divergence of male sexual traits between populations can be driven by sexual selection, and the weak cues in some populations might be unsexy (Dale et al. 1999; Lorch 2002; Morganscl et al. 2014). Signal reliability/honesty increases with increases in the intensity of the signal despite the rising cost (Zahavi 1977; Searcy and Nowicki 2005; Weaver et al. 2017). The coevolution of signal production and reception implies that if males emit strong signals, females must possess a powerful receiving system; for example, in the visual communication of medakas and the pheromonal communication of fruit flies, the emission and reception of a signal are controlled by a single gene that regulates both a sexually attractive trait in males and the sensory preferences or receptors of females (Fukamachi et al. 2009; Bousquet et al. 2012; Ng et al. 2014). In Drosophila, for example, the expression of a desaturase gene, desat1, in neural and non-neural tissues separately affects the perception and emission of sex pheromones (Bousquet et al. 2012). However, the coevolution of sex pheromones and their reception has not been empirically examined in mammals, even in the house mouse Mus musculus, which has the most extensively studied chemical communication system of any mammal (Smadja and Ganem 2002; Mucignat-Caretta et al. 2010; Hurst et al. 2017; Loire et al. 2017; Sheehan et al. 2019). Some mouse subspecies have been found to exhibit divergence in terms of pheromones, chemosensory responses, and mate choice, but the coevolution of pheromone emission and reception has not been examined (Smadja and Ganem 2002; Karn et al. 2010; Mucignat-Caretta et al. 2010; Hurst et al. 2017; Loire et al. 2017; Charlton et al. 2019; Sheehan et al. 2019).
In murid rodents, particularly mice and rats, the most vital sexual ornaments are pheromones, and male urine contains a large amount of major urinary proteins (MUPs) and volatile organic compounds (VOCs), both of which can act as sex pheromones to convey messages about genetic quality and genetic relatedness for female mate choice (Roberts and Gosling 2003; Roberts et al. 2010; Kumar et al. 2014; Zhang and Zhang 2014; Guo et al. 2015; Nelson et al. 2015; Fang et al. 2016; Lee et al. 2017; Guo et al. 2018; Zhang et al. 2019b). MUPs, which are synthesized by the liver and excreted into the urine, not only act as pheromones themselves but also bind and carry small VOC pheromones (Roberts et al. 2010; Kumar et al. 2014; Hurst et al. 2017; Guo et al. 2018; Gomez-Baena et al. 2019). Females have innate preferences for MUPs, whereas preferences for VOCs must be acquired through associative learning with MUPs; therefore, MUPs might be a better indicator of sexual attractiveness than VOCs (Roberts et al. 2010; Gomez-Baena et al. 2019; Zhang et al. 2019a,2019b). Olfaction-mediated mate choice relies on both specific pheromones and precisely matched receiving systems, and vomeronasal receptors (VRs) specifically receive pheromone signals and are likely subject to coadaptation with pheromones (Brennan and Zufall 2006; Teeter et al. 2008; Ibarra-Soria et al. 2017).
The brown rat Rattus norvegicus is a worldwide pest species, and similar to the house mouse, this species serves as a good model for examining the coevolution of pheromones and their reception because female rats display non-random mate choice based on specific attributes of male rats (Berdoy and Drickamer 2007; Kumar et al. 2014; Costa et al. 2016; Zhang et al. 2019b). In China, the brown rat has been differentiated into 4 subspecies marked by morphological, genetic, and genomic divergences (Wu 1982; Wang 2003; Musser and Carleton 2005; Teng et al. 2017). A pilot experiment showed extremely marked differences in urinary VOC and MUP pheromones between 2 parapatric rat subspecies, namely, R. n. humiliatus (Rnh) and R. n. caraco (Rnc); specifically, the Rnh subspecies, which lives mainly in Beijing and Hebei Province in North China, possesses abundant MUP pheromones, whereas the Rnc subspecies, which lives mainly in Northeast China, produces scarce amounts of MUP pheromones (Guo 2016; Zhang et al. 2019b). We recently reported that high pheromone levels are associated with reproductive success in Rnh males (Zhang et al. 2019b) and questioned whether this association also exists in Rnc males with low levels of MUPs. We also considered whether the divergence of MUP pheromones might be magnified by male–male competition to increase the effectiveness of the MUPs as male signals for female mate choice in the Rnc subspecies. Additionally, whether male pheromones coevolved/coadapted with female chemosensory reception in Rnc and Rnh rat populations remain unclear.
Here, we first investigated the relationships between male MUP pheromones and mating success in captive-bred adult Rnh and Rnc rats. Second, we established dominance–submission relationships in Rnc rats and explored the relationship among social status, pheromone levels, and mating success. We also investigated the pheromone reception of the two subspecies by examining the gene expression of VRs via a transcriptome analysis and testing the vomeronasal sensitivity to pheromones via calcium imaging (Leinders-Zufall et al. 2000; Sam et al. 2001; Loire et al. 2017). This study provides the first insights into the coevolution/coadaptation of the intensity of MUP pheromones and vomeronasal reception for mediating mating success.
Materials and Methods
Subjects
The ancestors of the captive-bred Rnh and Rnc rats were captured alive in rural areas of Beijing in North China and Harbin in Northeast China, respectively, and maintained as outbred colonies of approximately 300–400 individuals of each subspecies in our laboratory. For breeding, male–female pairs of the same subspecies were caged in plastic rat cages (37 × 26 × 17 cm) (Suzhou Fengshi Laboratory Animal Equipment Co., Ltd., Suzhou, China) with wood shavings for bedding (Beijing Keao Xieli Feed Co., Ltd., Beijing, China). Standard rat chow and tap water were provided ad libitum. The colonies were maintained on a 14-h light:10-h dark cycle (lights on at 19:00) at 23 ± 2 °C.
The subjects used in this study were 4 or 5 generations removed from the wild-caught stock and were caged in groups of 3 or 4 same-sex siblings of the same subspecies after being weaned at 4 weeks of age. All the males were 5–12 months of age, sexually naïve, caged individually for 2 weeks prior to initial urine collection, established as dominant or subordinate, and subjected to mate choice and paternity tests. All the females were 3–6 months of age and sexually naïve and had a 4- or 5-day estrous cycle. The phase of the estrous cycle was determined by vaginal smear cytology, and only females with regular estrous cycles were used in the study (Marcondes et al. 2002).
A total of 120 males and 60 females were used in the behavioral tests. First, 48 males of each subspecies served as urine donors. After sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) analysis (methods are described below), the 24 male rats with the highest relative abundances of MUPs were placed in the high-MUP group, and the 24 male rats with the lowest relative abundances of MUPs were placed in the low-MUP group. Males from the high- and low-MUP groups of the same subspecies and 24 females from either the same or the other subspecies were used for the assessments of olfactory preferences and mating success. Second, another 24 Rnc males were first subjected to dyadic encounters (using the methods described below) and then used for the assessment of mating success with 12 Rnc females.
A total of 26 females served as vomeronasal organ (VNO) and olfactory epithelium (OE) donors. Nine females of each subspecies were sacrificed for transcriptome sequencing of the OE and VNO, and 4 females of each subspecies were used for the calcium ion imaging of VNO slices.
Two-way tests of the chemosensory preference and mating success of the 2 subspecies
The chemosensory preference tests were performed under dim red light during the dark phase of the light cycle, and the experimenter was blind to the nature of the sample. Urine samples selected at random (the urine donors must have a similar body weight, <10% difference) from the high- and low-MUP groups were paired and presented to a female of either the same or other subspecies as previously described (Zhang and Zhang 2014). For each test, 1 estrous female subject was placed in the home cage. Two glass rods (length of 20 cm, diameter of 4 mm), each of which had been scented with 2 μL of urine from the high- and low-MUP groups of either the Rnc or Rnh subspecies, were simultaneously presented to the subject. The test lasted for 3 min starting from the time at which the subject first sniffed or licked the tip of a rod. The time that the female subject spent investigating each urine sample was recorded. Each subject was tested only once a day, and the tests were replicated with urine samples from different pairs of males for 4 consecutive days. The summed investigation times of each female toward each group across the 4 days were then analyzed.
The procedures used for the mating success tests have been previously described in detail (Zhang et al. 2019b). Each mating trio was composed of 1 female of either the Rnc or Rnh subspecies and 2 body weight-matched males selected at random from the high- or low-MUP group of either the Rnc or Rnh subspecies. The 2 males in a mating trio had similar body weights (<10% difference) and were from the same subspecies but were not siblings. The trials were performed in a 3-chamber apparatus constructed from 3 rat cages (37 × 26 × 17 cm), and the 2 choice cages were symmetrically connected to one of the long sides of the neutral cage via acrylic tubes (length of 50 cm and inner diameter of 7 cm). A sexually naïve female was placed in the neutral cage, and each of the pair of males with similar body weights was loosely tethered within one of the choice cages. Females could freely investigate all 3 cages, whereas the males could move freely within their own cages. The mating trios were kept in the apparatus until the females delivered. Most of the females delivered within a month. The mating trios in which the female did not get pregnant within 2 months were excluded from the study. Upon delivery, the females were separated from males, the pups were counted, and 3 mm of the tail tip was cut from each pup for the microsatellite analysis of paternity. After the pups were weaned, the adult subjects were anesthetized (45 mg/kg pentobarbital sodium), and 3 mm of the tail tip was collected from each adult.
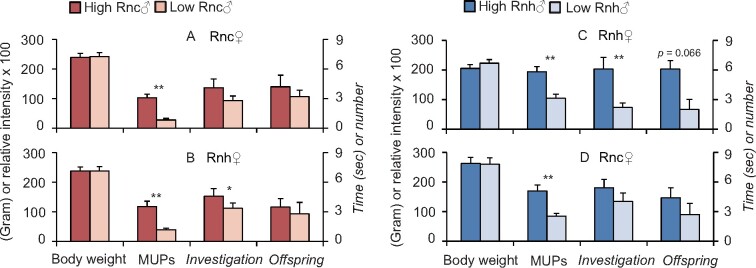
The mating trios were as follows. (1) Rnc females choosing between Rnc males belonging to the high-MUP group and Rnc males belonging to the low-MUP group (body weight: high-MUP males, 239.1 ± 42.48 g; low-MUP males, 241.9 ± 43.05 g; mean ± standard deviation [SD], P = 0.769, t = 0.302, df = 10, 11 pairs were analyzed, 1 nonpregnant mating trio was excluded; Figure 1A); (2) Rnh females choosing between Rnc males belonging to the high-MUP group and Rnc males belonging to the low-MUP group (body weight: high-MUP males, 238.5 ± 41.96 g; low-MUP males, 238.6 ± 45.34 g; mean ± SD, P = 0.976, t = 0.031, df = 9, 10 pairs were analyzed; 2 nonpregnant mating trios were excluded; Figure 1B); (3) Rnh female choosing between Rnh males belonging to the high-MUP group and Rnh males belonging to the low-MUP group (body weight: high-MUP males, 205.16 ± 42.15 g; low-MUP males, 222.7 ± 38.76 g; mean ± SD, P = 0.315, t = 1.065, df = 9, 10 pairs were analyzed; 2 nonpregnant mating trios were excluded; Figure 1C); and (4) Rnc females choosing between Rnh males belonging to the high-MUP group and Rnh males belonging to the low-MUP group (body weight: high-MUP males, 262.39 ± 58.06 g; low-MUP males, 260.51 ± 58.02 g; mean ± SD, P = 0.351, t = 1.000, df = 7, 8 pairs were analyzed; 4 nonpregnant mating trios were excluded; Figure 1D).
Figure 1.
Body weight, relative abundances of MUPs, olfactory preferences, and offspring of the paired high- and low-MUP males. (A) Paired high- and low-MUP Rnc males subjected to choice by Rnc females. (B) Paired Rnc males subjected to choice by Rnh females. (C) Paired high- and low-MUP Rnh males subjected to choice by Rnh females. (D) Paired Rnh males subjected to choice by Rnc females (mean ± standard error [SE], paired t-test or Wilcoxon signed-rank test, **P < 0.01).
Establishment of dominance–submission relationships and assessment of the mating success of the Rnc subspecies
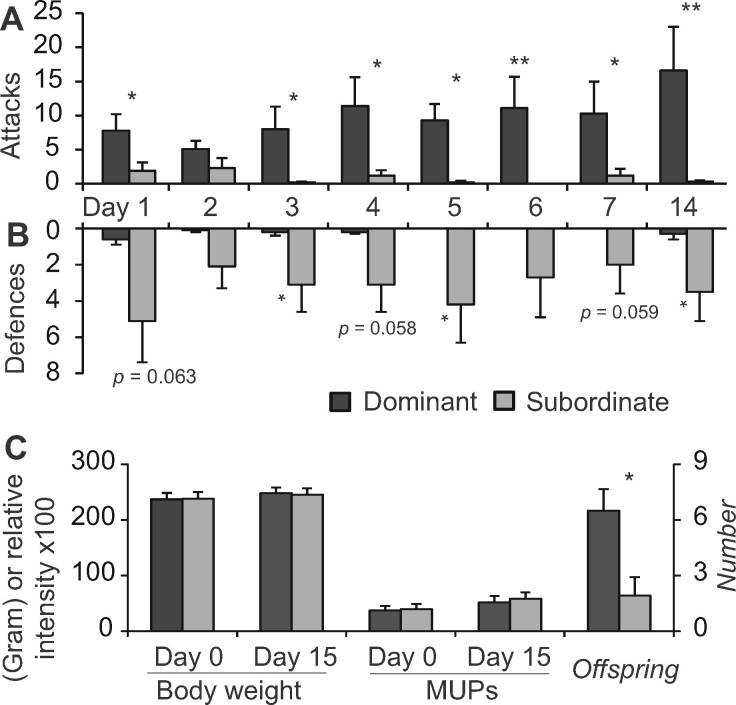
Staged dyadic encounters to establish dominance–submission relationships were conducted in a separate room under dim illumination during the dark period. A clean standard plastic cage served as a neutral area (105 × 35 × 40 cm). Two unrelated male Rnc rats with similar weights (236.9 ± 34.10 g vs. 238.1 ± 35.28 g, t = 0.569, df = 8, P = 0.586; Figure 2) were paired and placed symmetrically and simultaneously into the 2 sides of the arena. Each trial lasted 15 min after the initial aggressive or defensive action between paired males. All the rats were tested once daily on 14 successive days (Barnett 1958; Zook and Adams 1975; Pohorecky et al. 2008).
Figure 2.
Establishment of the dominance–submission relationships among paired Rnc males and body weight, relative abundance of MUPs and numbers of offspring sired by dominant and subordinate Rnc males. Frequency of attacks (A) and defenses (B) of paired Rnc males during 10-min dyadic encounters for 14 consecutive days (C). Body weights and relative MUP abundances of the paired males before and after 2 weeks of dyadic encounters and the numbers of offspring sired by the dominant and subordinate males (mean ± SE, paired t-test or Wilcoxon signed-rank test, *P < 0.05, **P < 0.01).
Aggressive and defensive behaviors were identified as follows: aggressive behaviors included the actions of chasing, biting, stepping on and mounting the opponent, and defensive behaviors included fleeing, upright posture, screaming, and ingratiating oneself with rivals (e.g., licking and allogrooming) (Barnett 1958; Zook and Adams 1975; Zhang et al. 2001). Each bout of action that had a duration ˂10 s was scored as 1 unit. On 14 successive days, the paired male that displayed more aggressive behaviors and fewer defensive behaviors was regarded as the dominant male, and its opponent was regarded as the subordinate male (Zhang et al. 2001; Fang et al. 2016). The dominance–submission relationship was further corroborated by individual activities; specifically, dominant males often appeared to behave boldly and run around, whereas subordinate males looked fearful and cowered (Barnett 1958).
After the establishment of dominance–submission relationships, the males were subjected to mating success tests (methods are described above), where each mating trio was composed of 1 female and a pair of dominant and subordinate males.
DNA extraction and microsatellite analysis for paternity testing
DNA was extracted from the rat tails using the phenol–chloroform method and checked with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Each rat was genotyped at 9 microsatellite loci. The forward primer of each locus was labeled with hexachloro-6-carboxy-fluorescine, 6-carboxyfluorescein or 5-carboxytetramethylrhodamine dye. The polymerase chain reaction (PCR) conditions were optimized. For D1wox31, the reaction mixtures contained 1.25 U of Takara Taq, 0.2 mM each dNTP, 1.5 mM MgCl2, 5 μL of PCR buffer (Takara, Dalian, China), 0.2 μM each primer, and 80–90 ng of template DNA in a total volume of 50 μL. For the other 8 loci, the reaction mixtures contained 0.8 U of Takara Taq, 0.15 mM each dNTP, 2 mM MgCl2, 2 μL of PCR buffer, 0.4 μM each primer, and 80–90 ng of template DNA in a total volume of 20 μL. The mixture was amplified using a PCR machine (EDC-810; Eastwin Life Sciences Inc., Beijing, China). The thermal cycle was programmed as follows: initial denaturation at 94 °C for 5 min followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at the Tm (Supplementary Table S1) for 30 s, and extension at 72 °C for 40 s (for D1wox31, 1 min) and a final extension at 72 °C for 10 min. Capillary electrophoresis was performed with an ABI 3130 Genetic Analyzer (Applied Biosystems, Waltham, MA). The alleles were analyzed using GeneMarker software (SoftGenetics, State College, PA, USA), and the allele size ranges were determined by comparison with the standard size of the GS500 LIZ based on the automatically scored peaks. The alleles from pups, mothers, and potential fathers were identified. Because the maternal alleles are known, the paternal alleles and the fathers can be identified (Heiberg et al. 2006; Zhang et al. 2019b).
Urine, VNO, and OE collection
Urine collection was performed during the dark phase of the light cycle and lasted for 8 h. Standard rat chow and water were freely available. Each rat was placed in a clean rat metabolic cage, and urine was collected in a tube immersed in an ice box. The metabolic cages were washed thoroughly with water and sterilized between collections. The urine samples were maintained at −20 °C prior to use. The VNO and OE used for RNA sequencing (RNA-seq) were dissected from 9 females of each subspecies. We euthanized the rats via cervical dislocation, opened the mouth with forceps, and visualized the palate. An incision was made in the upper part of the palate. We removed the palate membrane with microdissecting forceps, cut the upper and the lower parts of the nasal septum with iris scissors, and then dissected the VNO. The OE was located on both sides of the nasal septum. We eliminated the skin and facial muscles with scissors and removed the bone covering the nasal cavity with a rongeur. The OE was then delicately separated from the posterior nasal septum surface. All the procedures were conducted within 10 min after death, and the samples were rapidly frozen with liquid nitrogen.
Quantification of the levels of total MUPs via SDS–PAGE
The relative abundances of levels of MUPs were quantified by SDS–PAGE with a Mini-Protean system (Bio-Rad, Hercules, CA, USA) as previously described (Guo et al. 2015; Zhang et al. 2019b). The urine sample was mixed with SDS–PAGE loading buffer, boiled at 100 °C, fractionated on 15% SDS–PAGE gels, and then stained with Coomassie brilliant blue. To normalize the bands across gels, a specific sample was loaded on different gels. Gel images were acquired with a ChemiDoc MP system (Bio-Rad, Hercules, CA, USA), and the bands were quantified using ImageJ software (NIH, Bethesda, MD, USA).
Polymorphism of MUPs assessed by isoelectric focusing
Isoelectric focusing (IEF) was performed using a Model 111 Mini IEF Cell (Bio-Rad, Hercules, CA, USA). The rat urine samples were de-salted, freeze-dried, dissolved in deionized water, and electrophoresed in a 5% polyacrylamide gel containing 5% ampholytes (pH range of 3–7, SERVA Electrophoresis GmbH, Germany). The gels were fixed, stained, destained, and then imaged with a ChemiDoc MP system (Guo et al. 2018).
RNA-seq analysis and qPCR of the VNO and OE
RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Libraries were sequenced with the Illumina HiSeq X Ten platform. The index of the reference genome (rn6) was built using Bowtie version 2.2.3, and paired-end clean reads were aligned to the reference genome using TopHat version 2.0.12. HTSeq version 0.6.1 was used to count the reads. Differentially expressed genes (DEGs) were determined with the DESeq2 R package version 1.24.0. An adjusted P< 0.05 and a |log2 fold change| > 1 were used as the criteria for significance. g:Profiler was used to test the enrichment of DEGs in Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Hierarchical clustering was performed with the normalized read counts of the chemoreceptor genes using the pheatmap R package version 1.0.12. The Euclidean distance and complete linkage method were applied. The expression of receptors in individuals was analyzed by quantitative real-time PCR (qPCR) (Supplementary Table S2) (Ibarra-Soria et al. 2014; Ibarra-Soria et al. 2017). The RNA-seq data are available from the Sequence Read Archive of the National Center for Biotechnology Information (BioProject ID: PRJNA591253).
Calcium imaging
The pheromone-evoked responses in VR neurons (VRNs) were detected by the calcium imaging of VNO slides. Each animal was euthanized by cervical dislocation, and the head was placed in artificial cerebrospinal fluid (ACSF). VNOs were then separated from the nasal septum and embedded in low-melting agarose. The coronal slices were cut at a thickness of 200 μm with a vibratome (Leica VT1200S, Germany) (Brechbuhl et al. 2011; Liu et al. 2019), and the slices were incubated with loading solution (ACSF with 10 μM FURA-2 AM and 0.1% Pluronic F-127). The samples were illuminated with an ion imaging system (Nikon Ti-E, Japan), and the changes in the calcium ratio were monitored. For each imaging session, one of the 3 pheromones (10−5 M 2-heptanone, 10−7 M MUP13, or 10−7 M olfactory‐binding protein 3 [OBP3]) ( Guo et al. 2018) was delivered at a flow rate of 1 mL/min, and 50 mM KCl was applied to verify the viability of the cells at the end of the test (Zufall and Leinders-Zufall 2007; Brechbuhl et al. 2011; Liu et al. 2019).
Statistical analysis
The distributions of the raw data were analyzed using a Kolmogorov–Smirnov test. Parametric and nonparametric tests were used for normally and non-normally distributed data, respectively. We tested the differences in the body weights, relative abundances of MUP, aggressive and defensive behaviors and number of offspring between paired males using paired t-tests or Wilcoxon signed-rank tests and compared the calcium responses between subspecies using independent t tests or Mann–Whitney U tests. All statistical analyses were conducted using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA), and the significance level was set to P < 0.05.
Ethical standards
The capture of the wild rats was conducted under a permit from the Professional Committee of Rodent Control, China Society of Plant Protection. All the procedures performed in studies involving animals were approved by the Animal Use Committee of the Institute of Zoology, Chinese Academy of Sciences (approval number IOZ12017), and were in accordance with the ethical standards of the institution (Institutional Guidelines for Animal Use and Care at the Institute of Zoology, Chinese Academy of Sciences).
Results
MUPs and paternity success of males subjected to female choice
Within the Rnc subspecies, the high-MUP males had significantly higher MUP levels than the low-MUP males (P < 0.001, t = 8.436, df = 10). However, no significant differences were found in the investigation time of females toward male urine from the high- and low-MUP group or the number of pups sired by Rnc males from the 2 groups with choosy Rnc females of the same subspecies (urine: P = 0.161, t = 0.1.503, n = 12; offspring: P = 0.594, z = 0.534, n = 11, Figure 1A). Three litters were sired by high-MUP males, 4 litters were sired by low-MUP males, and 4 litters were sired by both high- and low-MUP males.
When cross-bred with Rnh females, the high-MUP Rnc males had significantly higher MUP levels than the low-MUP Rnc males (P = 0.001, t = 4.539, df = 9). Moreover, the females spent more time investigating the male urine of the high-MUP group than that of the low group, but the number of pups delivered by Rnh females that were sired by males from the 2 groups did not exhibit a significant difference (urine: P = 0.050, z = 1.961, n = 12; offspring: P = 0.813, z = 0.236, n = 12, Figure 1B). Seven litters were sired by high-MUP males, 3 litters were sired by low-MUP males, and 2 litters were sired by both high- and low-MUP males.
Within the Rnh subspecies, the high-MUP males had significantly higher MUP contents than the low-MUP males (P = 0.001, t = 5.284, df = 9). Moreover, the females spent more time investigating the male urine of the high group than that of the low group, and the number of pups from Rnh females sired by high-MUP Rnh males tended to be higher than that sired by the low-MUP Rnh counterparts (urine: P = 0.002, z = 3.059, n = 12; offspring: P = 0.066, z = 1.838, n = 10, marginal significance, Figure 1C). Seven litters were sired by high-MUP males, 1 litter was sired by a low-MUP male, and 2 litters were sired by both high- and low-MUP males.
When cross-bred with Rnc females, the high-MUP Rnh males had a significantly higher MUP content than the low-MUP Rnh males (P = 0.007, t = 3.807, df = 7); however, neither the time that the females spent investigating male urine of the high- and low-MUP groups nor the number of pups from Rnc females sired by males from these 2 groups exhibited a significant difference (urine: P = 0.158, t = 1.412, n = 12; offspring: P = 0.414, z = 0.817, n = 10, Figure 1D). Four litters were sired by high-MUP males, 2 litters were sired by low-MUP males, and 4 litters were sired by both high- and low-MUP males.
Social dominance–submission relationships and paternity success within the Rnc subspecies
Stable dominance–submission relationships between the paired males were established after 2 weeks of daily dyadic encounters. Dominant males displayed more aggressive behaviors (P1 = 0.034, z1 = 2.120, P2 = 1.109, z2 = 1.601, P3 = 0.017, z3 = 2.386, P4 = 0.033, z4 = 2.136, P5 = 0.012, z5 = 2.527, P6 = 0.008, z6 = 2.366, P7 = 0.012, z7 = 2.527, P14 = 0.012, z14 = 2.521, n = 10, paired t-test, the subscripts indicate the experimental day, Figure 2A) and fewer defensive behaviors (paired t-test: P1 = 0.063, z1 = 1.863, P2 = 0.109, z2 = 1.604, P3 = 0.027, z3 = 2.207, P4 = 0.058, z4 = 1.897, P5 = 0.026, z5 = 2.232, P6 = 0.109, z6 = 1.604, P7 = 0.059, z7 = 1.890, P14 = 0.042, z14 = 2.032, n = 10, Figure 2B) than their subordinate opponents. The MUP levels did not show differences between dominant and subordinate males either before or after 2 weeks of daily dyadic encounters (Day 0: P = 0.842, t = 0.206, df = 8, Day 15: P = 0.677, t = 0.432, df = 8). In addition, no differences were found between the MUP levels either before or after the dyadic encounters in either the dominant or subordinate males (dominant: P = 0.268, t = 1.189, df = 8, subordinate: P = 0.199, t = 1.399, df = 8). The body weights did not differ between dominant and subordinate males after 2 weeks of daily encounters (Day 0: P = 0.586, t = 0.569, df = 8, Day 15: P = 0.625, t = 0.502, df = 8, Figure 2C) but were significantly increased in dominant males (Day 0 vs. Day 15, P = 0.028, t = 2.083, df = 8) and showed an increasing trend in subordinate males (Day 0 vs. Day 15, P = 0.099, df = 8, t = 2.083, marginal significance).
In mating trios consisting of 1 Rnc female and 2 Rnc males with a dominant and subordinate status, the dominants sired more pups than their subordinate opponents (P = 0.049, z = 1.970, n = 12, Figure 2C). A total of 12 litters were produced; among these, 9 litters were all or mostly sired by the dominant males, and the others were all or mostly sired by the subordinate males. In addition, 9 of the litters had single paternity, and the others had dual paternity.
Comparison of MUPs between Rnh and Rnc males
The Rnh males had significantly higher MUP levels than the Rnc males, as revealed by SDS–PAGE (t = 5.053, df = 16.23, P < 0.001, Figure 3A,B). In the IEF analysis, 10 MUP bands were separated in the Rnh males within the pI range of rats (5.0–6.0), but only 5 MUP bands were obtained with the Rnc males. In particular, the bands of the Rnh males were extremely clear, but those from the Rnc males were barely visible (n = 13; Figure 3C).
Figure 3.
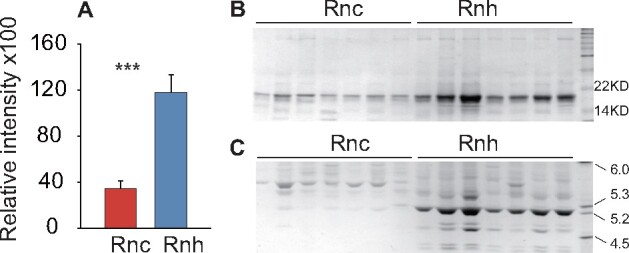
(A) Urinary relative abundance of MUPs in Rnc and Rnh males quantified by SDS–PAGE (mean ± SE, independent t-test, *P < 0.05, **P < 0.01). (B) SDS–PAGE gel image of Rnc and Rnh male urine samples. The MUPs are at ∼19 kDa. (C) The urine of Rnc and Rnh males was analyzed by IEF.
Differentiation of the olfactory transcriptomes between subspecies
The OE and VNO samples yielded an average of 54.96 million and 53.75 million clean reads, respectively. For each sample, >72% of the reads were uniquely mapped to the genome (Supplementary Tables S3 and S4). The variation in gene expression among biological replicate samples was assessed by Pearson correlation tests. For both the OE and VNO, the correlation values were highly significant (P < 2.20 × 10−16), and the correlation coefficients between samples from the same subspecies were higher than those found between samples from different subspecies (Supplementary Table S5).
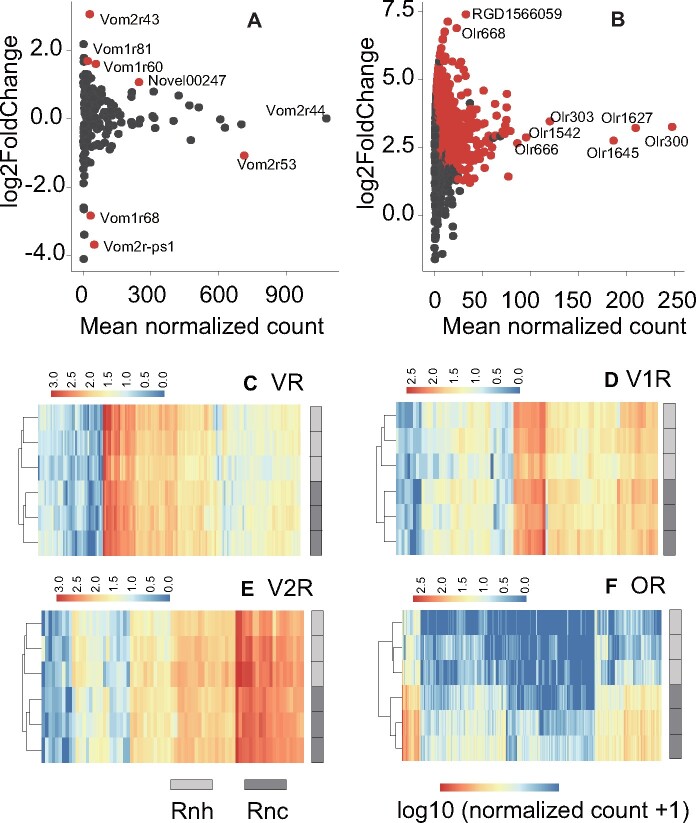
In the VNO, 628 DEGs were identified between the 2 subspecies, and these were enriched in 173 GO terms (adjusted P < 0.05). The top 3 most significant GO terms were immune response, extracellular region, and immune system process (Supplementary Figure S1A,B and Supplementary Tables S6 and S7). The DEGs were also enriched in 26 KEGG pathways, and the top 3 most significant of these pathways were cytokine–cytokine receptor interaction, influenza A, viral protein interaction with cytokine and cytokine receptor (Supplementary Table S7). The most highly expressed genes in the VNO were obp2a and LOC103691751 (mean normalized counts: 1,326,928.03 and 649,818.53, respectively), which encode odourant-binding proteins (Supplementary Figure S1A and Supplementary Table S6). Of the 180 VR genes (mean normalized count: 86.32) that were detected, vom2r44 had the highest expression levels (normalized count: 1,078.59), vom2r53 was the second most highly expressed VR (normalized count: 713.88), and 75% of the VRs had a normalized count ˂100. The top 15 VRs were all V2Rs, and these accounted for 48% of the total read counts. Eight VRs had expression levels that differed between subspecies; the expression levels of vom2r53, vom2r-ps1 (pseudogene), and vom1r68 in Rnh were higher than those in Rnc, whereas those of vom2r43, vom1r60, vom1r81, and Novel00247 were higher in Rnc than in Rnh (Figure 4A and Supplementary Table S6). A dendrogram of the VNO samples clearly grouped the samples by subspecies (Figures 4C,D and 5E).
Figure 4.
Scatter plot of the mean normalized counts (x-axis) of VR genes in the VNO (A) and OR genes in the OE (B) vs. the log2 fold change between Rnc and Rnh (y-axis). Genes with expression values that differed between subspecies are represented in red (|log2 fold change| > 1 and adjusted P < 0.05). Heatmap of the expression patterns of genes encoding vomeronasal receptors (C), vomeronasal Type 1 receptors (D), and vomeronasal Type 2 receptors (E) in the VNO and ORs in the OE (F). Hierarchical clustering was performed using the log10 (normalized read counts +1) with the R package pheatmap; the Euclidean distance and the complete linkage method were applied, and receptors with weak expression (normalized read counts < 1) were not included.
In the OE, 3,017 DEGs were found between the 2 subspecies and were enriched in 219 GO terms, and the top 3 most significant GO terms were nervous system process, sensory perception, and sensory perception of smell (Supplementary Figure S1C,D and Supplementary Tables S8, S9). The DEGs were enriched in the KEGG pathways related to olfactory transduction and cell adhesion molecules (Supplementary Table S9). The most highly expressed genes in the OE were bpifa1 and LOC690507 (similar to vomeromodulin) (mean normalized counts: 410,692.69 and 297,947.53, respectively), and bpifa1 was more highly expressed in Rnh than in Rnc (Supplementary Figure S1C and Supplementary Table S8). A total of 1,072 olfactory receptor (OR) genes were expressed (mean normalized count: 9.66); among these genes, olr300 exhibited the highest expression level (normalized count: 247.33), 75% had a normalized read count ˂10, and 388 were more highly expressed in Rnc than in Rnh (Figure 4B and Supplementary Table S8). The dendrogram of the OE samples clearly grouped the samples by subspecies (Figure 4F).
Differences in VNO sensitivity to pheromones between subspecies
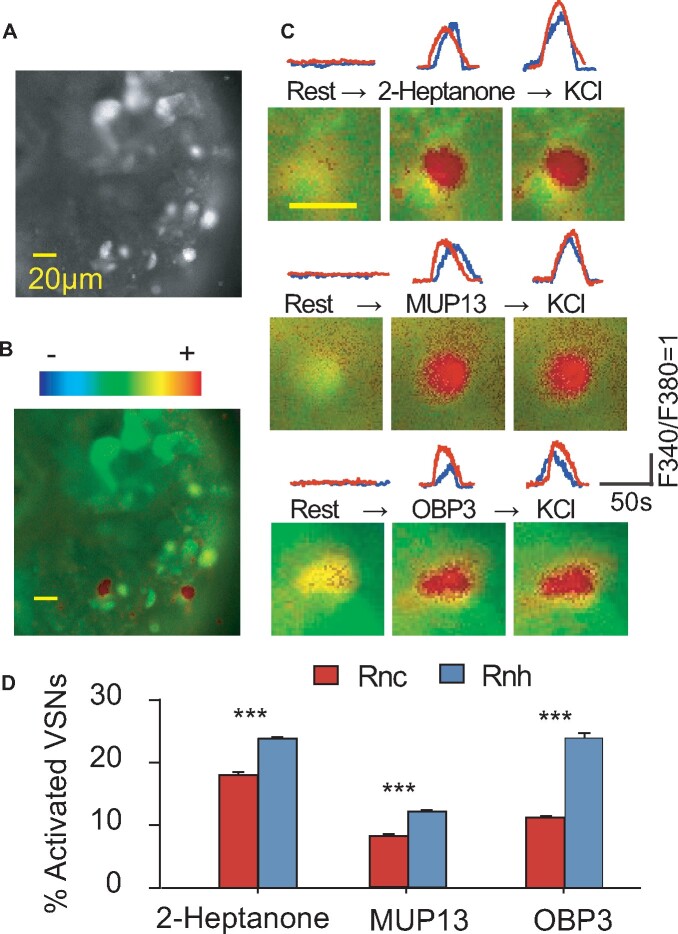
The VRN sensitivities to pheromones were detected by calcium imaging using VNO slides loaded with the calcium-sensitive dye Fura-2-AM, and the numbers of cells showing responses to both pheromones and the positive control potassium chloride or to potassium chloride alone were quantified (Figure 5). We analyzed 294.08 ± 16.62 cells from each individual female rat and 4 individuals of each subspecies and from each pheromone stimulus. The percentages of pheromone-activated cells in Rnh individuals were higher than those in Rnc individuals (2-heptanone: P < 0.001, t = 11.013, MUP13: P < 0.001, t = 11.789, OBP3: P < 0.001, t = 15.962, n = 4, Figure 5D).
Figure 5.
Pheromones that evoked intracellular Ca2+ elevation in vomeronasal sensory neurons (VRNs). The fluorescence images are displayed on gray (A) and pseudocolor scales (B). (C) Traces and images of VRNs responsive to 10−5 M 2-heptanone, 10−7 M MUP13, 10−7 M OBP3, and 50 mM KCl (positive control). (D) Comparisons of the percentage of pheromone-activated cells between Rnc and Rnh rats (independent t-test, n = 4 for each subspecies, ***P < 0.001). The scale bar in the images represents 20 µm.
Discussion
This study showed that the females of Rnh subspecies exhibited chemosensory preferences for males with high MUP levels. We also found no difference in offspring numbers between Rnc sires with high and low MUP levels, regardless of the subspecies of the dams. This finding suggests that the MUP levels are not related to paternity success in Rnc sires and differs from the results obtained with Rnh sires, which revealed that high MUP levels are related to high paternity success (Zhang et al. 2019b). Males with stronger sexually attractive traits are likely to achieve greater mating success, but attractive traits cannot always predict female mate choice decisions and mating success (Godin and Briggs 1996; Møller and Ninni 1998; Brooks 2000; Demary and Lewis 2007; Kehl et al. 2015; Zhang et al. 2019b). Weak signals cannot effectively deliver reliable information about the sexual attractiveness and genetic quality of male bearers (Møller et al. 1998; Johansson and Jones 2007). The pheromones were markedly lower in Rnc than in Rnh, which suggests that the urinary pheromones of Rnc males might exhibit the insufficient intensity and reliability for females to use in mate choice (Zahavi 1977; Møller et al. 1998; Searcy and Nowicki 2005; Johansson and Jones 2007; Weaver et al. 2017; Zhang et al. 2019b). Another possibility of explaining the inconsistent results between Rnh and Rnc might be that the trade-off between genetic compatibility and sexual attraction influences the disassociation between MUPs and mating success in Rnc males. In murids, male pheromones can serve as ‘sexual chemical ornaments’ to attract females independently of genetic compatibility, whereas genetic compatibility can influence female mating preferences only when male pheromones show small variations (Roberts and Gosling 2003; Zhang and Zhang 2014). Both the pheromone levels in males and their inter-individual differences appear to have a threshold above which the influence of sexual attractiveness would override genetic compatibility benefits; otherwise, sexual attractiveness would be overridden by inbreeding avoidance in rats (Zhang and Zhang 2014). In Rnh rats, the MUPs and VOCs can reliably reflect sexual attractiveness (Roberts et al. 2010; Zhang et al. 2019a, 2019b). Here, the levels of MUPs and VOCs in Rnc males were markedly lower than those in Rnh males and might thus be unable to convey information about sexual attractiveness and male quality to females, and the MUP levels in Rnc males are not associated with mating success (Searcy and Nowicki 2005; Weaver et al. 2017). Whether the MUPs signal the genetic compatibility and influence the mate choice of Rnc females remains to be investigated. In this study, the MUP levels showed significant differences between the high- and low-MUP groups of Rnc sires, but the difference in offspring numbers between the Rnc sires belonging to the high- and low-MUP groups (Wilcoxon signed-rank test: 2-tailed, P = 0.066, z = 1.838; one-tailed, P = 0.034, z = 1.836) was not as remarkable as those observed in our previous work (P = 0.039; Zhang et al. 2019b), possibly because the MUP levels between the paired males were not extremely different from those in our previous study. How MUP variations affect mating strategies and paternity success deserves further study.
We found that mating success in Rnc male rats was related to the dominance–submission relationship. Male–male competition can increase the honesty of secondary sexual signals by magnifying intermale signal differences after the establishment of a dominance–submission relationship and facilitate adaptive female choice (Candolin 2000; Meagher et al. 2000; Guo et al. 2015; Fang et al. 2016). In mice and rats, dominant males are more attractive, have priority access to females, and achieve greater reproductive success than subordinates (Gartner et al. 1981; D’Amato 1988; Wright et al. 2019). Male–male competition can magnify the interindividual differences in MUP pheromones, which can serve as an indicator of social status and mediate female mate choice in mice (Guo et al. 2015; Nelson et al. 2015; Lee et al. 2017; Thoss et al. 2019). However, in this study, no change in MUP levels was found during the establishment of dominance submission, which suggests that some other traits and biological processes related to social dominance instead of MUPs might mediate mate choice and paternity success in brown rats (Andersson and Simmons 2006; Wright et al. 2019; Zhang et al. 2019b). In mice, VOCs and preputial gland-excreted VOCs, in addition to urinary MUPs, are associated with dominance–submission relationships (Jemiolo et al. 1991; Nelson et al. 2015; Fang et al. 2016; Lee et al. 2017), and in laboratory lines of brown rats, the preputial glands of dominant males are larger and excrete more VOCs than those of subordinates (Pohorecky et al. 2008). Moreover, in both mice and rats, different ultrasonic vocalizations have been found between dominant and subordinate males and affect female preferences (Inagaki et al. 2005; Wang et al. 2011). We thus speculated that other signals, such as preputial gland-secreted VOCs, ultrasonic vocalizations and biological processes, such as courtship behaviors and sperm competition, could convey information about social status to mediate female mate choice and paternity success in Rnc (Inagaki et al. 2005; Pohorecky et al. 2008; Wang et al. 2011). Moreover, the occurrence of multiple paternities suggests that sperm precedence and competition and mating order, interval and duration might influence paternity success in dominant and subordinate Rnc males (Kraaijeveld-Smit et al. 2002; Lymbery et al. 2018; Lorch, 2002; Cordero Rivera et al. 2004; Hosken et al. 2008; Mank, 2009; Costa et al. 2016; Lymbery et al. 2018).
Because the differences in the MUP levels between Rnh males could elicit the chemosensory preferences of females for high- to low-MUP males and thus affect mating success in the Rnh subspecies but not in the Rnc subspecies, the chemosensory ability of Rnc females might be attenuated to coadapt with the scarce pheromones of Rnc males (Johansson and Jones 2007; Bousquet et al. 2012; Ng et al. 2014; Seike et al. 2015; Charlton et al. 2019; Zhang et al. 2019b). This conjecture was also confirmed by the low expression of several VRs and the attenuated vomeronasal sensitivity in Rnc female rats. The chemosensory receptor repertoires of V1Rs, VR family 2 genes (V2Rs) and ORs showed subspecies-specific transcriptome patterns between Rnc and Rnh females. Of the 3 diverged special pheromone receptors (V2Rs), vom2r53 and vom2r-ps1 (a V2R pseudogene) were upregulated and vom2r43 was downregulated in Rnh compared with Rnc females, and these differences in expression might be coadapted with the high abundance of pheromones in Rnh males and the scarcity of pheromones in Rnc males (Brennan and Zufall 2006; Roberts et al. 2010). Specifically, vom2r53, the second most highly expressed VR gene (9-fold higher than the mean VR read count), exhibited higher expression in Rnh than in Rnc and was the only DEG among the top 12 most highly expressed VRs. LOC690507 (similar to vomeromodulin, which is a pheromone transporter) was also more highly expressed in Rnh than in Rnc, which suggests that Rnh rats might exhibit higher sensitivity to pheromone stimuli (Khewgoodall et al. 1991). The volatile compound-detecting receptors (i.e., V1Rs and ORs) are able to receive both pheromones and environmental compounds (Leinders-Zufall et al. 2000; Sam et al. 2001; Boschat et al. 2002; Wang et al. 2006; Yoshikawa et al. 2013; Ibarra-Soria et al. 2017). A large number of OR and V1R genes were upregulated in Rnc females compared with Rnh females, and this finding could be partially explained by their adaptation to different environmental molecules. Coincidently, as revealed by calcium imaging, Rnc females had a smaller percentage of VRNs activated by 3 pheromone analogs (recombinant MUP13 and OBP3 and synthesized 2-heptanone) than Rnc females, which suggests that Rnc rats exhibit reduced vomeronasal sensitivity to pheromones. These results suggest that pheromone reception by females was structurally and functionally weakened to coadapt with the low intensity of MUP pheromones in males of the Rnc subspecies, which is consistent with the signal production–reception coevolution hypothesis (Leinders-Zufall et al. 2000; Sam et al. 2001; Andersson and Simmons 2006; Charlton et al. 2019).
Natural selection, sexual selection, and stochastic processes such as genetic drift and founder events are important evolutionary forces that lead to genetic and phenotypic divergence between populations (Dale et al. 1999; Morganscl et al. 2014). In some cases, sexual selection is stronger than natural selection in driving population divergence, particularly in male secondary sexual characteristics (Svensson et al. 2006). Divergences in pheromones, genetic architecture, and morphology (e.g., body size and fur coloration) have occurred between the brown rat subspecies Rnh and Rnc (Wu 1982; Teng et al. 2017; Yohe et al. 2020; Zhao et al. 2020). Sexual selection is based on genetic variation in mate choice-related traits, and thus, the interaction among genetic variation, mate choice, and male pheromones should be studied further (Andersson and Simmons 2006).
Supplementary Material
Acknowledgments
We are most grateful for Mr Jin-Hua Zhang’s assistance with the behavioral tests. We thank the editors and 3 anonymous reviewers for their constructive comments and suggestions, which helped us to improve the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 31672306 and 32070451 to Y.H.Z. and 31872227 to J.X.Z.] and grants from the Strategic Priority Research Program of the Chinese Academy of Sciences [grant number XDB11010400 to J.X.Z.]. The funders had no role in the design of the study, in the collection, analysis, and interpretation of the data, or in the writing of the manuscript.
Authors’ Contributions
J.X.Z. conceived of the study, participated in the data analysis, coordinated the study, and wrote the manuscript. Y.H. performed the behavioral tests, RNA-seq analysis and wrote the manuscript. L.Z. conducted the SDS–PAGE, IEF, and paternity tests. S.H.F. performed the calcium imaging analysis. Z.S.W. participated in the design of the study and the calcium imaging analysis. All the authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest related to this work.
Data Accessibility
The RNA-seq data are available from the NCBI (BioProject ID: PRJNA591253). The other data generated or analyzed during this study are included in this published article (and its supplementary information files).
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
References
- Andersson M, Simmons LW, 2006. Sexual selection and mate choice. Trends Ecol Evol 21:296–302. [DOI] [PubMed] [Google Scholar]
- Barnett SA, 1958. An analysis of social behaviour in wild rats. Proc Zool Soc Lond 1:107–152. [Google Scholar]
- Berdoy M, Drickamer LC, 2007. Comparative social organization and life history of Rattus and Mus. In: Wolff J, Sherman PW, editors. Rodent Societies: An Ecological and Evolutionary Perspective. Chicago (IL: ): University of Chicago Press, 380–393. [Google Scholar]
- Birkhead T, Møller A, 1993. Female control of paternity. Trends Ecol Evol 8:100–104. [DOI] [PubMed] [Google Scholar]
- Boschat C, Pelofi C, Randin O, Roppolo D, Luscher C. et al. , 2002. Pheromone detection mediated by a V1r vomeronasal receptor. Nat Neurosci 5:1261–1262. [DOI] [PubMed] [Google Scholar]
- Bousquet F, Nojima T, Houot B, Chauvel I, Chaudy S. et al. , 2012. Expression of a desaturase gene, desat1, in neural and nonneural tissues separately affects perception and emission of sex pheromones in Drosophila. Proc Natl Acad Sci U S A 109:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechbühl J, Luyet G, Moine F, Rodriguez I, Broillet MC, 2011. Imaging pheromone sensing in a mouse vomeronasal acute tissue slice preparation. J Vis Exp 58:e3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Zufall F, 2006. Pheromonal communication in vertebrates. Nature 444:308–315. [DOI] [PubMed] [Google Scholar]
- Brooks R, 2000. Negative genetic correlation between male sexual attractiveness and survival. Nature 406:67–70. [DOI] [PubMed] [Google Scholar]
- Candolin U, 2000. Male-male competition ensures honest signaling of male parental ability in the three-spined stickleback Gasterosteus aculeatus. Behav Ecol Sociobiol 49:57–61. [Google Scholar]
- Candolin U, 2003. The use of multiple cues in mate choice. Biol Rev 78:575–595. [DOI] [PubMed] [Google Scholar]
- Charlton BD, Owen MA, Swaisgood RR, 2019. Coevolution of vocal signal characteristics and hearing sensitivity in forest mammals. Nat Commun 10:2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero Rivera A, Andres JA, Cordoba-Aguilar A, Utzeri C, 2004. Postmating sexual selection: allopatric evolution of sperm competition mechanisms and genital morphology in calopterygid damselflies (Insecta: Odonata). Evolution 58:349–359. [DOI] [PubMed] [Google Scholar]
- Costa F, Richardson JL, Dion K, Mariani C, Pertile AC. et al. , 2016. Multiple paternity in the Norway rat Rattus norvegicus from urban slums in Salvador, Brazil. J Hered 107:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato FR, 1988. Effects of male social-status on reproductive success and on behavior in mice Mus musculus. J Comp Psychol 102:146–151. [DOI] [PubMed] [Google Scholar]
- Dale S, Slagsvold T, Lampe HM, Saetre GP, 1999. Population divergence in sexual ornaments: the white forehead patch of Norwegian pied flycatchers is small and unsexy. Evolution 53:1235–1246. [DOI] [PubMed] [Google Scholar]
- Demary KC, Lewis SM, 2007. Male courtship attractiveness and paternity success in Photinus greeni fireflies. Evolution 61:431–439. [DOI] [PubMed] [Google Scholar]
- Fang Q, Zhang YH, Shi YL, Zhang JH, Zhang JX, 2016. Individuality and transgenerational Inheritance of social dominance and sex pheromones in isogenic male mice. J Exp Zool B Mol Dev Evol 326:225–236. [DOI] [PubMed] [Google Scholar]
- Fukamachi S, Kinoshita M, Aizawa K, Oda S, Meyer A. et al. , 2009. Dual control by a single gene of secondary sexual characters and mating preferences in medaka. BMC Biol 7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner K, Wankel B, Gaudszuhn D, 1981. The hierarchy in copulatory competition and its correlation with paternity in grouped male laboratory rats. Z Tierpsychol 56:243–254. [Google Scholar]
- Godin JGJ, Briggs SE, 1996. Female mate choice under predation risk in the guppy. Anim Behav 51:117–130. [Google Scholar]
- Gomez-Baena G, Armstrong SD, Halstead JO, Prescott M, Roberts SA. et al. , 2019. Molecular complexity of the major urinary protein system of the Norway rat Rattus norvegicus . Sci Rep 9:10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Fang Q, Huo Y, Zhang Y, Zhang J, 2015. Social dominance–related major urinary proteins and the regulatory mechanism in mice. Integr Zool 10:543–554. [DOI] [PubMed] [Google Scholar]
- Guo HL, 2016. Interspecific chemical signals and behavioral interactions between rattus Tanezumi and R. norvegicus [master thesis]. [ Beijing, China: ]: University of Chinese Academy of Sciences, Chinese Academy of Sciences. [Google Scholar]
- Guo X, Guo H, Zhao L, Zhang YH, Zhang JX, 2018. Two predominant MUPs, OBP3 and MUP13, are male pheromones in rats. Front Zool 15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiberg AC, Leirs H, Siegismund HR, 2006. Reproductive success of bromadiolone-resistant rats in absence of anticoagulant pressure. Pest Manage Sci 62:862–871. [DOI] [PubMed] [Google Scholar]
- Hosken DJ, Taylor ML, Hoyle K, Higgins S, Wedell N, 2008. Attractive males have greater success in sperm competition. Curr Biol 18:R553–R554. [DOI] [PubMed] [Google Scholar]
- Hurst JL, Beynon RJ, Armstrong SD, Davidson AJ, Roberts SA. et al. , 2017. Molecular heterogeneity in major urinary proteins of Mus musculus subspecies: potential candidates involved in speciation. Sci Rep 7:44992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Soria X, Levitin MO, Saraiva LR, Logan DW, 2014. The olfactory transcriptomes of mice. PLoS Genet 10:e1004593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra-Soria X, Nakahara TS, Lilue JT, Jiang Y, Trimmer C. et al. , 2017. Variation in olfactory neuron repertoires is genetically controlled and environmentally modulated. eLife 6:e21476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H, Kuwahara M, Kikusui T, Tsubone H, 2005. The influence of social environmental condition on the production of stress-induced 22 kHz calls in adult male Wistar rats. Physiol Behav 84:17–22. [DOI] [PubMed] [Google Scholar]
- Janotova K, Stopka P, 2011. The level of major urinary proteins is socially regulated in wild mus musculus musculus. J Chem Ecol 37:647–656. [DOI] [PubMed] [Google Scholar]
- Jemiolo B, Xie TM, Novotny M, 1991. Sociosexual olfactory preference in female mice: attractiveness of synthetic chemosignals. Physiol Behav 50:1119–1122. [DOI] [PubMed] [Google Scholar]
- Johansson BG, Jones TM, 2007. The role of chemical communication in mate choice. Biol Rev 82:265–289. [DOI] [PubMed] [Google Scholar]
- Karn RC, Young JM, Laukaitis CM, 2010. A candidate subspecies discrimination system involving a vomeronasal receptor gene with different alleles fixed in M. m. domesticus and M. m. musculus. Genome Biol 11:P22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl T, Dublon IA, Fischer K, 2015. Young male mating success is associated with sperm number but not with male sex pheromone titres. Front Zool 12:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khewgoodall Y, Grillo M, Getchell ML, Danho W, Getchell TV. et al. , 1991. Vomeromodulin, a putative pheromone transporter: cloning, characterization, and cellular-localization of a novel glycoprotein of lateral nasal gland. FASEB J 5:2976–2982. [DOI] [PubMed] [Google Scholar]
- Kraaijeveld-Smit FJL, Ward SJ, Temple-Smith PD, Paetkau D, 2002. Factors influencing paternity success in Antechinus agilis: last-male sperm precedence, timing of mating and genetic compatibility. J Evol Biol 15:100–107. [Google Scholar]
- Kumar V, Vasudevan A, Soh LJT, Le Min C, Vyas A. et al. , 2014. Sexual attractiveness in male rats is associated with greater concentration of major urinary proteins. Biol Reprod 91:150. [DOI] [PubMed] [Google Scholar]
- Lee W, Khan A, Curley JP, 2017. Major urinary protein levels are associated with social status and context in mouse social hierarchies. Proc Biol Sci 284:20171570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Lane AP, Puche AC, Ma WD, Novotny MV. et al. , 2000. Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature 405:792–796. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang Y, Wang P, Guo X, Wu Y. et al. , 2019. Two preputial gland-secreted pheromones evoke sexually dimorphic neural pathways in the mouse vomeronasal system. Front Cell Neurosci 13:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loire E, Tusso S, Caminade P, Severac D, Boursot P. et al. , 2017. Do changes in gene expression contribute to sexual isolation and reinforcement in the house mouse? Mol Ecol 26:5189–5202. [DOI] [PubMed] [Google Scholar]
- Lorch PD, 2002. Understanding reversals in the relative strength of sexual selection on males and females: a role for sperm competition? Am Nat 159:645–657. [DOI] [PubMed] [Google Scholar]
- Lymbery RA, Kennington WJ, Evans JP, 2018. Multivariate sexual selection on ejaculate traits under sperm competition. Am Nat 192:94–104. [DOI] [PubMed] [Google Scholar]
- Møller AP, Barbosa A, Cuervo JJ, de Lope F, Merino S. et al. 1998. Sexual selection and tail streamers in the barn swallow. Proc R Soc B 265:409–414. [Google Scholar]
- Møller AP, Ninni P, 1998. Sperm competition and sexual selection: a meta-analysis of paternity studies of birds. Behav Ecol Sociobiol 43:345–358. [Google Scholar]
- Morganscl CookeGM, OrdTJ, . 2014. How populations differentiate despite gene flow: sexual and natural selection drive phenotypic divergence within a land fish, the Pacific leaping blenny. BMC Evol Biol 14. DOI: 10.1186/1471-2148-14-97 24884492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank JE, 2009. The evolution of heterochiasmy: the role of sexual selection and sperm competition in determining sex-specific recombination rates in eutherian mammals. Genet Res (Camb) 91:355–363. [DOI] [PubMed] [Google Scholar]
- Marcondes F, Bianchi F, Tanno A, 2002. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614. [DOI] [PubMed] [Google Scholar]
- Meagher S, Penn DJ, Potts WK, 2000. Male-male competition magnifies inbreeding depression in wild house mice. Proc Natl Acad Sci U S A 97:3324–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucignat-Caretta C, Redaelli M, Orsetti A, Perriat-Sanguinet M, Zagotto G. et al. , 2010. Urinary volatile molecules vary in males of the 2 European subspecies of the house mouse and their hybrids. Chem Sens 35:647–654. [DOI] [PubMed] [Google Scholar]
- Musser GG, Carleton MD, 2005. Mammal species of the world a taxonomic and geographic reference. In: Don EW, Dee Ann R editors. Mammal Species of the World: A Taxonomic and Geographic Reference. Baltimore (MD: ): Johns Hopkins University Press, 894–1531. [Google Scholar]
- Nelson AC, Cunningham CB, Ruff JS, Potts WK, 2015. Protein pheromone expression levels predict and respond to the formation of social dominance networks. J Evol Biol 28:1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SH, Shankar S, Shikichi Y, Akasaka K, Mori K. et al. , 2014. Pheromone evolution and sexual behavior in Drosophila are shaped by male sensory exploitation of other males. Proc Natl Acad Sci U S A 111:3056–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohorecky LA, Blakley GG, Ma EW, Soini HA, Wiesler D. et al. , 2008. Social housing influences the composition of volatile compounds in the preputial glands of male rats. Horm Behav 53:536–545. [DOI] [PubMed] [Google Scholar]
- Qvarnstrom A, Forsgren E, 1998. Should females prefer dominant males? Trends Ecol Evol 13:498–501. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH. et al. , 2010. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male's odour. BMC Biol 8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SC, Gosling LM, 2003. Genetic similarity and quality interact in mate choice decisions by female mice. Nat Genet 35:103–106. [DOI] [PubMed] [Google Scholar]
- Sam M, Vora S, Malnic B, Ma WD, Novotny MV. et al. , 2001. Neuropharmacology: odorants may arouse instinctive behaviours. Nature 412:142. [DOI] [PubMed] [Google Scholar]
- Searcy WA, Nowicki S, 2005. The Evolution of Animal Communication: Reliability and Deception in Signaling Systems. Princeton (NJ): Princeton University Press. [Google Scholar]
- Seike T, Nakamura T, Shimoda C, 2015. Molecular coevolution of a sex pheromone and its receptor triggers reproductive isolation in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A 112:4405–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan MJ, Campbell P, Miller CH, 2019. Evolutionary patterns of major urinary protein scent signals in house mice and relatives. Mol Ecol 28:3587–3601. [DOI] [PubMed] [Google Scholar]
- Smadja C, Ganem G, 2002. Subspecies recognition in the house mouse: a study of two populations from the border of a hybrid zone. Behav Ecol 13:312–320. [Google Scholar]
- Svensson EI, EroukhmanoffF, FribergM, . 2006. Effects of natural and sexual selection on adaptive population divergence and premating isolation in a damselfly. Evolution 60: 1242–1253. [PubMed] [Google Scholar]
- Taylor ML, Wedell N, Hosken DJ, 2007. The heritability of attractiveness. Curr Biol 17:R959–R960. [DOI] [PubMed] [Google Scholar]
- Teeter KC, Payseur BA, Harris LW, Bakewell MA, Thibodeau LM. et al. , 2008. Genome-wide patterns of gene flow across a house mouse hybrid zone. Genome Res 18:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HJ, Zhang YH, Shi CM, Mao FB, Cai WS. et al. , 2017. Population genomics reveals speciation and introgression between brown Norway rats and their sibling species. Mol Biol Evol 34:2214–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoss M, Luzynski KC, Enk VM, Razzazi–Fazeli E, Kwak J. et al. , 2019. Regulation of volatile and non-volatile pheromone attractants depends upon male social status. Sci Rep 9:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinghitella RM, Lackey ACR, Martin M, Dijkstra PD, Drury JP. et al. , 2018. On the role of male competition in speciation: a review and research agenda. Behav Ecol 29:783–797. [Google Scholar]
- Wang F, Zhu J, Zhu H, Zhang Q, Lin ZM. et al. , 2011. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science 334:693–697. [DOI] [PubMed] [Google Scholar]
- Wang YX, 2003. A Complete Checklists of Mammal Species and Subspecies in China. Beijing, China: China Forestry Publishing House. [Google Scholar]
- Wang ZS, Sindreu CB, Li V, Nudelman A, Chan GCK. et al. , 2006. Pheromone detection in male mice depends on signaling through the type 3 adenylyl cyclase in the main olfactory epithelium. J Neurosci 26:7375–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver RJ, Koch RE, Hill GE, 2017. What maintains signal honesty in animal colour displays used in mate choice? Phil Trans R Soc BPhil Trans R Soc B 372: 2016.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E, Galbany J, McFarlin SC, Ndayishimiye E, Stoinski TS. et al. , 2019. Male body size, dominance rank and strategic use of aggression in a group–living mammal. Anim Behav 151:87–102. [Google Scholar]
- Wu DL, 1982. Subspecies of the brown rat Rattus norvegicus Berkenhout in China. Acta Theriol Sin 2:107–112. [Google Scholar]
- Yohe LRFabbri MHanson M, Bhullar B-AS, 2020. Olfactory receptor gene evolution is unusually rapid across Tetrapoda and outpaces chemosensory phenotypic change. Curr Zool zoaa051. DOI: 010.1093/cz/zoaa1051 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K, Nakagawa H, Mori N, Watanabe H, Touhara K, 2013. An unsaturated aliphatic alcohol as a natural ligand for a mouse odorant receptor. Nat Chem Biol 9:160–162. [DOI] [PubMed] [Google Scholar]
- Zahavi A, 1977. The cost of honesty (further remarks on the handicap principle). J Theor Biol 67:603–605. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Zhang ZB, Wang ZW, 2001. Seasonal changes in and effects of familiarity on agonistic behaviors of rat-like hamsters Cricetulus triton. Ecol Res 16:309–317. [Google Scholar]
- Zhang YH, Tang MM, Guo X, Gao XR, Zhang JH. et al. , 2019a. Associative learning is necessary for airborne pheromones to activate sexual arousal–linked brain areas of female rats. Behav Ecol Sociobiol 73:75. [Google Scholar]
- Zhang YH, Zhang JX, 2014. A male pheromone-mediated trade-off between female preferences for genetic compatibility and sexual attractiveness in rats. Front Zool 11:73. [Google Scholar]
- Zhang YH, Zhao L, Guo X, Zhang JH, Zhang JX, 2019b. Sex pheromone levels are associated with paternity rate in brown rats. Behav Ecol Sociobiol 73:15. [Google Scholar]
- Zhao L, Zhang JX, Zhang YH, 2020. Genetic boundary and gene flow between 2 parapatric subspecies of brown rats. Curr Zool zoaa027. DOI: 10.1093/cz/zoaa027 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook JM, Adams DB, 1975. Competitive fighting in the rat. J Comp Physiol Psychol 88:418–423. [DOI] [PubMed] [Google Scholar]
- Zufall F, Leinders-Zufall T, 2007. Mammalian pheromone sensing. Curr Opin Neurobiol 17:483–489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data are available from the NCBI (BioProject ID: PRJNA591253). The other data generated or analyzed during this study are included in this published article (and its supplementary information files).