Abstract
Objective
Increased mortality from respiratory diseases was observed in epidemiological studies of patients with ulcerative colitis [UC] as a potentially underestimated extraintestinal manifestation. We therefore investigated the presence of pulmonary manifestations of inflammatory bowel disease [IBD] and the potential effect of tumour necrosis factor alpha [TNF-α] inhibitors on pulmonary function tests [PFT] in a prospective, longitudinal study.
Methods
In all, 92 consecutive patients with IBD (49 Crohn´s disease [CD], 43 UC) and 20 healthy controls were recruited. Fifty patients with IBD were in remission, and 42 had active disease with 22 of these being examined before and 6 weeks after initiating anti-TNF therapy. Pulmonary function tests [PFT] were evaluated using the Medical Research Council [MRC] dyspnoea index and a standardized body plethysmography. IBD activity was assessed using Harvey‐Bradshaw index for CD and partial Mayo score for UC. Data are presented as mean ± standard error of the mean [SEM].
Results
Patients with active IBD showed significant reduction of PFT. Forced expiration [Tiffeneau index] values [FEV1%] were significantly reduced in IBD patients with active disease [78.8 ± 1.1] compared with remission [86.1 ± 0.9; p = 0.0002] and with controls [87.3 ± 1.3; p = 0.001]. Treatment with anti-TNF induced a significant relief in obstruction [p = 0.003 for FEV1% in comparison with baseline levels]. The level of pulmonary obstruction significantly correlated with clinical inflammation scores [HBI or Mayo].
Conclusions: Patients
with active IBD present with significant obstructive abnormalities in their PFTs. Obstruction is related to inflammatory activity, with anti-TNF improving PFTs. Pulmonary obstruction and possibly chronic bronchopulmonary inflammation is an overlooked problem in active IBD that is probably obscured by intestinal symptoms.
Keywords: IBD, extraintestinal manifestation, biological therapy
What is known?
Extraintestinal manifestations are accepted complications in IBD patients, caused by systemic immune activation.
Increased mortality from respiratory diseases was observed in epidemiological studies of patients with ulcerative colitis [UC].
Pulmonary involvement may be underestimated in daily practice.
What is new here?
High numbers of patients with active IBD exhibit mild symptoms of respiratory impairment.
Patients with active IBD showed significant reduction of pulmonary function tests compared with IBD in remission and with healthy controls.
Treatment with anti-TNF induced a significant relief in obstruction.
1. Introduction
Inflammatory bowel disease [IBD]comprises two main subentities, Crohn’s disease [CD] and ulcerative colitis [UC], among some rarer forms including microscopic colitis, collagenous colitis, and indeterminate colitis. IBD is seen as a chronic inflammatory, systemic disorder that not only directly affects the gastrointestinal tract but also is associated with various extraintestinal manifestations [EIM] that can involve nearly any organ. The rate of EIM in patients with IBD varies from 6% to 47% and seems to increase with the duration of the intestinal disease.1 Vice versa, the majority of patients [>90%] with EIM shows significant intestinal inflammation, though EIM can precede the onset of the intestinal symptoms. The most common manifestations affect skin, joints, eyes, and the liver/biliary tract. Other manifestations have been described in the renal and respiratory systems.
It is debated whether EIM are always true reflections of the pathophysiology of IBD or whether they represent non-specific signs of general immune activation.2,3 Various pulmonary affections have been reported in patients with IBD, including small and large airway dysfunction as well as obstructive and interstitial lung disorders, comprising bronchial hyper-reagibility, bronchitis and bronchiectasis, inflammatory tracheal stenosis, and interstitial pneumonitis, as well as bronchiolitis obliterans organising pneumonia [BOOP].4 In 30–60% of patients with CD, lung involvement occurs on a subclinical, non-apparent level. Wallaert et al. revealed a lymphocyte alveolitis in approximately 50% of CD patients by bronchoalveolar lavage, without any clinically significant respiratory symptoms.5 Furthermore, abnormal pulmonary function tests [PFT] have been reported in 42% of IBD patients again without any respiratory symptoms, compared with an incidence of only 3% in asymptomatic healthy controls.6
The clinical relevance of pulmonary manifestation appears to be low. Severe focal inflammatory complications affecting the lungs, trachea, or the pleural serosa are rare and are usually linked to uncontrolled gastrointestinal inflammation. However, population-based cohort studies showed an increased mortality from respiratory disorders in patients with IBD.7–9 Patients with UC in a Swedish cohort showed an increased standardised mortality ratio of 1.5 due to obstructive respiratory diseases, especially bronchitis, emphysema, and asthma.10 Pathophysiological concepts for pulmonary involvement in IBD are limited11 and are only based on theoretical considerations. Since both the lungs and the gastrointestinal tract embryologically arise from the primitive gut, it was suggested that the inflammatory response to the gastrointestinal mucosa may cross-react with the lung surface.12,13 Furthermore, chronic intestinal inflammation is associated with an impairment of barrier function that could allow the entrance of antigens and bacteria into the organism that potentially activate immune response on a systemic level and especially the lungs.14 A pulmonary affection may also reflect the overlap between the genetic architecture of IBD and various chronic inflammatory lung diseases.15,16
Although there is observation of pulmonary pathology in patients with IBD, little is known about its frequency in systematic studies and its relationship to disease activity and specific biologic therapy. Therefore, the aim of this prospective study was to assess pulmonary function abnormalities in IBD patients in comparison with healthy controls and investigate the effect of a potent anti-inflammatory therapy (ie, use of tumour necrosis factor alpha [TNF-α] inhibitors) on pulmonary function.
In summary:
2. Methods
2.1. Patient selection criteria, clinical assessments, and management
Between May 2016 and August 2017, 92 consecutive patients with IBD were included. Patients and subjects were recruited at our IBD outpatient centre at the University Medical Center Schleswig Holstein, Campus Kiel, Kiel, Germany. The study was conducted as a prospective, non-interventional observational experiment. Ethical permission was granted prior to study conduct by the Ethics Committee of the Medical Faculty of the University of Schleswig-Holstein, Campus Kiel [reference number: A104/13]. The study was registered at ClinTrials.gov [NCT02694588]. Prior written informed consent for the inclusion into the study was obtained from all patients and control subjects.
Patients between 18 and 75 years of age with active IBD as well as those with IBD in remission were included in this study. Disease activity was assessed using the Harvey‐BradshawIndex [HBI] for CD17 and the partial Mayo [pMayo] score for UC patients.18,19 Clinical remission was defined as HBI index score <5 or a pMayo score <3 [with no subscore larger than 1], respectively. Volunteers, who were recruited from the health care personnel, served as healthy controls.
A detailed medical history was recorded in all patients and healthy controls (ie, age, sex, body mass index [BMI], duration of disease, present and past extraintestinal manifestations, medication, smoking and drinking habits).
A total of 22 patients with active IBD were recruited before initiation of anti-TNF-α therapy. These patients were assessed at baseline and after 6 weeks of treatment. Anti-TNF-α therapy was provided and initiated by an independent gastroenterologist at our IBD outpatient department. Indication for and treatment with anti-TNF was purely based on clinical indication and was not part of the study protocol.
Either adalimumab [ADA] [Humira®; Abbvie, Maidenhead, UK] or infliximab [IFX] [Remicade®; MSD Sharp Dome GmbH, Haar, Germany] was used based on clinical decision and according to the manufacturer`s specifications. Selection of the medication was at the discretion of the gastroenterologist in charge of the patient.
If concurrent corticosteroid therapy was used by the patient, only those were asked to participate who were on a stable dosage [prednisone <20 mg/day for at least 40 days; budesonide 9 mg/day] before initiation of biologic therapy. Steroid doses remained stable throughout the study period to avoid potential improvement in underlying asthma-type lung inflammation. Concurrent oral immunomodulators also remained constant during the anti-TNF-α treatment. In addition, recruited patients in remission under anti-TNF-α treatment had a stable dosage of the respective medication for at least 14 weeks.
Patients were selected to participate in this study using the following inclusion and exclusion criteria. Inclusion criteria were: histologically confirmed IBD, either active or in remission, written informed consent, ASA [American Society of Anaesthesiology] classification I-II, and stable or no medication with glucocorticoids as described above. Exclusion criteria were: known and/or clinically significant underlying chronic lung disease, ie, chronic obstructive lung disease, allergic asthma, lung cancer, history of or ongoing nicotine consumption/smoking, history of lung surgery, use of inhaled corticosteroids or anticholinergic or sympathomimetic drugs, systemic treatment with beta blockers or non-steroidal anti-inflammatory drugs [NSAID], previous anti-TNF-α treatment in active IBD, no stable dosage of anti-TNF-α treatment in IBD patients in remission [<14 weeks].
2.2. Pulmonary function tests
Patients underwent an assessment before initiation of therapy and 6 weeks thereafter. Pulmonary function was evaluated using the Medical Research Council [MRC] dyspnoea score and a standardized body plethysmography. The MRC is a five-graded clinical symptom score that provides a simple and valid method of categorising patients in terms of their disability due to pulmonary obstruction: Grade 0: no dyspnoea, not troubled by breathlessness except with strenuous exercise; Grade 1: slight dyspnoea, troubled by shortness of breath when hurrying on a level surface or walking up a slight hill; Grade 2: moderate dyspnoea, walk slower than normal based on age on a level surface due to breathlessness or has to stop for breath when walking on level surface at own pace; Grade 3: severe dyspnoea, stops for breath after walking 100 yards or after a few minutes on level surface; Grade 4: very severe dyspnoea, too breathless to leave the house or becoming breathless while dressing or undressing.20
2.3. Body plethysmography
Body plethysmography examinations [Master Screen Body, Vyaire Medical Inc., Mettawa, IL, USA] started with determining the lung function while breathing at rest, followed by the Shutter manoeuvre and continued with spirometric measurements. Assessment of the expiratory reserve volume [ERV] and inspiratory vital capacity [IVC] was performed after opening the shutter, by which total lung capacity [TLC] and residual volume [RV] were calculated. Thereafter, prolonged forced expiration was carried out with measurement of inspiratory vital capacity [VC], forced vital capacity [FVC], forced expiratory volume in one second [FEV1], maximal expiratory flow at 25%, 50%, and 75% [MEF25-75]. By these means, information on obstructive and restrictive abnormalities of the lung were obtained in one sequence of subsequent measurements.21 All data were calculated as percentages of the predicted values adjusted for sex, age, height, and weight.
2.4. Blood tests
PFT was scheduled in concordance with a venous blood sample being drawn for routine clinical assessment [full blood count, C-reactive protein]. Blood was drawn from volunteer controls too, and laboratory reports were supplied to them.
2.5. Endpoints and sample size calculation
The aim of this prospective study was to evaluate and quantify potential abnormal pulmonary function in patients with active IBD and with IBD in remission, and to obtain information about potential changes of clinical/subclinical pulmonary involvement in IBD patients undergoing anti-TNF-α therapy.
Obstructive lung disease is indicated by an impaired FEV1 <70% of the age- and weight-adjusted normal range according to the Global Initiative for Chronic Obstructive Lung Disease [GOLD].22 Therefore, an FEV1 <70% was defined as the primary endpoint. Significance level α was set at 5% and power was set at 90%. Given a dropout rate of 15%, at least 23 patients with active IBD had to be investigated to allow sufficient power of the study. Sample size was calculated using G*Power calculation software, version 3.1.9.2 [http://gpower.hhu.de]. Secondary parameters of analysis included peripheral airway obstruction [MEF 75‐25 %], changes in PFT, and clinical parameters of dyspnoea.
2.6. Statistical analysis
Statistical analysis was performed using the Mann‐Whitney-test for unpaired, non-parametric comparison or a contingency table with calculation of Fisher`s exact test, as appropriate. Intra-individual changes of the PFT during the course of therapy were compared using Wilcoxon matched-pair tests. A p-value of <0.05 was considered statistically significant. Statististical analyses were performed using SPSS 14 [IBM, Armon, NY, USA] and GraphPad Prism 6.0 [GraphPad, La Jolla, CA, USA]. Data on patient characteristics are presented as mean ± standard error of the mean [SEM] unless otherwise stated. Data of the MRC dyspnoea score and the spirometric data are presented as median/25th percentile/75th percentile [median/25/75].
3. Results
3.1. Patient characteristics
A total of 92 IBD patients, 49 with CD and 43, with UC were recruited into this study. Among the IBD patients, 50 were in remission and 42 presented with active disease; 22 IBD patients were scheduled for anti-TNF-α therapy. Age and sex distributions were not significantly different between the respective patient groups and healthy controls. Detailed patient characteristics are summarised in Table 1.
Table 1.
Patient characteristics.
| IBD total N = 92 | CD N = 49 | UC N = 43 | HC N = 20 | ||||
|---|---|---|---|---|---|---|---|
| Remission | Active | Remission | Active | Remisson | Active | ||
| N [%] | 50 | 42 | 26 | 23 | 24 | 19 | 20 |
| Age [years], mean ± SEM | 44.78 ± 1.7 | 49.4 ± 2.4 | 44.15 ± 2.5 | 47.65 ± 3.1 | 45.46 ± 2.2 | 51.53 ± 3.8 | 44.25 ± 2.9 |
| IBD activity score, median/25th percentile/75th percentile | - | - | 2/0/3 | 6/5/9 | 1/0/2 | 5/4/6 | - |
| C-reactive protein [mg/L], mean ± SEM | 4.9 ± 1.0 | 9.5 ± 1.7 | 5.3 ± 1.5 | 13.1 ± 2.8 | 3.4 ± 1.2 | 5.2 ± 0.9 | 1.2 ± 0.2 |
| BMI [kg/m2], mean ± SEM | 24.9 ± 0.5 | 25.6 ± 0.7 | 24.5 ± 0.7 | 25.2 ± 1.0 | 25.4 ± 0.6 | 26.0 ± 1.0 | 25.3 ± 0.6 |
| Smoking, N | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Known EIM of IBD, N [%] | 5 [10] | 13 [31] | 5 [19] | 5 [22] | 0 [0] | 8 [42] | - |
| Concomitant medication, N [%] | |||||||
| 5-ASA | 19 [38] | 14 [33] | 2 [8] | 2 [9] | 14 [58] | 12 [63] | - |
| Steroids | 8 [16] | 14 [33] | 6 [23] | 6 [26] | 2 [8] | 8 [42] | - |
| Budesonide | 7 [14] | 3 [7] | 1 [4] | 3 [13] | 6 [25] | 0 [0] | - |
| Azathioprine | 2 [4] | 6 [14] | 1 [4] | 2 [9] | 1 [4] | 4 [21] | - |
| Anti-TNF-α | 21 [42] | 0 [0] | 11 [42] | 0 [0] | 10 [42] | 0 [0] | - |
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; HC, healthy controls; SEM, standard error of the mean; BMI, body mass index; EIM, extraintestinal manifestations; 5-ASA, 5-aminosalicylic acid; TNF, tumour necrosis factor.
EIMs were observed in 31% [N = 13] of patients with active IBD and in 10% [N = 5] of patients with IBD in remission. Of note, 19% of patients with CD in clinical remission presented with EIM in contrast to none of the respective UC patients. The EIM manifestations in active UC comprised asymmetrical arthralgia [N= 6], erythema nodosum [N = 2], and iritis [N = 1]. The patient with iritis had accompanying arthralgia as a second EIM. In CD, five patients in remission and another five patients with active disease presented with arthralgia.
3.2. Medical Research Council dyspnoea score
Of patients with active IBD, 43% [18/42] had a positive MRC dyspnoea score [grade 1 or 2], whereas only 20% [10/50] of IBD patients in remission displayed a similar degree of clinical symptoms [healthy volunteers 0%] (Table 2). Higher MRC dyspnoea scores [3 or 4] were not recorded. Fisher`s exact test revealed a significantly higher rate of a positive MRC dyspnoea score in patients with active IBD compared with IBD in remission [p = 0.02]. In the CD population, a positive symptom score [grade 1 or 2] was found in 43% of patients [10/23] with active disease and in 15% [4/26] of patients in remission. This trend failed to reach statistical significance [p = 0.055] due to low sample size. In patients with UC, pulmonary symptoms were detected in 42% [8/19] of active patients and in 25% [6/24] of cases in remisson [p >0.05].
Table 2.
Medical research council dyspnoea score20 [* indicates significant differences between the groups].
| IBD | Crohn`s disease | Ulcerative colitis | ||||
|---|---|---|---|---|---|---|
| Active | Remission | Active | Remission | Active | Remission | |
| Positive score [>0]; N [%] | 18 [43]* | 10 [20]* | 10 [43] | 4 [15] | 8 [42] | 6 [25] |
| Negative score [= 0]; N [%] | 24 [57] | 40 [80] | 13 [57] | 22 [85] | 11 [58] | 18 [75] |
| Total | 42 | 50 | 23 | 26 | 19 | 24 |
IBD,inflammatory bowel disease.
3.3. Pulmonary function tests [PFTs]
Patients with active IBD showed significantly reduced respiratory function. Central or peripheral airway obstruction were observed in 31.9% of patients with active disease, in 6.9% of patients in remission, and in 5% of controls [p = 0.006 for comparison of active IBD vs IBD in remission]. Restrictive changes of pulmonary function tests were seen in 2.1% of patients with active disease, in 6.9% of patients in remission, and in 0% of controls [p >0.05 for comparison of active IBD vs IBD in remission].
Patients with active IBD exhibited a mean FEV1% level of 78.8 ± 1.1 compared with 86.1 ± 0.9 in IBD patients in remission [p = 0.0002]. The respective parameters of patients with active CD vs CD in remission [p = 0.009], and active UC vs UC in remission [p = 0.02], changed accordingly [Table 3]. FEV1% in patients with IBD in remission was comparable to healthy controls [FEV1% healthy controls = 87.3 ± 1.3; p = 0.6].
Table 3.
Comparison of pulmonary function tests [* indicates significant differences for the comparison between active IBD and IBD in remission.; + indicates significant differences for the comparison between active Crohn’s disease and Crohn’s disease in remission; # indicates significant differences for the comparison between active ulcerative colitis and ulcerative colitis in remission].
| IBD | Crohn`s disease | Ulcerative colitis | Controls | ||||
|---|---|---|---|---|---|---|---|
| Active | Remission | Active | Remission | Active | Remission | ||
| FEV1% [mean ± SEM] | 78.8 ± 1.1* | 86.1 ± 0.9* | 78.3 ± 1.6+ | 85.8 ± 1.3+ | 79.40 ± 1.6# | 86.5 ± 1.2# | 87.3 ± 1.3 |
| MEF75 [mean ± SEM] | 89.2 ± 3.4* | 104.3 ± 2.4* | 87.2 ± 4.8+ | 103.3 ± 3.2+ | 91.6 ± 4.9# | 105.3 ± 3.6# | 103.1 ± 3.8 |
| MEF50 [mean ± SEM] | 80.3 ± 4.3* | 99.9 ± 3.2* | 78.5 ± 6.0+ | 97.9 ± 4.0+ | 82.5 ± 6.4# | 102.0 ± 5.2# | 94.5 ± 4.0 |
| MEF25 [mean ± SEM] | 72.4 ± 4.3* | 93.8 ± 4.8* | 68.7 ± 6.0+ | 93.1 ± 6.6+ | 77.0 ± 6.2# | 94.7 ± 7.1# | 85.5 ± 4.5 |
| VC [mean ± SEM] | 92.4 ± 2.3 | 95.6 ± 1.8 | 91.0 ± 3.2 | 91.7 ± 2.3 | 94.1 ± 3.3 | 99.7 ± 2.5 | 104.7 ± 2.5 |
| TLC [mean ± SEM] | 99.6 ± 3.2 | 95.4 ± 2.2 | 101.8 ± 4.6 | 93.1 ± 3.5 | 96.9 ± 4.3 | 97.8 ± 2.7 | 102.4 ± 3.5 |
| RV [mean ± SEM] | 102.2 ± 8.5* | 88.0 ± 6.0* | 112.0 ± 14.1+ | 83.8 ± 9.9+ | 90.5 ± 7.4 | 92.5 ± 6.7 | 102.1 ± 8.0 |
SEM, standard error of the mean.
Parameters of peripheral airway obstruction [MEF 75-25] showed impaired values in active disease but not in remission [MEF75: IBDactive vs controls, p = 0.01; IBDactive vs IBDremission, p = 0.002; IBDremission vs controls, p >0.05] [Table 3].
Despite an overall reduction in vital capacity [VC] in IBD patients [VC IBDactive= 92.4 ± 2.3%; VC IBDremission= 95.6 ± 1.8%] compared with healthy controls [VCcontrols= 104.7 ± 2.5%; p = 0.02] no influence of disease activity was observed [active vs remission]. Similar observations were made for total lung capacity [TLC] in IBD patients [TLC IBD active and remission, p = 0.4], whereas TLC levels were significantly reduced in IBD patients compared with healthy controls [p = 0.005]. Residual volume [RV] was significantly impaired in IBD patients compared with healthy controls [p = 0.02]. Furthermore, a significantly lowered RV was observed in IBD patients in remission compared with active IBD. This difference was caused by the CD subgroup [RV CDactive= 112.0 ± 14.1%; RV CDremission= 83.8 ± 14.1%; p = 0.03], whereas the UC group exhibited no significant changes of the RV [RV UCactive= 90.5 ± 7.4%; RV UCremission= 92.5 ± 6.7%; p >0.05]. Detailed results of pulmonary functions tests are summarised in Table 3.
3.4. PFTs and correlation with disease activity
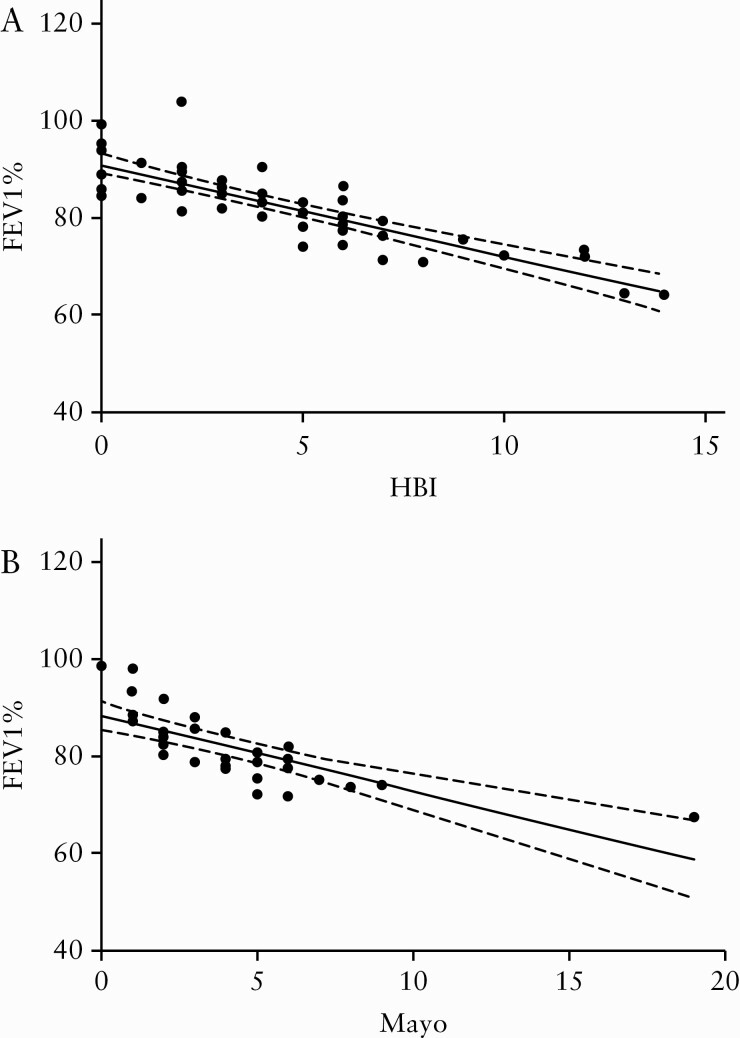
The clear association between abnormalities in PFT and disease activity [ie, remission versus active disease] prompted us to conduct correlation analyses using HBI and pMayo as comparators. Correlation analyses of PFT [FEV1% as an example] with HBI as well as Mayo score expressed a significant correlation between the level of inflammation and pulmonary obstruction [HBI vs FEV1%: r = -0.84, p <0.0001; Mayo vs FEV1%: r = -0.85, p <0.0001] [Figure 1].
Figure 1.
Correlation analysis of inflammation scores (HBI [A] and Mayo[B] vs FEV1%). Statistical analyses were carried by simple linear regression analysis. Solid lines indicate the best-fit regression, dashed lines indicate 95% confidence bands of the best-fit line.
3.5. Response of PFTs to anti-TNF-α therapy
Given the correlation between disease activity and impaired pulmonary function in IBD, we next investigated whether immunosuppressive therapy with anti-TNF-α antibodies can restore pulmonary function. At baseline, IBD patients scheduled for anti-TNF-α presented with an FEV1% level of 78.5 ± 1.8%. After 6 weeks of anti-TNF-α treatment, FEV1% improved significantly to 86.3 ± 1.9% [p <0.0001]. This significant improvement was also observed in the CD and UC subgroup analysis [CDbaseline= 77.0 ± 2.2% vs CD6weeks= 86.0 ± 3.0%; p = 0.002; UCbaseline= 80.2 ± 2.8% vs UCbaseline= 86.8 ± 2.6%; p = 0.009]. Parameters of peripheral airway obstruction changed accordingly. Further details are listed in Table 4.
Table 4.
Comparison of pulmonary function tests before and after 6 weeks of anti-TNF-alpha therapy [* indicates significant differences for the comparison between active IBD and IBD in remission.; + indicates significant differences for the comparison between active Crohn’s disease and Crohn’s disease in remission; # indicates significant differences for the comparison between active ulcerative colitis and ulcerative colitis in remission].
| IBD N = 22 | Crohn`s disease N = 12 | Ulcerative colitis N = 10 | ||||
|---|---|---|---|---|---|---|
| Baseline | TNF-α 6 weeks | Baseline | TNF-α 6 weeks | Baseline | TNF-α 6 weeks | |
| FEV1% [mean ± SEM] | 78.5 ± 1.8* | 86.3 ± 1.9* | 77.0 ± 2.2+ | 86.0 ± 3.0+ | 80.2 ± 2.8# | 86.8 ± 2.6# |
| MEF75 [mean ± SEM] | 87.2 ± 4.4* | 96.4 ± 4.6* | 83.5 ± 6.7+ | 97.0 ± 7.7+ | 91.3 ± 5.7# | 95.9 ± 5.1# |
| MEF50 [mean ± SEM] | 77.0 ± 6.7* | 93.2 ± 7.3* | 74.3 ± 10.3+ | 90.6 ± 11.5+ | 80.0 ± 8.8# | 96.1 ± 9.3# |
| MEF25 [mean ± SEM] | 73.5 ± 7.0* | 93.7 ± 8.8* | 66.7 ± 9.5+ | 90.0 ± 13.8+ | 81.0 ± 10.3# | 97.7 ± 11.0# |
| VC [mean ± SEM] | 88.2 ± 2.7 | 93.2 ± 3.2 | 86.8 ± 3.8 | 93.5 ± 5.2 | 89.9 ± 4.0 | 92.8 ± 3.8 |
| TLC [mean ± SEM] | 103.7 ± 5.7 | 98.1 ± 3.7 | 110.7 ± 8.3 | 100.8 ± 5.1 | 96.0 ± 7.2 | 95.2 ± 5.5 |
| RV [mean ± SEM] | 117.1 ± 15.4 | 104.3 ± 10.6 | 143.9 ± 24.8 | 117.7 ± 17.6 | 87.7 ± 13.1 | 89.5 ± 10.0 |
IBD, inflammatory bowel disease; TNF, tumour necrosis factor.
4. Discussion
The aim of this study was to evaluate clinical and subclinical pulmonary involvement in patients with active IBD and with IBD in remission in comparison with healthy controls. We further aimed to assess the effects of TNF-α inhibitors on pulmonary function.
A significant proportion of patients with active IBD displayed mild symptoms of respiratory dysfunction, as evidenced by low-grade MRC score abnormalities in 41.8% of patients with active IBD compared with 17% of those in remission. The majority of active patients showed significantly impaired PFT, indicating obstructive abnormalities in comparison with patients with IBD in remission and healthy controls.+
This observation is in agreement with previous observations. Godet et al. showed obstructive abnormalities in 23% of UC patients, and Mohamed-Hussein et al. recorded small airway obstruction in 58% of active UC patients.23,24 Furthermore, obstructive dysfunction and small airway obstruction was described by Yilmaz et al. in 51% of the IBD patients, with significant correlation to the activity of the underlying disease.25 These data are in line with our results and with a strong correlation between disease activity and the level of PFT impairment in UC as well as in CD patients. Of note, according to current guidelines of the German and European respiratory societies, no relevant changes of the VC are expected in mild or moderate bronchopulmonary obstruction; only the overall time to release the lung volume is mildly prolonged.26,27 Since our recruited IBD patients only exhibited mild and moderate changes of FEV1% and/or MEF, this could explain the finding that the VC did not change regardless of disease activity in IBD patients compared with IBD in remission. Nevertheless, in IBD patients undergoing anti-TNF-α treatment a significant improvement of VC was observed.
In the majority of published studies, pulmonary manifestation of IBD is restricted to subclinical changes of PFT parameters and only a small proportion of patients complain of clinically relevant pulmonary symptoms as potential EIM.28–31 However, pulmonary obstruction and chronic bronchopulmonary inflammation may be clinically underestimated due to reduced physical activity in active disease and may be overlooked. Ekbom et al. demonstrated in their population-based cohort study that the mortality of chronic obstructive pulmonary disease [COPD] patients developing CD is 2.72 times higher compared with healthy controls and even greater than the risk reported for smoking as a single risk factor.9 Conversely, COPD seems to be a relevant mortality factor in CD patients, with standardised mortality ratios ranging from 2.5 to 3.5,7,32 as well as in UC with an increased odds ratio of 1.5 (95% confidence interval [CI] = 1.1–2.2).9,10 The hypothesis of interorganic dependence is strongly supported by our current correlation analysis between the level of inflammation, as expressed by the respective inflammatory scores [HBI and Mayo], and the level of PFT impairment. Both analyses showed a highly significant correlation with a p-value of <0.0001 each.
Based on the available data, pulmonary dysfunction may be overlooked in IBD. The majority of IBD patients will present with subclinical pulmonary involvement [with respect to their level of physical activity]. However, taking the increased mortality rates into account, regular screening PFT assessment may be a relevant addition to the care of patients with complex forms of IBD.
In clinical practice one should carefully consider whether potential abnormalities are based on the underlying IBD or are induced by the biologic therapy itself, ie, as drug treatment intolerances or opportunistic infections caused by the immunosuppressive agents.33
Despite the obvious epidemiological coincidence, the pathophysiological link between obstructive lung diseases and IBD remains only partially understood and is widely discussed. The epithelial barriers of the respiratory and the gastrointestinal tract both consist of a highly vascularised luminal surface that is covered by a selective epithelial barrier and protecting superficial mucus layer. Underneath the epithelial surface, mucosa-associated lymphatic tissue [MALT] is present and regulates lymphocyte trafficking, antigen sampling, and mucosal host defence to protect from invading commensal bacteria and foreign pathogens.34–36
In our study, anti-inflammatory therapy with anti-TNF significantly improved obstructive abnormalities in IBD patients, indicating that the inflammatory response caused by pro-inflammatory cytokines may play an important role in the aetiopathogenesis of IBD-associated pulmonary involvement. This hypothesis is supported by studies in colitis models in rats. In 12 rats, a colitis was induced by either dextran sulphate sodium [DSS] or trinitrobenzene sulphonic acid [TNBS]. After induction of colitis, the colon and the lungs were assessed histopathologically with special focus on the role of vascular endothelial growth factor [VEGF] and TNF-α. Aydin et al. demonstrated an alveolar haemorrhage in the majority of rats with acute colitis, caused by significantly elevated concentrations of VEGF and TNF-α in pulmonary tissues in both the DSS and TNBS colitis groups compared with untreated control rats. These changes occurred simultaneously to the onset of inflammation in colonic tissue.15
In contrast, a multicentre, randomised, double-blind, placebo-controlled study assessing the effect of infliximab in patients with moderate to severe COPD subjects showed no treatment benefit as measured by the primary endpoint, the Chronic Respiratory Questionnaire total score. Similarly, no changes in secondary endpoints were reported, including prebronchodilator FEV1%, 6-min walk distance, transition dyspnoea index, or rate of moderate and severe exacerbations.37 These studies suggest that the observed pulmonary involvement in IBD may not be caused by a COPD-like disease but rather by a systemic inflammatory response. Our study results demonstrate for the first time that subclinical pulmonary involvement in IBD is responsive to anti-TNF therapy used to treat disease activity.
4.1. Limitations
Based on our data, we cannot exactly differentiate if the observed improvement of PFTs in patients with IBD is directly caused by the anti-inflammatory effects of anti-TNF on the pulmonary level or if these changes are due to a restored general condition of patients in response to therapy. These arguments need to be addressed by assessment of molecular changes in the bronchoalveolar fluid.
4.2. Conclusion
IBD patients with active disease present significant obstructive lung abnormalities in comparison with IBD patients in remission and healthy controls. Anti-inflammatory therapy with anti-TNF improves obstructive changes. Pulmonary obstruction and chronic bronchopulmonary inflammation may be overlooked in IBD patients and particularly in those with active disease. Further studies are necessary to determine whether and how pulmonary involvement should be treated.
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
The study was supported by internal funding, Medical Department I, University Medical Center Schleswig Holstein, Campus Kiel, Kiel, Germany.
Conflict ofInterests
NS, AK, SZ, SS report personal fees from Abbvie and MSD Sharp Dome GmbH for advisory work. All other authors have no conflict of interest to disclose.
Author contributions
ME: planning and conduct of the study, collecting and interpreting data, and drafting of the manuscript; JB: planning and conduct of the study, collecting and interpreting data, and drafting of the manuscript; JB: collecting and interpreting data and drafting of the manuscript; CC: collecting and interpreting data, drafting of the manuscript; RN: collecting and interpreting data, drafting of the manuscript; TB: collecting and interpreting data, drafting of the manuscript; SN: collecting and interpreting data, drafting of the manuscript; KA: collecting and interpreting data, drafting of the manuscript; SZ: collecting and interpreting data, drafting of the manuscript; SS: planning of the study, interpreting data,ww and drafting of the manuscript. All authors approved the final draft submitted.
References
- 1. Bernstein CN. Extraintestinal manifestations of inflammatory bowel disease. Curr Gastroenterol Rep 2001;3:477–83. [DOI] [PubMed] [Google Scholar]
- 2. Larsen S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis, and management. Ann Med 2010;42:97–114. [DOI] [PubMed] [Google Scholar]
- 3. Majewski S, Piotrowski W. Pulmonary manifestations of inflammatory bowel disease. Arch Med Sci 2015;11:1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao Y, Wang J, Liu Z, et al. Pulmonary dysfunction in 114 patients with inflammatory bowel disease. Medicine [Baltimore] 2017;96:e6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallaert B, Colombel J, Tonnel A, et al. Evidence of lymphocyte alveolitis in Crohn’s disease. Chest 1985;87:363–7. doi: 10.1378/chest.87.3.363. [DOI] [PubMed] [Google Scholar]
- 6. Herrlinger KR, Noftz MK, Dalhoff K, Ludwig D, Stange EF, Fellermann K. Alterations in pulmonary function in inflammatory bowel disease are frequent and persist during remission. Am J Gastroenterol 2002;97:377–81. doi: 10.1016/S0002-9270[01]04035-7. [DOI] [PubMed] [Google Scholar]
- 7. Duricova D, Pedersen N, Elkjaer M, Gamborg M, Munkholm P, Jess T. Overall and cause-specific mortality in Crohn’s disease: a meta-analysis of population-based studies. Inflamm Bowel Dis 2010;16:347–53. [DOI] [PubMed] [Google Scholar]
- 8. Jess T, Gamborg M, Munkholm P, Sørensen TI. Overall and cause-specific mortality in ulcerative colitis: meta-analysis of population-based inception cohort studies. Am J Gastroenterol 2007;102:609–17. [DOI] [PubMed] [Google Scholar]
- 9. Ekbom A, Brandt L, Granath F, Löfdahl CG, Egesten A. Increased risk of both ulcerative colitis and Crohn’s disease in a population suffering from COPD. Lung 2008;186:167–72. [DOI] [PubMed] [Google Scholar]
- 10. Ekbom A, Helmick CG, Zack M, Holmberg L, Adami HO. Survival and causes of death in patients with inflammatory bowel disease: a population-based study. Gastroenterology 1992;103:954–60. doi: 10.1016/0016-5085[92]90029-X. [DOI] [PubMed] [Google Scholar]
- 11. Storch I, Sachar D, Katz S. Pulmonary manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2003;9:104–15. [DOI] [PubMed] [Google Scholar]
- 12. Brown E, James K. The lung primordium an outpouching from the foregut! Evidence-based dogma or myth? J Pediatr Surg 2009;44:607–15. [DOI] [PubMed] [Google Scholar]
- 13. Billmyre KK, Hutson M, Klingensmith J. One shall become two: separation of the esophagus and trachea from the common foregut tube. Dev Dyn 2015;244:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pullman WE, Elsbury S, Kobayashi M, Hapel AJ, Doe WF. Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology 1992;102:529–37. [DOI] [PubMed] [Google Scholar]
- 15. Aydin B, Songur Y, Songur N, et al. Investigation of pulmonary involvement in inflammatory bowel disease in an experimental model of colitis. Korean J Intern Med 2016;31:853–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H, Liu JS, Peng SH, et al. Gut-lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J Gastroenterol 2013;19:6794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harvey RF, Jane Bradshaw M. Measuring Crohn’s disease activity. Lancet 1980;24:1134–5. doi: 10.1016/S0140-6736[80]91577-9. [DOI] [PubMed] [Google Scholar]
- 18. Higgins PDR, Schwartz M, Mapili J, Zimmermann EM. Is endoscopy necessary for the measurement of disease activity in ulcerative colitis? Am J Gastroenterol 2005;100(2):355–61. doi: 10.1111/j.1572-0241.2005.40641.x. [DOI] [PubMed] [Google Scholar]
- 19. Higgins PD, Schwartz M, Mapili J, Krokos I, Leung J, Zimmermann EM. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005;54(6):782–8; doi: 10.1136/gut.2004.056358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council [MRC] dyspnea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Criée CP, Sorichter S, Smith H, et al. Body plethysmography - its principles and clinical use. Respir. Med 2011;105:959–71. [DOI] [PubMed] [Google Scholar]
- 22. Vestbo J, Hurd S, Agusti A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65. [DOI] [PubMed] [Google Scholar]
- 23. Godet PG, Cowie R, Woodman RC, Sutherland LR. Pulmonary function abnormalities in patients with ulcerative colitis. Am J Gastroenterol 1997;92:1154–6. [PubMed] [Google Scholar]
- 24. Mohamed-Hussein AAR, Mohamed NAS, Ibrahim ME.. Changes in pulmonary function in patients with ulcerative colitis. Respir Med 2007; 101:977–82. doi: 10.1016/j.rmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 25. Yilmaz A, Demirci N, Hosgün D, et al. Pulmonary involvement in inflammatory bowel disease. World J. Gastroenterol 2010;17:4952–7. doi: 10.3748/wjg.v16.i39.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller MR, Hankinson J, Brusasco V, et al. ; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J 2005;26:319–38. [DOI] [PubMed] [Google Scholar]
- 27. Criée CP, Baur X, Berdel D, et al. Scientific guidelines for occupational medicine: guide to spirometry. ASU Int 2015;2015:200–15. doi: 10.17147/asui.2015-03-10-01. [DOI] [Google Scholar]
- 28. Desai D, Patil S, Udwadia Z, Maheshwari S, Abraham P, Joshi A. Pulmonary manifestations in inflammatory bowel disease: a prospective study. Indian J Gastroenterol 2011;30:225–8. [DOI] [PubMed] [Google Scholar]
- 29. Ateş F, Karincaoǧlu M, Hacievliyagil SS, Yalniz M, Seçkin Y. Alterations in the pulmonary function tests of inflammatory bowel diseases. Turkish J Gastroenterol 2011;22:293–299. doi: 10.4318/tjg.2011.0215. [DOI] [PubMed] [Google Scholar]
- 30. Neilly JB, Main AN, McSharry C, Murray J, Russell RI, Moran F. Pulmonary abnormalities in Crohn’s disease. Respir Med 1989;83:487–91. doi:10.1016/S0954-6111[89]80132–5. [DOI] [PubMed] [Google Scholar]
- 31. Wilcox P, Miller R, Miller G, et al. Airway involvement in ulcerative colitis. Chest 1987;92:18–22. [DOI] [PubMed] [Google Scholar]
- 32. Jess T, Loftus EV Jr, Harmsen WS, et al. Survival and cause specific mortality in patients with inflammatory bowel disease: a long term outcome study in Olmsted County, Minnesota, 1940-2004. Gut 2006;55:1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harbord M, Annese V, Vavricka SR, et al. ; European Crohn’s and Colitis Organisation. First European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2016;10:239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol 2012; 5:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kominsky DJ, Keely S, MacManus CF, et al. An endogenously anti-inflammatory role for methylation in mucosal inflammation identified through metabolite profiling. J Immunol 2011;186:6505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forchielli ML, Walker WA. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr 2005;93[Suppl 1]:S41–8. [DOI] [PubMed] [Google Scholar]
- 37. Rennard SI, Fogarty C, Kelsen S, et al. ; COPD Investigators. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:926–34. [DOI] [PubMed] [Google Scholar]